Synthesis, Properties, and Biological Activity Evaluation of Some Novel Naphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazines
Abstract
1. Introduction
2. Results
3. Discussion
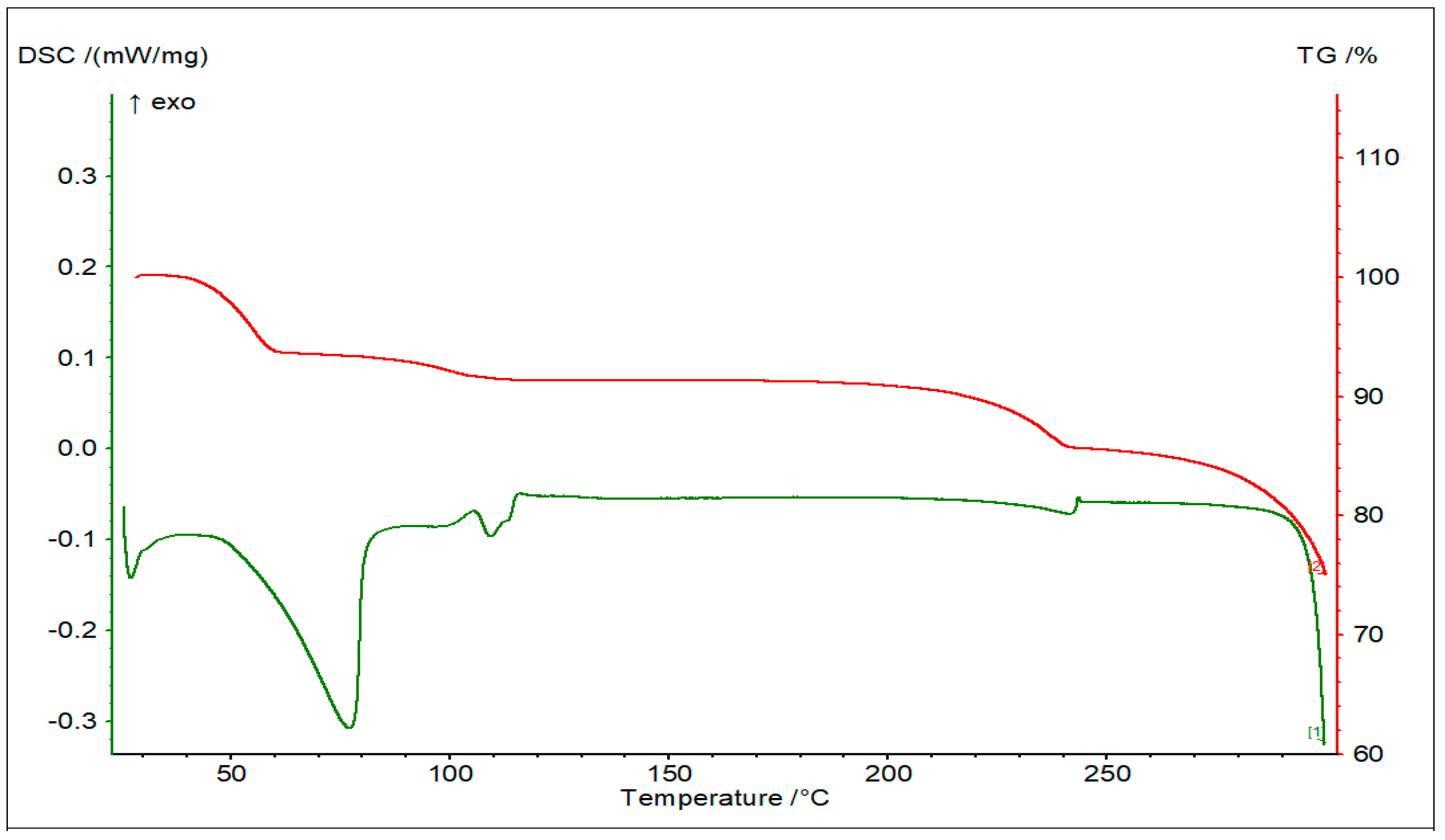
3.1. Thermal Analysis
3.2. Biological Activity Evaluation
3.3. Computational Studies
4. Materials and Methods
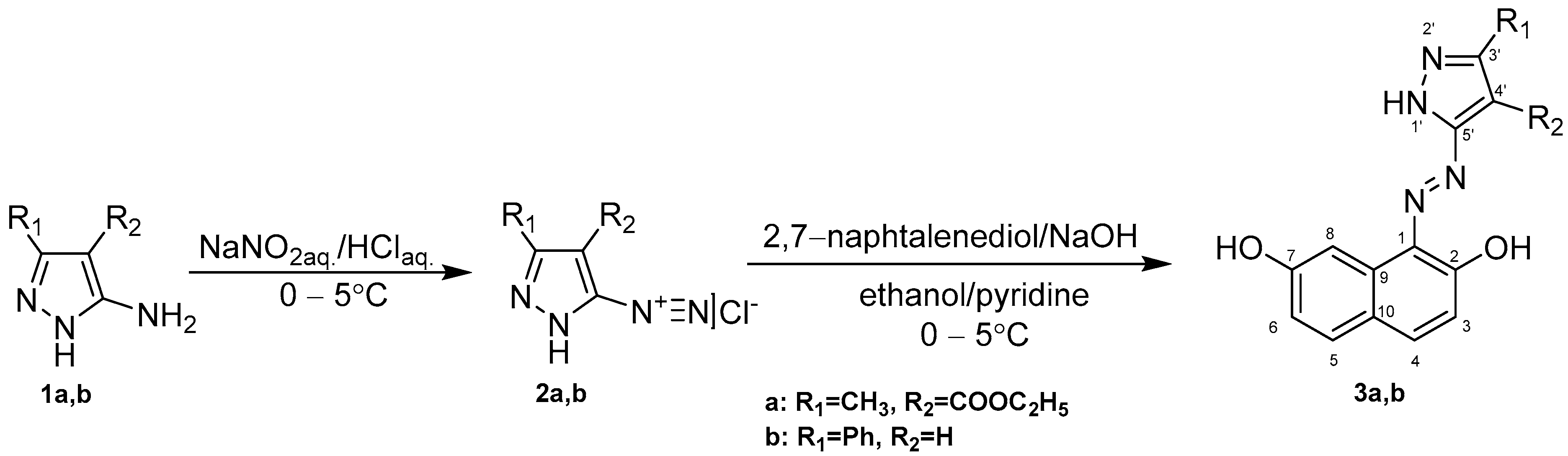
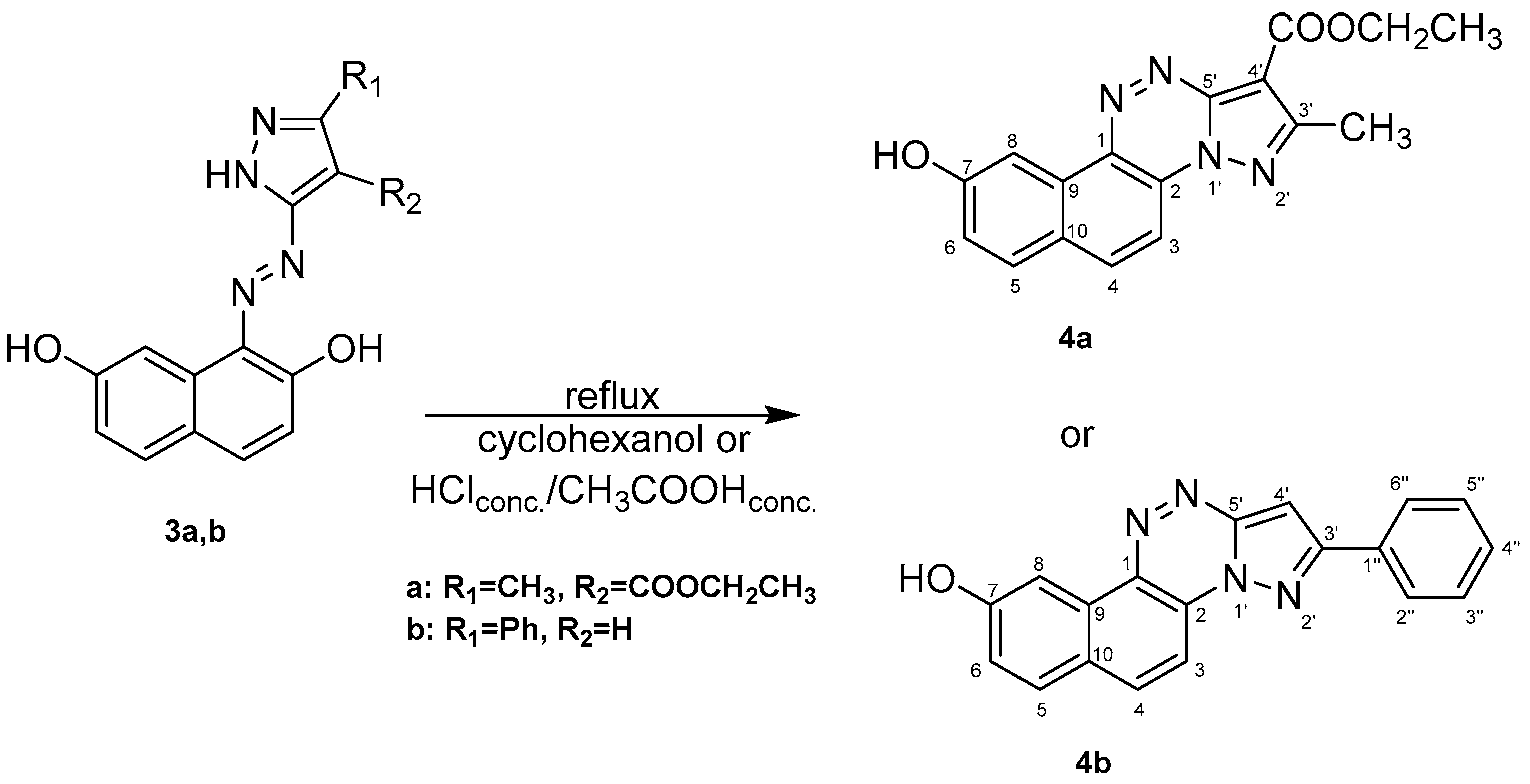
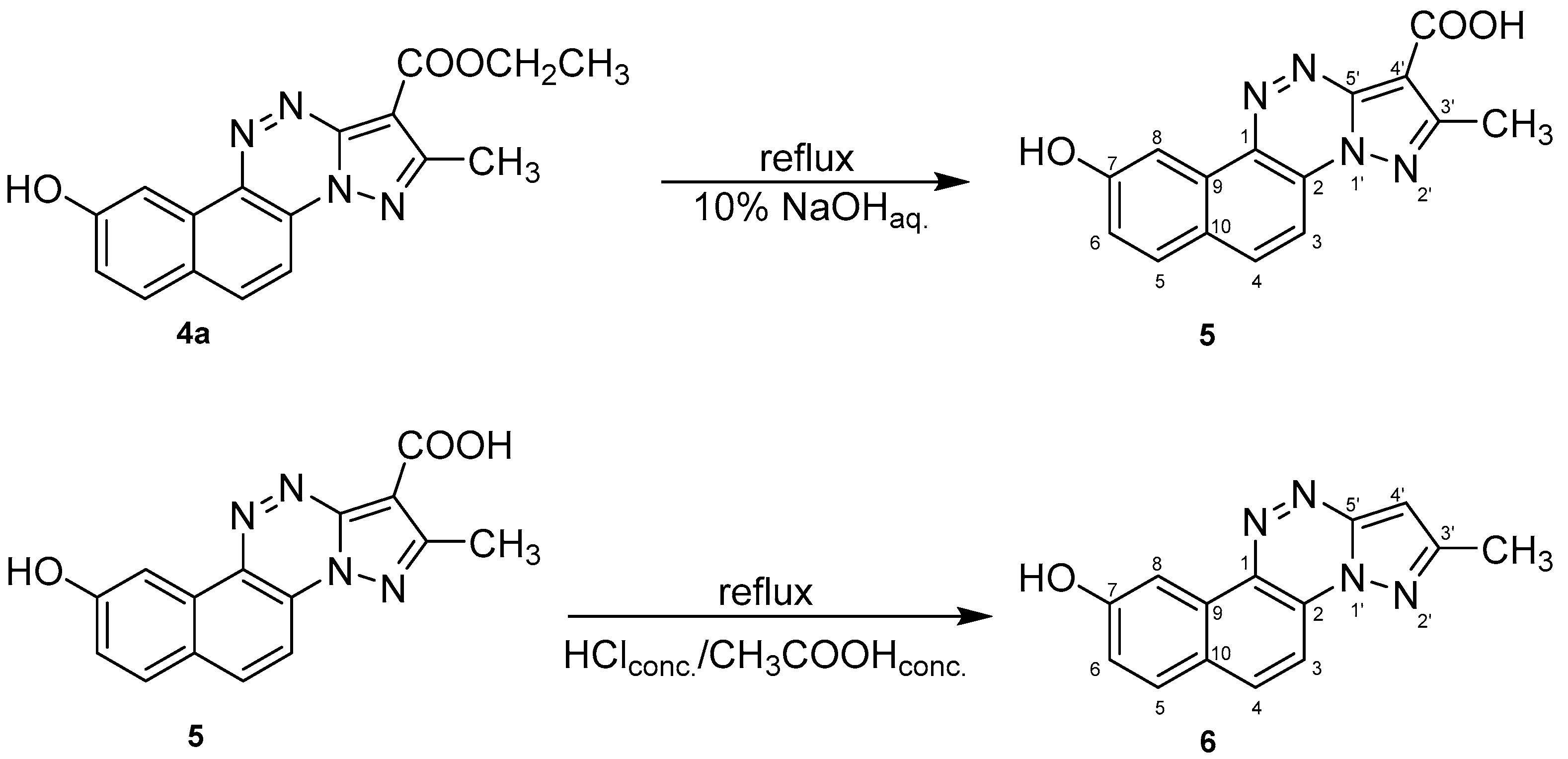
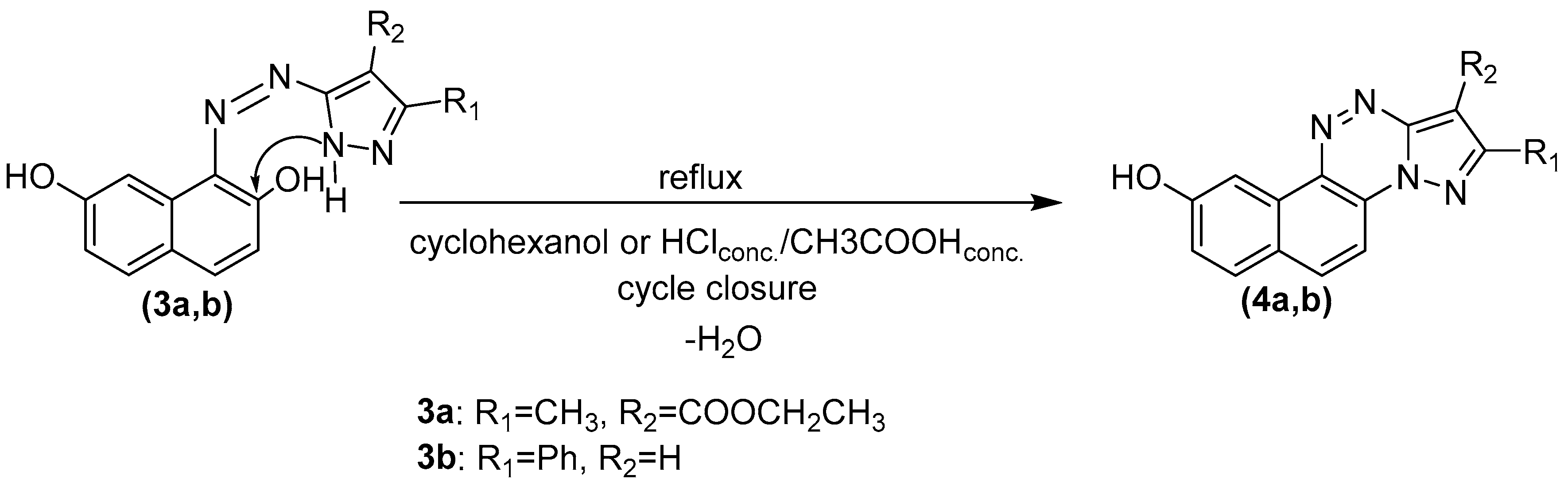
Compounds’ Synthesis Procedure
- 4-(ethoxycarbonyl)-3-methyl-1H-pyrazole-5-diazonium chloride synthesis (2a)
- 3-phenyl-1H-pyrazole-5-diazonium chloride synthesis (2b)
- Ethyl 5-((2,7-dihydroxynaphthalen-1-yl)diazenyl)-3-methyl-1H-pyrazole-4-carboxylate (3a)
- 1-((3-phenyl-1H-pyrazol-5-yl)diazenyl)naphthalene-2,7-diol synthesis (3b)
- Ethyl 9-hydroxy-2-methylnaphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazine-1-carboxylate (4a)
- 2-phenylnaphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazin-9-ol (4b)
- 9-hydroxy-2-methylnaphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazine-1-carboxylic acid (5)
