Serum Peroxiredoxins Reflect Oxidative Stress and Predict Renal Outcomes in Patients with Glomerulonephritis
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Baseline
4.2. Follow-Up
4.3. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Weisman, D.S.; Thavarajah, S.; Jaar, B.G. Prime time for chronic kidney disease. BMC Nephrol. 2023, 24, 295. [Google Scholar] [CrossRef] [PubMed]
- Kooman, J.P.; Kotanko, P.; Schols, A.M.; Shiels, P.G.; Stenvinkel, P. Chronic kidney disease and premature ageing. Nat. Rev. Nephrol. 2014, 10, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Mucha, K.; Foroncewicz, B.; Paczek, L. How to diagnose and follow patients with glomerulonephritis without kidney biopsy? Pol. Arch. Med. Wewn. 2016, 126, 471–473. [Google Scholar] [CrossRef][Green Version]
- Nihei, Y.; Suzuki, H.; Suzuki, Y. Current understanding of IgA antibodies in the pathogenesis of IgA nephropathy. Front. Immunol. 2023, 14, 1165394. [Google Scholar] [CrossRef] [PubMed]
- Truszewska, A.; Wirkowska, A.; Gala, K.; Truszewski, P.; Krzemień-Ojak, Ł.; Perkowska-Ptasińska, A.; Mucha, K.; Pączek, L.; Foroncewicz, B. Cell-free DNA profiling in patients with lupus nephritis. Lupus 2020, 29, 1759–1772. [Google Scholar] [CrossRef]
- Siegel, C.H.; Sammaritano, L.R. Systemic Lupus Erythematosus: A Review. JAMA 2024, 331, 1480–1491. [Google Scholar] [CrossRef]
- Lousa, I.; Reis, F.; Beirão, I.; Alves, R.; Belo, L.; Santos-Silva, A. New Potential Biomarkers for Chronic Kidney Disease Management—A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 43. [Google Scholar] [CrossRef]
- Krata, N.; Zagozdzon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Ther. Exp. 2018, 66, 211–220. [Google Scholar] [CrossRef]
