Atomistic-Level Insights into the Role of Mutations in the Engineering of PET Hydrolases: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
- (i)
- examined the enzymatic breakdown of PET using proteins bioengineered through site-directed mutagenesis;
- (ii)
- combined experimental results and MD simulations;
- (iii)
- were written in English.
- (i)
- involved plastic polymer substrates other than PET;
- (ii)
- focused on microbial degradation, genomics, chemical catalysis, and photocatalysis;
- (iii)
- examined the weathering of plastics and toxicity;
- (iv)
- incorporated only experimental or only computational approaches;
- (v)
- included only other bioengineering strategies (e.g., protein fusion technology, cell surface display, etc.).
2.3. Information Sources and Search Strategy
- “PET” OR “polyethylene terephthalate” OR “poly(ethylene terephthalate)” AND;
- protein OR enzym* AND;
- biodegrad* OR degrad* OR depolymeriz* OR bioconversion AND;
- hydrol* OR cataly* AND;
- plastic.
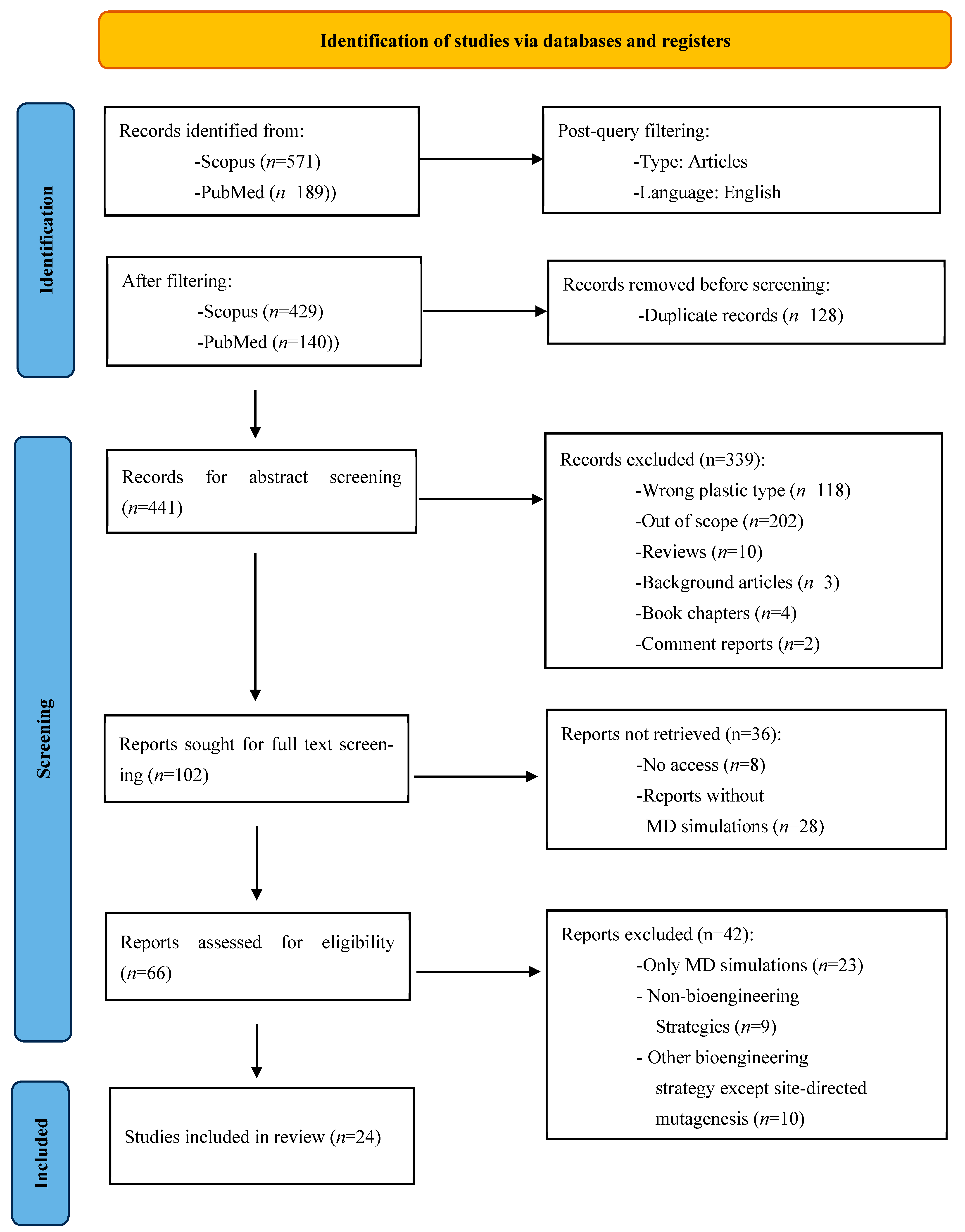
2.4. Screening and Selection Process
3. Results
3.1. Literature Search
- 8 articles were excluded due to inaccessible full texts;
- 28 articles employed computational methods that extended beyond the scope of MD simulations (e.g., Monte Carlo approaches, Markov models, quantum mechanics, or static molecular docking);
