Utility of Multicellular Spheroids for Investigating Mechanisms of Chemoresistance in Triple-Negative Breast Cancer
Abstract
1. Introduction
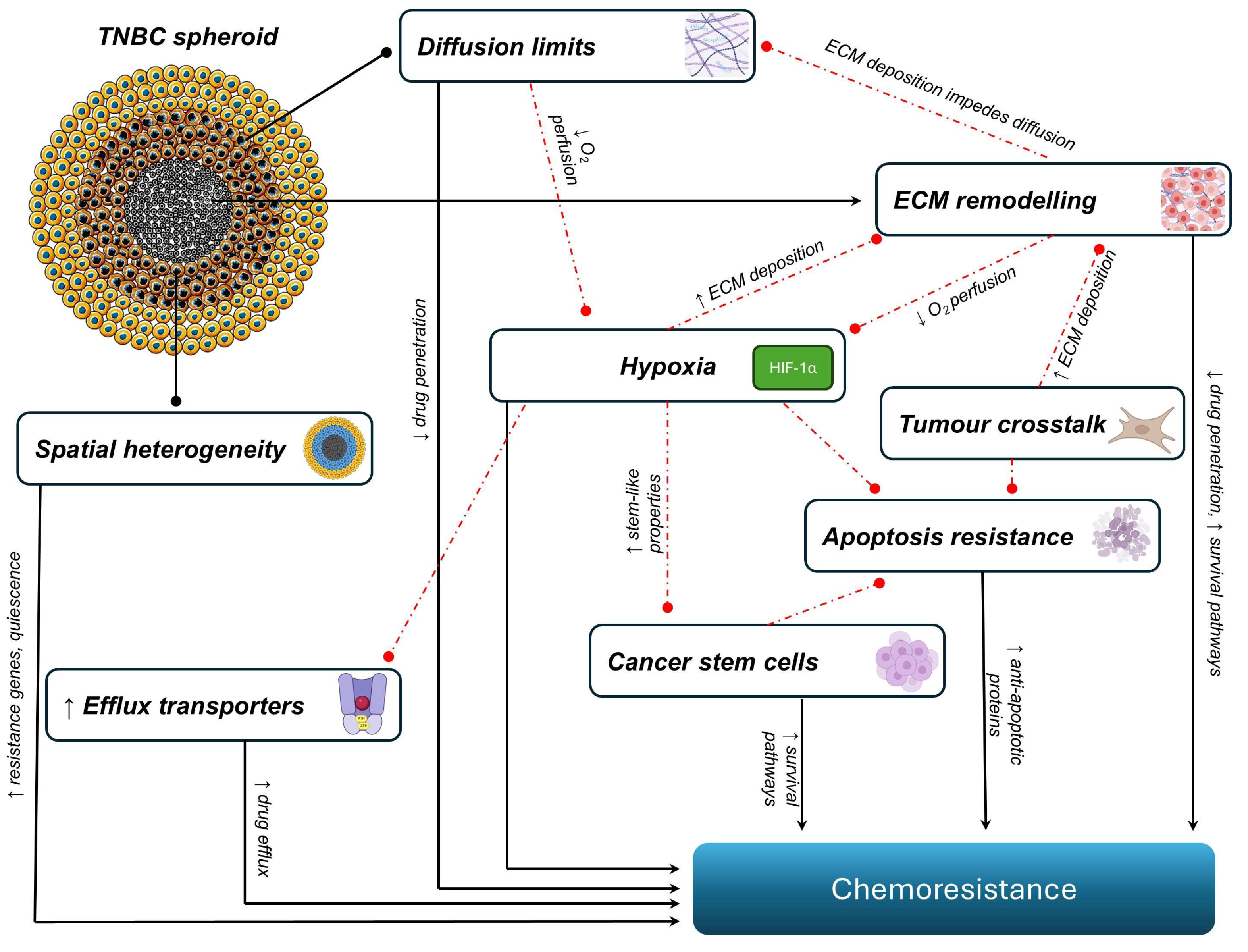
2. The Mechanisms of Chemoresistance in TNBC Spheroids
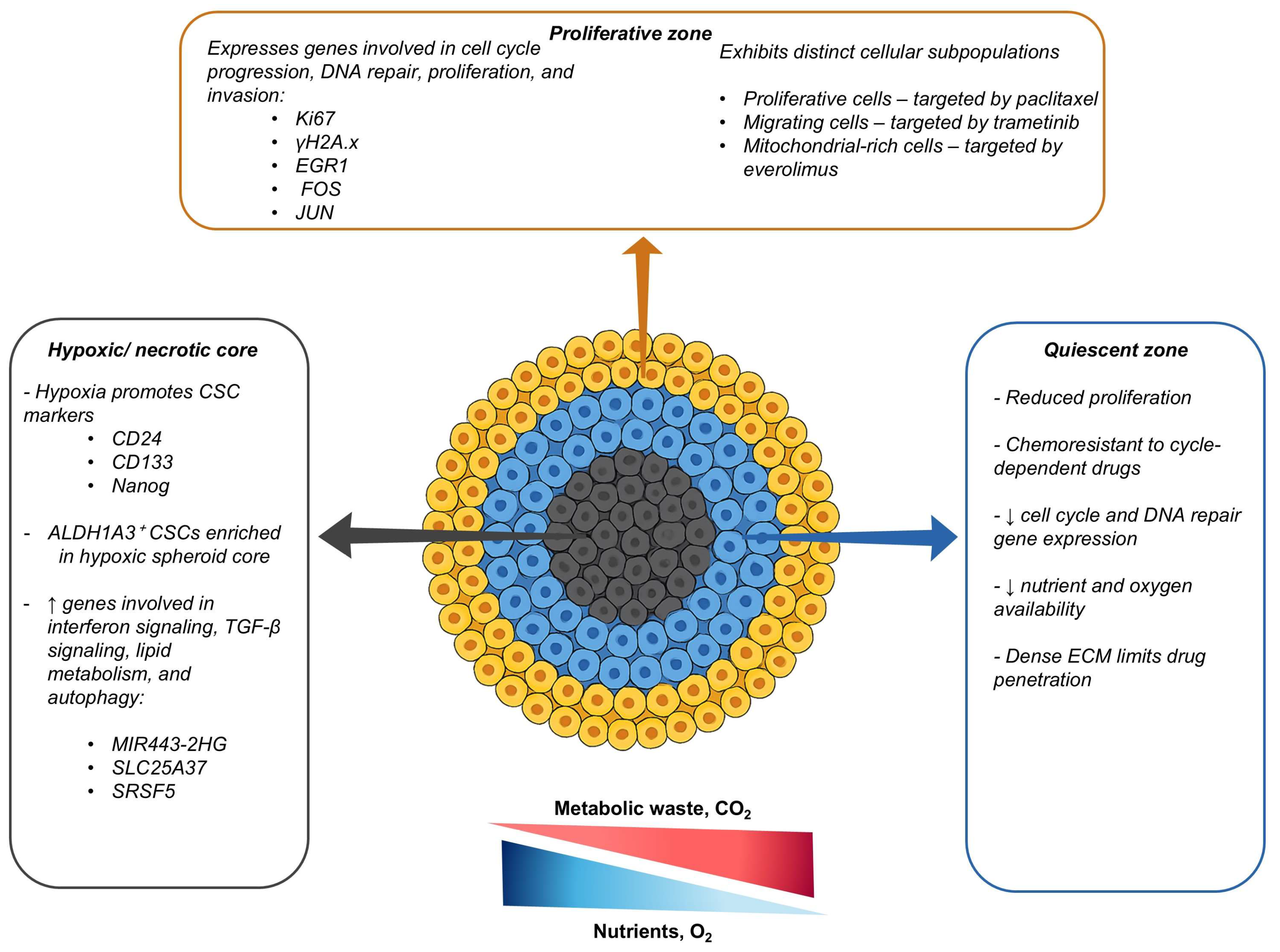
2.1. Spatial Heterogeneity
2.2. Diffusion Limits and Hypoxia
2.3. Extracellular Matrix Remodelling
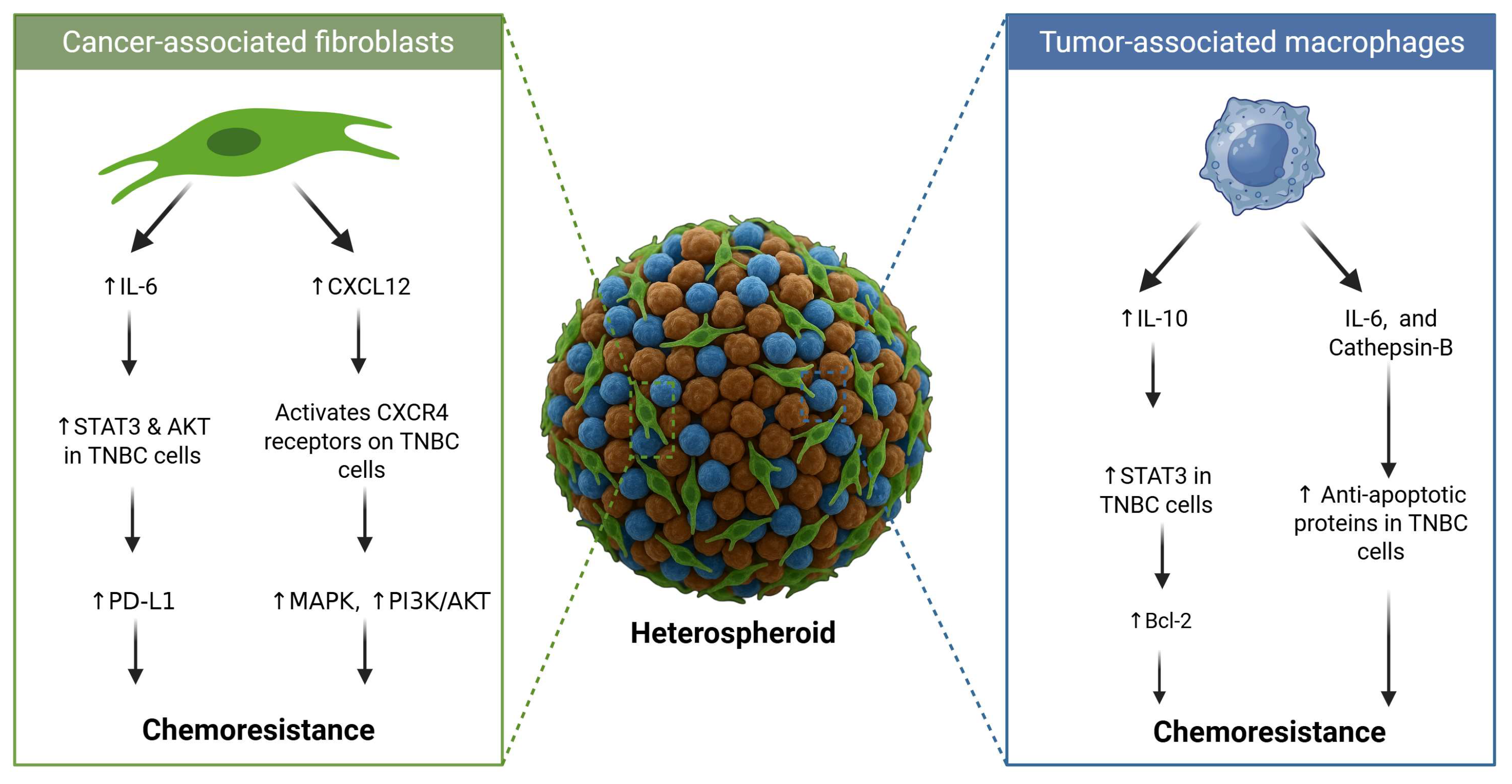
2.4. Tumour–Stroma Crosstalk and Microenvironmental Signalling
2.5. Drug Efflux
2.6. Apoptotic Resistance
2.7. Cancer Stem Cells and Pathway-Driven Chemoresistance
3. Strategies for Resensitising Chemoresistant TNBC Spheroids
4. Limitations and Future Opportunities in Using Spheroids as Models for Studying Chemoresistance in TNBC Spheroids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ABC | ATP-binding cassette |
| ASA | Acetylsalicylic acid |
| ALDH1A1 | Aldehyde dehydrogenase 1 family member A1 |
| ATP | Adenosine triphosphate |
| ATRA | All-trans retinoic acid |
| Bcl-2 | B-cell lymphoma 2 |
| Bcl-xL | B-cell lymphoma-extra-large |
| BCRP | Breast cancer resistance protein |
| CD | Cluster of differentiation |
| CAF | Cancer-associated fibroblast |
| CREB1 | cAMP response element-binding protein 1 |
| CSC | Cancer stem cell |
| CXCL12 | C-X-C motif chemokine ligand 12 |
| CXCR4 | Chemokine receptor Type 4 |
| DNA | Deoxyribonucleic acid |
| DR4 | Death receptor 4 |
| ECM | Extracellular matrix |
| EMT | Epithelial–mesenchymal transition |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| ERK5 | Extracellular signal-regulated kinase 5 |
| FZD8 | Frizzled class receptor 8 |
| FX | Fucoxanthin |
| HIF | Hypoxia-inducible factor |
| IL | Interleukin |
| LRP6 | Low-density lipoprotein receptor-related protein 6 |
| MAPK | Mitogen-activated protein kinase |
| MEK | MAPK/ERK kinase |
| MALDI | Matrix-assisted laser desorption/ionisation |
| MALDI MSI | MALDI mass spectrometry imaging |
| MCL-1 | Myeloid cell leukaemia 1 |
| MRI | Magnetic resonance imaging |
| NIR | Near-infrared |
| O2 | Oxygen |
| pCR | Pathological complete response |
| PD0325901 | MEK/ERK inhibitor |
| P-gp | P-glycoprotein |
| PI-103 | PI3K/AKT inhibitor |
| PI3K | Phosphoinositide 3-kinase |
| RASAL2 | RAS protein activator-like 2 |
| RNA | Ribonucleic acid |
| siRNA | Small interfering RNA |
| siTwist | SiRNA targeting Twist |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAM | Tumour-associated macrophage |
| TAZ | Transcriptional co-activator with PDZ-binding motif |
| TGF-β | Transforming growth factor-beta |
| TME | Tumour microenvironment |
| TNBC | Triple-negative breast cancer |
| USP22 | Ubiquitin-specific peptidase 22 |
| WAVE3 | Wiskott–Aldrich syndrome protein family member 3 |
| YAP | Yes-associated protein |
| T1/T2 | MRI relaxation times |
| Smart-seq3 | Single-cell RNA sequencing technique |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple Negative Breast Cancer: Pitfalls and Progress. npj Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review About Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef] [PubMed]
- Jie, H.; Ma, W.; Huang, C. Diagnosis, Prognosis, and Treatment of Triple-Negative Breast Cancer: A Review. Breast Cancer 2025, 17, 265–274. [Google Scholar] [CrossRef]
- Wahba, H.A.; El-Hadaad, H.A. Current Approaches in Treatment of Triple-Negative Breast Cancer. Cancer Biol. Med. 2015, 12, 106–116. [Google Scholar] [CrossRef]
- Sharma, P. Update on the Treatment of Early-Stage Triple-Negative Breast Cancer. Curr. Treat. Options Oncol. 2018, 19, 22. [Google Scholar] [CrossRef]
- Lebert, J.M.; Lester, R.; Powell, E.; Seal, M.; McCarthy, J. Advances in the Systemic Treatment of Triple-Negative Breast Cancer. Curr. Oncol. 2018, 25, S142–S150. [Google Scholar] [CrossRef]
- Abuhadra, N.; Stecklein, S.; Sharma, P.; Moulder, S. Early-stage Triple-negative Breast Cancer: Time to Optimize Personalized Strategies. Oncologist 2022, 27, 30–39. [Google Scholar] [CrossRef]
- Carey, L.A.; Dees, E.C.; Sawyer, L.; Gatti, L.; Moore, D.T.; Collichio, F.; Ollila, D.W.; Sartor, C.I.; Graham, M.L.; Perou, C.M. The Triple Negative Paradox: Primary Tumor Chemosensitivity of Breast Cancer Subtypes. Clin. Cancer Res. 2007, 13, 2329–2334. [Google Scholar] [CrossRef]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef]
- Nedeljkovic, M.; Damjanovic, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Barrett, J.S. Consideration of the Root Causes in Candidate Attrition During Oncology Drug Development. Clin. Pharmacol. Drug Dev. 2024, 13, 952–960. [Google Scholar] [CrossRef]
- Begley, C.G.; Ellis, L.M. Drug Development: Raise Standards for Preclinical Cancer Research. Nature 2012, 483, 531–533. [Google Scholar] [CrossRef]
- Foglizzo, V.; Cocco, E.; Marchiò, S. Advanced Cellular Models for Preclinical Drug Testing: From 2D Cultures to Organ-on-a-Chip Technology. Cancers 2022, 14, 3692. [Google Scholar] [CrossRef]
