Extracellular Adenosine in Gastric Cancer: The Role of GCSCs
Abstract
1. Introduction
2. Results
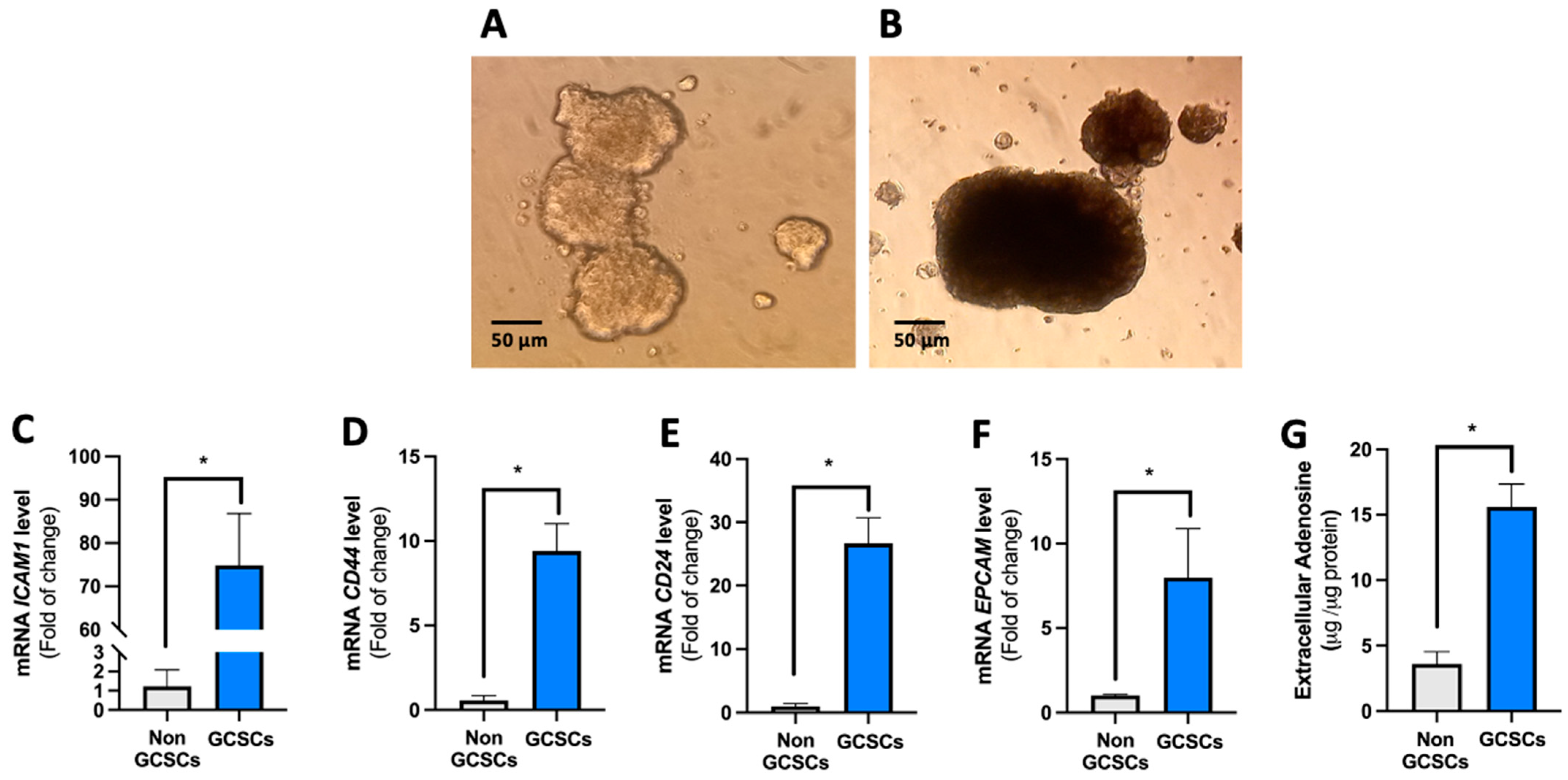
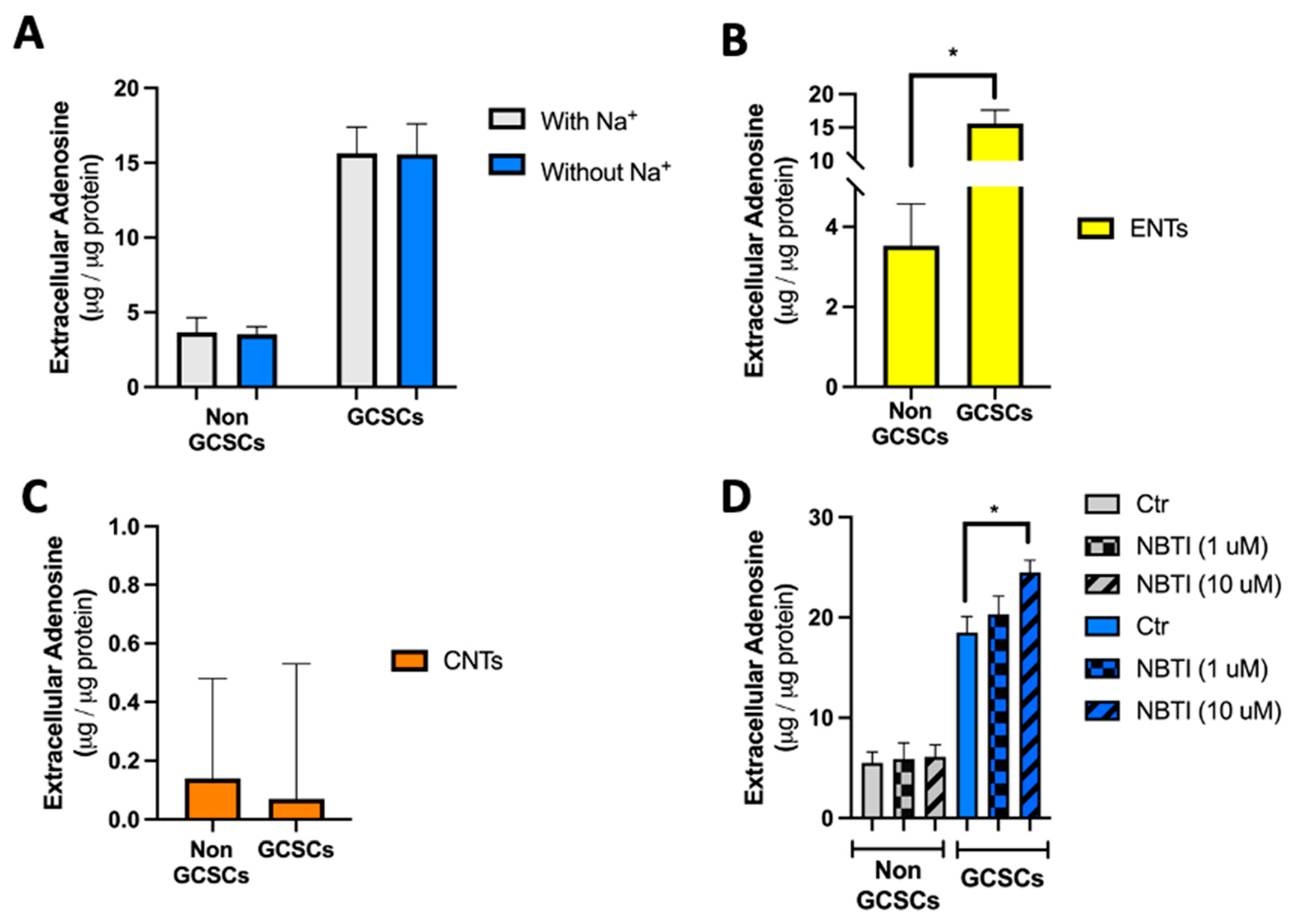
2.1. Gastric Cancer Stem Cells (GCSCs) Have an Intrinsically Increased Capability to Generate Extracellular Adenosine
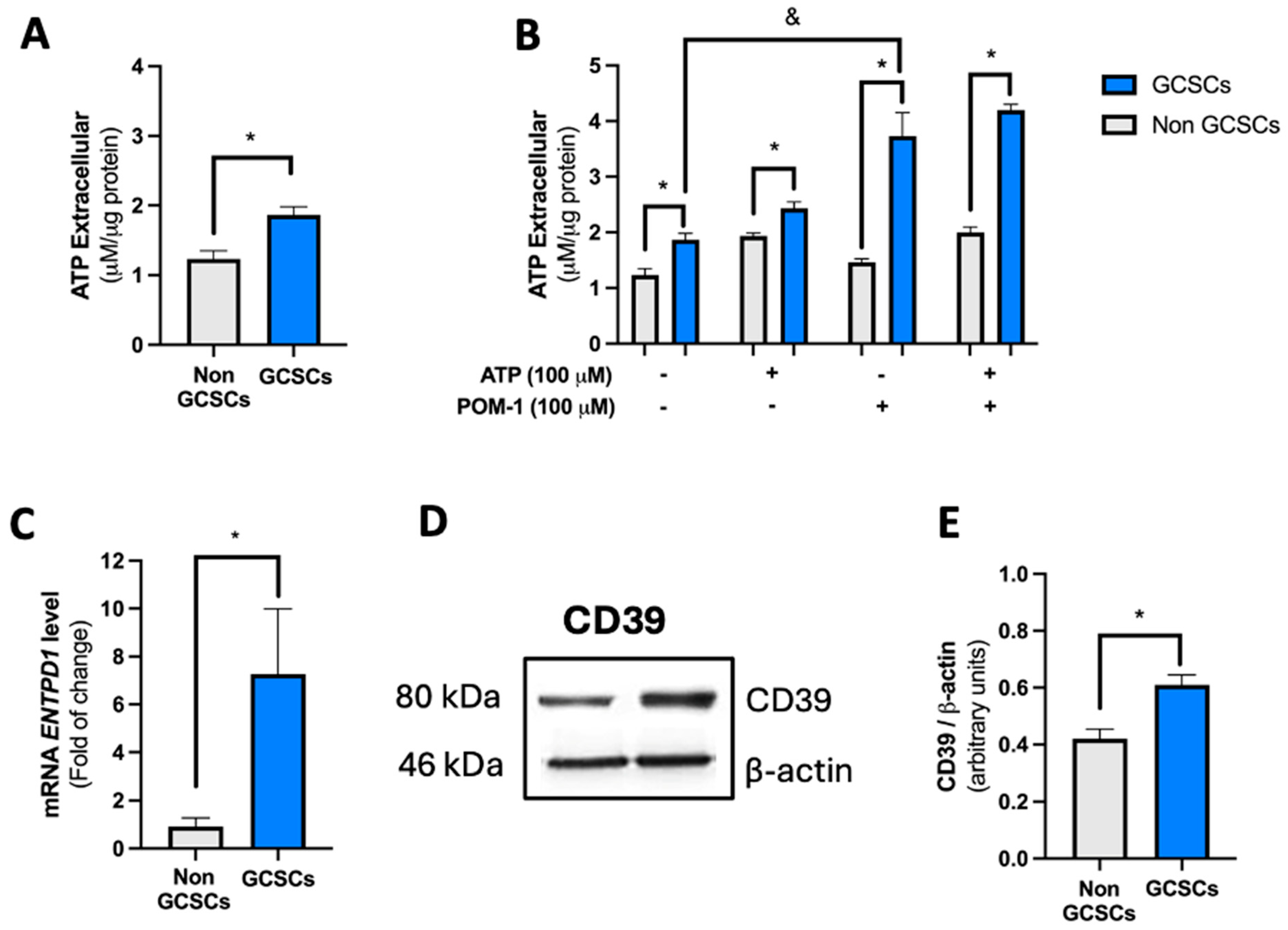
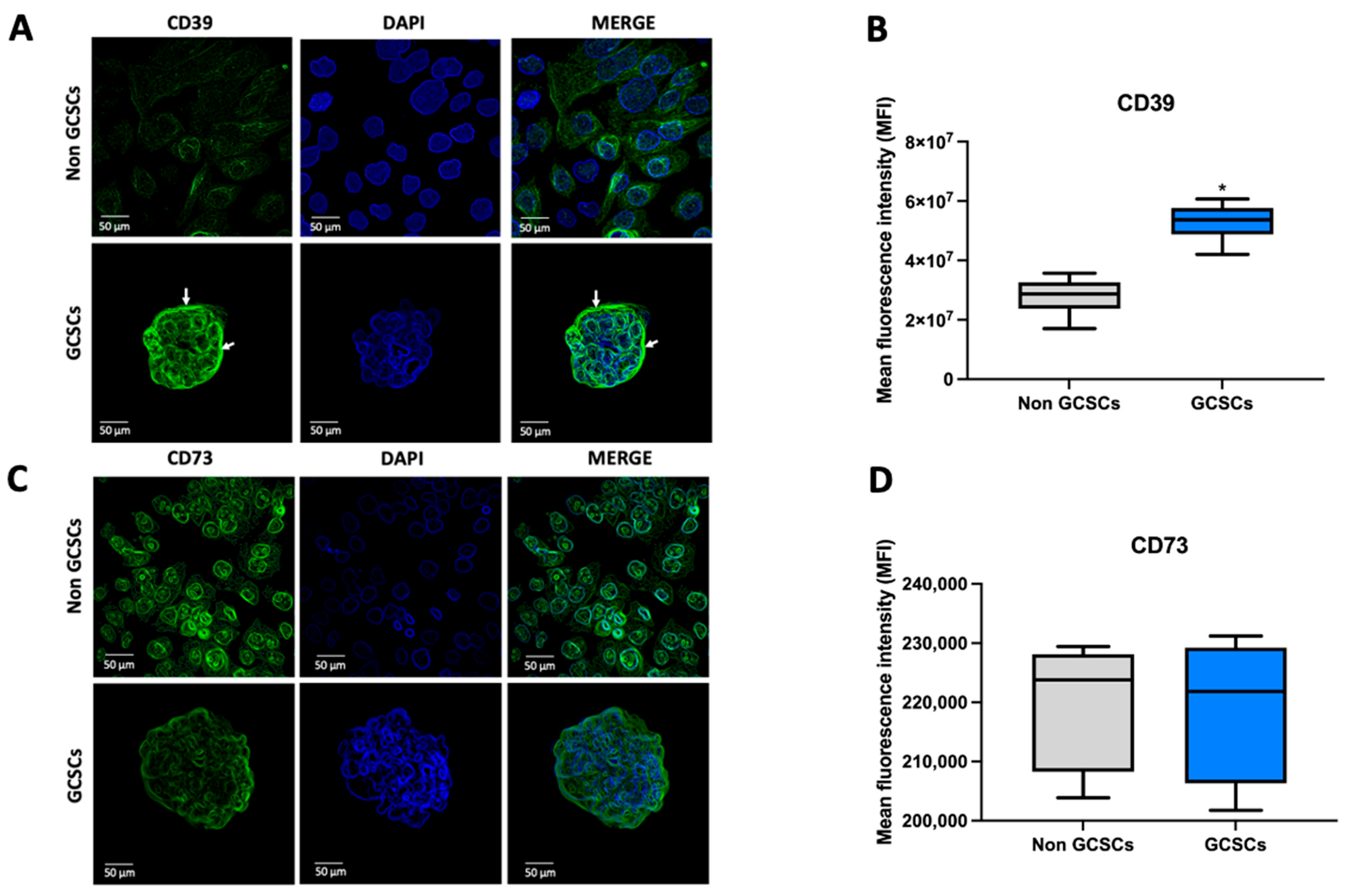
2.2. Gastric Cancer Stem Cells (GCSCs) Have a High Capacity to Degrade ATP
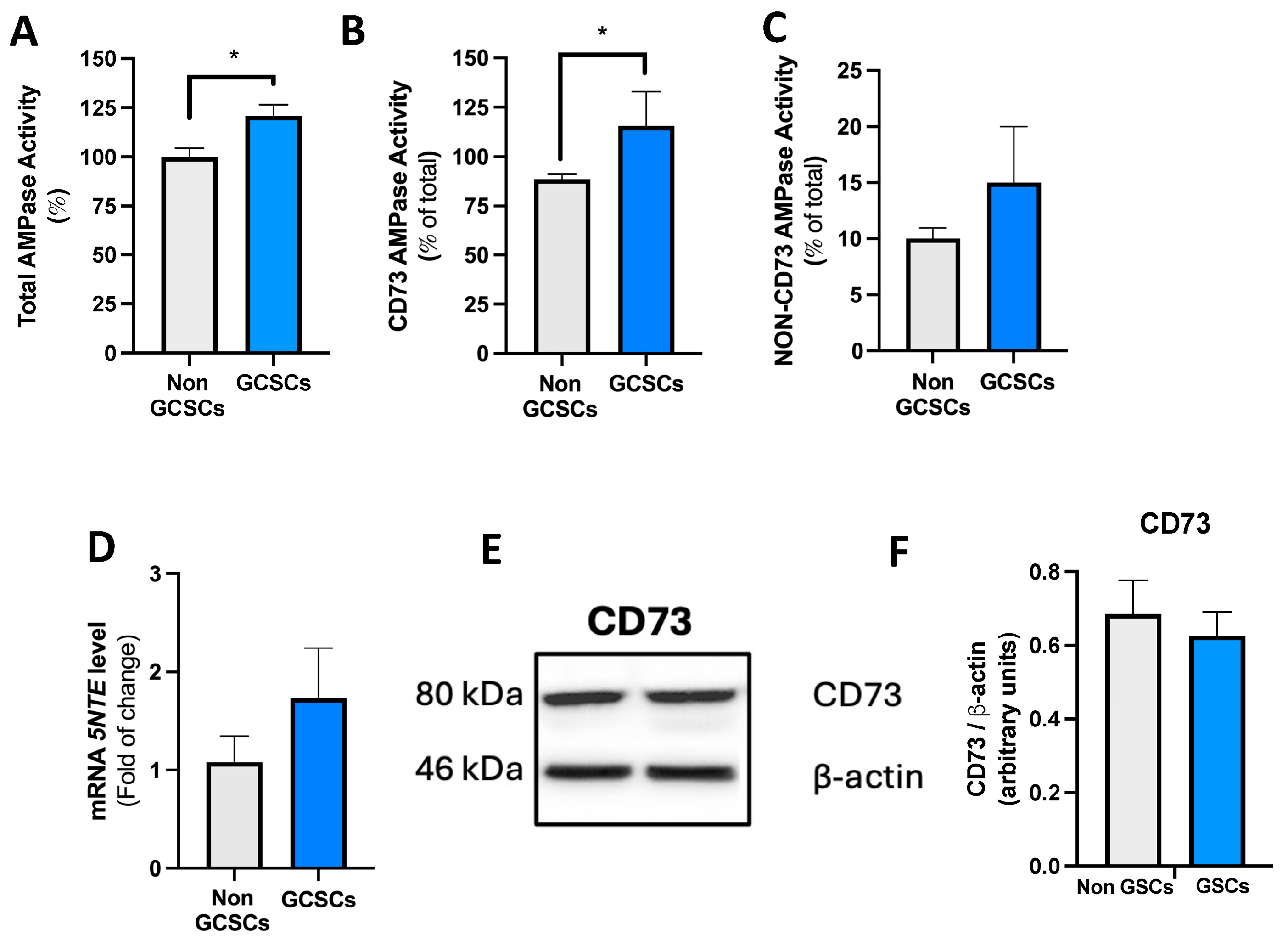
2.3. Gastric Cancer Stem Cells Present Greater AMPase Capacity than Non-GCSCs of the MKN-74 Cell Line
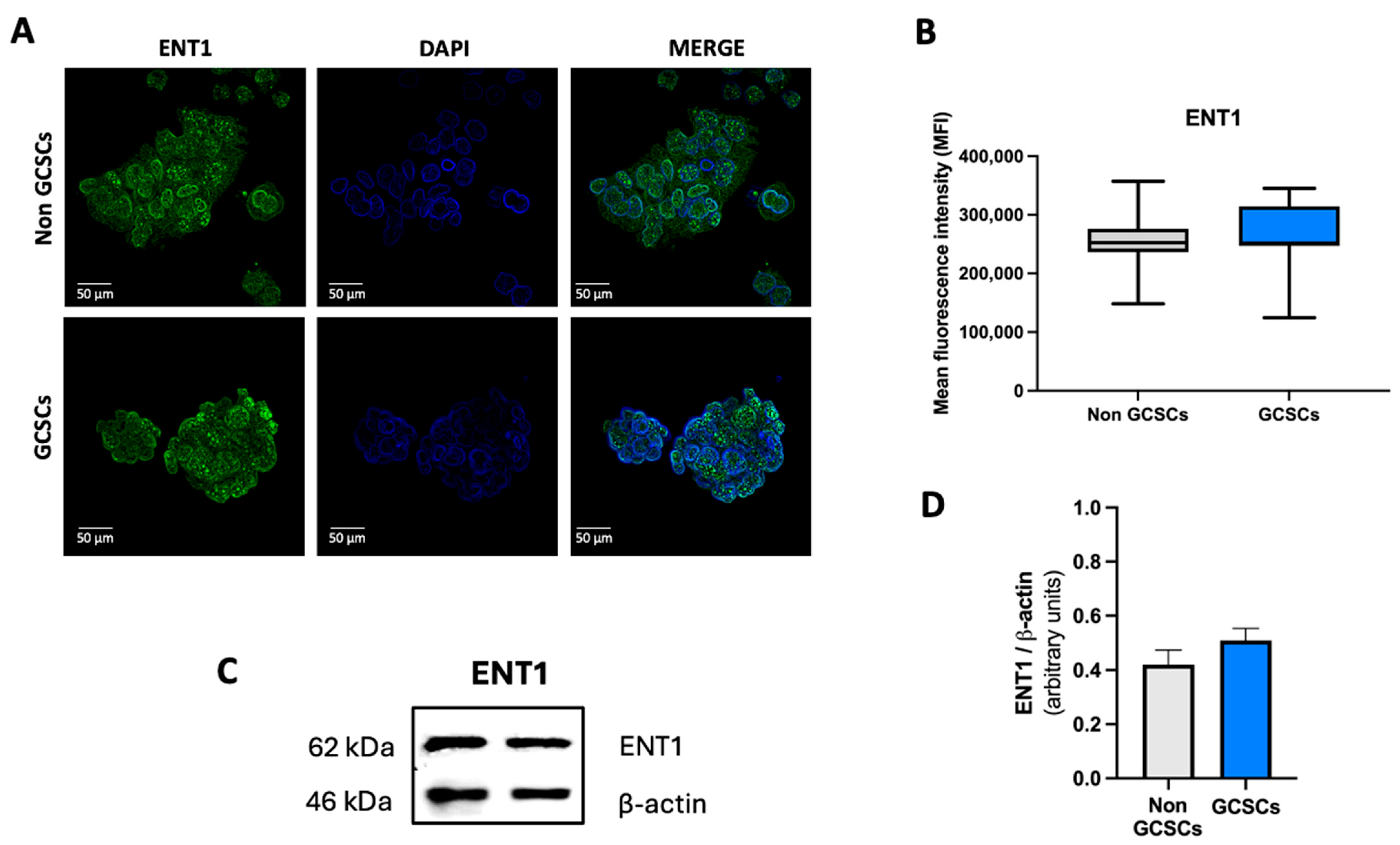
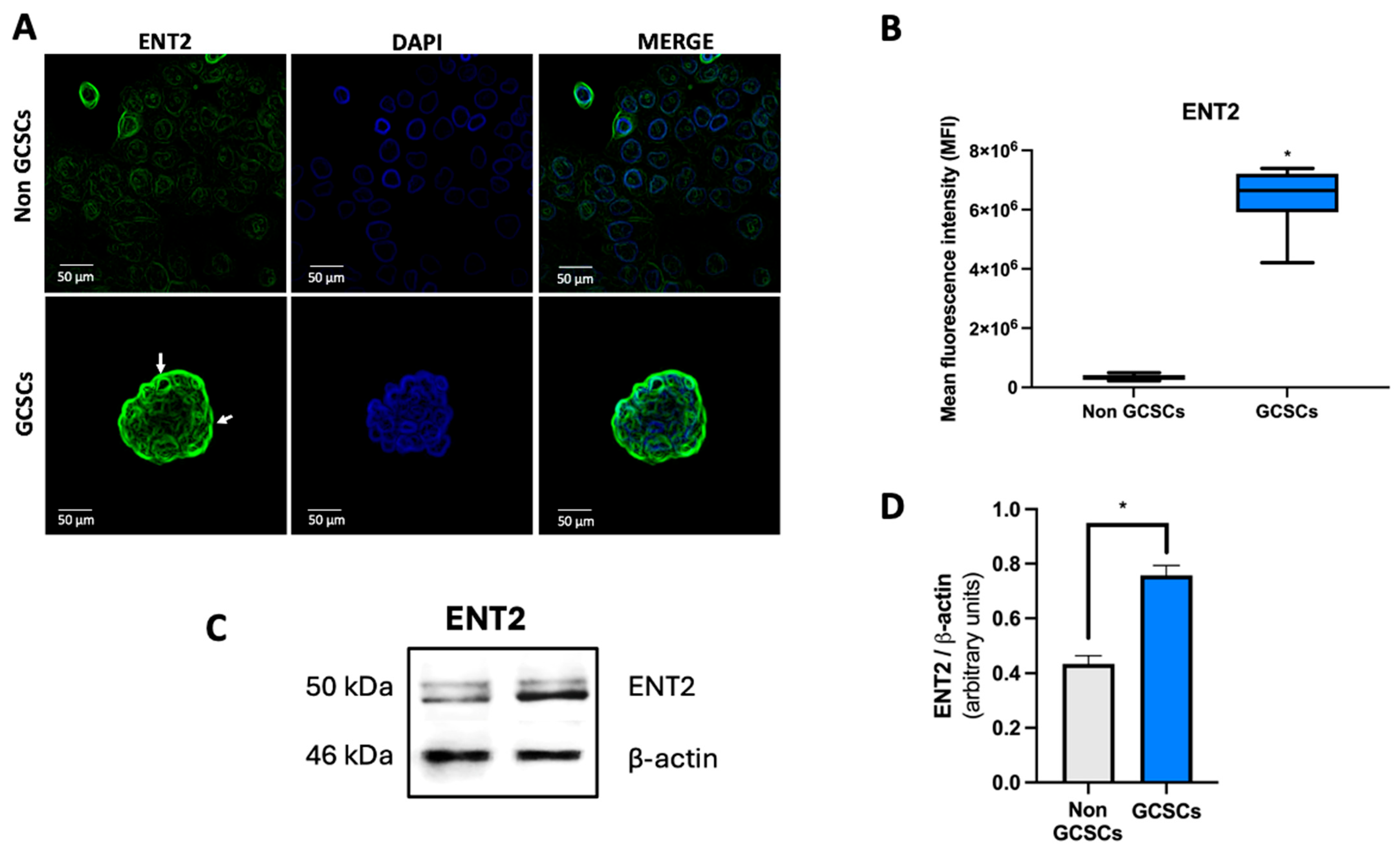
2.4. Gastric Cancer Stem Cells Have a Higher Level of ENT2 than Non-GCSCs of the MKN-74 Cell Line
3. Discussion
4. Materials and Methods
4.1. Pharmacological Agents
4.2. Cell Culture
4.3. Gastric Stem-like Cell Culture
4.4. ATP Quantification
4.5. Adenosine Quantification
4.6. Western Blots
4.7. RNA Extraction and qRT-PCR
4.8. Adenosine Accumulation
4.9. CD73 and PAP Activity
4.10. Immunofluorescence and Image Analysis
4.11. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC | Gastric Cancer |
| CSCs | Cancer Stem Cells |
| GCSCs | Gastric Cancer Stem Cells |
| ATP | Adenosine Triphosphate |
| AMP | Adenosine Monophosphate |
| ENT | Equilibrative Nucleoside Transporter |
| CNT | Concentrative Nucleoside Transporter |
References
- Wu, L.; Jang, S.J.; Shapiro, C.; Fazlollahi, L.; Wang, T.C.; Ryeom, S.W.; Moy, R.H. Diffuse Gastric Cancer: A Comprehensive Review of Molecular Features and Emerging Therapeutics. Target. Oncol. 2024, 19, 845–865. [Google Scholar] [CrossRef] [PubMed]
- Seeneevassen, L.; Zaafour, A.; Sifré, E.; Genevois, C.; Nguyen, T.L.; Pobiedonoscew, Y.; Giese, A.; Guignard, J.; Tiffon, C.; Rousseau, B.; et al. Targeting metastasis-initiating cancer stem cells in gastric cancer with leukaemia inhibitory factor. Cell Death Discov. 2024, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Mokadem, I.; Dijksterhuis, W.P.M.; van Putten, M.; Heuthorst, L.; de Vos-Geelen, J.M.; Haj Mohammad, N.; Nieuwenhuijzen, G.A.P.; van Laarhoven, H.W.M.; Verhoeven, R.H.A. Recurrence after preoperative chemotherapy and surgery for gastric adenocarcinoma: A multicenter study. Gastric Cancer 2019, 22, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Iwu, C.D.; Iwu-Jaja, C.J. Gastric Cancer Epidemiology: Current Trend and Future Direction. Hygiene 2023, 3, 256–268. [Google Scholar] [CrossRef]
