Isostrictiniin Alleviates LPS-Induced Acute Lung Injury via the Regulation of the Keap1-Nrf2/HO-1 and MAPK/NF-κB Signaling Pathways
Abstract
1. Introduction
2. Results
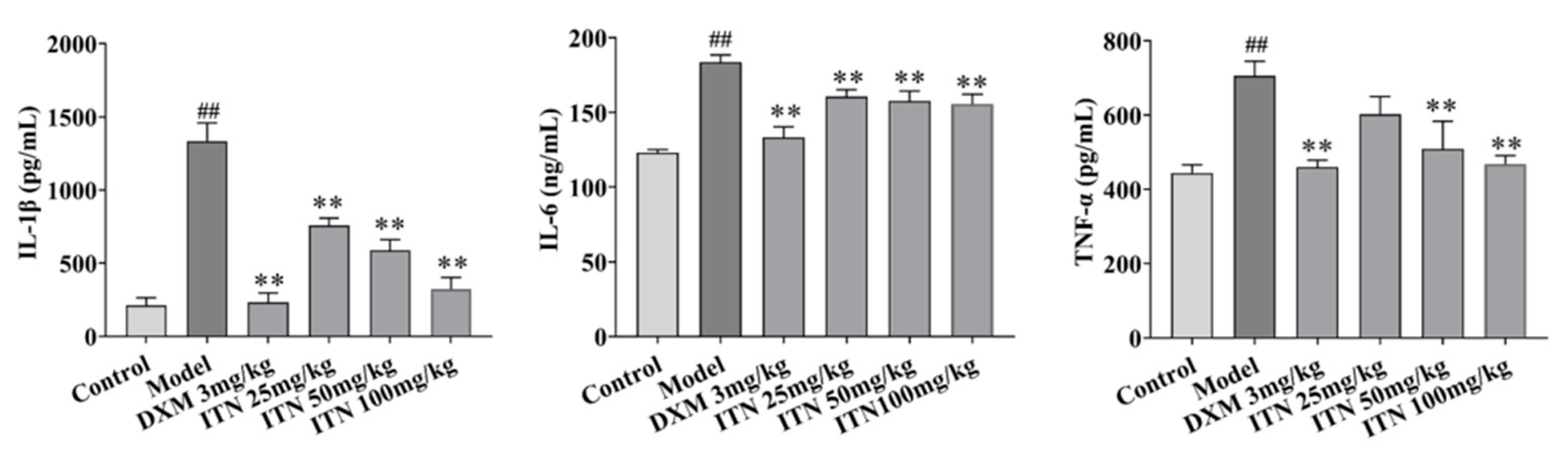
2.1. Impact of ITN on Inflammatory Responses in ALI Mice
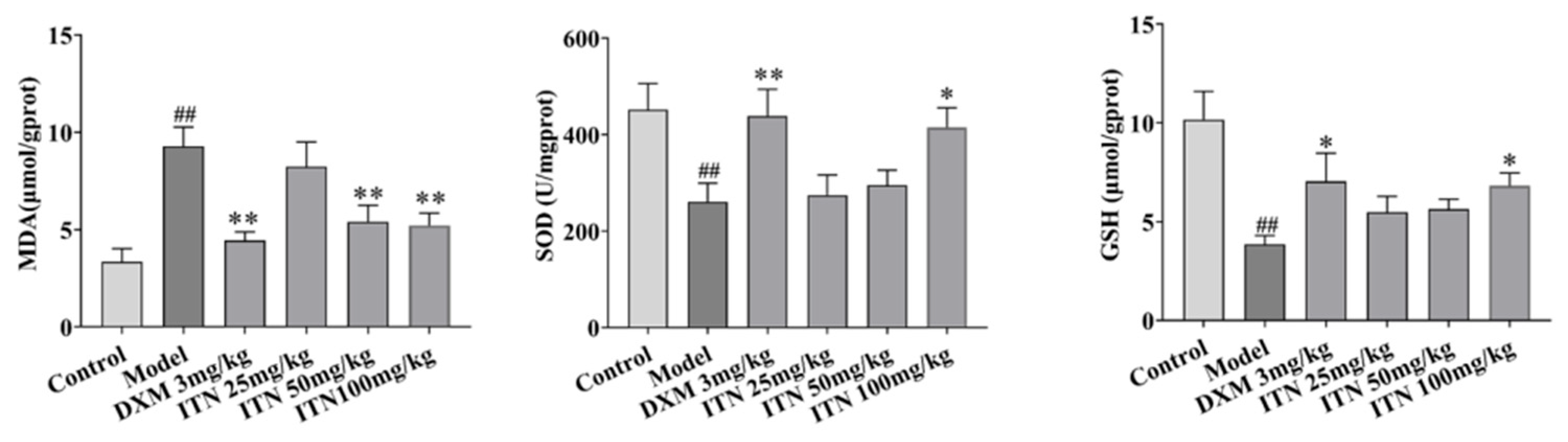
2.2. Effects of ITN on Lung Tissue MDA, SOD, and GSH in ALI Mice
2.3. Cell Viability
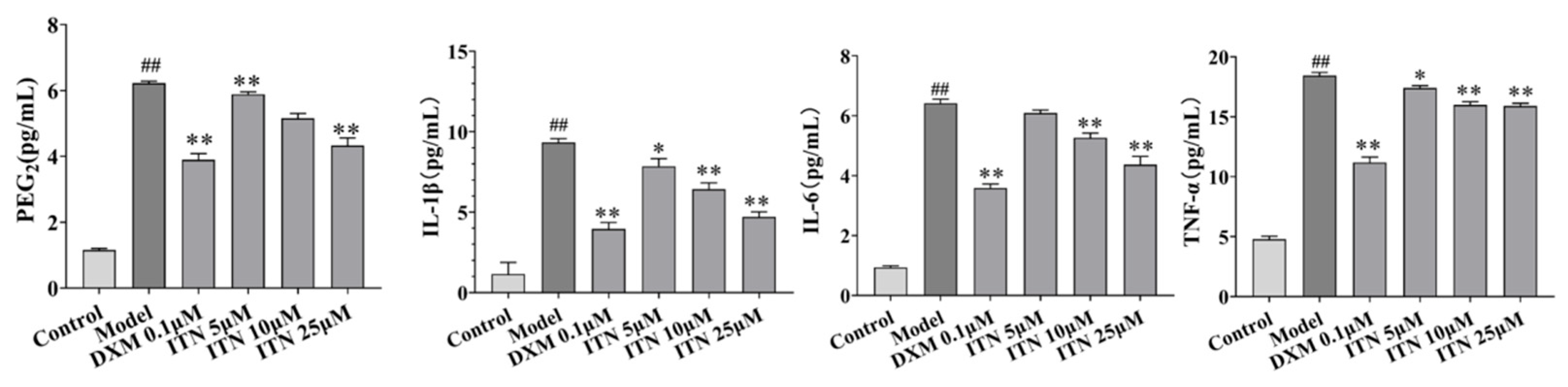
2.4. Effects of ITN on PGE2, IL-1β, IL-6, and TNF-α Levels in A549 Cells
2.5. Effects of ITN on Oxidative Stress in A549 Cells
2.6. Effects of ITN on Keap1-Nrf2/HO-1 Pathway in LPS-Induced A549 Cells
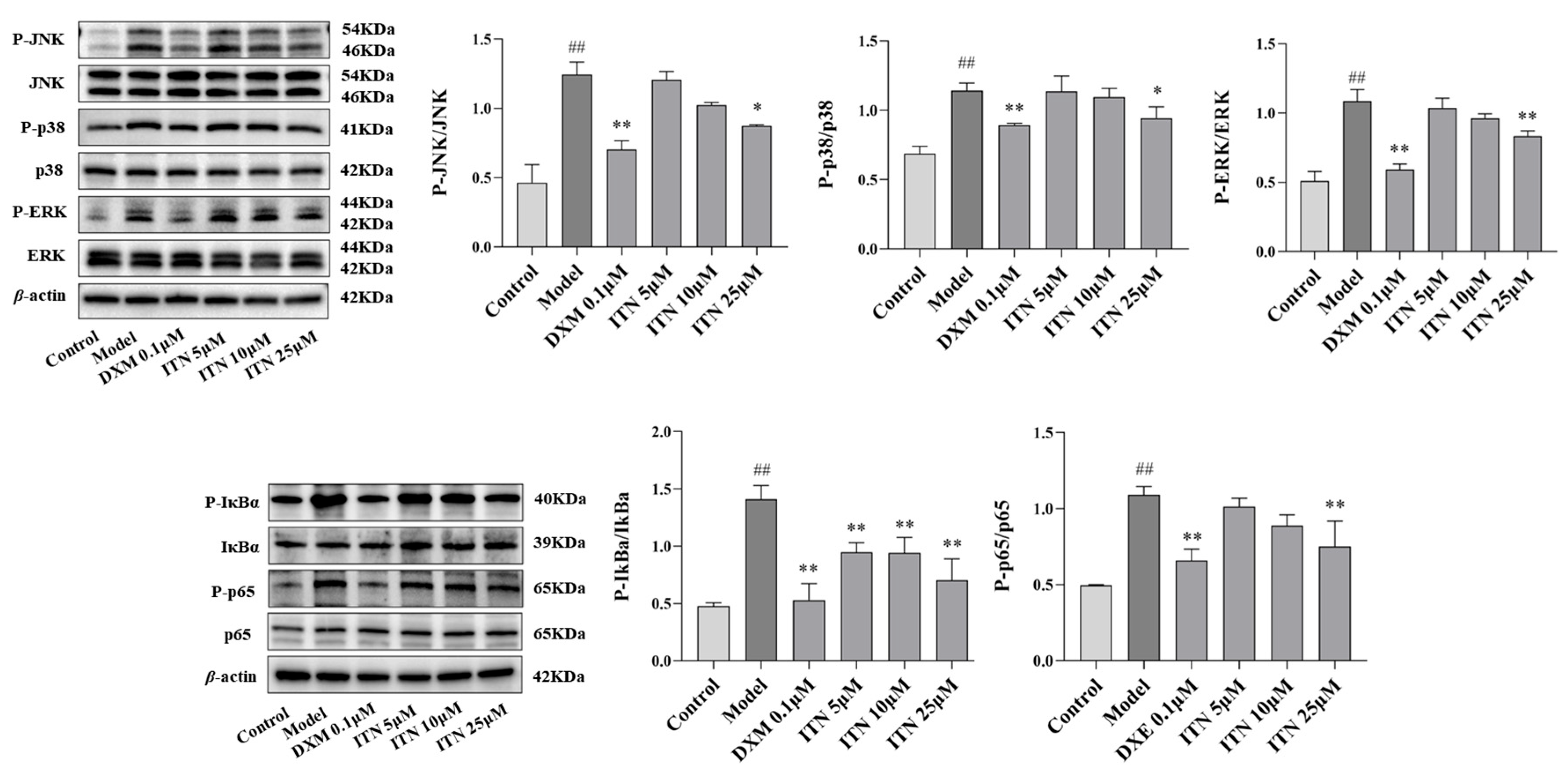
2.7. Effects of ITN on MAPK/NF-κB Pathway in LPS-Induced A549 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
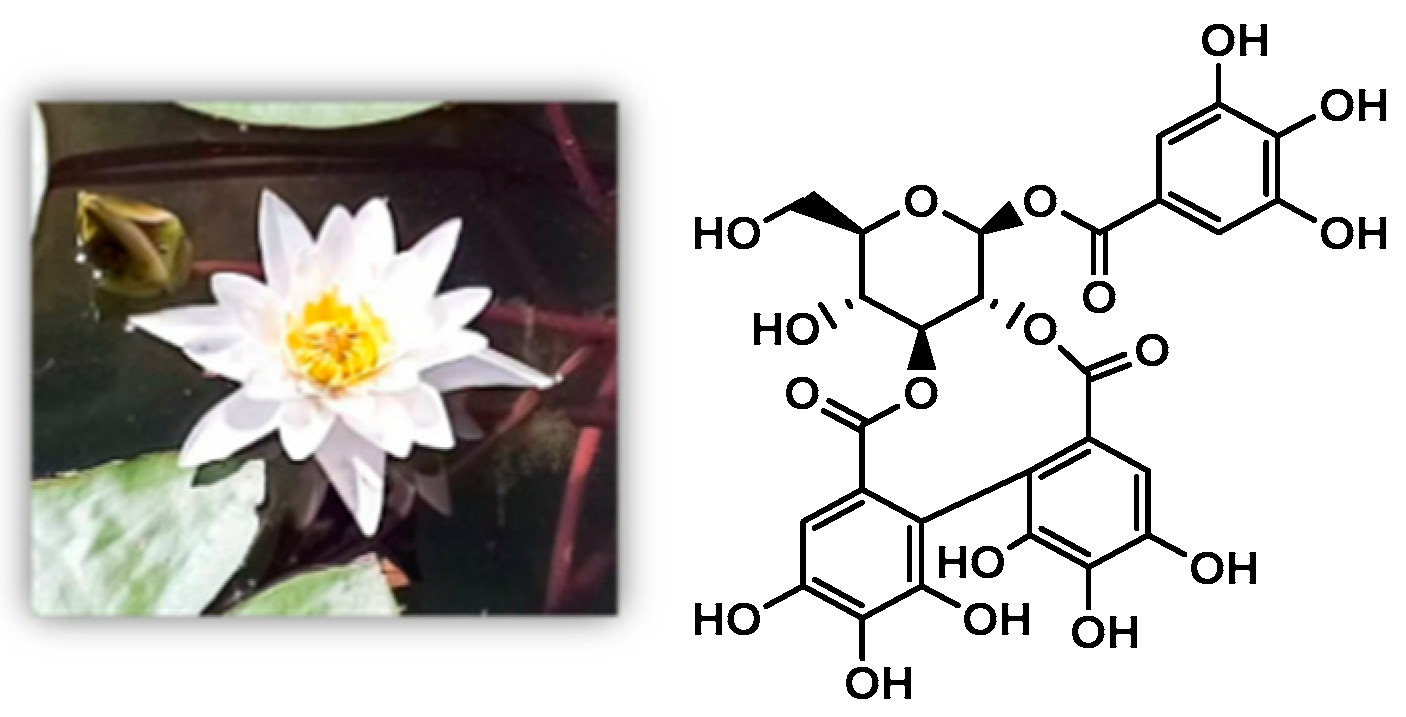
4.2. Plant Materials and Preparation of NCTP
4.3. Animal Experiment
4.3.1. Model Design
4.3.2. Lung Wet-to-Dry Ratio (W/D Ratio)
4.3.3. Cell Counts and Protein Contents of Bronchoalveolar Lavage Fluid (BALF) in Mice
4.3.4. Lung Tissue Pro-Inflammatory Cytokines and Oxidative Stress Indicators in Mice
4.3.5. Histopathological Analysis
4.4. Cell Experiments
4.4.1. Cell Culture and Treatment
4.4.2. CCK-8 Assay
4.4.3. Pro-Inflammatory Cytokines and Oxidative Stress Indicators in A549 Cells
4.4.4. Western Blot Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ITN | isostrictiniin |
| NCTP | polyphenol-enriched fraction from Nymphaea candida |
| ALI | acute lung injury |
| LPS | lipopolysaccharide |
| DXM | dexamethasone |
| BALF | bronchoalveolar lavage fluid |
| Neu | neutrophil |
| Lym | lymphocyte |
| WBC | while blood cell |
| MPO | myeloperoxidase |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| TNF-α | tumor necrosis factor-α |
| NO | nitric oxide |
| PGE2 | prostaglandin-2 |
| ROS | reactive oxygen species |
| MDA | malondialdehyde |