- 23 articles utilized MD simulations but lacked any accompanying experimental validation;
- 9 studies did not involve any protein engineering strategy;
- 10 studies applied alternative bioengineering techniques (e.g., protein fusion and surface display) not aligned with the site-directed mutagenesis criterion.
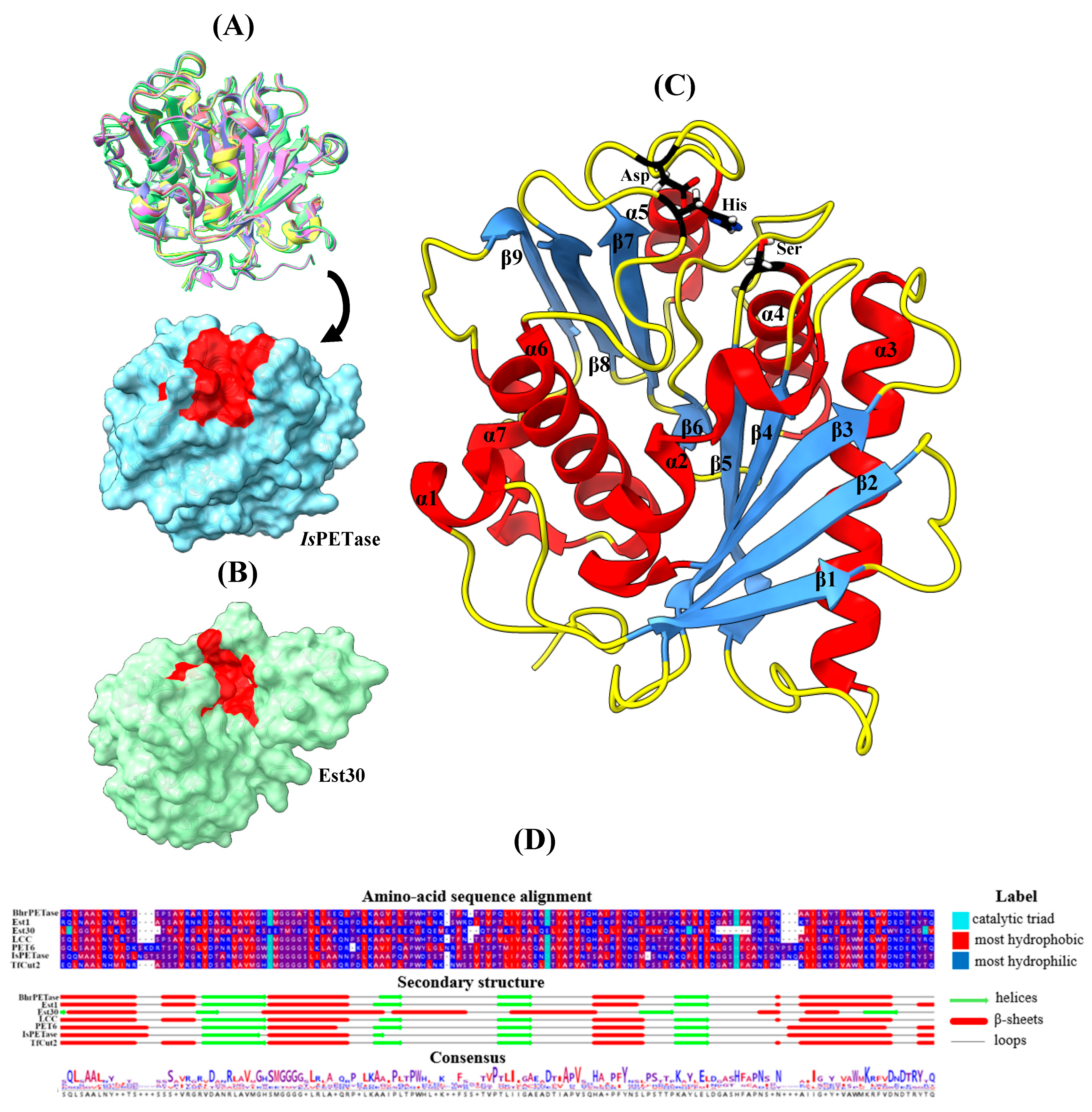
3.2. Characteristics of the Included Studies
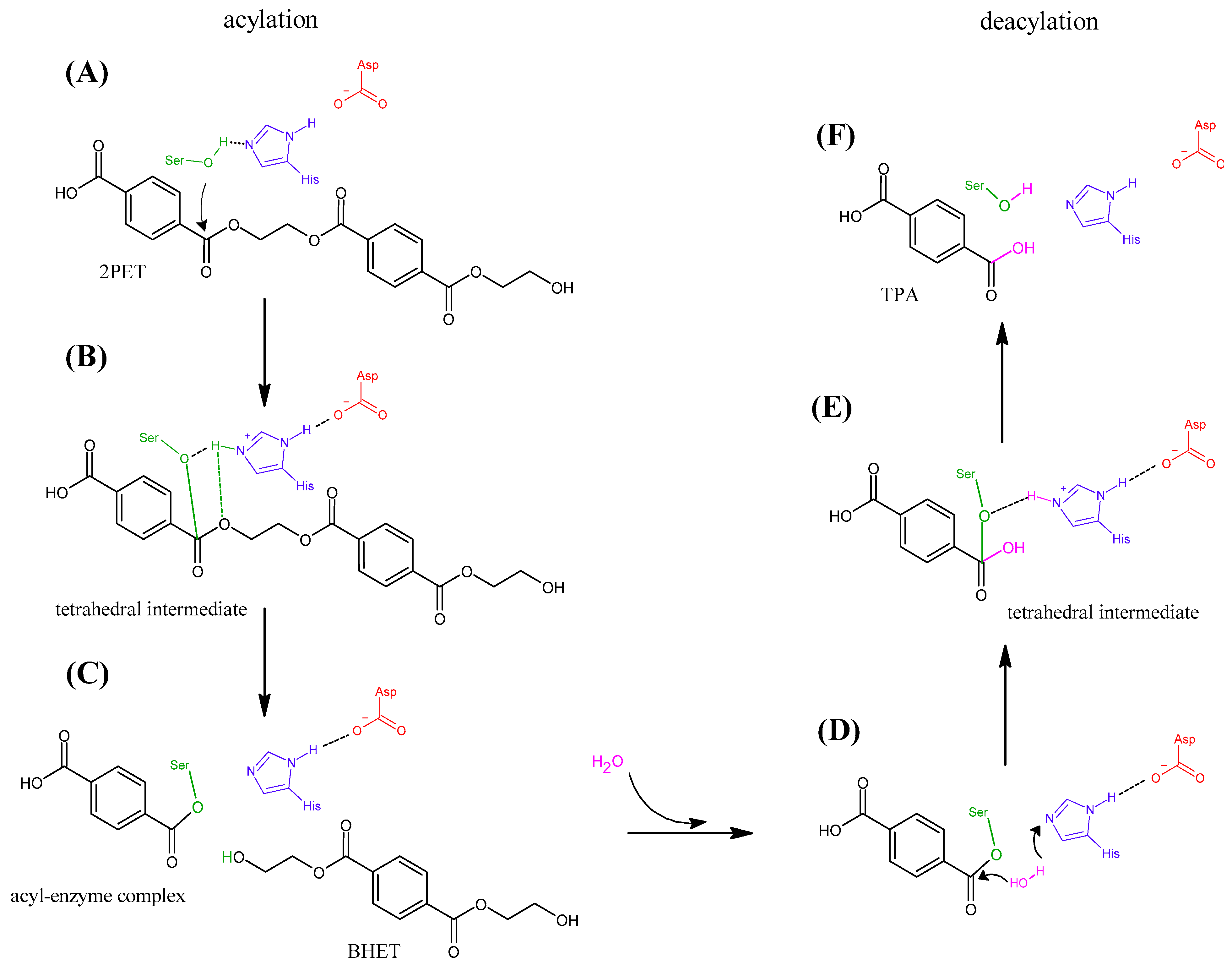
3.3. Catalytic Mechanism of PET Degradation
3.4. Insights into the Role of Mutations from MD Simulations
3.4.1. Improvement of Catalytic Activity
- A.
- Interactions between aromatic rings
- B.
- Modification of the hydrophobic pocket
- C.
- The Role of Glycine (Gly) and Phenylalanine (Phe) Substitutions in Catalytic Loops
3.4.2. Thermostability Improvement
- A.
- Formation of disulfide bonds
- B.
- Introduction of polar amino acids and the formation of salt bridges
- C.
- Hydrophobic residue mutations
- D.
- The role of Alanine (Ala) and Valine (Val)
- E.
- The role of Proline (Pro)
- F.
- The role of ions
4. Limitations and Future Work
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- PlasticsEurope. Plastics—The Fast Facts 2024. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2024/ (accessed on 24 July 2025).
