Photochemical Haze Formation on Titan and Uranus: A Comparative Review
Abstract
1. Introduction
- Section 2 will include a review of the atmospheric and radiative environments of the ice giants with an emphasis on Uranus and how they differ from Titan in the Saturnian system. In addition, I will survey the current state-of-the-art body of knowledge of their chemical inventories.
- Section 3 will include a review of the state of knowledge of low-energy (<50 eV) photochemistry-induced mechanisms on Titan and Uranus, covering observations and in situ measurements, experimental simulations of gas phase and condensed state chemistry, photochemical modeling, dicationic chemistry, and recent advances in quantum chemical calculations. Important aspects of branching ratio determination will also be discussed.
- Section 4 will be dedicated to negative ions. Discoveries pertaining to negative ion chemistry on Titan deserve their own section, as their participation in molecular and haze growth requiring photochemical and radiative processes has proven to be substantial.
- Section 5 will conclude with a summary of potential future investigations needed to probe Uranus and prepare for upcoming studies before future missions to the gas giants.
2. The Chemical and Radiative Environments of Titan and the Ice Giants
2.1. Ice Giants: General Considerations
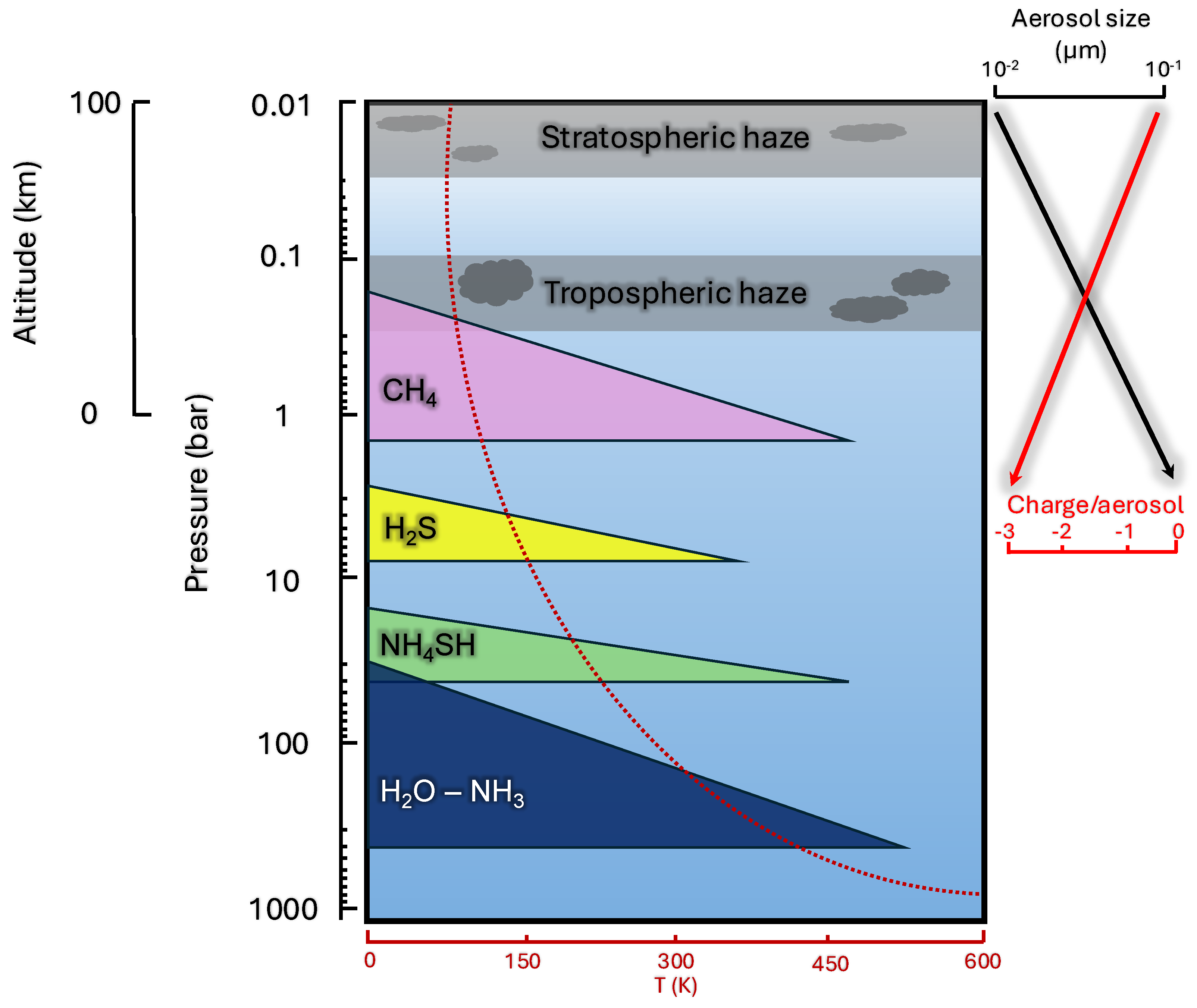
2.2. The Atmosphere of Uranus
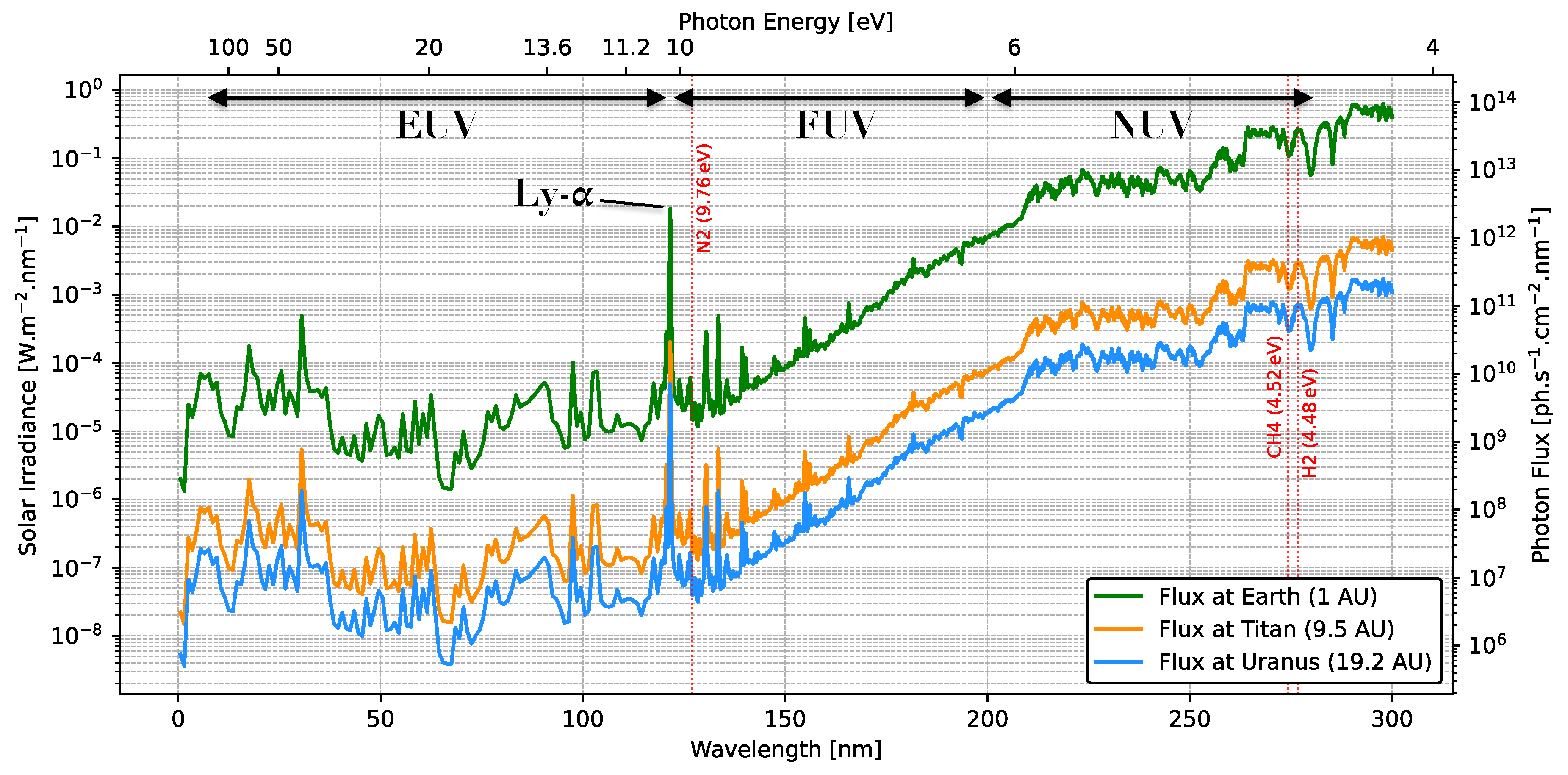
2.3. Chemical Inventory in Uranus
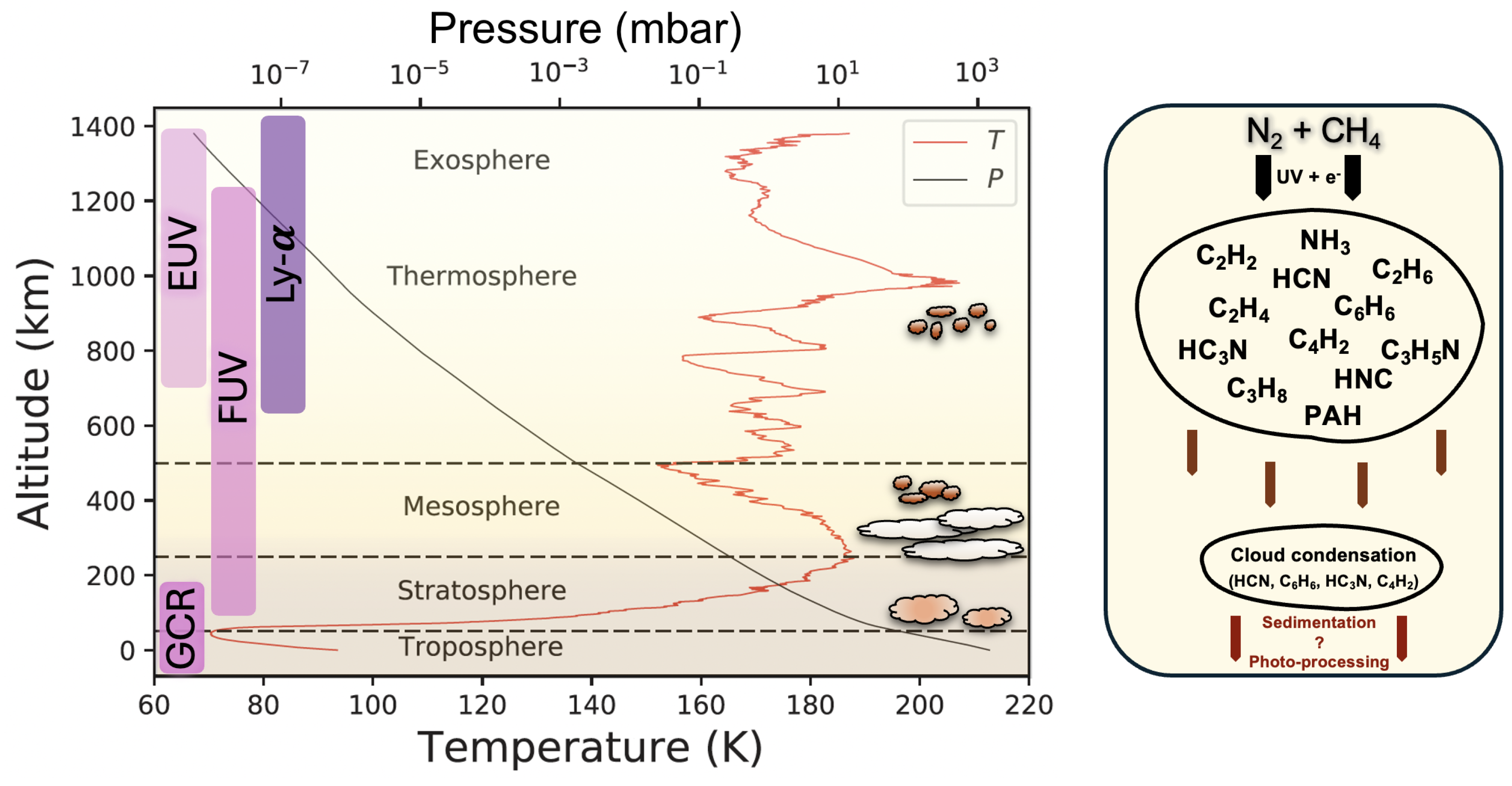
2.4. The Atmosphere of Titan
2.5. Chemical Inventory in Titan
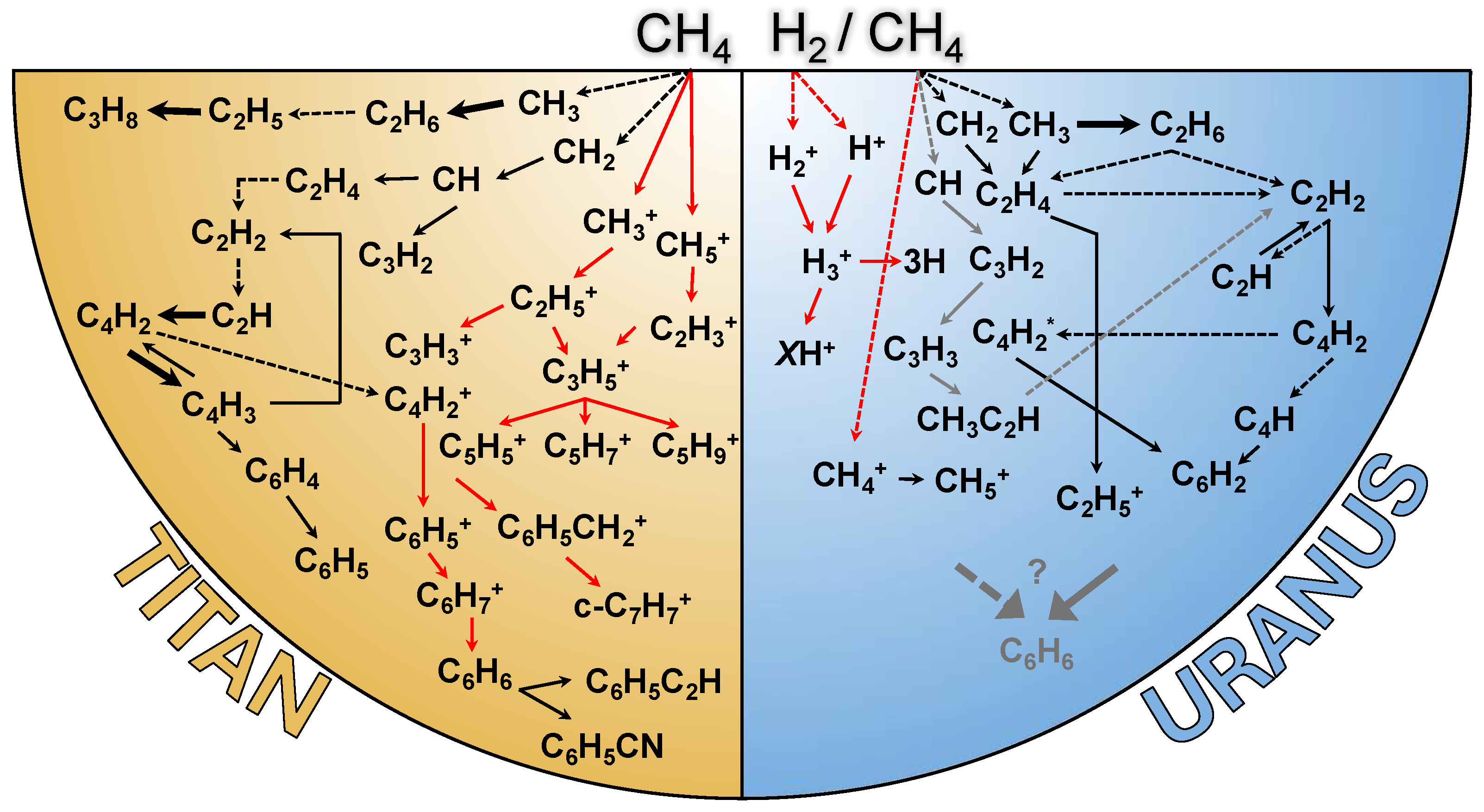
2.6. From Dynamics to Haze Stratification and Evolution
3. Photochemistry vs. Radiative Chemistry: Competing Processes and Role in Haze Formation
3.1. Fundamental Processes
- photodissociation:
- quenching:
- luminescence:
- photoisomerization:
- bimolecular reaction:
- hydrogen abstraction:
- Ionization:
- Ion dissociation:
- Ion-molecule reaction:
- Electron attachment:
- Fluorescence:
- Excimer formation:
