Pathophysiology of Status Epilepticus Revisited
Abstract
1. Introduction
2. Definition and Classification of Seizure Activity
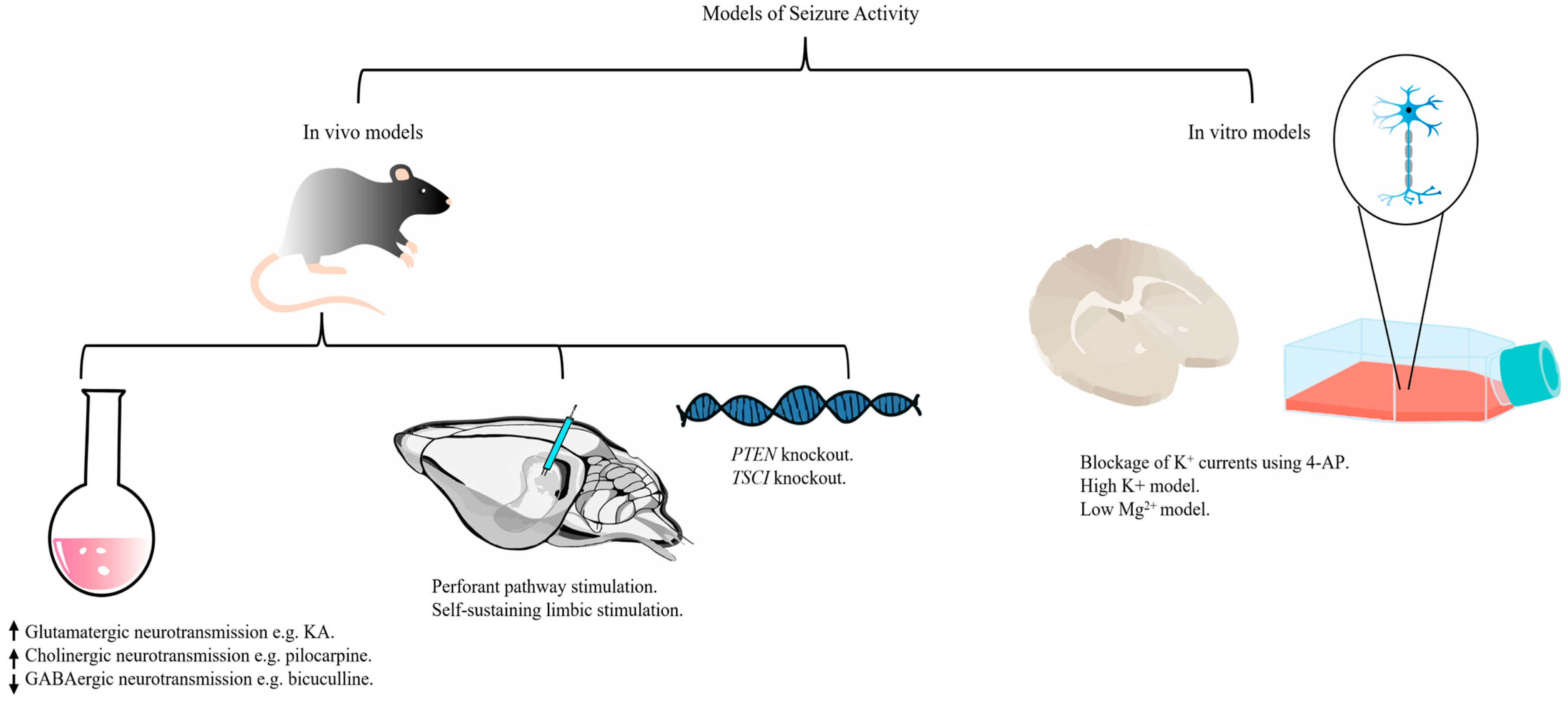
3. Models of Seizure Activity
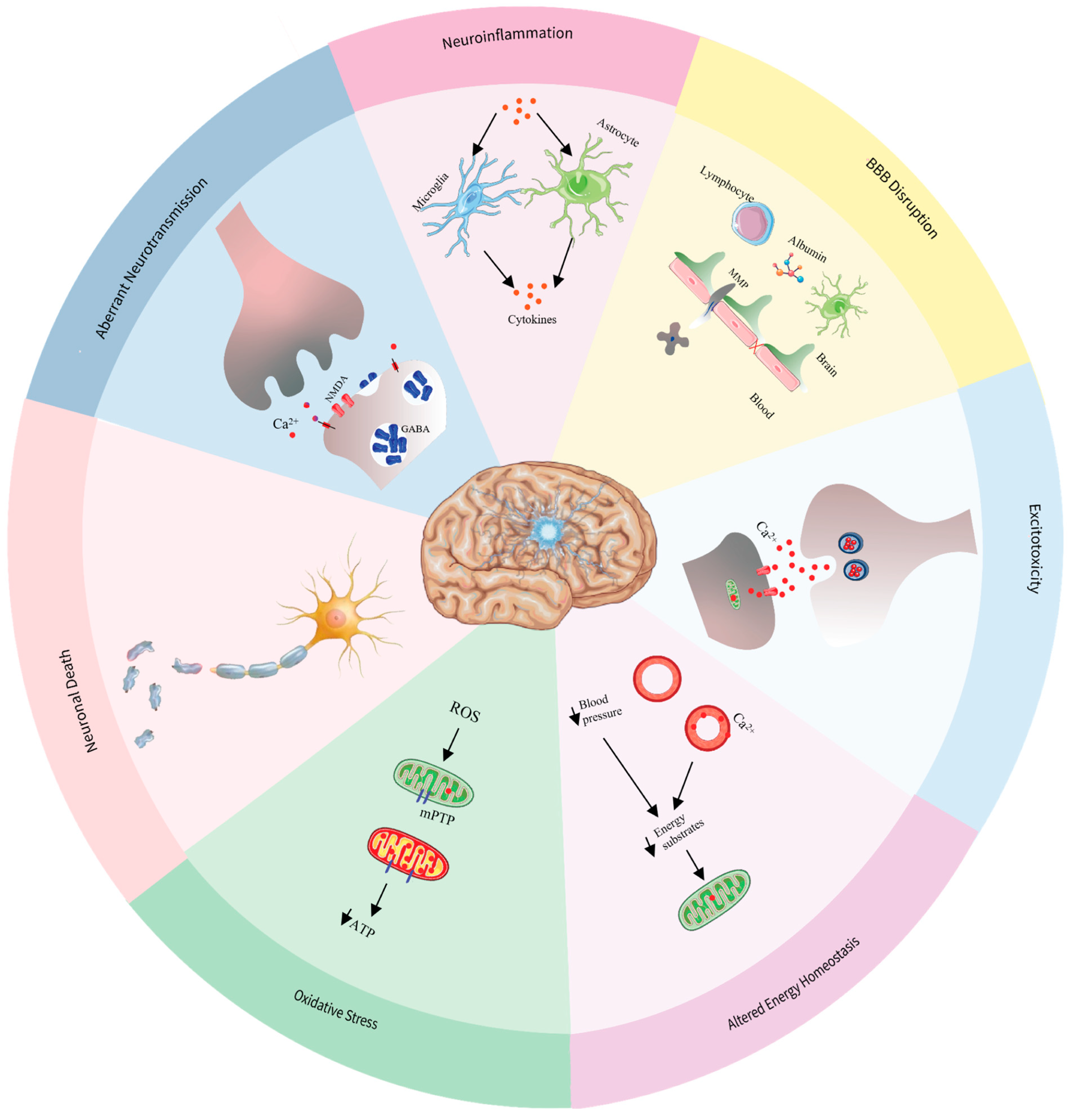
4. Pathophysiology of Status Epilepticus
4.1. Aberrant Neurotransmission
4.2. Neuroinflammation
4.3. Altered Energy Homeostasis
4.4. Excitotoxicity
4.5. Oxidative Stress and Mitochondrial Dysfunction
4.6. Neuronal Death
5. Mechanisms of Epileptogenesis
5.1. Aberrant Neurogenesis
5.2. Mossy Fiber Sprouting
5.3. Neuronal Death
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A Definition and Classification of Status Epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef]
- Betjemann, J.P.; Lowenstein, D.H. Status Epilepticus in Adults. Lancet Neurol. 2015, 14, 615–624. [Google Scholar] [CrossRef]
- Gutiérrez-Viedma, Á.; Parejo-Carbonell, B.; Romeral-Jiménez, M.; Sanz-Graciani, I.; Serrano-García, I.; Cuadrado, M.-L.; García-Morales, I. Therapy Delay in Status Epilepticus Extends Its Duration and Worsens Its Prognosis. Acta Neurol. Scand. 2021, 143, 281–289. [Google Scholar] [CrossRef]
- Dingledine, R.; Varvel, N.H.; Dudek, F.E. When and How Do Seizures Kill Neurons, and Is Cell Death Relevant to Epileptogenesis? In Issues in Clinical Epileptology: A View from the Bench; Scharfman, H.E., Buckmaster, P.S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 109–122. [Google Scholar] [CrossRef]
- Nelson, S.E.; Varelas, P.N. Status Epilepticus, Refractory Status Epilepticus, and Super-Refractory Status Epilepticus. Contin. Lifelong Learn. Neurol. 2018, 24, 1683. [Google Scholar] [CrossRef]
- Ascoli, M.; Ferlazzo, E.; Gasparini, S.; Mastroianni, G.; Citraro, R.; Roberti, R.; Russo, E. Epidemiology and Outcomes of Status Epilepticus. Int. J. Gen. Med. 2021, 14, 2965–2973. [Google Scholar] [CrossRef]
- Gao, Q.; Ou-Yang, T.; Sun, X.; Yang, F.; Wu, C.; Kang, T.; Kang, X.; Jiang, W. Prediction of Functional Outcome in Patients with Convulsive Status Epilepticus: The END-IT Score. Crit. Care 2016, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, N.; Giovannini, G.; Rossi, J.; Cioclu, M.C.; Meletti, S. Clinical Outcomes and Treatments Effectiveness in Status Epilepticus Resolved by Antiepileptic Drugs: A Five-Year Observational Study. Epilepsia Open 2020, 5, 166–175. [Google Scholar] [CrossRef]
- Fernández, I.S.; Goodkin, H.P.; Scott, R.C. Pathophysiology of Convulsive Status Epilepticus. Seizure-Eur. J. Epilepsy 2019, 68, 16–21. [Google Scholar] [CrossRef]
- Hocker, S. Systemic Complications of Status Epilepticus—An Update. Epilepsy Behav. 2015, 49, 83–87. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kuruba, R. Experimental Models of Status Epilepticus and Neuronal Injury for Evaluation of Therapeutic Interventions. Int. J. Mol. Sci. 2013, 14, 18284–18318. [Google Scholar] [CrossRef]
- Pradip Chauhan, M.; Shalom Elsy Philip, M.; Girish Chauhan, M.D.S.; Simmi Mehra, M. The Anatomical Basis of Seizures; Exon Publications: Brisbane, Australia, 2022; pp. 15–23. [Google Scholar] [CrossRef]
- Haut, S.R.; Shinnar, S.; Moshé, S.L. Seizure Clustering: Risks and Outcomes. Epilepsia 2005, 46, 146–149. [Google Scholar] [CrossRef]
- Haut, S.R. Seizure Clustering. Epilepsy Behav. 2006, 8, 50–55. [Google Scholar] [CrossRef]
- Penovich, P.E.; Glauser, T. Seizure Clusters: Practical Aspects and Clinical Strategies to Care for Patients in the Community. Epilepsia 2022, 63 (Suppl. 1), S3–S5. [Google Scholar] [CrossRef]
- Engel, J., Jr. A Proposed Diagnostic Scheme for People with Epileptic Seizures and with Epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia 2001, 42, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Hauser, W.A. Status Epilepticus and Antiepileptic Medication Levels. Neurology 1994, 44, 47. [Google Scholar] [CrossRef]
- Kantanen, A.-M.; Sairanen, J.; Kälviäinen, R. Incidence of the Different Stages of Status Epilepticus in Eastern Finland: A Population-Based Study. Epilepsy Behav. 2019, 101, 106413. [Google Scholar] [CrossRef]
- Nagarkatti, N.; Deshpande, L.S.; DeLorenzo, R.J. Development of the Calcium Plateau Following Status Epilepticus: Role of Calcium in Epileptogenesis. Expert Rev. Neurother. 2009, 9, 813. [Google Scholar] [CrossRef]
- Sperber, E.F.; Haas, K.Z.; Romero, M.T.; Stanton, P.K. Flurothyl Status Epilepticus in Developing Rats: Behavioral, Electrographic Histological and Electrophysiological Studies. Dev. Brain Res. 1999, 116, 59–68. [Google Scholar] [CrossRef]
- Heinrich, C.; Lähteinen, S.; Suzuki, F.; Anne-Marie, L.; Huber, S.; Häussler, U.; Haas, C.; Larmet, Y.; Castren, E.; Depaulis, A. Increase in BDNF-Mediated TrkB Signaling Promotes Epileptogenesis in a Mouse Model of Mesial Temporal Lobe Epilepsy. Neurobiol. Dis. 2011, 42, 35–47. [Google Scholar] [CrossRef]
- Niquet, J.; Nguyen, D.; de Araujo Furtado, M.; Lumley, L. Treatment of Cholinergic-induced Status Epilepticus with Polytherapy Targeting GABA and Glutamate Receptors. Epilepsia Open 2023, 8 (Suppl. 1), S117–S140. [Google Scholar] [CrossRef]
- Löscher, W. Mammalian Models of Status Epilepticus—Their Value and Limitations. Epilepsy Behav. 2024, 158, 109923. [Google Scholar] [CrossRef]
- Sloviter, R.S. Decreased Hippocampal Inhibition and a Selective Loss of Interneurons in Experimental Epilepsy. Science 1987, 235, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Koene, L.M.C.; van Grondelle, S.E.; Proietti Onori, M.; Wallaard, I.; Kooijman, N.H.R.M.; van Oort, A.; Schreiber, J.; Elgersma, Y. Effects of Antiepileptic Drugs in a New TSC/mTOR-Dependent Epilepsy Mouse Model. Ann. Clin. Transl. Neurol. 2019, 6, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Blundell, J.; Ogawa, S.; Kwon, C.-H.; Zhang, W.; Sinton, C.; Powell, C.M.; Parada, L.F. Pharmacological Inhibition of mTORC1 Suppresses Anatomical, Cellular, and Behavioral Abnormalities in Neural-Specific Pten Knock-out Mice. J. Neurosci. 2009, 29, 1773–1783. [Google Scholar] [CrossRef]
- Blanc-Sylvestre, N.; Bouchard, P.; Chaussain, C.; Bardet, C. Pre-Clinical Models in Implant Dentistry: Past, Present, Future. Biomedicines 2021, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Kovac, S.; Domijan, A.-M.; Walker, M.C.; Abramov, A.Y. Prolonged Seizure Activity Impairs Mitochondrial Bioenergetics and Induces Cell Death. J. Cell Sci. 2012, 125, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Postnikova, T.Y.; Amakhin, D.V.; Trofimova, A.M.; Zaitsev, A.V. Calcium-Permeable AMPA Receptors Are Essential to the Synaptic Plasticity Induced by Epileptiform Activity in Rat Hippocampal Slices. Biochem. Biophys. Res. Commun. 2020, 529, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Krsek, P.; Mikulecká, A.; Druga, R.; Hlinák, Z.; Kubová, H.; Mares, P. An Animal Model of Nonconvulsive Status Epilepticus: A Contribution to Clinical Controversies. Epilepsia 2001, 42, 171–180. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, F.; Li, W.; Yang, X.; Gao, Q.; Bi, L.; Jiang, Y.; Jiang, W. Nonconvulsive Status Epilepticus after Convulsive Status Epilepticus: Clinical Features, Outcomes, and Prognostic Factors. Epilepsy Res. 2018, 142, 53–57. [Google Scholar] [CrossRef]
