Interobserver Agreement in Immunohistochemical Evaluation of Folate Receptor Alpha (FRα) in Ovarian Cancer: A Multicentre Study
Abstract
1. Introduction
2. Results
2.1. Internal Control Adequacy
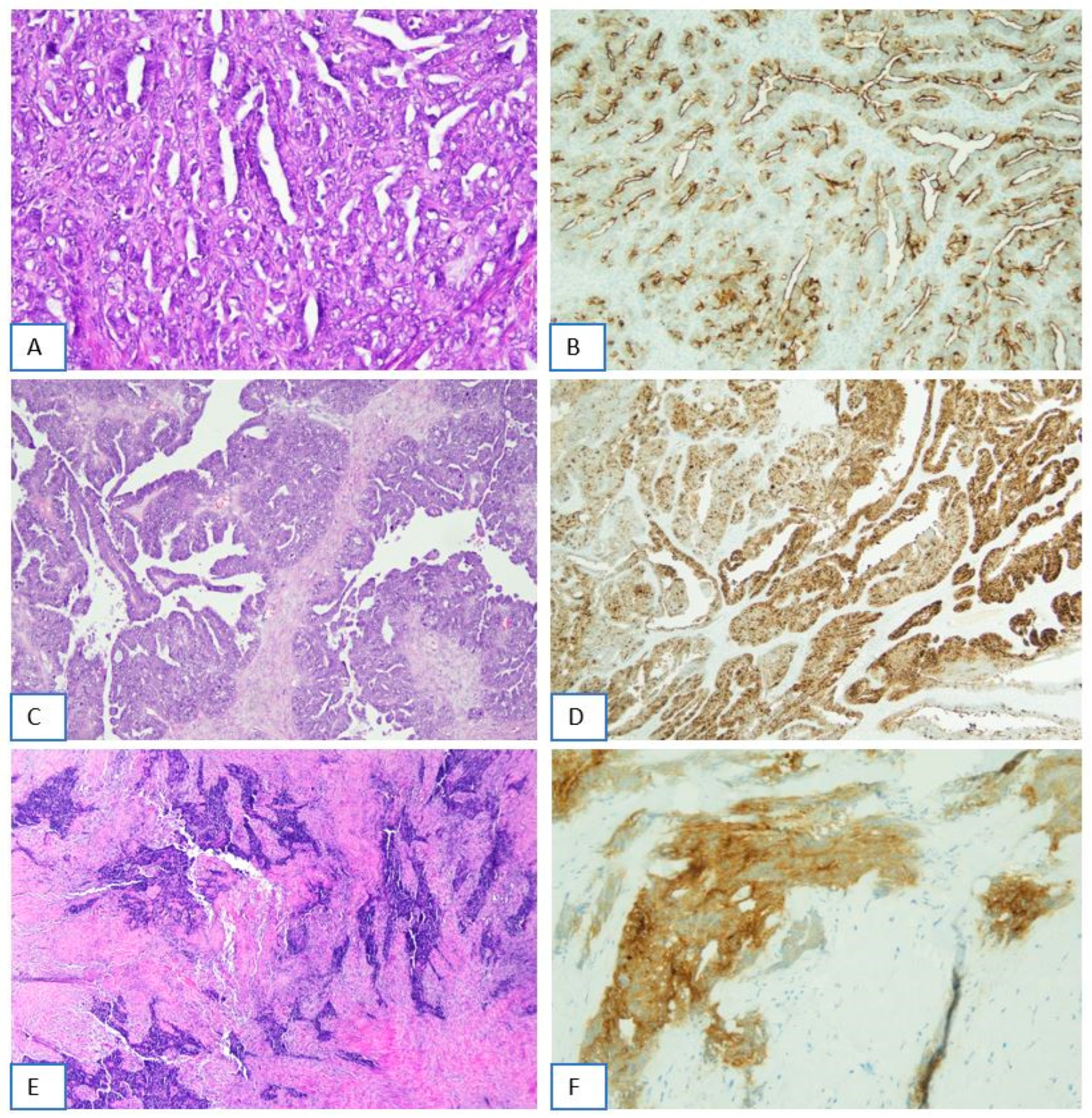
2.2. FRα Staining Intensity in Tumor Cells
2.3. Percentage of FRα-Positive Tumor Cells
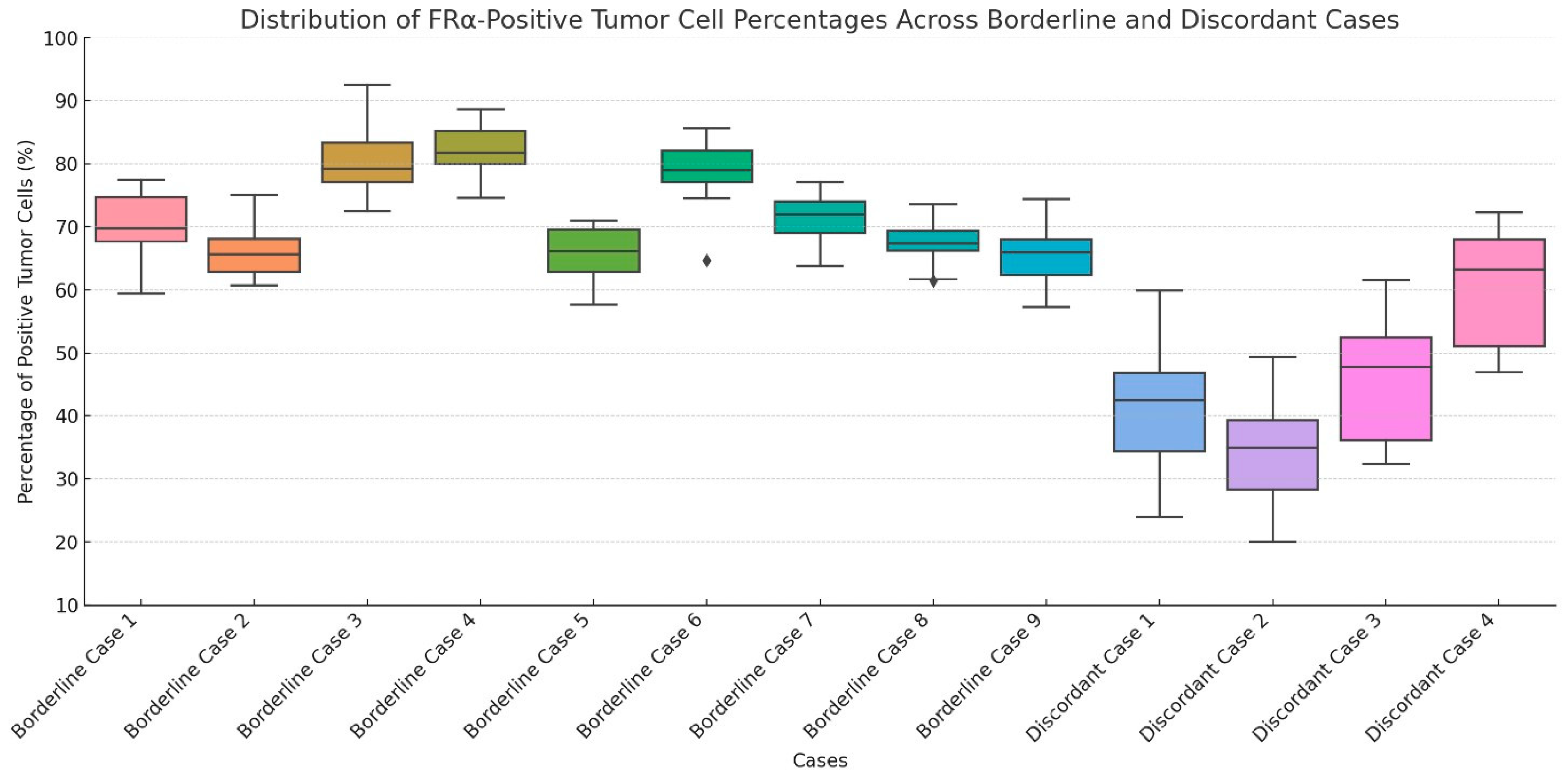
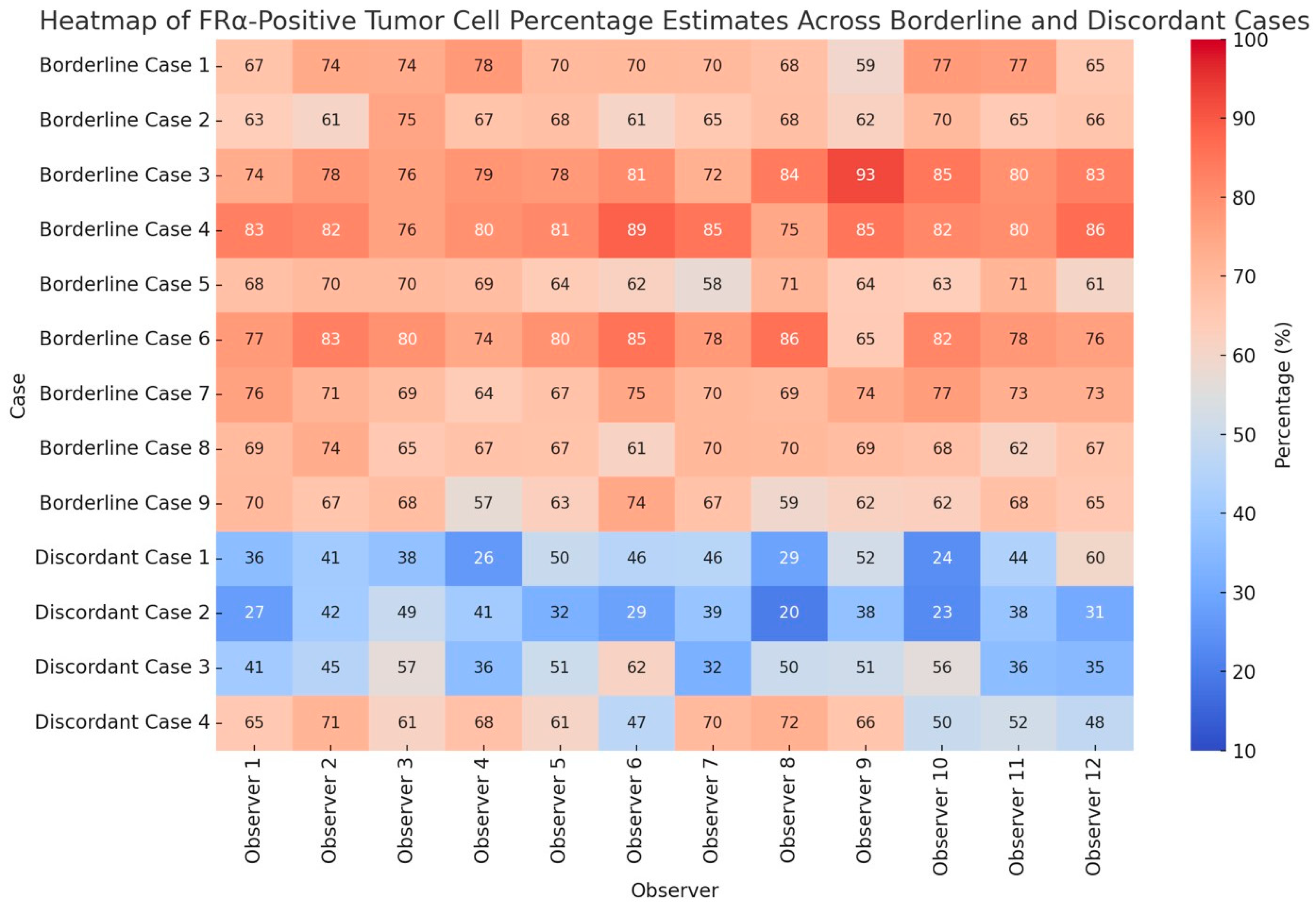
2.4. Overall FRα Positivity and Analysis of Borderline and Discordant Cases
3. Discussion
4. Materials and Methods
4.1. Case Selection and Immunohistochemistry
4.2. Reference Evaluation
4.3. Observer Evaluation
4.4. Assessment of Internal Control Adequacy
4.5. Assessment of FRα Staining Intensity in Tumor Cells
4.6. Assessment of the Percentage of FRα-Positive Tumor Cells
4.7. Assessment of Overall FRα Positivity and Definition of Borderline Cases
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webb, P.M.; Ibiebele, T.I.; Hughes, M.C.; Beesley, J.; van der Pols, J.C.; Chen, X.; Nagle, C.M.; Bain, C.J.; Chenevix-Trench, G. The Australian Cancer Study (Ovarian Cancer) and the Australian Ovarian Cancer Study Group Folate and related micronutrients, folate-metabolising genes and risk of ovarian cancer. Eur. J. Clin. Nutr. 2011, 65, 1133–1140. [Google Scholar] [CrossRef]
- Gonzalez, T.; Muminovic, M.; Nano, O.; Vulfovich, M. Folate Receptor Alpha—A Novel Approach to Cancer Therapy. Int. J. Mol. Sci. 2024, 25, 1046. [Google Scholar] [CrossRef]
- Scaranti, M.; Cojocaru, E.; Banerjee, S.; Banerji, U. Exploiting the folate receptor α in oncology. Nat. Rev. Clin. Oncol. 2020, 17, 349–359. [Google Scholar] [CrossRef]