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef]
- Duan, J.; Bolan, N.; Li, Y.; Ding, S.; Atugoda, T.; Vithanage, M.; Sarkar, B.; Tsang, D.C.W.; Kirkham, M.B. Weathering of microplastics and interaction with other coexisting constituents in terrestrial and aquatic environments. Water Res. 2021, 196, 117011. [Google Scholar] [CrossRef]
- Papavasiliou, C.; Mintis, D.G.; Tsoumanis, A.; Karaoli, A.; Lynch, I.; Krause, S.; Varsou, D.D.; Melagraki, G.; Kavousanakis, M.; Afantitis, A. MicroPlasticFate web application: Multimedia environmental fate modelling of microplastic particles via the enalos cloud platform. Bioresour. Technol. Rep. 2025, 30, 102157. [Google Scholar] [CrossRef]
- Blasing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Fu, D.; Qi, H.; Lan, C.Q.; Yu, H.; Ge, C. Micro-and nano-plastics in marine environment: Source, distribution and threats—A review. Sci. Total Environ. 2020, 698, 134254. [Google Scholar] [CrossRef] [PubMed]
- Chia, R.W.; Lee, J.-Y.; Kim, H.; Jang, J. Microplastic pollution in soil and groundwater: A review. Environ. Chem. Lett. 2021, 19, 4211–4224. [Google Scholar] [CrossRef]
- Yee, M.S.; Hii, L.W.; Looi, C.K.; Lim, W.M.; Wong, S.F.; Kok, Y.Y.; Tan, B.K.; Wong, C.Y.; Leong, C.O. Impact of Microplastics and Nanoplastics on Human Health. Nanomaterials 2021, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Manna, C.; Padha, S.; Verma, A.; Sharma, P.; Dhar, A.; Ghosh, A.; Bhattacharya, P. Micro (nano) plastics pollution and human health: How plastics can induce carcinogenesis to humans? Chemosphere 2022, 298, 134267. [Google Scholar] [CrossRef]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- North, E.J.; Halden, R.U. Plastics and environmental health: The road ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef]
- Lisk, D.J. Environmental implications of incineration of municipal solid waste and ash disposal. Sci. Total Environ. 1988, 74, 39–66. [Google Scholar] [CrossRef]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini-review. Clean. Eng. Technol. 2021, 2, 100083. [Google Scholar] [CrossRef]
- Zhang, Y.; Pedersen, J.N.; Eser, B.E.; Guo, Z. Biodegradation of polyethylene and polystyrene: From microbial deterioration to enzyme discovery. Biotechnol. Adv. 2022, 60, 107991. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.; Ahn, Y.R.; Kim, M.; Yu, S.; Kim, N.; Lim, S.Y.; Park, J.A.; Ha, S.J.; Lim, K.S.; et al. Recent advances in microbial and enzymatic engineering for the biodegradation of micro- and nanoplastics. RSC Adv. 2024, 14, 9943–9966. [Google Scholar] [CrossRef]
- Müller, R.J.; Schrader, H.; Profe, J.; Dresler, K.; Deckwer, W.D. Enzymatic degradation of poly (ethylene terephthalate): Rapid hydrolyse using a hydrolase from T. fusca. Macromol. Rapid Commun. 2005, 26, 1400–1405. [Google Scholar] [CrossRef]
- Son, H.F.; Cho, I.J.; Joo, S.; Seo, H.; Sagong, H.-Y.; Choi, S.Y.; Lee, S.Y.; Kim, K.-J. Rational protein engineering of thermo-stable PETase from Ideonella sakaiensis for highly efficient PET degradation. ACS Catal. 2019, 9, 3519–3526. [Google Scholar] [CrossRef]
- Nguyen, H.T.H.; Qi, P.; Rostagno, M.; Feteha, A.; Miller, S.A. The quest for high glass transition temperature bioplastics. J. Mater. Chem. A 2018, 6, 9298–9331. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Deng, B.; Yue, Y.; Yang, J.; Yang, M.; Xing, Q.; Peng, H.; Wang, F.; Li, M.; Ma, L.; Zhai, C. Improving the activity and thermostability of PETase from Ideonella sakaiensis through modulating its post-translational glycan modification. Commun. Biol. 2023, 6, 39. [Google Scholar] [CrossRef]
- Han, X.; Liu, W.; Huang, J.W.; Ma, J.; Zheng, Y.; Ko, T.P.; Xu, L.; Cheng, Y.S.; Chen, C.C.; Guo, R.T. Structural insight into catalytic mechanism of PET hydrolase. Nat. Commun. 2017, 8, 2106. [Google Scholar] [CrossRef]
- Chen, C.C.; Han, X.; Ko, T.P.; Liu, W.; Guo, R.T. Structural studies reveal the molecular mechanism of PET ase. FEBS J. 2018, 285, 3717–3723. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Liu, X.; Dong, S.; Tian, Y.e.; Qiao, Y.; Mitra, R.; Han, J.; Li, C.; Han, X.; et al. Computational Redesign of a PETase for Plastic Biodegradation under Ambient Condition by the GRAPE Strategy. ACS Catal. 2021, 11, 1340–1350. [Google Scholar] [CrossRef]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.L.; Smithson, R.; Kilbride, S.; Foster, J.; Hardy, F.J.; Ramachandran, S.; Tedstone, A.A.; Haigh, S.J.; Garforth, A.A.; Day, P.J.; et al. Directed evolution of an efficient and thermostable PET depolymerase. Nat. Catal. 2022, 5, 673–681. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, Y.; Sun, J.; Zhu, T.; Pang, H.; Li, C.; Geng, W.-C.; Wu, B. Computational redesign of a hydrolase for nearly complete PET depolymerization at industrially relevant high-solids loading. Nat. Commun. 2024, 15, 1417. [Google Scholar] [CrossRef] [PubMed]
- Sperger, T.; Sanhueza, I.A.; Schoenebeck, F. Computation and Experiment: A Powerful Combination to Understand and Predict Reactivities. Acc. Chem. Res. 2016, 49, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- da Costa, C.H.S.; Dos Santos, A.M.; Alves, C.N.; Marti, S.; Moliner, V.; Santana, K.; Lameira, J. Assessment of the PETase conformational changes induced by poly(ethylene terephthalate) binding. Proteins 2021, 89, 1340–1352. [Google Scholar] [CrossRef]
- Fecker, T.; Galaz-Davison, P.; Engelberger, F.; Narui, Y.; Sotomayor, M.; Parra, L.P.; Ramirez-Sarmiento, C.A. Active Site Flexibility as a Hallmark for Efficient PET Degradation by I. sakaiensis PETase. Biophys. J. 2018, 114, 1302–1312. [Google Scholar] [CrossRef]
- Jerves, C.; Neves, R.P.P.; Ramos, M.J.; da Silva, S.; Fernandes, P.A. Reaction Mechanism of the PET Degrading Enzyme PETase Studied with DFT/MM Molecular Dynamics Simulations. ACS Catal. 2021, 11, 11626–11638. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; da Costa, C.H.; Silva, P.H.; Skaf, M.S.; Lameira, J. Exploring the Reaction Mechanism of Polyethylene Terephthalate Biodegradation through QM/MM Approach. J. Phys. Chem. B 2024, 128, 7486–7499. [Google Scholar] [CrossRef]
- Joo, S.; Cho, I.J.; Seo, H.; Son, H.F.; Sagong, H.Y.; Shin, T.J.; Choi, S.Y.; Lee, S.Y.; Kim, K.J. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef]
- Waltmann, C.; Mills, C.E.; Wang, J.; Qiao, B.; Torkelson, J.M.; Tullman-Ercek, D.; Olvera de la Cruz, M. Functional enzyme-polymer complexes. Proc. Natl. Acad. Sci. USA 2022, 119, e2119509119. [Google Scholar] [CrossRef]
- Hollingsworth, S.A.; Dror, R.O. Molecular dynamics simulation for all. Neuron 2018, 99, 1129–1143. [Google Scholar] [CrossRef]
- Gao, R.; Pan, H.; Lian, J. Recent advances in the discovery, characterization, and engineering of poly(ethylene terephthalate) (PET) hydrolases. Enzym. Microb. Technol. 2021, 150, 109868. [Google Scholar] [CrossRef]
- Khairul Anuar, N.F.S.; Huyop, F.; Ur-Rehman, G.; Abdullah, F.; Normi, Y.M.; Sabullah, M.K.; Abdul Wahab, R. An overview into polyethylene terephthalate (PET) hydrolases and efforts in tailoring enzymes for improved plastic degradation. Int. J. Mol. Sci. 2022, 23, 12644. [Google Scholar] [CrossRef] [PubMed]
- Benavides Fernández, C.D.; Guzmán Castillo, M.P.; Quijano Pérez, S.A.; Carvajal Rodríguez, L.V. Microbial degradation of polyethylene terephthalate: A systematic review. SN Appl. Sci. 2022, 4, 263. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Zhang, L.; Wu, J.; Zeng, X.; Shi, X.; Liu, L.; Chen, J. A comprehensive review on enzymatic biodegradation of polyethylene terephthalate. Environ. Res. 2024, 240, 117427. [Google Scholar] [CrossRef] [PubMed]
- Mican, J.; Da’san MM, J.; Liu, W.; Weber, G.; Mazurenko, S.; Bornscheuer, U.T.; Damborsky, J.; Wei, R.; Bednar, D. Exploring new galaxies: Perspectives on the discovery of novel PET-degrading enzymes. Appl. Catal. B Environ. 2024, 342, 123404. [Google Scholar] [CrossRef]
- Liu, F.; Wang, T.; Yang, W.; Zhang, Y.; Gong, Y.; Fan, X.; Wang, G.; Lu, Z.; Wang, J. Current advances in the structural biology and molecular engineering of PETase. Front. Bioeng. Biotechnol. 2023, 11, 1263996. [Google Scholar] [CrossRef]
- Barclay, A.; Acharya, K.R. Engineering Plastic Eating Enzymes Using Structural Biology. Biomolecules 2023, 13, 1407. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- PubMed. National Library of Medicine, National Center for Biotechnology Information. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 2 September 2024).
