Abstract
The available literature data indicate that obestatin, a peptide derived from the preproghrelin precursor, may modulate neuroendocrine function, particularly in appetite regulation and somatotrophic/gonadotrophic pathways. This review synthesizes animal studies assessing the influence of obestatin on central neuroendocrine systems. Obestatin has been shown to affect the hypothalamic appetite-regulating center through neuropeptides such as neuropeptide Y and agouti-related peptide, yet findings remain inconsistent between species. In rodents, its effects on food intake and energy balance are inconclusive, whereas sheep models demonstrate significant alterations in orexigenic gene expression and peptide immunoreactivity. Regarding the somatotrophic axis, obestatin showed no significant effect on growth hormone (GH) secretion in rodents; however, in sheep, it modulated growth hormone-releasing hormone and somatostatin mRNA expression, elevated pituitary GH synthesis, and increased circulating GH levels. Studies involving the gonadotrophic axis demonstrated the presence of obestatin in Leydig and pituitary cells, with in vitro evidence suggesting its ability to modulate intracellular pathways implicated in gonadoliberin, luteinizing hormone, and follicle-stimulating hormone release. The collective findings discussed in this article indicate that obestatin interacts with multiple hypothalamic–pituitary axes, though its effects vary depending on species and experimental conditions. This review highlights the complexity of obestatin’s central actions and the need for further research to elucidate its functional relevance in neuroendocrine regulation.
1. Introduction
Both animals and humans live in a diverse and constantly changing environment that generates a vast array of stimuli. Maintaining a state of relative physiological balance is necessary for the proper functioning of all physiological processes. The integration and processing of incoming stimuli occurs in the central nervous system (CNS), which relays information to other organs and tissues. Effective coordination of functions at different levels relies on a communication network involving the circulatory, nervous, and endocrine pathways.
Availability of energy from the external environment is one of the crucial factors influencing proper metabolism, thermogenesis, the rate of cellular metabolism, as well as the development and function of the nervous system. Both short-term and chronic food deficiency result in the inhibition of the thyroid axis secretory activity, which consequently may lead to significant clinical consequences, including reduced metabolic processes, cardiac arrhythmias, reproductive abnormalities, and psychiatric symptoms such as depression or anxiety. Furthermore, controlling reproductive and growth processes is important, not only from the individual point of view but also from the maintenance of species. Reproduction, especially in females, is an energy-requiring process, so the relationship between the reproductive system functioning and the organism’s energy status is an important aspect. Both short-term and chronic food deficiency result in the inhibition of gonadotrophic and somatotrophic axes activity, which consequently leads to a lower number of offspring, temporary infertility, or stunted growth of the body. It is noteworthy that the activity of those axes is conditioned by a number of metabolic and nutritional signals, suggesting that common regulatory pathways are involved in the joint control of reproduction, growth, and energy balance [1].
New compounds, including hormones and neurotransmitters, continue to be discovered, and further studies confirm their involvement in the regulation of key physiological processes. One of them is obestatin, a 23-amino-acid (aa) peptide (2.5 kDa) encoded by the GHRL gene, whose translation produces a preproghrelin precursor protein. Subsequently, both ghrelin and obestatin peptides are generated through post-translational cleavage of preproghrelin [2]. Initial studies proposed an anorexigenic role for obestatin, demonstrating its ability to suppress food intake, reduce body weight, and delay gastric emptying [2,3,4]. However, subsequent reports presented varying results depending on obestatin administration route and dosage [5,6]. Obestatin was originally isolated from rat stomach mucosa cells [2], but subsequent studies showed that its synthesis was not limited to this organ. Huda et al. (2008) [7] demonstrated that blood obestatin levels remained stable after gastrectomy, confirming its production in other tissues. Immunohistochemical analyses have localized obestatin to multiple organs, including the duodenum, jejunum, large intestine, pancreas, liver, mammary glands, testicles (Leydig cells), and lungs, as well as in body fluids such as saliva and blood plasma [2,7,8,9].
The GHRL gene, encoding obestatin, is located on chromosome 3p26-p25 in humans and chromosome 4q4 in rats. The gene consists of four exons, three introns, and an additional small (20 bp) exon 0, responsible for encoding the 117 aa preproghrelin precursor [10,11]. Recent studies identified an additional exon (−1) and demonstrated that alternative splicing of exons −1, 3, and 4 generates functional obestatin [12,13,14]. Studies on the properties of obestatin initially indicated its affinity for the orphan G protein-coupled receptor 39 (GPR39) [2]. This receptor, a member of the rhodopsin-like receptor family, was first cloned by [15] as part of a family related to growth hormone secretagogue receptors (GHS-R). Analysis of GPR39 expression revealed the presence of two isoforms: the full-length GPR39-1a (423 aa), highly expressed in peripheral metabolic tissues (liver, pancreas, kidneys, adipose tissue), and the truncated GPR39-1b (284 aa), predominantly localized to the central nervous system [16]. However, subsequent GPR39 knockout mouse models showed no functional response to obestatin [17,18,19], challenging its role as the canonical obestatin receptor.
A study by Granata et al., (2008) [20] indicated potential obestatin binding to the glucagon-like peptide type 1 receptor (GLP-1R), involved in appetite regulation in the CNS; however, subsequent research by Unniappan et al., (2008) [21] failed to replicate these findings. These discrepancies in available data may result from the unresolved identification of the receptor and the limited information on specific agonists and antagonists of this peptide. Therefore, further research is necessary to identify the obestatin receptor and determine its location. Similar to ghrelin, obestatin appears capable of crossing the blood–brain barrier. Ref. [22] demonstrated this property through intravenous administration of radiolabeled obestatin, which showed both rapid brain uptake and concurrent metabolic degradation. This phenomenon is likely due to the short half-life of obestatin in blood plasma, estimated at approximately 22 min [22].
2. Neurohormonal Regulatory Networks
The hypothalamus integrates signals from both the body’s periphery and external environment. This small structure is limited by the ventral part of the lateral and ventral walls of the third ventricle of the brain, located between the optic chiasm anteriorly and the mammillary bodies posteriorly [23]. Its neuroanatomical organization comprises distinct clusters of neuronal cell bodies (perikarya) called hypothalamic nuclei. These nuclei and their neural projections form interconnected networks that regulate fundamental physiological processes, including body weight control, energy homeostasis, thermoregulation, reproduction, and growth.
Key hypothalamic nuclei include the paraventricular nucleus (PVN), suprachiasmatic nucleus (SCN), supraoptic nucleus (SON), ventromedial nucleus (VMN), dorsomedial nucleus (DMN), and arcuate nucleus (ARC). While these nuclei were initially considered to independently regulate distinct physiological processes, current evidence demonstrates significant functional overlap. Neuroanatomical studies demonstrate that brain areas governing food intake regulation substantially coincide with those controlling growth and reproductive processes (Figure 1). Furthermore, key nuclei comprising the appetite-regulating network, including the ARC, PVN, VMN, and DMN, colocalize with the area responsible for the synthesis of key neurohormones that modulate growth and reproduction [24,25]. The release of hypothalamic neuropeptides into the hypothalamic–pituitary portal circulation system occurs in the median eminence (ME). This structure is a periventricular organ composed of blood vessels from the hypothalamic–pituitary portal circulation system and nerve terminals from hypothalamic nuclei and other brain regions. Through this unique vascular-neural interface, the hypothalamus regulates pituitary secretion of key hormones, including those of the somatotrophic axis that control body growth and the gonadotrophic axis governing reproductive functions.
