Mosquito Exosomal Tetraspanin CD151 Facilitates Flaviviral Transmission and Interacts with ZIKV and DENV2 Viral Proteins
Abstract
1. Introduction
2. Results
2.1. Bioinformatic and Prediction Analysis of Tetraspanins Combed from the Aedes aegypti Genome
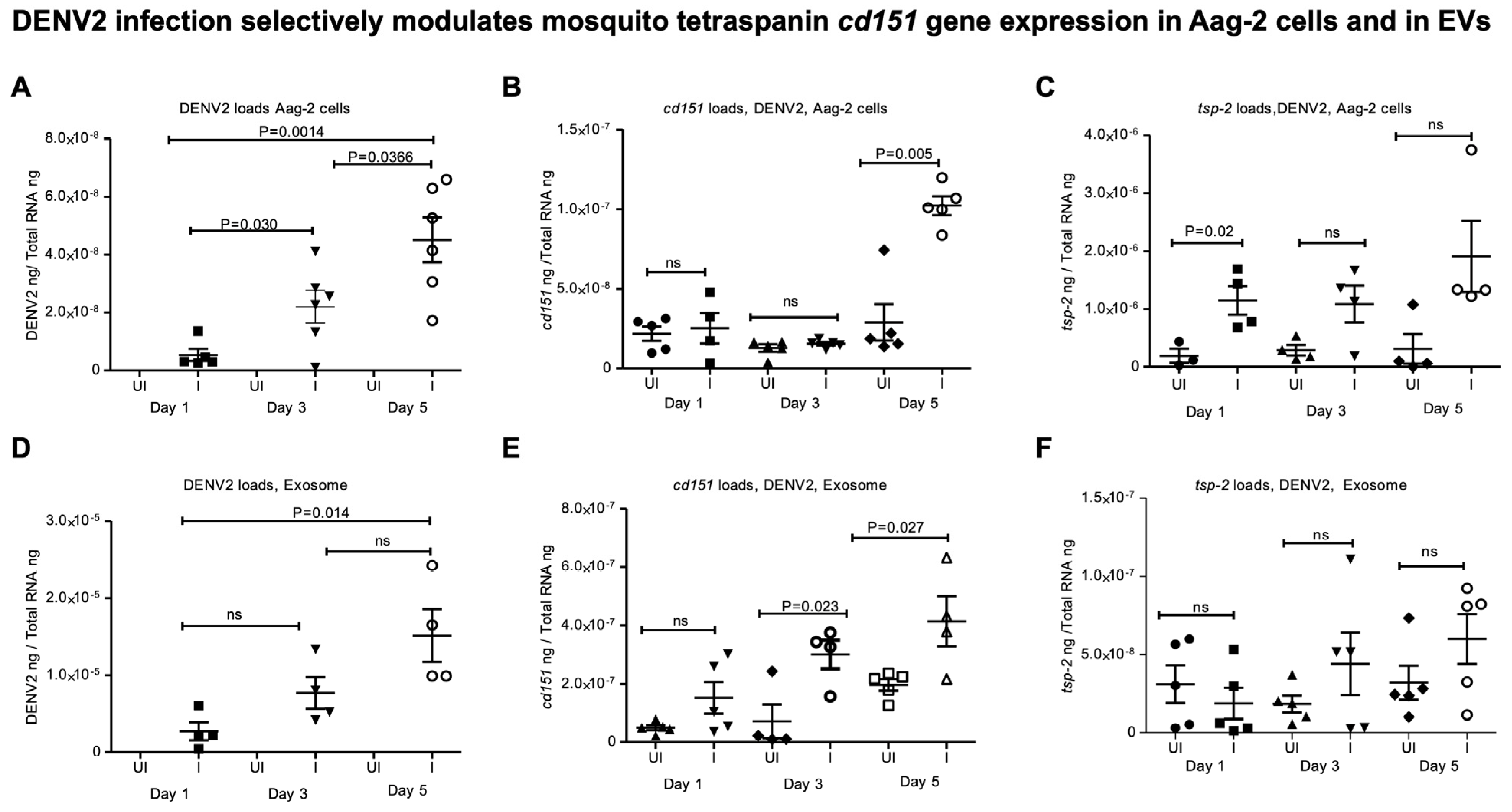
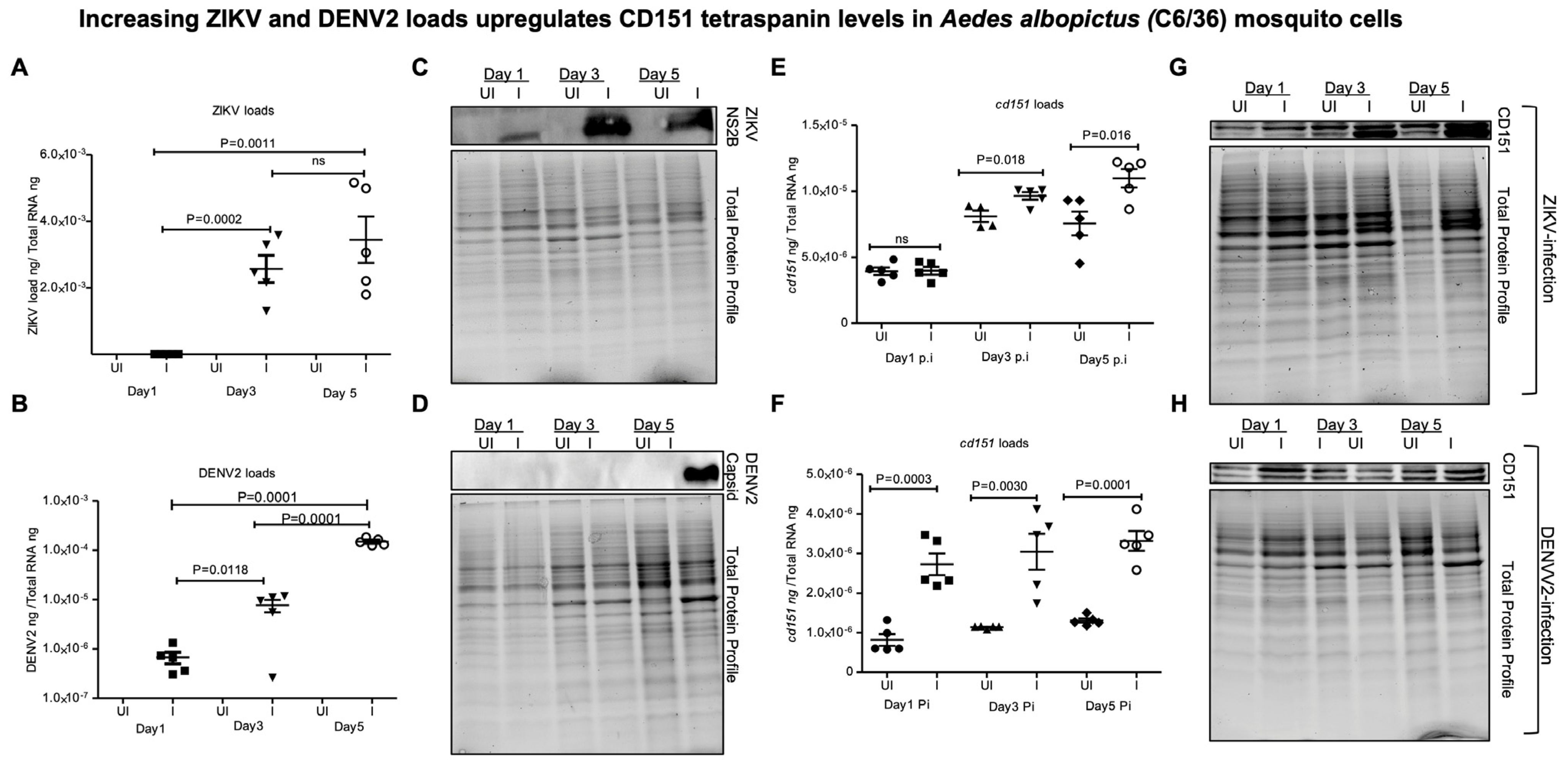
2.2. ZIKV and DENV2 Infection Selectively Modulates Tetraspanin cd151 Gene Expression in Mosquito Cells and Derived EVs
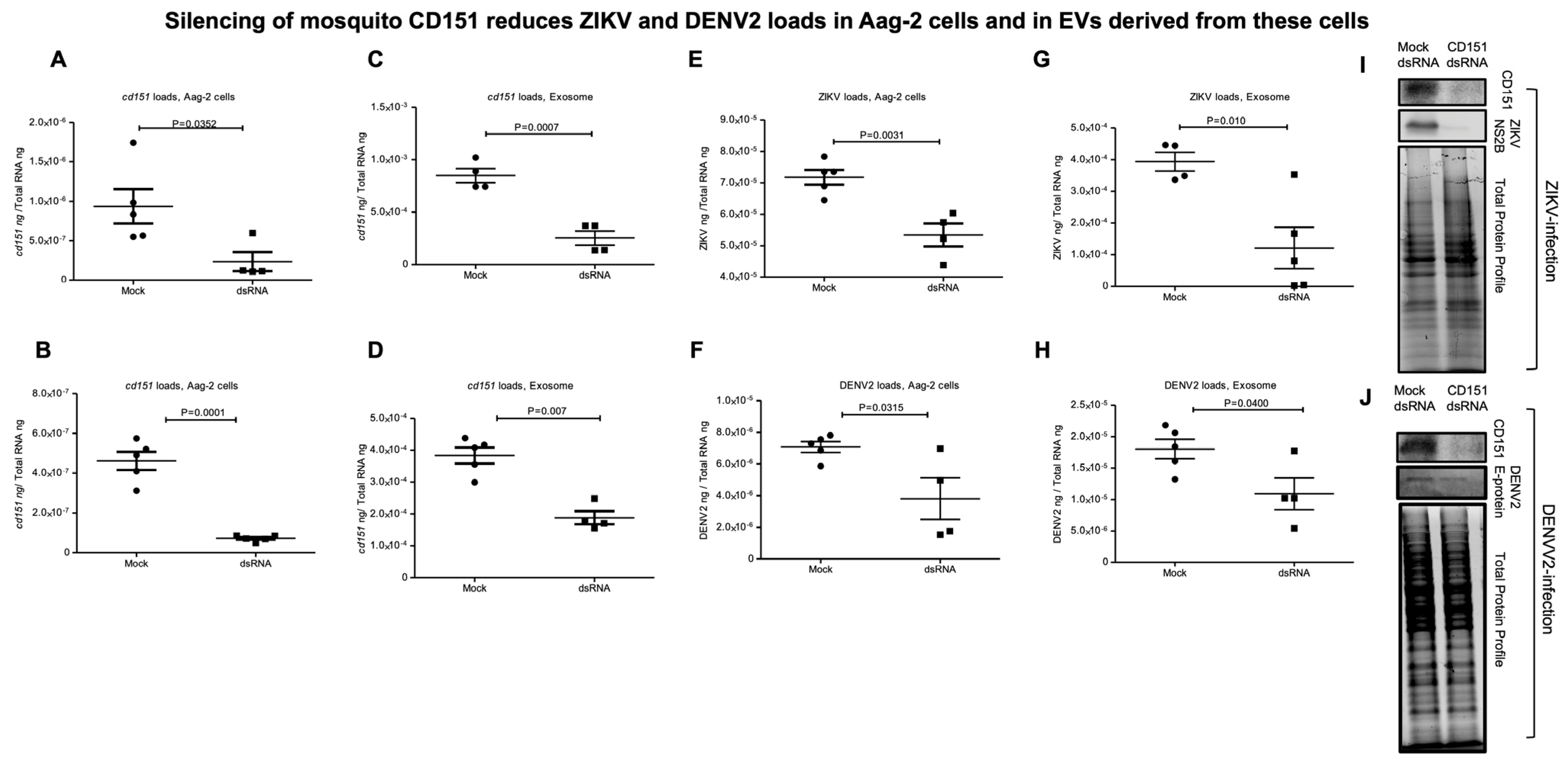
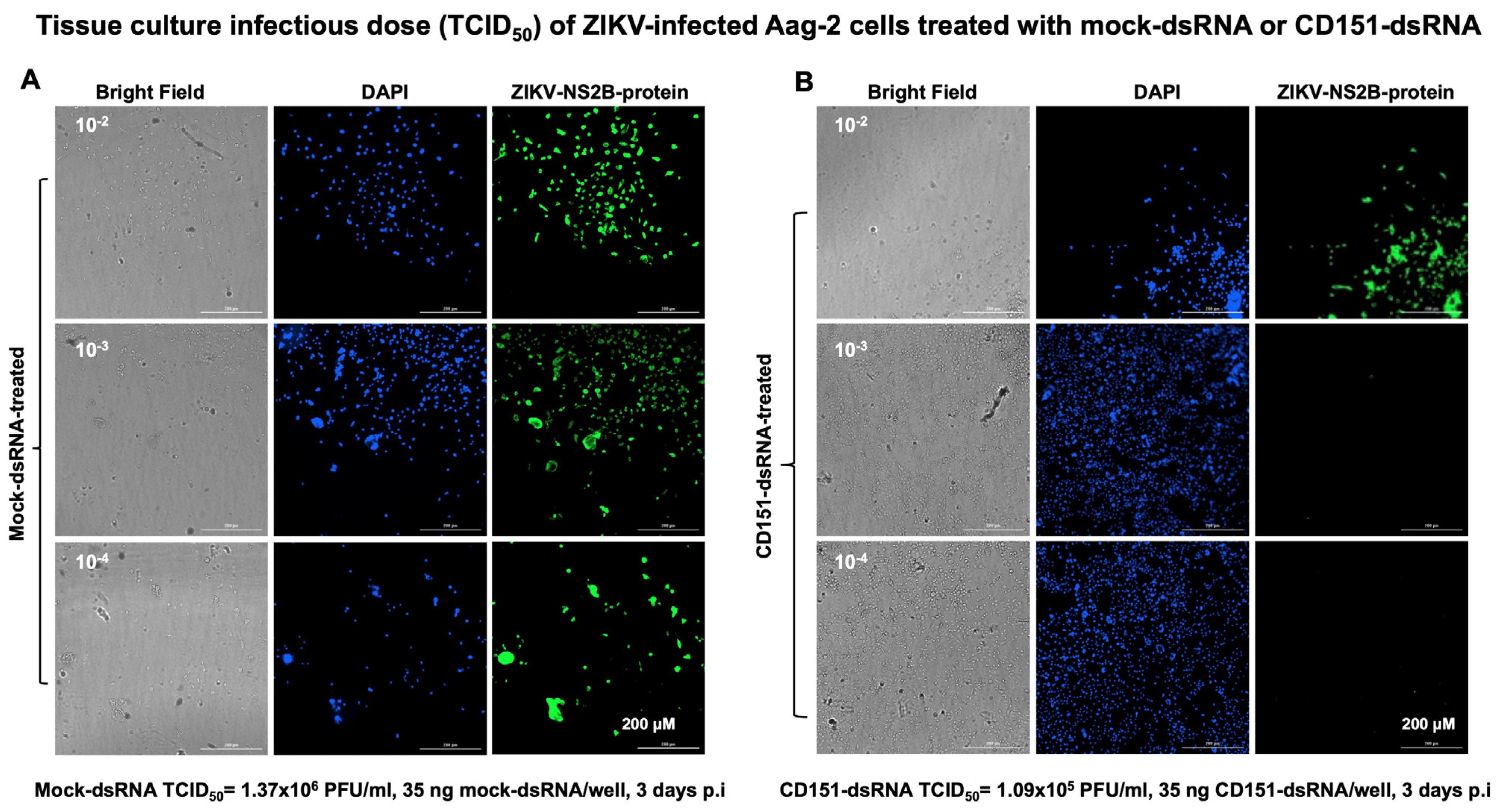
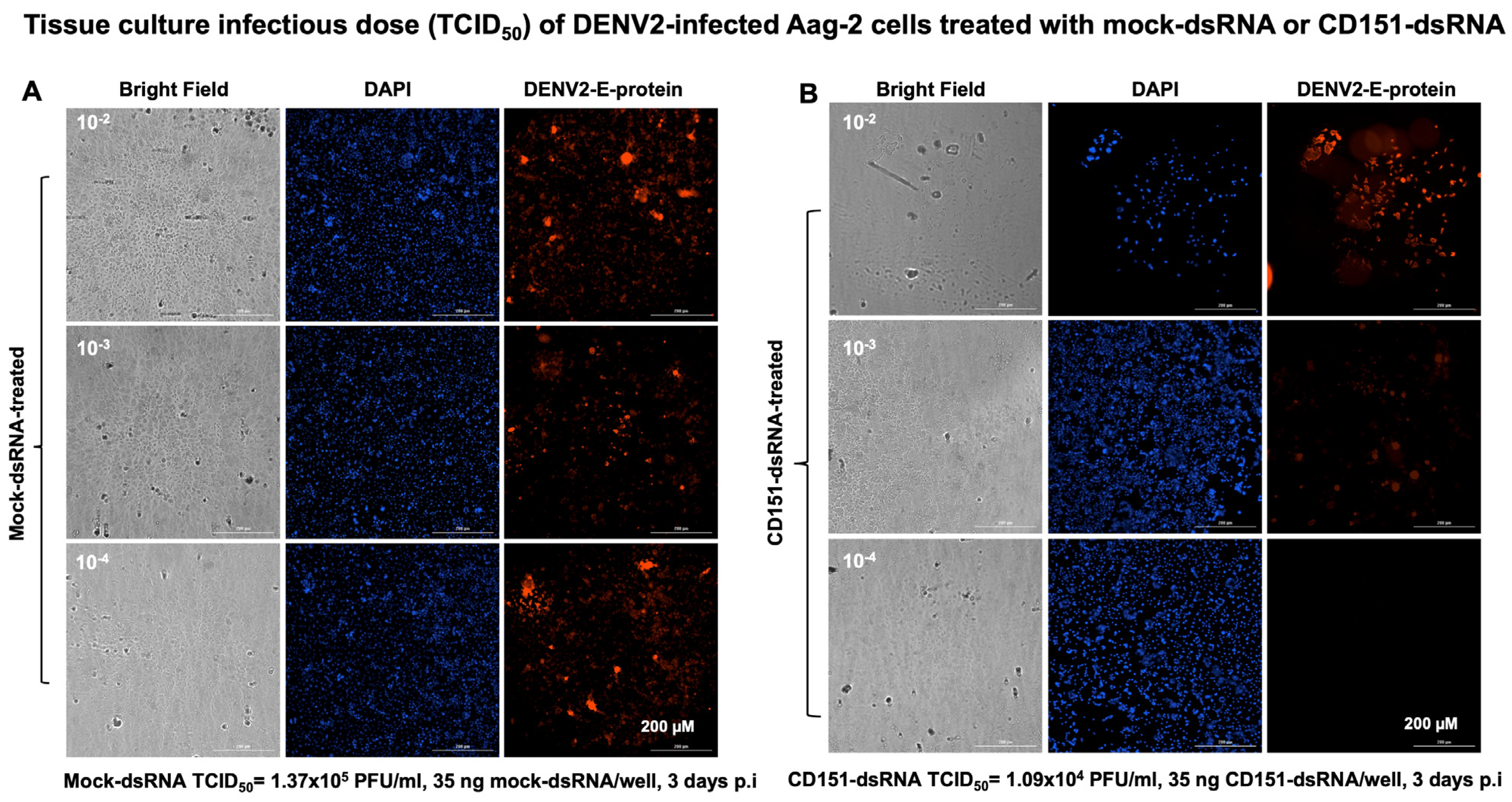
2.3. RNAi-Mediated Silencing of cd151 Reduces ZIKV and DENV2 Burden in Aag-2 Cells
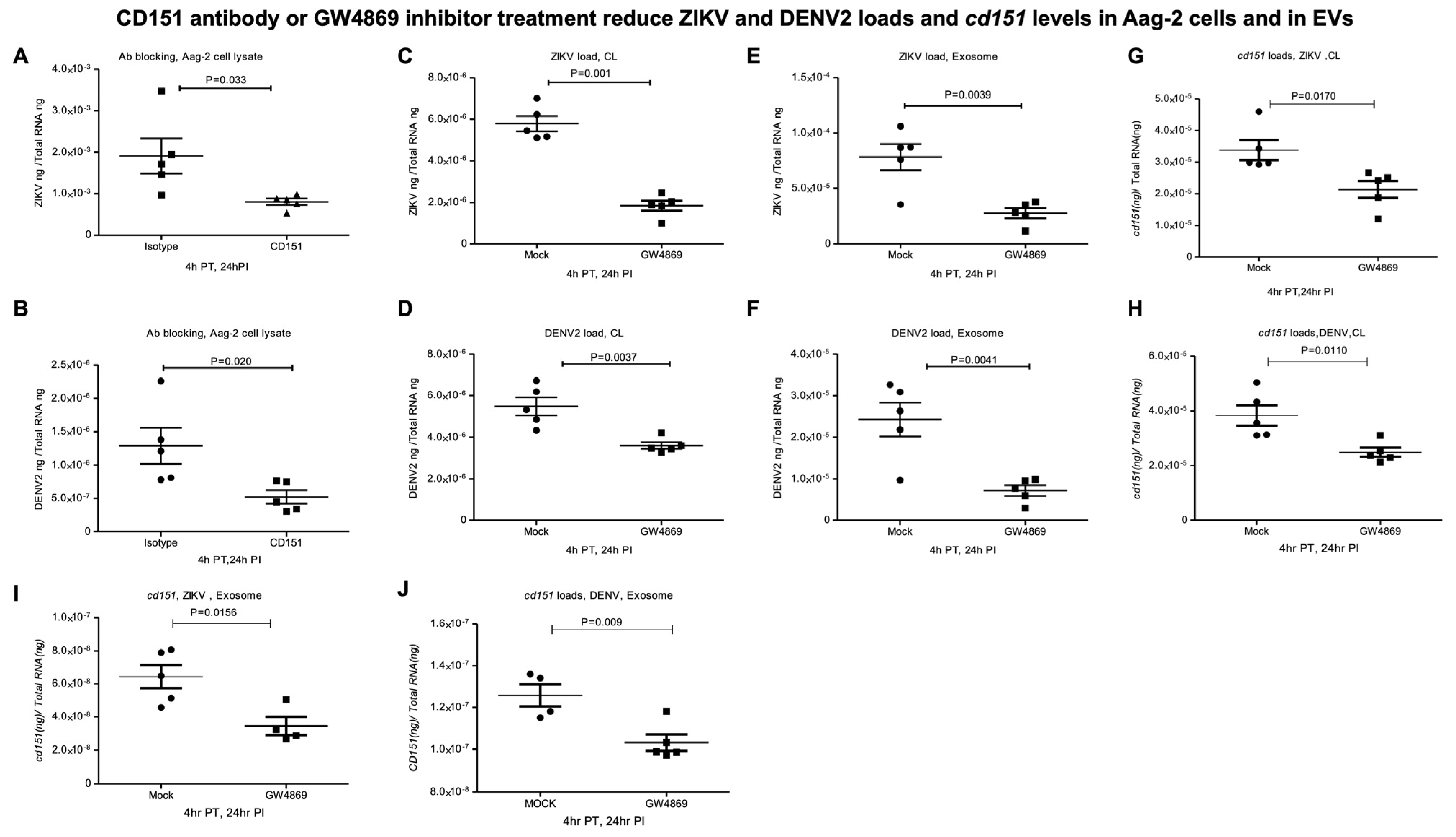
2.4. CD151 Antibody or GW4869 Treatment Reduces ZIKV and DENV2 Burden in Mosquito Cells
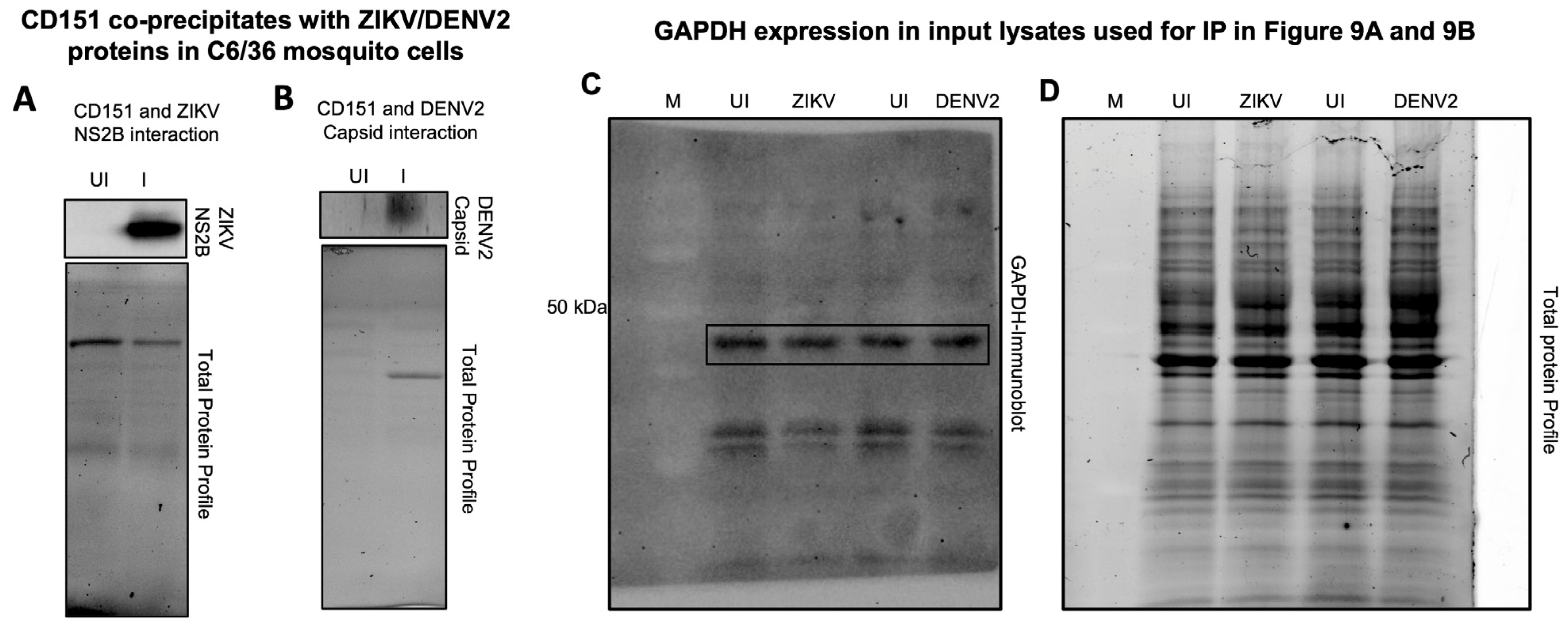
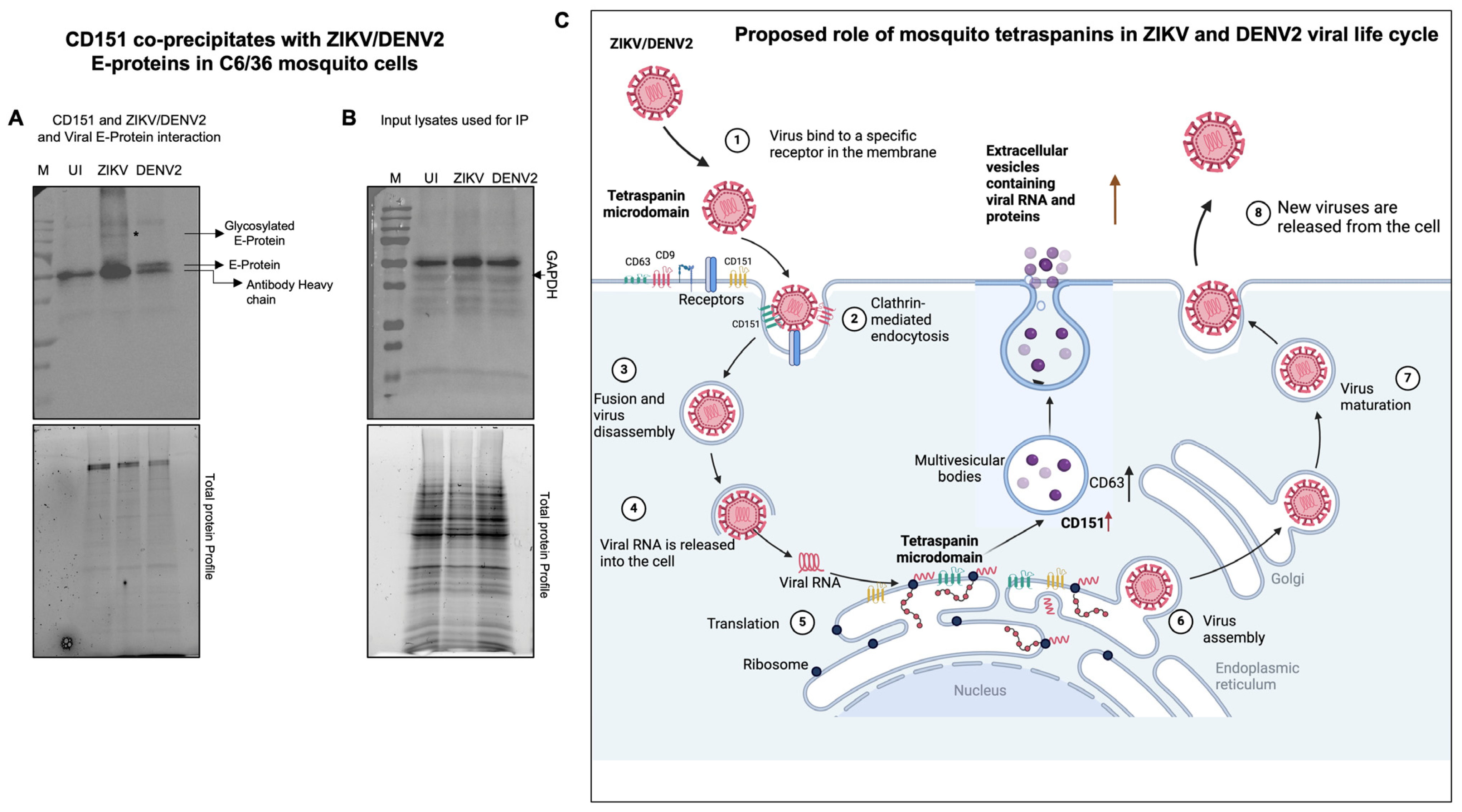
2.5. Tetraspanin CD151 Directly Interacts and Localizes with ZIKV NS2B or DENV2 Capsid or Viral Envelope Proteins in Mosquito Cells
3. Discussion
4. Materials and Methods
4.1. Mosquito Cell Culture and Viral Infection
4.2. Isolation of EVs
4.3. Total RNA Extraction, cDNA Synthesis, and QRT-PCR Analysis
4.4. Immunoblotting Analysis
4.5. dsRNA Synthesis and Transfection of Aag-2 Cells
4.6. Antibody Blocking and GW4869 Inhibition Studies
4.7. End-Point Viral Dilution or Yield Assay
4.8. MTT Assay
4.9. Immunoprecipitation
4.10. Immunofluorescence Analysis
4.11. Statistical Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrows, N.J.; Campos, R.K.; Liao, K.C.; Prasanth, K.R.; Soto-Acosta, R.; Yeh, S.C.; Schott-Lerner, G.; Pompon, J.; Sessions, O.M.; Bradrick, S.S.; et al. Biochemistry and Molecular Biology of Flaviviruses. Chem. Rev. 2018, 118, 4448–4482. [Google Scholar] [CrossRef]
