Application of Multiplatform Mass Spectrometry to the Study of Babesia divergens Metabolism and the Pathogenesis of Human Babesiosis
Abstract
1. Introduction
2. Results
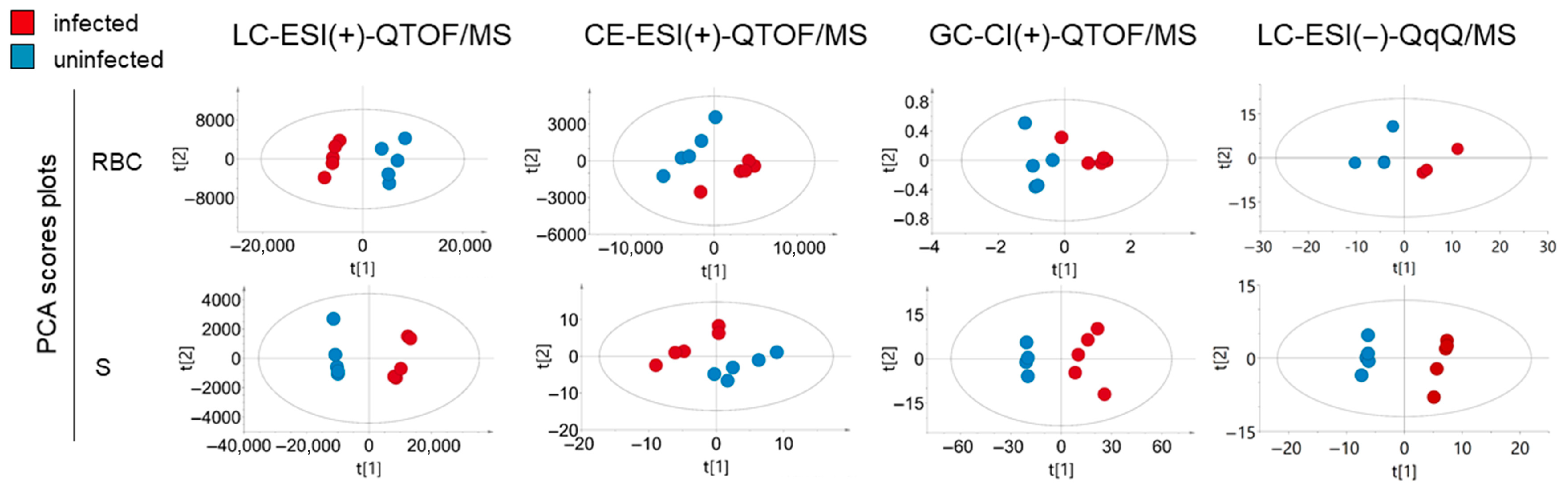
2.1. Metabolite Coverage and Univariate and Multivariate Analysis of Infected and Non-Infected RBCs and Supernatants
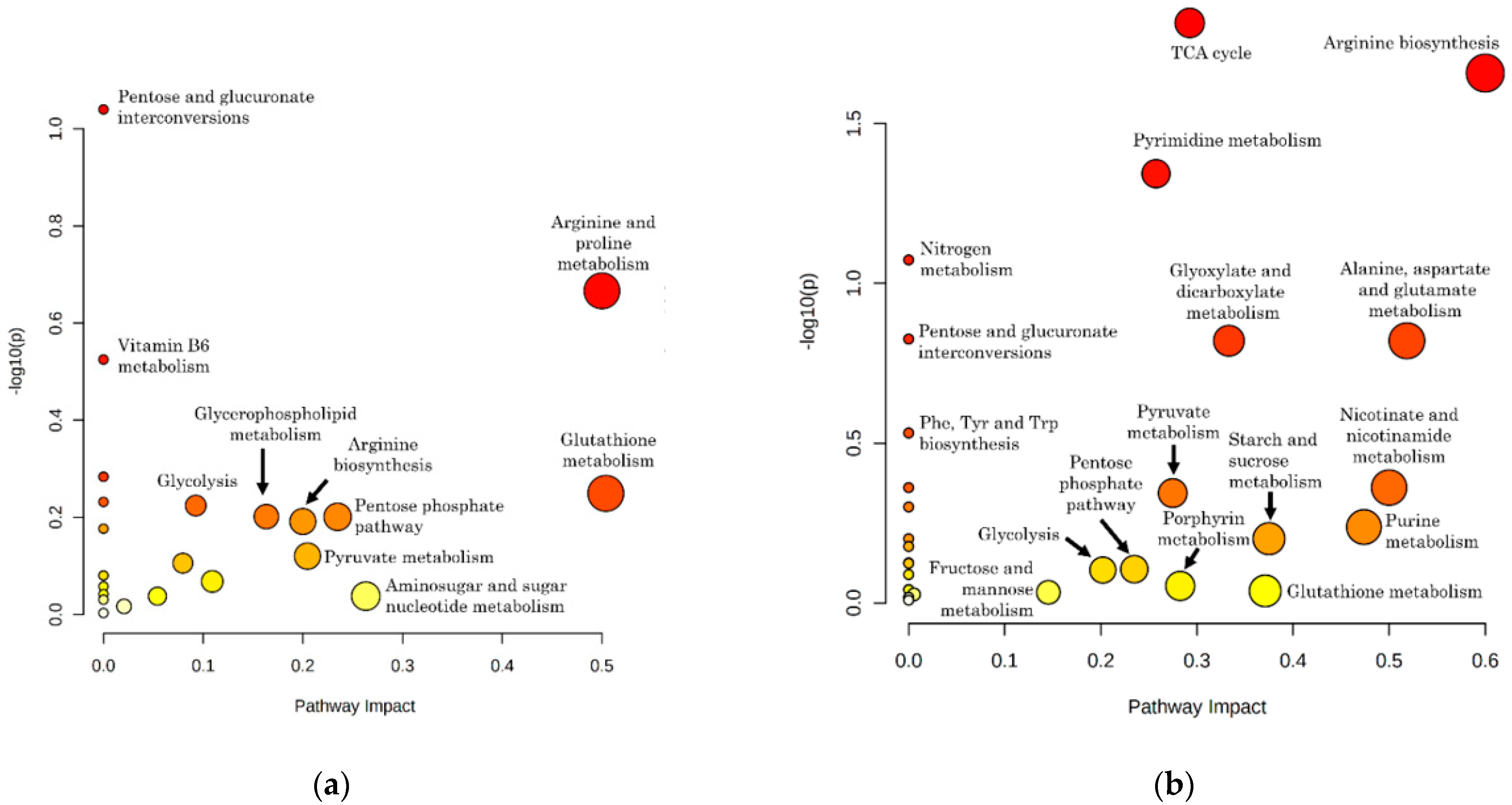
2.2. Representation and Significance of Altered Metabolic Pathways in Infected RBCs and Supernatants
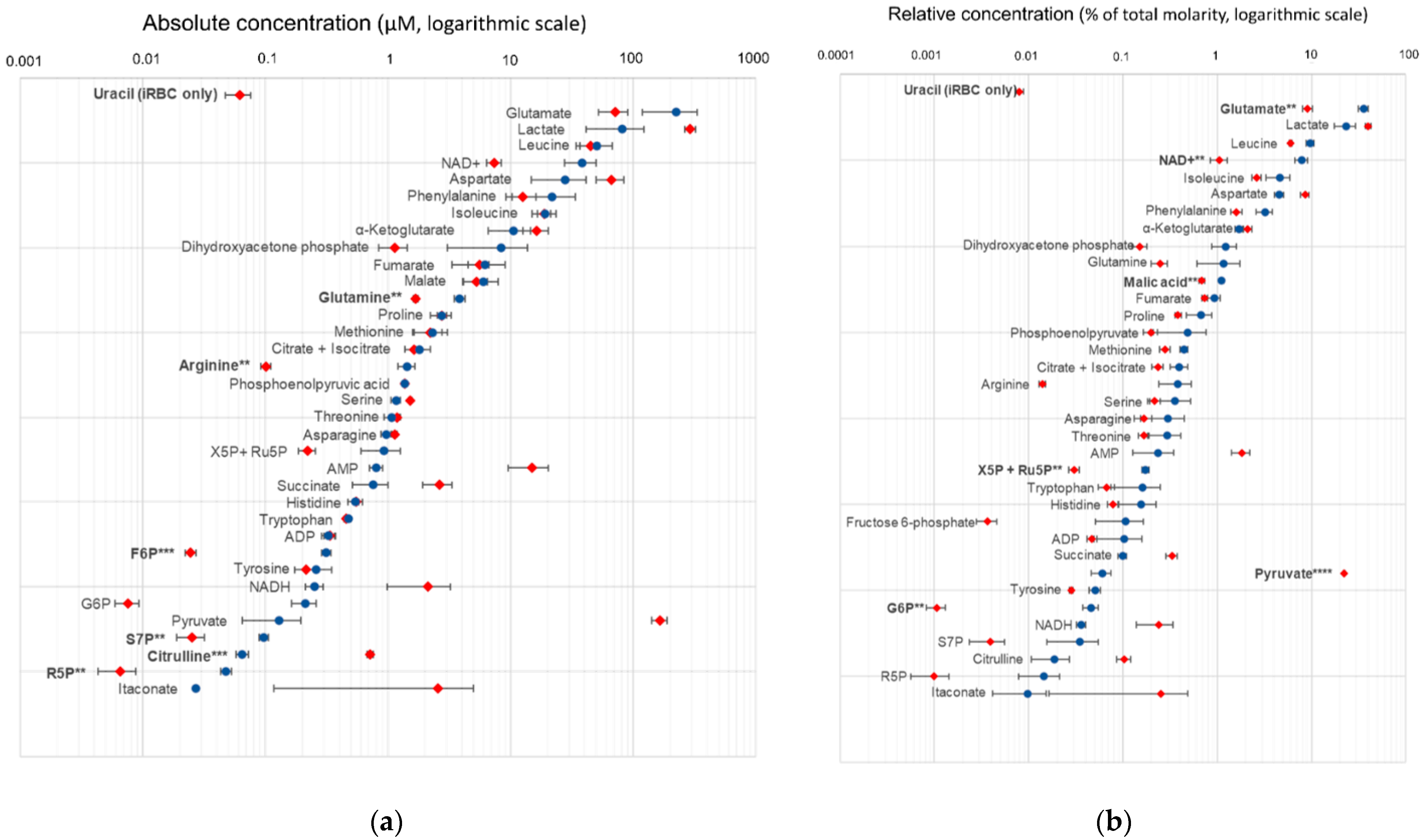
2.3. Absolute and Relative Concentrations of Metabolites in the B. divergens Intraerythrocytic Parasite–RBC System
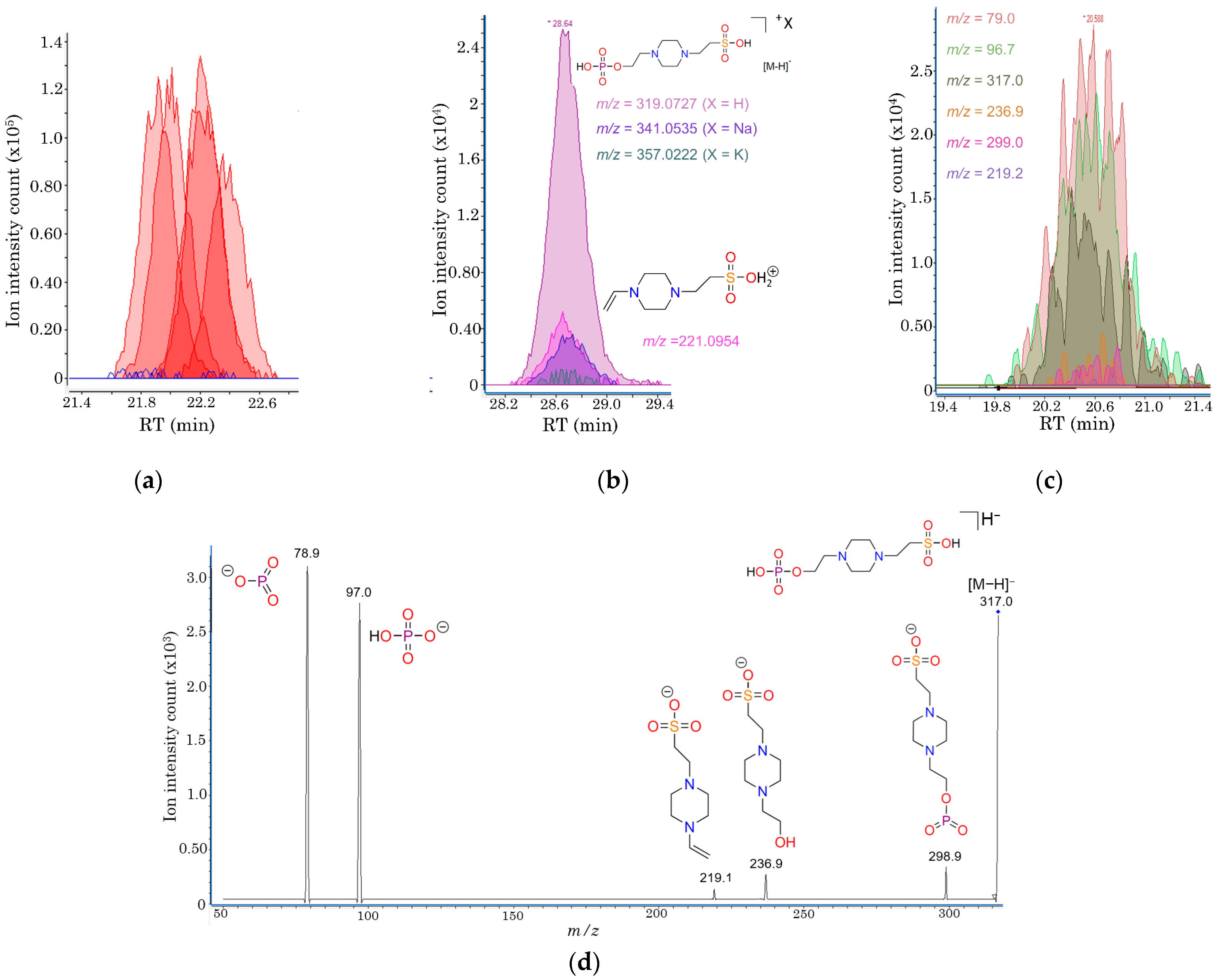
2.4. Structural Elucidation of Phosphorylated HEPES, a Novel Metabolite Uniquely Detected in iRBCs
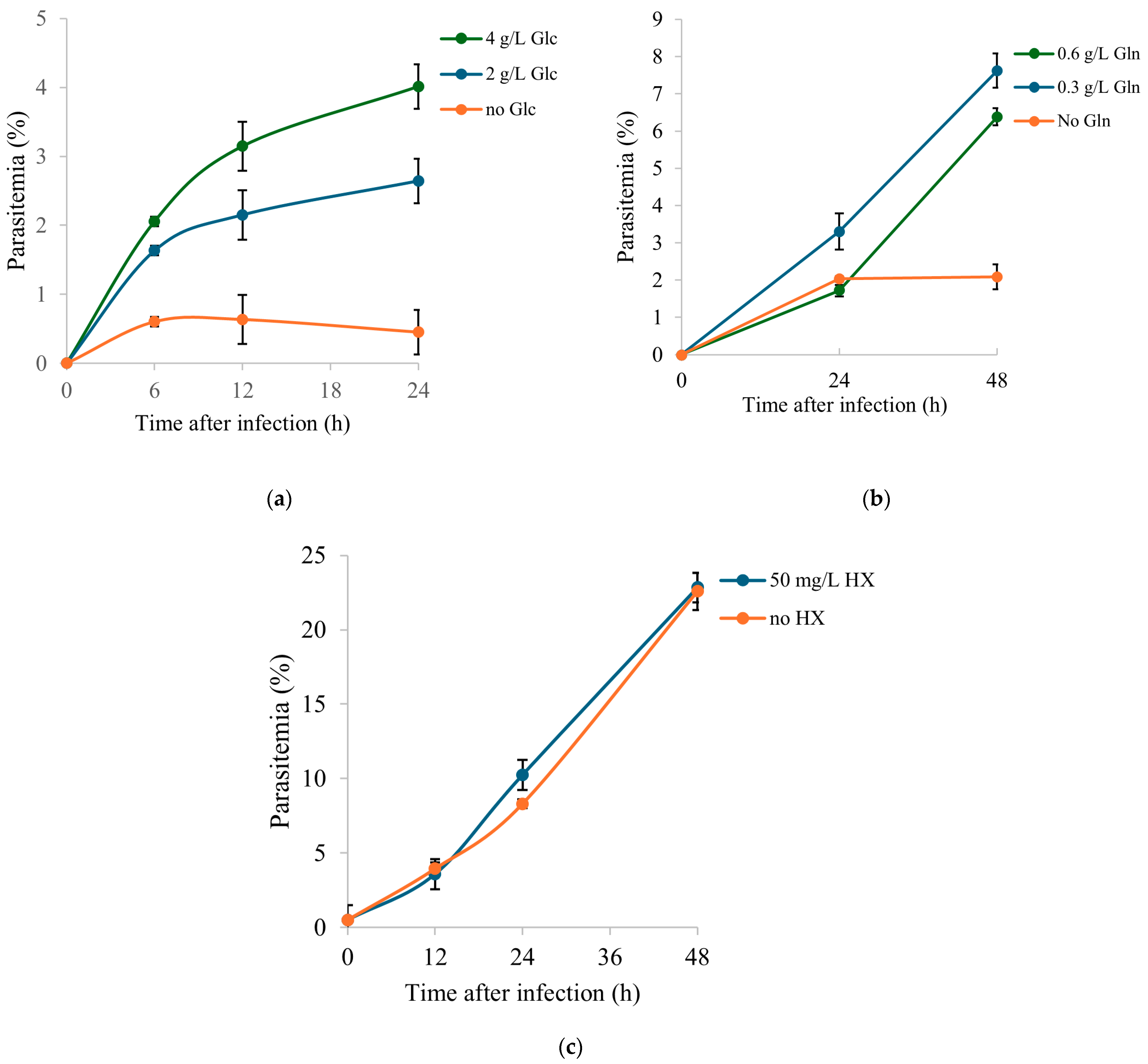
2.5. Functional Validation of Selected Extracellular Nutrients
3. Discussion
3.1. Discriminatory Profile and Potential Biomarker Utility of B. divergens-Induced Metabolic Alterations
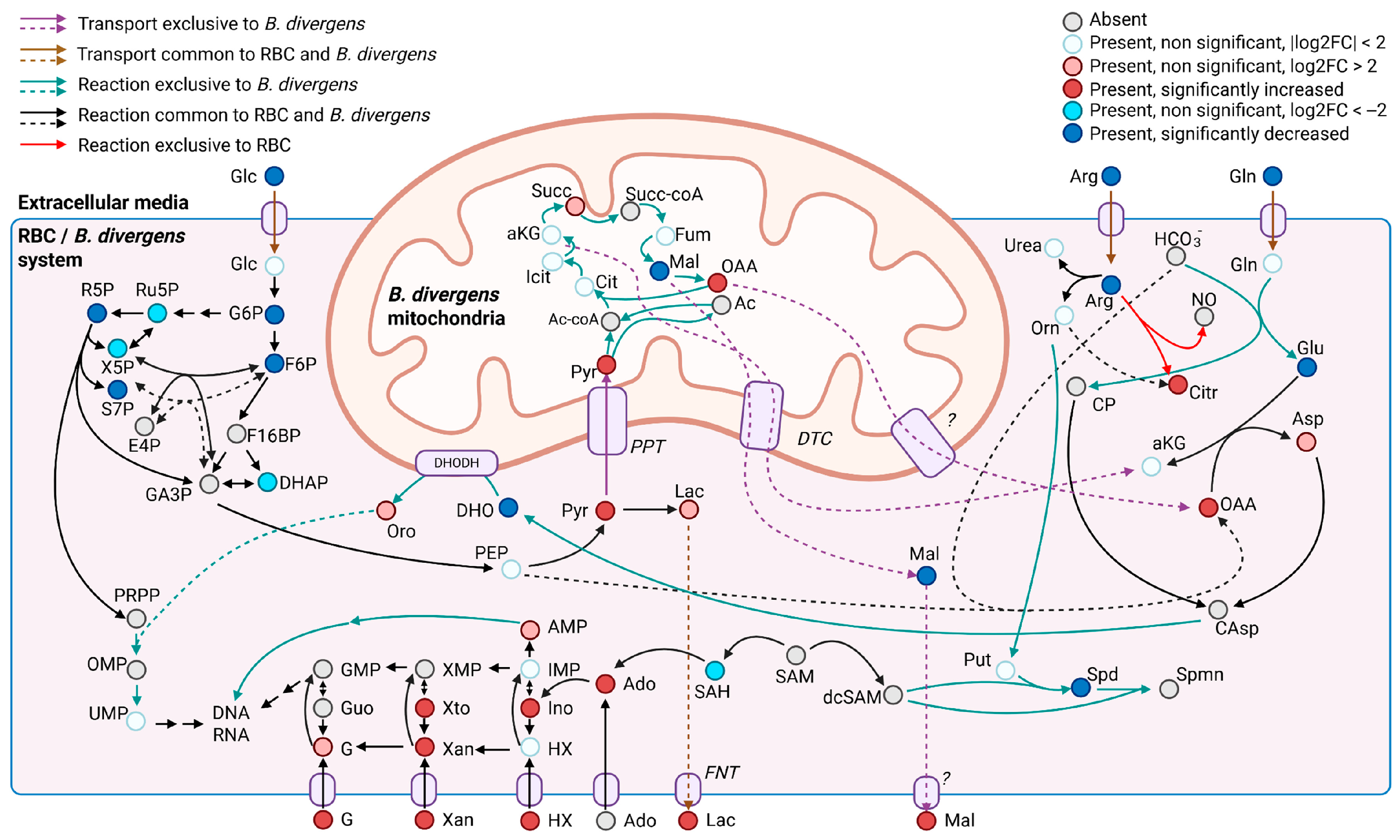
3.2. Putative Metabolic Pathway Structure and Potential Carbon and Nitrogen Sources of B. divergens
3.3. Relationship Between Oxidative Stress and B. divergens Metabolism
3.4. Involvement of Metabolic Alterations in the Pathophysiology of Human Babesiosis
3.5. Uncharacterized Phosphorylation of HEPES in B. divergens-Infected RBCs
4. Materials and Methods
4.1. Multiplatform Metabolomics Pipeline Structure
4.2. Reagents and Solutions
4.3. Babesia divergens Strains and Erythrocyte Infection
4.3.1. Ethics Statement
4.3.2. Parasite Propagation
4.4. Isolation of Intraerythrocytic Parasites and Supernatants from B. divergens In Vitro Cultures
4.5. Metabolite Extraction
4.6. CE-TOF/MS Analyses
4.7. GC-QTOF/MS Analyses
4.8. LC-QTOF/MS Analyses
4.9. LC-QqQ/MS Profiling of Polar Metabolites
4.10. LC-QqQ/MS Targeted Analysis of 2-(4-(2-(Phosphonooxy)ethyl)piperazinyl)-ethanesulfonic Acid (PEPES)
4.11. Parasite Growth Assays Using Extracellular Nutrients
4.12. Statistical Analysis
4.13. Metabolic Pathway Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; O’Bryan, J.; Krause, P.J. The Global Emergence of Human Babesiosis. Pathogens 2021, 10, 1447. [Google Scholar] [CrossRef]
- Bloch, E.M.; Krause, P.J.; Tonnetti, L. Preventing Transfusion-Transmitted Babesiosis. Pathogens 2021, 10, 1176. [Google Scholar] [CrossRef]
- Singh, P.; Vydyam, P.; Fang, T.; Estrada, K.; Gonzalez, L.M.; Grande, R.; Kumar, M.; Chakravarty, S.; Berry, V.; Ranwez, V.; et al. Insights into the Evolution, Virulence and Speciation of Babesia MO1 and Babesia divergens through Multiomics Analyses. Emerg. Microbes Infect. 2024, 13, 2386136. [Google Scholar] [CrossRef]
- Zintl, A.; Mulcahy, G.; Skerrett, H.E.; Taylor, S.M.; Gray, J.S. Babesia divergens, a Bovine Blood Parasite of Veterinary and Zoonotic Importance. Clin. Microbiol. Rev. 2003, 16, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Zintl, A.; Montero, E.; Hunfeld, K.-P.; Gray, J. Human Babesiosis in Europe. Pathogens 2021, 10, 1165. [Google Scholar] [CrossRef]