- Smalley, K.S.; Lioni, M.; Herlyn, M. Life Isn’t Flat: Taking Cancer Biology to the Next Dimension. Vitr. Cell. Dev. Biol.-Anim. 2006, 42, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Delarue, M.; Montel, F.; Vignjevic, D.; Prost, J.; Joanny, J.F.; Cappello, G. Compressive Stress Inhibits Proliferation in Tumor Spheroids through a Volume Limitation. Biophys. J. 2014, 107, 1821–1828. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Geraghty, R.J.; Capes-Davis, A.; Davis, J.M.; Downward, J.; Freshney, R.I.; Knezevic, I.; Lovell-Badge, R.; Masters, J.R.; Meredith, J.; Stacey, G.N.; et al. Guidelines for the Use of Cell Lines in Biomedical Research. Br. J. Cancer 2014, 111, 1021–1046. [Google Scholar] [CrossRef]
- Ncube, K.N.; Jurgens, T.; Steenkamp, V.; Cromarty, A.D.; van den Bout, I.; Cordier, W. Comparative Evaluation of the Cytotoxicity of Doxorubicin in Bt-20 Triple-Negative Breast Carcinoma Monolayer and Spheroid Cultures. Biomedicines 2023, 11, 1484. [Google Scholar] [CrossRef] [PubMed]
- Alemany-Ribes, M.; Semino, C.E. Bioengineering 3d Environments for Cancer Models. Adv. Drug Deliv. Rev. 2014, 79, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Griffith, L.G.; Swartz, M.A. Capturing Complex 3d Tissue Physiology in Vitro. Nat. Rev. Mol. Cell Biol. 2006, 7, 211–224. [Google Scholar] [CrossRef]
- Asghar, W.; El Assal, R.; Shafiee, H.; Pitteri, S.; Paulmurugan, R.; Demirci, U. Engineering Cancer Microenvironments for in Vitro 3-D Tumor Models. Mater Today 2015, 18, 539–553. [Google Scholar] [CrossRef]
- Huang, H.L.; Hsing, H.W.; Lai, T.C.; Chen, Y.W.; Lee, T.R.; Chan, H.T.; Lyu, P.C.; Wu, C.L.; Lu, Y.C.; Lin, S.T.; et al. Trypsin-Induced Proteome Alteration During Cell Subculture in Mammalian Cells. J. Biomed. Sci. 2010, 17, 36. [Google Scholar] [CrossRef]
- Wrzesinski, K.; Fey, S.J. After Trypsinisation, 3d Spheroids of C3a Hepatocytes Need 18 Days to Re-Establish Similar Levels of Key Physiological Functions to Those Seen in the Liver. Toxicol. Res. 2013, 2, 123–135. [Google Scholar] [CrossRef]
- Cordeiro, S.; Oliveira, B.B.; Valente, R.; Ferreira, D.; Luz, A.; Baptista, P.V.; Fernandes, A.R. Breaking the Mold: 3d Cell Cultures Reshaping the Future of Cancer Research. Front. Cell Dev. Biol. 2024, 12, 1507388. [Google Scholar] [CrossRef]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The Third Dimension Bridges the Gap between Cell Culture and Live Tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Kunz-Schughart, L.A.; Freyer, J.P.; Hofstaedter, F.; Ebner, R. The Use of 3-D Cultures for High-Throughput Screening: The Multicellular Spheroid Model. J. Biomol. Screen. 2004, 9, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas. JNCI J. Natl. Cancer Inst. 1971, 46, 113–120. [Google Scholar] [CrossRef]
- Huang, B.W.; Gao, J.Q. Application of 3d Cultured Multicellular Spheroid Tumor Models in Tumor-Targeted Drug Delivery System Research. J. Control. Release 2018, 270, 246–259. [Google Scholar] [CrossRef] [PubMed]
- Lagies, S.; Schlimpert, M.; Neumann, S.; Waldin, A.; Kammerer, B.; Borner, C.; Peintner, L. Cells Grown in Three-Dimensional Spheroids Mirror in Vivo Metabolic Response of Epithelial Cells. Commun. Biol. 2020, 3, 246. [Google Scholar] [CrossRef]
- Pradhan, S.; Sperduto, J.L.; Farino, C.J.; Slater, J.H. Engineered in Vitro Models of Tumor Dormancy and Reactivation. J. Biol. Eng. 2018, 12, 37. [Google Scholar] [CrossRef]
- Taubenberger, A.V.; Bray, L.J.; Haller, B.; Shaposhnykov, A.; Binner, M.; Freudenberg, U.; Guck, J.; Werner, C. 3D Extracellular Matrix Interactions Modulate Tumour Cell Growth, Invasion and Angiogenesis in Engineered Tumour Microenvironments. Acta Biomater. 2016, 36, 73–85. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Herrmann, D.; Conway, J.R.; Vennin, C.; Magenau, A.; Hughes, W.E.; Morton, J.P.; Timpson, P. Three-Dimensional Cancer Models Mimic Cell-Matrix Interactions in the Tumour Microenvironment. Carcinogenesis 2014, 35, 1671–1679. [Google Scholar] [CrossRef]
- Barbone, D.; Van Dam, L.; Follo, C.; Jithesh, P.V.; Zhang, S.D.; Richards, W.G.; Bueno, R.; Fennell, D.A.; Broaddus, V.C. Analysis of Gene Expression in 3D Spheroids Highlights a Survival Role for Ass1 in Mesothelioma. PLoS ONE 2016, 11, e0150044. [Google Scholar] [CrossRef] [PubMed]
- Ellero, A.A.; van den Bout, I.; Vlok, M.; Cromarty, A.D.; Hurrell, T. Continual Proteomic Divergence of Hepg2 Cells as a Consequence of Long-Term Spheroid Culture. Sci. Rep. 2021, 11, 10917. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. Multiplexing Spheroid Volume, Resazurin and Acid Phosphatase Viability Assays for High-Throughput Screening of Tumour Spheroids and Stem Cell Neurospheres. PLoS ONE 2014, 9, e103817. [Google Scholar] [CrossRef] [PubMed]
- Carragher, N.; Piccinini, F.; Tesei, A.; Trask, O.J., Jr.; Bickle, M.; Horvath, P. Concerns, Challenges and Promises of High-Content Analysis of 3d Cellular Models. Nat. Rev. Drug Discov. 2018, 17, 606. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; et al. Comparison of 2D- and 3D-Culture Models as Drug-Testing Platforms in Breast Cancer. Oncol. Rep. 2015, 33, 1837–1843. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic in Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef]
- Winnard, P.T., Jr.; Vesuna, F.; Muthukumar, S.; Raman, V. Divergent Organ-Specific Isogenic Metastatic Cell Lines Identified Using Multi-Omics Exhibit Differential Drug Sensitivity. PLoS ONE 2020, 15, e0242384. [Google Scholar] [CrossRef]
