The Interplay Between Oxidant/Antioxidant System, Transcription Factors, and Non-Coding RNA in Lung Cancer
Abstract
1. Introduction
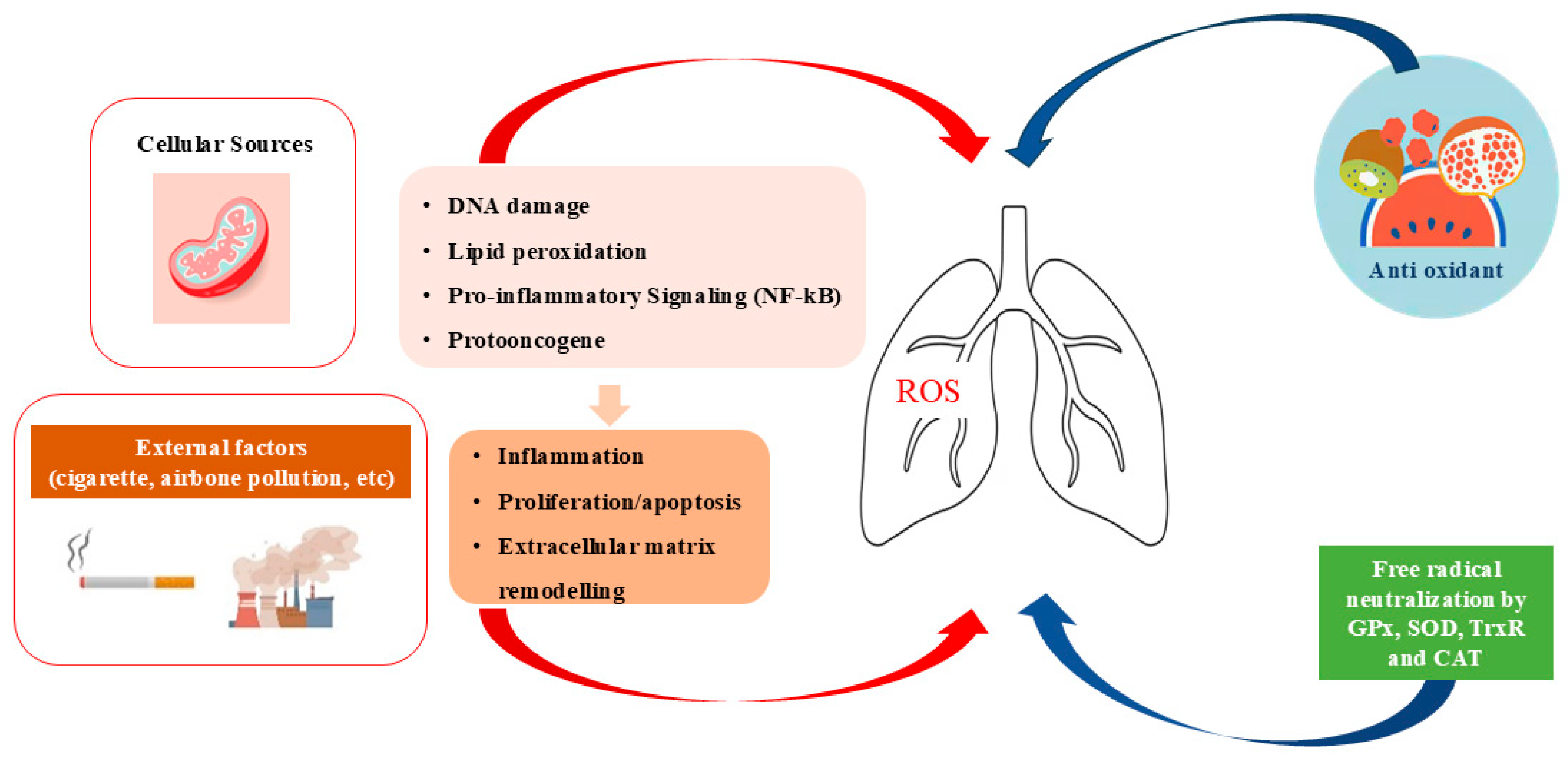
2. Oxidant/Antioxidant System
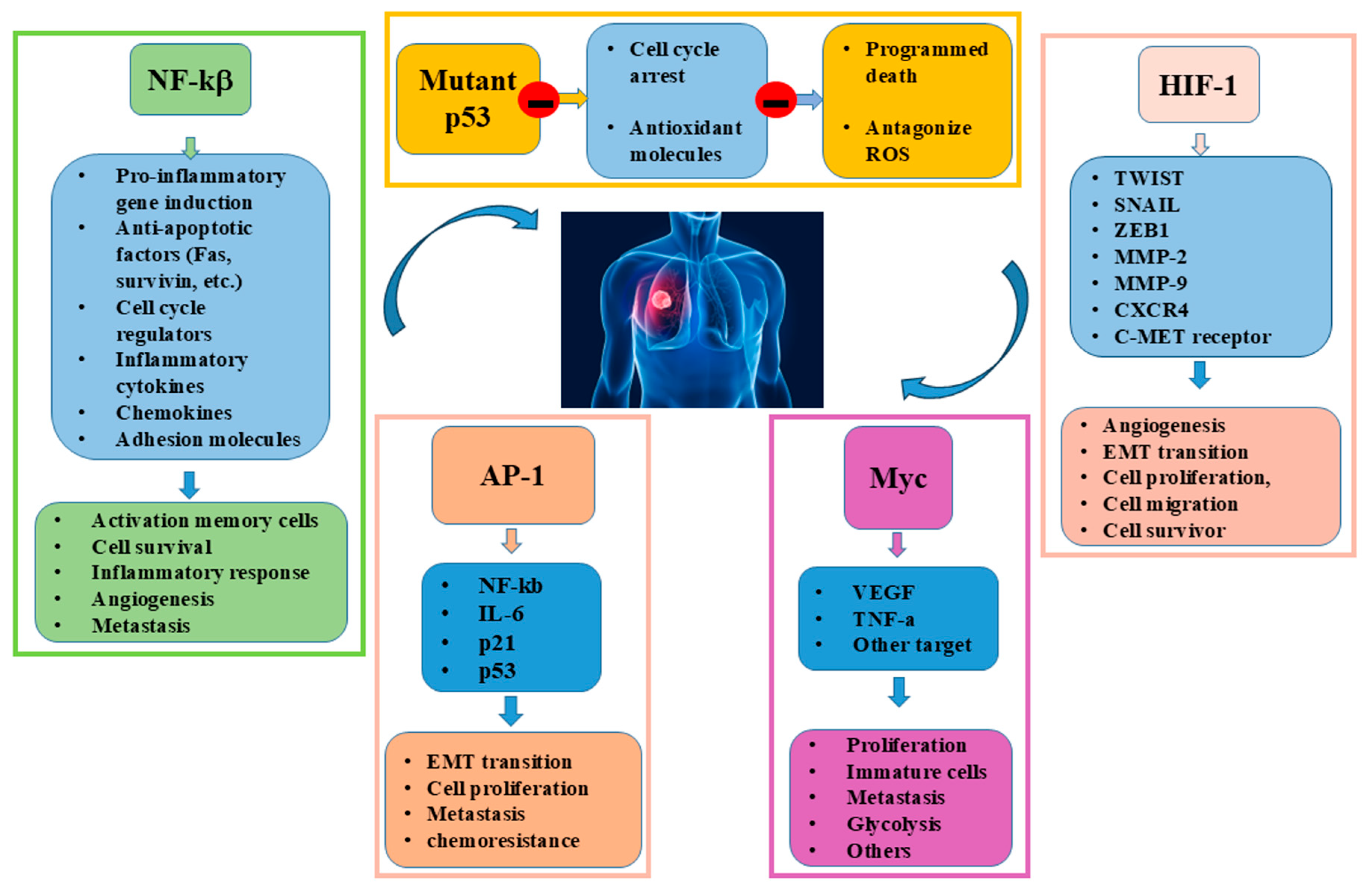
3. Transcription Factors in Lung Cancer
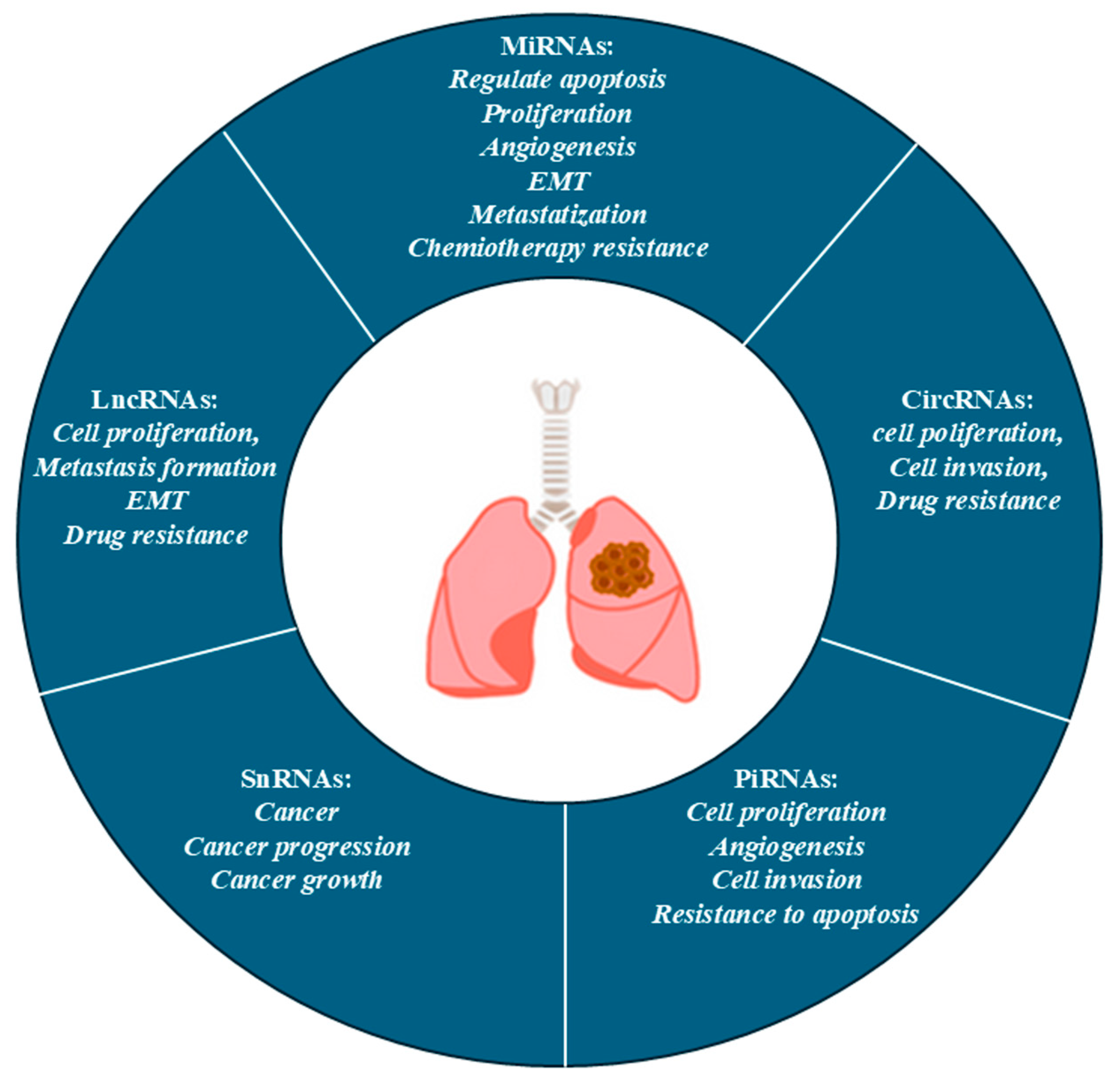
4. Role of nc-RNAs in Lung Cancer
4.1. MiRNA
4.2. LncRNAs
4.3. CircRNAs
4.4. PiRNA and Small nc-RNA
5. Interplay Between Oxidative Stress, nc-RNA, and TFs in Lung Cancer
5.1. Interplay and MYC
5.2. Interplay and p53
5.3. Interplay and NF-κB
5.4. Interplay and HIF-1α
5.5. Interplay of AP-1
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Araghi, M.; Mannani, R.; Heidarnejad Maleki, A.; Hamidi, A.; Rostami, S.; Safa, S.H.; Faramarzi, F.; Khorasani, S.; Alimohammadi, M.; Tahmasebi, S.; et al. Recent Advances in Non-Small Cell Lung Cancer Targeted Therapy; an Update Review. Cancer Cell Int. 2023, 23, 162. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Wang, X.; Li, J.; Li, W.; Wang, Z. Epigenetic Modifications in Early Stage Lung Cancer: Pathogenesis, Biomarkers, and Early Diagnosis. MedComm 2025, 6, e70080. [Google Scholar] [CrossRef]
- Gilyazova, I.; Gimalova, G.; Nizamova, A.; Galimova, E.; Ishbulatova, E.; Pavlov, V.; Khusnutdinova, E. Non-Coding RNAs as Key Regulators in Lung Cancer. Int. J. Mol. Sci. 2023, 25, 560. [Google Scholar] [CrossRef]
- Leng, X.; Zhang, M.; Xu, Y.; Wang, J.; Ding, N.; Yu, Y.; Sun, S.; Dai, W.; Xue, X.; Li, N.; et al. Non-Coding RNAs as Therapeutic Targets in Cancer and Its Clinical Application. J. Pharm. Anal. 2024, 14, 100947. [Google Scholar] [CrossRef]
- Chen, B.; Dragomir, M.P.; Yang, C.; Li, Q.; Horst, D.; Calin, G.A. Targeting Non-Coding RNAs to Overcome Cancer Therapy Resistance. Signal Transduct. Target. Ther. 2022, 7, 121. [Google Scholar] [CrossRef]
- Sun, Q.; Lei, X.; Yang, X. The Crosstalk between Non-Coding RNAs and Oxidative Stress in Cancer Progression. Genes Dis. 2025, 12, 101286. [Google Scholar] [CrossRef]
- D’Souza, L.C.; Mishra, S.; Chakraborty, A.; Shekher, A.; Sharma, A.; Gupta, S.C. Oxidative Stress and Cancer Development: Are Noncoding RNAs the Missing Links? Antioxid. Redox Signal. 2020, 33, 1209–1229. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Miserocchi, G.; Bocchini, M.; Cortesi, M.; Arienti, C.; De Vita, A.; Liverani, C.; Mercatali, L.; Bravaccini, S.; Ulivi, P.; Zanoni, M. Combining Preclinical Tools and Models to Unravel Tumor Complexity: Jump into the next Dimension. Front. Immunol. 2023, 14, 1171141. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Ding, C.; Wang, Y.; Lu, T.; Song, W. Plasma-Activated Medium Inhibited the Proliferation and Migration of Non-Small Cell Lung Cancer A549 Cells in 3D Culture. Int. J. Mol. Sci. 2024, 25, 13262. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Deng, Z.; Lei, C.; Ding, X.; Li, J.; Wang, C. The Role of Oxidative Stress in Tumorigenesis and Progression. Cells 2024, 13, 441. [Google Scholar] [CrossRef]