3.2. Observational Considerations: From Low-Mass to Intermediate-Mass Molecules
3.2.1. Low-Mass Species
3.2.2. Higher-Mass Species
3.2.3. Polycyclic Aromatic Hydrocarbons: Agents of Haze Growth?
3.3. Laboratory Experiments: Simulating Atmospheric Chemistry
- Atmospheric chemistry science: Multiple complementary laboratory experiments (Table 6) utilizing different sources of energy (plasmas, UV lamps, high-energy synchrotron beamlines) are substantial to probe specific chemical and photoionizing processes. In particular, questions surrounding the role of ion-molecule chemistry and haze growth on Titan have significantly benefitted from laboratory studies. Coupled with in situ or ex situ analyses such as high-resolution mass spectrometry, IR spectroscopy, electron microscopy, secondary ion mass spectrometry, X-ray photoemission spectroscopy, atmospheric-pressure photoionization, just to name a few, future measurements would provide much insights into the chemical composition of Uranian tholins and gas phase precursors. Furthermore, laboratory characterizations of photochemical products would help support in situ measurements by a future UOP and help quantify these precursors resulting from the photodissociation of CH4, NH3, etc.
- Cloud science: Laboratory measurements of the physical and optical properties of any Uranian laboratory-produced aerosols would directly provide valuable information to interpret cloud observations and the modeled scattering, nucleating, and size properties of the CCN. Their properties would then help address the role and interaction of clouds with condensable species. Moreover, studies of the photochemical evolution under low-energy (as well as of much higher-energy) photon irradiation remains critically unexplored.
- Chemical kinetics & thermodynamics: As outlined below, kinetic rates, branching ratios, and absorption cross-sections are fundamental properties that are needed to solve model degeneracies and inaccurate abundance retrievals (Table 7). Future theoretical calculations combined with experimental measurements are much needed.
3.4. Condensed Phase
| Molecule | Photochemical Products | br |
|---|---|---|
| + H | 0.42 | |
| 1CH2 + | 0.48 | |
| 3CH2 + 2H | <0.1 | |
| CH + + H or C + 2 | <0.1 | |
| → + h (fluorescence) | 0.8–0.9 | |
| H + H (predissociation) | 0.1–0.2 | |
| C2H2 | C2H + H | 0.3 |
| C2 + H2 | 0.1 | |
| C2H2* → C2H2 | 0.6 | |
| C2H2+ +e− | 0.84 a | |
| C2H4 | C2H2 + H2 | 0.58 b |
| C2H2 + 2H | 0.42 b | |
| C2H6 | C2H4 + H2 | 0.12 |
| C2H4 + 2H | 0.30 | |
| C2H2 → 2H2 | 0.25 | |
| CH4 +1CH2 | 0.25 | |
| 2CH3 | 0.08 | |
| CH3C2H | C3H3 + H | 0.56 c |
| C3H2 + H2 | 0.44 c | |
| C3H8 | C3H6 + H2 | 0.34 d |
| C2H6 + 1CH2 | 0.09 d | |
| C2H5 + CH3 | 0.35 d | |
| C2H4 + CH4 | 0.22 d | |
| C4H2 | C4H + H | 0.20 e |
| 2C2H | 0.03 e | |
| C2H2 + C2 | 0.10 e | |
| C4H2* | 0.67 e | |
| CH3CN | CH3 + CN | 0.20 f |
| CH2CN + H | 0.80 f | |
| H2S | H2 + S(1D) | <0.12 g |
| AROM | C6H6 + photoproducts | 0.1–0.3 h |
3.5. Branching Ratios
3.6. Dication Chemistry and Photo Double Ionization Processes
4. Negative Ion Chemistry and Haze Growth
4.1. Anions on Titan
| Species | Reaction | Rates (cm3 s−1) | Ref. |
|---|---|---|---|
| H− | CH4 + e− → H− + CH3 | () | [285] |
| H− + h → H + e | () | Miller-threshold Law | |
| CN− | H− + HCN → CN− + H2 | [286] | |
| CN− + H → HCN + e | [286] | ||
| C2H− | + → + | [282] | |
| H + H → C2H2 + e | [287] | ||
| C3N− | C3N + e → C3N− + h | [288] | |
| C3N− + H →HC3N | [289] | ||
| C5N− | CN− + HC5N → C5N− + HCN | Su-Chesnavich | |
| C5N− + H → HC5N | [289] | ||
| C4H− | C4H + e → C4H− + h | [288] | |
| C4H− + H → C2H2 + e | [287] | ||
| C6H− | H− + C6H2 → C6H− + H2 | Langevin | |
| C6H− + H → Products | [287] | ||
| OH− | H− + H2O → OH− + H2 | [282] | |
| OH− + h → OH + e | Miller-threshold Law | ||
| O− | H2O + e → O− + H2 | () | [290] |
| O− + h → O + e | () | Miller-threshold Law |
4.2. Anions on Uranus
4.3. Summary: Dissociative Electron Attachment (<20 eV)
5. Summary: Opportunities for Future Studies
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| UOP | Uranus Orbiter Probe |
| GCR | Galactic Cosmic Rays |
| UVS | Ultraviolet Spectrometer |
| NUV | Near Ultraviolet |
| FUV | Far Ultraviolet |
| VUV | Vacuum Ultraviolet |
| EUV | Extreme Ultraviolet |
| LIPM | Local Interplanetary Medium |
| ISRF | Interstellar Radiation Field |
| ISM | Interstellar Medium |
| JWST | James Webb Space Telescope |
| CRIR | Cosmic Ray Ionization Rate |
| INMS | Ion and Neutral Mass Spectrometer |
| CAPS | Cassini Plasma Spectrometer |
| SSI | Solar Spectrum Irradiance |
| CCN | Cloud Condensation Nuclei |
| DEA | Dissociative Electron Attachment |
| TNI | Temporary Negative Ions |
References
- National Academies of Sciences, Engineering, and Medicine. Origins, Worlds, and Life: A Decadal Strategy for Planetary Science and Astrobiology 2023–2032; National Academies Press: Washington, DC, USA, 2023. [Google Scholar] [CrossRef]
- Lebreton, J.P.; Matson, D.L. An overview of the Cassini mission. Il Nuovo Cimento C 1992, 15, 1137–1147. [Google Scholar] [CrossRef]
- Lebreton, J.P.; Matson, D.L. The Huygens probe: Science, payload and mission overview. Space Sci. Rev. 2002, 104, 59–100. [Google Scholar] [CrossRef]
- Lebreton, J.P.; Witasse, O.; Sollazzo, C.; Blancquaert, T.; Couzin, P.; Schipper, A.M.; Jones, J.B.; Matson, D.L.; Gurvits, L.I.; Atkinson, D.H.; et al. An overview of the descent and landing of the Huygens probe on Titan. Nature 2005, 438, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.H.; Lebreton, J.P.; Waite, J. Titan from Cassini-Huygens; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Lopes, R.M.C.; Elachi, C.; Müller-Wodarg, I.C.F.; Solomonidou, A. Titan After Cassini-Huygens; Springer: Berlin/Heidelberg, Germany, 2025. [Google Scholar]
- Cable, M.L.; Hörst, S.M.; Hodyss, R.; Beauchamp, P.M.; Smith, M.A.; Willis, P.A. Titan tholins: Simulating Titan organic chemistry in the Cassini-Huygens era. Chem. Rev. 2012, 112, 1882–1909. [Google Scholar] [CrossRef] [PubMed]
- Hörst, S.M. Titan’s Atmosphere and Climate. J. Geophys. Res. Planets 2017, 122, 432–482. [Google Scholar] [CrossRef]
- Nixon, C.A.; Lorenz, R.D.; Achterberg, R.K.; Buch, A.; Coll, P.; Clark, R.N.; Courtin, R.; Hayes, A.; Iess, L.; Johnson, R.E.; et al. Titan’s cold case files-Outstanding questions after Cassini-Huygens. Planet. Space Sci. 2018, 155, 50–72. [Google Scholar] [CrossRef]
- Nixon, C.A. The Composition and Chemistry of Titan’s Atmosphere. ACS Earth Space Chem. 2024, 8, 406–456. [Google Scholar] [CrossRef]
- Nixon, C.A.; Achterberg, R.K.; Ádámkovics, M.; Bézard, B.; Bjoraker, G.L.; Cornet, T.; Hayes, A.G.; Lellouch, E.; Lemmon, M.T.; López-Puertas, M.; et al. Titan Science with the James Webb Space Telescope. Astron. Soc. Pac. 2016, 128, 018009. [Google Scholar] [CrossRef]
- Coy, B.P.; Nixon, C.A.; Rowe-Gurney, N.; Achterberg, R.; Lombardo, N.A.; Fletcher, L.N.; Irwin, P. Spitzer IRS Observations of Titan as a Precursor to JWST MIRI Observations. Planet. Sci. J. 2023, 4, 114. [Google Scholar] [CrossRef]
- Goldreich, P.; Lithwick, Y.; Sari, R. Planet Formation by Coagulation: A Focus on Uranus and Neptune. Annu. Rev. Astron. Astrophys. 2004, 42, 549–601. [Google Scholar] [CrossRef]
- Helled, R.; Bodenheimer, P. The Formation of Uranus and Neptune: Challenges and Implications for Intermediate-mass Exoplanets. Astrophys. J. 2014, 789, 69. [Google Scholar] [CrossRef]
- Guillot, T. Uranus and Neptune are key to understand planets with hydrogen atmospheres. Exp. Astron. 2019, 54, 1027–1049. [Google Scholar] [CrossRef]
- Moses, I.; Allen, M. Nucleation and Aerosol Formation Neptune’s Atmosphere. Icarus 1992, 346, 318–346. [Google Scholar] [CrossRef]
- Orton, G.S.; Fletcher, L.N.; Moses, J.I.; Mainzer, A.K.; Hines, D.; Hammel, H.B.; Martin-Torres, F.J.; Burgdorf, M.; Merlet, C.; Line, M.R. Mid-Infrared Spectroscopy of Uranus from the Spitzer Infrared Spectrometer: 1. Determination of the Mean Temperature Structure of the Upper Troposphere and Stratosphere. Icarus 2014, 243, 494–513. [Google Scholar] [CrossRef]
- Moses, J.I.; Fletcher, L.N.; Greathouse, T.K.; Orton, G.S.; Hue, V. Seasonal stratospheric photochemistry on Uranus and Neptune. Icarus 2018, 307, 124–145. [Google Scholar] [CrossRef]
- Hueso, R.; Sánchez-Lavega, A. Atmospheric Dynamics and Vertical Structure of Uranus and Neptune’s Weather Layers. Space Sci. Rev. 2019, 215, 52. [Google Scholar] [CrossRef]
- Moses, J.I.; Cavalié, T.; Fletcher, L.N.; Roman, M.T. Atmospheric chemistry on Uranus and Neptune. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20190477. [Google Scholar] [CrossRef]
- Fletcher, L.N. The Atmosphere of Uranus. In Oxford Research Encyclopedia of Planetary Science; Oxford University Press: Oxford, UK, 2021. [Google Scholar]
- Lamy, L.; Prangé, R.; Hansen, K.C.; Clarke, J.T.; Zarka, P.; Cecconi, B.; Aboudarham, J.; André, N.; Branduardi-Raymont, G.; Gladstone, R.; et al. Earth-based detection of Uranus’ aurorae. Geophys. Res. Lett. 2012, 39, L07105. [Google Scholar] [CrossRef]
- Melin, H. The upper atmospheres of Uranus and Neptune. Philos. Trans. R. Soc. A 2020, 378, 20190478. [Google Scholar] [CrossRef]
- Broadfoot, A.L.; Atreya, S.K.; Bertaux, J.L.; Blamont, J.E.; Dessler, A.J.; Donahue, T.M.; Forrester, W.T.; Hall, D.T.; Herbert, F.; Holberg, J.B.; et al. Ultraviolet Spectrometer Observations of Neptune and Triton. Science 1989, 246, 1459–1466. [Google Scholar] [CrossRef]
- Moore, L.; Moses, J.I.; Melin, H.; Stallard, T.S.; O’Donoghue, J. Atmospheric implications of the lack of H3+ detection at Neptune. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2020, 378, 20200100. [Google Scholar] [CrossRef]
- Strobel, D.F.; Yelle, R.V.; Shemansky, D.E.; Atreya, S.K. The Upper Atmosphere of Uranus. In Uranus; The University of Arizona Press: Tucson, AZ, USA, 1991. [Google Scholar]
- Atreya, S.K.; Sandel, B.R.; Romani, P.N. Photochemistry and Vertical Mixing. In Uranus; The University of Arizona Press: Tucson, AZ, USA, 1991. [Google Scholar]
- Orton, G.S.; Baines, K.H.; Caldwell, J.; Romani, P.; Tokunaga, A.T.; West, R.A. Calibration of the 7- to 14-μm brightness spectra of Uranus and Neptune. Icarus 1990, 85, 257–265. [Google Scholar] [CrossRef]
- Encrenaz, T.; Feuchtgruber, H.; Atreya, S.K.; Bézard, B.; Lellouch, E.; Bishop, J.; Edgington, S.; De Graauw, T.; Griffin, M.; Kessler, M.F. ISO observations of Uranus: The stratospheric distribution of C2H2 and the eddy diffusion coefficient. Astron. Astrophys. 1998, 333, L43–L46. [Google Scholar]
- Pearl, J.; Conrath, B.; Hanel, R.; Pirraglia, J.; Coustenis, A. The albedo, effective temperature, and energy balance of Uranus, as determined from Voyager IRIS data. Icarus 1990, 84, 12–28. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Roman, M.; Zhang, X.; Jiang, X.; Fry, P.M.; Li, C.; Milcareck, G.; Sanchez-Lavega, A.; Perez-Hoyos, S.; et al. Internal Heat and Energy Imbalance of Uranus. arXiv 2025, arXiv:2502.20722. [Google Scholar] [CrossRef]