- Zhao, R.; Goldman, I.D. Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors. Mol. Asp. Med. 2013, 34, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, L.E. The role of folate receptor α in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Bax, H.J.; Chauhan, J.; Stavraka, C.; Santaolalla, A.; Osborn, G.; Khiabany, A.; Grandits, M.; López-Abente, J.; Palhares, L.C.G.F.; Hak, C.C.W.; et al. Folate receptor alpha in ovarian cancer tissue and patient serum is associated with disease burden and treatment outcomes. Br. J. Cancer 2022, 128, 342–353. [Google Scholar] [CrossRef]
- Della Corte, L.; Palumbo, M.; Ascione, M.; D’Angelo, G.; La Verde, M.; Ferrari, F.; Morra, I.; Bifulco, G. Impact on Global Health Status, Quality-Sexual Life and Chronic Fatigue State of Risk-Reducing Salpingo-Oophorectomy in Women Who Are BRCA1/2 Mutation Carriers: Experience from a Third-Level Italian Center. Gynecol. Obstet. Investig. 2025, 3, 1–10. [Google Scholar] [CrossRef]
- Moore, K.N.; Angelergues, A.; Konecny, G.E.; García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.-Y.; Moroney, J.W.; Colombo, N.; Roszak, A.; et al. Mirvetuximab Soravtansine in FRα-Positive, Platinum-Resistant Ovarian Cancer. N. Engl. J. Med. 2023, 389, 2162–2174. [Google Scholar] [CrossRef]
- Kong, B.; Zheng, W. Mirvetuximab soravtansine: Current and future applications. J. Hematol. Oncol. 2025, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.; Oaknin, A.; Matulonis, U.A.; Mantia-Smaldone, G.M.; Lim, P.C.; Castro, C.M.; Provencher, D.; Memarzadeh, S.; Method, M.; Wang, J.; et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol. Oncol. 2023, 170, 241–247. [Google Scholar] [CrossRef]