- ArulJothi, K.N.; Kumaran, K.; Senthil, S.; Nidhu, A.B.; Munaff, N.; Janitri, V.B.; Kirubakaran, R.; Singh, S.K.; Gupt, G.; Dua, K.; et al. Implications of Reactive Oxygen Species in Lung Cancer and Exploiting It for Therapeutic Interventions. Med. Oncol. 2022, 40, 43. [Google Scholar] [CrossRef]
- Hecht, F.; Zocchi, M.; Alimohammadi, F.; Harris, I.S. Regulation of Antioxidants in Cancer. Mol. Cell 2024, 84, 23–33. [Google Scholar] [CrossRef]
- Starlard-Davenport, A.; Palani, C.D.; Zhu, X.; Pace, B.S. Innovations in Drug Discovery for Sickle Cell Disease Targeting Oxidative Stress and NRF2 Activation-A Short Review. Int. J. Mol. Sci. 2025, 26, 4192. [Google Scholar] [CrossRef]
- Stading, R.; Chu, C.; Couroucli, X.; Lingappan, K.; Moorthy, B. Molecular Role of Cytochrome P4501A Enzymes Inoxidative Stress. Curr. Opin. Toxicol. 2020, 20–21, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Ornatowski, W.; Lu, Q.; Yegambaram, M.; Garcia, A.E.; Zemskov, E.A.; Maltepe, E.; Fineman, J.R.; Wang, T.; Black, S.M. Complex Interplay between Autophagy and Oxidative Stress in the Development of Pulmonary Disease. Redox Biol. 2020, 36, 101679. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Cordani, M.; Butera, G.; Dando, I.; Torrens-Mas, M.; Butturini, E.; Pacchiana, R.; Oppici, E.; Cavallini, C.; Gasperini, S.; Tamassia, N.; et al. Mutant P53 Blocks SESN1/AMPK/PGC-1α/UCP2 Axis Increasing Mitochondrial O2-· Production in Cancer Cells. Br. J. Cancer 2018, 119, 994–1008. [Google Scholar] [CrossRef]
- Cordani, M.; Butera, G.; Pacchiana, R.; Masetto, F.; Mullappilly, N.; Riganti, C.; Donadelli, M. Mutant P53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules 2020, 10, 361. [Google Scholar] [CrossRef]
- Nigam, M.; Mishra, A.P.; Deb, V.K.; Dimri, D.B.; Tiwari, V.; Bungau, S.G.; Bungau, A.F.; Radu, A.-F. Evaluation of the Association of Chronic Inflammation and Cancer: Insights and Implications. Biomed. Pharmacother. 2023, 164, 115015. [Google Scholar] [CrossRef]
- Theofani, E.; Xanthou, G. Autophagy: A Friend or Foe in Allergic Asthma? Int. J. Mol. Sci. 2021, 22, 6314. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several Lines of Antioxidant Defense against Oxidative Stress: Antioxidant Enzymes, Nanomaterials with Multiple Enzyme-Mimicking Activities, and Low-Molecular-Weight Antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox System in Health and Disease: The Latest Update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Yamada, R.; Sato, Y.; Togawa, R. Airway Smooth Muscle Regulated by Oxidative Stress in COPD. Antioxidants 2023, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Xing, F.; Hu, Q.; Qin, Y.; Xu, J.; Zhang, B.; Yu, X.; Wang, W. The Relationship of Redox With Hallmarks of Cancer: The Importance of Homeostasis and Context. Front. Oncol. 2022, 12, 862743. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Guo, J.; Xu, S.; Hou, Y.; Pan, F.; Guo, Z. Oxidative Stress Regulates CDH3 Expression in Lung Cancer Cells via OGG1-Mediated SP1 Binding. Antioxidants 2025, 14, 332. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Valko, R.; Liska, J.; Nepovimova, E.; Kuca, K.; Valko, M. Flavonoids and Their Role in Oxidative Stress, Inflammation, and Human Diseases. Chem.-Biol. Interact. 2025, 413, 111489. [Google Scholar] [CrossRef]
