Green Synthesis and Characterization of Silver Nanoparticles Using Artemisia terrae-albae Extracts and Evaluation of Their Cytogenotoxic Effects
Abstract
1. Introduction
2. Results and Discussion
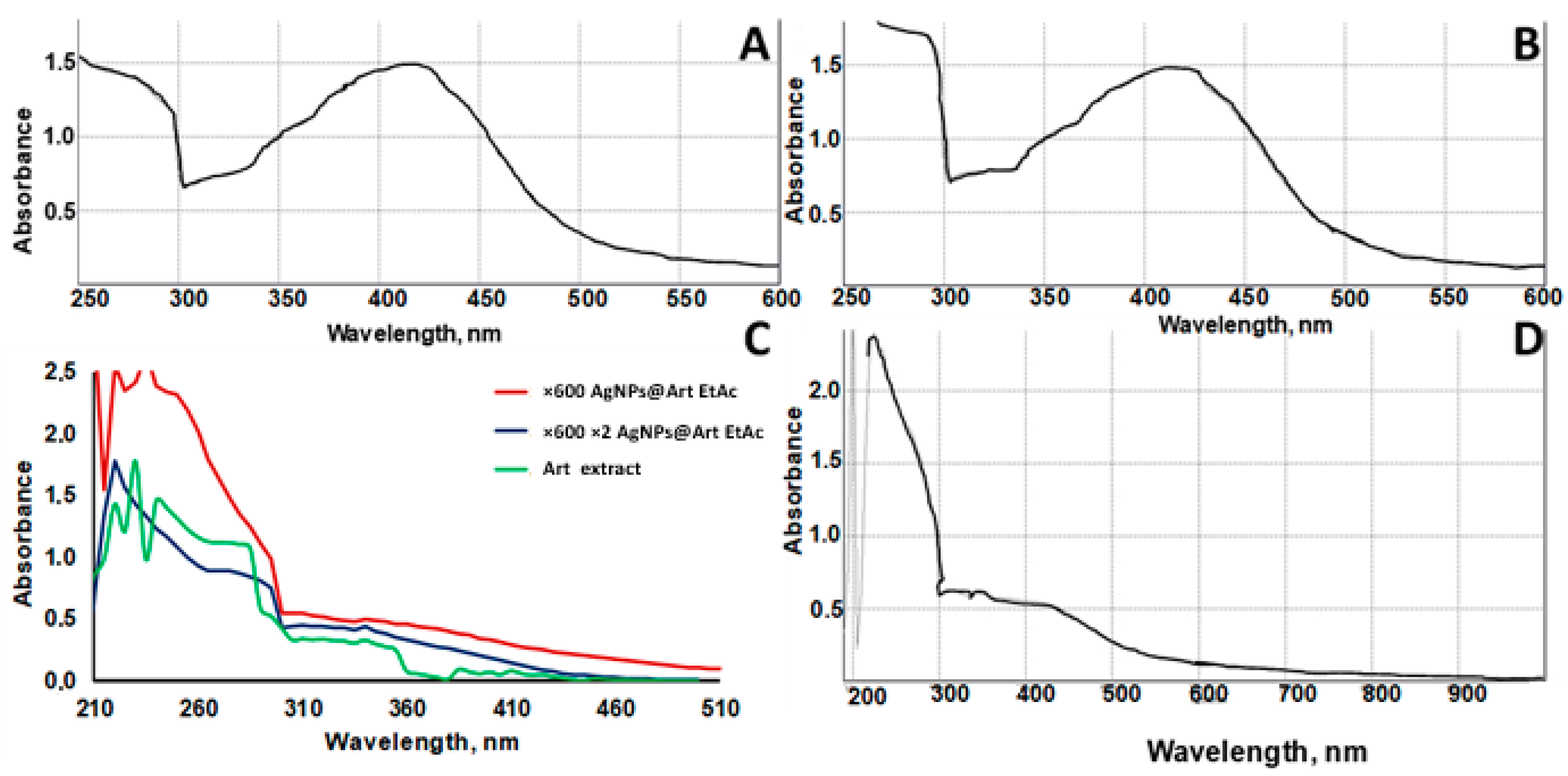
2.1. UV-Vis Absorbance Spectral Studies of AgNPs@Artemisia EtOH Diluted in Water
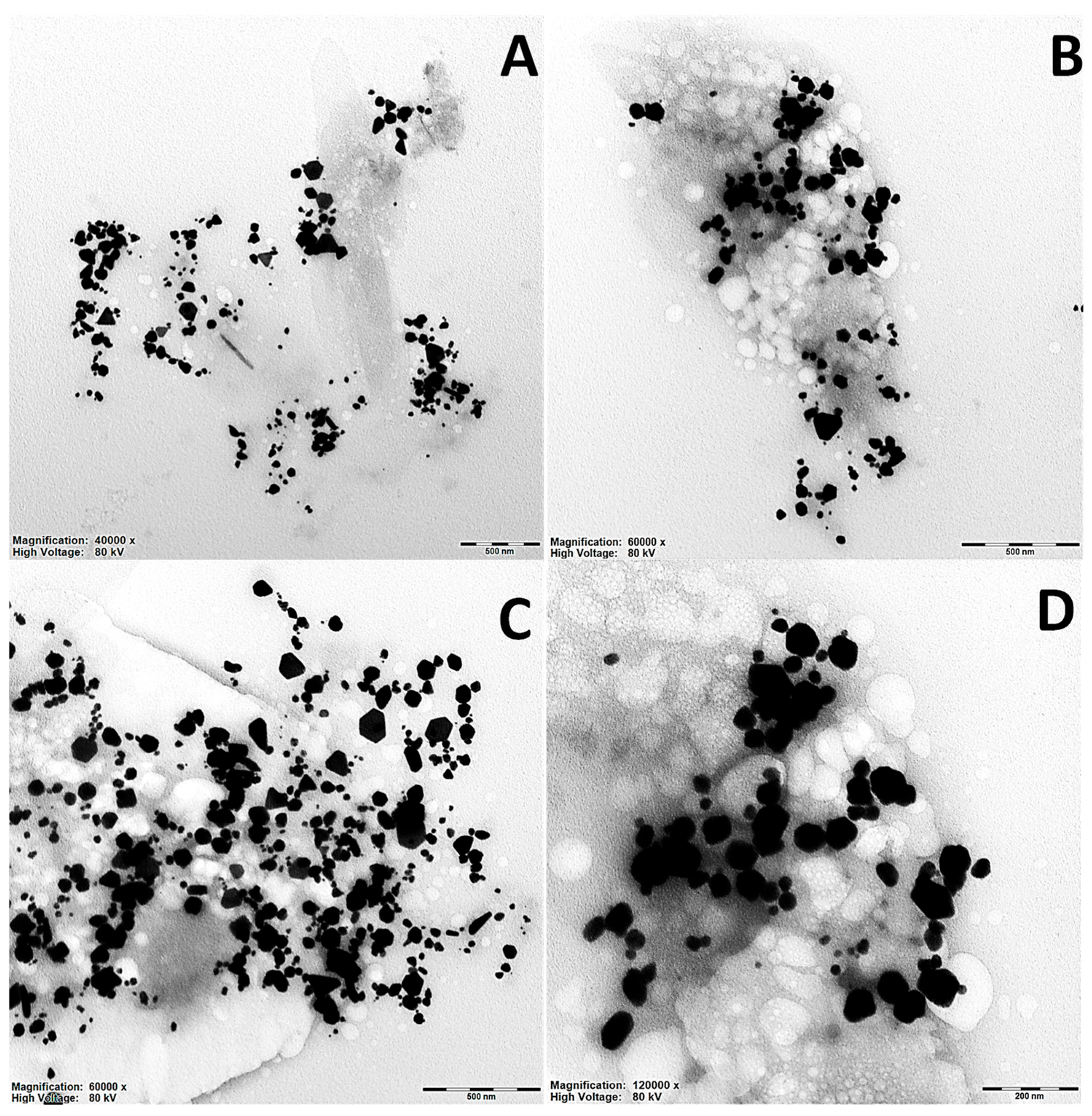