- Lv, R.-J.; Wang, Q.; Cui, T.; Zhu, F.; Shao, X.-Q. Status Epilepticus-Related Etiology, Incidence and Mortality: A Meta-Analysis. Epilepsy Res. 2017, 136, 12–17. [Google Scholar] [CrossRef]
- Debanne, D.; Mylonaki, K.; Musella, M.L.; Russier, M. Voltage-Gated Ion Channels in Epilepsies: Circuit Dysfunctions and Treatments. Trends Pharmacol. Sci. 2024, 45, 1018–1032. [Google Scholar] [CrossRef]
- Schindler, K.; Elger, C.E.; Lehnertz, K. Increasing Synchronization May Promote Seizure Termination: Evidence from Status Epilepticus. Clin. Neurophysiol. 2007, 118, 1955–1968. [Google Scholar] [CrossRef]
- Walker, M.C. Pathophysiology of Status Epilepticus. Neurosci. Lett. 2018, 667, 84–91. [Google Scholar] [CrossRef]
- Cash, S.S. Status Epilepticus as a System Disturbance: Is Status Epilepticus Due to Synchronization or Desynchronization? Epilepsia 2013, 54 (Suppl. 6), 37–39. [Google Scholar] [CrossRef]
- Bromfield, E.B.; Cavazos, J.E.; Sirven, J.I. Basic Mechanisms Underlying Seizures and Epilepsy. In An Introduction to Epilepsy [Internet]; American Epilepsy Society: Chicago, IL, USA, 2006. [Google Scholar]
- Sumadewi, K.T.; Harkitasari, S.; Tjandra, D.C. Biomolecular Mechanisms of Epileptic Seizures and Epilepsy: A Review. Acta Epileptol. 2023, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- de Curtis, M.; Suffczynski, P.; Lévesque, M.; Librizzi, L.; Uva, L.; Scalmani, P.; Gnatkovsky, V.; Avoli, M. Mechanisms Leading to Initiation, Development, and Termination of Focal Seizures. In Jasper’s Basic Mechanisms of the Epilepsies; Avoli, M., de Curtis, M., Bernard, C., Soltesz, I., Noebels, J.L., Avoli, M., Rogawski, M.A., Vezzani, A., Delgado-Escueta, A.V., Eds.; Oxford University Press: Oxford, UK, 2024; pp. 161–180. [Google Scholar] [CrossRef]
- Krishnan, G.P.; Bazhenov, M. Ionic Dynamics Mediate Spontaneous Termination of Seizures and Postictal Depression State. J. Neurosci. 2011, 31, 8870–8882. [Google Scholar] [CrossRef] [PubMed]
- Lado, F.A.; Moshé, S.L. How Do Seizures Stop? Epilepsia 2008, 49, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann-Eden, B.; Beghi, E.; Camfield, C.; Camfield, P. The First Seizure and Its Management in Adults and Children. Bmj 2006, 332, 339–342. [Google Scholar] [CrossRef]
- Timofeev, I.; Sejnowski, T.J.; Bazhenov, M.; Chauvette, S.; Grand, L.B. Age Dependency of Trauma-Induced Neocortical Epileptogenesis. Front. Cell Neurosci. 2013, 7, 154. [Google Scholar] [CrossRef]
- Bellot-Saez, A.; Kékesi, O.; Morley, J.W.; Buskila, Y. Astrocytic Modulation of Neuronal Excitability through K+ Spatial Buffering. Neurosci. Biobehav. Rev. 2017, 77, 87–97. [Google Scholar] [CrossRef]
- Alolayan, Y.S.; McKinley, K.; Bhatia, R.; Alkhachroum, A. Review and Updates on the Treatment of Refractory and Super Refractory Status Epilepticus. J. Clin. Med. 2021, 10, 3028. [Google Scholar] [CrossRef]
- Wasterlain, C. Fifty Years of Research on Status Epilepticus: Seizures Use Hippocampal Memory Circuits to Generate Status Epilepticus and Disrupt Brain Development. Epilepsy Behav. 2023, 141, 109142. [Google Scholar] [CrossRef]
- Vargas-Sánchez, K.; Mogilevskaya, M.; Rodríguez-Pérez, J.; Rubiano, M.G.; Javela, J.J.; González-Reyes, R.E. Astroglial Role in the Pathophysiology of Status Epilepticus: An Overview. Oncotarget 2018, 9, 26954–26976. [Google Scholar] [CrossRef]
- Vuu, I.; Patterson, E.E.; Wu, C.-Y.; Zolkowska, D.; Leppik, I.E.; Rogawski, M.A.; Worrell, G.A.; Kremen, V.; Cloyd, J.C.; Coles, L.D. Intravenous and Intramuscular Allopregnanolone for Early Treatment of Status Epilepticus: Pharmacokinetics, Pharmacodynamics, and Safety in Dogs. J. Pharmacol. Exp. Ther. 2022, 380, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Mazarati, A.; Bragin, A.; Baldwin, R.; Shin, D.; Wilson, C.; Sankar, R.; Naylor, D.; Engel, J., Jr.; Wasterlain, C.G. Epileptogenesis After Self-Sustaining Status Epilepticus. Epilepsia 2002, 43 (Suppl. 5), 74–80. [Google Scholar] [CrossRef] [PubMed]
- Kapur, J.; Macdonald, R.L. Rapid Seizure-Induced Reduction of Benzodiazepine and Zn2+ Sensitivity of Hippocampal Dentate Granule Cell GABAA Receptors. J. Neurosci. 1997, 17, 7532–7540. [Google Scholar] [CrossRef] [PubMed]
- Qashu, F.; Figueiredo, T.H.; Aroniadou-Anderjaska, V.; Apland, J.P.; Braga, M.F.M. Diazepam Administration after Prolonged Status Epilepticus Reduces Neurodegeneration in the Amygdala but Not in the Hippocampus during Epileptogenesis. Amino Acids 2010, 38, 189–197. [Google Scholar] [CrossRef]
- Wasterlain, C.G.; Fujikawa, D.G.; Penix, L.; Sankar, R. Pathophysiological Mechanisms of Brain Damage from Status Epilepticus. Epilepsia 1993, 34 (Suppl. 1), S37–S53. [Google Scholar] [CrossRef]
- Hasegawa, D.; Matsuki, N.; Fujita, M.; Ono, K.; Orima, H. Kinetics of Glutamate and γ-Aminobutyric Acid in Cerebrospinal Fluid in a Canine Model of Complex Partial Status Epilepticus Induced by Kainic Acid. J. Vet. Med. Sci. 2004, 66, 1555–1559. [Google Scholar] [CrossRef]
- Santos, V.R.; Tilelli, C.Q.; Fernandes, A.; de Castro, O.W.; Del-Vecchio, F.; Garcia-Cairasco, N. Different Types of Status Epilepticus May Lead to Similar Hippocampal Epileptogenesis Processes. IBRO Neurosci. Rep. 2023, 15, 68–76. [Google Scholar] [CrossRef]
- Kokaia, M.; Holmberg, K.; Nanobashvili, A.; Xu, Z.-Q.D.; Kokaia, Z.; Lendahl, U.; Hilke, S.; Theodorsson, E.; Kahl, U.; Bartfai, T.; et al. Suppressed Kindling Epileptogenesis in Mice with Ectopic Overexpression of Galanin. Proc. Natl. Acad. Sci. USA 2001, 98, 14006–14011. [Google Scholar] [CrossRef]
- Qian, X.; Ding, J.-Q.; Zhao, X.; Sheng, X.-W.; Wang, Z.-R.; Yang, Q.; Zheng, J.-J.; Zhong, J.-G.; Zhang, T.-Y.; He, S.-Q.; et al. Proteomic Analysis Reveals the Vital Role of Synaptic Plasticity in the Pathogenesis of Temporal Lobe Epilepsy. Neural Plast. 2022, 2022, 8511066. [Google Scholar] [CrossRef]
- Akbar, M.T.; Lundberg, A.M.C.; Liu, K.; Vidyadaran, S.; Wells, K.E.; Dolatshad, H.; Wynn, S.; Wells, D.J.; Latchman, D.S.; de Belleroche, J. The Neuroprotective Effects of Heat Shock Protein 27 Overexpression in Transgenic Animals against Kainate-Induced Seizures and Hippocampal Cell Death. J. Biol. Chem. 2003, 278, 19956–19965. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Yang, J.-L.; Chen, L.-J.; Zhang, Y.; Yang, M.-L.; Wu, Y.-Y.; Li, F.-Q.; Tang, M.-H.; Liang, S.-F.; Wei, Y.-Q. Comparative Proteomics and Correlated Signaling Network of Rat Hippocampus in the Pilocarpine Model of Temporal Lobe Epilepsy. Proteomics 2008, 8, 582–603. [Google Scholar] [CrossRef]
- de Oliveira, C.V.; Grigoletto, J.; Canzian, J.M.; Duarte, M.M.M.F.; Duarte, T.; Furian, A.F.; Oliveira, M.S. Effect of Atorvastatin on Behavioral Alterations and Neuroinflammation during Epileptogenesis. Epilepsy Behav. 2018, 78, 109–117. [Google Scholar] [CrossRef]
- Vezzani, A.; Di Sapia, R.; Kebede, V.; Balosso, S.; Ravizza, T. Neuroimmunology of Status Epilepticus. Epilepsy Behav. 2023, 140, 109095. [Google Scholar] [CrossRef]