- Di Carlo, E.; Sorrentino, C. Oxidative Stress and Age-Related Tumors. Antioxidants 2024, 13, 1109. [Google Scholar] [CrossRef]
- Zhaorigetu; Farrag, I.M.; Belal, A.; Badawi, M.H.; Al Abdelhady, A.A.; Galala, F.M.A.A.; El-Sharkawy, A.; El-Dahshan, A.A.; Mehany, A.B.M. Antiproliferative, Apoptotic Effects and Suppression of Oxidative Stress of Quercetin against Induced Toxicity in Lung Cancer Cells of Rats: In Vitro and In Vivo Study. J. Cancer 2021, 12, 5249–5259. [Google Scholar] [CrossRef] [PubMed]
- Papavassiliou, K.A.; Sofianidi, A.A.; Gogou, V.A.; Papavassiliou, A.G. Leveraging the ROS-TME Axis for Cancer Treatment. Antioxidants 2024, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Papavassiliou, K.A.; Anagnostopoulos, N.; Papavassiliou, A.G. Lung Cancer through Transcription Factors. Int. J. Mol. Sci. 2023, 24, 9461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ludden, C.M.; Cullen, A.J.; Tew, K.D.; Branco de Barros, A.L.; Townsend, D.M. Nuclear Factor Kappa B Expression in Non-Small Cell Lung Cancer. Biomed. Pharmacother. 2023, 167, 115459. [Google Scholar] [CrossRef]
- Khusnurrokhman, G.; Wati, F.F. Tumor-Promoting Inflammation in Lung Cancer: A Literature Review. Ann. Med. Surg. 2022, 79, 104022. [Google Scholar] [CrossRef]
- Sherwood, A.M.; Yasseen, B.A.; DeBlasi, J.M.; Caldwell, S.; DeNicola, G.M. Distinct Roles for the Thioredoxin and Glutathione Antioxidant Systems in Nrf2-Mediated Lung Tumor Initiation and Progression. Redox Biol. 2025, 83, 103653. [Google Scholar] [CrossRef]
- Tracewell, M.A.; Karlin, J.E.; Barnada, S.M.; McDuffie, E.L.; Scott, C.P.; Barta, J.A.; McMahon, S.B. Somatic P53 Mutations That Are Markedly Overrepresented in Lung Cancer Confer Resistance to ROS-Induced Cell Death. Carcinogenesis 2025, 46, bgaf027. [Google Scholar] [CrossRef]
- Chen, D.; Lu, S.; Huang, K.; Pearson, J.D.; Pacal, M.; Peidis, P.; McCurdy, S.; Yu, T.; Sangwan, M.; Nguyen, A.; et al. Cell Cycle Duration Determines Oncogenic Transformation Capacity. Nature 2025, 641, 1309–1318. [Google Scholar] [CrossRef]
- Wadowska, K.; Bil-Lula, I.; Trembecki, Ł.; Śliwińska-Mossoń, M. Genetic Markers in Lung Cancer Diagnosis: A Review. Int. J. Mol. Sci. 2020, 21, 4569. [Google Scholar] [CrossRef]
- Ortmann, B.M. Hypoxia-Inducible Factor in Cancer: From Pathway Regulation to Therapeutic Opportunity. BMJ Oncol. 2024, 3, e000154. [Google Scholar] [CrossRef]
