Abstract
Obesity is a complex, diverse, and multifactorial disease that has become a significant public health concern. It is a modifiable risk factor for developing type 2 diabetes (T2D). The current classification systems rely on anthropometric measurements, such as body mass index (BMI), which cannot capture the physiopathological diversity of this disease. This study aimed to analyze the metabolic signatures of obesity and diabetes using 1H-nuclear magnetic resonance (NMR). Obese patients with BMI ≥ 25 kg/m2 (according to the Asian cut-off value) with different diabetes status scheduled to undergo metabolic-bariatric surgery at three hospitals were prospectively recruited for this study. Plasma samples of 111 obese patients and 26 healthy controls were analyzed by 1H-NMR. When compared among groups with different diabetes statuses, four clusters with no differences in BMI but different metabolomics profiles were obtained. These clusters highlight intricate metabolic relationships associated with obesity and diabetes. This study demonstrated the benefits of using precision techniques like 1H-NMR to better early detection, substantially decreasing the risk of developing T2D and its related complications. This study is the first to report on metabolic markers and altered metabolic profiles of T2D and prediabetes among obese Malaysians with a BMI cut-off value for the Asian population.
1. Introduction
Obesity is a major, modifiable risk factor for developing type 2 diabetes (T2D), and nearly 90% of people with T2D are overweight or obese [1]. Excess body fat, especially abdominal fat, can disrupt the body’s metabolic processes, leading to insulin resistance and raised blood sugar levels [2,3]. Eventually, the pancreas may struggle to produce enough insulin, leading to high blood sugar levels and T2D [1]. Obesity also can lead to metabolic changes, including changes in blood sugar, lipid levels, and blood pressure, which increase the risk of heart disease and stroke [4]. Furthermore, obesity is associated with chronic inflammation, which can lead to blood vessel damage and insulin resistance [5]. Addressing obesity and weight management can assist, prevent, delay, and manage the progression of diabetes [1,6,7].
It has emerged as a major global health issue, where 537 million people worldwide are currently affected by diabetes as estimated by the International Diabetes Federation (IDF). By 2030, this number is expected to increase to 578 million, and by 2045, it will reach 783 million, reflecting a 46% rise over the next two decades [8]. Malaysia exhibits one of the highest prevalences of diabetes within the Western Pacific region [9]. The pooled prevalence of diabetes in Malaysia is at 14.39% (95% CI, 12.51–16.38%) among adults aged 18 and older, derived from 15 studies with a total of 103,063 participants [10].
The prevalence of diabetes in Malaysia has significantly increased over the years. The National Health and Morbidity Survey 2023 indicated that 15.6% of adults aged 18 and older were diagnosed with diabetes [11].
Multiple factors influence the onset of T2D, with genetic variants contributing, albeit to a limited extent. As of 2023, around 700 genetic variants linked to a higher risk of T2D have been discovered through genome-wide association studies (GWASs) [12]. Early intervention in T2D is essential to avert long-term complications, including kidney failure, heart disease, and blindness. Relying exclusively on traditional clinical risk factors such as fasting blood glucose, body mass index (BMI), glycated hemoglobin (HbA1c), and blood pressure may prove inadequate for early and precise prediction. Conventional risk factors, although beneficial, possess inherent limitations. Fasting blood glucose and HbA1c indicate glucose metabolism issues; however, they may not encompass the complete range of metabolic dysfunctions before the onset of T2D [13]. BMI and blood pressure are significant indicators; however, they do not offer a comprehensive understanding of the underlying metabolic changes [14,15]. Metabolomics, which examines the entire array of small-molecule metabolites in a biological system, provides a more thorough method for predicting T2D [16,17,18]. Metabolomic research has revealed particular serum metabolites linked to a heightened risk of T2D. Metabolites like blood acylcarnitines, branched-chain amino acids (BCAAs), and specific choline-containing phospholipids are included [19]. Models utilizing metabolomic data for machine learning demonstrate superior predictive accuracy for future T2D incidence compared to those relying exclusively on clinical risk factors [20]. A study with 543 non-diabetic participants demonstrated that a novel biomarker panel identified via metabolomics enhanced predictive performance [21]. Early detection via metabolomics facilitates timely interventions, substantially decreasing the risk of developing T2D and its related complications, evidenced by an area under the receiver operating characteristic curve (AUC) of 0.78, which is notably superior to the reference model based solely on clinical risk factors (AUC = 0.68) [21]. This integrated approach underscores the significance of metabolomics in improving the prediction and prevention of T2D, thereby reducing its economic and health impacts.
Nuclear magnetic resonance (NMR) is one of the main methods used in metabolomics, alongside gas chromatography-mass spectrometry (GC-MS) and liquid chromatography-mass spectrometry (LC-MS). NMR spectroscopy is used in diabetes metabolomics studies because it provides a non-destructive and quantitative method for analyzing metabolites in biological fluids, like plasma, serum, and urine. NMR offers advantages in terms of reproducibility, sample preparation simplicity, and the ability to handle diverse biofluids, making it suitable for large-scale studies [22,23].
It would be interesting how these metabolites are associated with our study groups. For example, trimethylamine N-oxide (TMAO), a metabolite produced from specific nutrients by the liver and gut bacteria, is increasingly studied for its connection to diabetes and related complications, with NMR spectroscopy having an important role in its measurement. While some studies suggest TMAO is linked to increased diabetes risk and complications, others indicate potential protective effects, and the overall role of TMAO in diabetes remains controversial [24]. Glycine, in particular, has been linked to insulin sensitivity and resistance, with lower glycine levels often observed in individuals with diabetes [25]. How these metabolites are associated with our study groups would be interesting.
Our study seeks to analyze the metabolic signatures of obesity and diabetes using 1H nuclear magnetic resonance (NMR) metabolomics, aiming to identify specific patterns that can aid in early diagnosis, risk prediction, and personalized treatment strategies independent of traditional clinical risk factors.
2. Results
2.1. Participants’ Characteristics
Table 1 presents the general characteristics of the 137 study participants. The bariatric surgery patients were stratified based on their HbA1c levels into three groups: obese non-diabetes, obese with prediabetes, and obese with diabetes. Additionally, 26 lean, healthy individuals were recruited as controls. The mean age of the participants was 36.3 (±9.1) years, and the majority were female (63.5%). The mean BMI was significantly higher in all obese groups compared to the healthy controls. Among the obese groups, those with prediabetes and diabetes had significantly higher BMI than the non-diabetes group (Table 1). Additionally, several metabolic risk factors, including HbA1c, triglycerides (TG), and HDL-C, showed significant differences across the groups. HbA1c and TG levels were highest, while HDL-C levels were lowest in the obese diabetes group, following a stepwise increase from healthy controls to obese non-diabetes, obese prediabetes, and obese diabetes. There were no significant differences in total cholesterol (TC) and LDL-C levels across the groups.

Table 1.
General characteristics of participants.
2.2. Overall Analysis
The 1H NMR spectra are illustrated in Supplementary Figures S1 and S2. ANOVA was carried out to identify metabolites that differed significantly between groups, i.e., obese only, obese prediabetes, obese diabetes vs. healthy control group. Figure 1 represents the distribution of metabolites based on their statistical significance, expressed as −log10(p). Overall, 47 metabolites were detected (Table S1). Among the analyzed metabolites, ten were highly significant (p < 0.001, Table 2) while 38 showed significant differences (p < 0.05). Nine metabolites were not significantly different (p > 0.05) and are depicted in gray. The color scale, ranging from yellow to red, reflects the level of significance, with red indicating the most statistically significant metabolites. Additionally, the size of each data point represents the magnitude of the effect, providing a visual representation of key metabolic alterations.
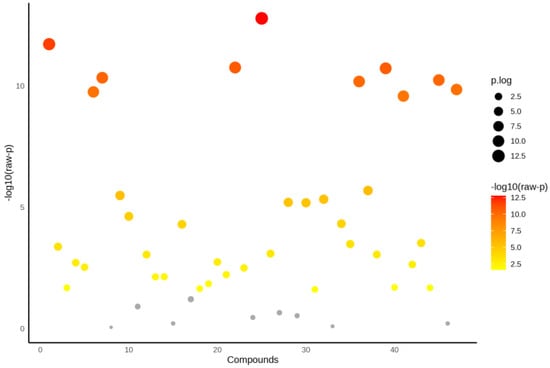
Figure 1.
ANOVA for all groups (Healthy control, obese only, obese prediabetes, obese diabetes).

Table 2.
List of highly significant metabolites across study groups (p < 0.001).
2.3. Fold Change, T-Test, and Volcano Plot Relative to Healthy Controls
- (i)
- Obese only
We observed significant upregulation of four metabolites in obese non-diabetes patients compared to healthy controls (Figure 2a). Specifically, acetate had a 3.9-fold increase, whereas 3-aminoisobutyrate, 3-hydroxybutyrate, and fucose had a 2.0–2.3-fold increase.
We identified nine significant metabolites differentiating the obese non-diabetes group from healthy controls (Figure 3a). Significantly, the metabolites with the highest significance levels included putrescine, 2-oxoglutarate, leucine, proline, and pyruvate.
To illustrate these results, a volcano plot was generated, depicting the correlation between fold change and statistical significance (Figure 4a). The volcano plot illustrates the relationship between log2 fold change (x-axis) and the negative log10 of the p-values (y-axis), with metabolites, namely 3-hydroxybutyrate, 3-aminoisobutyrate, and fucose in the upper regions indicating high statistical significance and their horizontal position reflecting the magnitude of change.
- (ii)
- Obese prediabetes
Significant upregulation of four metabolites was observed in obese prediabetes patients compared to healthy controls (Figure 2b). Specifically, 3-hydroxybutyrate, fucose, and 3-aminoisobutyrate exhibited a 2.3-fold increase, indicating their concentration is 2.3 times higher in the prediabetic group, while acetoacetate had a 2-fold increase.
A total of 30 significant metabolites were identified, with p < 0.05 indicating statistical significance (Figure 3b). Noteworthy metabolites with the highest significance included putrescine, glycine, pyruvate, 1,6-anhydro-D-glucose, and acetoacetate. The relationship between fold change and statistical significance is visualized by a volcano plot (Figure 4b).
The volcano plot effectively highlights key metabolites that warrant further investigation into their roles in metabolic pathways and their potential as therapeutic targets. Metabolites such as hydroxybutyrate, fucose, 3-aminoisobutyrate, and acetoacetate in the upper regions indicate high statistical significance, and their horizontal position reflects the magnitude of change.
- (iii)
- Obese diabetes
Our analysis revealed significant upregulation of four metabolites in obese diabetes patients relative to healthy controls (Figure 2c). In particular, fucose demonstrated a 2.95-fold increase, whereas 3-aminoisobutyrate, acetoacetate, and 3-hydroxybutyrate exhibited increases between 2.6- and 2.75-fold.
We conducted a t-test to compare metabolite levels between obese diabetes patients and healthy controls, identifying 37 significant metabolites with p < 0.05 (Figure 3c). The metabolites identified as having the highest significance included putrescine, glycine, 1,6-anhydro-D-glucose, glucose, and 4-hydroxyphenylacetate.
A volcano plot was also generated to visualize these differences, illustrating the relationship between fold change and statistical significance for the identified metabolites (Figure 4c). The volcano plot illustrates the relationship between log2 fold change (x-axis) and the negative log10 of the p-values (y-axis), with metabolites fucose, 3-aminoisobutyrate, acetoacetate, and 3-hydroxybutyrate in the upper regions indicating high statistical significance and their horizontal position reflecting the magnitude of change.
