The Effect of Intracellular Calcium Buffer Bapta on Epileptiform Activity of Hippocampal Neurons
Abstract
1. Introduction
2. Results
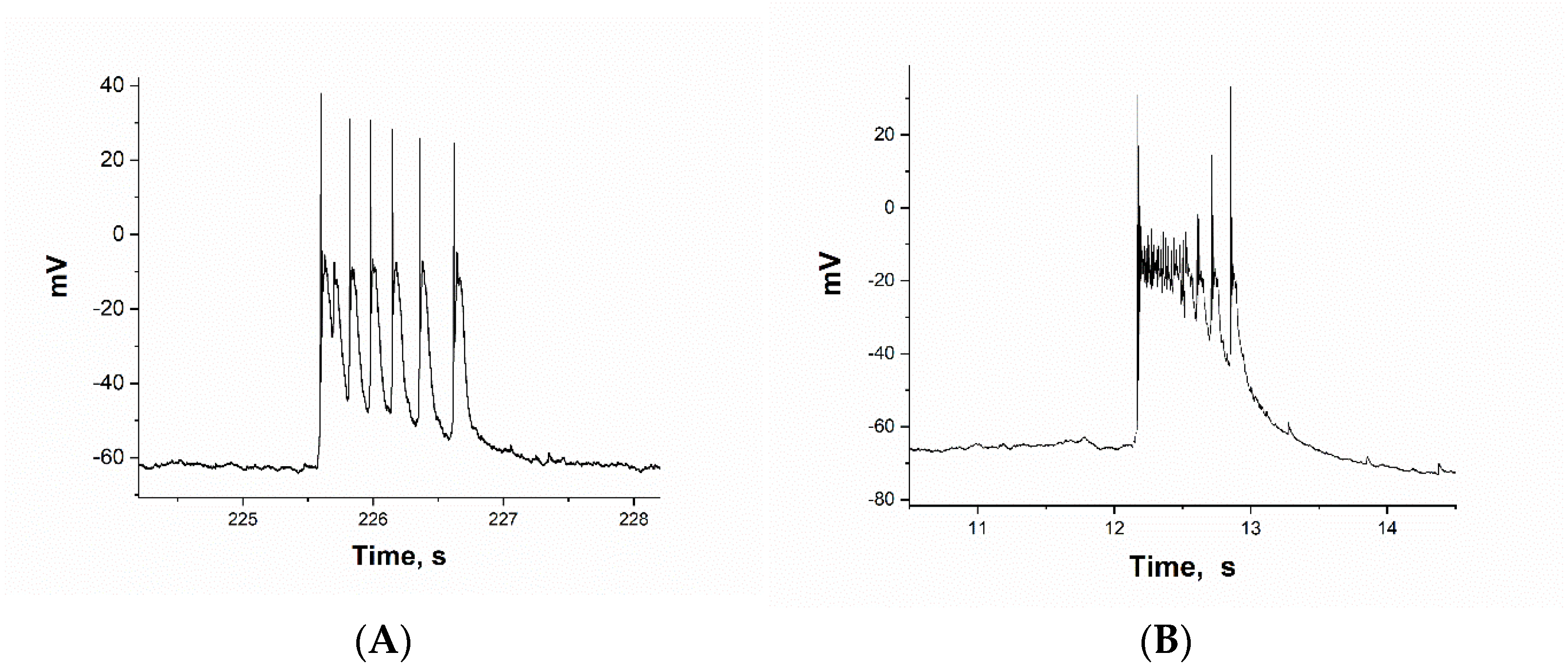
2.1. Selection of Neurons. ATPA-Sensitive GABAergic Neurons
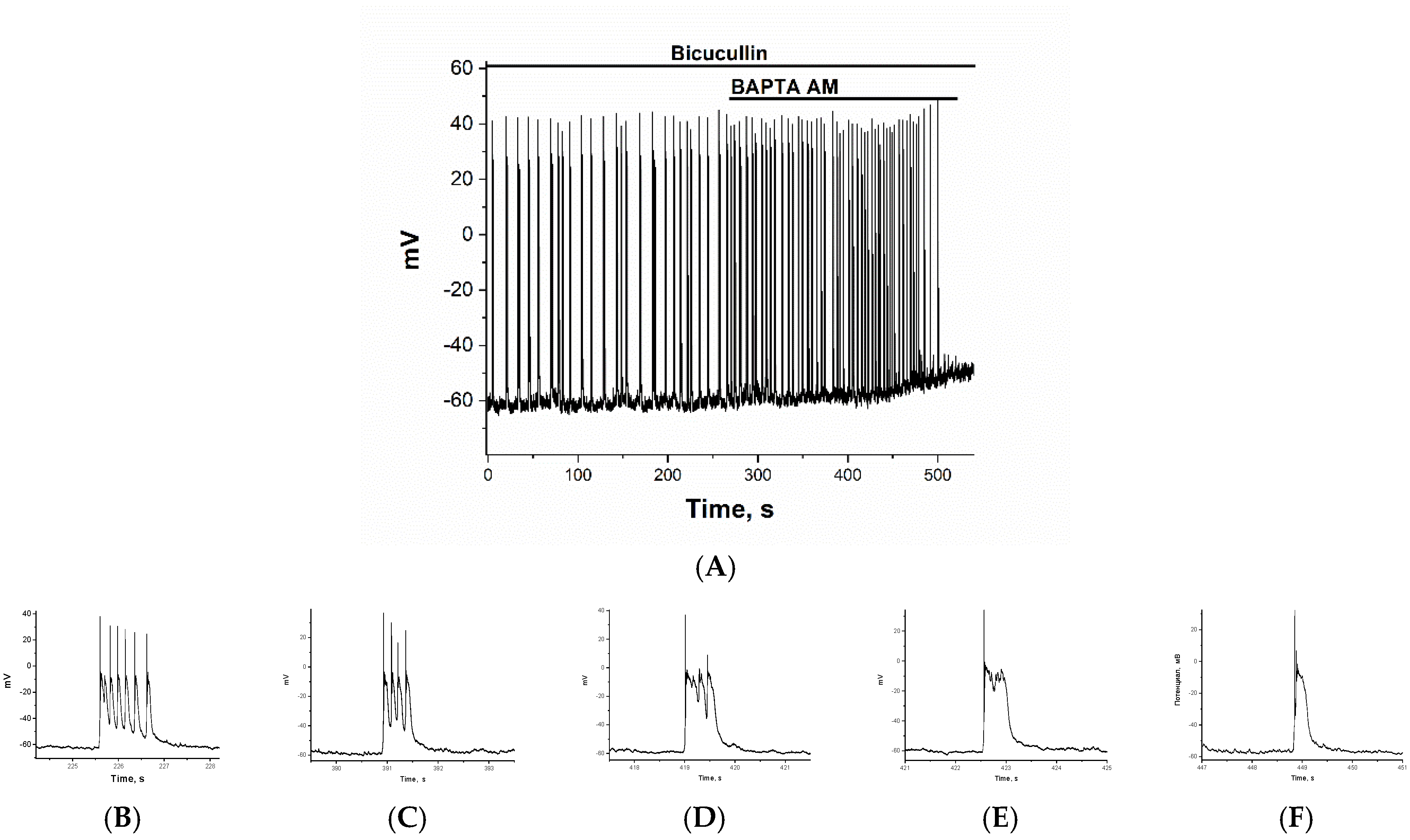
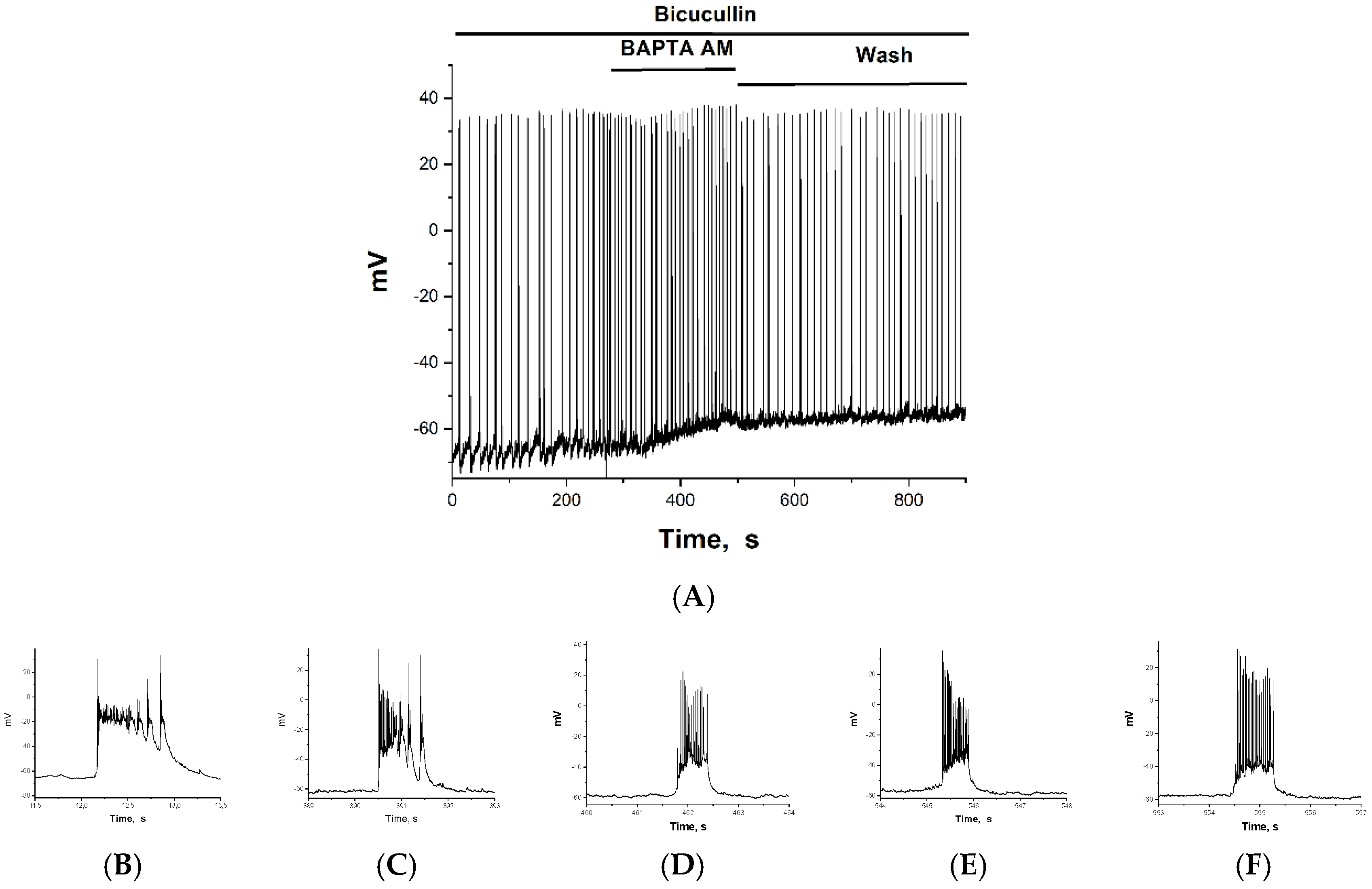
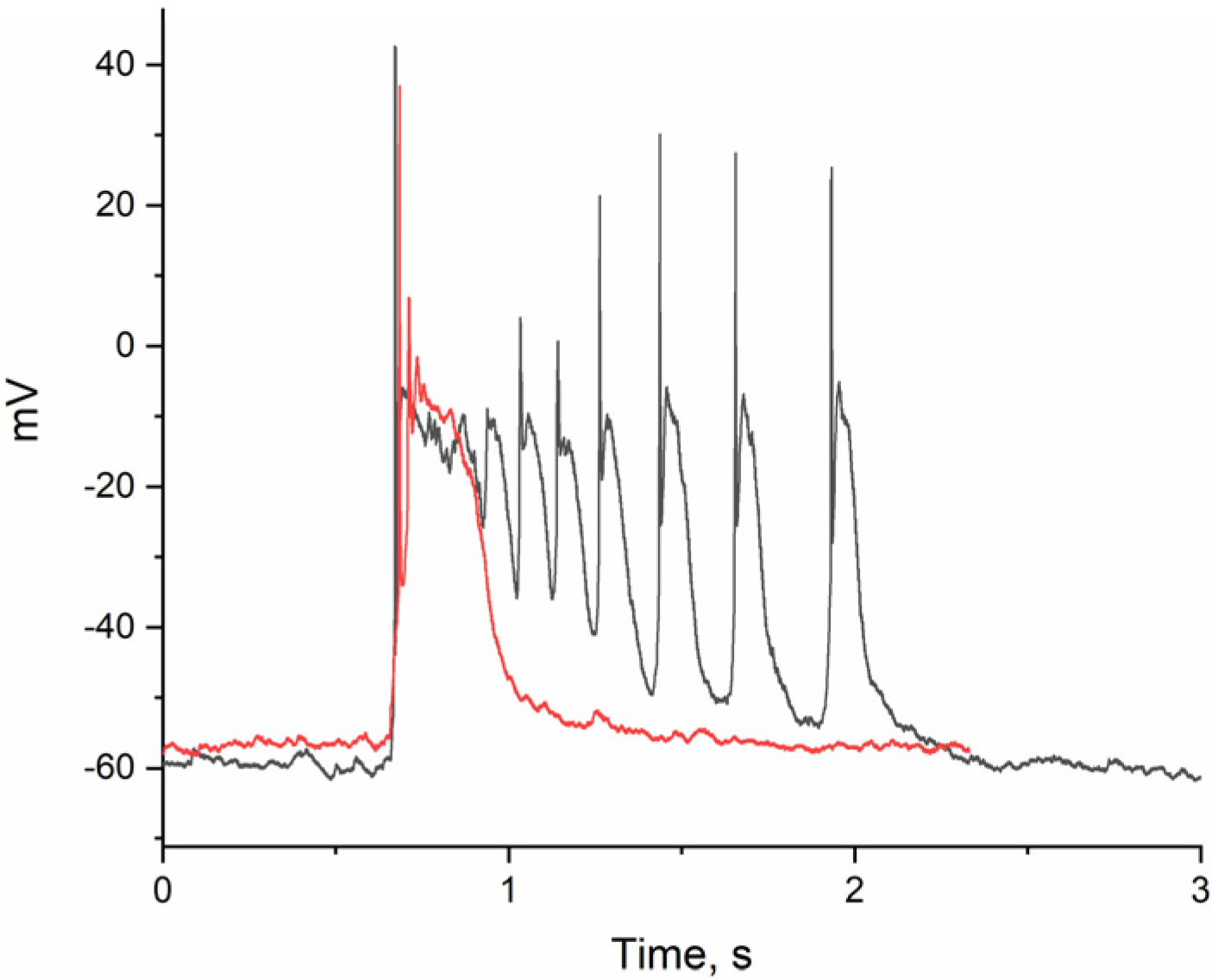
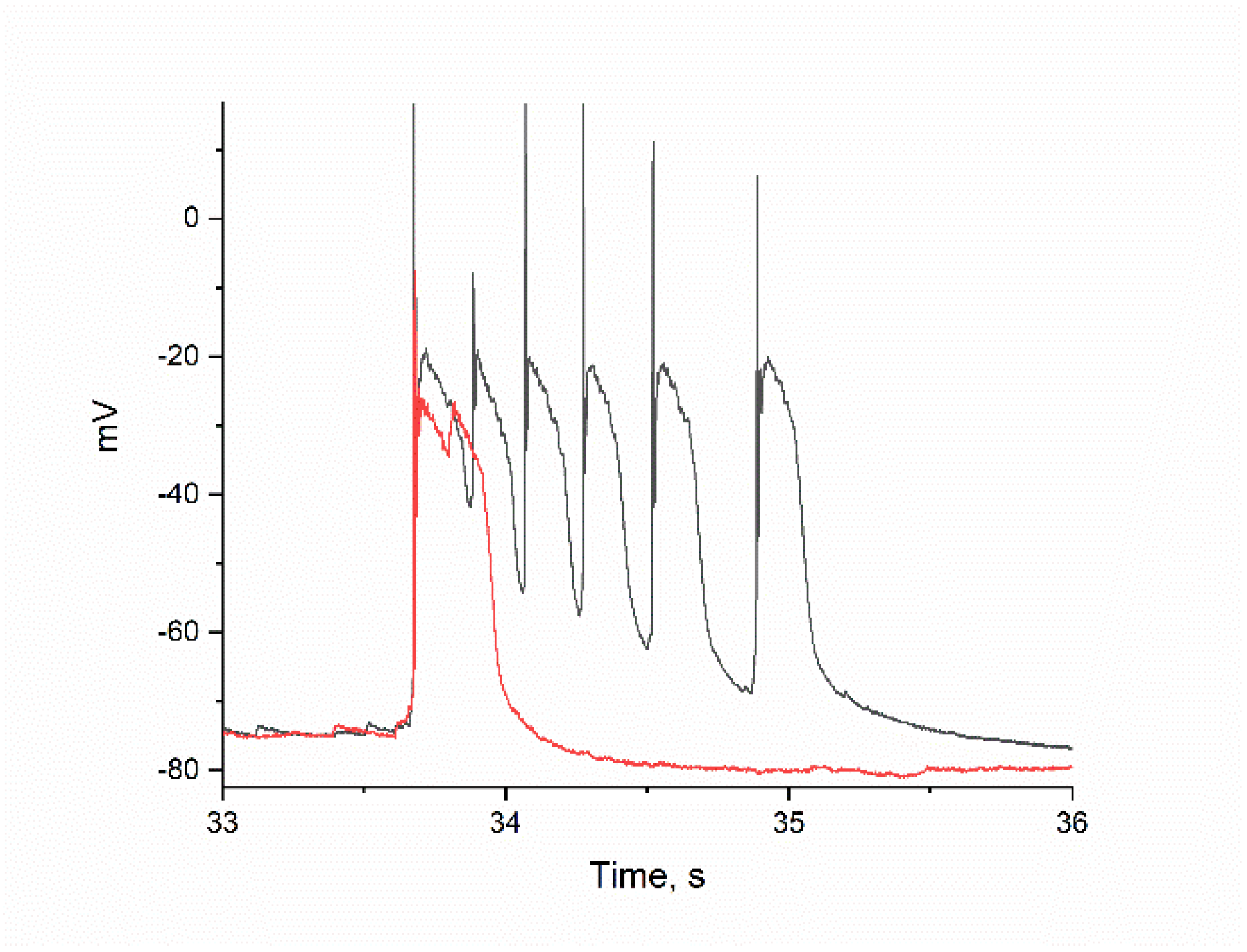
2.2. The Effect of BAPTA on PDS Clusters During Epileptiform Neuronal Activity
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Fluorescent [Ca2+]i Measurements
4.3. Electrophysiological Measurements
4.4. Reagents
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAPTA | 1,2-bis (o-aminophenoxy) ethane-N,N,N′,N’-tetraacetic acid) selective Ca2+ chelator |
| AD | Alzheimer’s disease |
| PD | Parkinson disease |
| GABA | γ-aminobutyric acid |
| DIV | days in vitro |
| AP | action potential |
| PDS | paroxysmal depolarization shift |
| ATPA | calcium-permeable kainate receptor agonist, (RS)-2-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid |
| CBPs | calcium-binding proteins |
| CaCaM | Calcium–Calmodulin complex |
| Kv7 | potassium channels |
References
- Teplov, I.Y.; Zinchenko, V.P.; Kosenkov, A.M.; Gaidin, S.G.; Nenov, M.N.; Sergeev, A.I. Involvement of NMDA and GABA(A) receptors in modulation of spontaneous activity in hippocampal culture: Interrelations between burst firing and intracellular calcium signal. Biochem. Biophys. Res. Commun. 2021, 553, 99–106. [Google Scholar] [CrossRef]
