The 3D Language of Cancer: Communication via Extracellular Vesicles from Tumor Spheroids and Organoids
Abstract
1. Introduction
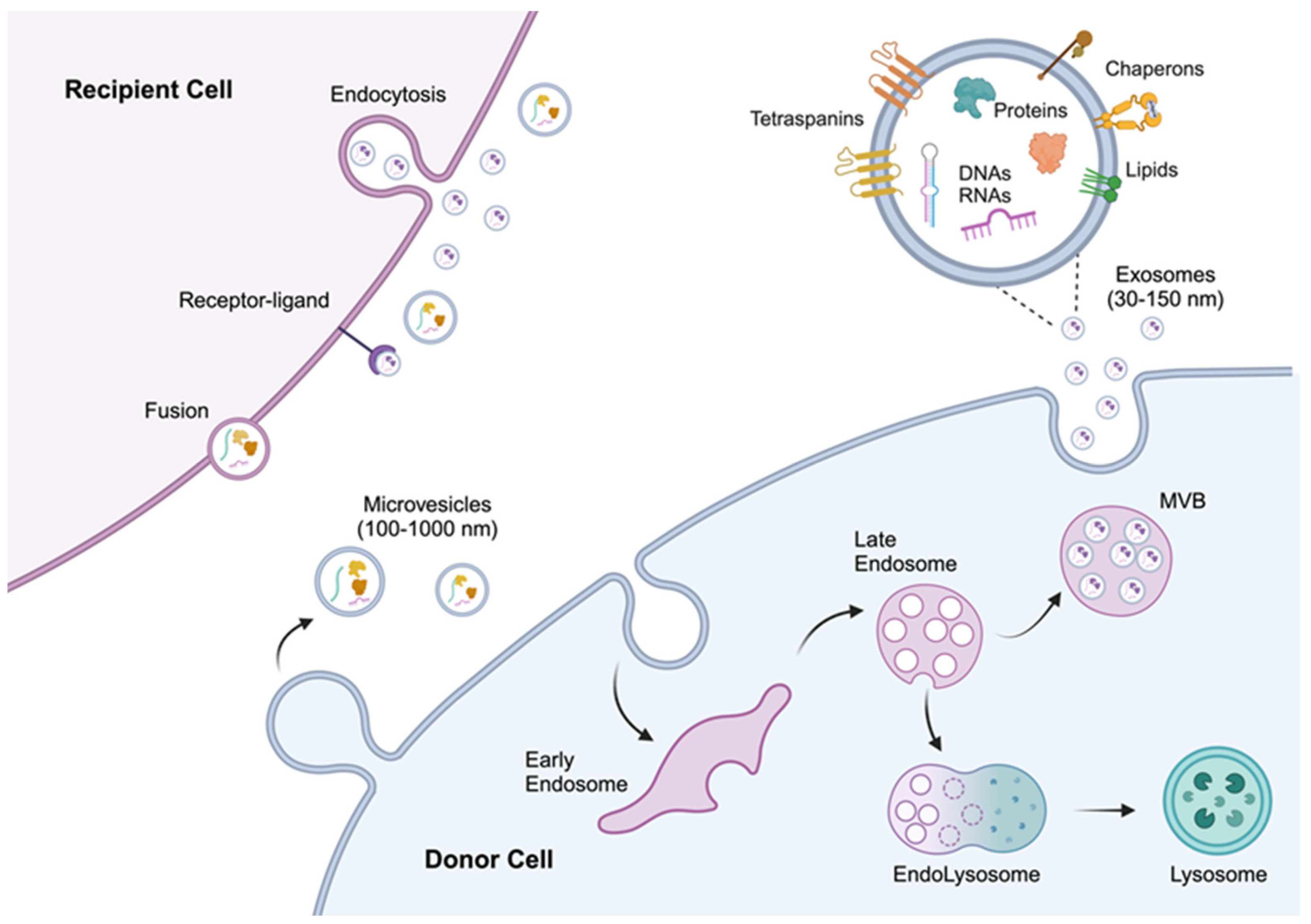
2. Biogenesis and Characteristics of Extracellular Vesicles
3. Isolation and Characterization Techniques
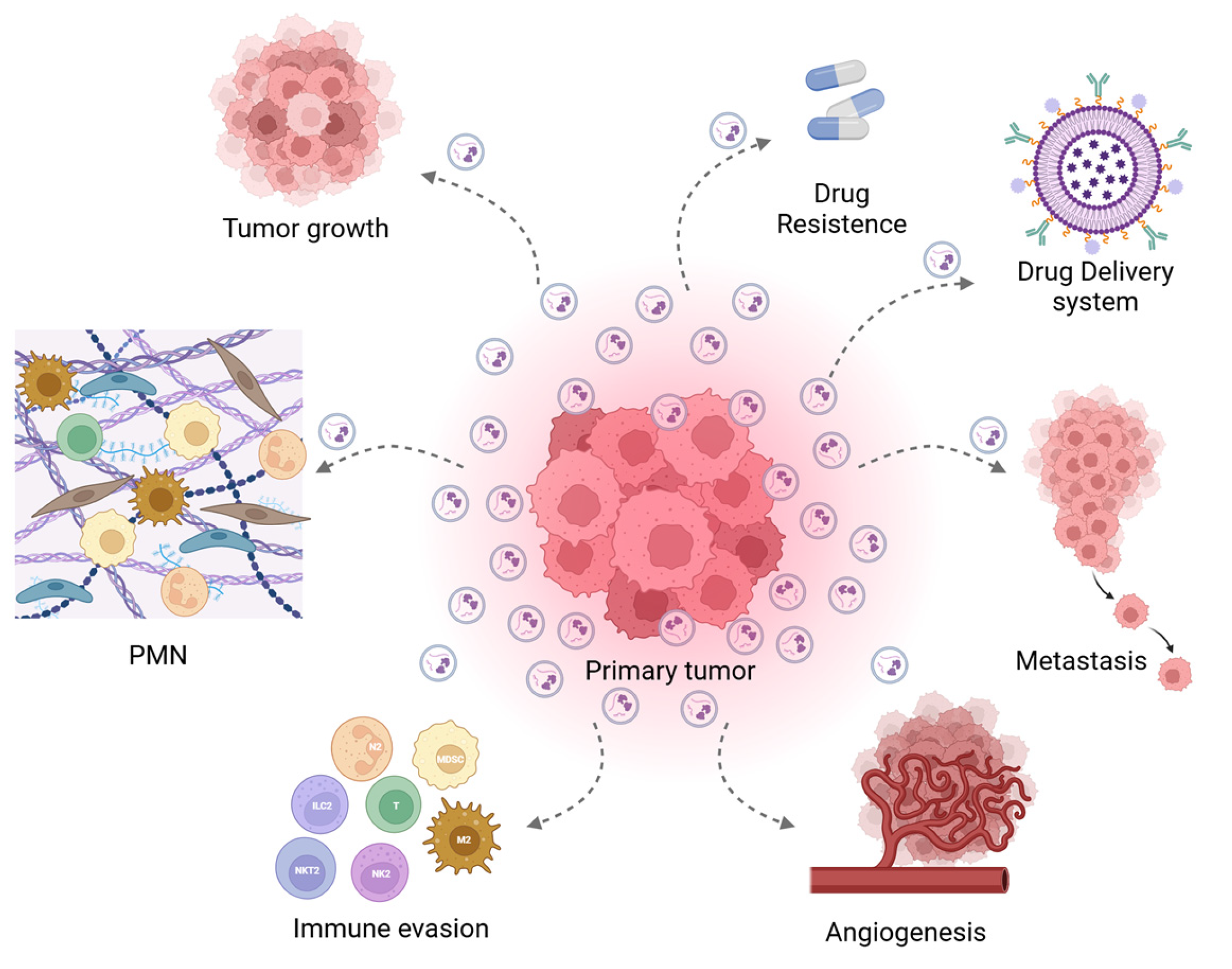
4. Tumor-Derived EVs
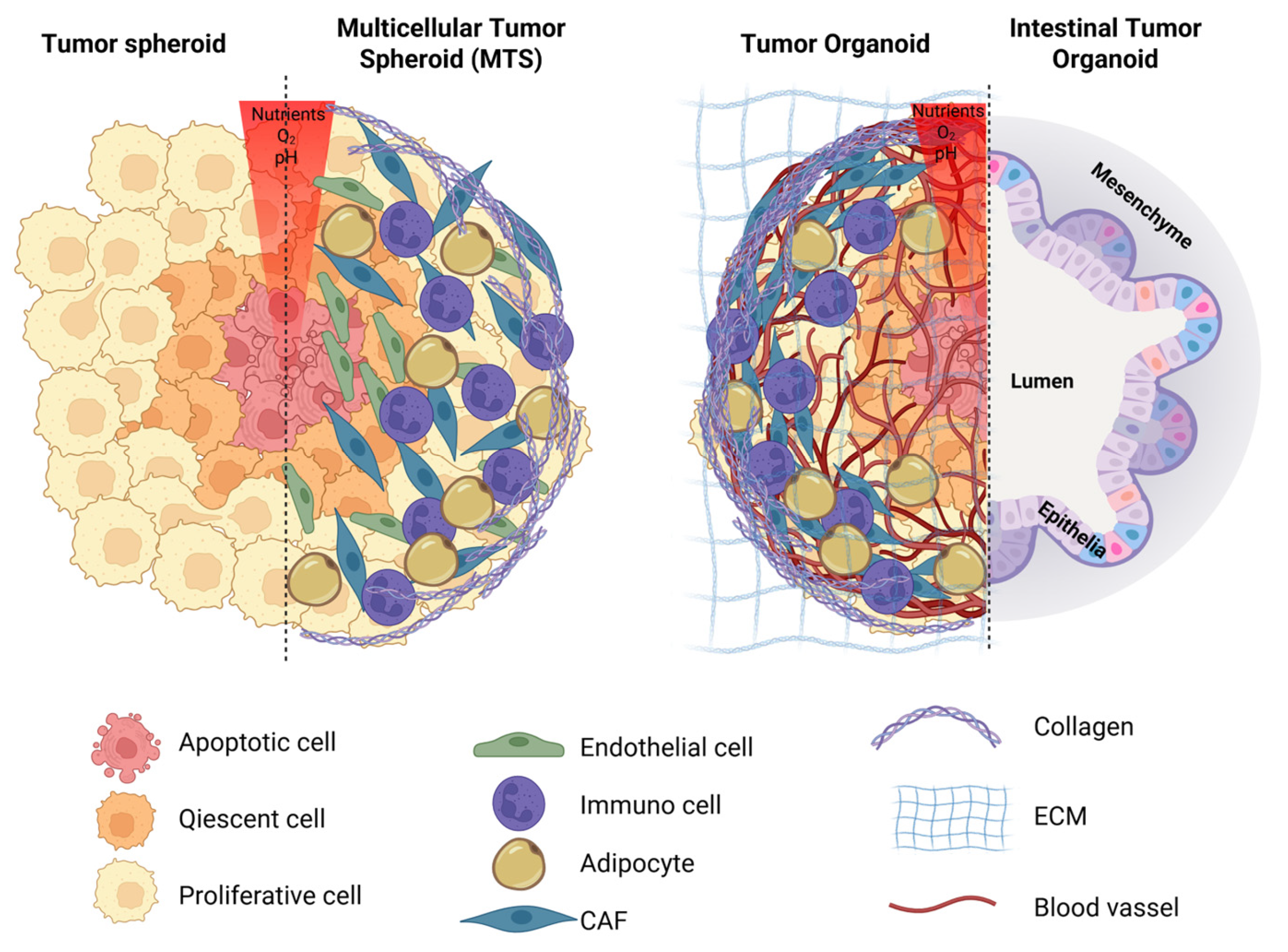
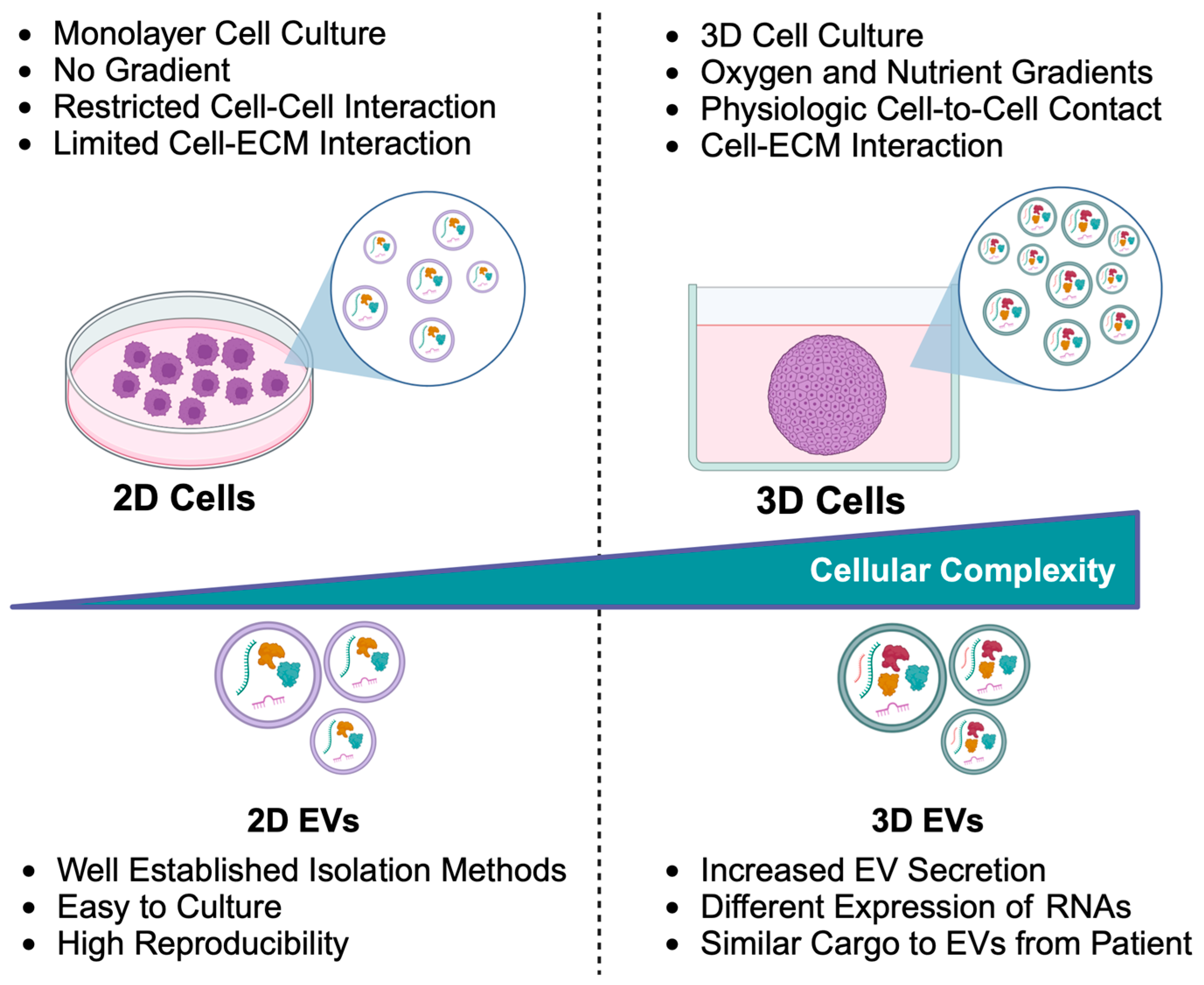
5. Tumor Spheroids and Organoids: An In Vitro Model
6. EVs from Tumor Spheroids
7. EVs from Tumor Organoids
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| 3D EVs | Tumor spheroid-derived extracellular vesicles |
| ARF6 | ADP-ribosylation factor 6 |
| ASCs | Adult tissue-resident stem cells |
| BMDC | Bone marrow-derived cells |
| CAF | Cancer-associated fibroblast |
| CEA | Carcinoembryonic antigen |
| CRC | Colorectal cancer |
| ECs | Endothelial cells |
| ESCRT | Endosomal Sorting Complex Required for Transport |
| ESCs | Embryonic stem cells |
| EVs | Extracellular vesicles |
| FAP | Familial adenomatous polyposis |
| FBS | Fetal bovine serum |
| Fc | Fragment crystallizable |
| FLT1 | Vascular endothelial growth factor receptor |
| GBM | Glioblastoma |
| GelMA | Gelatin methacrylate |
| GMP | Good manufacturing practice |
| HA | Hyaluronic acid |
| HSC | Hepatic stellate cell |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-13 | Interleukin-13 |
| ILV | Intraluminal vesicle |
| iPSCs | Induced pluripotent stem cells |
| MDSC | Myeloid-derived suppressor cell |
| METTL3 | Methyltransferase-like 3 |
| MIO NPs | Magnetic iron oxide nanoparticles |
| MMPs | Matrix metalloproteinases |
| MTS | Multicellular tumor spheroids |
| MVBs | Multivesicular bodies |
| MVs | Microvesicles |
| MSCs | Mesenchymal stromal cells |
| NGF | Nerve growth factor |
| NGFR | NGF receptor |
| NK | Natural Killer |
| PBMCs | Peripheral blood mononuclear cells |
| PD-1 | Death protein 1 |
| PDAC | Pancreatic ductal adenocarcinoma |
| PD-L1 | Programmed death-ligand 1 |
| PDOs | Patient-derived organoids |
| pHEMA | Poly(2-hydroxyethyl methacrylate) |
| PMN | Pre-metastatic niches |
| SNARE | SNAP receptor |
| SNAP | Soluble NSF [N-ethylmaleimide-Sensitive Factor] attachment protein |
| TAM | Tumor-associated macrophages |
| TEM | Transmission Electron Microscopy |
| TME | Tumor microenvironment |
| TMZ | Temozolomide |
| TNBC | Triple-negative breast cancer |
| T-reg | Regulatory T cells |
| VEGF | Vascular endothelial growth factor |
| WJ-MSCs | Wharton’s Jelly-derived MSCs |
References
- Raposo, G.; Stoorvogel, W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and Directions in Studying Cell–Cell Communication by Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Stahl, P.D.; Raposo, G. Extracellular Vesicles Shuffling Intercellular Messages: For Good or for Bad. Curr. Opin. Cell Biol. 2015, 35, 69–77. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Riches, A.; Campbell, E.; Borger, E.; Powis, S. Regulation of Exosome Release from Mammary Epithelial and Breast Cancer Cells-A New Regulatory Pathway. Eur. J. Cancer 2014, 50, 1025–1034. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Hagiwara, K.; Yoshioka, Y.; Takeshita, F.; Ochiya, T. Neutral Sphingomyelinase 2 (NSMase2)-Dependent Exosomal Transfer of Angiogenic Micrornas Regulate Cancer Cell Metastasis. J. Biol. Chem. 2013, 288, 10849–10859. [Google Scholar] [CrossRef]
- Cook, K.; Li, H. Advancing Extracellular Vesicle Production: Improving Physiological Relevance and Yield with 3D Cell Culture. Nanoscale 2025, 17, 15110–15131. [Google Scholar] [CrossRef]
- Khan, N.L.A.; Muhandiram, S.; Dissanayake, K.; Godakumara, K.; Midekessa, G.; Andronowska, A.; Heath, P.R.; Kodithuwakku, S.; Hart, A.R.; Fazeli, A. Effect of 3D and 2D Cell Culture Systems on Trophoblast Extracellular Vesicle Physico-Chemical Characteristics and Potency. Front. Cell Dev. Biol. 2024, 12, 1382552. [Google Scholar] [CrossRef]
- Wu, B.; Liu, D.A.; Guan, L.; Myint, P.K.; Chin, L.K.; Dang, H.; Xu, Y.; Ren, J.; Li, T.; Yu, Z.; et al. Stiff Matrix Induces Exosome Secretion to Promote Tumour Growth. Nat. Cell Biol. 2023, 25, 415–424. [Google Scholar] [CrossRef]
- Paniushkina, L.; Grueso-Navarro, E.; Cheng, X.; Nazarenko, I. Three-Dimensional Cell Models for Extracellular Vesicles Production, Isolation, and Characterization. Methods Enzymol. 2020, 645, 209–230. [Google Scholar] [CrossRef]
- Abuwatfa, W.H.; Pitt, W.G.; Husseini, G.A. Scaffold-Based 3D Cell Culture Models in Cancer Research. J. Biomed. Sci. 2024, 31, 7. [Google Scholar] [CrossRef]
- Krylova, S.V.; Feng, D. The Machinery of Exosomes: Biogenesis, Release, and Uptake. Int. J. Mol. Sci. 2023, 24, 1337. [Google Scholar] [CrossRef] [PubMed]
- Vats, S.; Galli, T. Role of SNAREs in Unconventional Secretion—Focus on the VAMP7-Dependent Secretion. Front. Cell Dev. Biol. 2022, 10, 884020. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef] [PubMed]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX Exosome Biogenesis and Budding into Multivesicular Bodies Are Controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef]
- Henne, W.M.; Stenmark, H.; Emr, S.D. Molecular Mechanisms of the Membrane Sculpting ESCRT Pathway. Cold Spring Harb. Perspect. Biol. 2013, 5, a016766. [Google Scholar] [CrossRef]
- Colombo, M.; Moita, C.; Van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Moita, L.F.; Théry, C.; Raposo, G. Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Raposo, G. The Cell Biology of Exosomes: Historical and Perspectives. In Emerging Concepts of Tumor Exosome–Mediated Cell-Cell Communication; Springer: New York, NY, USA, 2012; pp. 1–32. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brügger, B.; Simons, M. Ceramide Triggers Budding of Exosome Vesicles into Multivesicular Endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Edgar, J.R.; Eden, E.R.; Futter, C.E. Hrs- and CD63-Dependent Competing Mechanisms Make Different Sized Endosomal Intraluminal Vesicles. Traffic 2014, 15, 197–211. [Google Scholar] [CrossRef]
- Palmulli, R.; Couty, M.; Piontek, M.C.; Ponnaiah, M.; Dingli, F.; Verweij, F.J.; Charrin, S.; Tantucci, M.; Sasidharan, S.; Rubinstein, E.; et al. CD63 Sorts Cholesterol into Endosomes for Storage and Distribution via Exosomes. Nat. Cell Biol. 2024, 26, 1093–1109. [Google Scholar] [CrossRef]
- Fan, Y.; Pionneau, C.; Cocozza, F.; Boëlle, P.Y.; Chardonnet, S.; Charrin, S.; Théry, C.; Zimmermann, P.; Rubinstein, E. Differential Proteomics Argues against a General Role for CD9, CD81 or CD63 in the Sorting of Proteins into Extracellular Vesicles. J. Extracell. Vesicles 2023, 12, e12352. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Jeppesen, D.K.; Laurberg, J.R.; Boysen, A.T.; Bramsen, J.B.; Primdal-Bengtson, B.; Hendrix, A.; Lamy, P.; Dagnaes-Hansen, F.; Rasmussen, M.H.; et al. Cellular Disposal of MiR23b by RAB27-Dependent Exosome Release Is Linked to Acquisition of Metastatic Properties. Cancer Res. 2014, 74, 5758–5771. [Google Scholar] [CrossRef]
