Amphiregulin and Fibrosis: Existing Evidence and Future Directions
Abstract
1. Introduction
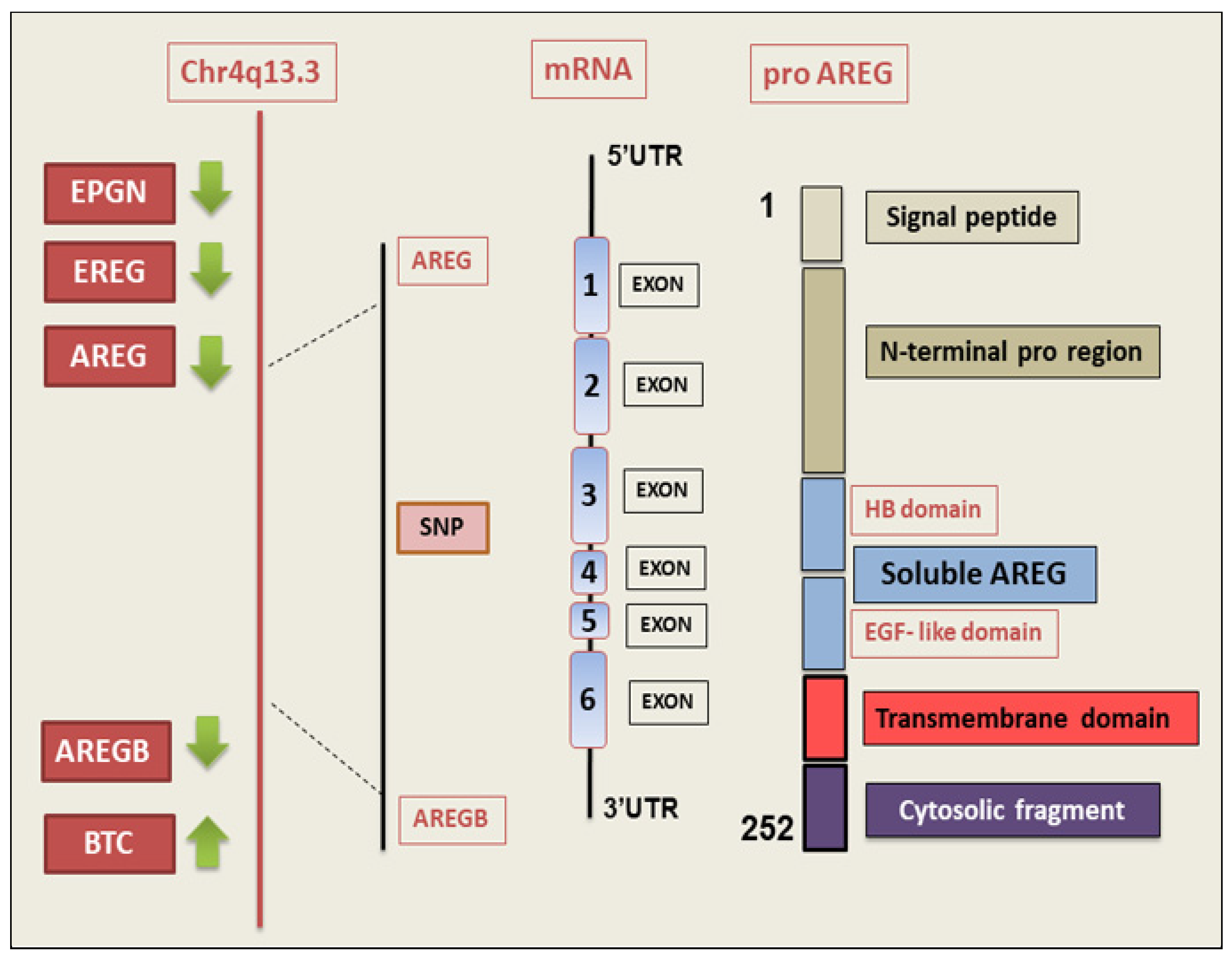
2. AREG Gene and Protein
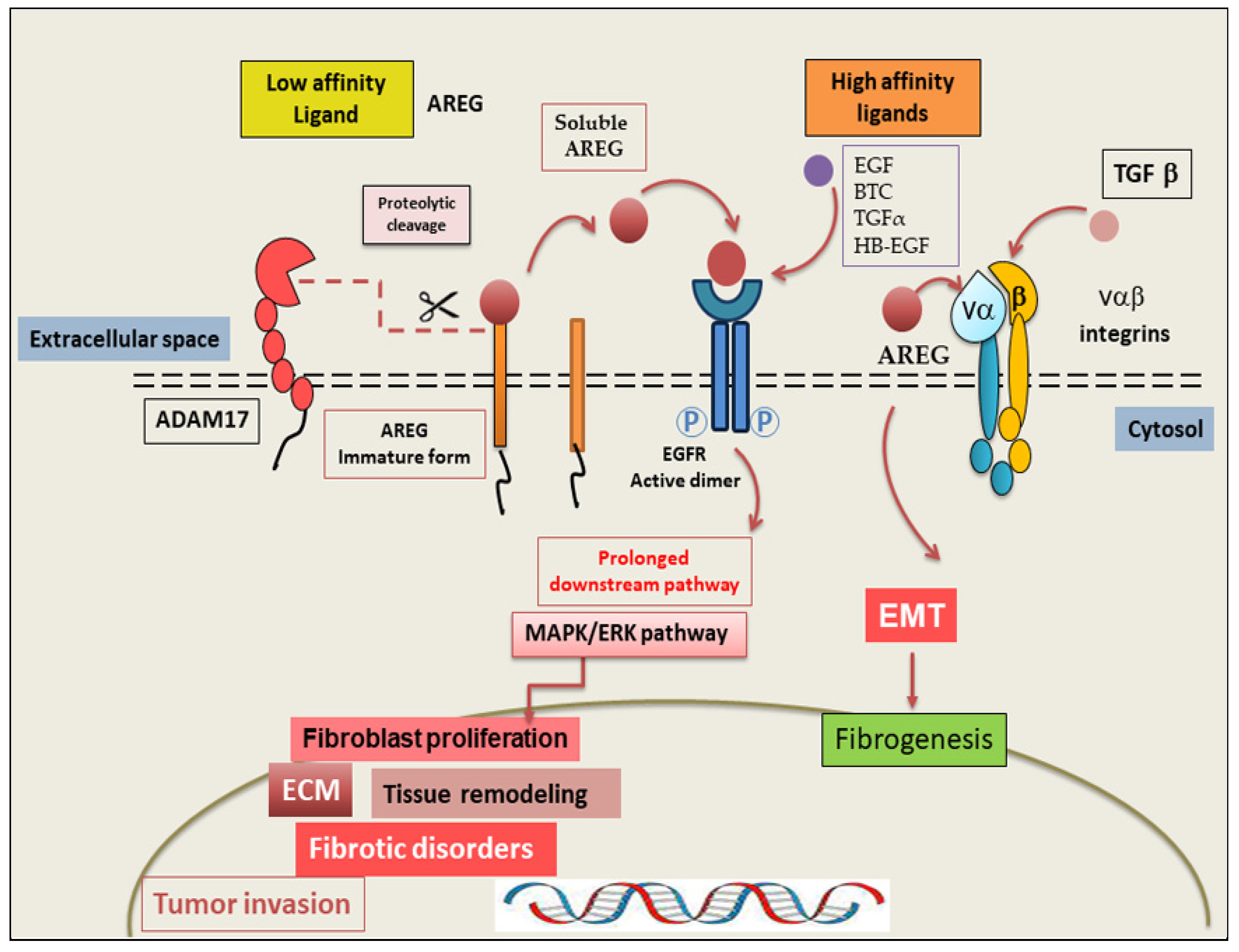
3. AREG: Mechanism of Action
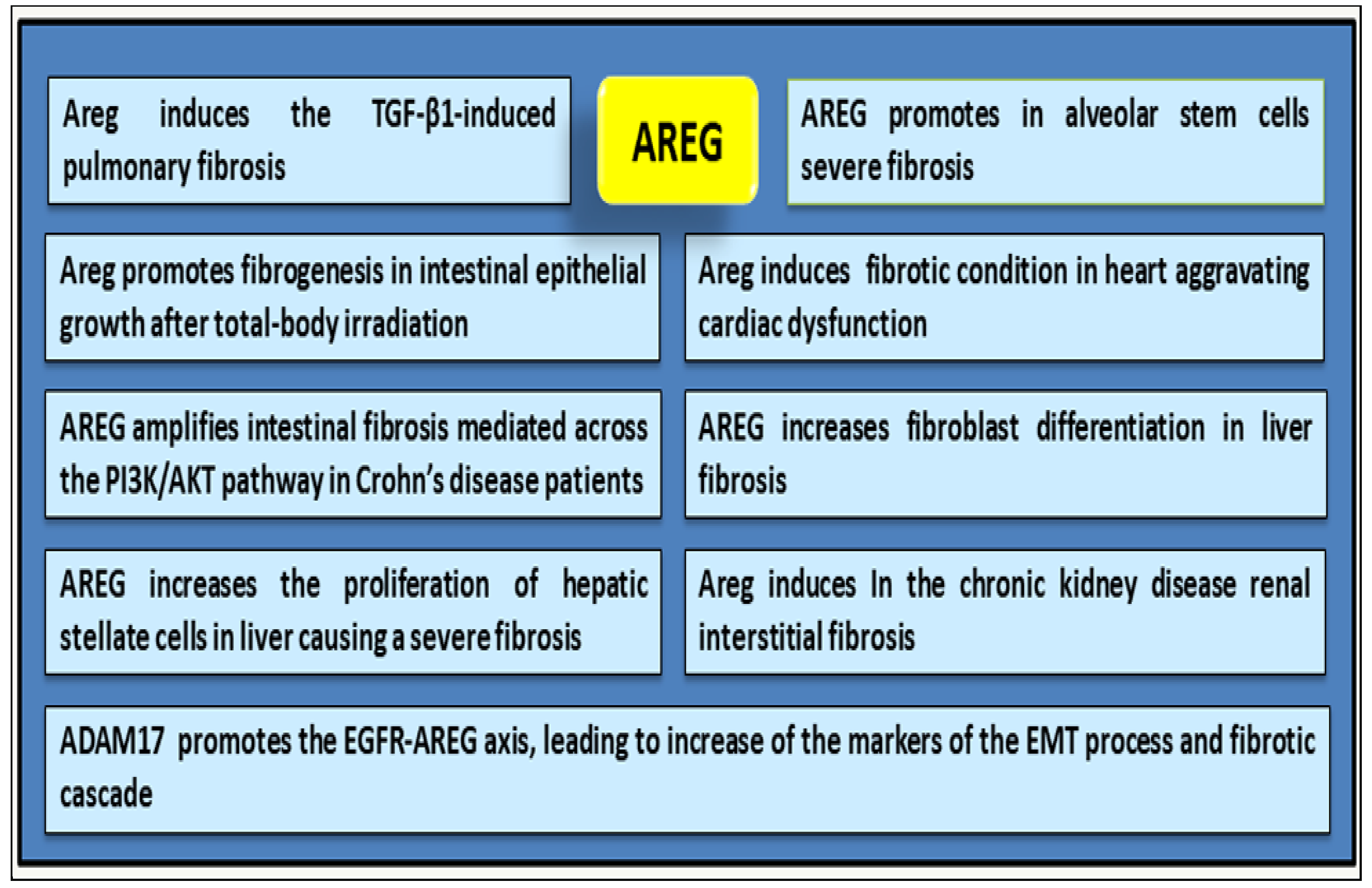
4. AREG as a Driver of Fibrotic Mechanisms
5. Analysis of the Correlation Between AREG Expression and Fibrosis in Autoimmune Diseases
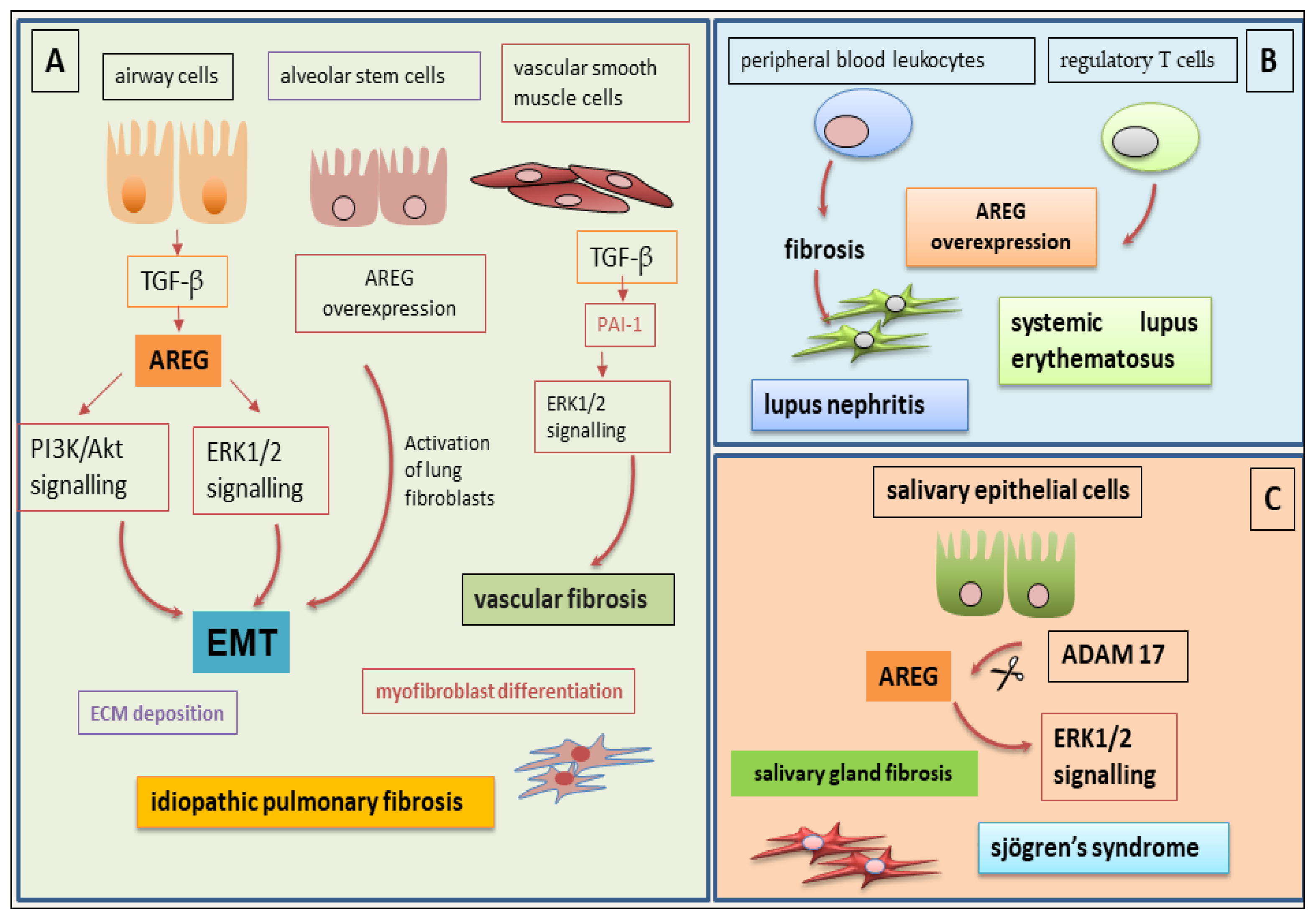
5.1. The Role of AREG in IPF-Related Fibrosis
5.2. AREG at the Basis of Fibrotic Phenomena in SLE
5.3. The Debated Role of AREG in Salivary Gland Fibrosis in SjD
6. Potential Application of Innovative Anti-AREG Therapies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar]
- Sisto, M.; Lisi, S. Towards a Unified Approach in Autoimmune Fibrotic Signalling Pathways. Int. J. Mol. Sci. 2023, 24, 9060. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lisi, S. Immune and Non-Immune Inflammatory Cells Involved in Autoimmune Fibrosis: New Discoveries. J. Clin. Med. 2023, 12, 3801. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Z.; Wang, G.; Geng, J.; Wu, H.; Liu, X.; Bin, E.; Sui, J.; Dai, H.; Tang, N. Sustained amphiregulin expression in intermediate alveolar stem cells drives progressive fibrosis. Cell Stem Cell 2024, 31, 1344–1358. [Google Scholar] [CrossRef]
- Savage, T.M.; Fortson, K.T.; de Los Santos-Alexis, K.; Oliveras-Alsina, A.; Rouanne, M.; Rae, S.S.; Gamarra, J.R.; Shayya, H.; Kornberg, A.; Cavero, R.; et al. Amphiregulin from regulatory T cells promotes liver fibrosis and insulin resistance in non-alcoholic steatohepatitis. Immunity 2024, 57, 303–318.e6. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Fang, S.; Gao, H.; Zhang, X.; Gu, D.; Liu, Y.; Wan, J.; Xie, J. A critical role of AREG for bleomycin-induced skin fibrosis. Cell Biosci. 2021, 11, 40. [Google Scholar] [CrossRef]
- Shoyab, M.; McDonald, V.L.; Bradley, J.G.; Todaro, G.J. Amphiregulin: A bifunctional growth-modulating glycoprotein produced by the phorbol 12-myristate 13-acetate-treated human breast adenocarcinoma cell line MCF-7. Proc. Natl. Acad. Sci. USA 1988, 85, 6528–6532. [Google Scholar] [CrossRef]
- Busser, B.; Sancey, L.; Brambilla, E.; Coll, J.L.; Hurbin, A. The multiple roles of amphiregulin in human cancer. Biochim. Biophys. Acta 2011, 1816, 119–131. [Google Scholar] [CrossRef]
- Nakanishi, T.; Koma, Y.-i.; Miyako, S.; Torigoe, R.; Yokoo, H.; Omori, M.; Yamanaka, K.; Ishihara, N.; Tsukamoto, S.; Kodama, T.; et al. AREG Upregulation in Cancer Cells via Direct Interaction with Cancer-Associated Fibroblasts Promotes Esophageal Squamous Cell Carcinoma Progression Through EGFR-Erk/p38 MAPK Signalling. Cells 2024, 13, 1733. [Google Scholar] [CrossRef]
- Zaiss, D.M.W.; Gause, W.C.; Osborne, L.C.; Artis, D. Emerging functions of amphiregulin in orchestrating immunity, inflammation, and tissue repair. Immunity 2015, 42, 216–226. [Google Scholar] [CrossRef]
