Cardio-Pulmonary Features of Long COVID: From Molecular and Histopathological Characteristics to Clinical Implications
Abstract
1. Introduction
- -
- Molecular mechanisms: endothelial dysfunction, immune activation, and coagulation abnormalities;
- -
- Histopathological findings in the heart and lung tissues;
- -
- Clinical manifestations including myocarditis and pulmonary fibrosis;
- -
- Implications for diagnosis, follow-up, and potential therapeutic strategies, including anticoagulants, vitamin D supplementation, and immunomodulatory agents.
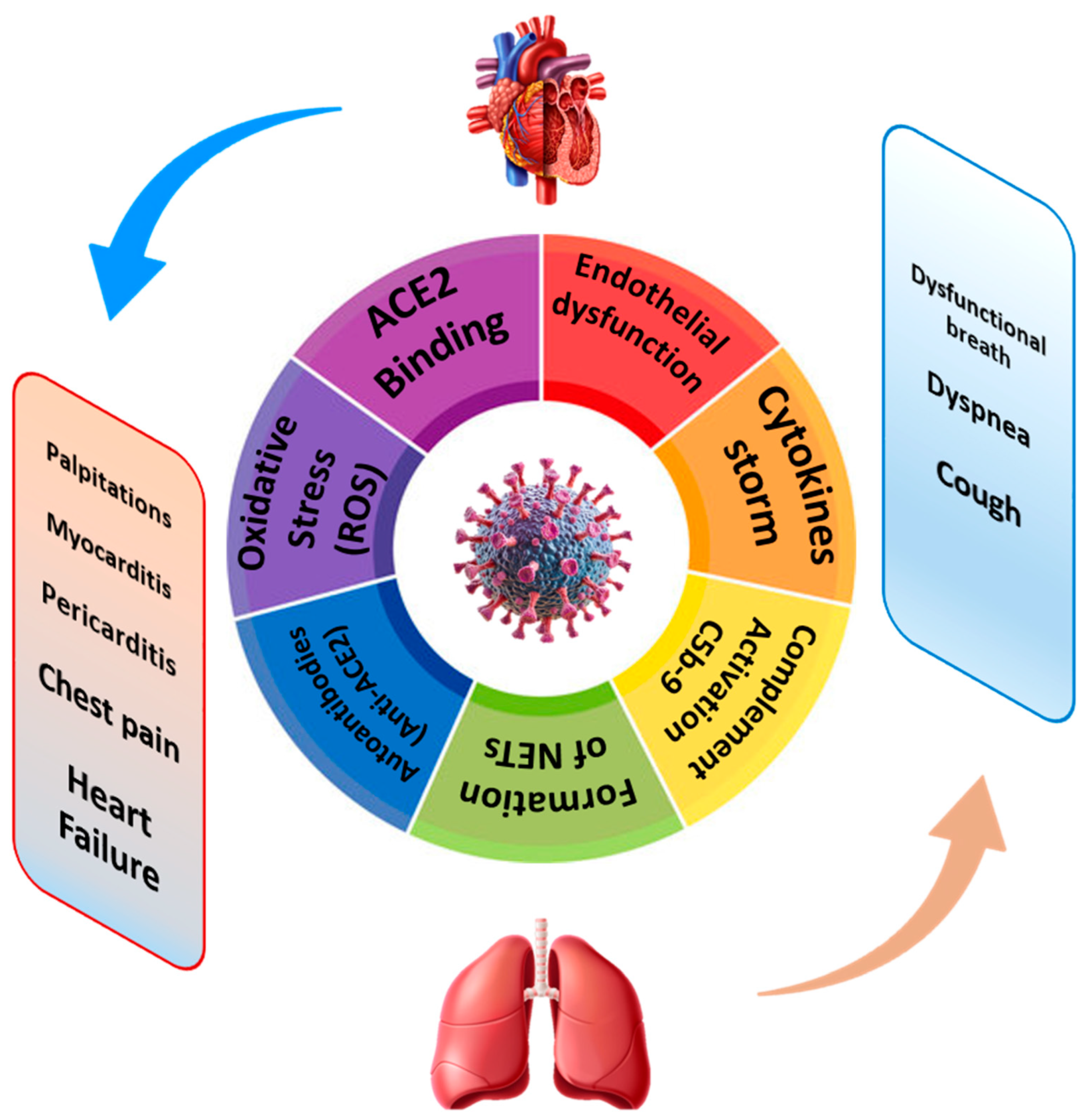
2. Molecular Pathways in Cardio-Pulmonary Long COVID: Two Sides of the Same Coin
2.1. COVID-19-Related Endothelial Dysfunction: A Common Pathway in Cardiac and Pulmonary Involvement
2.2. Immuno-Inflammatory Activation
Viral Endotheliitis and Interaction with the Immune System (NETs, IL-6)
