Two Decades of Disease Evolution and Biomarker-Guided Clinical Decision Making in Metastatic Prostate Cancer
Abstract
1. Introduction
2. Results
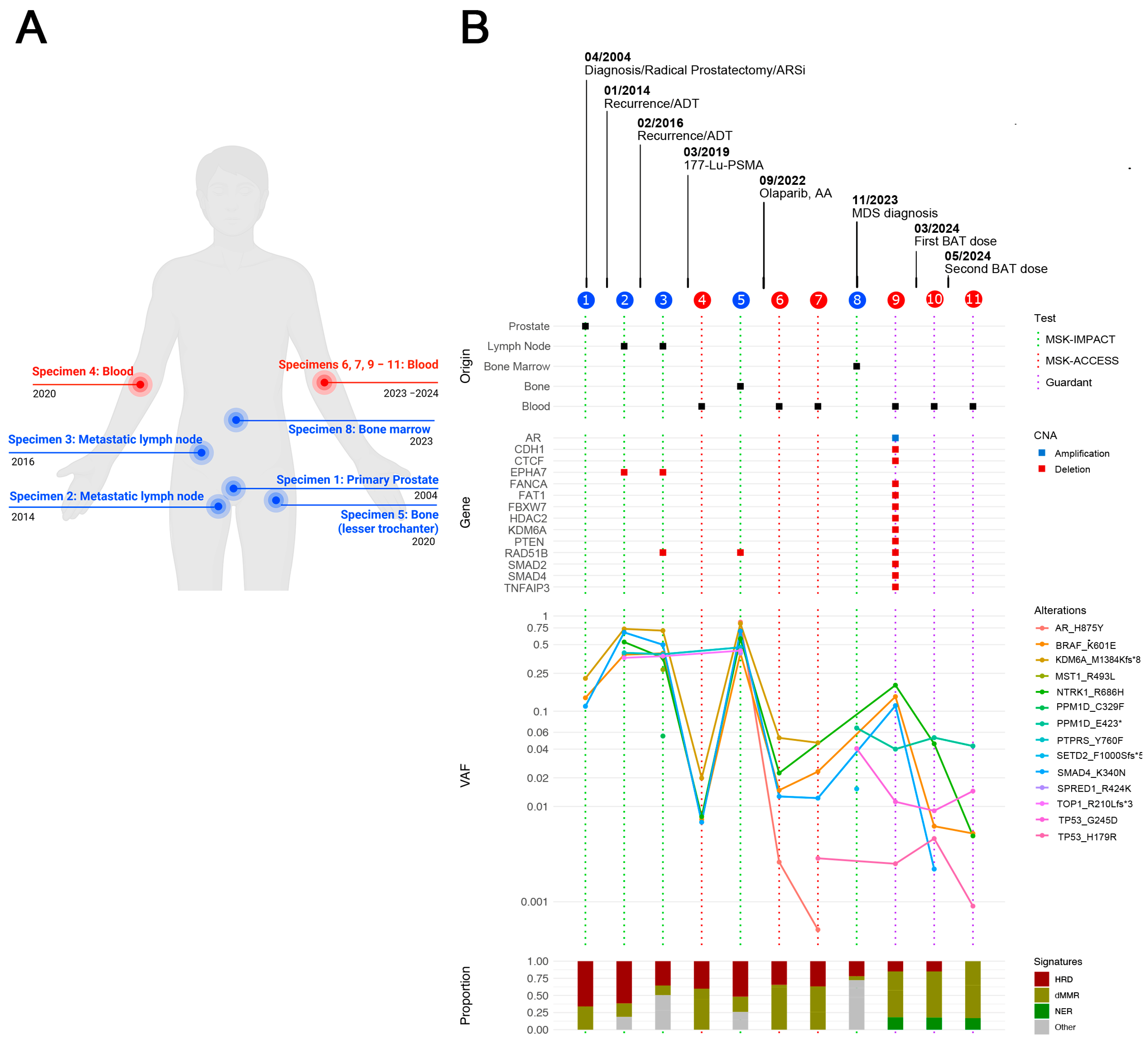
2.1. Clinical Case
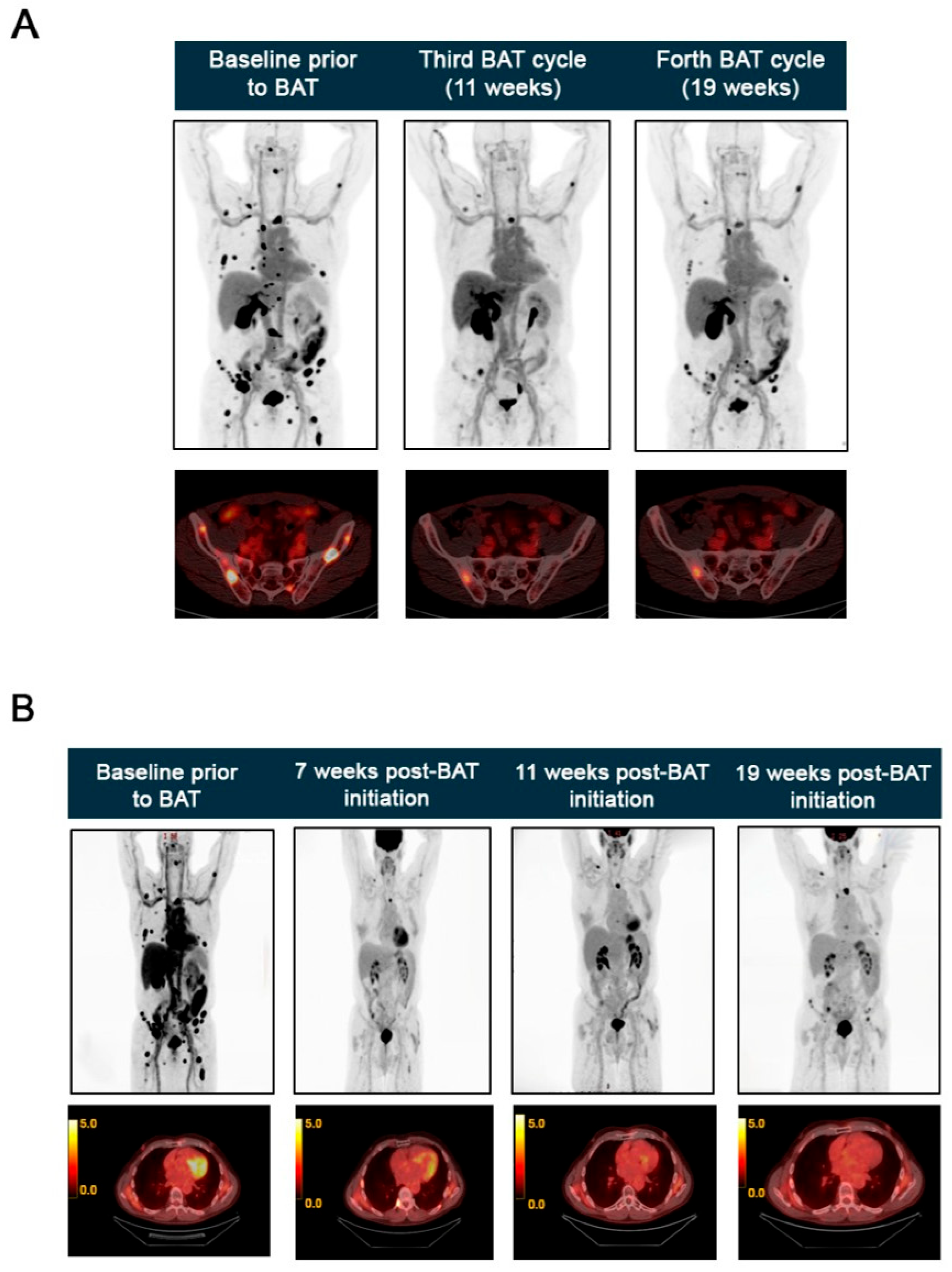
BAT Eliminates AR Amplification Detected by cfDNA Analysis
3. Discussion
4. Materials and Methods
4.1. Tumor Genomic Profiling with MSK-IMPACT
4.2. ctDNA Profiling
4.3. Mutational Signatures Analysis
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| mCRPC | Metastatic castration-resistant prostate cancer |
| AR | Androgen receptor |
| PSA | Prostate-specific antigen |
| BAT | Bipolar androgen therapy |
| ADT | Androgen deprivation therapy |
| ARSi | Androgen receptor signaling inhibitors |
| 177Lu-PSMA | 177Lutetium PSMA |
| SRT | Salvage radiotherapy |
| ctDNA | Circulating tumor DNA |
| cfDNA | Cell-free DNA |
| NGS | Next-generation sequencing |
| MSK-IMPACT | MSK-Integrated Mutation Profiling of Actionable Cancer Targets |
| MSK-ACCESS | Circulating cfDNA to Examine Somatic Status |
| VAF | Variant allele frequency |
| CNA | Copy number alteration |
| WB-MRI | Whole-body magnetic resonance imaging |
| IGRT | Image-guided radiation therapy |
| HRD | Homologous recombination deficiency |
| dMMR | DNA mismatch repair |
| NER | Nucleotide excision repair |
References
- Banerjee, S.; Booth, C.M.; Bruera, E.; Buchler, M.W.; Drilon, A.; Fry, T.J.; Ghobrial, I.M.; Gianni, L.; Jain, R.K.; Kroemer, G.; et al. Two decades of advances in clinical oncology-lessons learned and future directions. Nat. Rev. Clin. Oncol. 2024, 21, 771–780. [Google Scholar] [CrossRef]
- Grewal, K.; Dorff, T.B.; Mukhida, S.S.; Agarwal, N.; Hahn, A.W. Advances in Targeted Therapy for Metastatic Prostate Cancer. Curr. Treat. Options Oncol. 2025, 26, 465–475. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Freedland, S.J.; Davis, M.; Epstein, A.J.; Arondekar, B.; Ivanova, J.I. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2024, 27, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Vaishampayan, U. Therapy of Advanced Prostate Cancer: Targeting the Androgen Receptor Axis in Earlier Lines of Treatment. Target. Oncol. 2018, 13, 679–689. [Google Scholar] [CrossRef]