- Wang, G.; Li, J.; Bojmar, L.; Chen, H.; Li, Z.; Tobias, G.C.; Hu, M.; Homan, E.A.; Lucotti, S.; Zhao, F.; et al. Tumour Extracellular Vesicles and Particles Induce Liver Metabolic Dysfunction. Nature 2023, 618, 374–382. [Google Scholar] [CrossRef]
- Peche, V.S.; Pietka, T.A.; Jacome-Sosa, M.; Samovski, D.; Palacios, H.; Chatterjee-Basu, G.; Dudley, A.C.; Beatty, W.; Meyer, G.A.; Goldberg, I.J.; et al. Endothelial Cell CD36 Regulates Membrane Ceramide Formation, Exosome Fatty Acid Transfer and Circulating Fatty Acid Levels. Nat. Commun. 2023, 14, 4029. [Google Scholar] [CrossRef]
- Boshans, R.L.; Szanto, S.; van Aelst, L.; D’Souza-Schorey, C. ADP-Ribosylation Factor 6 Regulates Actin Cytoskeleton Remodeling in Coordination with Rac1 and RhoA. Mol. Cell. Biol. 2000, 20, 3685–3694. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, L.; Kawashima, M.; Sugimoto, M.; Sonomura, K.; Pu, F.; Li, W.; Takeda, M.; Goto, T.; Kawaguchi, K.; Sato, T.A.; et al. Discovery of Lipid Profiles in Plasma-Derived Extracellular Vesicles as Biomarkers for Breast Cancer Diagnosis. Cancer Sci. 2023, 114, 4020–4031. [Google Scholar] [CrossRef] [PubMed]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal Lipid Composition and the Role of Ether Lipids and Phosphoinositides in Exosome Biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Pfrieger, F.W.; Vitale, N. Thematic Review Series: Exosomes and Microvesicles: Lipids as Key Components of Their Biogenesis and Functions Cholesterol and the Journey of Extracellular Vesicles. J. Lipid Res. 2018, 59, 2255–2261. [Google Scholar] [CrossRef]
- Lauwers, E.; Wang, Y.C.; Gallardo, R.; Van der Kant, R.; Michiels, E.; Swerts, J.; Baatsen, P.; Zaiter, S.S.; McAlpine, S.R.; Gounko, N.V.; et al. Hsp90 Mediates Membrane Deformation and Exosome Release. Mol. Cell 2018, 71, 689–702.e9. [Google Scholar] [CrossRef]
- Nolte’T Hoen, E.N.M.; Buermans, H.P.J.; Waasdorp, M.; Stoorvogel, W.; Wauben, M.H.M.; ’T Hoen, P.A.C. Deep Sequencing of RNA from Immune Cell-Derived Vesicles Uncovers the Selective Incorporation of Small Non-Coding RNA Biotypes with Potential Regulatory Functions. Nucleic Acids Res. 2012, 40, 9272–9285. [Google Scholar] [CrossRef]
- Skog, J.; Würdinger, T.; van Rijn, S.; Meijer, D.H.; Gainche, L.; Curry, W.T.; Carter, B.S.; Krichevsky, A.M.; Breakefield, X.O. Glioblastoma Microvesicles Transport RNA and Proteins That Promote Tumour Growth and Provide Diagnostic Biomarkers. Nat. Cell Biol. 2008, 10, 1470–1476. [Google Scholar] [CrossRef]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R.; et al. Breast-Cancer-Secreted MiR-122 Reprograms Glucose Metabolism in Premetastatic Niche to Promote Metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef]
- Sruthi, T.V.; Edatt, L.; Raji, G.R.; Kunhiraman, H.; Shankar, S.S.; Shankar, V.; Ramachandran, V.; Poyyakkara, A.; Kumar, S.V.B. Horizontal Transfer of MiR-23a from Hypoxic Tumor Cell Colonies Can Induce Angiogenesis. J. Cell. Physiol. 2018, 233, 3498–3514. [Google Scholar] [CrossRef]
- Kulkarni, B.; Gondaliya, P.; Kirave, P.; Rawal, R.; Jain, A.; Garg, R.; Kalia, K. Exosome-Mediated Delivery of MiR-30a Sensitize Cisplatin-Resistant Variant of Oral Squamous Carcinoma Cells via Modulating Beclin1 and Bcl2. Oncotarget 2020, 11, 1832–1845. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.L.; Delevoye, C.; Gilles-Marsens, F.; Loew, D.; Dingli, F.; Guéré, C.; André, N.; Vié, K.; Van Niel, G.; Raposo, G. Exosomes Released by Keratinocytes Modulate Melanocyte Pigmentation. Nat. Commun. 2015, 6, 7506. [Google Scholar] [CrossRef]
- Li, B.; Cao, Y.; Sun, M.; Feng, H. Expression, Regulation, and Function of Exosome-Derived MiRNAs in Cancer Progression and Therapy. FASEB J. 2021, e21916. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Y.; Ma, L.; Chen, Y.; Liu, J.; Guo, Y.; Yu, T.; Zhang, L.; Zhu, L.; Shu, Y. Role of Exosomal Non-Coding RNAs from Tumor Cells and Tumor-Associated Macrophages in the Tumor Microenvironment. Mol. Ther. 2022, 30, 3133–3154. [Google Scholar] [CrossRef]
- Dou, Q.; Wang, J.; Yang, Y.; Zhuo, W. Roles of Exosome-Derived Non-Coding RNA in Tumor Micro-Environment and Its Clinical Application. J. Zhejiang Univ. (Med. Sci.) 2025, 52, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, A.; Gennebäck, N.; Hellman, U.; Ronquist, G. Cardiomyocyte Microvesicles Contain DNA/RNA and Convey Biological Messages to Target Cells. PLoS ONE 2012, 7, e34653. [Google Scholar] [CrossRef]
- Fernando, M.R.; Jiang, C.; Krzyzanowski, G.D.; Ryan, W.L. New Evidence That a Large Proportion of Human Blood Plasma Cell-Free DNA Is Localized in Exosomes. PLoS ONE 2017, 12, e0183915. [Google Scholar] [CrossRef]
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and Transfer of Mitochondrial DNA via Exosomes Regulate Escape from Dormancy in Hormonal Therapy-Resistant Breast Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075. [Google Scholar] [CrossRef]
- Konaka, H.; Kato, Y.; Hirano, T.; Tsujimoto, K.; Park, J.; Koba, T.; Aoki, W.; Matsuzaki, Y.; Taki, M.; Koyama, S.; et al. Secretion of Mitochondrial DNA via Exosomes Promotes Inflammation in Behçet’s Syndrome. EMBO J. 2023, 42, e112573. [Google Scholar] [CrossRef] [PubMed]
- D’Acunzo, P.; Kim, Y.; Ungania, J.M.; Pérez-González, R.; Goulbourne, C.N.; Levy, E. Isolation of Mitochondria-Derived Mitovesicles and Subpopulations of Microvesicles and Exosomes from Brain Tissues. Nat. Protoc. 2022, 17, 2517–2549. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular Vesicles in Cancer—Implications for Future Improvements in Cancer Care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Sriwastva, M.K.; Teng, Y.; Mu, J.; Xu, F.; Kumar, A.; Sundaram, K.; Malhotra, R.K.; Xu, Q.; Hood, J.L.; Zhang, L.; et al. An Extracellular Vesicular Mutant KRAS-Associated Protein Complex Promotes Lung Inflammation and Tumor Growth. J. Extracell. Vesicles 2023, 12, 12307. [Google Scholar] [CrossRef]
- Bhatta, B.; Luz, I.; Krueger, C.; Teo, F.X.; Lane, D.P.; Sabapathy, K.; Cooks, T. Cancer Cells Shuttle Extracellular Vesicles Containing Oncogenic Mutant P53 Proteins to the Tumor Microenvironment. Cancers 2021, 13, 2985. [Google Scholar] [CrossRef]
- Janpipatkul, K.; Panvongsa, W.; Worakitchanon, W.; Reungwetwattana, T.; Chairoungdua, A. Extracellular Vesicles from EGFR T790M/L858R-Mutant Non-Small Cell Lung Cancer Promote Cancer Progression. Anticancer. Res. 2022, 42, 3835–3844. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Tan, X.; Du, Y.; Wei, Y.; Liu, S. Extracellular Vesicle-Mediated Pre-Metastatic Niche Formation via Altering Host Microenvironments. Front. Immunol. 2024, 15, 1367373. [Google Scholar] [CrossRef]
- Li, M.X.; Hu, S.; Lei, H.H.; Yuan, M.; Li, X.; Hou, W.K.; Huang, X.J.; Xiao, B.W.; Yu, T.X.; Zhang, X.H.; et al. Tumor-Derived MiR-9-5p-Loaded EVs Regulate Cholesterol Homeostasis to Promote Breast Cancer Liver Metastasis in Mice. Nat. Commun. 2024, 15, 10539. [Google Scholar] [CrossRef]
