Noninvasive Assessment of Arterial Wall and Soluble ST2 in Patients with Type 2 Diabetes and Coronary Artery Disease
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of Patients
2.2. Assessment of Epicardial Coronary Flow and Myocardial Perfusion
2.3. Results of Measurements of Vascular Wall Condition Markers
2.4. Results of Biochemical Parameter Evaluation
2.5. The Relationship Between Biochemical Parameters and Markers Assessing the Condition of the Vascular Wall
2.6. The Influence of Sex on the Obtained Results
3. Discussion
4. Materials and Methods
4.1. Clinical Evaluation
4.2. Analyzed Parameters
4.2.1. sST2
4.2.2. Biochemical Tests
4.3. Coronary Angiography and Angiogram Analysis
4.4. Blood Pressure Assessment
4.5. ABI Assessment
4.6. Arterial Stiffness Assessment
4.7. Endothelial Function
4.8. Statistical Analysis
5. Conclusions
6. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABI | Ankle–brachial index |
| AGEs | Advanced glycation endproducts |
| ARIC | Atherosclerosis Risk in Communities |
| AS | Arterial stiffness |
| baPWV | Brachial ankle pulse wave velocity |
| CAD | Coronary artery disease |
| cfPWV | Carotid–femoral pulse wave velocity |
| crPWV | Carotid–radial pulse wave velocity |
| cTFC | Corrected TIMI frame count |
| CVD | Cardiovascular disease |
| Cx | Circumflex artery |
| EVA | Early vascular aging |
| faPWV | Femoral ankle pulse wave velocity |
| FHS | Framingham Heart Study |
| FLASH | Fluoroscopy-assisted scoring of myocardial hypoperfusion |
| Il33 | Interleukin 33 |
| LAD | Left anterior descending artery |
| MBG | Myocardial blush grade |
| MESA | Multi-Ethnic Study of Atherosclerosis |
| PAD | Peripheral artery disease |
| QuBE | Quantitative blush evaluator |
| RCA | Right coronary artery |
| RHI | Reactive hyperemia index |
| RIS | Radiology Information System |
| sST2 | Soluble suppression of tumorgenicity 2 |
| TGF-β | Transforming growth factor |
| TIMI | Thrombolysis in myocardial infarction |
| T2DM | Type 2 diabetes mellitus |
| VSCM | Vascular smooth muscle cell |
References
- Domingueti, C.P.; Dusse, L.M.S.A.; Carvalho, M.D.G.; De Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes Mellitus: The Linkage between Oxidative Stress, Inflammation, Hypercoagulability and Vascular Complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Eringa, E.C.; Serne, E.H.; Meijer, R.I.; Schalkwijk, C.G.; Houben, A.J.H.M.; Stehouwer, C.D.A.; Smulders, Y.M.; Van Hinsbergh, V.W.M. Endothelial Dysfunction in (Pre)Diabetes: Characteristics, Causative Mechanisms and Pathogenic Role in Type 2 Diabetes. Rev. Endocr. Metab. Disord. 2013, 14, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rask-Madsen, C.; King, G.L. Vascular Complications of Diabetes: Mechanisms of Injury and Protective Factors. Cell Metab. 2013, 17, 20–33. [Google Scholar] [CrossRef]
- Ergul, A. Endothelin-1 and Diabetic Complications: Focus on the Vasculature. Pharmacol. Res. 2011, 63, 477–482. [Google Scholar] [CrossRef]
- Stirban, A.; Gawlowski, T.; Roden, M. Vascular Effects of Advanced Glycation Endproducts: Clinical Effects and Molecular Mechanisms. Mol. Metab. 2014, 3, 94–108. [Google Scholar] [CrossRef]