- Arafeh, R.; Shibue, T.; Dempster, J.M.; Hahn, W.C.; Vazquez, F. The Present and Future of the Cancer Dependency Map. Nat. Rev. Cancer 2025, 25, 59–73. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple Negative Breast Cancer Cell Lines: One Tool in the Search for Better Treatment of Triple Negative Breast Cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Holliday, D.L.; Speirs, V. Choosing the Right Cell Line for Breast Cancer Research. Breast Cancer Res. 2011, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Blondeel, E.; Peirsman, A.; Vermeulen, S.; Piccinini, F.; De Vuyst, F.; Estêvão, D.; Al-Jamei, S.; Bedeschi, M.; Castellani, G.; Cruz, T.; et al. The Spheroid Light Microscopy Image Atlas for Morphometrical Analysis of Three-Dimensional Cell Cultures. Sci. Data 2025, 12, 283. [Google Scholar] [CrossRef]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D Tumor Spheroid Models for in Vitro Therapeutic Screening: A Systematic Approach to Enhance the Biological Relevance of Data Obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Ham, S.L.; Joshi, R.; Luker, G.D.; Tavana, H. Engineered Breast Cancer Cell Spheroids Reproduce Biologic Properties of Solid Tumors. Adv. Healthc. Mater. 2016, 5, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.S.; Tevis, K.M.; Blessing, W.A.; Colson, Y.L.; Zaman, M.H.; Grinstaff, M.W. Breast Cancer Spheroids Reveal a Differential Cancer Stem Cell Response to Chemotherapeutic Treatment. Sci. Rep. 2017, 7, 10382. [Google Scholar] [CrossRef]
- Cougnoux, A.; Mahmoud, L.; Johnsson, P.A.; Eroglu, A.; Gsell, L.; Rosenbauer, J.; Sandberg, R.; Hausser, J. Diffusion Smart-Seq3 of Breast Cancer Spheroids to Explore Spatial Tumor Biology and Test Evolutionary Principles of Tumor Heterogeneity. Sci. Rep. 2025, 15, 3811. [Google Scholar] [CrossRef]
- Mahmoud, L.; Cougnoux, A.; Bekiari, C.; Araceli Ruiz de Castroviejo Teba, P.; El Marrahi, A.; Panneau, G.; Gsell, L.; Hausser, J. Microscopy-Based Phenotypic Monitoring of Mda-Mb-231 Spheroids Allows the Evaluation of Phenotype-Directed Therapy. Exp. Cell Res. 2023, 425, 113527. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Chen, Z.; Feng, Y.; Wang, J.; Li, T.; Li, S.; Qin, X.; Wu, C.; Zheng, C.; et al. Polymeric Hybrid Nanomicelles for Cancer Theranostics: An Efficient and Precise Anticancer Strategy for the Codelivery of Doxorubicin/Mir-34a and Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2019, 11, 43865–43878. [Google Scholar] [CrossRef]
- Ohori-Morita, Y.; Ashry, A.; Niibe, K.; Egusa, H. Current Perspectives on the Dynamic Culture of Mesenchymal Stromal/Stem Cell Spheroids. Stem Cells Transl. Med. 2025, 14, szae093. [Google Scholar] [CrossRef]
- Wicks, E.E.; Semenza, G.L. Hypoxia-Inducible Factors: Cancer Progression and Clinical Translation. J. Clin. Investig. 2022, 132, e159839. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, H.; Siddiqui, A.; Karadkar, S.; Tayalia, P. In Vitro 3D Spheroid Model Preserves Tumor Microenvironment of Hot and Cold Breast Cancer Subtypes. Adv. Healthc. Mater. 2023, 12, e2300164. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.H.; Wang, P.; Zhang, H.Q.; Fang, W.G.; Tian, X.X. Extracellular Atp Promotes Breast Cancer Chemoresistance Via Hif-1alpha Signaling. Cell Death Dis. 2022, 13, 199. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, C.; Liu, C.; Li, H.; Wu, J.; Sun, C. Targeting Hypoxia-Inducible Factor-1alpha: A New Strategy for Triple-Negative Breast Cancer Therapy. Biomed. Pharmacother. 2022, 156, 113861. [Google Scholar] [CrossRef]
- Bigos, K.J.; Quiles, C.G.; Lunj, S.; Smith, D.J.; Krause, M.; Troost, E.G.; West, C.M.; Hoskin, P.; Choudhury, A. Tumour Response to Hypoxia: Understanding the Hypoxic Tumour Microenvironment to Improve Treatment Outcome in Solid Tumours. Front. Oncol. 2024, 14, 1331355. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Chen, C.; Huang, H.; Zhang, Z.; Zeng, F.; Zhang, S. Hypoxia-Inducible Factor in Breast Cancer: Role and Target for Breast Cancer Treatment. Front. Immunol. 2024, 15, 1370800. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, X.; Li, J.; Liu, W.; Pan, G.; Mao, A.; Liu, J.; Zhang, Q.; Rao, L.; Xie, X.; et al. Hypoxia Induces Docetaxel Resistance in Triple-Negative Breast Cancer Via the Hif-1α/Mir-494/Survivin Signaling Pathway. Neoplasia 2022, 32, 100821. [Google Scholar] [CrossRef]
- Shang, T.; Jia, Z.; Li, J.; Cao, H.; Xu, H.; Cong, L.; Ma, D.; Wang, X.; Liu, J. Unraveling the Triad of Hypoxia, Cancer Cell Stemness, and Drug Resistance. J. Hematol. Oncol. 2025, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Usmani, S.S.; Subbarayan, R.; Saini, R.; Pandey, P.K. Hypoxia: Syndicating Triple Negative Breast Cancer against Various Therapeutic Regimens. Front. Oncol. 2023, 13, 1199105. [Google Scholar] [CrossRef]
- Dekker, Y.; Le Dévédec, S.E.; Danen, E.H.J.; Liu, Q. Crosstalk between Hypoxia and Extracellular Matrix in the Tumor Microenvironment in Breast Cancer. Genes 2022, 13, 1585. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, Y.; Han, Y.; Cao, C.; Zhang, Z.; Li, L.; Xiao, C.; Guo, H.; Wang, L.; Han, L.; et al. Extracellular Matrix Physical Properties Govern the Diffusion of Nanoparticles in Tumor Microenvironment. Proc. Natl. Acad. Sci. USA 2023, 120, e2209260120. [Google Scholar] [CrossRef]