- Sromovsky, L.; Karkoschka, E.; Fry, P.; de Pater, I.; Hammel, H. The methane distribution and polar brightening on Uranus based on HST/STIS, Keck/NIRC2, and IRTF/SpeX observations through 2015. Icarus 2019, 317, 266–306. [Google Scholar] [CrossRef]
- Yelle, R.V.; Doose, L.R.; Tomasko, M.G.; Strobel, D.F. Analysis of Raman scattered LY-α emissions from the atmosphere of Uranus. Geophys. Res. Lett. 1987, 14, 483–486. [Google Scholar] [CrossRef]
- Herbert, F.; Sandel, B.R.; Yelle, R.V.; Holberg, J.B.; Broadfoot, A.L.; Shemansky, D.E.; Atreya, S.K.; Romani, P.N. The upper atmosphere of Uranus: EUV occultations observed by Voyager 2. J. Geophys. Res. Space Phys. 1987, 92, 15093–15109. [Google Scholar] [CrossRef]
- Yelle, R.V.; McConnell, J.C.; Strobel, D.F.; Doose, L.R. The far ultraviolet reflection spectrum of Uranus: Results from the Voyager encounter. Icarus 1989, 77, 439–456. [Google Scholar] [CrossRef]
- Barthélemy, M.; Lamy, L.; Menager, H.; Schulik, M.; Bernard, D.; Abgrall, H.; Roueff, E.; Cessateur, G.; Prange, R.; Lilensten, J. Dayglow and auroral emissions of Uranus in H2 FUV bands. Icarus 2014, 239, 160–167. [Google Scholar] [CrossRef]
- Waite, J.H.; Chandler, M.O.; Yelle, R.V.; Sandel, B.R.; Cravens, T.E. Superthermal electron processes in the upper atmosphere of Uranus: Aurora and electroglow. J. Geophys. Res. Space Phys. 1988, 93, 14295–14308. [Google Scholar] [CrossRef]
- Barthélemy, M.; Cessateur, G. Sensitivity of upper atmospheric emissions calculations to solar/stellar UV flux. J. Space Weather Space Clim. 2014, 4, A35. [Google Scholar] [CrossRef][Green Version]
- France, K.; Froning, C.S.; Linsky, J.L.; Roberge, A.; Stocke, J.T.; Tian, F.; Bushinsky, R.; Désert, J.M.; Mauas, P.; Vieytes, M.; et al. The Ultraviolet Radiation Environment Around M Dwarf Exoplanet Host Stars. Astrophys. J. 2013, 763, 149. [Google Scholar] [CrossRef]
- Linsky, J.L.; France, K.; Ayres, T. Computing Intrinsic LYα Fluxes of F5 V to M5 V Stars. Astrophys. J. 2013, 766, 69. [Google Scholar] [CrossRef]
- Linsky, J.L.; Redfield, S. Inferring Intrinsic Stellar EUV and Lyman-Alpha Fluxes and Their Effects on Exoplanet Atmospheres. Space Sci. Rev. 2024, 220, 32. [Google Scholar] [CrossRef]
- Meftah, M.; Sarkissian, A.; Keckhut, P.; Hauchecorne, A. The SOLAR-HRS New High-Resolution Solar Spectra for Disk-Integrated, Disk-Center, and Intermediate Cases. Remote Sens. 2023, 15, 3560. [Google Scholar] [CrossRef]
- Secchi, A. Resultats fournis par l’analyse spectrale de la lumiere d’Uranus, de l’étoile R des Gémeaus, et des taches solaires. Comptes Rendus 1869, 68, 761–765. [Google Scholar]
- Huggins, W. IV. Note on the spectrum of Uranus and the spectrum of comet I., 1871. Proc. R. Soc. Lond. 1871, 19, 488–491. [Google Scholar] [CrossRef]
- Chinnici, I. Contributions to Astrophysics and other Sciences. In Decoding the Stars: A Biography of Angelo Secchi, Jesuit and Scientist; BRILL: Leiden, The Netherlands, 2019; pp. 160–207. [Google Scholar] [CrossRef]
- Adel, A.; Slipher, V.M. The Constitution of the Atmospheres of the Giant Planets. Phys. Rev. 1934, 46, 902–906. [Google Scholar] [CrossRef]
- Herzberg, G.; Welsh, H.L.; Crawford, M.F.; F MacDonald, J.C.; Chisholm, D.A. Spectroscopic Evidence of Molecular Hydrogen in the Atmospheres of Uranus and Neptune. Astrophys. J. 1952, 115, 337–340. [Google Scholar] [CrossRef]
- Encrenaz, T.; Combes, M.; Atreya, S.K.; Romani, P.N.; Fricke, K.; Moore, V.; Hunt, G.; Wagener, R.; Caldwell, J.; Owen, T.; et al. A study of the upper atmosphere of Uranus using the IUE. Astron. Astrophys. 1986, 162, 317–322. [Google Scholar]
- Tyler, G.L.; Sweetnam, D.N.; Anderson, J.D.; Campbell, J.K.; Eshleman, V.R.; Hinson, D.P.; Levy, G.S.; Lindal, G.F.; Marouf, E.A.; Simpson, R.A. Voyager 2 Radio Science Observations of the Uranian System: Atmosphere, Rings, and Satellites. Science 1986, 233, 79–84. [Google Scholar] [CrossRef]
- Conrath, B.; Gautier, D.; Hanel, R.; Lindal, G.; Marten, A. The helium abundance of Uranus from Voyager measurements. J. Geophys. Res. Space Phys. 1987, 92, 15003–15010. [Google Scholar] [CrossRef]
- Encrenaz, T.; Lellouch, E.; Drossart, P.; Feuchtgruber, H.; Orton, G.S.; Atreya, S.K. First detection of CO in Uranus. Astron. Astrophys. 2004, 413, L5–L9. [Google Scholar] [CrossRef]
- Burgdorf, M.; Orton, G.; van Cleve, J.; Meadows, V.; Houck, J. Detection of new hydrocarbons in Uranus’ atmosphere by infrared spectroscopy. Icarus 2006, 184, 634–637. [Google Scholar] [CrossRef]
- Moreno, R.; Lellouch, E.; Cavalié, T.; Moullet, A. Detection of CS in Neptune’s atmosphere from ALMA observations. Astron. Astrophys. 2017, 608, L5. [Google Scholar] [CrossRef]
- Irwin, P.G.J.; Toledo, D.; Garland, R.; Teanby, N.A.; Fletcher, L.N.; Orton, G.A.; Bézard, B. Detection of hydrogen sulphide above the clouds in Uranus’ atmosphere. Nat. Astron. 2018, 2, 420–427. [Google Scholar] [CrossRef]
- Karkoschka, E.; Tomasko, M. The haze and methane distributions on Uranus from HST-STIS spectroscopy. Icarus 2009, 202, 287–309. [Google Scholar] [CrossRef]
- Lunine, J.I. The Atmospheres of Uranus and Neptune. Annu. Rev. Astron. Astrophys. 1993, 31, 217–263. [Google Scholar] [CrossRef]
- Irwin, P.G.J.; Teanby, N.A.; Fletcher, L.N.; Toledo, D.; Orton, G.S.; Wong, M.H.; Roman, M.T.; Pérez-Hoyos, S.; James, A.; Dobinson, J. Hazy Blue Worlds A Holistic Aerosol Model for Uranus and Neptune Including Dark Spots. JGR Planets 2022, 127, e2022JE007189. [Google Scholar] [CrossRef]
- Sánchez-Lavega, A.; Irwin, P.; García Muñoz, A. Dynamics and clouds in planetary atmospheres from telescopic observations. Astron. Astrophys. Rev. 2023, 31, 5. [Google Scholar] [CrossRef]
- Irwin, P.G.; Dobinson, J.; James, A.; Teanby, N.A.; Simon, A.A.; Fletcher, L.N.; Roman, M.T.; Orton, G.S.; Wong, M.H.; Toledo, D.; et al. Modelling the seasonal cycle of Uranus’s colourãnd magnitude,ãnd comparison with Neptune. Mon. Not. R. Astron. Soc. 2024, 527, 11521–11538. [Google Scholar] [CrossRef]
- Fegley, B.J.; Gautier, D.; Owen, T.; Prinn, R.G. Spectroscopy and Chemistry of the Atmosphere of Uranus. In Uranus; Bergstralh, J.T., Miner, E.D., Matthews, M.S., Eds.; The University of Arizona Press: Tucson, AZ, USA, 1991. [Google Scholar]
- Vorburger, A.; Wurz, P.; Helled, R.; Mousis, O. Mass Spectrometer Experiment for a Uranus Probe. Space Sci. Rev. 2024, 220, 64. [Google Scholar] [CrossRef]
- Molina-Cuberos, G.J.; Witasse, O.; Toledo, D.; Tripathi, S.N. The Low-Altitude Ionosphere of the Ice Giant Planets. J. Geophys. Res. Planets 2023, 128, e2022JE007568. [Google Scholar] [CrossRef]
- Mousis, O.; Atkinson, D.H.; Cavalié, T.; Fletcher, L.N.; Amato, M.J.; Aslam, S.; Ferri, F.; Renard, J.B.; Spilker, T.; Venkatapathy, E.; et al. Scientific rationale for Uranus and Neptune in situ explorations. Planet. Space Sci. 2018, 155, 12–40. [Google Scholar] [CrossRef]
- Apéstigue, V.; Toledo, D.; Irwin, P.G.; Rannou, P.; Gonzalo, A.; Martínez-Oter, J.; Ceballos-Cáceres, J.; Azcue, J.; Jiménez, J.J.; Sebastian, E.; et al. The Uranus Multi-Experiment Radiometer for Haze and Clouds Characterization. Space Sci. Rev. 2024, 220, 6. [Google Scholar] [CrossRef]
- Niemann, H.B.; Atreya, S.K.; Bauer, S.J.; Carignan, G.R.; Demick, J.E.; Frost, R.L.; Gautier, D.; Haberman, J.A.; Harpold, D.N.; Hunten, D.M.; et al. The abundances of constituents of Titan’s atmosphere from the GCMS instrument on the Huygens probe. Nature 2005, 438, 779–784. [Google Scholar] [CrossRef]
- Fletcher, L.; Orton, G.; Teanby, N.; Irwin, P.; Bjoraker, G. Methane and its isotopologues on Saturn from Cassini/CIRS observations. Icarus 2009, 199, 351–367. [Google Scholar] [CrossRef]
- Howett, C.; Irwin, P.; Teanby, N.; Simon-Miller, A.; Calcutt, S.; Fletcher, L.; De Kok, R. Meridional variations in stratospheric acetylene and ethane in the southern hemisphere of the saturnian atmosphere as determined from Cassini/CIRS measurements. Icarus 2007, 190, 556–572. [Google Scholar] [CrossRef]
- Guerlet, S.; Fouchet, T.; Bézard, B. Ethane, Acetylene and Propane distribution in Saturn’s Stratosphere from Cassini/CIRS Limb Observations. In Proceedings of the SF2A-2008: Proceedings of the Annual Meeting of the French Society of Astronomy and Astrophysics, Paris, France, 30 June–4 July 2008. [Google Scholar]
- Sylvestre, M.; Guerlet, S.; Fouchet, T.; Spiga, A.; Flasar, F.; Hesman, B.; Bjoraker, G. Seasonal changes in Saturn’s stratosphere inferred from Cassini/CIRS limb observations. Icarus 2015, 258, 224–238. [Google Scholar] [CrossRef]
- Vuitton, V.; Yelle, R.V.; Klippenstein, S.J.; Hörst, S.M.; Lavvas, P. Simulating the density of organic species in the atmosphere of Titan with a coupled ion-neutral photochemical model. Icarus 2019, 324, 120–197. [Google Scholar] [CrossRef]
- Hesman, B.E.; Bjoraker, G.L.; Sada, P.V.; Achterberg, R.K.; Jennings, D.E.; Romani, P.N.; Lunsford, A.W.; Fletcher, L.N.; Boyle, R.J.; Simon-Miller, A.A.; et al. Elusive Ethylene Detected in Saturn’s Northern Storm Region. Astrophys. J. 2012, 760, 24. [Google Scholar] [CrossRef]
- Guerlet, S.; Fouchet, T.; Bézard, B.; Moses, J.I.; Fletcher, L.N.; Simon-miller, A.A.; Flasar, F.M. Meridional distribution of CH3C2H and C4H2 in Saturn’s stratosphere from CIRS/Cassini limb and nadir observations. Icarus 2010, 209, 682–695. [Google Scholar] [CrossRef]
- Abbas, M.M.; LeClair, A.; Woodard, E.; Young, M.; Stanbro, M.; Flasar, M. Measurements of CO2 Distribution in Saturn’s Atmosphere by Cassini-Infrared Observations. In Proceedings of the 44th Lunar and Planetary Science Conference (LPSC), The Woodlands, TX, USA, 18–22 March 2013. [Google Scholar]
- Cavalié, T.; Billebaud, F.; Dobrijevic, M.; Fouchet, T.; Lellouch, E.; Encrenaz, T.; Brillet, J.; Moriarty-Schieven, G.; Wouterloot, J.; Hartogh, P. First observation of CO at 345GHz in the atmosphere of Saturn with the JCMT: New constraints on its origin. Icarus 2009, 203, 531–540. [Google Scholar] [CrossRef]
- Cavalié, T.; Hue, V.; Hartogh, P.; Moreno, R.; Lellouch, E.; Feuchtgruber, H.; Jarchow, C.; Cassidy, T.; Fletcher, L.N.; Billebaud, F.; et al. Herschel map of Saturn’s stratospheric water, delivered by the plumes of Enceladus. Astron. Astrophys. 2019, 630, A87. [Google Scholar] [CrossRef]
- Coustenis, A.; Jennings, D.E.; Jolly, A.; Bénilan, Y.; Nixon, C.A.; Vinatier, S.; Gautier, D.; Bjoraker, G.L.; Romani, P.N.; Carlson, R.C.; et al. Detection of C2HD and the D/H ratio on Titan. Icarus 2008, 197, 539–548. [Google Scholar] [CrossRef]
- Pierel, J.D.R.; Nixon, C.A.; Lellouch, E.; Fletcher, L.N.; Bjoraker, G.L.; Achterberg, R.K.; Bézard, B.; Hesman, B.E.; Irwin, P.G.J.; Flasar, F.M. D/H Ratios on Saturn and Jupiter from Cassini CIRS. Astron. J. 2017, 154, 178. [Google Scholar] [CrossRef]
- Atreya, S.K.; Ponthieu, J.J. Photolysis of Methane and the Ionosphere of Uranus. Planet. Space Sci. 1983, 31, 939. [Google Scholar] [CrossRef]
- Summers, M.E.; Strobel, D.F. Photochemistry of the Atmosphere of Uranus. Astrophys. J. 1989, 346, 495–508. [Google Scholar] [CrossRef]