- Moszczuk, B.; Krata, N.; Rudnicki, W.; Foroncewicz, B.; Cysewski, D.; Pączek, L.; Kaleta, B.; Mucha, K. Osteopontin-A Potential Biomarker for IgA Nephropathy: Machine Learning Application. Biomedicines 2022, 10, 734. [Google Scholar] [CrossRef]
- Kiryluk, K.; Sanchez-Rodriguez, E.; Zhou, X.J.; Zanoni, F.; Liu, L.; Mladkova, N.; Khan, A.; Marasa, M.; Zhang, J.Y.; Balderes, O.; et al. Genome-wide association analyses define pathogenic signaling pathways and prioritize drug targets for IgA nephropathy. Nat. Genet. 2023, 55, 1091–1105. [Google Scholar] [CrossRef]
- Pac, M.; Krata, N.; Moszczuk, B.; Wyczałkowska-Tomasik, A.; Kaleta, B.; Foroncewicz, B.; Rudnicki, W.; Pączek, L.; Mucha, K. NR3C1 Glucocorticoid Receptor Gene Polymorphisms Are Associated with Membranous and IgA Nephropathies. Cells 2021, 10, 3186. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Libetta, C.; Sepe, V.; Esposito, P.; Galli, F.; Dal Canton, A. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clin. Biochem. 2011, 44, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Tirichen, H.; Yaigoub, H.; Xu, W.; Wu, C.; Li, R.; Li, Y. Mitochondrial Reactive Oxygen Species and Their Contribution in Chronic Kidney Disease Progression Through Oxidative Stress. Front. Physiol. 2021, 12, 627837. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Canaud, B.; Eckardt, K.U.; Stenvinkel, P.; Wanner, C.; Zoccali, C. Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol. Dial. Transplant. 2003, 18, 1272–1280. [Google Scholar] [CrossRef]
- Perkins, A.; Nelson, K.J.; Parsonage, D.; Poole, L.B.; Karplus, P.A. Peroxiredoxins: Guardians against oxidative stress and modulators of peroxide signaling. Trends Biochem. Sci. 2015, 40, 435–445. [Google Scholar] [CrossRef]
- Rhee, S.G. Overview on Peroxiredoxin. Mol. Cells 2016, 39, 1–5. [Google Scholar] [CrossRef]
