Abstract
The problem of spreading harmful infections through contaminated surfaces has become more acute during the recent coronavirus pandemic. The design of self-cleaning materials, which can continuously decompose biological contaminants, is an urgent task for environmental protection and human health care. In this study, the surface of blended cotton/polyester fabric was functionalized with N-doped TiO2 (TiO2-N) nanoparticles using titanium(IV) isopropoxide as a binder to form durable photoactive coating and additionally decorated with Cu species to promote its self-cleaning properties. The photocatalytic ability of the material with photoactive coating was investigated in oxidation of acetone vapor, degradation of deoxyribonucleic acid (DNA) fragments of various lengths, and inactivation of PA136 bacteriophage virus and Candida albicans fungi under visible light and ultraviolet A (UVA) radiation. The kinetic aspects of inactivation and degradation processes were studied using the methods of infrared (IR) spectroscopy, polymerase chain reaction (PCR), double-layer plaque assay, and ten-fold dilution. The results of experiments showed that the textile fabric modified with TiO2-N photocatalyst exhibited photoinduced self-cleaning properties and provided efficient degradation of all studied contaminants under exposure to both UVA and visible light. Additional modification of the material with Cu species substantially improved its self-cleaning properties, even in the absence of light.
1. Introduction
The growing density of urban populations accelerates the spread of infectious diseases [1]. Research on pathogen transmission and disease control methods remains a critical focus for the scientific community. The COVID-19 pandemic highlighted the risks of surface-mediated infection transmission, underscoring the urgent need for materials capable of permanent surface decontamination [2,3]. Self-cleaning paints [4,5], window glasses [6,7], tiles, and filters [8] have been developed. Among these, self-cleaning textiles warrant special attention, as fabrics can harbor pathogenic microorganisms that remain infectious for long periods [9]. Antibacterial properties can be achieved via the decoration of a fabric’s surface with silver and copper nanoparticles [10,11]. The functionalization of fabrics with photocatalysts, such as titanium dioxide (TiO2) and zinc oxide (ZnO), is another promising approach to obtain self-cleaning textiles with a high oxidation activity in chemical degradation and microorganism disinfection [12]. TiO2-coated fabrics can completely decompose chemical pollutants [13,14,15] and provide inactivation of biological agents under exposure to light [16,17].
As TiO2 has a bandgap of 3.0–3.2 eV [18], the TiO2-coated fabrics can be activated under UV radiation only. ZnO is also commonly used as the photoactive component for the modification of textile materials to provide them with self-cleaning properties. Similarly to TiO2, the ZnO-coated fabrics can decompose contaminants under the radiation of UV region due to the wide bandgap of ZnO (3.2–3.4 eV) [19]. This fact limits the efficiency of solar light utilization because the UV region is less than 5% of the solar spectrum [20,21], while visible light is much safer to humans than UV light and makes up almost half of the radiation from the Sun. Thus, enhancing the efficiency of sunlight utilization is a crucial task, which can be addressed through the development of new photocatalysts with a visible-light response (e.g., metal sulfides [22,23], bismuth oxyhalides [24], and graphitic carbon nitride [25]) or modification of conventional photocatalysts to extend their action spectrum to the visible region [26].
TiO2 doping with nitrogen (TiO2-N) has emerged as a successful strategy of its modification that offers the extension of light absorption into the visible region (up 550 nm) and enhanced photocatalytic degradation activity toward various organic pollutants [27,28]. TiO2-N can be employed for modification of fabrics to provide them with self-cleaning properties under visible light. For instance, self-cleaning fabrics modified with TiO2-N were prepared via their impregnation with an alcohol mixture of TiO2-N nanosol, which was obtained by sol-gel method using titanium(IV) n-butoxide (Ti(OBu)4) [29], or titanium(IV) isopropoxide (Ti(iOPr)4) [30] and triethylamine ((C2H5)3N). These materials were thermally treated under air conditions at a temperature of no more than 150 °C because of the low thermal stability of the textile base [31]. However, highly active TiO2 photocatalysts are commonly formed at higher temperatures: above 300 °C for the treatment under air conditions [32] or 150–200 °C for hydrothermal treatment [33,34]. This means that the synthesis of highly active coating on the fabric surfaces cannot be realized due to the impossibility of treatment at high temperatures, and the development of low-temperature deposition methods is important.
Immobilization of well-crystalline photocatalyst particles onto the fabric surface is one of successful strategies for the preparation of photocatalytic materials with a high activity [35]. For instance, the fabric was impregnated with aqueous or alcoholic dispersions of TiO2-N, which was synthesized via high-temperature calcination of the prepared nanosol [36,37]. This fabrication method demonstrated superior photocatalytic activity of as-prepared materials relative to the methods described above [38]. However, the obtained photoactive fabric exhibited poor interfacial adhesion of the photoactive component to the fabric’s surface due to the lack of strong bonding between TiO2-N particles and textile functional groups on the surface of the fiber [39,40]. As a result, photocatalytic performance of material substantially decreased after washing procedures due to flushing of many titania particles. This problem can be solved by using a combined approach when the initially formed photocatalyst particles are fixed on the fabric using a binder, which provides strong bonding and increases coating stability [41,42]. Self-cleaning fabrics have been prepared using organic binders (e.g., carboxylic acids [43,44]), as well as inorganic compounds (e.g., silica [45]). However, carboxylic acids could be degraded during the photocatalytic oxidation process, thus resulting in a decrease in adhesion between photocatalytic particles and the surface of material and a decline of its photoactivity due to removal of particles from the fabric [46]. Inorganic substances can significantly improve the photocatalytic properties of fabric while being the binding material [47]. Addition of SiO2 into the impregnated composition was shown to enhance the mechanical stability of the photoactive coating by incorporating TiO2 into the silicon matrix [40,45], which also positively affected the rate of photocatalytic decolorization of dye stains [48]. Besides SiO2, amorphous TiO2 formed during the hydrolysis of titanium(IV) isopropoxide was used as a binder contributing to highly active cotton fabric with an uniform and stable photoactive coating [49].
In this study, we successfully prepared a self-cleaning textile based on a blended cotton/polyester fabric functionalized with TiO2-N nanoparticles using titanium(IV) isopropoxide as a binder and additionally decorated with Cu species. The photocatalytic ability of the material was investigated in oxidation of acetone vapor, degradation of DNA fragments of various lengths, and inactivation of PA136 bacteriophage and Candida albicans fungi under visible light and UVA radiation. The as-prepared self-cleaning fabric exhibited stability of photoactive coating and high performance in the inactivation of viruses and fungi. Additional modification of the material with Cu species substantially improved its self-cleaning properties, even in the absence of light.
2. Results and Discussion
Visible-light-active TiO2-N, known for its ability for complete mineralization of various pollutants [50], was employed as the photocatalytic component to obtain photoactive fabric with self-cleaning properties. TiO2-N was prepared via the pH-controlled precipitation of titania from an aqueous solution of TiOSO4 using ammonia as a precipitating agent, as well as a source of nitrogen, followed by the calcination of precipitate in air at an increased temperature. This method resulted in the formation of nanocrystalline anatase, which can absorb radiation in the region up to 540 nm due to the incorporation of nitrogen impurities from ammonia into the TiO2 lattice. The photoactive coating was deposited onto the fabric surface using the impregnation method. Titanium(IV) isopropoxide, which was hydrolyzed during the synthesis with a formation of TiO2 matrix, was used as a binder to anchor the TiO2-N particles onto the fabric surface. Since copper is known to enhance the photocatalytic properties of titania in both the pollutant degradation and the inactivation of biological objects [51], the textile material was additionally modified with Cu species by using copper acetate in the impregnating compound.
2.1. Material Characteristics
The SEM image shows that fibers of the initial fabric (IF) have a smooth surface (Figure 1a). The prepared PF-TN material exhibits a coating with fixed agglomerates of TiO2-N particles, which have a size of 1–5 µm (Figure 1b). The surface morphology of this coating is shown in Figure 1c: the observed lamellar structure can be attributed to the binder layer. The small particles in the layer can be attributed TiO2-N agglomerates with size of several hundred nanometers. Thus, the use of a titanium binder made it possible to reliably fix photocatalytic particles on the fabric fiber. Additional copper deposition did not change surface morphology (Figure 1d). This is to be expected, since when copper is applied in this way, it is distributed evenly and randomly, without leading to the formation of large particles, even on the surface of the titania photocatalyst [52]. Bright-field scanning transmission electron microscopy (BFSTEM) (Figure 1e) and energy dispersive X-ray (EDX) mapping (Figure 1f) images confirm this statement and show that copper is evenly distributed on the surface of coated fibers in the PF-TN-Cu material.
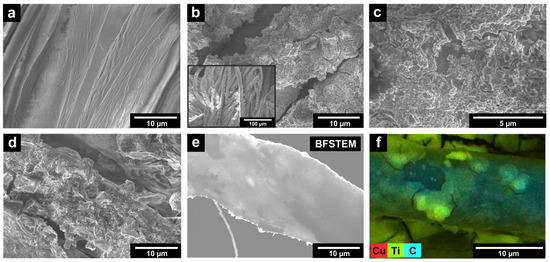
Figure 1.
SEM micrographs of (a) initial fabric (IF) and prepared photoactive (b,c) PF-TN and (d) PF-TN-Cu materials; (e) bright-field STEM image of coated fiber in PF-TN-Cu material; (f) copper distribution in PF-TN-Cu material according to EDX analysis.
The XPS data confirmed the successful fixation of the photocatalytic particles on the surface of the initial fabric sample (IF), which did not have any peaks in the Ti2p photoelectron spectral region (Figure 2a). The presence of TiO2 on the PF-TN surface is clearly evident by the presence of two characteristic peaks at 458.8 and 464.4 eV, which can be attributed to Ti2p3/2 and Ti2p1/2 of Ti4+ state [53].
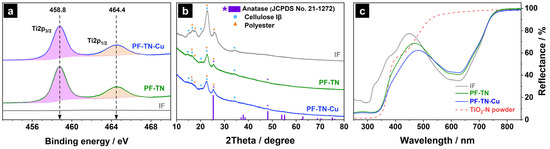
Figure 2.
(a) XPS spectra in the Ti2p region, (b) XRD patterns, and (c) UV–Vis diffuse reflectance spectra of the initial fabric (IF), photoactive fabric modified with TiO2-N (PF-TN), and photoactive fabric modified with TiO2-N and Cu species (PF-TN-Cu).