- Scopus. Available online: https://www.scopus.com/home.uri (accessed on 2 September 2024).
- Gusenbauer, M.; Haddaway, N.R. Which academic search systems are suitable for systematic reviews or meta-analyses? Evaluating retrieval qualities of Google Scholar, PubMed, and 26 other resources. Res. Synth. Methods 2020, 11, 181–217. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- von Haugwitz, G.; Han, X.; Pfaff, L.; Li, Q.; Wei, H.; Gao, J.; Methling, K.; Ao, Y.; Brack, Y.; Mican, J.; et al. Structural insights into (Tere) phthalate-Ester hydrolysis by a carboxylesterase and its role in promoting PET depolymerization. ACS Catal. 2022, 12, 15259–15270. [Google Scholar] [CrossRef]
- Mrigwani, A.; Pitaliya, M.; Kaur, H.; Kasilingam, B.; Thakur, B.; Guptasarma, P. Rational mutagenesis of Thermobifida fusca cutinase to modulate the enzymatic degradation of polyethylene terephthalate. Biotechnol. Bioeng. 2023, 120, 674–686. [Google Scholar] [CrossRef]
- Richter, P.K.; Blazquez-Sanchez, P.; Zhao, Z.; Engelberger, F.; Wiebeler, C.; Kunze, G.; Frank, R.; Krinke, D.; Frezzotti, E.; Lihanova, Y.; et al. Structure and function of the metagenomic plastic-degrading polyester hydrolase PHL7 bound to its product. Nat. Commun. 2023, 14, 1905. [Google Scholar] [CrossRef]
- Avilan, L.; Lichtenstein, B.R.; König, G.; Zahn, M.; Allen, M.D.; Oliveira, L.; Clark, M.; Bemmer, V.; Graham, R.; Austin, H.P.; et al. Concentration-dependent inhibition of mesophilic PETases on poly (ethylene terephthalate) can be eliminated by enzyme engineering. ChemSusChem 2023, 16, e202202277. [Google Scholar] [CrossRef]
- Lu, D.; Chen, Y.; Jin, S.; Wu, Q.; Wu, J.; Liu, J.; Wang, F.; Deng, L.; Nie, K. The evolution of cutinase Est1 based on the clustering strategy and its application for commercial PET bottles degradation. J. Environ. Manag. 2024, 368, 122217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H.; Luo, Z.; Yang, Z.; Zhang, Z.; Wang, P.; Li, M.; Zhang, Y.; Feng, Y.; Lu, D.; et al. Computational design of highly efficient thermostable MHET hydrolases and dual enzyme system for PET recycling. Commun. Biol. 2023, 6, 1135. [Google Scholar] [CrossRef] [PubMed]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Xu, G.; Miao, R.; Wu, N.; Zhang, W.; Yao, B.; Guan, F.; Huang, H.; Tian, J. Rational redesign of thermophilic PET hydrolase LCCICCG to enhance hydrolysis of high crystallinity polyethylene terephthalates. J. Hazard. Mater. 2023, 453, 131386. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Q.; Liu, P.; Yuan, Y.; Dian, L.; Wang, Q.; Liang, Q.; Su, T.; Qi, Q. Dynamic docking-assisted engineering of hydrolases for efficient PET depolymerization. ACS Catal. 2024, 14, 3627–3639. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, J.; You, S.; Lin, W.; Su, R.; Qi, W. Efficient thermophilic PET hydrolase enhanced by cross correlation-based accumulated mutagenesis strategy. Bioresour. Technol. 2024, 406, 130929. [Google Scholar] [CrossRef] [PubMed]
- Weigert, S.; Perez-Garcia, P.; Gisdon, F.J.; Gagsteiger, A.; Schweinshaut, K.; Ullmann, G.M.; Chow, J.; Streit, W.R.; Hocker, B. Investigation of the halophilic PET hydrolase PET6 from Vibrio gazogenes. Protein Sci. 2022, 31, e4500. [Google Scholar] [CrossRef]
- Meng, X.; Yang, L.; Liu, H.; Li, Q.; Xu, G.; Zhang, Y.; Guan, F.; Zhang, Y.; Zhang, W.; Wu, N.; et al. Protein engineering of stable IsPETase for PET plastic degradation by Premuse. Int. J. Biol. Macromol. 2021, 180, 667–676. [Google Scholar] [CrossRef]
- Guo, B.; Vanga, S.R.; Lopez-Lorenzo, X.; Saenz-Mendez, P.; Ericsson, S.R.; Fang, Y.; Ye, X.; Schriever, K.; Backstrom, E.; Biundo, A.; et al. Conformational selection in biocatalytic plastic degradation by PETase. ACS Catal. 2022, 12, 3397–3409. [Google Scholar] [CrossRef]
- Yin, Q.; You, S.; Zhang, J.; Qi, W.; Su, R. Enhancement of the polyethylene terephthalate and mono-(2-hydroxyethyl) terephthalate degradation activity of Ideonella sakaiensis PETase by an electrostatic interaction-based strategy. Bioresour. Technol. 2022, 364, 128026. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.E.; Garcia, M.D.; Perez-Castillo, Y.; Armijos-Jaramillo, V.; Casado, S.; Vizuete, K.; Debut, A.; Cerda-Mejia, L. Degradation of PET Bottles by an Engineered Ideonella sakaiensis PETase. Polymers 2023, 15, 1779. [Google Scholar] [CrossRef]
- Qu, Z.; Chen, K.; Zhang, L.; Sun, Y. Computation-Based Design of Salt Bridges in PETase for Enhanced Thermostability and Performance for PET Degradation. ChemBioChem 2023, 24, e202300373. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Zhang, L.; Sun, Y. Molecular Insights into the Enhanced Activity and/or Thermostability of PET Hydrolase by D186 Mutations. Molecules 2024, 29, 1338. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Nina, M.R.H.; Zhang, X.; Huang, H.; Fan, D.; Bai, Y. Discovery and characterization of two novel polyethylene terephthalate hydrolases: One from a bacterium identified in human feces and one from the Streptomyces genus. J. Hazard. Mater. 2024, 472, 134532. [Google Scholar] [CrossRef] [PubMed]
- Joho, Y.; Royan, S.; Caputo, A.T.; Newton, S.; Peat, T.S.; Newman, J.; Jackson, C.; Ardevol, A. Enhancing PET Degrading Enzymes: A Combinatory Approach. ChemBioChem 2024, 25, e202400084. [Google Scholar] [CrossRef] [PubMed]
- Then, J.; Wei, R.; Oeser, T.; Barth, M.; Belisario-Ferrari, M.R.; Schmidt, J.; Zimmermann, W. Ca2+ and Mg2+ binding site engineering increases the degradation of polyethylene terephthalate films by polyester hydrolases from Thermobifida fusca. Biotechnol. J. 2015, 10, 592–598. [Google Scholar] [CrossRef]