- Kubista, H.; Boehm, S.; Hotka, M. The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond. Int. J. Mol. Sci. 2019, 20, 577. [Google Scholar] [CrossRef]
- Kuijlaars, J.; Oyelami, T.; Diels, A.; Rohrbacher, J.; Versweyveld, S.; Meneghello, G.; Tuefferd, M.; Verstraelen, P.; Detrez, J.R.; Verschuuren, M.; et al. Sustained synchronized neuronal network activity in a human astrocyte co-culture system. Sci. Rep. 2016, 6, 36529. [Google Scholar] [CrossRef]
- Mohajerani, M.H.; Cherubini, E. Role of giant depolarizing potentials in shaping synaptic currents in the developing hippocampus. Crit. Rev. Neurobiol. 2006, 18, 13–23. [Google Scholar] [CrossRef]
- Mohajerani, M.H.; Sivakumaran, S.; Zacchi, P.; Aguilera, P.; Cherubini, E. Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3 CA1 connections in the hippocampus. Proc. Natl. Acad. Sci. USA 2007, 104, 13176–13181. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, P.; Zou, Q.; Liang, J.; Chen, Y.; Cai, Y.; Li, S.; Li, J.; Su, J.; Li, Q. Status of epilepsy in the tropics: An overlooked perspective. Epilepsia Open 2023, 8, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Polymeropoulos, M.H.; Higgins, J.J.; Golbe, L.I.; Johnson, W.G.; Ide, S.E.; Di Iorio, G.; Sanges, G.; Stenroos, E.S.; Pho, L.T.; Schaffer, A.A.; et al. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science 1996, 274, 1197–1199. [Google Scholar] [CrossRef] [PubMed]
