A Narrative Review of Theranostics in Neuro-Oncology: Advancing Brain Tumor Diagnosis and Treatment Through Nuclear Medicine and Artificial Intelligence
Abstract
1. Introduction
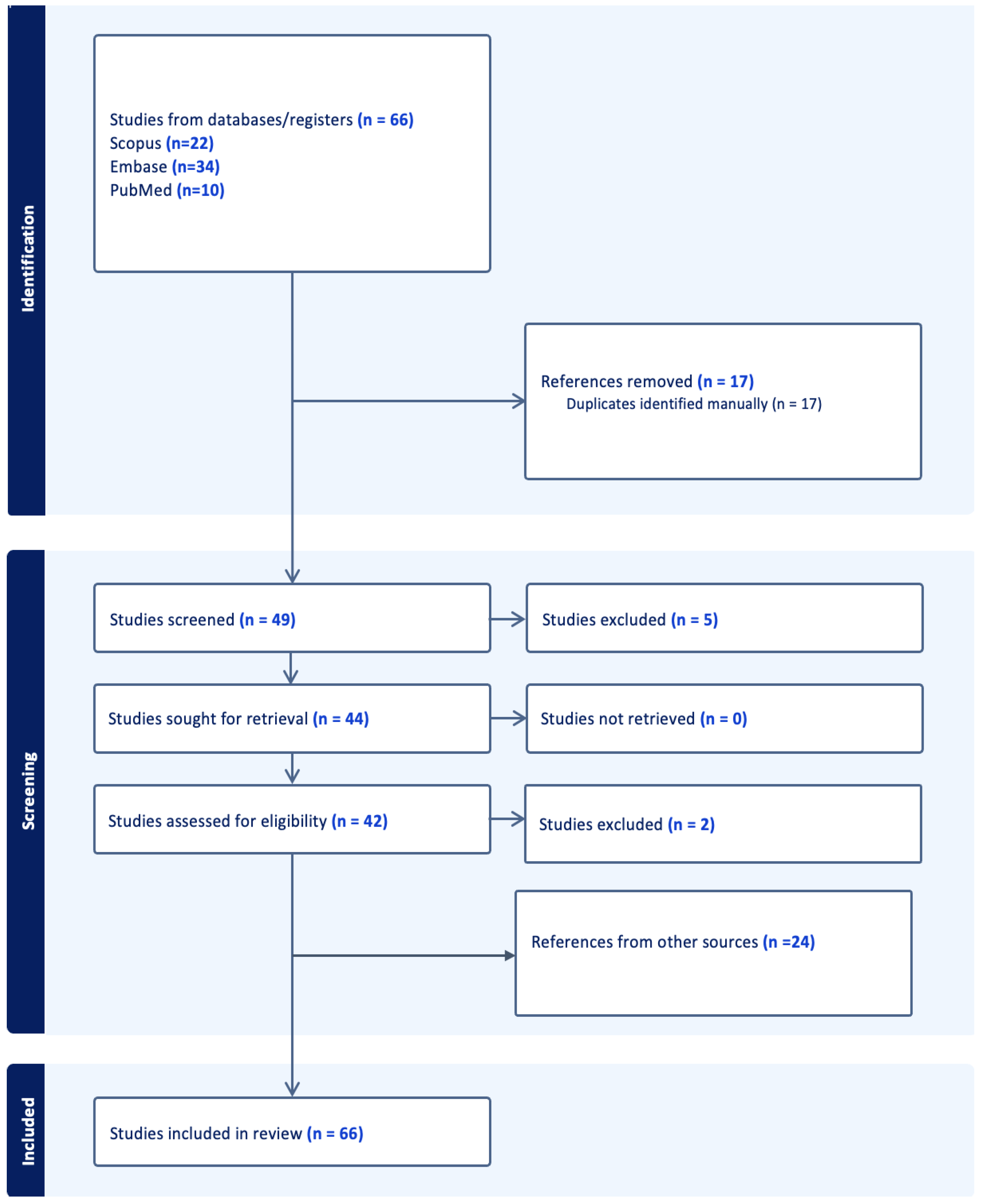
2. Materials and Methods
3. Results
4. Discussion
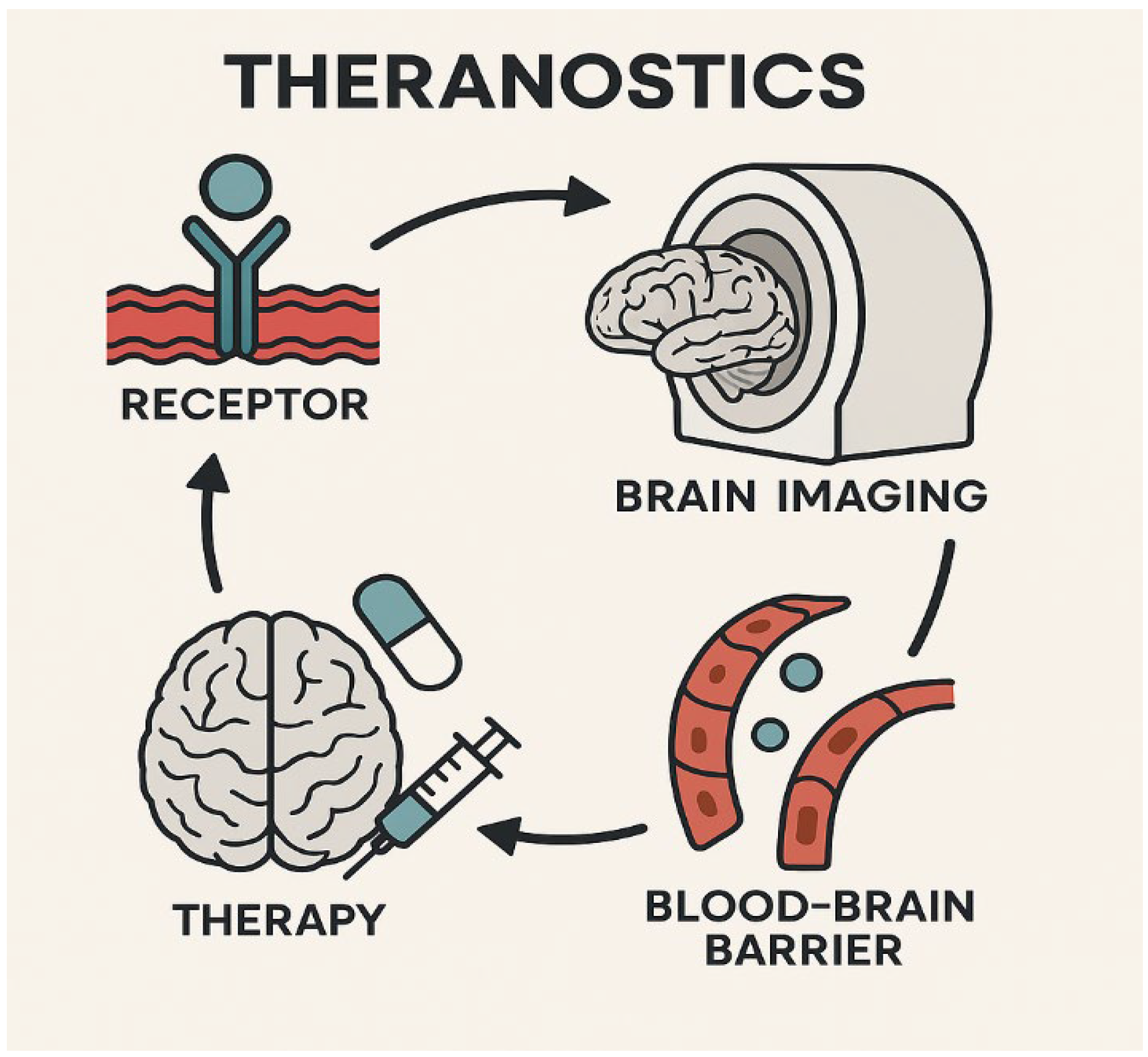
4.1. Theranostics in Neuro-Oncology
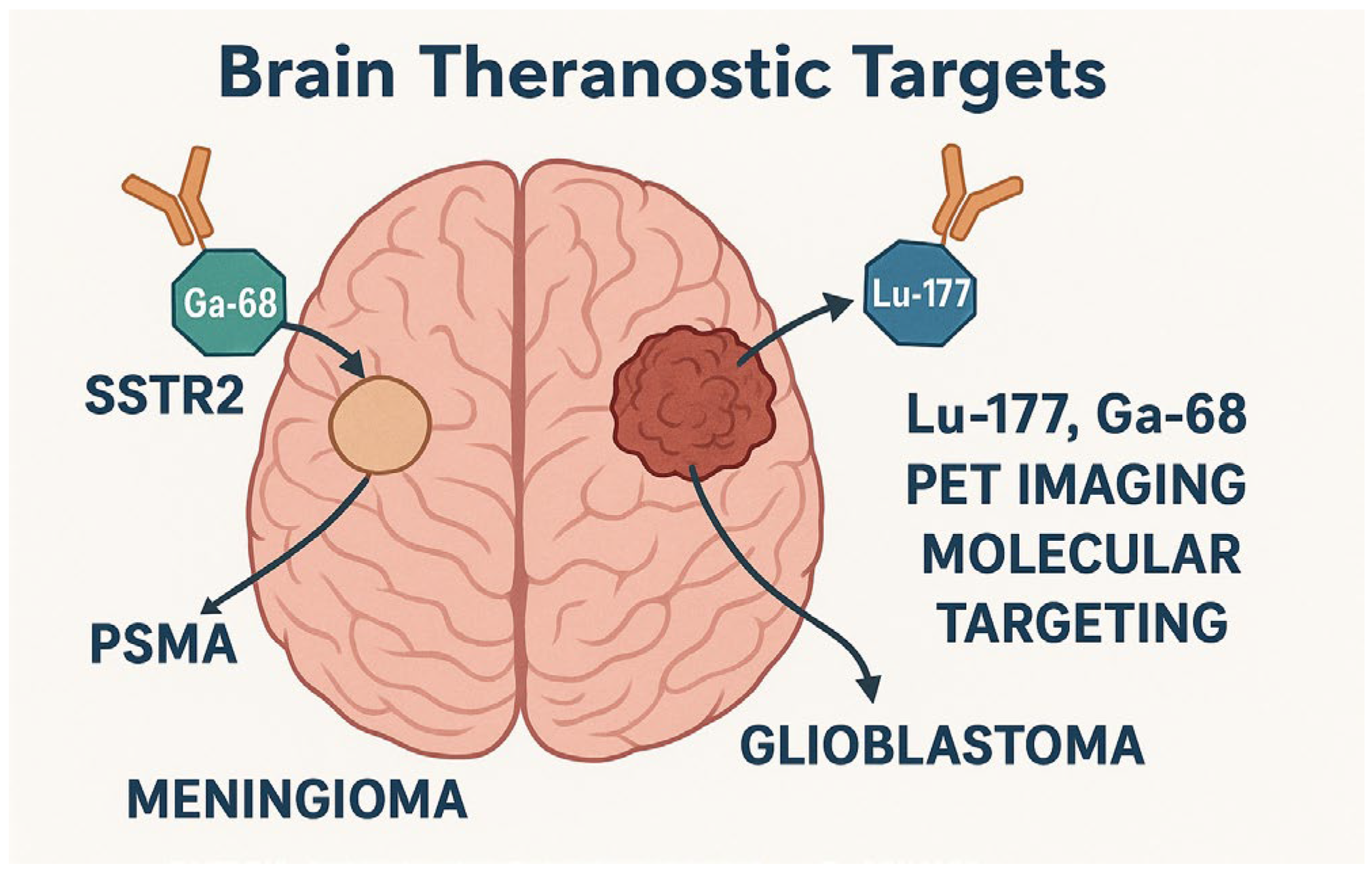
4.1.1. Overview of Theranostic Agents in Brain Tumors
| Agent/Approach | Target | Modality | Clinical Stage | Reference |
|---|---|---|---|---|
| 177Lu-DOTATATE | Somatostatin Receptors | PET + Therapy | Approved | [6,28] |
| PSMA Radioligands | PSMA+ gliomas | PET | Investigational | [23,29] |
| CXCR4 Radioligands | CXCR4 | SPECT/PET | Preclinical | [21] |
| Tau-targeting radiolabeled compounds | Tau protein in gliomas | SPECT | Preclinical | [26] |
| Gadolinium Nanoparticles | Tumor enhancement agents | MRI | Early Clinical | [30,31] |
| NIR-II Photothermal Nanoparticles | GBM cells | Optical + Thermal | Preclinical | [19] |
4.1.2. Evolving Clinical Applications of Theranostics
4.1.3. Unmet Needs and Limitations in Neuro-Theranostics
4.2. Artificial Intelligence in Neuro-Oncology Imaging
4.2.1. AI in Segmentation, Diagnosis, and Classification
4.2.2. Predictive Analytics: Prognosis and Response to Therapy
4.2.3. AI for Radiomics and PET/CT Fusion Imaging
4.2.4. Challenges in Clinical Adoption of AI in Neuro-Oncology Imaging
4.3. Integrating AI and Theranostics
4.3.1. Synergy Between AI and Theranostics: Predicting Tracer Uptake and Guiding Therapy
4.3.2. Precision Medicine: AI to Optimize Theranostic Protocols
4.3.3. Role of Multimodal Imaging and AI to Enhance Treatment Planning
4.3.4. Early-Phase Clinical Studies and Proof-of-Concept Applications in Theranostics
4.4. Current Gaps and Limitations
4.5. Future Perspectives
4.6. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| BBB | Blood–Brain Barrier |
| CNS | Central Nervous System |
| CNN | Convolutional Neural Network |
| CT | Computed Tomography |
| DL | Deep Learning |
| DNA | Deoxyribonucleic Acid |
| FAP | Fibroblast Activation Protein |
| FDG | Fluorodeoxyglucose |
| GBM | Glioblastoma Multiforme |
| HGG | High-Grade Glioma |
| IDH | Isocitrate Dehydrogenase |
| IHC | Immunohistochemistry |
| LITT | Laser Interstitial Thermal Therapy |
| LN | Lymph Node |
| LNM | Lymph Node Metastasis |
| Lu-177 | Lutetium-177 |