The surface Ti contents in PF-TN and PF-TN-Cu samples were determined as ca. 17% and 14%, respectively, considering the atomic scattering factors. The total Ti content determined by the ICP-OES method was 3.4 wt% and 2.9 wt% for PF-TN and PF-TN-Cu, respectively. A slight decrease in titanium amount in the PF-TN-Cu sample was observed on the surface as a result of the copper application procedure and a partial wash-out of TiO2 particles.
It is worth noting that no XPS signals are observed in the photoelectron N1s and Cu2p spectral regions of PF-TN-Cu material, although nitrogen and copper states are evident for the powdered Cu-modified TiO2-N photocatalyst (TN-Cu) by the means of the XPS method (see Figure S1 in the Supporting Information). This fact results from an extremely low content of these elements due to the predominance of fabric and TiO2 components in the composition of photoactive material. Nitrogen and copper contents in PF-TN-Cu are estimated as 0.01 and 0.03 wt%, respectively, and these values are much lower than the detection limit of the XPS method.
The XRD pattern of the IF sample had peaks of the initial fabric components only (Figure 2b). Thus, the high-intensity peaks at 15.2°, 16.6°, 20.5°, 22.7°, and 34.4°, which correspond to the , , , , and planes of cellulose Iβ, were detected because cotton was the main component of the fabric used [54]. At the same time, peaks at 17.6° and 25.6° attributed to the and planes of polyester (JCPDS No. 50-2275) proved the initial fabric composition [55]. No evidence of the presence of spandex was observed possibly due to its small content in the material (less than 2 wt%) and the low intensity of the corresponding signals being overloaded by other fabric peaks [56]. The peaks described above were observed for all synthesized samples.
The surface of modification of initial fabric with TiO2-N resulted in the appearance of additional peaks in the XRD pattern of PF-TN at 25.5°, 38.2°, 48.2°, 54.2°, 55.2°, and 63.0° (Figure 2b). They can be attributed to the , , , , , and planes of TiO2 anatase (JCPDS No. 21-1272) [57]. The XRD pattern of PF-TN-Cu material is completely consistent with the diffractogram of PF-TN, where the peaks described corresponded with the initial fabric and titanium dioxide. No copper compounds were observed due to their low content on the material’s surface, and they did not form crystallites. In the case of TiO2-N powder, the copper can be found in the forms of Cu0, CuxO, Cu2+ [58], but on the surface of fabric it predominantly presents in the form of CuxO [59].
UV–Vis diffuse reflectance spectra (Figure 2c) showed that all samples had two main regions of light absorption. The first significant decrease in the reflection below 400 nm can be attributed to polyester, which has an absorption edge at ca. 390 nm [60]. Another light absorption in the 500–750 nm range is caused by the presence of blue dye in the used fabric. An absorption in the range of wavelengths shorter than 450 nm increased for the PF-TN sample due to light absorption by the deposited TiO2-N [61]. The presence of Cu increased the absorption on the 350–500 nm for the PF-TN-Cu sample. Therefore, the results of analysis proved the successful formation of coating on the surface of cotton/polyester fibers.
2.2. Photocatalytic Oxidation
The self-cleaning properties were firstly investigated in a test reaction of acetone vapor oxidation under visible light and UVA radiation. The rate of CO2 formation was chosen as the measure of photocatalytic activity of samples (Figure 3). The photocatalytic activity of the IF sample did not exceed the detection limit and was mostly caused by the CO2 desorption from the fabric surface under irradiation. This confirms that non-modified fabric cannot oxidize such chemicals. The photocatalytic activity of PF-TN which equaled 0.1 μmol min−1 under visible light was caused by the presence of a photoactive component, namely TiO2-N, in its composition. The obtained value was significantly lower than that of TiO2-N powder (0.92 μmol min−1) used for fabric preparation. The activity reduction may be assigned to a low catalyst content in the fabric material (less than 4 wt% of titanium amount). The same behavior was observed for samples irradiated with UVA: the activity value was 0.36 μmol min−1 for the PF-TN sample and 1.39 μmol min−1 for the TN sample (TiO2-N powder).
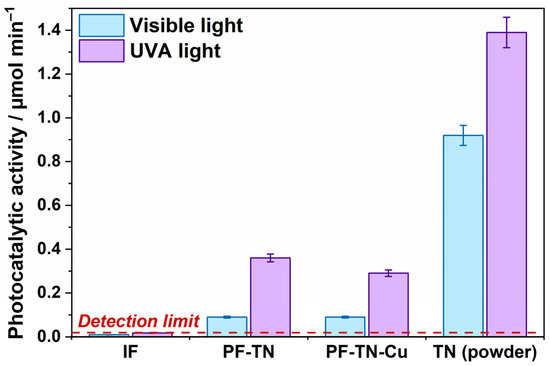
Figure 3.
Photocatalytic activity of synthesized fabric materials and TN powder under visible light and UVA radiation.
Regardless of the fact that the visible-light activity of Cu-modified TiO2-N (TN-Cu) photocatalyst with Cu loading of 1 wt% is ~30% higher than the activity of the initial TiO2-N (TN) sample (Figure S2 in the Supporting Information), the activity of PF-TN-Cu material similarly equals to 0.1 μmol min−1 under visible light as for PF-TN (Figure 3). This means no noticeable effect of Cu species on the material activity under visible light. The activity of TiO2-N powder is known to increase after copper modification due to improving the transfer of photogenerated holes to the photocatalyst surface [58]. Probably, the concentration of Cu species on the surface of TiO2-N crystallites in the case of PF-TN-Cu is lower than in the case of powdered TN-Cu photocatalyst because many Cu species are absorbed on the surface of fabric fibers and amorphous titania binder. As a result, the surface concentration of Cu species on the surface TiO2-N crystallites is not high enough to substantially improve the separation of charges photogenerated in TiO2-N crystallites and enhance the overall photocatalytic activity of the material. An additional argument in favor of copper adsorption on fabric fibers is the evidence of improved adsorption of copper ions in the presence of hydroxyl groups on fibers [62,63]. Under UVA radiation, PF-TN-Cu exhibits a slight decrease in the activity compared to PF-TN, but the observed activity is still high enough for productive oxidation of chemicals.
Note that application of the titanium(IV) isopropoxide binder in the impregnating dispersion provides incorporation of the photocatalyst’s nanoparticles into the titania matrix and their attachment to the surface of the fabric fibers. As a result, the as-prepared materials have a good stability toward washing procedures (Figure S3). Another aspect shown in our previous paper [49] is that the TiO2-coated fabrics exhibit steady-state photocatalytic activity under long-term irradiation for days. All these factors confirm their potential for practical application as self-cleaning textile fabrics.
2.3. DNA Degradation
The contaminant degradation rate can significantly change when changing the pollutant studied from chemicals to biological objects. The rate of methylene blue and Staphylococcus aureus bacteria removal was shown to change under light on the surface of fabric modified with Mn-doped TiO2 nanoparticles [64]. Simple and representative biological contaminants are nucleic acids, but they are rarely used to characterize self-cleaning properties of materials. However, the presence of deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) in the ambient air or on the surface of the material may interfere with obtaining representative results of any studies using highly sensitive microbiological methods such as PCR [65]. Thus, the study of nucleic acid (NA) degradation is of high importance.
The kinetics of nucleic acid degradation was studied using DNA fragments of various lengths under UVA radiation on the surface of the photoactive fabric (PF-TN) and initial fabric (IF) as the reference. The change in DNA concentration was calculated according to Equation (1) using the Cq values determined by the PCR method. The obtained kinetic curves of each fragment are shown in Figure 4a–d. DNA concentration remained constant on the IF surface and matched the initial DNA fragments’ concentration since the used unmodified fabric did not have any anti-contamination properties. An effect of UVA light on the DNA structure was observed on the IF surface (IF under UVA, Figure 4). The DNA concentration decreased by less than tenfold even after 360 min of UVA irradiation. This could be caused by DNA damage under ultraviolet irradiation even in the absence of titanium dioxide [66]. We observed the same pattern in our previous study [67]. Therefore, the stability of DNA on the initial fabric surface and a slight decrease in its concentration under irradiation highlights the ability of NA molecules to be stable on the fabric surface. This makes it possible to transfer them from contaminated fabric to other materials and surfaces.
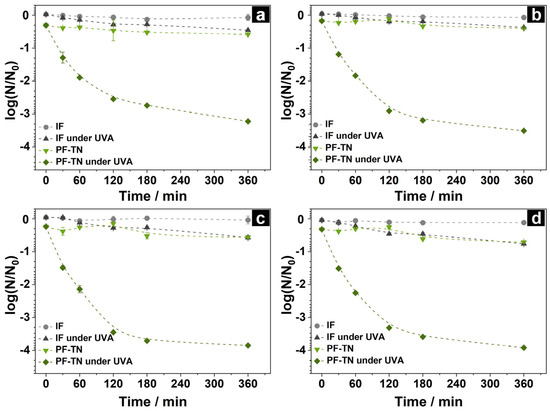
Figure 4.
Kinetic curves of DNA concentration change under UVA irradiation on the surface of initial fabric (IF) and photoactive fabric with TiO2-N (PF-TN) using DNA fragments with lengths of (a) 71 bp, (b) 126 bp, (c) 226 bp, and (d) 454 bp.
A much more noticeable influence of UVA light was observed for samples with photoactive coating. A small decrease in DNA concentration occurred on the non-irradiated material PF-TN (Figure 4): the decrease in concentration was about 10 % at point 0 min, and then concentration monotonically declined during 360 min and reached the value observed for the IF under UVA light. That may be caused by the growth of adsorption characteristics of fabric after modification with TiO2-N.