- Cariello, M.; Squilla, A.; Piacente, M.; Venutolo, G.; Fasano, A. Drug Resistance: The Role of Exosomal MiRNA in the Microenvironment of Hematopoietic Tumors. Molecules 2022, 28, 116. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Caires, H.R.; Bergantim, R.; Guimarães, J.E.; Vasconcelos, M.H. MiRNAs Mediated Drug Resistance in Hematological Malignancies. Semin. Cancer Biol. 2022, 83, 283–302. [Google Scholar] [CrossRef]
- Galati, D.; Solimando, A.G.; Reale, A.; Khong, T.; Spencer, A. Extracellular Vesicles and Their Roles in the Tumor Immune Microenvironment. J. Clin. Med. 2022, 11, 6892. [Google Scholar] [CrossRef] [PubMed]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A Comparison of Methods for the Isolation and Separation of Extracellular Vesicles from Protein and Lipid Particles in Human Serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Franklin, J.L.; Coffey, R.J. Comprehensive Isolation of Extracellular Vesicles and Nanoparticles. Nat. Protoc. 2023, 18, 1462–1487. [Google Scholar] [CrossRef] [PubMed]
- Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Greening, D.W.; Xu, R.; Ji, H.; Tauro, B.J.; Simpson, R.J. A Protocol for Exosome Isolation and Characterization: Evaluation of Ultracentrifugation, Density-Gradient Separation, and Immunoaffinity Capture Methods. Methods Mol. Biol. 2015, 1295, 179–209. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Krumeich, S.; Raposo, G.; Théry, C. Diverse Subpopulations of Vesicles Secreted by Different Intracellular Mechanisms Are Present in Exosome Preparations Obtained by Differential Ultracentrifugation. J. Extracell. Vesicles 2012, 1, 18397. [Google Scholar] [CrossRef]
- Gupta, S.; Rawat, S.; Arora, V.; Kottarath, S.K.; Dinda, A.K.; Vaishnav, P.K.; Nayak, B.; Mohanty, S. An Improvised One-Step Sucrose Cushion Ultracentrifugation Method for Exosome Isolation from Culture Supernatants of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 180. [Google Scholar] [CrossRef]
- Filipović, L.; Spasojević, M.; Prodanović, R.; Korać, A.; Matijaševic, S.; Brajušković, G.; de Marco, A.; Popović, M. Affinity-Based Isolation of Extracellular Vesicles by Means of Single-Domain Antibodies Bound to Macroporous Methacrylate-Based Copolymer. New Biotechnol. 2022, 69, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Stranska, R.; Gysbrechts, L.; Wouters, J.; Vermeersch, P.; Bloch, K.; Dierickx, D.; Andrei, G.; Snoeck, R. Comparison of Membrane Affinity-Based Method with Size-Exclusion Chromatography for Isolation of Exosome-like Vesicles from Human Plasma. J. Transl. Med. 2018, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Leijendekker, R.; Harding, C.V.; Melief, C.J.M.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Pascucci, L.; Scattini, G. Imaging Extracelluar Vesicles by Transmission Electron Microscopy: Coping with Technical Hurdles and Morphological Interpretation. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129648. [Google Scholar] [CrossRef]
- Nolan, J.P.; Duggan, E. Analysis of Individual Extracellular Vesicles by Flow Cytometry. Methods Mol. Biol. 2018, 1678, 79–92. [Google Scholar] [CrossRef]
- van der Vlist, E.J.; Nolte-’t Hoen, E.N.M.; Stoorvogel, W.; Arkesteijn, G.J.A.; Wauben, M.H.M. Fluorescent Labeling of Nano-Sized Vesicles Released by Cells and Subsequent Quantitative and Qualitative Analysis by High-Resolution Flow Cytometry. Nat. Protoc. 2012, 7, 1311–1326. [Google Scholar] [CrossRef]
- Pocsfalvi, G.; Stanly, C.; Vilasi, A.; Fiume, I.; Capasso, G.; Turiák, L.; Buzas, E.I.; Vékey, K. Mass Spectrometry of Extracellular Vesicles. Mass. Spectrom. Rev. 2016, 35, 3–21. [Google Scholar] [CrossRef]
- Acland, M.; Mittal, P.; Lokman, N.A.; Klingler-Hoffmann, M.; Oehler, M.K.; Hoffmann, P. Mass Spectrometry Analyses of Multicellular Tumor Spheroids. Proteom. Clin. Appl. 2018, 12, e1700124. [Google Scholar] [CrossRef]
- Ma, L.; Liu, Y.H.; Liu, C.; Wang, S.Q.; Ma, J.; Li, X.Q.; Ren, M.; Yang, T.T.; Liu, G.Z. LncRNA, MiRNA, and MRNA of Plasma and Tumor-Derived Exosomes of Cardiac Myxoma-Related Ischaemic Stroke. Sci. Data 2025, 12, 91. [Google Scholar] [CrossRef]
- Yuana, Y.; Sturk, A.; Nieuwland, R. Extracellular Vesicles in Physiological and Pathological Conditions. Blood Rev. 2013, 27, 31–39. [Google Scholar] [CrossRef]
- Chang, W.H.; Cerione, R.A.; Antonyak, M.A. Extracellular Vesicles and Their Roles in Cancer Progression. Methods Mol. Biol. 2021, 2174, 143–170. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, A.; Majkowska, I.; Nagase, H.; Di Liegro, I.; Troeberg, L. Microvesicles Shed by Oligodendroglioma Cells and Rheumatoid Synovial Fibroblasts Contain Aggrecanase Activity. Matrix Biol. 2012, 31, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Wandrey, M.; Jablonska, J.; Stauber, R.H.; Gül, D. Exosomes in Cancer Progression and Therapy Resistance: Molecular Insights and Therapeutic Opportunities. Life 2023, 13, 2033. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular Vesicles in Cancer: Exosomes, Microvesicles and the Emerging Role of Large Oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Tang, M.K.S.; Yue, P.Y.K.; Ip, P.P.; Huang, R.L.; Lai, H.C.; Cheung, A.N.Y.; Tse, K.Y.; Ngan, H.Y.S.; Wong, A.S.T. Soluble E-Cadherin Promotes Tumor Angiogenesis and Localizes to Exosome Surface. Nat. Commun. 2018, 9, e1700124. [Google Scholar] [CrossRef]
- Chen, S.; Chen, X.; Luo, Q.; Liu, X.; Wang, X.; Cui, Z.; He, A.; He, S.; Jiang, Z.; Wu, N.; et al. Retinoblastoma Cell-Derived Exosomes Promote Angiogenesis of Human Vesicle Endothelial Cells through MicroRNA-92a-3p. Cell Death Dis. 2021, 12, 695. [Google Scholar] [CrossRef]
- Wang, L.; Qiao, C.; Han, L.; Wang, X.; Miao, J.; Cao, L.; Huang, C.; Wang, J. HOXD3 Promotes the Migration and Angiogenesis of Hepatocellular Carcinoma via Modifying Hepatocellular Carcinoma Cells Exosome-Delivered CCR6 and Regulating Chromatin Conformation of CCL20. Cell Death Dis. 2024, 15, 221. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D. Exosomes in Cancer Development, Metastasis, and Immunity. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 455–468. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Taverna, S.; Flugy, A.; Saieva, L.; Kohn, E.C.; Santoro, A.; Meraviglia, S.; De Leo, G.; Alessandro, R. Role of Exosomes Released by Chronic Myelogenous Leukemia Cells in Angiogenesis. Int. J. Cancer 2012, 130, 2033–2043. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Wang, F.; Fang, Y.; Yang, Y.; Zhou, Q.; Yuan, W.; Gu, X.; Hu, J.; Yang, S. Pre-Metastatic Niche: Formation, Characteristics and Therapeutic Implication. Signal Transduct. Target. Ther. 2024, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Alečković, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; García-Santos, G.; Ghajar, C.M.; et al. Melanoma Exosomes Educate Bone Marrow Progenitor Cells toward a Pro-Metastatic Phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- García-Silva, S.; Benito-Martín, A.; Nogués, L.; Hernández-Barranco, A.; Mazariegos, M.S.; Santos, V.; Hergueta-Redondo, M.; Ximénez-Embún, P.; Kataru, R.P.; Lopez, A.A.; et al. Melanoma-Derived Small Extracellular Vesicles Induce Lymphangiogenesis and Metastasis through an NGFR-Dependent Mechanism. Nat. Cancer 2021, 2, 1387–1405. [Google Scholar] [CrossRef] [PubMed]