- Yang, Y.; Meng, W.-J.; Wang, Z.-Q. The origin of gastric cancer stem cells and their effects on gastric cancer: Novel therapeutic targets for gastric cancer. Front. Oncol. 2022, 12, 960539. [Google Scholar] [CrossRef]
- Chu, X.; Tian, W.; Ning, J.; Xiao, G.; Zhou, Y.; Wang, Z.; Zhai, Z.; Tanzhu, G.; Yang, J.; Zhou, R. Cancer stem cells: Advances in knowledge and implications for cancer therapy. Signal Transduct. Target. Ther. 2024, 9, 170. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, Q.; Guo, H.; Guan, H.; Hong, Z.; Shang, D. Deciphering drug resistance in gastric cancer: Potential mechanisms and future perspectives. Biomed. Pharmacother. 2024, 173, 116310. [Google Scholar] [CrossRef]
- Xia, C.; Yin, S.; To, K.K.W.; Fu, L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol. Cancer 2023, 22, 44. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Buccioni, M. Current Understanding of the Role of Adenosine Receptors in Cancer. Molecules 2024, 29, 3501. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, J.; Wang, J. The Immune Regulatory Role of Adenosine in the Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 14928. [Google Scholar] [CrossRef]
- Peñate, L.; Carrillo-Beltrán, D.; Spichiger, C.; Cuevas-Zhbankova, A.; Torres-Arévalo, Á.; Silva, P.; Richter, H.G.; Ayuso-Sacido, Á.; San Martín, R.; Quezada-Monrás, C. The Impact of A3AR Antagonism on the Differential Expression of Chemoresistance-Related Genes in Glioblastoma Stem-like Cells. Pharmaceuticals 2024, 17, 579. [Google Scholar] [CrossRef]
- Torres, Á.; Erices, J.I.; Sanchez, F.; Ehrenfeld, P.; Turchi, L.; Virolle, T.; Uribe, D.; Niechi, I.; Spichiger, C.; Rocha, J.D.; et al. Extracellular adenosine promotes cell migration/invasion of Glioblastoma Stem-like Cells through A3 Adenosine Receptor activation under hypoxia. Cancer Lett. 2019, 446, 112–122. [Google Scholar] [CrossRef]
- Shukla, S.; Dalai, P.; Agrawal-Rajput, R. Metabolic crosstalk: Extracellular ATP and the tumor microenvironment in cancer progression and therapy. Cell. Signal. 2024, 121, 111281. [Google Scholar] [CrossRef]
- Leone, R.D.; Emens, L.A. Targeting adenosine for cancer immunotherapy. J. Immunother. Cancer 2018, 6, 57. [Google Scholar] [CrossRef]