In the case of the UVA-irradiated PF-TN sample, the DNA concentration markedly fell during the initial 120 min, and then it slowly decreased the next 240 min to the 1000-fold decrease relative to the initial value (Figure 4). The significant fall of the DNA amount is caused by the production of reactive oxygen species (ROS), especially hydroxyl radicals (OH●), on the UVA-irradiated surface of titania, which can oxidize the nucleic acids [68]. Such oxidation of DNA leads to a decrease in its concentration due to the oxidative damage of nitrogenous bases as well as the accumulation of both single-strand breaks (SSBs) and double-strand breaks (DSBs) in DNA molecules followed by its complete degradation to carbon dioxide, phosphates, and nitrogen compounds according to the mechanisms described in our previous paper [67]. It is worth noting that an increase in the fragment length led to growth in the removal rate. The DNA concentration decreased by 1600, 3200, 7000, and 8100 times for the DNA lengths of 71, 126, 226, and 454 bp, respectively (Figure 4a–d). This is a consequence of the non-selective nature of TiO2-mediated photocatalytic oxidation under UVA irradiation [69] and, therefore, the increased oxidation probability for molecules with increased length [67].
Also, DNA degradation was studied using a modified method with three-point activity measurement under visible light: this will still allow obtaining relevant data on the DNA concentration changes, while requiring fewer resources. For this, the volume of applied DNA mixture was increased from 25 to 100 µL and the control experiment under UVA irradiation was performed to check out the effect of the contaminant volume. The DNA concentration change on the material surface under visible light was investigated after 3 and 8 h of irradiation to check out that the degradation pattern for DNA was the same for both the irradiation types as it was for chemical contaminations [70]. The relative decrease in DNA concentration was calculated by normalizing the DNA concentration on irradiated materials to those that were kept in the dark. The obtained threshold cycle values (Cq) are shown in Figure S4. It was shown that the DNA concentration on the surface of PF-TN decreased 210–360 times compared to the initial level after 3 h of UVA irradiation (Figure 5a). For the degradation kinetic curves under similar conditions for DNA fragments with lengths of 71–226 bp (Figure 4), these values were ca. 320–1510. This shows that the DNA degradation degree depends on the initial DNA concentration and that the fourfold increase in the amount of applied DNA (100 µL instead of 25 µL) decreases DNA degradation degree within the same period. Increasing the irradiation time up to 8 h allowed us to increase the DNA degradation degree significantly (DNA concentration 900–3000 times less than initial). So, the modified approach is shown to give a valid result by increasing the irradiation time.
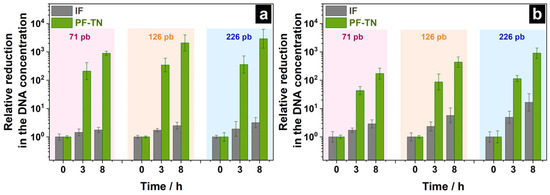
Figure 5.
Relative reduction in DNA concentration on the surface of initial fabric (IF) and PF-TN material under (a) UVA and (b) visible light.
The DNA concentration on the surface of IF material decreased 2–16 times after 8 h of irradiation under visible light which could be mostly caused by the thermal effect of the radiation from the visible-light LED on the DNA structure (Figure 5b). The photoactive fabric PF-TN significantly decreased the DNA amount on its surface: reduction by 170, 440, and 900 times after 8 h of lighting for DNA fragments with lengths of 71, 126, and 226 bp was observed, respectively. This confirms the dependence of the DNA degradation rate on nucleic acid length within the same mechanism for this process. Herewith, the obtained DNA degradation rate under 450-nm irradiation was 3–5 times less than that under UVA irradiation, which is consistent with the photocatalytic activity observed under visible and UVA LEDs (Figure 3). The results obtained show the ability of photoactive fabric PF-TN to provide DNA degradation on its surface under both UVA and visible light.
2.4. Antiviral Activity
The ability of self-cleaning photoactive materials to degrade the membranes and cell walls of microorganisms, including endospores, fungi, bacteria, and viruses, causing their death, holds a special interest on such materials because of the attractiveness for practical use [39,71]. Although numerous studies of photoactive materials and their antibacterial activity are presented in the literature [72,73], very few papers are dedicated to their antiviral activity [74]. Those publications are mostly focused on the destruction of enveloped viruses, such as influenza virus [42], SARS-CoV-2 coronavirus [75], murine coronavirus (MHV-3) [74], because these viruses can replicate themselves quickly in human society. It was proved that photoactive materials effectively inactivate influenza virus and completely destroy its structure down to RNA under UVA irradiation [42].
Non-enveloped viruses lack the additional lipid bilayer, and are therefore essentially different in their structure. The inactivation of this type of virus, e.g., human adenovirus (HAdV-5) [74] and human norovirus [76], has been also studied. Nevertheless, bacteriophage inactivation is understudied as yet. For this reason, the non-enveloped bacteriophage PA136 from the Myoviridae family containing a double-stranded DNA was selected as an object for this research [77].
The virus inactivation ability of prepared samples was studied as control, because it is crucial to prevent possible cell infection with any viruses. For this purpose, the virus suspension was applied to the surface of IF and PF-TN materials and exposed to radiation with visible light. A slight decrease in virus concentration on both the initial IF and photoactive fabric PF-TN was observed even in the absence of irradiation (Figure 6a): from 1.6 × 107 to 4.4 × 106 PFU mL−1, which is a four-fold decrease. This is about the same decrease as for the virus sample placed on the glass surface under the same conditions (≈3.6 times, Figure S5). This can be associated with the damage of the virus itself in the environmental conditions.
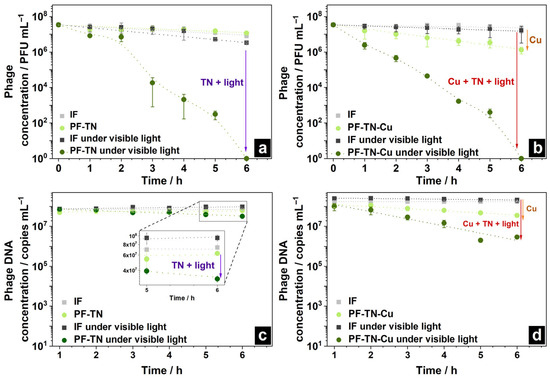
Figure 6.
Change in phage concentration on the surface of (a) PF-TN and (b) PF-TN-Cu materials; change in its DNA on (c) PF-TN and (d) PF-TN-Cu surfaces under visible light.
A slight reduction in bacteriophage concentration was noticed on the surface of initial fabric IF under visible light irradiation (Figure 6a): concentration decreased ca. 4.3 times under visible light (namely from 1.6 × 107 to 3.7 × 106 PFU mL−1), which is presumably caused by heating of the virus-supporting fabric under visible irradiation. In the case of the irradiated photoactive PF-TN sample, the phage concentration slightly decreased during the initial 2 h. Then, after 3 h of the experiment, a sharp decrease in the virus concentration occurred with inactivation of ca. 99.9% of virions. The observed induction period can be related to the need for accumulation of enough damage to inactivate the viral particle. Complete inactivation with a decrease in the virus amounts down to the detection limit occurred after 6 h. The same trend was observed in materials irradiated by UVA (Figure S6a). However, the phage concentration decreased significantly faster (more than 99.8% in 2 h), which may be associated with a higher photoactivity of materials under UVA described earlier (Figure 3). Thus, the synthesized materials demonstrate antiviral activity both under visible and UV irradiation, reducing the virus amount by more than 99.8% during 2–3 h of irradiation.
2.5. Effect of Cu Species on DNA Degradation and Antiviral Activity
The copper effect on antiviral properties of the materials obtained was additionally studied using the PF-TN-Cu sample (Figure 6b). The phage concentration decreased more than 10 times after 6 h even in the absence of radiation (inactivation degree more than 96% of virions instead of 53% earlier), which is caused by antimicrobial properties of copper itself [78]. In the case of irradiated PF-TN-Cu material, the decrease in the phage concentration after 2 h was shown to be 99.7 and 98.6% under visible and UVA radiation, respectively (Figure S6b). It is worth noting that phage concentration was almost unchanged in the absence of copper (Figure 6a) within the initial 2 h. So, this means that copper application onto the photoactive coating decreased the observed induction period. At the same time, the Cu addition did not affect the time of complete inactivation: the kinetic curves for materials with and without copper addition matched completely after more than 3 h of irradiation (Figure S7). Therefore, we may conclude that copper decreased the induction time due to its antimicrobial properties, while the overall antiviral effect exists because of catalytic oxidation on the irradiated photoactive surface.
In addition to virus inactivation process, the Cu-addition effect was studied for DNA degradation too. In the absence of copper, the DNA concentration hardly changed on unirradiated samples and on IF even under irradiation. However, a two-fold decrease in DNA concentration was observed on the PF-TN sample after 6 h of irradiation with visible light, confirming the existence of photocatalytic antiviral properties (Figure 6c). One can see that phage inactivation occurred much faster than DNA destruction: this is obvious because the bacteriophage DNA is protected by capsid proteins from oxidative effects (including the effect of the photocatalyst).
The degradation of phage structure also occurred over the samples considered under UVA irradiation (Figure S6c), which aligned well with the virus inactivation data and photocatalytic oxidation activity values. The PF-TN-Cu material led to an increase in the rate of DNA degradation by more than order of magnitude if compared to the PF-TN sample which was not modified with copper (Figure 6d and Figure S6d). Copper is known to be an effective antimicrobial agent, which can damage viral genomic DNA due to synergistic action of copper ion attack (binding and cross-linking between the strands of NAs) and ROS generation reaction [79]. Therefore, the fundamental antiviral effect of the materials considered is caused by photocatalytic oxidation over TiO2-N with irradiation by UVA or visible light while the copper presence is required for effective removal of contaminants on the material surface in the initial stages of the process.
2.6. Antifungal Properties
Fungi are other harmful biological contaminants, since they can cause a wide range of diseases [80,81]. In contrast to viruses, the fungi have a thick and durable cell wall protecting them from the negative environmental impact [82]; therefore, a much longer period is required for their removing from the surface of any material compared to the Gram-negative bacteria [83]. So, the antifungal properties of synthesized self-cleaning materials are strongly important characteristics, which are usually studied using Aspergius niger [84,85], Aspergillus flavus [86], Penicillium chrysogenum [86], and the most common object for studying the materials—the diploid fungus Candida albicans [87,88,89]. Therefore, this was the one used as the sample to study and compare the data obtained with the results of previous studies. Antifungal experiments were carried out on the initial fabric IF and the copper-modified photoactive material PF-TN-Cu, which had the highest activity and contaminant removal effect.