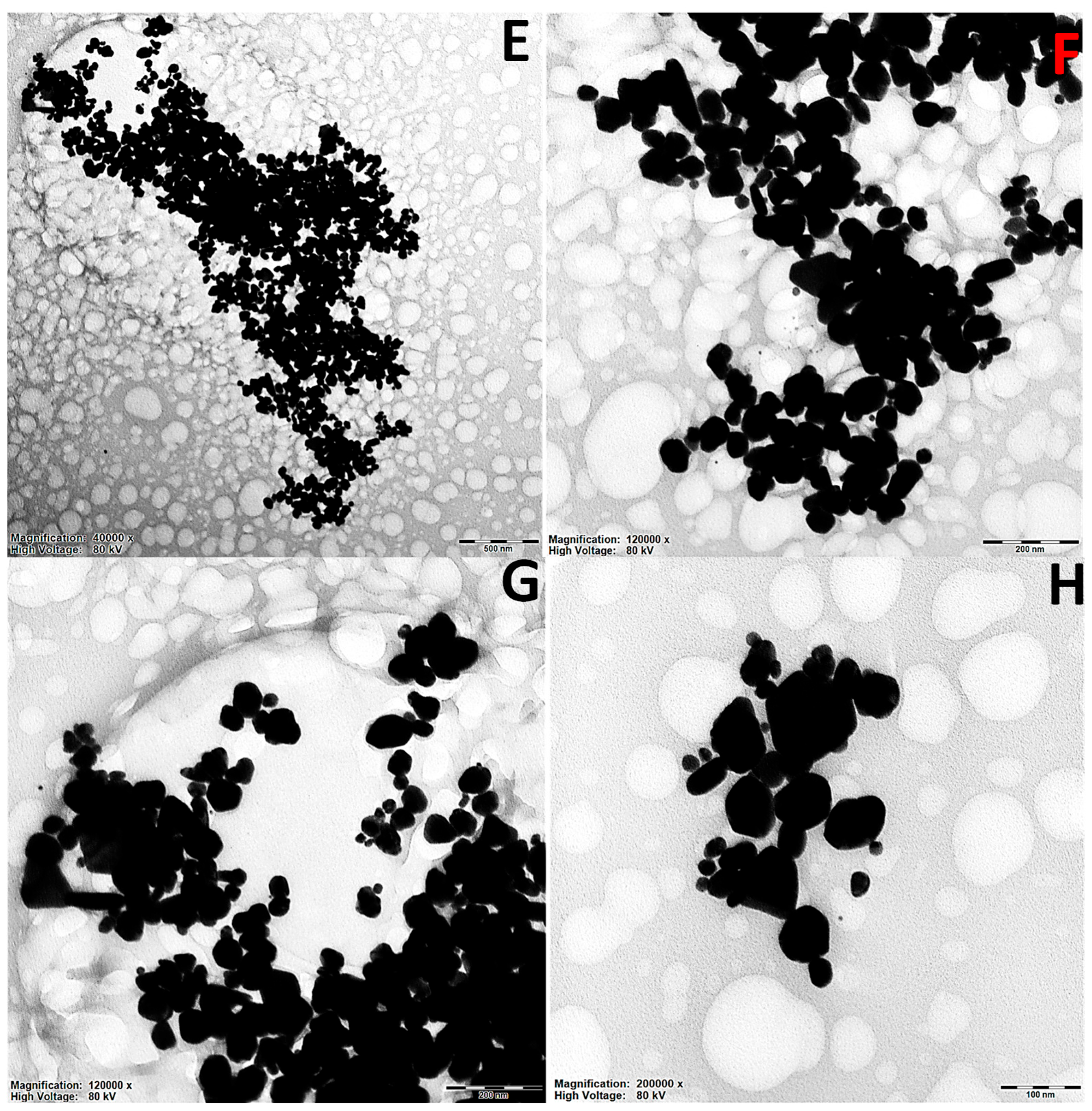
2.2. TEM Surface Morphological Studies of Artemisia EtOH/H2O and Artemisia EtOH/H2O/EtAc
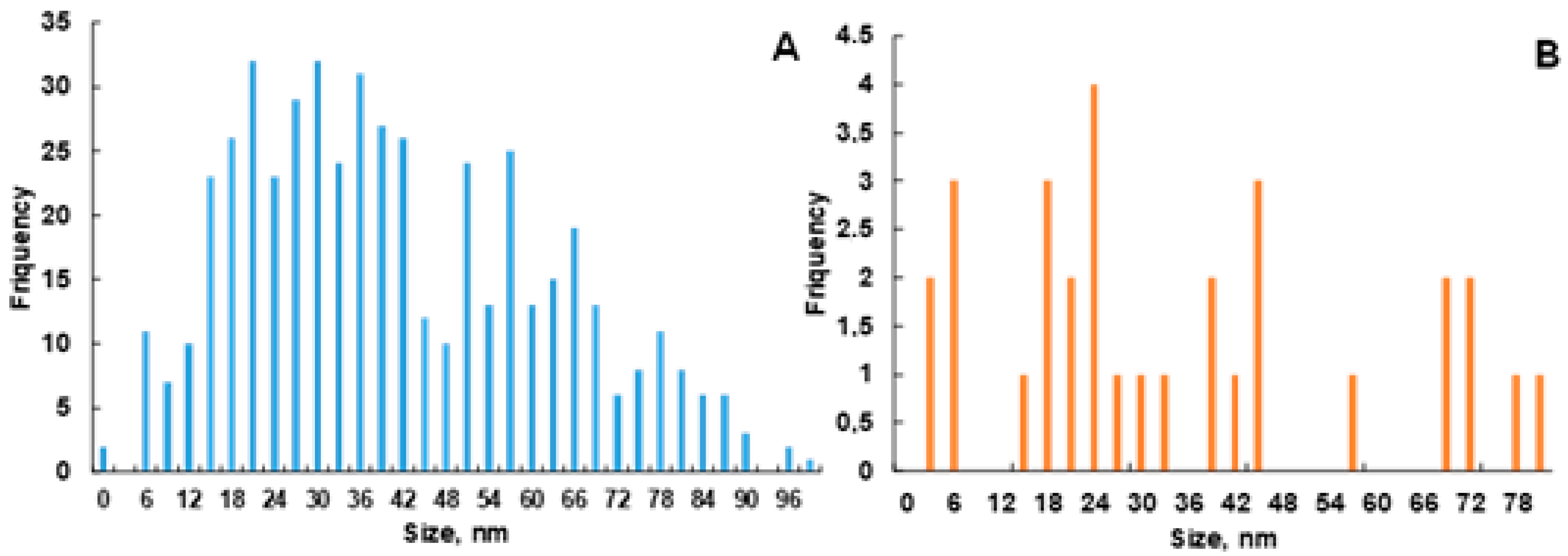
2.3. Surface Particle Distribution and Size Analysis of AgNPs@Artemisia EtOH/H2O and AgNPs@Artemisia EtOAc/H2O
2.4. Cytotoxicity Studies of AgNPs@Artemisia EtOH/H2O Extract and AgNPs@Artemisia EtOH/EtOAc Extract