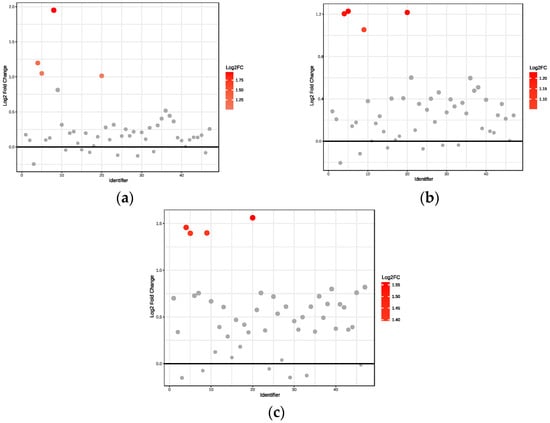
Figure 2.
Fold change relative to healthy controls: (a) Obese only, (b) Obese prediabetes, (c) Obese diabetes. Black horizontal line: Indicates the reference value for Log2 Fold Change (usually set at 0), representing no change between groups. Points above this line have increased expression (or abundance), and points below would have decreased expression, relative to the reference group.
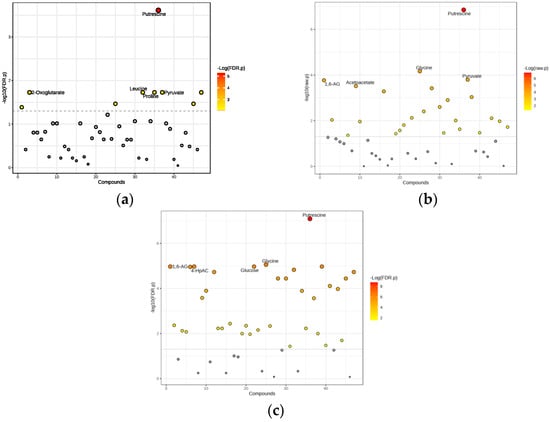
Figure 3.
Significant metabolites differentiating the obese diabetes group from healthy controls: (a) Obese only, (b) Obese prediabetes, (c) Obese diabetes. Gray dots: Represent features (such as metabolites or genes) that do not meet the threshold for significant change. These are considered not significantly different between groups, in contrast to the colored (red) dots, which indicate significant changes. 1,6-AG—1,6-Anhydro-β-D-glucose; 4-HpAC—4-Hydroxyphenylacetate.
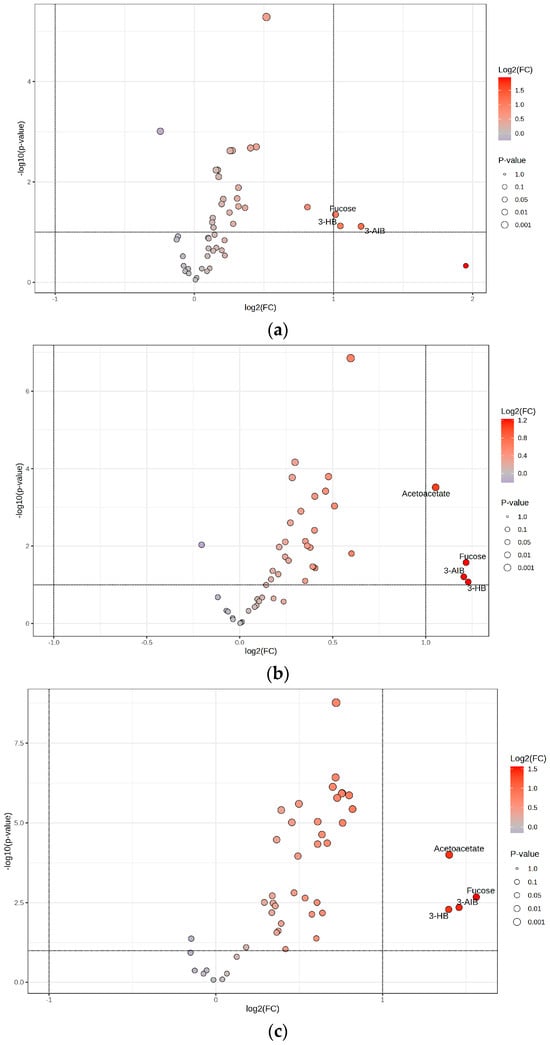
Figure 4.
Volcano plots for (a) Obese only, (b) Obese prediabetes, (c) Obese diabetes. Horizontal dotted line: Represents the significance cutoff for the p-value (commonly set at p = 0.05). Points above this line have p-values lower than the cutoff and are considered statistically significant. Vertical dotted lines: Represent the fold change thresholds (commonly log2(FC) = ±1, which corresponds to a 2-fold change). Points to the right or left of these lines have fold changes greater than or less than the set threshold, indicating substantial upregulation or downregulation. 3-AIB—3-Aminoisobutyrate; 3-HB—3-Hydroxybutyrate.
2.4. PLS-DA All Groups
The PLS-DA score plot (Figure 5) reveals substantial overlap among the four groups—healthy controls, obese only, obese prediabetes, and obese diabetes—indicating that their metabolic profiles are more similar than distinct.
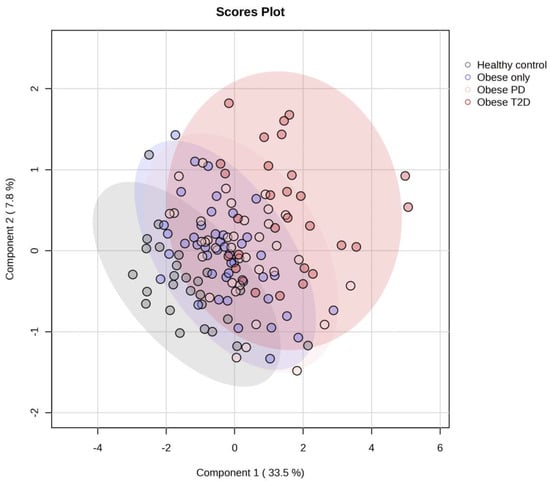
Figure 5.
PLS-DA score plot for all four groups. PD—prediabetes; T2D—type 2 diabetes.
2.5. OPLS-DA
As the use of PLS-DA is less effective when analyzing more than three groups due to increased complexity in interpretation, a higher risk of overfitting, and diminished discriminatory power, we then employed OPLS-DA (Orthogonal Partial Least Squares Discriminant Analysis) with healthy controls as a common reference group to address these issues. This approach allows for a clearer separation of the groups by disentangling group-predictive variation from unrelated variation, thereby enhancing the interpretability of the results and providing a more robust framework for identifying metabolic differences among the groups. These results represent OPLS-DA relative to healthy controls:
- (i)
- Obese only
We employed OPLS-DA to assess metabolic differences between obese only patients and healthy controls (Figure 6a). The model demonstrated a cumulative R2Y value of 0.337, indicating that 33.7% of the variance in the metabolite data was explained by group membership, along with a Q2 value of 0.235, suggesting reasonable predictive ability. The analysis identified five key metabolites with the highest Variable Importance in Projection (VIP) scores (Figure 7a): pyruvate, putrescine, π-methylhistidine, 2-oxoglutarate, and glycine. Notably, all these metabolites, except for 2-oxoglutarate, were upregulated in the obese only group compared to healthy controls. This distinct metabolic profile underscores the significance of our research, as it highlights potential biomarkers for obesity and emphasizes the complexity of metabolic interactions within these populations.
- (ii)
- Obese prediabetes
We also utilized OPLS-DA to evaluate the metabolic differences between obese prediabetes patients and healthy controls (Figure 6b). The model demonstrated a cumulative R2Y value of 0.4, indicating that 40% of the variance in the metabolite data could be explained by group membership, suggesting a moderate level of model fit. The Q2 value of 0.318 reflects the model’s predictive ability, indicating reasonable predictive power, although there is room for improvement. The analysis identified five key metabolites with the highest VIP scores (Figure 7b): acetoacetate, putrescine, Fucose, 3-aminoisobutyrate, and succinate. Notably, these metabolites were found to be upregulated in obese prediabetes patients compared to healthy controls.
- (iii)
- Obese diabetes
OPLS-DA is again used to assess metabolic differences between obese diabetes patients and healthy controls (Figure 6c). The model demonstrated a cumulative R2Y value of 0.57, indicating that 57% of the variance in the metabolite data was explained by group membership, along with a Q2 value of 0.547, suggesting strong predictive ability. The analysis identified five key metabolites with the highest VIP scores (Figure 7c): putrescine, glycine, 1,6-Anhydro-D-glucose, taurine, and glucose. Notably, all these metabolites were upregulated in the obese diabetes group compared to healthy controls. Putrescine is involved in cellular growth, while taurine plays a role in various physiological processes, underscoring their significance in metabolic pathways. Distinct metabolic profiles were observed between the two groups, although some overlap indicated shared metabolic features. Overall, our findings suggest that OPLS-DA effectively elucidates metabolic differences, highlighting potential biomarkers for obesity-related diabetes and emphasizing the complexity of metabolic interactions within these populations.
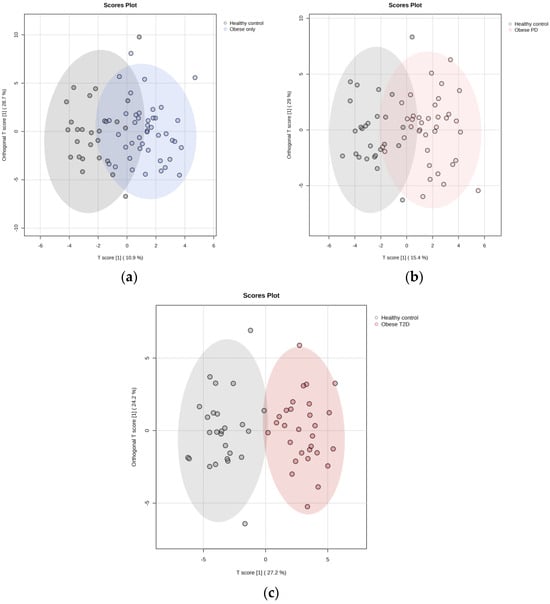
Figure 6.
OPLS-DA relative to healthy controls: (a) Obese only, (b) Obese prediabetes, (c) Obese diabetes. PD—prediabetes; T2D—type 2 diabetes.
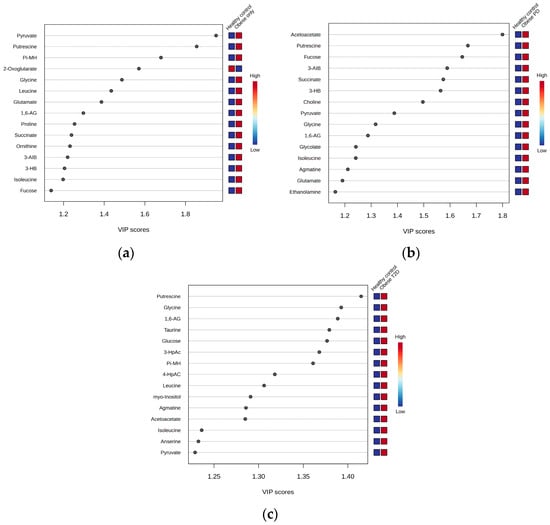
Figure 7.