- Krata, N.; Foroncewicz, B.; Zagożdżon, R.; Moszczuk, B.; Zielenkiewicz, M.; Pączek, L.; Mucha, K. Peroxiredoxins as Markers of Oxidative Stress in IgA Nephropathy, Membranous Nephropathy and Lupus Nephritis. Arch. Immunol. Ther. Exp. 2021, 70, 3. [Google Scholar] [CrossRef]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Kochi, M.; Kohagura, K.; Shiohira, Y.; Iseki, K.; Ohya, Y. Chronic kidney disease, inflammation, and cardiovascular disease risk in rheumatoid arthritis. J. Cardiol. 2018, 71, 277–283. [Google Scholar] [CrossRef]
- Yan, Z.; Shao, T. Chronic Inflammation in Chronic Kidney Disease. Nephron 2024, 148, 143–151. [Google Scholar] [CrossRef]
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, D.M. Inflammation in chronic kidney disease: Role in the progression of renal and cardiovascular disease. Pediatr. Nephrol. 2009, 24, 1445–1452. [Google Scholar] [CrossRef]
- Martinez-Moral, M.P.; Kannan, K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ. Int. 2019, 123, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; Quiroga, B.; García de Vinuesa, S.; Verdalles, U.; Reque, J.; Panizo, N.; Arroyo, D.; Santos, A.; Macías, N.; Luño, J. Intraindividual interleukin-6 variations on the cardiovascular prognosis of patients with chronic renal disease. Ren. Fail. 2012, 34, 1002–1009. [Google Scholar] [CrossRef]
- Wei, L.; Mao, S.; Liu, X.; Zhu, C. Association of systemic inflammation response index with all-cause mortality as well as cardiovascular mortality in patients with chronic kidney disease. Front. Cardiovasc. Med. 2024, 11, 1363949. [Google Scholar] [CrossRef] [PubMed]
- Yoshitomi, R.; Nakayama, M.; Sakoh, T.; Fukui, A.; Katafuchi, E.; Seki, M.; Tsuda, S.; Nakano, T.; Tsuruya, K.; Kitazono, T. High neutrophil/lymphocyte ratio is associated with poor renal outcomes in Japanese patients with chronic kidney disease. Ren. Fail. 2019, 41, 238–243. [Google Scholar] [CrossRef]
