Abstract
Among brain regions, the cerebellum (CBL) has traditionally been associated with motor control. However, increasing evidence from connectomics and functional imaging has expanded this view, revealing its involvement in a wide range of cognitive and integrative processes. Despite this emerging relevance, the CBL has received comparatively less attention in aging research, which has focused mainly on other central nervous system (CNS) regions such as the neocortex and hippocampus. This review synthesizes the current evidence on glial cell aging across the CNS, emphasizing how cerebellar circuits follow distinct trajectories in terms of cellular remodeling, transcriptional reprogramming, and structural vulnerability. Recent findings highlight that cerebellar astrocytes and microglia exhibit specific signatures related to aging compared to their cortical counterpart, including moderate reactivity, selective immune response, and spatial reorganization. Cerebellar white matter (WM) undergoes structural alteration, suggesting that oligodendroglial cells may undergo region-specific alterations, particularly within WM tracts, although these aspects remain underexplored. Despite the presence of glial remodeling, the CBL maintains a notable degree of structural and functional integrity during aging. This resilience may be the result of the CBL’s ability to maintain synaptic adaptability and homeostatic balance, supported by its highly organized and compartmentalized architecture. A better understanding of the dynamics of cerebellar glial cells in aging may provide new insight into the mechanisms of brain maintenance and identify potential biomarkers for healthy brain aging.
Keywords:
astrocytes heterogeneity; Bergmann glia; CBL; CNS-aging; glial cells; microglia; oligodendroglia; white matter 1. Introduction
Aging is a complex and multifactorial biological process that affects all tissues and organs, including the central nervous system (CNS), and leads to progressive changes in structure, cellular composition, and function. Although aging is not a disease per se, it is the main risk factor for neurodegenerative diseases (NDs) and age-associated cognitive and motor decline [1].
Understanding how the brain changes with age has become a major scientific and clinical challenge in light of the continuous increase in global life expectancy. Although neurons have long been considered the main focus of age-related research, recent evidence has shifted attention to the role of glial cells, which are now recognized as highly active and heterogeneous players in brain homeostasis. Astrocytes, oligodendroglia and microglia contribute to essential functions such as synaptic regulation, neurotransmitter recycling, immune surveillance, metabolic support, and maintenance of the blood–brain barrier (BBB) [2].
During aging, these glial cell populations undergo profound transcriptional, metabolic, and morphological remodeling, often preceding or amplifying neuronal dysfunction [3,4,5,6]. Among the CNS regions, the CBL has historically been studied in relation to motor control. However, recent advances in connectome and circuit complexity analysis have challenged the traditional notion of the CBL as a uniform and exclusive motor structure. Instead, it is now considered a highly complex region, characterized by a modular organization, specialized neuron types, dense feedback loops, and non-synaptic signaling mechanisms. Emerging evidence, including the identification of novel interneuron subtypes and ephaptic communication, suggests that the CBL engages in parallel, multilayered signal processing with a level of precision that is comparable to that observed in the cortex [7]. This complexity requires a specialized glial compartment capable of precise support and regulation of different microcircuits.
However, converging data from neuroimaging, injury analysis, and functional connectivity studies indicate the involvement of the CBL in non-motor functions such as working memory, attention, emotion regulation, and language [8,9,10].
Its anatomical connections with the prefrontal cortex, the thalamus, and the basal ganglia suggest an integrative role in sensorimotor and cognitive processes. While most aging studies have traditionally focused on the neocortical and hippocampal circuits, recent advances have pointed to the CBL as susceptible to structural and functional alterations with aging. These aspects deserve specific attention, also because the CBL is altered and possibly involved in age-related NDs.
This review aims to synthesize current knowledge on glial specialization and its alterations during aging, moving from general CNS principles to CBL-specific responses. Evidence from the literature shows that cerebellar glia, particularly astrocytes and microglia, exhibit earlier and more pronounced transcriptional changes related to aging than those of other regions of the CNS [3,4,11,12,13]. These findings suggest that the CBL does not simply mirror CNS aging, but rather follows its own trajectory, shaped by region-specific glia-neuron interactions and metabolic demands. For this reason, elucidating how glial aging contributes to cerebellar vulnerability may provide new insights into the cellular and molecular mechanisms of brain aging and its pathological trajectories and may offer identification of potential therapeutic targets.
2. Glial Cells in the CNS
Glia cells represent a heterogeneous and highly specialized population of non-neuronal elements within the CNS, where they perform essential roles in the structural, metabolic, and functional maintenance of neural tissue. Traditionally considered passive support elements, glial cells are now recognized as active and essential regulators of brain function, contributing to synaptic transmission, tissue homeostasis, immune signaling, and cerebrovascular dynamics [14]. They form an integrated and responsive network that ensures stability, plasticity, and adaptability of the CNS under physiological and pathological conditions.
The major classes of glial cells in the CNS include astrocytes, oligodendroglia, microglia, and ependymal cells. These cell populations originate from distinct embryological lineages.
Astrocytes, derived from radial glia cells (RGCs), represent the most abundant glial population and show significant morphological and molecular diversity. These cells play a role in the regulation of ions and neurotransmitter homeostasis, facilitating their recycling and supporting neuronal metabolism. They also contribute to the integrity and function of the BBB. In addition to their homeostatic functions, astrocytes exert fine modulatory control over synaptic activity through Ca2+-dependent signaling and the release of neurotransmitters (gliotransmission) [15].
Oligodendroglia, a cell lineage that includes oligodendrocyte precursor cells (OPCs) which mature into oligodendrocytes (OLs), classically responsible for myelination of multiple neuronal axons. This process enables efficient saltatory conduction in neurons and provides metabolic and plasticity support. OPCs persist in adulthood and maintain proliferative and differentiation potential in response to injury or demyelination [16].
Microglia, the resident macrophages of the CNS, derive from myeloid progenitors. They exhibit dynamic surveillance activity and are capable of rapid adaptive phenotypic changes in response to local perturbations. Microglia contribute to synaptic pruning, phagocytosis of apoptotic cells and cell debris, and modulation of inflammatory cascades [17].
Recently, border-associated macrophages (BAMs) have been identified as a new player in CNS immune cells. BAMs are located in leptomeninges and perivascular spaces, where they contribute to the regulation of tissue homeostasis and disease [18]. However, they will not be considered in detail in this review.
Ependymal cells, which line the ventricular system and the central canal of the spinal cord, are ciliated epithelial-like glial elements involved in cerebrospinal fluid (CSF) production and circulation. They contribute to CSF homeostasis by facilitating metabolite clearance and preserving the integrity of the periventricular niche. Although historically less studied, recent evidence suggests that ependymal cells contribute to adult neurogenesis, regulation, and maintenance of ventricular–parenchymal interfaces [19]. Nevertheless, due to the limited evidence on their role in aging, particularly in the CBL, and their non-parenchymal localization, these cells are not addressed further in this review.
2.1. Astrocytes
Among the CNS glial cell populations, astrocytes are known for their remarkable adaptability and dynamic properties. Recent advances in molecular profiling and high-resolution imaging have revealed that astrocytes constitute a highly heterogeneous population, encompassing developmental origin, region-specific transcriptional programs, functional specializations and structural adaptations.
Astrocyte identity is established early in development by restriction of the RGC lineage [20].
The ontogenetic patterning is a key determinant of the regional identity of astrocytes shaping their transcriptional programs according to local circuit demands, thus influencing their differential susceptibility to aging and pathology [21,22].
Traditionally, categorized into two major morphological classes: protoplasmic astrocytes, predominant in the gray matter (GM) and fibrous astrocytes (FAs), located in the white matter (WM), these cells differ in structure, distribution, and functional roles [23,24,25]. Protoplasmic astrocytes exhibit highly branched processes and occupy non-overlapping spatial domains in close association with neuronal synapses and blood vessels, playing crucial roles in synaptic modulation, neurotransmitter recycling, and neurovascular coupling [26,27]. Conversely, FAs possess elongated and less branched processes aligned with the axonal tracts and are mainly involved in maintaining the integrity of WM, ion homeostasis, and myelination support [27,28].
They interact with oligodendrocytes, the vasculature, and neurons in Ranvier nodes.
Interestingly, a cluster of astrocytes in the WM of the corpus callosum (CC), but not in the CBL, exhibit high expression of immediate early genes, such as c-fos and c-jun, related to RGCs, as well as others that are enriched in terms of gene ontology (GO) associated with cell proliferation. In vivo functional experiments confirm the proliferation of astrocytes in WM/CC, but not in cerebellar WM, indicating more complex region heterogeneity of these cells [27].
Beyond the classical dichotomy, recent advances in transcriptomic and spatial profiling studies have revealed a more complex landscape of astrocyte taxonomy. Translating Ribosome Affinity Purification (TRAP) and bulk RNA sequencing (seq) analysis have shown substantial transcriptional differences among astrocyte populations in different CNS regions, including the cortex, the striatum, the thalamus, and the brainstem, correlating with specific local functions [22,29]. Single cell (sc)RNA-seq has further refined this view, identifying distinct subpopulations of astrocytes even within individual regions. For example, in the adult mouse cortex and hippocampus, Batiuk et al. (2020) [30], identified five spatially segregated astrocyte subtypes (AST1–AST5) characterized by distinct gene expression profiles and laminar distribution specialized for local circuit demands such as synaptic function, ionic homeostasis, and immune response. Recent spatial transcriptomic and morphometric analysis in 13 CNS regions, including the CBL, demonstrates that only 20% of the identified genes are common in all regions, thus constituting the core genes for astrocytes [31].
They consist of genes for K+ homeostasis, neurotransmitter transport, cholesterol metabolism, and G-protein-coupled receptors. When clustering genes that are differentially expressed in one region compared to the other 12, the CBL results as a distinct cell group.
Interestingly, astrocytes from different regions possess a characteristic morphology associated with a specific molecular signature [31]. The region-specific expression of cytoskeletal and adhesion-related genes, such as Ezr, Fermt2, Lama2, and Sept4, has been shown to be associated with astrocyte morphometric parameters (territory size, arbor complexity, and spatial orientation).
Notably, the diversity of astrocytes extends to the subcellular level. Perisynaptic astrocytic processes (PAPs) unsheathe synaptic terminals and exhibit activity-dependent remodeling and locally specialized mRNA translation [32,33]. PAPs differ in transporter expression (Glt1, Aqp4), cytoskeletal composition, and mitochondrial density [34,35,36], enabling compartmentalized regulation of synaptic activity. These features establish PAPs as key effectors of astrocyte function and potential focal points of vulnerability related to aging.
Recent studies have highlighted that astrocytic identity and transcriptional activity are not determined autonomously by individual cells but instead are dynamically shaped by neuron-derived signals and synaptic activity. Spatial transcriptomic analyses of cortical regions have revealed that neuronal activation triggers the expression of localized astrocyte genes involved in glutamate transport, metabolic support, and ion homeostasis. Synergy between neurons and astrocytes is critical for circuit plasticity and memory consolidation [37] and appears to decline with age [34].
Functionally, astrocyte specialization is reflected in several critical physiological processes.
They modulate neuronal activity and synaptic plasticity through Ca2+-dependent signaling mechanisms. Intracellular Ca2+ dynamics, mediated by astrocytic channels and receptors such as RYR3, regulate gliotransmission and neuronal synchronization [38].
Astrocytes also support neuronal energetics by supplying glycolysis-derived lactate to active neurons [39,40]. Astrocytes are also crucial regulators of CNS immune responses. Under pathological or traumatic stimuli and during aging, they acquire reactive phenotypes dichotomized into neurotoxic A1, secreting pro-inflammatory cytokines, chemokines and complement components and neuroprotective A2 states, secreting neurotrophic factors [41]. This classification will certainly be overcome by the specific transcriptomic analyses that are beginning to broaden the heterogeneity of the functional states of these cells, as defined by the genes they express in different non-physiological contexts [42,43]. Finally, astrocytes contribute to cerebrovascular homeostasis.
Their perivascular endfeets interact with endothelial cells and blood vessel pericytes to regulate blood flow, BBB permeability, and nutrient delivery, forming a key component of the neurovascular unit (NVU) [38].
2.2. Oligodendroglia
Oligodendroglia is a cell lineage primarily enriched in WM, where they sustain lifelong myelin production. The regulated differentiation pathway of this lineage includes OPCs, pre-myelinating oligodendrocytes and OLs [44,45,46]. During differentiation, these cells undergo morphological changes: OPCs are prevalently bipolar, while mature cells become highly branched.
Distinct molecular signatures accompany this morphological heterogeneity at each stage of maturation, as revealed by scRNAseq or single nuclear RNA sequencing (snRNAseq) analysis [47].
OPCs are classically identified by the expression of the Pdgfrα and the proteoglycan NG2, while OLs characteristically express myelin-associated proteins such as MBP, MAG and MOG.
OPCs are distributed in both WM, often along the axonal tracts, and GM, near the neural soma.
WM OPCs differentiate into OLs more efficiently than their GM counterparts, many of which remain NG2+ progenitors [48], and in these two regions they acquire distinct morphological features and transcriptional profiles [49,50,51].
In the adult brain, OPCs maintain mitotic activity to preserve the pool of progenitors and are essential for myelin maintenance and regeneration, since new OLs are continuously generated by their differentiation [52]. This process occurs in response to neuronal activity associated with learning [53,54] and following myelin injury. In this context, OPCs became activated through interaction with microglia and astrocytes [55], proliferate and migrate to the site of injury where they differentiate into OLs [56]. Conversely, in the adult mouse brain, it has been demonstrated that OPCs are essential to maintain the homeostatic state of microglia [57]. OPCs modulate neural activity and contribute to synapse formation by releasing neuromodulatory factors. They are involved in synaptic pruning and axonal remodeling engulfing these neural portions [58,59]. OPCs also support the integrity of the BBB through interaction with endothelial cells, pericytes and astrocytes within the NVU [60].
Finally, these cells exhibit immunomodulatory properties, responding to brain insults and influencing immune cell activity [61]. The traditional view of OPCs as mere progenitors of OLs is challenged by mounting evidence that highlights their dynamic nature and multifaceted contributions in physiological and pathological contexts [16]. scRNAseq has revealed a transcriptional continuum that spans OPCs, pre-myelinating and OLs reflecting progressive states of differentiation [47,62]. Furthermore, mature OLs can be subdivided into transcriptionally distinct populations with regional heterogeneity in terms of their abundance and response to injury [47,50,62]. Recently, Heo et al. (2025) [47] extended the profiling to the human brain, showing that cycling, committed, differentiating, and quiescent OPCs exhibit conserved gene expression signatures with their murine counterpart. This suggests evolutionary conservation in the molecular programs that guide the maturation of OPCs.
In addition to organizing myelin sheaths around axons, OLs play fundamental roles in modulating their plasticity which is important to adapt neural circuits to experiences and acquisition of new skills underpinning learning and memory. Modifications of myelin throughout the life are very dynamic and regulated and include changes in myelin sheath thickness, distribution, and length of the Ranvier nodes [54,63,64]. Alterations in these processes may contribute to age-related cognitive decline.
OLs, in addition to their traditional function in myelination, also provide metabolic support and regulate neuronal activity through their complex interaction with neurons, other glial cells, and the microenvironment [65,66]. For instance, these cells contribute to the maintenance of extracellular ion homeostasis, especially K+, through the activation of the voltage-gated ion channel [67] in collaboration with astrocytes. In turn, astrocytes, which highly express genes that control cholesterol metabolism, support OLs in axonal myelination providing this fundamental myelin component, especially during the stage of remyelination [68,69].
2.3. Microglia
Microglia, involved in the immune surveillance of neural tissue, develop during embryogenesis from hematopoietic precursors in the yolk sac instead of the neuroectoderm [70,71]. Thereafter, these cells colonize the CNS before differentiation of the BBB and appearance of the other glial cells.
Microglia are considered long-lived and self-renewing cells that exhibit region-specific turnover rates in the brain in both mice and humans [72,73]. These characteristics result in the accumulation of age-related damage over time and the development of a senescence phenotype in these innate immune cells. In the developing and early postnatal brain, microglia exhibit an amoeboid morphology, are highly mobile and, through phagocytosis of dying neurons and secretion of multiple factors, contribute to modeling the neural architecture. Adult cells acquire a ramified and flexible morphology, which is suitable for dynamically surveying their surroundings and interacting with neighboring cells.
They actively participate in the maintenance of CNS homeostasis and the shaping of neural circuits throughout life [74]. For instance, they regulate synaptic plasticity through different signaling pathways, and alteration of these processes can result in aberrant neural functionality [75].
Furthermore, recent studies with leveraging advanced single-cell technologies have revealed the presence of multiple microglial states in both mouse and human brains associated with development, aging, and disease processes, accompanied by the acquisition of distinct morphologies [76,77,78,79,80,81]. These cells can adapt their states in response to a multitude of cues. Consequently, it is a simplistic approach to consider only the “resting” and “activated” polarization scheme (reviewed in ref. [17,82]). As is also evident in other glial cells, specifically astrocytes and oligodendroglia, microglial states are determined by a complex interplay of intrinsic (species, sex, genetic background, age) and extrinsic factors (spatial location and interactions with other cells, local environment). These factors dynamically influence the cells, making it more difficult to gain a deeper understanding of their roles in health, aging, and disease.
3. Cerebellar Glial Cells
The glial cells of the CBL display distinctive functional and morphological characteristics.
The CBL is anatomically divided into ten lobules (I–X) and exhibits a trilaminar cortical organization. This consists of a subpial molecular layer (ML), which is rich in interneurons, glial processes, and a network of neuronal extensions, a Purkinje cell layer (PCL), with a monolayer of GABAergic Purkinje cells (PCs); and a granular layer (GL), which contain densely packed granule cells (GCs).
Neuroanatomical and clinical studies support also a functional division of the CBL into a sensorimotor territory, encompassing the anterior lobe and lobule VIII, and a cognitive territory, including posterior lobules VI–VII, particularly Crus II, which are connected to prefrontal and posterior parietal cortices [8,83].