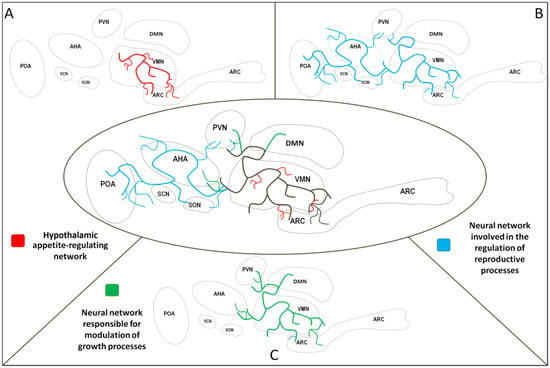
Figure 1.
Selected neural networks in the hypothalamus. Shared area of neural networks—black lines; neural network involved in the regulation of energy homeostasis—red lines (A). Neural network involved in the regulation of reproductive processes—blue lines (B). Neural network responsible for the modulation of growth processes—green lines (C). PVN—paraventricular nucleus, VMN—ventromedial nucleus, DMN—dorsomedial nucleus, ARC—arcuate nucleus, POA—preoptic area, AHA—anterior hypothalamic area, SCN—suprachiasmatic nucleus, SON—supraoptic nucleus.
Numerous studies have demonstrated that the body’s energy status is a key factor in the proper functioning of the somatotrophic and gonadotrophic axes [26,27]. Both insufficient energy (malnutrition, nutrient deficiencies) and excess energy states (overweight, obesity) disrupt these endocrine systems. Ongoing research in many centers worldwide aims to clarify the mechanisms that link the body’s energy status with the regulation of growth and reproductive functions.
3. Effect of Obestatin on Neurohormonal Network Activity
3.1. Obestatin’s Influence on Body Energy Status/Appetite-Regulating Network
The mediobasal hypothalamus (MBH) contains a few specialized neuronal populations that play a critical role in metabolic regulation and energy homeostasis [28]. Among others, two main neuronal subpopulations have been identified in the ARC: the first coexpresses neuropeptide Y (NPY) and agouti-related peptide (AgRP; NPY/AgRP neurons), while the second coexpresses cocaine- and amphetamine-regulated transcript (CART) and α-melanotropin (α-MSH; CART/α-MSH neurons). These neurons send their axonal projections to multiple hypothalamic regions, including the PVN, VMN, POA, and AHA [29,30,31,32], and are involved in the regulation of food intake and energy expenditure [33,34].
NPY represents one of the most abundant peptides in the brain, with its primary hypothalamic synthesis occurring in the ARC [35]. Recognized as one of the most potent orexigenic factors, NPY strongly stimulates appetite and food intake through its actions on Y1 and Y5 receptors, which belong to the G protein-coupled receptor family [36,37]. The expression of NPY is dynamically regulated by the body’s energy status: under conditions of optimal nutrition, the amount of NPY in nerve cells decreases, while starvation and/or malnutrition elevate its secretion [26,38,39,40]. AgRP also exerts potent orexigenic effects, though unlike NPY, its expression appears restricted to the ARC [41]. Similar to NPY, AgRP expression is dynamically regulated by energy status. Central administration of AgRP stimulates hyperphagia while reducing energy consumption, ultimately causing weight gain. Mechanistically, AgRP functions as an endogenous antagonist of melanocortin receptors MC3R and MC4R, counteracting the appetite-suppressing effects of α-MSH [39,42].
The α-MSH peptide is produced through post-translational processing of the precursor protein pro-opiomelanocortin (POMC). In contrast to the previously described peptides, α-MSH is an anorexigenic hormone that reduces food intake [43] through activation of melanocortin receptors MC3R and MC4R, which are predominantly expressed in the PVN [44]. As with NPY and AgRP, the expression of the Pomc gene and α-MSH protein is also associated with the nutritional status of the organism: starvation decreases α-MSH levels, while refeeding increases its production [45]. CART is another extremely important peptide associated with the regulation of energy status in ARC neurons [46]. This peptide exhibits anorexigenic properties similar to α-MSH. Studies show that fasting reduces CART secretion, while restored food intake stimulates its release [47,48,49]. Although no specific CART receptor has been identified, in vitro evidence suggests interactions with G protein-coupled receptors [50].
Both NPY/AgRP and CART/α-MSH neurons express receptors for several peripheral peptides and proteins involved in energy regulation, including leptin and ghrelin [42]. Moreover, direct connections have been identified between these neuronal populations and neurons responsible for the secretion of SOM, GHRH, and GnRH hormones. This suggests that NPY/AgRP and CART/α-MSH neurons, particularly NPY neurons, play a central role in transmitting information about energy status and integrating it with the functioning of the somatotrophic and gonadotrophic axes.
The literature data indicate that obestatin may modulate the activity of the hypothalamic appetite-regulating network, but the outcomes vary depending on the species (Figure 2). Studies in rats demonstrated that obestatin induces appetite suppression, body weight reduction, and delays gastric emptying [2]. Surprisingly, subsequent investigations in rodents found no significant effect of peripherally or centrally administered obestatin on food intake, energy expenditure, or body weight [51,52]. In addition, research on intraventricular administration of obestatin in rats showed no changes in hypothalamic mRNA expression of NPY and AgRP [4]. In sheep, intracerebroventricular administration of obestatin increased mRNA expression of NPY, AGRP, and NPY1R in MBH neurons. On the other hand, the same study reported decreased NPY immunoreactivity in ARC nucleus perikarya and nerve fibers, along with reduced NPY nerve fiber density in the PEV nucleus [53].
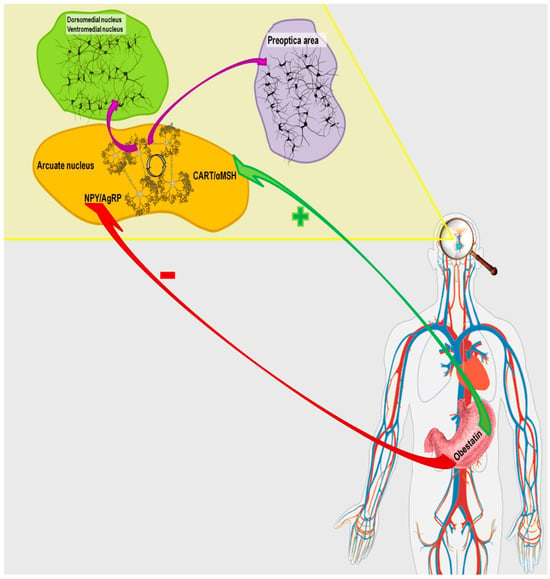
Figure 2.
Pathway of obestatin action in the hypothalamic network regulating the body’s energy homeostasis.
3.2. Obestatin and Somatotrophic Axis
The hypothalamic regions involved in regulating the somatotrophic axis include the anterior hypothalamic area (AHA) as well as the PVN, DMN, VMN, and ARC. This axis (hypothalamus–pituitary–peripheral tissue cells) is primarily controlled by two antagonistic neurohormones: somatostatin (SRIF) and growth-hormone-releasing hormone (GHRH). The antagonistic interplay between GHRH and SRIF results in the pulsatile release of growth hormone (GH) from pituitary somatotrophic cells into circulation [40,54,55,56]. The direct effects of SRIF and GHRH on pituitary somatotrophs are mediated by their specific receptors: GHRH receptor increases intracellular cAMP levels, triggering signaling pathways that enhance GH gene expression, while SRIF receptor inhibits GH production through cAMP suppression [57].