- Siddhartha, R.; Garg, M. Interplay Between Extracellular Matrix Remodeling and Angiogenesis in Tumor Ecosystem. Mol. Cancer Ther. 2023, 22, 291–305. [Google Scholar] [CrossRef]
- Kong, J.; Tian, H.; Zhang, F.; Zhang, Z.; Li, J.; Liu, X.; Li, X.; Liu, J.; Li, X.; Jin, D.; et al. Extracellular Vesicles of Carcinoma-Associated Fibroblasts Creates a Pre-Metastatic Niche in the Lung through Activating Fibroblasts. Mol. Cancer 2019, 18, 175. [Google Scholar] [CrossRef]
- González-Callejo, P.; Gener, P.; Díaz-Riascos, Z.V.; Conti, S.; Cámara-Sánchez, P.; Riera, R.; Mancilla, S.; García-Gabilondo, M.; Peg, V.; Arango, D.; et al. Extracellular Vesicles Secreted by Triple-Negative Breast Cancer Stem Cells Trigger Premetastatic Niche Remodeling and Metastatic Growth in the Lungs. Int. J. Cancer 2023, 152, 2153–2165. [Google Scholar] [CrossRef]
- Costa-Silva, B.; Aiello, N.M.; Ocean, A.J.; Singh, S.; Zhang, H.; Thakur, B.K.; Becker, A.; Hoshino, A.; Mark, M.T.; Molina, H.; et al. Pancreatic Cancer Exosomes Initiate Pre-Metastatic Niche Formation in the Liver. Nat. Cell Biol. 2015, 17, 816–826. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 Contributes to Immunosuppression and Is Associated with Anti-PD-1 Response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Zhu, G.; Cao, B.; Liang, X.; Li, L.; Hao, Y.; Meng, W.; He, C.; Wang, L.; Li, L. Small Extracellular Vesicles Containing MiR-192/215 Mediate Hypoxia-Induced Cancer-Associated Fibroblast Development in Head and Neck Squamous Cell Carcinoma. Cancer Lett. 2021, 506, 11–22. [Google Scholar] [CrossRef]
- Li, Y.; Gao, S.; Hu, Q.; Wu, F. Functional Properties of Cancer Epithelium and Stroma-Derived Exosomes in Head and Neck Squamous Cell Carcinoma. Life 2022, 12, 757. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, Y.; Zhang, Y.; Li, H.; Li, L.; Wu, Y.; Chen, X.; Qiu, L.; Han, J.; Wang, Z. Colorectal Cancer-Derived Extracellular Vesicles Induce Liver Premetastatic Immunosuppressive Niche Formation to Promote Tumor Early Liver Metastasis. Signal Transduct. Target. Ther. 2023, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Nittayaboon, K.; Leetanaporn, K.; Sangkhathat, S.; Roytrakul, S.; Navakanitworakul, R. Proteomic Analysis of Butyrate-Resistant Colorectal Cancer-Derived Exosomes Reveals Potential Resistance to Anti-Cancer Drugs. Discov. Med. 2024, 36, 1306. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.N.d.S.; Otake, A.H.; Cardim, S.G.B.; da Silva, F.I.; Ikoma Sakamoto, M.M.; Furuya, T.K.; Uno, M.; Pasini, F.S.; Chammas, R. Extracellular Vesicles Shedding Promotes Melanoma Growth in Response to Chemotherapy. Sci. Rep. 2019, 9, 14482. [Google Scholar] [CrossRef]
- Yang, E.; Wang, L.; Jin, W.; Liu, X.; Wang, Q.; Wu, Y.; Tan, Y.; Wang, Y.; Cui, X.; Zhao, J.; et al. PTRF/Cavin-1 Enhances Chemo-Resistance and Promotes Temozolomide Efflux through Extracellular Vesicles in Glioblastoma. Theranostics 2022, 12, 4330–4347. [Google Scholar] [CrossRef]
- Serratì, S.; Guida, M.; Di Fonte, R.; De Summa, S.; Strippoli, S.; Iacobazzi, R.M.; Quarta, A.; De Risi, I.; Guida, G.; Paradiso, A.; et al. Circulating Extracellular Vesicles Expressing PD1 and PD-L1 Predict Response and Mediate Resistance to Checkpoint Inhibitors Immunotherapy in Metastatic Melanoma. Mol. Cancer 2022, 21, 20. [Google Scholar] [CrossRef]
- Yu, Z.L.; Liu, J.Y.; Chen, G. Small Extracellular Vesicle PD-L1 in Cancer: The Knowns and Unknowns. npj Precis. Oncol. 2022, 6, 42. [Google Scholar] [CrossRef]
- Kang, S.H.; Oh, S.Y.; Lee, K.Y.; Lee, H.J.; Kim, M.S.; Kwon, T.G.; Kim, J.W.; Lee, S.T.; Choi, S.Y.; Hong, S.H. Differential Effect of Cancer-Associated Fibroblast-Derived Extracellular Vesicles on Cisplatin Resistance in Oral Squamous Cell Carcinoma via MiR-876-3p. Theranostics 2024, 14, 460–479. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Ruan, Z. GATA3 Encapsulated by Tumor-Associated Macrophage-Derived Extracellular Vesicles Promotes Immune Escape and Chemotherapy Resistance of Ovarian Cancer Cells by Upregulating the CD24/Siglec-10 Axis. Mol. Pharm. 2023, 20, 971–986. [Google Scholar] [CrossRef]
- Ning, J.; Hou, X.; Hao, J.; Zhang, W.; Shi, Y.; Huang, Y.; Ruan, X.; Zheng, X.; Gao, M. METTL3 Inhibition Induced by M2 Macrophage-Derived Extracellular Vesicles Drives Anti-PD-1 Therapy Resistance via M6A-CD70-Mediated Immune Suppression in Thyroid Cancer. Cell Death Differ. 2023, 30, 2265–2279. [Google Scholar] [CrossRef]
- Wiklander, O.P.B.; Mamand, D.R.; Mohammad, D.K.; Zheng, W.; Jawad Wiklander, R.; Sych, T.; Zickler, A.M.; Liang, X.; Sharma, H.; Lavado, A.; et al. Antibody-Displaying Extracellular Vesicles for Targeted Cancer Therapy. Nat. Biomed. Eng. 2024, 8, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Chen, J.; Li, Q.; Li, Y.; Zhang, L.; Zhida, L.; Yuan, F.; Zhang, R. Tumor-Derived Extracellular Vesicle Drug Delivery System for Chemo-Photothermal-Immune Combination Cancer Treatment. iScience 2024, 27, 108833. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, K.; Ranjbar, M.; Pirpour Tazehkand, A.; Asgharian, P.; Montazersaheb, S.; Tarhriz, V.; Ghasemnejad, T. Evaluation of Exosomal Non-Coding RNAs in Cancer Using High-Throughput Sequencing. J. Transl. Med. 2022, 20, 30. [Google Scholar] [CrossRef]
- Li, L.; Zhang, L.; Montgomery, K.C.; Jiang, L.; Lyon, C.J.; Hu, T.Y. Advanced Technologies for Molecular Diagnosis of Cancer: State of Pre-Clinical Tumor-Derived Exosome Liquid Biopsies. Mater. Today Bio 2023, 18, 100538. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Yang, X.; Jiang, Y.; Li, A.; Cong, J.; Li, Y.; Xie, Q.; Xu, C.; Liu, D. Identification of Faecal Extracellular Vesicles as Novel Biomarkers for the Non-Invasive Diagnosis and Prognosis of Colorectal Cancer. J. Extracell. Vesicles 2023, 12, 12300. [Google Scholar] [CrossRef]
- Yin, H.; Xie, J.; Xing, S.; Lu, X.; Yu, Y.; Ren, Y.; Tao, J.; He, G.; Zhang, L.; Yuan, X.; et al. Machine Learning-Based Analysis Identifies and Validates Serum Exosomal Proteomic Signatures for the Diagnosis of Colorectal Cancer. Cell Rep. Med. 2024, 5, 101689. [Google Scholar] [CrossRef]
- Yousafzai, N.A.; El Khalki, L.; Wang, W.; Szpendyk, J.; Sossey-Alaoui, K. Advances in 3D Culture Models to Study Exosomes in Triple-Negative Breast Cancer. Cancers 2024, 16, 883. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Reis, R.L.; Pirraco, R.P. Modelling the Complex Nature of the Tumor Microenvironment: 3D Tumor Spheroids as an Evolving Tool. J. Biomed. Sci. 2024, 31, 13. [Google Scholar] [CrossRef]