- Järvelä, J.T.; Lopez-Picon, F.R.; Holopainen, I.E. Age-Dependent Cyclooxygenase-2 Induction and Neuronal Damage after Status Epilepticus in the Postnatal Rat Hippocampus. Epilepsia 2008, 49, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like Receptor 4 and High-Mobility Group Box-1 Are Involved in Ictogenesis and Can Be Targeted to Reduce Seizures. Nat. Med. 2010, 16, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lehtimäki, K.A.; Peltola, J.; Koskikallio, E.; Keränen, T.; Honkaniemi, J. Expression of Cytokines and Cytokine Receptors in the Rat Brain after Kainic Acid-Induced Seizures. Mol. Brain Res. 2003, 110, 253–260. [Google Scholar] [CrossRef]
- Vezzani, A.; Conti, M.; De Luigi, A.; Ravizza, T.; Moneta, D.; Marchesi, F.; De Simoni, M.G. Interleukin-1beta Immunoreactivity and Microglia Are Enhanced in the Rat Hippocampus by Focal Kainate Application: Functional Evidence for Enhancement of Electrographic Seizures. J. Neurosci. 1999, 19, 5054–5065. [Google Scholar] [CrossRef]
- Vizuete, A.F.K.; Hennemann, M.M.; Gonçalves, C.A.; de Oliveira, D.L. Phase-Dependent Astroglial Alterations in Li–Pilocarpine-Induced Status Epilepticus in Young Rats. Neurochem. Res. 2017, 42, 2730–2742. [Google Scholar] [CrossRef] [PubMed]
- van den Munckhof, B.; de Vries, E.E.; Braun, K.P.J.; Boss, H.M.; Willemsen, M.A.; van Royen-Kerkhof, A.; de Jager, W.; Jansen, F.E. Serum Inflammatory Mediators Correlate with Disease Activity in Electrical Status Epilepticus in Sleep (ESES) Syndrome. Epilepsia 2016, 57, e45–e50. [Google Scholar] [CrossRef]
- Freund, Y.; Bloom, B.; Bokobza, J.; Baarir, N.; Laribi, S.; Harris, T.; Navarro, V.; Bernard, M.; Pearse, R.; Riou, B.; et al. Predictive Value of S100-B and Copeptin for Outcomes Following Seizure: The BISTRO International Cohort Study. PLoS ONE 2015, 10, e0122405. [Google Scholar] [CrossRef][Green Version]
- Gurnett, C.A.; Landt, M.; Wong, M. Analysis of Cerebrospinal Fluid Glial Fibrillary Acidic Protein after Seizures in Children. Epilepsia 2003, 44, 1455–1458. [Google Scholar] [CrossRef]
- Rizzi, M.; Perego, C.; Aliprandi, M.; Richichi, C.; Ravizza, T.; Colella, D.; Velískŏvá, J.; Moshé, S.L.; De Simoni, M.G.; Vezzani, A. Glia Activation and Cytokine Increase in Rat Hippocampus by Kainic Acid-Induced Status Epilepticus during Postnatal Development. Neurobiol. Dis. 2003, 14, 494–503. [Google Scholar] [CrossRef]
- Foiadelli, T.; Santangelo, A.; Costagliola, G.; Costa, E.; Scacciati, M.; Riva, A.; Volpedo, G.; Smaldone, M.; Bonuccelli, A.; Clemente, A.M.; et al. Neuroinflammation and Status Epilepticus: A Narrative Review Unraveling a Complex Interplay. Front. Pediatr. 2023, 11, 1251914. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, L.; Peng, W.; Mao, X. Glial Polarization in Neurological Diseases: Molecular Mechanisms and Therapeutic Opportunities. Ageing Res. Rev. 2025, 104, 102638. [Google Scholar] [CrossRef]
- Virgilio, F.D.; Ben, D.D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Beamer, E.; Morgan, J.; Alves, M.; Menéndez Méndez, A.; Morris, G.; Zimmer, B.; Conte, G.; de Diego-Garcia, L.; Alarcón-Vila, C.; Yiu Ng, N.K.; et al. Increased Expression of the ATP-Gated P2X7 Receptor Reduces Responsiveness to Anti-Convulsants during Status Epilepticus in Mice. Br. J. Pharmacol. 2022, 179, 2986–3006. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Durillo, M.; Frenguelli, B.G. Antagonism of P2X7 Receptors Enhances Lorazepam Action in Delaying Seizure Onset in an in Vitro Model of Status Epilepticus. Neuropharmacology 2023, 239, 109647. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Nguyen, N.T.; Alves, M.; de Diego-Garcia, L.; Kenny, A.; Nicke, A.; Henshall, D.C.; Jimenez-Mateos, E.M.; Engel, T. P2X7 Receptor-Dependent microRNA Expression Profile in the Brain Following Status Epilepticus in Mice. Front. Mol. Neurosci. 2020, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Zhang, X.; Zhang, Y.; Wu, Y.; Song, Y.; Wang, X.; Chen, T.; Liu, W.; Peng, B.; Yin, J.; et al. Neurotoxic A1 Astrocytes Promote Neuronal Ferroptosis via CXCL10/CXCR3 Axis in Epilepsy. Free Radic. Biol. Med. 2023, 195, 329–342. [Google Scholar] [CrossRef]
- Vezzani, A.; Ravizza, T.; Bedner, P.; Aronica, E.; Steinhäuser, C.; Boison, D. Astrocytes in the Initiation and Progression of Epilepsy. Nat. Rev. Neurol. 2022, 18, 707–722. [Google Scholar] [CrossRef]
- Jiang, G.-T.; Shao, L.; Kong, S.; Zeng, M.-L.; Cheng, J.-J.; Chen, T.-X.; Han, S.; Yin, J.; Liu, W.-H.; He, X.-H.; et al. Complement C3 Aggravates Post-Epileptic Neuronal Injury Via Activation of TRPV1. Neurosci. Bull. 2021, 37, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Activation of Mitochondrial Transient Receptor Potential Vanilloid 1 Channel Contributes to Microglial Migration. Glia 2015, 63, 1870–1882. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-T.; Wu, S.-X.; Zhang, H.; Kuang, F. Inhibition of MyD88 Signaling Skews Microglia/Macrophage Polarization and Attenuates Neuronal Apoptosis in the Hippocampus After Status Epilepticus in Mice. Neurotherapeutics 2018, 15, 1093–1111. [Google Scholar] [CrossRef] [PubMed]
- Meskinimood, S.; Rahimi, N.; Faghir-Ghanesefat, H.; Gholami, M.; Sharifzadeh, M.; Dehpour, A.R. Modulatory Effect of Opioid Ligands on Status Epilepticus and the Role of Nitric Oxide Pathway. Epilepsy Behav. 2019, 101. [Google Scholar] [CrossRef]
- Engel, J.; Pitkänen, A. Biomarkers for epileptogenesis and its treatment. Neuropharmacology 2020, 167, 107735. [Google Scholar] [CrossRef]
- Jia, X.; Wang, Q.; Ji, J.; Lu, W.; Liu, Z.; Tian, H.; Guo, L.; Wang, Y. Mitochondrial Transplantation Ameliorates Hippocampal Damage Following Status Epilepticus. Anim. Models Exp. Med. 2023, 6, 41–50. [Google Scholar] [CrossRef]
- Alyu, F.; Dikmen, M. Inflammatory Aspects of Epileptogenesis: Contribution of Molecular Inflammatory Mechanisms. Acta Neuropsychiatr. 2017, 29, 1–16. [Google Scholar] [CrossRef]
- Roseti, C.; van Vliet, E.A.; Cifelli, P.; Ruffolo, G.; Baayen, J.C.; Di Castro, M.A.; Bertollini, C.; Limatola, C.; Aronica, E.; Vezzani, A.; et al. GABAA Currents Are Decreased by IL-1β in Epileptogenic Tissue of Patients with Temporal Lobe Epilepsy: Implications for Ictogenesis. Neurobiol. Dis. 2015, 82, 311–320. [Google Scholar] [CrossRef]
- Noe, F.M.; Polascheck, N.; Frigerio, F.; Bankstahl, M.; Ravizza, T.; Marchini, S.; Beltrame, L.; Banderó, C.R.; Löscher, W.; Vezzani, A. Pharmacological Blockade of IL-1β/IL-1 Receptor Type 1 Axis during Epileptogenesis Provides Neuroprotection in Two Rat Models of Temporal Lobe Epilepsy. Neurobiol. Dis. 2013, 59, 183–193. [Google Scholar] [CrossRef]
- Marchi, N.; Fan, Q.; Ghosh, C.; Fazio, V.; Bertolini, F.; Betto, G.; Batra, A.; Carlton, E.; Najm, I.; Granata, T.; et al. Antagonism of Peripheral Inflammation Reduces the Severity of Status Epilepticus. Neurobiol. Dis. 2009, 33, 171–181. [Google Scholar] [CrossRef]
- Rincón-López, C.; Tlapa-Pale, A.; Medel-Matus, J.-S.; Martínez-Quiroz, J.; Rodríguez-Landa, J.F.; López-Meraz, M.-L. Interleukin-1β Increases Neuronal Death in the Hippocampal Dentate Gyrus Associated with Status Epilepticus in the Developing Rat. Neurologia 2017, 32, 587–594. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, Y.; Liu, K.; Chen, J.; Lai, N.; Fei, F.; Shi, J.; Xu, C.; Wang, S.; Nishibori, M.; et al. HMGB1 Is a Therapeutic Target and Biomarker in Diazepam-Refractory Status Epilepticus with Wide Time Window. Neurotherapeutics 2020, 17, 710–721. [Google Scholar] [CrossRef]