- Gray, J.; Kahl, O.; Zintl, A. What Do We Still Need to Know about Ixodes Ricinus? Ticks Tick Borne Dis. 2021, 12, 101682. [Google Scholar] [CrossRef]
- Jalovecka, M.; Hajdusek, O.; Sojka, D.; Kopacek, P.; Malandrin, L. The complexity of piroplasms life cycles. Front. Cell Infect. Microbiol. 2018, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Gray, J.S.; Hunfeld, K.-P. Human babesiosis in Europe: What clinicians need to know. Infection 2021, 49, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, E.; González, L.M.; Luque, D.; Gray, J.; Montero, E. Kinetics of the Invasion and Egress Processes of Babesia divergens, Observed by Time-Lapse Video Microscopy. Sci. Rep. 2018, 8, 14116. [Google Scholar] [CrossRef] [PubMed]
- Cursino-Santos, J.R.; Singh, M.; Senaldi, E.; Manwani, D.; Yazdanbakhsh, K.; Lobo, C.A. Altered Parasite Life-Cycle Processes Characterize Babesia divergens Infection in Human Sickle Cell Anemia. Haematologica 2019, 104, 2189–2199. [Google Scholar] [CrossRef]
- Renard, I.; Ben Mamoun, C. Treatment of Human Babesiosis: Then and Now. Pathogens 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Rickard, M.D. Carbohydrate metabolism in Babesia rodhaini: Differences in the metabolism of normal and infected rat erythrocytes. Exp. Parasitol. 1969, 25, 16–31. [Google Scholar] [CrossRef]
- Barry, D.N. Metabolism of Babesia parasites in vitro. Glucose and energy metabolism of B. bovis. Aust. J. Exp. Biol. Med. Sci. 1984, 62, 53–61. [Google Scholar] [CrossRef]
- Mackenzie, N.E.; Johnson, J.; Burton, G.; Wagner, G.G.; Scott, A.I. 13C NMR studies of glycolysis in intra- and extra-erythrocytic Babesia microti. Mol. Biochem. Parasitol. 1984, 13, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shikano, S.; Shimada, T.; Nakada, K.; Hashiguchi, R.; Ono, K. Enzyme activities related to glucose metabolism in Babesia microti and Babesia rodhaini. J. Vet. Med. Sci. 1995, 57, 93–97. [Google Scholar] [CrossRef][Green Version]
- Yamasaki, M.; Hossain, M.A.; Jeong, J.R.; Chang, H.S.; Satoh, H.; Yamato, O.; Maede, Y. Babesia gibsoni-specific isoenzymes related to energy metabolism of the parasite in infected erythrocytes. J. Parasitol. 2003, 89, 1142–1146. [Google Scholar] [CrossRef]
- Matias, C.; Nott, S.E.; Bagnara, A.S.; O’Sullivan, W.J.; Gero, A.M. Purine salvage and metabolism in Babesia bovis. Parasitol. Res. 1990, 76, 207–213. [Google Scholar] [CrossRef]
- Hassan, H.F.; Phillips, R.S.; Coombs, G.H. Purine-metabolizing enzymes in Babesia divergens. Parasitol. Res. 1987, 73, 121–125. [Google Scholar] [CrossRef]
- Cornillot, E.; Hadj-Kaddour, K.; Dassouli, A.; Noel, B.; Ranwez, V.; Vacherie, B.; Augagneur, Y.; Brès, V.; Duclos, A.; Randazzo, S.; et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res. 2012, 40, 9102–9114. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Liu, J.; Liu, A.; He, X.; Xu, J.; Li, Z.; Zhao, S.; Li, Y.; Yin, H.; et al. Comparative analysis of apicoplast genomes of Babesia infective to small ruminants in China. Parasit. Vectors 2019, 12, 312. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, L.; Jiang, F.; Li, M.; Zhan, X.; Huang, Y.; Wang, S.; Du, X.; He, L.; Zhao, J. Annotation and characterization of Babesia gibsoni apicoplast genome. Parasit. Vectors 2020, 13, 209. [Google Scholar] [CrossRef]