Growth hormone is a major regulator of peripheral growth processes. This 23 kDa polypeptide exhibits high interspecies sequence and structural homology, reflecting its fundamental biological role [58]. Despite this homology, GH half-life varies between species, from approximately 6 min in rats to about 25 min in humans [59,60]. Pulsatile GH secretion from pituitary somatotrophs results from alternating SRIF and GHRH release at the median eminence. GH exerts its effects on peripheral tissues either directly or indirectly through insulin-like growth factors (IGF) type 1 and 2 (IGF-1, IGF-2) [61,62]. The main effect of GH, mediated by IGF-1 and IGF-2, is the stimulation of body mass gain by promoting chondrogenesis and osteogenesis in bone cartilage. GH also directly regulates carbohydrate metabolism by inducing hepatic glycogenolysis and increasing glucose release. In adipose tissue, GH enhances lipolysis and suppresses lipogenesis, ultimately elevating plasma free fatty acid concentrations.
Current understanding of obestatin’s effects on GH regulation is primarily based on rodent studies (Figure 3). Initial research focused on explaining the potential effect of obestatin on GH secretion from pituitary somatotrophic cells [2]. In vivo studies in rats showed no effect of obestatin on either spontaneous or ghrelin-induced GH release [63]. Similarly, intraperitoneal administration of obestatin did not affect the change in GH levels in 10-day-old rats [5]. Moreover, Zizzari et al. (2007) [64] demonstrated that obestatin antagonizes ghrelin-dependent GH release in vivo. In vitro studies performed on rat cell lines or pituitary explants showed no effect of obestatin on either spontaneous or ghrelin-dependent GH release [2,64]. Contrasting findings were reported by Pazos et al., (2009) [65], who demonstrated obestatin-induced GH release in GC cell lines derived from mouse somatotrophic tumors.
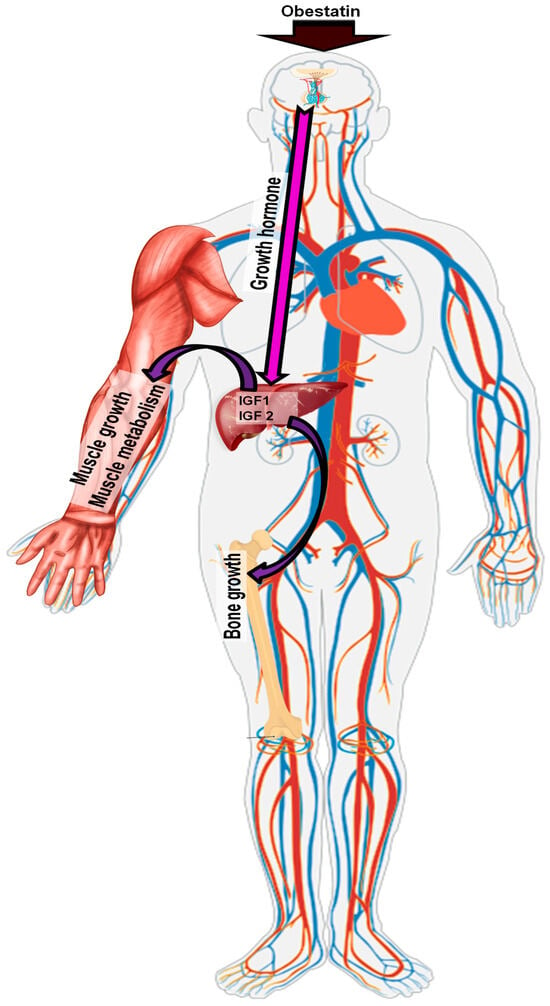
Figure 3.
Pathway of obestatin action in the somatotrophic axis.
Recent research has identified a potential role of obestatin in the hypothalamus. Specifically, experiments on mouse hypothalamus explants have demonstrated that obestatin inhibits ghrelin-dependent GHRH release, while not altering the activity of SOM neurons [66,67]. Food restrictions in mice and rats cause reduced secretory activity of somatotrophic pituitary cells and a significant decrease in blood GH levels. In ruminants, however, similar conditions result in increased GH synthesis and release [68]. These changes are largely attributed to the removal of the inhibitory effect of SOM [26,69]. Nevertheless, it should be noted that in ruminants, the mechanism regulating growth processes differs from that in rodents, which are monogastric animals with distinct eating habits and gastrointestinal physiology. A study on sheep subjected to intraventricular administration of obestatin to the third ventricle of the brain demonstrated that this peptide could regulate somatotrophic axis activity at the hypothalamic level. Intraventricular obestatin administration inhibited SOM mRNA expression in both the AHA and ME, while simultaneously stimulating GHRH transcription in the MBH. These hypothalamic effects were accompanied by significant endocrine changes, including increased GH mRNA production, elevated numbers of GH-immunoreactive cells in the pituitary, and higher circulating GH levels characterized by enhanced pulse frequency (Table 1) [6].
3.3. Obestatin and Gonadotrophic Axis
Gonadoliberin (GnRH), produced primarily in POA neurons, serves as the key regulator of the gonadotrophic axis. In sheep, approximately 70% of GnRH neurons project to the median eminence inner layer [70], where the peptide is released into the hypothalamic–pituitary portal circulation to control pituitary gonadotropin secretion. GnRH is released in a pulsatile manner, mainly controlled by the GnRH pulse generator system, which includes ARC neurons coexpressing kisspeptin (Kiss), neurokinin B (NKB), and dynorphin (Dyn)—collectively termed KNDy neurons [71]. Increased Kiss release from KNDy neuron terminals in the ME stimulates GnRH secretion into the local blood vessels [72]. Kiss release itself is modulated through auto- and paracrine mechanisms by NKB and Dyn [73]. Specifically, NKB stimulates the synthesis and release of Kiss, whereas Dyn inhibits these processes [71]. GnRH acts directly on pituitary gonadotrophs through specific G protein-coupled receptors (GnRHR). While multiple GnRHR types have been identified in various animal taxa, they all belong to the G protein-coupled receptor family [74], with GnRH receptor type I (GnRH1R) and type II receptor (GnRH2R) being the main receptors in mammals [75,76,77]. GnRHR activation primarily stimulates luteinizing hormone (LH) secretion by pituitary gonadotrophs, with secondary stimulation of follicle-stimulating hormone (FSH) release [78].
LH secretion occurs in a pulsatile pattern directly following GnRH pulses. In females, LH regulates estrogen production, influencing the development of the corpus luteum, progesterone synthesis, and ovulation. In males, LH is responsible for testosterone secretion and the development of secondary sexual traits (according to Pierzchała-Koziec, 2005 [79]). Meanwhile, FSH regulation involves two distinct mechanisms: a constitutive pathway that operates independently of GnRH pulses and a pulse-dependent pathway. The complexity of these two mechanisms may be additionally modulated by locally acting pituitary factors, including inhibins, activins, or follistatins, which form autocrine/paracrine regulatory networks for FSH control [78,80].
Current research has demonstrated that obestatin is produced in the male gonads by Leydig cells and is also present in pituitary cells [9,81]. In vitro studies on mouse, rat, and porcine cell lines have revealed that obestatin stimulates the secretion of both cAMP and the cellular signaling protein ERK1/2, while inhibiting myogen-activated kinase (MAPK). Additionally, the increase in cAMP has been associated with the activation of the cellular cAMP/protein kinase A-dependent pathway (PKA; [2,82,83,84]). The cAMP/PKA pathway, ERK1/2, and MAPK are known to mediate numerous hormonal signals, including intracellular signaling pathways involved in the synthesis and secretion of GnRH, LH, and FSH [83,85,86]. In vitro studies in porcine granulosa cells showed that obestatin stimulated progesterone secretion into the culture medium, without affecting estradiol and testosterone secretion [83]. However, contrasting results were obtained in human luteal cells, where obestatin reduced progesterone and prostaglandin (E2 and F2α) secretion [87]. Other in vitro studies using mouse cell lines, as well as in vivo experiments in mice, found no effect of obestatin on prolactin, LH, or FSH release from pituitary gonadotropes (Figure 4) [88].
