High-Protein Diet Prevents Glucocorticoid-Induced Fat Mass Accumulation and Hyperglycemia
Abstract
1. Introduction
2. Results
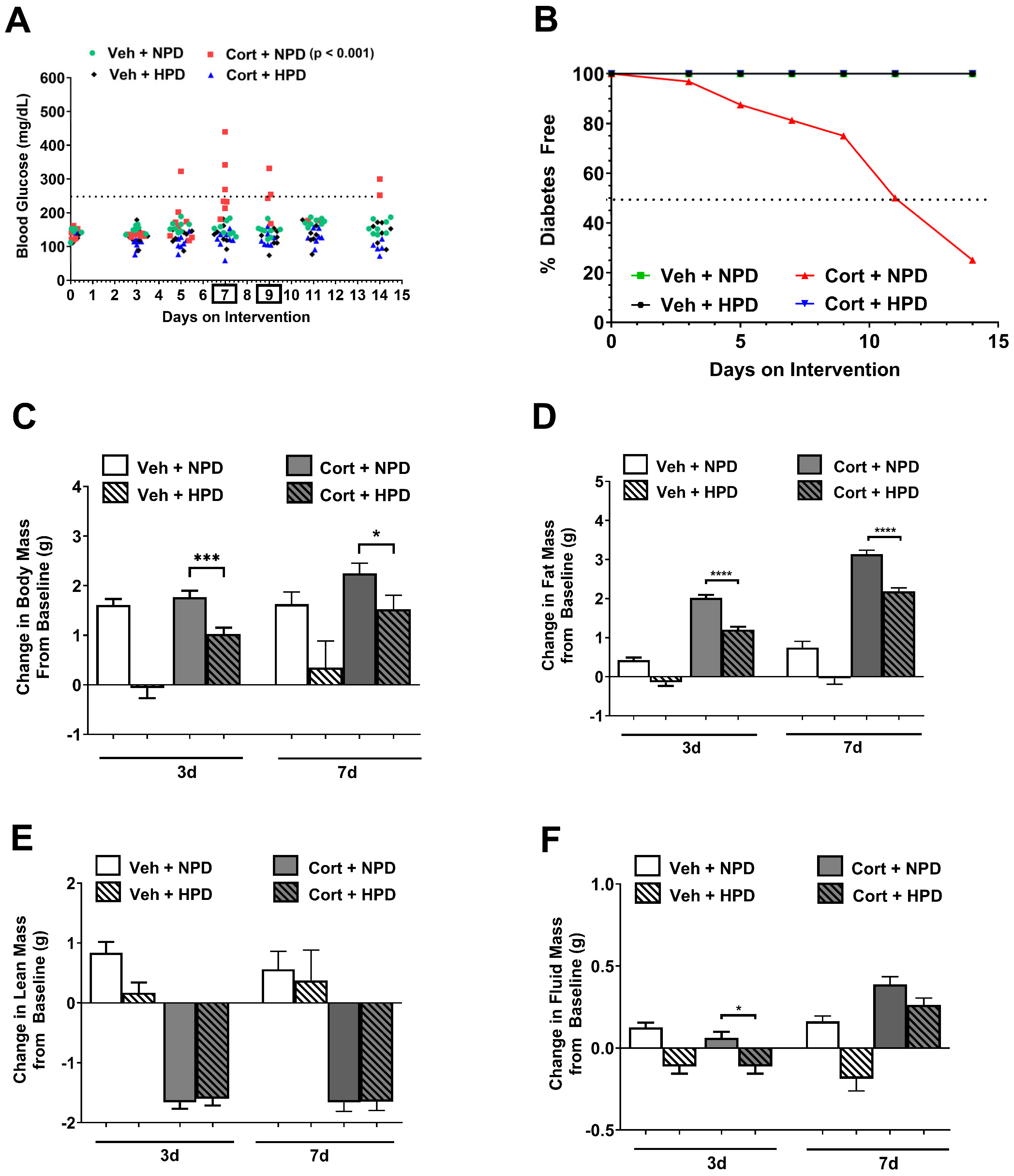
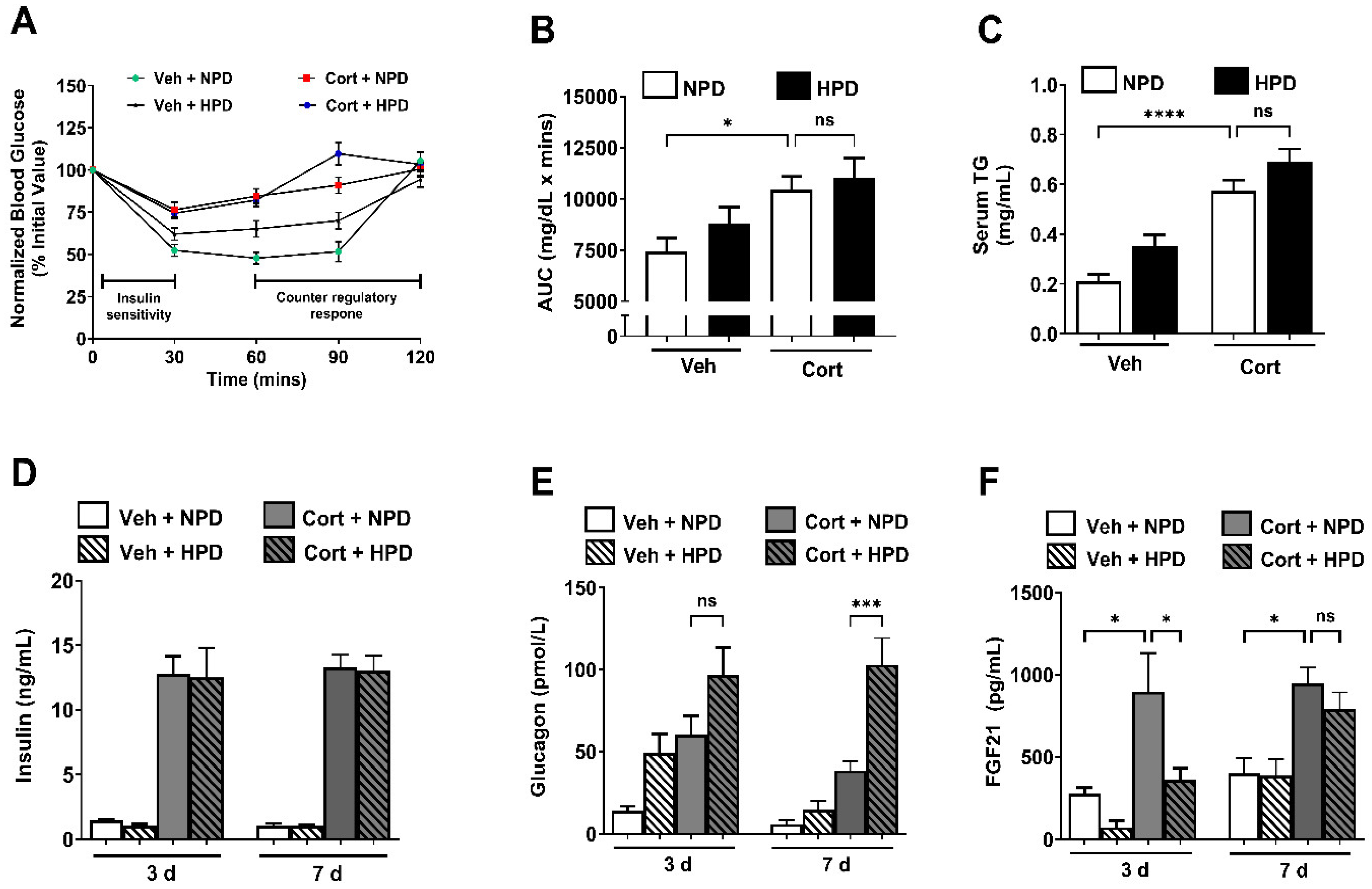
2.1. High-Protein Diet Protects Against Glucocorticoid-Induced Hyperglycemia in Male Mice
2.2. High-Protein Diet Enhances Circulating Glucagon and Suppresses Acute Upregulation of FGF21 During Corticosterone Exposure in Male Mice
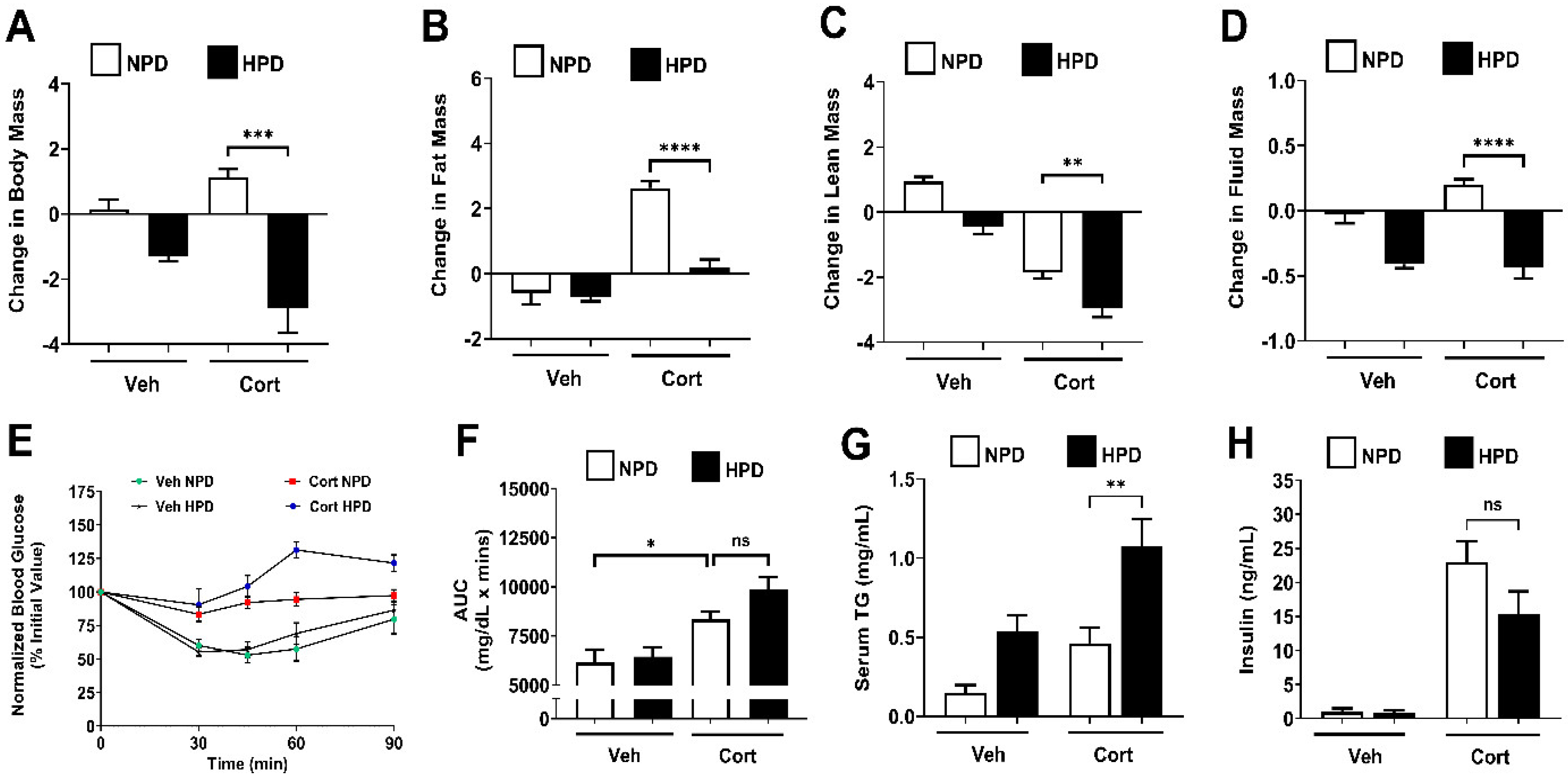
2.3. High-Protein Diet Prevents Fat Mass Accumulation in Female Mice
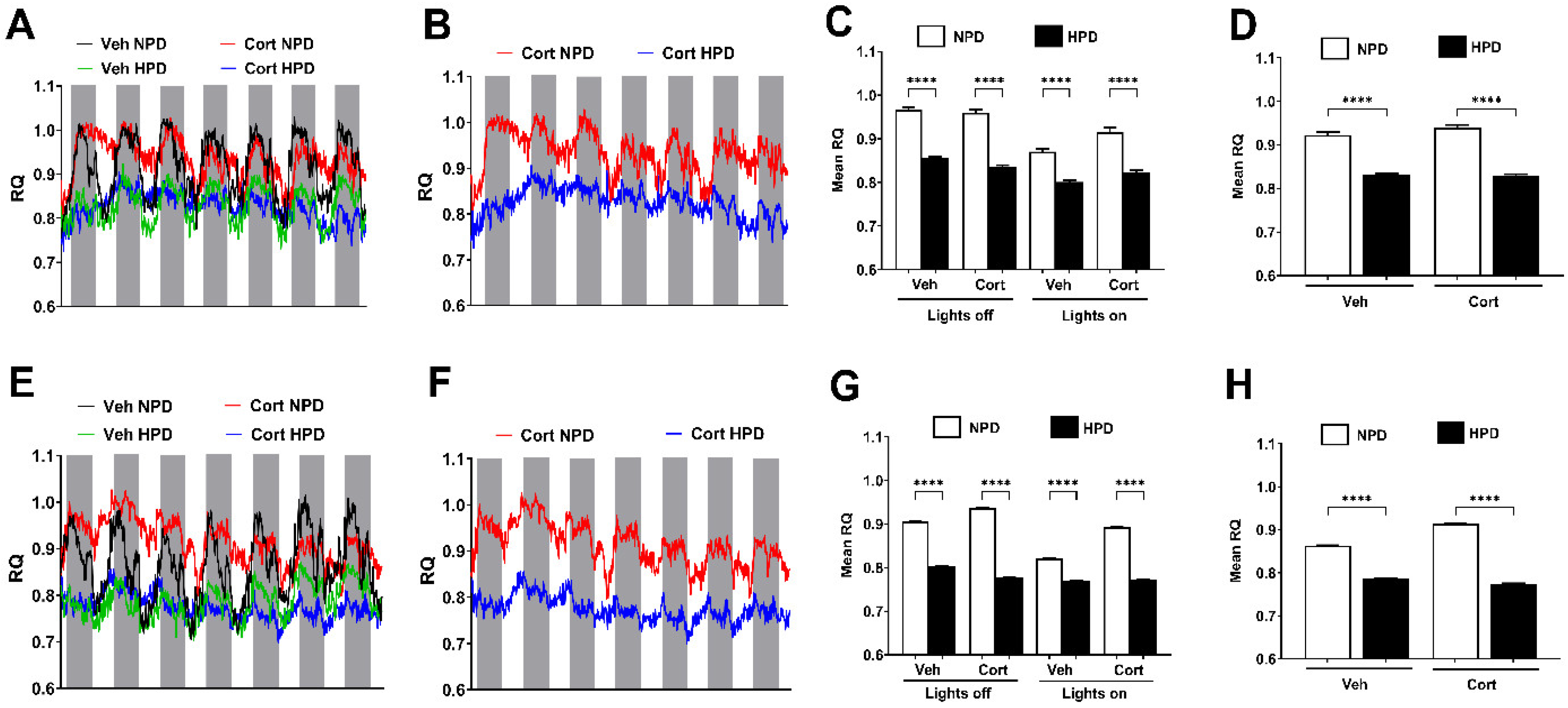
2.4. Whole-Body Fuel Usage in Male and Female Mice Is Altered in Response to Diet
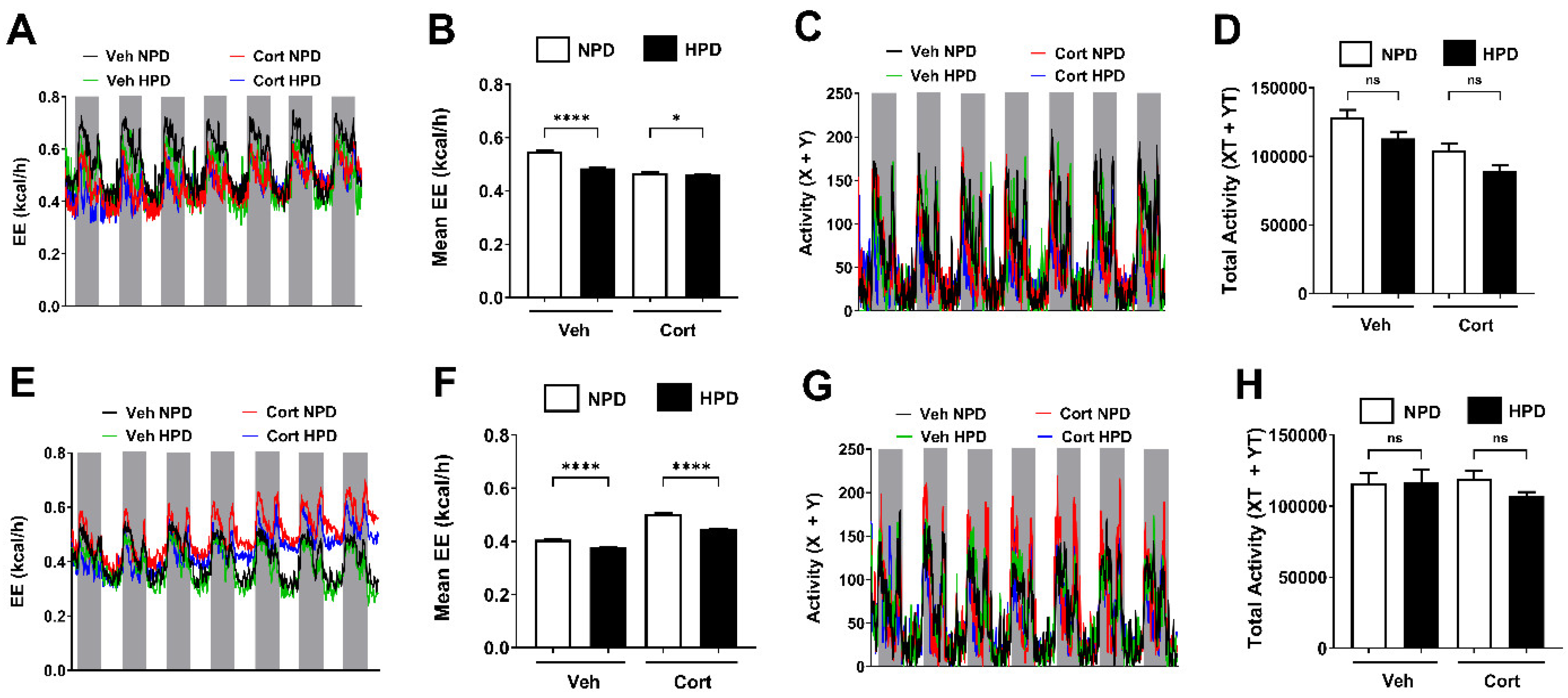
2.5. Energy Expenditure (EE) in Male and Female Mice Is Driven by Changes in Protein Content Within the Diet
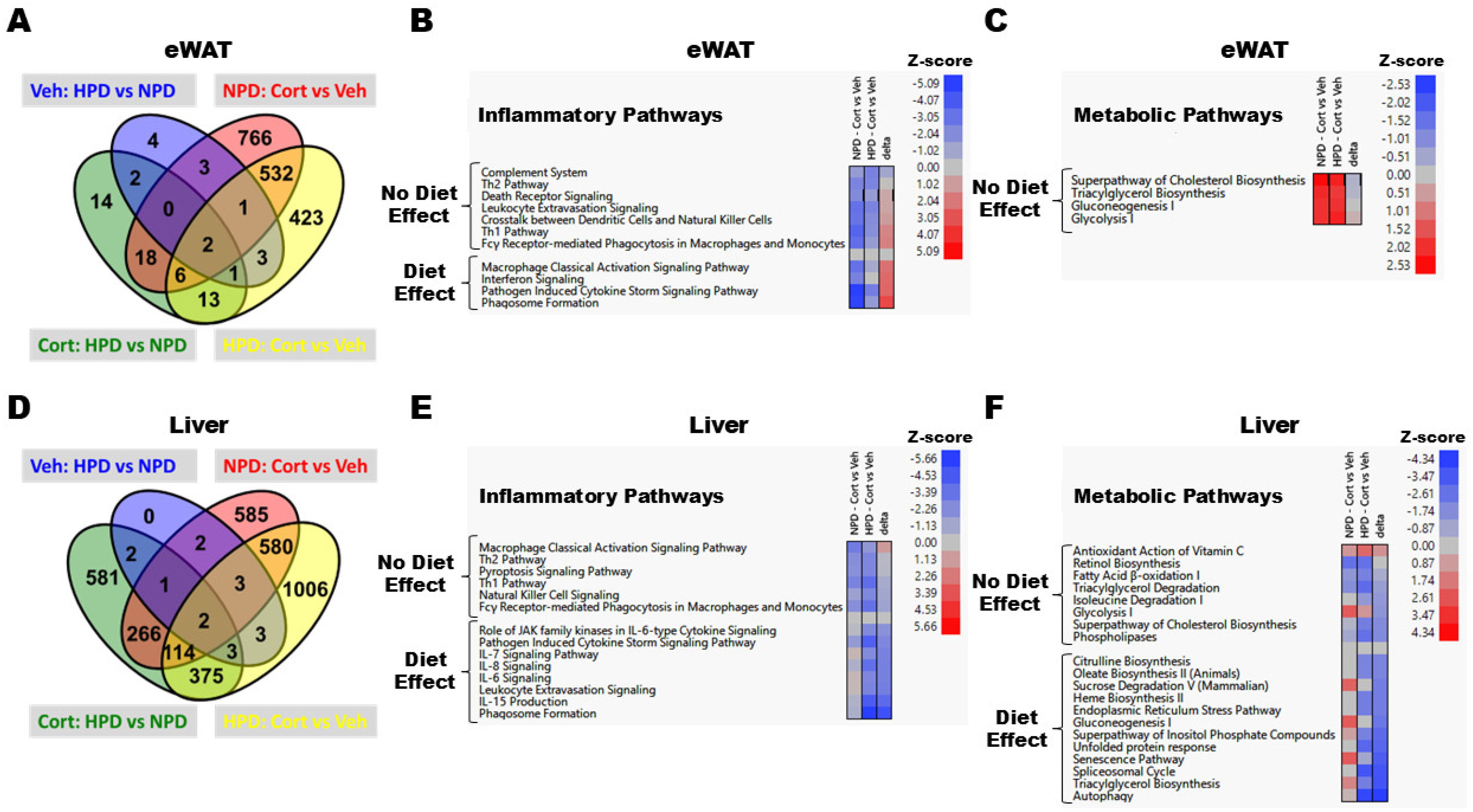
2.6. RNA-Seq Reveals Distinct Changes in Transcript Abundance in eWAT and Liver
3. Discussion
4. Materials and Methods
4.1. Experimental Mice and Reagents
4.2. Blood Glucose, Body Composition, and Metabolic Cage Analysis
4.3. Insulin Tolerance Test (ITT) and Serum ELISAs
4.4. RNA Isolation, cDNA Synthesis, and RNA-Seq
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPD | High-Protein Diet |
| NPD | Normal-Protein Diet |
| GC | Glucocorticoid |
| Cort | Corticosterone |
| FGF21 | Fibroblast Growth Factor 21 |
| Veh | Vehicle |
| AUC | Area Under the Curve |
| ITT | Insulin Tolerance Test |
| RQ | Respiratory Quotient |
| EE | Energy Expenditure |
| eWAT | epididymal White Adipose Tissue |
| GR | Glucocorticoid Receptor |
| NMR | Nuclear Magnetic Resonance |
References