- Bauer, M.; Tournier, N.; Langer, O. Imaging P-Glycoprotein Function at the Blood–Brain Barrier as a Determinant of the Variability in Response to Central Nervous System Drugs. Clin. Pharmacol. Ther. 2019, 105, 1061–1064. [Google Scholar] [CrossRef]
- Joseph, S.A.; Lynd-Balta, E.; O’Banion, M.K.; Rappold, P.M.; Daschner, J.; Allen, A.; Padowski, J. Enhanced Cyclooxygenase-2 Expression in Olfactory-Limbic Forebrain Following Kainate-Induced Seizures. Neuroscience 2006, 140, 1051–1065. [Google Scholar] [CrossRef] [PubMed]
- Strauss, K.I.; Marini, A.M. Cyclooxygenase-2 Inhibition Protects Cultured Cerebellar Granule Neurons from Glutamate-Mediated Cell Death. J. Neurotrauma 2002, 19, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Serrano, G.E.; Lelutiu, N.; Rojas, A.; Cochi, S.; Shaw, R.; Makinson, C.D.; Wang, D.; FitzGerald, G.A.; Dingledine, R. Ablation of Cyclooxygenase-2 in Forebrain Neurons Is Neuroprotective and Dampens Brain Inflammation after Status Epilepticus. J. Neurosci. 2011, 31, 14850–14860. [Google Scholar] [CrossRef] [PubMed]
- Holtman, L.; van Vliet, E.A.; van Schaik, R.; Queiroz, C.M.; Aronica, E.; Gorter, J.A. Effects of SC58236, a Selective COX-2 Inhibitor, on Epileptogenesis and Spontaneous Seizures in a Rat Model for Temporal Lobe Epilepsy. Epilepsy Res. 2009, 84, 56–66. [Google Scholar] [CrossRef]
- Polascheck, N.; Bankstahl, M.; Löscher, W. The COX-2 Inhibitor Parecoxib Is Neuroprotective but Not Antiepileptogenic in the Pilocarpine Model of Temporal Lobe Epilepsy. Exp. Neurol. 2010, 224, 219–233. [Google Scholar] [CrossRef]
- Moscovicz, F.; Taborda, C.; Fernández, F.; Borda, N.; Auzmendi, J.; Lazarowski, A. Ironing out the Links: Ferroptosis in Epilepsy and SUDEP. Epilepsy Behav. 2024, 157, 109890. [Google Scholar] [CrossRef]
- Sui, H.; Zhou, S.; Wang, Y.; Liu, X.; Zhou, L.; Yin, P.; Fan, Z.; Li, Q. COX-2 Contributes to P-Glycoprotein-Mediated Multidrug Resistance via Phosphorylation of c-Jun at Ser63/73 in Colorectal Cancer. Carcinogenesis 2011, 32, 667–675. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, N.; Chen, Y.; Zhang, K.; Ma, H.-Y.; Di, Q. HMGB1 Regulates P-Glycoprotein Expression in Status Epilepticus Rat Brains via the RAGE/NF-κB Signaling Pathway. Mol. Med. Rep. 2017, 16, 1691–1700. [Google Scholar] [CrossRef]
- Brandt, C.; Bethmann, K.; Gastens, A.M.; Löscher, W. The Multidrug Transporter Hypothesis of Drug Resistance in Epilepsy: Proof-of-Principle in a Rat Model of Temporal Lobe Epilepsy. Neurobiol. Dis. 2006, 24, 202–211. [Google Scholar] [CrossRef]
- Holtman, L.; van Vliet, E.A.; Edelbroek, P.M.; Aronica, E.; Gorter, J.A. Cox-2 Inhibition Can Lead to Adverse Effects in a Rat Model for Temporal Lobe Epilepsy. Epilepsy Res. 2010, 91, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Moerman, L.; Wyffels, L.; Slaets, D.; Raedt, R.; Boon, P.; De Vos, F. Antiepileptic Drugs Modulate P-Glycoproteins in the Brain: A Mice Study with 11C-Desmethylloperamide. Epilepsy Res. 2011, 94, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-E.; Ryu, H.J.; Kang, T.-C. Status Epilepticus Induces Vasogenic Edema via Tumor Necrosis Factor-α/ Endothelin-1-Mediated Two Different Pathways. PLoS ONE 2013, 8, e74458. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A. Blood–Brain Barrier Dysfunction, Status Epilepticus, Seizures, and Epilepsy: A Puzzle of a Chicken and Egg? Epilepsia 2011, 52 (Suppl. 8), 19–20. [Google Scholar] [CrossRef]
- Marchi, N.; Angelov, L.; Masaryk, T.; Fazio, V.; Granata, T.; Hernandez, N.; Hallene, K.; Diglaw, T.; Franic, L.; Najm, I.; et al. Seizure-Promoting Effect of Blood–Brain Barrier Disruption. Epilepsia 2007, 48, 732–742. [Google Scholar] [CrossRef]
- Correale, J.; Rabinowicz, A.L.; Heck, C.N.; Smith, T.D.; Loskota, W.J.; DeGiorgio, C.M. Status Epilepticus Increases CSF Levels of Neuron-Specific Enolase and Alters the Blood-Brain Barrier. Neurology 1998, 50, 1388–1391. [Google Scholar] [CrossRef]
- Löscher, W.; Friedman, A. Structural, Molecular, and Functional Alterations of the Blood-Brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int. J. Mol. Sci. 2020, 21, 591. [Google Scholar] [CrossRef]
- Swissa, E.; Serlin, Y.; Vazana, U.; Prager, O.; Friedman, A. Blood–Brain Barrier Dysfunction in Status Epileptics: Mechanisms and Role in Epileptogenesis. Epilepsy Behav. 2019, 101, 106285. [Google Scholar] [CrossRef]
- Mendes, N.F.; Pansani, A.P.; Carmanhães, E.R.F.; Tange, P.; Meireles, J.V.; Ochikubo, M.; Chagas, J.R.; da Silva, A.V.; Monteiro de Castro, G.; Le Sueur-Maluf, L. The Blood-Brain Barrier Breakdown During Acute Phase of the Pilocarpine Model of Epilepsy Is Dynamic and Time-Dependent. Front. Neurol. 2019, 10, 382. [Google Scholar] [CrossRef]
- Giovannini, G.; Meletti, S. Fluid Biomarkers of Neuro-Glial Injury in Human Status Epilepticus: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 12519. [Google Scholar] [CrossRef]
- Lenz, M.; Shimon, M.B.; Benninger, F.; Neufeld, M.Y.; Shavit-Stein, E.; Vlachos, A.; Maggio, N. Systemic Thrombin Inhibition Ameliorates Seizures in a Mouse Model of Pilocarpine-Induced Status Epilepticus. J. Mol. Med. 2019, 97, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; He, J.C.; Wu, J.W.; Zhan, S.Q.; Zhang, G.L.; Wu, H.Q.; Zhang, R.; Gao, Z.; Ren, H.W. Losartan Inhibits Development of Spontaneous Recurrent Seizures by Preventing Astrocyte Activation and Attenuating Blood-Brain Barrier Permeability Following Pilocarpine-Induced Status Epilepticus. Brain Res. Bull. 2019, 149, 251–259. [Google Scholar] [CrossRef]
- Eguchi, S.; Kawai, T.; Scalia, R.; Rizzo, V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension 2018, 71, 804–810. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Marchi, N. Neurovascular Unit Dysfunction as a Mechanism of Seizures and Epilepsy during Aging. Epilepsia 2022, 63, 1297. [Google Scholar] [CrossRef] [PubMed]
- Vinet, J.; Costa, A.-M.; Salinas-Navarro, M.; Leo, G.; Moons, L.; Arckens, L.; Biagini, G. A Hydroxypyrone-Based Inhibitor of Metalloproteinase-12 Displays Neuroprotective Properties in Both Status Epilepticus and Optic Nerve Crush Animal Models. Int. J. Mol. Sci. 2018, 19, 2178. [Google Scholar] [CrossRef]
- Broekaart, D.W.; Bertran, A.; Jia, S.; Korotkov, A.; Senkov, O.; Bongaarts, A.; Mills, J.D.; Anink, J.J.; Seco, J.; Baayen, J.C.; et al. The Matrix Metalloproteinase Inhibitor IPR-179 Has Antiseizure and Antiepileptogenic Effects. J. Clin. Investig. 2021, 131, e138332. [Google Scholar] [CrossRef]
- Pijet, B.; Konopka, A.; Rejmak, E.; Stefaniuk, M.; Khomiak, D.; Bulska, E.; Pikul, S.; Kaczmarek, L. The Matrix Metalloproteinase Inhibitor Marimastat Inhibits Seizures in a Model of Kainic Acid-Induced Status Epilepticus. Sci. Rep. 2020, 10, 21314. [Google Scholar] [CrossRef]
- de Melo, I.S.; Pacheco, A.L.D.; dos Santos, Y.M.O.; Figueiredo, L.M.; Nicacio, D.C.S.P.; Cardoso-Sousa, L.; Duzzioni, M.; Gitaí, D.L.G.; Tilelli, C.Q.; Sabino-Silva, R.; et al. Modulation of Glucose Availability and Effects of Hypo- and Hyperglycemia on Status Epilepticus: What We Do Not Know Yet? Mol. Neurobiol. 2021, 58, 505–519. [Google Scholar] [CrossRef]