- Cuesta, I.; González, L.M.; Estrada, K.; Grande, R.; Zaballos, A.; Lobo, C.A.; Barrera, J.; Sanchez-Flores, A.; Montero, E. High-Quality Draft Genome Sequence of Babesia divergens, the Etiological Agent of Cattle and Human Babesiosis. Genome Announc. 2014, 2, e01194-14. [Google Scholar] [CrossRef]
- González, L.M.; Estrada, K.; Grande, R.; Jiménez-Jacinto, V.; Vega-Alvarado, L.; Sevilla, E.; de la Barrera, J.; Cuesta, I.; Zaballos, Á.; Bautista, J.M.; et al. Comparative and functional genomics of the protozoan parasite Babesia divergens highlighting the invasion and egress processes. PLoS Negl. Trop. Dis. 2019, 13, e0007680. [Google Scholar] [CrossRef]
- Olszewski, K.L.; Morrisey, J.M.; Wilinski, D.; Burns, J.M.; Vaidya, A.B.; Rabinowitz, J.D.; Llinás, M. Host-parasite interactions revealed by Plasmodium falciparum metabolomics. Cell Host Microbe 2009, 5, 191–199. [Google Scholar] [CrossRef]
- Leopold, S.J.; Ghose, A.; Allman, E.L.; Kingston, H.W.F.; Hossain, A.; Dutta, A.K.; Plewes, K.; Chotivanich, K.; Day, N.P.J.; Tarning, J.; et al. Identifying the Components of Acidosis in Patients With Severe Plasmodium falciparum Malaria Using Metabolomics. J. Infect. Dis. 2019, 219, 1766–1776. [Google Scholar] [CrossRef] [PubMed]
- Sana, T.R.; Gordon, D.B.; Fischer, S.M.; Tichy, S.E.; Kitagawa, N.; Lai, C.; Gosnell, W.L.; Chang, S.P.; Turrens, J.F. Global mass spectrometry based metabolomics profiling of erythrocytes infected with Plasmodium falciparum. PLoS ONE 2013, 8, e60840. [Google Scholar] [CrossRef]
- Allman, E.L.; Painter, H.J.; Samra, J.; Carrasquilla, M.; Llinás, M. Metabolomic Profiling of the Malaria Box Reveals Antimalarial Target Pathways. Antimicrob. Agents Chemother. 2016, 60, 6635–6649. [Google Scholar] [CrossRef]
- Beri, D.; Singh, M.; Rodriguez, M.; Goyal, N.; Rasquinha, G.; Liu, Y.; An, X.; Yazdanbakhsh, K.; Lobo, C.A. Global Metabolomic Profiling of Host Red Blood Cells Infected with Babesia divergens Reveals Novel Antiparasitic Target Pathways. Microbiol. Spectr. 2023, 11, e0468822. [Google Scholar] [CrossRef]
- Ord, R.L.; Lobo, C.A. Human Babesiosis: Pathogens, Prevalence, Diagnosis and Treatment. Curr. Clin. Microbiol. Rep. 2015, 2, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.W.; Gero, A.M.; O’Sullivan, W.J. Enzymes of de novo pyrimidine biosynthesis in Babesia rodhaini. J. Protozool. 1983, 30, 36–40. [Google Scholar] [CrossRef]
- Gero, A.M.; O’Sullivan, W.J.; Wright, I.G.; Mahoney, D.F. The enzymes of pyrimidine biosynthesis in Babesia bovis and Babesia bigemina. Aust. J. Exp. Biol. Med. Sci. 1983, 6, 239–243. [Google Scholar] [CrossRef]
- Kamyingkird, K.; Cao, S.; Masatani, T.; Moumouni, P.F.; Vudriko, P.; Mousa, A.A.; Terkawi, M.A.; Nishikawa, Y.; Igarashi, I.; Xuan, X. Babesia bovis dihydroorotate dehydrogenase (BboDHODH) is a novel molecular target of drug for bovine babesiosis. J. Vet. Med. Sci. 2014, 76, 323–330. [Google Scholar] [CrossRef][Green Version]