- Weikum, E.R.; Knuesel, M.T.; Ortlund, E.A.; Yamamoto, K.R. Glucocorticoid receptor control of transcription: Precision and plasticity via allostery. Nat. Rev. Mol. Cell Biol. 2017, 18, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.R. Steroid receptor regulated transcription of specific genes and gene networks. Annu. Rev. Genet. 1985, 19, 209–252. [Google Scholar] [CrossRef] [PubMed]
- Ronchetti, S.; Ayroldi, E.; Ricci, E.; Gentili, M.; Migliorati, G.; Riccardi, C. A Glance at the Use of Glucocorticoids in Rare Inflammatory and Autoimmune Diseases: Still an Indispensable Pharmacological Tool? Front. Immunol. 2020, 11, 613435. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulos, T.P.; Tentolouris, N.K.; Bertsias, G.K.; Boumpas, D.T. Steroid-induced diabetes in rheumatologic patients. Clin. Exp. Rheumatol. 2013, 32, 126–130. [Google Scholar]
- Fong, A.C.; Cheung, N.W. The high incidence of steroid-induced hyperglycaemia in hospital. Diabetes Res. Clin. Pract. 2013, 99, 277–280. [Google Scholar] [CrossRef]
- Mills, E.; Devendra, S. Steroid-induced hyperglycaemia in primary care. Lond. J. Prim. Care 2015, 7, 103–106. [Google Scholar] [CrossRef]
- Gulliford, M.C.; Charlton, J.; Latinovic, R. Risk of diabetes associated with prescribed glucocorticoids in a large population. Diabetes Care 2006, 29, 2728–2729. [Google Scholar] [CrossRef]
- Tamez-Perez, H.E.; Quintanilla-Flores, D.L.; Rodriguez-Gutierrez, R.; Gonzalez-Gonzalez, J.G.; Tamez-Pena, A.L. Steroid hyperglycemia: Prevalence, early detection and therapeutic recommendations: A narrative review. World J. Diabetes 2015, 6, 1073–1081. [Google Scholar] [CrossRef]
