Role of Dyadic Proteins in Proper Heart Function and Disease
Abstract
1. Introduction
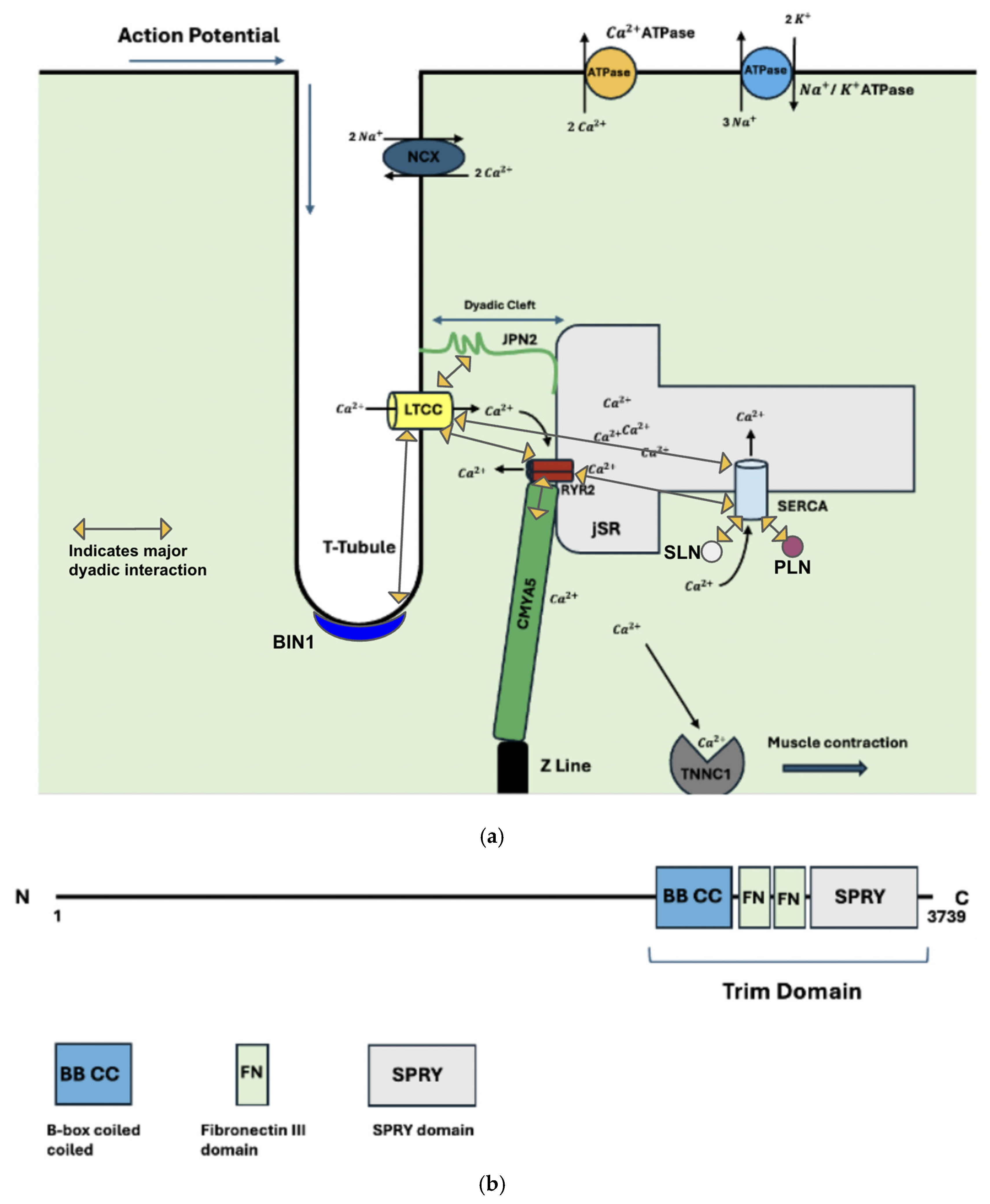
2. The Dyad
2.1. Structural Proteins of the Dyad
2.2. Functional Dyadic Proteins
2.3. Dyadic Protein Interactions
3. Mutations
Dyadic Mutations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gómez, A.M.; Valdivia, H.H.; Cheng, H.; Lederer, M.R.; Santana, L.F.; Cannell, M.B.; McCune, S.A.; Altschuld, R.A.; Lederer, W.J. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science 1997, 276, 800–806. [Google Scholar] [CrossRef]
- Basit, H.; Brito, D.; Sharma, S. Hypertrophic Cardiomyopathy; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430788/ (accessed on 17 November 2024).