| MGMT | O6-Methylguanine-DNA Methyltransferase |
| MRI | Magnetic Resonance Imaging |
| NIR | Near-Infrared |
| NIR-II | Second Near-Infrared Window |
| OCT | Optical Coherence Tomography |
| PET | Positron Emission Tomography |
| PSMA | Prostate-Specific Membrane Antigen |
| RLT | Radioligand Therapy |
| SPECT | Single Photon Emission Computed Tomography |
| TAU | Tubulin-Associated Unit |
| WHO | World Health Organization |
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Schaff, L.R.; Mellinghoff, I.K. Glioblastoma and Other Primary Brain Malignancies in Adults: A Review. JAMA 2023, 329, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Tolboom, N.; Verger, A.; Albert, N.L.; Fraioli, F.; Guedj, E.; Traub-Weidinger, T.; Gempt, J.; Pioch, M.; Lassmann, M.; Preusser, M.; et al. Theranostics in Neurooncology: Heading Toward New Horizons. J. Nucl. Med. 2024, 65, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lawal, I.O.; Abubakar, S.O.; Ndlovu, H.; Mokoala, K.M.G.; More, S.S.; Sathekge, M.M. Advances in Radioligand Theranostics in Oncology. Mol. Diagn. Ther. 2024, 28, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Lecocq, Q.; De Vlaeminck, Y.; Hanssens, H.; D’Huyvetter, M.; Raes, G.; Goyvaerts, C.; Caveliers, V.; Lahoutte, T.; Xavier, C.; Vanhove, C.; et al. Theranostics in Immuno-Oncology Using Nanobody Derivatives. Theranostics 2019, 9, 7772–7791. [Google Scholar] [CrossRef]
- Shooli, H.; Nemati, R.; Ahmadzadehfar, H.; Aboian, M.; Jafari, E.; Jokar, N.; Farzin, H.; Ebrahimi, S.A.; Movahedi, M.; Alejo, C.D.; et al. Theranostics in Brain Tumors. PET Clin. 2021, 16, 397–418. [Google Scholar] [CrossRef]
- Seifert, R.; Alberts, I.L.; Afshar-Oromieh, A.; Rahbar, K. Prostate Cancer Theranostics. PET Clin. 2021, 16, 391–396. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Kobayashi, N.; Takano, S.; Kato, I.; Endo, K.; Inoue, T. Neuroendocrine Tumor Theranostics. Cancer Sci. 2022, 113, 1930–1938. [Google Scholar] [CrossRef]
- Mittra, E.S. Neuroendocrine Tumor Therapy: 177Lu-DOTATATE. Am. J. Roentgenol. 2018, 211, 278–285. [Google Scholar] [CrossRef]
- Galldiks, N.; Lohmann, P.; Friedrich, M.; Werner, J.M.; Stetter, I.; Wollring, M.M.; Hutterer, M.; Holzgreve, A.; Kocher, M.; Hinz, R.; et al. PET Imaging of Gliomas: Status Quo and Quo Vadis? Neuro Oncol. 2024, 26, S185–S198. [Google Scholar] [CrossRef]