- Zaltieri, M.; Longhena, F.; Pizzi, M.; Missale, C.; Spano, P.; Bellucci, A. Mitochondrial Dysfunction and α-Synuclein Synaptic Pathology in Parkinson’s Disease: Who’s on First? Park. Dis. 2015, 2015, 108029. [Google Scholar] [CrossRef] [PubMed]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Petrović, G. Faktori rizika u pojavi cerebrovaskularnog insulta [Risk factors for development of cerebrovascular stroke]. Med. Pregl. 2000, 53, 207–214. [Google Scholar]
- Ngugi, A.K.; Bottomley, C.; Kleinschmidt, I.; Sander, J.W.; Newton, C.R. Estimation of the burden of active and life-time epilepsy: A meta-analytic approach. Epilepsia 2010, 51, 883–890. [Google Scholar] [CrossRef]
- Bacci, A.; Verderio, C.; Pravettoni, E.; Matteoli, M. Synaptic and intrinsic mechanisms shape synchronous oscillations in hippocampal neurons in culture. Eur. J. Neurosci. 1999, 11, 389–397. [Google Scholar] [CrossRef]
- Yáñez, M.; Gil-Longo, J.; Campos-Toimil, M. Calcium binding proteins. Adv. Exp. Med. Biol. 2012, 740, 461–482. [Google Scholar]
- Carapeto, A.P.; Marcuello, C.; Faísca, P.F.N.; Rodrigues, M.S. Morphological and Biophysical Study of S100A9 Protein Fibrils by Atomic Force Microscopy Imaging and Nanomechanical Analysis. Biomolecules 2024, 14, 1091. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, Y.; Cheng, J.; Tao, J. Ion channels in epilepsy: Blasting fuse for neuronal Hyperexcitability. In Epilepsy—Advances in Diagnosis and Therapy; Al-Zwaini, I.J., Albadri, B.A.-H.M., Eds.; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Zinchenko, V.P.; Turovsky, E.A.; Turovskaya, M.V.; Berezhnov, A.V.; Sergeev, A.I.; Dynnik, V.V. NAD causes dissociation of neural networks into subpopulations of neurons by inhibiting the network synchronous hyperactivity evoked by ammonium ions. Biochem. Moscow Suppl. Ser. A 2016, 10, 118–125. [Google Scholar] [CrossRef]
- Schwaller, B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010, 2, a004051. [Google Scholar] [CrossRef]
- Lukas, W.; Jones, K.A. Cortical neurons containing calretinin are selectively resistant to calcium overload and excitotoxicity in vitro. Neuroscience 1994, 61, 307–316. [Google Scholar] [CrossRef]
- Kang, K.R.; Kim, J.; Ryu, B.; Lee, S.G.; Oh, M.S.; Baek, J.; Ren, X.; Canavero, S.; Kim, C.Y.; Chung, H.M. BAPTA, a calcium chelator, neuroprotects injured neurons in vitro and promotes motor recovery after spinal cord transection in vivo. CNS Neurosci. Ther. 2021, 27, 919–929. [Google Scholar] [CrossRef]
- Laryushkin, D.P.; Maiorov, S.A.; Zinchenko, V.P.; Mal’tseva, V.N.; Gaidin, S.G.; Kosenkov, A.M. Of the Mechanisms of Paroxysmal Depolarization Shifts: Generation and Maintenance of Bicuculline- Induced Paroxysmal Activity in Rat Hippocampal Cell Cultures. Int. J. Mol. Sci. 2023, 24, 10991. [Google Scholar] [CrossRef]