- Teskey, G.C.; Tran, C.H.T. Neurovascular Coupling in Seizures. Neuroglia 2021, 2, 36–47. [Google Scholar] [CrossRef]
- Lourenço, C.F.; Ledo, A.; Caetano, M.; Barbosa, R.M.; Laranjinha, J. Age-Dependent Impairment of Neurovascular and Neurometabolic Coupling in the Hippocampus. Front. Physiol. 2018, 9, 913. [Google Scholar] [CrossRef]
- Farrell, J.S.; Colangeli, R.; Dong, A.; George, A.G.; Addo-Osafo, K.; Kingsley, P.J.; Morena, M.; Wolff, M.D.; Dudok, B.; He, K.; et al. In Vivo Endocannabinoid Dynamics at the Timescale of Physiological and Pathological Neural Activity. Neuron 2021, 109, 2398–2403.e4. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.S.; Gaxiola-Valdez, I.; Wolff, M.D.; David, L.S.; Dika, H.I.; Geeraert, B.L.; Rachel Wang, X.; Singh, S.; Spanswick, S.C.; Dunn, J.F.; et al. Postictal Behavioural Impairments Are Due to a Severe Prolonged Hypoperfusion/Hypoxia Event That Is COX-2 Dependent. eLife 2016, 5, e19352. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, D.G. Programmed Mechanisms of Status Epilepticus-Induced Neuronal Necrosis. Epilepsia Open 2023, 8 (Suppl. 1), S25–S34. [Google Scholar] [CrossRef] [PubMed]
- Helbok, R.; Claassen, J. Multimodal Invasive Monitoring in Status Epilepticus: What Is the Evidence It Has a Place? Epilepsia 2013, 54 (Suppl. 6), 57–60. [Google Scholar] [CrossRef]
- Engelhorn, T.; Doerfler, A.; Weise, J.; Baehr, M.; Forsting, M.; Hufnagel, A. Cerebral Perfusion Alterations during the Acute Phase of Experimental Generalized Status Epilepticus: Prediction of Survival By Using Perfusion-Weighted MR Imaging and Histopathology. Am. J. Neuroradiol. 2005, 26, 1563–1570. [Google Scholar]
- Costa, M.S.; Rocha, J.B.T.; Perosa, S.R.; Cavalheiro, E.A.; da Graça Naffah-Mazzacoratti, M. Pilocarpine-Induced Status Epilepticus Increases Glutamate Release in Rat Hippocampal Synaptosomes. Neurosci. Lett. 2004, 356, 41–44. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular Mechanisms of Calcium-Dependent Neurodegeneration in Excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- Sennvik, K.; Benedikz, E.; Fastbom, J.; Sundström, E.; Winblad, B.; Ankarcrona, M. Calcium Ionophore A23187 Specifically Decreases the Secretion of β-Secretase Cleaved Amyloid Precursor Protein during Apoptosis in Primary Rat Cortical Cultures. J. Neurosci. Res. 2001, 63, 429–437. [Google Scholar] [CrossRef]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Pal, S.; Sun, D.; Limbrick, D.; Rafiq, A.; DeLorenzo, R.J. Epileptogenesis Induces Long-Term Alterations in Intracellular Calcium Release and Sequestration Mechanisms in the Hippocampal Neuronal Culture Model of Epilepsy. Cell Calcium 2001, 30, 285–296. [Google Scholar] [CrossRef]
- Meldrum, B.S. Excitotoxicity and Selective Neuronal Loss in Epilepsy. Brain Pathol. 1993, 3, 405–412. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore—Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells 2023, 12, 1273. [Google Scholar] [CrossRef]
- Verma, M.; Lizama, B.N.; Chu, C.T. Excitotoxicity, Calcium and Mitochondria: A Triad in Synaptic Neurodegeneration. Transl. Neurodegener. 2022, 11, 3. [Google Scholar] [CrossRef]
- Xiong, T.-Q.; Chen, L.-M.; Tan, B.-H.; Guo, C.-Y.; Li, Y.-N.; Zhang, Y.-F.; Li, S.-L.; Zhao, H.; Li, Y.-C. The Effects of Calcineurin Inhibitor FK506 on Actin Cytoskeleton, Neuronal Survival and Glial Reactions after Pilocarpine-Induced Status Epilepticus in Mice. Epilepsy Res. 2018, 140, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Oreiro-García, M.T.; Vázquez-Illanes, M.D.; Sierra-Paredes, G.; Sierra-Marcuño, G. Changes in Extracellular Amino Acid Concentrations in the Rat Hippocampus after in Vivo Actin Depolymerization with Latrunculin A. Neurochem. Int. 2007, 50, 734–740. [Google Scholar] [CrossRef]
- Freire-Cobo, C.; Sierra-Paredes, G.; Freire, M.; Sierra-Marcuño, G. The Calcineurin Inhibitor Ascomicin Interferes with the Early Stage of the Epileptogenic Process Induced by Latrunculin A Microperfusion in Rat Hippocampus. J. Neuroimmune Pharmacol. 2014, 9, 654–667. [Google Scholar] [CrossRef]
- DeLorenzo, R.J.; Pal, S.; Sombati, S. Prolonged Activation of the N-Methyl-d-Aspartate Receptor–Ca2+ Transduction Pathway Causes Spontaneous Recurrent Epileptiform Discharges in Hippocampal Neurons in Culture. Proc. Natl. Acad. Sci. USA 1998, 95, 14482–14487. [Google Scholar] [CrossRef]
- Sy, G.; Opitz, T.; Mv, B.; Rs, Z. Status Epilepticus Decreases Glutamate Receptor 2 mRNA and Protein Expression in Hippocampal Pyramidal Cells before Neuronal Death. Proc. Natl. Acad. Sci. USA 2000, 97, 3631–3636. [Google Scholar] [CrossRef]
- Friedman, L.K.; Pellegrini-Giampietro, D.E.; Sperber, E.F.; Bennett, M.V.; Moshe, S.L.; Zukin, R.S. Kainate-Induced Status Epilepticus Alters Glutamate and GABAA Receptor Gene Expression in Adult Rat Hippocampus: An in Situ Hybridization Study. J. Neurosci. 1994, 14, 2697–2707. [Google Scholar] [CrossRef]
- Oguro, K.; Oguro, N.; Kojima, T.; Grooms, S.Y.; Calderone, A.; Zheng, X.; Bennett, M.V.L.; Zukin, R.S. Knockdown of AMPA Receptor GluR2 Expression Causes Delayed Neurodegeneration and Increases Damage by Sublethal Ischemia in Hippocampal CA1 and CA3 Neurons. J. Neurosci. 1999, 19, 9218–9227. [Google Scholar] [CrossRef]
- Raza, M.; Blair, R.E.; Sombati, S.; Carter, D.S.; Deshpande, L.S.; DeLorenzo, R.J. Evidence That Injury-Induced Changes in Hippocampal Neuronal Calcium Dynamics during Epileptogenesis Cause Acquired Epilepsy. Proc. Natl. Acad. Sci. USA 2004, 101, 17522–17527. [Google Scholar] [CrossRef]
- Shekh-Ahmad, T.; Kovac, S.; Abramov, A.Y.; Walker, M.C. Reactive Oxygen Species in Status Epilepticus. Epilepsy Behav. 2019, 101, 106410. [Google Scholar] [CrossRef]
- Jové, M.; Mota-Martorell, N.; Obis, È.; Sol, J.; Martín-Garí, M.; Ferrer, I.; Portero-Otín, M.; Pamplona, R. Lipid Adaptations against Oxidative Challenge in the Healthy Adult Human Brain. Antioxidants 2023, 12, 177. [Google Scholar] [CrossRef]
- Patel, M.; Liang, L.P.; Roberts, L.J. Enhanced Hippocampal F2-Isoprostane Formation Following Kainate-Induced Seizures. J. Neurochem. 2001, 79, 1065–1069. [Google Scholar] [CrossRef]
- Fuchs, M.; Viel, C.; Lehto, A.; Lau, H.; Klein, J. Oxidative Stress in Rat Brain during Experimental Status Epilepticus: Effect of Antioxidants. Front. Pharmacol. 2023, 14, 1233184. [Google Scholar] [CrossRef]
- Mishra, V.; Shuai, B.; Kodali, M.; Shetty, G.A.; Hattiangady, B.; Rao, X.; Shetty, A.K. Resveratrol Treatment after Status Epilepticus Restrains Neurodegeneration and Abnormal Neurogenesis with Suppression of Oxidative Stress and Inflammation. Sci. Rep. 2015, 5, 17807. [Google Scholar] [CrossRef] [PubMed]
- Cock, H.R.; Tong, X.; Hargreaves, I.P.; Heales, S.J.R.; Clark, J.B.; Patsalos, P.N.; Thom, M.; Groves, M.; Schapira, A.H.V.; Shorvon, S.D.; et al. Mitochondrial Dysfunction Associated with Neuronal Death Following Status Epilepticus in Rat. Epilepsy Res. 2002, 48, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, H. Inhibitory Effect of Astaxanthin on Oxidative Stress-Induced Mitochondrial Dysfunction—A Mini-Review. Nutrients 2018, 10, 1137. [Google Scholar] [CrossRef]