- Lavaud, P.; Dumont, C.; Thibault, C.; Albiges, L.; Baciarello, G.; Colomba, E.; Flippot, R.; Fuerea, A.; Loriot, Y.; Fizazi, K. Next-generation androgen receptor inhibitors in non-metastatic castration-resistant prostate cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920978134. [Google Scholar] [CrossRef]
- Chan, J.M.; Zaidi, S.; Love, J.R.; Zhao, J.L.; Setty, M.; Wadosky, K.M.; Gopalan, A.; Choo, Z.N.; Persad, S.; Choi, J.; et al. Lineage plasticity in prostate cancer depends on JAK/STAT inflammatory signaling. Science 2022, 377, 1180–1191. [Google Scholar] [CrossRef]
- Jamroze, A.; Liu, X.; Tang, D.G. Treatment-induced stemness and lineage plasticity in driving prostate cancer therapy resistance. Cancer Heterog. Plast. 2024, 1, 5. [Google Scholar] [CrossRef]
- Mu, P.; Zhang, Z.; Benelli, M.; Karthaus, W.R.; Hoover, E.; Chen, C.C.; Wongvipat, J.; Ku, S.Y.; Gao, D.; Cao, Z.; et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017, 355, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Rebello, R.J.; Oing, C.; Knudsen, K.E.; Loeb, S.; Johnson, D.C.; Reiter, R.E.; Gillessen, S.; Van der Kwast, T.; Bristow, R.G. Prostate cancer. Nat. Rev. Dis. Primers 2021, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; McKay, R.; Abida, W.; Aggarwal, R.; Alumkal, J.; Alva, A.; Feng, F.; Gao, X.; Graff, J.; Hussain, M.; et al. Accelerating precision medicine in metastatic prostate cancer. Nat. Cancer 2020, 1, 1041–1053. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Chen, E.; Cario, C.L.; Leong, L.; Lopez, K.; Marquez, C.P.; Li, P.S.; Oropeza, E.; Tenggara, I.; Cowan, J.; Simko, J.P.; et al. Cell-Free DNA Detection of Tumor Mutations in Heterogeneous, Localized Prostate Cancer Via Targeted, Multiregion Sequencing. JCO Precis. Oncol. 2021, 5, 710–725. [Google Scholar] [CrossRef]
- Herberts, C.; Annala, M.; Sipola, J.; Ng, S.W.S.; Chen, X.E.; Nurminen, A.; Korhonen, O.V.; Munzur, A.D.; Beja, K.; Schonlau, E.; et al. Deep whole-genome ctDNA chronology of treatment-resistant prostate cancer. Nature 2022, 608, 199–208. [Google Scholar] [CrossRef]
- Fonseca, N.M.; Maurice-Dror, C.; Herberts, C.; Tu, W.; Fan, W.; Murtha, A.J.; Kollmannsberger, C.; Kwan, E.M.; Parekh, K.; Schonlau, E.; et al. Prediction of plasma ctDNA fraction and prognostic implications of liquid biopsy in advanced prostate cancer. Nat. Commun. 2024, 15, 1828. [Google Scholar] [CrossRef]