- Neves, L.; Cross, H.F.; Loureiro, L.; Akça, A.; Hommel, M.; Trees, A.J. Addition of hypoxanthine to culture media allows in vitro cultivation of Babesia bovis and B. bigemina at reduced serum concentrations. Parasitology 2001, 123, 357–363. [Google Scholar] [CrossRef]
- Irvin, A.D.; Young, E.R. Further studies on the uptake of tritiated nucleic acid precursors by Babesia spp. of cattle and mice. Int. J. Parasitol. 1979, 9, 109–114. [Google Scholar] [CrossRef]
- Cobbold, S.A.; Llinás, M.; Kirk, K. Sequestration and metabolism of host cell arginine by the intraerythrocytic malaria parasite Plasmodium falciparum. Cell Microbiol. 2016, 18, 820–830. [Google Scholar] [CrossRef]
- Kropf, P.; Fuentes, J.M.; Fähnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Müller, I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005, 19, 1000–1002. [Google Scholar] [CrossRef]
- Alkaitis, M.S.; Wang, H.; Ikeda, A.K.; Rowley, C.A.; MacCormick, I.J.; Chertow, J.H.; Billker, O.; Suffredini, A.F.; Roberts, D.J.; Taylor, T.E.; et al. Decreased Rate of Plasma Arginine Appearance in Murine Malaria May Explain Hypoargininemia in Children with Cerebral Malaria. J. Infect. Dis. 2016, 214, 1840–1849. [Google Scholar] [CrossRef][Green Version]
- Weinberg, J.B.; Lopansri, B.K.; Mwaikambo, E.; Granger, D.L. Arginine, nitric oxide, carbon monoxide, and endothelial function in severe malaria. Curr. Opin. Infect. Dis. 2008, 21, 468–475. [Google Scholar] [CrossRef]
- Xu, F.; Zou, L.; Lin, Q.; Ong, C.N. Use of liquid chromatography/tandem mass spectrometry and online databases for identification of phosphocholines and lysophosphatidylcholines in human red blood cells. Rapid Commun. Mass. Spectrom. 2009, 23, 3243–3254. [Google Scholar] [CrossRef]
- Singh, P.; Alaganan, A.; More, K.R.; Lorthiois, A.; Thiberge, S.; Gorgette, O.; Blisnick, M.G.; Guglielmini, J.; Aguilera, S.S.; Touqui, L.; et al. Role of a patatin-like phospholipase in Plasmodium falciparum gametogenesis and malaria transmission. Proc. Natl. Acad. Sci. USA 2019, 116, 17498–17508. [Google Scholar] [CrossRef]
- Flammersfeld, A.; Lang, C.; Flieger, A.; Pradel, G. Phospholipases during membrane dynamics in malaria parasites. Int. J. Med. Microbiol. 2018, 308, 129–141. [Google Scholar] [CrossRef]
- Lehane, A.M.; Saliba, K.J.; Allen, R.J.; Kirk, K. Choline uptake into the malaria parasite is energized by the membrane potential. Biochem. Biophys. Res. Commun. 2004, 320, 311–317. [Google Scholar] [CrossRef]
- Mi-Ichi, F.; Kano, S.; Mitamura, T. Oleic acid is indispensable for intraerythrocytic proliferation of Plasmodium falciparum. Parasitology 2007, 134, 1671–1677. [Google Scholar] [CrossRef]
- Efferth, T.; Schwarzl, S.M.; Smith, J.; Osieka, R. Role of glucose-6-phosphate dehydrogenase for oxidative stress and apoptosis. Cell Death Differ. 2006, 13, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef]
- Tiwari, S.; Sharma, N.; Sharma, G.P.; Mishra, N. Redox interactome in malaria parasite Plasmodium falciparum. Parasitol. Res. 2021, 120, 423–434. [Google Scholar] [CrossRef]