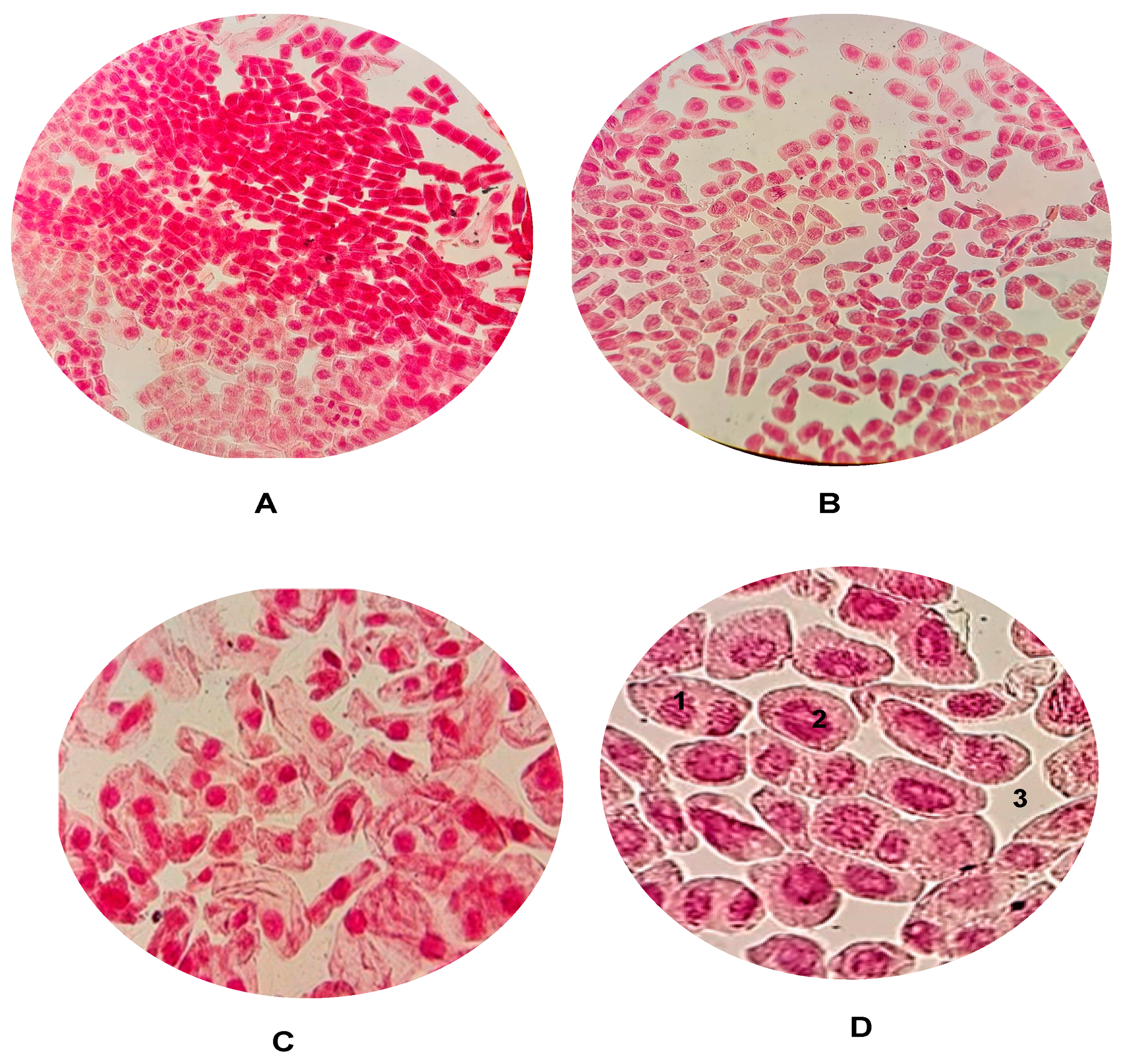
2.5. Cell Mitosis Studies of AgNPs@Artemisia EtOH/H2O Extract and AgNPs@Artemisia EtOH/EtOAc Extract
2.6. Cytogenotoxicity Studies of AgNPs@Artemisia EtOH extract/H2O and AgNPs@Artemisia EtOH extract/EtOAc
3. Materials and Methods
3.1. Reagents
3.2. AgNP Synthesis
3.3. TEM Analysis of AgNPs
3.4. Methodology for Determining Chromosomal Aberrations
- The average number of roots;
- Root length;
- The distribution of cells in the root tip of Allium cepa.
- MI—mitotic index;
- P—total number of cells in prophase;
- M—total number of cells in metaphase;
- A—total number of cells in anaphase;
- T—total number of cells in telophase;
- N—total number of analyzed cells.
