Early Roots of Childhood Obesity: Risk Factors, Mechanisms, and Prevention Strategies
Abstract
1. Introduction
2. Methods
2.1. Literature Search Strategy and Selection Criteria
2.2. Data Extraction
2.3. Narrative Synthesis
3. Preconceptional Factors and Transgenerational Risk Factors
3.1. Maternal Pre-Pregnancy BMI
3.2. Excessive Gestational Weight Gain
3.3. Hyperglycemia and Diabetes in Pregnancy
3.4. Maternal Nutrition
3.4.1. Excessive Maternal Nutrition
3.4.2. Poor Maternal Nutrition
3.5. Caesarean Section
3.6. Maternal Smoking
3.7. Exposure to Environmental Obesogens
3.8. Maternal Psychosocial Stress
4. Effect of Birth Weight and Being Born IUGR/SGA on Weight Gain and Metabolism
5. First 2 Years of Life: Evidence on How Nutritional, Environmental, and Behavioral Factors Affect Adipose Tissue and Metabolism in Future Life
5.1. Breastfeeding and Formula Feeding
5.2. Complementary Feeding
5.3. Physical Activity and Screen Time Exposures
5.4. Socioeconomic Factors
5.5. Gut Microbiome
5.6. Exposure to Endocrine-Disrupting Chemicals
6. Discussion and Conclusions: How Adverse Trajectories Could or Can Be Changed
6.1. Preconception–Pregnancy
6.2. Infancy and Early Childhood
- Fresh food is preferable to processed and canned foods.
- It is preferable to choose chemical-free food.
- Glass or ceramic containers should replace plastic ones when heating food in a microwave oven.
- The consumption of fatty dairy or meat products should be reduced.
- Products such as makeup, perfume, and skin care should be free of phthalates, parabens, triclosan, and other chemicals.
- It is preferable to opt for ecological household cleaning products.
- Flame retardant-treated furniture should be avoided.
- Indoor environments should be ventilated regularly.
- Alternatives to plastic toys are preferred.
Author Contributions
Funding
Conflicts of Interest
References
- Sahoo, K.; Sahoo, B.; Choudhury, A.K.; Sofi, N.Y.; Kumar, R.; Bhadoria, A.S. Childhood obesity: Causes and consequences. J. Family Med. Prim. Care 2015, 4, 187–192. [Google Scholar] [CrossRef]
- Smith, J.D.; Fu, E.; Kobayashi, M.A. Prevention and management of childhood obesity and its psychological and health comorbidities. Annu. Rev. Clin. Psychol. 2020, 16, 351–378. [Google Scholar] [CrossRef]
- Johansson, S.; Villamor, E.; Altman, M.; Bonamy, A.-K.E.; Granath, F.; Cnattingius, S. Maternal overweight and obesity in early pregnancy and risk of infant mortality: A population based cohort study in Sweden. BMJ 2014, 349, g6572. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Lyu, J.; Liu, Z.; Zhou, S.; Ji, Y.; Wang, H. Association of gestational hypertension and preeclampsia with offspring adiposity: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 906781. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Huang, C.; Zhao, M.; Lee, P.M.Y.; Zhang, C.; Yu, Y.; Xi, B.; Li, J. Maternal hypertensive disorders during pregnancy and the risk of offspring diabetes mellitus in childhood, adolescence, and early adulthood: A nationwide population-based cohort study. BMC Med. 2023, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Patro Golab, B.; Santos, S.; Voerman, E.; Lawlor, D.A.; Jaddoe, V.W.V.; Gaillard, R.; MOCO Study Group Authors. Influence of maternal obesity on the association between common pregnancy complications and risk of childhood obesity: An individual participant data meta-analysis. Lancet Child. Adolesc. Health 2018, 2, 812–821. [Google Scholar] [CrossRef]
- Meek, C.L. An unwelcome inheritance: Childhood obesity after diabetes in pregnancy. Diabetologia 2023, 66, 1961–1970. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Langley-Evans, S.C.; McMullen, S. Developmental origins of adult disease. Med. Princ. Pract. 2010, 19, 87–98. [Google Scholar] [CrossRef]
- Zhu, Z.; Cao, F.; Li, X. Epigenetic programming and fetal metabolic programming. Front. Endocrinol. 2019, 10, 764. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Dłuski, D.F.; Wolińska, E.; Skrzypczak, M. Epigenetic changes in gestational diabetes mellitus. Int. J. Mol. Sci. 2021, 22, 7649. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-H.; Tan, Y.; Bajinka, O. The influence of maternal diet on offspring’s gut microbiota in early life. Arch. Gynecol. Obstet. 2024, 309, 1183–1190. [Google Scholar] [CrossRef]
- Barker, D.J.P. The developmental origins of adult disease. J. Am. Coll. Nutr. 2004, 23, 588S–595S. [Google Scholar] [CrossRef]