- Zieman, S.J.; Kass, D.A. Advanced Glycation Endproduct Crosslinking in the Cardiovascular System Potential Therapeutic Target for Cardiovascular Disease. Drugs 2004, 64, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Łoboz-Rudnicka, M.; Kruszyńska, E.; Sołtowska, A.; Łoboz-Grudzień, K.; Jaroch, J. Sztywność Naczyń: Patofizjologia, Implikacje Kliniczne, Metody Oceny. Folia Cardiol. 2022, 17, 243–250. [Google Scholar] [CrossRef]
- Husmann, M.; Jacomella, V.; Thalhammer, C.; Amann-Vesti, B.R. Markers of Arterial Stiffness in Peripheral Arte-rial Disease. Vasa-Eur. J. Vasc. Med. 2015, 44, 341–348. [Google Scholar]
- Muhammad, I.F.; Borné, Y.; Östling, G.; Kennbäck, C.; Gottsäter, M.; Persson, M.; Nilsson, P.M.; Engström, G. Arterial Stiffness and Incidence of Diabetes: A Population-Based Cohort Study. Diabetes Care 2017, 40, 1739–1745. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Laurent, S.; Cunha, P.G.; Olsen, M.H.; Rietzschel, E.; Franco, O.H.; Ryliškyte, L.; Strazhesko, I.; Vlachopoulos, C.; Chen, C.H.; et al. Characteristics of Healthy Vascular Ageing in Pooled Population-Based Cohort Studies: The Global Metabolic Syndrome and Artery Research Consortium. J. Hypertens. 2018, 36, 2340–2349. [Google Scholar] [CrossRef]
- Cuende, J.I.; Cuende, N.; Calaveras-Lagartos, J. How to Calculate Vascular Age with the SCORE Project Scales: A New Method of Cardiovascular Risk Evaluation. Eur. Heart J. 2010, 31, 2351–2358. [Google Scholar] [CrossRef]
- Gao, L.; Lu, D.; Xia, G.; Zhang, H. The Relationship between Arterial Stiffness Index and Coronary Heart Disease and Its Severity. BMC Cardiovasc. Disord. 2021, 21, 527. [Google Scholar] [CrossRef]
- Yannoutsos, A.; Ahouah, M.; Tubiana, C.D.; Topouchian, J.; Safar, M.E.; Blacher, J. Aortic Stiffness Improves the Prediction of Both Diagnosis and Severity of Coronary Artery Disease. Hypertens. Res. 2018, 41, 118–125. [Google Scholar] [CrossRef]
- Chiha, J.; Mitchell, P.; Gopinath, B.; Burlutsky, G.; Plant, A.; Kovoor, P.; Thiagalingam, A. Prediction of Coronary Artery Disease Extent and Severity Using Pulse Wave Velocity. PLoS ONE 2016, 11, e0168598. [Google Scholar] [CrossRef]
- Mitchell, G.F.; Hwang, S.J.; Vasan, R.S.; Larson, M.G.; Pencina, M.J.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J. Arterial Stiffness and Cardiovascular Events: The Framingham Heart Study. Circulation 2010, 121, 505–511. [Google Scholar] [CrossRef]
- Sehestedt, T.; Jeppesen, J.; Hansen, T.W.; Wachtell, K.; Ibsen, H.; Torp-Petersen, C.; Hildebrandt, P.; Olsen, M.H. Risk Prediction Is Improved by Adding Markers of Subclinical Organ Damage to SCORE. Eur. Heart J. 2010, 31, 883–891. [Google Scholar] [CrossRef]
- Barbu, E.; Popescu, M.R.; Popescu, A.C.; Balanescu, S.M. Inflammation as A Precursor of Atherothrombosis, Diabetes and Early Vascular Aging. Int. J. Mol. Sci. 2022, 23, 963. [Google Scholar] [CrossRef]