2.3. Coagulation Disorders: Is COVID-19 a Thrombotic Disease?
2.3.1. Molecular Mechanisms in the Persistent Prothrombotic State
- -
- PAI-1, which blocks fibrinolysis;
- -
- Tissue factor (TF), reinforcing coagulation;
- -
2.3.2. Persistence of D-Dimer and Hemostatic Markers Clinical Implications: Late Thromboembolic Events & Subclinical Ischemia
2.3.3. Clinical Implications: Late Thromboembolic Events & Subclinical Ischemia
3. Histopathological Evidence: Myocardial and Pulmonary Findings
4. Myocarditis in Long COVID: Pathophysiology and Clinical Manifestation
5. Pulmonary Involvement in Long COVID
6. Conclusions
Limitations and Strengths
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 8 July 2025).
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 2021, 38, 101019. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273, Erratum in JAMA Cardiol. 2020, 5, 1308. [Google Scholar] [CrossRef]
- Rajpal, S.; Tong, M.S.; Borchers, J.; Zareba, K.M.; Obarski, T.P.; Simonetti, O.P.; Daniels, C.J. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021, 6, 116–118. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Brauninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.P.; et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef] [PubMed]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef] [PubMed]
- Buja, L.M.; Wolf, D.A.; Zhao, B.; Akkanti, B.; McDonald, M.; Lelenwa, L.; Reilly, N.; Ottaviani, G.; Elghetany, M.T.; Trujillo, D.O.; et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020, 48, 107233. [Google Scholar] [CrossRef]
- Bharat, A.; Querrey, M.; Markov, N.S.; Kim, S.; Kurihara, C.; Garza-Castillon, R.; Manerikar, A.; Shilatifard, A.; Tomic, R.; Politanska, Y.; et al. Lung transplantation for patients with severe COVID-19. Sci. Transl. Med. 2020, 12, eabe4282. [Google Scholar] [CrossRef]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Yalavarthi, S.; Shi, H.; Gockman, K.; Zuo, M.; Madison, J.A.; Blair, C.; Weber, A.; Barnes, B.J.; Egeblad, M.; et al. Neutrophil extracellular traps in COVID-19. JCI Insight 2020, 5, e138999. [Google Scholar] [CrossRef]
- Manne, B.K.; Denorme, F.; Middleton, E.A.; Portier, I.; Rowley, J.W.; Stubben, C.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.; et al. Platelet gene expression and function in patients with COVID-19. Blood 2020, 136, 1317–1329. [Google Scholar] [CrossRef]
- Maruhashi, T.; Higashi, Y. Pathophysiological Association of Endothelial Dysfunction with Fatal Outcome in COVID-19. Int. J. Mol. Sci. 2021, 22, 5131. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Thomas, D.; Noishiki, C.; Gaddam, S.; Wu, D.; Manhas, A.; Liu, Y.; Tripathi, D.; Kathale, N.; Adkar, S.S.; Garhyan, J.; et al. CCL2-mediated endothelial injury drives cardiac dysfunction in long COVID. Nat. Cardiovasc. Res. 2024, 3, 1249–1265. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Furst, J.; Schulze-Rothe, S.; Wallukat, A.; Honicke, A.S.; Muller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Myall, K.J.; Mukherjee, B.; Castanheira, A.M.; Lam, J.L.; Benedetti, G.; Mak, S.M.; Preston, R.; Thillai, M.; Dewar, A.; Molyneaux, P.L.; et al. Persistent Post-COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Ann. Am. Thorac. Soc. 2021, 18, 799–806. [Google Scholar] [CrossRef]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial dysfunction in long COVID: Mechanisms, consequences, and potential therapeutic approaches. Geroscience 2024, 46, 5267–5286. [Google Scholar] [CrossRef]
- Cheon, I.S.; Li, C.; Son, Y.M.; Goplen, N.P.; Wu, Y.; Cassmann, T.; Wang, Z.; Wei, X.; Tang, J.; Li, Y.; et al. Immune signatures underlying post-acute COVID-19 lung sequelae. Sci. Immunol. 2021, 6, eabk1741. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Luscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef]