- Plava, J.; Cehakova, M.; Kuniakova, M.; Trnkova, L.; Cihova, M.; Bohac, M.; Danisovic, L. The Third Dimension of Tumor Microenvironment—The Importance of Tumor Stroma in 3D Cancer Models. Exp. Biol. Med. 2023, 248, 1347–1358. [Google Scholar] [CrossRef]
- El Harane, S.; Zidi, B.; El Harane, N.; Krause, K.H.; Matthes, T.; Preynat-Seauve, O. Cancer Spheroids and Organoids as Novel Tools for Research and Therapy: State of the Art and Challenges to Guide Precision Medicine. Cells 2023, 12, 1001. [Google Scholar] [CrossRef]
- Kirsh, S.M.; Pascetta, S.A.; Uniacke, J. Spheroids as a 3D Model of the Hypoxic Tumor Microenvironment. Methods Mol. Biol. 2023, 2614, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Laurent, J.; Frongia, C.; Cazales, M.; Mondesert, O.; Ducommun, B.; Lobjois, V. Multicellular Tumor Spheroid Models to Explore Cell Cycle Checkpoints in 3D. BMC Cancer 2013, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Riffle, S.; Pandey, R.N.; Albert, M.; Hegde, R.S. Linking Hypoxia, DNA Damage and Proliferation in Multicellular Tumor Spheroids. BMC Cancer 2017, 17, 338. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Calar, K.; De La Puente, P. Mimicking Tumor Hypoxia and Tumor-Immune Interactions Employing Three-Dimensional in Vitro Models. J. Exp. Clin. Cancer Res. 2020, 39, 75. [Google Scholar] [CrossRef]
- Godet, I.; Doctorman, S.; Wu, F.; Gilkes, D.M. Detection of Hypoxia in Cancer Models: Significance, Challenges, and Advances. Cells 2022, 11, 686. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Lee, M.; Kim, Y.; Yu, S.J.; Lee, S.Y.; Son, J.G.; Lee, T.G.; Cho, Y.; Shin, J.H.; Lee, E.; Im, S.G. Surface Hydrophobicity Modulates the Key Characteristics of Cancer Spheroids through the Interaction with the Adsorbed Proteins. Adv. Funct. Mater. 2021, 31, 2100775. [Google Scholar] [CrossRef]
- Malhão, F.; Macedo, A.C.; Ramos, A.A.; Rocha, E. Morphometrical, Morphological, and Immunocytochemical Characterization of a Tool for Cytotoxicity Research: 3D Cultures of Breast Cell Lines Grown in Ultra-Low Attachment Plates. Toxics 2022, 10, 415. [Google Scholar] [CrossRef]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-Dimensional in Vitro Culture Models in Oncology Research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef]
- Gai, T.; Zhang, Y.; Li, G.; Zhou, F.; He, C.; Wang, X.; Su, J. Engineered Hydrogel Microspheres for Spheroids and Organoids Construction. Chem. Eng. J. 2024, 498, 155131. [Google Scholar] [CrossRef]
- Lo Cicero, A.; Campora, S.; Lo Buglio, G.; Cinà, P.; Lo Pinto, M.; Scilabra, S.D.; Ghersi, G. Enhancing Therapeutic Efficacy through Degradation of Endogenous Extracellular Matrix in Primary Breast Tumor Spheroids. FEBS J. 2025, 292, 3494–3507. [Google Scholar] [CrossRef]
- Abbas, Z.N.; Al-Saffar, A.Z.; Jasim, S.M.; Sulaiman, G.M. Comparative Analysis between 2D and 3D Colorectal Cancer Culture Models for Insights into Cellular Morphological and Transcriptomic Variations. Sci. Rep. 2023, 13, 18380. [Google Scholar] [CrossRef] [PubMed]
- de Visser, K.E.; Joyce, J.A. The Evolving Tumor Microenvironment: From Cancer Initiation to Metastatic Outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Vakhshiteh, F.; Bagheri, Z.; Soleimani, M.; Ahvaraki, A.; Pournemat, P.; Alavi, S.E.; Madjd, Z. Heterotypic Tumor Spheroids: A Platform for Nanomedicine Evaluation. J. Nanobiotechnol. 2023, 21, 249. [Google Scholar] [CrossRef] [PubMed]
- Sheng, N.; Shindo, K.; Ohuchida, K.; Shinkawa, T.; Zhang, B.; Feng, H.; Yamamoto, T.; Moriyama, T.; Ikenaga, N.; Nakata, K.; et al. TAK1 Promotes an Immunosuppressive Tumor Microenvironment through Cancer-Associated Fibroblast Phenotypic Conversion in Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2024, 30, 5138–5153. [Google Scholar] [CrossRef]
- Cortini, M.; Macchi, F.; Reggiani, F.; Vitale, E.; Lipreri, M.V.; Perut, F.; Ciarrocchi, A.; Baldini, N.; Avnet, S. Endogenous Extracellular Matrix Regulates the Response of Osteosarcoma 3D Spheroids to Doxorubicin. Cancers 2023, 15, 1221. [Google Scholar] [CrossRef]
- Lazzari, G.; Nicolas, V.; Matsusaki, M.; Akashi, M.; Couvreur, P.; Mura, S. Multicellular Spheroid Based on a Triple Co-Culture: A Novel 3D Model to Mimic Pancreatic Tumor Complexity. Acta Biomater. 2018, 78, 296–307. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Heydari, Z.; Moeinvaziri, F.; Agarwal, T.; Pooyan, P.; Shpichka, A.; Maiti, T.K.; Timashev, P.; Baharvand, H.; Vosough, M. Organoids: A Novel Modality in Disease Modeling. Bio-Des. Manuf. 2021, 4, 689–716. [Google Scholar] [CrossRef]
- Thorel, L.; Perréard, M.; Florent, R.; Divoux, J.; Coffy, S.; Vincent, A.; Gaggioli, C.; Guasch, G.; Gidrol, X.; Weiswald, L.B.; et al. Patient-Derived Tumor Organoids: A New Avenue for Preclinical Research and Precision Medicine in Oncology. Exp. Mol. Med. 2024, 56, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Hillion, K.; Mahe, M.M. Redesigning Hydrogel Geometry for Enhanced Organoids. Nat. Methods 2022, 19, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue Extracellular Matrix Hydrogels as Alternatives to Matrigel for Culturing Gastrointestinal Organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.Y.; Du, Y.X.; Cao, H.M.; Su, L.Y.; Su, X.L.; Li, X. The Biological Macromolecules Constructed Matrigel for Cultured Organoids in Biomedical and Tissue Engineering. Colloids Surf. B Biointerfaces 2025, 247, 114435. [Google Scholar] [CrossRef]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic Alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Tan, R.; Zhang, Z.; Ding, P.; Liu, Y.; Liu, H.; Lu, M.; Chen, Y.G. A Growth Factor-Reduced Culture System for Colorectal Cancer Organoids. Cancer Lett. 2024, 588, 216737. [Google Scholar] [CrossRef]
- Urbischek, M.; Rannikmae, H.; Foets, T.; Ravn, K.; Hyvönen, M.; de la Roche, M. Organoid Culture Media Formulated with Growth Factors of Defined Cellular Activity. Sci. Rep. 2019, 9, 6193. [Google Scholar] [CrossRef]
- Pinto, B.; Henriques, A.C.; Silva, P.M.A.; Bousbaa, H. Three-Dimensional Spheroids as In Vitro Preclinical Models for Cancer Research. Pharmaceutics 2020, 12, 1186. [Google Scholar] [CrossRef]
- Zhu, S.; Yin, J.; Lu, X.; Jiang, D.; Chen, R.; Cui, K.; He, W.; Huang, N.; Xu, G. Influence of Experimental Variables on Spheroid Attributes. Sci. Rep. 2025, 15, 9751. [Google Scholar] [CrossRef]
- Živković, Z.; Opačak-Bernardi, T. An Overview on Spheroid and Organoid Models in Applied Studies. Sci 2025, 7, 27. [Google Scholar] [CrossRef]
- Nwokoye, P.N.; Abilez, O.J. Bioengineering Methods for Vascularizing Organoids. Cell Rep. Methods 2024, 4, 100779. [Google Scholar] [CrossRef]
- Druzhkova, I.; Nikonova, E.; Ignatova, N.; Koryakina, I.; Zyuzin, M.; Mozherov, A.; Kozlov, D.; Krylov, D.; Kuznetsova, D.; Lisitsa, U.; et al. Effect of Collagen Matrix on Doxorubicin Distribution and Cancer Cells’ Response to Treatment in 3D Tumor Model. Cancers 2022, 14, 5487. [Google Scholar] [CrossRef]