- Kim, J.; Song, S.H.; Oh, T.R.; Suh, S.H.; Choi, H.S.; Kim, C.S.; Ma, S.K.; Kim, S.W.; Bae, E.H. Prognostic role of the neutrophil-to-lymphocyte ratio in patients with chronic kidney disease. Korean J. Intern. Med. 2023, 38, 725–733. [Google Scholar] [CrossRef]
- Kamińska, J.; Stopiński, M.; Mucha, K.; Jędrzejczak, A.; Gołębiowski, M.; Niewczas, M.A.; Pączek, L.; Foroncewicz, B. IL 6 but not TNF is linked to coronary artery calcification in patients with chronic kidney disease. Cytokine 2019, 120, 9–14. [Google Scholar] [CrossRef]
- Kamińska, J.; Stopiński, M.; Mucha, K.; Pac, M.; Gołębiowski, M.; Niewczas, M.A.; Pączek, L.; Foroncewicz, B. Circulating Osteoprotegerin in Chronic Kidney Disease and All-Cause Mortality. Int. J. Gen. Med. 2021, 14, 2413–2420. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhu, H.; Danelisen, I. Role of Peroxiredoxins in Protecting Against Cardiovascular and Related Disorders. Cardiovasc. Toxicol. 2020, 20, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Jung, I.A.; Kim, S.W. Peroxiredoxin 5 Acts as a Negative Regulator of the Sodium-Chloride Cotransporter Involved in Alleviating Angiotensin II-Induced Hypertension. Antioxidants 2025, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Tang, B.; Zhang, C. Signaling pathways of chronic kidney diseases, implications for therapeutics. Signal Transduct. Target. Ther. 2022, 7, 182. [Google Scholar] [CrossRef]
- Geertsema, S.; Geertsema, P.; Kieneker, L.M.; Abdulle, A.E.; la Bastide-van Gemert, S.; Bakker, S.J.L.; Dullaart, R.P.F.; Dijkstra, G.; Gansevoort, R.T.; Faber, K.N.; et al. Serum peroxiredoxin-4, a biomarker of oxidative stress, associates with new-onset chronic kidney disease: A population-based cohort study. Redox Biol. 2024, 77, 103408. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Lu, R.; Lv, X.; Lei, Q.; Tang, D.; Dai, Q.; Deng, Z.; Liao, X.; Tu, S.; et al. Peroxiredoxin 1 aggravates acute kidney injury by promoting inflammation through Mincle/Syk/NF-κB signaling. Kidney Int. 2023, 104, 305–323. [Google Scholar] [CrossRef]