- Goshua, G.; Pine, A.B.; Meizlish, M.L.; Chang, C.H.; Zhang, H.; Bahel, P.; Baluha, A.; Bar, N.; Bona, R.D.; Burns, A.J.; et al. Endotheliopathy in COVID-19-associated coagulopathy: Evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020, 7, e575–e582. [Google Scholar] [CrossRef] [PubMed]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Fogarty, H.; Townsend, L.; Ni Cheallaigh, C.; Bergin, C.; Martin-Loeches, I.; Browne, P.; Bacon, C.L.; Gaule, R.; Gillett, A.; Byrne, M.; et al. COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 2020, 189, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; O’Donnell, J.S.; Sharif, K.; Emery, P.; Bridgewood, C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2020, 2, e437–e445. [Google Scholar] [CrossRef]
- Miller, M.R.; Raftis, J.B.; Langrish, J.P.; McLean, S.G.; Samutrtai, P.; Connell, S.P.; Wilson, S.; Vesey, A.T.; Fokkens, P.H.B.; Boere, A.J.F.; et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease. ACS Nano 2017, 11, 4542–4552, Erratum in ACS Nano 2017, 11, 10623–10624. [Google Scholar] [CrossRef]
- Rovas, A.; Osiaevi, I.; Buscher, K.; Sackarnd, J.; Tepasse, P.R.; Fobker, M.; Kuhn, J.; Braune, S.; Gobel, U.; Tholking, G.; et al. Microvascular dysfunction in COVID-19: The MYSTIC study. Angiogenesis 2021, 24, 145–157. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Shouman, K.; Vanichkachorn, G.; Cheshire, W.P.; Suarez, M.D.; Shelly, S.; Lamotte, G.J.; Sandroni, P.; Benarroch, E.E.; Berini, S.E.; Cutsforth-Gregory, J.K.; et al. Autonomic dysfunction following COVID-19 infection: An early experience. Clin. Auton. Res. 2021, 31, 385–394. [Google Scholar] [CrossRef]
- Kruger, A.; Vlok, M.; Turner, S.; Venter, C.; Laubscher, G.J.; Kell, D.B.; Pretorius, E. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc. Diabetol. 2022, 21, 190. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Fogarty, H.; Dyer, A.; Martin-Loeches, I.; Bannan, C.; Nadarajan, P.; Bergin, C.; O’Farrelly, C.; Conlon, N.; Bourke, N.M.; et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J. Thromb. Haemost. 2021, 19, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Knight, R.; Walker, V.; Ip, S.; Cooper, J.A.; Bolton, T.; Keene, S.; Denholm, R.; Akbari, A.; Abbasizanjani, H.; Torabi, F.; et al. Association of COVID-19 with Major Arterial and Venous Thrombotic Diseases: A Population-Wide Cohort Study of 48 Million Adults in England and Wales. Circulation 2022, 146, 892–906. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Luscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef]
- Tziolos, N.R.; Ioannou, P.; Baliou, S.; Kofteridis, D.P. Long COVID-19 Pathophysiology: What Do We Know So Far? Microorganisms 2023, 11, 2458. [Google Scholar] [CrossRef]
- Paidas, M.J.; Cosio, D.S.; Ali, S.; Kenyon, N.S.; Jayakumar, A.R. Long-Term Sequelae of COVID-19 in Experimental Mice. Mol. Neurobiol. 2022, 59, 5970–5986. [Google Scholar] [CrossRef]
- Blagova, O.; Lutokhina, Y.; Kogan, E.; Kukleva, A.; Ainetdinova, D.; Novosadov, V.; Rud, R.; Savina, P.; Zaitsev, A.; Fomin, V. Chronic biopsy proven post-COVID myoendocarditis with SARS-CoV-2 persistence and high level of antiheart antibodies. Clin. Cardiol. 2022, 45, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, Z. An update: The emerging evidence of complement involvement in COVID-19. Med. Microbiol. Immunol. 2021, 210, 101–109. [Google Scholar] [CrossRef]
- Murphy, M.C.; Little, B.P. Chronic Pulmonary Manifestations of COVID-19 Infection: Imaging Evaluation. Radiology 2023, 307, e222379. [Google Scholar] [CrossRef]
- Kianzad, A.; Meijboom, L.J.; Nossent, E.J.; Roos, E.; Schurink, B.; Bonta, P.I.; van den Berk, I.A.H.; Britstra, R.; Stoker, J.; Vonk Noordegraaf, A.; et al. COVID-19: Histopathological correlates of imaging patterns on chest computed tomography. Respirology 2021, 26, 869–877. [Google Scholar] [CrossRef]