- Hooper, G.W.; Ansari, S.; Johnson, J.M.; Ginat, D.T. Advances in the Radiological Evaluation of and Theranostics for Glioblastoma. Cancers 2023, 15, 4162. [Google Scholar] [CrossRef]
- Salgues, B.; Graillon, T.; Horowitz, T.; Chinot, O.; Padovani, L.; Taïeb, D.; Sans, V.; Carpentier, A.; Genin, J.; Le Bars, D.; et al. Somatostatin Receptor Theranostics for Refractory Meningiomas. Curr. Oncol. 2022, 29, 5550–5565. [Google Scholar] [CrossRef]
- Di Nunno, V.; Fordellone, M.; Minniti, G.; Asioli, S.; Conti, A.; Mazzatenta, D.; Iorio, A.; Pompucci, A.; Monfardini, L.; Carboni, N.; et al. Machine Learning in Neuro-Oncology: Toward Novel Development Fields. J. Neurooncol. 2022, 159, 333–346. [Google Scholar] [CrossRef]
- Dana, D.; Gadhiya, S.; St Surin, L.; Li, D.; Naaz, F.; Ali, Q.; Fatima, A. Deep Learning in Drug Discovery and Medicine; Scratching the Surface. Molecules 2018, 23, 2384. [Google Scholar] [CrossRef] [PubMed]
- Alongi, P.; Arnone, A.; Vultaggio, V.; Fraternali, A.; Versari, A.; Casali, C.; Bonanno, L.; Palaia, R.; Pasqualetti, F.; Fara, A.; et al. Artificial Intelligence Analysis Using MRI and PET Imaging in Gliomas: A Narrative Review. Cancers 2024, 16, 407. [Google Scholar] [CrossRef] [PubMed]
- Hajianfar, G.; Shiri, I.; Maleki, H.; Oveisi, N.; Haghparast, A.; Abdollahi, H.; Sharifi, M. Noninvasive O6 Methylguanine-DNA Methyltransferase Status Prediction in Glioblastoma Multiforme Cancer Using Magnetic Resonance Imaging Radiomics Features: Univariate and Multivariate Radiogenomics Analysis. World Neurosurg. 2019, 132, e140–e161. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Meißner, A.-K.; Kocher, M.; Bauer, E.K.; Werner, J.-M.; Fink, G.R.; Delbridge, C.; Summer, L.; Stumpp, P.; Bauer, S.; et al. Feature-Based PET/MRI Radiomics in Patients with Brain Tumors. Neuro Oncol. Adv. 2020, 2, iv15–iv21. [Google Scholar] [CrossRef]
- Christenson, C.; Wu, C.; Hormuth, D.A.; Huang, S.; Bao, A.; Brenner, A.; Allen, B.; Kung, H.F.; Yong, W.H.; Radishev, V.; et al. Predicting the Spatio-Temporal Response of Recurrent Glioblastoma Treated with Rhenium-186 Labelled Nanoliposomes. Brain Multiphys. 2023, 5, 100084. [Google Scholar] [CrossRef]