- Lau, E.; McCoy, P.; Reeves, F.; Chow, K.; Clarkson, M.; Kwan, E.M.; Packwood, K.; Northen, H.; He, M.; Kingsbury, Z.; et al. Detection of ctDNA in plasma of patients with clinically localised prostate cancer is associated with rapid disease progression. Genome Med. 2020, 12, 72. [Google Scholar] [CrossRef]
- Sweeney, C.J.; Petry, R.; Xu, C.; Childress, M.; He, J.; Fabrizio, D.; Gjoerup, O.; Morley, S.; Catlett, T.; Assaf, Z.J.; et al. Circulating Tumor DNA Assessment for Treatment Monitoring Adds Value to PSA in Metastatic Castration Resistant Prostate Cancer. Clin. Cancer Res. 2024, 30, 4115–4122. [Google Scholar] [CrossRef]
- Combes, A.D.; Palma, C.A.; Calopedos, R.; Wen, L.; Woo, H.; Fulham, M.; Leslie, S. PSMA PET-CT in the Diagnosis and Staging of Prostate Cancer. Diagnostics 2022, 12, 2594. [Google Scholar] [CrossRef] [PubMed]
- Sartor, A.O. PSMA-targeted radiotherapy in metastatic castration-resistant prostate cancer. Clin. Adv. Hematol. Oncol. 2021, 19, 494–496. [Google Scholar] [PubMed]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Filippi, L.; Urso, L.; Schillaci, O.; Evangelista, L. [(18)F]-FDHT PET for the Imaging of Androgen Receptor in Prostate and Breast Cancer: A Systematic Review. Diagnostics 2023, 13, 2613. [Google Scholar] [CrossRef]
- Cysouw, M.C.F.; Kramer, G.M.; Heijtel, D.; Schuit, R.C.; Morris, M.J.; van den Eertwegh, A.J.M.; Voortman, J.; Hoekstra, O.S.; Oprea-Lager, D.E.; Boellaard, R. Sensitivity of (18)F-fluorodihydrotestosterone PET-CT to count statistics and reconstruction protocol in metastatic castration-resistant prostate cancer. EJNMMI Res. 2019, 9, 70. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Marconcini, R.; Galli, L.; Antonuzzo, A.; Bursi, S.; Roncella, C.; Fontanini, G.; Sensi, E.; Falcone, A. Metastatic BRAF K601E-mutated melanoma reaches complete response to MEK inhibitor trametinib administered for over 36 months. Exp. Hematol. Oncol. 2017, 6, 6. [Google Scholar] [CrossRef]
- Fallah, J.; Agrawal, S.; Gittleman, H.; Fiero, M.H.; Subramaniam, S.; John, C.; Chen, W.; Ricks, T.K.; Niu, G.; Fotenos, A.; et al. FDA Approval Summary: Lutetium Lu 177 Vipivotide Tetraxetan for Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2023, 29, 1651–1657. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Sandhu, S. [(177)Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer-Author’s reply. Lancet Oncol. 2018, 19, e373. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, Y.I. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer: A Meta-analysis. Clin. Nucl. Med. 2018, 43, 728–734. [Google Scholar] [CrossRef]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schafers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Eifer, M.; Sutherland, D.E.K.; Goncalves, I.; Buteau, J.P.; Au, L.; Azad, A.A.; Emmett, L.; Kong, G.; Kostos, L.; Ravi Kumar, A.S.; et al. Therapy-Related Myeloid Neoplasms After [(177)Lu]Lu-PSMA Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer: A Case Series. J. Nucl. Med. 2025, 66, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Kusne, Y.; Lasho, T.; Finke, C.; Elsabbagh, Z.; McCue, S.; Hobday, T.; Starr, J.; Bekaii-Saab, T.; Halfdanarson, T.R.; Patnaik, M.M.; et al. Clonal Hematopoiesis in Patients With Neuroendocrine Tumor Treated With Lutetium-177 and the Risk of Thrombocytopenia: A Prospective Study. JCO Precis. Oncol. 2024, 8, e2400143. [Google Scholar] [CrossRef] [PubMed]
- Rose Brannon, A.; Jayakumaran, G.; Diosdado, M.; Patel, J.; Razumova, A.; Hu, Y.; Meng, F.; Haque, M.; Sadowska, J.; Murphy, B.J.; et al. Enhanced specificity of clinical high-sensitivity tumor mutation profiling in cell-free DNA via paired normal sequencing using MSK-ACCESS. Nat. Commun. 2021, 12, 3770. [Google Scholar] [CrossRef]
- Abida, W.; Cheng, M.L.; Armenia, J.; Middha, S.; Autio, K.A.; Vargas, H.A.; Rathkopf, D.; Morris, M.J.; Danila, D.C.; Slovin, S.F.; et al. Analysis of the Prevalence of Microsatellite Instability in Prostate Cancer and Response to Immune Checkpoint Blockade. JAMA Oncol. 2019, 5, 471–478. [Google Scholar] [CrossRef]
- Petljak, M.; Alexandrov, L.B.; Brammeld, J.S.; Price, S.; Wedge, D.C.; Grossmann, S.; Dawson, K.J.; Ju, Y.S.; Iorio, F.; Tubio, J.M.C.; et al. Characterizing Mutational Signatures in Human Cancer Cell Lines Reveals Episodic APOBEC Mutagenesis. Cell 2019, 176, 1282–1294.e20. [Google Scholar] [CrossRef]
- Denmeade, S.; Antonarakis, E.S.; Markowski, M.C. Bipolar androgen therapy (BAT): A patient’s guide. Prostate 2022, 82, 753–762. [Google Scholar] [CrossRef]
- Azad, A.A.; Volik, S.V.; Wyatt, A.W.; Haegert, A.; Le Bihan, S.; Bell, R.H.; Anderson, S.A.; McConeghy, B.; Shukin, R.; Bazov, J.; et al. Androgen Receptor Gene Aberrations in Circulating Cell-Free DNA: Biomarkers of Therapeutic Resistance in Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2015, 21, 2315–2324. [Google Scholar] [CrossRef]