- Richardson, D.L.; Moore, K.N.; Vergote, I.; Gilbert, L.; Martin, L.P.; Mantia-Smaldone, G.M.; Castro, C.M.; Provencher, D.; Matulonis, U.A.; Stec, J.; et al. Phase 1b study of mirvetuximab soravtansine, a folate receptor alpha (FRα)–targeting antibody-drug conjugate, in combination with carboplatin and bevacizumab in patients with platinum-sensitive ovarian cancer. Gynecol. Oncol. 2024, 185, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Lorusso, D.; Oaknin, A.; Cecere, S.C.; Denys, H.; Colombo, N.; van Gorp, T.; A Konner, J.; Marin, M.R.; Harter, P.; et al. Mirvetuximab soravtansine in folate receptor alpha (FRα)–high platinum-resistant ovarian cancer: Final overall survival and post hoc sequence of therapy subgroup results from the SORAYA trial. Int. J. Gynecol. Cancer 2024, 34, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Secord, A.A.; Lewin, S.; Murphy, C.; Cecere, S.; Barquín, A.; Gálvez-Montosa, F.; Mathews, C.; Konecny, G.; Ray-Coquard, I.; Oaknin, A.; et al. The efficacy and safety of mirvetuximab soravtansine in FRα-positive, third-line and later, recurrent platinum-sensitive ovarian cancer: The single-arm phase II PICCOLO trial. Ann. Oncol. 2024, 36, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, T.; Moore, K.N.; Konecny, G.E.; Leary, A.; García-García, Y.; Banerjee, S.; Lorusso, D.; Lee, J.