- Tezcan, O.; Elshafei, A.S.; Benderski, K.; Rama, E.; Wagner, M.; Moeckel, D.; Pola, R.; Pechar, M.; Etrych, T.; von Stillfried, S.; et al. Effect of Cellular and Microenvironmental Multidrug Resistance on Tumor-Targeted Drug Delivery in Triple-Negative Breast Cancer. J. Control. Release 2023, 354, 784–793. [Google Scholar] [CrossRef]
- Bae, I.Y.; Choi, W.; Oh, S.J.; Kim, C.; Kim, S.H. Timp-1-Expressing Breast Tumor Spheroids for the Evaluation of Drug Penetration and Efficacy. Bioeng. Transl. Med. 2022, 7, e10286. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Nishita, M. Integrins in Cancer Drug Resistance: Molecular Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 3143. [Google Scholar] [CrossRef] [PubMed]
- Lovitt, C.J.; Shelper, T.B.; Avery, V.M. Doxorubicin Resistance in Breast Cancer Cells Is Mediated by Extracellular Matrix Proteins. BMC Cancer 2018, 18, 41. [Google Scholar] [CrossRef]
- Botes, M.; Jurgens, T.; Riahi, Z.; Visagie, M.; van Janse Vuuren, R.; Joubert, A.M.; van den Bout, I. A Novel Non-Sulphamoylated 2-Methoxyestradiol Derivative Causes Detachment of Breast Cancer Cells by Rapid Disassembly of Focal Adhesions. Cancer Cell Int. 2018, 18, 188. [Google Scholar] [CrossRef]
- Ferreira, L.P.; Gaspar, V.M.; Mendes, L.; Duarte, I.F.; Mano, J.F. Organotypic 3D Decellularized Matrix Tumor Spheroids for High-Throughput Drug Screening. Biomaterials 2021, 275, 120983. [Google Scholar] [CrossRef]
- Kim, D.; Yu, Y.; Jung, K.S.; Kim, Y.H.; Kim, J.J. Tumor Microenvironment Can Predict Chemotherapy Response of Patients with Triple-Negative Breast Cancer Receiving Neoadjuvant Chemotherapy. Cancer Res. Treat. 2024, 56, 162–177. [Google Scholar] [CrossRef]
- Chuangchot, N.; Jamjuntra, P.; Yangngam, S.; Luangwattananun, P.; Thongchot, S.; Junking, M.; Thuwajit, P.; Yenchitsomanus, P.T.; Thuwajit, C. Enhancement of Pd-L1-Attenuated Car-T Cell Function through Breast Cancer-Associated Fibroblasts-Derived Il-6 Signaling Via Stat3/Akt Pathways. Breast Cancer Res. 2023, 25, 86. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.L.; Thakuri, P.S.; Plaster, M.; Li, J.; Luker, K.E.; Luker, G.D.; Tavana, H. Three-Dimensional Tumor Model Mimics Stromal - Breast Cancer Cells Signaling. Oncotarget 2018, 9, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Emami, F.; Banstola, A.; Jeong, J.-H.; Yook, S. Cetuximab-Anchored Gold Nanorod Mediated Photothermal Ablation of Breast Cancer Cell in Spheroid Model Embedded with Tumor Associated Macrophage. J. Ind. Eng. Chem. 2022, 106, 177–188. [Google Scholar] [CrossRef]
- Tevis, K.M.; Cecchi, R.J.; Colson, Y.L.; Grinstaff, M.W. Mimicking the Tumor Microenvironment to Regulate Macrophage Phenotype and Assessing Chemotherapeutic Efficacy in Embedded Cancer Cell/Macrophage Spheroid Models. Acta Biomater. 2017, 50, 271–279. [Google Scholar] [CrossRef]
- Emami, F.; Pathak, S.; Nguyen, T.T.; Shrestha, P.; Maharjan, S.; Kim, J.O.; Jeong, J.H.; Yook, S. Photoimmunotherapy with Cetuximab-Conjugated Gold Nanorods Reduces Drug Resistance in Triple Negative Breast Cancer Spheroids with Enhanced Infiltration of Tumor-Associated Macrophages. Cancer Cell Int. 2021, 329, 645–664. [Google Scholar] [CrossRef]
- Modi, A.; Roy, D.; Sharma, S.; Vishnoi, J.R.; Pareek, P.; Elhence, P.; Sharma, P.; Purohit, P. Abc Transporters in Breast Cancer: Their Roles in Multidrug Resistance and Beyond. J. Drug Target. 2022, 30, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Goisnard, A.; Daumar, P.; Dubois, C.; Aubel, C.; Roux, M.; Depresle, M.; Gauthier, J.; Vidalinc, B.; Penault-Llorca, F.; Mounetou, E.; et al. Lightspot((R))-Fl-1 Fluorescent Probe: An Innovative Tool for Cancer Drug Resistance Analysis by Direct Detection and Quantification of the P-Glycoprotein (P-Gp) on Monolayer Culture and Spheroid Triple Negative Breast Cancer Models. Cancers 2021, 13, 4050. [Google Scholar] [CrossRef]
- Foutadakis, S.; Kordias, D.; Vatsellas, G.; Magklara, A. Identification of New Chemoresistance-Associated Genes in Triple-Negative Breast Cancer by Single-Cell Transcriptomic Analysis. Int. J. Mol. Sci. 2024, 25, 6853. [Google Scholar] [CrossRef]
- Kumar, P.; Salve, R.; Paknikar, K.M.; Gajbhiye, V. Nucleolin Aptamer Conjugated Msnps-Plr-Peg Multifunctional Nanoconstructs for Targeted Co-Delivery of Anticancer Drug and Sirna to Counter Drug Resistance in Tnbc. Int. J. Biol. Macromol. 2023, 229, 600–614. [Google Scholar] [CrossRef]
- De Blasio, A.; Pratelli, G.; Drago-Ferrante, R.; Saliba, C.; Baldacchino, S.; Grech, G.; Tesoriere, G.; Scerri, C.; Vento, R.; Di Fiore, R. Loss of Mcl1 Function Sensitizes the Mda-Mb-231 Breast Cancer Cells to Rh-Trail by Increasing Dr4 Levels. J. Cell. Physiol. 2019, 234, 18432–18447. [Google Scholar] [CrossRef]
- Man, K.F.; Darweesh, O.; Hong, J.; Thompson, A.; O’Connor, C.; Bonaldo, C.; Melkonyan, M.N.; Sun, M.; Patel, R.; Ellisen, L.W.; et al. Creb1-Bcl2 Drives Mitochondrial Resilience in Ras Gap-Dependent Breast Cancer Chemoresistance. Oncogene 2025, 44, 1093–1105. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Marshall, J.R.; Messing, J.A.; Hsu, J.W.; King, M.R. Trail-Mediated Apoptosis in Breast Cancer Cells Cultured as 3D Spheroids. PLoS ONE 2014, 9, e111487. [Google Scholar] [CrossRef]