- Virág, L.; Szabó, É.; Gergely, P.; Szabó, C. Peroxynitrite-Induced Cytotoxicity: Mechanism and Opportunities for Intervention. Toxicol. Lett. 2003, 140–141, 113–124. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Fikry, H.; Saleh, L.A.; Mahmoud, F.A.; Gawad, S.A.; Abd-Alkhalek, H.A. CoQ10 Targeted Hippocampal Ferroptosis in a Status Epilepticus Rat Model. Cell Tissue Res. 2024, 396, 371–397. [Google Scholar] [CrossRef]
- Moradi, F.; Eslami, F.; Rahimi, N.; Koohfar, A.; Shayan, M.; Maadani, M.; Ghasemi, M.; Dehpour, A.R. Modafinil Exerts Anticonvulsive Effects against Lithium-Pilocarpine-Induced Status Epilepticus in Rats: A Role for Tumor Necrosis Factor-α and Nitric Oxide Signaling. Epilepsy Behav. 2022, 130, 108649. [Google Scholar] [CrossRef] [PubMed]
- Pauletti, A.; Terrone, G.; Shekh-Ahmad, T.; Salamone, A.; Ravizza, T.; Rizzi, M.; Pastore, A.; Pascente, R.; Liang, L.-P.; Villa, B.R.; et al. Targeting Oxidative Stress Improves Disease Outcomes in a Rat Model of Acquired Epilepsy. Brain 2019, 142, e39. [Google Scholar] [CrossRef] [PubMed]
- Salo, H.; Qu, H.; Mitsiou, D.; Aucott, H.; Han, J.; Zhang, X.; Aulin, C.; Erlandsson Harris, H. Disulfide and Fully Reduced HMGB1 Induce Different Macrophage Polarization and Migration Patterns. Biomolecules 2021, 11, 800. [Google Scholar] [CrossRef] [PubMed]
- Ambrogini, P.; Minelli, A.; Galati, C.; Betti, M.; Lattanzi, D.; Ciffolilli, S.; Piroddi, M.; Galli, F.; Cuppini, R. Post-Seizure α-Tocopherol Treatment Decreases Neuroinflammation and Neuronal Degeneration Induced by Status Epilepticus in Rat Hippocampus. Mol. Neurobiol. 2014, 50, 246–256. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Peng, J.; Yin, K.; Wang, J.-H. Potential Health-Promoting Effects of Astaxanthin: A High-Value Carotenoid Mostly from Microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Bae, M.; Lee, Y.; Park, Y.-K.; Shin, D.-G.; Joshi, P.; Hong, S.-H.; Alder, N.; Koo, S.I.; Lee, J.-Y. Astaxanthin Attenuates the Increase in Mitochondrial Respiration during the Activation of Hepatic Stellate Cells. J. Nutr. Biochem. 2019, 71, 82–89. [Google Scholar] [CrossRef]
- Margraf, N.G.; Dargvainiene, J.; Theel, E.; Leypoldt, F.; Lieb, W.; Franke, A.; Berger, K.; Kuhle, J.; Kuhlenbaeumer, G. Neurofilament Light (NfL) as Biomarker in Serum and CSF in Status Epilepticus. J. Neurol. 2023, 270, 2128–2138. [Google Scholar] [CrossRef]
- Alkhachroum, A.; Der-Nigoghossian, C.A.; Rubinos, C.; Claassen, J. Markers in Status Epilepticus Prognosis. J. Clin. Neurophysiol. 2020, 37, 422. [Google Scholar] [CrossRef]
- Custers, M.-L.; Vande Vyver, M.; Kaltenböck, L.; Barbé, K.; Bjerke, M.; Van Eeckhaut, A.; Smolders, I. Neurofilament Light Chain: A Possible Fluid Biomarker in the Intrahippocampal Kainic Acid Mouse Model for Chronic Epilepsy? Epilepsia 2023, 64, 2200–2211. [Google Scholar] [CrossRef]
- Rabinowicz, A.L.; Correale, J.D.; Bracht, K.A.; Smith, T.D.; DeGiorgio, C.M. Neuron-Specific Enolase Is Increased After Nonconvulsive Status Epilepticus. Epilepsia 1995, 36, 475–479. [Google Scholar] [CrossRef]
- Shacka, J.J.; Lu, J.; Xie, Z.-L.; Uchiyama, Y.; Roth, K.A.; Zhang, J. Kainic Acid Induces Early and Transient Autophagic Stress in Mouse Hippocampus. Neurosci. Lett. 2007, 414, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; He, M.; Zhao, D.; Wang, Y.; Ma, C.; Liang, H.; Wang, W.; Min, D.; Xue, L.; Guo, F. Mechanism of Cell Death Pathways in Status Epilepticus and Related Therapeutic Agents. Biomed. Pharmacother. 2022, 149, 112875. [Google Scholar] [CrossRef] [PubMed]
- Furtado, M.A.; Castro, O.W.; Del Vecchio, F.; de Oliveira, J.A.C.; Garcia-Cairasco, N. Study of Spontaneous Recurrent Seizures and Morphological Alterations after Status Epilepticus Induced by Intrahippocampal Injection of Pilocarpine. Epilepsy Behav. 2011, 20, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Naudí, A.; Cabré, R.; Dominguez-Gonzalez, M.; Ayala, V.; Jové, M.; Mota-Martorell, N.; Piñol-Ripoll, G.; Gil-Villar, M.P.; Rué, M.; Portero-Otín, M.; et al. Region-Specific Vulnerability to Lipid Peroxidation and Evidence of Neuronal Mechanisms for Polyunsaturated Fatty Acid Biosynthesis in the Healthy Adult Human Central Nervous System. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2017, 1862, 485–495. [Google Scholar] [CrossRef]
- Bartsch, T.; Döhring, J.; Reuter, S.; Finke, C.; Rohr, A.; Brauer, H.; Deuschl, G.; Jansen, O. Selective Neuronal Vulnerability of Human Hippocampal CA1 Neurons: Lesion Evolution, Temporal Course, and Pattern of Hippocampal Damage in Diffusion-Weighted MR Imaging. J. Cereb. Blood Flow Metab. 2015, 35, 1836–1845. [Google Scholar] [CrossRef]
- Hanin, A.; Lambrecq, V.; Denis, J.A.; Imbert-Bismut, F.; Rucheton, B.; Lamari, F.; Bonnefont-Rousselot, D.; Demeret, S.; Navarro, V. Cerebrospinal Fluid and Blood Biomarkers of Status Epilepticus. Epilepsia 2020, 61, 6–18. [Google Scholar] [CrossRef]
- Sloviter, R.S.; Bumanglag, A.V. Defining “Epileptogenesis” and Identifying “Antiepileptogenic Targets” in Animal Models of Acquired Temporal Lobe Epilepsy Is Not as Simple as It Might Seem. Neuropharmacology 2013, 69, 3–15. [Google Scholar] [CrossRef]
- Kralic, J.E.; Ledergerber, D.A.; Fritschy, J.-M. Disruption of the Neurogenic Potential of the Dentate Gyrus in a Mouse Model of Temporal Lobe Epilepsy with Focal Seizures. Eur. J. Neurosci. 2005, 22, 1916–1927. [Google Scholar] [CrossRef]
- Wolff, M.D.; Farrell, J.S.; Scantlebury, M.H.; Teskey, G.C. Dynamic Oxygen Changes during Status Epilepticus and Subsequent Endogenous Kindling. Epilepsia 2020, 61, 1515–1527. [Google Scholar] [CrossRef]
- Sloviter, R.S.; Zappone, C.A.; Harvey, B.D.; Frotscher, M. Kainic Acid-Induced Recurrent Mossy Fiber Innervation of Dentate Gyrus Inhibitory Interneurons: Possible Anatomical Substrate of Granule Cell Hyper-Inhibition in Chronically Epileptic Rats. J. Comp. Neurol. 2006, 494, 944–960. [Google Scholar] [CrossRef]
- Shapiro, L.A.; Ribak, C.E. Integration of Newly Born Dentate Granule Cells into Adult Brains: Hypotheses Based on Normal and Epileptic Rodents. Brain Res. Rev. 2005, 48, 43–56. [Google Scholar] [CrossRef]
- Morimoto, K.; Fahnestock, M.; Racine, R.J. Kindling and Status Epilepticus Models of Epilepsy: Rewiring the Brain. Prog. Neurobiol. 2004, 73, 1–60. [Google Scholar] [CrossRef]
- Hattiangady, B.; Rao, M.S.; Shetty, A.K. Chronic Temporal Lobe Epilepsy Is Associated with Severely Declined Dentate Neurogenesis in the Adult Hippocampus. Neurobiol. Dis. 2004, 17, 473–490. [Google Scholar] [CrossRef]
- Hanin, A.; Denis, J.A.; Frazzini, V.; Cousyn, L.; Imbert-Bismut, F.; Rucheton, B.; Bonnefont-Rousselot, D.; Marois, C.; Lambrecq, V.; Demeret, S.; et al. Neuron Specific Enolase, S100-Beta Protein and Progranulin as Diagnostic Biomarkers of Status Epilepticus. J. Neurol. 2022, 269, 3752–3760. [Google Scholar] [CrossRef]
- Cha, B.H.; Akman, C.; Silveira, D.C.; Liu, X.; Holmes, G.L. Spontaneous Recurrent Seizure Following Status Epilepticus Enhances Dentate Gyrus Neurogenesis. Brain Dev. 2004, 26, 394–397. [Google Scholar] [CrossRef]