- Alarcón, S.; Toro, M.D.; Villarreal, C.; Melo, R.; Fernández, R.; Ayuso Sacido, A.; Uribe, D.; San Martín, R.; Quezada, C. Decreased Equilibrative Nucleoside Transporter 1 (ENT1) Activity Contributes to the High Extracellular Adenosine Levels in Mesenchymal Glioblastoma Stem-Like Cells. Cells 2020, 9, 1914. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Pérez-Torras, S. Nucleoside transporter proteins as biomarkers of drug responsiveness and drug targets. Front. Pharmacol. 2015, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, S.; Okumura, T.; Tu, S.; Wang, S.S.W.; Shibata, W.; Vigneshwaran, R.; Gordon, S.A.K.; Shimada, Y.; Wang, T.C. Identification of Gastric Cancer Stem Cells Using the Cell Surface Marker CD44. Stem Cells 2009, 27, 1006–1020. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Lu, H.; Samanta, D.; Salman, S.; Lu, Y.; Semenza, G.L. Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc. Natl. Acad. Sci. USA 2018, 115, E9640–E9648. [Google Scholar] [CrossRef]
- Hu, C.-T.; Lin, C.-F.; Shih, H.-M.; You, R.-I.; Wu, W.-S.; Chen, Y.-C. Blockade of Src signaling prevented stemness gene expression and proliferation of patient-derived gastric cancer stem cells. Tzu Chi Med. J. 2025, 37, 65–71. [Google Scholar] [CrossRef]
- Yu, M.; Fei, B.; Chu, S. Targeting HNRNPA2B1 to Overcome Chemotherapy Resistance in Gastric Cancer Stem Cells: Mechanisms and Therapeutic Potential. J. Biol. Chem. 2025, 301, 108234. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, T.; Yuan, Y.; Li, H.; Shi, W.; Zheng, L.; Li, X.; Zhang, W. Tumor microenvironment of cancer stem cells: Perspectives on cancer stem cell targeting. Genes Dis. 2024, 11, 101043. [Google Scholar] [CrossRef]
- Torres, A.; Vargas, Y.; Uribe, D.; Jaramillo, C.; Gleisner, A.; Salazar-Onfray, F.; López, M.N.; Melo, R.; Oyarzún, C.; Martín, R.S.; et al. Adenosine A3 receptor elicits chemoresistance mediated by multiple resistance-associated protein-1 in human glioblastoma stem-like cells. Oncotarget 2016, 7, 67373–67386. [Google Scholar] [CrossRef]
- Arab, S.; Alizadeh, A.; Asgharzade, S. Tumor-resident adenosine-producing mesenchymal stem cells as a potential target for cancer treatment. Clin. Exp. Med. 2021, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Compagno, M.; Belisario, D.C.; Bracci, C.; Genova, T.; Mussano, F.; Vitale, M.; Horenstein, A.; Malavasi, F.; Ferracini, R.; et al. CD73/Adenosine Pathway Involvement in the Interaction of Non-Small Cell Lung Cancer Stem Cells and Bone Cells in the Pre-Metastatic Niche. Int. J. Mol. Sci. 2022, 23, 5126. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.-D.; Uribe, D.; Delgado, J.; Niechi, I.; Alarcón, S.; Erices, J.I.; Melo, R.; Fernández-Gajardo, R.; Salazar-Onfray, F.; San Martín, R.; et al. A2B Adenosine Receptor Enhances Chemoresistance of Glioblastoma Stem-Like Cells under Hypoxia: New Insights into MRP3 Transporter Function. Int. J. Mol. Sci. 2022, 23, 9022. [Google Scholar] [CrossRef] [PubMed]