- 2-methylnaphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazin-9-ol (6)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ledenyova, I.V.; Didenko, V.V.; Shikhaliev, K.S. Chemistry of Pyrazole-3(5)-Diazonium Salts (Review)*. Chem. Heterocycl. Compd. 2014, 50, 1214–1243. [Google Scholar] [CrossRef]
- Yıldırım, F.; Demirçalı, A.; Karcı, F.; Bayrakdar, A.; Taşlı, P.T.; Kart, H.H. New Coumarin-Based Disperse Disazo Dyes: Synthesis, Spectroscopic Properties and Theoretical Calculations. J. Mol. Liq. 2016, 223, 557–565. [Google Scholar] [CrossRef]
- Ok, S.; Altun, A.; Kasimogullari, R.; Sen, E. Role of the H-Bond Interactions in the Colloidal Mixtures of Pyrazole-Based Bioactive Dyes and Poly(Vinyl Alcohol). J. Chem. Eng. Data 2013, 58, 3521–3527. [Google Scholar] [CrossRef]
- Constantinescu, C.; Matei, A.; Ionita, I.; Ion, V.; Marascu, V.; Dinescu, M.; Vasiliu, C.; Emandi, A. Azo-Derivatives Thin Films Grown by Matrix-Assisted Pulsed Laser Evaporation for Non-Linear Optical Applications. Appl. Surf. Sci. 2014, 302, 69–73. [Google Scholar] [CrossRef]
- Karabacak, Ç.; Tilki, T.; Tuncer, B.Ö.; Cengiz, M. Antimicrobial Pyrazole Dyes: Synthesis, Characterization, and Absorption Characteristics. Res. Chem. Intermed. 2015, 41, 1985–1999. [Google Scholar] [CrossRef]