- Pannone, G.; Caponio, V.C.A.; De Stefano, I.S.; Ramunno, M.A.; Meccariello, M.; Agostinone, A.; Pedicillo, M.C.; Troiano, G.; Zhurakivska, K.; Cassano, T.; et al. Lung histopathological findings in COVID-19 disease—A systematic review. Infect. Agent. Cancer 2021, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Jeican, I.I.; Inisca, P.; Gheban, D.; Anton, V.; Lazar, M.; Vica, M.L.; Mironescu, D.; Rebeleanu, C.; Crivii, C.B.; Aluas, M.; et al. Histopathological Lung Findings in COVID-19 B.1.617.2 SARS-CoV-2 Delta Variant. J. Pers. Med. 2023, 13, 279. [Google Scholar] [CrossRef] [PubMed]
- Culebras, M.; Loor, K.; Sansano, I.; Persiva, O.; Clofent, D.; Polverino, E.; Felipe, A.; Osorio, J.; Munoz, X.; Alvarez, A.; et al. Histological Findings in Transbronchial Cryobiopsies Obtained From Patients After COVID-19. Chest 2022, 161, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Salai, G.; Tekavec-Trkanjec, J.; Kovacevic, I.; Tomasovic-Loncaric, C.; Pacic, A.; Vergles, M.; Ljubicic, D.; Cvetkovic-Kucic, D.; Luksic, I.; Barsic, B. Lung long distance: Histopathological changes in lung tissue after COVID-19 pneumonia. Croat. Med. J. 2024, 65, 501–509. [Google Scholar] [CrossRef]
- Deng, Q.; Hu, B.; Zhang, Y.; Wang, H.; Zhou, X.; Hu, W.; Cheng, Y.; Yan, J.; Ping, H.; Zhou, Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020, 311, 116–121. [Google Scholar] [CrossRef]
- Carsana, L.; Sonzogni, A.; Nasr, A.; Rossi, R.S.; Pellegrinelli, A.; Zerbi, P.; Rech, R.; Colombo, R.; Antinori, S.; Corbellino, M.; et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: A two-centre descriptive study. Lancet Infect. Dis. 2020, 20, 1135–1140. [Google Scholar] [CrossRef]
- Fedorov, D.N.; Korosteleva, P.A.; Zybin, D.I.; Popov, M.A.; Tyurina, V.M.; Varlamov, A.V. Morphological and immunohistochemical characteristics of changes in the bronchopulmonary lymph nodes in patients with a new COVID-19 coronavirus infection (based on autopsy results). Alm. Clin. Med. 2020, 48, 37–42. [Google Scholar] [CrossRef]
- Gerayeli, F.V.; Eddy, R.L.; Sin, D.D. A proposed approach to pulmonary long COVID: A viewpoint. Eur. Respir. J. 2024, 64, 2302302. [Google Scholar] [CrossRef]
- Son, K.; Jamil, R.; Chowdhury, A.; Mukherjee, M.; Venegas, C.; Miyasaki, K.; Zhang, K.; Patel, Z.; Salter, B.; Yuen, A.C.Y.; et al. Circulating anti-nuclear autoantibodies in COVID-19 survivors predict long COVID symptoms. Eur. Respir. J. 2023, 61, 2200970. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Alcaide, P.; Asselbergs, F.W.; Brundel, B.; Camici, G.G.; Martins, P.D.C.; Ferdinandy, P.; Fontana, M.; Girao, H.; Gnecchi, M.; et al. Long COVID and the cardiovascular system-elucidating causes and cellular mechanisms in order to develop targeted diagnostic and therapeutic strategies: A joint Scientific Statement of the ESC Working Groups on Cellular Biology of the Heart and Myocardial and Pericardial Diseases. Cardiovasc. Res. 2023, 119, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Rohun, J.; Dorniak, K.; Faran, A.; Kochanska, A.; Zacharek, D.; Danilowicz-Szymanowicz, L. Long COVID-19 Myocarditis and Various Heart Failure Presentations: A Case Series. J. Cardiovasc. Dev. Dis. 2022, 9, 427. [Google Scholar] [CrossRef]
- Morris, S.B.; Schwartz, N.G.; Patel, P.; Abbo, L.; Beauchamps, L.; Balan, S.; Lee, E.H.; Paneth-Pollak, R.; Geevarughese, A.; Lash, M.K.; et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1450–1456. [Google Scholar] [CrossRef]