- Poletto, G.; Cecchin, D.; Bartoletti, P.; Venturini, F.; Realdon, N.; Evangelista, L. Radionuclide Delivery Strategies in Tumor Treatment: A Systematic Review. Curr. Issues Mol. Biol. 2022, 44, 3267–3282. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Gu, J.; Roy, S.; Jin, J.; Kuang, T.; Li, Y.; Zhang, M.; Shi, C.; Xia, X.; et al. Bright NIR-II Emissive Cyanine Dye-Loaded Lipoprotein-Mimicking Nanoparticles for Fluorescence Imaging-Guided and Targeted NIR-II Photothermal Therapy of Subcutaneous Glioblastoma. J. Nanobiotechnol. 2024, 22, 788. [Google Scholar] [CrossRef]
- Mishra, S.; Bhatt, T.; Kumar, H.; Jain, R.; Shilpi, S.; Jain, V. Nanoconstructs for Theranostic Application in Cancer: Challenges and Strategies to Enhance the Delivery. Front. Pharmacol. 2023, 14, 1101320. [Google Scholar] [CrossRef]
- Roustaei, H.; Norouzbeigi, N.; Vosoughi, H.; Aryana, K. A Dataset of [(68)Ga]Ga-Pentixafor PET/CT Images of Patients with High-Grade Glioma. Data Brief. 2023, 48, 109236. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Sánchez, M.; Ferro-Flores, G.; Jiménez-Mancilla, N.; Ocampo-García, B.; Bravo-Villegas, G.; Luna-Gutiérrez, M.; Juárez-Mercado, K.; Chiu, L.-L.; Meléndez-Alafort, L.; Santiago-Guarneros, D.; et al. Synthesis and Preclinical Evaluation of the 99mTc-/177Lu-CXCR4-L Theranostic Pair for In Vivo Chemokine-4 Receptor-Specific Targeting. J. Radioanal. Nucl. Chem. 2020, 324, 21–32. [Google Scholar] [CrossRef]
- Li, L.; Cao, R.; Chen, K.; Qu, C.; Qian, K.; Lin, J.; Liu, Y.; Zhang, W.; Liu, L.; Wang, J.; et al. Development of an FAP-Targeted PET Probe Based on a Novel Quinolinium Molecular Scaffold. Bioconjug. Chem. 2024, 35, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Tubre, T.; Hacking, S.; Alexander, A.; Brickman, A.; Delalle, I.; Elinzano, H.; Yao, X.; Choi, E.; Agostini, M.; Gupta, N.; et al. Prostate-Specific Membrane Antigen Expression in Meningioma: A Promising Theranostic Target. J. Neuropathol. Exp. Neurol. 2022, 81, 1008–1017. [Google Scholar] [CrossRef]
- Callari, M.; Sola, M.; Magrin, C.; Rinaldi, A.; Bolis, M.; Paganetti, P.; Tagliabue, E.; Lau, M.; Gatti, L.; Baseggio, L.; et al. Cancer-Specific Association Between Tau (MAPT) and Cellular Pathways, Clinical Outcome, and Drug Response. Sci. Data 2023, 10, 637. [Google Scholar] [CrossRef]
- Gargini, R.; Segura-Collar, B.; Herránz, B.; García-Escudero, V.; Romero-Bravo, A.; Núñez, F.J.; González-Cao, M.; Velasco, G.; Barbazán, J.; Vázquez-Barquero, A.; et al. The IDH-TAU-EGFR Triad Defines the Neovascular Landscape of Diffuse Gliomas. Sci. Transl. Med. 2020, 12, eaax1501. [Google Scholar] [CrossRef]