- Al-Zinkee, J.M.M.; Jarad, A.J. Synthesis, Characterization and Microbial Efficiency of Azo Dye LigandComplexes with Some Metal Ions. Res. J. Phar. Bio. Chem. Sci. 2018, 9, 144–155. [Google Scholar]
- Bartwal, G.; Aggarwal, K.; Khurana, J.M. A Highly Selective pH Switchable Colorimetric Fluorescent Rhodamine Functionalized Azo-Phenol Derivative for Thorium Recognition up to Nano Molar Level in Semi-Aqueous Media: Implication towards Multiple Logic Gates. J. Hazard. Mater. 2018, 360, 51–61. [Google Scholar] [CrossRef]
- Reimlinger, H.; Overstraeten, A.V.; Viehe, H.G. Über das 3(5)-Diazo-pyrazol. Chem. Berichte 1961, 94, 1036–1041. [Google Scholar] [CrossRef]
- Vilarrasa, J.; Granados, R. Diazo-, Azo- and Azidoazoles, and Related Compounds. 1. Synthesis of Naphthoazolo-as-Triazines from Diazoazoles and 2-Naphthol. J. Heterocycl. Chem. 1974, 11, 867–872. [Google Scholar] [CrossRef]
- Deeb, A.; El-Mobayed, M.; Essawy, A.E.-N.; El-Hamid, A.A.; El-Hamid, A.A. Preparation of Naphtho[2,1-e]Pyrazolo[5,1-c][1,2,4]Triazine, Dipyrazolo[5,1-c:3’,4’-e][1,2,4]Triazines and Pyrazolo[5,1-c][1,2,4]Triazine Derivatives. Collect. Czechoslov. Chem. Commun. 1990, 55, 2790–2794. [Google Scholar] [CrossRef]