- Michalek, R.D.; Crump, K.E.; Weant, A.E.; Hiltbold, E.M.; Juneau, D.G.; Moon, E.Y.; Yu, D.Y.; Poole, L.B.; Grayson, J.M. Peroxiredoxin II regulates effector and secondary memory CD8+ T cell responses. J. Virol. 2012, 86, 13629–13641. [Google Scholar] [CrossRef]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Soja, J.; Gancarz, M.; Wojtunik-Kulesza, K.; Markut-Miotła, E.; Oniszczuk, A. The efficacy of black chokeberry fruits against cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 6541. [Google Scholar] [CrossRef]
- Mulka-Gierek, M.; Krata, N.; Foroncewicz, B.; Pączek, L.; Mucha, K. The Different Patterns of Over-the-Counter Nonsteroidal Anti-Inflammatory Drugs or Analgesics Use in Patients with Chronic Kidney Disease and the General Population. Healthcare 2022, 10, 2035. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Glassock, R.J.; Nath, K.A.; Sethi, S.; Fervenza, F.C. A Proposal for a Serology-Based Approach to Membranous Nephropathy. J. Am. Soc. Nephrol. 2017, 28, 421–430. [Google Scholar] [CrossRef]
- Patel, M.; Clarke, A.M.; Bruce, I.N.; Symmons, D.P. The prevalence and incidence of biopsy-proven lupus nephritis in the UK: Evidence of an ethnic gradient. Arthritis Rheum. 2006, 54, 2963–2969. [Google Scholar] [CrossRef]
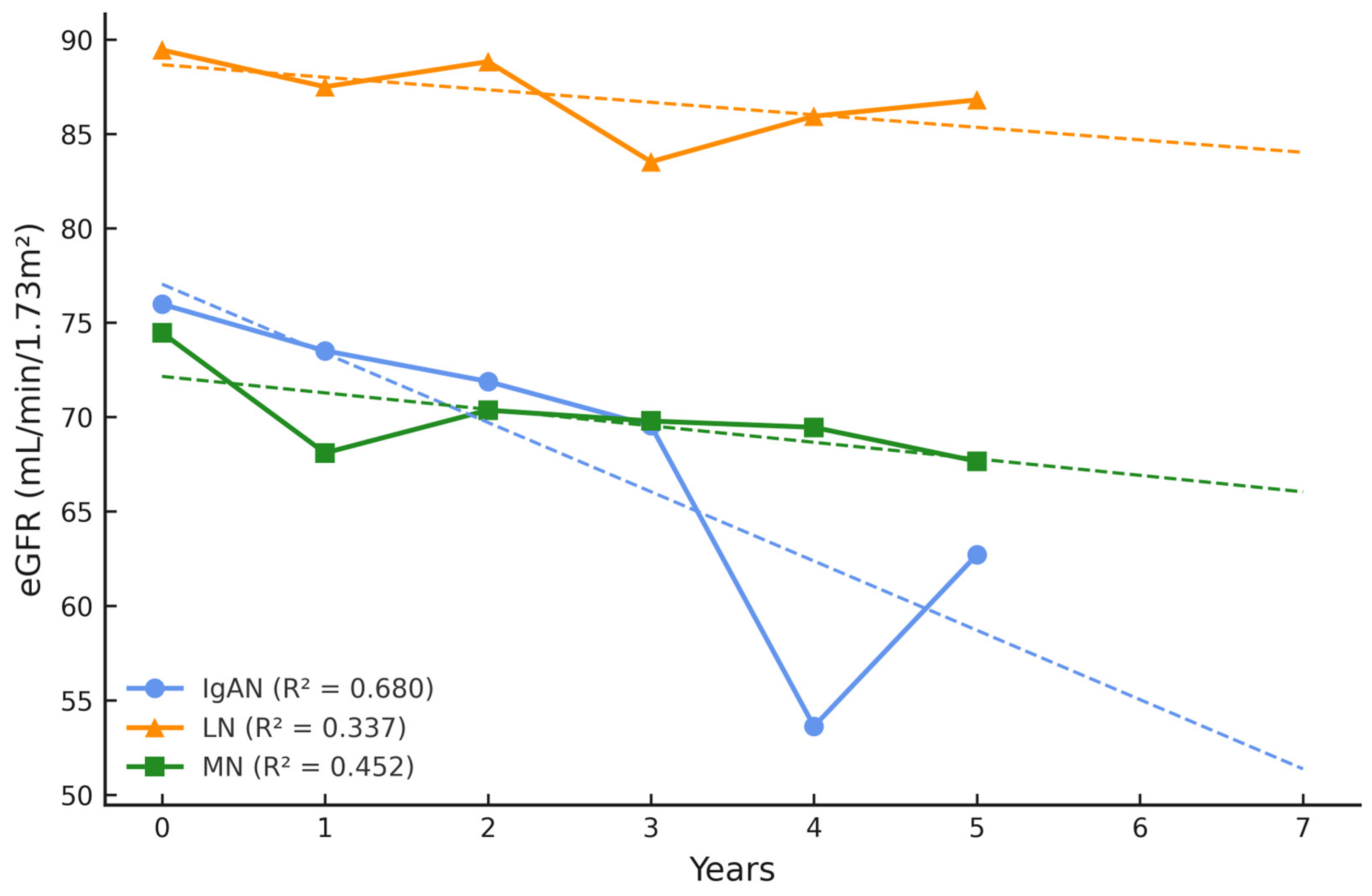
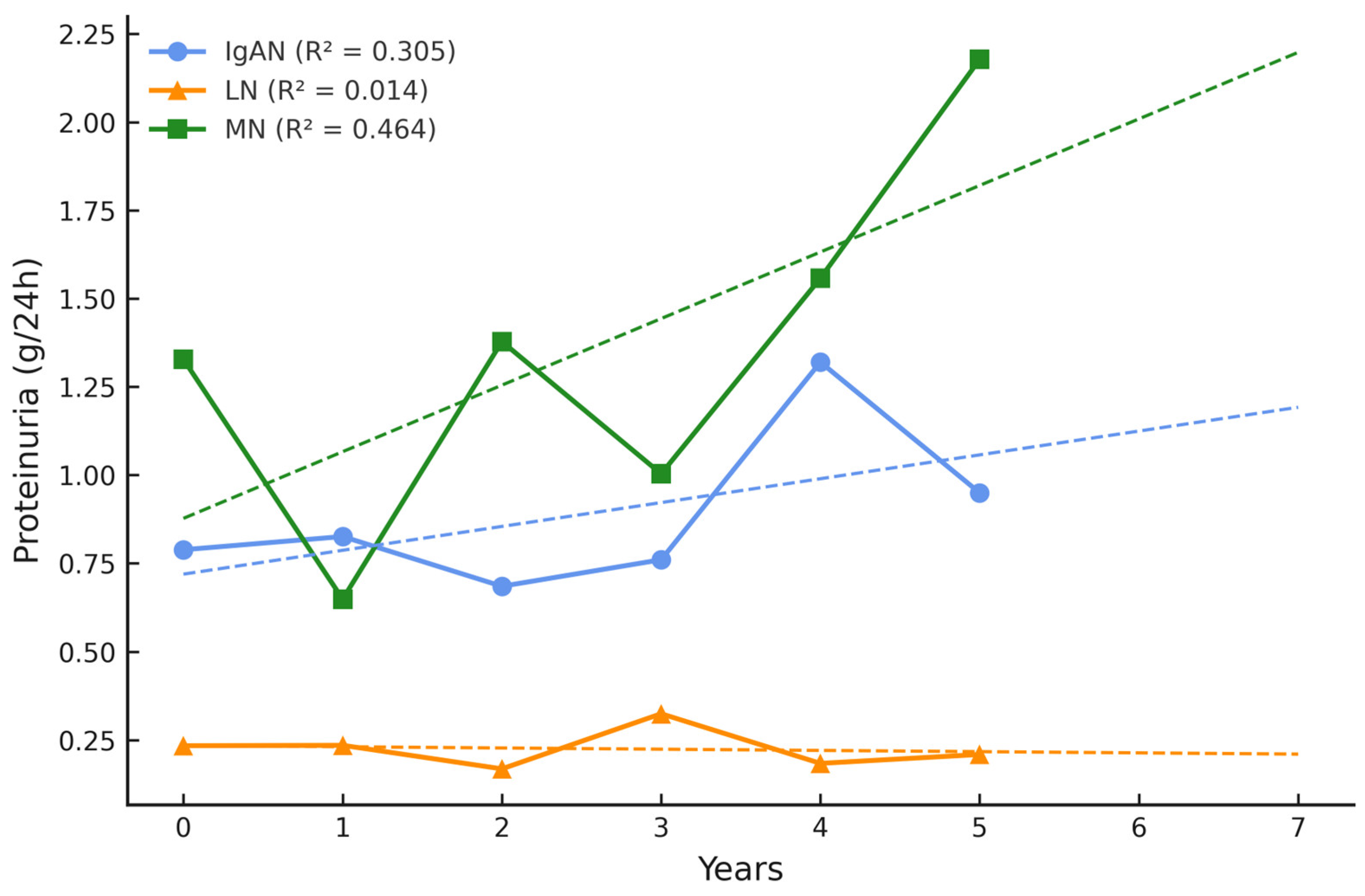

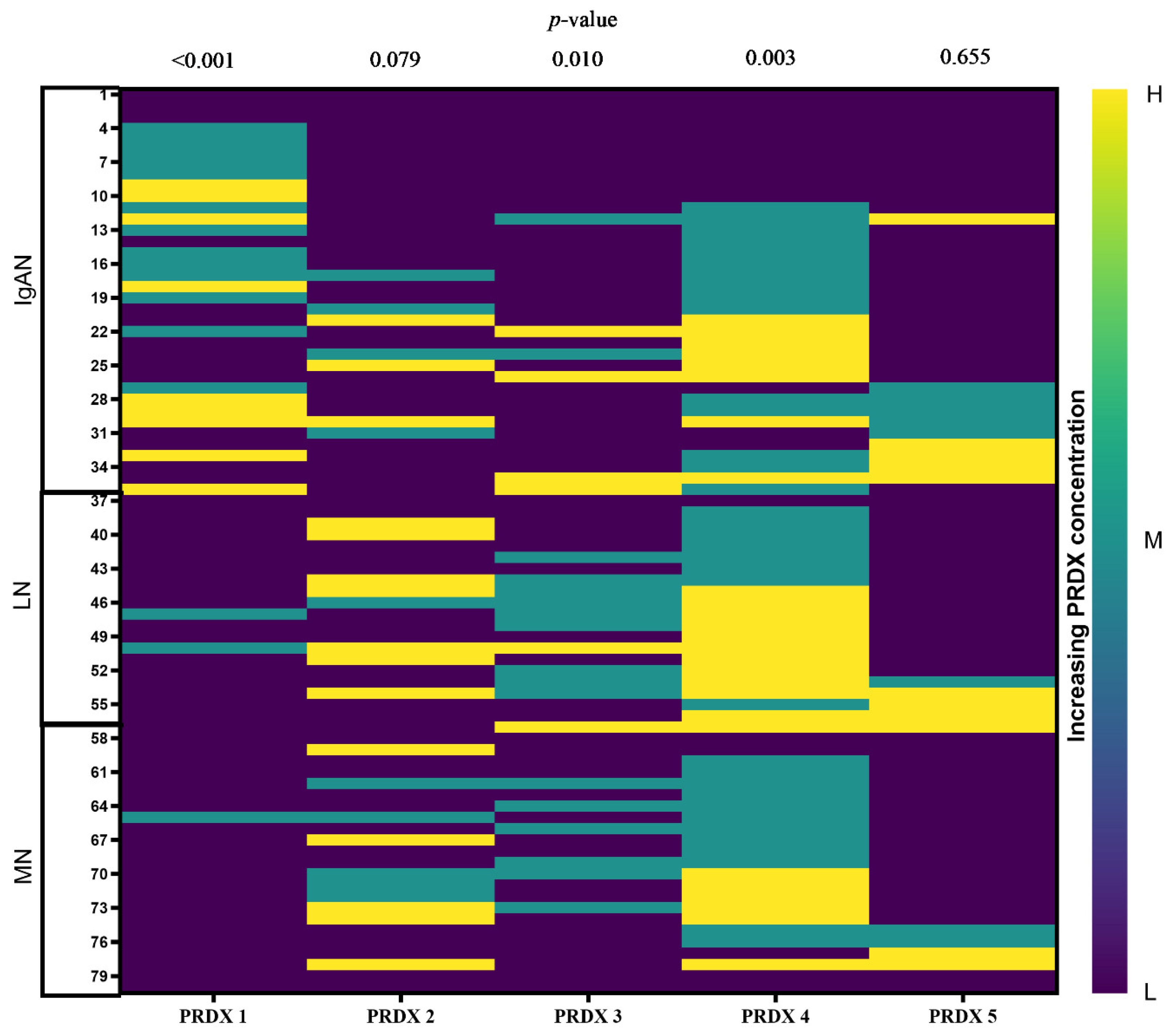
| GN, n = 80 | IgAN, n = 36 | LN, n = 21 | MN, n = 23 | p-Value |
|---|---|---|---|---|
| Gender (F/M) | 20/16 | 16/5 | 10/13 | 0.024 |
| Age, years | 47.11 (13.0) | 48.90 (12.1) | 58.96 (13.9) | 0.010 |