| SOD | superoxide dismutase |
| GSH | glutathione |
| GSSG | oxidized glutathione |
| ELISA | enzyme-linked immunosorbent assay |
| MAPK | mitogen-activated protein kinase |
| JNK | c-Jun N-terminal kinase |
| ERK | extracellular regulated protein kinases |
| NF-κB | nuclear factor kappa |
| IκBα | inhibitor of NF-κB |
| Keap1 | kelch-like ECH-associated protein 1 |
| HO-1 | heme oxygenase-1 |
| SEM | standard error of mean |
| ANOVA | analysis of variance |
References
- Yichen, L.I.; Huang, L.; Jilang, L.I.; Siyuan, L.I.; Jianzhen, L.V.; Zhong, G.; Ming, G.; Shilin, Y.; Shan, H.; Wenhui, H. Targeting TLR4 and regulating the Keap1/Nrf2 pathway with andrographolide to suppress inflammation and ferroptosis in LPS-induced acute lung injury. China J. Nat. Med. 2024, 22, 914–928. [Google Scholar] [CrossRef]
- Sun, B.; Lei, M.; Zhang, J.; Kang, H.; Liu, H.; Zhou, F. Acute lung injury caused by sepsis: How does it happen? Front. Med. 2023, 10, 1289194. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, K.; Xie, L. Progress in preclinical studies of macrophage autophagy in the regulation of ALI/ARDS. Front. Immunol. 2022, 13, 922702. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, K.; Xie, L. Advances in the use of exosomes for the treatment of ALI/ARDS. Front. Immunol. 2022, 13, 971189. [Google Scholar] [CrossRef]
- Nguyen, D.; Jeon, H.; Lee, J. Tissue factor links inflammation, thrombosis, and senescence in COVID-19. Sci. Rep. 2022, 12, 19842. [Google Scholar] [CrossRef]
- Liskova, A.; Samec, M.; Koklesova, L.; Samuel, S.M.; Zhai, K.; Al-Ishaq, R.K.; Abotaleb, M.; Nosal, V.; Kajo, K.; Ashrafizadeh, M. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed. Pharmacother. 2021, 138, 111430. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, J.; Lei, Y.; Chen, Z.; Wu, L.; Lin, C. Frontiers and hotspots evolution in cytokine storm: A bibliometric analysis from 2004 to 2022. Heliyon 2024, 10, e30955. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Xu, M.Y.; Deng, Z.H.; Zhao, Y.; Yang, M.M.; Liu, Y.Y.; Yuan, R.; Sun, Y.; Zhang, H.; et al. Regulation of inflammatory cytokine storms by mesenchymal stem cells. Front. Immunol. 2021, 12, 726909. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Ding, M.; Zhu, P.; Huang, H.L.; Zhuang, Q.; Shen, J.; Cai, Y.F.; Zhao, M.Y.; He, Q.N. New insights into the Nrf-2/HO-1 signaling axis and its application in pediatric respiratory diseases. Oxid. Med. Cell. Longev. 2019, 2019, 3214196. [Google Scholar] [CrossRef]
- Guo, J.; Jin, X.; Xue, H.; Zhang, L.; Zhang, Q.; LU, Q.; Guo, L.; Sun, Q. Effect of phillygenin on inflammatory response of A549 cells induced by lipopolysaccharide and normal human plasma. Chin. Pharm. Bull. 2023, 39, 503–511. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Liu, Z.L.; Zhang, Y.X.; Lin, H.J.; Zhang, Z.J. Lipoxin A4 Ameliorates Lipopolysaccharide-induced A549 cell Injury through upregulation of N-myc downstream- regulated gene-1. China Med. J. 2018, 131, 1342–1348. [Google Scholar] [CrossRef]
- Tosun, M.; Olmez, H.; Unver, E.; Arslan, Y.K.; Cimen, F.K.; Ozcicek, A.; Aktas, M.; Suleyman, H. Oxidative and pro-inflammatory lung injury induced by desflurane inhalation in rats and the protective effect of rutin. Adv. Clin. Exp. Med. 2021, 30, 941–948. [Google Scholar] [CrossRef]
- Wang, S.; Liu, J.Q.; Dong, J.; Fan, Z.Q.; Wang, F.G.; Wu, P.; Li, X.J.; Kou, R.R.; Chen, F. Allyl methyl trisulfide protected against LPS-induced acute lung injury in mice via inhibition of the NF-κB and MAPK pathways. Front. Pharmacol. 2022, 13, 919898. [Google Scholar] [CrossRef]
- Li, Y.Y.; Qin, S.Y.; Li, Q.; Song, S.J.; Xiao, W.; Yao, G.D. Jinzhen Oral Liquid alleviates lipopolysaccharide-induced acute lung injury through modulating TLR4/MyD88/NF-κB pathway. Phytomedicine 2023, 114, 154744. [Google Scholar] [CrossRef]