- Aung, A.; Kumar, V.; Theprungsirikul, J.; Davey, S.K.; Varghese, S. An Engineered Tumor-on-a-Chip Device with Breast Cancer–Immune Cell Interactions for Assessing T-Cell Recruitment. Cancer Res. 2020, 80, 263–275. [Google Scholar] [CrossRef]
- Wan, L.; Neumann, C.A.; Leduc, P.R. Tumor-on-a-Chip for Integrating a 3D Tumor Microenvironment: Chemical and Mechanical Factors. Lab. Chip 2020, 20, 873–888. [Google Scholar] [CrossRef]
- Li, W.; Zhou, Z.; Zhou, X.; Khoo, B.L.; Gunawan, R.; Chin, Y.R.; Zhang, L.; Yi, C.; Guan, X.; Yang, M. 3D Biomimetic Models to Reconstitute Tumor Microenvironment In Vitro: Spheroids, Organoids, and Tumor-on-a-Chip. Adv. Healthc. Mater. 2023, 12, 2202609. [Google Scholar] [CrossRef]
- Park, S.E.; Georgescu, A.; Huh, D. Organoids-on-a-Chip. Science 2019, 364, 960–965. [Google Scholar] [CrossRef]
- Collins, T.; Pyne, E.; Christensen, M.; Iles, A.; Pamme, N.; Pires, I.M. Spheroid-on-Chip Microfluidic Technology for the Evaluation of the Impact of Continuous Flow on Metastatic Potential in Cancer Models in Vitro. Biomicrofluidics 2021, 15, 44103. [Google Scholar] [CrossRef]
- Uzabakiriho, P.C.; Jiajun, F.; Nguchu, B.A.; Iqbal, S.; Manishimwe, C.; Shaw, P. Spheroid-on-a-Chip Platforms for Tumor Microenvironment and Drug Development. Adv. Mater. Technol. 2025, 15, 2401821. [Google Scholar] [CrossRef]
- Goertzen, C.; Eymael, D.; Magalhaes, M. Three-Dimensional Quantification of Spheroid Degradation-Dependent Invasion and Invadopodia Formation. Biol. Proced. Online 2018, 20, 20. [Google Scholar] [CrossRef]
- Karve, K.; Poon, S.; Prinos, P.; Ailles, L. 3D Spheroid Invasion Assay for High-Throughput Screening of Small-Molecule Libraries. Methods Mol. Biol. 2023, 2706, 201–214. [Google Scholar] [CrossRef]
- Lamichhane, A.; Tavana, H. Three-Dimensional Tumor Models to Study Cancer Stemness-Mediated Drug Resistance. Cell. Mol. Bioeng. 2024, 17, 107–119. [Google Scholar] [CrossRef]
- Liao, J.; Qian, F.; Tchabo, N.; Mhawech-Fauceglia, P.; Beck, A.; Qian, Z.; Wang, X.; Huss, W.J.; Lele, S.B.; Morrison, C.D.; et al. Ovarian Cancer Spheroid Cells with Stem Cell-Like Properties Contribute to Tumor Generation, Metastasis and Chemotherapy Resistance through Hypoxia-Resistant Metabolism. PLoS ONE 2014, 9, e84941. [Google Scholar] [CrossRef]
- Ishtiah, A.A.; Yahaya, B.H. The Enrichment of Breast Cancer Stem Cells from MCF7 Breast Cancer Cell Line Using Spheroid Culture Technique. Methods Mol. Biol. 2022, 2429, 475–484. [Google Scholar] [CrossRef]
- Elberskirch, L.; Knoll, T.; Königsmark, R.; Renner, J.; Wilhelm, N.; von Briesen, H.; Wagner, S. Microfluidic 3D Intestine Tumor Spheroid Model for Efficient in Vitro Investigation of Nanoparticular Formulations. J. Drug Deliv. Sci. Technol. 2021, 63, 102496. [Google Scholar] [CrossRef]
- Petreus, T.; Cadogan, E.; Hughes, G.; Smith, A.; Pilla Reddy, V.; Lau, A.; O’Connor, M.J.; Critchlow, S.; Ashford, M.; Oplustil O’Connor, L. Tumour-on-Chip Microfluidic Platform for Assessment of Drug Pharmacokinetics and Treatment Response. Commun. Biol. 2021, 4, 1001. [Google Scholar] [CrossRef]
- Wang, Y.; Jeon, H. 3D Cell Cultures toward Quantitative High-Throughput Drug Screening. Trends Pharmacol. Sci. 2022, 43, 569–581. [Google Scholar] [CrossRef]
- Zanoni, M.; Pignatta, S.; Arienti, C.; Bonafè, M.; Tesei, A. Anticancer Drug Discovery Using Multicellular Tumor Spheroid Models. Expert. Opin. Drug Discov. 2019, 14, 289–301. [Google Scholar] [CrossRef]
- Nayak, P.; Bentivoglio, V.; Varani, M.; Signore, A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers 2023, 15, 4846. [Google Scholar] [CrossRef]
- Saito, Y.; Muramatsu, T.; Kanai, Y.; Ojima, H.; Sukeda, A.; Hiraoka, N.; Arai, E.; Sugiyama, Y.; Matsuzaki, J.; Uchida, R.; et al. Establishment of Patient-Derived Organoids and Drug Screening for Biliary Tract Carcinoma. Cell Rep. 2019, 27, 1265–1276.e4. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-Derived Organoids Model Treatment Response of Metastatic Gastrointestinal Cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Calpe, B.; Kovacs, W.J. High-Throughput Screening in Multicellular Spheroids for Target Discovery in the Tumor Microenvironment. Expert. Opin. Drug Discov. 2020, 15, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhao, D.K.; Yin, H.M.; Tian, T.Y.; Kang, J.K.; Shen, S.; Wang, J. Combinatorial Screening of Nanomedicines in Patient-Derived Cancer Organoids Facilitates Efficient Cancer Therapy. Nano Today 2025, 61, 102665. [Google Scholar] [CrossRef]
- Zuo, J.; Fang, Y.; Wang, R.; Liang, S. High-Throughput Solutions in Tumor Organoids: From Culture to Drug Screening. Stem Cells 2025, 43, sxae070. [Google Scholar] [CrossRef]
- Kim, M.; Yun, H.W.; Park, D.Y.; Choi, B.H.; Min, B.H. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 427. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Bulati, M.; Gallo, A.; Zito, G.; Busà, R.; Iannolo, G.; Cuscino, N.; Castelbuono, S.; Carcione, C.; Centi, C.; Martucci, G.; et al. 3D Culture and Interferon-γ Priming Modulates Characteristics of Mesenchymal Stromal/Stem Cells by Modifying the Expression of Both Intracellular and Exosomal MicroRNAs. Biology 2023, 12, 1063. [Google Scholar] [CrossRef]
- Rovere, M.; Reverberi, D.; Arnaldi, P.; Palamà, M.E.F.; Gentili, C. Spheroid Size Influences Cellular Senescence and Angiogenic Potential of Mesenchymal Stromal Cell-Derived Soluble Factors and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2023, 11, 1297644. [Google Scholar] [CrossRef]
- Xie, L.; Mao, M.; Zhou, L.; Zhang, L.; Jiang, B. Signal Factors Secreted by 2D and Spheroid Mesenchymal Stem Cells and by Cocultures of Mesenchymal Stem Cells Derived Microvesicles and Retinal Photoreceptor Neurons. Stem Cells Int. 2017, 2017, 2730472. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.J.; Bae, E.H.; Ryu, J.S.; Kaur, G.; Kim, H.J.; Kim, J.Y.; Barreda, H.; Jung, S.Y.; Choi, J.M.; et al. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol. Ther. 2020, 28, 1628–1644. [Google Scholar] [CrossRef]
- Rocha, S.; Carvalho, J.; Oliveira, P.; Voglstaetter, M.; Schvartz, D.; Thomsen, A.R.; Walter, N.; Khanduri, R.; Sanchez, J.-C.; Keller, A.; et al. 3D Cellular Architecture Affects MicroRNA and Protein Cargo of Extracellular Vesicles. Adv. Sci. 2019, 6, 1800948. [Google Scholar] [CrossRef]
- Hu, S.; Li, Z.; Cores, J.; Huang, K.; Su, T.; Dinh, P.U.; Cheng, K. Needle-Free Injection of Exosomes Derived from Human Dermal Fibroblast Spheroids Ameliorates Skin Photoaging. ACS Nano 2019, 13, 11273–11282. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, Z.; Lutz, H.; Huang, K.; Su, T.; Cores, J.; Dinh, P.U.C.; Cheng, K. Dermal Exosomes Containing MiR-218-5p Promote Hair Regeneration by Regulating β-Catenin Signaling. Sci. Adv. 2020, 6, eaba1685. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.U.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of Lung Spheroid Cell Secretome and Exosomes Promotes Lung Repair in Pulmonary Fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Thippabhotla, S.; Zhong, C.; He, M. 3D Cell Culture Stimulates the Secretion of in Vivo like Extracellular Vesicles. Sci. Rep. 2019, 9, 13012. [Google Scholar] [CrossRef]