- Zaiss, D.M.W. Amphiregulin as a driver of tissue fibrosis. Am. J. Transplant. 2020, 20, 631–632. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A.; Staros, J.V. Evolutionary analysis of the ErbB receptor and ligand families. J. Mol. Evol. 2000, 50, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.A.; Staros, J.V. Insights into the evolution of the ErbB receptor family and their ligands from sequence analysis. BMC Evol. Biol. 2006, 6, 79. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sebio, A.; Páez, D.; Salazar, J.; Berenguer-Llergo, A.; Paré-Brunet, L.; Lasa, A.; Del Río, E.; Tobeña, M.; Martín-Richard, M.; Baiget, M.; et al. Intergenic polymorphisms in the amphiregulin gene region as biomarkers in metastatic colorectal cancer patients treated with anti-EGFR plus irinotecan. Pharmacogenom. J. 2014, 14, 256–262. [Google Scholar] [CrossRef]
- Berasain, C.; Avila, M.A. Amphiregulin. Semin. Cell Dev. Biol. 2014, 28, 31–41. [Google Scholar] [CrossRef]
- Levano, K.S.; Kenny, P.A. Clarification of the C-terminal proteolytic processing site of human Amphiregulin. FEBS Lett. 2012, 586, 3500–3502. [Google Scholar] [CrossRef]
- Gephart, J.D.; Singh, B.; Higginbotham, J.N.; Franklin, J.L.; Gonzalez, A.; Fölsch, H.; Coffey, R.J. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic 2011, 12, 1793–1804. [Google Scholar] [CrossRef]
- Fukuda, H.; Nishida-Fukuda, H.; Nakayama, H.; Inoue, H.; Higashiyama, S. Monoubiquitination of pro-amphiregulin regulates its endocytosis and ectodomain shedding. Biochem. Biophys. Res. Commun. 2012, 420, 315–320. [Google Scholar] [CrossRef]
- Sahin, U.; Weskamp, G.; Kelly, K.; Zhou, H.M.; Higashiyama, S.; Peschon, J.; Hartmann, D.; Saftig, P.; Blobel, C.P. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 2004, 164, 769–779. [Google Scholar] [CrossRef]
- Singh, B.; Carpenter, G.; Coffey, R.J. EGF receptor ligands: Recent advances. F1000Research 2016, 5, 2270. [Google Scholar] [CrossRef]
- Berasain, C.; Ujue Latasa, M.; Urtasun, R.; Goñi, S.; Elizalde, M.; Garcia-Irigoyen, O.; Azcona, M.; Prieto, J.; Avila, M.A. Epidermal Growth Factor Receptor (EGFR) Crosstalks in Liver Cancer. Cancers 2011, 3, 2444–2461. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.L.; Meise, K.S.; Plowman, G.D.; Coffey, R.J.; Dempsey, P.J. Cell surface ectodomain cleavage of human amphiregulin precursor is sensitive to a metalloprotease inhibitor. Release of a predominant N-glycosylated 43-kDa soluble form. J. Biol. Chem. 1998, 273, 17258–17268. [Google Scholar] [CrossRef]
- Willmarth, N.E.; Ethier, S.P. Autocrine and juxtacrine effects of amphiregulin on the proliferative, invasive, and migratory properties of normal and neoplastic human mammary epithelial cells. J. Biol. Chem. 2006, 281, 37728–37737. [Google Scholar] [CrossRef] [PubMed]
- Shoyab, M.; Plowman, G.D.; McDonald, V.L.; Bradley, J.G.; Todaro, G.J. Structure and function of human amphiregulin: A member of the epidermal growth factor family. Science 1989, 243, 1074–1076. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gureasko, J.; Shen, K.; Cole, P.A.; Kuriyan, J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell 2006, 125, 1137–1149. [Google Scholar] [CrossRef]
- Thirukkumaran, O.M.; Kluba, M.; Hofkens, J.; Mizuno, H. Autophosphorylation of EGFR at Y954 Facilitated Homodimerization and Enhanced Downstream Signals. Biophys. J. 2020, 119, 2127–2137. [Google Scholar] [CrossRef]
- Macdonald-Obermann, J.L.; Pike, L.J. Different epidermal growth factor (EGF) receptor ligands show distinct kinetics and biased or partial agonism for homodimer and heterodimer formation. J. Biol. Chem. 2014, 289, 26178–26188. [Google Scholar] [CrossRef]