- Weintraub, R.G.; Semsarian, C.; Macdonald, P. Dilated cardiomyopathy. Lancet 2017, 390, 400–414. [Google Scholar] [CrossRef]
- Lu, F.; Ma, Q.; Xie, W.; Liou, C.L.; Zhang, D.; Sweat, M.E.; Jardin, B.D.; Naya, F.J.; Guo, Y.; Cheng, H.; et al. CMYA5 Establishes Cardiac Dyad Architecture and Positioning. Nat. Commun. 2022, 13, 2185. [Google Scholar] [CrossRef]
- Poulet, C.; Sanchez-Alonso, J.; Swiatlowska, P.; Mouy, F.; Lucarelli, C.; Alvarez-Laviada, A.; Gross, P.; Terracciano, C.; Houser, S.; Gorelik, J. Junctophilin-2 tethers T-tubules and recruits functional L-type calcium channels to lipid rafts in adult cardiomyocytes. Cardiovasc. Res. 2021, 117, 149–161. [Google Scholar] [CrossRef]
- Takeshima, H.; Komazaki, S.; Nishi, M.; Iino, M.; Kangawa, K. Junctophilins: A Novel Family of Junctional Membrane Complex Proteins. Mol. Cell 2000, 6, 11–22. [Google Scholar] [CrossRef]
- Garbino, A.; Wehrens, X.H.T. Emerging role of junctophilin-2 as a regulator of calcium handling in the heart. Acta Pharmacol. Sin. 2010, 31, 1019–1021. [Google Scholar] [CrossRef]
- van Oort, R.J.; Garbino, A.; Wang, W.; Dixit, S.S.; Landstrom, A.P.; Gaur, N.; De Almeida, A.C.; Skapura, D.G.; Rudy, Y.; Burns, A.R.; et al. Disrupted junctional membrane complexes and hyperactive ryanodine receptors after acute junctophilin knockdown in mice. Circulation 2011, 123, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Casal, E.; Federici, L.; Zhang, W.; Fernandez-Recio, J.; Priego, E.M.; Miguel, R.N.; DuHadaway, J.B.; Prendergast, G.C.; Luisi, B.F.; Laue, E.D. The crystal structure of the BAR domain from human Bin1/amphiphysin II and its implications for molecular recognition. Biochemistry 2006, 45, 12917–12928. [Google Scholar] [CrossRef][Green Version]
- Peter, B.J.; Kent, H.M.; Mills, I.G.; Vallis, Y.; Butler, P.J.; Evans, P.R.; McMahon, H.T. BAR domains as sensors of membrane curvature: The amphiphysin BAR structure. Science 2004, 303, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Boglarka, Z.; Evelina, E.; Bastien, M.; Luc, N.; Mátyás, P.; Zsuzsanna, D.; Soren, O.; Gilles, T.; Jocelyn, L.; Gergo, G. Uncovering the BIN1-SH3 interactome underpinning centronuclear myopathy. eLife 2024, 13, RP95397. [Google Scholar] [CrossRef]
- Bodi, I.; Mikala, G.; Koch, S.E.; Akhter, S.A.; Schwartz, A. The L-type calcium channel in the heart: The beat goes on. J. Clin. Investig. 2005, 115, 3306–3317. [Google Scholar] [CrossRef]
- Catterall, W.A.; Perez-Reyes, E.; Snutch, T.P.; Striessnig, J. International Union of Pharmacology. XLVIII. nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 2005, 57, 411–425. [Google Scholar] [CrossRef]
- Lipscombe, D.; Helton, T.D.; Xu, W. L-Type Calcium Channels: The Low Down. J. Neurophysiol. 2004, 92, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Wahl-Schott, C.; Baumann, L.; Cuny, H.; Eckert, C.; Griessmeier, K.; Biel, M. Switching off calcium-dependent inactivation in L-type calcium channels by an autoinhibitory domain. Proc. Natl. Acad. Sci. USA 2006, 103, 15657–15662. [Google Scholar] [CrossRef]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef]
- Tikhonov, D.B.; Zhorov, B.S. Structural model for dihydropyridine binding to L-type calcium channels. J. Biol. Chem. 2009, 284, 19006–19017. [Google Scholar] [CrossRef]
- Bauerová-Hlinková, V.; Hajdúchová, D.; Bauer, J.A. Structure and Function of the Human Ryanodine Receptors and Their Association with Myopathies-Present State, Challenges, and Perspectives. Molecules 2020, 25, 4040. [Google Scholar] [CrossRef] [PubMed]