Variable Importance in Projection (VIP) scores: (a) Obese only, (b) Obese prediabetes, (c) Obese diabetes. PD—prediabetes; T2D—type 2 diabetes; 1,6-AG—1,6-Anhydro-β-D-glucose; 3-AIB—3-Aminoisobutyrate; 3-HB—3 Hydroxybutyrate; 3-HpAC—3-Hydroxyphenylacetate; 4-HpAC—4-Hydroxyphenylacetate; Pi-MH—π-methylhystidine.
2.6. Hierarchal Clustering Analysis (HCA)
Hierarchical clustering analysis was conducted to explore the metabolic profiles of healthy controls, obese only, obese prediabetes, and obese diabetes (Figure 8).
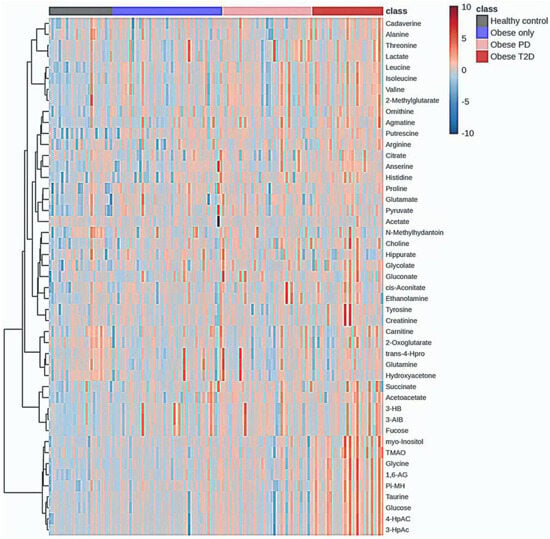
Figure 8.
Hierarchal Clustering Analysis (HCA). PD—prediabetes; T2D—type 2 diabetes; 1,6-AG—1,6-Anhydro-β-D-glucose; 3-AIB—3-Aminoisobutyrate; 3-HB—3 Hydroxybutyrate; 3-HpAC—3-Hydroxyphenylacetate; 4-HpAC—4-Hydroxyphenylacetate; trans-4-Hpro—trans-4-Hydroxy-L-proline; TMAO—Trimethylamine N-oxide; Pi-MH—π-methylhystidine.
The analysis revealed significant metabolic differences among these groups. Healthy controls exhibited distinct metabolic signatures compared to the obese groups; however, some subjects within the healthy control group showed an overlap in metabolic characteristics with the obese groups. These individuals were identified as outliers, indicating that while they generally belong to the healthy control group, their metabolic profiles resemble those of individuals with obesity.
Notably, there was some overlap in metabolic characteristics between the obese only and obese prediabetes groups, while the obese diabetes group displayed unique metabolic alterations associated with diabetes. The accompanying heatmap visually represented metabolite expression levels across the groups, indicating that certain metabolites were consistently upregulated in the obese diabetes group compared to the others.
The integration of the average results from HCA reveals a distinct group of metabolites that are massively upregulated in the obese diabetes group (Figure 9), including putrescine, glycine, 1,6-Anhydro-D-glucose, taurine, glucose, tyrosine, myoinositol, cadaverine, 3-hydroxyphenylacetate, 4-hydroxyphenylacetate, TMAO, creatinine, and alanine.
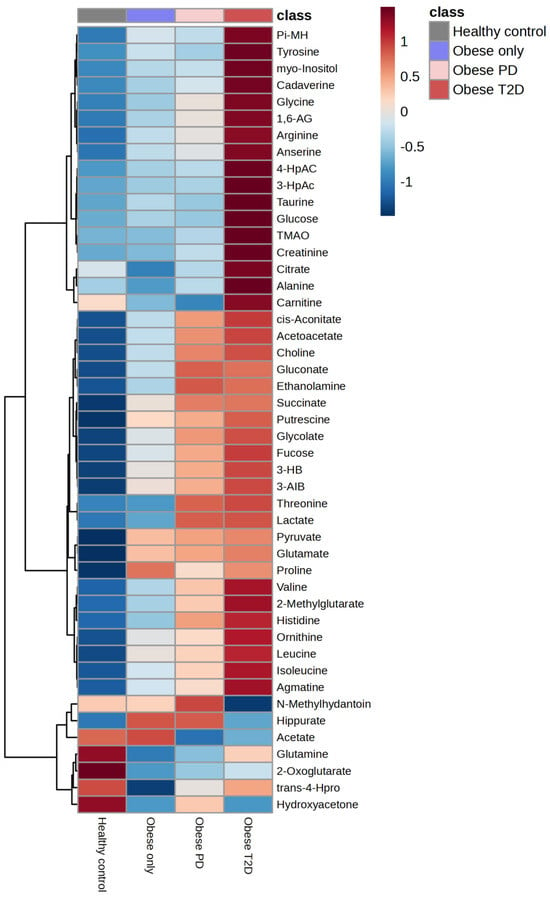
Figure 9.
The integration of the average results from hierarchical clustering analysis. PD—prediabetes; T2D—type 2 diabetes; 1,6-AG—1,6-Anhydro-β-D-glucose; 3-AIB—3-Aminoisobutyrate; 3-HB—3 Hydroxybutyrate; 3-HpAC—3-Hydroxyphenylacetate; 4-HpAC—4-Hydroxyphenylacetate; trans-4-Hpro—trans-4-Hydroxy-L-proline; TMAO—Trimethylamine N-oxide; Pi-MH—π-methylhystidine.
Conversely, several metabolites, including succinate, putrescine, amino isobutyrate, pyruvate, and glutamate, were strongly downregulated in the healthy control group.
Correlation heatmap of this study is presented in Figure 10. Glycine, 1,6-anhydro-D-glucose, myo-inositol, π-methylhistidine, 4-hydroxyphenylacetate, 3-hydroxyphenylacetate, taurine, glucose, and TMAO form Cluster 1, characterized by strong positive correlations with one another. This clustering suggests that these metabolites may be interconnected within metabolic pathways, indicating their potential collective role in the metabolic profile observed in our study. Additionally, several smaller clusters were identified: Cluster 2, comprising valine, 2-methylglutarate, leucine, and isoleucine; Cluster 3, consisting of 3-hydroxybutyrate, 3-aminoisobutyrate, and fucose; and Cluster 4, which includes trans-4-hydroxy-L-proline, glutamine, and hydroxyacetone.
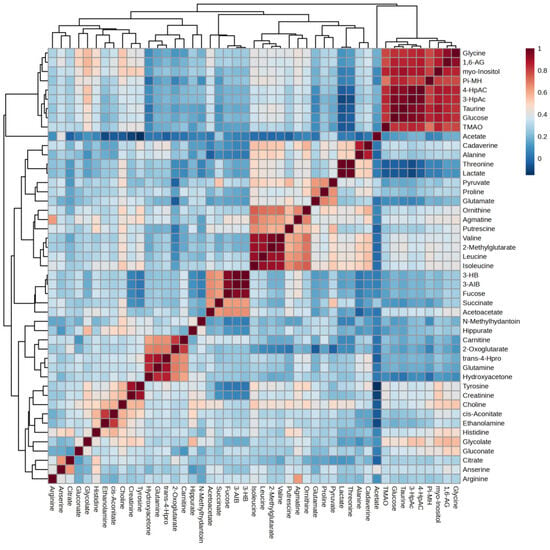
Figure 10.
Correlation heatmap. 1,6-AG—1,6-Anhydro-β-D-glucose; 3-AIB—3-Aminoisobutyrate; 3-HB—3 Hydroxybutyrate; 3-HpAC—3-Hydroxyphenylacetate; 4-HpAC—4-Hydroxyphenylacetate; trans-4-Hpro—trans-4-Hydroxy-L-proline; TMAO—Trimethylamine N-oxide; Pi-MH—π-methylhystidine.
Each cluster also exhibited strong correlations among their respective metabolites, further highlighting the intricate relationships within the metabolic network associated with obesity and diabetes. We will discuss these four clusters further in the Discussion session.
3. Discussion
Our study, to our knowledge, is the first to unveil predictive metabolic markers and altered metabolic profiles of T2D and prediabetes among obese individuals in Malaysia with a BMI cut-off value for the Asian population.
The strong overlap among the four groups in the OPLS-DA, particularly among the obese categories, highlights the complexity of metabolic responses and indicates that differentiating between these conditions based solely on metabolite profiles may be challenging, underscoring the need for further research in this area. While distinct metabolic profiles were observed between the obese prediabetes group and healthy controls, some overlap indicated that specific metabolites may not be exclusively altered in one group. This overlap suggests that while there are clear metabolic disturbances associated with obesity and prediabetes, there may also be shared metabolic features present in healthy individuals. Overall, our findings suggest that OPLS-DA is an effective method for elucidating metabolic differences between these groups, providing valuable insights into the underlying pathophysiological mechanisms contributing to obesity-related conditions. The identification of these key upregulated metabolites not only highlights potential biomarkers for obesity and prediabetes but also emphasizes the complexity of metabolic interactions within these populations.
Putrescine, one of the metabolites upregulated in the obese diabetes group compared to healthy controls, is involved in cellular growth, while taurine plays a role in various physiological processes, underscoring their significance in metabolic pathways. Distinct metabolic profiles were observed between the two groups, although some overlap indicated shared metabolic features. Overall, our findings suggest that OPLS-DA effectively elucidates metabolic differences, highlighting potential biomarkers for obesity-related diabetes and emphasizing the complexity of metabolic interactions within these populations.
For HCA, our finding reinforces the notion of metabolic dysregulation in obesity-related diabetes. Overall, these results from hierarchical clustering analysis emphasize the metabolic distinctions between healthy individuals and various stages of obesity and diabetes, while highlighting potential biomarkers for further investigation into obesity-related metabolic disturbances. Significant upregulation in the obese diabetes group, alongside the downregulation observed in healthy controls, underscores the metabolic alterations associated with obesity and diabetes, providing insights into potential biomarkers for further investigation into obesity-related metabolic disturbances.
The correlation heatmap analysis has unveiled four distinct metabolite clusters, providing a comprehensive view of the metabolic interactions across all study groups. This approach, in line with our study’s aim, offers a more integrated analysis than individual analytical methods. Focusing on the correlation heatmap can provide a more holistic interpretation of the metabolomic data, offering insights into the complex metabolic alterations associated with obesity and diabetes in the Malaysian population. These findings could potentially revolutionize diabetes management strategies.
Through the correlation heatmap, four clusters were formed. The most significant cluster, Cluster 1: Glycine, 1,6-anhydro-D-glucose, myo-inositol, methylhistidine, 4-hydroxyphenylacetate, 3-hydroxyphenylacetate, taurine, glucose, and trimethylamine N-oxid (TMAO), characterized by strong positive correlations with one another. This clustering suggests that these metabolites may be interconnected within metabolic pathways, indicating their potential collective role in the metabolic profile observed in our study. Additionally, several smaller clusters were identified: Cluster 2, comprising valine, 2-methylglutarate, leucine, and isoleucine. Next, Cluster 3, consisting of 3-hydroxybutyrate, 3-amino isobutyrate, and fucose; and finally, Cluster 4, which includes trans-4-hydroxy-L-proline, glutamine, and hydroxyacetone. Each cluster also exhibited strong correlations among their respective metabolites, further highlighting the intricate relationships within the metabolic network associated with obesity and diabetes. We summarize these metabolites in Table 3. Some of the metabolites will be discussed below.