- Abdelaziz, G.; Shamsel-Din, H.A.; Sarhan, M.O.; Gizawy, M.A. Tau Protein Targeting Via Radioiodinated Azure A for Brain Theranostics: Radiolabeling, Molecular Docking, In Vitro and In Vivo Biological Evaluation. J. Label. Compd. Radiopharm. 2020, 63, 33–42. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, J.; Cheng, Y.; Xiong, Z.; Tang, Y.; Guo, L.; Zhao, H.; Wu, J.; Song, X.; Wu, J.; et al. Chlorotoxin-Conjugated Multifunctional Dendrimers Labeled with Radionuclide 131I for Single Photon Emission Computed Tomography Imaging and Radiotherapy of Gliomas. ACS Appl. Mater. Interfaces 2015, 7, 19798–19808. [Google Scholar] [CrossRef]
- Hänscheid, H.; Lapa, C.; Buck, A.K.; Lassmann, M.; Werner, R.A. Dose Mapping after Endoradiotherapy with 177-Lu-DOTATATE/DOTATOC by a Single Measurement after 4 Days. J. Nucl. Med. 2018, 59, 75–81. [Google Scholar] [CrossRef]
- Pruis, I.J.; Van Doormaal, P.J.; Balvers, R.K.; Van Den Bent, M.J.; Harteveld, A.A.; De Jong, L.C.; Jansen, G.H.; Futterer, J.J.; Van Dijk, L.V.; van Laarhoven, H.W.; et al. Potential of PSMA-Targeting Radioligand Therapy for Malignant Primary and Secondary Brain Tumours Using Super-Selective Intra-Arterial Administration: A Single Centre, Open Label, Non-Randomised Prospective Imaging Study. eBioMedicine 2024, 102, 105068. [Google Scholar] [CrossRef]
- Biau, J.; Durando, X.; Boux, F.; Molnar, I.; Moreau, J.; Leyrat, B.; Just, N.; Tessonnier, L.; Moisan, A.; Gligorov, J.; et al. NANO-GBM Trial of AGuIX Nanoparticles with Radiotherapy and Temozolomide in the Treatment of Newly Diagnosed Glioblastoma: Phase 1b Outcomes and MRI-Based Biodistribution. Clin. Transl. Radiat. Oncol. 2024, 48, 100833. [Google Scholar] [CrossRef]
- Carmona, A.; Roudeau, S.; L’Homel, B.; Pouzoulet, F.; Bonnet-Boissinot, S.; Prezado, Y.; Desbrée, A.; Moubarek, M.S.; Curie, S.; Lemaire, A.; et al. Heterogeneous Intratumoral Distribution of Gadolinium Nanoparticles Within U87 Human Glioblastoma Xenografts Unveiled by Micro-PIXE Imaging. Anal. Biochem. 2017, 523, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Advanced Accelerator Applications. [177Lu]-NeoB in Patients with Advanced Solid Tumors and with [68Ga]-NeoB Lesion Uptake (NeoRay); ClinicalTrials.gov Identifier: NCT03872778; National Library of Medicine (US): Bethesda, MD, USA, 2025. Available online: https://clinicaltrials.gov/study/NCT03872778 (accessed on 9 June 2025).