- Percário, S.; Moreira, D.R.; Gomes, B.A.; Ferreira, M.E.; Gonçalves, A.C.; Laurindo, P.S.; Vilhena, T.C.; Dolabela, M.F.; Green, M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012, 13, 16346–16372. [Google Scholar] [CrossRef]
- Mbanefo, E.C.; Ahmed, A.M.; Titouna, A.; Elmaraezy, A.; Trang, N.T.; Phuoc Long, N.; Anh, N.H.; Nghi, T.D.; Hung, B.T.; Van Hieu, M.; et al. Association of glucose-6-phosphate dehydrogenase deficiency and malaria: A systematic review and meta-analysis. Sci. Rep. 2017, 7, 45963. [Google Scholar] [CrossRef]
- Ginsburg, H.; Atamna, H.; Shalmiev, G.; Kanaani, J.; Krugliak, M. Resistance of glucose-6-phosphate dehydrogenase deficiency to malaria: Effects of fava bean hydroxypyrimidine glucosides on Plasmodium falciparum growth in culture and on the phagocytosis of infected cells. Parasitology 1996, 113, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Usanga, E.A.; Luzzatto, L. Adaptation of Plasmodium falciparum to glucose 6-phosphate dehydrogenase-deficient host red cells by production of parasite-encoded enzyme. Nature 1985, 313, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.L.; Scopes, D.A.; Sodeinde, O.; Mason, P.J. Glucose-6-phosphate dehydrogenase-6-phosphogluconolactonase. A novel bifunctional enzyme in malaria parasites. Eur. J. Biochem. 2001, 268, 2013–2019. [Google Scholar] [CrossRef] [PubMed]
- Thien, H.V.; Kager, P.A.; Sauerwein, H.P. Hypoglycemia in falciparum malaria: Is fasting an unrecognized and insufficiently emphasized risk factor? Trends Parasitol. 2006, 22, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Madrid, L.; Lanaspa, M.; Maculuve, S.A.; Bassat, Q. Malaria-associated hypoglycaemia in children. Expert. Rev. Anti Infect. Ther. 2015, 13, 267–277. [Google Scholar] [CrossRef]
- Rubach, M.P.; Zhang, H.; Florence, S.M.; Mukemba, J.P.; Kalingonji, A.R.; Anstey, N.M.; Yeo, T.W.; Lopansri, B.K.; Thompson, J.W.; Mwaikambo, E.D.; et al. Kinetic and Cross-Sectional Studies on the Genesis of Hypoargininemia in Severe Pediatric Plasmodium falciparum malaria. Infect. Immun. 2019, 87, e00655-18. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Buffet, P.A.; Ciceron, L.; Milon, G.; Mercereau-Puijalon, O.; Safeukui, I. Reduced erythrocyte deformability associated with hypoargininemia during Plasmodium falciparum malaria. Sci. Rep. 2014, 4, 3767. [Google Scholar] [CrossRef]
- Costa, B.P.; Martins, P.; Verissimo, C.; Simões, M.; Tomé, M.; Grazina, M.; Pimentel, J.; Sousa, F.C. Glutaminemia prognostic significance in critical surgical patients—An analysis of plasma aminogram profile. Nutr. Hosp. 2017, 34, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Vilmont, M.; Azoulay, M.; Frappier, F. Metabolism of glutamine in erythrocytes infected with the human malaria parasite: Plasmodium falciparum. Ann. Parasitol. Hum. Comp. 1990, 65, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, C.J.; Burchmore, R.J.; Krishna, S. Hexose permeation pathways in Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. USA 2000, 97, 9931–9936. [Google Scholar] [CrossRef]