- Romani, P.N.; Bishop, J.; Bézard, B.; Atreya, S. Methane Photochemistry on Neptune: Ethane and Acetylene Mixing Ratios and Haze Production. Icarus 1993, 106, 442–463. [Google Scholar] [CrossRef]
- Lellouch, E.; Romani, P.N.; Rosenqvist, J. The vertical Distribution and Origin of HCN in Neptune’s Atmosphere. Icarus 1994, 108, 112–136. [Google Scholar] [CrossRef]
- Dobrijevic, M.; Cavali, T.; Hbrard, E.; Billebaud, F.; Hersant, F.; Selsis, F. Key reactions in the photochemistry of hydrocarbons in Neptune’s stratosphere. Planet. Space Sci. 2010, 58, 1555–1566. [Google Scholar] [CrossRef]
- Cavalié, T.; Moreno, R.; Lellouch, E.; Hartogh, P.; Venot, O.; Orton, G.S.; Jarchow, C.; Encrenaz, T.; Selsis, F.; Hersant, F.; et al. The first submillimeter observation of CO in the stratosphere of Uranus. Astron. Astrophys. 2014, 562, A33. [Google Scholar] [CrossRef]
- Moses, J.I.; Poppe, A.R. Dust ablation on the giant planets: Consequences for stratospheric photochemistry. Icarus 2017, 297, 33–58. [Google Scholar] [CrossRef]
- Milcareck, G.; Guerlet, S.; Montmessin, F.; Spiga, A.; Leconte, J.; Millour, E.; Clément, N.; Fletcher, L.N.; Roman, M.T.; Lellouch, E.; et al. Radiative-convective models of the atmospheres of Uranus and Neptune: Heating sources and seasonal effects. Astron. Astrophys. 2024, 686, A303. [Google Scholar] [CrossRef]
- Waite, J.H.; Young, D.; Cravens, T.E.; Coates, A.J.; Crary, F.J.; Magee, B.; Westlake, J. The Process of Tholin Formation in Titan’s Upper Atmosphere. Science 2007, 316, 870–875. [Google Scholar] [CrossRef] [PubMed]
- Krasnopolsky, V.A. Chemical composition of Titan’s atmosphere and ionosphere: Observations and the photochemical model. Icarus 2014, 236, 83–91. [Google Scholar] [CrossRef]
- Plainaki, C.; Lilensten, J.; Radioti, A.; Andriopoulou, M.; Milillo, A.; Nordheim, T.A.; Dandouras, I.; Coustenis, A.; Grassi, D.; Mangano, V.; et al. Planetary space weather: Scientific aspects and future perspectives. J. Space Weather Space Clim. 2016, 6, A31. [Google Scholar] [CrossRef]
- Crary, F.J.; Magee, B.A.; Mandt, K.; Waite, J.H.; Westlake, J.; Young, D.T. Heavy ions, temperatures and winds in Titan’s ionosphere: Combined Cassini CAPS and INMS observations. Planet. Space Sci. 2009, 57, 1847–1856. [Google Scholar] [CrossRef]
- Cui, J.; Galand, M.; Yelle, R.V.; Vuitton, V.; Wahlund, J.; Lavvas, P.P. Diurnal variations of Titan’s ionosphere. J. Geophys. Res. 2009, 114, 1–20. [Google Scholar] [CrossRef]
- Cui, J.; Yelle, R.V.; Vuitton, V.; Waite, J.H.; Kasprzak, W.T.; Gell, D.A.; Niemann, H.B.; Müller-Wodarg, I.C.F.; Borggren, N.; Fletcher, G.G.; et al. Analysis of Titan’s neutral upper atmosphere from Cassini Ion Neutral Mass Spectrometer measurements. Icarus 2009, 200, 581–615. [Google Scholar] [CrossRef]
- Dubois, D. Study of Titan’s Upper and Lower Atmosphere: An Experimental Approach. Ph.D. Thesis, Université Paris Saclay, Orsay, France, 2018. [Google Scholar]
- Broadfoot, A.L.; Sandel, B.R.; Shemansky, D.; Holberg, J.B.; Smith, G.R. Extreme Ultraviolet Observations from Voyager 1 Encounter with Saturn. Science 1981, 212, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Keller, C.N.; Cravens, T.E.; Gan, L. A model of the ionosphere of Titan. J. Geophys. Res. Space Phys. 1992, 97, 12117–12135. [Google Scholar] [CrossRef]
- Yung, Y.L.; Allen, M.; Pinto, J. Photochemistry of the atmosphere of Titan: Comparison between model and observations. Astrophys. J. 1984, 212, 465–506. [Google Scholar] [CrossRef]
- Singhal, R.P.; Haider, S.A. Some Molecular Nitrogen Emissions from Titan-Solar EUV and Magnetospheric Interaction. Indian J. Radio Space Phys. 1986, 15, 46–52. [Google Scholar]
- Yung, Y.L. An update of nitrile photochemistry on Titan. Icarus 1987, 72, 468–472. [Google Scholar] [CrossRef]
- Ip, W.H. Titan’s upper ionosphere. Astrophys. J. 1990, 362, 354–363. [Google Scholar] [CrossRef]
- Gan, L.; Keller, C.N.; Cravens, T.E. Electrons in the Ionosphere of Titan. J. Geophys. Res. 1992, 97, 12137–12151. [Google Scholar] [CrossRef]
- Keller, C.N.; Anicich, V.G.; Cravens, T.E. Model of Titan’s ionosphere with detailed hydrocarbon ion chemistry. Planet. Space Sci. 1998, 46, 1157–1174. [Google Scholar] [CrossRef]
- Lavvas, P.P.; Coustenis, A.; Vardavas, I.M. Coupling photochemistry with haze formation in Titan’s atmosphere, Part I: Model description. Planet. Space Sci. 2008, 56, 67–99. [Google Scholar] [CrossRef]
- Thuillier, G.; Floyd, L.; Woods, T.; Cebula, R.; Hilsenrath, E.; Hersé, M.; Labs, D. Solar irradiance reference spectra for two solar active levels. Adv. Space Res. 2004, 34, 256–261. [Google Scholar] [CrossRef]
- Lavvas, P.; Galand, M.; Yelle, R.V.; Heays, A.N.; Lewis, B.R.; Lewis, G.R.; Coates, A.J. Energy deposition and primary chemical products in Titan’s upper atmosphere. Icarus 2011, 213, 233–251. [Google Scholar] [CrossRef]
- Couturier-Tamburelli, I.; Gudipati, M.S.; Lignell, A.; Jacovi, R.; Piétri, N. Spectroscopic studies of non-volatile residue formed by photochemistry of solid C4N2: A model of condensed aerosol formation on Titan. Icarus 2014, 234, 81–90. [Google Scholar] [CrossRef]
- Hébrard, E. Incertitudes Photochimiques Dans les Modèles de L’atmosphère de Titan: Revue et Conséquences. Ph.D. Thesis, Université Paris-Diderot-Paris, Paris, France, 2006. [Google Scholar]
- Anderson, C.M.; Samuelson, R.E.; Nna-Mvondo, D. Organic Ices in Titan’s Stratosphere. Space Sci. Rev. 2018, 214, 125. [Google Scholar] [CrossRef]
- Barth, E.L. Modeling survey of ices in Titan’s stratosphere. Planet. Space Sci. 2017, 137, 20–31. [Google Scholar] [CrossRef]
- Anderson, C.M.; Samuelson, R.E.; Yung, Y.L.; McLain, J.L. Solid-state chemistry as a formation mechanism for Titan’s stratospheric C4N2 ice clouds. Geophys. Res. Lett. 2016, 43, 3088–3094. [Google Scholar] [CrossRef]
- Nna-Mvondo, D.; Anderson, C.; Samuelson, R.E. CIRS-Observed Titan’s Stratospheric Ice Clouds Studied in the Laboratory; American Astronomical Society: Washington, DC, USA, 2018. [Google Scholar]
- De Kok, R.J.; Teanby, N.A.; Maltagliati, L.; Irwin, P.G.J.; Vinatier, S. HCN ice in Titan’s high-altitude southern polar cloud. Nature 2014, 514, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, S.; Schmitt, B.; Bézard, B.; Rannou, P.; Dauphin, C.; de Kok, R.; Jennings, D.E.; Flasar, F.M. Study of Titan’s fall southern stratospheric polar cloud composition with Cassini/CIRS: Detection of benzene ice. Icarus 2018, 310, 89–104. [Google Scholar] [CrossRef]
- Rodriguez, S.; Le Mouélic, S.; Rannou, P.; Tobie, G.; Baines, K.H.; Barnes, J.W.; Griffith, C.A.; Hirtzig, M.; Pitman, K.M.; Sotin, C.; et al. Global circulation as the main source of cloud activity on Titan. Nature 2009, 459, 678–682. [Google Scholar] [CrossRef]
- Lora, J.M.; Lunine, J.I.; Russell, J.L. GCM simulations of Titan’s middle and lower atmosphere and comparison to observations. Icarus 2015, 250, 516–528. [Google Scholar] [CrossRef]
- Barth, E. Planetcarma: A new framework for studying the microphysics of planetary atmospheres. Atmosphere 2020, 11, 1064. [Google Scholar] [CrossRef]
- Dubois, D.; Iraci, L.T.; Barth, E.L.; Salama, F.; Vinatier, S.; Sciamma-O’Brien, E. Investigating the condensation of benzene (C6H6) in Titan’s South polar cloud system with a combination of laboratory, observational, and modeling tools. Planet. Sci. J. 2021, 2, 121. [Google Scholar] [CrossRef]
- de Batz de Trenquelléon, B.; Rosset, L.; d’Ollone, J.V.; Lebonnois, S.; Rannou, P.; Burgalat, J.; Vinatier, S. The New Titan Planetary Climate Model. I. Seasonal Variations of the Thermal Structure and Circulation in the Stratosphere. Planet. Sci. J. 2025, 6, 78. [Google Scholar] [CrossRef]
- Gudipati, M.S.; Couturier-Tamburelli, I.; Fleury, B.; Lignell, A.; Jacovi, R. Photochemical Evolution of Condensed Organics in Titan’s Atmosphere and on the Surface. In Astrobiology Science Conference 2015 (AbSciCon 2015): Habitability, Habitable Worlds, and Life; USRA Houston: Chicago, IL, USA, 2015. [Google Scholar]
- Dubois, D.; Gudipati, M.S.; Henderson, B.; Carrasco, N.; Fleury, B.; Couturier-Tamburelli, I. Photochemistry of HCN Ice on Tholins Simulated in Titan’s Lower Atmosphere Conditions. EPSC 2017 2017, 11, 9–10. [Google Scholar]
- Couturier-Tamburelli, I.; Piétri, N.; Gudipati, M. Simulation of Titan’s atmospheric photochemistry Formation of non-volatile residue from polar nitrile ices. Astron. Astrophys. 2015, 578, A111. [Google Scholar] [CrossRef]
- Couturier-Tamburelli, I.; Piétri, N.; Letty, V.L.; Chiavassa, T.; Gudipati, M. UV–Vis Light-induced Aging of Titan’s Haze and Ice. Astrophys. J. 2018, 852, 117. [Google Scholar] [CrossRef]
- Mouzay, J.; Henry, K.; Couturier-Tamburelli, I.; Danger, G.; Piétri, N.; Chiavassa, T. Photochemistry of benzene (C6H6) hydrogen cyanide (HCN) co-condensed ices part 1: A source of solid-state production of volatile nitrile compounds in Titan’s stratosphere. Icarus 2021, 368, 114595. [Google Scholar] [CrossRef]
- Wilson, E.H.; Atreya, S.K. Current state of modeling the photochemistry of Titan’s mutually dependent atmosphere and ionosphere. J. Geophys. Res. E Planets 2004, 109. [Google Scholar] [CrossRef]
- Hébrard, E.; Dobrijevic, M.; Loison, J.C.; Bergeat, A.; Hickson, K.M. Neutral production of hydrogen isocyanide (HNC) and hydrogen cyanide (HCN) in Titan’s upper atmosphere. Astron. Astrophys. 2012, 541, A21. [Google Scholar] [CrossRef]
- Hickson, K.M.; Loison, J.C.; Cavalié, T.; Hébrard, E.; Dobrijevic, M. The evolution of infalling sulfur species in Titan’s atmosphere. Astron. Astrophys. 2014, 572, A58. [Google Scholar] [CrossRef][Green Version]
- Loison, J.C.; Hébrard, E.; Dobrijevic, M.; Hickson, K.M.; Caralp, F.; Hue, V.; Gronoff, G.; Venot, O.; Bénilan, Y. The neutral photochemistry of nitriles, amines and imines in the atmosphere of Titan. Icarus 2015, 247, 218–247. [Google Scholar] [CrossRef]
- Dobrijevic, M.; Loison, J.C.; Hickson, K.M.; Gronoff, G. 1D-coupled photochemical model of neutrals, cations and anions in the atmosphere of Titan. Icarus 2016, 268, 313–339. [Google Scholar] [CrossRef]
- Mukundan, V.; Bhardwaj, A. A Model for Negative Ion Chemistry in Titan’s Ionosphere. Astrophys. J. 2018, 856, 168. [Google Scholar] [CrossRef]
- Fox, J.L.; Yelle, R.V. Hydrocarbon ions in the ionosphere of Titan. Geophys. Res. Lett. 1997, 24, 2179–2182. [Google Scholar] [CrossRef]
- Lavvas, P.; Yelle, R.V.; Koskinen, T.; Bazin, A.; Vuitton, V.; Vigren, E.; Galand, M.; Wellbrock, A.; Coates, A.J.; Wahlund, J.E.; et al. Aerosol growth in Titan’s ionosphere. Proc. Natl. Acad. Sci. USA 2013, 110, 2729–2734. [Google Scholar] [CrossRef]
- Loison, J.C.; Dobrijevic, M.; Hickson, K.M.; Heays, A.N. The photochemical fractionation of oxygen isotopologues in Titan’s atmosphere. Icarus 2017, 291, 17–30. [Google Scholar] [CrossRef]
- Nixon, C.A.; Bézard, B.; Cornet, T.; Coy, B.P.; de Pater, I.; Es-Sayeh, M.; Hammel, H.B.; Lellouch, E.; Lombardo, N.A.; López-Puertas, M.; et al. The atmosphere of Titan in late northern summer from JWST and Keck observations. Nat. Astron. 2025, 9, 969–981. [Google Scholar] [CrossRef]