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tareq, M.; Khadrawy, Y.A.; Rageh, M.M.; Mohammed, H.S. Dose-dependent biological toxicity of green synthesized silver nanoparticles in rat’s brain. Sci. Rep. 2022, 12, 22642. [Google Scholar] [CrossRef] [PubMed]
- Habeeb-Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Muthupandian, S. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.S.; Alsubhi, N.S.; Felimban, A.I. Green synthesis of silver nanoparticles using medicinal plants: Characterization and application. J. Radiat. Res. Appl. Sci. 2022, 15, 109–124. [Google Scholar] [CrossRef]
- Manzoor, S.I.; Jabeen, F.; Patel, R.; Alam Rizvi, M.M.; Imtiyaz, K.; Malik, M.A.; Dar, T.A. Green synthesis of biocompatible silver nanoparticles using Trillium govanianum rhizome extract: Comprehensive biological evaluation and in silico analysis. Mater. Adv. 2025, 6, 682–702. [Google Scholar] [CrossRef]
- Dutt, Y.; Pandey, R.P.; Dutt, M.; Gupta, A.; Vibhuti, A.; Raj, V.S.; Chang, C.-M.; Priyadarshini, A. Synthesis and biological characterization of phyto-fabricated silver nanoparticles from Azadirachta indica. J. Biomed. Nanotechnol. 2022, 18, 2022–2057. [Google Scholar] [CrossRef]
- Korcan, S.E.; Kahraman, T.; Acikbas, Y.; Liman, R.; Ciğerci, İ.H.; Konuk, M.; Ocak, İ. Cyto–genotoxicity, antibacterial, and antibiofilm properties of green synthesized silver nanoparticles using Penicillium toxicarium. Microsc. Res. Tech. 2021, 84, 2530–2543. [Google Scholar] [CrossRef]
- Patel, R.R.; Singh, S.K.; Singh, M. Green synthesis of silver nanoparticles: Methods, biological applications, delivery and toxicity. Mater. Adv. 2023, 4, 1831–1849. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Mater. Today Proc. 2023, 81, 859–863. [Google Scholar] [CrossRef]
- Ekiert, H.; Klimek-Szczykutowicz, M.; Rzepiela, A.; Klin, P.; Szopa, A. Artemisia species with high biological values as a potential source of medicinal and cosmetic raw materials. Molecules 2022, 27, 6427. [Google Scholar] [CrossRef]
- Swift, L.H.; Golsteyn, R.M. Genotoxic anti-cancer agents and their relationship to DNA, damage, mitosis, and checkpoint adaptation in proliferating cancer cells. Int. J. Mol. Sci. 2014, 15, 3403–3431. [Google Scholar] [CrossRef]
- Alhmoud, J.F.; Woolley, J.F.; Al Moustafa, A.E.; Malki, M.I. DNA damage/repair management in cancers. Cancers 2020, 12, 1050. [Google Scholar] [CrossRef] [PubMed]
- Kisby, G.E.; Wilson, D.M., III; Spencer, P.S. Introducing the role of genotoxicity in neurodegenerative diseases and neuropsychiatric disorders. Int. J. Mol. Sci. 2024, 25, 7221. [Google Scholar] [CrossRef]
- Shah, S.U. Importance of genotoxicity & S2A guidelines for genotoxicity testing for pharmaceuticals. IOSR J. Pharm. Biol. Sci. 2012, 1, 43–54. [Google Scholar] [CrossRef]
- Al-Shoeb, M.A.; Rahman, M.H.; Chakraborty, S.; Faisal, M.; Waise, T.M.Z.; Hassan, F.; Kabir, M.F.; Hossain, M.A. Cytogenetic assays of genotoxic effects and cancer risk of chromium on tannery workers in Bangladesh. J. Biol. 2014, 2, 39–46. [Google Scholar]
- Wijeyaratne, W.M.; Wadasinghe, L.G. Allium cepa bio-assay to assess the water and sediment cytogenotoxicity in a tropical stream subjected to multiple point and nonpoint source pollutants. J. Toxicol. 2019, 2019, 5420124. [Google Scholar] [CrossRef]
- Jena, G.; Kaul, C.L.; Poduri, R. Genotoxicity testing, a regulatory requirement for drug discovery and development: Impact of ICH guidelines. Indian J. Pharmacol. 2002, 34, 86–99. [Google Scholar]
- Vieira, C.; Marcon, C.; Droste, A. Phytotoxic and cytogenotoxic assessment of glyphosate on Lactuca sativa L. Braz. J. Biol. 2024, 84, 2–8. [Google Scholar] [CrossRef]
- Amini, S.M.; Akbari, A. Metal nanoparticles synthesis through natural phenolic acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef]
- Giri, S.; Grimshaw, A.; Bal, S.; Godby, K.; Kharel, P.; Djulbegovic, B.; Costa, L. Evaluation of daratumumab for the treatment of multiple myeloma in patients with high-risk cytogenetic factors: A systematic review and meta-analysis. JAMA Oncol. 2020, 6, 1759–1765. [Google Scholar] [CrossRef]