-Y.; Moroney, J.W.; Caruso, G.; et al. Patient-reported outcomes from the MIRASOL trial evaluating mirvetuximab soravtansine versus chemotherapy in patients with folate receptor α-positive, platinum-resistant ovarian cancer: A randomised, open-label, phase 3 trial. Lancet Oncol. 2025, 26, 503–515. [Google Scholar] [CrossRef]
- Previs, R.A.; Strickland, K.C.; Wallen, Z.; Ko, H.; Green, M.; Cooper, M.; Lyon, E.; Biorn, M.; Armetta, J.; Quarles, R.; et al. Analysis of real world FRα testing in ovarian, fallopian tube, and primary peritoneal cancers. Gynecol. Oncol. 2024, 192, 102–110. [Google Scholar] [CrossRef]
- James, R.L.; Sisserson, T.; Cai, Z.; Dumas, M.E.; Inge, L.J.; Ranger-Moore, J.; Mason, A.; Sloss, C.M.; McArthur, K. Development of an FRα Companion Diagnostic Immunohistochemical Assay for Mirvetuximab Soravtansine. Arch. Pathol. Lab. Med. 2024, 148, 1226–1233. [Google Scholar] [CrossRef]
- Deutschman, E.; Fulton, R.; Sloss, C.M. Evaluation of Laboratory-Derived Immunohistochemical Assays for Folate Receptor α Expression in Epithelial Ovarian Cancer and Comparison With a Companion Diagnostic. Arch. Pathol. Lab. Med. 2025. ahead of print. [Google Scholar] [CrossRef]
- Lawson, B.C.; Marques-Piubelli, M.L.; Westin, S.N.; Malpica, A. Folate Receptor Immunohistochemical Staining and Gynecologic Tumors: Initial Experience with 216 Cases. Int. J. Gynecol. Pathol. 2024, 44, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zannoni, G.F.; Santoro, A.; d’Amati, A.; D’Alessandris, N.; Scaglione, G.; Padial Urtueta, B.; Valente, M.; Narducci, N.; Addante, F.; Spadola, S.; et al. Folate Receptor Alpha in Advanced Epithelial Ovarian Cancer: Diagnostic Role and Therapeutic Implications of a Clinically Validated Biomarker. Int. J. Mol. Sci. 2025, 26, 5222. [Google Scholar] [CrossRef]