- Gannon, C.; Dando, N. Dose-sensitive steroid-induced hyperglycaemia. Palliat. Med. 2010, 24, 737–739. [Google Scholar] [CrossRef]
- Hwang, J.L.; Weiss, R.E. Steroid-induced diabetes: A clinical and molecular approach to understanding and treatment. Diabetes Metab. Res. Rev. 2014, 30, 96–102. [Google Scholar] [CrossRef]
- Ha, Y.; Lee, K.H.; Jung, S.; Lee, S.W.; Lee, S.K.; Park, Y.B. Glucocorticoid-induced diabetes mellitus in patients with systemic lupus erythematosus treated with high-dose glucocorticoid therapy. Lupus 2011, 20, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Schacke, H.; Docke, W.D.; Asadullah, K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol. Ther. 2002, 96, 23–43. [Google Scholar] [CrossRef] [PubMed]
- Skytte, M.J.; Samkani, A.; Petersen, A.D.; Thomsen, M.N.; Astrup, A.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Thomsen, H.S.; Madsbad, S.; et al. A carbohydrate-reduced high-protein diet improves HbA(1c) and liver fat content in weight stable participants with type 2 diabetes: A randomised controlled trial. Diabetologia 2019, 62, 2066–2078. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Mangano, K.M.; Hannan, M.T.; Kiel, D.P.; McLean, R.R. Higher Protein Intake Is Associated with Higher Lean Mass and Quadriceps Muscle Strength in Adult Men and Women. J. Nutr. 2015, 145, 1569–1575. [Google Scholar] [CrossRef]
- Kim, J.E.; O’Connor, L.E.; Sands, L.P.; Slebodnik, M.B.; Campbell, W.W. Effects of dietary protein intake on body composition changes after weight loss in older adults: A systematic review and meta-analysis. Nutr. Rev. 2016, 74, 210–224. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.M.; Bowles, N.P.; Weil, Z.M.; Pfaff, D.W.; McEwen, B.S. Endocrine and physiological changes in response to chronic corticosterone: A potential model of the metabolic syndrome in mouse. Endocrinology 2010, 151, 2117–2127. [Google Scholar] [CrossRef]