- Chong, H.Y.; Leow, C.Y.; Abdul Majeed, A.B.; Leow, C.H. Flavivirus infection—A review of immunopathogenesis, immunological response, and immunodiagnosis. Virus Res. 2019, 274, 197770. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- van den Elsen, K.; Quek, J.P.; Luo, D. Molecular Insights into the Flavivirus Replication Complex. Viruses 2021, 13, 956. [Google Scholar] [CrossRef]
- Diani, E.; Lagni, A.; Lotti, V.; Tonon, E.; Cecchetto, R.; Gibellini, D. Vector-Transmitted Flaviviruses: An Antiviral Molecules Overview. Microorganisms 2023, 11, 2427. [Google Scholar] [CrossRef]
- Naderian, R.; Eslami, M.; Ahmad, S.; Paraandavaji, E.; Yaghmayee, S.; Soltanipur, M.; Naderian, R.; Pajand, O.; Tajdini, P.; Alizadeh, A.; et al. Efficacy, Immune Response, and Safety of Dengue Vaccines in Adolescents: A Systematic Review. Rev. Med. Virol. 2025, 35, e70035. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.M.; Mendes, R.P.G.; Silva, P.G.D.; Chaves, E.J.F.; Pena, L.J. Vaccines Against Urban Epidemic Arboviruses: The State of the Art. Viruses 2025, 17, 382. [Google Scholar] [CrossRef] [PubMed]
- Saez-Llorens, X.; DeAntonio, R.; Low, J.G.H.; Kosalaraksa, P.; Dean, H.; Sharma, M.; Tricou, V.; Biswal, S. TAK-003: Development of a tetravalent dengue vaccine. Expert Rev. Vaccines 2025, 24, 324–338. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.K.; Langenburg, T. A Perspective on Current Flavivirus Vaccine Development: A Brief Review. Viruses 2023, 15, 860. [Google Scholar] [CrossRef]
- Kim, Y.C.; Reyes-Sandoval, A. Recent Developments in Vaccines against Flaviviruses and Alphaviruses. Vaccines 2023, 11, 448. [Google Scholar] [CrossRef]
- Slon Campos, J.L.; Mongkolsapaya, J.; Screaton, G.R. The immune response against flaviviruses. Nat. Immunol. 2018, 19, 1189–1198. [Google Scholar] [CrossRef]