- Lezcano, C.; Jungbluth, A.; Busam, K. Comparison of immunohistochemistry for PRAME with cytogenetic test results in the evaluation of challenging melanocytic tumors. Am. J. Surg. Pathol. 2020, 44, 893. [Google Scholar] [CrossRef]
- Vardiman, J.; Hyjek, E. World Health Organization classification, evaluation, and genetics of the myeloproliferative neoplasm variants. Hematol. Am. Soc. Hematol. Educ. Program 2011, 1, 250–256. [Google Scholar] [CrossRef]
- Fragouli, E.; Munne, S.; Wells, D. The cytogenetic constitution of human blastocysts: Insights from comprehensive chromosome screening strategies. Hum. Reprod. Updat. 2019, 25, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sahoo, T.; Schonberg, S.; Kopita, K.; Ross, L.; Patek, K.; Strom, C. Discordant noninvasive prenatal testing and cytogenetic results: A study of 109 consecutive cases. Genet. Med. 2015, 17, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2019 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2019, 94, 1149–1167. [Google Scholar] [CrossRef] [PubMed]
- Kassa, B.A. Cytotoxicity and genotoxicity evaluation of municipal wastewater discharged into the head of Blue Nile River using the Allium cepa test. Sci. Afr. 2021, 13, e00911. [Google Scholar] [CrossRef]
- Küçük, D.; Liman, R. Cytogenetic and genotoxic effects of 2-chlorophenol on Allium cepa L. root meristem cells. Environ. Sci. Pollut. Res. 2018, 25, 36117–36123. [Google Scholar] [CrossRef]
- Alabi, O.A.; Atanda, H.C.; Olumurewa, J.A. Cytogenotoxicity of the aqueous extract of Parquetina nigrescens leaf using Allium cepa assay. Protoplasma 2022, 259, 1417–1425. [Google Scholar] [CrossRef]
- Palácio, S.M.; de Almeida, J.C.B.; de Campos, É.A.; Veit, M.T.; Ferreira, L.K.; Deon, M.T.M. Silver nanoparticles effect on Artemia salina and Allium cepa organisms: Influence of test dilution solutions on toxicity and particles aggregation. Ecotoxicology 2021, 30, 836–850. [Google Scholar] [CrossRef]
- Saha, N.; Gupta, S. Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J. Hazard. Mater. 2017, 330, 18–28. [Google Scholar] [CrossRef]
- Scherer, M.D.; Sposito, J.C.; Falco, W.F.; Grisolia, A.B.; Andrade, L.H.; Lima, S.M.; Machado, G.; Nascimento, V.A.; Gonçalves, D.A.; Wender, H.; et al. Cytotoxic and genotoxic effects of silver nanoparticles on meristematic cells of Allium cepa roots: A close analysis of particle size dependence. Sci. Total Environ. 2019, 660, 459–467. [Google Scholar] [CrossRef]
- Yekeen, T.; Azeez, M.; Akinboro, A.; Lateef, A.; Asafa, T.; Oladipo, I.; Ajibola, A. Safety evaluation of green synthesized Cola nitida pod, seed and seed shell extract-mediated silver nanoparticles (AgNPs) using an Allium cepa assay. J. Taibah Univ. Sci. 2017, 11, 895–909. [Google Scholar] [CrossRef]
- Zhurinov, M.; Berillo, D.; Bazhykova, K.B.; Rakhimov, K.D.; Bekezhanova, T. An estimation of the antiviral activity and toxicity of biologically active substances obtained from the raw materials of Artemisia cina Berg in vitro and in vivo. Molecules 2023, 28, 5413. [Google Scholar] [CrossRef]
- Zhangabay, Z.; Berillo, D. Antimicrobial and antioxidant activity of AgNPs stabilized with Calendula officinalis flower extract. Results Surf. Interfaces 2023, 11, 100–109. [Google Scholar] [CrossRef]
- Rajakumar, G.; Rahuman, A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 2011, 3, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and nanostructure Synthesis and controlled growth methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, B.-H. Silver nanoparticles: Synthesis and application for nanomedicine. Int. J. Mol. Sci. 2019, 20, 865. [Google Scholar] [CrossRef]
- Han, C.; Zhang, G.; Mei, Y.; Shan, Z.; Shi, K.; Zhou, S.; Shao, H. Chemical profile of Artemisia vulgaris L. essential oil and its phytotoxic, insecticidal, and antimicrobial activities. S. Afr. J. Bot. 2023, 162, 20–28. [Google Scholar] [CrossRef]