- El-Shafei, A.; Fadda, A.A.; Khalil, A.M.; Ameen, T.A.E.; Badria, F.A. Synthesis, Antitumor Evaluation, Molecular Modeling and Quantitative Structure–Activity Relationship (QSAR) of Some Novel Arylazopyrazolodiazine and Triazine Analogs. Bioorganic Med. Chem. 2009, 17, 5096–5105. [Google Scholar] [CrossRef]
- Elmoghayar, M.R.H.; Ibrahim, M.K.A.; El-Sakka, I.; Elghandour, A.H.H.; Elnagdi, M.H. Pyrimidine Derivatives and Related Compounds, XI: Synthesis of Some New Mercaptopyrazolo[1,5-a]Pyrimidines and Mercaptopyrazolo[1,5-c]-as-Triazines. Arch. Pharm. 1983, 316, 697–702. [Google Scholar] [CrossRef]
- Abdel-Razik, H.H.; Fadda, A.A. Synthesis Of 1-(P-tosyl)pyrazolo[1,5-a]-pyrimidines And Pyrazolo [1,5-c]-[1,2,4]Triazine Derivatives. Synth. Commun. 2001, 31, 3547–3556. [Google Scholar] [CrossRef]
- Neidlein, R.; Jäschke, U. Synthesen von Pyrazolo-1,2,4-Triazin-Derivaten Des 1,6-Methano[10]Annulens. Chem. Berichte 1988, 121, 1359–1361. [Google Scholar] [CrossRef]
- Bamberger, E. Ueber Anhydrisirung von β-Naphtol-Azofarbstoffen. Berichte Dtsch. Chem. Ges. 1899, 32, 1797–1802. [Google Scholar] [CrossRef]
- Dziomko, V.M.; Berestevich, B.K. Synthesis of O-Azobispyrazole Derivatives. Chem. Heterocycl. Compd. 1978, 14, 313–318. [Google Scholar] [CrossRef]
- Dziomko, V.M.; Berestevich, B.K. Synthesis of O-Bisazo-1H-Pyrazoles and Study of Their Cyclization to Give Compounds with a 1,2,4-Triazine Ring. Chem. Heterocycl. Compd. 1979, 15, 657–663. [Google Scholar] [CrossRef]
- Castillon, S.; Vilarrasa, J. A 200-MHz Proton NMR Study of the Naphtho[2,1-e]Tetrazolo[5,1-c]-as-Triazine/3-Azidonaphtho[2,1-e]-as-Triazine/Naphtho[2,1-e]Tetrazolo[1,5-b]-as-Triazine Equilibrium. J. Org. Chem. 1982, 47, 3168–3169. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side Effects and Toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Holcombe, L.J.; O′Gara, F.; Morrissey, J.P. Implications of Interspecies Signaling for Virulence of Bacterial and Fungal Pathogens. Future Microbiol. 2011, 6, 799–817. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida Auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Rybak, M.J. The Pharmacokinetic and Pharmacodynamic Properties of Vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S35–S39. [Google Scholar] [CrossRef]
- M100 | Performance Standards for Antimicrobial Susceptibility Testing. Available online: https://clsi.org/shop/standards/m100/ (accessed on 4 August 2025).