- Lawrensia, S.; Henrina, J.; Cahyadi, A. Multisystem inflammatory syndrome in adults: A systematic review and meta-analysis of the rheumatological spectrum of complications post COVID-19 infection. Rev. Colomb. Reumatol. 2022, 29, S17–S24. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Wu, K.; Van Name, J.; Xi, L. Cardiovascular abnormalities of long-COVID syndrome: Pathogenic basis and potential strategy for treatment and rehabilitation. Sports Med. Health Sci. 2024, 6, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Bai, F.; Pu, J.; Che, W.; Chen, J.; Chen, M.; Chen, W.; Chen, X.; Chen, Y.; Cheng, X.; Cheng, X.; et al. 2023 Chinese expert consensus on the impact of COVID-19 on the management of cardiovascular diseases. Cardiol. Plus 2023, 8, 82–102. [Google Scholar] [CrossRef]
- Roca-Fernandez, A.; Wamil, M.; Telford, A.; Carapella, V.; Borlotti, A.; Monteiro, D.; Thomaides-Brears, H.; Kelly, M.; Dennis, A.; Banerjee, R.; et al. Cardiac abnormalities in Long COVID 1-year post-SARS-CoV-2 infection. Open Heart 2023, 10, e002241. [Google Scholar] [CrossRef]
- Huang, L.; Li, X.; Gu, X.; Zhang, H.; Ren, L.; Guo, L.; Liu, M.; Wang, Y.; Cui, D.; Wang, Y.; et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: A longitudinal cohort study. Lancet Respir. Med. 2022, 10, 863–876. [Google Scholar] [CrossRef]
- Steinbeis, F.; Thibeault, C.; Doellinger, F.; Ring, R.M.; Mittermaier, M.; Ruwwe-Glosenkamp, C.; Alius, F.; Knape, P.; Meyer, H.J.; Lippert, L.J.; et al. Severity of respiratory failure and computed chest tomography in acute COVID-19 correlates with pulmonary function and respiratory symptoms after infection with SARS-CoV-2: An observational longitudinal study over 12 months. Respir. Med. 2022, 191, 106709. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Antoniou, K.M.; Vasarmidi, E.; Russell, A.M.; Andrejak, C.; Crestani, B.; Delcroix, M.; Dinh-Xuan, A.T.; Poletti, V.; Sverzellati, N.; Vitacca, M.; et al. European Respiratory Society statement on long COVID follow-up. Eur. Respir. J. 2022, 60, 2102174. [Google Scholar] [CrossRef]
- Yelin, D.; Ghantous, N.; Awwad, M.; Daitch, V.; Kalfon, T.; Mor, M.; Buchrits, S.; Shafir, Y.; Shapira-Lichter, I.; Leibovici, L.; et al. Pulmonary diffusing capacity among individuals recovering from mild to moderate COVID-19: A cross-sectional study. Sci. Rep. 2024, 14, 26767, Erratum in Sci. Rep. 2024, 14, 30533. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.B.; Carbone, T.; Holtzclaw, A.W.; Huprikar, N.A.; Wagner, R.; Morris, M.J. Obesity-related Changes in Diffusing Capacity and Transfer Coefficient of the Lung for Carbon Monoxide and Resulting Patterns of Abnormality across Reference Equations. Ann. Am. Thorac. Soc. 2023, 20, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Flor, N.; Leidi, F.; Casella, F.; Mariani, L.; Piazza, M.; Del Medico, M.; Cogliati, C.B. Two-years chest-CT follow-up after severe COVID-19 pneumonia. Intern. Emerg. Med. 2023, 18, 1243–1245. [Google Scholar] [CrossRef]
- Lloyd-Jones, G.; Alcock, R.; Oudkerk, M. COVID-19 lung disease is a pulmonary vasculopathy. Clin. Radiol. 2024, 79, e975–e978. [Google Scholar] [CrossRef]
- Elyazed, T.I.A.; Alsharawy, L.A.; Salem, S.E.; Helmy, N.A.; El-Hakim, A. Effect of home-based pulmonary rehabilitation on exercise capacity in post COVID-19 patients: A randomized controlled trail. J. Neuroeng. Rehabil. 2024, 21, 40. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Oliva, E.; Martinez-Pozas, O.; Cuenca-Zaldivar, J.N.; Villafane, J.H.; Jimenez-Ortega, L.; Sanchez-Romero, E.A. Efficacy of Pulmonary Rehabilitation in Post-COVID-19: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dai, B.; Hou, Y.; Zhang, L.; Liu, J.; Hou, H.; Song, D.; Wang, S.; Li, X.; Zhao, H.; et al. Effect of pulmonary rehabilitation for patients with long COVID-19: A systematic review and meta-analysis of randomized controlled trials. Ther. Adv. Respir. Dis. 2025, 19, 17534666251323482. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]