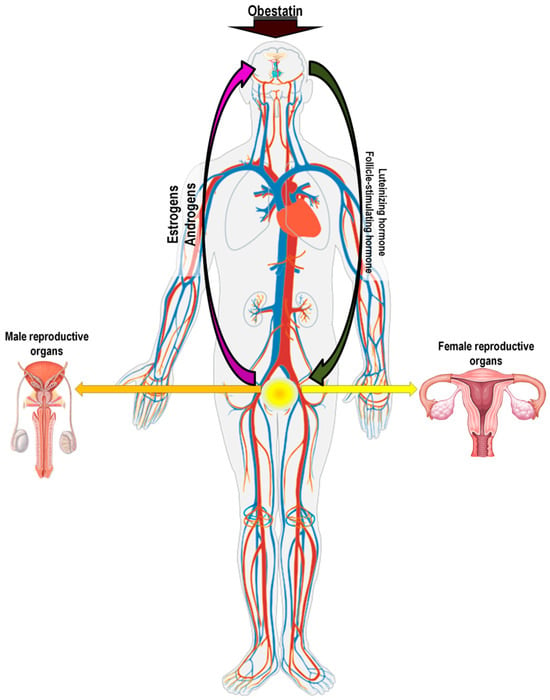
Figure 4.
Pathway of obestatin action in the gonadotrophic axis.

Table 1.
The influence of obestatin on the somatotrophic axis.
Table 1.
The influence of obestatin on the somatotrophic axis.
| Authors | Research Model | Materials and Methods | Outcome |
|---|---|---|---|
| Feng et al., 2011 [66] | Male mice | Incubation of hypothalamic explants | No effect of obestatin on spontaneous and ghrelin-induced reduction in somatostatin release from hypothalamic nerve cells. No effect on the activity of GHRH neurons and blocking ghrelin-induced GHRH release. |
| Hassouna et al., 2012 [67] | Male mice | Intraperitoneal injection in vivo | Obestatin abolished the stimulatory effect of ghrelin on the activity of GHRH neurons. Reduction in GH concentration as a result of obestatin eliminating the stimulatory effect of ghrelin. |
| Nogueiras et al., 2007 [4] | Male rats | Intravenous administration | Obestatin does not affect the release of GH into the blood. |
| Yamamoto et al., 2007 [63] | Male rats | Intravenous/intracerebroventricular administration | Obestatin does not affect the release of GH into the blood. |
| Bresciani et al., 2006 [5] | Male rats | Intraperitoneal injection | Obestatin does not affect the release of GH into the blood. |
| Zizzari et al., 2007 [64] | Male mice and rats | Intraperitoneal injection/intravenous injection | Obestatin antagonizes ghrelin-dependent GH release in vitro. |
| Zhang et al., 2005 [2] | Male rats | 48 h fasting | No effect of obestatin on spontaneous and ghrelin-dependent GH release. |
| Pazos et al., 2009 [65] | GC cells line | Medium supplemented with obestatin | Obestatin stimulated GH release into the culture medium, but only within the first 15 min of incubation. |
| Luque et al., 2014 [88] | Pituitary cells from baboons and mice | Medium supplemented with obestatin | 24 h incubation of cells in medium supplemented with obestatin resulted in decreased GH mRNA expression and reduced GH release. |
| Wójcik-Gładysz et al., 2018 [6] | Female sheep | Intracerebroventricular infusions | The mechanism of obestatin action seems to rely simultaneously on the stimulation of GHRH and the restraint of somatostatin output, which results in the enhanced release of GH from pituitary somatotrophic cells to the peripheral circulation. |
Research in sheep has revealed that obestatin exerts multi-level control over reproductive neuroendocrine function. When administered intracerebroventricularly, obestatin displayed region-specific regulation of GnRH neurons, stimulating GnRH mRNA expression in the POA, while suppressing it in the ME. This was accompanied by altered GnRH peptide distribution in ME nerve terminals, suggesting effects on both synthesis and release mechanisms. The peptide also modified GnRH pulse generator activity, indicating direct neuromodulatory actions on GnRH neuronal networks. Additionally, obestatin treatment triggered opposing effects in key neuropeptide systems by upregulating kisspeptin (Kiss) mRNA while downregulating prodynorphin (pDyn) expression. Paradoxically, despite increased kisspeptin transcription, immunohistochemical analysis revealed decreased kisspeptin immunoreactivity in both ARC perikarya and ME nerve fibers. These changes in GnRH regulation were further supported by subsequent research examining obestatin and its effects on LH and FSH release into the bloodstream. Obestatin decreased LHβ mRNA expression while increasing LH accumulation in gonadotropic cells, ultimately reducing circulating LH concentrations. Interestingly, the same study reported an increase in both FSHβ mRNA expression and FSH immunoreactivity in pituitary cells, although the mean blood FSH concentration remained unchanged (Table 2) [89,90].

Table 2.
The influence of obestatin on the gonadotrophic axis.
4. Summary and Future Research Directions
The physiological role of obestatin, a peptide hormone derived from the same precursor as ghrelin, remains complex and incompletely understood. Findings related to the somatotrophic axis activity suggest a limited or inhibitory role of obestatin in GH regulation in monogastric animals, such as rodents, based on both in vivo and in vitro studies. In contrast, results in ruminants, especially sheep, reveal a remarkably different pattern. When administered directly to the brain, obestatin inhibits somatostatin mRNA expression, simultaneously increasing GHRH transcript levels, leading to elevated pituitary GH synthesis and secretion into peripheral blood. Obestatin also appears to influence the activity of the hypothalamic–pituitary–gonadal axis. While in vitro studies have reported varied effects on steroid hormone release, in vivo sheep experiments indicated that obestatin modulates LH and FSH expression and secretion. Additionally, obestatin affects the GnRH pulse generator, thereby regulating GnRH neuronal activity. Furthermore, obestatin appears to interact with the hypothalamic appetite-regulating network, although its effects show species-specific variation. In rodents, obestatin may suppress appetite, whereas in sheep, it upregulates the expression of orexigenic peptides (NPY and AgRP).
The current literature lacks information on the potential therapeutic effects of obestatin in the neuroendocrine systems discussed in this review, namely the appetite regulation system and the somatotrophic and gonadotrophic axes. Nonetheless, there are some findings that suggest this peptide may be involved in therapeutic applications across various physiological systems. In a study conducted on rats with type 2 diabetes, it was demonstrated that obestatin administered over a period of 30 days had a modulatory effect on blood insulin and glucose concentrations. These findings suggest that obestatin represents a promising therapeutic target for the treatment of metabolic disorders, including diabetes and obesity [91,92]. However, future research could be expanded to investigate how obestatin interacts with the hypothalamic center involved in the regulation of food intake, particularly in the context of diabetes and feeding disorders like obesity. Interestingly, and relevant to the topic of obesity, obestatin appears to have an influence on atherosclerotic cardiovascular disease. It shows that obestatin may have a cardioprotective role, could impact on controlling blood pressure, and also enhances papillary muscle contractility and beta-adrenergic responsiveness, but only in type 1 diabetic rats [93]. Other studies have demonstrated that obestatin administration in rats fed a high-fat diet can protect against non-alcoholic fatty liver disease [94]. More interestingly, obestatin also shows neuroprotective properties and, by improving the function of dopaminergic neurons, it alleviates the progression and symptoms of Parkinson’s disease [95]. Since this peptide demonstrates properties influencing memory retention, vasodilation, and neuronal survival, further studies could be extended to investigate its effects on the neuroendocrine systems as well as the reward system. Interestingly, a therapeutic application for obestatin has also been found in muscle regeneration through the stimulation of satellite stem cells and myofiber hypertrophy [96].