- Vora, A.; Zhou, W.; Londono-Renteria, B.; Woodson, M.; Sherman, M.B.; Colpitts, T.M.; Neelakanta, G.; Sultana, H. Arthropod EVs mediate dengue virus transmission through interaction with a tetraspanin domain containing glycoprotein Tsp29Fb. Proc. Natl. Acad. Sci. USA 2018, 115, E6604–E6613. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Neelakanta, G.; Sultana, H. Tetraspanins as Potential Therapeutic Candidates for Targeting Flaviviruses. Front. Immunol. 2021, 12, 630571. [Google Scholar] [CrossRef]
- Hemler, M.E. Targeting of tetraspanin proteins--potential benefits and strategies. Nat. Rev. Drug. Discov. 2008, 7, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Kummer, D.; Steinbacher, T.; Schwietzer, M.F.; Thölmann, S.; Ebnet, K. Tetraspanins: Integrating cell surface receptors to functional microdomains in homeostasis and disease. Med. Microbiol. Immunol. 2020, 209, 397–405. [Google Scholar] [CrossRef]
- Susa, K.J.; Kruse, A.C.; Blacklow, S.C. Tetraspanins: Structure, dynamics, and principles of partner-protein recognition. Trends Cell Biol. 2023, 34, 509–522. [Google Scholar] [CrossRef]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Biol. 2017, 5, 34. [Google Scholar] [CrossRef]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Florin, L.; Lang, T. Tetraspanin Assemblies in Virus Infection. Front. Immunol. 2018, 9, 1140. [Google Scholar] [CrossRef]
- Martin, F.; Roth, D.M.; Jans, D.A.; Pouton, C.W.; Partridge, L.J.; Monk, P.N.; Moseley, G.W. Tetraspanins in viral infections: A fundamental role in viral biology? J. Virol. 2005, 79, 10839–10851. [Google Scholar] [CrossRef]
- N Monk, P.; J Partridge, L. Tetraspanins-gateways for infection. Infect. Disord. Drug Targets 2012, 12, 4–17. [Google Scholar] [CrossRef]