- Pacak, K.; Taieb, D.; Lin, F.I.; Jha, A. Approach to the Patient: Concept and Application of Targeted Radiotherapy in the Paraganglioma Patient. J. Clin. Endocrinol. Metab. 2024, 109, 2366–2388. [Google Scholar] [CrossRef]
- Poot, A.J.; Lam, M.G.E.H.; Van Noesel, M.M. The Current Status and Future Potential of Theranostics to Diagnose and Treat Childhood Cancer. Front. Oncol. 2020, 10, 578286. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chen, Z.; Park, S.W.; Lai, J.H.C.; Chan, K.W.Y. Molecular Imaging of Brain Tumors and Drug Delivery Using CEST MRI: Promises and Challenges. Pharmaceutics 2022, 14, 451. [Google Scholar] [CrossRef]
- Rai, A.; Shah, K.; Dewangan, H.K. Review on the Artificial Intelligence-Based Nanorobotics Targeted Drug Delivery System for Brain-Specific Targeting. Curr. Pharm. Des. 2023, 29, 3519–3531. [Google Scholar] [CrossRef] [PubMed]
- Khalighi, S.; Reddy, K.; Midya, A.; Pandav, K.B.; Madabhushi, A.; Abedalthagafi, M. Artificial Intelligence in Neuro-Oncology: Advances and Challenges in Brain Tumor Diagnosis, Prognosis, and Precision Treatment. NPJ Precis. Onc. 2024, 8, 80. [Google Scholar] [CrossRef]
- Preetha, R.; Jasmine Pemeena Priyadarsini, M.; Nisha, J.S. Brain Tumor Segmentation Using Multi-Scale Attention U-Net with EfficientNetB4 Encoder for Enhanced MRI Analysis. Sci. Rep. 2025, 15, 9914. [Google Scholar]
- Qutaish, M.Q.; Sullivant, K.E.; Burden-Gulley, S.M.; Lu, H.; Roy, D.; Wang, J.; Leikan, S.; Hitron, A.; Raza, S.; Bhadri, V.; et al. Cryo-Image Analysis of Tumor Cell Migration, Invasion, and Dispersal in a Mouse Xenograft Model of Human Glioblastoma Multiforme. Mol. Imaging Biol. 2012, 14, 572–583. [Google Scholar] [CrossRef][Green Version]
- Zhuang, Z.; Lin, J.; Wan, Z.; Weng, J.; Yuan, Z.; Xie, Y.; Lu, Q.; Yang, N.; Chen, C.; Zhou, J.; et al. Radiogenomic Profiling of Global DNA Methylation Associated with Molecular Phenotypes and Immune Features in Glioma. BMC Med. 2024, 22, 352. [Google Scholar] [CrossRef]
- Huang, Z.; Zou, S.; Wang, G.; Chen, Z.; Shen, H.; Wang, H.; Chen, Y.; Chen, J.; Zhang, K.; Chen, X.; et al. ISA-Net: Improved Spatial Attention Network for PET-CT Tumor Segmentation. Comput. Methods Programs Biomed. 2022, 226, 107129. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.; Ma, Y.; Yang, H. A Multi-Scale Interpretability-Based PET-CT Tumor Segmentation Method. Mathematics 2025, 13, 1139. [Google Scholar] [CrossRef]
- Marquis, H.; Deidda, D.; Gillman, A.; Willowson, K.P.; Gholami, Y.; Hioki, T.; Tsushima, Y.; Tran-Gia, J.; Bailey, D.L.; Schmidtlein, C.R. Theranostic SPECT Reconstruction for Improved Resolution: Application to Radionuclide Therapy Dosimetry. EJNMMI Phys. 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Marquis, H.; Willowson, K.P.; Schmidtlein, C.R.; Bailey, D.L. Investigation and Optimization of PET-Guided SPECT Reconstructions for Improved Radionuclide Therapy Dosimetry Estimates. Front. Nucl. Med. 2023, 3, 1124283. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Gao, E.; Liu, S.; Guo, R.; Dong, G.; Tang, X.; Mei, Y.; Liu, C.; Zhang, Q.; Chen, W.; et al. RMAP-ResNet: Segmentation of Brain Tumor OCT Images Using Residual Multicore Attention Pooling Networks for Intelligent Minimally Invasive Theranostics. Biomed. Signal Process. Control 2024, 90, 105805. [Google Scholar] [CrossRef]
- Phillips, W.T.; Goins, B.; Bao, A.; Vargas, D.; Ghaghada, K.; Salem, R.; Helfferich, J.; Negrete, R.; Mahdavi, M.; Phillips, M.S.; et al. Rhenium-186 Liposomes as Convection-Enhanced Nanoparticle Brachytherapy for Treatment of Glioblastoma. Neuro Oncol. 2012, 14, 416–425. [Google Scholar] [CrossRef]