- Niechi, I.; Erices, J.I.; Carrillo-Beltrán, D.; Uribe-Ojeda, A.; Torres, Á.; Rocha, J.D.; Uribe, D.; Toro, M.A.; Villalobos-Nova, K.; Gaete-Ramírez, B.; et al. Cancer Stem Cell and Aggressiveness Traits Are Promoted by Stable Endothelin-Converting Enzyme-1c in Glioblastoma Cells. Cells 2023, 12, 506. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Zhao, X.; Xiao, J.; Bi, J.; Li, X.-Y.; Chen, G.; Lu, L. Review immune response of targeting CD39 in cancer. Biomark. Res. 2023, 11, 63. [Google Scholar] [CrossRef]
- Kaplinsky, N.; Williams, K.; Watkins, D.; Adams, M.; Stanbery, L.; Nemunaitis, J. Regulatory role of CD39 and CD73 in tumor immunity. Future Oncol. 2024, 20, 1367–1380. [Google Scholar] [CrossRef]
- Liu, B.; Cao, Y.; Li, Y.; Ma, H.; Yang, M.; Zhang, Q.; Li, G.; Zhang, K.; Wu, Y.; Zhou, Y.; et al. Glioma Stem Cells Upregulate CD39 Expression to Escape Immune Response through SOX2 Modulation. Cancers 2022, 14, 783. [Google Scholar] [CrossRef]
- Reyna-Jeldes, M.; De la Fuente-Ortega, E.; Cerda, D.; Velázquez-Miranda, E.; Pinto, K.; Vázquez-Cuevas, F.G.; Coddou, C. Purinergic P2Y2 and P2X4 Receptors Are Involved in the Epithelial-Mesenchymal Transition and Metastatic Potential of Gastric Cancer Derived Cell Lines. Pharmaceutics 2021, 13, 1234. [Google Scholar] [CrossRef]
- Qian, Y.; Wang, X.; Liu, Y.; Li, Y.; Colvin, R.A.; Tong, L.; Wu, S.; Chen, X. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett. 2014, 351, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Wang, X.; Li, Y.; Cao, Y.; Chen, X. Extracellular ATP a New Player in Cancer Metabolism: NSCLC Cells Internalize ATP In Vitro and In Vivo Using Multiple Endocytic Mechanisms. Mol. Cancer Res. 2016, 14, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, L.; de Andrade Mello, P.; Mao, C.; Near, R.; Csizmadia, E.; Chan, L.L.-Y.; Enjyoji, K.; Gao, W.; Zhao, H.; et al. Glycoengineered anti-CD39 promotes anticancer responses by depleting suppressive cells and inhibiting angiogenesis in tumor models. J. Clin. Investig. 2022, 132, e157431. [Google Scholar] [CrossRef]