3.1. Cluster 1
3.1.1. Glycine
Glycine has been suggested as a conditionally essential amino acid. In metabolic disorders associated with obesity, T2D, and non-alcoholic fatty liver disease (NAFLD), lower circulating glycine levels have been consistently observed. Glycine supplementation has beneficial effects, as suggested in clinical studies [26]. Individuals with obesity and T2D tend to have lower glycine levels, suggesting that glycine depletion might be a factor in the development of diabetes [19].
3.1.2. Myo-Inositol
Myo-inositol, a type of inositol, has shown promise in improving cardiometabolic factors, including lipid profiles and liver function, and reducing BMI in obese individuals, potentially through mechanisms like improved insulin sensitivity and thyroid function [27,28]. Myo-inositol was shown to possess insulin-mimetic properties and efficiently lower post-prandial blood glucose [29]. In an animal model study, abnormalities in myo-inositol metabolism are associated with T2D in mice fed a high-fat diet [30]. Myo-inositol urinary excretion was strongly correlated with glucose urinary excretion. Interestingly, some research suggests that myo-inositol supplementation may help prevent gestational diabetes mellitus in overweight and obese pregnant women [31]. A recent large-scale cohort study of obese individuals in China found a potential obesity-linked bacterium, Megamonas. This research suggests potential strategies for future obesity management by illustrating how the bacterium degrades intestinal myo-inositol, enhances lipid absorption, and contributes to obesity [32].
3.1.3. Trimethylamine N-Oxide (TMAO)
TMAO, a gut microbiota-derived metabolite, has been associated with an elevated risk of obesity and cardiovascular disease, potentially acting as a novel risk factor. Studies suggest that elevated TMAO levels are associated with obesity and related metabolic issues, such as insulin resistance and increased risk of cardiovascular disease [33,34]. TMAO’s role in metabolic pathways and its impact on gut microbiota composition may contribute to the progress of obesity and related metabolic disorders [35]. The composition of the diet, particularly the TMAO precursors intake like choline, carnitine, and betaine, can influence TMAO levels. Interestingly, elevated levels of serum TMAO levels were positively associated with the risk of diabetic kidney disease in T2D patients [36].
3.1.4. Taurine
Metabolomic studies have shown that taurine supplementation can improve lipid metabolism and reduce the risk of metabolic syndrome in people with obesity. Taurine may also help prevent and manage T2D [37,38]. Taurine administration prevented weight gain and dyslipidemia, and alleviated lipid deposition in the liver and adipose tissue in hyperlipidemic mice [39]. Furthermore, taurine reduces the risk of metabolic syndrome [40].
3.2. Cluster 2
Branched-Chain Amino Acids (BCAAs)
BCAAs are essential amino acids that change along with consuming a protein-containing meal, including valine, leucine, and isoleucine [41]. Circulating BCAAs have emerged as strongly and positively associated with adiposity [42]. Studies have also shown a positive association between increased circulating BCAAs (valine, leucine, and isoleucine) and insulin resistance (IR) in obese or diabetic patients [43]. Isoleucine and valine levels are higher among the obese patients with T2D compared to healthy individuals [44,45]. In addition, BCAA metabolites’ downstream accumulation may cause mitochondrial dysfunction [46]. Meanwhile, elevated BCAA levels are associated with insulin resistance [47], diabetes [48], and coronary artery disease [49] However, the physiological mechanisms underlying the regulation of circulating BCAA concentrations remain unknown [50,51,52,53]. There are two potential mechanisms explaining how BCAAs might contribute to insulin resistance in T2D and obesity [46]. First, excess dietary BCAAs activate the mammalian rapamycin complex 1 (mTORC1) signaling target, leading to insulin resistance and T2D. Second, it has been suggested that increased BCAAs could serve as a practical biomarker of impaired metabolism, offering potential for clinical applications.
3.3. Cluster 3
3.3.1. 3-Hydroxybutyrate (3HB)
3HB, a ketone body, has gained attention for its potential role in obesity and related metabolic disorders, with research suggesting it may influence adipocyte function and gut microbiota and potentially reduce insulin resistance [54,55]. It has been shown that the 3HB ameliorates insulin resistance by inhibiting PPARγ Ser273 phosphorylation in T2D mice [56]. 3HB levels increase in people with diabetes and prediabetes [57].
3.3.2. 3-Amino Isobutyrate
3-amino isobutyrate (also known as β-aminoisobutyric acid or BAIBA) is a catabolic metabolite of thymine and valine in skeletal muscle [58,59]. During exercise, BAIBA is released by contracting human skeletal muscle and lowers insulin release from INS-1 832/3 cells by mediating mitochondrial energy metabolism. It is a possible mediator of insulin secretion [60]. During exercise, 3-aminoisobutyric acid is secreted by muscle cells into the blood. Once this molecule interacts with adipocytes, it may induce signaling pathways regulating fats, insulin, and cholesterol metabolism.
3.3.3. Fucose
L-fucose, the main form of fucose found in nature, has shown promising anti-obesity effects, potentially by suppressing lipid accumulation and improving insulin sensitivity, and may be a novel strategy for treating obesity and related diseases. L-fucose suppresses lipid accumulation via the AMPK Pathway in 3T3-L1 adipocytes [61]. L-fucose can improve the gut microbiota’s obesity-driven dysbiosis, potentially by decreasing the relative abundance of obesity-related intestinal bacteria [62]. Fucose might be a novel strategy to treat HFD-induced obesity and fatty liver [63].
3.4. Cluster 4
3.4.1. trans-4-Hydroxy-L-proline
While there is no direct, strong scientific evidence linking trans-4-hydroxy-L-proline (t4Hyp) to obesity, it is a valuable chiral building block used in pharmaceuticals and cosmetics, and its production is being explored through metabolic engineering. However, some patents mention t4Hyp as a potential anti-obesity agent, though this is not widely supported by scientific evidence (US Patent application No. US20060264498A1) [64].
3.4.2. Glutamine
Research suggests a link between glutamine, a non-essential amino acid, and obesity, with studies indicating that glutamine metabolism is altered in obese individuals and may play a role in inflammation within fat tissue, potentially impacting obesity-related conditions [65]. Some studies suggest that glutamine supplementation may reduce obesity and pro-inflammatory markers and improve insulin sensitivity in animal models and overweight/obese humans. Oral glutamine supplementation reduces obesity, and pro-inflammatory markers, improves insulin sensitivity in rats, and reduces waist circumference in overweight and obese human [66].
3.4.3. Hydroxyacetone
Hydroxyacetone (acetol) is a metabolite of acetone found in people with diabetic ketoacidosis [67]. It can bind to and modify proteins, which may contribute to the progress of diabetes complications. Hydroxyacetone is an organic compound that can be produced by reducing methylglyoxal. Diets rich in vegetables, whole grains, fruits, and lean protein contribute to normal metabolism and low levels of methylglyoxal production. In stark contrast, a western diet of processed food, refined grains, and red meat leads to metabolic dysfunction, increasing methylglyoxal levels, and is associated with several pathologies, including obesity, heart disease, diabetes, and cancer [68]

Table 3.
Summary table of metabolites identified from four clusters.
Table 3.
Summary table of metabolites identified from four clusters.
| Metabolites | Author | Populations | Platform | Findings |
|---|---|---|---|---|
| Cluster 1 | ||||
| Glycine | Thalacker-Mercer, A.E., et al. [69] | 124 adults (60 African American and 63 European American) | Hyperinsulinemic-euglycemic clamp | Concentration of glycine was correlated to glucose disposal rate (GDR) |
| Cheng, S., et al. [70] | Framingham Heart Study (n = 1015); Malmö Diet & Cancer Study (n = 746) | Liquid chromatography-tandem mass spectrometry (LC-MS) | The criteria for metabolic syndrome were met by 45% individuals | |
| Floegel, A., et al. [19] | (EPIC)-Potsdam study (2282 individuals of the subcohort and 800 individuals with incident T2D) | Tandem mass spectrometry (FIA-MS/MS) | Glycine levels tend to be lower in individuals with obesity and T2D | |
| Takashina, C., et al. [71] | Ninety-four healthy Japanese volunteers aged 20–60 years | HPLC | The 2 h or fasting plasma glucose levels or HOMA-IR were positively correlated with glutamate, valine, and tyrosine levels but negatively correlated with glutamine, citrulline, and glycine levels | |
| Tulipani, S., et al. [72] | 64 adults; sex-matched groups for BMI and risk of developing T2D | LC- and flow injection analysis-(electrospray ionization) mass spectrometry (FIA-ESI-MS/MS) | Glycine concentration is negatively associated with fasting insulin and HOMA-IR (R = −0.51, R = −0.49, respectively; both p < 0.0033) | |
| Wijayatunga, N.N., et al. [73] | 20 patients undergoing RYGB surgery, pre- and 6 months post-surgery | NMR | Serum glycine was significantly elevated at 6 months post-surgery compared with pre-surgery (n = 8, p < 0.05) | |
| 1,6-anhydro-D-glucose | Kawaguchi, T., et al. [74] | Five male patients with NAFLD; a randomized, single-blinded controlled interventional study | LC-time-of-flight mass spectrometry (TOFMS) | Isomaltulose improved insulin resistance in NAFLD patients |
| myo-inositol | Croze, M.L., et al. [30] | High-fat diet (HFD) was fed to C57BL/6 male mouse for 1 month (n = 10) | HPLC | Inosituria, which is myo-inositol urinary excretion, was correlated with glycosuria in mice (R2 0.915, linear regression) |
| Wu, C., et al. [32] | Chinese cohort of 631 obese subjects and 374 normal-weight controls | Shotgun metagenomic sequencing | Identified a Megamonas-dominated, enterotype-like cluster enriched in obese subjects, where Megamonas rupellensis possessed genes for myo-inositol degradation, which is a myo-inositol degrader. This function enhances lipid absorption and obesity | |
| Sikes, K.J., et al. [75] | 90 mice | Gas Chromatography Mass Spectrometry (GCMS) | Untargeted metabolomics analysis identifies creatine, myo-inositol, and lipid pathway modulation in a murine model of tendinopathy | |
| 4-hydroxyphenylacetate | Wang, P., et al. [76] | 24 mice, 16 weeks | GC-MS | 4-HPA was sufficient to reverse obesity and glucose intolerance in HFD-fed mice. Mechanistically, 4-HPA treatment markedly regulates SIRT1 signaling pathways and induces the expression of beige fat and thermogenesis-specific markers in white adipose tissue (WAT) |
| Osborn, L.J., et al. [77] | Male C57BL/6 mice | High-performance liquid chromatography tandem mass spectrometry (LC-MS/MS) | a single gut microbial flavonoid catabolite, 4-hydroxyphenylacetic acid (4-HPAA), is sufficient to reduce diet-induced cardiometabolic disease (CMD) burden in mice | |
| Chen, W., et al. [78] | 32 male C57BL mice | Urine metabolomic analysis by UHPLC-Q-TOF/MS | Microbial phenolic metabolites 3-(3′,4′-dihydroxyphenyl) propanoic acid and 3′,4′-dihydroxyphenylacetic acid prevent obesity in mice fed with high-fat diet | |
| 3-hydroxyphenylacetate | Zhang, Y., et al. [56] | 28 of 6-week-old male ICR/KM mice, high-fat diet | 1H-NMR | High-fat diet significantly reduced the 3-hydroxyphenylacetate concentrations in the cecum contents of mice |