- Deguchi, E.; Lin, S.; Hirayama, D.; Matsuda, K.; Tanave, A.; Sumiyama, K.; Tsukiji, S.; Otani, T.; Furuse, M.; Sorkin, A.; et al. Low-affinity ligands of the epidermal growth factor receptor are long-range signal transmitters in collective cell migration of epithelial cells. Cell Rep. 2024, 43, 114986. [Google Scholar] [CrossRef]
- Schramm, F.; Schaefer, L.; Wygrecka, M. EGFR Signalling in Lung Fibrosis. Cells 2022, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.R.; Son, B.; Lee, C.G.; Park, H.-O. Amphiregulin in Fibrotic Diseases and Cancer. Preprint 2025, 2025061303. [Google Scholar] [CrossRef]
- Zhou, Y.; Lee, J.Y.; Lee, C.M.; Cho, W.K.; Kang, M.J.; Koff, J.L.; Yoon, P.O.; Chae, J.; Park, H.O.; Elias, J.A.; et al. Amphiregulin, an epidermal growth factor receptor ligand, plays an essential role in the pathogenesis of transforming growth factor-beta-induced pulmonary fibrosis. J. Biol. Chem. 2012, 287, 41991–42000. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Marshall, J.F. The role of integrins in TGF beta activation in the tumour stroma. Cell Tissue Res. 2016, 365, 657–673. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; Ten Dijke, P. TGF-beta-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef]
- Lofgren, K.A.; Sreekumar, S.; Jenkins, E.C., Jr.; Ernzen, K.J.; Kenny, P.A. Anti-tumor efficacy of an MMAE conjugated antibody targeting cell surface TACE/ADAM17-cleaved Amphiregulin in breast cancer. Antib. Ther. 2021, 4, 252–261. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, N. EGFR signalling in renal fibrosis. Kidney Int. 2014, 4, 70–74. [Google Scholar] [CrossRef]
- Ding, L.; Liu, T.; Wu, Z.; Hu, B.; Nakashima, T.; Ullenbruch, M.; Gonzalez De Los Santos, F.; Phan, S.H. Bone Marrow CD11c+ Cell-Derived Amphiregulin Promotes Pulmonary Fibrosis. J. Immunol. 2016, 197, 303–312. [Google Scholar] [CrossRef]
- Ji, M.; Liu, Y.; Zuo, Z.; Xu, C.; Lin, L.; Li, Y. Downregulation of amphiregulin improves cardiac hypertrophy via attenuating oxidative stress and apoptosis. Biol. Direct. 2022, 17, 21. [Google Scholar] [CrossRef]
- Son, B.; Kim, T.R.; Park, J.H.; Yun, S.I.; Choi, H.; Choi, J.W.; Jeon, C.; Park, H.O. SAMiRNA Targeting Amphiregulin Alleviate Total-Body-Irradiation-Induced Renal Fibrosis. Radiat. Res. 2022, 197, 471–479. [Google Scholar] [CrossRef]
- Shao, J.; Sheng, H. Amphiregulin promotes intestinal epithelial regeneration: Roles of intestinal subepithelial myofibroblasts. Endocrinology 2010, 151, 3728–3737. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, L.; Lin, J.; Wang, M.; Li, J.; Guo, Q.; Jiao, C.; Tang, N.; Ma, J.; Zhang, H.; et al. Inflammatory monocyte-derived amphiregulin mediates intestinal fibrosis in Crohn’s disease by activating PI3K/AKT. Mucosal Immunol. 2025, 25, S1933. [Google Scholar] [CrossRef] [PubMed]
- Maranatha, D.; Hasan, H.; Bakhtiar, A.; Widyoningroem, A.; Aryati. Association of TNF-α, TGF-β1, amphiregulin, IL-2, and EGFR WITH pulmonary fibrosis in COVID-19. J. Infect. Public Health 2022, 15, 1072–1075. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Lin, J.; Li, J.; Wang, M.; Yu, J.; Sun, J.; Tang, N.; Jiao, C.; Ma, J.; et al. Treg and intestinal myofibroblasts-derived amphiregulin induced by TGF-β mediates intestinal fibrosis in Crohn’s disease. J. Transl. Med. 2025, 23, 452. [Google Scholar] [CrossRef]