PCs constitute the sole output of the cerebellar cortex, projecting to the deep cerebellar nuclei (DCN), which are embedded within the WM and are involved in highly organized feedback loops.
These neurons integrate excitatory input from climbing fibers (CFs), which originate in the inferior olive (IO) and parallel fibers (PFs) from GCs which in turn are activated by mossy fibers (MFs) arising from different brain regions.
The output of PCs is finely regulated by local inhibitory interneurons located in the ML, which are themselves activated by collaterals of CFs and PFs. Beyond this laminar structure, the CBL exhibits a highly ordered modular organization, defined by parasagittal longitudinal zones. These modules, molecularly marked by the expression of Aldolase C (Aldoc, or Zebrin II), reveal an alternating Zebrin-positive (Z+) and Zebrin-negative (Z−) bands of PCs, corresponding to distinct functional modules with specific input-output relationships and physiological roles [84].
This modular architecture supports distinct sensorimotor and cognitive tasks and aligns with region-specific vulnerability patterns during aging.
Alongside its modular neuronal architecture, the CBL contains a distinct and regionally specialized glial compartment, including astrocytes, oligodendroglia, and microglia that are key elements in the broad array of cerebellar functions.
3.1. Astrocytes
The spatial and functional complexity of the CBL is paralleled by a rich and regionally diverse glial landscape, in which astrocytes and their specialized subtypes play essential roles in synaptic regulation, ionic homeostasis, neurovascular coupling, and local circuit modulation [31,85,86]. Within the cerebellar cortex, astrocytes can be categorized into at least four morpho-functionally distinct populations that reflect the laminar organization of the CBL.
Bergmann glia (BG), located in PCL, possess elongated radial processes that extend through the ML and interact closely with PC dendrites [87]. The term Velate astrocytes (VAs) is used to refer to a specific type of astrocyte that is found in the GL. FAs, which occupy the WM and provide structural and metabolic support to myelinated axons, are also present.
Vascular astrocytes, which are distributed across the cortical and subcortical regions, interface with the microvasculature to support BBB integrity and neurovascular coupling [38,85,88,89]. In addition to their morpho-functional diversity, molecular profiling studies demonstrate that cerebellar astrocytes are transcriptionally distinct from their telencephalic counterparts. This distinction is characterized by a unique signature including genes prevalently expressed by BG, such as the regulator of glutamate transport Sept4 [31].
Among cerebellar astrocytic populations, BG have been the most extensively characterized in terms of molecular identity, morphology, and function. These cells in PCL form overlapping territories and functional microdomains, performing critical roles throughout life. Both BG and other astrocytes originate from RGC progenitors in the ventricular zone (VZ) [85]. While BG retain their radial morphology and settle in PCL, other astrocytes migrate to the ML or GL. During cerebellar development, BG provide structural scaffolding for the expanding cerebellar plate and support the migration of GCs to the ML [90,91]. Postnatally, they contribute to the growth and remodeling of PC dendrites, as well as the stable formation of synaptic connections [92,93,94].
In adulthood, BG remain functionally active by maintaining structural integrity and regulating the synaptic microenvironment [95,96].
Their processes are enriched with AQP4 which is involved in the regulation of cellular volume, and KIR4.1 which is involved in ion buffering [86].
Furthermore, BG express the glutamate transporters GLAST, and GLT-1 as well as Ca2+-permeable AMPA receptors (AMPAR) and GABA-A receptors which enables them to respond dynamically to glutamate and GABA during synaptic activity [92,97].
BG processes tightly unsheathe PC synapses, thus contributing to ion buffering, neurotransmitter clearance, and glutamine production [95].
Ultrastructural analyses have revealed that BG PAPs alter their position in response to synaptic stimulation. This indicates a dynamic interaction with cerebellar synapses that facilitated the clearance of glutamate spill-out, enabling a higher level of regulation of this neurotransmitter than in other regions of the brain [98]. In vivo Ca2+ imaging reveals that BG exhibit localized and wave-like Ca2+ transients modulated by PF input, noradrenaline and AMPA receptors.
snRNAseq across all cerebellar lobules revealed a region-specific transcriptional heterogeneity in BG cells. A subpopulation, identified by specific genes as well as Mybpc1 and Wif1 [99], is enriched in lobules VI, IX, and X, suggesting that BG may exhibit distinct molecular profiles depending on lobular identity [87]. Such heterogeneity may reflect differential functional roles and vulnerability to aging.
Furthermore, topographic studies show that BG are organized into parasagittal domains aligned with PC compartments which are defined by markers such as Hsp25 [99,100]. This supports the notion of a shared modular organization between glia and neurons [100]. Additionally, the identity of astrocytes is shaped by the presence and activity of neurons rather than being determined autonomously. Neuronal input regulates the maturation of BG via NOTCH signaling, ultimately affecting neurotransmitter uptake and metabolic functions [101,102]. This pathway is conserved in human development, but declines with age, suggesting a deterioration in neuron-astrocyte cooperation over time. Emerging evidence supports the notion that the coordinated interactions between PCs, BG, parenchymal astrocytes, and oligodendroglia are essential for proper cerebellar maturation and long-term structural resilience. For instance, the secretion of SONIC HEDGEHOG (SHH) by PCs induces the diversification of the molecular profiles of BG and VAs which express the SHH receptors (PTCH1/2) differentially [89].
VAs are morphologically distinct from BG displaying a stellate morphology. Structurally, they do not ensheathe individual synapses, but instead surround GC clusters and wrap around glomeruli (MF terminals, Golgi neuron boutons and granule dendrites), without penetrating them [97,103]. VAs display spontaneous Ca2+ activity and Ca2+ waves, which are stimulated by purinergic signaling [97], suggesting that they modulate cerebellar processing in a fundamentally distinct manner to BG. However, their exact roles remain to be defined.
Overall, cerebellar astrocytes, and BG in particular, emerge as transcriptionally and functionally specialized glial subtypes that are tightly integrated into modular cerebellar circuits and are actively shaped by neuronal signals. Their unique roles in synaptic and metabolic regulation highlight the need to explore astrocyte diversity and vulnerability in the aging CBL in more detail.
3.2. Oligodendroglia
The majority of cerebellar OPCs in mice, determined by Olig2 expression, originate from precursors in the neuroepithelial region of the caudal midbrain and the ventral rhombomer1 and migrate to the cerebellar primordium [104,105]. In this first wave of cells, new OPCs are added from the cerebellar VZ and the fourth ventricle after birth. In humans, Zhong et al. (2023) [106] use scRNAseq to reconstruct the developmental OPC pathway and identify cells positive for Olig1 and Pdgfra in one of the progenitors clusters isolated from the VZ, the same germinal niche of PCs, astrocytes, and BG.
OLs begin to differentiate in the regions surrounding the DCN, which comprise a mixture of WM and GM. They then move progressively to the periphery in parallel with the formation of the cortical lobes, occupying both the axial WM and the cortical layers [86]. Mature OLs and myelination appeared during the first postnatal days. These newborn cells predominantly myelinate calbindin-positive PC axons in a retrograde manner (from their terminal field to the cell body) [86], and this specificity is maintained in mature WM [107]. The molecular cues and signaling pathways that guide the selective myelination of PCs from OLs during cerebellar development and in the adult remain to be clarified.
Myelination of PC axons is necessary for precise synaptic transmission to DCN neurons. In a rat model in which genetic deletion of MBP is followed by severe myelin deficiency, the loss of PC action potential (AP) synchronization is evident, followed by hyperexcitability of postsynaptic DCN neurons [108]. Furthermore, compact myelin is also fundamental to the health of PC axons and to the synapse maintenance. In the same rat model, fewer functional synapses are observed, in addition to anatomical alterations in the presynaptic terminals and a reduction in the number of active zones [108]. These findings have significant implications for understanding the cellular and synaptic basis of motor deficits in demyelinating diseases such as Multiple Sclerosis (MS), where cerebellar involvement and synaptic pathology have been observed in DCN [109].
Using rabies virus-mediated single oligodendrocyte labeling, and transgenic mouse lines, Battulga et al. (2024) [107] show that the differentiation of OPCs in adult WM and GL is mainly directed toward sheathing the afferent CFs from IO and MFs from different regions of the brain.
PFs from GCs in ML are never myelinated [110], even though OPCs are dispersed in this region and extend numerous processes among PC dendrites. These ML processes receive direct synapses from PFs and CFs [111]. It has been suggested that these innervations may provide an inhibitory signal to prevent further differentiation of OPCs in this region of the cerebellar cortex devoid of myelin [111].
PCs are the only output of the CBL, and their neuronal afferents are uniquely organized, receiving thousands of PFs from GCs and a single CFs from the IO. Uncorrected activity in these circuits can affect other connected brain structures, ultimately affecting motor and cognitive behavior. In this regard, the importance of myelin plasticity in neural circuit homeostasis is becoming evident [64,112]. An example of this adaptive response has been demonstrated in CFs.
These axons provide instructive error signals in various forms of learning and adaptive motor response by coding sensory input and generating synchronized signals in a small number of PCs [113,114]. Different CFs must also ensure synchronization if the length of their axons varies in relation to the distance and folding of different folia. To compensate for this, axons are myelinated to a different extent by OLs with longer internodal lengths, and fewer Ranvier nodes, making the conduction time uniform [115]. This response is crucial for the coordinate movements and could be affected by age alongside the reduced ability of older OPCs to differentiate and myelinate,
As in other CNS regions, OLs play a key role in maintaining neural circuits and long-term neuronal integrity. Furthermore, PCs have an exceptionally high metabolic demand due to the high number of excitatory synaptic connections. OLs support axonal energy metabolism by detecting axonal spiking and increasing glycolytic activity, suppling lactate and pyruvate in synergy with astrocytes [66,116,117]. This OL activity decreases with age, probably contributing to axonal abnormalities and increased vulnerability to excitotoxicity in PCs during cerebellar aging [118].
3.3. Microglia
Microglia in mouse and human are characterized by molecular heterogeneity in different CNS regions [119,120]. In the CBL, these cells are distributed across the cerebellar layers and contribute to multiple essential functions (reviewed in [121]). During development, microglia shape cerebellar connectivity by clearing apoptotic PCs [122] and regulating the elimination of CF-PC synapses [123]. Like other glial cell populations, cerebellar microglia exhibit specific characteristics. They are less densely localized than in other CNS regions, presenting decreased cell volume, less ramified morphology, and reduced branching sites. Conversely, cerebellar cells have a higher lysosomal content, often engulfed by nuclear debris, and exhibit high clearance activity efficiently phagocytizing apoptotic cells in vitro [124].
Different high-throughput approaches reveal that cerebellar microglia are characterized by differential gene expression patterns compared to other microglial cell populations residing in different CNS regions, in both mice and humans [11,78,80,119,120,124].
Grabert et al. (2016) [11] define the adult mouse cerebellar microglia as a cell population in a more immune-alert state, characterized by a higher expression of genes involved in pathogen/self-recognition, cell adhesion, chemotaxis, signaling integration, antigen presentation, and microbial killing/sequestration. Additionally, Ayata et al., (2018) [124], using a microglia-specific TRAP approach that ensures non-specific activation of microglia, shows that cerebellar cell population is characterized by a reduction in homeostatic microglia genes, which are normally expressed in a “physiological context”. Instead, genes for endocytosis, phagocytosis, and apoptotic cell clearance are upregulated.
The spatial heterogeneity of adult CNS microglia populations remains a subject of debate, partly due to the artifactual activation caused by the cell isolation technique and the selection of the marker genes used to enrich microglia in various scRNAseq studies. Li et al. (2019) [120] reported that several genes previously attributed to regional microglial heterogeneity [11] were also expressed by non-microglial cells. Nonetheless, cerebellar microglial heterogeneity has also been investigated through other approaches, including electrophysiological measurement and the assessment of morphological and epigenetic features. In vivo imaging studies in mice have demonstrated that cerebellar microglia exhibit distinct morphological and dynamic profiles compared to their cortical counterpart with region-specific phenotypes across cerebellar layers.
Nevertheless, these cells have a markedly less ramified morphology compared to their cortical counterparts, they respond to focal laser-induced injury with speed and directionality comparable to those of cortical microglia [125].
Morphological analyses have also identified differences in microglial density and process motility in the cerebellar cortex [125,126]. While the functional implications of these layer-specific patterns remain unclear, microglia in ML and PCL closely contact PC dendrites and the soma, suggesting an active role in monitoring these key neuronal elements.
The specific identity of cerebellar microglia is likely stated by local developmental cues and persists in adulthood, supporting the plasticity of cerebellar circuits. This regional specificity of the cerebellar microglia is also evident in aging. Importantly, these cells exhibit higher basal turnover rates and are more reactive to pathological stimuli compared to microglia in other regions of the CNS [11,73]. Inflammatory signaling within cerebellar microglia has been shown to enhance PC dendritic excitability, in contrast to the reduction in intrinsic excitability observed in cortical neurons under similar conditions [127]. Aberrant immune responses in PCs are also mediated by the activation of BG [128], leading to altered functional connectivity and behavioral changes, including deficits in social interaction, exploratory activity, and the emergence of repetitive behaviors [127].
4. CNS Aging
4.1. Hallmarks of CNS Aging
The aging of the CNS is a multifactorial and progressive process, marked by cumulative alterations at the anatomical, functional, cellular, and molecular levels. These alterations affect cognitive performance, synaptic plasticity, and neuroglial homeostasis. Macroscopic signs of CNS aging, such as regional atrophy and impaired connectivity, reflect underlying biological dysfunctions and often precede overt pathology. The most evident sign of atrophy is progressive reductions in both GM and WM volume, cortical thinning, sulci widening, and ventricular enlargement [129].
Although neuronal loss appears limited in many regions of the brain, extensive structural remodeling is observed, including dendritic retraction, spine loss, and synaptic reorganization, particularly affecting long-range projection neurons. These morphological changes are correlated with decreased expression of synaptic proteins and neurotransmitter receptors, ultimately impairing synaptic efficacy and interregional communication [130].
WM is particularly vulnerable to age-related degeneration. Magnetic resonance imaging (MRI) studies consistently report selective alterations in anterior brain regions, including the prefrontal cortex, anterior CC, and thalamic radiation, tracts critical to cognitive processing [131,132]. These tracts are among the last to undergo full myelination during neurodevelopment and among the first to degenerate with aging. Advanced diffusion tensor imaging (DTI) analyses in older individuals reveal a progressive reduction in fractional anisotropy, an established marker of microstructural integrity of WM, particularly affecting major associative pathways [133,134]. This alteration of WM leads to compromised cortico-cortical and cortico-subcortical connectivity which is associated with slower information processing and cognitive decline [135,136].
Functionally, aging is associated with a progressive loss of synchrony across the major brain networks. Resting-state functional MRI (rs-fMRI) studies consistently demonstrate that communication between brain regions becomes less coordinated with age. In parallel, aging is commonly associated with slower processing speed, reduced working memory capacity, impaired attention control, and diminished executive function, while vocabulary and semantic memory remain relatively preserved [137]. In animal models, aging is accompanied by spatial navigation deficits, motor impairment, and increased anxiety-like behavior [138]. These structural and behavioral changes reflect a progressive decline in the homeostatic capacity of brain networks and are accompanied by a series of deeply interconnected molecular and cellular hallmarks [137,138].
A growing body of evidence suggests that brain aging reflects a complex interplay between intrinsic genetic programs and extrinsic influences such as environmental exposures, leading to cumulative damage in both the neuronal and glial compartments. As reviewed by Lopez-Oudin (2023) [139], the major biological domains involved in this decline include genomic instability and progressive telomeric shortening, epigenetic changes, mitochondrial dysfunction and oxidative stress, disrupted Ca2+ signaling, impaired autophagy and protein homeostasis (proteostasis), neuroinflammation, vascular dysregulation, cellular senescence and reduced neurogenesis.
Together, these mechanisms impair intercellular communication and metabolic regulation, ultimately compromising the structural and functional resilience of neural tissue.
Age-associated mitochondrial alterations include the accumulation of somatic mitochondrial (mt)DNA mutations, decreased oxidative phosphorylation and increased reactive oxygen species (ROS) production, promoting redox imbalance, protein oxidation and activation of pro-inflammatory cascades [140,141]. These alterations compromise synaptic transmission and neuronal excitability, particularly in regions with high energy demand, such as the hippocampus and CBL. Impaired mitophagy, due to reduced activity of regulators such as PINK1, PARKIN, and NIX, further exacerbates mitochondrial dysfunction. Notably, experimental reactivation of mitophagy in aged mice and transgenic models of AD reduces protein aggregation and restores cognitive function [142].
Autophagy, a fundamental process responsible for the degradation and recycling of damaged proteins and organelles, declines markedly with age, contributing to altered proteostasis and chronic cellular stress. Neurons and glial cells exhibit reduced autophagosome biogenesis and lysosomal activity during aging, leading to progressive accumulation of misfolded proteins, dysfunctional organelles, and oxidative byproducts. For instance, defective autophagy is involved in the accumulation of neurotoxic proteins such as AMYLOID b, TAU, α-SYNUCLEIN, and TDP-43 [137,142]. Transcriptomic and proteomic studies report downregulation of key autophagy-related genes (e.g., Atg7, Beclin-1) and decreased lysosomal acidification in aged neurons and glia.
Importantly, astrocytes and microglia also exhibit reduced autophagic flux, which contributes to aberrant synaptic pruning, accumulation of lipid droplets, and chronic activation [142].
Ca2+ homeostasis is also disrupted during aging. Alterations include reduced buffering capacity, downregulation of RYRs and IP3 receptors, and impaired mitochondrial Ca2+ reuptake [137,143].
These changes alter synaptic plasticity, long-term potentiation (LTP), and neuronal network synchronization. Aged astrocytes display a reduced frequency and amplitude of spontaneous Ca2+ transients, further weakening the communication between glia and neuron [144].