- Burke, S.J.; Batdorf, H.M.; Eder, A.E.; Karlstad, M.D.; Burk, D.H.; Noland, R.C.; Floyd, Z.E.; Collier, J.J. Oral Corticosterone Administration Reduces Insulitis but Promotes Insulin Resistance and Hyperglycemia in Male Nonobese Diabetic Mice. Am. J. Pathol. 2017, 187, 614–626. [Google Scholar] [CrossRef]
- Burke, S.J.; Batdorf, H.M.; Huang, T.Y.; Jackson, J.W.; Jones, K.A.; Martin, T.M.; Rohli, K.E.; Karlstad, M.D.; Sparer, T.E.; Burk, D.H.; et al. One week of continuous corticosterone exposure impairs hepatic metabolic flexibility, promotes islet beta-cell proliferation, and reduces physical activity in male C57BL/6J mice. J. Steroid Biochem. Mol. Biol. 2019, 195, 105468. [Google Scholar] [CrossRef]
- Ruzzin, J.; Wagman, A.S.; Jensen, J. Glucocorticoid-induced insulin resistance in skeletal muscles: Defects in insulin signalling and the effects of a selective glycogen synthase kinase-3 inhibitor. Diabetologia 2005, 48, 2119–2130. [Google Scholar] [CrossRef]
- Qi, D.; Rodrigues, B. Glucocorticoids produce whole body insulin resistance with changes in cardiac metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E654–E667. [Google Scholar] [CrossRef]
- Clore, J.N.; Thurby-Hay, L. Glucocorticoid-induced hyperglycemia. Endocr. Pract. 2009, 15, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Linden, M.A.; Burke, S.J.; Pirzadah, H.A.; Huang, T.Y.; Batdorf, H.M.; Mohammed, W.K.; Jones, K.A.; Ghosh, S.; Campagna, S.R.; Collier, J.J.; et al. Pharmacological inhibition of lipolysis prevents adverse metabolic outcomes during glucocorticoid administration. Mol. Metab. 2023, 74, 101751. [Google Scholar] [CrossRef] [PubMed]