| Glucose | Li, M., et al. [79] | Virgin Wistar rats, supplemented with taurine | Autoanalyser | Maternal taurine supplementation may ameliorate the adverse effects observed in offspring following a maternal obesogenic diet |
| Trimethylamine N-oxid (TMAO) | Zhang, Q., et al. [80] | 28 of 6-week-old male ICR/KM mice, high-fat diet | 1H-NMR | High-fat-diet-induced obese mice supplemented with fibers have lower levels of TMAO, compared to control subjects |
| Barrea, L., et al. [34] | 330 adult Caucasians subjects (20–63 years) of both genders | HPLC/MS | TMAO levels increased along with BMI and were positively associated with VAI and FLI, independently | |
| Lee, S.J., et al. [81] | 38 obese patients (18 with and 20 without T2D) who underwent bariatric surgery | UPLC/TQ-MS | TMAO increased more than twofold in patients with T2D after surgery, but not in patients without T2D | |
| Li, X., et al. [82] | Sixteen M. mulatta (six months old) were fed a control diet or a HFHC diet for 18 months | 1H-NMR | The diet rich in fish affects the concentration of TMAO, also the high-fat and high-calorie diet increases the levels of serum TMAO | |
| Cluster 2 | ||||
| valine | Ho, J.E., et al. [83] | 2383 Framingham Offspring cohort participants | LC-MS | Valine was associated with 4 metabolic traits: BMI, HOMA-IR, HDL, and triglycerides; circulating branched-chain amino acids, like valine, have emerged as strongly and positively associated with adiposity |
| Tai, E.S., et al. [84] | 263 non-obese, Asian-Indian and Chinese men, cross-sectional study in Singapore | Tandem MS (MS/MS) | Insulin resistance (IR) was correlated with increased levels of valine | |
| Wang, T.J., et al. [48] | The Framingham Offspring Study, 2422, free of diabetes and cardiovascular disease, underwent routine oral glucose tolerance testing (OGTT), >35 years | LC-MS | 2422 individuals with normal glucose levels followed for 12 years, 201 developed diabetes Valine had highly significant associations with future diabetes | |
| 2-methylglutarate | Wijayatunga, N.N., et al. [73] | 20 patients undergoing RYGB surgery pre and 6 months post-surgery | 1H-NMR | Serum 2-methylglutarate was significantly reduced, at 6 months post-surgery compared with pre-surgery (n = 8, p < 0.05) |
| Leucine | Ozcariz, E., et al. [85] | 1387 individuals from the general population | 1H-NMR | Leucine was found to be significantly different between clusters of metabolic profiles of cardiometabolic risk in patients with obesity |
| Isoleucine | Yu, D., et al. [86] | male C57BL/6J mice | Quadrupole-orbitrap mass spectrometer | Reducing isoleucine or valine rapidly restores metabolic health to diet-induced obese mice. A low isoleucine diet reprograms liver and adipose metabolism, increasing hepatic insulin sensitivity |
| Trautman M.E. et al., 2024 [87] | C57BL/6J and DBA/2J mice | Quadrupole-orbitrap mass spectrometer | Reducing dietary levels of isoleucine rapidly improves the metabolic health of diet-induced obese male C57BL/6J mice | |
| Cluster 3 | ||||
| 3-hydroxybutyrate (3-OHB) | Zhang, Y., et al. [56] | Four-week-old male C57BL/6 mice | Glucose uptake assay | 3OHB improves glucose tolerance, reduces fasting blood glucose level, and ameliorates insulin resistance in T2D mice through hydroxycarboxylic acid receptor 2 (HCAR2) |
| Jung, J., et al. [88] | Mice (db/db) were fed normal chow, high-fat, or ketogenic diets | Determination of Reactive Oxygen Species (ROS) | in vivo and in vitro models show that 3-OHB delays the progression of diabetic nephropathy by augmenting autophagy and inhibiting oxidative stress | |
| 3-aminoisobutyrate | Yousri, N.A., et al. [89] | 996 individuals in Qatar | ultra-performance liquid chromatography (UPLC), mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer | 3-aminoisobutyrate was in the T2D top most significant metabolites from the total of 229 metabolites, sorted by pathway |
| Kistner, S., et al. [90] | 255 healthy women and men, urine samples | 1H-NMR | 15–30 min following an incremental cycling test, valine derivative 3-aminoisobutyrate increased | |
| Cluster 4 | ||||
| trans-4-hydroxy-L-proline | Kenéz, Á., et al. [91] | Twenty horses of various breeds, ages, and body weights | LC-MS/MS | Due to trans-4-hydroxy-l-proline, and high concentration of glycine, collagen has been shown to stimulate insulin secretion and stabilize blood sugar levels in individuals with T2D Lower plasma trans-4-hydroxyproline is associated with insulin dysregulation in horses |
| Glutamine | Floegel, A., et al. [19] | 27,548 participants from the general population in Germany; 35–65 years of age | FIA-MS/MS | Glutamine improved insulin sensitivity |
| Stancáková, A., et al. [92] | 9369 non-diabetic or newly diagnosed T2D men from the population-based study (METSIM Study) (mean ± SD age, 57 ± 7 years; BMI, 27.0 ± 4.0 kg/m2) | 1H-NMR | Elevated fasting and 2 h plasma glucose levels were associated with increasing levels of several amino acids and reduced levels of glutamine | |
| Palmer, N.D., et al. [93] | 196 subjects European American, Hispanic, and African American Insulin Resistance Atherosclerosis Study (IRAS) | Mass spectrometry | the glutamine-to-glutamate ratio was found to predict insulin resistance after 5 years in participants | |
| Hydroxyacetone | Reichard, G.A., et al. [67] | Patients with diabetes ketoacidosis (DKA) | Gas chromatograph equipped with a flame ionization detector | Acetol (1-hydroxyacetone), a possible metabolite of acetone, was detected in plasma of the patient during diabetic ketoacidosis |
| Schumacher, D., et al. [94] | 15 diabetes patients 15 healthy controls | LC-MS/MS | Diabetic patients without complications had the highest content of hydroxyacetone |
4. Materials and Methods
4.1. Study Design
This is a multicenter cross-sectional study of patients with obesity and with different diabetes statuses who were scheduled for metabolic-bariatric surgery at the iHEAL Medical Centre KL, Sunway Medical Centre Velocity KL, Sunway Medical Centre Selangor, and KPJ Damansara Specialist Hospital, Malaysia. Anthropometric measurements were recorded and blood samples were collected pre-operatively.
Study participants were obese patients who were grouped into either obese only, prediabetes, or T2D. The diagnostic criteria were based on the HbA1c values [95] (Table 4). For comparison, healthy individuals (n = 26) were recruited as a control group.

Table 4.
Diagnostic values for prediabetes and type 2 diabetes (T2D) based on HbA1c values (Malaysia Clinical Practice Guidelines, 2020) [95].
This study involved 137 participants categorized into four groups: healthy controls (n = 26), obese only patients (n = 45), obese prediabetic patients (n = 37), and obese diabetic patients (n = 29). These participants except healthy controls were part of a cohort screened for bariatric surgery in multiple hospitals in the Klang Valley, Malaysia.
4.2. Individual Inclusion and Exclusion Criteria
Participants in the study were recruited based on the criteria outlined below.
- Inclusion Criteria
The inclusion criteria for the subjects are as follows: both men and women; age should be between 18 and 65 years; body mass index (BMI) ≥ 25 kg/m2 (Asian BMI: WHO/IASO/IOTF, 2000); and scheduled for metabolic surgery. Obese patients who fulfilled the criteria and have been diagnosed with prediabetes or T2D were also eligible. The inclusion criteria for healthy individuals are as follows: between 18 and 65 years of age; men and women; normal range BMI between 18.5 and 22.9 kg/m2; and without prediabetes, diabetes, or any related comorbidities. Written informed consent must be signed by all subjects.
- Exclusion Criteria
Patients and healthy individuals in the control group who were using medications that could impact their body weight at the time of recruitment, or who had medical conditions such as acromegaly, Cushing’s syndrome, heart failure, Crohn’s disease, or alcohol dependence, were excluded from the study. Individuals with a normal BMI of 18.5–22.9 kg/m2 but who present with comorbidities were also excluded.
4.3. Blood Biochemical Profile
Plasma samples (400 μL each) were collected from each participant. Before blood collection, the subjects were asked to fast overnight, which was estimated to be between 8 and 12 h. HbA1c was measured to determine the diabetes status. The blood samples were then centrifuged at 1500× g for 20 min. After centrifugation, the plasma was aliquoted into a few tubes and stored at −80 °C until further analysis. Lipid profiles were measured using chemistry analyzer.
4.4. Sample Preparation for Metabolomic Analysis
Two milliliters of blood were collected in lithium heparin tubes because other anticoagulants, such as EDTA and citrate, produce additional high-intensity signals in the NMR spectrum. The plasma samples were prepared according to published protocols for NMR metabolomic analysis [96]. This preparation ensured that the samples were ready for targeted and untargeted NMR metabolomics approaches.
4.5. Spectra Acquisition
The 1D 1H-NMR spectra were collected at a temperature of 26 °C using a JNM-ECZ-600 R 600 MHz spectrometer (JEOL, Tokyo, Japan). The combination of PRESAT and the Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence was used to suppress water signals and broad protein resonances. NMR spectra with a spectral width of 12 ppm were acquired using 128 scans and a 660 s acquisition time [96]. After acquiring the NMR spectra, they were processed using the Chenomx NMR suite software, version 9.2.
4.6. Metabolite Identification
Chenomx NMR suite software, version 9.2 (Chenomx Inc., Edmonton, AB, Canada) was used to identify and quantify different groups’ metabolites by identifying the sections of the spectrum (ppm) responsible. This was accomplished by comparing the corresponding peak’s location, intensity, and linewidth with the 600 MHz Human Metabolome Database (HMDB) metabolites library. The area under the peak corresponded to the metabolites’ relative concentrations [96]. This study integrated targeted and untargeted NMR metabolomics, utilizing the advantages of both methodologies. The untargeted method offers a broad perspective of the metabolome, facilitating the identification of new biomarkers and metabolic pathways. The targeted method provides accurate quantification of specific metabolites, thereby improving the comprehension of their functions in metabolic processes. This integrated approach provides a comprehensive analysis of the metabolic changes linked to obesity, prediabetes, and diabetes. This dual approach enables a comprehensive understanding of the metabolic landscape and facilitates the identification of key biomarkers.
4.7. Data Pretreatment
The metabolomic data were analyzed using MetaboAnalyst 6.0 web application (https://www.metaboanalyst.ca/, (accessed on 15 August 2024), a comprehensive web-based tool suite for metabolomic data analysis. The raw data were uploaded to MetaboAnalyst 6.0 web application and subjected to data pretreatment. This included normalization to constant sum, data transformation using log normalization, and data scaling using Pareto scaling to ensure the data were properly prepared for downstream analysis.
4.8. Statistical Analysis
Several statistical and machine learning methods were employed using MetaboAnalyst 6.0 web application:
ANOVA for All Groups: One-way analysis of variance (ANOVA) was performed to identify significant differences among all groups. Post hoc analyses were also conducted to determine the specific group differences.
Fold Change Analysis Relative to Healthy Controls: Fold change analysis was used to compare the absolute value changes between the healthy control group and the other groups. This analysis helped identify metabolites that were significantly upregulated or downregulated relative to the healthy controls.