- Zinchenko, V.P.; Kosenkov, A.M.; Gaidin, S.G.; Sergeev, A.I.; Dolgacheva, L.P.; Tuleukhanov, S.T. Properties of GABAergic Neurons Containing Calcium-Permeable Kainate and AMPA-Receptors. Life 2021, 11, 1309. [Google Scholar] [CrossRef]
- Maiorov, S.A.; Kairat, B.K.; Berezhnov, A.V.; Zinchenko, V.P.; Gaidin, S.G.; Kosenkov, A.M. Peculiarities of ion homeostasis in neurons containing calcium-permeable AMPA receptors. Arch. Biochem. Biophys. 2024, 754, 109951. [Google Scholar] [CrossRef]
- Gaidin, S.G.; Kosenkov, A.M.; Zinchenko, V.P.; Kairat, B.K.; Malibayeva, A.E.; Tuleukhanov, S.T. Identification of Neurons Containing Calcium-Permeable AMPA and Kainate Receptors Using Ca2+ Imaging. Bio Protocl 2025, 15, e5199. [Google Scholar] [CrossRef]
- Maiorov, S.A.; Zinchenko, V.P.; Gaidin, S.G.; Kosenkov, A.M. Potential mechanism of GABA secretion in response to the activation of GluK1-containing kainate receptors. Neurosci. Res. 2021, 171, 27–33. [Google Scholar] [CrossRef]
- Sneyers, F.; Speelman-Rooms, F.; Verhelst, S.H.L.; Bootman, M.D.; Bultynck, G. Cellular effects of BAPTA: Are they only about Ca2+ chelation? Biochim. Biophys. Acta Mol. Cell Res. 2023, 1871, 119589. [Google Scholar] [CrossRef]
- Tang, Q.; Jin, M.W.; Xiang, J.Z.; Dong, M.Q.; Sun, H.Y.; Lau, C.P.; Li, G.R. The membrane permeable calcium chelator BAPTA-AM directly blocks human ether a-go-go-related gene potassium channels stably expressed in HEK 293 cells. Biochem. Pharmacol. 2007, 74, 1596–1607. [Google Scholar] [CrossRef]