Cellular senescence occurs in both neuronal and glial populations characterized by expression of markers such as p16INK4A and senescence associated β galactosidase (SA-β-gal), secretion of senescence-associated secretory phenotype (SASP) factors, and DNA damage [37,143]. PCs in CBL and cortical neurons exhibit senescence-like features, including chromatin remodeling, telomere attrition, and nuclear envelope disruption. Senescent glia, especially astrocytes and microglia, promote low-grade neuroinflammation known as ‘inflammaging’. This condition contributes to synaptic loss, neuronal death, and impaired myelin regeneration [37,137]. Crosstalk between senescent glial cells, which involves cytokines, chemokines, and complement proteins, reinforces the organization of a pro-degenerative environment [3,41].
Vascular dysfunction contributes to aging-related decline. Structural changes in the NVU include mislocalization of astrocyte endfeets, reduced coverage of pericytes, and endothelial tight junction breakdown, resulting in BBB leakage [145]. These alterations allow infiltration of immune cells and alter neurovascular coupling, thus fueling metabolic dysregulation and neuroinflammation [146].
At the epigenetic level, single cell transcriptomic and epigenomic studies have shown widespread age-related changes in gene expression and chromatin organization. Glial cells show greater transcriptional responsiveness than neurons [37]. Hallmarks include DNA hypomethylation, altered histone modifications, and decreased chromatin accessibility, with functional consequences for glial reactivity, immune signaling, and energy metabolism [143].
Importantly, aging trajectories vary across brain regions. High-throughput single cell and spatial omics approaches reveal that aging signatures emerge earlier or more intensely in subcortical structures such as the CBL, hippocampus, and hypothalamus, compared to neocortical areas [5,37]. These observations highlight the region- and cell-type-specific nature of CNS aging, shaped by intrinsic developmental programs, local circuit properties, and extrinsic factors.
4.2. Glial Cell Aging
For decades, cognitive and motor decline associated with brain aging has been primarily attributed to progressive neuronal loss. However, quantitative and morphological studies have challenged this view, showing that neuronal numbers remain relatively stable in most brain regions during healthy aging [81,147,148]. Instead, increasing evidence points to functional deterioration of glial cells as a primary driver of brain vulnerability [137,143]. During aging, glial cells undergo profound cell type-specific and region-dependent changes that compromise their ability to maintain neural homeostasis. Recent transcriptomic studies reveal that glia, particularly in vulnerable regions such as the hippocampus, CBL, and hypothalamus, exhibit stronger and earlier transcriptional responses to aging compared to neurons [3,37]. These findings have led to a conceptual change: glia are no longer viewed as passive victims of aging, but as active contributors to age-related brain dysfunction. Understanding how glial aging shapes the CNS environment is therefore crucial for elucidating the mechanisms of brain aging and for the development of targeted interventions.
4.2.1. Astrocytes
Astrocytes, historically considered passive support cells, are now recognized as active regulators of synaptic function, metabolic homeostasis and neuronal viability, CNS immunity, and brain resilience. During aging, they undergo substantial and regionally heterogeneous transformations that extend beyond morphological alterations to encompass transcriptional reprogramming and loss of homeostatic function. Although their overall number appears to be relatively preserved in most brain regions [147,148], astrocytes display marked molecular and functional changes [149] shaped by intrinsic aging processes and prolonged exposure to systemic and local stressors.
Bulk RNA-seq and snRNA-seq studies in rodents and humans have consistently identified astrocytes as the most transcriptionally responsive cell types during aging. Declining astrocytes downregulate genes involved in ion buffering (e.g., Kcnj10/Kir4.1), glutamate uptake (Slc1a2, Slc1a3), and cholesterol biosynthesis, while upregulating genes associated with immune activation, complement pathways (e.g., C3, C4b), and interferon signaling [3,5,149].
These changes indicate a shift toward a low-grade reactive state, distinct from the classical astrogliosis observed in acute injury.
Three-dimensional morphometric analyses of astrocytes in both human postmortem samples and rodent models have revealed age-associated structural remodeling. In the cortex and hippocampus, aged astrocytes exhibit reduced arbor complexity, retraction of fine processes, and decreased territorial coverage [150,151]. Astrocyte metabolism is also impaired by aging. Transcriptomic and lipidomic analyses of astrocytes from aged hippocampus and cortex reveal accumulation of lipid droplets, altered fatty acid metabolism, and impaired cholesterol biosynthesis, ultimately leading to reduced energetic support for neurons [3,152]. These effects are most pronounced in astrocytes located near WM or blood vessels, where metabolic demands are higher [149].
Furthermore, age-related mitochondrial dysfunction, characterized by reduced oxidative phosphorylation and increased mitochondrial fragmentation, further compromises astrocytic capacity to buffer redox stress and maintain neuron–glia metabolic coupling [153,154].
Alterations in Ca2+ signaling are another hallmark of astrocyte aging. In the hippocampus and CBL of aged rodents, astrocytes display a reduced frequency and amplitude of spontaneous Ca2+ transients, delayed responses to synaptic input, and dysregulated expression of Ca2+-handling proteins [144]. These deficits likely impair gliotransmission and compromise astrocytic modulation of synaptic activity.
In summary, astrocyte aging is a complex and multifactorial process characterized by transcriptional remodeling, reduced morphological complexity, metabolic dysfunction, and loss of neuroprotective functions. These changes do not occur uniformly, but are shaped by region, species, methodological variables, and environmental influences.
4.2.2. WM and Oligodendroglia
One of the most evident alterations associated with CNS aging is WM atrophy, which is linked to myelin degeneration and reduced myelin renewal. These changes are correlated with motor and cognitive impairments [155,156,157]. Since OPCs are primarily responsible for myelin production and maintenance, numerous studies have investigated their age-related changes over time [158,159].
Several findings indicate that while OPCs remain numerically stable with age, they become functionally impaired and more heterogeneous [47,160]. Their mitotic activity decreases [161], and they exhibit a reduced ability to differentiate into OLs, reflecting the decreased ability of the CNS to regenerate myelin in old age. Older OPCs display slower differentiation rates and diminished responsiveness to pro-differentiation stimuli, partly due to decreased paracrine signaling and altered expression of critical receptors such as GPR17 and APJ [162,163].
In addition, quiescent adult OPC population undergo transcriptional remodeling with age, characterized by increased activation of immune and death cell pathway such as STAT1 and TGFβ signaling, and downregulation of cell cycle regulators such as MYC and KRAS [47].
OLs, which are highly long-lived cells, also exhibit age-related alterations [52,164]. Allen et al. (2023) [81] demonstrate through a single cell spatial transcriptomic study of the frontal cortex and striatum that the abundance of OLs identified by Olig-2 expression decreased in old animals, while increasing the amount of those identified by Olig-3 expression. These OLs are characterized by a transcriptional signature marked by upregulation of inflammatory and innate immune response genes such as C4b and interleukin 33 (Il33), and downregulation of myelin-associated transcripts, including Mbp, Mog, and Mag [81].
Conversely, a human study by Duffy et al. (2023) [165] which analyzes two cortical regions (entorhinal cortex and middle temporal gyrus) and two subcortical regions (putamen and subventricular zone) relevant for NDs, does not reveal a significant change in the proportions of oligodendroglia cells with age. However, the transcriptional alterations in aged human OLs are consistent with those observed in mice.
In addition, the functionality of oligodendroglia declines with aging, reflecting the decrease in myelin production, and the appearance of thinner and shorter internodes. The mechanisms underlying these changes are complex and involve many biological aspects.
Ols are particularly susceptible to oxidative stress due to their high metabolic activity, which leads to prolonged exposure to cytotoxic molecules such as ROS. These cells have also been shown to have reduced antioxidant levels compared to astrocytes and that these levels decline with age [166,167]. Mitochondrial dysfunction further exacerbates this vulnerability [168].
Oxidative damage affects all major macromolecules, including DNA, leading to mutations and genomic instability. As with neurons, both OLs and OPCs accumulate DNA damage with age due to inefficient DNA repair mechanisms [169]. Oxidative stress can also induce senescence in OPCs and OLs through multiple mechanisms, a process thoroughly reviewed by Spaas et al. (2021) [170].
This senescent phenotype is the result of intrinsic metabolic stress and the perturbed niche.
For instance, senescence is documented in other glial cells during aging [171], particularly microglia, which closely interact with OPCs and OLs in the maintenance of myelin.
Furthermore, Gomez et al. (2024) [172] demonstrate that the region-specific emergence of senescent OPCs in the WM of aged mice is not uniform throughout the brain. Specifically, they demonstrate the existence of regional phenotypes by comparing WM OPCs from the hippocampal fimbria with GM cells from the dental gyrus. Aged OPCs show increased cell size, reduced density, and closer proximity to microglia. These OPCs also exhibit a shared gene expression profile marked by upregulation of genes associated with senescence and OL disease (Serp3n, Nol3, Skap2), suggesting functional vulnerability and altered cell–cell interactions. This evidence provides useful targets for future investigations [172].
Epigenetic regulation of gene expression, which plays a crucial role in lineage specification during development [173], is also affected by aging in oligodendroglia. Human OLs exhibit significant hypomethylation of CpG islands with age, reflecting a global relaxation of epigenetic control [174].
Similarly, aged OPCs from the rat spinal cord display reduced expression of Dnmt1 [175].
These alterations likely invalidate the transcriptional program necessary for remyelination. In aged mice, impaired recruitment of HDACs to inhibitory transcription factor promoters, such as Sox2 and Hes5, results in increased expression of these factors, which affects the differentiation of OPCs [176].
These epigenetic modifications may be reversible and influenced by the niche environment.
Aging is associated with increased extracellular matrix stiffness [177], and the accumulation of inhibitory molecules, such as hyaluronan and chondroitin sulfate proteoglycans, which impair the proliferation and differentiation of OPCs [178].
Cell–cell interactions are also recognized as important regulators of myelin health.
Microglia, astrocytes, and OLs could be considered a functional unit [69], with astrocytes and OLs physically interacting through connexins [179]. With age, both astrocytes and microglia adopt a senescence-associated secretory phenotype, as illustrated before, which creates a pro-inflammatory microenvironment detrimental to OL viability and function.
Recent studies (reviewed in [155]) have also revealed an increase in the infiltration of adaptive immune cells, particularly cytotoxic CD8+ T cells, in aged WM, especially in areas close to the lateral ventricles [180]. These cells are often located near OLs and microglia and trigger an IFNg-responsive state in both cells, ultimately leading to myelin loss. These findings underscore the complexity of cell–cell interactions in aging and their contribution to impaired OPC differentiation and OL function.
4.2.3. Microglia
Recent studies have revealed that microglia are highly dynamic and plastic cells that can undergo transitions along a continuum of functional states in response to intrinsic and extrinsic cues.
Aging has emerged as a key factor in modulating this plasticity [78,83,181]. Transcriptomic, proteomic, and morphological analyses have been instrumental in identifying aging-associated features of microglia. Given the involvement of these cells in the pathogenesis of several NDs, multiple transcriptomic studies have been performed in both mouse models and postmortem human tissue. Olah et al. (2018) [182] provided the first transcriptomic atlas of an older human dorsolateral prefrontal cortex, showing that microglia exhibit a distinct gene expression profile enriched for pathways related to DNA damage, telomere maintenance, and phagocytosis.
Importantly, many of these genes overlap with those found in Alzheimer disease (AD) and MS-associated profiles [182]. Interestingly, these transcriptomic changes are accompanied by parallel proteomic changes. Subsequent studies confirmed that aged microglia exhibit a pro-inflammatory transcriptional signature along with downregulation of homeostatic genes, including those involved in phagocytosis. These changes impair the clearance of apoptotic cells and myelin debris, a hallmark of aging [183,184,185].
Age-associated alterations in myelin contribute to the emergence of distinct microglia phenotypes in WM, known as WM-associated microglia (WAM). This state shares molecular features with disease-associated microglia (DAM) [186]. WAM are typically found in clusters or nodules engulfed with aberrant myelin and may initially exert adaptive and protective functions.
Successively, prolonged microglia activation promotes CD8+ T cell recruitment, triggering axonal degeneration and myelin loss [155] (see also Section 3.2). A hallmark of aged microglia is the acquisition of dystrophic morphology characterized by reduced ramification, cytoplasmic swelling, and fragmentation [187]. Although this morphology is primarily associated with pathology rather than aging per se [188], it frequently overlaps with the molecular features of senescence.
These features include increased p16INK4A expression, lipofuscin accumulation, SA-β-Gal activity and SASP components. Sex-specific microglial aging trajectories have also been described (reviewed in [189]). Sex differences in the microglia transcriptome have been linked to differential regulation of the immune process, cytokine-mediated signaling pathway, and phagocytosis. Female cells undergo a progressive aging process, whereas male microglia show a more abrupt transition [190]. The underlying mechanisms are not well understood, but they may contribute to sex-biased vulnerability to NDs. Finally, there is an ongoing debate about the need for a unified system for classifying and interpreting microglial states in different brain regions and conditions [17,83,181,191].
Table 1 schematizes the most relevant evidence synthesizing the altered functions of glial cells in the CNS aging.

Table 1.
Main Functions and aging-related changes in CNS glial cell types.
5. Cerebellar Aging
5.1. Hallmarks of Cerebellar Aging
With aging, the CBL undergoes different anatomical, molecular, and functional changes that affect balance, coordination, cognitive flexibility, and associative learning [192,193,194]. Although the overall loss of cerebellar volume appears to be less pronounced than that observed in the cortex, increasing evidence indicates that aging affects the CBL in a region-specific and cell-type-specific manner.
Early postmortem stereological analysis shows that the human CBL exhibits selective structural changes, with pronounced degeneration in the anterior lobe, a region involved primarily in motor control [195]. These changes included a ~40% reduction in GCs and PCs, a loss of cortical and WM volume, and a reduction in PC soma size in the absence of nuclear loss.
Wang et al. (2024) [196], integrating volumetric and morphological analyses and applying a specific algorithm to divide the organ into different subregions, obtain a more complete picture of cerebellar aging. The authors confirm a progressive reduction in cerebellar volume with age, including deep WM. Atrophy was most pronounced in vermis X, the lateral regions of the cerebellar hemispheres, the I-III lobules, and the medial portions of the posterior lobe.
Notably, although men generally have larger volumes than women in most regions of the CBL, they show slightly more pronounced age-related atrophy, particularly in the right crus II [196].
Cross-sectional data limit our understanding of temporal dynamics. However, few longitudinal studies of healthy individuals have also reported cerebellar shrinkage and region-specific atrophy [197,198]. Complementary approaches highlight the associations between preserved cerebellar structure and cognitive performance. For instance, Feng et al. (2025) [199] discovered that, among all regions of the brain examined, the VIIb lobule of the right CBL showed the strongest correlation with preserved common executive functions in aging adults.
Age-related functional reorganization has also been observed. Functional connectivity MRI (fcMRI) studies demonstrate that reduced volumes in Crus I/II and Vermis VI are associated with impaired working memory, attention, and executive function [200]. Task-based fcMRI reveals that older adults exhibit more diffuse and less distinct cerebellar activation patterns.
Cognitive tasks recruit motor-related regions (e.g., lobule V) and motor tasks engage cognitive regions (e.g., Crus I/II), indicating a form of compensatory scaffolding [201,202] that is similar to that observed in the prefrontal cortex [193]. Furthermore, aging results in less lateralized and more symmetric cerebellar activation, reflecting reduced topographic precision and efficiency of cerebellar-cortical integration [203]. Despite these indications of vulnerability, the CBL maintains a notable degree of structural and functional resilience and appears to resist the development of specific alterations in the early stage of NDs such as AD [201,204]. Very recent proteomic profiling of AD tissues indicates that the CBL displays higher expression levels of synaptotropic genes compared to the cerebral regions. This elevated expression is believed to confer neuroprotection, which can protect the CBL during dementia or AD progression [205].
All structural and functional alterations observed in CBL aging are accompanied by specific cellular changes.
PCs, which have high metabolic demands, are particularly susceptible to the effects of aging. They exhibit dendritic atrophy, spine loss, and reduced electrophysiological precision [206,207]. Woodruff-Pak et al. (2010) [207] observe selective PC loss, particularly in the vermis. Donofrio et al. (2025) [118] recently demonstrated that PC loss in aged mice occurs in a parasagittal pattern. They also found evidence of regionally selective PC loss in human postmortem samples.
Cerebellar aging involves not only neuronal atrophy but also changes in the cellular phenotype of neural tissue that could precede death.
Mitochondrial dysfunction is a recurring hallmark in the cerebellar compartments. Electron microscopy in rodents reveals that mitochondrial alterations occur early and selectively in specific types of cerebellar cells. In PCs, aging is associated with a reduced number of mitochondria, [208]. Functional impairments are also evident. The activity of cytochrome c oxidase (complex IV) declines significantly in an older CBL, [209]. Increased oxidative stress and reduced respiratory efficiency have also been described in neurons and astrocytes [210].
Ca2+ signaling is another critical domain affected by aging. PCs rely on tightly regulated intracellular Ca2+ signaling to maintain excitability, synaptic integration, and motor coordination.
In parallel, BG in aged mice exhibits increased spontaneous Ca2+ waves, which can perturb PC firing, particularly in lobule VI and the anterior vermis [211]. Notably, BG modulates PC excitability through Ca2+-dependent K+ uptake, which contributes to regulation of PC bistability and firing dynamics, highlighting the importance of glial Ca2+ signaling in shaping cerebellar output [212].
Autophagy dysfunction contributes to the accumulation of intracellular debris. Furthermore, autophagy dysfunction is emerging as a hallmark of age-related cerebellar diseases, such as Spinocerebellar Ataxias (SCAs) and mitochondrial disorders, where defective autophagy exacerbates PC degeneration [213,214]. Aging-related myelin atrophy is evident in human cerebellar WM, particularly within the cerebellar peduncles and DCN [215]. These changes are a consequence of the alterations that oligodendroglia undergo. They will be discussed in the following.