- Abida, W.; Armenia, J.; Gopalan, A.; Brennan, R.; Walsh, M.; Barron, D.; Danila, D.; Rathkopf, D.; Morris, M.; Slovin, S.; et al. Prospective Genomic Profiling of Prostate Cancer Across Disease States Reveals Germline and Somatic Alterations That May Affect Clinical Decision Making. JCO Precis. Oncol. 2017, 1, 1–16. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Sena, L.A.; Wang, H.; Antonarakis, E.S.; Markowski, M.C. Bipolar Androgen Therapy Followed by Androgen Receptor Inhibition as Sequential Therapy for Prostate Cancer. Oncologist 2023, 28, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Wang, H.; Sullivan, R.; Rifkind, I.; Sinibaldi, V.; Schweizer, M.T.; Teply, B.A.; Ngomba, N.; Fu, W.; Carducci, M.A.; et al. A Multicohort Open-label Phase II Trial of Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur. Urol. 2021, 79, 692–699. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Wang, H.; Agarwal, N.; Smith, D.C.; Schweizer, M.T.; Stein, M.N.; Assikis, V.; Twardowski, P.W.; Flaig, T.W.; Szmulewitz, R.Z.; et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J. Clin. Oncol. 2021, 39, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Taplin, M.E.; Aggarwal, R.; Sena, L.A.; Wang, H.; Qi, H.; Lalji, A.; Sinibaldi, V.; Carducci, M.A.; Paller, C.J.; et al. Bipolar androgen therapy plus nivolumab for patients with metastatic castration-resistant prostate cancer: The COMBAT phase II trial. Nat. Commun. 2024, 15, 14. [Google Scholar] [CrossRef]
- Teply, B.A.; Kachhap, S.; Eisenberger, M.A.; Denmeade, S.R. Extreme Response to High-dose Testosterone in BRCA2- and ATM-mutated Prostate Cancer. Eur. Urol. 2017, 71, 499. [Google Scholar] [CrossRef] [PubMed]
- Lam, H.M.; Nguyen, H.M.; Labrecque, M.P.; Brown, L.G.; Coleman, I.M.; Gulati, R.; Lakely, B.; Sondheim, D.; Chatterjee, P.; Marck, B.T.; et al. Durable Response of Enzalutamide-resistant Prostate Cancer to Supraphysiological Testosterone Is Associated with a Multifaceted Growth Suppression and Impaired DNA Damage Response Transcriptomic Program in Patient-derived Xenografts. Eur. Urol. 2020, 77, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Markowski, M.C.; Kachhap, S.; De Marzo, A.M.; Sena, L.A.; Luo, J.; Denmeade, S.R.; Antonarakis, E.S. Molecular and Clinical Characterization of Patients With Metastatic Castration Resistant Prostate Cancer Achieving Deep Responses to Bipolar Androgen