- Loehr, L.R.; Meyer, M.L.; Poon, A.K.; Selvin, E.; Palta, P.; Tanaka, H.; Pankow, J.S.; Wright, J.D.; Griswold, M.E.; Wagenknecht, L.E.; et al. Prediabetes and Diabetes Are Associated With Arterial Stiffness in Older Adults: The ARIC Study. Am. J. Hypertens. 2016, 29, 1038–1045. [Google Scholar] [CrossRef]
- Chen, J.Y.; Chou, C.H.; Lee, Y.L.; Tsai, W.C.; Lin, C.C.; Huang, Y.Y.; Chen, J.H. Association of Central Aortic Pres-sures Indexes with Development of Diabetes Mellitus in Essential Hypertension. Am. J. Hypertens. 2010, 23, 1069–1073. [Google Scholar] [CrossRef]
- Cooper, M.E. Importance of Advanced Glycation End Products in Diabetes-Associated Cardiovascular and Renal Disease. Am. J. Hypertens. 2004, 17, S31–S38. [Google Scholar] [CrossRef]
- McEniery, C.M.; Wilkinson, I.B.; Johansen, N.B.; Witte, D.R.; Singh-Manoux, A.; Kivimaki, M.; Tabak, A.G.; Brun-ner, E.J.; Shipley, M.J. Nondiabetic Glucometabolic Status and Progression of Aortic Stiffness: The Whitehall II Study. Diabetes Care 2017, 40, 599–606. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Leite, N.C.; Cardoso, C.R.L.; Salles, G.F. Correlates of Aortic Stiffness Progression in Patients with Type 2 Diabetes: Importance of Glycemic Control-The Rio de Janeiro Type 2 Diabetes Cohort Study. Diabetes Care 2015, 38, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Alikhanova, N.; Musakhanova, C.; Nazarova, N.; Davronov, R.; Takhirova, F.; Akramova, G.; Abboskhujaeva, L.; Munavvara, S.; Trigulova, R.; Kayumova, D.; et al. Arterial Stiffness in Patients with Type 2 Diabetes Mellitus at Different Stages of Diabetic Nephropathy. Endocr. Abstr. 2023, 90, EP291. [Google Scholar] [CrossRef]
- Castelli, R.; Gidaro, A.; Casu, G.; Merella, P.; Profili, N.I.; Donadoni, M.; Maioli, M.; Delitala, A.P. Aging of the Arterial System. Int. J. Mol. Sci. 2023, 24, 6910. [Google Scholar] [CrossRef]
- Marreiros, C.; Viegas, C.; Simes, D. Targeting a Silent Disease: Vascular Calcification in Chronic Kidney Disease. Int. J. Mol. Sci. 2022, 23, 16114. [Google Scholar] [CrossRef]
- O’Rourke, M.F.; Franklin, S.S. Arterial Stiffness: Reflections on the Arterial Pulse. Eur. Heart J. 2006, 27, 2497–2498. [Google Scholar] [CrossRef]
- Stanek, A.; Grygiel-Górniak, B.; Brożyna-Tkaczyk, K.; Myśliński, W.; Cholewka, A.; Zolghadri, S. The Influence of Dietary Interventions on Arterial Stiffness in Overweight and Obese Subjects. Nutrients 2023, 15, 1440. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; Lee, J.P.; Lim, W.H.; Seo, J.B.; Zo, J.H.; Kim, M.A.; Kim, S.H. Association between the Level of Serum Soluble ST2 and Invasively Measured Aortic Pulse Pressure in Patients Undergoing Coronary Angiography. Medicine 2019, 98, e14215. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Martínez, E.; Miana, M.; Jurado-López, R.; Rousseau, E.; Rossignol, P.; Zannad, F.; Cachofeiro, V.; López-Andrés, N. A Role for Soluble ST2 in Vascular Remodeling Associated with Obesity in Rats. PLoS ONE 2013, 8, e79176. [Google Scholar] [CrossRef]
- Miller, A.M.; Xu, D.; Asquith, D.L.; Denby, L.; Li, Y.; Sattar, N.; Baker, A.H.; McInnes, I.B.; Liew, F.Y. IL-33 Reduces the Development of Atherosclerosis. J. Exp. Med. 2008, 205, 339–346. [Google Scholar] [CrossRef]