- Chen, X.Q.; Guo, Z.Y.; Wang, L.; Yan, Z.F.; Jin, C.X.; Huang, Q.S.; Kong, D.M.; Rao, D.M.; Wu, J. Directional-path modification strategy enhances PET hydrolase catalysis of plastic degradation. J. Hazard. Mater. 2022, 433, 128816. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Li, Z.; Zhang, P.; Contreras, F.; Ji, Y.; Schwaneberg, U. Deep learning guided enzyme engineering of Thermobifida fusca cutinase for increased PET depolymerization. Chin. J. Catal. 2023, 50, 229–238. [Google Scholar] [CrossRef]
- Ding, K.; Levitskaya, Z.; Sana, B.; Pasula, R.R.; Kannan, S.; Adam, A.; Sundaravadanam, V.V.; Verma, C.; Lim, S.; Ghadessy, J.F. Modulation of PETase active site flexibility and activity on morphologically distinct polyethylene terephthalate substrates by surface charge engineering. Biochem. Eng. J. 2024, 209, 109420. [Google Scholar] [CrossRef]
- Long, J.Z.; Cravatt, B.F. The metabolic serine hydrolases and their functions in mammalian physiology and disease. Chem. Rev. 2011, 111, 6022–6063. [Google Scholar] [CrossRef]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Neitzel, J.J. Enzyme catalysis: The serine proteases. Nat. Educ. 2010, 3, 21. [Google Scholar]
- Radisky, E.S.; Lee, J.M.; Lu, C.-J.K.; Koshland, D.E., Jr. Insights into the serine protease mechanism from atomic resolution structures of trypsin reaction intermediates. Proc. Natl. Acad. Sci. USA 2006, 103, 6835–6840. [Google Scholar] [CrossRef] [PubMed]
- García-Meseguer, R.; Ortí, E.; Tuñón, I.; Ruiz-Pernía, J.J.; Aragó, J. Insights into the Enhancement of the Poly (ethylene terephthalate) Degradation by FAST-PETase from Computational Modeling. J. Am. Chem. Soc. 2023, 145, 19243–19255. [Google Scholar] [CrossRef]
- Wang, N.; Li, Y.; Zheng, M.; Dong, W.; Zhang, Q.; Wang, W. BhrPETase catalyzed polyethylene terephthalate depolymerization: A quantum mechanics/molecular mechanics approach. J. Hazard. Mater. 2024, 477, 135414. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, S.; Yamato, S.; Kanaya, E.; Kim, J.J.; Koga, Y.; Takano, K.; Kanaya, S. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. Appl. Environ. Microbiol. 2012, 78, 1556–1562. [Google Scholar] [CrossRef]
- Kitadokoro, K.; Thumarat, U.; Nakamura, R.; Nishimura, K.; Karatani, H.; Suzuki, H.; Kawai, F. Crystal structure of cutinase Est119 from Thermobifida alba AHK119 that can degrade modified polyethylene terephthalate at 1.76 Å resolution. Polym. Degrad. Stab. 2012, 97, 771–775. [Google Scholar] [CrossRef]
- Roth, C.; Wei, R.; Oeser, T.; Then, J.; Follner, C.; Zimmermann, W.; Strater, N. Structural and functional studies on a thermostable polyethylene terephthalate degrading hydrolase from Thermobifida fusca. Appl. Microbiol. Biotechnol. 2014, 98, 7815–7823. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.F.; Ewis, H.E.; Abdelal, A.T.; Lu, C.D.; Harrison, R.W.; Weber, I.T. Covalent reaction intermediate revealed in crystal structure of the Geobacillus stearothermophilus carboxylesterase Est30. J. Mol. Biol. 2004, 342, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.G.; Thompson, J.D.; Gibson, T.J. Using CLUSTAL for multiple sequence alignments. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1996; Volume 266, pp. 383–402. [Google Scholar]
- Clamp, M.; Cuff, J.; Searle, S.M.; Barton, G.J. The Jalview Java alignment editor. Bioinformatics 2004, 20, 426–427. [Google Scholar] [CrossRef]
- Peracchi, A. Enzyme catalysis: Removing chemically ‘essential’ residues by site-directed mutagenesis. Trends Biochem. Sci. 2001, 26, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, Y.; Lu, Z.; Shan, R.; Sun, D.; Li, J.; Li, P. Switchable enzyme mimics based on self-assembled peptides for polyethylene terephthalate degradation. J. Colloid Interface Sci. 2023, 646, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar]
- Sonnendecker, C.; Oeser, J.; Richter, P.K.; Hille, P.; Zhao, Z.; Fischer, C.; Lippold, H.; Blazquez-Sanchez, P.; Engelberger, F.; Ramirez-Sarmiento, C.A.; et al. Low Carbon Footprint Recycling of Post-Consumer PET Plastic with a Metagenomic Polyester Hydrolase. ChemSusChem 2022, 15, e202101062. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yan, Y.; Zhang, C.; Dalby, P.A. Two strategies to engineer flexible loops for improved enzyme thermostability. Sci. Rep. 2017, 7, 41212. [Google Scholar] [CrossRef]
- Qi, X.; Wu, Y.; Zhang, S.-T.; Yin, C.-F.; Ji, M.; Liu, Y.; Xu, Y.; Zhou, N.-Y. The unique salt bridge network in GlacPETase: A key to its stability. Appl. Environ. Microbiol. 2024, 90, e02242-23. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Suzuki, Y. Protein thermostabilization by proline substitutions. J. Mol. Catal. B Enzym. 1998, 4, 167–180. [Google Scholar] [CrossRef]
- Prajapati, R.S.; Das, M.; Sreeramulu, S.; Sirajuddin, M.; Srinivasan, S.; Krishnamurthy, V.; Ranjani, R.; Ramakrishnan, C.; Varadarajan, R. Thermodynamic effects of proline introduction on protein stability. Proteins 2007, 66, 480–491. [Google Scholar] [CrossRef]
- MacArthur, M.W.; Thornton, J.M. Influence of proline residues on protein conformation. J. Mol. Biol. 1991, 218, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Lemkul, J.A. Robustness in biomolecular simulations: Addressing challenges in data generation, analysis, and curation. Cell Rep. Phys. Sci. 2025, 6, 102566. [Google Scholar] [CrossRef]
- Yan, X.E.; Ayaz, P.; Zhu, S.J.; Zhao, P.; Liang, L.; Zhang, C.H.; Wu, Y.C.; Li, J.L.; Choi, H.G.; Huang, X.; et al. Structural Basis of AZD9291 Selectivity for EGFR T790M. J. Med. Chem. 2020, 63, 8502–8511. [Google Scholar] [CrossRef]