- McBean, B.; Abou Zeidane, R.; Lichtman-Mikol, S.; Hauk, B.; Speers, J.; Tidmore, S.; Flores, C.L.; Rana, P.S.; Pisano, C.; Liu, M.; et al. Melk as a Mediator of Stemness and Metastasis in Aggressive Subtypes of Breast Cancer. Int. J. Mol. Sci. 2025, 26, 2245. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.B.; Wright, T.D.; Barnes, V.; Chakrabarty, S.; Matossian, M.D.; Lexner, E.; Ucar, D.A.; Miele, L.; Flaherty, P.T.; Burow, M.E.; et al. Diverse and Converging Roles of Erk1/2 and Erk5 Pathways on Mesenchymal to Epithelial Transition in Breast Cancer. Transl. Oncol. 2021, 14, 101046. [Google Scholar] [CrossRef]
- Talukdar, P.D.; Pramanik, K.; Gatti, P.; Mukherjee, P.; Ghosh, D.; Roy, H.; Germain, M.; Chatterji, U. Precise Targeting of Transcriptional Co-Activators Yap/Taz Annihilates Chemoresistant Brcscs by Alteration of Their Mitochondrial Homeostasis. Signal Transduct. Target. Ther. 2025, 10, 61. [Google Scholar] [CrossRef]
- Ramamoorthy, P.; Dandawate, P.; Jensen, R.A.; Anant, S. Celastrol and Triptolide Suppress Stemness in Triple Negative Breast Cancer: Notch as a Therapeutic Target for Stem Cells. Biomedicines 2021, 9, 482. [Google Scholar] [CrossRef]
- Moreira, M.P.; Franco, E.P.; Barros, B.A.F.; Anjos, B.R.D.; Almada, D.G.; Barbosa, I.N.T.; Braga, L.D.C.; Cassali, G.D.; Silva, L.M. Standard Chemotherapy Impacts on in Vitro Cellular Heterogeneity in Spheroids Enriched with Cancer Stem Cells (Cscs) Derived from Triple-Negative Breast Cancer Cell Line. Biochem. Biophys. Res. Commun. 2024, 734, 150765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, R.; Zhang, J. Usp22 Contributes to Chemoresistance, Stemness, and Emt Phenotype of Triple-Negative Breast Cancer Cells by Egulating the Warburg Effect Via C-Myc Deubiquitination. Clin. Breast Cancer 2023, 23, 162–175. [Google Scholar] [CrossRef]
- Wang, W.; Rana, P.S.; Markovic, V.; Sossey-Alaoui, K. The Wave3/Beta-Catenin Oncogenic Signaling Regulates Chemoresistance in Triple Negative Breast Cancer. Breast Cancer Res. 2023, 25, 31. [Google Scholar] [CrossRef]
- Nigjeh, S.E.; Yeap, S.K.; Nordin, N.; Kamalideghan, B.; Ky, H.; Rosli, R. Citral Induced Apoptosis in Mda-Mb-231 Spheroid Cells. BMC Complement. Med. Ther. 2018, 18, 56. [Google Scholar] [CrossRef]
- Venkatesh, J.; Rishi, A.K.; Reddy, K.B. Novel Strategies to Target Chemoresistant Triple-Negative Breast Cancer. Genes. Cancer 2020, 11, 95–105. [Google Scholar] [CrossRef]
- Bhatavdekar, O.; Godet, I.; Gilkes, D.; Sofou, S. The Rate of Cisplatin Dosing Affects the Resistance and Metastatic Potential of Triple Negative Breast Cancer Cells, Independent of Hypoxia. Pharmaceutics 2022, 14, 2184. [Google Scholar] [CrossRef] [PubMed]
- Sudhakaran, M.; Parra, M.R.; Stoub, H.; Gallo, K.A.; Doseff, A.I. Apigenin by Targeting Hnrnpa2 Sensitizes Triple-Negative Breast Cancer Spheroids to Doxorubicin-Induced Apoptosis and Regulates Expression of Abcc4 and Abcg2 Drug Efflux Transporters. Biochem. Pharmacol. 2020, 182, 114259. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Dong, W.; Xu, Y.; Li, L.; Yu, X.; Pang, Y.; Chan, L.; Deng, Y.; Qian, C. Targeting Mitochondrial Dynamics by Azd5363 in Triple-Negative Breast Cancer Mda-Mb-231 Cell-Derived Spheres. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2545–2553. [Google Scholar] [CrossRef]
- Lee, J.; Galloway, R.; Grandjean, G.; Jacob, J.; Humphries, J.; Bartholomeusz, C.; Goodstal, S.; Lim, B.; Bartholomeusz, G.; Ueno, N.T.; et al. Comprehensive Two- and Three-Dimensional Rnai Screening Identifies Pi3k Inhibition as a Complement to Mek Inhibitor As703026 for Combination Treatment of Triple-Negative Breast Cancer. J. Cancer 2015, 6, 1306–1319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Z.; Tang, Y.; Liu, Y.; Chen, Z.; Feng, Y.; Hu, H.; Liu, H.; Chen, G.; Lu, Y.; Hu, Y.; et al. Co-Delivery of Fucoxanthin and Twist Sirna Using Hydroxyethyl Starch-Cholesterol Self-Assembled Polymer Nanoparticles for Triple-Negative Breast Cancer Synergistic Therapy. J. Adv. Res. 2025, 70, 463–479. [Google Scholar] [CrossRef]
- Edvall, C.; Kale, N.; Tani, S.; Ambhore, S.; Hossain, R.; Ozoude, C.; Van Horsen, K.; Mohammad, J.; Tuvin, D.M.; Kalathingal, S.; et al. Hypoxia-Responsive Polymersomes for Stemness Reduction in Patient-Derived Solid Tumor Spheroids. ACS Appl. Bio Mater. 2025, 8, 2916–2926. [Google Scholar] [CrossRef]
- Rad, S.K.; Yeo, K.K.L.; Li, R.; Wu, F.; Liu, S.; Nourmohammadi, S.; Murphy, W.M.; Tomita, Y.; Price, T.J.; Ingman, W.V.; et al. Enhancement of Doxorubicin Efficacy by Bacopaside Ii in Triple-Negative Breast Cancer Cells. Biomolecules 2025, 15, 55. [Google Scholar] [CrossRef]
- Sambi, M.; Samuel, V.; Qorri, B.; Haq, S.; Burov, S.V.; Markvicheva, E.; Harless, W.; Szewczuk, M.R. A Triple Combination of Metformin, Acetylsalicylic Acid, and Oseltamivir Phosphate Impacts Tumour Spheroid Viability and Upends Chemoresistance in Triple-Negative Breast Cancer. Drug Des. Devel. Ther. 2020, 14, 1995–2019. [Google Scholar] [CrossRef]