Although neurovascular and glymphatic dysfunctions are increasingly recognized as contributing factors to brain aging, specific data on the CBL remain limited. Only a few rodent studies have characterized cerebellar aging at the glial-vascular interface. Aged mice exhibit significant upregulation of KIR4.1 and AQP4 in cerebellar astrocytes [216] as well as a notable increase in spontaneous Ca2+ waves in BG, accompanied by local tissue hypoxia [211]. Together, these findings support the hypothesis that cerebellar NVU may become progressively dysregulated with age, potentially altering metabolic homeostasis and glial-vascular signaling.
5.2. Glial Cells Aging
Research on cerebellar aging has historically focused on neurons, particularly PCs. However, accumulating evidence now suggests that neuronal dysfunction in the aging CBL reflects broader changes in the cellular microenvironment. Specifically, signs of oxidative stress, mitochondrial dysfunction, and impaired homeostasis have been observed not only in neurons but also in cerebellar glial populations [205,207,217]. In some cases, these glial changes appear to precede or exacerbate neuronal vulnerability, raising the hypothesis that glial senescence may be a primary driver of cerebellar aging. Given their critical roles in synaptic regulation, immune surveillance, metabolic support, and myelin maintenance, cerebellar glial cells may represent a focal point in aging research.
The following sections explore how specific glial subtypes undergo structural and functional changes with age and how these alterations may contribute to cerebellar decline.
5.2.1. Astrocytes
Astrocytic aging in the CBL is increasingly recognized as a contributing factor to cellular and network-level dysfunction. A growing body of evidence indicates that aged astrocytes undergo a progressive remodeling of gene expression, morphology, and signaling capacity. Among them, BG exhibits altered Ca2+ dynamics, metabolic fragility, and early signs of reactive transformation.
One of the most comprehensive data sets is provided by Boisvert et al. (2018) [3], who analyzed astrocytes from multiple regions of the brain, including the CBL, in young and old mice using RiboTag RNA-seq. Transcriptomic profiling reveals that cerebellar astrocytes exhibit the most extensive changes in age-related gene expression between all regions analyzed, with more than 400 differentially expressed genes. These include upregulation of reactivity-associated markers, such as Gfap, Serp3n, as well as immune-related transcripts, such as C3, C4b, consistent with a shift towards a primed, moderate inflammatory state. They do not show upregulation of key interferon-responsive cytokines (e.g., Cxcl10 and Il6), nor do they significantly induce phagocytic receptors such as Mertk or Megf10 [3]. Instead, the older CBL appears to adopt a ‘permissive’ environment for synaptic remodeling, inducing in astrocytes the increased expression of Sparc, Thbs2, and Tgfβ2, without acquisition of a neurotoxic state similar to A1 [3]. More recent transcriptomic studies support these findings, reporting evidence of neuroimmune priming in aging astrocytes, including upregulation of MHC-I components (e.g., H2-K1, Tap1) and interferon-stimulated genes [13,218].
While this profile does not fully align with the canonical A1 signature [41], it shares several pro-inflammatory features and may represent a partially reactive CBL-specific state. In addition, Tsai et al. (2025) [13] also report a reduction in spatial proximity between aged microglia and astrocyte subtypes, suggesting altered glial communication. Although BG morphology was not directly assessed in that study, the findings support a model of multicellular remodeling in the older CBL. Despite substantial transcriptional changes, the core astrocytic homeostatic genes, including Slc1a2 (Glt1), Aqp4, Gja1 (connexin-43), Glul, and Kcnj10 (Kir4.1), remain largely stable, indicating preserved capacity for synaptic support and metabolic regulation. In contrast, genes involved in cholesterol biosynthesis pathways are significantly downregulated (e.g., Hmgcr, Fdps, Sqle), whereas cholesterol transport genes (e.g., ApoE and Abcg1) are upregulated, suggesting an adaptive remodeling of membrane metabolism in the aging CBL.
Taken together, these findings indicate that although aging induces significant transcriptomic remodeling in cerebellar astrocytes, many core functions are preserved. These age-related traits should be interpreted considering the intrinsic astrocyte identity of the CBL, which differs from that of other regions of the brain in adulthood. Even in adult mice, cerebellar astrocytes differ substantially from cortical populations, exhibiting higher basal expression of genes involved in synaptic regulation (e.g., Thbs1-4, Sparcl1, and C3) and structural components (e.g., Gfap, Aqp4, Vim), while maintaining stable levels of key transporters (Slc1a2, Slc1a3, and Slc6a11) and metabolic enzymes (Glul). This distinct molecular signature likely contributes to their differential vulnerability and response to aging [3]. These transcriptomic findings are supported by morphological and physiological data from rodent models. In aged rats, cerebellar astrocytes show features of cellular senescence, including hypertrophy and hyperplasia, increased GFAP immunoreactivity and lipofuscin accumulation, hallmarks of astrocytic senescence [219].
Although some studies report modest increases in GFAP in cortical and subcortical regions with aging [220], similar changes in the CBL have yet to be consistently demonstrated. Nevertheless, the absence of overt astrocyte loss or gliopathy suggests the presence of region-specific, low-grade astrogliosis in the aging CBL. In this context, BG may play a central role in preserving cerebellar architecture. Although the aging trajectory of BG remains poorly defined, recent scRNAseq analyses reveal that BG are enriched in genes involved in synaptic organization, glutamate homeostasis, and metabolic coupling with neurons [27]. This unique transcriptional profile suggests that any age-related perturbation, such as oxidative stress, energy deprivation, or neurotransmitter imbalance, is more likely to impact BG, potentially triggering early astroglial dysfunction in the cerebellar cortex. This hypothesis is consistent with the astroglia alterations observed in ML of a murine model of aging induced by chronic exposure to D-galactose that causes oxidative stress along with significant PC degeneration and cortical thinning.
Due to their highly specialized morphology, radial organization and proximity to PCs, BGs are likely involved in early remodeling during aging. Spatial transcriptomic data show that BG form a distinct transcriptional cluster enriched in lobules VI, IX, and X of the mouse CBL, reinforcing this hypothesis [87]. These lobules are characterized by high metabolic demand and functional complexity. In particular, the same posterior regions show early signs of morphological remodeling and functional decline in the CBL aging of both humans and rodents and are involved in sensorimotor and cognitive integration [194]. This regional enrichment suggests that BG may be differentially susceptible to age-related metabolic, synaptic, or vascular stressors, even in the absence of obvious morphological degeneration.
Experimental models provide functional insights into BG behavior under stress. In acute metabolic stress, such as oxygen and glucose deprivation, these cells respond rapidly with membrane depolarization and Ca2+ influx, but exhibit limited recovery, indicating metabolic fragility [221]. Chronic alterations in BG Ca2+ dynamics, such as increased spontaneous transients and prolonged responses, have been linked to impaired PC bistability and reduced firing precision, particularly in motor-related lobules such as lobule VI and the anterior vermis [211,222]. Several lines of evidence suggest that aging may alter the fine-tuned interaction between BG and PCs. In aged mice, BG exhibits up to 20-fold increase in spontaneous Ca2+ transients under hypoxic conditions, which is interpreted as a sign of impaired metabolic and signaling homeostasis.
In SCAs, BG exhibit early dysfunction, including reduced expression of KIR4.1, GLAST, and adhesion molecules, leading to impaired glutamate clearance and excitotoxic stress in PCs [223,224]. Notably, glial reactivity and glia-synaptic disruption often precede neuronal degeneration, supporting the idea that astrocytic pathology makes a primary, cell-autonomous contribution to cerebellar disorders and aging.
5.2.2. WM and Oligodendroglia
Cerebellar WM undergoes structural alteration during aging. However, data on the extent and distribution of these changes are inconsistent due to differences in experimental design and age ranges in the studies [225,226,227]. WM loss related to aging is frequently accompanied by the emergence of focal lesions that appear hyperintense on MRI (WM hyperintensity, WMH). In humans, DTI studies have documented age-related reductions in the integrity of cerebellar WM, especially in the fronto-cerebellar tracts [134]. Both the number and the volume of WMHs have been correlated with changes in brain connectivity, including in cerebellar networks [228,229].
Despite the growing interest in the involvement of the cerebellar WM in NDs and in late-onset SCA, notably type 6, which, unlike most hereditary ataxias, is largely restricted to the CBL [230], the response of oligodendroglial cells during healthy aging remains poorly understood.
While the role of adaptive immune reactions in myelin pathology has been explored in other regions of the brain, the CBL has received limited attention in this context. Nonetheless, it is known to be susceptible to autoimmune disorders [231], and one proposed mechanism involves the distinctive features of the blood–cerebrospinal fluid barrier (BCSFB), which may allow immune cell infiltration via the choroid plexus of the fourth ventricle and the pial vasculature between folia.
5.2.3. Microglia
Age-related microglia dystrophy is particularly evident in the CBL, as shown in murine models, where these cells exhibit process thickening, deramification of processes, and hypertrophy of the cell body. Such changes are especially evident in the WM of the inferior cerebellar peduncles, where microglia aggregate and show markedly thickened processes [232]. Beyond morphological alterations, cerebellar microglia also undergo substantial transcriptional remodeling with aging among different cell types.
Hahn et al. (2023) [12] identified a common aging signature (CAS) in mouse brain RNA, comprising 82 genes primarily related to immune and inflammatory pathways, many of which are involved in microglial regulation. The CBL showed one of the highest rates of changes in CAS expression, comparable to the striatum and caudate putamen and more pronounced than those of the cortex. Notably, CAS-related gene expression increased in aged brain regions, reaching a greater extent in cerebellar microglia [12].
These findings are consistent with previous data from Grabert et al. (2016) [11], who reported that adult cerebellar microglia exhibit increased immunological responsiveness and sensitivity to aging compared to those of other brain regions. This is characterized by elevated expression of interferon-responsive genes and cytokine-mediated signaling pathways.
Very recently, Tsai et al. (2025) [13], analyzing sorted cerebellar microglia isolated from aged mice, obtain an RNA signature coincident with a neuroprotective functional state [13] corresponding to upregulation of ApoE, Stat1, Axl, alongside the downregulation of genes involved in lipid droplet accumulation, features characteristic of AD-related degeneration [233].
Interestingly, applying multiplexed error-robust fluorescence in situ hybridization (MERFISH), to localize genes relevant for aging, neuroinflammation and cellular stress, Tsai et al. (2025) [13] further reveal a distinct, age-related, spatial redistribution of microglia in the CBL. In aged mice, microglia are predominantly localized within GL, in proximity to GCs, and expressed genes such as Cbln1, Cdk2, and Cxcl2, suggesting a region-specific adaptive response to aging. The molecular phenotype of aged microglia may initially suggest a protective state that supports debris clearance and homeostatic maintenance. However, with time and sustained stress, this phenotype may shift toward a more reactive and damage-prone state. Notably, many of these ‘activated’ molecular traits are also observed in aging cerebellar astrocytes [3,4,32], raising the hypothesis of a coordinated multicellular neuroinflammatory response in the older CBL.
The CBL is increasingly recognized as a critical node in NDs [234]. In the early stages of NDs, microglia proliferate and exert neuroprotective functions. However, as the disease progresses, microglia adopt a pro-inflammatory phenotype [235,236,237,238], leading to increased neuronal vulnerability and degeneration. Several evidence show an increased number of microglia cells in the CBL of AD patients [239,240,241], as well as in relevant animal models and this cells express Trem2, a gene involved in the resiliency to AD pathogenesis in CNS [242]. Nonetheless, although the CBL appears to be more resilient to neurodegeneration than other regions of the brain, the precise mechanisms underlying this resistance need to be fully elucidated.
Table 2 summarizes the most significant findings regarding the altered functions of glial cells in CBL aging.

Table 2.
Main functions and aging-related changes in CBL glial cell types.
6. Conclusions
This review highlights region-specific vulnerabilities and unexpected resilience capacities during CBL aging. While brain aging is determined by the cumulative impact of environmental, biological, and behavioral exposures, collectively called the ‘exposome’, the CBL follows a distinct trajectory, marked by selective glial remodeling and relative structural preservation.
As illustrated in Figure 1, which schematizes the main glial alterations in the adult and older CNS, aging affects glial cell populations in a coordinated yet regionally diversified manner. These general changes are particularly relevant when comparing the cerebellum to other CNS regions.
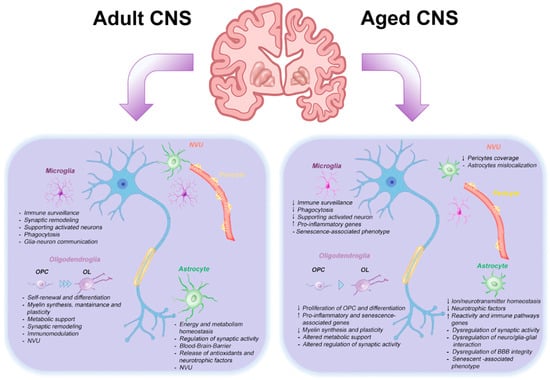
Figure 1.
Hallmarks of CNS aging. The glial cells’ characteristics in adult and healthy older CNSs are schematized. NVU: neurovascular unit; OPC: oligodendrocyte progenitor cell; OL: mature oligodendrocyte.
The highly laminar architecture, dense synaptic organization, and functional compartmentalization across the lobules provide a uniquely defined anatomical context in which interactions between glia and neurons can be examined with spatial precision. These features make the CBL a valuable system for investigating how glial cells mediate protective versus maladaptive responses across subregions involved in motor and cognitive functions.
Comparative analyses reveal significant differences in glial aging between the CBL and other regions of the CNS. As summarized in Figure 2, cerebellar astrocytes, especially BG, display a distinct transcriptional profile characterized by moderate reactivity and partial immune priming without transitioning to a fully neurotoxic state. BG undergo early and localized remodeling, particularly in Ca2+ dynamics and synaptic regulation, while maintaining key homeostatic functions, suggesting controlled adaptation rather than degeneration.
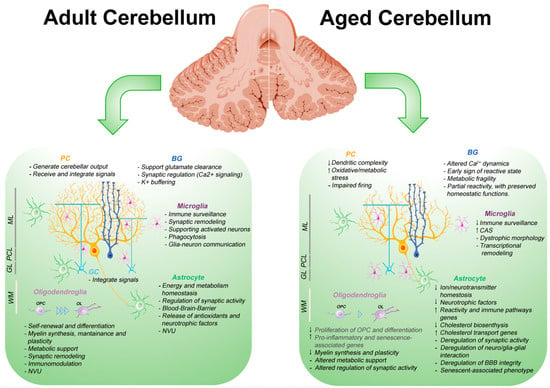
Figure 2.
Hallmarks of the CBL aging. The glial cells characteristics in adult and healthy aged CBL are schematized. Cortical layers of the mature CBL are labeled on the right as follows: ML: molecular layer; PCL: Purkinje cell layer; GL: granule layer; WM: white matter. PC: Purkinje cell; GC: granule cell; BG: Bergman Glia; OPC: oligodendrocyte progenitor cell; OL: mature oligodendrocyte; NVU: Neurovascular unit; CAS: common aging signature. Statements in gray are speculative and derived from pathological models or extrapolated from cortical data, due to limited evidence in the cerebellum.
Furthermore, microglial aging in the CBL is distinct. These cells exhibit an accelerated expression of CAS and undergo spatial redistribution, especially in GL. This may reflect enhanced neuroimmune surveillance or an adaptive effort to preserve tissue integrity.
Oligodendroglial changes are less well defined, but initial findings suggest region-specific vulnerabilities in WM integrity, with potential implications for NDs.
Despite these cellular changes, the CBL maintains substantial anatomical and functional integrity throughout its lifetime. Neuroimaging studies demonstrated that there is a reduction in circuit segregation and topographic specificity with age, along with increased recruitment of compensatory networks, supporting the notion of a cerebellar cognitive reserve.
However, there are still important questions to answer. The boundary between adaptive glial remodeling and functional decline remains unclear. The reversibility of these glial states and their potential as therapeutic targets are open questions, as are the roles of sex differences and lobular specificity in shaping aging trajectories. To address these issues, future research should integrate spatially resolved, multi-omics approaches with advanced human-relevant models, including human induced pluripotent stem cells (hiPSC)-derived cerebellar glial cells, cerebellar organoids, long-term glia-neurons co-cultures, and chimeric systems. These tools will be essential to clarify how glial cells contribute to cerebellar aging. Finally, recent evidence indicates that the CBL displays increased baseline expression of synaptotropic and neuroprotective genes and exhibits relative preservation in early AD, suggesting the importance of studying the role of glial networks in supporting resilience. Identification of CBL-specific biomarkers of glial aging has the potential to develop new strategies for the preservation of brain function in aging populations.
A concise summary box of the main concepts discussed in this review is provided below.
Author Contributions
G.L.S. and D.F. contributed to the writing and editing of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
CBL: Cerebellum; CNS: Central Nervous System; WM: White matter; GM: gray matter; ND: Neurodegenerative Disease; BBB: Blood–brain barrier; RGC: Radial glia cell; OPC: Oligodendrocyte Precursor Cell; OL: Oligodendrocyte; BAM: Border-Associated Macrophage; CSF: Cerebrospinal Fluid; CC: Corpus Callosum; GO: Gene Ontology; TRAP: Translating Ribosome Affinity Purification; scRNA-seq: single cell RNA-sequencing; snRNA-seq: single nuclear RNA-sequencing; FA: Fibrous Astrocyte; PAP: Perisynaptic Astrocytic Process; NVU: Neurovascular Unit; ML: Molecular Layer; PCL: Purkinje Cell Layer; PC: Purkinje Cell; GL: Granular Layer; GC Granule Cell; DCN: Deep Cerebellar Nuclei; CF: Climbing Fiber; IO: Inferior Olive; PF: Parallel Fiber; MF: Mossy Fiber; BG: Bergmann Glia; VA: Velate astrocyte; VZ: Ventricular Zone; SHH: Sonic Hedgehog; AP: action potential; MS: Multiple Sclerosis; MRI: Magnetic Resonance Imaging; DTI: Diffusion Tensor Imaging; rs-fMRI: resting-state functional MRI; fcMRI: functional connectivity MRI; mt: mitochondrial; ROS: Reactive Oxygen Species; LTP: Long-Term Potentiation; SASP: Senescence-Associated Secretory Phenotype; AD: Alzheimer Disease; WAM: WM-associated microglia; DAM: Disease-Associated Microglia; SCA: SpinoCerebellar Ataxia; WMH: WM hyperintensity; BCSFB: Blood–Cerebrospinal Fluid Barrier; CAS: Common Aging Signature; MERFISH: Multiplexed Error-Robust Fluorescence In Situ Hybridization; hiPSC: human induced Pluripotent Stem Cell.