- Hassell, A.M.; An, G.; Bledsoe, R.K.; Bynum, J.M.; Carter, H.L., 3rd; Deng, S.J.; Gampe, R.T.; Grisard, T.E.; Madauss, K.P.; Nolte, R.T.; et al. Crystallization of protein-ligand complexes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Wienen-Schmidt, B.; Oebbeke, M.; Ngo, K.; Heine, A.; Klebe, G. Two methods, one goal: Structural differences between cocrystallization and crystal soaking to discover ligand binding poses. ChemMedChem 2021, 16, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Kan, Y.; He, L.; Luo, Y.; Bao, R. IsPETase is a novel biocatalyst for poly (ethylene terephthalate)(PET) hydrolysis. ChemBioChem 2021, 22, 1706–1716. [Google Scholar] [CrossRef] [PubMed]
- Doddam, L.S.; Cabeza de Vaca, I.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef]
| Enzyme | Organism | Mutation | Short-Name | Ref. |
|---|---|---|---|---|
| BhrPETase | Bacterium HR29 | - | BhrPETase_WT | [32] |
| His218Ser/Phe222Ile | BhrPETase_M2 | |||
| BhrPETase_M2/Asp238Lys | BhrPETase_M3 | |||
| BhrPETase_M3/Ala251Cys/Ala281Cys | BhrPETase_M5 | |||
| BhrPETase_M5/Ala209Arg | BhrPETase_M6 | |||
| BhrPETase_M6/Trp104Leu/Phe243Thr | TurboPETase | |||
| Est1 | Thermobifida alba AHK119 | - | Est1_WT | [57] |
| Asn213Met | Est1_N213M | |||
| Est1_N213M/Thr215Pro | Est1_N213M/T215P | |||
| Est1_N213M/T215P/Ser115Pro | Est1_MPP | |||
| Est1_MPP/Gln93Ala/Leu91Trp | Est1_5M | |||
| Est30 | Geobacillus stearothermophilus | - | Est30_WT | [58] |
| Gly130Leu | Est30_M1 | |||
| Ile171Lys | Est30_M2 | |||
| Est30_M2/Gly130Leu | Est30_M8 | |||
| Est30_M8/Met127Ser | Est30_M14 | |||
| LCC | Metagenomic | - | LCC_WT | [59] |
| Phe243Ile | LCC_Μ1 | |||
| Asp238Cys/Ser283Cys | LCC_M2 | |||
| LCC_Μ1/Asp238Cys/Ser283Cys | LCC_ICC | |||
| LCC_ICC/Tyr127Gly | LCC_ICCG | |||
| LCC_ICCG/Ser32Leu/Asp18Thr/Ser98Arg/ Thr157Pro/Glu173Gln/Asn213Pro | LCCICCG_I6M | [60] | ||
| LCC_ICCG/His218Tyr | LCC_ICCG_Μ1-H218Υ | [61] | ||
| LCC_ICCG_Μ1-H218Y/Asn248Asp | LCC-A2 | |||
| LCC-A2/Ser247Ala | LCC-A3 | |||
| LCC_ICCG/His183Tyr | LCC_ICCG_Μ1-H183Υ | [62] | ||
| LCC_ICCG_Μ1-H183Υ/Leu124Gly | LCC_ICCG_M2-L124G | |||
| LCC_ICCG_M2-L124G/Ser29Ala | LCC-YGA | |||
| PET6 | Vibrio gazogenes | - | PET6_WT | [63] |
| Val91Thr/Ser92Ala | PET6-VSTA | |||
| IsPETase | Ideonella sakaiensis 201-F6 | - | IsPETase_WT | [64] |
| Trp159His | IsPETase_M1a | |||
| Phe229Tyr | IsPETase_F229Y | |||
| IsPETase_M1a/Phe229Tyr | IsPETase_M2a | |||
| Ser238Ala | IsPETase_S238A | [65] | ||
| Tyr87Glu | IsPETase_Y87E | |||
| Ile139Arg | IsPETase_M1b | [66] | ||
| IsPETase_M1b/Asp157Glu | IsPETase_M2b | |||
| Ser92Lys | IsPETase_M1c | |||
| IsPETase_M1c/Arg251Ala | IsPETase_M2c | |||
| IsPETase_M1c/Asp157Glu | IsPETase_M2d | |||
| Ile208Val | IsPETase_I208V | [67] | ||
| Ser238Tyr Asn212Ala | IsPETase_S238Y IsPETase_S238Y | |||
| Ile168Arg/Ser188Asp | IsPETase_M2e | [68] | ||
| Ile168Arg/Ser188Glu | IsPETase_M2f | |||
| Asp186Val | IsPETase_D186V | [69] | ||
| Asp186Ala | IsPETase_D186A | |||
| Asp186Asn | IsPETase_D186N | |||
| Asp186His | IsPETase_D186H | |||
| PpPETase | Pseudomonas paralcaligenes MRCP1333 | - | PpPETase_WT | [70] |
| Tyr239Arg | PpPETase_M1 | |||
| PpPETase_M1/Phe244Gly | PpPETase_M2 | |||
| PpPETase_M2/Tyr250Gly | PpPETase_M3 | |||
| PsPETase | Piscinibacter sakaiensis | - | PsPETase_WT | [71] |
| Asp186Ala | PETaseA | |||
| PETaseA/Asn233Cys/Ser282Cys | PETaseACC | |||
| PETaseACC/Ala179Cys/Ser136Glu/Ser214Thr | PETaseACCET | |||
| PETaseACCET/Lys95Asn | PETaseACCETN (Combi-PETase) | |||
| ScPETase | Streptomyces calvus DSM 41452 | - | ScPETase_WT | [70] |
| Ala212Cys/Thr249Cys | ScPETase_M2 | |||
| ScPETase_M1/Asn195His | ScPETase_M3 | |||
| ScPETase_M2/Asn243Lys | ScPETase_M4 | |||
| TfCut2 | Thermobifida fusca KW3 | - | TfCut2_WT | [72] |
| Ca2+/Mg2+ | TfCut2_cations | |||
| His184Ser/Phe209Ile | TfCut2_M2a | [73] | ||
| TfCut2_M2a/Gln92Gly | TfCut2_M3a | |||
| TfCut2_M3a/Ile213Lys | TfCut2_4Mz | |||
| Leu32Glu/Ser113Glu | TfCut2_M2b | [74] | ||
| TfCut2_M2b/Thr237Gln | TfCut2_M3b | |||
| V3 PETase | Engineered | Gly119Gln/Asn233Cys/Ser238Trp/Ser282Cys | V3 PETase_WT | [75] |
| Lys95Ala | V3 PETase_M1 | |||
| V3 PETase_M1/Arg132Asn | V3 PETase_M2 | |||
| V3 PETase_M2/Arg280Ala | V3 PETase_M3 |
| Enzyme | Mutants | Substrate | Conditions | Degradation Result | Ref. | ||
|---|---|---|---|---|---|---|---|
| BhrPETase | His218Ser/Phe222Ile/Ala209Arg/ Asp238Lys/Ala251Cys/ Ala281Cys/Trp104Leu/ Phe243Thr (TurboPETase) | −12 °C | 84 °C | Pre-treated PET flakes (11.1% cryst.) | 65 °C, pH 8.0, 8 h | 98.2% depolymerization | [32] |
| Est1 | Asn213Met/Thr215Pro/Ser115Pro/ Gln93Ala/Leu91Trp (Est_5M) | __ | __ | Used PET plastic waste (9.1% cryst.) | 65 °C, pH 8.0, 72 h | 65-fold improvement, 90% degradation | [57] |
| Est30 | Ile171Lys/Met127Ser/Gly130Leu | −10.89 °C | 63.18 °C | MHET | 50 °C, pH 7.5, 10 min | 96.3-fold increase in catalytic efficiency | [58] |
| LCC | Tyr127Gly/Asp238Cys/Phe243Ile/ Ser283Cys (LCC_ICCG) | +9.3 °C | 94.0 °C | Used PET plastic waste | 72 °C, pH 8.0, 10 h | >90% depolymerization | [59] |
| LCC_ICCG | Ser32Leu/Asp18Thr/Ser98Arg/ Thr157Pro/Glu173Gln/Asn213Pro (LCCICCG_I6M) | +1.04 °C | 96.13 °C | PET bottle powder (31.30% cryst.) | 80 °C, pH 8.0, 24 h | 264% increase in soluble products (3.64-fold increase) | [60] |
| His218Tyr/Asn248Asp (LCC-A2) His218Tyr/Asn248Asp/Ser247Ala (LCC-A3) | +1.11 °C +0.61 °C | 95.25 °C 94.75 °C | Amorphous PET powder (cryst. 8.17%) | 72 °C, pH 8.0, 6 h | 40.54% increase in product release 39.46% increase in product release | [61] | |