- Ludvik, B.; Clodi, M.; Kautzky-Willer, A.; Capek, M.; Hartter, E.; Pacini, G.; Prager, R. Effect of dexamethasone on insulin sensitivity, islet amyloid polypeptide and insulin secretion in humans. Diabetologia 1993, 36, 84–87. [Google Scholar] [CrossRef]
- Tappy, L.; Randin, D.; Vollenweider, P.; Vollenweider, L.; Paquot, N.; Scherrer, U.; Schneiter, P.; Nicod, P.; Jequier, E. Mechanisms of dexamethasone-induced insulin resistance in healthy humans. J. Clin. Endocrinol. Metab. 1994, 79, 1063–1069. [Google Scholar] [CrossRef]
- Binnert, C.; Ruchat, S.; Nicod, N.; Tappy, L. Dexamethasone-induced insulin resistance shows no gender difference in healthy humans. Diabetes Metab. 2004, 30, 321–326. [Google Scholar] [CrossRef]
- Qi, D.; Pulinilkunnil, T.; An, D.; Ghosh, S.; Abrahani, A.; Pospisilik, J.A.; Brownsey, R.; Wambolt, R.; Allard, M.; Rodrigues, B. Single-dose dexamethasone induces whole-body insulin resistance and alters both cardiac fatty acid and carbohydrate metabolism. Diabetes 2004, 53, 1790–1797. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Munzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- Laeger, T.; Albarado, D.C.; Burke, S.J.; Trosclair, L.; Hedgepeth, J.W.; Berthoud, H.R.; Gettys, T.W.; Collier, J.J.; Munzberg, H.; Morrison, C.D. Metabolic Responses to Dietary Protein Restriction Require an Increase in FGF21 that Is Delayed by the Absence of GCN2. Cell Rep. 2016, 16, 707–716. [Google Scholar] [CrossRef]