- Atreya, S.; Donahue, T. Ionospheric models of Saturn, Uranus, and Neptune. Icarus 1975, 24, 358–362. [Google Scholar] [CrossRef]
- Atreya, S.K.; Romani, P.N. Photochemistry and Clouds of Jupiter, Saturn and Uranus. Recent Adv. Planet. Meteorol. 1985, 17, 68. [Google Scholar]
- Chandler, M.O.; Waite, J.H. The Ionosphere of Uranus: A Myriad of Possibilities. Geophys. Res. Lett. 1986, 13, 6–9. [Google Scholar] [CrossRef]
- Atreya, S.K. Atmospheres and Ionospheres of the Outer Planets and Their Satellites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1986; Volume 15. [Google Scholar]
- Pollack, J.B.; Rages, K.; Pope, S.K.; Tomasko, M.G.; Romani, P.N.; Atreya, S.K. Nature of the stratospheric haze on Uranus: Evidence for condensed hydrocarbons. J. Geophys. Res. Space Phys. 1987, 92, 15037–15065. [Google Scholar] [CrossRef]
- Waite, J.H.; Cravens, T.E. Current Review of the Jupiter, Saturn, and Uranus Ionospheres. Adv. Space Res. 1987, 7, 119–134. [Google Scholar] [CrossRef]
- Atreya, S.K. Uranus Photochemistry and Prospects for Voyager 2 at Neptune. Adv. Space Res 1990, 10, 119. [Google Scholar] [CrossRef]
- Trafton, L.M.; Geballe, T.R.; Miller, S.; Tennyson, J.; Ballester, G.E. Detection of H3+ from Uranus. Astrophys. J. 1993, 405, 761–766. [Google Scholar] [CrossRef]
- Moses, J.I.; Fouchet, T.; Bézard, B.; Gladstone, G.R.; Lellouch, E.; Feuchtgruber, H. Photochemistry and diffusion in Jupiter’s stratosphere: Constraints from ISO observations and comparisons with other giant planets. J. Geophys. Res. Planets 2005, 110, E08001. [Google Scholar] [CrossRef]
- Strobel, D.F. Photochemistry in outer solar system atmospheres. Space Sci. Rev. 2005, 116, 155–170. [Google Scholar] [CrossRef]
- Loison, J.C.; Dobrijevic, M.; Hickson, K.M. The photochemical production of aromatics in the atmosphere of Titan. Icarus 2019, 329, 55–71. [Google Scholar] [CrossRef]
- Melin, H.; Fletcher, L.N.; Stallard, T.S.; Miller, S.; Trafton, L.M.; Moore, L.; O’Donoghue, J.; Vervack, J.J.; Russo, N.D.; Lamy, L.; et al. The H3+ ionosphere of Uranus: Decades-long cooling and local-time morphology. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2019, 377, 20180408. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Murga, M.S.; Ananikov, V.P. Role of Acetylene in the Chemical Evolution of Carbon Complexity. ACS Earth Space Chem. 2024, 8, 798–856. [Google Scholar] [CrossRef]
- Joshi, S.; Roth, L.; Gladstone, R.; Ivchenko, N.; Pryor, W.; Lamy, L. Uranus-hydrogen upper atmosphere: Insights from pre- and post-equinox HST Lyman-α images. Astron. Astrophys. 2025, 693, A231. [Google Scholar] [CrossRef]
- Dobrijevic, M.; Loison, J.C.; Hue, V.; Cavalié, T.; Hickson, K.M. 1D photochemical model of the ionosphere and the stratosphere of Neptune. Icarus 2020, 335, 113375. [Google Scholar] [CrossRef]
- Guillot, T. Condensation of Methane, Ammonia, and Water and the Inhibition of Convection in Giant Planets. Science 1995, 269, 1697–1699. [Google Scholar] [CrossRef] [PubMed]
- Leconte, J.; Selsis, F.; Hersant, F.; Guillot, T. Condensation-inhibited convection in hydrogen-rich atmospheres: Stability against double-diffusive processes and thermal profiles for Jupiter, Saturn, Uranus, and Neptune. Astron. Astrophys. 2017, 598, A98. [Google Scholar] [CrossRef]
- Rannou, P.; Coutelier, M.; Rivière, E.; Lebonnois, S.; Rey, M.; Maltagliati, L. Convection behind the Humidification of Titan’s Stratosphere. Astrophys. J. 2021, 922, 239. [Google Scholar] [CrossRef]
- Dobrijevic, M.; Loison, J.C.; Hickson, K.M.; Dobrijevic, M.; Loison, J.C.; Hickson, K.M. The eddy diffusion coefficient in the atmosphere of Titan: Models comparison. 2016; Unpublished paper. [Google Scholar] [CrossRef]
- Petrie, S.; Bohme, D.K. Ions in space. Mass Spectrom. Rev. 2007, 26, 258–280. [Google Scholar] [CrossRef] [PubMed]
- Arumainayagam, C. Photochemistry. In Encyclopedia of Astrobiology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Arumainayagam, C.R. Radiation Chemistry. In Encyclopedia of Astrobiology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–6. [Google Scholar] [CrossRef]
- Regina, A.; Paranjothy, M. Theoretical Investigation of Bimolecular Carbon Chain Growth Reactions in the Interstellar Media. J. Phys. Chem. 2023, 128, 2409–2416. [Google Scholar] [CrossRef]
- Dobrijevic, M.; Hébrard, E.; Loison, J.C.; Hickson, K.M. Coupling of oxygen, nitrogen, and hydrocarbon species in the photochemistry of titan’s atmosphere. Icarus 2014, 228, 324–346. [Google Scholar] [CrossRef]
- Hrodmarsson, H.R.; Aleman, I.; Candian, A.; Wiersma, S.; Palotás, J.; Dubois, D.; Sidhu, A.; Loru, D.; Sundarajan, P.; Sciamma-O’Brien, E.; et al. The AstroPAH 10 Years of Science Review. Space Sci. Rev. 2025, 221, 42. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Production of low-energy electrons by ionizing radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Arumainayagam, C.R.; Lee, H.L.; Nelson, R.B.; Haines, D.R.; Gunawardane, R.P. Low-energy electron-induced reactions in condensed matter. Surf. Sci. Rep. 2010, 65, 1–44. [Google Scholar] [CrossRef]
- Shulenberger, K.E.; Zhu, J.L.; Tran, K.; Abdullahi, S.; Belvin, C.; Lukens, J.; Peeler, Z.; Mullikin, E.; Cumberbatch, H.M.; Huang, J.; et al. Electron-Induced Radiolysis of Astrochemically Relevant Ammonia Ices. ACS Earth Space Chem. 2019, 3, 800–810. [Google Scholar] [CrossRef]
- Wu, Q.T.; Anderson, H.; Watkins, A.K.; Arora, D.; Barnes, K.; Padovani, M.; Shingledecker, C.N.; Arumainayagam, C.R.; Battat, J.B. Role of Low-Energy (<20 eV) Secondary Electrons in the Extraterrestrial Synthesis of Prebiotic Molecules. ACS Earth Space Chem. 2024, 8, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.C.; Rivas, N.; Tran, A.A.; Verish, C.A.; Arumainayagam, C.R. The role of low-energy (≤20 eV) electrons in astrochemistry. Surf. Sci. 2016, 652, 26–32. [Google Scholar] [CrossRef]
- Stelmach, K.B.; Neveu, M.; Vick-Majors, T.J.; Mickol, R.L.; Chou, L.; Webster, K.D.; Tilley, M.; Zacchei, F.; Escudero, C.; Flores Martinez, C.L.; et al. Secondary Electrons as an Energy Source for Life. Astrobiology 2018, 18, 73–85. [Google Scholar] [CrossRef]
- TP, R.K.; Bjornsson, R.; Barth, S.; Ingólfsson, O. Formation and decay of negative ion states up to 11 eV above the ionization energy of the nanofabrication precursor HFeCo3 (CO) 12. Chem. Sci. 2017, 8, 5949–5952. [Google Scholar] [CrossRef]
- Ryszka, M.; Alizadeh, E.; Li, Z.; Ptasińska, S. Low-energy electron-induced dissociation in gas-phase nicotine, pyridine, and methyl-pyrrolidine. J. Chem. Phys. 2017, 147, 094303. [Google Scholar] [CrossRef]
- Bredehöft, J.H. Electron-induced chemistry in the condensed phase. Atoms 2019, 7, 33. [Google Scholar] [CrossRef]
- Larsson, M.; Geppert, W.D.; Nyman, G. Ion chemistry in space. Rep. Prog. Phys. 2012, 75, 066901. [Google Scholar] [CrossRef]
- Arumainayagam, C.R.; Garrod, R.T.; Boyer, M.; Hay, A.; Tong Bao, S.; Campbell, J.; Wang, A.; Nowak, C.M.; Arumainayagam, M.R.; Hodge, P.J. Extraterrestrial Prebiotic Molecules: Photochemistry vs. Radiation Chemistry of Interstellar Ices. Chem. Soc. Rev. 2019, 48, 2293–2314. [Google Scholar] [CrossRef]
- Jackson, W.; Halpern, J.B.; Lin, C.S. Multiphoton ultraviolet photochemistry. Chem. Phys. Lett. 1978, 55, 254–258. [Google Scholar] [CrossRef]
- Jackson, W.M.; Bao, Y.; Urdahl, R.S. Implications of C2H photochemistry on the modeling of C2 distributions in comets. J. Geophys. Res. Planets 1991, 96, 17569–17572. [Google Scholar] [CrossRef]
- Carney, T.E.; Baer, T. The mechanism for multiphoton ionization of H2S. J. Chem. Phys. 1981, 75, 4422–4429. [Google Scholar] [CrossRef]
- Achiba, Y.; Sato, K.; Shobatake, K.; Kimura, K. The mechanism for photofragmentation of H2S revealed by multiphoton ionization photoelectron spectroscopy. J. Chem. Phys. 1982, 77, 2709–2714. [Google Scholar] [CrossRef]
- Ashfold, M.; Dixon, R. Multiphoton ionisation spectroscopy of H2S: A reinvestigation of the 1B1-1A1 band at 139.1 nm. Chem. Phys. Lett. 1982, 93, 5–10. [Google Scholar] [CrossRef]
- Chacko, R.; Barik, S.; Banhatti, S.; Aravind, G. Multiphoton ionization and dissociation of polycyclic aromatic hydrocarbon molecules of astrophysical interest. Phys. Rev. A 2022, 105, 032804. [Google Scholar] [CrossRef]
- Menzel, D.; Gomer, R. Electron-impact desorption of carbon monoxide from tungsten. J. Chem. Phys. 1964, 41, 3329–3351. [Google Scholar] [CrossRef]
- Gans, B.; Boyé-Péronne, S.; Broquier, M.; Delsaut, M.; Douin, S.; Fellows, C.E.; Halvick, P.; Loison, J.C.; Lucchese, R.R.; Gauyacq, D. Photolysis of methane revisited at 121.6 nm and at 118.2 nm: Quantum yields of the primary products, measured by mass spectrometry. Phys. Chem. Chem. Phys. 2011, 13, 8140. [Google Scholar] [CrossRef]
- Boduch, P.; Dartois, E.; De Barros, A.L.; Da Silveira, E.F.; Domaracka, A.; Lv, X.Y.; Palumbo, M.E.; Pilling, S.; Rothard, H.; Duarte, E.S.; et al. Radiation effects in astrophysical ices. J. Phys. Conf. Ser. 2015, 629, 012008. [Google Scholar] [CrossRef]
- Hrušák, J.; Paidarová, I. Step Towards Modeling the Atmosphere of Titan: State-Selected Reactions of O+ with Methane. Orig. Life Evol. Biosph. 2016, 46, 419–424. [Google Scholar] [CrossRef]
- Gudipati, M.S.; Jacovi, R.; Couturier-Tamburelli, I.; Lignell, A.; Allen, M. Photochemical activity of Titan’s low-altitude condensed haze. Nat. Commun. 2013, 4, 1648. [Google Scholar] [CrossRef]
- Boyer, M.; Atkinson, K.E.; Arumainayagam, C.R. Low-Energy Electrons: Fundamentals and Applications; Pan Stanford Publishing: Singapore, 2019; p. 417. [Google Scholar]
- Arumainayagam, C.R.; Herbst, E.; Heays, A.N.; Mullikin, E.; Farrah, M.; Mavros, M.G. Extraterrestrial Photochemistry: Principles and Applications. In Prebiotic Photochemistry; The Royal Society of Chemistry: London, UK, 2021. [Google Scholar] [CrossRef]
- Yung, Y.L.; DeMore, W.B. Photochemistry of Planetary Atmospheres; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Plane, J.M.C.; Yung, Y.L.; DeMore, W.B. Photochemistry of Planetary Atmospheres. J. Atmos. Chem. 2001, 39, 215–216. [Google Scholar] [CrossRef]
- Trafton, L. On the possible detection of H2 in titan’s atmosphere. Astrophys. J. 1972, 5, 285–293. [Google Scholar] [CrossRef]
- Miller, S.; Achilleos, N.; Ballester, G.E.; Geballe, T.R.; Joseph, R.D.; Prangé, R.; Rego, D.; Stallard, T.; Tennyson, J.; Trafton, L.M.; et al. The role of H3+ in planetary atmospheres. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2000, 358, 2485–2502. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, B.; Yang, C.L.; Wei, L.; Wang, B.; Han, J.; Yu, W.; Qi, Y.; Zou, Y.; Chen, L.; et al. Formation of H3+ from ethane dication induced by electron impact. Commun. Chem. 2020, 3, 160. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Sandhu, S.; Shaik, M.; Stamm, J.; Sandhu, J.; Das, R.; Hetherington, C.V.; Levine, B.G.; Dantus, M. What is the Mechanism of H3+ Formation from Cyclopropane? J. Phys. Chem. A 2023, 127, 8633–8638. [Google Scholar] [CrossRef]