- Azizipour, N.; Avazpour, R.; Weber, M.H.; Sawan, M.; Ajji, A.; Rosenzweig, D.H. Uniform Tumor Spheroids on Surface-Optimized Microfluidic Biochips for Reproducible Drug Screening and Personalized Medicine. Micromachines 2022, 13, 587. [Google Scholar] [CrossRef] [PubMed]
- Niepel, M.; Hafner, M.; Duan, Q.; Wang, Z.; Paull, E.O.; Chung, M.; Lu, X.; Stuart, J.M.; Golub, T.R.; Subramanian, A.; et al. Common and Cell-Type Specific Responses to Anti-Cancer Drugs Revealed by High Throughput Transcript Profiling. Nat. Commun. 2017, 8, 1186. [Google Scholar] [CrossRef] [PubMed]
- Wrzesinski, K.; Frandsen, H.S.; Calitz, C.; Gouws, C.; Korzeniowska, B.; Fey, S.J. Clinostat 3D Cell Culture: Protocols for the Preparation and Functional Analysis of Highly Reproducible, Large, Uniform Spheroids and Organoids. Methods Mol. Biol. 2021, 2273, 17–62. [Google Scholar] [CrossRef]
- Gupta, S.K.; Torrico Guzman, E.A.; Meenach, S.A. Coadministration of a Tumor-Penetrating Peptide Improves the Therapeutic Efficacy of Paclitaxel in a Novel Air-Grown Lung Cancer 3D Spheroid Model. Int. J. Cancer 2017, 141, 2143–2153. [Google Scholar] [CrossRef]
- Fevre, R.; Mary, G.; Vertti-Quintero, N.; Durand, A.; Tomasi, R.F.; Del Nery, E.; Baroud, C.N. Combinatorial Drug Screening on 3d Ewing Sarcoma Spheroids Using Droplet-Based Microfluidics. iScience 2023, 26, 106651. [Google Scholar] [CrossRef]
- Daster, S.; Amatruda, N.; Calabrese, D.; Ivanek, R.; Turrini, E.; Droeser, R.A.; Zajac, P.; Fimognari, C.; Spagnoli, G.C.; Iezzi, G.; et al. Induction of Hypoxia and Necrosis in Multicellular Tumor Spheroids Is Associated with Resistance to Chemotherapy Treatment. Oncotarget 2017, 8, 1725–1736. [Google Scholar] [CrossRef]
- Wrzesinski, K.; Alnøe, S.; Jochumsen, H.H.; Mikkelsen, K.; Bryld, T.D.; Vistisen, J.S.; Alnøe, P.W.; Fey, S.J. A Purpose-Built System for Culturing Cells as in Vivo Mimetic 3D Structures. In Biomechanics and Functional Tissue Engineering; Haidar, Z.S., Abdurakhmonov, I.Y., Barkaoui, A., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Kim, C.; Zhu, Z.; Barbazuk, W.B.; Bacher, R.L.; Vulpe, C.D. Time-Course Characterization of Whole-Transcriptome Dynamics of Hepg2/C3a Spheroids and Its Toxicological Implications. Toxicol. Lett. 2024, 401, 125–138. [Google Scholar] [CrossRef]
- Ngalonkulu, O. Establishing a Cisplatin-Resistant Triple Negative Breast Cancer Spheroid Model. Master’s Thesis, University of the Free State, Bloemfontein, South Africa, 2024. [Google Scholar]
- Tajaldini, M.; Saeedi, M.; Amiriani, T.; Amiriani, A.H.; Sedighi, S.; Mohammad Zadeh, F.; Dehghan, M.; Jahanshahi, M.; Zanjan Ghandian, M.; Khalili, P.; et al. Cancer-Associated Fibroblasts (Cafs) and Tumor-Associated Macrophages (Tams); Where Do They Stand in Tumorigenesis and How They Can Change the Face of Cancer Therapy? Eur. J. Pharmacol. 2022, 928, 175087. [Google Scholar] [CrossRef]
- Wan, Z.; Floryan, M.A.; Coughlin, M.F.; Zhang, S.; Zhong, A.X.; Shelton, S.E.; Wang, X.; Xu, C.; Barbie, D.A.; Kamm, R.D. New Strategy for Promoting Vascularization in Tumor Spheroids in a Microfluidic Assay. Adv. Heal. Mater. 2023, 12, e2201784. [Google Scholar] [CrossRef]
- Yakavets, I.; Jenard, S.; Francois, A.; Maklygina, Y.; Loschenov, V.; Lassalle, H.P.; Dolivet, G.; Bezdetnaya, L. Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening. J. Clin. Med. 2019, 8, 1686. [Google Scholar] [CrossRef]
- Desigaux, T.; Comperat, L.; Dusserre, N.; Stachowicz, M.L.; Lea, M.; Dupuy, J.W.; Vial, A.; Molinari, M.; Fricain, J.C.; Paris, F.; et al. 3D Bioprinted Breast Cancer Model Reveals Stroma-Mediated Modulation of Extracellular Matrix and Radiosensitivity. Bioact. Mater. 2024, 42, 316–327. [Google Scholar] [CrossRef]
- Hong, S.; Song, J.M. 3D Bioprinted Drug-Resistant Breast Cancer Spheroids for Quantitative in Situ Evaluation of Drug Resistance. Acta Biomater. 2022, 138, 228–239. [Google Scholar] [CrossRef]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An Overview of Extrusion-Based Bioprinting with a Focus on Induced Shear Stress and Its Effect on Cell Viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Zandrini, T.; Florczak, S.; Levato, R.; Ovsianikov, A. Breaking the Resolution Limits of 3D Bioprinting: Future Opportunities and Present Challenges. Trends Biotechnol. 2023, 41, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Singh, Y.P.; Celik, N.; Yeo, M.; Rizk, E.; Hayes, D.J.; Ozbolat, I.T. High-Throughput Bioprinting of Spheroids for Scalable Tissue Fabrication. Nat. Commun. 2024, 15, 10083. [Google Scholar] [CrossRef]
- Jamieson, L.; Harrison, D.J.; Campbell, C. Chemical Analysis of Multicellular Tumour Spheroids. Analyst 2015, 140, 3910–3920. [Google Scholar] [CrossRef]
- Marx, V. Method of the Year: Spatially Resolved Transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef]
- Ning, K.; Xie, Y.; Sun, W.; Feng, L.; Fang, C.; Pan, R.; Li, Y.; Yu, L. Non-Destructive in Situ Monitoring of Structural Changes of 3d Tumor Spheroids During the Formation, Migration, and Fusion Process. eLife 2025, 13, RP101886. [Google Scholar] [CrossRef]