It was shown that the IF did not adsorb fungi cells on its surface at all and the cell concentration completely coincided with initial one (Figure 7, point 0 min). Furthermore, the concentration insignificantly changed from 1.1 × 105 to 0.8 × 105 and to 0.6 × 105 CFU mL−1 during 150 min in the dark and under UVA irradiation, respectively (Figure 7a, grey and black lines). This means that Candida albicans remained viable on the IF surface in the dark as well as under UVA light. Perelshtein et al. showed equality of Candida albicans concentration after incubation on a cotton surface for 1 and 3 h without light [90] meaning the cells were viable on the surface of non-modified fabrics. Additionally, the cell concentration was not changed on the surface of UVA-irradiated pristine fabric because UVA irradiation is ineffective for destruction of this type of contaminants [91].
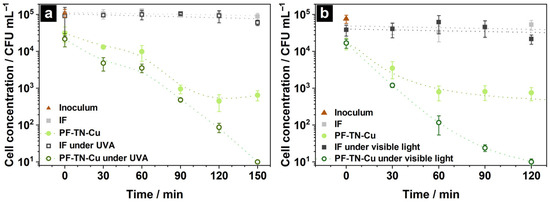
Figure 7.
Kinetic changes in cell concentration of Candida Albicans on the surface of initial fabric IF and photoactive material PF-TN-Cu under (a) UVA irradiation and (b) visible light.
The presence of photoactive coating in the PF-TN-Cu sample significantly increased the Candida albicans adsorption (Figure 7a). Such an effect was noted also during the bacteria destruction studies that proved the adsorption of more than 70% of E. coli cells occurred on the cotton surface (Figure S8). When the fungus cells were kept on the photoactive fabric surface during 60–90 min in the dark, the concentration of cells evenly decreased down to 9.6 × 102 CFU mL−1. This behavior of the kinetic curve can be explained by the presence of copper, which has antimicrobial properties [78]. However, cell count remained about 3.5 × 102–5.2 × 102 CFU mL−1 even after 120 min and was stable during the next 2 h (Figure 7b). So, neither the high adsorption by TiO2 nor the antimicrobial properties of Cu were enough to inactivate the fungi cells completely. However, the light irradiation improved the antifungal properties of PF-TN-Cu material. Thus, the cell concentration monotonically decreased down to the detection limit (less than 10 CFU mL−1) under UVA irradiation during 150 min, which was caused by the formation of ROS on the surface of TiO2-coated material [92]. Such a high antifungal ability is already known [93,94]. As in the case of UVA irradiation, the Candida albicans was also inactivated on the PF-TN-Cu photoactive material irradiated with visible light (Figure 7b). The inactivation period under visible light is only 120 min compared to 150 min under UVA, which might be caused by the 20-times higher irradiation density from the visible-light LED. Indeed, a significant decrease in CFU of Candida auris was observed with the rise of the visible light dose [95]. As shown by Lozano-Rosas et al., the presence of Cu on the TiO2 surface reduced the number of Candida albicans cells and the visible light irradiation enhanced antifungal properties of photocatalyst [96]. Therefore, the obtained self-cleaning fabric materials can inactivate even such high-resisting objects because of the occurrence of the TiO2-N photoactive layer and copper with pronounced antimicrobial properties.
The obtained photoactive fabrics functionalized with TiO2-N can provide degradation of various biological contaminants on their surface under visible light or UVA radiation. The self-cleaning ability of materials under visible light shows promise for their usage in creating fabric products, such as medical coats and curtains, for medical and scientific institutions that require high levels of environmental cleanliness. The possibility of their practical application in the mentioned areas is also provided by the low cytotoxicity of used functional components toward human skin cells, even with prolonged contact [37,97]. Although photoactive fabrics functionalized with a nanocrystalline photocatalyst have a potential risk of leaching the photocatalyst’s nanoparticles [98], the proposed preparation technique solves this problem via use of the titanium(IV) isopropoxide binder, which forms a titania matrix and incorporates the photocatalyst’s particles. Thus, they do not present as single nanoobjects, and the as-functionalized photoactive fabrics exhibit low environmental impact.
3. Materials and Methods
3.1. Synthesis of Materials
The pieces of a blue-colored fabric consisting of 55% cotton, 43% polyester, and 2% spandex (155 g m−2, Jiangsu Xinghe Textile Co., Ltd., Wujiang, China) were used as substrates to obtain self-cleaning textile samples. All fabric pieces were preliminarily washed in an aqueous solution of Triton X-100 nonionic surfactant (Helicon, Moscow, Russia) and laundered with deionized water and isopropyl alcohol (CH3CH(OH)CH3, AO EKOS-1, Moscow, Russia) followed by drying at 60 °C. Titanium(IV) isopropoxide (Ti(OC3H7)4; >98%; Acros Organics, Waltham, MA, USA) dissolved in isopropyl alcohol was employed to anchor the photocatalyst’s nanoparticles on the fabric surface. Titanium(IV) oxysulfate dihydrate (TiOSO4∙2H2O, AO Vekton, Moscow, Russia) and aqueous solution of ammonia (NH4OH, 25%, AO Reachem, Moscow, Russia) were used to synthesize nitrogen-doped titanium dioxide (TiO2-N) which served as the visible-light photocatalyst. Copper(II) acetate (Cu(OAc)2) monohydrate (Cu(CH3COO)2∙H2O, 99%, AO Vekton, Russia) was employed to modify the material’s surface with copper species. All reagents were high purity grade and used without further purification.
3.2. TiO2-N Synthesis
Nitrogen-doped titanium dioxide (TN) was synthesized according to our previously published technique [58], in which the titanium(IV) oxysulfate dihydrate and an aqueous solution of ammonia were added dropwise into deionized water under a constantly maintained pH of 7 (Figure 8a). After aging for two days under continuous stirring, the sediment was washed with deionized water and then calcined under air atmosphere in an electric oven at 450 °C for 3 h. The as-obtained obtained powder was milled using a Retsch MM500 nano mixer mill (Retsch GmbH, Haan, Germany) using zirconium oxide balls (0.1 mm) and isopropyl alcohol, followed by drying at 70 °C.
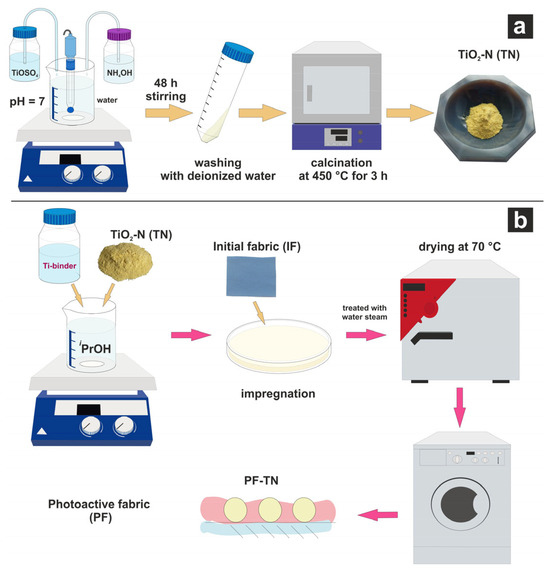
Figure 8.
Synthesis schemes of (a) TiO2-N photocatalyst (TN) and (b) photoactive fabric (PF-TN).
3.3. Synthesis of Photoactive Fabric
Photoactive fabric modified with TiO2-N nanoparticles (PF-TN) was prepared by the impregnation method using titanium(IV) isopropoxide as a binder [42]. The scheme of PF-TN synthesis is shown in Figure 8b. Briefly, a piece of the initial fabric (IF) was impregnated with a suspension of TN, titanium(IV) isopropoxide, and isopropyl, pressed, and dried at room temperature. Actual quantities of used components are listed in Table 1. After that, the second layer of the photoactive coating was deposited using the same technique to increase the thickness of photoactive coating and, consequently, the photoactivity of the material. Finally, the material was treated with water steam, dried at 70 °C, and washed with water followed by drying at 70 °C.

Table 1.
Composition of the suspension used for impregnating fabric pieces.
The copper-modified material (PF-TN-Cu) was synthesized by addition of copper(II) acetate monohydrate to the impregnated composition described above. The amount of added Cu(OAc)2 was estimated on the basis of achieving a copper loading of 1 wt% relative to the mass of the TiO2-N component (Table 1) because it was regarded as an optimum value [58]. The other parameters of the synthesis procedure were kept the same. Table 1 shows the composition of each sample, and the quantities of the components used.
3.4. Material Characterization
A JSM-6460 LV microscope (JEOL, Tokyo, Japan) was used to investigate the surface morphology of samples by scanning electron microscopy (SEM). Scanning transmission electron microscopy (STEM) images were obtained in the bright-field (BF) mode using a Regulus 8230 field-emission scanning electron microscope (FE-SEM, Hitachi, Tokyo, Japan) at an accelerating voltage up to 30 kV. Element distribution was scanned using a Tescan Solaris microscope (TESCAN GROUP, Brno-Kohoutovice, Brno, Czech Republic). X-ray powder diffraction (XRD) analysis was performed using an ARL X’TRA diffractometer (Thermo, Basel, Switzerland) equipped with a CuKα radiation source and a Mythen2 X 1D linear detector (Detris, Baden-Daettwil, Switzerland) to determine the phase composition. The UV–Vis diffuse reflectance spectra (DRS) of the prepared materials were recorded in the range of 250–850 nm with a resolution of 1 nm using a Cary 300 UV–Vis spectrophotometer (Agilent, Santa Clara, CA, USA) equipped with a DRA-30I diffuse reflectance accessory; polytetrafluoroethylene was used as a reflectance standard. The surface state of Ti was determined by X-ray photoelectron spectroscopy (XPS) using a SPECS spectrometer (SPECS Surface Nano Analysis GmbH, Berlin, Germany) equipped with a PHOIBOS-150-MCD-9 analyzer (AlKα radiation, hν = 1486.6 eV, 150 W). The peak positions of Au4f7/2 and Cu2p3/2 with binding energies equal to 84.0 eV and 932.67 eV, respectively, were used for instrument calibration. Ti content in the samples was measured by inductively coupled plasma optical emission spectroscopy (ICP-OES) using an OPTIMA 4300 DV spectrometer (PerkinElmer, Springfield, IL, USA).