- McKee, C.; Sigala, B.; Soeda, J.; Mouralidarane, A.; Morgan, M.; Mazzoccoli, G.; Rappa, F.; Cappello, F.; Cabibi, D.; Pazienza, V.; et al. Amphiregulin activates human hepatic stellate cells and is upregulated in non alcoholic steatohepatitis. Sci. Rep. 2015, 5, 8812. [Google Scholar] [CrossRef]
- Fujiwara, A.; Takemura, K.; Tanaka, A.; Matsumoto, M.; Katsuyama, M.; Okanoue, T.; Yamaguchi, K.; Itoh, Y.; Iwata, K.; Amagase, K.; et al. Carfilzomib shows therapeutic potential for reduction of liver fibrosis by targeting hepatic stellate cell activation. Sci. Rep. 2024, 14, 19288. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, W.M.; Sun, H.Y.; Peng, Y.; Huang, R.J.; Chen, C.Y.; Zhang, H.D.; Zhou, S.A.; Wu, H.P.; Tang, D.; et al. Hepatocyte-derived liver progenitor-like cells attenuate liver cirrhosis via induction of apoptosis in hepatic stellate cells. Hepatol. Commun. 2025, 9, e0614. [Google Scholar] [CrossRef]
- Liu, T.; De Los Santos, F.G.; Ding, L.; Wu, Z.; Phan, S.H. Amphiregulin Promotes Fibroblast Activation in Pulmonary Fibrosis. FASEB J. 2016, 30, 50.6. [Google Scholar] [CrossRef]
- Cheng, W.H.; Kao, S.Y.; Chen, C.L.; Yuliani, F.S.; Lin, L.Y.; Lin, C.H.; Chen, B.C. Amphiregulin induces CCN2 and fibronectin expression by TGF-beta through EGFR-dependent pathway in lung epithelial cells. Respir. Res. 2022, 23, 381. [Google Scholar] [CrossRef]
- Son, S.S.; Hwang, S.; Park, J.H.; Ko, Y.; Yun, S.I.; Lee, J.H.; Son, B.; Kim, T.R.; Park, H.O.; Lee, E.Y. In vivo silencing of amphiregulin by a novel effective Self-Assembled-Micelle inhibitory RNA ameliorates renal fibrosis via inhibition of EGFR signals. Sci. Rep. 2021, 11, 2191. [Google Scholar] [CrossRef] [PubMed]
- Tito, C.; Masciarelli, S.; Colotti, G.; Fazi, F. EGF receptor in organ development, tissue homeostasis and regeneration. J. Biomed. Sci. 2025, 32, 24. [Google Scholar] [CrossRef]
- Melderis, S.; Warkotsch, M.T.; Dang, J.; Hagenstein, J.; Ehnold, L.I.; Herrnstadt, G.R.; Niehus, C.B.; Feindt, F.C.; Kylies, D.; Puelles, V.G.; et al. The Amphiregulin/EGFR axis protects from Lupus nephritis via downregulation of pathogenic CD4+ T helper cell responses. J. Autoimmun. 2022, 129, 102829. [Google Scholar] [CrossRef] [PubMed]
- Dreschers, S.; Platen, C.; Oppermann, L.; Doughty, C.; Ludwi, G.A.; Babendreyer, A.; Orlikowsky, T.W. EGF-Receptor against Amphiregulin (AREG) Influences Costimulatory Molecules on Monocytes and T Cells and Modulates T-Cell Responses. J. Immunol. Res. 2023, 2023, 8883045. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, G.; Cerveró-Varona, A.; Perugini, M.; Sulcanese, L.; Iannetta, A.; Haidar-Montes, A.A.; Stöckl, J.; Canciello, A.; Berardinelli, P.; Russo, V.; et al. Amphiregulin orchestrates the paracrine immune-suppressive function of amniotic-derived cells through its interplay with COX-2/PGE2/EP4 axis. iScience 2024, 27, 110508. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Cho, S.J.; Lee, C.G.; Homer, R.J.; Elias, J.A. Transforming growth factor (TGF)-beta1 stimulates pulmonary fibrosis and inflammation via a Bax-dependent, bid-activated pathway that involves matrix metalloproteinase-12. J. Biol. Chem. 2007, 282, 7723–7732. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Aashaq, S.; Batool, A.; Mir, S.A.; Beigh, M.A.; Andrabi, K.I.; Shah, Z.A. TGF-β signalling: A recap of SMAD-independent and SMAD-dependent pathways. J. Cell Physiol. 2022, 237, 59–85. [Google Scholar] [CrossRef]