- Zinchenko, V.P.; Teplov, I.Y.; Kosenkov, A.M.; Gaidin, S.G.; Kairat, B.K.; Tuleukhanov, S.T. Participation of calcium-permeable AMPA receptors in the regulation of epileptiform activity of hippocampal neurons. Front. Synaptic Neurosci. 2024, 16, 1349984. [Google Scholar] [CrossRef]
- Gomis-Perez, C.; Soldovieri, M.V.; Malo, C.; Ambrosino, P.; Taglialatela, M.; Areso, P.; Villarroel, A. Differential Regulation of PI(4,5)P2 Sensitivity of Kv7.2 and Kv7.3 Channels by Calmodulin. Front. Mol. Neurosci. 2017, 10, 117. [Google Scholar] [CrossRef]
- Yang, X.; Chen, S.; Zhang, S.; Shi, S.; Zong, R.; Gao, Y.; Guan, B.; Gamper, N.; Gao, H. Intracellular zinc protects Kv7 K+ channels from Ca2+/calmodulin-mediated inhibition. J. Biol. Chem. 2023, 299, 102819. [Google Scholar] [CrossRef]
- Watkins, C.S.; Mathie, A. Effects on K+ currents in rat cerebellar granule neurones of a membrane-permeable analogue of the calcium chelator BAPTA. Br. J. Pharmacol. 1996, 118, 1772–1778. [Google Scholar] [CrossRef]
- Maiorov, S.A.; Laryushkin, D.P.; Kritskaya, K.A.; Zinchenko, V.P.; Gaidin, S.G.; Kosenkov, A.M. The Role of Ion Channels and Intracellular Signaling Cascades in the Inhibitory Action of WIN 55,212-2 upon Hyperexcitation. Brain Sci. 2024, 14, 668. [Google Scholar] [CrossRef]