- Melin, H.; Moore, L.; Fletcher, L.N.; Hammel, H.B.; O’Donoghue, J.; Stallard, T.S.; Milam, S.N.; Roman, M.; King, O.R.T.; Rowe-Gurney, N.; et al. Discovery of H3+ and infrared aurorae at Neptune with JWST. Nat. Astron. 2025, 9, 666–671. [Google Scholar] [CrossRef]
- Waite, J.H.; Niemann, H.B.; Yelle, R.V.; Kasprzak, W.T.; Cravens, T.E.; Luhmann, J.G.; McNutt, R.L.; Ip, W.H.; Gell, D.; De La Haye, V.; et al. Ion Neutral Mass Spectrometer Results from the First Flyby of Titan. Science 2005, 85, 195–209. [Google Scholar] [CrossRef]
- Lorenz, R.D.; Imanaka, H.; McKay, C.P.; Makel, D.; Hunter, G.P.; Trainer, M.G.; Osiander, R.; Mastandrea, A.; Barnes, J.W.; Turtle, E.P. Hydrogen sensing in Titan’s atmosphere: Motivations and techniques. Planet. Space Sci. 2019, 174, 1–7. [Google Scholar] [CrossRef]
- López-Puertas, M.; Dinelli, B.M.; Adriani, A.; Funke, B.; García-Comas, M.; Moriconi, M.L.; D’Aversa, E.; Boersma, C.; Allamandola, L.J. Large Abundances of Polycyclic Aromatic Hydrocarbons in Titan’s Upper Atmosphere. Astrophys. J. 2013, 770, 132. [Google Scholar] [CrossRef]
- Coustenis, A.; Schmitt, B.; Khanna, R.K.; Trotta, F. Plausible condensates in Titan’s stratosphere from Voyager infrared spectra. Planet. Space Sci. 1999, 47, 1305–1329. [Google Scholar] [CrossRef]
- Griffith, C.A.; Penteado, P.; Rannou, P.; Brown, R.; Boudon, V.; Baines, K.H.; Clark, R.; Drossart, P.; Buratti, B.; Nicholson, P.; et al. Evidence for a polar ethane cloud on Titan. Science 2006, 313, 1620–1622. [Google Scholar] [CrossRef]
- Drexel, H.; Senn, G.; Fiegele, T.; Scheier, P.; Stamatovic, A.; Mason, N.J.; Märk, T.D. Dissociative electron attachment to hydrogen. J. Phys. At. Mol. Opt. Phys. 2001, 34, 1415. [Google Scholar] [CrossRef]
- Gans, B.; Peng, Z.; Carrasco, N.; Gauyacq, D.; Lebonnois, S.; Pernot, P. Impact of a new wavelength-dependent representation of methane photolysis branching ratios on the modeling of Titan’s atmospheric photochemistry. Icarus 2013, 223, 330–343. [Google Scholar] [CrossRef]
- Hrodmarsson, H.R.; Van Dishoeck, E.F. Photodissociation and photoionization of molecules of astronomical interest: Updates to the Leiden photodissociation and photoionization cross section database. Astron. Astrophys. 2023, 675, A25. [Google Scholar] [CrossRef]
- Gillett, F.C. Further Observations of the 8–13 Micron Spectrum of Titan. Astrophys. J. 1975, 201, 41–43. [Google Scholar] [CrossRef]
- Hickson, K.M.; Bray, C.; Loison, J.C.; Dobrijevic, M. A Kinetic Study of the N(2D) + C2H4 Reaction at Low Temperature. Phys. Chem. Chem. Phys. 2020, 22, 14026–14035. [Google Scholar] [CrossRef] [PubMed]
- Lignell, A.; Tenelanda-Osorio, L.I.; Gudipati, M.S. Visible-light photoionization of aromatic molecules in water-ice: Organic chemistry across the universe with less energy. Chem. Phys. Lett. 2021, 778, 138814. [Google Scholar] [CrossRef]
- Coates, A.J.; Crary, F.J.; Lewis, G.R.; Young, D.T.; Waite, J.H.; Sittler, E.C. Discovery of heavy negative ions in Titan’s ionosphere. Geophys. Res. Lett. 2007, 34, L22103. [Google Scholar] [CrossRef]
- Bézard, B.; Drossart, P.; Encrenaz, T.; Feuchtgruber, H. Benzene on the Giant Planets. Icarus 2001, 154, 492–500. [Google Scholar] [CrossRef]
- Dubois, D.; Sciamma-O’Brien, E.; Iraci, L.T.; Barth, E.; Salama, F.; Vinatier, S. C6H6 Condensation on Titan’s Stratospheric Aerosols: An Integrated Laboratory, Modeling and Experimental Approach. Proc. Int. Astron. Union 2019, 15, 189–192. [Google Scholar] [CrossRef]
- Vuitton, V.; Yelle, R.V.; Cui, J. Formation and distribution of benzene on Titan. J. Geophys. Res. E Planets 2008, 113, 1–18. [Google Scholar] [CrossRef]
- Mebel, A.M.; Kislov, V.V.; Kaiser, R.I. Photoinduced mechanism of formation and growth of polycyclic aromatic hydrocarbons in low-temperature environments via successive ethynyl radical additions. J. Am. Chem. Soc. 2008, 130, 13618–13629. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.S.; Wilson, A.V.; Kaiser, R.I.; Mayhall, N.J.; Head-Gordon, M.; Tielens, A.G. On the formation of silacyclopropenylidene (c-SiC2H2) and its role in the organosilicon chemistry in the interstellar medium. Astrophys. J. 2013, 770, 33. [Google Scholar] [CrossRef][Green Version]
- Esposito, V.; Alessandrini, S.; Dubois, D.; Fortenberry, R. A Catalytic Pathway for the Formation of Cyanobenzene in Nitrogen-rich Environments and the Spectroscopy of the Reactive Intermediates. Planet. Sci. J. 2025, 6, 113. [Google Scholar] [CrossRef]
- Sanchez, R.; Ferris, J.P.; Orgel, L.E. Conditions for Purine Synthesis: Did Prebiotic Synthesis Occur at Low Temperatures? Science 1966, 36, 677–686. [Google Scholar] [CrossRef]
- Gupta, S.; Ochiai, E.; Ponnamperuma, C. Organic synthesis in the atmosphere of Titan. Nature 1981, 293, 725–727. [Google Scholar] [CrossRef]
- Khare, B.N.; Sagan, C.; Zumberge, J.E.; Sklarew, D.S.; Nagy, B. Organic solids produced by electrical discharge in reducing atmospheres: Tholin molecular analysis. Icarus 1981, 48, 290–297. [Google Scholar] [CrossRef]
- Scattergood, T.W.; McKay, C.P.; Borucki, W.J.; Giver, L.P.; van Ghyseghem, H.; Parris, J.E.; Miller, S.L. Production of organic compounds in plasmas: A comparison among electric sparks, laser-induced plasmas, and UV light. Icarus 1989, 81, 413–428. [Google Scholar] [CrossRef]
- Thompson, W.R.; Henry, T.J.; Schwartz, J.M.; Khare, B.N.; Sagan, C. Plasma discharge in N2 + CH4 at low pressures: Experimental results and applications to Titan. Icarus 1991, 90, 57–73. [Google Scholar] [CrossRef]
- McDonald, G.D.; Reid Thompson, W.; Heinrich, M.; Khare, B.N.; Sagan, C. Chemical Investigation of Titan and Triton Tholins. Icarus 1994, 108, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Coll, P.; Coscia, D.; Gazeau, M.C.; de Vanssay, E.; Guillemin, J.C.; Raulin, F. Organic chemistry in Titan’s atmosphere: New data from laboratory simulations at low temperature. Adv. Space Res. 1995, 16, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Navarro-González, R.; Ramírez, S.I. Corona discharge of Titan’s troposphere. Adv. Space Res. 1997, 19, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, S.; Coll, P.; da Silva, A.; Navarro-González, R.; Lafait, J.; Raulin, F. Complex Refractive Index of Titan’s Aerosol Analogues in the 200–900 nm Domain. Icarus 2002, 156, 515–529. [Google Scholar] [CrossRef][Green Version]
- Imanaka, H.; Khare, B.N.; Elsila, J.E.; Bakes, E.L.O.; McKay, C.P.; Cruikshank, D.P.; Sugita, S.; Matsui, T.; Zare, R.N. Laboratory experiments of Titan tholin formed in cold plasma at various pressures: Implications for nitrogen-containing polycyclic aromatic compounds in Titan haze. Icarus 2004, 168, 344–366. [Google Scholar] [CrossRef]
- Ramírez, S.I.; Navarro-González, R.; Coll, P.; Raulin, F. Organic chemistry induced by corona discharges in Titan’s troposphere: Laboratory simulations. Adv. Space Res. 2005, 36, 274–280. [Google Scholar] [CrossRef]
- Szopa, C.; Cernogora, G.; Boufendi, L.; Correia, J.J.; Coll, P. PAMPRE: A dusty plasma experiment for Titan’s tholins production and study. Planet. Space Sci. 2006, 54, 394–404. [Google Scholar] [CrossRef]
- Ricketts, C.L.; Contreras, C.S.; Walker, R.L.; Salama, F. The coupling of a reflectron time-of-flight mass spectrometer with a cosmic simulation chamber: A powerful new tool for laboratory astrophysics. Intern. J. Mass Spectrom. 2011, 300, 26–30. [Google Scholar] [CrossRef][Green Version]
- Carrasco, N.; Gautier, T.; Es-Sebbar, E.T.; Pernot, P.; Cernogora, G. Volatile products controlling Titan’s tholins production. Icarus 2012, 219, 230–240. [Google Scholar] [CrossRef]
- Hörst, S.M.; Tolbert, M.A. In situ measurements of the size and density of titan aerosol analogs. Astrophys. J. Lett. 2013, 770, L10. [Google Scholar] [CrossRef]
- Sciamma-O’Brien, E.; Ricketts, C.L.; Salama, F. The Titan haze simulation experiment on cOsmic: Probing titan’s atmospheric chemistry at low temperature. Icarus 2014, 243, 325–336. [Google Scholar] [CrossRef]
- Cunha De Miranda, B.; Garcia, G.A.; Gaie-Levrel, F.; Mahjoub, A.; Gautier, T.; Fleury, B.; Nahon, L.; Pernot, P.; Carrasco, N. Molecular Isomer Identification of Titan’s Tholins Organic Aerosols by Photoelectron/photoion Coincidence Spectroscopy Coupled to VUV Synchrotron Radiation. J. Phys. Chem. A 2016, 120, 6529–6540. [Google Scholar] [CrossRef]
- Dubois, D.; Carrasco, N.; Petrucciani, M.; Vettier, L.; Tigrine, S.; Pernot, P. In situ investigation of neutrals involved in the formation of Titan tholins. Icarus 2019, 317, 182–196. [Google Scholar] [CrossRef]
- Dubois, D.; Carrasco, N.; Jovanovic, L.; Vettier, L.; Gautier, T.; Westlake, J. Positive ion chemistry in an N2-CH4 plasma discharge: Key precursors to the growth of Titan tholins. Icarus 2020, 338, 113437. [Google Scholar] [CrossRef]
- Perrin, Z.; Carrasco, N.; Chatain, A.; Jovanovic, L.; Vettier, L.; Ruscassier, N.; Cernogora, G. An Atmospheric Origin for HCN-Derived Polymers on Titan. Processes 2021, 9, 965. [Google Scholar] [CrossRef]
- He, C.; Serigano, J.; Hörst, S.M.; Radke, M.; Sebree, J.A. Titan Atmospheric Chemistry Revealed by Low-Temperature N2–CH4 Plasma Discharge Experiments. ACS Earth Space Chem. 2022, 6, 2295–2304. [Google Scholar] [CrossRef]
- Dodonova, N.Y. Activation of nitrogen by vacuum ultraviolet radiation. Russ. J. Phys. Chem. 1966, 40, 523. [Google Scholar]
- Sagan, C.; Khare, B.N. Experimental Jovian Photochemistry: Initial Results. Astrophys. J. 1971, 15, 563–569. [Google Scholar] [CrossRef]
- Chang, S.; Scattergood, T.; Aronowitz, S.; Flores, J. Organic Chemistry on Titan. Rev. Geophys. Space Phys. 1979, 17, 1923–1933. [Google Scholar] [CrossRef]
- Ferris, J.; Tran, B.; Joseph, J.; Vuitton, V.; Briggs, R.; Force, M. The role of photochemistry in Titan’s atmospheric chemistry. Adv. Space Res. 2005, 36, 251–257. [Google Scholar] [CrossRef]
- Vuitton, V.; Doussin, J.F.; Bénilan, Y.; Raulin, F.; Gazeau, M.C. Experimental and theoretical study of hydrocarbon photochemistry applied to Titan stratosphere. Icarus 2006, 185, 287–300. [Google Scholar] [CrossRef]
- Imanaka, H.; Smith, M.A. EUV photochemical production of unsaturated hydrocarbons: Implications to EUV photochemistry in Titan and Jovian planets. J. Phys. Chem. A 2009, 113, 11187–11194. [Google Scholar] [CrossRef]
- Carrasco, N.; Giuliani, A.; Correia, J.J.; Cernogora, G. VUV photochemistry simulation of planetary upper atmosphere using synchrotron radiation. J. Synchrotron Radiat. 2013, 20, 587–589. [Google Scholar] [CrossRef]
- Tigrine, S.; Carrasco, N.; Vettier, L.; Cernogora, G. A microwave plasma source for VUV atmospheric photochemistry. J. Phys. D Appl. Phys. 2016, 49, 395202. [Google Scholar] [CrossRef]
- Gautier, T.; Sebree, J.A.; Li, X.; Pinnick, V.T.; Grubisic, A.; Loeffler, M.J.; Getty, S.A.; Trainer, M.G.; Brinckerhoff, W.B. Influence of trace aromatics on the chemical growth mechanisms of Titan aerosol analogues. Planet. Space Sci. 2017, 140, 27–34. [Google Scholar] [CrossRef]
- Carrasco, N.; Tigrine, S.; Gavilan, L.; Nahon, L.; Gudipati, M.S. The evolution of Titan’s high-altitude aerosols under ultraviolet irradiation. Nat. Astron. 2018, 2, 489–494. [Google Scholar] [CrossRef]