- Awasthi, V.; Chauhan, R.; Chattopadhyay, D.; Das, J. Effect of L-arginine on the growth of Plasmodium falciparum and immune modulation of host cells. J. Vector Borne Dis. 2017, 54, 139–145. [Google Scholar] [CrossRef]
- Mancio-Silva, L.; Slavic, K.; Grilo Ruivo, M.T.; Grosso, A.R.; Modrzynska, K.K.; Vera, I.M.; Sales-Dias, J.; MacPherson, C.R.; Crozet, P.; Adamo, M.; et al. Nutrient sensing modulates malaria parasite virulence. Nature 2017, 547, 213–216. [Google Scholar] [CrossRef]
- Gupta, S.; Seydel, K.; Miranda-Roman, M.A.; Feintuch, C.M.; Saidi, A.; Kim, R.S.; Birbeck, G.L.; Taylor, T.; Daily, J.P.; Motta, A. Extensive alterations of blood metabolites in pediatric cerebral malaria. PLoS ONE 2017, 12, e0175686. [Google Scholar] [CrossRef]
- Brancucci, N.M.B.; Gerdt, J.P.; Wang, C.; De Niz, M.; Philip, N.; Adapa, S.R.; Zhang, M.; Hitz, E.; Niederwieser, I.; Boltryk, S.D.; et al. Lysophosphatidylcholine Regulates Sexual Stage Differentiation in the Human Malaria Parasite Plasmodium falciparum. Cell 2017, 171, 1532–1544.e15. [Google Scholar] [CrossRef]
- Wegiel, B.; Hauser, C.J.; Otterbein, L.E. Heme as a danger molecule in pathogen recognition. Free Radic. Biol. Med. 2015, 89, 651–661. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-inflammatory Actions of Heme and Other Hemoglobin-Derived DAMPs. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Ferreira, A.; Balla, J.; Jeney, V.; Balla, G.; Soares, M.P. A central role for free heme in the pathogenesis of severe malaria: The missing link? J. Mol. Med. 2008, 86, 1097–1111. [Google Scholar] [CrossRef]
- Pettus, B.J.; Chalfant, C.E.; Hannun, Y.A. Sphingolipids in inflammation: Roles and implications. Curr. Mol. Med. 2004, 4, 405–418. [Google Scholar] [CrossRef]
- Habib, A.; Tabata, M. Oxidative DNA damage induced by HEPES (2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid) buffer in the presence of Au(III). J. Inorg. Biochem. 2004, 98, 1696–1702. [Google Scholar] [CrossRef]
- Tadolini, B. Iron autoxidation in Mops and Hepes buffers. Free Radic. Res. Commun. 1987, 4, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Gorenflot, A.; Moubri, K.; Precigout, E.; Carcy, B.; Schetters, T.P. Human babesiosis. Ann. Trop. Med. Parasitol. 1998, 92, 489–501. [Google Scholar] [CrossRef]
- Dettmer, K.; Nürnberger, N.; Kaspar, H.; Gruber, M.A.; Almstetter, M.F.; Oefner, P.J. Metabolite extraction from adherently growing mammalian cells for metabolomics studies: Optimization of harvesting and extraction protocols. Anal. Bioanal. Chem. 2011, 399, 1127–1139. [Google Scholar] [CrossRef]
- Garcia, A.; Naz, S.; Barbas, C. Metabolite fingerprinting by capillary electrophoresis-mass spectrometry. Methods Mol. Biol. 2014, 1198, 107–123. [Google Scholar] [PubMed]