- Vignali, P.D.A.; DePeaux, K.; Watson, M.J.; Ye, C.; Ford, B.R.; Lontos, K.; McGaa, N.K.; Scharping, N.E.; Menk, A.V.; Robson, S.C.; et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nat. Immunol. 2023, 24, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Qian, Y.; Evers, M.; Nielsen, C.M.; Chen, X. Cancer Stem Cell Formation Induced and Regulated by Extracellular ATP and Stanniocalcin-1 in Human Lung Cancer Cells and Tumors. Int. J. Mol. Sci. 2022, 23, 14770. [Google Scholar] [CrossRef]
- Cai, X.-Y.; Wang, X.-F.; Li, J.; Dong, J.-N.; Liu, J.-Q.; Li, N.-P.; Yun, B.; Xia, R.-L. Overexpression of CD39 and high tumoral CD39+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 14757–14764. [Google Scholar]
- Shen, Y.; Qiu, Y.; Duan, Z.; Li, Y.; Wang, Y.; Zhang, Y.; Zhu, B.; Yu, X.; Tan, X.; Chen, W.; et al. CD39hi identifies an exhausted tumor-reactive CD8+ T cell population associated with tumor progression in human gastric cancer. Pharmacol. Res. 2024, 202, 107122. [Google Scholar] [CrossRef]
- Shao, L.; Wang, X.; Yu, Q.; Gong, J.; Zhang, X.; Zhou, Y. In lung adenocarcinoma, low expression of the cell surface extracellular nucleotidase CD39 is related to immune infiltration and a poor prognosis. J. Thorac. Dis. 2022, 14, 4938–4950. [Google Scholar] [CrossRef]
- Künzli, B.M.; Berberat, P.O.; Giese, T.; Csizmadia, E.; Kaczmarek, E.; Baker, C.; Halaceli, I.; Büchler, M.W.; Friess, H.; Robson, S.C. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am. J. Physiol. Liver Physiol. 2007, 292, G223–G230. [Google Scholar] [CrossRef]
- Bach, N.; Winzer, R.; Tolosa, E.; Fiedler, W.; Brauneck, F. The Clinical Significance of CD73 in Cancer. Int. J. Mol. Sci. 2023, 24, 11759. [Google Scholar] [CrossRef]
- Li, H.; Xie, P.; Li, P.; Du, Y.; Zhu, J.; Yuan, Y.; Wu, C.; Shi, Y.; Huang, Z.; Wang, X.; et al. CD73/NT5E is a Potential Biomarker for Cancer Prognosis and Immunotherapy for Multiple Types of Cancers. Adv. Biol. 2023, 7, 2200263. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Zhang, J.; Cao, H.; Xiao, G.; Wang, Z.; Zhang, X.; Zhang, N.; Wu, W.; Zhang, H.; Wang, Q.; et al. Identification of CD73 as a Novel Biomarker Encompassing the Tumor Microenvironment, Prognosis, and Therapeutic Responses in Various Cancers. Cancers 2022, 14, 5663. [Google Scholar] [CrossRef] [PubMed]
- Lupia, M.; Angiolini, F.; Bertalot, G.; Freddi, S.; Sachsenmeier, K.F.; Chisci, E.; Kutryb-Zajac, B.; Confalonieri, S.; Smolenski, R.T.; Giovannoni, R.; et al. CD73 Regulates Stemness and Epithelial-Mesenchymal Transition in Ovarian Cancer-Initiating Cells. Stem Cell Rep. 2018, 10, 1412–1425. [Google Scholar] [CrossRef]