- Croasdell Lucchini, A.; Gachanja, N.N.; Rossi, A.G.; Dorward, D.A.; Lucas, C.D. Epithelial cells and inflammation in pulmonary wound repair. Cells 2021, 10, 339. [Google Scholar] [CrossRef]
- Li, D.H.; Yang, L.; Wang, W.; Song, C.K.; Xiong, R.; Pan, S.Z.; Li, N.; Geng, Q. Eriocitrin attenuates sepsis-induced acute lung injury in mice by regulating MKP1/MAPK pathway mediated-glycolysis. Int. Immunopharmacol. 2023, 118, 110021. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta-Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Ulasov, A.V.; Rosenkranz, A.A.; Georgiev, G.P.; Sobolev, A.S. Nrf2/Keap1/ARE signaling: Towards specific regulation. Life Sci. 2022, 291, 120111. [Google Scholar] [CrossRef] [PubMed]
- Kavianpour, M.; Saleh, M.; Verdi, J. The role of mesenchymal stromal cells in immune modulation of COVID-19: Focus on cytokine storm. Stem Cell Res. Ther. 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Belhaj, A.; Dewachter, L.; Kerbaul, F.; Brimioulle, S.; Dewachter, C.; Naeije, R.; Rondelet, B. Heme oxygenase-1 and inflammation in experimental right ventricular failure on prolonged overcirculation-induced pulmonary hypertension. PLoS ONE 2013, 8, e69470. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.; Lu, K.M.; Sun, F.L.; Tan, S.J.; Zhang, X.; Sheng, W.; Hao, W.M.; Liu, M.; Lv, W.H.; Han, W. Panaxydol attenuates ferroptosis against LPS-induced acute lung injury in mice by Keap1-Nrf2/HO-1 pathway. J. Transl. Med. 2021, 19, 96. [Google Scholar] [CrossRef]
- Wang, X.; Gai, Y.N.; Li, B.B.; Huang, L.L. Andalucin from Artemisia lannta suppresses the neuroinflammation via the promotion of Nrf2-mediated HO-1 levels by blocking the p65–p300 interaction in LPS-activated BV2 microglia. Phytomedicine 2018, 51, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, C.Y.; Li, J.F.; Yao, Y.H.; Zhao, J. Protective Effects of Isostrictiniin Against High-Fat, High-Sugar Diet-Induced Steatosis in MASLD Mice via Regulation of the AMPK/SREBP-1c/ACC Pathway. Nutrients 2024, 16, 3876. [Google Scholar] [CrossRef]