- Vera, N.; Acuña-Gallardo, S.; Grünenwald, F.; Caceres-Verschae, A.; Realini, O.; Acuña, R.; Lladser, A.; Illanes, S.E.; Varas-Godoy, M. Small Extracellular Vesicles Released from Ovarian Cancer Spheroids in Response to Cisplatin Promote the Pro-Tumorigenic Activity of Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 4972. [Google Scholar] [CrossRef]
- Giusti, I.; Poppa, G.; D’Ascenzo, S.; Esposito, L.; Vitale, A.R.; Calvisi, G.; Dolo, V. Cancer Three-Dimensional Spheroids Mimic In Vivo Tumor Features, Displaying “Inner” Extracellular Vesicles and Vasculogenic Mimicry. Int. J. Mol. Sci. 2022, 23, 11782. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Zhang, Y.; Ni, X.; Zhang, G.; Cui, X.; Liu, M.; Xu, C.; Zhang, Q.; Zhu, H.; et al. ZIP4 Promotes Muscle Wasting and Cachexia in Mice With Orthotopic Pancreatic Tumors by Stimulating RAB27B-Regulated Release of Extracellular Vesicles From Cancer Cells. Gastroenterology 2019, 156, 722–734.e6. [Google Scholar] [CrossRef]
- Jeon, T.J.; Kim, O.H.; Kang, H.; Lee, H.J. Preadipocytes Potentiate Melanoma Progression and M2 Macrophage Polarization in the Tumor Microenvironment. Biochem. Biophys. Res. Commun. 2024, 721, 150129. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Guo, J.; Fei, X.; Yu, L.; Ma, S.; Wang, J.; Wu, Y.; Guo, J.; Fei, X.; et al. Adipocyte-Derived Exosomes Promote Lung Cancer Metastasis by Increasing MMP9 Activity via Transferring MMP3 to Lung Cancer Cells. Oncotarget 2017, 8, 81880–81891. [Google Scholar] [CrossRef]
- Donzelli, J.; Proestler, E.; Riedel, A.; Nevermann, S.; Hertel, B.; Guenther, A.; Gattenlöhner, S.; Savai, R.; Larsson, K.; Saul, M.J. Small Extracellular Vesicle-Derived MiR-574-5p Regulates PGE2-Biosynthesis via TLR7/8 in Lung Cancer. J. Extracell. Vesicles 2021, e12143. [Google Scholar] [CrossRef]
- Mullen, S.; Movia, D. The Role of Extracellular Vesicles in Non-Small-Cell Lung Cancer, the Unknowns, and How New Approach Methodologies Can Support New Knowledge Generation in the Field. Eur. J. Pharm. Sci. 2023, 188, 106516. [Google Scholar] [CrossRef]
- Relucenti, M.; Francescangeli, F.; De Angelis, M.L.; D’Andrea, V.; Miglietta, S.; Donfrancesco, O.; Li, X.; Chen, R.; Zeuner, A.; Familiari, G. A Different Exosome Secretion Pattern Characterizes Patient-Derived Colorectal Cancer Multicellular Spheroids and Their Mouse Xenografts. Biology 2022, 11, 1427. [Google Scholar] [CrossRef]
- Al Hrout, A.; Levesque, M.P.; Chahwan, R. Investigating the Tumor-Immune Microenvironment through Extracellular Vesicles from Frozen Patient Biopsies and 3D Cultures. Front. Immunol. 2023, 14, 1176175. [Google Scholar] [CrossRef] [PubMed]
- Schuster, M.; Braun, F.K.; Chiang, D.M.L.; Ludwig, C.; Meng, C.; Grätz, C.; Kirchner, B.; Proescholdt, M.; Hau, P.; Steinlein, O.K.; et al. Extracellular Vesicles Secreted by 3D Tumor Organoids Are Enriched for Immune Regulatory Signaling Biomolecules Compared to Conventional 2D Glioblastoma Cell Systems. Front. Immunol. 2024, 15, 1388769. [Google Scholar] [CrossRef] [PubMed]
- Santoro, J.; Carrese, B.; Peluso, M.S.; Coppola, L.; D’Aiuto, M.; Mossetti, G.; Salvatore, M.; Smaldone, G. Influence of Breast Cancer Extracellular Vesicles on Immune Cell Activation: A Pilot Study. Biology 2023, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, C.; Vaché, J.; Brunel, A.; Mahouche, I.; Raymond, A.A.; Dupuy, J.W.; Petrel, M.; Bioulac-Sage, P.; Perrais, D.; Dugot-Senant, N.; et al. Emerging Role of Oncogenic SS-Catenin in Exosome Biogenesis as a Driver of Immune Escape in Hepatocellular Carcinoma. eLife 2024, RP95191. [Google Scholar] [CrossRef]
- Eguchi, T.; Sogawa, C.; Okusha, Y.; Uchibe, K.; Iinuma, R.; Ono, K.; Nakano, K.; Murakami, J.; Itoh, M.; Arai, K.; et al. Organoids with Cancer Stem Cell-like Properties Secrete Exosomes and HSP90 in a 3D Nanoenvironment. PLoS ONE 2018, 13, e0191109. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ma, J.; Sun, T.; Zhou, Q.; Wang, W.; Wang, G.; Wu, P.; Wang, H.; Jiang, L.; et al. Exosomal CircRNAs: Biogenesis, Effect and Application in Human Diseases. Mol. Cancer 2019, 18, 116. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, L.; Hua, F.; Min, Z.; Zhan, Y.; Zhang, W.; Yao, J. Exosomal CircCARM1 from Spheroids Reprograms Cell Metabolism by Regulating PFKFB2 in Breast Cancer. Oncogene 2022, 41, 2012–2025. [Google Scholar] [CrossRef]
- Xu, F.; Wang, K.; Zhu, C.; Fan, L.; Zhu, Y.; Wang, J.F.; Li, X.; Liu, Y.; Zhao, Y.; Zhu, C.; et al. Tumor-Derived Extracellular Vesicles as a Biomarker for Breast Cancer Diagnosis and Metastasis Monitoring. iScience 2024, 27, 109506. [Google Scholar] [CrossRef]
- Mathew, M.; Zade, M.; Mezghani, N.; Patel, R.; Wang, Y.; Momen-Heravi, F. Extracellular Vesicles as Biomarkers in Cancer Immunotherapy. Cancers 2020, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Bockorny, B.; Paul, I.; Akshinthala, D.; Frappart, P.O.; Gandarilla, O.; Bose, A.; Sanchez-Gonzalez, V.; Rouse, E.E.; Lehoux, S.D.; et al. PDX-Derived Organoids Model in Vivo Drug Response and Secrete Biomarkers. JCI Insight 2020, 5, e135544. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-Dried and GMP-Compliant Pharmaceuticals Containing Exosomes for Acellular Mesenchymal Stromal Cell Immunomodulant Therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Son, J.P.; Kim, E.H.; Shin, E.K.; Kim, D.H.; Sung, J.H.; Oh, M.J.; Cha, J.M.; Chopp, M.; Bang, O.Y. Mesenchymal Stem Cell-Extracellular Vesicle Therapy for Stroke: Scalable Production and Imaging Biomarker Studies. Stem Cells Transl. Med. 2023, 12, 459–473. [Google Scholar] [CrossRef]
- Zeöld, A.; Sándor, G.O.; Kiss, A.; Soós, A.Á.; Tölgyes, T.; Bursics, A.; Szűcs, Á.; Harsányi, L.; Kittel, Á.; Gézsi, A.; et al. Shared Extracellular Vesicle MiRNA Profiles of Matched Ductal Pancreatic Adenocarcinoma Organoids and Blood Plasma Samples Show the Power of Organoid Technology. Cell. Mol. Life Sci. 2021, 78, 3005–3020. [Google Scholar] [CrossRef]
- Xiao, W.; Pahlavanneshan, M.; Eun, C.Y.; Zhang, X.; DeKalb, C.; Mahgoub, B.; Knaneh-Monem, H.; Shah, S.; Sohrabi, A.; Seidlits, S.K.; et al. Matrix Stiffness Mediates Pancreatic Cancer Chemoresistance through Induction of Exosome Hypersecretion in a Cancer Associated Fibroblasts-Tumor Organoid Biomimetic Model. Matrix Biol. Plus 2022, 14, 100111. [Google Scholar] [CrossRef]
- Xu, K.; Shen, R.; Zhang, L.; Gao, X.; Wang, X.; Zhang, C.; Chen, X.; Wang, X. Pancreatic Cancer-Derived Extracellular Vesicles Enriched with MiR-223-5p Promote Skeletal Muscle Wasting Associated with Cachexia. Adv. Sci. 2025. [Google Scholar] [CrossRef]