| His183Tyr/Leu124Gly/Ser29Ala (LCC-YGA) | __ | __ | Amorphous PET films (cryst. 8%) | 70 °C, pH 8.0, 5 h | 107% enhancement in hydrolytic activity (2.1-fold improvement) | [62] | |
| PET6 | Val91Thr/Ser92Ala (PET6-VSTA) | −0.5 to −1 °C | (56.7–57.2) °C | Used PET plastic waste (cryst. 10%) | 50 °C, pH 8.5, 1M NaCl | 63% increase in product release | [63] |
| IsPETase | Trp159His/Phe229Tyr | +10.4 °C | 61.2 °C | Amorphous PET | 40 °C, pH 9.5, 24 h | 40-fold increase in total product concentration | [64] |
| Ser238Ala Tyr87Glu | +0 °C +12 °C | 42 °C 54 °C | PET film | 30 °C, pH 7.2, 72 h | ~55% increase in product release No product release | [65] | |
| Ser92Lys/Arg251Ala Ile139Arg | +5.42 °C +8.71 °C | 53.08 °C 56.37 °C | PET film (22.3% crystallinity) | 40 °C, pH 9.0, 24 h | 2.9-fold increase in degradation activity 3.6-fold increase in degradation activity | [66] | |
| Ile208Val Ser238Tyr Asn212Ala | __ | __ | PET film (30.88% cryst.) | 30 °C, pH 9.4, 72 h | No effect on degradation activity 3.3-fold increase in degradation activity 1.4-fold increase in degradation activity. | [67] | |
| Ile168Arg/Ser188Asp Ile168Arg/Ser188Glu | +7.4 °C +8.7 °C | 50.2 °C 51.5 °C | Amorphous PET film (8.1% cryst.) | 40 °C, pH 9.0, 6 d | 3.8-fold increase in product concentration 4.3-fold increase in product concentration | [68] | |
| Asp186Val Asp186Ala Asp186Asn Asp186His | +12.91 °C +11.84 °C +8.89 °C +8.76 °C | 59.49 °C 58.42 °C 55.47 °C 55.34 °C | PET film (28.34% cryst.) | 40 °C, pH 9.0, 6 d | 2.49-fold increase in degradation activity 3.62-fold increase in degradation activity 3.69-fold increase in degradation activity 3.43-fold increase in degradation activity | [69] | |
| PpPETase | Tyr239Arg/Phe244Gly/Tyr250Gly | __ | __ | PET powder | 30 °C, pH 7.0, 24 h | 3.1-fold increase in product release | [70] |
| PsPETase | Asp186Ala/Asn233Cys/Ser282Cys/ Ala179Cys/Ser136Glu/ Ser214Thr/Lys95Asn (Combi-PETase) | +27.2 °C | 70.4 °C | PET particles bottle (45% cryst.) | 50 °C, pH 9.0, 28 h | 4.25-fold increase in degradation activity | [71] |
| ScPETase | Ala212Cys/Thr249Cys/Asn195His/ Asn243Lys | __ | __ | PET powder | 30 °C, pH 7.0, 24 h | 1.9-fold increase in product release | [70] |
| TfCut2 | His184Ser/Gln92Gly/Phe209Ile/ Ile213Lys (4Mz) | __ | __ | Amorphous PET film | 60 °C, pH 8.0, 96 h | 90% degradation rate, 30-fold improvement in catalytic efficiency | [73] |
| Leu32Glu/Ser113Glu/Thr237Gln | −0.6 °C | 72.2 °C | PET powder (>40% cryst.) | 65 °C, pH 8.5, 48 h | 5.3-fold depolymerization improvement | [74] | |
| V3 PETase | Lys95Ala/Arg132Asn/Arg280Ala | +3.5 °C | 61.25 °C | Pre-treated PET bottle (9% cryst.) | 40 °C, pH 8.0, 72 h | 3-fold improvement in product release, 100% degradation | [75] |
| Enzyme | Mutants | RMSF | Hydrogen Bonds | Catalytic Distance | Binding Affinity | Impact a | Ref. |
|---|---|---|---|---|---|---|---|
| BhrPETase | His218Ser/Phe222Ile/ Ala209Arg/Asp238Lys/ Ala251Cys/Ala281Cys/ Trp104Leu/Phe243Thr (TurboPETase) | Increased at β7-α5 and β8-α6 loop | __ | Ser-PET decreased from 4.88 Å to 4.15 Å | __ | Activity | [32] |
| Est1 | Asn213Met/Thr215Pro/ Ser115Pro/Gln93Ala/ Leu91Trp (Est_5M) | Decreased at β4-α3, α3-b5, and β8-α6 loops | __ | __ | __ | Activity | [57] |
| Est30 | Ile171Lys/Met127Ser/ Gly130Leu | __ | __ | Ser-PET < 3.0 Å increased from 11.66% to 34.45% | __ | Activity | [58] |
| LCC | Tyr127Gly/Asp238Cys/ Phe243Ile/Ser283Cys (LCC_ICCG) | __ | Increased from 15.2% to 90% of the simulation time between catalytic residues | Ser-His decreased from ~4 Å to 2.8 Å | __ | Activity | [59] |
| LCCICCG | Ser32Leu/Asp18Thr/ Ser98Arg/Thr157Pro/ Glu173Gln/Asn213Pro (LCCICCG_I6M) | Decreased at β8-α6 loop Increased at β7-α5 loop | __ | __ | __ | Stability Activity | [60] |
| His218Tyr/Asn248Asp (LCC-A2) His218Tyr/Asn248Asp/ Ser247Ala (LCC-A3) | __ | Number of bonds between protein–PET increased from 2.33 to 3.76 (LCC-A2) and 4.79 (LCC-A3) | __ | __ | Activity | [61] | |
| His183Tyr/Leu124Gly/ Ser29Ala (LCC-YGA) | Increased at β1-β2 loop and β5 strand | __ | __ | __ | Activity | [62] | |
| PET6 | Val91Thr/Ser92Ala (PET6-VSTA) | __ | __ | His-PET contact frequency increased from 18% to 64% | __ | Activity | [63] |
| IsPETase | Trp159His/Phe229Tyr | Decreased between β7-α5 loop and β8 strand | Increased the number of bonds within the enzyme | __ | __ | Stability | [64] |
| Ser238Ala Tyr87Glu | __ | __ | Ser-His and His-Asp < 3.5 Å increased from 10.1% to 12.8% (Tyr87Glu) and 75.4% (Ser238Ala) | __ | Activity | [65] | |
| Ser92Lys/Arg251Ala Ile139Arg | __ | __ | Ser-PET decreased from 5.1 Å to 3.4 Å (Ser92Lys/Arg251Ala) and 4.0 Å (Ile139Arg) | __ | Activity | [66] | |
| Ile208Val, Ser238Tyr, Asn212Ala | __ | __ | __ | −25.50 kcal/mol −25.50 kcal/mol −28.36 kcal/mol (−21.20 kcal/mol for wild-type) | Activity | [67] | |
| Ile168Arg/Ser188Asp Ile168Arg/Ser188Glu | Decreased at α4 helix and β6-β7 loop | __ | __ | __ | Stability | [68] | |
| Asp186Val, Asp186Ala, Asp186Asn, Asp186His | Decreased at β6-β7 and β7-α5 loops and a5 helix | Occupancy rates increased between Asn/His186-Ser187/188 and between β6-β7 loop and α5/α6 helices | __ | −65.27kJ/mol −84.83kJ/mol −93.23 kJ/mol −90.98 kJ/mol (−88.23 kJ/mol for wild-type) | Stability | [69] | |
| PpPETase | Tyr239Arg/Phe244Gly/ Tyr250Gly | Increased at β7-α5, β8-α6 loops, and α6 helix | __ | __ | __ | Activity | [70] |
| PsPETase | Asp186Ala/Asn233Cys/ Ser282Cys/Ala179Cys/ Ser136Glu/Ser214Thr/ Lys95Asn (Combi-PETase) | __ | Break of bond between Ser214Thr and Pro184 | __ | __ | Activity | [71] |