- Song, L.; Ye, W.; Cui, Y.; Lu, J.; Zhang, Y.; Ding, N.; Hu, W.; Pei, H.; Yue, Z.; Zhou, G. Ecto-5′-nucleotidase (CD73) is a biomarker for clear cell renal carcinoma stem-like cells. Oncotarget 2017, 8, 31977–31992. [Google Scholar] [CrossRef] [PubMed]
- García-Rocha, R.; Monroy-García, A.; Carrera-Martínez, M.; Hernández-Montes, J.; Don-López, C.A.; Weiss-Steider, B.; Monroy-Mora, K.A.; de los Ángeles Ponce-Chavero, M.; Montesinos-Montesinos, J.J.; Escobar-Sánchez, M.L.; et al. Evidence that cervical cancer cells cultured as tumorspheres maintain high CD73 expression and increase their protumor characteristics through TGF-β production. Cell Biochem. Funct. 2022, 40, 760–772. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, Z.; Cao, Y.; Hu, J.; Min, M. CD73 is a hypoxia-responsive gene and promotes the Warburg effect of human gastric cancer cells dependent on its enzyme activity. J. Cancer 2021, 12, 6372–6382. [Google Scholar] [CrossRef]
- Ju, F.; Atyah, M.M.; Horstmann, N.; Gul, S.; Vago, R.; Bruns, C.J.; Zhao, Y.; Dong, Q.-Z.; Ren, N. Characteristics of the cancer stem cell niche and therapeutic strategies. Stem Cell Res. Ther. 2022, 13, 233. [Google Scholar] [CrossRef]
- Wielgat, P.; Trofimiuk, E.; Czarnomysy, R.; Braszko, J.J.; Car, H. Sialic acids as cellular markers of immunomodulatory action of dexamethasone on glioma cells of different immunogenicity. Mol. Cell. Biochem. 2019, 455, 147–157. [Google Scholar] [CrossRef]
- Antonioli, L.; Yegutkin, G.G.; Pacher, P.; Blandizzi, C.; Haskó, G. Anti-CD73 in Cancer Immunotherapy: Awakening New Opportunities. Trends Cancer 2016, 2, 95–109. [Google Scholar] [CrossRef]
- Alcedo, K.P.; Guerrero, A.; Basrur, V.; Fu, D.; Richardson, M.L.; McLane, J.S.; Tsou, C.; Nesvizhskii, A.I.; Welling, T.H.; Lebrilla, C.B.; et al. Tumor-Selective Altered Glycosylation and Functional Attenuation of CD73 in Human Hepatocellular Carcinoma. Hepatol. Commun. 2019, 3, 1400–1414. [Google Scholar] [CrossRef]
- Beavis, P.A.; Stagg, J.; Darcy, P.K.; Smyth, M.J. CD73: A potent suppressor of antitumor immune responses. Trends Immunol. 2012, 33, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kaur, T.; Weadick, B.; Mace, T.A.; Desai, K.; Odom, H.; Govindarajan, R. Nucleoside transporters and immunosuppressive adenosine signaling in the tumor microenvironment: Potential therapeutic opportunities. Pharmacol. Ther. 2022, 240, 108300. [Google Scholar] [CrossRef]