3.5. Photocatalytic Activity
The ability of samples to oxidize volatile organic compounds under exposure to light was investigated according to the test method described in our previous papers [28,49]. Briefly, each fabric sample (9 cm2) was placed into the continuous-flow photoreactor and irradiated sequentially with two light-emitting diodes (LEDs) providing blue light (λmax = 450 nm) or UVA light (λmax = 365 nm). In each case, the sample was located directly under the LED at a distance of 9 cm, and the measured irradiance of the sample was 160 mW cm−2 and 10 mW cm−2 for blue light and UVA light, respectively. The powdered samples were distributed on a glass surface of 9 cm2 to obtain an area density of 30 mg cm−2. Acetone vapor (CH3COCH3, Mosreaktiv LLC, Moskow, Russia) with a concentration of 30–32 μmol L−1 was used as a testing oxidizing compound. Volume flow rate of humidified air (relative humidity of 20%) was 0.065 L min−1. The amount of carbon dioxide (CO2) formed as the final product of complete oxidation of acetone was determined using in situ IR spectroscopy on a Nicolet 380 FTIR spectrometer (Thermo Scientific, Waltham, MA, USA) in the range of 2200–2400 cm−1 related to the stretching vibrations of C–O [99]. The photocatalytic activity of each sample was calculated by multiplying the concertation of evolved CO2 and the flow rate.
3.6. Degradation of Biological Contaminants
Degradation of biological contaminates on the surface of fabric materials was studied using the fragments of DNA molecules of various lengths, PA136 virus bacteriophage, and Candida albicans fungi under exposure to radiation from a low-pressure mercury lamp (AeroLife LLC, Moscow, Russia) providing UVA light (λmax = 365 nm, 3 mW cm−2) or two LEDs providing UVA light (λmax = 370 nm, 5 mW cm−2) and blue light (λmax = 450 nm, 30–80 mW cm−2), respectively. The samples were located directly under the light source at a distance of 11–15 cm, and the actual values of measured irradiance are mentioned in the corresponding sections below. All experiments were carried out at a room temperature of 23–25 °C and relative humidity of 20% simultaneously using photoactive fabric materials and the initial fabric as a control. In addition, a part of the samples was irradiated with light, while another part was kept in the dark to estimate the self-cleaning ability of materials without light and to investigate the effect of irradiation on the self-cleaning properties. The zero-time point was used to illustrate the adsorption properties of materials. Each experimental point in the kinetic plots was the result of averaging at least three independent repeats, and the experimental error was evaluated as the standard deviation.
3.6.1. DNA Degradation
The investigation of nucleic acid degradation was carried out with a mixture of four DNA fragments, namely HSPA8 (454 base pairs (bp)), GAPDH (226 bp), 28-2.2 (126 bp), and 18-1702 (71 bp). Initial concentration () of each fragment was adjusted to 0.125 μg mL−1. The kinetic curves of DNA concentration were determined by the polymerase chain reaction (PCR) method. An amount of 25 µL of DNA mixture was placed on the surface of a 1 cm2 piece of fabric and irradiated with UVA (5 mW cm−2) for 360 min. Then, the remaining DNA was extracted by the fabric incubation in a 1 mL of 0.01 M Tris-HCl (pH = 8.0, Biolabmix LLC, Novosibirsk, Russia) for 30 min, then the obtained samples were kept at 4 °C. The specific primers (Table 2) were added into the washed solution to determine the amount of DNA. The 28-2.1 PCR fragment corresponding to the sequence from 4502 to 4548 nucleotide of 28S ribosomal RNA was employed as an internal control to evaluate the presence of inhibitors. The threshold cycle value (Cq) was measured using a LightCycler® 96 System and LightCycler 96 software version 1.1.0.1320 (Roche Diagnostics, Rotkreuz, Switzerland). The PCR protocol is described in detail in the Supporting Information. The experimental Cq value was used to quantify the decrease in DNA concentration for all samples:
where is the DNA concentration on the material surface, is the initial DNA concentration, is the threshold cycle value of the sample, and is the threshold cycle value of initial DNA.

Table 2.
Sequences of the forward (F) and reverse (R) primers for each DNA fragment and DNA of PA136 bacteriophage.
The rate of DNA degradation due to photocatalytic oxidation under UVA and visible light was studied using the method described above with some corrections. The initial volume of DNA solution containing GAPDH, 28-2.2, and 18-1702 fragments was 100 µL and the material was irradiated with UVA (5 mW cm−2) or blue light (80 mW cm−2) for 0 h, 3 h, and 8 h. The experimental Cq of the sample irradiated with light and the one kept without light were marked as Cqwith light and Cqwithout light, respectively. The decrease in DNA concentration (E) showing the rate of DNA degradation was evaluated as follows using the obtained ΔCq values (see Figure S4):
3.6.2. Antiviral Activity
The antiviral activity of materials was investigated using PA136 bacteriophage (phage) from the collection of extremophilic microorganisms and typical cultures (EMTCs) of the Institute of Chemical Biology and Fundamental Medicine of the Siberian Branch of the Russian Academy of Sciences (ICBFM SB RAS). This bacteriophage was preliminarily isolated in the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of the Russian Academy of Sciences. It is a non-enveloped virus containing genomic double-stranded DNA and multiplying in Pseudomonas aeruginosa cells. Pieces of fabric samples 4 cm2 in size were placed in a Petri dish followed by adding 1 mL of bacteriophage suspension with concentration of 107 plaque-forming units per mL (PFU mL−1) in 0.9 wt% saline solution (Renewal, Novosibirsk, Russia) onto the sample surface. Additionally, a bacteriophage suspension of equal value was placed in a Petri dish without samples to control the phage viability under experimental conditions (Figure S5). Then, the samples were placed on a cooling agent to eliminate overheating, covered with quartz glass to avoid evaporation, and irradiated with UVA (5 mW cm−2) or blue light (60 mW cm−2) for 0–6 h. Probes of 60 µL were taken every hour after mixing the suspension by pipetting to analyze bacteriophage infectivity and changes in DNA concentration.
Bacteriophage inactivation was studied using Pseudomonas aeruginosa bacteria from the collection of EMTCs of the ICBFM SB RAS by double-layer plaque assay (Gratia method) [100]. The concentration of the surviving phage was determined by titration with a tenfold step up to the fifth dilution in saline solution. An amount of 5 µL of each dilution of the solution was placed on agar with bacteria culture and incubated at 37 °C for 18–20 h. After that, phage concentration was calculated using the resulting plaque amount. The phage concentration (K) in PFU mL−1 was calculated as follows [101]:
where is the number of negative colonies in Petri dish in PFU, is the volume of the seeded sample in mL, n is the dilution number of the preparation, for which the phage particles are calculated.
DNA concentration change on the material surface was investigated simultaneously with the virus inactivation study to prove the destruction of all bacteriophage structures. To accomplish that, special primers (Table 2) were added to the wash-off taken before PCR. Then, Cq was determined by the PCR method. The DNA amount was determined based on the linear Cq and log of DNA phage concentration dependency (Figure S9). It was plotted based on the correlation of the initial concentration of bacteriophage suspension in the range of 5 × 102–5 × 109 PFU mL−1 (1 PFU mL−1 equals 1 DNA copy in mL−1) and its measured Cq value. This allowed us to determine the DNA concentration in the samples from the Cq under similar conditions.
3.6.3. Antifungal Properties
Candida albicans ATCC 10231 was used to study the antifungal properties of the materials. An amount of 10 mL of cell suspension with concentration of ca. 5 × 105 colony-forming units per mL (CFU mL−1) of the Candida albicans night culture were deposited on samples of 1 cm2, placed in a Petri dish, then covered with a quartz glass to prevent the evaporation. The Petri dishes were placed on a cooling agent to eliminate the heating effect of the optical irradiation. The samples were irradiated by UV light from a mercury lamp (320–400 nm, 3 mW cm−2, AeroLife, Moscow, Russia) or blue light (30 mW cm−2) for 0–240 min. Then, every sample fragment was placed in 5 mL of 0.9 wt% saline solution (Renewal, Russia) for 2 h to allow cell desorption, after which it was stirred for 15 s. The number of cells was evaluated using the 10-fold dilution method [102]. An amount of 100 μL of aliquot was picked and diluted in 0.9 wt% saline solution, then spotted on Sabouraud agar (bioMérieux, Craponne, France) and incubated at 37 °C. After 48 h, colonies were counted to define the CFU mL−1.
4. Conclusions
Photoactive textile material based on blended cotton/polyester fabric was successfully synthesized via a simple impregnation method using TiO2-N nanoparticles as a visible-light-active photocatalyst and titanium(IV) isopropoxide as a binder. The addition of this binder, which was hydrolyzed during the synthesis with a formation of amorphous TiO2 matrix, allowed for anchoring TiO2-N particles onto the fabric surface. The as-prepared self-cleaning fabric exhibited stability of photoactive coating and high photocatalytic performance in the oxidation of volatile organic compounds and the degradation of biological contaminants under both UVA and visible light due to the presence of TiO2-N in the composition. The possibility of nucleic acid degradation under light was studied using DNA fragments of various lengths. The kinetic curves of DNA concentration changes showed that the concentration of each DNA fragment rapidly decreased by 103 times during 2–3 h on the surface of the photoactive material under UVA irradiation. This proved the high efficiency of NA degradation under light. The presence of TiO2-N photocatalyst allowed us to decompose DNA molecules under visible light too. The rate of DNA degradation under UVA was higher than under visible light, which agreed with the photocatalytic activity of these materials in the oxidation of acetone vapor. Study of the degradation of PA136 virus bacteriophage confirmed the antiviral properties of the synthesized material. The virus concentration decreased by more than 99.8% under irradiation with visible light or UVA for 2–3 h. The addition of copper(II) acetate into the impregnating compound shortened the induction period and enhanced the antiviral activity of the material, even in the absence of light, thus showing that Cu species can substantially improve the self-cleaning properties of photoactive textiles. The kinetic curves of the Candida albicans cell concentration on the photoactive surface demonstrated a rapid decrease in fungi amount under UVA and visible light. It was proved that these self-cleaning fabric materials can inactivate even such resistant biological objects because of the both photoactive layer with TiO2-N and Cu species, which themselves have antimicrobial properties.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms26157550/s1.