- Seephan, S.; Sasaki, S.-I.; Wattanathamsan, O.; Singharajkomron, N.; He, K.; Ucche, S.; Kungsukool, S.; Petchjorm, S.; Chantaravisoot, N.; Wongkongkathep, P.; et al. CAMSAP3 Negatively Regulates Lung Cancer Cell Invasion and Angiogenesis through Nucleolin/HIF-1α MRNA Complex Stabilization. Life Sci. 2023, 322, 121655. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic Microenvironment in Cancer: Molecular Mechanisms and Therapeutic Interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Kao, T.-W.; Bai, G.-H.; Wang, T.-L.; Shih, I.-M.; Chuang, C.-M.; Lo, C.-L.; Tsai, M.-C.; Chiu, L.-Y.; Lin, C.-C.; Shen, Y.-A. Novel Cancer Treatment Paradigm Targeting Hypoxia-Induced Factor in Conjunction with Current Therapies to Overcome Resistance. J. Exp. Clin. Cancer Res. 2023, 42, 171. [Google Scholar] [CrossRef]
- Jiang, Y.-X.; Yang, S.-W.; Li, P.-A.; Luo, X.; Li, Z.-Y.; Hao, Y.-X.; Yu, P.-W. The Promotion of the Transformation of Quiescent Gastric Cancer Stem Cells by IL-17 and the Underlying Mechanisms. Oncogene 2017, 36, 1256–1264. [Google Scholar] [CrossRef]
- Panneerselvam, J.; Jin, J.; Shanker, M.; Lauderdale, J.; Bates, J.; Wang, Q.; Zhao, Y.D.; Archibald, S.J.; Hubin, T.J.; Ramesh, R. IL-24 Inhibits Lung Cancer Cell Migration and Invasion by Disrupting the SDF-1/CXCR4 Signaling Axis. PLoS ONE 2015, 10, e0122439. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Li, M.; Wu, X.; Setrerrahmane, S.; Xu, H. Integrins as Attractive Targets for Cancer Therapeutics. Acta Pharm. Sinica B 2021, 11, 2726–2737. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, R.; Brown, J.S.; Gatenby, R.A.; Ibrahim-Hashim, A. A Gene for All Seasons: The Evolutionary Consequences of HIF-1 in Carcinogenesis, Tumor Growth and Metastasis. Semin. Cancer Biol. 2024, 102–103, 17–24. [Google Scholar] [CrossRef]
- Mollaoglu, G.; Guthrie, M.R.; Böhm, S.; Brägelmann, J.; Can, I.; Ballieu, P.M.; Marx, A.; George, J.; Heinen, C.; Chalishazar, M.D.; et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017, 31, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Wallbillich, N.J.; Lu, H. Role of C-Myc in Lung Cancer: Progress, Challenges, and Prospects. Chin. Med. J. Pulm. Crit. Care Med. 2023, 1, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; O’Grady, S.; Tang, M.; Crown, J. MYC as a Target for Cancer Treatment. Cancer Treat. Rev. 2021, 94, 102154. [Google Scholar] [CrossRef]
- Massó-Vallés, D.; Beaulieu, M.-E.; Soucek, L. MYC, MYCL, and MYCN as Therapeutic Targets in Lung Cancer. Expert Opin. Ther. Targets 2020, 24, 101–114. [Google Scholar] [CrossRef]
- Cargill, K.R.; Stewart, C.A.; Park, E.M.; Ramkumar, K.; Gay, C.M.; Cardnell, R.J.; Wang, Q.; Diao, L.; Shen, L.; Fan, Y.-H.; et al. Targeting MYC-Enhanced Glycolysis for the Treatment of Small Cell Lung Cancer. Cancer Metab. 2021, 9, 33. [Google Scholar] [CrossRef]
- Xue, X.; Li, Z.; Zhao, J.; Zhao, Z.; Li, Z.; Li, Y.; Liu, Y.; He, H. Advances in the Relationship between AP-1 and Tumorigenesis, Development and Therapy Resistance. Discov. Oncol. 2025, 16, 61. [Google Scholar] [CrossRef]
- Rasmi, R.R.; Sakthivel, K.M.; Guruvayoorappan, C. NF-ΚB Inhibitors in Treatment and Prevention of Lung Cancer. Biomed. Pharmacother. 2020, 130, 110569. [Google Scholar] [CrossRef]