- New, C.; Lee, Z.Y.; Tan, K.S.; Wong, A.H.; Wang, Y.; Tran, T. Tetraspanins: Host Factors in Viral Infections. Int. J. Mol. Sci. 2021, 22, 11609. [Google Scholar] [CrossRef]
- van Spriel, A.B.; Figdor, C.G. The role of tetraspanins in the pathogenesis of infectious diseases. Microbes Infect. 2010, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, M.J.; Harris, H.J.; McKeating, J.A. Hepatitis C virus entry and the tetraspanin CD81. Biochem. Soc. Trans. 2011, 39, 532–536. [Google Scholar] [CrossRef]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Palacios-Rápalo, S.N.; Cordero-Rivera, C.D.; Farfan-Morales, C.N.; Hurtado-Monzón, A.M.; Gallardo-Flores, C.E.; Alcaraz-Estrada, S.L.; Salas-Benito, J.S.; et al. The Regulation of Flavivirus Infection by Hijacking Exosome-Mediated Cell-Cell Communication: New Insights on Virus-Host Interactions. Viruses 2020, 12, 765. [Google Scholar] [CrossRef]
- Yang, C.-F.; Tu, C.-H.; Lo, Y.-P.; Cheng, C.-C.; Chen, W.-J. Involvement of tetraspanin C189 in cell-to-cell spreading of the dengue virus in C6/36 cells. PLoS Neglected Trop. Dis. 2015, 9, e0003885. [Google Scholar] [CrossRef]
- Zhu, Y.-Z.; Luo, Y.; Cao, M.-M.; Liu, Y.; Liu, X.-Q.; Wang, W.; Wu, D.-G.; Guan, M.; Xu, Q.-Q.; Ren, H.; et al. Significance of palmitoylation of CD81 on its association with tetraspanin-enriched microdomains and mediating hepatitis C virus cell entry. Virology 2012, 429, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hochdorfer, D.; Florin, L.; Sinzger, C.; Lieber, D. Tetraspanin CD151 Promotes Initial Events in Human Cytomegalovirus Infection. J. Virol. 2016, 90, 6430–6442. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, S.N.; Nkosi, D.; Conlon, M.M.; York, S.B.; Liu, X.; Tremblay, D.C.; Meckes, D.G., Jr. CD63 Regulates Epstein-Barr Virus LMP1 Exosomal Packaging, Enhancement of Vesicle Production, and Noncanonical NF-κB Signaling. J. Virol. 2017, 91, e02251-16. [Google Scholar] [CrossRef]