- Staicu, M.; Brundige, M.L.; Datta, S.; Laguio-Vila, M. Safety of Stopping Antibiotics Prescribed “Just in Case”–Comparison of Mortality, Readmissions and Clostridium Difficile in Patients with Accepted Stewardship Interventions Compared with Declined. Open Forum Infect. Dis. 2017, 4 (Suppl. S1), S482. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action and Resistance of Older and Newer Fluoroquinolones. Clin. Infect. Dis. 2000, 31 (Suppl. S2), S24–S28. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Esmaeili, R.; Moghimi, S.; Oghabi Bakhshaiesh, T.; Eslami-S, Z.; Majidzadeh-A, K.; Safavi, M.; Emami, S.; Foroumadi, A. Synthesis and Biological Evaluation of 4-Amino-5-Cinnamoylthiazoles as Chalcone-like Anticancer Agents. Eur. J. Med. Chem. 2018, 145, 404–412. [Google Scholar] [CrossRef]
- Zyrianov, Y. Distribution-Based Descriptors of the Molecular Shape. J. Chem. Inf. Model. 2005, 45, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank. Available online: https://www.rcsb.org/structure/3GCW (accessed on 3 August 2025).[Green Version]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Beyer, H.; Wolter, G. Über Thiazole, XXIX. Mitteil.: Über die Kondensationsprodukte von Thiosemicarbazid mit α-Chloracetessigester und eine neuartige Ringverengung des 2-Amino-5-methyl-6-carbäthoxy-1.3.4-thiodiazins zum 3-Methyl-4-carbäthoxy-5-amino-pyrazol. Chem. Berichte 1956, 89, 1652–1658. [Google Scholar] [CrossRef]
- Ege, G.; Heck, R.; Gilbert, K.; Irngartinger, H.; Huber-Patz, U.; Rodewald, H. Reactions with Diazoazoles. Part VI. Unequivocal Synthesis of 3-Methyl-3h-Azolotetrazoles. Correction of the Formerly Described 3-Methylazolotetrazoles in Favour of Mesoionic 2-Methylazolotetrazoles. J. Heterocycl. Chem. 1983, 20, 1629–1639. [Google Scholar] [CrossRef]
- Coenen, M.; Faust, J.; Ringel, C.; Mayer, R. Synthesen mit Trichloracetonitril. J. Prakt. Chem. 1965, 27, 239–250. [Google Scholar] [CrossRef]
- Burcă, I.; Badea, V.; Deleanu, C.; Bercean, V.-N. 5-((8-Hydroxyquinolin-5-Yl)Diazenyl)-3-Methyl-1H-Pyrazole-4-Carboxylic Acid. Molbank 2021, 2021, M1238. [Google Scholar] [CrossRef]
- Burcă, I.; Diaconescu, A.-M.; Badea, V.; Péter, F. 5-((4-(-Phenyldiazenyl)Phenyl)Diazenyl)Quinolin-8-Ol. Molbank 2023, 2023, M1701. [Google Scholar] [CrossRef]
- Bercean, V.-N.; Badea, V.; Nutiu, R. Compusi azoici derivati din 1H-3-metil-4-etoxicarbonil-5-aminopirazoli si fenoli. Rev. Chim. 2002, 53, 508–511. [Google Scholar]

| Microorganism | Compound | ||||||
|---|---|---|---|---|---|---|---|
| Amphotericin B [19,20,21] | (3a) | (4a) | (3b) | (4b) | (5) | (6) | |
| C. albicans Conc./% inhibition | 50.0 μM/ 95 ± 3% | 50.0 μM/ 4.55% | 50.0 μM/ −0.78% | 50.0 μM/ 8.88% | 50.0 μM/ 2.05% | 50.0 μM/ −1.88% | 50.0 μM/ 14.95% |
| C. auris Conc./% inhibition | 50.0 μM/ 90 ± 4% | 50.0 μM/ 0.96% | 50.0 μM/ 6.24% | 50.0 μM/ 34.00% | 50.0 μM/ 7.67% | 50.0 μM/ 3.31% | 50.0 μM/ 2.62% |
| A. fumigatus Conc./% inhibition | 50.0 μM/ 85 ± 4% | 50.0 μM/ 5.38% | 50.0 μM/ −14.14% | 50.0 μM/ −9.79% | 50.0 μM/ −16.18% | 50.0 μM/ −15.78% | 50.0 μM/ −6.41% |
| Microorganism | Compound | ||||||
|---|---|---|---|---|---|---|---|
| Vancomycin [22,23] | (3a) | (4a) | (3b) | (4b) | (5) | (6) | |
| E. faecalis ATCC Conc./% inhibition | 50.0 μM/ 90 ± 4% | 50.0 μM/ −7.04% | 50.0 μM/ −1.84% | 50.0 μM/ 6.85% | 50.0 μM/ 1.41% | 50.0 μM/ −1.43% | 50.0 μM/ 0.09% |
| S. aureus Conc./% inhibition | 50.0 μM/ 92 ± 3% | 50.0 μM/ −6.08% | 50.0 μM/ 5.32% | 50.0 μM/ −8.47% | 50.0 μM/ 8.10% | 50.0 μM/ 0.35% | 50.0 μM/ −6.69% |