| Molecular aspects | |
| Endothelial dysfunction | Reduced nitric oxide (NO), oxidative stress via NOX2, upregulation of adhesion molecules (VCAM-1, ICAM-1), elevated von Willebrand factor (vWF), angiopoietin-2 (Ang-2), soluble thrombomodulin |
| Chronic inflammation | Persistent elevation of IL-6, IL-1β, TNF-α, CRP; activation of inflammatory pathways (NF-κB, JAK/STAT, MAPK) |
| Immune system overactivation | Ongoing activation of CD14+ monocytes, persistent dysregulated CD8+ T-cells, dysfunctional NK cells |
| Autoimmunity | Presence of autoantibodies against GPCRs, ACE2, endothelial antigens, adrenergic and muscarinic receptors |
| Molecular mimicry | Cross-reactivity between viral antigens and host cardiac proteins leading to immune attack |
| Epigenetic & metabolic dysfunction | Mitochondrial impairment, altered microRNA expression, sustained activation of interferon-stimulated genes |
| Fibrotic remodeling | Activation of TGF-β pathway promoting fibrosis in lungs and heart |
| Prothrombotic state | Elevated PAI-1, Factor VIII, tissue factor; platelet hyperactivation and persistent D-dimer levels; resistant microthrombi |
| Pathophysiological aspects | |
| Cardiac damage | Myocarditis, subclinical inflammation, myocardial fibrosis, reduced ventricular compliance, arrhythmias |
| Pulmonary involvement | Interstitial lung disease, alveolar damage, persistent inflammation, fibrosis, microangiopathy |
| Vascular injury | Endothelial inflammation and dysfunction, microvascular thrombosis in multiple organs |
| Dysautonomia | Autonomic nervous system imbalance: inappropriate sinus tachycardia, low blood pressure, impaired vascular tone |
| Viral persistence | Detection of SARS-CoV-2 RNA or proteins in myocardial or lung tissue months after infection |
| Clinical aspects | |
| Cardio-pulmonary symptoms | Fatigue, dyspnea on exertion, chest pain, desaturation, palpitations, exercise intolerance |
| Systemic symptoms | Cognitive dysfunction (“brain fog”), anxiety, depression, sleep disorders, arthralgia |
| Vascular complications | Risk of late thromboembolic events (e.g., pulmonary embolism, stroke), subclinical ischemia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cimmino, G.; D’Elia, S.; Morello, M.; Titolo, G.; Luisi, E.; Solimene, A.; Serpico, C.; Conte, S.; Natale, F.; Loffredo, F.S.; et al. Cardio-Pulmonary Features of Long COVID: From Molecular and Histopathological Characteristics to Clinical Implications. Int. J. Mol. Sci. 2025, 26, 7668. https://doi.org/10.3390/ijms26167668
Cimmino G, D’Elia S, Morello M, Titolo G, Luisi E, Solimene A, Serpico C, Conte S, Natale F, Loffredo FS, et al. Cardio-Pulmonary Features of Long COVID: From Molecular and Histopathological Characteristics to Clinical Implications. International Journal of Molecular Sciences. 2025; 26(16):7668. https://doi.org/10.3390/ijms26167668
Chicago/Turabian StyleCimmino, Giovanni, Saverio D’Elia, Mariarosaria Morello, Gisella Titolo, Ettore Luisi, Achille Solimene, Chiara Serpico, Stefano Conte, Francesco Natale, Francesco S. Loffredo, and et al. 2025. "Cardio-Pulmonary Features of Long COVID: From Molecular and Histopathological Characteristics to Clinical Implications" International Journal of Molecular Sciences 26, no. 16: 7668. https://doi.org/10.3390/ijms26167668
APA StyleCimmino, G., D’Elia, S., Morello, M., Titolo, G., Luisi, E., Solimene, A., Serpico, C., Conte, S., Natale, F., Loffredo, F. S., Bianco, A., & Golino, P. (2025). Cardio-Pulmonary Features of Long COVID: From Molecular and Histopathological Characteristics to Clinical Implications. International Journal of Molecular Sciences, 26(16), 7668. https://doi.org/10.3390/ijms26167668