- Niora, M.; Dufva, M.; Jauffred, L.; Berg-Sørensen, K. Tumor Spheroid Uptake of Fluorescent Nanodiamonds Is Limited by Mass Density: A 4d Light-Sheet Assay. Chem. Biomed. Imaging 2025, 3, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Charlet-Faure, C.; Thulesen, A.P.; Rogowska-Wrzesinska, A. Advancements in 3d Spheroid Imaging: Optimised Cryosectioning and Immunostaining Techniques. MethodsX 2023, 11, 102415. [Google Scholar] [CrossRef]
- Edwards, S.J.; Carannante, V.; Kuhnigk, K.; Ring, H.; Tararuk, T.; Hallbook, F.; Blom, H.; Onfelt, B.; Brismar, H. High-Resolution Imaging of Tumor Spheroids and Organoids Enabled by Expansion Microscopy. Front. Mol. Biosci. 2020, 7, 208. [Google Scholar] [CrossRef]
- Diosdi, A.; Piccinini, F.; Boroczky, T.; Dobra, G.; Castellani, G.; Buzas, K.; Horvath, P.; Harmati, M. Single-Cell Light-Sheet Fluorescence 3d Images of Tumour-Stroma Spheroid Multicultures. Sci. Data 2025, 12, 492. [Google Scholar] [CrossRef]
- Lopez, A.; Holbrook, J.H.; Kemper, G.E.; Lukowski, J.K.; Andrews, W.T.; Hummon, A.B. Tracking Drugs and Lipids: Quantitative Mass Spectrometry Imaging of Liposomal Doxorubicin Delivery and Bilayer Fate in Three-Dimensional Tumor Models. Anal. Chem. 2024, 96, 9254–9261. [Google Scholar] [CrossRef]
- Liu, Y.; Johnson, J.; Zhang, H.; Huang, P.; Li, L. Direct Transfer of Multicellular Tumor Spheroids Grown in Agarose Microarrays for High-Throughput Mass Spectrometry Imaging Analysis. Anal. Bioanal. Chem. 2025, 417, 3021–3031. [Google Scholar] [CrossRef]
- Wissmann, R.; Martirosian, P.; Danalache, M.; Grozinger, G.; Schick, F.; Elser, S. Imaging Cell Spheroid Clusters: An Mri Protocol for Non-Invasive Standardized Characterization. Heliyon 2025, 11, e41803. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Perez, P.; Xiao, C.; Sanles-Sobrido, M.; Rovira-Esteva, M.; Conesa, J.J.; Mulens-Arias, V.; Jaque, D.; Rivera-Gil, P. Multiphoton Imaging of Melanoma 3d Models with Plasmonic Nanocapsules. Acta Biomater. 2022, 142, 308–319. [Google Scholar] [CrossRef]
- Zhong, X.; Gao, C.; Li, H.; He, Y.; Fei, P.; Chen, Z.; Gu, Z.; Zhu, D.; Yu, T. Macs-W: A Modified Optical Clearing Agent for Imaging 3D Cell Cultures. J. Innov. Opt. Health Sci. 2023, 17, 2350018. [Google Scholar] [CrossRef]
- Hari, N.; Patel, P.; Ross, J.; Hicks, K.; Vanholsbeeck, F. Optical Coherence Tomography Complements Confocal Microscopy for Investigation of Multicellular Tumour Spheroids. Sci. Rep. 2019, 9, 10601. [Google Scholar] [CrossRef] [PubMed]
- Beller, N.C.; Lukowski, J.K.; Ludwig, K.R.; Hummon, A.B. Spatial Stable Isotopic Labeling by Amino Acids in Cell Culture: Pulse-Chase Labeling of Three-Dimensional Multicellular Spheroids for Global Proteome Analysis. Anal. Chem. 2021, 93, 15990–15999. [Google Scholar] [CrossRef]
| Pathway | Pathway Function | Mechanism of Resistance | Model Used | Reference |
|---|---|---|---|---|
| ERK1/2 and ERK5 | Regulates the EMT and survival | ERK5 activation regulates the survival of anoikis-resistant spheroids, contributing to chemoresistance. | Spheroids (MDA-MB-231, BT-549) | [86] |
| Hippo (YAP/TAZ) | Promotes tissue-specific progenitor cells during renewal and regeneration and facilitates cell proliferation | YAP/TAZ maintains stemness properties, regulates redox homeostasis, and modulates mitochondrial dynamics, leading to chemoresistance to paclitaxel. | Mammospheres (MDA-MB-231, MDA-MB-468, and 4T1) | [87] |
| Notch1 | Maintains a CSC phenotype | Notch signalling promotes resistance to targeted or cytotoxic therapies by enriching a small population of CSCs. | Mammospheres (MDA-MB-231, BT20) | [88] |
| STAT3 | Maintains stemness | STAT3 enhances CSC survival and promotes chemoresistance to doxorubicin. | Mammospheres (BT-549) | [89] |
| USP22 | Promotes glycolysis, the EMT, and CSC traits | USP22 promotes glycolysis via c-Myc deubiquitination, which enhances stemness and the EMT phenotype, leading to chemoresistance. | Mammospheres (BT-549, MDA-MB-231) | [90] |
| WAVE3/β-catenin | Stabilises β-catenin; sustains CSC survival | WAVE3 prevents β-catenin degradation and maintains stemness after exposure to cisplatin, doxorubicin, and paclitaxel. | Mammospheres (MDA-MB-231) | [91] |
| Wnt/β-catenin | Is involved in CSC maintenance and cell proliferation | Hyperactive Wnt signalling and the downregulation of tumour suppressor genes cause high levels of self-renewal and dysregulated proliferation, leading to chemoresistance. | Spheroids (MDA-MB-231) | [92] |
| Wnt/FZD8 | Is involved in CSC maintenance and growth | Wnt signalling through the FZD8 and LRP6 receptors leads to the enrichment of cisplatin-resistant CSCs. | Mammospheres (MDA-MB-468, MDA-MB-231, CRL-2335) | [93] |
| Chemoresistance Mechanism | Intervention Drug(s) | Resensitisation Mechanism | Reference(s) |
|---|---|---|---|
| Limited diffusion | Doxorubicin and miR-34a-loaded hybrid micelles | Improved drug penetration and distribution throughout the spheroids. | [53] |
| Hypoxia | TH-302 (hypoxia-activated prodrug) | TH-302 is activated in hypoxic regions, releasing a DNA crosslinker that targets doxorubicin-resistant hypoxic cells. | [49] |
| Spatial heterogeneity | Paclitaxel, everolimus, trametinib | This combination targets cells with multiple phenotypes. | [52] |