- Greene, D.L.; Hoshi, N. Modulation of Kv7 channels and excitability in the brain. Cell. Mol. Life Sci. 2017, 74, 495–508. [Google Scholar] [CrossRef]
- Zaydman, M.A.; Cui, J. PIP2 regulation of KCNQ channels: Biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front. Physiol. 2014, 5, 195. [Google Scholar] [CrossRef]
- Taylor, K.C.; Sanders, C.R. Regulation of KCNQ/Kv7 family voltage-gated K+ channels by lipids. Biochim. Biophys. Acta Biomembr. 2017, 1859, 586–597. [Google Scholar] [CrossRef]
- van der Horst, J.; Greenwood, I.A.; Jepps, T.A. Cyclic AMP-Dependent Regulation of Kv7 Voltage-Gated Potassium Channels. Front. Physiol. 2020, 11, 727. [Google Scholar] [CrossRef] [PubMed]
- Hladnik, A.; Džaja, D.; Darmopil, S.; Jovanov-Milošević, N.; Petanjek, Z. Spatio-temporal extension in site of origin for cortical calretinin neurons in primates. Front. Neuroanat. 2014, 8, 50. [Google Scholar] [CrossRef]
- Gaidin, S.G.; Maiorov, S.A.; Laryushkin, D.P.; Zinchenko, V.P.; Kosenkov, A.M. A novel approach for vital visualization and studying of neurons containing Ca2+ -permeable AMPA receptors. J. Neurochem. 2023, 164, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Laryushkin, D.P.; Maiorov, S.A.; Zinchenko, V.P.; Gaidin, S.G.; Kosenkov, A.M. Role of L-Type Voltage-Gated Calcium Channels in Epileptiform Activity of Neurons. Int. J. Mol. Sci. 2021, 22, 10342. [Google Scholar] [CrossRef] [PubMed]