- Gungor, O.; Unal, H.U.; Guclu, A.; Gezer, M.; Eyileten, T.; Guzel, F.B.; Altunoren, O.; Erken, E.; Oguz, Y.; Kocyigit, I.; et al. IL-33 and ST2 Levels in Chronic Kidney Disease: Associations with Inflammation, Vascular Abnormalities, Cardiovascular Events, and Survival. PLoS ONE 2017, 12, e0178939. [Google Scholar] [CrossRef]
- Hasan, A.; Aldhahi, W. Soluble Suppression of Tumorigenicity 2 Is Directly Correlated with Glycated Hemoglobin in Individuals with an Average Glycemia in the Normal/Prediabetes Range. Diabetes Metab. Syndr. Obes. 2020, 13, 2711–2718. [Google Scholar] [CrossRef]
- Pannier, B.; Guérin, A.P.; Marchais, S.J.; Safar, M.E.; London, G.M. Stiffness of Capacitive and Conduit Arteries: Prognostic Significance for End-Stage Renal Disease Patients. Hypertension 2005, 45, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Kim, K.-S.; Nam, C.-W.; Han, S.-W.; Hur, S.-H.; Kim, Y.-N.; Kim, K.-B.; Lee, J.-B. Clinical Implication of Carotid-Radial Pulse Wave Velocity for Patients with Coronary Artery Disease. Orig. Artic. Korean Circ. J. 2006, 36, 565–572. [Google Scholar] [CrossRef]
- Tsuchikura, S.; Shoji, T.; Kimoto, E.; Shinohara, K.; Hatsuda, S.; Koyama, H.; Emoto, M.; Nishizawa, Y. Central versus Peripheral Arterial Stiffness in Association with Coronary, Cerebral and Peripheral Arterial Disease. Atherosclerosis 2010, 211, 480–485. [Google Scholar] [CrossRef]
- Liang, X.; Li, D.; Wang, Z.; Cheng, Y.; Mou, K.; Ye, C.; Duan, Y.; Yang, Y. Aortic Stiffness Measured by Carotid Femoral-Pulse Wave Velocity at Different Stages of Normal Glucose, Prediabetes, and Diabetes Mellitus: A Systematic Review and Meta-Analysis. Rev. Cardiovasc. Med. 2024, 25, 339. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.; Tattersall, M.C.; Gepner, A.D.; Korcarz, C.E.; Kaufman, J.; Colangelo, L.A.; Liu, K.; Stein, J.H. Sex Differences in Predictors of Longitudinal Changes in Carotid Artery Stiffness: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 478–484. [Google Scholar] [CrossRef]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The Protective Role of Estrogen and Estrogen Receptors in Cardiovascular Disease and the Controversial Use of Estrogen Therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Barton, M. Cholesterol and Atherosclerosis. Curr. Opin. Lipidol. 2013, 24, 214–220. [Google Scholar] [CrossRef]
- Zaydun, G.; Tomiyama, H.; Hashimoto, H.; Arai, T.; Koji, Y.; Yambe, M.; Motobe, K.; Hori, S.; Yamashina, A. Menopause Is an Independent Factor Augmenting the Age-Related Increase in Arterial Stiffness in the Early Postmenopausal Phase. Atherosclerosis 2006, 184, 137–142. [Google Scholar] [CrossRef]
- Waddell, T.K.; Dart, A.M.; Gatzka, C.D.; Cameron, J.D.; Kingwell, B.A. Women Exhibit a Greater Age-Related Increase in Proximal Aortic Stiffness than Men. J. Hypertens. 2001, 19, 2205–2212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Q.; Zheng, R.; Zhao, Z.; Li, M.; Wang, T.; Xu, M.; Lu, J.; Wang, S.; Lin, H.; et al. Sex Differences in the Risk of Arterial Stiffness among Adults with Different Glycemic Status and Modifications by Age. J. Diabetes 2023, 15, 121–132. [Google Scholar] [CrossRef]