Author Contributions
Conceptualization, D.K.; methodology, V.M. and G.S.; software, M.L.; validation, E.Z., Y.K. and A.B.; formal analysis, G.S.; investigation, M.S., E.Z., Y.K. and A.B.; resources, V.M. and G.S.; data curation, E.Z.; writing—original draft preparation, M.S.; writing—review and editing, M.L. and D.S.; visualization, M.S. and M.L.; supervision, D.S.; project administration, D.K.; funding acquisition, D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Russian Federation within the project FWUR-2024-0036.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Alexey Salanov, Evgeny Suprun, Igor Prosvirin, and Svetlana Cherepanova for helping in the characterization of fabric materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CFU | Colony-forming unit |
| DNA | Deoxyribonucleic acid |
| DSB | Double-strand break |
| NA | Nucleic acid |
| PFU | Plaque-forming unit |
| ROS | Reactive oxygen species |
| RNA | Ribonucleic acid |
| SSB | Single-strand break |
| UVA | Ultraviolet A |
References
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef]
- Ashokkumar, S.; Kaushik, N.K.; Han, I.; Uhm, H.S.; Park, J.S.; Cho, G.S.; Oh, Y.-J.; Shin, Y.O.; Choi, E.H. Persistence of Coronavirus on Surface Materials and Its Control Measures Using Nonthermal Plasma and Other Agents. Int. J. Mol. Sci. 2023, 24, 14106. [Google Scholar] [CrossRef]
- Querido, M.M.; Aguiar, L.; Neves, P.; Pereira, C.C.; Teixeira, J.P. Self-Disinfecting Surfaces and Infection Control. Colloids Surf. B Biointerfaces 2019, 178, 8–21. [Google Scholar] [CrossRef]
- Ganesh, V.A.; Raut, H.K.; Nair, A.S.; Ramakrishna, S. A Review on Self-Cleaning Coatings. J. Mater. Chem. 2011, 21, 16304. [Google Scholar] [CrossRef]
- Ragesh, P.; Anand Ganesh, V.; Nair, S.V.; Nair, A.S. A Review on ‘Self-Cleaning and Multifunctional Materials. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Paolini, R.; Borroni, D.; Pedeferri, M.; Diamanti, M.V. Self-Cleaning Building Materials: The Multifaceted Effects of Titanium Dioxide. Constr. Build. Mater. 2018, 182, 126–133. [Google Scholar] [CrossRef]
- Zhang, M.; Feng, S.; Wang, L.; Zheng, Y. Lotus Effect in Wetting and Self-Cleaning. Biotribology 2016, 5, 31–43. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic Disinfection Using Titanium Dioxide: Spectrum and Mechanism of Antimicrobial Activity. Appl. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef]
- Koca, O.; Altoparlak, U.; Ayyildiz, A.; Kaynar, H. Persistence of Nosocomial Pathogens on Various Fabrics. Eurasian J. Med. 2012, 44, 28–31. [Google Scholar] [CrossRef]
- Xing, H.; Cheng, J.; Tan, X.; Zhou, C.; Fang, L.; Lin, J. Ag Nanoparticles-Coated Cotton Fabric for Durable Antibacterial Activity: Derived from Phytic Acid–Ag Complex. J. Text. Inst. 2020, 111, 855–861. [Google Scholar] [CrossRef]
- Xu, Q.; Ke, X.; Ge, N.; Shen, L.; Zhang, Y.; Fu, F.; Liu, X. Preparation of Copper Nanoparticles Coated Cotton Fabrics with Durable Antibacterial Properties. Fibers Polym. 2018, 19, 1004–1013. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Lauruengtana, V.; Surassmo, S.; Ruktanonchai, U. Antibacterial Effect of Apatite-Coated Titanium Dioxide for Textiles Applications. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 240–249. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent Advances in TiO2-Functionalized Textile Surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Karaseva, I.P.; Uvaev, V.V.; Kozlov, D.V.; Parmon, V.N. Effect of Preparation Method of Functionalized Textile Materials on Their Photocatalytic Activity and Stability under UV Irradiation. Chem. Eng. J. 2013, 224, 114–120. [Google Scholar] [CrossRef]
- Behzadnia, A.; Montazer, M.; Rashidi, A.; Rad, M.M. Sonosynthesis of Nano TiO2 on Wool Using Titanium Isopropoxide or Butoxide in Acidic Media Producing Multifunctional Fabric. Ultrason. Sonochemistry 2014, 21, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad Spectrum Microbicidal Activity of Photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
- Truong, P.L.; Kidanemariam, A.; Park, J. A Critical Innovation of Photocatalytic Degradation for Toxic Chemicals and Pathogens in Air. J. Ind. Eng. Chem. 2021, 100, 19–39. [Google Scholar] [CrossRef]
- Du, Z.; Cheng, C.; Tan, L.; Lan, J.; Jiang, S.; Zhao, L.; Guo, R. Enhanced Photocatalytic Activity of Bi2WO6/TiO2 Composite Coated Polyester Fabric under Visible Light Irradiation. Appl. Surf. Sci. 2018, 435, 626–634. [Google Scholar] [CrossRef]
- Bogdan, J.; Zarzyńska, J.; Pławińska-Czarnak, J. Comparison of Infectious Agents Susceptibility to Photocatalytic Effects of Nanosized Titanium and Zinc Oxides: A Practical Approach. Nanoscale Res. Lett. 2015, 10, 309. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Li, Q.; Zhang, X.; Shang, J.K. Antifungal Activity and Mechanism of Palladium-Modified Nitrogen-Doped Titanium Oxide Photocatalyst on Agricultural Pathogenic Fungi Fusarium Graminearum. ACS Appl. Mater. Interfaces 2013, 5, 10953–10959. [Google Scholar] [CrossRef]
- Shakeel, N.; Piwoński, I.; Iqbal, P.; Kisielewska, A. Green Synthesis of Titanium Dioxide Nanoparticles: Physicochemical Characterization and Applications: A Review. Int. J. Mol. Sci. 2025, 26, 5454. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhang, L.; Yu, J. In Situ Irradiated XPS Investigation on S-Scheme TiO2@ZnIn2S4 Photocatalyst for Efficient Photocatalytic CO2 Reduction. Small 2021, 17, 2103447. [Google Scholar] [CrossRef] [PubMed]
- Markovskaya, D.V.; Cherepanova, S.V.; Gerasimov, E.Y.; Zhurenok, A.V.; Selivanova, A.V.; Selishchev, D.S.; Kozlova, E.A. The Influence of the Sacrificial Agent Nature on Transformations of the Zn(OH)2/Cd0.3Zn0.7S Photocatalyst during Hydrogen Production under Visible Light. RSC Adv. 2020, 10, 1341–1350. [Google Scholar] [CrossRef] [PubMed]
- Zulkiflee, A.; Khan, M.M.; Harunsani, M.H. Bismuth Oxyhalides: Recent Progress and Its Applications in Photocatalysis, Hydrogen Production, Antibacterial Studies, and Sensors. Mater. Sci. Semicond. Process. 2023, 163, 107547. [Google Scholar] [CrossRef]
- Qi, K.; Liu, S.; Zada, A. Graphitic Carbon Nitride, a Polymer Photocatalyst. J. Taiwan Inst. Chem. Eng. 2020, 109, 111–123. [Google Scholar] [CrossRef]
- Weon, S.; He, F.; Choi, W. Status and Challenges in Photocatalytic Nanotechnology for Cleaning Air Polluted with Volatile Organic Compounds: Visible Light Utilization and Catalyst Deactivation. Environ. Sci. Nano 2019, 6, 3185–3214. [Google Scholar] [CrossRef]
- Acharya, R.; Pani, P. Visible Light Susceptible Doped TiO2 Photocatalytic Systems: An Overview. Mater. Today Proc. 2022, 67, 1276–1282. [Google Scholar] [CrossRef]
- Lyulyukin, M.; Kovalevskiy, N.; Bukhtiyarov, A.; Kozlov, D.; Selishchev, D. Kinetic Aspects of Benzene Degradation over TiO2-N and Composite Fe/Bi2WO6/TiO2-N Photocatalysts under Irradiation with Visible Light. Int. J. Mol. Sci. 2023, 24, 5693. [Google Scholar] [CrossRef]
- Wu, D.; Long, M. Low-Temperature Synthesis of N-TiO2 Sol and Characterization of N-TiO2 Coating on Cotton Fabrics. Surf. Coat. Technol. 2012, 206, 3196–3200. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Zebarjad, S.M.; Janghorban, K. Investigation of Mechanical Characteristics of Functionalized Cotton Textiles by N-Doped TiO2 Nanoparticles. Mater. Chem. Phys. 2018, 218, 239–245. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Z.; Zhang, X.; Mao, N. Photocatalytic Effects of Wool Fibers Modified with Solely TiO2 Nanoparticles and N-Doped TiO2 Nanoparticles by Using Hydrothermal Method. Chem. Eng. J. 2014, 254, 106–114. [Google Scholar] [CrossRef]
- Wang, L.; Shen, Y.; Xu, L.; Cai, Z.; Zhang, H. Thermal Crystallization of Low-Temperature Prepared Anatase Nano-TiO2 and Multifunctional Finishing of Cotton Fabrics. J. Text. Inst. 2016, 107, 651–662. [Google Scholar] [CrossRef]
- Wu, D.; Long, M.; Cai, W.; Chen, C.; Wu, Y. Low Temperature Hydrothermal Synthesis of N-Doped TiO2 Photocatalyst with High Visible-Light Activity. J. Alloys Compd. 2010, 502, 289–294. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Wang, S.; Ding, H.; Zhao, Y.; Li, Y.; Wang, W. Fabrication of Protective Textile with N-Doped TiO2 Embedded Citral Microcapsule Coating and Its Air Purification Properties. Fibers Polym. 2020, 21, 334–342. [Google Scholar] [CrossRef]