- Uppaluri, K.R.; Challa, H.J.; Gaur, A.; Jain, R.; Krishna Vardhani, K.; Geddam, A.; Natya, K.; Aswini, K.; Palasamudram, K.; K, S.M. Unlocking the Potential of Non-Coding RNAs in Cancer Research and Therapy. Transl. Oncol. 2023, 35, 101730. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Bu, P. Non-Coding RNA in Cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Sweef, O.; Zaabout, E.; Bakheet, A.; Halawa, M.; Gad, I.; Akela, M.; Tousson, E.; Abdelghany, A.; Furuta, S. Unraveling Therapeutic Opportunities and the Diagnostic Potential of MicroRNAs for Human Lung Cancer. Pharmaceutics 2023, 15, 2061. [Google Scholar] [CrossRef]
- Pan, Z.; Tian, Y.; Niu, G.; Cao, C. Role of MicroRNAs in Remodeling the Tumor Microenvironment (Review). Int. J. Oncol. 2020, 56, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Fu, J.; Mantareva, V.; Blažević, I.; Wu, Y.; Wen, D.; Battulga, T.; Wang, Y.; Zhang, J. The Role of Tumor-Derived Exosomal LncRNA in Tumor Metastasis. Cancer Gene Ther. 2025, 32, 273–285. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A.; et al. Long Non-Coding RNAs: Definitions, Functions, Challenges and Recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Gencel-Augusto, J.; Wu, W.; Bivona, T.G. Long Non-Coding RNAs as Emerging Targets in Lung Cancer. Cancers 2023, 15, 3135. [Google Scholar] [CrossRef]
- Ge, X.; Shen, Z.; Yin, Y. Comprehensive Review of LncRNA-Mediated Therapeutic Resistance in Non-Small Cell Lung Cancer. Cancer Cell Int. 2024, 24, 369. [Google Scholar] [CrossRef]
- Pi, Y.-N.; Qi, W.-C.; Xia, B.-R.; Lou, G.; Jin, W.-L. Long Non-Coding RNAs in the Tumor Immune Microenvironment: Biological Properties and Therapeutic Potential. Front. Immunol. 2021, 12, 697083. [Google Scholar] [CrossRef]
- Duréndez-Sáez, E.; Torres-Martinez, S.; Calabuig-Fariñas, S.; Meri-Abad, M.; Ferrero-Gimeno, M.; Camps, C. Exosomal MicroRNAs in Non-Small Cell Lung Cancer. Transl. Cancer Res. 2021, 10, 3128–3139. [Google Scholar] [CrossRef]
- Cao, J.; Feng, B.; Xv, Y.; Yu, J.; Cao, S.; Ma, C. Continued Attention: The Role of Exosomal Long Non-Coding RNAs in Tumors over the Past Three Years. Int. Immunopharmacol. 2025, 144, 113666. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Sun, N.; He, J. Exosome-Derived LncRNAs in Lung Cancer. Front. Oncol. 2020, 10, 1728. [Google Scholar] [CrossRef]
- Chen, R.; Xu, X.; Qian, Z.; Zhang, C.; Niu, Y.; Wang, Z.; Sun, J.; Zhang, X.; Yu, Y. The Biological Functions and Clinical Applications of Exosomes in Lung Cancer. Cell. Mol. Life Sci. 2019, 76, 4613–4633. [Google Scholar] [CrossRef]
- Babayev, M.; Silveyra, P. Role of Circular RNAs in Lung Cancer. Front. Genet. 2024, 15, 1346119. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, E.; Remiszewski, P.; Stec, R. The Role of CircHIPK3 in Tumorigenesis and Its Potential as a Biomarker in Lung Cancer. Cells 2024, 13, 1483. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Zhang, Y.; Ding, J.; Yang, Q.; Xie, H.; Gao, X. CircHIPK3 Acts as Competing Endogenous RNA and Promotes Non-Small-Cell Lung Cancer Progression through the MiR-107/BDNF Signaling Pathway. BioMed Res. Int. 2020, 2020, 6075902. [Google Scholar] [CrossRef]