List of Full Gene and Protein Names
| Genes | |
| Abcg1 | ATP Binding Cassette Subfamily G Member 1 |
| Aldoc (Zebrin II) | Aldolase C |
| Atg7 | Autophagy Related 7 |
| Beclin-1 | Beclin 1 |
| C3 | Complement Component 3 |
| C4b | Complement Component 4B |
| Cbln1 | Precerebellin 1 Precursor |
| Cdk2 | Cyclin Dependent Kinase 2 |
| C-fos | FBJ Murine Osteosarcoma Viral Oncogene |
| C-jun | JUN Proto-Oncogene |
| Cxcl10 | C-X-C Motif Chemokine Ligand 10 |
| Cxcl2 | C-X-C Motif Chemokine Ligand 2 |
| Fdps | Farnesyl Diphosphate Synthase |
| Gfap | Glial Fibrillary Acidic Protein |
| Glul | Glutamate-Ammonia Ligase |
| H2-K1 | Histocompatibility 2, K1, K Region |
| Hes5 | Hes family bHLH transcription factor 5 |
| Hmgcr | 3-Hydroxy-3-Methylglutaryl-CoA Reductase |
| Il33 | Interleukin 33 |
| Il6 | Interleukin 6 |
| Kcnj10 | Potassium Inwardly Rectifying Channel Subfamily J Member 10 |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog |
| Lama2 | Laminin Subunit Alpha 2 |
| Megf10 | Multiple EGF Like Domains 10 |
| Mertk | MER Proto-Oncogene, Tyrosine Kinase |
| Mbp | Myelin Basic Protein |
| Mag | Myelin Associated Glycoprotein |
| Mog | Myelin Oligodendrocyte Glycoprotein |
| Mybpc1 | Myosin Binding Protein C1 |
| Nol3 | Nucleolar Protein 3 |
| Olig1 | Oligodendrocyte Transcription Factor 1 |
| Olig-2 | Oligodendrocyte Transcription Factor 2 |
| Olig-3 | Oligodendrocyte Transcription Factor 3 |
| Pdgfr-a | Platelet Derived Growth Factor Receptor Alpha |
| Sept4 | Septin 4 |
| Serp3n | Serpin Family 3 Member N |
| SHH | Sonic Hedgehog |
| Skap2 | Src Kinase Associated Phosphoprotein 2 |
| Slc1a2 | Solute Carrier Family 1 Member 2 (EAAT2) |
| Slc1a3 | Solute Carrier Family 1 Member 3 (EAAT1) |
| Slc6a11 | Solute Carrier Family 6 Member 11 |
| Sox2 | SRY-Box Transcription Factor 2 |
| Sparc | Secreted Protein Acidic and Rich in Cysteine |
| Sqle | Squalene Epoxidase |
| Tgfβ2 | Transforming Growth Factor Beta 2 |
| Thbs1–4 | Thrombospondin 1 to 4 |
| Thbs2 | Thrombospondin 2 |
| Vim | Vimentin |
| Wif1 | WNT Inhibitory Factor 1 |
| Zebrin II | see Aldoc |
| Proteins | |
| AMPAR | α-Amino-3-Hydroxy-5-Methyl-4-Isoxazolepropionic Acid Receptor |
| APJ | Apelin Receptor |
| ApoE | Apolipoprotein E |
| AQP4 | Aquaporin 4 |
| C3 | Complement Component 3 |
| C4b | Complement Component 4B |
| CALB | Calbindin |
| GABA | Gamma-Aminobutyric Acid |
| GLAST | Glutamate Aspartate Transporter |
| GLT-1 | Glutamate Transporter 1 |
| GJA1 | Gap Junction Alpha-1 Protein (Connexin-43) |
| GPR17 | G Protein-Coupled Receptor 17 |
| HDACs | Histone Deacetylases |
| IP3 | Inositol 1,4,5-trisphosphate |
| KIR4.1 | Inward Rectifier Potassium Channel 4.1 |
| MAG | Myelin-Associated Glycoprotein |
| MBP | Myelin Basic Protein |
| MOG | Myelin Oligodendrocyte Glycoprotein |
| MYC | MYC Proto-Oncogene |
| NG2 | Neural/Glial Antigen 2 |
| NIX | BNIP3-Like Protein |
| NOTCH | Notch Receptor |
| P16INK4A | Cyclin Dependent Kinase Inhibitor 2A |
| PARKIN | E3 Ubiquitin-Protein Ligase Parkin |
| PDGFRα | Platelet Derived Growth Factor Receptor Alpha |
| PINK1 | PTEN-Induced Kinase 1 |
| PTCH1/2 | Patched 1 and 2 Receptors |
| RYR3 | Ryanodine Receptor 3 |
| SA-β-Gal | Senescence-Associated β-Galactosidase |
| SHH | Sonic Hedgehog |
| STAT1 | Signal Transducer and Activator of Transcription 1 |
| TAP1 | Transporter Associated with Antigen Processing 1 |
| TDP-43 | TAR DNA Binding Protein 43 |
| TAU | Microtubule-Associated Protein Tau |
| TGFβ | Transforming Growth Factor Beta |
References
- Oveisgharan, S.; Wang, T.; Barnes, L.L.; Schneider, J.A.; Bennett, D.A.; Buchman, A.S. The Time Course of Motor and Cognitive Decline in Older Adults and Their Associations with Brain Pathologies: A Multicohort Study. Lancet Healthy Longev. 2024, 5, E336–E345. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, X.; Zhang, Y.; Zheng, X.; Cepeda, C.; Wang, Y.; Duan, S.; Tong, X. Interactions of Glial Cells with Neuronal Synapses, from Astrocytes to Microglia and Oligodendrocyte Lineage Cells. Glia 2023, 71, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Boisvert, M.M.; Erikson, G.A.; Shokhirev, M.N.; Allen, N.J. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep. 2018, 22, 269–285. [Google Scholar] [CrossRef]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal Aging Induces A1-like Astrocyte Reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef]
- Soreq, L.; Rose, J.; Soreq, E.; Hardy, J.; Trabzuni, D.; Cookson, M.R.; Smith, C.; Ryten, M.; Patani, R.; Ule, J. Major Shifts in Glial Regional Identity Are a Transcriptional Hallmark of Human Brain Aging. Cell Rep. 2017, 18, 557–570. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Zhang, M.; Wen, J.; Wang, S.; Yuan, M.; Sun, H.; Shu, L.; Yang, X.; Pu, Y.; Cai, Z. Microglia in Brain Aging: An Overview of Recent Basic Science and Clinical Research Developments. J. Biomed. Res. 2024, 38, 122–136. [Google Scholar] [CrossRef]
- Jun, S.; Park, H.; Kim, M.; Kang, S.; Kim, T.; Kim, D.; Yamamoto, Y.; Tanaka-Yamamoto, K. Increased Understanding of Complex Neuronal Circuits in the Cerebellar Cortex. Front. Cell. Neurosci. 2024, 18, 1487362. [Google Scholar] [CrossRef]
- Habas, C.; Kamdar, N.; Nguyen, D.; Prater, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct Cerebellar Contributions to Intrinsic Connectivity Networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef]
- Buckner, R.L. The CBL and Cognitive Function: 25 Years of Insight from Anatomy and Neuroimaging. Neuron 2013, 80, 807–815. [Google Scholar] [CrossRef]
- Zhang, P.; Duan, L.; Ou, Y.; Ling, Q.; Cao, L.; Qian, H.; Zhang, J.; Wang, J.; Yuan, X. The CBL and Cognitive Neural Networks. Front. Hum. Neurosci. 2023, 17, 1197459. [Google Scholar] [CrossRef]
- Grabert, K.; Michoel, T.; Karavolos, M.H.; Clohisey, S.; Baillie, J.K.; Stevens, M.P.; Freeman, T.C.; Summers, K.M.; McColl, B.W. Microglial Brain Region-Dependent Diversity and Selective Regional Sensitivities to Aging. Nat. Neurosci. 2016, 19, 504–516. [Google Scholar] [CrossRef]
- Hahn, O.; Foltz, A.G.; Atkins, M.; Kedir, B.; Moran-Losada, P.; Guldner, I.H.; Munson, C.; Kern, F.; Pálovics, R.; Lu, N.; et al. Atlas of the Aging Mouse Brain Reveals White Matter as Vulnerable Foci. Cell 2023, 186, 4117–4133.e22. [Google Scholar] [CrossRef] [PubMed]
- Tsai, A.P.; Henze, D.E.; Lopez, E.R.; Haberberger, J.; Dong, C.; Lu, N.; Atkins, M.; Costa, E.K.; Farinas, A.; Oh, H.S.-H.; et al. Spatial and Molecular Insights into Microglial Roles in Cerebellar Aging. Biorxiv 2025, 2025, 640978. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Ho, M.S.; Zorec, R.; Parpura, V. The Concept of Neuroglia. Adv. Exp. Med. Biol. 2019, 1175, 1–13. [Google Scholar] [CrossRef]
- Won, W.; Bhalla, M.; Lee, J.-H.; Lee, C.J. Astrocytes as Key Regulators of Neural Signaling in Health and Disease. Annu. Rev. Neurosci. 2025, 48, 251–276. [Google Scholar] [CrossRef]
- Buchanan, J.; Cheadle, L. Many. Lives Oligodendrocyte Precursor Cell. Annu. Rev. Neurosci. 2025, 48, 425–444. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, L.; Nazlie Mohebiany, A.; Premereur, J.; Polanco Miquel, P.; Bijnens, B.; Van de Walle, P.; Fattorelli, N.; Mancuso, R. Microglia Heterogeneity, Modeling and Cell-State Annotation in Development and Neurodegeneration. Nat. Neurosci. 2025, 28, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Vara-Pérez, M.; Movahedi, K. Border-Associated Macrophages as Gatekeepers of Brain Homeostasis and Immunity. Immunity 2025, 58, 1085–1100. [Google Scholar] [CrossRef]
- Deng, S.; Gan, L.; Liu, C.; Xu, T.; Zhou, S.; Guo, Y.; Zhang, Z.; Yang, G.-Y.; Tian, H.; Tang, Y. Roles of Ependymal Cells in the Physiology and Pathology of the Central Nervous System. Aging Dis. 2023, 14, 468–483. [Google Scholar] [CrossRef]
- Markey, K.M.; Saunders, J.C.; Smuts, J.; von Reyn, C.R.; Garcia, A.D.R. Astrocyte Development—More Questions than Answers. Front. Cell Dev. Biol. 2023, 11, 1063843. [Google Scholar] [CrossRef]
- Khakh, B.S.; Deneen, B. The Emerging Nature of Astrocyte Diversity. Annu. Rev. Neurosci. 2019, 42, 187–207. [Google Scholar] [CrossRef]
- Westergard, T.; Rothstein, J.D. Astrocyte Diversity: Current Insights and Future Directions. Neurochem. Res. 2020, 45, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.H.; Raff, M.C. Fibrous and Protoplasmic Astrocytes Are Biochemically and Developmentally Distinct. J. Neurosci. 1984, 4, 585–592. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of Astrocyte Functions and Phenotypes in Neural Circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Köhler, S.; Winkler, U.; Hirrlinger, J. Heterogeneity of Astrocytes in Grey and White Matter. Neurochem. Res. 2021, 46, 3–14. [Google Scholar] [CrossRef]
- Bushong, E.A.; Martone, M.E.; Jones, Y.Z.; Ellisman, M.H. Protoplasmic Astrocytes in CA1 Stratum Radiatum Occupy Separate Anatomical Domains. J. Neurosci. 2002, 22, 183–192. [Google Scholar] [CrossRef]
- Bocchi, R.; Thorwirth, M.; Simon-Ebert, T.; Koupourtidou, C.; Clavreul, S.; Kolf, K.; Della Vecchia, P.; Bottes, S.; Jessberger, S.; Zhou, J.; et al. Astrocyte Heterogeneity Reveals Region-Specific Astrogenesis in the White Matter. Nat. Neurosci. 2025, 28, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Osório, M.J.; Kress, B.; Sanggaard, S.; Nedergaard, M. White Matter Astrocytes in Health and Disease. Neuroscience 2014, 276, 161–173. [Google Scholar] [CrossRef]
- Chai, H.; Diaz-Castro, B.; Shigetomi, E.; Monte, E.; Octeau, J.C.; Yu, X.; Cohn, W.; Rajendran, P.S.; Vondriska, T.M.; Whitelegge, J.P.; et al. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 2017, 95, 531–549.e9. [Google Scholar] [CrossRef] [PubMed]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of Region-Specific Astrocyte Subtypes at Single Cell Resolution. Nat. Commun. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular Basis of Astrocyte Diversity and Morphology across the CNS in Health and Disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Holt, M.G. Astrocyte Heterogeneity and Interactions with Local Neural Circuits. Essays Biochem. 2023, 67, 93–106. [Google Scholar] [CrossRef]
- Hasel, P.; Dando, O.; Jiwaji, Z.; Baxter, P.; Todd, A.C.; Heron, S.; Márkus, N.M.; McQueen, J.; Hampton, D.W.; Torvell, M.; et al. Neurons and Neuronal Activity Control Gene Expression in Astrocytes to Regulate Their Development and Metabolism. Nat. Commun. 2017, 8, 15132. [Google Scholar] [CrossRef]
- Sakers, K.; Lake, A.M.; Khazanchi, R.; Ouwenga, R.; Vasek, M.J.; Dani, A.; Dougherty, J.D. Astrocytes Locally Translate Transcripts in Their Peripheral Processes. Proc. Natl. Acad. Sci. USA 2017, 114, E3830–E3838. [Google Scholar] [CrossRef] [PubMed]
- Mazaré, N.; Oudart, M.; Moulard, J.; Cheung, G.; Tortuyaux, R.; Mailly, P.; Mazaud, D.; Bemelmans, A.-P.; Boulay, A.-C.; Blugeon, C.; et al. Local Translation in Perisynaptic Astrocytic Processes Is Specific and Changes after Fear Conditioning. Cell Rep. 2020, 32, 108076. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Liu, Z.; Jiang, X.; Chen, M.B.; Dong, H.; Liu, J.; Südhof, T.C.; Quake, S.R. Spatial Transcriptomics Reveal Neuron-Astrocyte Synergy in Long-Term Memory. Nature 2024, 627, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Vasile, F.; Dossi, E.; Rouach, N. Human Astrocytes: Structure and Functions in the Healthy Brain. Brain Struct. Funct. 2017, 222, 2017–2029. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Sweet Sixteen for ANLS. J. Cereb. Blood Flow. Metab. 2012, 32, 1152–1166. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2022, 12, 825816. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Cameron, E.G.; Nahmou, M.; Toth, A.B.; Heo, L.; Tanasa, B.; Dalal, R.; Yan, W.; Nallagatla, P.; Xia, X.; Hay, S.; et al. A Molecular Switch for Neuroprotective Astrocyte Reactivity. Nature 2024, 626, 574–582. [Google Scholar] [CrossRef]
- Elbaz, B.; Popko, B. Molecular Control of Oligodendrocyte Development. Trends Neurosci. 2019, 42, 263–277. [Google Scholar] [CrossRef]
- Nishiyama, A.; Shimizu, T.; Sherafat, A.; Richardson, W.D. Life-Long Oligodendrocyte Development and Plasticity. Semin. Cell Dev. Biol. 2021, 116, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Nishiyama, A.; Hughes, E.G. Features, Fates, and Functions of Oligodendrocyte Precursor Cells. Cold Spring Harb. Perspect. Biol. 2024, 16, a041425. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.; Kim, A.A.; Neumann, B.; Doze, V.N.; Xu, Y.K.T.; Mironova, Y.A.; Slosberg, J.; Goff, L.A.; Franklin, R.J.M.; Bergles, D.E. Transcriptional Profiles of Mouse Oligodendrocyte Precursor Cells across the Lifespan. Nat. Aging 2025, 5, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.A.; Futia, G.L.; Stockton, M.E.; Budoff, S.A.; Ramirez, A.N.; Ozbay, B.; Tzang, O.; Kilborn, K.; Poleg-Polsky, A.; Restrepo, D.; et al. Long-Term in Vivo Three-Photon Imaging Reveals Region-Specific Differences in Healthy and Regenerative Oligodendrogenesis. Nat. Neurosci. 2024, 27, 846–861. [Google Scholar] [CrossRef]
- Sherafat, A.; Pfeiffer, F.; Nishiyama, A. Shaping of Regional Differences in Oligodendrocyte Dynamics by Regional Heterogeneity of the Pericellular Microenvironment. Front. Cell. Neurosci. 2021, 15, 721376. [Google Scholar] [CrossRef]
- Hilscher, M.M.; Langseth, C.M.; Kukanja, P.; Yokota, C.; Nilsson, M.; Castelo-Branco, G. Spatial and Temporal Heterogeneity in the Lineage Progression of Fine Oligodendrocyte Subtypes. BMC Biol. 2022, 20, 122. [Google Scholar] [CrossRef]
- Osorio, M.J.; Mariani, J.N.; Zou, L.; Schanz, S.J.; Heffernan, K.; Cornwell, A.; Goldman, S.A. Glial Progenitor Cells of the Adult Human White and Grey Matter Are Contextually Distinct. Glia 2023, 71, 524–540. [Google Scholar] [CrossRef]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.-J.; Cossell, L.; Attwell, D.; Tohyama, K.; Richardson, W.D. Oligodendrocyte Dynamics in the Healthy Adult CNS: Evidence for Myelin Remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef]
- McKenzie, I.A.; Ohayon, D.; Li, H.; de Faria, J.P.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor Skill Learning Requires Active Central Myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef]