Therapy. Clin. Genitourin. Cancer 2022, 20, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, M.T.; Gulati, R.; Yezefski, T.; Cheng, H.H.; Mostaghel, E.; Haffner, M.C.; Patel, R.A.; De Sarkar, N.; Ha, G.; Dumpit, R.; et al. Bipolar androgen therapy plus olaparib in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023, 26, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Prosz, A.; Duan, H.; Tisza, V.; Sahgal, P.; Topka, S.; Klus, G.T.; Borcsok, J.; Sztupinszki, Z.; Hanlon, T.; Diossy, M.; et al. Nucleotide excision repair deficiency is a targetable therapeutic vulnerability in clear cell renal cell carcinoma. Sci. Rep. 2023, 13, 20567. [Google Scholar] [CrossRef]
- Nishiyama, A.; Sato, S.; Sakaguchi, H.; Kotani, H.; Yamashita, K.; Ohtsubo, K.; Nanjo, S.; Yano, S.; Mizuguchi, K.; Ikeda, H.; et al. Challenges in the treatment of BRAF K601E-mutated lung carcinoma: A case report of rapid response and resistance to dabrafenib and trametinib. Front. Oncol. 2024, 14, 1374594. [Google Scholar] [CrossRef]
- Johnson, D.B.; Zhao, F.; Noel, M.; Riely, G.J.; Mitchell, E.P.; Wright, J.J.; Chen, H.X.; Gray, R.J.; Li, S.; McShane, L.M.; et al. Trametinib Activity in Patients with Solid Tumors and Lymphomas Harboring BRAF Non-V600 Mutations or Fusions: Results from NCI-MATCH (EAY131). Clin. Cancer Res. 2020, 26, 1812–1819. [Google Scholar] [CrossRef]
- Schreck, K.C.; de la Fuente, M.I.; Rine, J.; Kline, I.; Shepherd, S.P.; Rodón, J.; Sherman, R.Y.E. FORTE: A Phase II master protocol assessing plixorafenib for BRAF-altered cancers. Cancer Res. 2025, 85 (8_Suppl. 2), CT247. [Google Scholar] [CrossRef]
- Dai, C.; Dehm, S.M.; Sharifi, N. Targeting the Androgen Signaling Axis in Prostate Cancer. J. Clin. Oncol. 2023, 41, 4267–4278. [Google Scholar] [CrossRef]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef]
- Sandhu, S.; Joshua, A.M.; Emmett, L.; Crumbaker, M.; Bressel, M.; Huynh, R.; Banks, P.D.; Wallace, R.; Hamid, A.; Inderjeeth, A.J.; et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 2023, 41 (Suppl. 16), 5005. [Google Scholar] [CrossRef]
- Hallqvist, A.; Brynjarsdottir, E.; Krantz, T.; Sjogren, M.; Svensson, J.; Bernhardt, P. (177)Lu-DOTATATE in Combination with PARP Inhibitor Olaparib Is Feasible in Patients with Somatostatin-Positive Tumors: Results from the LuPARP Phase I Trial. J. Nucl. Med. 2025, 66, 707–712. [Google Scholar] [CrossRef]