- Yang, J.; Yang, C.; Li, P. Circ-IARS Depletion Inhibits the Progression of Non-Small-Cell Lung Cancer by Circ-IARS/MiR-1252-5p/HDGF CeRNA Pathway. Open Med. 2023, 18, 20220613. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Qin, H.; Ponnusamy, M.; Chen, Y.; Lin, Z. PIWI-interacting RNA in Cancer: Molecular Mechanisms and Possible Clinical Implications (Review). Oncol. Rep. 2021, 46, 209. [Google Scholar] [CrossRef]
- Dong, X.; Ding, S.; Yu, M.; Niu, L.; Xue, L.; Zhao, Y.; Xie, L.; Song, X.; Song, X. Small Nuclear RNAs (U1, U2, U5) in Tumor-Educated Platelets Are Downregulated and Act as Promising Biomarkers in Lung Cancer. Front. Oncol. 2020, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Mourksi, N.-E.-H.; Morin, C.; Fenouil, T.; Diaz, J.-J.; Marcel, V. SnoRNAs Offer Novel Insight and Promising Perspectives for Lung Cancer Understanding and Management. Cells 2020, 9, 541. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Chen, Z.; Wu, G.; Guo, W.; Li, Y. A potential therapeutic target in lung cancer. Int. J. Oncol. 2025, 67, 67. [Google Scholar] [CrossRef]
- Xia, H.; Sun, S.; Wang, B.; Wang, T.; Liang, C.; Li, G.; Huang, C.; Qi, D.; Chu, X. miR-143 Inhibits NSCLC Cell Growth and Metastasis by Targeting Limk1. Int. J. Mol. Sci. 2014, 15, 11973–11983. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Terroba, E.; Plasek-Hegde, L.M.; Chiotakakos, I.; Li, V.; de Miguel, F.J.; Robles-Oteiza, C.; Tyagi, A.; Politi, K.; Zamudio, J.R.; Dimitrova, N. Overexpression of Malat1 drives metastasis through inflammatory reprogramming of the tumor microenvironment. Sci. Immunol. 2024, 9, eadh5462. [Google Scholar] [CrossRef]
- Qiao, X.; Ding, Y.; Wu, D.; Zhang, A.; Yin, Y.; Wang, Q.; Wang, W.; Kang, J. The roles of long noncoding RNA-mediated macrophage polarization in respiratory diseases. Front. Immunol. 2023, 13, 1110774. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Y.; Jiang, P. Circular RNA HIPK3 exerts oncogenic properties through suppression of miR-124 in lung cancer. Biochem. Biophys. Res. Commun. 2018, 506, 455–462. [Google Scholar] [CrossRef]
- Li, D.; Luo, Y.; Gao, Y.; Yang, Y.; Wang, Y.; Xu, Y.; Tan, S.; Zhang, Y.; Duan, J. piR-651 promotes tumor formation in non-small cell lung carcinoma through the upregulation of cyclin D1 and CDK4. Int. J. Mol. Med. 2016, 38, 927–936. [Google Scholar] [CrossRef]
- Yan, Y.; Ren, Y.; Bao, Y.; Wang, Y. RNA splicing alterations in lung cancer pathogenesis and therapy. Cancer Pathog. Ther. 2023, 1, 272–283. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Wang, J.; Ao, X.; Xue, J. Non-Coding RNAs in Lung Cancer: Molecular Mechanisms and Clinical Applications. Front. Oncol. 2023, 13, 1256537. [Google Scholar] [CrossRef]