- Laver, D.R. Regulation of the RyR channel gating by Ca2+ and Mg2. Biophys. Rev. 2018, 10, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Potenza, D.M.; Janicek, R.; Fernandez-Tenorio, M.; Camors, E.; Ramos-Mondragón, R.; Valdivia, H.H.; Niggli, E. Phosphorylation of the ryanodine receptor 2 at serine 2030 is required for a complete β-adrenergic response. J. Gen. Physiol. 2019, 151, 131–145. [Google Scholar] [CrossRef]
- Brohus, M.; Søndergaard, M.T.; Wayne Chen, S.R.; van Petegem, F.; Overgaard, M.T. Ca2+-dependent calmodulin binding to cardiac ryanodine receptor (RyR2) calmodulin-binding domains. Biochem. J. 2019, 476, 193–209. [Google Scholar] [CrossRef]
- Sacchetto, R.; Bertipaglia, I.; Giannetti, S.; Cendron, L.; Mascarello, F.; Damiani, E.; Carafoli, E.; Zanotti, G. Crystal structure of sarcoplasmic reticulum Ca2+-ATPase (SERCA) from bovine muscle. J. Struct. Biol. 2012, 178, 38–44. [Google Scholar] [CrossRef]
- Tsunekawa, N.; Ogawa, H.; Tsueda, J.; Akiba, T.; Toyoshima, C. Mechanism of the E2 to E1 transition in Ca2+ pump revealed by crystal structures of gating residue mutants. Proc. Natl. Acad. Sci. USA 2018, 115, 12722–12727. [Google Scholar] [CrossRef]
- Periasamy, M.; Bhupathy, P.; Babu, G.J. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc. Res. 2007, 77, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Hof, I.E.; van der Heijden, J.F.; Kranias, E.G.; Sanoudou, D.; de Boer, R.A.; van Tintelen, J.P.; van der Zwaag, P.A.; Doevendans, P.A. Prevalence and cardiac phenotype of patients with a phospholamban mutation. Neth. Heart J. 2019, 27, 64–69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bal, N.C.; Maurya, S.K.; Sopariwala, D.H.; Sahoo, S.K.; Gupta, S.C.; Shaikh, S.A.; Pant, M.; Rowland, L.A.; Bombardier, E.; Goonasekera, S.A.; et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 2012, 18, 1575–1579. [Google Scholar] [CrossRef]
- Bal, N.C.; Periasamy, M. Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2020, 375, 20190135. [Google Scholar] [CrossRef]
- MacLennan, D.H.; Kranias, E.G. Phospholamban: A crucial regulator of cardiac contractility. Nat. Rev. Mol. Cell Biol. 2003, 4, 566–577. [Google Scholar] [CrossRef]
- Sato, D.; Uchinoumi, H.; Bers, D.M. Increasing SERCA function promotes initiation of calcium sparks and breakup of calcium waves. J. Physiol. 2021, 599, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Choi, D.W. Na+-Ca2+Exchange Currents in Cortical Neurons: Concomitant Forward and Reverse Operation and Effect of Glutamate. Eur. J. Neurosci. 1997, 9, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Gudrun, A.; Karin, R. Sipido, Targeting calcium handling in arrhythmias. EP Eur. 2008, 10, 1364–1369. [Google Scholar] [CrossRef]
- Ren, X.; Philipson, K.D. The topology of the cardiac Na+/Ca2+ exchanger, NCX1. J. Mol. Cell. Cardiol. 2013, 57, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zeng, W.; Han, Y.; John, S.; Ottolia, M.; Jiang, Y. Structural mechanisms of the human cardiac sodium-calcium exchanger NCX1. Nat. Commun. 2023, 14, 6181. [Google Scholar] [CrossRef]
- Repke, K.R.; Schön, R.; Henke, W.; Schönfeld, W.; Streckenbach, B.; Dittrick, F. Experimental and theoretical examination of the flip-flop model of (Na, K)-ATPase function. Ann. N. Y. Acad. Sci. 1974, 242, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Li, H.; Zeng, W.; Sauer, D.B.; Belmares, R.; Jiang, Y. Structural insight into the ion-exchange mechanism of the sodium/calcium exchanger. Science 2012, 335, 686–690. [Google Scholar] [CrossRef]