Obestatin has emerged as a multifunctional peptide involved in growth, reproduction, and appetite regulation. However, research has yielded inconsistent results depending on species, administration routes, and experimental conditions. These discrepancies underscore the complexity of obestatin’s function and emphasize the need for further research to fully elucidate its neuroendocrine mechanisms and physiological significance.
Author Contributions
M.S.: writing—original draft, review and editing, conceptualization, figure preparation. A.W.-G.: critical revision of the manuscript for important intellectual content. A.G.: critical revision of the manuscript for important intellectual content. B.J.P.: writing—original draft, review and editing, figure preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wójcik-Gładysz, A.; Szlis, M. Hypothalamo-gastrointestinal axis—Role in food intake regulation. J. Anim. Feed Sci. 2016, 25, 97–108. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J.W. Medicine: Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef]
- Green, B.D.; Irwin, N.; Flatt, P.R. Direct and indirect effects of obestatin peptides on food intake and the regulation of glucose homeostasis and insulin secretion in mice. Peptides 2007, 28, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Nogueiras, R.; Pfluger, P.; Tovar, S.; Arnold, M.; Mitchell, S.; Morris, A.; Perez-Tilve, D.; Vázquez, M.J.; Wiedmer, P.; Castañeda, T.R.; et al. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology 2007, 148, 21–26. [Google Scholar] [CrossRef]
- Bresciani, E.; Rapetti, D.; Donà, F.; Bulgarelli, I.; Tamiazzo, L.; Locatelli, V.; Torsello, A. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J. Endocrinol. Investig. 2006, 29, RC16–RC18. [Google Scholar] [CrossRef]
- Wójcik-Gładysz, A.; Szlis, M.; Misztal, A.; Przybył, B.J.; Polkowska, J. Obestatin stimulates the somatotrophic axis activity in sheep. Brain Res. 2018, 1678, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Huda, M.S.B.; Durham, B.H.; Wong, S.P.; Deepak, D.; Kerrigan, D.; McCulloch, P.; Ranganath, L.; Pinkney, J.; Wilding, J.P.H. Plasma obestatin levels are lower in obese and post-gastrectomy subjects, but do not change in response to a meal. Int. J. Obes. 2008, 32, 129–135. [Google Scholar] [CrossRef]
- Zhang, J.V.; Jahr, H.; Luo, C.-W.; Klein, C.; Van Kolen, K.; Ver Donck, L.; De, A.; Baart, E.; Li, J.; Moechars, D.; et al. Obestatin Induction of Early-Response Gene Expression in Gastrointestinal and Adipose Tissues and the Mediatory Role of G Protein-Coupled Receptor, GPR39. Mol. Endocrinol. 2008, 22, 1464–1475. [Google Scholar] [CrossRef]
- Dun, S.L.; Brailoiu, G.C.; Brailoiu, E.; Yang, J.; Chang, J.K.; Dun, N.J. Distribution and biological activity of obestatin in the rat. J. Endocrinol. 2006, 191, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kanamoto, N.; Akamizu, T.; Tagami, T.; Hataya, Y.; Moriyama, K.; Takaya, K.; Hosoda, H.; Kojima, M.; Kangawa, K.; Nakao, K. Genomic structure and characterization of the 5′-flanking region of the human ghrelin gene. Endocrinology 2004, 145, 4144–4153. [Google Scholar] [CrossRef]
- Nakai, N.; Kaneko, M.; Nakao, N.; Fujikawa, T.; Nakashima, K.; Ogata, M.; Tanaka, M. Identification of promoter region of ghrelin gene in human medullary thyroid carcinoma cell line. Life Sci. 2004, 75, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Seim, I.; Collet, C.; Herington, A.C.; Chopin, L.K. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genom. 2007, 8, 298. [Google Scholar] [CrossRef] [PubMed]
- Seim, I.; Amorim, L.; Walpole, C.; Carter, S.; Chopin, L.K.; Herington, A.C. Ghrelin gene-related peptides: Multifunctional endocrine/autocrine modulators in health and disease. Clin. Exp. Pharmacol. Physiol. 2010, 37, 125–131. [Google Scholar] [CrossRef]
- Soares, J.-B.; Leite-Moreira, A.F. Ghrelin, des-acyl ghrelin and obestatin: Three pieces of the same puzzle. Peptides 2008, 29, 1255–1270. [Google Scholar] [CrossRef]
- McKee, K.K.; Tan, C.P.; Palyha, O.C.; Liu, J.; Feighner, S.D.; Hreniuk, D.L.; Smith, R.G.; Howard, A.D.; Van der Ploeg, L.H.T. Cloning and Characterization of Two Human G Protein-Coupled Receptor Genes (GPR38 and GPR39) Related to the Growth Hormone Secretagogue and Neurotensin Receptors. Genomics 1997, 46, 426–434. [Google Scholar] [CrossRef]
- Egerod, K.L.; Holst, B.; Petersen, P.S.; Hansen, J.B.; Mulder, J.; Hökfelt, T.; Schwartz, T.W. GPR39 Splice Variants Versus Antisense Gene LYPD1: Expression and Regulation in Gastrointestinal Tract, Endocrine Pancreas, Liver, and White Adipose Tissue. Mol. Endocrinol. 2007, 21, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Moechars, D.; Depoortere, I.; Moreaux, B.; de Smet, B.; Goris, I.; Hoskens, L.; Daneels, G.; Kass, S.; Ver Donck, L.; Peeters, T.; et al. Altered Gastrointestinal and Metabolic Function in the GPR39-Obestatin Receptor-Knockout Mouse. Gastroenterology 2006, 131, 1131–1141. [Google Scholar] [CrossRef]
- Tremblay, F.; Perreault, M.; Klaman, L.D.; Tobin, J.F.; Smith, E.; Gimeno, R.E. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology 2007, 148, 501–506. [Google Scholar] [CrossRef]
- Szlis, M.; Przybył, B.J.; Pałatyńska, K.; Wójcik-Gładysz, A. QRFP43 modulates the activity of the hypothalamic appetite regulatory centre in sheep. J. Anim. Feed Sci. 2024, 33, 281–286. [Google Scholar] [CrossRef]
- Granata, R.; Settanni, F.; Gallo, D.; Trovato, L.; Biancone, L.; Cantaluppi, V.; Nano, R.; Annunziata, M.; Campiglia, P.; Arnoletti, E. Obestatin Promotes Survival of Pancreatic bCells and Human Islets and Induces Expression of Genes Involved in the Regulation of b-Cell Mass and Function. Diabetes 2008, 57, 967–979. [Google Scholar] [CrossRef]
- Unniappan, S.; Speck, M.; Kieffer, T.J. Metabolic effects of chronic obestatin infusion in rats. Peptides 2008, 29, 1354–1361. [Google Scholar] [CrossRef]
- Pan, W.; Tu, H.; Kastin, A.J. Differential BBB interactions of three ingestive peptides: Obestatin, ghrelin, and adiponectin. Peptides 2006, 27, 911–916. [Google Scholar] [CrossRef] [PubMed]