- Hong, H.X.; Gao, M.; Zhou, M.; Wang, A.; Hua, R.M.; Ma, Z.W.; Wang, Y.C.; Xu, Y.W.; Bai, Y.; Huang, G.D.; et al. Ethyl acetate extract of Nymphaea candida Presl: A potential anti-depressant and neuroprotective treatment strategy. Biomed Pharmacother. 2024, 179, 117304. [Google Scholar] [CrossRef]
- Sun, Y.; Qi, Z.Y.; Xu, Y.H.; Li, C.Y.; Zhao, J.; Liu, T. Anti-inflammatory, analgesic, antitussive and antipyretic activities of polyphenol-enriched fraction from Nymphaea candida. J. Ethnopharmacol. 2024, 324, 117789. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qi, X.; Xu, L.; Sun, Y.; Chen, Y.; Yao, Y.; Zhao, J. Preventive effect of the total polyphenols from Nymphaea candida on sepsis-induced acute lung injury in mice via gut microbiota and NLRP3, TLR-4/NF-κB pathway. Int. J. Mol. Sci. 2024, 25, 4276. [Google Scholar] [CrossRef]
- Qiao, X.Y.; Yin, J.; Zheng, Z.H.; Li, L.G.; Feng, X.J. Endothelial cell dynamics in sepsis-induced acute lung injury and acute respiratory distress syndrome: Pathogenesis and therapeutic implications. Cell Commun. Signal. 2024, 22, 241. [Google Scholar] [CrossRef]
- Ding, N.; Li, P.B.; Li, H.Q.; Lei, Y.L.; Zhang, Z.Z. The ROCK-ezrin signaling pathway mediates LPS-induced cytokine production in pulmonary alveolar epithelial cells. Cell Commun. Signal. 2022, 20, 65. [Google Scholar] [CrossRef]
- Xiong, L.W.; Liu, Y.; Wang, Y.; Zhao, H.W.; Song, X.C.; Fan, W.J.; Zhang, L.F.; Zhang, Y.Q. The protective effect of Lonicera japonica Thunb. against lipopolysaccharide-induced acute lung injury in mice: Modulation of inflammation, oxidative stress, and ferroptosis. J. Ethnopharmacol. 2024, 331, 118333. [Google Scholar] [CrossRef]