- Yang, Y.C.; Kao, L.S. Regulation of sodium-calcium exchanger activity by creatine kinase. Adv. Exp. Med. Biol. 2013, 961, 163–173. [Google Scholar] [CrossRef]
- Bossuyt, J.; Taylor, B.E.; James-Kracke, M.; Hale, C.C. Evidence for cardiac sodium-calcium exchanger association with caveolin-3. FEBS Lett. 2002, 511, 113–117. [Google Scholar] [CrossRef]
- Lu, F.; Liou, C.; Ma, Q.; Wu, Z.; Xue, B.; Xia, Y.; Xia, S.; Trembley, M.A.; Ponek, A.; Xie, W.; et al. Virally delivered CMYA5 enhances the assembly of cardiac dyads. Nat. Biomed. Eng. 2025, 9, 730–741. [Google Scholar] [CrossRef]
- Hall, D.D.; Hiroshi, T.; Song, L.-S. Structure, Function, and Regulation of the Junctophilin Family. Annu. Rev. Physiol. 2023, 86, 123–147. [Google Scholar] [CrossRef]
- Gross, P.; Johnson, J.; Romero, C.M.; Eaton, D.M.; Poulet, C.; Sanchez-Alonso, J.; Lucarelli, C.; Ross, J.; Gibb, A.A.; Garbincius, J.F.; et al. Interaction of the Joining Region in Junctophilin-2 With the L-Type Ca 2+ Channel Is Pivotal for Cardiac Dyad Assembly and Intracellular Ca2+ Dynamics. Circ. Res. 2021, 128, 92–114. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.T.; Smyth, J.W.; Gao, D.; Chu, K.Y.; Vogan, J.M.; Fong, T.S.; Jensen, B.C.; Colecraft, H.M.; Shaw, R.M. BIN1 localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010, 8, e1000312. [Google Scholar] [CrossRef]
- Laitinen, P.J.; Brown, K.M.; Piippo, K.; Swan, H.; Devaney, J.M.; Brahmbhatt, B.; Donarum, E.A.; Marino, M.; Tiso, N.; Viitasalo, M.; et al. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation 2001, 103, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Miotto, M.C.; Reiken, S.; Wronska, A.; Yuan, Q.; Dridi, H.; Liu, Y.; Weninger, G.; Tchagou, C.; Marks, A.R. Structural basis for ryanodine receptor type 2 leak in heart failure and arrhythmogenic disorders. Nat. Commun. 2024, 15, 8080. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.J.; Lee, W.S.; Chien, Y.C.; Chen, T.Y.; Yang, K.T. The link between abnormalities of calcium handling proteins and catecholaminergic polymorphic ventricular tachycardia. Tzu Chi Med. J. 2021, 33, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Baltogiannis, G.G.; Lysitsas, D.N.; di Giovanni, G.; Ciconte, G.; Sieira, J.; Conte, G.; Kolettis, T.M.; Chierchia, G.B.; de Asmundis, C.; Brugada, P. CPVT: Arrhythmogenesis, Therapeutic Management, and Future Perspectives. A Brief Review of the Literature. Front. Cardiovasc. Med. 2019, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Bezzerides, V.J.; Caballero, A.; Wang, S.; Ai, Y.; Hylind, R.J.; Lu, F.; Heims-Waldron, D.A.; Chambers, K.D.; Zhang, D.; Abrams, D.J.; et al. Gene Therapy for Catecholaminergic Polymorphic Ventricular Tachycardia by Inhibition of Ca2+/Calmodulin-Dependent Kinase II. Circulation 2019, 140, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Balcazar, D.; Regge, V.; Santalla, M.; Meyer, H.; Paululat, A.; Mattiazzi, A.; Ferrero, P. SERCA is critical to control the Bowditch effect in the heart. Sci. Rep. 2018, 8, 12447. [Google Scholar] [CrossRef]
- Sikkel, M.B.; Hayward, C.; MacLeod, K.T.; Harding, S.E.; Lyon, A.R. SERCA2a gene therapy in heart failure: An anti-arrhythmic positive inotrope. Br. J. Pharmacol. 2014, 171, 38–54. [Google Scholar] [CrossRef]