- Bonde, S.; Ekdahl, C.T.; Lindvall, O. Long-Term Neuronal Replacement in Adult Rat Hippocampus after Status Epilepticus despite Chronic Inflammation. Eur. J. Neurosci. 2006, 23, 965–974. [Google Scholar] [CrossRef]
- Jessberger, S.; Zhao, C.; Toni, N.; Clemenson, G.D.; Li, Y.; Gage, F.H. Seizure-Associated, Aberrant Neurogenesis in Adult Rats Characterized with Retrovirus-Mediated Cell Labeling. J. Neurosci. 2007, 27, 9400–9407. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, D.P.; Hintz, T.M.; Pierce, J.P.; Scharfman, H.E. Stereological Methods Reveal the Robust Size and Stability of Ectopic Hilar Granule Cells after Pilocarpine-Induced Status Epilepticus in the Adult Rat. Eur. J. Neurosci. 2006, 24, 2203–2210. [Google Scholar] [CrossRef]
- Pierce, J.P.; McCloskey, D.P.; Scharfman, H.E. Morphometry of Hilar Ectopic Granule Cells in the Rat. J. Comp. Neurol. 2011, 519, 1196–1218. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Z.; Nusslock, R. Exercise-Mediated Neurogenesis in the Hippocampus via BDNF. Front. Neurosci. 2018, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-H.; Chu, K.; Lee, S.-T.; Kim, J.; Sinn, D.-I.; Kim, J.-M.; Park, D.-K.; Lee, J.-J.; Kim, S.U.; Kim, M.; et al. Cyclooxygenase-2 Inhibitor, Celecoxib, Inhibits the Altered Hippocampal Neurogenesis with Attenuation of Spontaneous Recurrent Seizures Following Pilocarpine-Induced Status Epilepticus. Neurobiol. Dis. 2006, 23, 237–246. [Google Scholar] [CrossRef]
- Hüttmann, K.; Sadgrove, M.; Wallraff, A.; Hinterkeuser, S.; Kirchhoff, F.; Steinhäuser, C.; Gray, W.P. Seizures Preferentially Stimulate Proliferation of Radial Glia-like Astrocytes in the Adult Dentate Gyrus: Functional and Immunocytochemical Analysis. Eur. J. Neurosci. 2003, 18, 2769–2778. [Google Scholar] [CrossRef]
- Jin, W. Regulation of BDNF-TrkB Signaling and Potential Therapeutic Strategies for Parkinson’s Disease. J. Clin. Med. 2020, 9, 257. [Google Scholar] [CrossRef]
- Benini, R.; Roth, R.; Khoja, Z.; Avoli, M.; Wintermark, P. Does Angiogenesis Play a Role in the Establishment of Mesial Temporal Lobe Epilepsy? Int. J. Dev. Neurosci. 2016, 49, 31–36. [Google Scholar] [CrossRef]
- Canto, A.M.; Matos, A.H.B.; Godoi, A.B.; Vieira, A.S.; Aoyama, B.B.; Rocha, C.S.; Henning, B.; Carvalho, B.S.; Pascoal, V.D.B.; Veiga, D.F.T.; et al. Multi-Omics Analysis Suggests Enhanced Epileptogenesis in the Cornu Ammonis 3 of the Pilocarpine Model of Mesial Temporal Lobe Epilepsy. Hippocampus 2021, 31, 122–139. [Google Scholar] [CrossRef]
- Yuan, P.; Han, W.; Xie, L.; Cheng, L.; Chen, H.; Chen, J.; Jiang, L. The Implications of Hippocampal Neurogenesis in Adolescent Rats after Status Epilepticus: A Novel Role of Notch Signaling Pathway in Regulating Epileptogenesis. Cell Tissue Res. 2020, 380, 425–433. [Google Scholar] [CrossRef]
- Rich, S.; Chameh, H.M.; Lefebvre, J.; Valiante, T.A. Loss of Neuronal Heterogeneity in Epileptogenic Human Tissue Impairs Network Resilience to Sudden Changes in Synchrony. Cell Rep. 2022, 39, 110863. [Google Scholar] [CrossRef]
- Mathern, G.W.; Leite, J.P.; Babb, T.L.; Pretorius, J.K.; Kuhlman, P.A.; Mendoza, D.; Fried, I.; Sakamoto, A.C.; Assirati, J.A.; Adelson, P.D.; et al. Aberrant Hippocampal Mossy Fiber Sprouting Correlates with Greater NMDAR2 Receptor Staining. Neuroreport 1996, 7, 1029. [Google Scholar] [CrossRef] [PubMed]
- Ransom, C.B.; Blumenfeld, H. 23-Acquired Epilepsy: Cellular and Molecular Mechanisms. In Molecular Neurology; Waxman, S.G., Ed.; Academic Press: San Diego, CA, USA, 2007; pp. 347–370. [Google Scholar] [CrossRef]
- Buckmaster, P.S.; Ingram, E.A.; Wen, X. Inhibition of the Mammalian Target of Rapamycin Signaling Pathway Suppresses Dentate Granule Cell Axon Sprouting in a Rodent Model of Temporal Lobe Epilepsy. J. Neurosci. 2009, 29, 8259–8269. [Google Scholar] [CrossRef] [PubMed]
- Crino, P.B. Mechanistic Target of Rapamycin (mTOR) Signaling in Status Epilepticus. Epilepsy Behav. 2019, 101, 106550. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, H.; Yang, J.; Wu, J.; McMahon, J.; Lin, Y.; Cao, Z.; Gruenthal, M.; Huang, Y. Pharmacological Inhibition of the Mammalian Target of Rapamycin Pathway Suppresses Acquired Epilepsy. Neurobiol. Dis. 2010, 40, 193–199. [Google Scholar] [CrossRef]
- LaSarge, C.L.; Danzer, S.C. Mechanisms Regulating Neuronal Excitability and Seizure Development Following mTOR Pathway Hyperactivation. Front. Mol. Neurosci. 2014, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Fabrizi, C.; Frati, A.; Fornai, F. mTOR-Related Cell-Clearing Systems in Epileptic Seizures, an Update. Int. J. Mol. Sci. 2020, 21, 1642. [Google Scholar] [CrossRef] [PubMed]
- Wong, M. Rapamycin for Treatment of Epilepsy: Antiseizure, Antiepileptogenic, Both, or Neither? Epilepsy Curr. 2011, 11, 66–68. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Forte, G.; Holtman, L.; den Burger, J.C.G.; Sinjewel, A.; de Vries, H.E.; Aronica, E.; Gorter, J.A. Inhibition of Mammalian Target of Rapamycin Reduces Epileptogenesis and Blood–Brain Barrier Leakage but Not Microglia Activation. Epilepsia 2012, 53, 1254–1263. [Google Scholar] [CrossRef]
- Zeng, L.-H.; Rensing, N.R.; Wong, M. The Mammalian Target of Rapamycin Signaling Pathway Mediates Epileptogenesis in a Model of Temporal Lobe Epilepsy. J. Neurosci. 2009, 29, 6964–6972. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Li, X.; Wang, Y.; Wei, D.; Jiang, W. Fingolimod (FTY720) Inhibits Neuroinflammation and Attenuates Spontaneous Convulsions in Lithium-Pilocarpine Induced Status Epilepticus in Rat Model. Pharmacol. Biochem. Behav. 2012, 103, 187–196. [Google Scholar] [CrossRef]
- Issa, N.P.; Nunn, K.C.; Wu, S.; Haider, H.A.; Tao, J.X. Putative Roles for Homeostatic Plasticity in Epileptogenesis. Epilepsia 2023, 64, 539–552. [Google Scholar] [CrossRef]
- González, O.C.; Krishnan, G.P.; Chauvette, S.; Timofeev, I.; Sejnowski, T.; Bazhenov, M. Modeling of Age-Dependent Epileptogenesis by Differential Homeostatic Synaptic Scaling. J. Neurosci. 2015, 35, 13448–13462. [Google Scholar] [CrossRef]
- Burman, R.J.; Selfe, J.S.; Lee, J.H.; van den Berg, M.; Calin, A.; Codadu, N.K.; Wright, R.; Newey, S.E.; Parrish, R.R.; Katz, A.A.; et al. Excitatory GABAergic Signalling Is Associated with Benzodiazepine Resistance in Status Epilepticus. Brain 2019, 142, 3482–3501. [Google Scholar] [CrossRef]
- Niquet, J.; Baldwin, R.; Norman, K.; Suchomelova, L.; Lumley, L.; Wasterlain, C.G. Midazolam–Ketamine Dual Therapy Stops Cholinergic Status Epilepticus and Reduces Morris Water Maze Deficits. Epilepsia 2016, 57, 1406–1415. [Google Scholar] [CrossRef]
- Niquet, J.; Baldwin, R.; Suchomelova, L.; Lumley, L.; Eavey, R.; Wasterlain, C.G. Treatment of Experimental Status Epilepticus with Synergistic Drug Combinations. Epilepsia 2017, 58, e49–e53. [Google Scholar] [CrossRef] [PubMed]