- Lee, C.G.; Cho, S.J.; Kang, M.J.; Chapoval, S.P.; Lee, P.J.; Noble, P.W.; Yehualaeshet, T.; Lu, B.; Flavell, R.A.; Milbrandt, J.; et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J. Exp. Med. 2004, 200, 377–789. [Google Scholar] [CrossRef]
- Kang, H.R.; Lee, C.G.; Homer, R.J.; Elias, J.A. Semaphorin 7A plays a critical role in TGF-beta1-induced pulmonary fibrosis. J. Exp. Med. 2007, 204, 1083–1093. [Google Scholar] [CrossRef]
- Kang, H.R.; Lee, J.Y.; Lee, C.G. TGF-β1 as a therapeutic target for pulmonary fibrosis and COPD. Expert Rev. Clin. Pharmacol. 2008, 1, 547–558. [Google Scholar] [CrossRef]
- Samarakoon, R.; Higgins, P.J. Integration of non-SMAD and SMAD signalling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells. Thromb. Haemost. 2008, 100, 976–983. [Google Scholar] [CrossRef]
- Liu, M.; Yang, S.C.; Sharma, S.; Luo, J.; Cui, X.; Peebles, K.A.; Huang, M.; Sato, M.; Ramirez, R.D.; Shay, J.W.; et al. EGFR signalling is required for TGF-beta 1 mediated COX-2 induction in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2007, 37, 578–588. [Google Scholar] [CrossRef]
- Wang, S.W.; Oh, C.K.; Cho, S.H.; Hu, G.; Martin, R.; Demissie-Sanders, S.; Li, K.; Moyle, M.; Yao, Z. Amphiregulin expression in human mast cells and its effect on the primary human lung fibroblasts. J. Allergy Clin. Immunol. 2005, 115, 287–294. [Google Scholar] [CrossRef]
- Schuger, L.; Johnson, G.R.; Gilbride, K.; Plowman, G.D.; Mandel, R. Amphiregulin in lung branching morphogenesis: Interaction with heparan sulfate proteoglycan modulates cell proliferation. Development 1996, 122, 1759–1767. [Google Scholar] [CrossRef]
- Ishii, Y.; Fujimoto, S.; Fukuda, T. Gefitinib prevents bleomycin-induced lung fibrosis in mice. Am. J. Respir. Crit. Care Med. 2006, 174, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, J.; Harada, C.; Kawaguchi, T.; Suetsugu, S.; Maeyama, T.; Inoshima, I.; Hamada, N.; Kuwano, K.; Nakanishi, Y. Amphiregulin attenuates bleomycin-induced pneumopathy in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 298, L131–L138. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, M.; Kang, H.R.; Homer, R.J.; Chapoval, S.P.; Cho, S.J.; Lee, B.J.; Elias, J.A.; Lee, C.G. P21 regulates TGF-beta1-induced pulmonary responses via a TNF-alpha-signalling pathway. Am. J. Respir. Cell Mol. Biol. 2008, 38, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, B.; Faulkner, K. Overview of the tolerability of gefitinib (IRESSA) monotherapy: Clinical experience in non-small-cell lung cancer. Drug Saf. 2004, 27, 1081–1092. [Google Scholar] [CrossRef]
- Ding, B.S.; Nolan, D.J.; Guo, P.; Babazadeh, A.O.; Cao, Z.; Rosenwaks, Z.; Crystal, R.G.; Simons, M.; Sato, T.N.; Worgall, S.; et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell 2011, 147, 539–553. [Google Scholar] [CrossRef]
- Ciarloni, L.; Mallepell, S.; Brisken, C. Amphiregulin is an essential mediator of estrogen receptor alpha function in mammary gland development. Proc. Natl. Acad. Sci. USA 2007, 104, 5455–5460. [Google Scholar] [CrossRef]