- Corcoran, N.M.; Clarkson, M.J.; Stuchbery, R.; Hovens, C.M. Molecular Pathways: Targeting DNA Repair Pathway Defects Enriched in Metastasis. Clin. Cancer Res. 2016, 22, 3132–3137. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, X.A.; Zhang, N.; Wang, J. Evolving insights: How DNA repair pathways impact cancer evolution. Cancer Biol. Med. 2020, 17, 805–827. [Google Scholar] [CrossRef]
- Cheng, D.T.; Mitchell, T.N.; Zehir, A.; Shah, R.H.; Benayed, R.; Syed, A.; Chandramohan, R.; Liu, Z.Y.; Won, H.H.; Scott, S.N.; et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J. Mol. Diagn. 2015, 17, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Barzi, A.; Weipert, C.M.; Espenschied, C.R.; Raymond, V.M.; Wang-Gillam, A.; Nezami, M.A.; Gordon, E.J.; Mahadevan, D.; Mody, K. ERBB2 (HER2) amplifications and co-occurring KRAS alterations in the circulating cell-free DNA of pancreatic ductal adenocarcinoma patients and response to HER2 inhibition. Front. Oncol. 2024, 14, 1339302. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, L.B.; Kim, J.; Haradhvala, N.J.; Huang, M.N.; Tian Ng, A.W.; Wu, Y.; Boot, A.; Covington, K.R.; Gordenin, D.A.; Bergstrom, E.N.; et al. The repertoire of mutational signatures in human cancer. Nature 2020, 578, 94–101. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erazo, T.; Moiso, E.; Aras, O.; Scher, H.I. Two Decades of Disease Evolution and Biomarker-Guided Clinical Decision Making in Metastatic Prostate Cancer. Int. J. Mol. Sci. 2025, 26, 7593. https://doi.org/10.3390/ijms26157593
Erazo T, Moiso E, Aras O, Scher HI. Two Decades of Disease Evolution and Biomarker-Guided Clinical Decision Making in Metastatic Prostate Cancer. International Journal of Molecular Sciences. 2025; 26(15):7593. https://doi.org/10.3390/ijms26157593
Chicago/Turabian StyleErazo, Tatiana, Enrico Moiso, Omer Aras, and Howard I. Scher. 2025. "Two Decades of Disease Evolution and Biomarker-Guided Clinical Decision Making in Metastatic Prostate Cancer" International Journal of Molecular Sciences 26, no. 15: 7593. https://doi.org/10.3390/ijms26157593
APA StyleErazo, T., Moiso, E., Aras, O., & Scher, H. I. (2025). Two Decades of Disease Evolution and Biomarker-Guided Clinical Decision Making in Metastatic Prostate Cancer. International Journal of Molecular Sciences, 26(15), 7593. https://doi.org/10.3390/ijms26157593