| Time from diagnosis, years | 15.6 (10.3) | 9.2 (4.4) | 16.1 (7.9) | 0.002 |
| Serum creatinine, mg/dL at baseline | 1.22 (0.6) | 0.88 (0.3) | 1.12 (0.5) | 0.079 |
| Serum creatinine, mg/dL at follow-up | 1.63 (1.2) | 0.90 (0.3) | 1.35 (1.1) | 0.005 |
| eGFR (mL/min/1.73 m2) at baseline | 75.98 (31.0) | 89.46 (28.7) | 74.46 (25.7) | 0.181 |
| eGFR (mL/min/1.73 m2) at follow-up | 62.71 (30.6) | 86.81 (25.8) | 67.65 (27.7) | 0.013 |
| Δ eGFR (mL/min/1.73 m2) | −13.27 ↓ | −2.65 ↓ | −6.81 ↓ | n.a. |
| eGFR slope (mean) | −0.21 | −0.02 | −0.07 | n.a. |
| 24 h urine protein (g/24 h) at baseline | 0.79 (0.6) | 0.23 (0.2) | 1.33 (2.2) | 0.305 |
| 24 h urine protein (g/24 h) at follow-up | 0.95 (0.99) | 0.21 (0.2) | 2.18 (2.8) | 0.760 |
| Δ 24 h urine protein (g/24 h) | 0.16 ↑ | −0.02 ↓ | 0.85 ↑ | n.a. |
| Medications, n | ||||
| ACEI | 29 | 16 | 15 | 0.410 |
| ARB | 8 | 3 | 11 | 0.029 |
| Immunosuppression | 3 | 15 | 12 | <0.001 |
| Steroids | 11 | 7 | 17 | 0.002 |
| Comorbidities, n | ||||
| Hypertension | 32 | 20 | 22 | 0.435 |
| Diabetes mellitus | 2 | 0 | 2 | 0.409 |
| Anemia | 1 | 6 | 1 | 0.040 |
| Atherosclerosis | 1 | 3 | 6 | 0.029 |
| Dyslipidemia | 23 | 7 | 21 | <0.001 |
| Parameter | Partial Regression Coefficient (β) | Standard Error (S.E.) | p-Value |
| Age | −0.216 | 0.116 | 0.067 |
| Gender | −0.198 | 0.101 | 0.055 |
| HT | −0.248 | 0.106 | 0.022 |
| Anemia | −0.049 | 0.102 | 0.633 |
| Atherosclerosis | −0.018 | 0.109 | 0.867 |
| Dyslipidemia | −0.224 | 0.103 | 0.034 |
| PRDX Level | PRDX 1 | PRDX 2 | PRDX 3 | PRDX 4 | PRDX 5 |
|---|---|---|---|---|---|
| Low | 0.043 | 0.001 | 0.036 | 0.184 | 0.007 |
| Medium | 0.441 | 0.870 | 0. 270 | 0.050 | n.a. |
| High | n.a. | 0.052 | 0.772 | 0.313 | 0.640 |
| PRDX Level | PRDX 1 | PRDX 2 | PRDX 3 | PRDX 4 | PRDX 5 |
|---|---|---|---|---|---|