- Yang, P.; Feng, P.; Tian, G.; Zhao, G.; Yuan, G.; Pan, Y. Integrative Machine Learning and Bioinformatics Analysis Unveil Key Genes for Precise Glioma Classification and Prognosis Evaluation. Comput. Biol. Chem. 2025, 119, 108510. [Google Scholar] [CrossRef]
- Chiu, F.Y.; Yen, Y. Imaging Biomarkers for Clinical Applications in Neuro-Oncology: Current Status and Future Perspectives. Biomark. Res. 2023, 11, 35. [Google Scholar] [CrossRef]
- Sansone, G.; Vivori, N.; Vivori, C.; Di Stefano, A.L.; Picca, A. Basic Premises: Searching for New Targets and Strategies in Diffuse Gliomas. Clin. Transl. Imaging 2022, 10, 517–534. [Google Scholar] [CrossRef]
- Timilehin, O. PET-CT and MRI: A Powerful Combination for Brain Tumor Assessment. 2025. Available online: https://www.researchgate.net/publication/389279006_PET-CT_and_MRI_A_Powerful_Combination_for_Brain_Tumor_Assessment (accessed on 9 June 2025).
- Cui, S.; Traverso, A.; Niraula, D.; Zou, J.; Luo, Y.; Owen, D.; Lu, W.; Siddiqui, S.; Snyder, K.; Tsai, K.L.; et al. Interpretable Artificial Intelligence in Radiology and Radiation Oncology. Br. J. Radiol. 2023, 96, 20230142. [Google Scholar] [CrossRef]
- Saboury, B.; Bradshaw, T.; Boellaard, R.; Buvat, I.; Dutta, J.; Hatt, M.; Thomassen, A.; Nooijen, P.T.; Vinjamuri, S.; Bahri, M.A.; et al. Artificial Intelligence in Nuclear Medicine: Opportunities, Challenges, and Responsibilities Toward a Trustworthy Ecosystem. J. Nucl. Med. 2023, 64, 188–196. [Google Scholar] [CrossRef]
- Lavielle, A.; Boux, F.; Deborne, J.; Pinaud, N.; Dufort, S.; Verry, C.; Ferrand, J.; Menei, P.; Bernis, G.; Chérel, M.; et al. T1 Mapping From MPRAGE Acquisitions: Application to the Measurement of the Concentration of Nanoparticles in Tumors for Theranostic Use. J. Magn. Reson. Imaging 2023, 58, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, L.; Paikray, S.K.; Tripathy, N.S.; Fernandes, D.; Dilnawaz, F. Advancements in Nanotheranostics for Glioma Therapy. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 2587–2608. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.V.; Chandrasekar, V.; Janapareddy, P.; Mathews, D.E.; Laux, P.; Luch, A.; Saleh, N.; Valsesia, A. Emerging Application of Nanorobotics and Artificial Intelligence to Cross the BBB: Advances in Design, Controlled Maneuvering, and Targeting of the Barriers. ACS Chem. Neurosci. 2021, 12, 1835–1853. [Google Scholar] [CrossRef]
- Monti, S.; Truppa, M.E.; Albanese, S.; Mancini, M. Radiomics and Radiogenomics in Preclinical Imaging on Murine Models: A Narrative Review. J. Pers. Med. 2023, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Stępień, E.Ł.; Rząca, C.; Moskal, P. Radiovesicolomics-New Approach in Medical Imaging. Front. Physiol. 2022, 13, 996985. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, D.; Sarabia-Estrada, R.; Guerrero-Cázares, H.; Li, D.; Quiñones-Hinojosa, A.; Huang, P.; Xing, L. Theranostic OCT Microneedle for Fast Ultrahigh-Resolution Deep-Brain Imaging and Efficient Laser Ablation In Vivo. Sci. Adv. 2020, 6, eaaz9664. [Google Scholar] [CrossRef]
- Foster, A.; Nigam, S.; Tatum, D.S.; Raphael, I.; Xu, J.; Kumar, R.; Salem, A.; Kalas, T.; Karschnia, P.; Bette, S.; et al. Novel Theranostic Agent for PET Imaging and Targeted Radiopharmaceutical Therapy of Tumour-Infiltrating Immune Cells in Glioma. eBioMedicine 2021, 71, 103571. [Google Scholar] [CrossRef]
- Albert, N.L.; Le Rhun, E.; Minniti, G.; Mair, M.J.; Galldiks, N.; Tolboom, N.; Rushing, E.J.; Senetta, R.; Brandes, A.A.; Dhermain, F.; et al. Translating the Theranostic Concept to Neuro-Oncology: Disrupting Barriers. Lancet Oncol. 2024, 25, e441–e451. [Google Scholar] [CrossRef]
- Ayalew, B.D.; Abdullah Khan, S.M.; Alemayehu, Z.G.; Teferi, M.G.; Aboye, B.T.; Abdalla, A.; Atnafie, S.; Ahmed, Z.; Oumer, S.; Ibrahim, M. Role of Emerging Theranostic Technologies in Precision Oncology: Revolutionizing Cancer Diagnosis and Treatment. Oncologie 2025, 27, 229–238. [Google Scholar] [CrossRef]