| ECM remodelling | Fucoxanthin and twist siRNA (siTwist) | Reduces the deposition of collagen, leading to better drug penetration. | [98] |
| Tumour crosstalk (TAMs) | Cetuximab-conjugated gold nanorods + NIR irradiation | Causes polarisation of pro-tumoural TAMs (M2-like) to an antitumoural phenotype (M1-like) | [75] |
| Tumour crosstalk (CAFs) | Fucoxanthin and siTwist | Targeting the Twist gene (important for CAF activation) and using FX (multi-target effects) resensitise the tumour microenvironment. | [98] |
| Drug efflux | Doxorubicin and ATRA | ATRA inhibits efflux pumps, leading to an increased intracellular doxorubicin concentration. | [99] |
| Bacopaside II | Bacopaside II increases intracellular doxorubicin accumulation by inhibiting ABC transporters like ABCC3, which are overexpressed in resistant TNBC spheroids. | [100] | |
| Resistance to apoptosis | Apigenin and doxorubicin | Apigenin sensitises TNBC spheroids to doxorubicin-induced apoptosis by triggering DNA damage, activating the caspase-9-mediated intrinsic apoptotic pathway, and increasing caspase-3 activity. | [95] |
| CSCs and signalling | ASA + metformin + oseltamivir phosphate | The reduction in the CD44/CD24 ratio and ALDH1A1 expression reverses stemness. | [101] |
| PD0325901 (MEK/ERK inhibitor) PI-103 (PI3K/AKT inhibitor) | Inhibition of MAPK and PI3K activation reverses paclitaxel resistance in spheroids. | [74] |
| Technique | Applications | Principle | Reference(s) |
|---|---|---|---|
| Consecutive Cryosectioning | Immunofluorescence imaging of spheroids | Spheroids are embedded into a freezing medium, frozen, and sectioned into thin slices using a cryotome; this enables high-resolution imaging of the internal architecture with improved section integrity and a reduced layer overlap. | [123] |
| Expansion Microscopy | Nanoscale-resolution imaging of tumour spheroids | Spheroids are embedded into a swellable polymer gel, enzymatically digested the sample, and physically expanded to achieve super-resolution imaging with conventional microscopy. | [124] |
| Light-Sheet Fluorescence Microscopy | Three-dimensional imaging of large spheroids | Spheroids are illuminated with a thin sheet of light for optical sectioning. Each plane is captured with a camera to rapidly acquire high-resolution volumetric fluorescence images with minimal photobleaching. | [125] |
| MALDI MSI | Spatial metabolomic/lipidomic profiling | Matrix-assisted laser desorption/ionisation is performed on thin spheroid sections; enhanced MALDI with trapped ion mobility is used to obtain high-resolution maps of lipids and metabolites across the spheroid. | [126,127] |
| MRI | Non-invasive, label-free 3D characterisation of spheroid clusters | Used 3T MRI with quantitative mapping is used to assess spheroids’ structure, viability, and extracellular matrix composition over time without disrupting the sample. | [128] |
| Multiphoton Microscopy | Multimodal imaging and therapeutic monitoring | The two-photon luminescence and X-ray contrast properties of plasmonic nanocapsules with gold nanoislands and fluorescent payloads are used to image spheroids. | [129] |
| Optical Clearing | Three-dimensional imaging of large spheroids | Reduces light scattering by matching the refractive indices, allowing deeper and clearer imaging of spheroids. | [130] |
| Optical Coherence Tomography | Label-free 3D live imaging of spheroids | Uses low-coherence interferometry to generate cross-sectional images of the spheroids. | [131] |
| Serial Trypsinisation | Spatial proteomics, transcriptomics, and metabolomics | Enzymatically disassociates various layers in spheroids, which can be isolated and analysed using downstream assays. | [132] |
| Diffusion Smart-seq3 | Spatial single-cell transcriptomics in spheroids | Diffuses dye into the spheroid, labelling cells by their radial position; sorted cells undergo deep Smart-seq3xpress single-cell RNA-seq to map the gene expression from the core to the periphery. | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ncube, K.N.; van den Bout, I.; Willers, C.; Gouws, C.; Cordier, W. Utility of Multicellular Spheroids for Investigating Mechanisms of Chemoresistance in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2025, 26, 7503. https://doi.org/10.3390/ijms26157503
Ncube KN, van den Bout I, Willers C, Gouws C, Cordier W. Utility of Multicellular Spheroids for Investigating Mechanisms of Chemoresistance in Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2025; 26(15):7503. https://doi.org/10.3390/ijms26157503
Chicago/Turabian StyleNcube, Keith N., Iman van den Bout, Clarissa Willers, Chrisna Gouws, and Werner Cordier. 2025. "Utility of Multicellular Spheroids for Investigating Mechanisms of Chemoresistance in Triple-Negative Breast Cancer" International Journal of Molecular Sciences 26, no. 15: 7503. https://doi.org/10.3390/ijms26157503
APA StyleNcube, K. N., van den Bout, I., Willers, C., Gouws, C., & Cordier, W. (2025). Utility of Multicellular Spheroids for Investigating Mechanisms of Chemoresistance in Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 26(15), 7503. https://doi.org/10.3390/ijms26157503