- Mok, C.C.; To, C.H.; Ma, K.M. Changes in body composition after glucocorticoid therapy in patients with systemic lupus erythematosus. Lupus 2008, 17, 1018–1022. [Google Scholar] [CrossRef]
- van Raalte, D.H.; Diamant, M. Steroid diabetes: From mechanism to treatment? Neth. J. Med. 2014, 72, 62–72. [Google Scholar]
- Pennings, B.; Boirie, Y.; Senden, J.M.; Gijsen, A.P.; Kuipers, H.; van Loon, L.J. Whey protein stimulates postprandial muscle protein accretion more effectively than do casein and casein hydrolysate in older men. Am. J. Clin. Nutr. 2011, 93, 997–1005. [Google Scholar] [CrossRef]
- Vu, J.P.; Luong, L.; Parsons, W.F.; Oh, S.; Sanford, D.; Gabalski, A.; Lighton, J.R.; Pisegna, J.R.; Germano, P.M. Long-Term Intake of a High-Protein Diet Affects Body Phenotype, Metabolism, and Plasma Hormones in Mice. J. Nutr. 2017, 147, 2243–2251. [Google Scholar] [CrossRef]
- Gower, B.A.; Goss, A.M. A lower-carbohydrate, higher-fat diet reduces abdominal and intermuscular fat and increases insulin sensitivity in adults at risk of type 2 diabetes. J. Nutr. 2015, 145, 177S–183S. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.C.; Nuttall, F.Q. Effect of a high-protein, low-carbohydrate diet on blood glucose control in people with type 2 diabetes. Diabetes 2004, 53, 2375–2382. [Google Scholar] [CrossRef] [PubMed]
- Dorans, K.S.; Bazzano, L.A.; Qi, L.; He, H.; Chen, J.; Appel, L.J.; Chen, C.S.; Hsieh, M.H.; Hu, F.B.; Mills, K.T.; et al. Effects of a Low-Carbohydrate Dietary Intervention on Hemoglobin A1c: A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2238645. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Despres, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.D.; Nunez, M.V.; Chen, G.X.; Keim, N.L.; Havel, P.J. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015, 101, 1144–1154. [Google Scholar] [CrossRef]