- Xia, S.; Lu, X.; Wang, W.; Pan, X.; Cui, J.; Wang, S.; Wang, Z. The Regulatory Role and Therapeutic Potential of Long Non-Coding RNA in Non-Small Cell Lung Cancer. J. Cancer 2025, 16, 1137–1148. [Google Scholar] [CrossRef]
- Ao, Y.-Q.; Gao, J.; Jiang, J.-H.; Wang, H.-K.; Wang, S.; Ding, J.-Y. Comprehensive Landscape and Future Perspective of Long Noncoding RNAs in Non-Small Cell Lung Cancer: It Takes a Village. Mol. Ther. 2023, 31, 3389–3413. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long Non-Coding RNA HOTAIR Reprograms Chromatin State to Promote Cancer Metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Gupta, S.C.; Awasthee, N.; Rai, V.; Chava, S.; Gunda, V.; Challagundla, K.B. Long Non-Coding RNAs and Nuclear Factor-ΚB Crosstalk in Cancer and Other Human Diseases. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188316. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Tran, N. MiRNA Interplay: Mechanisms and Consequences in Cancer. Dis. Models Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Priya Dharshini, L.C.; Vishnupriya, S.; Sakthivel, K.M.; Rasmi, R.R. Oxidative Stress Responsive Transcription Factors in Cellular Signalling Transduction Mechanisms. Cell. Signal. 2020, 72, 109670. [Google Scholar] [CrossRef] [PubMed]
- Desind, S.Z.; Iacona, J.R.; Yu, C.Y.; Mitrofanova, A.; Lutz, C.S. PACER LncRNA Regulates COX-2 Expression in Lung Cancer Cells. Oncotarget 2022, 13, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, J.; Cao, W.; Xiao, X.; Liang, L.; Liu-Smith, F.; Wang, W.; Liu, H.; Zhou, P.; Ouyang, R.; et al. C-Myc/MiR-150/EPG5 Axis Mediated Dysfunction of Autophagy Promotes Development of Non-Small Cell Lung Cancer. Theranostics 2019, 9, 5134–5148. [Google Scholar] [CrossRef]
- García-Caballero, D.; Hart, J.R.; Vogt, P.K. Long Non-Coding RNAs as “MYC Facilitators”. Pathophysiology 2023, 30, 389–399. [Google Scholar] [CrossRef]
- Di Agostino, S. The Impact of Mutant P53 in the Non-Coding RNA World. Biomolecules 2020, 10, 472. [Google Scholar] [CrossRef]
- Napoli, M.; Flores, E.R. The P53 Family Reaches the Final Frontier: The Variegated Regulation of the Dark Matter of the Genome by the P53 Family in Cancer. RNA Biol. 2020, 17, 1636–1647. [Google Scholar] [CrossRef]
- Deka, K.; Li, Y. Transcriptional Regulation during Aberrant Activation of NF-ΚB Signalling in Cancer. Cells 2023, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Markopoulos, G.S.; Roupakia, E.; Tokamani, M.; Alabasi, G.; Sandaltzopoulos, R.; Marcu, K.B.; Kolettas, E. Roles of NF-ΚB Signaling in the Regulation of MiRNAs Impacting on Inflammation in Cancer. Biomedicines 2018, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Elsakka, E.G.E.; Midan, H.M.; Abulsoud, A.I.; Fathi, D.; Abdelmaksoud, N.M.; Abdel Mageed, S.S.; Zaki, M.B.; Abd-Elmawla, M.A.; Rizk, N.I.; Elrebehy, M.A.; et al. Emerging Insights: MiRNA Modulation of Ferroptosis Pathways in Lung Cancer. Exp. Cell Res. 2024, 442, 114272. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gao, H.; Xu, R.; Wang, H.; Mei, J.; Liu, C. The Interplay between HIF-1α and Noncoding RNAs in Cancer. J. Exp. Clin. Cancer Res. 2020, 39, 27. [Google Scholar] [CrossRef]