T-test Relative to Healthy Controls: T-tests were conducted to compare the metabolite levels between the healthy control group and each of the other groups. This analysis provided insights into the statistical significance of the differences observed.
OPLS-DA Relative to Healthy Controls: Orthogonal Partial Least Squares-Discriminant Analysis (OPLS-DA) was performed to identify metabolites that discriminated between the healthy control group and the other groups. This method helped in understanding the metabolic differences and identifying potential biomarkers.
4.9. Multivariate Analysis
PLS-DA VIP Score for All Groups: Partial Least Squares-Discriminant Analysis (PLS-DA) was used to identify the most important metabolites contributing to the separation between groups. The Variable Importance in Projection (VIP) scores were calculated to rank the metabolites based on their contribution to the model.
4.10. Clustering and Visualization
Hierarchical cluster analysis (HCA) and Heatmap: Hierarchical clustering was performed to group samples based on their metabolic profiles. Heatmaps were generated to visualize the clustering results and to identify patterns in the data.
Correlation Heatmap: Correlation analysis was conducted to identify significant correlations between metabolites. A correlation heatmap was generated to visualize these correlations, providing insights into the metabolic networks and relationships.
By using MetaboAnalyst 6.0 web application, we were able to perform a comprehensive analysis of the metabolomic data, including data pretreatment, statistical analysis, multivariate analysis, and visualization, which helped in identifying key metabolites and understanding the metabolic differences among the study groups.
4.11. Study Strengths
This study’s strengths lie in its comprehensive methodology, employing both targeted and untargeted NMR metabolomics approaches across multiple patient groups, and its use of advanced statistical analyses to identify key metabolites associated with obesity and diabetes.
4.12. Study Limitations
This study has some limitations that should be considered. First, the sample size is small, especially in the control group. However, we have sufficient samples for analysis [97]. Secondly, some unmeasured potential confounding factors (e.g., lifestyle or dietary intake) might have influenced our findings. Food intake influences metabolite levels, and gut microbiota plays a key role in modifying the metabolome and maintaining metabolic homeostasis [98]. Unfortunately, we do not have this data. However, the results based on diabetes status groups are a reasonable basis for diabetes exploration in metabolomics. Another limitation is the mean age difference between the healthy controls and obese groups. Several age-associated metabolites could reflect the changes in metabolisms during aging [99]. However, the younger, fitter group of healthy controls may be a good group to compare with. Hence, considering all limitations, our study results should be interpreted cautiously.
5. Conclusions
This study suggests that the use of 1H NMR metabolomics, in conjunction with conventional diabetes screening tools, could help to identify the mechanisms of diabetes and its progression by providing insights into underlying metabolic pathways. Identification of these metabolites may lead to more effective diabetes management for obese individuals. The metabolites identified in our study that may indicate the risk associated with T2D include fucose, 3-aminoisobutyrate, acetoacetate, and 3-hydroxybutyrate. These metabolites were significantly upregulated (by more than 2.6-fold) in obese patients with diabetes compared to healthy controls. This approach should incorporate precision medicine, utilizing genomic, and metabolomic biomarkers to personalize interventions, improve risk prediction, and initiate both lifestyle and pharmacological treatments.
Additionally, it is important to highlight those individuals classified as obese, specifically those with a BMI greater than 25 kg/m2—without prediabetes or diabetes according to Asian criteria—show significant divergences in metabolite levels compared to healthy controls. Therefore, more actions need to be taken at both community and individual levels to combat obesity and prevent diabetes-related comorbidities.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms26136216/s1.
Author Contributions
Conceptualization, M.F. and M.N.M.N.; methodology, M.F., Z.N.H., T.G., and M.N.M.N.; database, M.N.M.N., L.A.Z., N.A.Z.A., and N.S.Y.; validation, M.F.; formal analysis, M.N.M.N. and L.A.Z.; surgeries, T.G., S.Y.L., Z.N.H., and H.A.; investigation, T.G., S.Y.L., Z.N.H., H.A., N.A.Z.A., T.Y.Z., F.H.M., P.Y.T., and M.S.D.; laboratory analysis, M.N.M.N. and N.S.Y.; resources, M.F.; data curation, L.A.Z. and N.A.Z.A.; writing—original draft preparation, M.F. and M.N.M.N.; writing—review and editing, M.F., Z.N.H., T.G., T.Y.Z., F.H.M., P.Y.T., M.S.D., L.A.Z., N.A.Z.A., and M.N.M.N.; visualization, M.F.; supervision, M.F.; project administration, M.F. and N.S.Y.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Health Malaysia, grant number NMRR-20-2496-56353.
Institutional Review Board Statement
This study is in accordance with the 2013 Declaration of Helsinki and was approved by the Malaysia Research Ethic Committee (MREC) (NMRR-20-2496-56353) on 21 April 2021. All data are will be saved and associated with pseudonymized patient identification numbers. Research Electronic Data Capture (REDCap) is used for data handling. Trial registration number, NCT05322551.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
We thank the Director General of Health Malaysia for his permission to publish this manuscript. We want to extend special thanks to our laboratory technical staff for their excellent recruitment processes, specimen collection, laboratory assistance, and data entry: Siti Norfizah, Siti Mastura, Siti Azrinnah, Stephanie, Nur Iffah, and Siti Haslina; We would like to thank Lee Bee Hoon for financial management. Moreover, we thank all the ward and OT nurses who provided research assistance at the study sites.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why Does Obesity Cause Diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef]
- Karpe, F.; Dickmann, J.R.; Frayn, K.N. Fatty acids, obesity, and insulin resistance: Time for a reevaluation. Diabetes 2011, 60, 2441–2449. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef]
- Sam, S.; Mazzone, T. Adipose tissue changes in obesity and the impact on metabolic function. Transl. Res. 2014, 164, 284–292. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef]
- Grant, B.; Sandelson, M.; Agyemang-Prempeh, B.; Zalin, A. Managing obesity in people with type 2 diabetes. Clin. Med. 2021, 21, e327–e331. [Google Scholar] [CrossRef]
- Ruze, R.; Liu, T.; Zou, X.; Song, J.; Chen, Y.; Xu, R.; Yin, X.; Xu, Q. Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments. Front. Endocrinol. 2023, 14, 1161521. [Google Scholar] [CrossRef]
- Magliano, D.; Boyko, E.J. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2021. [Google Scholar]
- Ganasegeran, K.; Hor, C.P.; Jamil, M.F.A.; Suppiah, P.D.; Noor, J.M.; Hamid, N.A.; Chuan, D.R.; Manaf, M.R.A.; Ch’ng, A.S.H.; Looi, I. Mapping the Scientific Landscape of Diabetes Research in Malaysia (2000–2018): A Systematic Scientometrics Study. Int. J. Environ. Res. Public. Health 2021, 18, 318. [Google Scholar] [CrossRef]
- Akhtar, S.; Nasir, J.A.; Ali, A.; Asghar, M.; Majeed, R.; Sarwar, A. Prevalence of type-2 diabetes and prediabetes in Malaysia: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263139. [Google Scholar] [CrossRef]
- Institute for Public Health (IKU). National Health and Morbidity Survey (NHMS) 2023: Non-Communicable. Diseases and Healthcare Demand: Technical Report; Institute for Public Health: Shah Alam, Malaysia, 2024. [Google Scholar]
- Imamura, M.; Maeda, S. Perspectives on genetic studies of type 2 diabetes from the genome-wide association studies era to precision medicine. J. Diabetes Investig. 2024, 15, 410–422. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Lin, W. The Association between Body Mass Index and Glycohemoglobin (HbA1c) in the US Population’s Diabetes Status. Int. J. Environ. Res. Public. Health 2024, 21, 517. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Xu, W.; Ye, D.; Bai, X.; Wu, M.; Zhang, C.; Shi, R. Association Between Glycated Hemoglobin and the Lipid Profile at the Central Yunnan Plateau: A Retrospective Study. Diabetes Metab. Syndr. Obes. Targets Ther. 2024, 17, 2975–2981. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef] [PubMed]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef]
- Shahisavandi, M.; Wang, K.; Ghanbari, M.; Ahmadizar, F. Exploring Metabolomic Patterns in Type 2 Diabetes Mellitus and Response to Glucose-Lowering Medications—Review. Genes 2023, 14, 1464. [Google Scholar] [CrossRef]
- Floegel, A.; Stefan, N.; Yu, Z.; Mühlenbruch, K.; Drogan, D.; Joost, H.-G.; Fritsche, A.; Häring, H.-U.; Hrabě de Angelis, M.; Peters, A.; et al. Identification of Serum Metabolites Associated With Risk of Type 2 Diabetes Using a Targeted Metabolomic Approach. Diabetes 2013, 62, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Leiherer, A.; Muendlein, A.; Mink, S.; Mader, A.; Saely, C.H.; Festa, A.; Fraunberger, P.; Drexel, H. Machine Learning Approach to Metabolomic Data Predicts Type 2 Diabetes Mellitus Incidence. Int. J. Mol. Sci. 2024, 25, 5331. [Google Scholar] [CrossRef]
- Peddinti, G.; Cobb, J.; Yengo, L.; Froguel, P.; Kravić, J.; Balkau, B.; Tuomi, T.; Aittokallio, T.; Groop, L. Early metabolic markers identify potential targets for the prevention of type 2 diabetes. Diabetologia 2017, 60, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Emwas, A.-H.; Roy, R.; McKay, R.T.; Tenori, L.; Saccenti, E.; Gowda, G.A.N.; Raftery, D.; Alahmari, F.; Jaremko, L.; Jaremko, M.; et al. NMR Spectroscopy for Metabolomics Research. Metabolites 2019, 9, 123. [Google Scholar] [CrossRef]