- McNamara, R.P.; Dittmer, D.P. Extracellular vesicles in virus infection and pathogenesis. Curr. Opin. Virol. 2020, 44, 129–138. [Google Scholar] [CrossRef]
- Perez-Hernandez, D.; Gutiérrez-Vázquez, C.; Jorge, I.; López-Martín, S.; Ursa, A.; Sánchez-Madrid, F.; Vázquez, J.; Yáñez-Mó, M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J. Biol. Chem. 2013, 288, 11649–11661. [Google Scholar] [CrossRef]
- Latanova, A.; Karpov, V.; Starodubova, E. Extracellular Vesicles in Flaviviridae Pathogenesis: Their Roles in Viral Transmission, Immune Evasion, and Inflammation. Int. J. Mol. Sci. 2024, 25, 2144. [Google Scholar] [CrossRef]
- Okada-Tsuchioka, M.; Kajitani, N.; Omori, W.; Kurashige, T.; Boku, S.; Takebayashi, M. Tetraspanin heterogeneity of small extracellular vesicles in human biofluids and brain tissue. Biochem. Biophys. Res. Commun. 2022, 627, 146–151. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Y.; Jiang, C.; Qu, R.; Wren, J.D.; Georgescu, C.; Wang, X.; Reuter, D.N.; Liu, B.; Giles, C.B.; et al. CD151 Maintains Endolysosomal Protein Quality to Inhibit Vascular Inflammation. Circ. Res. 2024, 134, 1330–1347. [Google Scholar] [CrossRef]
- Ciambella, C.; Witt, H.; Dickinson, C.M.; Smith, M.L.; Coburn, N.; Messina, N.; Heffernan, D.S.; Kim, M.; Reichner, J.S. Inhibition of Integrin Vla-3 and Tetraspanin Cd151 Protects against Neutrophil-Mediated Endothelial Damage. Shock 2024, 62, 165–172. [Google Scholar] [CrossRef]