- Pastor-Anglada, M.; Pérez-Torras, S. Who Is Who in Adenosine Transport. Front. Pharmacol. 2018, 9, 627. [Google Scholar] [CrossRef]
- Nishimura, M.; Naito, S. Tissue-specific mRNA Expression Profiles of Human ATP-binding Cassette and Solute Carrier Transporter Superfamilies. Drug Metab. Pharmacokinet. 2005, 20, 452–477. [Google Scholar] [CrossRef]
- Pennycooke, M.; Chaudary, N.; Shuralyova, I.; Zhang, Y.; Coe, I.R. Differential Expression of Human Nucleoside Transporters in Normal and Tumor Tissue. Biochem. Biophys. Res. Commun. 2001, 280, 951–959. [Google Scholar] [CrossRef]
- Chen, H.-P.; Chen, C.-I.; Liu, K.-W.; Chen, T.-J.; Tian, Y.-F.; Kuo, Y.-H.; Li, W.-S.; Tsai, H.-H.; Wu, L.-C.; Yeh, C.-F.; et al. High SLC28A2 expression endows an inferior survival for rectal cancer patients managed by neoadjuvant CCRT. Pathol. Res. Pract. 2022, 239, 154158. [Google Scholar] [CrossRef]
- Ye, C.; Han, K.; Lei, J.; Zeng, K.; Zeng, S.; Ju, H.; Yu, L. Inhibition of histone deacetylase 7 reverses concentrative nucleoside transporter 2 repression in colorectal cancer by up-regulating histone acetylation state. Br. J. Pharmacol. 2018, 175, 4209–4217. [Google Scholar] [CrossRef]
- Naes, S.M.; Ab-Rahim, S.; Mazlan, M.; Rahman, A.A.; Zhang, D.M. Equilibrative Nucleoside Transporter 2: Properties and Physiological Roles. Biomed Res. Int. 2020, 2020, 5197626. [Google Scholar] [CrossRef]
- Tandio, D.; Vilas, G.; Hammond, J.R. Bidirectional transport of 2-chloroadenosine by equilibrative nucleoside transporter 4 (hENT4): Evidence for allosteric kinetics at acidic pH. Sci. Rep. 2019, 9, 13555. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, K.; Nakagawa, F.; Kobunai, T.; Takechi, T. Trifluridine/tipiracil overcomes the resistance of human gastric 5-fluorouracil-refractory cells with high thymidylate synthase expression. Oncotarget 2018, 9, 13438–13450. [Google Scholar] [CrossRef] [PubMed]
- Spratlin, J.; Sangha, R.; Glubrecht, D.; Dabbagh, L.; Young, J.D.; Dumontet, C.; Cass, C.; Lai, R.; Mackey, J.R. The Absence of Human Equilibrative Nucleoside Transporter 1 Is Associated with Reduced Survival in Patients With Gemcitabine-Treated Pancreas Adenocarcinoma. Clin. Cancer Res. 2004, 10, 6956–6961. [Google Scholar] [CrossRef] [PubMed]
- Altaweraqi, R.A.; Yao, S.Y.; Smith, K.M.; Cass, C.E.; Young, J.D. HPLC reveals novel features of nucleoside and nucleobase homeostasis, nucleoside metabolism and nucleoside transport. Biochim. Biophys. Acta (BBA)—Biomembr. 2020, 1862, 183247. [Google Scholar] [CrossRef]