- Jay, F.F.; Vaidya, M.; Porada, S.M.; Andrukhova, O.; Schneider, M.R.; Erben, R.G. Amphiregulin lacks an essential role for the bone anabolic action of parathyroid hormone. Mol. Cell Endocrinol. 2015, 417, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, T.R.; Kim, S.H.; Kim, I.H.; Ko, Y.; Yun, S.; Lee, I.C.; Park, H.O.; Kim, J.C. Genotoxicity evaluation of self-assembled-micelle inhibitory RNA-targeting amphiregulin (SAMiRNA-AREG), a novel siRNA nanoparticle for the treatment of fibrotic disease. Drug Chem. Toxicol. 2022, 45, 2109–2115. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Kim, T.R.; Kim, S.H.; Kim, I.H.; Lim, J.O.; Park, J.H.; Yun, S.; Lee, I.C.; Park, H.O.; Kim, J.C. Four-Week Repeated Intravenous Dose Toxicity of Self-Assembled-Micelle Inhibitory RNA-Targeting Amphiregulin in Mice. Int. J. Toxicol. 2021, 40, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.R.; Kim, H.Y.; Kim, I.H.; Kim, K.C.; Ko, Y.; Park, J.H.; Yun, S.; Lee, I.C.; Kim, S.H.; Park, H.O. Safety pharmacology of self-assembled-micelle inhibitory RNA-targeting amphiregulin (SAMiRNA-AREG), a novel siRNA nanoparticle platform. Toxicol Rep. 2021, 8, 839–845. [Google Scholar] [CrossRef]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. Rev. Dis. Primers 2016, 2, 16039. [Google Scholar] [CrossRef]
- Anders, H.J.; Saxena, R.; Zhao, M.H.; Parodis, I.; Salmon, J.E.; Mohan, C. Lupus nephritis. Nat. Rev. Dis. Primers 2020, 6, 7. [Google Scholar] [CrossRef]
- Melderis, S.; Wiech, T.; Iking-Konert, C.; Steinmetz, O.M. Lupusnephritis [Lupus nephritis]. Z. Rheumatol. 2018, 77, 593–608. [Google Scholar] [CrossRef]
- Ishii, T.; Onda, H.; Tanigawa, A.; Ohshima, S.; Fujiwara, H.; Mima, T.; Katada, Y.; Deguchi, H.; Suemura, M.; Miyake, T.; et al. Isolation and expression profiling of genes upregulated in the pe-ripheral blood cells of systemic lupus erythematosus patients. DNA Res. 2005, 12, 429–439. [Google Scholar] [CrossRef]
- Ko, J.H.; Kim, H.J.; Jeong, H.J.; Lee, H.J.; Oh, J.Y. Mesenchymal Stem and Stromal Cells Harness Macrophage-Derived Amphiregulin to Maintain Tissue Homeostasis. Cell Rep. 2020, 30, 3806–3820.e6. [Google Scholar] [CrossRef]
- Minutti, C.M.; Modak, R.V.; Macdonald, F.; Li, F.; Smyth, D.J.; Dorward, D.A.; Blair, N.; Husovsky, C.; Muir, A.; Giampazolias, E.; et al. A Macrophage-Pericyte Axis Directs Tissue Restoration via Amphiregulin-Induced Transforming Growth Factor Beta Activation. Immunity 2019, 50, 645–654.e6. [Google Scholar] [CrossRef]
- Ehnold, L.I.; Melderis, S.; Hagenstein, J.; Warkotsch, M.T.; Laas, V.; Feindt, F.C.; Wu, H.; Huber, T.B.; Grahammer, F.; Steinmetz, O.M. Treg derived Amphiregulin protects from murine Lupus nephritis via tissue reparative effects. Sci. Rep. 2025, 15, 7776. [Google Scholar] [CrossRef]