- Gaidin, S.G.; Zinchenko, V.P.; Kosenkov, A.M. Mechanisms of ammonium-induced neurotoxicity. Neuroprotective effect of alpha-2 adrenergic agonists. Arch. Biochem. Biophys. 2020, 693, 108593. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zinchenko, V.P.; Teplov, I.Y.; Tyurin, F.V.; Malibayeva, A.E.; Kairat, B.K.; Tuleukhanov, S.T. The Effect of Intracellular Calcium Buffer Bapta on Epileptiform Activity of Hippocampal Neurons. Int. J. Mol. Sci. 2025, 26, 7596. https://doi.org/10.3390/ijms26157596
Zinchenko VP, Teplov IY, Tyurin FV, Malibayeva AE, Kairat BK, Tuleukhanov ST. The Effect of Intracellular Calcium Buffer Bapta on Epileptiform Activity of Hippocampal Neurons. International Journal of Molecular Sciences. 2025; 26(15):7596. https://doi.org/10.3390/ijms26157596
Chicago/Turabian StyleZinchenko, V. P., I. Yu. Teplov, F. V. Tyurin, A. E. Malibayeva, B. K. Kairat, and S. T. Tuleukhanov. 2025. "The Effect of Intracellular Calcium Buffer Bapta on Epileptiform Activity of Hippocampal Neurons" International Journal of Molecular Sciences 26, no. 15: 7596. https://doi.org/10.3390/ijms26157596
APA StyleZinchenko, V. P., Teplov, I. Y., Tyurin, F. V., Malibayeva, A. E., Kairat, B. K., & Tuleukhanov, S. T. (2025). The Effect of Intracellular Calcium Buffer Bapta on Epileptiform Activity of Hippocampal Neurons. International Journal of Molecular Sciences, 26(15), 7596. https://doi.org/10.3390/ijms26157596