- Ivănescu, B.; Burlec, A.F.; Crivoi, F.; Roșu, C.; Corciovă, A. Secondary metabolites from Artemisia genus as biopesticides and innovative nano-based application strategies. Molecules 2021, 26, 3061. [Google Scholar] [CrossRef]
- Mukinda, J.T.; Syce, J.A. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. J. Ethnopharmacol. 2007, 112, 138–144. [Google Scholar] [CrossRef]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. The effects of Artemisia annua L. and Achillea millefolium L. crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L.(Lepidoptera: Pieridae). Pestic. Biochem. Physiol. 2011, 99, 244–249. [Google Scholar] [CrossRef]
- Singh, N.P.; Ferreira, J.F.; Park, J.S.; Lai, H.C. Cytotoxicity of ethanolic extracts of Artemisia annua to Molt-4 human leukemia cells. Planta Medica 2011, 77, 1788–1793. [Google Scholar] [CrossRef]
- Emami, S.A.; Rabe, S.Z.T.; Ahi, A.; Mahmoudi, M.; Tabasi, N. Study the cytotoxic and pro-apoptotic activity of Artemisia annua extracts. Pharmacologyonline 2009, 3, 1062–1069. [Google Scholar]
- Cetkovic, T.; Haveric, A.; Klacar, L.C.; Omanovic, M.H.; Haveric, S. In vitro assessment of genotoxic and cytotoxic effects of Artemisia annua L. tincture. Genet. Appl. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Jakovljević, M.R.; Grujičić, D.; Vukajlović, J.T.; Marković, A.; Milutinović, M.; Stanković, M.; Vuković, N.; Vukić, M.; Milošević-Djordjević, O. In vitro study of genotoxic and cytotoxic activities of methanol extracts of Artemisia vulgaris L. and Artemisia alba Turra. S. Afr. J. Bot. 2020, 132, 117–126. [Google Scholar] [CrossRef]
- Abderrahman, S.M.; Jamal Shbailat, S. Genotoxic and cytotoxic effects of Artemisia herba-alba on mammalian cells. Caryologia 2014, 67, 265–272. [Google Scholar] [CrossRef]
- Gaulden, M. Hypothesis: Some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosome stickiness, which causes chromosome aberrations. Mutagenesis 1987, 2, 357–365. [Google Scholar] [CrossRef]
- Evseeva, T.; Geras’kin, S.; Shuktomova, I.; Taskaev, A. Genotoxicity and cytotoxicity assay of water sampled from the underground nuclear explosion site in the north of the Perm region (Russia). J. Environ. Radioact. 2005, 80, 59–74. [Google Scholar] [CrossRef]
- Luo, L.; Werner, K.; Gollin, S.; Saunders, W. Cigarette smoke induces anaphase bridges and genomic imbalances in normal cells. Mutat. Res. 2004, 554, 375–385. [Google Scholar] [CrossRef]
- Leme, D.; Marin-Morales, M. Allium cepa test in environmental monitoring: A review on its application. Mutat. Res. 2009, 682, 71–81. [Google Scholar] [CrossRef]
- Choudhary, S.; Ansari, M.; Khan, Z.; Gupta, H. Cytotoxic action of lead nitrate on cytomorphology of Trigonella foenum-graecum L. Turk. J. Biol. 2012, 36, 267–273. [Google Scholar] [CrossRef]
- Haq, I.; Kumari, V.; Kumar, S.; Raj, A.; Lohani, M.; Bhargava, R. Evaluation of the phytotoxic and genotoxic potential of pulp and paper mill effluent using Vigna radiata and Allium cepa. Adv. Biol. 2016, 10, 8065736. [Google Scholar] [CrossRef]
- Kontsevaya, I.; Tolkacheva, T. Improving bioassay technique based Allium test. Vestn. Vitebsk. Gos. Univ. 2012, 6, 57–65. (In Russian) [Google Scholar]
- Barberio, A.; Voltolini, J.; Mello, M. Standardization of bulb and root sample sizes for the Allium cepa test. Ecotoxicology 2011, 20, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Dyusebaeva, M.A.; Berillo, D.A.; Berganayeva, A.E.; Berganayeva, G.E.; Ibragimova, N.A.; Jumabayeva, S.M.; Kudaibergenov, N.Z.; Kanapiyeva, F.M.; Kirgizbayeva, A.A.; Vassilina, G.K. Antimicrobial activity of silver nanoparticles stabilized by liposoluble extract of Artemisia terrae-albae. Processes 2023, 11, 3041. [Google Scholar] [CrossRef]
- Ibragimova, N.; Lyu, M.; Dyusambaev, K. The need for regulation of medicinal products in environmental objects to ensure the biological safety of the republic of Kazakhstan. Pharm. Kazakhstan 2023, 4, 205–213. [Google Scholar] [CrossRef]