- Pease-Raissi, S.E.; Chan, J.R. Building a (w)Rapport between Neurons and Oligodendroglia: Reciprocal Interactions Underlying Adaptive Myelination. Neuron 2021, 109, 1258–1273. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.A.; Miron, V.E. Microglia Regulation of Central Nervous System Myelin Health and Regeneration. Nat. Rev. Immunol. 2024, 24, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.J.M.; Ffrench-Constant, C. Remyelination in the CNS: From Biology to Therapy. Nat. Rev. Neurosci. 2008, 9, 839–855. [Google Scholar] [CrossRef]
- Liu, Y.; Aguzzi, A. NG2 Glia Are Required for Maintaining Microglia Homeostatic State. Glia 2020, 68, 345–355. [Google Scholar] [CrossRef]
- Fang, L.-P.; Bai, X. Oligodendrocyte Precursor Cells: The Multitaskers in the Brain. Pflügers Arch. 2023, 475, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Buchanan, J.; Elabbady, L.; Collman, F.; Jorstad, N.L.; Bakken, T.E.; Ott, C.; Glatzer, J.; Bleckert, A.A.; Bodor, A.L.; Brittain, D.; et al. Oligodendrocyte Precursor Cells Ingest Axons in the Mouse Neocortex. Proc. Natl. Acad. Sci. USA 2022, 119, e2202580119. [Google Scholar] [CrossRef]
- Pfeiffer, F. Reciprocal Interactions between Oligodendrocyte Precursor Cells and the Neurovascular Unit in Health and Disease. Cells 2022, 11, 1954. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Yang, Q.; Gu, H.; Yin, Y.; Li, Y.; Hou, J.; Chen, R.; Sun, Q.; Sun, Y.; et al. NG2 Glia Regulate Brain Innate Immunity via TGF-Β2/TGFBR2 Axis. BMC Med. 2019, 17, 204. [Google Scholar] [CrossRef]
- Marques, S.; Van Bruggen, D.; Vanichkina, D.P.; Floriddia, E.M.; Munguba, H.; Väremo, L.; Giacomello, S.; Falcão, A.M.; Meijer, M.; Björklund, Å.K.; et al. Transcriptional Convergence of Oligodendrocyte Lineage Progenitors during Development. Dev. Cell 2018, 46, 504–517.e7. [Google Scholar] [CrossRef]
- Chapman, T.W.; Hill, R.A. Myelin Plasticity in Adulthood and Aging. Neurosci. Lett. 2020, 715, 134645. [Google Scholar] [CrossRef]
- Bonetto, G.; Belin, D.; Káradóttir, R.T. Myelin: A Gatekeeper of Activity-Dependent Circuit Plasticity? Science 2021, 374, eaba6905. [Google Scholar] [CrossRef]
- Lee, Y.; Morrison, B.M.; Li, Y.; Lengacher, S.; Farah, M.H.; Hoffman, P.N.; Liu, Y.; Tsingalia, A.; Jin, L.; Zhang, P.-W.; et al. Oligodendroglia Metabolically Support Axons and Contribute to Neurodegeneration. Nature 2012, 487, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Saab, A.S.; Tzvetavona, I.D.; Trevisiol, A.; Baltan, S.; Dibaj, P.; Kusch, K.; Möbius, W.; Goetze, B.; Jahn, H.M.; Huang, W.; et al. Oligodendroglial NMDA Receptors Regulate Glucose Import and Axonal Energy Metabolism. Neuron 2016, 91, 119–132. [Google Scholar] [CrossRef]
- Larson, V.A.; Mironova, Y.; Vanderpool, K.G.; Waisman, A.; Rash, J.E.; Agarwal, A.; Bergles, D.E. Oligodendrocytes Control Potassium Accumulation in White Matter and Seizure Susceptibility. eLife 2018, 7, e34829. [Google Scholar] [CrossRef] [PubMed]
- Camargo, N.; Goudriaan, A.; van Deijk, A.-L.F.; Otte, W.M.; Brouwers, J.F.; Lodder, H.; Gutmann, D.H.; Nave, K.-A.; Dijkhuizen, R.M.; Mansvelder, H.D.; et al. Oligodendroglial Myelination Requires Astrocyte-Derived Lipids. PLoS Biol. 2017, 15, e1002605. [Google Scholar] [CrossRef]
- Molina-Gonzalez, I.; Holloway, R.K.; Jiwaji, Z.; Dando, O.; Kent, S.A.; Emelianova, K.; Lloyd, A.F.; Forbes, L.H.; Mahmood, A.; Skripuletz, T.; et al. Astrocyte-Oligodendrocyte Interaction Regulates Central Nervous System Regeneration. Nat. Commun. 2023, 14, 3372. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Gong, Y.; Huang, T.; Lee, C.Z.W.; Bian, L.; Bai, Z.; Shi, H.; Zeng, Y.; Liu, C.; He, J.; et al. Deciphering Human Macrophage Development at Single-Cell Resolution. Nature 2020, 582, 571–576. [Google Scholar] [CrossRef]
- Dermitzakis, I.; Manthou, M.E.; Meditskou, S.; Tremblay, M.-È.; Petratos, S.; Zoupi, L.; Boziki, M.; Kesidou, E.; Simeonidou, C.; Theotokis, P. Origin and Emergence of Microglia in the CNS—An Interesting (Hi)Story of an Eccentric Cell. Curr. Issues Mol. Biol. 2023, 45, 2609–2628. [Google Scholar] [CrossRef]
- Silvin, A.; Qian, J.; Ginhoux, F. Brain Macrophage Development, Diversity and Dysregulation in Health and Disease. Cell. Mol. Immunol. 2023, 20, 1277–1289. [Google Scholar] [CrossRef]
- Tay, T.L.; Mai, D.; Dautzenberg, J.; Fernández-Klett, F.; Lin, G.; Sagar; Datta, M.; Drougard, A.; Stempfl, T.; Ardura-Fabregat, A.; et al. A New Fate Mapping System Reveals Context-Dependent Random or Clonal Expansion of Microglia. Nat. Neurosci. 2017, 20, 793–803. [Google Scholar] [CrossRef]
- Réu, P.; Khosravi, A.; Bernard, S.; Mold, J.E.; Salehpour, M.; Alkass, K.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; et al. The Lifespan and Turnover of Microglia in the Human Brain. Cell Rep. 2017, 20, 779–784. [Google Scholar] [CrossRef]
- Sierra, A.; Paolicelli, R.C.; Kettenmann, H. Cien Años de Microglía: Milestones in a Century of Microglial Research. Trends Neurosci. 2019, 42, 778–792. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Dorman, L.C.; Pan, S.; Vainchtein, I.D.; Han, R.T.; Nakao-Inoue, H.; Taloma, S.E.; Barron, J.J.; Molofsky, A.B.; Kheirbek, M.A.; et al. Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 2020, 182, 388–403.e15. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, D.; Skola, D.; Coufal, N.G.; Holtman, I.R.; Schlachetzki, J.C.M.; Sajti, E.; Jaeger, B.N.; O’Connor, C.; Fitzpatrick, C.; Pasillas, M.P.; et al. An Environment-Dependent Transcriptional Network Specifies Human Microglia Identity. Science 2017, 356, eaal3222. [Google Scholar] [CrossRef] [PubMed]
- Sankowski, R.; Böttcher, C.; Masuda, T.; Geirsdottir, L.; Sagar; Sindram, E.; Seredenina, T.; Muhs, A.; Scheiwe, C.; Shah, M.J.; et al. Mapping Microglia States in the Human Brain through the Integration of High-Dimensional Techniques. Nat. Neurosci. 2019, 22, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Hammond, T.R.; Dufort, C.; Dissing-Olesen, L.; Giera, S.; Young, A.; Wysoker, A.; Walker, A.J.; Gergits, F.; Segel, M.; Nemesh, J.; et al. Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity 2019, 50, 253–271.e6. [Google Scholar] [CrossRef]
- Böttcher, C.; Schlickeiser, S.; Sneeboer, M.A.M.; Kunkel, D.; Knop, A.; Paza, E.; Fidzinski, P.; Kraus, L.; Snijders, G.J.L.; Kahn, R.S.; et al. Human Microglia Regional Heterogeneity and Phenotypes Determined by Multiplexed Single-Cell Mass Cytometry. Nat. Neurosci. 2019, 22, 78–90. [Google Scholar] [CrossRef]
- Allen, W.E.; Blosser, T.R.; Sullivan, Z.A.; Dulac, C.; Zhuang, X. Molecular and Spatial Signatures of Mouse Brain Aging at Single-Cell Resolution. Cell 2023, 186, 194–208.e18. [Google Scholar] [CrossRef]
- Martins-Ferreira, R.; Calafell-Segura, J.; Leal, B.; Rodríguez-Ubreva, J.; Martínez-Saez, E.; Mereu, E.; Pinho, E.; Costa, P.; Laguna, A.; Ballestar, E. The Human Microglia Atlas (HuMicA) Unravels Changes in Disease-Associated Microglia Subsets across Neurodegenerative Conditions. Nat. Commun. 2025, 16, 739. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Murphy, T.H. Decoding State-Dependent Cortical-Cerebellar Cellular Functional Connectivity in the Mouse Brain. Cell Rep. 2024, 43, 114348. [Google Scholar] [CrossRef] [PubMed]
- Apps, R.; Hawkes, R. Cerebellar Cortical Organization: A One-Map Hypothesis. Nat. Rev. Neurosci. 2009, 10, 670–681. [Google Scholar] [CrossRef]
- Buffo, A.; Rossi, F. Origin, Lineage and Function of Cerebellar Glia. Prog. Neurobiol. 2013, 109, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Kozareva, V.; Martin, C.; Osorno, T.; Rudolph, S.; Guo, C.; Vanderburg, C.; Nadaf, N.; Regev, A.; Regehr, W.G.; Macosko, E. A Transcriptomic Atlas of Mouse Cerebellar Cortex Comprehensively Defines Cell Types. Nature 2021, 598, 214–219. [Google Scholar] [CrossRef]
- Reichenbach, A.; Siegel, A.; Rickmann, M.; Wolff, J.R.; Noone, D.; Robinson, S.R. Distribution of Bergmann Glial Somata and Processes: Implications for Function. J. Hirnforsch. 1995, 36, 509–517. [Google Scholar]
- Farmer, W.T.; Abrahamsson, T.; Chierzi, S.; Lui, C.; Zaelzer, C.; Jones, E.V.; Bally, B.P.; Chen, G.G.; Théroux, J.-F.; Peng, J.; et al. Neurons Diversify Astrocytes in the Adult Brain through Sonic Hedgehog Signaling. Science 2016, 351, 849–854. [Google Scholar] [CrossRef]
- Gomez, C.M. The CBL in Health and Disease. Neurosci. Lett. 2019, 688, 1. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.; Tang, X.; Zhao, M.; Liang, F.; Xu, P.; Hou, B.; Xing, Y.; Bao, X.; Fan, X. Bergmann Glia Function in Granule Cell Migration During CBL Development. Mol. Neurobiol. 2013, 47, 833–844. [Google Scholar] [CrossRef]
- De Zeeuw, C.I.; Hoogland, T.M. Reappraisal of Bergmann Glial Cells as Modulators of Cerebellar Circuit Function. Front. Cell. Neurosci. 2015, 9, 246. [Google Scholar] [CrossRef]
- Lippman, J.J.; Lordkipanidze, T.; Buell, M.E.; Yoon, S.O.; Dunaevsky, A. Morphogenesis and Regulation of Bergmann Glial Processes during Purkinje Cell Dendritic Spine Ensheathment and Synaptogenesis. Glia 2008, 56, 1463–1477. [Google Scholar] [CrossRef]
- Yamada, K.; Watanabe, M. Cytodifferentiation of Bergmann Glia and Its Relationship with Purkinje Cells. Anat. Sci. Int. 2002, 77, 94–108. [Google Scholar] [CrossRef]
- Müller, T.; Kettenmann, H. Physiology of Bergmann Gllal Cells. In International Review of Neurobiology; Bradley, R.J., Harris, R.A., Eds.; Academic Press: Palm Bay, FL, USA, 1995; Volume 38, pp. 341–359. [Google Scholar]
- Ottersen, O.P.; Chaudhry, F.A.; Danbolt, N.C.; Laake, J.H.; Nagelhus, E.A.; Storm-Mathisen, J.; Torp, R. Chapter 6 Molecular Organization of Cerebellar Glutamate Synapses. In Progress in Brain Research; De Zeeuw, C.I., Strata, P., Voogd, J., Eds.; The CBL: From Structure to Control; Elsevier: Amsterdam, The Netherlands, 1997; Volume 114, pp. 97–107. [Google Scholar]
- Hoogland, T.M.; Kuhn, B. Recent Developments in the Understanding of Astrocyte Function in the CBL In Vivo. Cerebellum 2010, 9, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Tao-Cheng, J.-H. Ultrastructural Characterization of Peri-Synaptic Astrocytic Processes around Cerebellar Purkinje Spines under Resting and Stimulated Conditions. Mol. Brain 2025, 18, 28. [Google Scholar] [CrossRef] [PubMed]
- Rodriques, S.G.; Stickels, R.R.; Goeva, A.; Martin, C.A.; Murray, E.; Vanderburg, C.R.; Welch, J.; Chen, L.M.; Chen, F.; Macosko, E.Z. Slide-Seq: A Scalable Technology for Measuring Genome-Wide Expression at High Spatial Resolution. Science 2019, 363, 1463–1467. [Google Scholar] [CrossRef]
- Reeber, S.L.; Arancillo, M.K.V.; Sillitoe, R.V. Bergmann Glia Are Patterned into Topographic Molecular Zones in the Developing and Adult Mouse CBL. Cerebellum 2018, 17, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, T.C. Interactions between Purkinje Neurones and Bergmann Glia. Cerebellum 2006, 5, 116–126. [Google Scholar] [CrossRef]
- Eiraku, M.; Tohgo, A.; Ono, K.; Kaneko, M.; Fujishima, K.; Hirano, T.; Kengaku, M. DNER Acts as a Neuron-Specific Notch Ligand during Bergmann Glial Development. Nat. Neurosci. 2005, 8, 873–880. [Google Scholar] [CrossRef]
- Chan-Palay, V.; Palay, S.L. High Voltage Electron Microscopy of Rapid Golgi Preparations. Neurons and Their Processes in the Cerebellar Cortex of Monkey and Rat. Z. Anat. Entwicklungsgeschichte 1972, 137, 125–153. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, P.; Parras, C.; Guillemot, F.; Rossi, F.; Wassef, M. Origins and Control of the Differentiation of Inhibitory Interneurons and Glia in the CBL. Dev. Biol. 2009, 328, 422–433. [Google Scholar] [CrossRef]
- Hashimoto, R.; Hori, K.; Owa, T.; Miyashita, S.; Dewa, K.; Masuyama, N.; Sakai, K.; Hayase, Y.; Seto, Y.; Inoue, Y.U.; et al. Origins of Oligodendrocytes in the CBL, Whose Development Is Controlled by the Transcription Factor, Sox9. Mech. Dev. 2016, 140, 25–40. [Google Scholar] [CrossRef]
- Zhong, S.; Wang, M.; Huang, L.; Chen, Y.; Ge, Y.; Zhang, J.; Shi, Y.; Dong, H.; Zhou, X.; Wang, B.; et al. Single-Cell Epigenomics and Spatiotemporal Transcriptomics Reveal Human Cerebellar Development. Nat. Commun. 2023, 14, 7613. [Google Scholar] [CrossRef]
- Battulga, B.; Osanai, Y.; Yamazaki, R.; Shinohara, Y.; Ohno, N. Axonal Selectivity of Myelination by Single Oligodendrocytes Established During Development in Mouse Cerebellar White Matter. Glia 2025, 73, 873–886. [Google Scholar] [CrossRef]
- Barron, T.; Saifetiarova, J.; Bhat, M.A.; Kim, J.H. Myelination of Purkinje Axons Is Critical for Resilient Synaptic Transmission in the Deep Cerebellar Nucleus. Sci. Rep. 2018, 8, 1022. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Barrantes-Freer, A.; Lohrberg, M.; Antel, J.P.; Prineas, J.W.; Palkovits, M.; Wolff, J.R.; Brück, W.; Stadelmann, C. Synaptic Pathology in the Cerebellar Dentate Nucleus in Chronic Multiple Sclerosis. Brain Pathol. 2017, 27, 737–747. [Google Scholar] [CrossRef]
- Wyatt, K.D.; Tanapat, P.; Wang, S.S.-H. Speed Limits in the CBL: Constraints from Myelinated and Unmyelinated Parallel Fibers. Eur. J. Neurosci. 2005, 21, 2285–2290. [Google Scholar] [CrossRef]
- Lin, S.; Huck, J.H.J.; Roberts, J.D.B.; Macklin, W.B.; Somogyi, P.; Bergles, D.E. Climbing Fiber Innervation of NG2-Expressing Glia in the Mammalian CBL. Neuron 2005, 46, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.A.; Li, A.M.; Grutzendler, J. Lifelong Cortical Myelin Plasticity and Age-Related Degeneration in the Live Mammalian Brain. Nat. Neurosci. 2018, 21, 683–695. [Google Scholar] [CrossRef]
- Heffley, W.; Song, E.Y.; Xu, Z.; Taylor, B.N.; Hughes, M.A.; McKinney, A.; Joshua, M.; Hull, C. Coordinated Cerebellar Climbing Fiber Activity Signals Learned Sensorimotor Predictions. Nat. Neurosci. 2018, 21, 1431–1441. [Google Scholar] [CrossRef]
- Silva, N.T.; Ramírez-Buriticá, J.; Pritchett, D.L.; Carey, M.R. Climbing Fibers Provide Essential Instructive Signals for Associative Learning. Nat. Neurosci. 2024, 27, 940–951. [Google Scholar] [CrossRef]
- Lang, E.J.; Rosenbluth, J. Role of Myelination in the Development of a Uniform Olivocerebellar Conduction Time. J. Neurophysiol. 2003, 89, 2259–2270. [Google Scholar] [CrossRef] [PubMed]