- Torngren, K.; Rylance, R.; Björk, J.; Engström, G.; Frantz, S.; Marko-Varga, G.; Melander, O.; Nihlen, U.; Olsson, H.; Planck, M.; et al. Association of Coronary Calcium Score with Endothelial Dysfunction and Arterial Stiffness. Atherosclerosis 2020, 313, 70–75. [Google Scholar] [CrossRef]
- Young, A.; Garcia, M.; Sullivan, S.M.; Liu, C.; Moazzami, K.; Ko, Y.A.; Shah, A.J.; Kim, J.H.; Pearce, B.; Uphoff, I.; et al. Impaired Peripheral Microvascular Function and Risk of Major Adverse Cardiovascular Events in Patients with Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1801–1809. [Google Scholar] [CrossRef]
- Cooper, L.L.; Wang, N.; Beiser, A.S.; Romero, J.R.; Aparicio, H.J.; Lioutas, V.A.; Benjamin, E.J.; Larson, M.G.; Vasan, R.S.; Mitchell, G.F.; et al. Digital Peripheral Arterial Tonometry and Cardiovascular Disease Events: The Fram-ingham Heart Study. Stroke 2021, 52, 2866–2873. [Google Scholar] [CrossRef]
- Tajima, E.; Sakuma, M.; Tokoi, S.; Matsumoto, H.; Saito, F.; Watanabe, R.; Toyoda, S.; Abe, S.; Inoue, T. The Com-parison of Endothelial Function between Conduit Artery and Microvasculature in Patients with Coronary Artery Disease. Cardiol. J. 2020, 27, 38–46. [Google Scholar] [CrossRef]
- Venuraju, S.; Jeevarethinam, A.; Mehta, V.S.; Ruano, S.; Dumo, A.; Nair, D.; Rosenthal, M.; Darko, D.; Cohen, M.; Rakhit, R.; et al. Predicting Severity of Coronary Artery Disease in Patients with Diabetes Using Endothelial Function Measured with Peripheral Arterial Tonometry: PROCEED Study. Angiology 2019, 70, 613–620. [Google Scholar] [CrossRef]
- Holm, H.; Kennbäck, C.; Laucyte-Cibulskiene, A.; Nilsson, P.M.; Jujic, A. The Impact of Prediabetes and Diabetes on Endothelial Function in a Large Population-Based Cohort. Blood Press 2024, 33, 2298309. [Google Scholar] [CrossRef]
- van der Heijden, D.J.; van Leeuwen, M.A.H.; Janssens, G.N.; Lenzen, M.J.; van de Ven, P.M.; Eringa, E.C.; van Royen, N. Body Mass Index Is Associated with Microvascular Endothelial Dysfunction in Patients with Treated Metabolic Risk Factors and Suspected Coronary Artery Disease. J. Am. Heart Assoc. 2017, 6, e006082. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; He, J.; Cao, H.; Hu, X. Predictive Value of Abnormal Ankle-Brachial Index in Patients with Diabetes: A Meta-Analysis. Diabetes Res. Clin. Pract. 2021, 174, 108723. [Google Scholar] [CrossRef] [PubMed]
- Fowkes, G.; Fowkes, F.G.R.; Murray, G.D.; Butcher, I.; Heald, C.L.; Lee, R.J.; Chambless, L.E.; Folsom, A.R.; Hirsch, A.T.; Dramaix, M.; et al. Ankle Brachial Index Combined with Framingham Risk Score to Predict Cardiovascular Events and Mortality: A Meta-Analysis. JAMA 2008, 300, 197–208. [Google Scholar] [CrossRef]
- Clairotte, C.; Retout, S.; Potier, L.; Roussel, R.; Escoubet, B. Automated Ankle-Brachial Pressure Index Measurement by Clinical Staff for Peripheral Arterial Disease Diagnosis in Nondiabetic and Diabetic Patients. Diabetes Care 2009, 32, 1231–1236. [Google Scholar] [CrossRef]