| ScPETase | Ala212Cys/Thr249Cys/ Asn195His/ Asn243Lys | Increased at the mutation’s locations | __ | __ | __ | Stability | [70] |
| TfCut2 | His184Ser/Gln92Gly/ Phe209Ile/Ile213Lys (4Mz) | __ | __ | Ser-PET decreased from 4.6 Å to 3.8 Å | __ | Activity | [73] |
| Leu32Glu/Ser113Glu/ Thr237Gln | __ | __ | Ser-PET decreased from 8.2 Å to 3.7 Å | −81.80 kJ/mol (−64.31 kJ/mol for wild-type) | Activity | [74] | |
| V3 PETase | Lys95Ala/Arg132Asn/ Arg280Ala | Decreased at the active site region | __ | __ | __ | Activity | [75] |
| Enzyme | Starting Variant | Added Mutation a | Activity | Stability | Ref. |
|---|---|---|---|---|---|
| BhrPETase | BhrPETase_WT | His218Ser/Phe222Ile | ↑ b | ↓ b | [32] |
| BhrPETase_M2 | Asp238Lys | ↓ | ↑ | ||
| BhrPETase_M3 | Ala251Cys/Ala281Cys | ↓ | ↑ | ||
| BhrPETase_M5 | Ala209Arg | ↓ | ↑ | ||
| BhrPETase_M6 | Trp104Leu/Phe243Thr | ↑ | ↓ | ||
| Est1 | Est1_WT | Asn213Met | ↑ | - | [57] |
| Est1_N213M | Thr215Pro | ↑ | - | ||
| Est1_N213M/T215P | Ser115Pro | - | ↑ | ||
| Est1_MPP | Leu91Trp | ↑ | - | ||
| Est1_MPP/L91W | Gln93Ala | ↑ | - | ||
| Est30 | Est_WT | Ile171Lys | ↑ | ↓ | [58] |
| Est30_M2 | Gly130Leu | ↑ | ↑ | ||
| Est30_M8 | Met127Ser | ↑ | ↓ | ||
| LCC | LCC_WT | Phe243Ile | ↑ | ↓ | [59] |
| LCC_Μ1 | Asp238Cys/Ser283Cys | ↓ | ↑ | ||
| LCC_ICC | Tyr127Gly | ↓ | ↑ | ||
| LCC_ICCG | Asn213Pro | ↑ (75 °C) | ↑ | [60] | |
| LCC_ICCG | Thr157Pro | ↑ (75 °C) | ↑ | ||
| LCC_ICCG | Glu173Gln | ↑ (75 °C) | - | ||
| LCC_ICCG | His218Tyr | ↑ | - | [61] | |
| LCC_ICCG_Μ1-H218Y | Asn248Asp | ↑ | - | ||
| LCC-A2 | Ser247Ala | No change | - | ||
| LCC_ICCG | His183Tyr | ↑ | - | [62] | |
| LCC_ICCG_Μ1-H183Υ | Leu124Gly | ↑ | - | ||
| PET6 | PET6_WT | Val91Thr/Ser92Ala | ↑ | - | [63] |
| IsPETase | IsPETase_WT | Trp159His | ↑ | ↑ | [64] |
| IsPETase_M1a | Phe229Tyr | ↑ | ↑ | ||
| IsPETase_WT | Ser238Ala | ↑ | No change | [65] | |
| IsPETase_WT | Tyr87Glu | ↓ | ↑ | ||
| IsPETase_WT | Ile139Arg | ↑ | ↑ | [66] | |
| IsPETase_M1b | Asp157Glu | ↓ | ↓ | ||
| IsPETase_WT | Ser92Lys | ↑ | ↑ | ||
| IsPETase_M1c | Arg251Ala | ↑ | ↑ | ||
| IsPETase_M1c | Asp157Glu | ↓ | ↓ | ||
| IsPETase_WT | Ile208Val | ↓ | - | [67] | |
| IsPETase_WT | Ser238Tyr | ↑ | - | ||
| IsPETase_WT | Asn212Ala | ↑ | - | ||
| IsPETase_WT | Ile168Arg/Ser188Asp | ↑ | ↑ | [68] | |
| IsPETase_WT | Ile168Arg/Ser188Glu | ↑ | ↑ | ||
| IsPETase_WT | Asp186Val | ↑ | ↑ | [69] | |
| IsPETase_WT | Asp186Ala | ↑ | ↑ | ||
| IsPETase_WT | Asp186Asn | ↑ | ↑ | ||
| IsPETase_WT | Asp186His | ↑ | ↑ | ||
| PpPETase | PpPETase_WT | Tyr239Arg | ↑ | - | [70] |
| PpPETase_M1 | Phe244Gly/Tyr250Gly | ↑ | - | ||
| PsPETase | PsPETase_WT | Asp186Ala | ↓ | ↑ | [71] |
| PETaseA | Asn233Cys/Ser282Cys | ↑ | ↑ | ||
| PETaseACC | Ala179Cys/Ser136Glu/Ser214Thr | ↑ | ↑ | ||
| PETaseACCET | Lys95Asn | ↑ | ↑ | ||
| ScPETase | ScPETase_WT | Ala212Cys/Thr249Cys | ↑ | - | [70] |
| ScPETase_M2 | Asn195His/Asn243Lys | ↑ | - | ||
| TfCut2 | TfCut2_WT | Ca2+/Mg2+ | ↑ | ↑ | [72] |
| TfCut2_WT | His184Ser/Phe209Ile | ↑ | - | [73] | |
| TfCut2_M2a | Gln92Gly /Ile213Lys | ↑ | - | ||
| TfCut2_WT | Leu32Glu/Ser113Glu | ↑ | ↑ | [74] | |
| TfCut2_M2b | Thr237Gln | ↑ | ↑ | ||
| V3 PETase | V3 PETase_WT | Lys95Ala | ↓ (film) ↑ (powder) | ↑ | [75] |
| V3 PETase_M1 | Arg132Asn | ↑ | ↓ | ||
| V3 PETase_M2 | Arg280Ala | No change | ↑ |
| Enzyme | Ionic Concentration | Substrate | Conditions | Degradation Properties | Ref. | |
|---|---|---|---|---|---|---|
| IsPETase | 50 mM NaCl 1 M NaCl | 46.2 °C 52.7 °C | Used PET plastic waste (cryst. 10%) | 30–50 °C, pH 8.5 | 21–45 μM at 30–50 °C Decreased at 50 °C 5.6 μM at 30 °C 0.9 μM at 50 °C | [63] |
| PET6 | 50 mM NaCl 1 M NaCl | 49.8 °C 57.7 °C | Used PET plastic waste (cryst. 10%) | 30–50 °C, pH 8.5 | 0.02–0.2 μM at 30–50 °C 0.08 μM at 40 °C 1.1 μΜ at 50 °C | [63] |
| TfCut2 | None 10 mM MgCl2 10 mM CaCl2 | 71.2 °C 82.0 °C 83.8 °C | PET film | 65 °C, pH 8.5 | none ~7% weight loss 12.6% weight loss | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaoli, A.; Tzoupis, H.; Papavasileiou, K.D.; Papadiamantis, A.G.; Mintis, D.G.; Kiranoudis, C.T.; Lynch, I.; Melagraki, G.; Afantitis, A. Atomistic-Level Insights into the Role of Mutations in the Engineering of PET Hydrolases: A Systematic Review. Int. J. Mol. Sci. 2025, 26, 7682. https://doi.org/10.3390/ijms26167682
Karaoli A, Tzoupis H, Papavasileiou KD, Papadiamantis AG, Mintis DG, Kiranoudis CT, Lynch I, Melagraki G, Afantitis A. Atomistic-Level Insights into the Role of Mutations in the Engineering of PET Hydrolases: A Systematic Review. International Journal of Molecular Sciences. 2025; 26(16):7682. https://doi.org/10.3390/ijms26167682
Chicago/Turabian StyleKaraoli, Athina, Haralampos Tzoupis, Konstantinos D. Papavasileiou, Anastasios G. Papadiamantis, Dimitris G. Mintis, Chris T. Kiranoudis, Iseult Lynch, Georgia Melagraki, and Antreas Afantitis. 2025. "Atomistic-Level Insights into the Role of Mutations in the Engineering of PET Hydrolases: A Systematic Review" International Journal of Molecular Sciences 26, no. 16: 7682. https://doi.org/10.3390/ijms26167682
APA StyleKaraoli, A., Tzoupis, H., Papavasileiou, K. D., Papadiamantis, A. G., Mintis, D. G., Kiranoudis, C. T., Lynch, I., Melagraki, G., & Afantitis, A. (2025). Atomistic-Level Insights into the Role of Mutations in the Engineering of PET Hydrolases: A Systematic Review. International Journal of Molecular Sciences, 26(16), 7682. https://doi.org/10.3390/ijms26167682