- Zaiss, D.M.; van Loosdregt, J.; Gorlani, A.; Bekker, C.P.; Gröne, A.; Sibilia, M.; van Bergen en Henegouwen, P.M.; Roovers, R.C.; Coffer, P.J.; Sijts, A.J. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity 2013, 38, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Wachtendorf, S.; Jonin, F.; Ochel, A.; Heinrich, F.; Westendorf, A.M.; Tiegs, G.; Neumann, K. The ST2+ Treg/amphiregulin axis protects from immune-mediated hepatitis. Front. Immunol. 2024, 15, 1351405. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirahara, K.; Kiuchi, M.; Wada, T.; Ichikawa, T.; Kanno, T.; Okano, M.; Kokubo, K.; Onodera, A.; Sakurai, D.; et al. Amphiregulin-Producing Pathogenic Memory T Helper 2 Cells Instruct Eosinophils to Secrete Osteopontin and Facilitate Airway Fibrosis. Immunity 2018, 49, 134–150.e6. [Google Scholar] [CrossRef] [PubMed]
- Lisi, S.; Sisto, M.; Lofrumento, D.D.; Cucci, L.; Frassanito, M.A.; Mitolo, V.; D’Amore, M. Pro-inflammatory role of Anti-Ro/SSA autoantibodies through the activation of Furin-TACE-amphiregulin axis. J. Autoimmun. 2010, 35, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Yamane, S.; Ishida, S.; Hanamoto, Y.; Kumagai, K.; Masuda, R.; Tanaka, K.; Shiobara, N.; Yamane, N.; Mori, T.; Juji, T.; et al. Proinflammatory role of Amphiregulin, an epidermal growth factor family member whose expression is augmented in rheumatoid arthritis patients. J. Inflamm. 2008, 5, 5. [Google Scholar] [CrossRef]
- Cook, P.W.; Piepkorn, M.; Clegg, C.H.; Plowman, G.D.; DeMay, J.M.; Brown, J.R.; Pittelkow, M.R. Transgenic expression of the human amphiregulin gene induces a psoriasis-like phenotype. J. Clin. Investig. 1997, 100, 2286–2294. [Google Scholar] [CrossRef]
- Kefaloyianni, E.; Keerthi Raja, M.R.; Schumacher, J.; Muthu, M.L.; Krishnadoss, V.; Waikar, S.S.; Herrlich, A. Proximal Tubule-Derived Amphiregulin Amplifies and Integrates Profibrotic EGF Receptor Signals in Kidney Fibrosis. J. Am. Soc. Nephrol. 2019, 30, 2370–2383. [Google Scholar] [CrossRef]
- Basheva-Kraeva, Y.M.; Kraev, K.I.; Uchikov, P.A.; Kraeva, M.I.; Hristov, B.K.; Stoyanova, N.S.; Mitkova-Hristova, V.T.; Ivanov, B.; Karamitev, S.S.; Koleva, N.; et al. Seronegative Sicca Syndrome: Diagnostic Considerations and Management Strategies. Life 2025, 15, 966. [Google Scholar] [CrossRef]
- Gorgoulis, V.; Giatromanolaki, A.; Iliopoulos, A.; Kanavaros, P.; Aninos, D.; Ioakeimidis, D.; Kontomerkos, T.; Karameris, A. EGF and EGF-r immunoexpression in Sjögren’s syndrome secondary to rheumatoid arthritis. Correlation with EBV expression? Clin. Exp. Rheumatol. 1993, 11, 623–627. [Google Scholar]
- Nakamura, H.; Kawakami, A.; Ida, H.; Koji, T.; Eguchi, K. Epidermal growth factor inhibits Fas-mediated apoptosis in salivary epithelial cells of patients with primary Sjögren’s syndrome. Clin. Exp. Rheumatol. 2007, 25, 831–837. [Google Scholar] [PubMed]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef] [PubMed]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-kappaB and IkappaB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sisto, M.; Lisi, S.; D’Amore, M.; Lofrumento, D.D. The metalloproteinase ADAM17 and the epidermal growth factor receptor (EGFR) signalling drive the inflammatory epithelial response in Sjögren’s syndrome. Clin. Exp. Med. 2015, 15, 215–225. [Google Scholar] [CrossRef]
- Sisto, M.; Lisi, S.; Lofrumento, D.D.; Ingravallo, G.; Mitolo, V.; D’Amore, M. Expression of pro-inflammatory TACE-TNF-α-amphiregulin axis in Sjögren’s syndrome salivary glands. Histochem. Cell Biol. 2010, 134, 345–353. [Google Scholar] [CrossRef]
- Lisi, S.; D’Amore, M.; Sisto, M. ADAM17 at the interface between inflammation and autoimmunity. Immunol. Lett. 2014, 162, 159–169. [Google Scholar] [CrossRef]