- Wang, F.; Fu, X.Z.; Wu, X.W.; Zhang, J.H.; Zhu, J.H.; Zou, Y.; Li, J.B. Bone marrow derived M2 macrophages protected against lipopolysaccharide-induced acute lung injury through inhibiting oxidative stress and inflammation by modulating neutrophils and T lymphocytes responses. Int. Immunopharmacol. 2018, 61, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Meng, M. Digitoflavone (DG) attenuates LPS-induced acute lung injury through reducing oxidative stress and inflammatory response dependent on the suppression of TXNIP/NLRP3 and NF-κB. Biomed. Pharmacother. 2017, 94, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Liu, C.C.; Li, Y.S.; Lee, P.Y.; Liu, P.L.; Wu, P.C.; Lin, T.C.; Chen, C.S.; Chiu, C.C.; Lai, Y.H. Punicalagin attenuates LPS-induced inflammation and ROS production in microglia by inhibiting the MAPK/NF-κB signaling pathway and NLRP3 inflammasome activation. J. Inflamm. Res. 2022, 15, 5347–5359. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.P.; Ji, F.P.; Yan, R.J.; Jiao, J.Z.; Wang, W.; Zhang, M.M.; Li, F.H.; Zhao, Y.Y.; Chang, Z.J.; Yan, S.G.; et al. Reyanning mixture inhibits M1 macrophage polarization through the glycogen synthesis pathway to improve lipopolysaccharide- induced acute lung injury. J. Ethnopharmacol. 2024, 328, 118005. [Google Scholar] [CrossRef]
- Vaka, R.; Khan, S.; Ye, B.; Risha, Y.; Parent, S.; Courtman, D.; Stewart, D.J.; Davis, D.R. Direct comparison of different therapeutic cell types susceptibility to inflammatory cytokines associated with COVID-19 acute lung injury. Stem. Cell Res. Ther. 2022, 13, 20. [Google Scholar] [CrossRef]
- Xiao, J.J.; Hou, F.; Wang, H.; Wang, R.X.; Liu, Y.; Wu, X.Y.; Xie, L.X. Monocyte-derived macrophages induce alveolar macrophages death via TNF-α in acute lung injury. Immun. Inflamm. Dis. 2024, 12, e70081. [Google Scholar] [CrossRef]