- Funfschilling, U.; Supplie, L.M.; Mahad, D.; Boretius, S.; Saab, A.S.; Edgar, J.; Brinkmann, B.G.; Kassmann, C.M.; Tzvetanova, I.D.; Mobius, W.; et al. Glycolytic Oligodendrocytes Maintain Myelin and Long-Term Axonal Integrity. Nature 2012, 485, 517–521. [Google Scholar] [CrossRef]
- Looser, Z.J.; Faik, Z.; Ravotto, L.; Zanker, H.S.; Jung, R.B.; Werner, H.B.; Ruhwedel, T.; Möbius, W.; Bergles, D.E.; Barros, L.F.; et al. Oligodendrocyte–Axon Metabolic Coupling Is Mediated by Extracellular K+ and Maintains Axonal Health. Nat. Neurosci. 2024, 27, 433–448. [Google Scholar] [CrossRef]
- Donofrio, S.G.; Brandenburg, C.; Brown, A.M.; Lin, T.; Lu, H.-C.; Sillitoe, R.V. Cerebellar Purkinje Cell Stripe Patterns Reveal a Differential Vulnerability and Resistance to Cell Loss during Normal Aging in Mice. Biorxiv 2025, 2025, 634923. [Google Scholar]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Böttcher, C.; Amann, L.; Sagar; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and Temporal Heterogeneity of Mouse and Human Microglia at Single-Cell Resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef]
- Li, Q.; Barres, B.A. Microglia and Macrophages in Brain Homeostasis and Disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Stoessel, M.B.; Majewska, A.K. Little Cells of the Little Brain: Microglia in Cerebellar Development and Function. Trends Neurosci. 2021, 44, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Marín-Teva, J.L.; Dusart, I.; Colin, C.; Gervais, A.; van Rooijen, N.; Mallat, M. Microglia Promote the Death of Developing Purkinje Cells. Neuron 2004, 41, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Abe, M.; Morimoto, C.; Iida, T.; Okabe, S.; Sakimura, K.; Hashimoto, K. Microglia Permit Climbing Fiber Elimination by Promoting GABAergic Inhibition in the Developing CBL. Nat. Commun. 2018, 9, 2830. [Google Scholar] [CrossRef]
- Ayata, P.; Badimon, A.; Strasburger, H.J.; Duff, M.K.; Montgomery, S.E.; Loh, Y.-H.E.; Ebert, A.; Pimenova, A.A.; Ramirez, B.R.; Chan, A.T.; et al. Epigenetic Regulation of Brain Region-Specific Microglia Clearance Activity. Nat. Neurosci. 2018, 21, 1049–1060. [Google Scholar] [CrossRef]
- Stowell, R.D.; Wong, E.L.; Batchelor, H.N.; Mendes, M.S.; Lamantia, C.E.; Whitelaw, B.S.; Majewska, A.K. Cerebellar Microglia Are Dynamically Unique and Survey Purkinje Neurons in Vivo. Dev. Neurobiol. 2018, 78, 627–644. [Google Scholar] [CrossRef]
- Vela, J.M.; Dalmau, I.; González, B.; Castellano, B. Morphology and Distribution of Microglial Cells in the Young and Adult Mouse CBL. J. Comp. Neurol. 1995, 361, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Kim, M.; Imai, H.; Itakura, Y.; Ohtsuki, G. Microglia-Triggered Plasticity of Intrinsic Excitability Modulates Psychomotor Behaviors in Acute Cerebellar Inflammation. Cell Rep. 2019, 28, 2923–2938.e8. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.G.; Jang, S.-S.; Kim, S.H.; Hwang, E.M.; Min, J.O.; Kim, H.Y.; Kim, Y.S.; Ryu, C.; Chung, G.; Kim, Y.; et al. TNF-α Increases the Intrinsic Excitability of Cerebellar Purkinje Cells through Elevating Glutamate Release in Bergmann Glia. Sci. Rep. 2018, 8, 11589. [Google Scholar] [CrossRef]
- Blinkouskaya, Y.; Caçoilo, A.; Gollamudi, T.; Jalalian, S.; Weickenmeier, J. Brain Aging Mechanisms with Mechanical Manifestations. Mech. Ageing Dev. 2021, 200, 111575. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Magnus, T. Aging and Neuronal Vulnerability. Nat. Rev. Neurosci. 2006, 7, 278–294. [Google Scholar] [CrossRef]
- Gunning-Dixon, F.M.; Brickman, A.M.; Cheng, J.C.; Alexopoulos, G.S. Aging of Cerebral White Matter: A Review of MRI Findings. Int. J. Geriatr. Psychiatry 2009, 24, 109–117. [Google Scholar] [CrossRef]
- Cox, S.R.; Ritchie, S.J.; Tucker-Drob, E.M.; Liewald, D.C.; Hagenaars, S.P.; Davies, G.; Wardlaw, J.M.; Gale, C.R.; Bastin, M.E.; Deary, I.J. Ageing and Brain White Matter Structure in 3513 UK Biobank Participants. Nat. Commun. 2016, 7, 13629. [Google Scholar] [CrossRef]
- Bennett, I.J.; Madden, D.J. Disconnected Aging: Cerebral White Matter Integrity and Age-Related Differences in Cognition. Neuroscience 2014, 276, 187–205. [Google Scholar] [CrossRef]
- Sullivan, E.V.; Pfefferbaum, A. Diffusion Tensor Imaging and Aging. Neurosci. Biobehav. Rev. 2006, 30, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Peters, A. The Effects of Normal Aging on Myelin and Nerve Fibers: A Review. J. Neurocytol. 2002, 31, 581–593. [Google Scholar] [CrossRef]
- Simons, M.; Nave, K.-A. Oligodendrocytes: Myelination and Axonal Support. Cold Spring Harb. Perspect. Biol. 2016, 8, a020479. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent Metabolic Switching, Neuroplasticity and Brain Health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef]
- Radulescu, C.I.; Cerar, V.; Haslehurst, P.; Kopanitsa, M.; Barnes, S.J. The Aging Mouse Brain: Cognition, Connectivity and Calcium. Cell Calcium 2021, 94, 102358. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 617588. [Google Scholar] [CrossRef]
- Palmer, J.E.; Wilson, N.; Son, S.M.; Obrocki, P.; Wrobel, L.; Rob, M.; Takla, M.; Korolchuk, V.I.; Rubinsztein, D.C. Autophagy, Aging, and Age-Related Neurodegeneration. Neuron 2025, 113, 29–48. [Google Scholar] [CrossRef]
- Azam, S.; Haque, M.E.; Balakrishnan, R.; Kim, I.-S.; Choi, D.-K. The Ageing Brain: Molecular and Cellular Basis of Neurodegeneration. Front. Cell Dev. Biol. 2021, 9, 683459. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Gomes, S.; Viana, J.F.; Nascimento, D.S.M.; Correia, J.S.; Sardinha, V.M.; Caetano, I.; Sousa, N.; Pinto, L.; Oliveira, J.F. The Role of Astrocytic Calcium Signaling in the Aged Prefrontal Cortex. Front. Cell. Neurosci. 2018, 12, 379. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The Blood-Brain Barrier in Aging and Neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Zhao, Z.; Montagne, A.; Nelson, A.R.; Zlokovic, B.V. Blood-Brain Barrier: From Physiology to Disease and Back. Physiol. Rev. 2019, 99, 21–78. [Google Scholar] [CrossRef]
- Pelvig, D.P.; Pakkenberg, H.; Stark, A.K.; Pakkenberg, B. Neocortical Glial Cell Numbers in Human Brains. Neurobiol. Aging 2008, 29, 1754–1762. [Google Scholar] [CrossRef]
- Fabricius, K.; Jacobsen, J.S.; Pakkenberg, B. Effect of Age on Neocortical Brain Cells in 90+ Year Old Human Females—A Cell Counting Study. Neurobiol. Aging 2013, 34, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gildea, H.K.; Liddelow, S.A. Mechanisms of Astrocyte Aging in Reactivity and Disease. Mol. Neurodegener. 2025, 20, 21. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Yeh, C.-Y.; Terzieva, S.; Olabarria, M.; Kulijewicz-Nawrot, M.; Verkhratsky, A. Complex and Region-Specific Changes in Astroglial Markers in the Aging Brain. Neurobiol. Aging 2014, 35, 15–23. [Google Scholar] [CrossRef]
- Popov, A.; Brazhe, A.; Denisov, P.; Sutyagina, O.; Li, L.; Lazareva, N.; Verkhratsky, A.; Semyanov, A. Astrocyte Dystrophy in Ageing Brain Parallels Impaired Synaptic Plasticity. Aging Cell 2021, 20, e13334. [Google Scholar] [CrossRef]
- Palmer, A.L.; Ousman, S.S. Astrocytes and Aging. Front. Aging Neurosci. 2018, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yuan, Z.; Yang, S.; Zhu, Y.; Xue, M.; Zhang, J.; Leng, L. Brain Energy Metabolism: Astrocytes in Neurodegenerative Diseases. CNS Neurosci. Ther. 2023, 29, 24–36. [Google Scholar] [CrossRef]
- Araujo, A.P.B.; Vargas, G.; Hayashide, L.d.S.; Matias, I.; Andrade, C.B.V.; de Carvalho, J.J.; Gomes, F.C.A.; Diniz, L.P. Aging Promotes an Increase in Mitochondrial Fragmentation in Astrocytes. Front. Cell. Neurosci. 2024, 18, 1496163. [Google Scholar] [CrossRef] [PubMed]
- Groh, J.; Feng, R.; Yuan, X.; Liu, L.; Klein, D.; Hutahaean, G.; Butz, E.; Wang, Z.; Steinbrecher, L.; Neher, J.; et al. Microglia Activation Orchestrates CXCL10-Mediated CD8+ T Cell Recruitment to Promote Aging-Related White Matter Degeneration. Nat. Neurosci. 2025, 28, 1160–1173. [Google Scholar] [CrossRef]
- Wang, F.; Ren, S.-Y.; Chen, J.-F.; Liu, K.; Li, R.-X.; Li, Z.-F.; Hu, B.; Niu, J.-Q.; Xiao, L.; Chan, J.R.; et al. Myelin Degeneration and Diminished Myelin Renewal Contribute to Age-Related Deficits in Memory. Nat. Neurosci. 2020, 23, 481–486. [Google Scholar] [CrossRef]
- Coelho, A.; Fernandes, H.M.; Magalhães, R.; Moreira, P.S.; Marques, P.; Soares, J.M.; Amorim, L.; Portugal-Nunes, C.; Castanho, T.; Santos, N.C.; et al. Signatures of White-Matter Microstructure Degradation during Aging and Its Association with Cognitive Status. Sci. Rep. 2021, 11, 4517. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, N.; Xiao, L.; Wang, F.; Li, T. Replenishing the Aged Brains: Targeting Oligodendrocytes and Myelination? Front. Aging Neurosci. 2021, 13, 760200. [Google Scholar] [CrossRef]
- Sams, E.C. Oligodendrocytes in the Aging Brain. Neuronal Signal 2021, 5, NS20210008. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, S.O.; Sitnikov, S.; Kamen, Y.; Evans, K.A.; Kronenberg-Versteeg, D.; Dietmann, S.; de Faria, O.; Agathou, S.; Káradóttir, R.T. Oligodendrocyte Progenitor Cells Become Regionally Diverse and Heterogeneous with Age. Neuron 2019, 101, 459–471.e5. [Google Scholar] [CrossRef]
- Psachoulia, K.; Jamen, F.; Young, K.M.; Richardson, W.D. Cell Cycle Dynamics of NG2 Cells in the Postnatal and Ageing Brain. Neuron Glia Biol. 2009, 5, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Muramatsu, R.; Kato, Y.; Sharma, B.; Uyeda, A.; Tanabe, S.; Fujimura, H.; Kidoya, H.; Takakura, N.; Kawahara, Y.; et al. Age-Dependent Decline in Remyelination Capacity Is Mediated by Apelin-APJ Signaling. Nat. Aging 2021, 1, 284–294. [Google Scholar] [CrossRef]
- Rivera, A.D.; Pieropan, F.; Chacon-De-La-Rocha, I.; Lecca, D.; Abbracchio, M.P.; Azim, K.; Butt, A.M. Functional Genomic Analyses Highlight a Shift in Gpr17-Regulated Cellular Processes in Oligodendrocyte Progenitor Cells and Underlying Myelin Dysregulation in the Aged Mouse Cerebrum. Aging Cell 2021, 20, e13335. [Google Scholar] [CrossRef]
- Tripathi, R.B.; Jackiewicz, M.; McKenzie, I.A.; Kougioumtzidou, E.; Grist, M.; Richardson, W.D. Remarkable Stability of Myelinating Oligodendrocytes in Mice. Cell Rep. 2017, 21, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.F.; Ding, J.; Langston, R.G.; Shah, S.I.; Nalls, M.A.; Scholz, S.W.; Whitaker, D.T.; Auluck, P.K.; Marenco, S.; Gibbs, J.R.; et al. Divergent Patterns of Healthy Aging across Human Brain Regions at Single-Cell Resolution Reveal Links to Neurodegenerative Disease. Biorxiv 2023. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Thorburne, S.K.; Hertz, L. Peroxide-Scavenging Deficit Underlies Oligodendrocyte Susceptibility to Oxidative Stress. Glia 1998, 22, 371–378. [Google Scholar] [CrossRef]
- Thorburne, S.K.; Juurlink, B.H. Low Glutathione and High Iron Govern the Susceptibility of Oligodendroglial Precursors to Oxidative Stress. J. Neurochem. 1996, 67, 1014–1022. [Google Scholar] [CrossRef]
- Guo, Y.; Guan, T.; Shafiq, K.; Yu, Q.; Jiao, X.; Na, D.; Li, M.; Zhang, G.; Kong, J. Mitochondrial Dysfunction in Aging. Ageing Res. Rev. 2023, 88, 101955. [Google Scholar] [CrossRef]
- Tse, K.-H.; Herrup, K. DNA Damage in the Oligodendrocyte Lineage and Its Role in Brain Aging. Mech. Ageing Dev. 2017, 161, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative Stress and Impaired Oligodendrocyte Precursor Cell Differentiation in Neurological Disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef]
- Sikora, E.; Bielak-Zmijewska, A.; Dudkowska, M.; Krzystyniak, A.; Mosieniak, G.; Wesierska, M.; Wlodarczyk, J. Cellular Senescence in Brain Aging. Front. Aging Neurosci. 2021, 13, 646924. [Google Scholar] [CrossRef]
- Gomez, P.T.; Carver, C.M.; Rodriguez, S.L.; Wang, L.; Zhang, X.; Schafer, M.J. Aging and Senescent Fates of Oligodendrocyte Precursor Cells in the Mouse Brain. Npj Aging 2024, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Tiane, A.; Schepers, M.; Rombaut, B.; Hupperts, R.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. From OPC to Oligodendrocyte: An Epigenetic Journey. Cells 2019, 8, 1236. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Mendizabal, I.; Yi, S.V. Human Brain Aging Is Associated with Dysregulation of Cell Type Epigenetic Identity. GeroScience 2025, 47, 3759–3770. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.; Xiao, B.; Guo, X.; Zheng, Q.; Wu, B. Age-Related Changes in the Global DNA Methylation Profile of Oligodendrocyte Progenitor Cells Derived from Rat Spinal Cords. Curr. Med. Sci. 2019, 39, 67–74. [Google Scholar] [CrossRef]
- Shen, S.; Sandoval, J.; Swiss, V.A.; Li, J.; Dupree, J.; Franklin, R.J.M.; Casaccia-Bonnefil, P. Age-Dependent Epigenetic Control of Differentiation Inhibitors Is Critical for Remyelination Efficiency. Nat. Neurosci. 2008, 11, 1024–1034. [Google Scholar] [CrossRef]
- Segel, M.; Neumann, B.; Hill, M.F.E.; Weber, I.P.; Viscomi, C.; Zhao, C.; Young, A.; Agley, C.C.; Thompson, A.J.; Gonzalez, G.A.; et al. Niche Stiffness Underlies the Ageing of Central Nervous System Progenitor Cells. Nature 2019, 573, 130–134. [Google Scholar] [CrossRef]
- Richard, A.D.; Tian, X.-L.; El-Saadi, M.W.; Lu, X.-H. Erasure of Striatal Chondroitin Sulfate Proteoglycan–Associated Extracellular Matrix Rescues Aging-Dependent Decline of Motor Learning. Neurobiol. Aging 2018, 71, 61–71. [Google Scholar] [CrossRef]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef]
- Kaya, T.; Mattugini, N.; Liu, L.; Ji, H.; Cantuti-Castelvetri, L.; Wu, J.; Schifferer, M.; Groh, J.; Martini, R.; Besson-Girard, S.; et al. CD8+ T Cells Induce Interferon-Responsive Oligodendrocytes and Microglia in White Matter Aging. Nat. Neurosci. 2022, 25, 1446–1457. [Google Scholar] [CrossRef]
- Carr, L.; Mustafa, S.; Collins-Praino, L.E. The Hallmarks of Ageing in Microglia. Cell. Mol. Neurobiol. 2025, 45, 45. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Patrick, E.; Villani, A.-C.; Xu, J.; White, C.C.; Ryan, K.J.; Piehowski, P.; Kapasi, A.; Nejad, P.; Cimpean, M.; et al. A Transcriptomic Atlas of Aged Human Microglia. Nat. Commun. 2018, 9, 539. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, N.; Yu, B.; Zhang, W.; Wan, J. Transcriptomic Profiling of Microglia and Astrocytes throughout Aging. J. Neuroinflammation 2020, 17, 97. [Google Scholar] [CrossRef]