| Low | 0.937 | 0.025 | 0.025 | 0.286 | 0.005 |
| Medium | n.a. | 0.342 | 0.670 | 0.010 | n.a. |
| High | n.a. | 0.019 | 0.319 | 0.227 | n.a. |
| Level | PRDX 1 (pg/mL) | PRDX 2 (ng/mL) | PRDX 3 (ng/mL) | PRDX 4 (pg/mL) | PRDX 5 (ng/mL) |
|---|---|---|---|---|---|
| Low | ≤15.6 | ≤0.156 | ≤0.312 | ≤78 | ≤0.78 |
| Medium | 15.7–30.46 | 0.157–0.94 | 0.312–0.55 | 78–376.18 | 0.78–2.04 |
| High | >30.46 | >0.94 | >0.55 | >376.18 | >2.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiewiórska-Krata, N.; Moszczuk, B.; Tańska, J.; Knioła, E.; Grywalska, E.; Pączek, L.; Foroncewicz, B.; Mucha, K. Serum Peroxiredoxins Reflect Oxidative Stress and Predict Renal Outcomes in Patients with Glomerulonephritis. Int. J. Mol. Sci. 2025, 26, 7708. https://doi.org/10.3390/ijms26167708
Wiewiórska-Krata N, Moszczuk B, Tańska J, Knioła E, Grywalska E, Pączek L, Foroncewicz B, Mucha K. Serum Peroxiredoxins Reflect Oxidative Stress and Predict Renal Outcomes in Patients with Glomerulonephritis. International Journal of Molecular Sciences. 2025; 26(16):7708. https://doi.org/10.3390/ijms26167708
Chicago/Turabian StyleWiewiórska-Krata, Natalia, Barbara Moszczuk, Julia Tańska, Emilia Knioła, Ewelina Grywalska, Leszek Pączek, Bartosz Foroncewicz, and Krzysztof Mucha. 2025. "Serum Peroxiredoxins Reflect Oxidative Stress and Predict Renal Outcomes in Patients with Glomerulonephritis" International Journal of Molecular Sciences 26, no. 16: 7708. https://doi.org/10.3390/ijms26167708
APA StyleWiewiórska-Krata, N., Moszczuk, B., Tańska, J., Knioła, E., Grywalska, E., Pączek, L., Foroncewicz, B., & Mucha, K. (2025). Serum Peroxiredoxins Reflect Oxidative Stress and Predict Renal Outcomes in Patients with Glomerulonephritis. International Journal of Molecular Sciences, 26(16), 7708. https://doi.org/10.3390/ijms26167708