- Byun, Y.; Choi, Y.-C.; Jeong, Y.; Lee, G.; Yoon, S.; Jeong, Y.; Yoon, J.; Baek, K. MiR-200c Downregulates HIF-1α and Inhibits Migration of Lung Cancer Cells. Cell. Mol. Biol. Lett. 2019, 24, 28. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Kim, H.H.; Abusaliya, A.; Vetrivel, P.; Ha, S.E.; Park, M.Y.; Lee, H.J.; Kim, G.S. Structural and Functional Properties of Activator Protein-1 in Cancer and Inflammation. Evid.-Based Complement. Altern. Med. 2022, 2022, 9797929. [Google Scholar] [CrossRef]
| Class | ncRNA | Phenotype in Lung Cancer | Reference |
|---|---|---|---|
| miRNA | miR-21 | Oncogenic: ↑ proliferation, migration, invasion; ↓ apoptosis; promotes EMT via PTEN/Akt/GSK3β; confers chemoresistance | [76] |
| miRNA | miR-143 | Tumor suppressor: ↓ proliferation, migration, invasion; inhibits angiogenesis (in non-lung models); ↓ tumor growth in NSCLC models | [77] |
| lncRNA | MALAT1 | Oncogenic: promotes metastasis via ECM remodeling, motility gene regulation, and immunomodulation (e.g., via CCL2) | [78] |
| lncRNA | HOTAIR | Oncogenic: induces M2 macrophage polarization, creating immunosuppressive TME | [79] |
| circRNA | circHIPK3 | Oncogenic: sponges miR-124 → ↑ STAT3/CDK4 & CDK6 → ↑ proliferation in NSCLC | [80] |
| circRNA | circ-IARS | Oncogenic (exosomal): promotes malignancy via circ-IARS/miR-1252-5p/HDGF axis | [72] |
| piRNA | piR-651 | Oncogenic: overexpressed in NSCLC; ↑ proliferation and invasion via Cyclin D1/CDK4 | [81] |
| snRNA | U1, U2, U4, U5, U6 | Spliceosome components: mutations/alterations → aberrant splicing of oncogenes/tumor suppressors | [82] |
| Legend: ↑ = increase | ↓ = decrease | EMT = epithelial–mesenchymal transition | TME = tumor microenvironment | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sano, C.; D’Anna, C.; Montalbano, A.M.; Gjomarkaj, M.; Profita, M. The Interplay Between Oxidant/Antioxidant System, Transcription Factors, and Non-Coding RNA in Lung Cancer. Int. J. Mol. Sci. 2025, 26, 7679. https://doi.org/10.3390/ijms26167679
Di Sano C, D’Anna C, Montalbano AM, Gjomarkaj M, Profita M. The Interplay Between Oxidant/Antioxidant System, Transcription Factors, and Non-Coding RNA in Lung Cancer. International Journal of Molecular Sciences. 2025; 26(16):7679. https://doi.org/10.3390/ijms26167679
Chicago/Turabian StyleDi Sano, Caterina, Claudia D’Anna, Angela Marina Montalbano, Mark Gjomarkaj, and Mirella Profita. 2025. "The Interplay Between Oxidant/Antioxidant System, Transcription Factors, and Non-Coding RNA in Lung Cancer" International Journal of Molecular Sciences 26, no. 16: 7679. https://doi.org/10.3390/ijms26167679
APA StyleDi Sano, C., D’Anna, C., Montalbano, A. M., Gjomarkaj, M., & Profita, M. (2025). The Interplay Between Oxidant/Antioxidant System, Transcription Factors, and Non-Coding RNA in Lung Cancer. International Journal of Molecular Sciences, 26(16), 7679. https://doi.org/10.3390/ijms26167679