- Niquet, J.; Baldwin, R.; Norman, K.; Suchomelova, L.; Lumley, L.; Wasterlain, C.G. Simultaneous Triple Therapy for the Treatment of Status Epilepticus. Neurobiol. Dis. 2017, 104, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Glauser, T.; Shinnar, S.; Gloss, D.; Alldredge, B.; Arya, R.; Bainbridge, J.; Bare, M.; Bleck, T.; Dodson, W.E.; Garrity, L.; et al. Evidence-Based Guideline: Treatment of Convulsive Status Epilepticus in Children and Adults: Report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016, 16, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Niquet, J.; Lumley, L.; Baldwin, R.; Rossetti, F.; Schultz, M.; de Araujo Furtado, M.; Suchomelova, L.; Naylor, D.; Franco-Estrada, I.; Wasterlain, C.G. Early Polytherapy for Benzodiazepine-Refractory Status Epilepticus. Epilepsy Behav. 2019, 101, 106367. [Google Scholar] [CrossRef]
- Nguyen, D.A.; Stone, M.F.; Schultz, C.R.; de Araujo Furtado, M.; Niquet, J.; Wasterlain, C.G.; Lumley, L.A. Evaluation of Midazolam-Ketamine-Allopregnanolone Combination Therapy against Cholinergic-Induced Status Epilepticus in Rats. J. Pharmacol. Exp. Ther. 2024, 388, 376–385. [Google Scholar] [CrossRef]
- Vaitkevicius, H.; Husain, A.M.; Rosenthal, E.S.; Rosand, J.; Bobb, W.; Reddy, K.; Rogawski, M.A.; Cole, A.J. First-in-man Allopregnanolone Use in Super-refractory Status Epilepticus. Ann. Clin. Transl. Neurol. 2017, 4, 411–414. [Google Scholar] [CrossRef]
- Vaitkevicius, H.; Ramsay, R.E.; Swisher, C.B.; Husain, A.M.; Aimetti, A.; Gasior, M. Intravenous Ganaxolone for the Treatment of Refractory Status Epilepticus: Results from an Open-label, Dose-finding, Phase 2 Trial. Epilepsia 2022, 63, 2381–2391. [Google Scholar] [CrossRef]
- Kanes, S.; Rosenthal, E.; Vaitkevicius, H.; Claassen, J.; Wainwright, M.; Hoffman, E.; Baird, M.; Quirk, M.; Colquhoun, H. 547-SSE-201 for Super-Refractory Status Epilepticus: Response and Relationship to Underlying Patient Characteristics (S14.003). Neurology 2016, 86 (Suppl. 16), S14.003. [Google Scholar] [CrossRef]
- Singh, R.K.; Singh, R.; Stewart, A.; Van Poppel, K.; Klinger, S.; Hulihan, J.; Van Heusen, H.; Vaitkevicius, H.; Gasior, M. Intravenous Ganaxolone in Pediatric Super-Refractory Status Epilepticus: A Single Hospital Experience. Epilepsy Behav. Rep. 2022, 20, 100567. [Google Scholar] [CrossRef]
- Perez, D.Q.; Espiritu, A.I.; Jamora, R.D.G. Perampanel in Achieving Status Epilepticus Cessation: A Systematic Review. Epilepsy Behav. 2022, 128, 108583. [Google Scholar] [CrossRef] [PubMed]
- Mazarati, A.M.; Wasterlain, C.G. N-Methyl-d-Asparate Receptor Antagonists Abolish the Maintenance Phase of Self-Sustaining Status Epilepticus in Rat. Neurosci. Lett. 1999, 265, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D. Rescue Therapies for Seizure Emergencies: Current and Future Landscape. Neurol. Sci. 2021, 42, 4017–4027. [Google Scholar] [CrossRef]
- Zolkowska, D.; Wu, C.-Y.; Rogawski, M.A. Intranasal Allopregnanolone Confers Rapid Seizure Protection: Evidence for Direct Nose-to-Brain Delivery. Neurotherapeutics 2021, 18, 544–555. [Google Scholar] [CrossRef]
- Narang, B.; Barve, K.; Wairkar, S. Thermosensitive, Mucoadhesive Brivaracetam Nasal Gel: A Promising Strategy for Targeted Relief of Epilepsy. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025. [Google Scholar] [CrossRef]
- Bitsika, V.; Duveau, V.; Simon-Areces, J.; Mullen, W.; Roucard, C.; Makridakis, M.; Mermelekas, G.; Savvopoulos, P.; Depaulis, A.; Vlahou, A. High-Throughput LC–MS/MS Proteomic Analysis of a Mouse Model of Mesiotemporal Lobe Epilepsy Predicts Microglial Activation Underlying Disease Development. J. Proteome Res. 2016, 15, 1546–1562. [Google Scholar] [CrossRef]
- van Vliet, E.A.; Zibell, G.; Pekcec, A.; Schlichtiger, J.; Edelbroek, P.M.; Holtman, L.; Aronica, E.; Gorter, J.A.; Potschka, H. COX-2 Inhibition Controls P-Glycoprotein Expression and Promotes Brain Delivery of Phenytoin in Chronic Epileptic Rats. Neuropharmacology 2010, 58, 404–412. [Google Scholar] [CrossRef]
- Iannetti, P.; Spalice, A.; Parisi, P. Calcium-Channel Blocker Verapamil Administration in Prolonged and Refractory Status Epilepticus. Epilepsia 2005, 46, 967–969. [Google Scholar] [CrossRef]
- Mathy, F.-X.; Dohin, E.; Bonfitto, F.; Pelgrims, B. Drug–Drug Interaction between Levetiracetam and Non-Vitamin K Antagonist Anticoagulants. Eur. Heart J. 2019, 40, 1571. [Google Scholar] [CrossRef]
- Rejdak, K.; Pikulicka, A.; Piekarska, M.; Pacek, K.; Płachta, K. Inflammation as Treatment Target for Status Epilepticus. Curr. Neuropharmacol. 2023, 21, 708–714. [Google Scholar] [CrossRef]
- Farias-Moeller, R.; Hanin, A.; Ahsan, S.; Brooks, J.; Caganap, S.D.; Czeisler, B.; Cheuret, E.; Cocuzzo, B.; Hahn, C.D.; Kyureghyan, H.; et al. Intrathecal Dexamethasone as a FIRES Extinguisher: A 12-Patient Clinical Experience with Usage of Intrathecal Dexamethasone for Febrile Infection-Related Epilepsy Syndrome. Neurocrit. Care 2025. [Google Scholar] [CrossRef]
- Fisher, K.; Cokley, J. Intrathecal Steroids: A More Targeted Approach to Central Nervous System Inflammation. Neurology 2024, 103, S136–S137. [Google Scholar] [CrossRef]
- Palacios-Mendoza, M.; Gómez, A.; Prieto, J.; Barrios, J.C.; Orera, M.; Massot-Tarrús, A. Response to Anakinra in New-Onset Refractory Status Epilepticus: A Clinical Case. Seizure 2022, 94, 92–94. [Google Scholar] [CrossRef]
- Kenney-Jung, D.L.; Kahoud, R.J.; Vezzani, A.; LaFrance-Corey, R.G.; Ho, M.-L.; Muskardin, T.W.; Gleich, S.J.; Wirrell, E.C.; Howe, C.L.; Payne, E.T. Super-Refractory Status Epilepticus and Febrile Infection-Related Epilepsy Syndrome Treated with Anakinra. Ann. Neurol. 2016, 80, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Liddell, J.R.; Hilton, J.B.W.; Kysenius, K.; Billings, J.L.; Nikseresht, S.; McInnes, L.E.; Hare, D.J.; Paul, B.; Mercer, S.W.; Belaidi, A.A.; et al. Microglial Ferroptotic Stress Causes Non-Cell Autonomous Neuronal Death. Mol. Neurodegener. 2024, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.-Y.; Zhou, H.-H.; Jin, W.-L. Ferroptosis Induction in Pentylenetetrazole Kindling and Pilocarpine-Induced Epileptic Seizures in Mice. Front. Neurosci. 2019, 13, 721. [Google Scholar] [CrossRef]
- Leppik, I.E.; Patterson, E.N.; Coles, L.D.; Craft, E.M.; Cloyd, J.C. Canine Status Epilepticus: A Translational Platform for Human Therapeutic Trials. Epilepsia 2011, 52 (Suppl. 8), 31–34. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshehri, R.S.; Alrawaili, M.S.; Zawawi, B.M.H.; Alzahrany, M.; Habib, A.H. Pathophysiology of Status Epilepticus Revisited. Int. J. Mol. Sci. 2025, 26, 7502. https://doi.org/10.3390/ijms26157502
Alshehri RS, Alrawaili MS, Zawawi BMH, Alzahrany M, Habib AH. Pathophysiology of Status Epilepticus Revisited. International Journal of Molecular Sciences. 2025; 26(15):7502. https://doi.org/10.3390/ijms26157502
Chicago/Turabian StyleAlshehri, Rawiah S., Moafaq S. Alrawaili, Basma M. H. Zawawi, Majed Alzahrany, and Alaa H. Habib. 2025. "Pathophysiology of Status Epilepticus Revisited" International Journal of Molecular Sciences 26, no. 15: 7502. https://doi.org/10.3390/ijms26157502
APA StyleAlshehri, R. S., Alrawaili, M. S., Zawawi, B. M. H., Alzahrany, M., & Habib, A. H. (2025). Pathophysiology of Status Epilepticus Revisited. International Journal of Molecular Sciences, 26(15), 7502. https://doi.org/10.3390/ijms26157502