- Berry, J.L.; Ugelow, M.S.; Tolbert, M.A.; Browne, E.C. Chemical Composition of Gas-Phase Positive Ions during Laboratory Simulations of Titan’s Haze Formation. ACS Earth Space Chem. 2019, 3, 202–211. [Google Scholar] [CrossRef]
- Bourgalais, J.; Carrasco, N.; Vettier, L.; Pernot, P. Low-Pressure EUV Photochemical Experiments: Insight on the Ion Chemistry Occurring in Titan’s Atmosphere. J. Geophys. Res. Space Phys. 2019, 124, 9214–9228. [Google Scholar] [CrossRef]
- Bourgalais, J.; Carrasco, N.; Vettier, L.; Comby, A.; Descamps, D.; Petit, S.; Blanchet, V.; Gaudin, J.; Mairesse, Y.; Marty, B. Aromatic Formation Promoted by Ion-Driven Radical Pathways in EUV Photochemical Experiments Simulating Titan’s Atmospheric Chemistry. J. Phys. Chem. A 2021, 125, 3159–3168. [Google Scholar] [CrossRef]
- Carrasco, N.; Bourgalais, J.; Vettier, L.; Pernot, P.; Giner, E.; Spezia, R. A missing link in the nitrogen-rich organic chain on Titan. Astron. Astrophys. 2022, 663, A165. [Google Scholar] [CrossRef]
- Curtis, D.B.; Hatch, C.D.; Hasenkopf, C.A.; Toon, O.B.; Tolbert, M.A.; McKay, C.P.; Khare, B.N. Laboratory studies of methane and ethane adsorption and nucleation onto organic particles: Application to Titan’s clouds. Icarus 2008, 195, 792–801. [Google Scholar] [CrossRef]
- Sciamma-O’Brien, E.; Upton, K.T.; Salama, F. The Titan Haze Simulation (THS) experiment on COSmIC. Part II. Ex-situ analysis of aerosols produced at low temperature. Icarus 2017, 289, 214–226. [Google Scholar] [CrossRef]
- Couturier-Tamburelli, I.; Toumi, A.; Piétri, N.; Chiavassa, T. Behaviour of solid phase ethyl cyanide in simulated conditions of Titan. Icarus 2018, 300, 477–485. [Google Scholar] [CrossRef]
- Fleury, B.; Gudipati, M.S.; Couturier-Tamburelli, I.; Carrasco, N. Photoreactivity of condensed acetylene on Titan aerosols analogues. Icarus 2019, 321, 358–366. [Google Scholar] [CrossRef]
- Selliez, L.; Maillard, J.; Cherville, B.; Gautier, T.; Thirkell, L.; Gaubicher, B. High resolution mass spectrometry for future space missions: Comparative analysis of complex organic matter with LAb- CosmOrbitrap and LDI-FTICR. Rapid Commun. Mass Spectrom. 2019, 34, e8645. [Google Scholar] [CrossRef]
- Mouzay, J.; Couturier-Tamburelli, I.; Piétri, N.; Chiavassa, T. Experimental simulation of Titan’s stratospheric photochemistry: Benzene (C6H6) ices. J. Geophys. Res. Planets 2020, 126, e2020JE006566. [Google Scholar] [CrossRef]
- Couturier-Tamburelli, I.; Danger, G.; Mouzay, J.; Piétri, N. Photochemistry of benzene (C6H6) hydrogen cyanide (HCN) co-condensed ices part 2: Formation of aerosols analogues of titan’s atmosphere. Icarus 2025, 438, 116626. [Google Scholar] [CrossRef]
- Imanaka, H.; Smith, M.A. Role of photoionization in the formation of complex organic molecules in Titan’s upper atmosphere. Geophys. Res. Lett. 2007, 34, 1–5. [Google Scholar] [CrossRef]
- Thissen, R.; Vuitton, V.; Lavvas, P.; Lemaire, J.; Dehon, C.; Dutuit, O.; Smith, M.A.; Turchini, S.; Catone, D.; Yelle, R.V.; et al. Laboratory studies of molecular growth in the titan ionosphere. J. Phys. Chem. A 2009, 113, 11211–11220. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Gautier, T.; Carrasco, N.; Pernot, P.; Giuliani, A.; Mahjoub, A.; Correia, J.J.; Buch, A.; Bénilan, Y.; Szopa, C.; et al. Titan’s atmosphere simulation experiment using continuum UV-VUV synchrotron radiation. J. Geophys. Res. Planets 2013, 118, 778–788. [Google Scholar] [CrossRef]
- Tigrine, S.; Carrasco, N.; Bozanic, D.K.; Garcia, G.A.; Nahon, L. FUV Photoionization of Titan Atmospheric Aerosols. Astrophys. J. 2018, 867, 164. [Google Scholar] [CrossRef]
- Anderson, C.M.; Samuelson, R.E.; Mclain, J.L.; Dworkin, J.P. The SPECTRAL Ice Chamber: Application to Titan’s Stratospheric Ice Clouds. Astrophys. J. 2018, 865, 62. [Google Scholar] [CrossRef]
- Sciamma-O’Brien, E.; Roush, T.L.; Rannou, P.; Dubois, D.; Salama, F. First Optical Constants of Laboratory-generated Organic Refractory Materials (Tholins) Produced in the NASA Ames COSmIC Facility from the Visible to the Near Infrared (0.4–1.6 μm): Application to Titan’s Aerosols. Planet. Sci. J. 2023, 4, 121. [Google Scholar] [CrossRef]
- Hudson, R.L.; Yarnall, Y.Y.; Gerakines, P.A. Benzene Vapor Pressures at Titan Temperatures: First Microbalance Results. Planet. Sci. J. 2022, 3, 121. [Google Scholar] [CrossRef]
- Coupeaud, A.; Kołos, R.; Aycard, J.P.; Pie, N. Photochemical Synthesis of the Cyanodiacetylene HC5N: A Cryogenic Matrix Experiment. J. Phys. Chem. 2006, 110, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Couturier-Tamburelli, I.; Piétri, N.; Crépin, C.; Turowski, M.; Guillemin, J.C.; Kołos, R. Synthesis and spectroscopy of cyanotriacetylene (HC7N) in solid argon. J. Chem. Phys. 2014, 140, 044329. [Google Scholar] [CrossRef] [PubMed]
- Mouzay, J.; Piétri, N.; Couturier-Tamburelli, I.; Chiavassa, T. UV irradiation of benzene in N2 matrix: A relevant study for titan’s chemistry. J. Mol. Struct. 2021, 1237, 130296. [Google Scholar] [CrossRef]
- Carrasco, N.; Dutuit, O.; Thissen, R.; Banaszkiewicz, M.; Pernot, P. Uncertainty analysis of bimolecular reactions in Titan ionosphere chemistry model. Planet. Space Sci. 2007, 55, 141–157. [Google Scholar] [CrossRef][Green Version]
- Carrasco, N.; Pernot, P. Modeling of branching ratio uncertainty in chemical networks by dirichlet distributions. J. Phys. Chem. A 2007, 111, 3507–3512. [Google Scholar] [CrossRef]
- Carrasco, N.; Alcaraz, C.; Dutuit, O.; Plessis, S.; Thissen, R.; Vuitton, V.; Yelle, R.; Pernot, P. Sensitivity of a Titan ionospheric model to the ion-molecule reaction parameters. Planet. Space Sci. 2008, 56, 1644–1657. [Google Scholar] [CrossRef]
- Heays, A.N.; Bosman, A.D.; Van Dishoeck, E.F. Photodissociation and photoionisation of atoms and molecules of astrophysical interest. Astron. Astrophys. 2017, 602, A105. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Y.; Hansen, C.S.; Yang, J.; Chang, Y.; Yu, Y.; Cheng, G.; Chen, Z.; He, Z.; Yu, S.; et al. Ultraviolet photolysis of H2S and its implications for SH radical production in the interstellar medium. Nat. Commun. 2020, 11, 1547. [Google Scholar] [CrossRef]
- Chang, Y.; Ashfold, M.N.; Yuan, K.; Yang, X. Exploring the vacuum ultraviolet photochemistry of astrochemically important triatomic molecules. Natl. Sci. Rev. 2023, 10, nwad158. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Moore, B.M.; Borengasser, Q.D.; Renshaw, K.T.; Johnson, R.; Kanaherarachchi, A.C.; Broderick, B.M. VUV Processing of Nitrile Ice: Direct Comparison of Branching in Ice and TPD Spectra. Acs Earth Space Chem. 2025. [Google Scholar] [CrossRef]
- Mebel, A.M.; Hayashi, M.; Jackson, W.M.; Wrobel, J.; Green, M.; Xu, D.; Lin, S.H. Branching ratios of C2 products in the photodissociation of C2H at 193 nm. J. Chem. Phys. 2001, 114, 9821–9831. [Google Scholar] [CrossRef]
- Loison, J.C.; Bergeat, A.; Caralp, F.; Hannachi, Y. Rate constants and H atom branching ratios of the gas-phase reactions of methylidyne CH(X2II) radical with a series of alkanes. J. Phys. Chem. A 2006, 110, 13500–13506. [Google Scholar] [CrossRef]
- Carrasco, N.; Plessis, S.; Pernot, P. Towards a reduction of the bimolecular reaction model for Titan ionosphere. Int. J. Chem. Kinet. 2008, 40, 699–709. [Google Scholar] [CrossRef]
- Lilensten, J.; Witasse, O.; Simon, C.; Soldi-Lose, H.; Dutuit, O.; Thissen, R.; Alcaraz, C. Prediction of a N2++ layer in the upper atmosphere of Titan. Geophys. Res. Lett. 2005, 32, 1–4. [Google Scholar] [CrossRef]
- Thissen, R.; Witasse, O.; Dutuit, O.; Wedlund, C.S.; Gronoff, G.; Lilensten, J. Doubly-charged ions in the planetary ionospheres: A review. Phys. Chem. Chem. Phys. 2011, 13, 18264. [Google Scholar] [CrossRef]
- Matsubara, T. A model of ionization-induced reactions in CH4/N2 clusters in Titan’s atmosphere: Theoretical insights into mono- and divalent states. Bull. Chem. Soc. Jpn. 2024, 97, uoae047. [Google Scholar] [CrossRef]
- Matsubara, T. Theoretical Insights into a Novel Ion-Ion Reaction of Methane in the Initial Stages of Hydrocarbon Growth in Space. Acs Earth Space Chem. 2024, 8, 2557–2573. [Google Scholar] [CrossRef]
- Hargreaves, J. The Solar-Terrestrial Environment. In The Solar-Terrestrial Environment; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Chaizy, P.; Rème, H.; Sauvaud, J.A.; d’Uston, C.; Lin, R.P.; Larson, D.E.; Mitchell, D.L.; Anderson, K.A.; Carlson, C.W.; Korth, A.; et al. Negative ions in the coma of comet Halley. Nature 1991, 349, 393–396. [Google Scholar] [CrossRef]
- Coates, A.J.; Jones, G.H.; Lewis, G.R.; Wellbrock, A.; Young, D.T.; Crary, F.J.; Johnson, R.E.; Cassidy, T.A.; Hill, T.W. Negative ions in the Enceladus plume. Icarus 2010, 206, 618–622. [Google Scholar] [CrossRef]
- Coates, A.J.; Wellbrock, A.; Lewis, G.R.; Jones, G.H.; Young, D.T.; Crary, F.J.; Waite, J.H. Heavy negative ions in Titan’s ionosphere: Altitude and latitude dependence. Planet. Space Sci. 2009, 57, 1866–1871. [Google Scholar] [CrossRef]
- Wellbrock, A.; Coates, A.J.; Jones, G.H.; Lewis, G.R.; Waite, J.H. Cassini CAPS-ELS observations of negative ions in Titan’s ionosphere: Trends of density with altitude. Geophys. Res. Lett. 2013, 40, 4481–4485. [Google Scholar] [CrossRef]
- Desai, R.T.; Coates, A.J.; Wellbrock, A.; Vuitton, V.; Crary, F.J.; González-Caniulef, D.; Shebanits, O.; Jones, G.H.; Lewis, G.R.; Waite, J.H.; et al. Carbon chain anions and the growth of complex organic molecules in Titan’s ionosphere. Astrophys. J. Lett. 2017, 844, L18. [Google Scholar] [CrossRef]
- Vuitton, V.; Lavvas, P.; Yelle, R.V.; Galand, M.; Wellbrock, A.; Lewis, G.R.; Coates, A.J.; Wahlund, J.E. Negative ion chemistry in Titan’s upper atmosphere. Planet. Space Sci. 2009, 57, 1558–1572. [Google Scholar] [CrossRef]
- Marif, H.; Lilensten, J. Suprathermal electron moments in the ionosphere. J. Space Weather Space Clim. 2020, 10, 22. [Google Scholar] [CrossRef]
- Wang, Z.; Su, Z.; Liu, N.; Dai, G.; Zheng, H.; Wang, Y.; Wang, S. Suprathermal Electron Evolution Under the Competition Between Plasmaspheric Plume Hiss Wave Heating and Collisional Cooling. Geophys. Res. Lett. 2020, 47, e2020GL089649. [Google Scholar] [CrossRef]
- Millar, T.J.; Walsh, C.; Field, T.A. Negative ions in space. Chem. Rev. 2017, 117, 1765–1795. [Google Scholar] [CrossRef]
- Martinez, O.; Yang, Z.; Demarais, N.J.; Snow, T.P.; Bierbaum, V.M. Gas-phase reactions of hydride anion, H-. Astrophys. J. 2010, 720, 173–177. [Google Scholar] [CrossRef]
- Pirim, C.; Gann, R.; McLain, J.; Orlando, T. Electron-molecule chemistry and charging processes on organic ices and Titan’s icy aerosol surrogates. Icarus 2015, 258, 109–119. [Google Scholar] [CrossRef]
- Harada, N.; Herbst, E. Modeling Carbon Chain Anions in L1527. Astrophys. J. 2008, 685, 272–280. [Google Scholar] [CrossRef]
- Rawat, P.; Prabhudesai, V.S.; Rahman, M.; Ram, N.B.; Krishnakumar, E. Absolute cross sections for dissociative electron attachment to NH3 and CH4. Int. J. Mass Spectrom. 2008, 277, 96–102. [Google Scholar] [CrossRef]