- Harashima, K.; Hayashi, J.; Miwa, T.; Tsunoda, T. Long-Term Pioglitazone Therapy Improves Arterial Stiffness in Patients with Type 2 Diabetes Mellitus. Metabolism 2009, 58, 739–745. [Google Scholar] [CrossRef]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide in Mice with Experimental Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef]
- Zhang, B.; He, K.; Chen, W.; Cheng, X.; Cui, H.; Zhong, W.; Li, S.; Wang, L. Alagebrium (ALT-711) Improves the Anti-Hypertensive Efficacy of Nifedipine in Diabetic-Hypertensive Rats. Hypertens. Res. 2014, 37, 901–907. [Google Scholar] [CrossRef]
- Travers, J.G.; Tharp, C.A.; Rubino, M.; McKinsey, T.A. Therapeutic Targets for Cardiac Fibrosis: From Old School to next-Gen. J. Clin. Investig. 2022, 132, e148554. [Google Scholar] [CrossRef] [PubMed]
- Biesbroek, P.S.; Roos, S.T.; Van Hout, M.; Van Der Gragt, J.; Teunissen, P.F.A.; De Waard, G.A.; Knaapen, P.; Kamp, O.; Van Royen, N. Fluoroscopy Assisted Scoring of Myocardial Hypoperfusion (FLASH) Ratio as a Novel Predictor of Mortality after Primary PCI in STEMI Patients. Int. J. Cardiol. 2016, 202, 639–645. [Google Scholar] [CrossRef]
- Vogelzang, M.; Vlaar, P.J.; Svilaas, T.; Amo, D.; Nijsten, M.W.N.; Zijlstra, F. Computer-Assisted Myocardial Blush Quantification after Percutaneous Coronary Angioplasty for Acute Myocardial Infarction: A Substudy from the TAPAS Trial. Eur. Heart J. 2009, 30, 594–599. [Google Scholar] [CrossRef]
- Brunner-La Rocca, H.-P. Towards Applicability of Measures of Arterial Stiffness in Clinical Routine. Eur. Heart J. 2010, 31, 2320–2322. [Google Scholar] [CrossRef]
- Axtell, A.L.; Gomari, F.A.; Cooke, J.P. Assessing Endothelial Vasodilator Function with the Endo-PAT 2000. J. Vis. Exp. 2010, 44, e2167. [Google Scholar] [CrossRef]
- Ingelfinger, J.; Mosteller, F.; Thibodeau, L.; Ware, J. Biostatistics in Clinical Medicine, 2nd ed.; McGraw-Hill Companies: New York, NY, USA, 1987. [Google Scholar]
- Knapp, R.; Miller, M.I. Clinical Epidemiology and Biostatistics, 1st ed.; Springer: Baltimore, MD, USA, 1992. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; Iowa State: Ames, IA, USA, 1980. [Google Scholar]
- Dong, Y.; Peng, C.-Y.J. Principled Missing Data Methods for Researchers. Springerplus 2013, 2, 222. [Google Scholar] [CrossRef] [PubMed]



| T2DM (−) n = 42 | T2DM (+) n = 58 | Significance | |
|---|---|---|---|
| Sex, women/men | 28/14 | 28/30 | NS |
| Age, years (X ± SD) | 66.5 ± 7.0 | 69.4 ± 7.5 | NS |
| Height, m (X ± SD) | 1.69 ± 0.08 | 1.64 ± 0.09 | p < 0.05 |
| Body mass, kg (X ± SD) | 80.8 ± 12.6 | 86.9 ± 16.6 | p < 0.05 |
| BMI 1, kg/m2 (X ± SD) | 28.4 ± 4.7 | 33.0 ± 6.3 | p < 0.001 |
| Active smokers, n (%) | 10 (23.8) | 12 (20.7) | NS |
| Former smokers, n (%) | 24 (57.1) | 20 (34.5) | p < 0.05 |
| Family health history, n (%) | 14 (33.3) | 32 (55.2) | NS |
| Concomitant diseases | |||
| Chronic coronary syndrome, n (%) | 38 (90.5) | 52 (89.7) | NS |
| Hyperlipidemia, n (%) | 30 (71.4) | 50 (86.2) | NS |
| Arterial hypertension, n (%) | 34 (80.9) | 54 (93.1) | NS |