- Burke, S.J.; Batdorf, H.M.; Martin, T.M.; Burk, D.H.; Noland, R.C.; Cooley, C.R.; Karlstad, M.D.; Johnson, W.D.; Collier, J.J. Liquid Sucrose Consumption Promotes Obesity and Impairs Glucose Tolerance Without Altering Circulating Insulin Levels. Obesity 2018, 26, 1188–1196. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy, W.S., Jr.; et al. Management of Type 1 Diabetes with a Very Low-Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- LaPierre, M.P.; Abraham, M.A.; Yue, J.T.; Filippi, B.M.; Lam, T.K. Glucagon signalling in the dorsal vagal complex is sufficient and necessary for high-protein feeding to regulate glucose homeostasis in vivo. EMBO Rep. 2015, 16, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.J.; Thombare, K.; Sarsenbayeva, A.; Kamble, P.G.; Almby, K.; Lundqvist, M.; Eriksson, J.W. Direct effects of glucagon on glucose uptake and lipolysis in human adipocytes. Mol. Cell Endocrinol. 2020, 503, 110696. [Google Scholar] [CrossRef] [PubMed]
- Wernstedt Asterholm, I.; Tao, C.; Morley, T.S.; Wang, Q.A.; Delgado-Lopez, F.; Wang, Z.V.; Scherer, P.E. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014, 20, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.M.; Burke, S.J.; Batdorf, H.M.; Burk, D.H.; Ghosh, S.; Dupuy, S.D.; Karlstad, M.D.; Collier, J.J. ICAM-1 Abundance Is Increased in Pancreatic Islets of Hyperglycemic Female NOD Mice and Is Rapidly Upregulated by NF-kappaB in Pancreatic beta-Cells. J. Immunol. 2022, 209, 569–581. [Google Scholar] [CrossRef]