- Jankovicova, J.; Frolikova, M.; Palenikova, V.; Valaskova, E.; Cerny, J.; Secova, P.; Bartokova, M.; Horovska, L.; Manaskova-Postlerova, P.; Antalikova, J.; et al. Expression and distribution of CD151 as a partner of alpha6 integrin in male germ cells. Sci. Rep. 2020, 10, 4374. [Google Scholar] [CrossRef]
- Liu, L.; He, B.; Liu, W.M.; Zhou, D.; Cox, J.V.; Zhang, X.A. Tetraspanin CD151 promotes cell migration by regulating integrin trafficking. J. Biol. Chem. 2007, 282, 31631–31642. [Google Scholar] [CrossRef]
- Marni, R.; Malla, M.; Chakraborty, A.; Malla, R. Proteomic profiling and ROC analysis identify CD151 and ELAVL1 as potential therapy response markers for the antiviral drug in resistant TNBC. Life Sci. 2023, 320, 121534. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Khanal, S.; Neelakanta, G.; Sultana, H. Tick-Borne Flavivirus Inhibits Sphingomyelinase (IsSMase), a Venomous Spider Ortholog to Increase Sphingomyelin Lipid Levels for Its Survival in Ixodes scapularis Ticks. Front. Cell. Infect. Microbiol. 2020, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Yan, Y.; Tan, K.S.; Tan, S.S.L.; Seet, J.E.; Arumugam, T.V.; Chow, V.T.K.; Wang, Y.; Tran, T. CD151, a novel host factor of nuclear export signaling in influenza virus infection. J. Allergy Clin. Immunol. 2018, 141, 1799–1817. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Hoffmann, H.H. Flavivirus-host interactions: An expanding network of proviral and antiviral factors. Curr. Opin. Virol. 2022, 52, 71–77. [Google Scholar] [CrossRef]
- Wang, T.; Fang, L.; Zhao, F.; Wang, D.; Xiao, S. Exosomes Mediate Intercellular Transmission of Porcine Reproductive and Respiratory Syndrome Virus. J. Virol. 2018, 92, e01734-17. [Google Scholar] [CrossRef]
- Morris-Love, J.; Gee, G.V.; O’Hara, B.A.; Assetta, B.; Atkinson, A.L.; Dugan, A.S.; Haley, S.A.; Atwood, W.J. JC Polyomavirus Uses Extracellular Vesicles To Infect Target Cells. mBio 2019, 10, e00379-19. [Google Scholar] [CrossRef]