- Martin, L.P.; Konner, J.A.; Moore, K.N.; Seward, S.M.; Matulonis, U.A.; Perez, R.P.; Su, Y.; Berkenblit, A.; Ruiz-Soto, R.; Birrer, M.J. Characterization of folate receptor alpha (FRα) expression in archival tumor and biopsy samples from relapsed epithelial ovarian cancer patients: A phase I expansion study of the FRα-targeting antibody-drug conjugate mirvetuximab soravtansine. Gynecol. Oncol. 2017, 147, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Dilawari, A.; Shah, M.; Ison, G.; Gittleman, H.; Fiero, M.H.; Shah, A.; Hamed, S.S.; Qiu, J.; Yu, J.; Manheng, W.; et al. FDA Approval Summary: Mirvetuximab Soravtansine-Gynx for FRα-Positive, Platinum-Resistant Ovarian Cancer. Clin. Cancer Res. 2023, 29, 3835–3840. [Google Scholar] [CrossRef] [PubMed]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, V.; Ullah, E.; Bychkov, A.; Song, A.H.; Walk, E.E.; Louis, P.; Rasool, G.; Singh, R.S.; Mahmood, F.; Bui, M.M.; et al. Generative Artificial Intelligence in Anatomic Pathology. Arch. Pathol. Lab. Med. 2025, 149, 298–318. [Google Scholar] [CrossRef] [PubMed]
- Humphries, M.P.; Maxwell, P.; Salto-Tellez, M. QuPath: The global impact of an open source digital pathology system. Comput. Struct. Biotechnol. J. 2021, 19, 852–859. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannoni, G.F.; Angelico, G.; d’Amati, A.; D’Alessandris, N.; Scaglione, G.; Urtueta, B.P.; Ferrara, G.; Caliò, A.; Campisi, P.; De Leo, A.; et al. Interobserver Agreement in Immunohistochemical Evaluation of Folate Receptor Alpha (FRα) in Ovarian Cancer: A Multicentre Study. Int. J. Mol. Sci. 2025, 26, 7687. https://doi.org/10.3390/ijms26167687
Zannoni GF, Angelico G, d’Amati A, D’Alessandris N, Scaglione G, Urtueta BP, Ferrara G, Caliò A, Campisi P, De Leo A, et al. Interobserver Agreement in Immunohistochemical Evaluation of Folate Receptor Alpha (FRα) in Ovarian Cancer: A Multicentre Study. International Journal of Molecular Sciences. 2025; 26(16):7687. https://doi.org/10.3390/ijms26167687
Chicago/Turabian StyleZannoni, Gian Franco, Giuseppe Angelico, Antonio d’Amati, Nicoletta D’Alessandris, Giulia Scaglione, Belen Padial Urtueta, Gerardo Ferrara, Anna Caliò, Paola Campisi, Antonio De Leo, and et al. 2025. "Interobserver Agreement in Immunohistochemical Evaluation of Folate Receptor Alpha (FRα) in Ovarian Cancer: A Multicentre Study" International Journal of Molecular Sciences 26, no. 16: 7687. https://doi.org/10.3390/ijms26167687
APA StyleZannoni, G. F., Angelico, G., d’Amati, A., D’Alessandris, N., Scaglione, G., Urtueta, B. P., Ferrara, G., Caliò, A., Campisi, P., De Leo, A., Guerini Rocco, E., Iuzzolino, M., Lerda, L., Paolini, B., Punzi, A., Vinci, M., Troncone, G., & Santoro, A. (2025). Interobserver Agreement in Immunohistochemical Evaluation of Folate Receptor Alpha (FRα) in Ovarian Cancer: A Multicentre Study. International Journal of Molecular Sciences, 26(16), 7687. https://doi.org/10.3390/ijms26167687