- Stan, M.S.; Nica, I.C.; Dinischiotu, A.; Varzaru, E.; Iordache, O.G.; Dumitrescu, I.; Popa, M.; Chifiriuc, M.C.; Pircalabioru, G.G.; Lazar, V.; et al. Photocatalytic, Antimicrobial and Biocompatibility Features of Cotton Knit Coated with Fe-N-Doped Titanium Dioxide Nanoparticles. Materials 2016, 9, 789. [Google Scholar] [CrossRef]
- Stan, M.S.; Badea, M.A.; Pircalabioru, G.G.; Chifiriuc, M.C.; Diamandescu, L.; Dumitrescu, I.; Trica, B.; Lambert, C.; Dinischiotu, A. Designing Cotton Fibers Impregnated with Photocatalytic Graphene Oxide/Fe, N-Doped TiO2 Particles as Prospective Industrial Self-Cleaning and Biocompatible Textiles. Mater. Sci. Eng. C 2019, 94, 318–332. [Google Scholar] [CrossRef]
- Vero, N.; Hribernik, S.; Andreozzi, P.; Sfiligoj-Smole, M. Homogeneous Self-Cleaning Coatings on Cellulose Materials Derived from TIP/TiO2 P25. Fibers Polym. 2009, 10, 716–723. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, J.; Sun, L.; Wang, X. A Review on the Application of Photocatalytic Materials on Textiles. Text. Res. J. 2015, 85, 1104–1118. [Google Scholar] [CrossRef]
- Han, Z.; Chang, V.W.C.; Zhang, L.; Tse, M.S.; Tan, O.K.; Hildemann, L.M. Preparation of TiO2-Coated Polyester Fiber Filter by Spray-Coating and Its Photocatalytic Degradation of Gaseous Formaldehyde. Aerosol Air Qual. Res. 2012, 12, 1327–1335. [Google Scholar] [CrossRef]
- Dhineshbabu, N.; Arunmetha, S.; Manivasakan, P.; Karunakaran, G.; Rajendran, V. Enhanced Functional Properties of Cotton Fabrics Using TiO2/SiO2 Nanocomposites. J. Ind. Text. 2016, 45, 674–692. [Google Scholar] [CrossRef]
- Selishchev, D.; Stepanov, G.; Sergeeva, M.; Solovyeva, M.; Zhuravlev, E.; Komissarov, A.; Richter, V.; Kozlov, D. Inactivation and Degradation of Influenza A Virus on the Surface of Photoactive Self-Cleaning Cotton Fabric Functionalized with Nanocrystalline TiO2. Catalysts 2022, 12, 1298. [Google Scholar] [CrossRef]
- Montazer, M.; Hashemikia, S. Textile with Immobilised Nano Titanium Dioxide for Repeated Discoloration of CI Reactive Black 5 under UV-A. Color. Technol. 2012, 128, 403–409. [Google Scholar] [CrossRef]
- Yuranova, T.; Laub, D.; Kiwi, J. Synthesis, Activity and Characterization of Textiles Showing Self-Cleaning Activity under Daylight Irradiation. Catal. Today 2007, 122, 109–117. [Google Scholar] [CrossRef]
- Carlo, G.D.; Liotta, L.F.; Calogero, G.; Giuliani, C.; Ingo, G.M. Green Cleaning Procedures Based on Titania-Doped Cotton Textiles: Effect of Titania Textural Properties. J. Nanosci. Nanotechnol. 2017, 17, 3842–3847. [Google Scholar] [CrossRef]
- Meilert, K.T.; Laub, D.; Kiwi, J. Photocatalytic Self-Cleaning of Modified Cotton Textiles by TiO2 Clusters Attached by Chemical Spacers. J. Mol. Catal. A Chem. 2005, 237, 101–108. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A.; Wang, X. Self-Cleaning and Superhydrophilic Wool by TiO2/SiO2 Nanocomposite. Appl. Surf. Sci. 2013, 275, 397–402. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A.; Sun, L.; Wang, X. Visible and UV Functionality of TiO2 Ternary Nanocomposites on Cotton. Appl. Surf. Sci. 2014, 321, 447–456. [Google Scholar] [CrossRef]
- Solovyeva, M.; Selishchev, D.; Cherepanova, S.; Stepanov, G.; Zhuravlev, E.; Richter, V.; Kozlov, D. Self-Cleaning Photoactive Cotton Fabric Modified with Nanocrystalline TiO2 for Efficient Degradation of Volatile Organic Compounds and DNA Contaminants. Chem. Eng. J. 2020, 388, 124167. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Zhang, M.; Yang, J.; Zhang, Z. Enhanced Visible Light Photocatalytic Activity of N-Doped TiO2 in Relation to Single-Electron-Trapped Oxygen Vacancy and Doped-Nitrogen. Appl. Catal. B Environ. 2010, 100, 84–90. [Google Scholar] [CrossRef]
- Wojcieszak, D.; Mazur, M.; Kaczmarek, D.; Poniedziałek, A.; Osękowska, M. An Impact of the Copper Additive on Photocatalytic and Bactericidal Properties of TiO2 Thin Films. Mater. Sci.-Pol. 2017, 35, 421–426. [Google Scholar] [CrossRef]
- Gribov, E.; Koshevoy, E.; Fazliev, T.; Lyulyukin, M.; Kozlov, D.; Selishchev, D. Effect of Surface Fe- and Cu-Species on the Flat-Band Potential and Photoelectrocatalytic Properties of N-Doped TiO2. J. Photochem. Photobiol. A Chem. 2025, 464, 116342. [Google Scholar] [CrossRef]
- Sen, S.K.; Riga, J.; Verbist, J. 2s and 2p X-Ray Photoelectron Spectra of Ti4+ Ion in TiO2. Chem. Phys. Lett. 1976, 39, 560–564. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Lionetto, F.; Corcione, C.E.; Rizzo, A.; Maffezzoli, A. Production and Characterization of Polyethylene Terephthalate Nanoparticles. Polymers 2021, 13, 3745. [Google Scholar] [CrossRef]
- Ma, Z.; Xu, R.; Wang, W.; Yu, D. A Wearable, Anti-Bacterial Strain Sensor Prepared by Silver Plated Cotton/Spandex Blended Fabric for Human Motion Monitoring. Colloids Surf. A Physicochem. Eng. Asp. 2019, 582, 123918. [Google Scholar] [CrossRef]
- Wang, P.; Dong, Y.; Li, B.; Li, Z.; Bian, L. A Sustainable and Cost Effective Surface Functionalization of Cotton Fabric Using TiO2 Hydrosol Produced in a Pilot Scale: Condition Optimization, Sunlight-Driven Photocatalytic Activity and Practical Applications. Ind. Crops Prod. 2018, 123, 197–207. [Google Scholar] [CrossRef]
- Kovalevskiy, N.; Svintsitskiy, D.; Cherepanova, S.; Yakushkin, S.; Martyanov, O.; Selishcheva, S.; Gribov, E.; Kozlov, D.; Selishchev, D. Visible-Light-Active N-Doped TiO2 Photocatalysts: Synthesis from TiOSO4, Characterization, and Enhancement of Stability Via Surface Modification. Nanomaterials 2022, 12, 4146. [Google Scholar] [CrossRef]
- Román, L.E.; Uribe, C.; Paraguay-Delgado, F.; Sutjianto, J.G.; Navarrete-López, A.M.; Gomez, E.D.; Solís, J.L.; Gómez, M.M. Physical and Surface Chemical Analysis of High-Quality Antimicrobial Cotton Fabrics Functionalized with CuOx Grown In Situ from Different Copper Salts: Experimental and Theoretical Approach. ACS Appl. Mater. Interfaces 2025, 17, 1869–1882. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, Z.; Jiang, W.; Zhang, G.; Tan, Y.; Guan, Y.; Zhou, L.; Cui, L.; Choi, S.W.; Li, M.-X. Preparation of Y2O3/TiO2-Loaded Polyester Fabric and Its Photocatalytic Properties under Visible Light Irradiation. Polymers 2022, 14, 2760. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, C.; Zhang, M.; Yang, J.; Zhang, Z. Visible Light Active N-Doped TiO2 Prepared from Different Precursors: Origin of the Visible Light Absorption and Photoactivity. Appl. Catal. B Environ. 2011, 104, 268–274. [Google Scholar] [CrossRef]
- Radetić, M.; Marković, D. Nano-Finishing of Cellulose Textile Materials with Copper and Copper Oxide Nanoparticles. Cellulose 2019, 26, 8971–8991. [Google Scholar] [CrossRef]
- Prabhakar, P.; Sen, R.K.; Patel, M.; Shruti; Dwivedi, N.; Singh, S.; Kumar, P.; Chouhan, M.; Yadav, A.K.; Mondal, D.P.; et al. Development of Copper Impregnated Bio-Inspired Hydrophobic Antibacterial Nanocoatings for Textiles. Colloids Surf. B Biointerfaces 2022, 220, 112913. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Papadopoulou, E.L.; Suarato, G.; Binas, V.D.; Kiriakidis, G.; Gounaki, I.; Moira, O.; Venieri, D.; Bayer, I.S.; Athanassiou, A. Fabrication of Visible Light-Induced Antibacterial and Self-Cleaning Cotton Fabrics Using Manganese Doped TiO2 Nanoparticles. ACS Appl. Bio Mater. 2018, 1, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Champlot, S.; Berthelot, C.; Pruvost, M.; Bennett, E.A.; Grange, T.; Geigl, E.-M. An Efficient Multistrategy DNA Decontamination Procedure of PCR Reagents for Hypersensitive PCR Applications. PLoS ONE 2010, 5, e13042. [Google Scholar] [CrossRef]
- Karran, P.; Brem, R. Protein Oxidation, UVA and Human DNA Repair. DNA Repair 2016, 44, 178–185. [Google Scholar] [CrossRef]
- Solovyeva, M.; Stepanov, G.; Zhuravlev, E.; Kozlov, D.; Zharkov, D.; Dvornikova, A.; Selishchev, D. Mechanism of DNA and RNA Degradation over a Photoactive TiO2@SiO2-Coated Fabric. Int. J. Biol. Macromol. 2025, 318, 145089. [Google Scholar] [CrossRef]
- Hirakawa, K.; Mori, M.; Yoshida, M.; Oikawa, S.; Kawanishi, S. Photo-Irradiated Titanium Dioxide Catalyzes Site Specific DNA Damage via Generation of Hydrogen Peroxide. Free Radic. Res. 2004, 38, 439–447. [Google Scholar] [CrossRef]
- Ren, Y.; Guo, D.; Zhao, Z.; Chen, P.; Li, F.; Yao, J.; Jiang, H.; Liu, Y. Singlet Oxygen Mediated Photocatalytic Antimonite Decontamination in Water Using Nanoconfined TiO2. Chem. Eng. J. 2022, 435, 134832. [Google Scholar] [CrossRef]