- van der Grein, S.G.; Defourny, K.A.Y.; Rabouw, H.H.; Galiveti, C.R.; Langereis, M.A.; Wauben, M.H.M.; Arkesteijn, G.J.A.; van Kuppeveld, F.J.M.; Nolte-‘t Hoen, E.N.M. Picornavirus infection induces temporal release of multiple extracellular vesicle subsets that differ in molecular composition and infectious potential. PLoS Pathog. 2019, 15, e1007594. [Google Scholar] [CrossRef]
- Mardi, N.; Haiaty, S.; Rahbarghazi, R.; Mobarak, H.; Milani, M.; Zarebkohan, A.; Nouri, M. Exosomal transmission of viruses, a two-edged biological sword. Cell Commun. Signal. 2023, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef] [PubMed]
- York, S.B.; Sun, L.; Cone, A.S.; Duke, L.C.; Cheerathodi, M.R.; Meckes, D.G. Zika Virus Hijacks Extracellular Vesicle Tetraspanin Pathways for Cell-to-Cell Transmission. mSphere 2021, 6, e0019221. [Google Scholar] [CrossRef] [PubMed]
- Bukong, T.N.; Momen-Heravi, F.; Kodys, K.; Bala, S.; Szabo, G. Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90. PLOS Pathog. 2014, 10, e1004424. [Google Scholar] [CrossRef]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; de Ruiter, P.E.; Willemsen, R.; Demmers, J.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef]
- Bayzid, M.; Bhowmick, B.; Ahmed, W.; Neelakanta, G.; Sultana, H. Pharmacological Agent GW4869 inhibits tick-borne Langat virus replication to affect Extracellular vesicles secretion. Viruses 2025, 17, 969. [Google Scholar] [CrossRef]
- Dahmane, S.; Rubinstein, E.; Milhiet, P.E. Viruses and tetraspanins: Lessons from single molecule approaches. Viruses 2014, 6, 1992–2011. [Google Scholar] [CrossRef]