- Sotowska-Brochocka, J. Fizjologia Zwierząt—Zagadnienia Wybrane, 1st ed.; Wydawnictwa Uniwersytetu Warszawskiego: Warszawa, Polska, 2001; p. 448. ISBN 83-235-0193-9. [Google Scholar]
- Barrett, K.E.; Barman, S.M.; Brooks, H.L.; Yuan, J.X.-J. Hypothalamic Regulation of Hormonal Functions. In Ganong’s Review of Medical Physiology, 26th ed.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Funahashi, H.; Takenoya, F.; Guan, J.L.; Kageyama, H.; Yada, T.; Shioda, S. Hypothalamic neuronal networks and feeding-related peptides involved in the regulation of feeding. Anat. Sci. Int. 2003, 78, 123–138. [Google Scholar] [CrossRef]
- Gładysz, A.; Krejči, P.; Šimůnek, J.; Polkowska, J. Effects of central infusions of neuropeptide Y on the somatotropic axis in sheep fed on two levels of protein. Acta Neurobiol. Exp. 2001, 61, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Tillet, Y.; Picard, S.; Bruneau, G.; Ciofi, P.; Wańkowska, M.; Wójcik-Gładysz, A.; Polkowska, J. Hypothalamic arcuate neuropeptide Y-neurons decrease periventricular somatostatin-neuronal activity before puberty in the female lamb: Morphological arguments. J. Chem. Neuroanat. 2010, 40, 265–271. [Google Scholar] [CrossRef]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef]
- Kim, M.S.; Rossi, M.; Abusnana, S.; Sunter, D.; Morgan, D.G.A.; Small, C.J.; Edwards, C.M.; Heath, M.M.; Stanley, S.A.; Seal, L.J.; et al. Hypothalamic localization of the feeding effect of agouti-related peptide and alpha-melanocyte-stimulating hormone. Diabetes 2000, 49, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.; Wynne, K.; McGowan, B.; Bloom, S. Hormonal Regulation of Food Intake. Physiol. Rev. 2005, 85, 1131–1158. [Google Scholar] [CrossRef]
- Simpson, K.A.; Martin, N.M.; Bloom, S.R. Hypothalamic regulation of food intake and clinical therapeutic applications Regulação hipotalâmica da ingestão alimentar e suas aplicações terapêuticas clínicas. Arq. Bras. Endocrinol. Metabol. 2009, 53, 120–128. [Google Scholar] [CrossRef]
- Wojtulewicz, K.; Tomczyk, M.; Wójcik, M.; Bochenek, J.; Antushevich, H.; Krawczyńska, A.; Załȩcki, M.; Herman, A.P. Circadian and seasonal changes in the expression of clock genes in the ovine pars tuberalis. J. Anim. Feed Sci. 2023, 32, 363–371. [Google Scholar] [CrossRef]
- Wynne, K.; Stanley, S.; McGowan, B.; Bloom, S.R. Appetite control. J. Endocrinol. 2005, 184, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Farooqi, I.S.; O’Rahilly, S. The Hormonal Control of Food Intake. Cell 2007, 129, 251–262. [Google Scholar] [CrossRef]
- Tatemoto, K.; Carlquist, M.; Mutt, V. Neuropeptide Y—A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 1982, 296, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Mitchell, N.F.; Lin, S.; Macia, L.; Yulyaningsih, E.; Baldock, P.A.; Enriquez, R.F.; Zhang, L.; Shi, Y.-C.; Zolotukhin, S.; et al. Y1 and Y5 Receptors Are Both Required for the Regulation of Food Intake and Energy Homeostasis in Mice. PLoS ONE 2012, 7, e40191. [Google Scholar] [CrossRef]
- Sohn, J.W.; Elmquist, J.K.; Williams, K.W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 2013, 36, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.P.; Dube, M.G.; Pu, S.; Xu, B.; Horvath, T.L.; Kalra, P.S. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr. Rev. 1999, 20, 68–100. [Google Scholar]
- Lopaschuk, G.D.; Ussher, J.R.; Jaswal, J.S. Targeting Intermediary Metabolism in the Hypothalamus as a Mechanism to Regulate Appetite. Pharmacol. Rev. 2010, 62, 237–264. [Google Scholar] [CrossRef]
- Szczepkowska, A.; Bochenek, J.; Wójcik, M.; Tomaszewska-Zaremba, D.; Antushevich, H.; Tomczyk, M.; Skipor, J.; Herman, A.P. Effect of caffeine on adenosine and ryanodine receptor gene expression in the hypothalamus, pituitary, and choroid plexus in ewes under basal and LPS challenge conditions. J. Anim. Feed Sci. 2023, 32, 17–25. [Google Scholar] [CrossRef]
- Cowley, M.A.; Smith, R.G.; Diano, S.; Tschöp, M.; Pronchuk, N.; Grove, K.L.; Strasburger, C.J.; Bidlingmaier, M.; Esterman, M.; Heiman, M.L.; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 37, 649–661. [Google Scholar] [CrossRef]
- Schneeberger, M.; Gomis, R.; Claret, M. Hypothalamic and brainstem neuronal circuits controlling homeostatic energy balance. J. Endocrinol. 2014, 220, T25–T46. [Google Scholar] [CrossRef]
- Germano, C.M.R.; de Castro, M.; Rorato, R.; Laguna, M.T.C.; Antunes-Rodrigues, J.; Elias, C.F.; Elias, L.L.K. Time course effects of adrenalectomy and food intake on cocaine- and amphetamine-regulated transcript expression in the hypothalamus. Brain Res. 2007, 1166, 55–64. [Google Scholar] [CrossRef]
- Mercer, A.J.; Hentges, S.T.; Meshul, C.K.; Low, M.J. Unraveling the Central Proopiomelanocortin Neural Circuits. Front. Neurosci. 2013, 7, 19. [Google Scholar] [CrossRef]
- Benoit, S.C.; Schwartz, M.W.; Lachey, J.L.; Hagan, M.M.; Rushing, P.A.; Blake, K.A.; Yagaloff, K.A.; Kurylko, G.; Franco, L.; Danhoo, W.; et al. A Novel Selective Melanocortin-4 Receptor Agonist Reduces Food Intake in Rats and Mice without Producing Aversive Consequences. J. Neurosci. 2000, 20, 3442–3448. [Google Scholar] [CrossRef]
- Douglass, J.; McKinzie, A.; Couceyro, P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 1995, 15, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Robson, A.J.; Rousseau, K.; Loudon, A.S.I.; Ebling, F.J.P. Cocaine and Amphetamine-Regulated Transcript mRNA Regulation in the Hypothalamus in Lean and Obese Rodents. J. Neuroendocrinol. 2002, 14, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Wortley, K.E.; Anderson, K.D.; Garcia, K.; Murray, J.D.; Malinova, L.; Liu, R.; Moncrieffe, M.; Thabet, K.; Cox, H.J.; Yancopoulos, G.D.; et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc. Natl. Acad. Sci. USA 2004, 101, 8227–8232. [Google Scholar] [CrossRef]
- Archer, Z.A.; Rayner, D.V.; Duncan, J.S.; Bell, L.M.; Mercer, J.G. Introduction of a High-Energy Diet Acutely Up-Regulates Hypothalamic Cocaine and Amphetamine-Regulated Transcript, Mc4R and Brown Adipose Tissue Uncoupling Protein-1 Gene Expression in Male Sprague-Dawley Rats. J. Neuroendocrinol. 2005, 17, 10–17. [Google Scholar] [CrossRef]