- Selishchev, D.S.; Filippov, T.N.; Lyulyukin, M.N.; Kozlov, D.V. Uranyl-Modified TiO2 for Complete Photocatalytic Oxidation of Volatile Organic Compounds under UV and Visible Light. Chem. Eng. J. 2019, 370, 1440–1449. [Google Scholar] [CrossRef]
- Dworniczek, E.; Franiczek, R.; Kowal, K.; Buzalewicz, I.; Podbielska, H.; Tofail, S.A.M. Photocatalytic and Antimicrobial Activity of Titania Nanoparticles. In Electrically Active Materials for Medical Devices; Imperial College Press: London, UK, 2016; pp. 193–208. ISBN 978-1-78326-986-0. [Google Scholar]
- Pakdel, E.; Daoud, W.A.; Wang, X. Effect of the Photoreduction Process on the Self-Cleaning and Antibacterial Activity of Au-Doped TiO2 Colloids on Cotton Fabric. ACS Appl. Mater. Interfaces 2024, 16, 25221–25235. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Amparán, M.A.; Martínez-Cornejo, V.; Cedeño-Caero, L.; Hernandez-Hernandez, K.A.; Cadena-Nava, R.D.; Alonso-Núñez, G.; Moyado, S.F. Characterization and Photocatalytic Activity of TiO2 Nanoparticles on Cotton Fabrics, for Antibacterial Masks. Appl. Nanosci. 2022, 12, 4019–4032. [Google Scholar] [CrossRef] [PubMed]
- Souza, D.C.; Amorim, S.M.; Cadamuro, R.D.; Fongaro, G.; Peralta, R.A.; Peralta, R.M.; Puma, G.L.; Moreira, R.F. Hydrophobic Cellulose-Based and Non-Woven Fabrics Coated with Mesoporous TiO2 and Their Virucidal Properties under Indoor Light. Carbohydr. Polym. Technol. Appl. 2022, 3, 100182. [Google Scholar] [CrossRef]
- da Silva, D.J.; Duran, A.; Cabral, A.D.; Fonseca, F.L.A.; Bueno, R.F.; Wang, S.H.; Rosa, D.S. Delta SARS-CoV-2 Inactivation and Bactericidal Performance of Cotton Wipes Decorated with TiO2/Ag Nanoparticles like Brazilian Heavy-Fruited Myrciaria cauliflora. Mater. Today Commun. 2022, 33, 104288. [Google Scholar] [CrossRef]
- Moon, E.W.; Lee, H.-W.; Rok, J.H.; Ha, J.-H. Photocatalytic Inactivation of Viral Particles of Human Norovirus by Cu-Doped TiO2 Non-Woven Fabric under UVA-LED Wavelengths. Sci. Total Environ. 2020, 749, 141574. [Google Scholar] [CrossRef]
- Chechushkov, A.; Kozlova, Y.; Baykov, I.; Morozova, V.; Kravchuk, B.; Ushakova, T.; Bardasheva, A.; Zelentsova, E.; Allaf, L.A.; Tikunov, A.; et al. Influence of Caudovirales Phages on Humoral Immunity in Mice. Viruses 2021, 13, 1241. [Google Scholar] [CrossRef]
- Fan, X.; Yahia, L.; Sacher, E. Antimicrobial Properties of the Ag, Cu Nanoparticle System. Biology 2021, 10, 137. [Google Scholar] [CrossRef]
- Govind, V.; Bharadwaj, S.; Sai Ganesh, M.R.; Vishnu, J.; Shankar, K.V.; Shankar, B.; Rajesh, R. Antiviral Properties of Copper and Its Alloys to Inactivate COVID-19 Virus: A Review. Biometals 2021, 34, 1217–1235. [Google Scholar] [CrossRef]
- Köhler, J.R.; Hube, B.; Puccia, R.; Casadevall, A.; Perfect, J.R. Fungi That Infect Humans. Microbiol. Spectr. 2017, 5, 813–843. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Tennakoon, D.S.; Jayatunga, D.P.W.; Hongsanan, S.; Xie, N. Humans vs. Fungi: An Overview of Fungal Pathogens against Humans. Pathogens 2024, 13, 426. [Google Scholar] [CrossRef]
- Latgé, J.-P. The Cell Wall: A Carbohydrate Armour for the Fungal Cell. Mol. Microbiol. 2007, 66, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Behzadnia, A.; Montazer, M.; Rashidi, A.; Mahmoudi Rad, M. Rapid Sonosynthesis of N-Doped Nano TiO2 on Wool Fabric at Low Temperature: Introducing Self-cleaning, Hydrophilicity, Antibacterial/Antifungal Properties with Low Alkali Solubility, Yellowness and Cytotoxicity. Photochem. Photobiol. 2014, 90, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Brzeziński, S.; Kamińska, I. Multifunctional Nanocoating Finishing of Polyester/Cotton Woven Fabric by the Sol-Gel Method. Text. Res. J. 2018, 88, 946–956. [Google Scholar] [CrossRef]
- Rilda, Y.; Mahardika, G.; Alif, A.; Agustien, A.; Djamaan, A. Antifungal Property of Cotton Fabric Textile: Modification of Cotton Fiber Functions by Coating Compounds of TiO2-SiO2/Chitosan. Pharma Chem. 2016, 8, 124–131. [Google Scholar]
- Al-Etaibi, A.M.; El-Apasery, M.A. Nano TiO2 Imparting Multifunctional Performance on Dyed Polyester Fabrics with Some Disperse Dyes Using High Temperature Dyeing as an Environmentally Benign Method. Int. J. Environ. Res. Public Health 2020, 17, 1377. [Google Scholar] [CrossRef]
- Behzadnia, A.; Montazer, M.; Rad, M.M. Simultaneous Sonosynthesis and Sonofabrication of N-Doped ZnO/TiO2 Core–Shell Nanocomposite on Wool Fabric: Introducing Various Properties Specially Nano Photo Bleaching. Ultrason. Sonochemistry 2015, 27, 10–21. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Vodnik, V.; Potkonjak, B.; Jovančić, P.; Nedeljković, J.M.; Radetić, M. Multifunctional PES Fabrics Modified with Colloidal Ag and TiO2 Nanoparticles. Polym. Adv. Technol. 2011, 22, 2244–2249. [Google Scholar] [CrossRef]
- Saraswati, M.; Levi Permadani, R. The Innovation of Antimicrobial and Self-Cleaning Using Ag/TiO2 Nanocomposite Coated on Cotton Fabric for Footwear Application. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 12091. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Grinblat, J.; Gedanken, A. A One-Step Process for the Antimicrobial Finishing of Textiles with Crystalline TiO2 Nanoparticles. Chem.—Eur. J. 2012, 18, 4575–4582. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Antifungal Activity of TiO2 Photocatalysis against Penicillium expansum in Vitro and in Fruit Tests. Int. J. Food Microbiol. 2006, 107, 99–103. [Google Scholar] [CrossRef]
- Ma, H.; Brennan, A.; Diamond, S.A. Photocatalytic Reactive Oxygen Species Production and Phototoxicity of Titanium Dioxide Nanoparticles Are Dependent on the Solar Ultraviolet Radiation Spectrum. Environ. Toxic Chem. 2012, 31, 2099–2107. [Google Scholar] [CrossRef]
- Scacchetti, F.A.P.; Pinto, E.; Soares, G. A Multifunctional Cotton Fabric Using TiO2 and PCMs: Introducing Thermal Comfort and Self-Cleaning Properties. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 122011. [Google Scholar] [CrossRef]
- Nazari, A. Superior Self-Cleaning and Antimicrobial Properties on Cotton Fabrics Using Nano Titanium Dioxide along with Green Walnut Shell Dye. Fibers Polym. 2019, 20, 2503–2509. [Google Scholar] [CrossRef]
- Gierke, A.-M.; Hessling, M. Photoinactivation by UVA Radiation and Visible Light of Candida Auris Compared to Other Fungi. Photochem. Photobiol. Sci. 2024, 23, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Rosas, R.; Ramos-Garcia, R.; Salazar-Morales, M.F.; Robles-Águila, M.J.; Spezzia-Mazzocco, T. Evaluation of Antifungal Activity of Visible Light-Activated Doped TiO2 Nanoparticles. Photochem. Photobiol. Sci. 2024, 23, 823–837. [Google Scholar] [CrossRef]
- Nica, I.C.; Stan, M.S.; Dinischiotu, A.; Popa, M.; Chifiriuc, M.C.; Lazar, V.; Pircalabioru, G.G.; Bezirtzoglou, E.; Iordache, O.G.; Varzaru, E.; et al. Innovative Self-Cleaning and Biocompatible Polyester Textiles Nano-Decorated with Fe–N-Doped Titanium Dioxide. Nanomaterials 2016, 6, 214. [Google Scholar] [CrossRef]
- Busi, E.; Maranghi, S.; Corsi, L.; Basosi, R. Environmental Sustainability Evaluation of Innovative Self-Cleaning Textiles. J. Clean. Prod. 2016, 133, 439–450. [Google Scholar] [CrossRef]
- Lyulyukin, M.; Filippov, T.; Cherepanova, S.; Solovyeva, M.; Prosvirin, I.; Bukhtiyarov, A.; Kozlov, D.; Selishchev, D. Synthesis, Characterization and Visible-Light Photocatalytic Activity of Solid and TiO2-Supported Uranium Oxycompounds. Nanomaterials 2021, 11, 1036. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of Bacteriophages by Double Agar Overlay Plaque Assay. In Bacteriophages: Methods and Protocols, Volume 1: Isolation, Characterization, and Interactions; Clokie, M.R.J., Kropinski, A.M., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 69–76. ISBN 978-1-60327-164-6. [Google Scholar]
- Fedorov, E.; Samokhin, A.; Kozlova, Y.; Kretien, S.; Sheraliev, T.; Morozova, V.; Tikunova, N.; Kiselev, A.; Pavlov, V. Short-Term Outcomes of Phage-Antibiotic Combination Treatment in Adult Patients with Periprosthetic Hip Joint Infection. Viruses 2023, 15, 499. [Google Scholar] [CrossRef]
- Palma, F.; Acunzo, M.; Della Marca, R.; Dell’Annunziata, F.; Folliero, V.; Chianese, A.; Zannella, C.; Franci, G.; De Filippis, A.; Galdiero, M. Evaluation of Antifungal Spectrum of Cupferron against Candida albicans. Microb. Pathog. 2024, 194, 106835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).