- Chen, I.C.; Wang, S.C.; Chen, Y.T.; Tseng, H.H.; Liu, P.L.; Lin, T.C.; Wu, H.E.; Chen, Y.R.; Tseng, Y.H.; Hsu, J.H.; et al. Corylin ameliorates LPS-induced acute lung injury via suppressing the MAPKs and IL-6/STAT3 signaling pathways. Pharmaceuticals 2021, 14, 1046. [Google Scholar] [CrossRef]
- Dai, S.Y.; Ji, J.; Li, R.R.; Gao, L.; He, X.Y. Stellate Ganglion block attenuates LPS-induced acute lung injury by activating Sirt3 regulation of oxidative stress and inflammation. Biomedicines 2024, 12, 1148. [Google Scholar] [CrossRef]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Sfriso, M.M.; Mazzatenta, A.; Macchi, V.; De Caro, R.; Di Giulio, C. Effects of hyperoxic exposure on signal transduction pathways in the lung. Respir. Physiol. Neurobiol. 2015, 209, 106–114. [Google Scholar] [CrossRef]
- Han, S.; Gao, H.W.; Chen, S.R.; Wang, Q.Q.; Li, X.X.; Du, L.J.; Li, J.; Luo, Y.Y.; Li, J.X.; Zhao, L.C.; et al. Procyanidin A1 alleviates inflammatory response induced by LPS through NF-κB, MAPK, and Nrf2/HO-1 pathways in RAW264. 7 cells. Sci. Rep. 2019, 9, 15087. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Huang, C.; Bian, E.; Lei, T.; Lv, X.J.; Li, J. NLRC5 negatively regulates inflammatory responses in LPS-induced acute lung injury through NF-κB and p38 MAPK signal pathways. Toxicol. Appl. Pharmacol. 2020, 5, 115150. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Derler, M.; Basílio, J.; Schmid, J.A. NF-κB in monocytes and macrophages–an inflammatory master regulator in multitalented immune cells. Front. Immunol. 2023, 14, 1134661. [Google Scholar] [CrossRef]
- Yang, H.H.; Lv, H.M.; Li, H.J.; Ci, X.X.; Peng, L.P. Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun. Signal. 2019, 17, 1–15. [Google Scholar] [CrossRef]
- Zhu, X.; Hua, E.S.; Tu, Q.F.; Liu, M.; Xu, L.Q.; Feng, J. Foxq1 promotes alveolar epithelial cell death through tle1-mediated Inhibition of the NF-κB signaling pathway. Am. J. Respir. Cell. Mol. Biol. 2024, 71, 53–65. [Google Scholar] [CrossRef]