- Lin, Y.; Hall, R.A.; Kuhar, M.J. CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: Identification of PACAP 6-38 as a CART receptor antagonist. Neuropeptides 2011, 45, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Gourcerol, G.; St-Pierre, D.H.; Taché, Y. Lack of obestatin effects on food intake: Should obestatin be renamed ghrelin-associated peptide (GAP)? Regul. Pept. 2007, 141, 1–7. [Google Scholar] [CrossRef]
- Mondal, M.S.; Toshinai, K.; Ueno, H.; Koshinaka, K.; Nakazato, M. Characterization of obestatin in rat and human stomach and plasma, and its lack of acute effect on feeding behavior in rodents. J. Endocrinol. 2008, 198, 339–346. [Google Scholar] [CrossRef]
- Szlis, M.; Polkowska, J.; Skrzeczyńska, E.; Przybył, B.J.; Wójcik-Gładysz, A. Does obestatin modulate the hypothalamic appetite-regulating network in peripubertal sheep? J. Anim. Physiol. Anim. Nutr. 2018, 102, 690–700. [Google Scholar] [CrossRef] [PubMed]
- G S Tannenbaum, N.L. The interrelationship of growth hormone (GH)-releasing factor and somatostatin in generation of the ultradian rhythm of GH secretion. Endocrinology 1984, 115, 1952–1957. [Google Scholar] [CrossRef]
- Hartman, M.L.; Veldhuis, J.D.; Thorner, M.O. Normal Control of Growth Hormone Secretion. Horm. Res. 1993, 40, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Plotsky, P.M.; Vale, W. Patterns of Growth Hormone-Eeleasing Factor and Somatostatin Secretion into the Hypophysial-Portal Circulation of the Rat. Science 1985, 230, 461–463. [Google Scholar] [CrossRef]
- Theill, L.E.; Karin, M. Transcriptional control of GH expression and anterior pituitary development. Endocr. Rev. 1993, 14, 670–689. [Google Scholar]
- Goffin, V.; Shiverick, K.T.; Kelly, P.A.; Martial, J.A. Sequence-Function Relationships Within the Expanding. Endocr. Rev. 1996, 17, 385–410. [Google Scholar]
- Badger, T.M.; Millard, W.J.; Owens, S.M.; Larovere, J.; O’Sullivan, D. Effects of gonadal steroids on clearance of growth hormone at steady state in the rat. Endocrinology 1991, 128, 1065–1072. [Google Scholar] [CrossRef]
- Holl, R.W.; Schwarz, U.; Schauwecker, P.; Benz, R.; Veldhuis, J.D.; Heinze, E. Diurnal variation in the elimination rate of human growth hormone (GH): The half-life of serum GH is prolonged in the evening, and affected by the source of the hormone, as well as by body size and serum estradiol. J. Clin. Endocrinol. Metab. 1993, 77, 216–220. [Google Scholar] [PubMed]
- Spagnoli, A.; Rosenfeld, R.G. The mechanisms by which growth hormone brings about growth: The relative contributions of growth hormone and insulin-like growth factors. Endocrinol. Metab. Clin. N. Am. 1996, 25, 615–631. [Google Scholar] [CrossRef]
- Daughaday, W.H.; Trivedi, B.; Kapadia, M. Measurement of insulin-like growth factor ii by a specific radioreceptor assay in serum of normal individuals, patients with abnormal growth hormone secretion, and patients with tumor-associated hypoglycemia. J. Clin. Endocrinol. Metab. 1981, 53, 289–294. [Google Scholar] [CrossRef]
- Yamamoto, D.; Ikeshita, N.; Daito, R.; Herningtyas, E.H.; Toda, K.; Takahashi, K.; Iida, K.; Takahashi, Y.; Kaji, H.; Chihara, K.; et al. Neither intravenous nor intracerebroventricular administration of obestatin affects the secretion of GH, PRL, TSH and ACTH in rats. Regul. Pept. 2007, 138, 141–144. [Google Scholar] [CrossRef]
- Zizzari, P.; Longchamps, R.; Epelbaum, J.; Bluet-Pajot, M.T. Obestatin partially affects ghrelin stimulation of food intake and growth hormone secretion in rodents. Endocrinology 2007, 148, 1648–1653. [Google Scholar] [CrossRef]
- Pazos, Y.; Álvarez, C.J.P.; Camiña, J.P.; Al-Massadi, O.; Seoane, L.M.; Casanueva, F.F. Role of obestatin on growth hormone secretion: An in vitro approach. Biochem. Biophys. Res. Commun. 2009, 390, 1377–1381. [Google Scholar] [CrossRef]
- Feng, D.D.; Yang, S.K.; Loudes, C.; Simon, A.; Al-Sarraf, T.; Culler, M.; Alvear-Perez, R.; Llorens-Cortes, C.; Chen, C.; Epelbaum, J.; et al. Ghrelin and obestatin modulate growth hormone-releasing hormone release and synaptic inputs onto growth hormone-releasing hormone neurons. Eur. J. Neurosci. 2011, 34, 732–744. [Google Scholar] [CrossRef]
- Hassouna, R.; Zizzari, P.; Viltart, O.; Yang, S.K.; Gardette, R.; Videau, C.; Badoer, E.; Epelbaum, J.; Tolle, V. A Natural Variant of Obestatin, Q90L, Inhibits Ghrelin’s Action on Food Intake and GH Secretion and Targets NPY and GHRH Neurons in Mice. PLoS ONE 2012, 7, e51135. [Google Scholar] [CrossRef]
- Carro, E.; Señaris, R.; Peino, R.; Leal-Cerro, A.; Popovic, V.; Micic, D.; Dieguez, C.; Casanueva, F.F. Neuropharmacological assessment of growth hormone secretion. Acta Paediatr. Int. J. Paediatr. Suppl. 1997, 86, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.B.; Cummins, J.T.; Francis, H.; Sudbury, A.W.; Mccloud, P.I.; Clarke, I.J. Effect of restricted feeding on the relationship between hypophysial portal concentrations of growth hormone (GH)-releasing factor and somatostatin, and jugular concentrations of GH in ovariectomized ewes. Endocrinology 1991, 128, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Kochman, K.; Przekop, F.; Okrasa, S. Ośrodkowa regulacja neuralna procesów rozrodczych. In Biologia Rozrodu Zwierząt: Fizjologiczna Regulacja Procesów Rozrodczych Samicy; Krzymowski, T., Ed.; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Polska, 2007; pp. 21–46. ISBN 83-7299-437-4. [Google Scholar]
- Navarro, V.M.; Gottsch, M.L.; Chavkin, C.; Okamura, H.; Clifton, D.K.; Steiner, R.A. Regulation of Gonadotropin-Releasing Hormone Secretion by Kisspeptin/Dynorphin/Neurokinin B Neurons in the Arcuate Nucleus of the Mouse. J. Neurosci. 2009, 29, 11859–11866. [Google Scholar] [CrossRef]
- García-Galiano, D.; Van Ingen Schenau, D.; Leon, S.; Krajnc-Franken, M.A.M.; Manfredi-Lozano, M.; Romero-Ruiz, A.; Navarro, V.M.; Gaytan, F.; Van Noort, P.I.; Pinilla, L.; et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology 2012, 153, 316–328. [Google Scholar] [CrossRef]