- de Lopes, K.P.; Snijders, G.J.L.; Humphrey, J.; Allan, A.; Sneeboer, M.A.M.; Navarro, E.; Schilder, B.M.; Vialle, R.A.; Parks, M.; Missall, R.; et al. Genetic Analysis of the Human Microglial Transcriptome across Brain Regions, Aging and Disease Pathologies. Nat. Genet. 2022, 54, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Jin, Y.; Zhang, Y.; Wu, J.; Xu, Z.; Huang, Y.; Cai, L.; Gao, S.; Liu, T.; et al. Transcriptional and Epigenetic Decoding of the Microglial Aging Process. Nat. Aging 2023, 3, 1288–1311. [Google Scholar] [CrossRef] [PubMed]
- Safaiyan, S.; Besson-Girard, S.; Kaya, T.; Cantuti-Castelvetri, L.; Liu, L.; Ji, H.; Schifferer, M.; Gouna, G.; Usifo, F.; Kannaiyan, N.; et al. White Matter Aging Drives Microglial Diversity. Neuron 2021, 109, 1100–1117.e10. [Google Scholar] [CrossRef] [PubMed]
- Streit, W.J.; Sammons, N.W.; Kuhns, A.J.; Sparks, D.L. Dystrophic Microglia in the Aging Human Brain. Glia 2004, 45, 208–212. [Google Scholar] [CrossRef]
- Shahidehpour, R.K.; Higdon, R.E.; Crawford, N.G.; Neltner, J.H.; Ighodaro, E.T.; Patel, E.; Price, D.; Nelson, P.T.; Bachstetter, A.D. Dystrophic Microglia Are Associated with Neurodegenerative Disease and Not Healthy Aging in the Human Brain. Neurobiol. Aging 2021, 99, 19–27. [Google Scholar] [CrossRef]
- Lynch, M.A. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front. Aging Neurosci. 2022, 14, 868448. [Google Scholar] [CrossRef]
- Pan, K.; Garaschuk, O. The Role of Intracellular Calcium-Store-Mediated Calcium Signals in in Vivo Sensor and Effector Functions of Microglia. J. Physiol. 2023, 601, 4203–4215. [Google Scholar] [CrossRef]
- Sankowski, R.; Prinz, M. A Dynamic and Multimodal Framework to Define Microglial States. Nat. Neurosci. 2025, 28, 1372–1380. [Google Scholar] [CrossRef]
- Bernard, J.A.; Peltier, S.J.; Wiggins, J.L.; Jaeggi, S.M.; Buschkuehl, M.; Fling, B.W.; Kwak, Y.; Jonides, J.; Monk, C.S.; Seidler, R.D. Disrupted Cortico-Cerebellar Connectivity in Older Adults. Neuroimage 2013, 83, 103–119. [Google Scholar] [CrossRef]
- Filip, P.; Gallea, C.; Lehéricy, S.; Lungu, O.; Bareš, M. Neural Scaffolding as the Foundation for Stable Performance of Aging. Cerebellum 2019, 18, 500–510. [Google Scholar] [CrossRef]
- McElroy, C.L.; Wang, B.; Zhang, H.; Jin, K. CBL and Aging: Update and Challenges. Aging Dis. 2024, 15, 2345–2360. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.B.; Gundersen, H.J.G.; Pakkenberg, B. Aging of the Human CBL: A Stereological Study. J. Comp. Neurol. 2003, 466, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Teng, Y.; Liu, T.; Tang, Y.; Liang, W.; Wang, W.; Li, Z.; Xia, Q.; Xu, F.; Liu, S. Morphological Changes in the CBL during Aging: Evidence from Convolutional Neural Networks and Shape Analysis. Front. Aging Neurosci. 2024, 16, 1359320. [Google Scholar] [CrossRef]
- Raz, N.; Schmiedek, F.; Rodrigue, K.M.; Kennedy, K.M.; Lindenberger, U.; Lövdén, M. Differential Brain Shrinkage Over Six Months Shows Limited Association with Cognitive Practice. Brain Cogn. 2013, 82, 171–180. [Google Scholar] [CrossRef]
- Han, S.; An, Y.; Carass, A.; Prince, J.L.; Resnick, S.M. Longitudinal Analysis of Regional CBL Volumes during Normal Aging. Neuroimage 2020, 220, 117062. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Xiao, W.; Li, Y.; Zhao, X. Exploring the Neural Mechanisms Linking Healthy Aging and Cognitive Maintenance: Insights from Mendelian Randomization and Mediation Analyses. Cereb. Cortex 2025, 35, bhaf006. [Google Scholar] [CrossRef]
- Hicks, T.H.; Magalhães, T.N.C.; Jackson, T.B.; Ballard, H.K.; Herrejon, I.A.; Bernard, J.A. Functional and Structural Cerebellar-Behavior Relationships in Aging. Biorxiv 2024, 230, 10. [Google Scholar] [CrossRef]
- Liang, K.J.; Carlson, E.S. Resistance, Vulnerability and Resilience: A Review of the Cognitive CBL in Aging and Neurodegenerative Diseases. Neurobiol. Learn. Mem. 2020, 170, 106981. [Google Scholar] [CrossRef]
- Mitoma, H.; Kakei, S.; Yamaguchi, K.; Manto, M. Physiology of Cerebellar Reserve: Redundancy and Plasticity of a Modular Machine. Int. J. Mol. Sci. 2021, 22, 4777. [Google Scholar] [CrossRef]
- Bernard, J.A.; Nguyen, A.D.; Hausman, H.K.; Maldonado, T.; Ballard, H.K.; Jackson, T.B.; Eakin, S.M.; Lokshina, Y.; Goen, J.R.M. Shaky Scaffolding: Age Differences in Cerebellar Activation Revealed through Activation Likelihood Estimation Meta-analysis. Hum. Brain Mapp. 2020, 41, 5255–5281. [Google Scholar] [CrossRef]
- Bernard, J.A. Don’t Forget the Little Brain: A Framework for Incorporating the CBL into the Understanding of Cognitive Aging. Neurosci. Biobehav. Rev. 2022, 137, 104639. [Google Scholar] [CrossRef]
- Baghel, B.; Kumari, B.; Bhattacharjee, A.; Sharma, S.; Roy, P.K. High Resilience of Human CBL in Alzheimer’s Disease: Reciprocal Coupling of Cellular Anabolic and Catabolic Fluxes Enable Intensive Neuroprotection. 3 Biotech 2025, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhu, Q.; Hua, T. Aging of Cerebellar Purkinje Cells. Cell Tissue Res. 2010, 341, 341–347. [Google Scholar] [CrossRef]
- Woodruff-Pak, D.S.; Foy, M.R.; Akopian, G.G.; Lee, K.H.; Zach, J.; Nguyen, K.P.T.; Comalli, D.M.; Kennard, J.A.; Agelan, A.; Thompson, R.F. Differential Effects and Rates of Normal Aging in CBL and Hippocampus. Proc. Natl. Acad. Sci. USA 2010, 107, 1624–1629. [Google Scholar] [CrossRef] [PubMed]
- Fattoretti, P.; Bertoni-Freddari, C.; Caselli, U.; Paoloni, R.; Meier-Ruge, W. Morphologic Changes in Cerebellar Mitochondria during Aging. Anal. Quant. Cytol. Histol. 1996, 18, 205–208. [Google Scholar]
- Bertoni-Freddari, C.; Fattoretti, P.; Giorgetti, B.; Solazzi, M.; Balietti, M.; Di Stefano, G.; Casoli, T. Decay of Mitochondrial Metabolic Competence in the Aging CBL. Ann. N. Y. Acad. Sci. 2004, 1019, 29–32. [Google Scholar] [CrossRef]
- Balietti, M.; Fattoretti, P.; Giorgetti, B.; Casoli, T.; Di Stefano, G.; Platano, D.; Aicardi, G.; Lattanzio, F.; Bertoni-Freddari, C. Effect of Two Medium Chain Triglycerides-Supplemented Diets on Synaptic Morphology in the Cerebellar Cortex of Late-Adult Rats. Microsc. Res. Tech. 2009, 72, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Mathiesen, C.; Brazhe, A.; Thomsen, K.; Lauritzen, M. Spontaneous Calcium Waves in Bergman Glia Increase with Age and Hypoxia and May Reduce Tissue Oxygen. J. Cereb. Blood Flow. Metab. 2013, 33, 161–169. [Google Scholar] [CrossRef]
- Wang, F.; Xu, Q.; Wang, W.; Takano, T.; Nedergaard, M. Bergmann Glia Modulate Cerebellar Purkinje Cell Bistability via Ca2+-Dependent K+ Uptake. Proc. Natl. Acad. Sci. USA 2012, 109, 7911–7916. [Google Scholar] [CrossRef]
- Marcelo, A.; Afonso, I.T.; Afonso-Reis, R.; Brito, D.V.C.; Costa, R.G.; Rosa, A.; Alves-Cruzeiro, J.; Ferreira, B.; Henriques, C.; Nobre, R.J.; et al. Autophagy in Spinocerebellar Ataxia Type 2, a Dysregulated Pathway, and a Target for Therapy. Cell Death Dis. 2021, 12, 1117. [Google Scholar] [CrossRef]
- Estrada, R.; Galarraga, J.; Orozco, G.; Nodarse, A.; Auburger, G. Spinocerebellar Ataxia 2 (SCA2): Morphometric Analyses in 11 Autopsies. Acta Neuropathol. 1999, 97, 306–310. [Google Scholar] [CrossRef]
- Matsusue, E.; Fujii, S.; Kanasaki, Y.; Kaminou, T.; Ohama, E.; Ogawa, T. Cerebellar Lesions in Multiple System Atrophy: Postmortem MR Imaging-Pathologic Correlations. Am. J. Neuroradiol. 2009, 30, 1725–1730. [Google Scholar] [CrossRef]
- Gupta, R.K.; Kanungo, M. Glial Molecular Alterations with Mouse Brain Development and Aging: Up-Regulation of the Kir4.1 and Aquaporin-4. Age 2013, 35, 59–67. [Google Scholar] [CrossRef][Green Version]
- Popa-Wagner, A.; Sandu, R.E.; Cristin, C.; Uzoni, A.; Welle, K.A.; Hryhorenko, J.R.; Ghaemmaghami, S. Increased Degradation Rates in the Components of the Mitochondrial Oxidative Phosphorylation Chain in the CBL of Old Mice. Front. Aging Neurosci. 2018, 10, 32. [Google Scholar] [CrossRef]
- Labarta-Bajo, L.; Allen, N.J. Astrocytes in Aging. Neuron 2025, 113, 109–126. [Google Scholar] [CrossRef]
- Sabbatini, M.; Barili, P.; Bronzetti, E.; Zaccheo, D.; Amenta, F. Age-Related Changes of Glial Fibrillary Acidic Protein Immunoreactive Astrocytes in the Rat Cerebellar Cortex. Mech. Ageing Dev. 1999, 108, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lynch, A.M.; Murphy, K.J.; Deighan, B.F.; O’Reilly, J.; Gun’ko, Y.K.; Cowley, T.R.; Gonzalez-Reyes, R.E.; Lynch, M.A. The Inpact of Glial Activation in the Aging Brain. Aging Dis. 2010, 32, 262–278. [Google Scholar] [CrossRef]
- Helleringer, R.; Chever, O.; Daniel, H.; Galante, M. Oxygen and Glucose Deprivation Induces Bergmann Glia Membrane Depolarization and Ca2+ Rises Mainly Mediated by K+ and ATP Increases in the Extracellular Space. Front. Cell. Neurosci. 2017, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Nanclares, C.; Noriega-Prieto, J.A.; Labrada-Moncada, F.E.; Cvetanovic, M.; Araque, A.; Kofuji, P. Altered Calcium Signaling in Bergmann Glia Contributes to Spinocerebellar Ataxia Type-1 in a Mouse Model of SCA1. Neurobiol. Dis. 2023, 187, 106318. [Google Scholar] [CrossRef]
- Revuelta, M.; Scheuer, T.; Chew, L.-J.; Schmitz, T. Glial Factors Regulating White Matter Development and Pathologies of the CBL. Neurochem. Res. 2020, 45, 643–655. [Google Scholar] [CrossRef]
- Cerrato, V. Cerebellar Astrocytes: Much More Than Passive Bystanders In Ataxia Pathophysiology. J. Clin. Med. 2020, 9, 757. [Google Scholar] [CrossRef]
- Escalona, P.R.; McDonald, W.M.; Doraiswamy, P.M.; Boyko, O.B.; Husain, M.M.; Figiel, G.S.; Laskowitz, D.; Ellinwood, E.H.; Krishnan, K.R. In Vivo Stereological Assessment of Human Cerebellar Volume: Effects of Gender and Age. Am. J. Neuroradiol. 1991, 12, 927–929. [Google Scholar] [PubMed]
- Jernigan, T.L.; Archibald, S.L.; Fennema-Notestine, C.; Gamst, A.C.; Stout, J.C.; Bonner, J.; Hesselink, J.R. Effects of Age on Tissues and Regions of the Cerebrum and CBL. Neurobiol. Aging 2001, 22, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Walhovd, K.B.; Fjell, A.M.; Reinvang, I.; Lundervold, A.; Dale, A.M.; Eilertsen, D.E.; Quinn, B.T.; Salat, D.; Makris, N.; Fischl, B. Effects of Age on Volumes of Cortex, White Matter and Subcortical Structures. Neurobiol. Aging 2005, 26, 1261–1270. [Google Scholar] [CrossRef]
- Hoogendam, Y.Y.; van der Lijn, F.; Vernooij, M.W.; Hofman, A.; Niessen, W.J.; van der Lugt, A.; Ikram, M.A.; van der Geest, J.N. Older Age Relates to Worsening of Fine Motor Skills: A Population-Based Study of Middle-Aged and Elderly Persons. Front. Aging Neurosci. 2014, 6, 259. [Google Scholar] [CrossRef]
- Smith, E.E.; O’Donnell, M.; Dagenais, G.; Lear, S.A.; Wielgosz, A.; Sharma, M.; Poirier, P.; Stotts, G.; Black, S.E.; Strother, S.; et al. Early Cerebral Small Vessel Disease and Brain Volume, Cognition, and Gait. Ann. Neurol. 2015, 77, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Schöls, L.; Bauer, P.; Schmidt, T.; Schulte, T.; Riess, O. Autosomal Dominant Cerebellar Ataxias: Clinical Features, Genetics, and Pathogenesis. Lancet Neurol. 2004, 3, 291–304. [Google Scholar] [CrossRef]
- Hampe, C.S.; Mitoma, H. A Breakdown of Immune Tolerance in the CBL. Brain Sci. 2022, 12, 328. [Google Scholar] [CrossRef]
- Hart, A.D.; Wyttenbach, A.; Hugh Perry, V.; Teeling, J.L. Age Related Changes in Microglial Phenotype Vary between CNS Regions: Grey versus White Matter Differences. Brain Behav. Immun. 2012, 26, 754–765. [Google Scholar] [CrossRef]
- Tsai, A.P.; Dong, C.; Lin, P.B.-C.; Oblak, A.L.; Viana Di Prisco, G.; Wang, N.; Hajicek, N.; Carr, A.J.; Lendy, E.K.; Hahn, O.; et al. Genetic Variants of Phospholipase C-Γ2 Alter the Phenotype and Function of Microglia and Confer Differential Risk for Alzheimer’s Disease. Immunity 2023, 56, 2121–2136.e6. [Google Scholar] [CrossRef]
- Haney, M.S.; Pálovics, R.; Munson, C.N.; Long, C.; Johansson, P.K.; Yip, O.; Dong, W.; Rawat, E.; West, E.; Schlachetzki, J.C.M.; et al. APOE4/4 Is Linked to Damaging Lipid Droplets in Alzheimer’s Disease Microglia. Nature 2024, 628, 154–161. [Google Scholar] [CrossRef]
- Yang, C.; Liu, G.; Chen, X.; Le, W. CBL in Alzheimer’s Disease and Other Neurodegenerative Diseases: An Emerging Research Frontier. MedComm 2024, 5, e638. [Google Scholar] [CrossRef]
- Sarlus, H.; Heneka, M.T. Microglia in Alzheimer’s Disease. J. Clin. Investig. 2017, 127, 3240–3249. [Google Scholar] [CrossRef]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Hung, Y.-S.; Fuh, J.-L.; Chen, N.-J.; Chu, Y.-S.; Chen, S.-C.; Fann, M.-J.; Wong, Y.-H. Efficient Conversion of Human Induced Pluripotent Stem Cells into Microglia by Defined Transcription Factors. Stem Cell Rep. 2021, 16, 1363–1380. [Google Scholar] [CrossRef]
- Tuddenham, J.F.; Taga, M.; Haage, V.; Marshe, V.S.; Roostaei, T.; White, C.; Lee, A.J.; Fujita, M.; Khairallah, A.; Zhang, Y.; et al. A Cross-Disease Resource of Living Human Microglia Identifies Disease-Enriched Subsets and Tool Compounds Recapitulating Microglial States. Nat. Neurosci. 2024, 27, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Singh-Bains, M.K.; Linke, V.; Austria, M.D.R.; Tan, A.Y.S.; Scotter, E.L.; Mehrabi, N.F.; Faull, R.L.M.; Dragunow, M. Altered Microglia and Neurovasculature in the Alzheimer’s Disease CBL. Neurobiol. Dis. 2019, 132, 104589. [Google Scholar] [CrossRef]
- Krbot, K.; Hermann, P.; Skorić, M.K.; Zerr, I.; Sepulveda-Falla, D.; Goebel, S.; Matschke, J.; Krasemann, S.; Glatzel, M. Distinct Microglia Profile in Creutzfeldt-Jakob Disease and Alzheimer’s Disease Is Independent of Disease Kinetics. Neuropathology 2018, 38, 591–600. [Google Scholar] [CrossRef]
- Strobel, S.; Grünblatt, E.; Riederer, P.; Heinsen, H.; Arzberger, T.; Al-Sarraj, S.; Troakes, C.; Ferrer, I.; Monoranu, C.M. Changes in the expression of genes related to neuroinflammation over the course of sporadic Alzheimer’s disease progression: CX3CL1, TREM2, and PPARγ. J. Neural Transm. 2015, 122, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).