- Naes, S.M.; Ab-Rahim, S.; Mazlan, M.; Hashim, N.A.A.; Rahman, A.A. Increased ENT2 expression and its association with altered purine metabolism in cell lines derived from different stages of colorectal cancer. Exp. Ther. Med. 2023, 25, 212. [Google Scholar] [CrossRef]
- Iwasa, S.; Nakajima, T.E.; Nakamura, K.; Takashima, A.; Kato, K.; Hamaguchi, T.; Yamada, Y.; Shimada, Y. First-line fluorouracil-based chemotherapy for patients with severe peritoneal disseminated gastric cancer. Gastric Cancer 2012, 15, 21–26. [Google Scholar] [CrossRef]
- Digklia, A.; Wagner, A.D. Advanced gastric cancer: Current treatment landscape and future perspectives. World J. Gastroenterol. 2016, 22, 2403–2414. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) resistance and the new strategy to enhance the sensitivity against cancer: Implication of DNA repair inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Anglada, M.; Pérez-Torras, S. Emerging Roles of Nucleoside Transporters. Front. Pharmacol. 2018, 9, 606. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.S.; Zhang, Y.; López Quiñones, A.J.; Hu, T.; Singh, D.K.; Stevens, J.; Prasad, B.; Park, J.R.; Wang, J. The Plasma Membrane Monoamine Transporter is Highly Expressed in Neuroblastoma and Functions as an mIBG Transporter. J. Pharmacol. Exp. Ther. 2023, 387, 239–248. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdivia, S.; Añazco, C.; Riquelme, C.; Carrasco, M.C.; Alarcón, A.; Alarcón, S. Extracellular Adenosine in Gastric Cancer: The Role of GCSCs. Int. J. Mol. Sci. 2025, 26, 7594. https://doi.org/10.3390/ijms26157594
Valdivia S, Añazco C, Riquelme C, Carrasco MC, Alarcón A, Alarcón S. Extracellular Adenosine in Gastric Cancer: The Role of GCSCs. International Journal of Molecular Sciences. 2025; 26(15):7594. https://doi.org/10.3390/ijms26157594
Chicago/Turabian StyleValdivia, Sharin, Carolina Añazco, Camila Riquelme, María Constanza Carrasco, Andrés Alarcón, and Sebastián Alarcón. 2025. "Extracellular Adenosine in Gastric Cancer: The Role of GCSCs" International Journal of Molecular Sciences 26, no. 15: 7594. https://doi.org/10.3390/ijms26157594
APA StyleValdivia, S., Añazco, C., Riquelme, C., Carrasco, M. C., Alarcón, A., & Alarcón, S. (2025). Extracellular Adenosine in Gastric Cancer: The Role of GCSCs. International Journal of Molecular Sciences, 26(15), 7594. https://doi.org/10.3390/ijms26157594