| Experiment Number | Number of Bulbs | Number of Roots | The Length of the Roots cm | Number of Roots M ± m | The Length of the Roots, cm M ± m |
|---|---|---|---|---|---|
| Control (distilled water) | |||||
| 1 | 5 | 10 | 1.6 | 15.2 ± 5.07 | 3.48 ± 3.11 |
| 2 | 5 | 11 | 1.9 | ||
| 3 | 5 | 20 | 2.7 | ||
| 4 | 5 | 21 | 2.2 | ||
| 5 | 5 | 14 | 9.0 | ||
| AgNPs@Artemisia EtOH/H2O diluted by water ×40 (70 + 35 H2O) | |||||
| 1 | 5 | 8 | 0.4 | 16.4 ± 6.88 | 0.58 ± 0.13 |
| 2 | 5 | 12 | 0.6 | ||
| 3 | 5 | 19 | 0.5 | ||
| 4 | 5 | 26 | 0.7 | ||
| 5 | 5 | 17 | 0.7 | ||
| AgNPs@Artemisia EtOH/EtOAc extract diluted by water ×45 (73) | |||||
| 1 | 5 | 18 | 0.9 | 17.6 ± 6.27 | 0.84 ± 0.17 |
| 2 | 5 | 9 | 0.7 | ||
| 3 | 5 | 26 | 1.1 | ||
| 4 | 5 | 15 | 0.7 | ||
| 5 | 5 | 20 | 0.8 | ||
| Experiment Number | The Number of Cells Analyzed | The Number of Cells in | Mitotic Index (%) | |||
|---|---|---|---|---|---|---|
| Prophase | Metaphase | Anaphase | Telophase | |||
| Control (distilled water) | ||||||
| 1 | 684 | 664 | 12 | 6 | 2 | 68.4 |
| 2 | 648 | 638 | 7 | 3 | 0 | 64.8 |
| 3 | 657 | 642 | 10 | 4 | 1 | 65.7 |
| 4 | 634 | 623 | 8 | 3 | 0 | 53.4 |
| 5 | 695 | 681 | 8 | 5 | 1 | 69.5 |
| M ± m | 664 ± 25 | 650 ± 23 | 9 ± 2 | 4 ± 1.3 | 0.8 ± 0.8 | 64.4 ± 6.4 |
| AgNPs@Artemisia EtOH/H2O diluted by water ×40 (70 + 35 H2O) | ||||||
| 1 | 243 | 243 | 0 | 0 | 0 | 24.3 |
| 2 | 237 | 237 | 0 | 0 | 0 | 23.7 |
| 3 | 219 | 218 | 1 | 0 | 0 | 21.9 |
| 4 | 285 | 283 | 2 | 0 | 0 | 28.5 |
| 5 | 267 | 266 | 1 | 0 | 0 | 26.7 |
| M ± m | 250 ± 26 | 249 ± 25 | 0.8 ± 0.8 | 0 | 0 | 25.0 ± 2.6 |
| AgNPs@Artemisia EtOH/EtOAc extract diluted by water ×45 (73) | ||||||
| 1 | 320 | 320 | 0 | 0 | 0 | 32.0 |
| 2 | 409 | 404 | 3 | 1 | 1 | 40.9 |
| 3 | 346 | 343 | 2 | 1 | 0 | 34.6 |
| 4 | 389 | 388 | 1 | 0 | 0 | 38.9 |
| 5 | 332 | 331 | 1 | 0 | 0 | 33.2 |
| M ± m | 359 ± 38 | 357 ± 37 | 1.4 ±1.1 | 0.4 ± 0.5 | 0.2 ± 0.4 | 35.8 ± 3.6 |
| Experimental Group | Mitotic Phase Index, % | Mitotic Phase Index | |||
|---|---|---|---|---|---|
| PI | MI | AI | TI | ||
| Control | 97.89 | 1.36 | 0.60 | 0.12 | - |
| AgNPs@Artemisia EtOH/H2O ×40 (70 + 35 H2O) | 99.60 | 0. 32 | 0 | 0 | 0.39 |
| AgNPs@Artemisia EtOH/EtOAc extract ×45 (73 nm) | 99.44 | 0.39 | 0.11 | 0.06 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyusebaeva, M.; Berillo, D.; Yesbussinova, Z.; Ibragimova, N.; Shepilov, D.; Sydykbayeva, S.; Almabekova, A.; Chinibayeva, N.; Adeloye, A.O.; Berganayeva, G. Green Synthesis and Characterization of Silver Nanoparticles Using Artemisia terrae-albae Extracts and Evaluation of Their Cytogenotoxic Effects. Int. J. Mol. Sci. 2025, 26, 7499. https://doi.org/10.3390/ijms26157499
Dyusebaeva M, Berillo D, Yesbussinova Z, Ibragimova N, Shepilov D, Sydykbayeva S, Almabekova A, Chinibayeva N, Adeloye AO, Berganayeva G. Green Synthesis and Characterization of Silver Nanoparticles Using Artemisia terrae-albae Extracts and Evaluation of Their Cytogenotoxic Effects. International Journal of Molecular Sciences. 2025; 26(15):7499. https://doi.org/10.3390/ijms26157499
Chicago/Turabian StyleDyusebaeva, Moldyr, Dmitriy Berillo, Zhansaya Yesbussinova, Nailya Ibragimova, Daniil Shepilov, Sandugash Sydykbayeva, Almagul Almabekova, Nurzhan Chinibayeva, Adewale Olufunsho Adeloye, and Gulzat Berganayeva. 2025. "Green Synthesis and Characterization of Silver Nanoparticles Using Artemisia terrae-albae Extracts and Evaluation of Their Cytogenotoxic Effects" International Journal of Molecular Sciences 26, no. 15: 7499. https://doi.org/10.3390/ijms26157499
APA StyleDyusebaeva, M., Berillo, D., Yesbussinova, Z., Ibragimova, N., Shepilov, D., Sydykbayeva, S., Almabekova, A., Chinibayeva, N., Adeloye, A. O., & Berganayeva, G. (2025). Green Synthesis and Characterization of Silver Nanoparticles Using Artemisia terrae-albae Extracts and Evaluation of Their Cytogenotoxic Effects. International Journal of Molecular Sciences, 26(15), 7499. https://doi.org/10.3390/ijms26157499