- Mackay, G.I.; Betowski, L.D.; Payzant, J.D.; Schiff, I.; Bohme, D.K. Proton-Transfer Reactions with HCN and CH3CN Rate Constants at 297 K for Proton-Transfer Reactions with HCN and CH3CN. Comparisons with Classical Theories and Exothermicity. J. Phys. Chem. 1976, 80, 2919–2922. [Google Scholar] [CrossRef]
- Barckholtz, C.; Snow, T.P.; Bierbaum, V.M. Reactions of Cn- and CnH- with Atomic and Molecular Hydrogen. Astrophys. J. 2001, 547, 171–174. [Google Scholar] [CrossRef]
- Herbst, E.; Osamura, Y. Calculations on the Formation Rates and Mechanisms for CnH Anions in Interstellar and Circumstellar Media. Astrophys. J. 2008, 679, 1670. [Google Scholar] [CrossRef]
- Yang, Z.; Cole, C.A.; Martinez, O., Jr.; Carpenter, M.Y.; Snow, T.P.; Bierbaum, V.M. Experimental and Theoretical Studies of Reactions Between H Atoms and Nitrogen-Containing Carbanions. Astrophys. J. 2011, 739, 19. [Google Scholar] [CrossRef]
- Itikawa, Y.; Mason, N. Cross sections for electron collisions with water molecules. J. Phys. Chem. Ref. Data 2005, 34, 1–22. [Google Scholar] [CrossRef]
- Horvath, G.; Aranda-Gonzalvo, Y.; Mason, N.J.; Zahoran, M.; Matejcik, S. Negative ions formed in N2/CH4/Ar discharge—A simulation of Titan’s atmosphere chemistry. Eur. Phys. J. Appl. Phys. 2009, 49, 13105. [Google Scholar] [CrossRef]
- Dubois, D.; Carrasco, N.; Bourgalais, J.; Vettier, L.; Desai, R.; Wellbrock, A.; Coates, A. Nitrogen-containing Anions and Tholin Growth in Titan’s Ionosphere: Implications for Cassini CAPS-ELS Observations. Astrophys. J. Lett. 2019, 872, L31. [Google Scholar] [CrossRef]
- Dubois, D.; Sciamma-O’Brien, E.; Fortenberry, R. The Fundamental Vibrational Frequencies and Spectroscopic Constants of the Dicyanoamine Anion, NCNCN− (C2N3−): Quantum Chemical Analysis for Astrophysical and Planetary Environments. Astrophys. J. 2019, 883, 109. [Google Scholar] [CrossRef]
- Bierbaum, V.M. Anions in Space and in the Laboratory. Proc. Int. Astron. Union 2011, 7, 383–389. [Google Scholar] [CrossRef]
- Capone, L.A.; Whitten, R.C.; Prasad, S.S.; Dubach, J. The ionospheres of Saturn, Uranus, and Neptune. Astrophys. J. 1977, 215, 977–983. [Google Scholar] [CrossRef]
- Romani, P.N.; Atreya, S.K. Methane Photochemistry and Haze Production on Neptune. Icarus 1988, 74, 424–445. [Google Scholar] [CrossRef]
- Bezard, B.; Feuchtgruber, H.; Encrenaz, T. Observations of Hydrocarbons in the Giant Planets; The Universe as Seen by ISO: Paris, France, 1999. [Google Scholar]
- Mao, M.; Benedikt, J.; Consoli, A.; Bogaerts, A. New pathways for nanoparticle formation in acetylene dusty plasmas: A modelling investigation and comparison with experiments. J. Phys. D Appl. Phys. 2008, 41, 225201. [Google Scholar] [CrossRef]
- Krishnakumar, E.; Denifl, S.; Cadez, I.; Markelj, S.; Mason, N.J. Dissociative electron attachment cross sections for H2 and D2. Phys. Rev. Lett. 2011, 106, 243201. [Google Scholar] [CrossRef]
- Song, M.Y.; Yoon, J.S.; Cho, H.; Karwasz, G.P.; Kokoouline, V.; Nakamura, Y.; Tennyson, J. Cross sections for electron collisions with acetylene. J. Phys. Chem. Ref. Data 2017, 46, 013106. [Google Scholar] [CrossRef]
- Čadež, I.; Markelj, S.; Rupnik, Z. Low energy H− production by electron collision with small hydrocarbons. Eur. Phys. J. D 2012, 66, 73. [Google Scholar] [CrossRef]
- Szymańska, E.; Mason, N.J.; Krishnakumar, E.; Matias, C.; Mauracher, A.; Scheier, P.; Denifl, S. Dissociative electron attachment and dipolar dissociation in ethylene. Int. J. Mass Spectrom. 2014, 365–366, 356–364. [Google Scholar] [CrossRef]
- Janečková, R.; May, O.; Fedor, J. Dissociative electron attachment to methylacetylene and dimethylacetylene: Symmetry versus proximity. Phys. Rev. A—At. Mol. Opt. Phys. 2012, 86, 052702. [Google Scholar] [CrossRef]
- May, O.; Fedor, J.; Ibǎnescu, B.C.; Allan, M. Absolute cross sections for dissociative electron attachment to acetylene and diacetylene. Phys. Rev. A—At. Mol. Opt. Phys. 2008, 77, 040701. [Google Scholar] [CrossRef]
- May, O.; Kubala, D.; Allan, M. Absolute cross sections for dissociative electron attachment to HCN and DCN. Phys. Rev. A 2010, 82, 010701. [Google Scholar] [CrossRef]
- Tanzer, K.; Pelc, A.; Huber, S.E.; Czupyt, Z.; Denifl, S. Low energy electron attachment to cyanamide (NH2CN) Low energy electron attachment to cyanamide (NH2CN). J. Chem. Phys. 2015, 142, 034301. [Google Scholar] [CrossRef]
- Pelc, A.; Huber, S.E.; Matias, C.; Czupyt, Z.; Denifl, S. Formation of Negative Ions upon Dissociative Electron Attachment to the Astrochemically Relevant Molecule Aminoacetonitrile. J. Phys. Chem. A 2016, 120, 903–910. [Google Scholar] [CrossRef]
- Fiquet-Fayard, F.; Ziesel, J.P.; Azria, R.; Tronc, M.; Chiari, J. Formation of HS− and DS− by Dissociative Attachment in H2S, HDS, and D2S. J. Chem. Phys. 1972, 56, 2540–2548. [Google Scholar] [CrossRef]
- Rao, M.V.V.S.; Srivastava, S.K. Electron Impact Ionization and Attachment Cross Sections for H2S. J. Geophys. Res. 1993, 98, 13137–13145. [Google Scholar] [CrossRef]
- Simon, A.; Cohen, I.; Hedman, M.; Hofstadter, M.; Mandt, K.; Nimmo, F. Uranus Flagship Science-Driven Tour Design: Community Input Poll. arXiv 2025, arXiv:2505.05514. [Google Scholar] [CrossRef]

| Region 1 | Region 2 | Region 3 | |
|---|---|---|---|
| Near/Far-UV (400–121.6 nm) | Lyman- (121.6 nm) | EUV/VUV (121.6–25 nm) | |
| Energy range (eV) | 3.1–10.2 | 10.2 | 10.2–49.6 |
| Mass () | Solar Constant (W m−2) | EUV Intensity (kR), 90–110 nm | T (1 bar Level) in Kelvin | Mean Molecular Weight | |
|---|---|---|---|---|---|
| Titan | 0.023 | 14.8 | 0.21 | 94 | 27.8 |
| Saturn | 95.2 | 14.8 | 0.21 | 134–145 | 2.0 |
| Uranus | 14.5 | 3.7 | 0.05 | 76–86 | 2.3 |
| Atoms | C | N | O | Other | ||||
|---|---|---|---|---|---|---|---|---|
| Titan | Uranus | Titan | Uranus | Titan | Uranus | Titan | Uranus | |
| 1 | CH4 | CH4 | HCN, HNC, CH3CN, HC3N, C3H3N, C3H5N, C4H3N | - | H2O, CO | CO | - | H2, H2S |
| 2 | C2H2, C2H4, C2H6 | C2H2, C2H6 | N2, C2N2 | - | CO2 | CO2 | - | - |
| 3 | C3H2, C3H4, C3H6, C3H8 | C3H4 | - | - | - | - | - | - |
| 4 | C4H2 | C4H2 | - | - | - | - | - | - |
| 5 | - | - | - | - | - | - | - | - |
| 6 | C6H6 | - | - | - | - | - | - | - |
| Stratosphere | ||||
|---|---|---|---|---|
| Species | Titan | Saturn | Uranus | Ref. |
| CH4 | 1–2% | 16 ppm | [17,65,66] | |
| C2H2 | 0.25 ppm | [67,68,69,70] | ||
| C2H4 | < | [17,70,71] | ||
| C2H6 | 0.13 ppm | [17,67,68,69,70] | ||
| C3H4 | 0.36 ppb | [17,70,72] | ||
| C4H2 | 0.13 ppb | [17,70,72] | ||
| CO2 | 0.08 ppb | [17,70,73] | ||
| CO | 6 ppb | [17,70,74] | ||
| H2O | 1.1 ppb | 3.8 ppb | [20,70,75] | |
| D/H (in H2/C2H2) | [20,76,77] | |||
| Characteristics | Photochemistry | Radiation-Induced Chemistry | Examples |
|---|---|---|---|
| Energy source | Ly-; UV continuum | EUV/X-rays; energetic particles | 100–400 nm; secondary electrons; ions |
| Primary effect | Photodissociation; photoionization; electronic excitation | Ionization; radiolysis; dissociative electron attachment | + → + H → H− + CH3+ |
| Key products | Radicals; small hydrocarbons | Ions (e.g., ); complex organics; electrons | H2+, CH3, CH3+, C2H3+, C3H4+ |
| Timescales | ns–hours (daylight-driven) | fs–ns (instantaneous, flux-dependent) | O → H• + OH• (spur reactions) |
| Temperature dependence | Strong (Arrhenius kinetics) | Weak (governed by particle flux) | CH4 + H → CH3 + H2 |
| Electron transfer | Charge transfer | Ionization cascades; secondary electron emission | O+ + CH4 |
| Quantum effects | Electronic transitions; spin-forbidden pathways | Ro-vibrational excitation; plasmon resonances (ices) | singlet-triplet absorption |
| Observables | Dayglow emissions; gas abundances | Auroral X-rays; Lyman-Werner band emissions; mass spectra of ices | mass spectra, IR-UV spectra |
| Altitude/region | Stratosphere; ionosphere (day side) | Thermosphere; polar auroral zones; interstellar ices | + → + H |
| Desorption yields | Low–moderate (UV-photon dependent) | High (sputtering by >100 eV electrons) | CO + e− → CO(ads) → CO(g) |
| Multiphoton effects | Rare | Dominant (ionization cascades; track formation) | → + e− (15.6 eV) |
| Category 1 | Main Processes | Energy Source | References |
|---|---|---|---|
| Gas phase | Ionization, dissociation, radical chemistry | Plasma discharges | [206,207,208,209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226] |
| Photolysis, radical, excitation, SE | FUV–Ly-–EUV | [220,227,228,229,230,231,232,233,234,235,236,237,238,239,240] | |
| Tholins/ice | Condensation, solid-state photochemistry, SE | FUV/VUV | [104,119,120,121,178,198,241,242,243,244,245,246,247] |
| Synchrotron | Ionization, dissociation, excitation, SE | EUV-VUV Target wavelength | [232,233,236,248,249,250,251] |
| Reaction | Photochemical Pathway | Quantum Yield () |
|---|---|---|
| N+ + CH4 | CH3+ + NH | 0.50 |
| CH4+ + N | 0.05 | |
| H2CN+ + H2 | 0.10 | |
| HCN+ + NH + H | 0.36 | |
| N2+ + CH4 | CH2+ + N + H2 | 0.09 |
| CH3+ + N + H | 0.91 | |
| N2H+ + CH3 | - | |
| CH3N2+ | N2CH2+ + H | 0.01 |
| Atom | H production channels | H atom yield |
| H | CH + CH4 | 1.00 |
| CH + C2H6 | 0.22 | |
| CH + C2H6 | 0.14 | |
| CH + C3H8 | 0.19 | |
| CH + C4H10 | 0.14 |
| Parent | Fragment | Resonance Position (eV) | Cross-Section Peak (cm2) | Refs. |
|---|---|---|---|---|
| CH4 | CH2− | 10.4 | [127] | |
| H− | 9.8 | [127] | ||
| H2 | H− | 4.0 | [299] | |
| 14 | [299] | |||
| D2 | D− | 14.0 | [299] | |
| C2H2 | C2H− | 2.8 | [300] | |
| C2− | 8.3 | [300] | ||
| H− | 7.9 | [300] | ||
| C2H4 | H− | 10.5 | [301,302] | |
| CH− | 9.8 | ion yield | [302] | |
| C2H− | 9.8 | ion yield | [302] | |
| C2H2− | 1.6 | ion yield | [302] | |
| C2H3− | 7.0 | ion yield | [302] | |
| C2H6 | H− | 9.2 | ion yield | [301] |
| C3H4 | C3H3− | 3.4 | [303] | |
| C3H8 | H− | 8.6 | ion yield | [301] |
| C4H2 | C4H− | 2.5 | [304] | |
| 5.3 | [304] | |||
| C4H6 | H− | 4.0 | [303] | |
| C6H2 | C6H− | 2.8 | , est. | [278,298,304] |
| HCN | CN− | 1.9 | [305] | |
| DCN | CN− | 1.9 | [305] | |
| NH3 | H− | 5.7 | [285] | |
| NH2− | 5.9 | [285] | ||
| CH2N2 | CN− | 6.4 | [306] | |
| C2H4N2 | CN− | 1.9 | [307] | |
| H2S | HS− | 1.6 | [308,309] | |
| S− | 9.7 | [308,309] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubois, D. Photochemical Haze Formation on Titan and Uranus: A Comparative Review. Int. J. Mol. Sci. 2025, 26, 7531. https://doi.org/10.3390/ijms26157531
Dubois D. Photochemical Haze Formation on Titan and Uranus: A Comparative Review. International Journal of Molecular Sciences. 2025; 26(15):7531. https://doi.org/10.3390/ijms26157531
Chicago/Turabian StyleDubois, David. 2025. "Photochemical Haze Formation on Titan and Uranus: A Comparative Review" International Journal of Molecular Sciences 26, no. 15: 7531. https://doi.org/10.3390/ijms26157531
APA StyleDubois, D. (2025). Photochemical Haze Formation on Titan and Uranus: A Comparative Review. International Journal of Molecular Sciences, 26(15), 7531. https://doi.org/10.3390/ijms26157531