- Marousez, L.; Lesage, J.; Eberlé, D. Epigenetics: Linking early postnatal nutrition to obesity programming? Nutrients 2019, 11, 2966. [Google Scholar] [CrossRef]
- Galvan-Martinez, D.H.; Bosquez-Mendoza, V.M.; Ruiz-Noa, Y.; Ibarra-Reynoso, L.D.R.; Barbosa-Sabanero, G.; Lazo-de-la-Vega-Monroy, M.-L. Nutritional, pharmacological, and environmental programming of NAFLD in early life. Am. J. Physiol. Gastrointest. Liver Physiol. 2023, 324, G99–G114. [Google Scholar] [CrossRef]
- He, S.; Stein, A.D. Early-Life Nutrition Interventions and Associated Long-Term Cardiometabolic Outcomes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2021, 12, 461–489. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Anaclerio, F.; Moscogiuri, L.A.; Konstantinidou, F.; Stuppia, L.; Gatta, V. Exploring Maternal Diet-Epigenetic-Gut Microbiome Crosstalk as an Intervention Strategy to Counter Early Obesity Programming. Curr. Issues Mol. Biol. 2024, 46, 4358–4378. [Google Scholar] [CrossRef]
- Shock, T.; Badang, L.; Ferguson, B.; Martinez-Guryn, K. The interplay between diet, gut microbes, and host epigenetics in health and disease. J. Nutr. Biochem. 2021, 95, 108631. [Google Scholar] [CrossRef]
- Faienza, M.F.; Urbano, F.; Lassandro, G.; Valente, F.; D’Amato, G.; Portincasa, P.; Giordano, P. The Cardiovascular Disease (CVD) Risk Continuum from Prenatal Life to Adulthood: A Literature Review. Int. J. Environ. Res. Public Health 2022, 19, 8282. [Google Scholar] [CrossRef]
- Veenendaal, M.V.; Painter, R.C.; de Rooij, S.R.; Bossuyt, P.M.; van der Post, J.A.; Gluckman, P.D.; Hanson, M.A.; Roseboom, T.J. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG 2013, 120, 548–553. [Google Scholar] [CrossRef]
- Rajamoorthi, A.; LeDuc, C.A.; Thaker, V.V. The metabolic conditioning of obesity: A review of the pathogenesis of obesity and the epigenetic pathways that “program” obesity from conception. Front. Endocrinol. 2022, 13, 1032491. [Google Scholar] [CrossRef]
- Vickers, M.H. Utility of preclinical models of altered maternal nutrition to support the developmental origins of health and disease hypothesis. Clin. Sci. 2022, 136, 711–714. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Lillycrop, K.A.; Vickers, M.H.; Pleasants, A.B.; Phillips, E.S.; Beedle, A.S.; Burdge, G.C.; Hanson, M.A. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc. Natl. Acad. Sci. USA 2007, 104, 12796–12800. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.; Zimmet, P.; Forrester, T. Losing the war against obesity: The need for a developmental perspective. Sci. Transl. Med. 2011, 3, 93–119. [Google Scholar] [CrossRef]
- Hüls, A.; Wright, M.N.; Bogl, L.H.; Kaprio, J.; Lissner, L.; Molnár, D.; Moreno, L.A.; De Henauw, S.; Siani, A.; Veidebaum, T.; et al. Polygenic risk for obesity and its interaction with lifestyle and sociodemographic factors in European children and adolescents. Int. J. Obes. 2021, 45, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Trandafir, L.M.; Temneanu, O.R. Pre and post-natal risk and determination of factors for child obesity. J. Med. Life 2016, 9, 386–391. [Google Scholar]
- Cristian, A.; Tarry-Adkins, J.L.; Aiken, C.E. The uterine environment and childhood obesity risk: Mechanisms and predictions. Curr. Nutr. Rep. 2023, 12, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Josey, M.J.; McCullough, L.E.; Hoyo, C.; Williams-DeVane, C. Overall gestational weight gain mediates the relationship between maternal and child obesity. BMC Public Health 2019, 19, 1062. [Google Scholar] [CrossRef] [PubMed]
- Dello Russo, M.; Ahrens, W.; De Vriendt, T.; Marild, S.; Molnar, D.; Moreno, L.A.; Reeske, A.; Veidebaum, T.; Kourides, Y.A.; Barba, G.; et al. Gestational weight gain and adiposity, fat distribution, metabolic profile, and blood pressure in offspring: The IDEFICS project. Int. J. Obes. 2013, 37, 914–919. [Google Scholar] [CrossRef]
- Champion, M.L.; Harper, L.M. Gestational weight gain: Update on outcomes and interventions. Curr. Diab Rep. 2020, 20, 11. [Google Scholar] [CrossRef]
- Voerman, E.; Santos, S.; Patro Golab, B.; Amiano, P.; Ballester, F.; Barros, H.; Bergström, A.; Charles, M.A.; Chatzi, L.; Chevrier, C.; et al. Maternal body mass index, gestational weight gain, and the risk of overweight and obesity across childhood: An individual participant data meta-analysis. PLoS Med. 2019, 16, e1002744. [Google Scholar] [CrossRef]