| Ischemic stroke, n (%) | 4 (9.5) | 4 (6.9) | NS |
| CKD 2, n (%) | 0 (0) | 12 (20.7) | p < 0.01 |
| Drugs | |||
| ACEI 3, n (%) | 36 (85.0) | 40 (69.0) | NS |
| ARB 4, n (%) | 6 (14.3) | 14 (24.1) | NS |
| CCB 5, n (%) | 12 (28.4) | 32 (55.2) | p < 0.05 |
| Beta-blocker, n (%) | 38 (90.5) | 52 (89.7) | NS |
| Diuretic, n (%) | 14 (33.3) | 32 (60.3) | NS |
| Potassium-sparing diuretic, n (%) | 8 (19.0) | 12 (20.7) | NS |
| ASA 6, n (%) | 36 (85.0) | 56 (95.6) | NS |
| Clopidogrel, n (%) | 22 (52.4) | 32 (60.3) | NS |
| Ticagrelor, n (%) | 4 (9.5) | 6 (10.3) | NS |
| Fibrate, n (%) | 0 (0) | 12 (20.7) | p < 0.01 |
| Statin, n (%) | 40 (95.2) | 50 (86.2) | NS |
| OAC 7, n (%) | 2 (4.8) | 2 (3.4) | NS |
| Metformin, n (%) | 0 | 42 | |
| Sulfonyl 8, n (%) | 0 | 18 | |
| SGLT2 inhibitor, n (%) | 2 | 10 | |
| Insulin, n (%) | 0 | 24 |
| T2DM (−) n = 42 | T2DM (+) n = 58 | Significance | |
|---|---|---|---|
| Number of narrowed coronary arteries, n (%) | |||
| 1 | 18 (42.9) | 23 (39.7) | NS |
| 2 | 14 (33.3) | 16 (27.6) | |
| 3 | 10 (23.8) | 19 (32.7) | |
| LAD 1 | |||
| cTFC, X ± SD | 26.3 ± 8.4 | 24.6 ± 9.3 | NS |
| FLASH, mL/min, X ± SD | 48.4 ± 15.7 | 50.3 ± 19.8 | NS |
| QuBE, arb. units X ± SD | 3.5 ± 2.3 | 3.8 ± 3.6 | NS |
| Cx 2 | |||
| cTFC, X ± SD | 18.4 ± 6.5 | 19.8 ± 7.5 | NS |
| FLASH, mL/min, X ± SD | 42.5 ± 14.9 | 40.4 ± 16.8 | NS |
| QuBE, arb. units X ± SD | 3.1 ± 4.3 | 2.9 ± 2.6 | NS |
| RCA 3 | |||
| cTFC, X ± SD | 20.1 ± 7.5 | 18.8 ± 8.7 | NS |
| FLASH, mL/min, X ± SD | 44.7 ± 18.2 | 47.1 ± 18.4 | NS |
| QuBE, arb. units X ± SD | 4.2 ± 3.5 | 4.9 ± 3.1 | NS |
| T2DM (−) n = 42 | T2DM (+) n = 58 | Significance | |
|---|---|---|---|
| SBP 1, mmHg, X ± SD | 138.6 ± 19.4 | 143.2 ± 19.0 | NS |
| DBP 2, mmHg, X ± SD | 80.0 ± 10.4 | 80.9 ± 10.3 | NS |
| ABI 3 right leg, X ± SD | 1.23 ± 0.11 | 1.22 ± 0.18 | NS |
| ABI 3 left leg, X ± SD | 1.25 ± 0.12 | 1.22 ± 0.21 | NS |
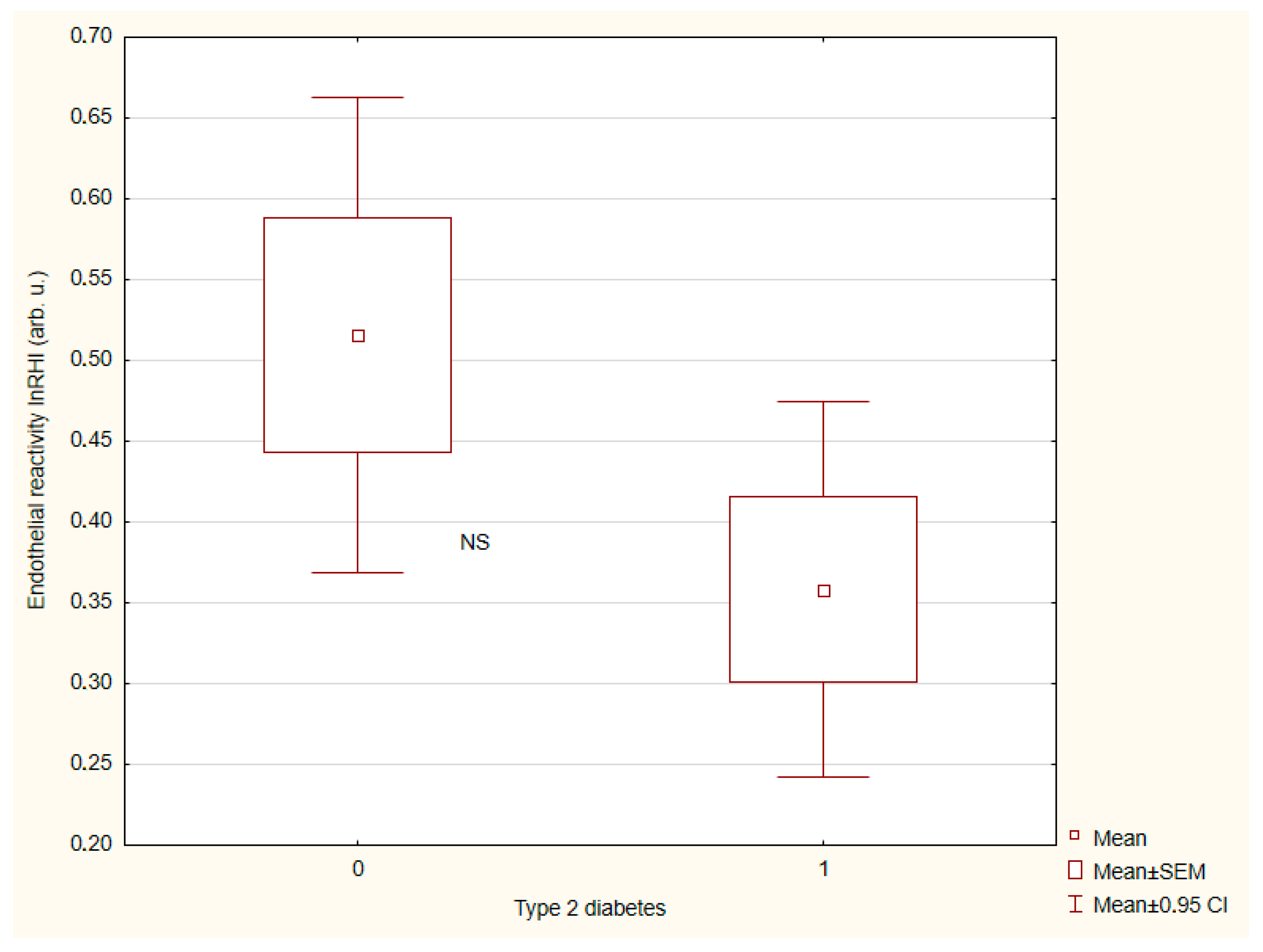
| LnRHI 4, X ± SD | 0.52 ± 0.47 | 0.36 ± 0.4 | NS |
| cfPWV 5, m/s, X ± SD | 9.9 ± 2.7 | 10.8 ± 2.1 | <0.05 |
| CrPWV 6, m/s, X ± SD | 9.3 ± 2.7 | 9.0 ± 2.1 | NS |
| Vascular age, years, X ± SD | 61.3 ± 15.4 | 70.0 ± 12.3 | <0.05 |
| T2DM (−) n = 42 | T2DM (+) n = 58 | Significance | |
|---|---|---|---|
| HbA1C, %, X ± SD | 5.8 ± 0.1 | 8.2 ± 1.3 | p < 0.001 |
| Creatinine, µmol/L, X ± SD | 73.2 ± 15.0 | 88.9 ± 36.2 | p < 0.05 |
| eGFR, mL/min, X ± SD | 82.1 ± 7.7 | 69.0 ± 21.0 | p < 0.001 |
| sST2, ng/mL, X ± SD | 22.2 ± 6.6 | 34.7 ± 27.7 | p < 0.01 |
| Dependent Variable | Independent Variable | β | t | P |
|---|---|---|---|---|
| cfPWV | HbA1C | 0.18 | 1.68 | NS |
| Creatinine | 1.12 | 2.65 | p < 0.01 | |
| eGFR | −0.94 | −2.14 | p < 0.01 | |
| sST2 | 0.11 | 1.09 | NS | |
| Regression Summary | ||||
| Multiple R = 0.47 | ||||
| Multiple R2 = 0.16 | ||||
| Adjusted R2 = 0.09 | ||||
| p < 0.05 | ||||
| Vascular age | HbA1C | 0.15 | 1.35 | NS |
| Creatinine | 0.45 | 1.9 | p < 0.05 | |
| eGFR | −0.53 | −2.25 | p < 0.05 | |
| sST2 | 0.53 | 2.32 | p < 0.05 | |
| Regression Summary | ||||
| Multiple R = 0.36 | ||||