| Microorganism | Compound | ||||||
|---|---|---|---|---|---|---|---|
| Ciprofloxacin [24,25] | (3a) | (4a) | (3b) | (4b) | (5) | (6) | |
| P. aeruginosa Conc./% growth inhibition | 50.0 μM/ 60 ± 6% | 50.0 μM/ 12.70% | 50.0 μM/ −2.82% | 50.0 μM/ −10.40% | 50.0 μM/ −5.30% | 50.0 μM/ −7.33% | 50.0 μM/ 18.70% |
| E.coli Conc./% growth inhibition | 50.0 μM/ 80 ± 5% | 50.0 μM/ −13.99% | 50.0 μM/ −16.42% | 50.0 μM/ −12.23% | 50.0 μM/ −6.73% | 50.0 μM/ −9.66% | 50.0 μM/ −11.13% |
| K. pneumoniae Conc./% inhibition | 50.0 μM/ 65 ± 6% | 50.0 μM/ −32.00% | 50.0 μM/ −19.00% | 50.0 μM/ −12.00% | 50.0 μM/ 14.00% | 50.0 μM/ −12.00% | 50.0 μM/ −38.00% |
| A. baumannii Conc./% inhibition | 50.0 μM/ 40 ± 7% | 50.0 μM/ −22.02% | 50.0 μM/ −10.33% | 50.0 μM/ −15.05% | 50.0 μM/ −10.33% | 50.0 μM/ −20.61% | 50.0 μM/ 0.37% |
| Cell Line | Compound | ||||||
|---|---|---|---|---|---|---|---|
| Etoposide [26] | (3a) | (4a) | (3b) | (4b) | (5) | (6) | |
| Cell line permanent Hep-G2 Conc./% inhibition | 10.0 μM/ 61.7 ± 6% | 10,000.0 nM/ 3.5 h 22.50% | 10,000.0 nM/ 3.5 h 3.60% | 10,000.0 nM/ 3.5 h 62.60% | 10,000.0 nM/ 3.5 h 11.20% | 10,000.0 nM/ 3.5 h 7.60% | 10,000.0 nM/ 3.5 h 11.60% |
| Compound | HOMO Orbitals | LUMO Orbitals |
| (4a) |  −0.23377 a.u. | 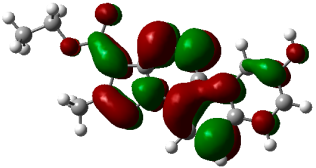 −0.12297 a.u. |
| (4b) |  −0.22842 a.u. | 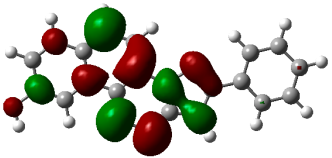 −0.11719 a.u. |
| (5) |  −0.23467 a.u. | 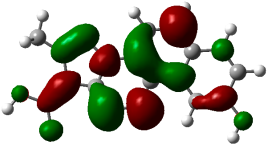 −0.12458 a.u. |
| (6) | 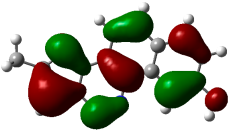 −0.22615 a.u. | 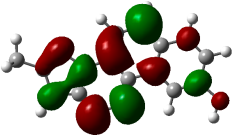 −0.11606 a.u. |
| Compound | HL Gap (eV) | Chemical Potential μ (eV) | Chemical Hardness η (eV) | Electrophilicity ω (eV) |
|---|---|---|---|---|
| (4a) | 3.014 | −4.852 | 1.507 | 7.810 |
| (4b) | 3.025 | −4.651 | 1.512 | 7.153 |
| (5) | 2.994 | −4.886 | 1.497 | 7.974 |
| (6) | 2.994 | −4.654 | 1.497 | 7.235 |
| Excited State (ES) | Transitions | Excitation Energy (eV) | λ (nm) | Oscillator Strength |
|---|---|---|---|---|
| ES 1 | HOMO → LUMO | 2.5629 | 483.76 | 0.4863 |
| ES 2 | HOMO-4 → LUMO HOMO-2 → LUMO | 2.9587 | 419.05 | 0.0050 |
| ES 3 | HOMO-1 → LUMO | 3.043 | 407.44 | 0.1301 |
| ES 4 | HOMO-2 → LUMO HOMO → LUMO+1 | 3.7235 | 332.97 | 0.0679 |
| ES 5 | HOMO-5 → LUMO HOMO-3 → LUMO HOMO → LUMO+1 | 3.7684 | 329.01 | 0.007 |
| Excited State (ES) | Transitions | Excitation Energy (eV) | λ (nm) | Oscillator Strength |
|---|---|---|---|---|
| ES 1 | HOMO → LUMO | 2.5027 | 495.39 | 0.3015 |
| ES 2 | HOMO-1 → LUMO | 2.7663 | 448.20 | 0.2777 |
| ES 3 | HOMO-4 → LUMO | 3.2085 | 386.42 | 0.0065 |
| ES 4 | HOMO-2 → LUMO HOMO → LUMO+1 | 3.3853 | 366.24 | 0.0303 |
| ES 5 | HOMO-5 → LUMO HOMO-3 → LUMO HOMO → LUMO+1 | 3.6563 | 339.10 | 0.046 |
| Excited State (ES) | Transitions | Excitation Energy (eV) | λ (nm) | Oscillator Strength |
|---|---|---|---|---|
| ES 1 | HOMO → LUMO | 2.4376 | 508.63 | 0.4355 |
| ES 2 | HOMO-1 → LUMO | 2.9759 | 416.62 | 0.1676 |
| ES 3 | HOMO-4 → LUMO HOMO-2 → LUMO | 3.0207 | 410.45 | 0.0051 |
| ES 4 | HOMO-3 → LUMO HOMO → LUMO+1 | 3.6602 | 338.74 | 0.0767 |
| ES 5 | HOMO-4 → LUMO HOMO-2 → LUMO | 3.7586 | 329.87 | 0.0006 |
| Excited State (ES) | Transitions | Excitation Energy (eV) | λ (nm) | Oscillator Strength |
|---|---|---|---|---|
| ES 1 | HOMO → LUMO | 2.5980 | 477.23 | 0.463 |
| ES 2 | HOMO-1 → LUMO | 2.9713 | 417.28 | 0.0408 |
| ES 3 | HOMO-2 → LUMO | 3.1708 | 391.02 | 0.0066 |
| ES 4 | HOMO-3 → LUMO | 3.6919 | 335.82 | 0.0106 |