- Lyon, A.R.; Bannister, M.L.; Collins, T.; Pearce, E.; Sepehripour, A.H.; Dubb, S.S.; Garcia, E.; O’Gara, P.; Liang, L.; Kohlbrenner, E.; et al. SERCA2a Gene Transfer Decreases Sarcoplasmic Reticulum Calcium Leak and Reduces Ventricular Arrhythmias in a Model of Chronic Heart Failure. Circ. Arrhythmia Electrophysiol. 2011, 4, 362–372. [Google Scholar] [CrossRef]
- Greenberg, B.; Butler, J.; Felker, G.M.; Ponikowski, P.; Voors, A.A.; Desai, A.S.; Barnard, D.; Bouchard, A.; Jaski, B.; Lyon, A.R.; et al. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): A randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet 2016, 387, 1178–1186. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Weisleder, N.; Batalden, K.B.; Bos, J.M.; Tester, D.J.; Ommen, S.R.; Wehrens, X.H.; Claycomb, W.C.; Ko, J.K.; Hwang, M.; et al. Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J. Mol. Cell. Cardiol. 2007, 42, 1026–1035. [Google Scholar] [CrossRef]
- Bazgir, F.; Nau, J.; Nakhaei-Rad, S.; Amin, E.; Wolf, M.J.; Saucerman, J.J.; Lorenz, K.; Ahmadian, M.R. The Microenvironment of the Pathogenesis of Cardiac Hypertrophy. Cells 2023, 12, 1780. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.O.; Chiang, D.Y.; Wang, W.; Beavers, D.L.; Dixit, S.S.; Skapura, D.G.; Landstrom, A.P.; Song, L.S.; Ackerman, M.J.; Wehrens, X.H. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc. Res. 2013, 100, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, H.-P.; Fairweather, D.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated cardiomyopathy. Nat. Rev. Dis. Primers 2019, 5, 32. [Google Scholar] [CrossRef]
- Roux-Buisson, N.; Gandjbakhch, E.; Donal, E.; Probst, V.; Deharo, J.C.; Chevalier, P.; Klug, D.; Mansencal, N.; Delacretaz, E.; Cosnay, P.; et al. Prevalence and significance of rare RYR2 variants in arrhythmogenic right ventricular cardiomyopathy/dysplasia: Results of a systematic screening. Heart Rhythm. 2014, 11, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Laury-Kleintop, L.D.; Mulgrew, J.R.; Heletz, I.; Nedelcoviciu, R.A.; Chang, M.Y.; Harris, D.M.; Koch, W.J.; Schneider, M.D.; Muller, A.J.; Prendergast, G.C. Cardiac-Specific Disruption of Bin1 in Mice Enables a Model of Stress- and Age-Associated Dilated Cardiomyopathy. J. Cell. Biochem. 2015, 116, 2541–2551. [Google Scholar] [CrossRef]
- Gambardella, J.; Wang, X.J.; Ferrara, J.; Morelli, M.B.; Santulli, G. Cardiac BIN1 Replacement Therapy Ameliorates Inotropy and Lusitropy in Heart Failure by Regulating Calcium Handling. JACC Basic Transl. Sci. 2020, 5, 579–581. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liou, C.; Chin, M.T. Role of Dyadic Proteins in Proper Heart Function and Disease. Int. J. Mol. Sci. 2025, 26, 7478. https://doi.org/10.3390/ijms26157478
Liou C, Chin MT. Role of Dyadic Proteins in Proper Heart Function and Disease. International Journal of Molecular Sciences. 2025; 26(15):7478. https://doi.org/10.3390/ijms26157478
Chicago/Turabian StyleLiou, Carter, and Michael T. Chin. 2025. "Role of Dyadic Proteins in Proper Heart Function and Disease" International Journal of Molecular Sciences 26, no. 15: 7478. https://doi.org/10.3390/ijms26157478
APA StyleLiou, C., & Chin, M. T. (2025). Role of Dyadic Proteins in Proper Heart Function and Disease. International Journal of Molecular Sciences, 26(15), 7478. https://doi.org/10.3390/ijms26157478