| Multiple R2 = 0.13 | ||||
| Adjusted R2 = 0.09 | ||||
| p < 0.01 |
| Men n = 56 | Women n = 44 | Significance | |
|---|---|---|---|
| SBP, mmHg, X ± SD | 137.4 ± 16.4 | 146.2 ± 21.2 | p < 0.01 |
| DBP, mmHg, X ± SD | 79.6 ± 8.3 | 81.7 ± 12.3 | NS |
| ABI right leg, X ± SD | 1.22 ± 0.15 | 1.23 ± 0.16 | NS |
| ABI left leg, X ± SD | 1.23 ± 0.17 | 1.24 ± 0.19 | NS |
| Endothelial reactivity, lnRHI, X ± SD | 0.44 ± 0.39 | 0.42 ± 0.49 | NS |
| cfPWV, m/s, X ± SD | 10.2 ± 2.6 | 10.7 ± 2.1 | NS |
| CrPWV, m/s, X ± SD | 9.1 ± 1.7 | 9.1 ± 1.5 | NS |
| Vascular age, years, X ± SD | 63.9 ± 15.9 | 69.1 ± 10.9 | NS |
| HbA1C, %, X ± SD | 7.0 ± 1.4 | 7.5 ± 1.7 | NS |
| Creatinine, µmol/L, X ± SD | 89.7 ± 31.6 | 71.8 ± 24.8 | p < 0.005 |
| eGFR, mL/min X ± SD | 75.3 ± 16.7 | 73.5 ± 19.6 | NS |
| sST2, ng/mL, X ± SD | 28.2 ± 10.8 | 31.1 ± 31.3 | NS |
| SBP, mmHg, X ± SD | 137.4 ± 16.4 | 146.2 ± 21.2 | p < 0.01 |
| Men | Women | Significance | |||
|---|---|---|---|---|---|
| T2DM (−) n = 28 (1) | T2DM (+) n = 28 (2) | T2DM (−) n = 14 (3) | T2DM (+) n = 30 (4) | Kruskal-Willis ANOVA | |
| SBP, mmHg, X ± SD | 132.3 ± 14.4 | 142.8 ± 17.0 | 151.1 ± 21.8 | 143.5 ± 20.8 | p < 0.01 (p < 0.005 1 vs. 3) |
| DBP, mmHg, X ± SD | 79.0 ± 8.7 | 80.2 ± 8.0 | 81.9 ± 13.2 | 81.6 ± 12.1 | NS |
| ABI right leg, X ± SD | 1.24 ± 0.11 | 1.2 ± 0.18 | 1.21 ± 0.1 | 1.24 ± 0.19 | NS |
| ABI left leg, X ± SD | 1.29 ± 0.13 | 1.17 ± 0.17 | 1.18 ± 0.08 | 1.27 ± 0.22 | NS |
| Endothelium reactivity, lnRHI, X ± SD | 0.47 ± 0.42 | 0.4 ± 0.35 | 0.63 ± 0.54 | 0.33 ± 0.44 | NS NS |
| cfPWV, m/s, X ± SD | 9.5 ± 2.9 | 11.0 ± 2.0 | 10.8 ± 2.1 | 10.7 ± 2.1 | p < 0.01 (p < 0.01 1 vs. 2 and p < 0.05 1 vs. 4) |
| crPWV, m/s, X ± SD | 9.2 ± 1.4 | 9.0 ± 1.9 | 9.4 ± 1.6 | 9.0 ± 1.6 | NS |
| Vascular age, years, X ± SD | 57.1 ± 14.8 | 71.3 ± 13.8 | 69.7 ± 12.4 | 68.7 ± 10.2 | p < 0.01 (p < 0.01 1 vs. 2 and p < 0.05 1 vs. 4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radzik, E.; Schulz, M.; Przywara-Chowaniec, B.; Tomasik, A. Noninvasive Assessment of Arterial Wall and Soluble ST2 in Patients with Type 2 Diabetes and Coronary Artery Disease. Int. J. Mol. Sci. 2025, 26, 7561. https://doi.org/10.3390/ijms26157561
Radzik E, Schulz M, Przywara-Chowaniec B, Tomasik A. Noninvasive Assessment of Arterial Wall and Soluble ST2 in Patients with Type 2 Diabetes and Coronary Artery Disease. International Journal of Molecular Sciences. 2025; 26(15):7561. https://doi.org/10.3390/ijms26157561
Chicago/Turabian StyleRadzik, Edyta, Marcin Schulz, Brygida Przywara-Chowaniec, and Andrzej Tomasik. 2025. "Noninvasive Assessment of Arterial Wall and Soluble ST2 in Patients with Type 2 Diabetes and Coronary Artery Disease" International Journal of Molecular Sciences 26, no. 15: 7561. https://doi.org/10.3390/ijms26157561
APA StyleRadzik, E., Schulz, M., Przywara-Chowaniec, B., & Tomasik, A. (2025). Noninvasive Assessment of Arterial Wall and Soluble ST2 in Patients with Type 2 Diabetes and Coronary Artery Disease. International Journal of Molecular Sciences, 26(15), 7561. https://doi.org/10.3390/ijms26157561