- Sul, O.J.; Ra, S.W. Quercetin prevents LPS-induced oxidative stress and inflammation by modulating NOX2/ROS/NF-kB in lung epithelial cells. Molecules 2021, 26, 6949. [Google Scholar] [CrossRef]





| Group | Grades | Analysis | |||
|---|---|---|---|---|---|
| - | + | ++ | +++ | ||
| Control | 5 | 1 | 0 | 0 | 0.1667 ± 0.167 |
| Model | 0 | 0 | 4 | 2 | 2.3333 ± 0.211 ## |
| DXM, 3 mg/kg | 0 | 5 | 1 | 0 | 1.1667 ± 0.167 ** |
| ITN, 25 mg/kg | 0 | 4 | 2 | 0 | 1.3333 ± 0.211 ** |
| ITN, 50 mg/kg | 0 | 4 | 2 | 0 | 1.3333 ± 0.211 ** |
| ITN, 100 mg/kg | 0 | 5 | 1 | 0 | 1.1667 ± 0.167 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, W.; Sun, Y.; Tuohudaali, W.; Li, C.; Yao, Y.; Zhao, J. Isostrictiniin Alleviates LPS-Induced Acute Lung Injury via the Regulation of the Keap1-Nrf2/HO-1 and MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2025, 26, 5912. https://doi.org/10.3390/ijms26125912
Ding W, Sun Y, Tuohudaali W, Li C, Yao Y, Zhao J. Isostrictiniin Alleviates LPS-Induced Acute Lung Injury via the Regulation of the Keap1-Nrf2/HO-1 and MAPK/NF-κB Signaling Pathways. International Journal of Molecular Sciences. 2025; 26(12):5912. https://doi.org/10.3390/ijms26125912
Chicago/Turabian StyleDing, Wanting, Yuan Sun, Wulipan Tuohudaali, Chenyang Li, Yuhan Yao, and Jun Zhao. 2025. "Isostrictiniin Alleviates LPS-Induced Acute Lung Injury via the Regulation of the Keap1-Nrf2/HO-1 and MAPK/NF-κB Signaling Pathways" International Journal of Molecular Sciences 26, no. 12: 5912. https://doi.org/10.3390/ijms26125912
APA StyleDing, W., Sun, Y., Tuohudaali, W., Li, C., Yao, Y., & Zhao, J. (2025). Isostrictiniin Alleviates LPS-Induced Acute Lung Injury via the Regulation of the Keap1-Nrf2/HO-1 and MAPK/NF-κB Signaling Pathways. International Journal of Molecular Sciences, 26(12), 5912. https://doi.org/10.3390/ijms26125912