- Kawasaki, S.; Kawamoto, S.; Yokoi, N.; Connon, C.; Minesaki, Y.; Kinoshita, S.; Okubo, K. Up-regulated gene expression in the conjunctival epithelium of patients with Sjögren’s syndrome. Exp. Eye Res. 2003, 77, 17–26. [Google Scholar] [CrossRef]
- Qi, W.; Tian, J.; Wang, G.; Yan, Y.; Wang, T.; Wei, Y.; Wang, Z.; Zhang, G.; Zhang, Y.; Wang, J. Advances in cellular and molecular pathways of salivary gland damage in Sjögren’s syndrome. Front. Immunol. 2024, 15, 1405126. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Zhao, L.; Guo, D.; Yi, H.; Yang, L.; Liu, X.; Sun, D.; Nian, H.; Wei, R. Human umbilical cord mesenchymal stem cells alleviate ongoing autoimmune dacryoadenitis in rabbits via polarizing macrophages into an anti-inflammatory phenotype. Exp. Eye Res. 2020, 191, 107905. [Google Scholar] [CrossRef]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-mesenchymal transitions: The importance of changing cells state in development and disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Sisto, M.; Lorusso, L.; Ingravallo, G.; Tamma, R.; Ribatti, D.; Lisi, S. The TGF-β1 sig-naling pathway as an attractive target in the fibrosis pathogenesis of sjögren’s syndrome. Mediat. Inflamm. 2018, 2018, 1965935. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Park, K.H.; Kwon, J.L.; Park, H.O.; Kim, J. Analysis of Self-Assembled Micelle Inhibitory RNA (SAMiRNA) Drug Using Ion-Pairing Reversed-Phase Liquid Chromatography Combined with Mass Spectrometry. J. Am. Soc. Mass. Spectrom. 2024, 35, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Yoon, P.O.; Park, J.W.; Lee, C.M.; Kim, S.H.; Kim, H.N.; Ko, Y.; Bae, S.J.; Yun, S.; Park, J.H.; Kwon, T.; et al. Self-assembled Micelle Interfering RNA for Effective and Safe Targeting of Dysregulated Genes in Pulmonary Fibrosis. J. Biol. Chem. 2016, 291, 6433–6446. [Google Scholar] [CrossRef] [PubMed]
- Ponticos, M.; Holmes, A.M.; Shi-wen, X.; Leoni, P.; Khan, K.; Rajkumar, V.S.; Hoyles, R.K.; Bou-Gharios, G.; Black, C.M.; Denton, C.P.; et al. Pivotal role of connective tissue growth factor in lung fibrosis: MAPK-dependent transcriptional activation of type I collagen. Arthritis Rheum. 2009, 60, 2142–2155. [Google Scholar] [CrossRef]
- Luetteke, N.C.; Qiu, T.H.; Fenton, S.E.; Troyer, K.L.; Riedel, R.F.; Chang, A.; Lee, D.C. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development 1999, 126, 2739–2750. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisto, M.; Lisi, S. Amphiregulin and Fibrosis: Existing Evidence and Future Directions. Int. J. Mol. Sci. 2025, 26, 7678. https://doi.org/10.3390/ijms26167678
Sisto M, Lisi S. Amphiregulin and Fibrosis: Existing Evidence and Future Directions. International Journal of Molecular Sciences. 2025; 26(16):7678. https://doi.org/10.3390/ijms26167678
Chicago/Turabian StyleSisto, Margherita, and Sabrina Lisi. 2025. "Amphiregulin and Fibrosis: Existing Evidence and Future Directions" International Journal of Molecular Sciences 26, no. 16: 7678. https://doi.org/10.3390/ijms26167678
APA StyleSisto, M., & Lisi, S. (2025). Amphiregulin and Fibrosis: Existing Evidence and Future Directions. International Journal of Molecular Sciences, 26(16), 7678. https://doi.org/10.3390/ijms26167678