| ES 5 | HOMO-6 → LUMO HOMO-3 → LUMO HOMO-1→LUMO+1 HOMO → LUMO+1 | 4.1250 | 300.56 | 0.096 |
| Compound | Connolly Accessible Area (Å2) | Connolly Solvent-Excluded Volume (Å3) | Ovality |
|---|---|---|---|
| (3a) | 591.485 | 273.689 | 1.554 |
| (3b) | 581.006 | 257.233 | 1.560 |
| (4a) | 561.954 | 257.552 | 1.526 |
| (4b) | 541.314 | 242.699 | 1.514 |
| (5) | 500.441 | 221.625 | 1.470 |
| (6) | 461.324 | 196.261 | 1.433 |
| Compound | Binding Energies (kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | E9 | |
| (3a) | −8.2 | −7.8 | −7.4 | −7.3 | −7.2 | −7.0 | −6.9 | −6.9 | −6.9 |
| (3b) | −9.7 | −9.5 | −9.3 | −9.3 | −9.0 | −9.0 | −8.2 | −8.0 | −8.0 |
| (4a) | −8.9 | −8.3 | −8.3 | −8.1 | −7.4 | −7.0 | −6.7 | −6.2 | −6.2 |
| (4b) | −10.2 | −9.7 | −9.5 | −9.5 | −9.5 | −9.0 | −8.4 | −8.3 | −7.8 |
| (5) | −8.9 | −8.7 | −8.3 | −7.3 | −7.1 | −6.9 | −6.6 | −6.5 | −6.5 |
| (6) | −8.8 | −8.6 | −8.5 | −8.3 | −8.2 | −8.0 | −7.9 | −7.8 | −7.6 |
| Compound | E (kcal/mol) |
|---|---|
| (3a) | −7.29 |
| (3b) | −8.89 |
| (4a) | −7.67 |
| (4b) | −9.10 |
| (5) | −7.42 |
| (6) | −8.19 |
| Compound | Hydrogen Bonds | Atoms in Close Contact Interactions |
|---|---|---|
| (3a) | Naphtol OH- Asp360 (2.122 Å) | Naphthaline residue: Pro331; Val460; Trp461 Pyrazol residue: Ile416; Pro438 Azo group: Arg456; Asp360 |
| (3b) | - | Naphthaline residue: Arg357; Val460; Trp461 Pyrazol residue: Asp360 Phenyl moiety: Leu440; Pro438; Asp651 |
| (4a) | - | Naphthaline residue: Arg357; Thr459 Pyrazolo-triazine residue: Pro438 Methyl moiety: Leu440; Val650 |
| (4b) | - | Naphthaline residue: Arg357; Thr459; Trp461 Pyrazolo-triazine residue: Pro438; Asn439 Phenyl moiety: Leu440; Val650; Asp651; Lys421 |
| (5) | - | Naphthaline residue: Arg357; Thr459; Trp461 Pyrazolo-triazine residue: Val435; Pro348 Methyl moiety: Val650 |
| (6) | Naphtol OH- Glu4260 (1.833 Å) | Naphthaline residue: Trp461; Tyr648 Pyrazolo-triazine residue: Leu440; Val650; Arg458 |
| Compound | Molar Concentration |
|---|---|
| (3a) | 7.052 × 10−5 M |
| (3b) | 5.415 × 10−5 M |
| (4a) | 5.150 × 10−5 M |
| (4b) | 7.044 × 10−5 M |
| (5) | 6.796 × 10−5 M |
| (6) | 1.578 × 10−4 M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burcă, I.; Bercean, V.-N.; Rusu, G.-I.; Pop, R.; Diaconescu, A.-M.; Badea, V.; Péter, F. Synthesis, Properties, and Biological Activity Evaluation of Some Novel Naphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazines. Int. J. Mol. Sci. 2025, 26, 7681. https://doi.org/10.3390/ijms26167681
Burcă I, Bercean V-N, Rusu G-I, Pop R, Diaconescu A-M, Badea V, Péter F. Synthesis, Properties, and Biological Activity Evaluation of Some Novel Naphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazines. International Journal of Molecular Sciences. 2025; 26(16):7681. https://doi.org/10.3390/ijms26167681
Chicago/Turabian StyleBurcă, Ion, Vasile-Nicolae Bercean, Gerlinde-Iuliana Rusu, Raluca Pop, Alexandra-Mihaela Diaconescu, Valentin Badea, and Francisc Péter. 2025. "Synthesis, Properties, and Biological Activity Evaluation of Some Novel Naphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazines" International Journal of Molecular Sciences 26, no. 16: 7681. https://doi.org/10.3390/ijms26167681
APA StyleBurcă, I., Bercean, V.-N., Rusu, G.-I., Pop, R., Diaconescu, A.-M., Badea, V., & Péter, F. (2025). Synthesis, Properties, and Biological Activity Evaluation of Some Novel Naphtho[2,1-e]pyrazolo[5,1-c][1,2,4]triazines. International Journal of Molecular Sciences, 26(16), 7681. https://doi.org/10.3390/ijms26167681