- Hernandez-Baixauli, J.; Quesada-Vázquez, S.; Mariné-Casadó, R.; Gil Cardoso, K.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Baselga-Escudero, L. Detection of Early Disease Risk Factors Associated with Metabolic Syndrome: A New Era with the NMR Metabolomics Assessment. Nutrients 2020, 12, 806. [Google Scholar] [CrossRef]
- Jaworska, K.; Kuś, M.; Ufnal, M. TMAO and diabetes: From the gut feeling to the heart of the problem. Nutr. Diabetes 2025, 15, 21. [Google Scholar] [CrossRef]
- Lin, H.-T.; Cheng, M.-L.; Lo, C.-J.; Lin, G.; Lin, S.-F.; Yeh, J.-T.; Ho, H.-Y.; Lin, J.-R.; Liu, F.-C. 1H Nuclear Magnetic Resonance (NMR)-Based Cerebrospinal Fluid and Plasma Metabolomic Analysis in Type 2 Diabetic Patients and Risk Prediction for Diabetic Microangiopathy. J. Clin. Med. 2019, 8, 874. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Dehghani, A.; Faghfouri, A.H.; Radkhah, N.; Naemi Kermanshahi, M.; Hamedi Kalajahi, F.; Mohammadzadeh Honarvar, N.; Ghoreishi, Z.; Ostadrahimi, A.; Ebrahimi Mamaghani, M. Inositol supplementation and body mass index: A systematic review and meta-analysis of randomized clinical trials. Obes. Sci. Pract. 2022, 8, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Arefhosseini, S.; Roshanravan, N.; Tutunchi, H.; Rostami, S.; Khoshbaten, M.; Ebrahimi-Mameghani, M. Myo-inositol supplementation improves cardiometabolic factors, anthropometric measures, and liver function in obese patients with non-alcoholic fatty liver disease. Front. Nutr. 2023, 10, 1092544. [Google Scholar] [CrossRef]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef]
- Croze, M.L.; Géloën, A.; Soulage, C.O. Abnormalities in myo-inositol metabolism associated with type 2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef]
- Mashayekh-Amiri, S.; Mohammad-Alizadeh-Charandabi, S.; Abdolalipour, S.; Mirghafourvand, M. Myo-inositol supplementation for prevention of gestational diabetes mellitus in overweight and obese pregnant women: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2022, 14, 93. [Google Scholar] [CrossRef]
- Wu, C.; Yang, F.; Zhong, H.; Hong, J.; Lin, H.; Zong, M.; Ren, H.; Zhao, S.; Chen, Y.; Shi, Z.; et al. Obesity-enriched gut microbe degrades myo-inositol and promotes lipid absorption. Cell Host Microbe 2024, 32, 1301–1314.e9. [Google Scholar] [CrossRef]
- Dehghan, P.; Farhangi, M.A.; Nikniaz, L.; Nikniaz, Z.; Asghari-Jafarabadi, M. Gut microbiota-derived metabolite trimethylamine N-oxide (TMAO) potentially increases the risk of obesity in adults: An exploratory systematic review and dose-response meta- analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e12993. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Di Somma, C.; Laudisio, D.; Maisto, M.; de Alteriis, G.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine-N-oxide (TMAO) as Novel Potential Biomarker of Early Predictors of Metabolic Syndrome. Nutrients 2018, 10, 1971. [Google Scholar] [CrossRef]
- Mihuta, M.S.; Paul, C.; Borlea, A.; Roi, C.M.; Pescari, D.; Velea-Barta, O.-A.; Mozos, I.; Stoian, D. Connections between serum Trimethylamine N-Oxide (TMAO), a gut-derived metabolite, and vascular biomarkers evaluating arterial stiffness and subclinical atherosclerosis in children with obesity. Front. Endocrinol. 2023, 14, 1253584. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhu, Z.; Huang, Z.; Zhou, J. Elevated serum trimethylamine oxide levels as potential biomarker for diabetic kidney disease. Endocr. Connect. 2023, 12, e220542. [Google Scholar] [CrossRef]
- Ahmed, K.; Choi, H.-N.; Yim, J.-E. The Impact of Taurine on Obesity-Induced Diabetes Mellitus: Mechanisms Underlying Its Effect. Endocrinol. Metab. 2023, 38, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, J.; Wang, H.; Yu, H.; Wan, K.; Ma, F.; Wang, R. Effect of Long-Term Taurine Supplementation on the Lipid and Glycaemic Profile in Adults with Overweight or Obesity: A Systematic Review and Meta-Analysis. Nutrients 2025, 17, 55. [Google Scholar] [CrossRef]
- Guo, X.; Ou, T.; Yang, X.; Song, Q.; Zhu, L.; Mi, S.; Zhang, J.; Zhang, Y.; Chen, W.; Guo, J. Untargeted metabolomics based on ultra-high performance liquid chromatography-mass spectrometry/MS reveals the lipid-lowering mechanism of taurine in hyperlipidemia mice. Front. Nutr. 2024, 11, 1367589. [Google Scholar] [CrossRef]
- Tzang, C.-C.; Chi, L.-Y.; Lin, L.-H.; Lin, T.-Y.; Chang, K.-V.; Wu, W.-T.; Özçakar, L. Taurine reduces the risk for metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Nutr. Diabetes 2024, 14, 29. [Google Scholar] [CrossRef]
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261S–267S. [Google Scholar] [CrossRef]
- Tobias, D.K.; Hazra, A.; Lawler, P.R.; Chandler, P.D.; Chasman, D.I.; Buring, J.E.; Lee, I.-M.; Cheng, S.; Manson, J.E.; Mora, S. Circulating branched-chain amino acids and long-term risk of obesity-related cancers in women. Sci. Rep. 2020, 10, 16534. [Google Scholar] [CrossRef]
- Zhao, X.; Gang, X.; Liu, Y.; Sun, C.; Han, Q.; Wang, G. Using Metabolomic Profiles as Biomarkers for Insulin Resistance in Childhood Obesity: A Systematic Review. J. Diabetes Res. 2016, 2016, 8160545. [Google Scholar] [CrossRef]
- Xu, F.; Tavintharan, S.; Sum, C.F.; Woon, K.; Lim, S.C.; Ong, C.N. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. J. Clin. Endocrinol. Metab. 2013, 98, E1060–E1065. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mangelinckx, S.; Adams, A.; Wang, Z.-T.; Li, W.-L.; De Kimpe, N. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat. Prod. Commun. 2015, 10, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Halama, A.; Aye, M.M.; Dargham, S.R.; Kulinski, M.; Suhre, K.; Atkin, S.L. Metabolomics of Dynamic Changes in Insulin Resistance Before and After Exercise in PCOS. Front. Endocrinol. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Larson, M.G.; Vasan, R.S.; Cheng, S.; Rhee, E.P.; McCabe, E.; Lewis, G.D.; Fox, C.S.; Jacques, P.F.; Fernandez, C.; et al. Metabolite Profiles and the Risk of Developing Diabetes. Nat. Med. 2011, 17, 448–453. [Google Scholar] [CrossRef]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef]
- Lee, C.C.; Watkins, S.M.; Lorenzo, C.; Wagenknecht, L.E.; Il’yasova, D.; Chen, Y.-D.I.; Haffner, S.M.; Hanley, A.J. Branched-Chain Amino Acids and Insulin Metabolism: The Insulin Resistance Atherosclerosis Study (IRAS). Diabetes Care 2016, 39, 582–588. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Lin, M.; Imamura, F.; Cai, W.; Wang, L.; Feng, J.-P.; Ruan, Y.; Tang, J.; Wang, F.; Yang, H.; et al. Serum metabolomics profiles in response to n-3 fatty acids in Chinese patients with type 2 diabetes: A double-blind randomised controlled trial. Sci. Rep. 2016, 6, 29522. [Google Scholar] [CrossRef]
- Newbern, D.; Gumus Balikcioglu, P.; Balikcioglu, M.; Bain, J.; Muehlbauer, M.; Stevens, R.; Ilkayeva, O.; Dolinsky, D.; Armstrong, S.; Irizarry, K.; et al. Sex Differences in Biomarkers Associated With Insulin Resistance in Obese Adolescents: Metabolomic Profiling and Principal Components Analysis. J. Clin. Endocrinol. Metab. 2014, 99, 4730–4739. [Google Scholar] [CrossRef]
- Würtz, P.; Soininen, P.; Kangas, A.J.; Rönnemaa, T.; Lehtimäki, T.; Kähönen, M.; Viikari, J.S.; Raitakari, O.T.; Ala-Korpela, M. Branched-Chain and Aromatic Amino Acids Are Predictors of Insulin Resistance in Young Adults. Diabetes Care 2013, 36, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Pawan, G.L.; Semple, S.J. Effect of 3-hydroxybutyrate in obese subjects on very-low-energy diets and during therapeutic starvation. Lancet Lond. Engl. 1983, 1, 15–17. [Google Scholar] [CrossRef]
- Nishitani, S.; Fukuhara, A.; Tomita, I.; Kume, S.; Shin, J.; Okuno, Y.; Otsuki, M.; Maegawa, H.; Shimomura, I. Ketone body 3-hydroxybutyrate enhances adipocyte function. Sci. Rep. 2022, 12, 10080. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Liu, X.; Chen, X.; Zhang, S.; Chen, Y.; Chen, J.; Chen, J.; Wu, F.; Chen, G.-Q. 3-Hydroxybutyrate ameliorates insulin resistance by inhibiting PPARγ Ser273 phosphorylation in type 2 diabetic mice. Signal Transduct. Target. Ther. 2023, 8, 190. [Google Scholar] [CrossRef]
- Mahendran, Y.; Vangipurapu, J.; Cederberg, H.; Stancáková, A.; Pihlajamäki, J.; Soininen, P.; Kangas, A.J.; Paananen, J.; Civelek, M.; Saleem, N.K.; et al. Association of ketone body levels with hyperglycemia and type 2 diabetes in 9,398 Finnish men. Diabetes 2013, 62, 3618–3626. [Google Scholar] [CrossRef]
- Vemula, H.; Kitase, Y.; Ayon, N.J.; Bonewald, L.; Gutheil, W.G. Gaussian and linear deconvolution of LC-MS/MS chromatograms of the eight aminobutyric acid isomers. Anal. Biochem. 2017, 516, 75–85. [Google Scholar] [CrossRef]
- Yi, X.; Yang, Y.; Li, T.; Li, M.; Yao, T.; Hu, G.; Wan, G.; Chang, B. Signaling metabolite β-aminoisobutyric acid as a metabolic regulator, biomarker, and potential exercise pill. Front. Endocrinol. 2023, 14, 1192458. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.P.; Karstoft, K.; Vigelsø, A.; Gram, M.; Helge, J.W.; Dela, F.; Pappan, K.; O’Neil, D.; Dunn, W.; Solomon, T.P.J. Beta-aminoisobutyric acid is released by contracting human skeletal muscle and lowers insulin release from INS-1 832/3 cells by mediating mitochondrial energy metabolism. Metab. Open 2020, 7, 100053. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Otaki, S.; Kominami, Y.; Watanabe, S.; Ito, M.; Aizawa, T.; Akahori, Y.; Ushio, H. L-Fucose Suppresses Lipid Accumulation via the AMPK Pathway in 3T3-L1 Adipocytes. Nutrients 2023, 15, 503. [Google Scholar] [CrossRef]
- Zuo, J.; Zhang, Y.; Wu, Y.; Liu, J.; Wu, Q.; Shen, Y.; Jin, L.; Wu, M.; Ma, Z.; Tong, H. Sargassum fusiforme fucoidan ameliorates diet-induced obesity through enhancing thermogenesis of adipose tissues and modulating gut microbiota. Int. J. Biol. Macromol. 2022, 216, 728–740. [Google Scholar] [CrossRef]