- Gil-de-la-Fuente, A.; Godzien, J.; Saugar, S.; Garcia-Carmona, R.; Badran, H.; Wishart, D.S.; Barbas, C.; Otero, A. CEU Mass Mediator 3.0: A Metabolite Annotation Tool. J. Proteome Res. 2019, 18, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Mamani-Huanca, M.; de la Fuente, A.G.; Otero, A.; Gradillas, A.; Godzien, J.; Barbas, C.; López-Gonzálvez, Á. Enhancing confidence of metabolite annotation in Capillary Electrophoresis-Mass Spectrometry untargeted metabolomics with relative migration time and in-source fragmentation. J. Chromatogr. A 2021, 1635, 461758. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.B.; Troyer, C.; Mairinger, T.; Ortmayr, K.; Neubauer, S.; Koellensperger, G.; Hann, S. Isotopologue analysis of sugar phosphates in yeast cell extracts by gas chromatography chemical ionization time-of-flight mass spectrometry. Anal. Bioanal. Chem. 2015, 407, 2865–2875. [Google Scholar] [CrossRef]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass spectral and retention index libraries for metabolomics based on quadrupole and time-of-flight gas chromatography/mass spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef]
- The NIST Mass Spectrometry Data Center. NIST/NIH/EPA Mass Spectral Library, Standard Reference Database, NIST 17 [L Spectral Library]; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2017. [Google Scholar]
- Rojo, D.; Canuto, G.A.; Castilho-Martins, E.A.; Tavares, M.F.; Barbas, C.; López-Gonzálvez, Á.; Rivas, L. A Multiplatform Metabolomic Approach to the Basis of Antimonial Action and Resistance in Leishmania infantum. PLoS ONE 2015, 10, e0130675. [Google Scholar] [CrossRef]
- Guijas, C.; Montenegro-Burke, J.R.; Domingo-Almenara, X.; Palermo, A.; Warth, B.; Hermann, G.; Koellensperger, G.; Huan, T.; Uritboonthai, W.; Aisporna, A.E.; et al. METLIN: A Technology Platform for Identifying Knowns and Unknowns. Anal. Chem. 2018, 90, 3156–3164. [Google Scholar] [CrossRef]
- Ruttkies, C.; Schymanski, E.L.; Wolf, S.; Hollender, J.; Neumann, S. MetFrag relaunched: Incorporating strategies beyond in silico fragmentation. J. Cheminform 2016, 8, 3. [Google Scholar] [CrossRef]
- Fernández-García, M.; Mesquita, I.; Ferreira, C.; Araújo, M.; Saha, B.; Rey-Stolle, M.F.; García, A.; Silvestre, R.; Barbas, C. Induces Multiple Dynamic Responses in the Metabolome Associated with Amastigote Differentiation and Maturation Inside the Human Macrophage. J. Proteome Res. 2023, 22, 2256–2270. [Google Scholar] [CrossRef]
- Cursino-Santos, J.R.; Singh, M.; Pham, P.; Rodriguez, M.; Lobo, C.A. Babesia Divergens Builds a Complex Population Structure Composed of Specific Ratios of Infected Cells to Ensure a Prompt Response to Changing Environmental Conditions: Babesia: Biological Clocks and Developmental Model. Cell Microbiol. 2016, 18, 859–874. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]

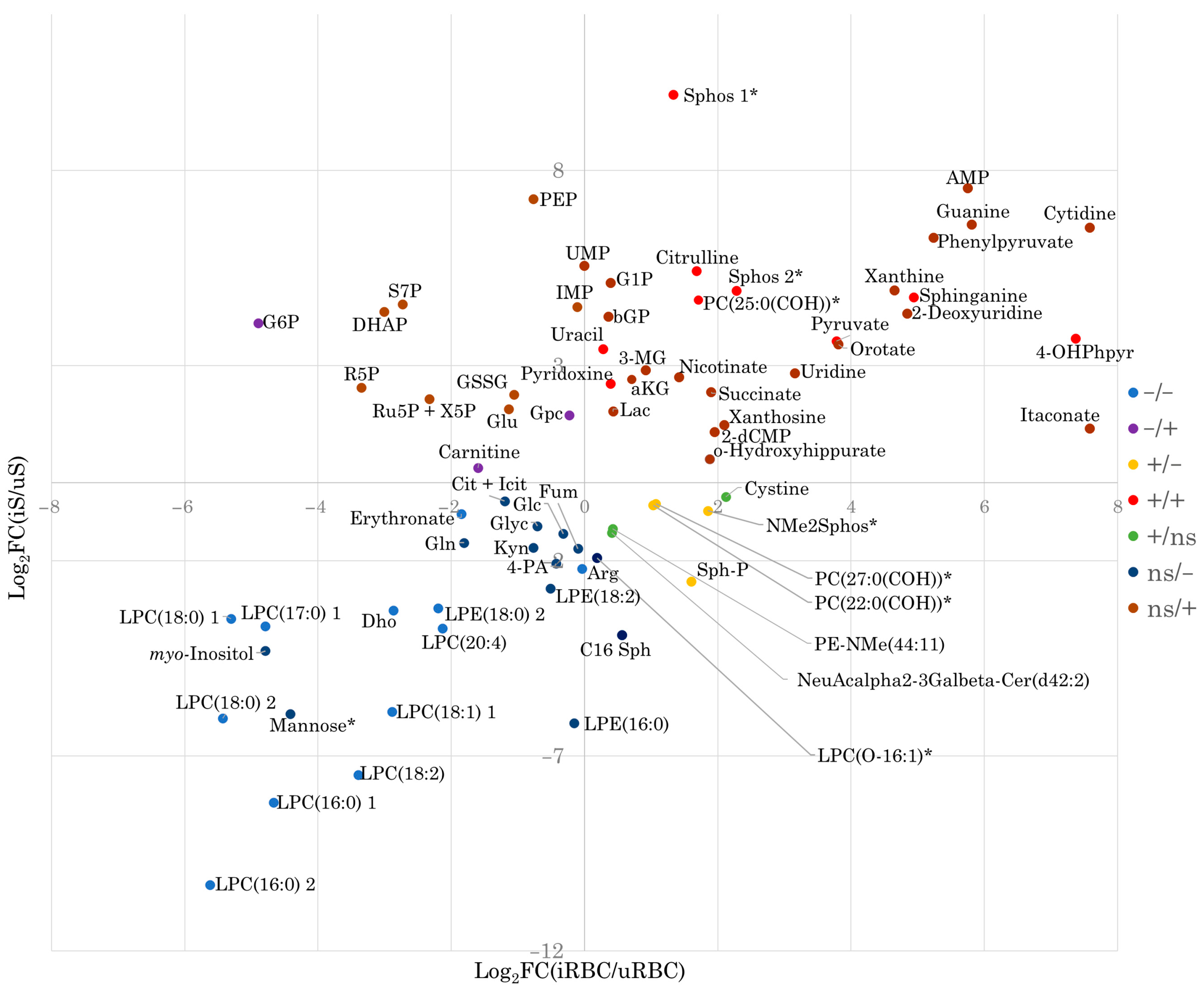
| Group | CORA Enriched Metabolite Sets (p < 0.05) | Enriched Pathway Terms (p < 0.05) |
|---|---|---|
| +/+ | amines; pyridoxines; alpha-keto acids and derivatives; phenylpyruvic acid derivatives; carboximidic acids; benzenediols; pyrimidines and pyrimidine derivatives; amino acids, peptides, and analogs | - |
| +/ns | amino acids, peptides and analogs; purine 2′-deoxyribonucleosides; phosphate esters; linoleic acid derivatives | - |