- Zhang, S.; Kodys, K.; Babcock, G.J.; Szabo, G. CD81/CD9 tetraspanins aid plasmacytoid dendritic cells in recognition of hepatitis C virus-infected cells and induction of interferon-alpha. Hepatology 2013, 58, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Hantak, M.P.; Qing, E.; Earnest, J.T.; Gallagher, T. Tetraspanins: Architects of Viral Entry and Exit Platforms. J. Virol. 2019, 93, e01429-17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Woodson, M.; Sherman, M.B.; Neelakanta, G.; Sultana, H. Exosomes mediate Zika virus transmission through SMPD3 neutral Sphingomyelinase in cortical neurons. Emerg. Microbes Infect. 2019, 8, 307–326. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neupane, D.; Bayzid, M.; Neelakanta, G.; Sultana, H. Mosquito Exosomal Tetraspanin CD151 Facilitates Flaviviral Transmission and Interacts with ZIKV and DENV2 Viral Proteins. Int. J. Mol. Sci. 2025, 26, 7394. https://doi.org/10.3390/ijms26157394
Neupane D, Bayzid M, Neelakanta G, Sultana H. Mosquito Exosomal Tetraspanin CD151 Facilitates Flaviviral Transmission and Interacts with ZIKV and DENV2 Viral Proteins. International Journal of Molecular Sciences. 2025; 26(15):7394. https://doi.org/10.3390/ijms26157394
Chicago/Turabian StyleNeupane, Durga, Md Bayzid, Girish Neelakanta, and Hameeda Sultana. 2025. "Mosquito Exosomal Tetraspanin CD151 Facilitates Flaviviral Transmission and Interacts with ZIKV and DENV2 Viral Proteins" International Journal of Molecular Sciences 26, no. 15: 7394. https://doi.org/10.3390/ijms26157394
APA StyleNeupane, D., Bayzid, M., Neelakanta, G., & Sultana, H. (2025). Mosquito Exosomal Tetraspanin CD151 Facilitates Flaviviral Transmission and Interacts with ZIKV and DENV2 Viral Proteins. International Journal of Molecular Sciences, 26(15), 7394. https://doi.org/10.3390/ijms26157394