- Buenafe, A.C.; Dorrell, C.; Reddy, A.P.; Klimek, J.; Marks, D.L. Proteomic Analysis Distinguishes Extracellular Vesicles Produced by Cancerous versus Healthy Pancreatic Organoids. Sci. Rep. 2022, 12, 3556. [Google Scholar] [CrossRef]
- Nagai, H.; Kuroha, M.; Handa, T.; Karasawa, H.; Ohnuma, S.; Naito, T.; Moroi, R.; Kanazawa, Y.; Shiga, H.; Hamada, S.; et al. Comprehensive Analysis of MicroRNA Profiles in Organoids Derived from Human Colorectal Adenoma and Cancer. Digestion 2021, 102, 860–869. [Google Scholar] [CrossRef]
- Szvicsek, Z.; Oszvald, Á.; Szabó, L.; Sándor, G.O.; Kelemen, A.; Soós, A.Á.; Pálóczi, K.; Harsányi, L.; Tölgyes, T.; Dede, K.; et al. Extracellular Vesicle Release from Intestinal Organoids Is Modulated by Apc Mutation and Other Colorectal Cancer Progression Factors. Cell. Mol. Life Sci. 2019, 76, 2463–2476. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.A.; Sogawa, C.; Okusha, Y.; Kawai, H.; Oo, M.W.; Elseoudi, A.; Lu, Y.; Nagatsuka, H.; Kubota, S.; Satoh, A.; et al. Knockout of MMP3 Weakens Solid Tumor Organoids and Cancer Extracellular Vesicles. Cancers 2020, 12, 1260. [Google Scholar] [CrossRef]
- Sasaki, A.; Kuroha, M.; Tosa, M.; Takahashi, S.; Oomori, S.; Nomura, E.; Kikuchi, T.; Onodera, M.; Sato, Y.; Miyazawa, T.; et al. Evaluation of Organoid-Derived Exosomal MicroRNA as Liquid Biopsy for Colorectal Cancer: A Multicenter Cross-Sectional Study. Clin. Transl. Sci. 2025, 18, e70270. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Mathivanan, S.; Ji, H.; Simpson, R.J. Two Distinct Populations of Exosomes Are Released from LIM1863 Colon Carcinoma Cell-Derived Organoids. Mol. Cell. Proteom. 2013, 12, 587–598. [Google Scholar] [CrossRef]
- Sándor, G.O.; Soós, A.Á.; Lörincz, P.; Rojkó, L.; Harkó, T.; Bogyó, L.; Tölgyes, T.; Bursics, A.; Buzás, E.I.; Moldvay, J.; et al. Wnt Activity and Cell Proliferation Are Coupled to Extracellular Vesicle Release in Multiple Organoid Models. Front. Cell Dev. Biol. 2021, 9, 670825. [Google Scholar] [CrossRef]
| Tumor | Cancer Cells | 3D Model | 3D EV Isolation Method | Finding | References |
|---|---|---|---|---|---|
| Gastric cancer | MKN74 MKN45 | Spheroid | Differential ultracentrifugation | Upregulation of microRNAs and downregulation of proteins in 3D EVs | [172] |
| Cervical cancer | Hela | Spheroid | Filtration | EV small RNAs | [176] |
| Ovarian cancer | HeyA8 Ovcar3 | Spheroid | Differential ultracentrifugation | Pro-angiogenetic role | [177] |
| Ovarian cancer | CABA I | Spheroid | Differential ultracentrifugation | “Inner” EVs | [178] |
| Pancreatic cancer | AsPC-1 BxPC-3 | Spheroid | Isolation kit | ZIP4 knockdown reduced EVs’ pancreatic cancer release | [179] |
| Melanoma | B16 | Spheroid | N/A | Influence of preadipocytes in melanoma growth | [180] |
| Lung cancer | 3LL A549 | Spheroid | Differential ultracentrifugation | Modulation of tumor microenvironment | [181] |
| Lung cancer | A549 H1650 2106T | Spheroid | Differential ultracentrifugation | Role of miR-574-5p in prostaglandin H2 regulation | [182] |
| Colorectal cancer | Primary | Spheroid | N/A | Multilayer spheroids release more EVs | [184] |
| Glioblastoma | IDH wild-type (CNS WHO grade 4)-derived models (BTIC10, -13, -131, -18, -129, -155) | Organoid | Precipitation and immunoaffinity | Comparison between EVs released from 2D and 3D models | [186] |
| Breast cancer | HS578T BT474 | Spheroid | Differential ultracentrifugation | Effects of EVs breast cancer on PBMC from healthy donors | [187] |
| Hepatocellular carcinoma | HepG2 Huh7 | Spheroid | Differential ultracentrifugation | β-catenin decreases EV release and immune cell infiltration | [188] |
| Prostatic adenocarcinoma | PC-3 | Spheroid | Differential ultracentrifugation Filtration | Secretion of HSP90 and EpCAM | [189] |
| Breast cancer | MDA-MB-231 | Spheroid | Differential ultracentrifugation | circCARM1 promotes breast cancer proliferation and glycolysis | [191] |
| Pancreatic ductal adenocarcinoma | Primary cells | Spheroid | Filtration and ultracentrifugation | New biomarkers | [194] |
| Pancreatic ductal adenocarcinoma | PDAC cell lines (derived from primary tumors) | Organoid | Differential ultracentrifugation | miRNA EVs released with matched patient plasma and extracellular matrix remodeling | [197] |
| Pancreatic ductal adenocarcinoma | Mouse-derived organoids | Organoid | Isolation kit | 3D biomimetic PDAC model with integrated CAF | [198] |
| Pancreatic ductal adenocarcinoma | Patient-derived organoids | Organoid | Differential ultracentrifugation | Absorption of miRNA in PDAC-derived EVs by skeletal muscles and the role in cachexia | [199] |
| Pancreatic ductal adenocarcinoma | Patient-derived organoids | Organoid | Isolation kit | Differences between EVs from PDAC organoids and healthy pancreatic organoids | [200] |
| Colorectal cancer | Patient-derived organoids | Organoid | Differential ultracentrifugation | The role of miR-1246 in promoting proliferation | [201] |
| Colorectal cancer | Mouse- and patient-derived organoids | Organoid | Isolation kit | APC mutation and collagen deposition enhance EV release | [202] |
| Colorectal cancer | LuM1 cell line | Organoid | Differential centrifugation and concentration | MMP3 knockout led to the additional release of EVs from organoids | [203] |
| Colorectal cancer | Patient-derived organoids | Organoid | Differential ultracentrifugation and filtration | miR-4284, miR-5100, miR-1246, miR-1290 elevated | [204] |
| Colorectal cancer | Human colon carcinoma LIM1863 cells | Organoid | Isolation kit | EVs isolated from apical and basolateral region have distinct proteomic profiles | [205] |
| Pancreatic ductal adenocarcinoma Lung bronchiolar Lung adenocarcinoma | Human PDAC organoids Mouse pancreas ductal and lung organoids Human bronchiolar and LUAD organoids | Organoid | Differential ultracentrifugation | Wnt signaling is tightly coupled to cell proliferation and EV secretion in lung adenocarcinoma but disrupted in PDAC | [206] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campora, S.; Lo Cicero, A. The 3D Language of Cancer: Communication via Extracellular Vesicles from Tumor Spheroids and Organoids. Int. J. Mol. Sci. 2025, 26, 7104. https://doi.org/10.3390/ijms26157104
Campora S, Lo Cicero A. The 3D Language of Cancer: Communication via Extracellular Vesicles from Tumor Spheroids and Organoids. International Journal of Molecular Sciences. 2025; 26(15):7104. https://doi.org/10.3390/ijms26157104
Chicago/Turabian StyleCampora, Simona, and Alessandra Lo Cicero. 2025. "The 3D Language of Cancer: Communication via Extracellular Vesicles from Tumor Spheroids and Organoids" International Journal of Molecular Sciences 26, no. 15: 7104. https://doi.org/10.3390/ijms26157104
APA StyleCampora, S., & Lo Cicero, A. (2025). The 3D Language of Cancer: Communication via Extracellular Vesicles from Tumor Spheroids and Organoids. International Journal of Molecular Sciences, 26(15), 7104. https://doi.org/10.3390/ijms26157104