- Ducote, M.P.; Cothern, C.R.; Batdorf, H.M.; Fontenot, M.S.; Martin, T.M.; Iftesum, M.; Gartia, M.R.; Noland, R.C.; Burk, D.H.; Ghosh, S.; et al. Pancreatic expression of CPT1A is essential for whole body glucose homeostasis by supporting glucose-stimulated insulin secretion. J. Biol. Chem. 2025, 301, 108187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burke, S.J.; Batdorf, H.M.; Ducote, M.P.; Martin, T.M.; Karlstad, M.D.; Noland, R.C.; Ghosh, S.; Morrison, C.D.; Collier, J.J. High-Protein Diet Prevents Glucocorticoid-Induced Fat Mass Accumulation and Hyperglycemia. Int. J. Mol. Sci. 2025, 26, 4212. https://doi.org/10.3390/ijms26094212
Burke SJ, Batdorf HM, Ducote MP, Martin TM, Karlstad MD, Noland RC, Ghosh S, Morrison CD, Collier JJ. High-Protein Diet Prevents Glucocorticoid-Induced Fat Mass Accumulation and Hyperglycemia. International Journal of Molecular Sciences. 2025; 26(9):4212. https://doi.org/10.3390/ijms26094212
Chicago/Turabian StyleBurke, Susan J., Heidi M. Batdorf, Maggie P. Ducote, Thomas M. Martin, Michael D. Karlstad, Robert C. Noland, Sujoy Ghosh, Christopher D. Morrison, and J. Jason Collier. 2025. "High-Protein Diet Prevents Glucocorticoid-Induced Fat Mass Accumulation and Hyperglycemia" International Journal of Molecular Sciences 26, no. 9: 4212. https://doi.org/10.3390/ijms26094212
APA StyleBurke, S. J., Batdorf, H. M., Ducote, M. P., Martin, T. M., Karlstad, M. D., Noland, R. C., Ghosh, S., Morrison, C. D., & Collier, J. J. (2025). High-Protein Diet Prevents Glucocorticoid-Induced Fat Mass Accumulation and Hyperglycemia. International Journal of Molecular Sciences, 26(9), 4212. https://doi.org/10.3390/ijms26094212