- Li, Q.; Millar, R.P.; Clarke, I.J.; Smith, J.T. Evidence that neurokinin B controls basal gonadotropin-releasing hormone secretion but is not critical for estrogen-positive feedback in sheep. Neuroendocrinology 2015, 101, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, C.A.; Chen, C.C.; Coetsee, M.; Mamputha, S.; Whitlock, K.E.; Bredenkamp, N.; Grosenick, L.; Fernald, R.D.; Illing, N. Expression, structure, function, and evolution of gonadotropin-releasing hormone (GnRH) receptors GnRH-R1SHS and GnRH-R2PEY in the teleost, Astatotilapia burtoni. Endocrinology 2007, 148, 5060–5071. [Google Scholar] [CrossRef]
- Fromme, B.J.; Katz, A.A.; Roeske, R.W.; Millar, R.P.; Flanagan, C.A. Role of Aspartate 7.32(302) of the human gonadotropin-releasing hormone receptor in stabilizing a high-affinity ligand conformation. Mol. Pharmacol. 2001, 60, 1280–1287. [Google Scholar] [CrossRef]
- Millar, R.P.; Lu, Z.L.; Pawson, A.J.; Flanagan, C.A.; Morgan, K.; Maudsley, S.R. Gonadotropin-releasing hormone receptors. Endocr. Rev. 2004, 25, 235–275. [Google Scholar] [CrossRef]
- Cheng, C.K.; Leung, P.C.K. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-II, and their receptors in humans. Endocr. Rev. 2005, 26, 283–306. [Google Scholar] [CrossRef]
- Krzymowski, T. Biologia Rozrodu Zwierząt: Fizjologiczne Regulacje Procesów Rozrodczych Samicy; Wydawnictwo Uniwersytetu Warmińsko-Mazurskiego: Olsztyn, Polska, 2007; pp. 95–131. [Google Scholar]
- Pierzchała-Koziec, K. Wydzielanie wewnętrzne. In Fizjologia Zwierząt; Krzymowski, T., Przała, J., Eds.; Państwowe Wydawnictwo Rolnicze i Leśne: Warszawa, Polska, 2005; pp. 159–197. [Google Scholar]
- Bilezikjian, L.M.; Blount, A.L.; Leal, A.M.O.; Donaldson, C.J.; Fischer, W.H.; Vale, W.W. Autocrine/paracrine regulation of pituitary function by activin, inhibin and follistatin. Mol. Cell. Endocrinol. 2004, 225, 29–36. [Google Scholar] [CrossRef]
- Volante, M.; Rosas, R.; Ceppi, P.; Rapa, I.; Cassoni, P.; Wiedenmann, B.; Settanni, F.; Granata, R.; Papotti, M. Obestatin in human neuroendocrine tissues and tumours: Expression and effect on tumour growth. J. Pathol. 2009, 218, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Granata, R.; Gallo, D.; Luque, R.M.; Baragli, A.; Scarlatti, F.; Grande, C.; Gesmundo, I.; Cordoba-Chacon, J.; Bergandi, L.; Settanni, F.; et al. Obestatin regulates adipocyte function and protects against diet-induced insulin resistance and inflammation. FASEB J. 2012, 26, 3393–3411. [Google Scholar] [CrossRef] [PubMed]
- Mészárosová, M.; Sirotkin, A.V.; Grossmann, R.; Darlak, K.; Valenzuela, F. The effect of obestatin on porcine ovarian granulosa cells. Anim. Reprod. Sci. 2008, 108, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Roszkowicz-Ostrowska, K.; Młotkowska, P.; Marciniak, E.; Szlis, M.; Barszcz, M.; Misztal, T. Activation of BDNF–TrkB Signaling in Specific Structures of the Sheep Brain by Kynurenic Acid. Cells 2024, 13, 1928. [Google Scholar] [CrossRef]
- Hunzicker-Dunn, M.; Maizels, E.T. FSH signaling pathways in immature granulosa cells that regulate target gene expression: Branching out from protein kinase A. Cell. Signal. 2006, 18, 1351–1359. [Google Scholar] [CrossRef]
- Perrett, R.M.; McArdle, C.A. Molecular mechanisms of gonadotropin-releasing hormone signaling: Integrating cyclic nucleotides into the network. Front. Endocrinol. 2013, 4, 180. [Google Scholar] [CrossRef] [PubMed]
- Romani, F.; Lanzone, A.; Tropea, A.; Familiari, A.; Scarinci, E.; Sali, M.; Delogu, G.; Catino, S.; Apa, R. In Vitro effect of unacylated ghrelin and obestatin on human luteal cell function. Fertil. Steril. 2012, 97, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Luque, R.M.; Córdoba-Chaćon, J.; Ibañez-Costa, A.; Gesmundo, I.; Grande, C.; Gracia-Navarro, F.; Tena-Sempere, M.; Ghigo, E.; Gahete, M.D.; Granata, R.; et al. Obestatin plays an opposite role in the regulation of pituitary somatotrope and corticotrope function in female primates and male/female mice. Endocrinology 2014, 155, 1407–1417. [Google Scholar] [CrossRef]
- Wójcik-Gładysz, A.; Szlis, M.; Przybył, B.J.; Polkowska, J. Obestatin may affect the GnRH/KNDy gene network in sheep hypothalamus. Res. Vet. Sci. 2019, 123, 51–58. [Google Scholar] [CrossRef]
- Szlis, M.; Wójcik-Gładysz, A.; Przybył, B.J. Central obestatin administration affect the LH and FSH secretory activity in peripubertal sheep. Theriogenology 2020, 145, 10–17. [Google Scholar] [CrossRef]
- Al-Halbouni, S.; Homsi, S.; Koshji, N. Evaluation the Effect of Chronic Obestatin Therapy on the Serum Glucose, Insulin And Lipid Levels in Type 2 Diabetic Rats. Open Public Health J. 2023, 16, 1–8. [Google Scholar] [CrossRef]
- Wojciechowicz, T.; Skrzypski, M.; Koodziejski, P.A.; Szczepankiewicz, D.; Pruszyskaoszmaek, E.; Kaczmarek, P.; Strowski, M.Z.; Nowak, K.W. Obestatin stimulates differentiation and regulates lipolysis and leptin secretion in rat preadipocytes. Mol. Med. Rep. 2015, 12, 8169–8175. [Google Scholar] [CrossRef]
- Bora, R.R.; Prasad, R.; Khatib, M.N. Cardio-Protective Role of a Gut Hormone Obestatin: A Narrative Review. Cureus 2023, 15, e37972. [Google Scholar] [CrossRef]
- Khaleel, E.F.; Abdel-Aleem, G.A. Obestatin protects and reverses nonalcoholic fatty liver disease and its associated insulin resistance in rats via inhibition of food intake, enhancing hepatic adiponectin signaling, and blocking ghrelin acylation. Arch. Physiol. Biochem. 2019, 125, 64–78. [Google Scholar] [CrossRef]
- Villarreal, D.; Pradhan, G.; Zhou, Y.; Xue, B.; Sun, Y. Diverse and Complementary Effects of Ghrelin and Obestatin. Biomolecules 2022, 12, 517. [Google Scholar] [CrossRef]
- Gurriarán-Rodríguez, U.; Santos-Zas, I.; González-Sánchez, J.; Beiroa, D.; Moresi, V.; Mosteiro, C.S.; Lin, W.; Viñuela, J.E.; Señarís, J.; García-Caballero, T.; et al. Action of obestatin in skeletal muscle repair: Stem cell expansion, muscle growth, and microenvironment remodeling. Mol. Ther. 2015, 23, 1003–1021. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).