| ns/+ | carbohydrates and carbohydrate conjugates *; purines and purine derivatives *; purine ribonucleotides *; pyrimidines and pyrimidine derivatives; pyrimidine ribonucleosides; glycosylamines; alpha-hydroxyacids and derivatives; purine ribonucleosides; amino acids, peptides and analogs; phenylpyruvic acid derivatives; pyrimidine deoxyribonucleotides; gamma-ketoacids and derivatives; pyrimidine ribonucleotides; beta-hydroxyacids and derivatives; phenylmethylamines; dicarboxylic acids and derivatives; phosphate esters; pyridinedicarboxylic acids and derivatives | carbon fixation by Calvin cycle *; pyrimidine metabolism; pentose and glucuronate interconversions; citrate cycle (TCA cycle); purine metabolism; pentose phosphate pathway |
| -/- | amino acids, peptides, and analogs; glycerophosphocolines | - |
| -/ns | Carbohydrates and carbohydrate conjugates *; amino acids, peptides, and analogs; benzoic acids and derivatives | - |
| ns/- | Carbohydrates and carbohydrate conjugates *; tricarboxylic acids and derivatives *; alpha-hydroxyacids and derivatives; sphingoid base analogs; dicarboxylic acids and derivatives; pyridinecarboxylic acids and derivatives; alcohols and polyols | glyoxylate and dicarboxylate metabolism *; citrate cycle (TCA cycle); alaninine, aspartate, and glutamate metabolism |
| +/- | Phosphosphingolipids; amines | - |
| -/+ | Quaternary ammonium salts | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-García, M.; Gonzalez, L.M.; Sevilla, E.; Gil, A.; Santos-Oliveira, H.; Revuelta, B.; Barbas, C.; Rey-Stolle, M.F.; Montero, E.; García, A. Application of Multiplatform Mass Spectrometry to the Study of Babesia divergens Metabolism and the Pathogenesis of Human Babesiosis. Int. J. Mol. Sci. 2025, 26, 7677. https://doi.org/10.3390/ijms26167677
Fernández-García M, Gonzalez LM, Sevilla E, Gil A, Santos-Oliveira H, Revuelta B, Barbas C, Rey-Stolle MF, Montero E, García A. Application of Multiplatform Mass Spectrometry to the Study of Babesia divergens Metabolism and the Pathogenesis of Human Babesiosis. International Journal of Molecular Sciences. 2025; 26(16):7677. https://doi.org/10.3390/ijms26167677
Chicago/Turabian StyleFernández-García, Miguel, Luis Miguel Gonzalez, Elena Sevilla, Aitor Gil, Henrique Santos-Oliveira, Belen Revuelta, Coral Barbas, Mª Fernanda Rey-Stolle, Estrella Montero, and Antonia García. 2025. "Application of Multiplatform Mass Spectrometry to the Study of Babesia divergens Metabolism and the Pathogenesis of Human Babesiosis" International Journal of Molecular Sciences 26, no. 16: 7677. https://doi.org/10.3390/ijms26167677
APA StyleFernández-García, M., Gonzalez, L. M., Sevilla, E., Gil, A., Santos-Oliveira, H., Revuelta, B., Barbas, C., Rey-Stolle, M. F., Montero, E., & García, A. (2025). Application of Multiplatform Mass Spectrometry to the Study of Babesia divergens Metabolism and the Pathogenesis of Human Babesiosis. International Journal of Molecular Sciences, 26(16), 7677. https://doi.org/10.3390/ijms26167677