- Davis, L.; Smith, A.-L.; Aldridge, M.D.; Foulkes, J.; Peet, C.; Wan, S.; Basu, S.; Capala, J.; Eslick, E.M.; Hacker, T.A.; et al. Personalisation of Molecular Radiotherapy through Optimisation of Theragnostics. J. Pers. Med. 2020, 10, 174. [Google Scholar] [CrossRef]
- Katsoulakis, E.; Wang, Q.; Wu, H.; Shahriyari, L.; Fletcher, R.; Liu, J.; Shaban-Nejad, A.; Vachharajani, V.; Massey, S.; Jones, M.; et al. Digital Twins for Health: A Scoping Review. NPJ Digit. Med. 2024, 7, 77. [Google Scholar] [CrossRef]
- Fathi Kazerooni, A.; Akbari, H.; Hu, X.; Bommineni, V.; Grigoriadis, D.; Toorens, E.; Stoyanova, R.; Han, S.; Kalpathy-Cramer, J.; Sood, S.; et al. The Radiogenomic and Spatiogenomic Landscapes of Glioblastoma and Their Relationship to Oncogenic Drivers. Commun. Med. 2025, 5, 55. [Google Scholar] [CrossRef]
| AI Application | Clinical Utility | Imaging Modality | AI Model Type | Reference(s) |
|---|---|---|---|---|
| Tumor segmentation | Precise tumor boundary detection | MRI, PET-CT | CNN, U-Net, EfficientNet | [38,39,41] |
| Genetic/molecular prediction | Predict MGMT, IDH status from imaging | MRI | Radiogenomics, XGBoost | [16,17,42] |
| Treatment response prediction | Forecast therapy outcomes | PET/MRI | ML models, deep learning | [13,37,40] |
| Radiotherapy planning assistance | Optimize dosing/target volume | MRI, PET | Explainable AI, Radiomics | [43,44,45] |
| Theranostic agent matching | Match tracers to biomarker profiles | PET-CT | Decision support AI | [28,29,46] |
| Challenge | Implication | Potential Solutions |
|---|---|---|
| Blood–brain barrier | Limits drug delivery | AI-designed nanocarriers, focused ultrasound |
| Lack of large annotated datasets | Hinders ML/AI model development | Federated learning, multicenter collaborations |
| Inter-modality variability | Reduces reproducibility | Standardized imaging protocols, harmonization AI |
| Regulatory approval of AI systems | Slows clinical translation | Transparent validation pipelines, explainable AI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, R.C.; Papageorgiou, P.S.; Pitsillos, R.; Woodward, A.; Papageorgiou, S.G.; Solomou, E.E.; Georgiou, M.F. A Narrative Review of Theranostics in Neuro-Oncology: Advancing Brain Tumor Diagnosis and Treatment Through Nuclear Medicine and Artificial Intelligence. Int. J. Mol. Sci. 2025, 26, 7396. https://doi.org/10.3390/ijms26157396
Christodoulou RC, Papageorgiou PS, Pitsillos R, Woodward A, Papageorgiou SG, Solomou EE, Georgiou MF. A Narrative Review of Theranostics in Neuro-Oncology: Advancing Brain Tumor Diagnosis and Treatment Through Nuclear Medicine and Artificial Intelligence. International Journal of Molecular Sciences. 2025; 26(15):7396. https://doi.org/10.3390/ijms26157396
Chicago/Turabian StyleChristodoulou, Rafail C., Platon S. Papageorgiou, Rafael Pitsillos, Amanda Woodward, Sokratis G. Papageorgiou, Elena E. Solomou, and Michalis F. Georgiou. 2025. "A Narrative Review of Theranostics in Neuro-Oncology: Advancing Brain Tumor Diagnosis and Treatment Through Nuclear Medicine and Artificial Intelligence" International Journal of Molecular Sciences 26, no. 15: 7396. https://doi.org/10.3390/ijms26157396
APA StyleChristodoulou, R. C., Papageorgiou, P. S., Pitsillos, R., Woodward, A., Papageorgiou, S. G., Solomou, E. E., & Georgiou, M. F. (2025). A Narrative Review of Theranostics in Neuro-Oncology: Advancing Brain Tumor Diagnosis and Treatment Through Nuclear Medicine and Artificial Intelligence. International Journal of Molecular Sciences, 26(15), 7396. https://doi.org/10.3390/ijms26157396