- Wu, G.; Niu, M.; Tang, W.; Hu, J.; Wei, G.; He, Z.; Chen, Y.; Jiang, Y.; Chen, P. l-Fucose ameliorates high-fat diet-induced obesity and hepatic steatosis in mice. J. Transl. Med. 2018, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Shirai, A. Anti-obesity Agent. US Application No. US20060264498A1, 26 March 2004. [Google Scholar]
- Petrus, P.; Lecoutre, S.; Dollet, L.; Wiel, C.; Sulen, A.; Gao, H.; Tavira, B.; Laurencikiene, J.; Rooyackers, O.; Checa, A.; et al. Glutamine Links Obesity to Inflammation in Human White Adipose Tissue. Cell Metab. 2020, 31, 375–390.e11. [Google Scholar] [CrossRef]
- Abboud, K.Y.; Reis, S.K.; Martelli, M.E.; Zordão, O.P.; Tannihão, F.; de Souza, A.Z.Z.; Assalin, H.B.; Guadagnini, D.; Rocha, G.Z.; Saad, M.J.A.; et al. Oral Glutamine Supplementation Reduces Obesity, Pro-Inflammatory Markers, and Improves Insulin Sensitivity in DIO Wistar Rats and Reduces Waist Circumference in Overweight and Obese Humans. Nutrients 2019, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Reichard, G.A.; Skutches, C.L.; Hoeldtke, R.D.; Owen, O.E. Acetone metabolism in humans during diabetic ketoacidosis. Diabetes 1986, 35, 668–674. [Google Scholar] [CrossRef]
- Hernandez-Castillo, C.; Shuck, S.C. Diet and Obesity-Induced Methylglyoxal Production and Links to Metabolic Disease. Chem. Res. Toxicol. 2021, 34, 2424–2440. [Google Scholar] [CrossRef] [PubMed]
- Thalacker-Mercer, A.E.; Ingram, K.H.; Guo, F.; Ilkayeva, O.; Newgard, C.B.; Garvey, W.T. BMI, RQ, Diabetes, and Sex Affect the Relationships Between Amino Acids and Clamp Measures of Insulin Action in Humans. Diabetes 2014, 63, 791–800. [Google Scholar] [CrossRef]
- Cheng, S.; Rhee, E.P.; Larson, M.G.; Lewis, G.D.; McCabe, E.L.; Shen, D.; Palma, M.J.; Roberts, L.D.; Dejam, A.; Souza, A.L.; et al. Metabolite Profiling Identifies Pathways Associated with Metabolic Risk in Humans. Circulation 2012, 125, 2222–2231. [Google Scholar] [CrossRef]
- Takashina, C.; Tsujino, I.; Watanabe, T.; Sakaue, S.; Ikeda, D.; Yamada, A.; Sato, T.; Ohira, H.; Otsuka, Y.; Oyama-Manabe, N.; et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. 2016, 13, 5. [Google Scholar] [CrossRef]
- Tulipani, S.; Palau-Rodriguez, M.; Miñarro Alonso, A.; Cardona, F.; Marco-Ramell, A.; Zonja, B.; Lopez de Alda, M.; Muñoz-Garach, A.; Sanchez-Pla, A.; Tinahones, F.J.; et al. Biomarkers of Morbid Obesity and Prediabetes by Metabolomic Profiling of Human Discordant Phenotypes. Clin. Chim. Acta Int. J. Clin. Chem. 2016, 463, 53–61. [Google Scholar] [CrossRef]
- Wijayatunga, N.N.; Sams, V.G.; Dawson, J.A.; Mancini, M.L.; Mancini, G.J.; Moustaid-Moussa, N. Roux-en-Y gastric bypass surgery alters serum metabolites and fatty acids in patients with morbid obesity. Diabetes Metab. Res. Rev. 2018, 34, e3045. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Nakano, D.; Oriishi, T.; Torimura, T. Effects of isomaltulose on insulin resistance and metabolites in patients with non-alcoholic fatty liver disease: A metabolomic analysis. Mol. Med. Rep. 2018, 18, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Sikes, K.J.; McConnell, A.; Serkova, N.; Cole, B.; Frisbie, D. Untargeted metabolomics analysis identifies creatine, myo-inositol, and lipid pathway modulation in a murine model of tendinopathy. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2022, 40, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, R.; Zhao, W.; Zhao, Y.; Wang, D.; Zhao, S.; Ge, Z.; Ma, Y.; Zhao, X. Gut microbiota-derived 4-hydroxyphenylacetic acid from resveratrol supplementation prevents obesity through SIRT1 signaling activation. Gut Microbes 2025, 17, 2446391. [Google Scholar] [CrossRef] [PubMed]
- Osborn, L.J.; Schultz, K.; Massey, W.; DeLucia, B.; Choucair, I.; Varadharajan, V.; Banerjee, R.; Fung, K.; Horak, A.J.; Orabi, D.; et al. A gut microbial metabolite of dietary polyphenols reverses obesity-driven hepatic steatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2202934119. [Google Scholar] [CrossRef]
- Chen, W.; Liu, R.; Zhu, X.; Lu, Q.; Yang, H.; Liu, R. Microbial phenolic metabolites 3-(3′,4′-dihydroxyphenyl)propanoic acid and 3′,4′-dihydroxyphenylacetic acid prevent obesity in mice fed with high-fat diet. Food Sci. Hum. Wellness 2024, 13, 327–338. [Google Scholar] [CrossRef]
- Li, M.; Reynolds, C.M.; Sloboda, D.M.; Gray, C.; Vickers, M.H. Effects of Taurine Supplementation on Hepatic Markers of Inflammation and Lipid Metabolism in Mothers and Offspring in the Setting of Maternal Obesity. PLoS ONE 2013, 8, e76961. [Google Scholar] [CrossRef][Green Version]
- Zhang, Q.; Cheng, J.; Jiang, X.; Tang, J.; Zhu, C.; Chen, H.; Laghi, L. Metabolomic Characteristics of Cecum Contents in High-Fat-Diet-Induced Obese Mice Intervened with Different Fibers. Foods 2023, 12, 1403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, S.J.; Park, Y.S.; Kim, Y.-J.; Han, S.-U.; Hwang, G.-S.; Han, Y.; Heo, Y.; Ha, E.; Ha, T.K. Changes in Trimethylamine-N-oxide Levels in Obese Patients following Laparoscopic Roux-en-Y Gastric Bypass or Sleeve Gastrectomy in a Korean Obesity Surgical Treatment Study (KOBESS). J. Clin. Med. 2021, 10, 5091. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Liu, J.; Yang, G.; Zhao, J.; Liao, G.; Shi, M.; Yuan, Y.; He, S.; Lu, Y.; et al. Serum metabolic variables associated with impaired glucose tolerance induced by high-fat-high-cholesterol diet in Macaca mulatta. Exp. Biol. Med. 2012, 237, 1310–1321. [Google Scholar] [CrossRef]
- Ho, J.E.; Larson, M.G.; Ghorbani, A.; Cheng, S.; Chen, M.-H.; Keyes, M.; Rhee, E.P.; Clish, C.B.; Vasan, R.S.; Gerszten, R.E.; et al. Metabolomic Profiles of Body Mass Index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS ONE 2016, 11, e0148361. [Google Scholar] [CrossRef]
- Tai, E.S.; Tan, M.L.S.; Stevens, R.D.; Low, Y.L.; Muehlbauer, M.J.; Goh, D.L.M.; Ilkayeva, O.R.; Wenner, B.R.; Bain, J.R.; Lee, J.J.M.; et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010, 53, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Ozcariz, E.; Guardiola, M.; Amigó, N.; Valdés, S.; Oualla-Bachiri, W.; Rehues, P.; Rojo-Martinez, G.; Ribalta, J. H-NMR metabolomics identifies three distinct metabolic profiles differentially associated with cardiometabolic risk in patients with obesity in the Di@bet.es cohort. Cardiovasc. Diabetol. 2024, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Richardson, N.E.; Green, C.L.; Spicer, A.B.; Murphy, M.E.; Flores, V.; Jang, C.; Kasza, I.; Nikodemova, M.; Wakai, M.H.; et al. The adverse metabolic effects of branched-chain amino acids are mediated by isoleucine and valine. Cell Metab. 2021, 33, 905–922.e6. [Google Scholar] [CrossRef] [PubMed]
- Trautman, M.E.; Green, C.L.; MacArthur, M.R.; Chaiyakul, K.; Alam, Y.H.; Yeh, C.Y.; Babygirija, R.; James, I.; Gilpin, M.; Zelenovskiy, E.; et al. Dietary isoleucine content defines the metabolic and molecular response to a Western diet. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jung, J.; Park, W.Y.; Kim, Y.J.; Kim, M.; Choe, M.; Jin, K.; Seo, J.H.; Ha, E. 3-Hydroxybutyrate Ameliorates the Progression of Diabetic Nephropathy. Antioxidants 2022, 11, 381. [Google Scholar] [CrossRef]
- Yousri, N.A.; Suhre, K.; Yassin, E.; Al-Shakaki, A.; Robay, A.; Elshafei, M.; Chidiac, O.; Hunt, S.C.; Crystal, R.G.; Fakhro, K.A. Metabolic and Metabo-Clinical Signatures of Type 2 Diabetes, Obesity, Retinopathy, and Dyslipidemia. Diabetes 2022, 71, 184–205. [Google Scholar] [CrossRef]
- Kistner, S.; Rist, M.J.; Döring, M.; Dörr, C.; Neumann, R.; Härtel, S.; Bub, A. An NMR-Based Approach to Identify Urinary Metabolites Associated with Acute Physical Exercise and Cardiorespiratory Fitness in Healthy Humans—Results of the KarMeN Study. Metabolites 2020, 10, 212. [Google Scholar] [CrossRef]
- Kenéz, Á.; Warnken, T.; Feige, K.; Huber, K. Lower plasma trans-4-hydroxyproline and methionine sulfoxide levels are associated with insulin dysregulation in horses. BMC Vet. Res. 2018, 14, 146. [Google Scholar] [CrossRef]
- Stancáková, A.; Civelek, M.; Saleem, N.K.; Soininen, P.; Kangas, A.J.; Cederberg, H.; Paananen, J.; Pihlajamäki, J.; Bonnycastle, L.L.; Morken, M.A.; et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9369 Finnish men. Diabetes 2012, 61, 1895–1902. [Google Scholar] [CrossRef]
- Palmer, N.D.; Stevens, R.D.; Antinozzi, P.A.; Anderson, A.; Bergman, R.N.; Wagenknecht, L.E.; Newgard, C.B.; Bowden, D.W. Metabolomic Profile Associated With Insulin Resistance and Conversion to Diabetes in the Insulin Resistance Atherosclerosis Study. J. Clin. Endocrinol. Metab. 2015, 100, E463–E468. [Google Scholar] [CrossRef]
- Schumacher, D.; Morgenstern, J.; Oguchi, Y.; Volk, N.; Kopf, S.; Groener, J.B.; Nawroth, P.P.; Fleming, T.; Freichel, M. Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol. Metab. 2018, 18, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health Malaysia. Clinical Practice Guideline—Management of Type 2 Diabetes Mellitus, 6th ed.; Ministry of Health Malaysia: Putrajaya, Malaysia, 2020. [Google Scholar]
- Saparuddin, F.; Mohd Nawi, M.N.; Ahmad Zamri, L.; Mansor, F.; Md Noh, M.F.; Omar, M.A.; Abdul Aziz, N.S.; Wahab, N.A.; Mediani, A.; Rajab, N.F.; et al. Metabolite, Biochemical, and Dietary Intake Alterations Associated with Lifestyle Interventions in Obese and Overweight Malaysian Women. Nutrients 2024, 16, 3501. [Google Scholar] [CrossRef] [PubMed]
- Billoir, E.; Navratil, V.; Blaise, B.J. Sample size calculation in metabolic phenotyping studies. Brief. Bioinform. 2015, 16, 813–819. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; Gibney, M.J.; Brennan, L. Dietary intake patterns are reflected in metabolomic profiles: Potential role in dietary assessment studies. Am. J. Clin. Nutr. 2011, 93, 314–321. [Google Scholar] [CrossRef]
- Tan, J.K.; Zakaria, S.N.A.; Gunasekaran, G.; Abdul Sani, N.F.; Nasaruddin, M.L.; Jaafar, F.; Abu Bakar, Z.H.; Amir Hamzah, A.I.Z.; Nor Aripin, K.N.; Mohd Rani, M.D.; et al. Metabolomics Profiling of Age-Associated Metabolites in Malay Population. Oxid. Med. Cell. Longev. 2023, 2023, 4416410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).