- Badon, S.E.; Quesenberry, C.P.; Xu, F.; Avalos, L.A.; Hedderson, M.M. Gestational weight gain, birthweight and early-childhood obesity: Between- and within-family comparisons. Int. J. Epidemiol. 2020, 49, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Liu, H.; Wang, L.; Song, Q.; Sun, D.; Li, W.; Leng, J.; Gao, R.; Hu, G.; Qi, L. Maternal gestational diabetes mellitus modifies the relationship between genetically determined body mass index during pregnancy and childhood obesity. Mayo Clin. Proc. 2020, 95, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tylavsky, F.A.; Han, J.C.; Kocak, M.; Fowke, J.H.; Davis, R.L.; Lewinn, K.; Bush, N.R.; Zhao, Q. Maternal metabolic factors during pregnancy predict early childhood growth trajectories and obesity risk: The CANDLE Study. Int. J. Obes. 2019, 43, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Mannino, A.; Sarapis, K.; Moschonis, G. The Effect of Maternal Overweight and Obesity Pre-Pregnancy and During Childhood in the Development of Obesity in Children and Adolescents: A Systematic Literature Review. Nutrients 2022, 14, 5125. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Liao, X.P.; Yu, Y.; Marc, I.; Dubois, L.; Abdelouahab, N.; Bouchard, L.; Wu, Y.T.; Ouyang, F.; Huang, H.F.; Fraser, W.D. Prenatal determinants of childhood obesity: A review of risk factors. Can. J. Physiol. Pharmacol. 2019, 97, 147–154. [Google Scholar] [CrossRef]
- Orós, M.; Lorenzo, M.; Serna, M.C.; Siscart, J.; Perejón, D.; Salinas-Roca, B. Obesity in Pregnancy as a Risk Factor in Maternal and Child Health-A Retrospective Cohort Study. Metabolites 2024, 14, 56. [Google Scholar] [CrossRef]
- Lowe, W.L.; Lowe, L.P.; Kuang, A.; Catalano, P.M.; Nodzenski, M.; Talbot, O.; Tam, W.-H.; Sacks, D.A.; McCance, D.; Linder, B.; et al. Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome Follow-up Study. Diabetologia 2019, 62, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; McIntyre, H.D.; Cruickshank, J.K.; McCance, D.R.; Dyer, A.R.; Metzger, B.E.; Lowe, L.P.; Trimble, E.R.; Coustan, D.R.; Hadden, D.R.; et al. The hyperglycemia and adverse pregnancy outcome study: Associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012, 35, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Josefson, J.L.; Catalano, P.M.; Lowe, W.L.; Scholtens, D.M.; Kuang, A.; Dyer, A.R.; Lowe, L.P.; Metzger, B.E. The joint associations of maternal bmi and glycemia with childhood adiposity. J. Clin. Endocrinol. Metab. 2020, 105, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, G.L.; De Los Santos, S.; Méndez-Sánchez, D.; Reyes-Castro, L.A.; Ibáñez, C.A.; Canto, P.; Zambrano, E. High-fat diet consumption by male rat offspring of obese mothers exacerbates adipose tissue hypertrophy and metabolic alterations in adult life. Br. J. Nutr. 2022, 130, 787–792. [Google Scholar] [CrossRef]
- Jones, H.N.; Woollett, L.A.; Barbour, N.; Prasad, P.D.; Powell, T.L.; Jansson, T. High-fat diet before and during pregnancy causes marked up-regulation of placental nutrient transport and fetal overgrowth in C57/BL6 mice. FASEB J. 2009, 23, 271–278. [Google Scholar] [CrossRef]
- Rosario, F.J.; Kanai, Y.; Powell, T.L.; Jansson, T. Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity 2015, 23, 1663–1670. [Google Scholar] [CrossRef]
- Masuyama, H.; Mitsui, T.; Nobumoto, E.; Hiramatsu, Y. The effects of high-fat diet exposure in utero on the obesogenic and diabetogenic traits through epigenetic changes in adiponectin and leptin gene expression for multiple generations in female mice. Endocrinology 2015, 156, 2482–2491. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Q.; Zhang, L.; Maricelli, J.W.; Rodgers, B.D.; Zhu, M.J.; Du, M. Maternal high-fat diet during lactation impairs thermogenic function of brown adipose tissue in offspring mice. Sci. Rep. 2016, 6, 34345. [Google Scholar] [CrossRef]
- Butruille, L.; Marousez, L.; Pourpe, C.; Oger, F.; Lecoutre, S.; Catheline, D.; Görs, S.; Metges, C.C.; Guinez, C.; Laborie, C.; et al. Maternal high-fat diet during suckling programs visceral adiposity and epigenetic regulation of adipose tissue stearoyl-CoA desaturase-1 in offspring. Int. J. Obes. 2019, 43, 2381–2393. [Google Scholar] [CrossRef]
- Lemes, S.F.; de Souza, A.C.; Payolla, T.B.; Versutti, M.D.; de Fátima da Silva Ramalho, A.D.; Mendes-da-Silva, C.; Souza, C.M.; Milanski, M.; Torsoni, A.S.; Torsoni, M.A. Maternal consumption of high-fat diet in mice alters hypothalamic Notch pathway, NPY cell population and food intake in offspring. Neuroscience 2018, 371, 1–15. [Google Scholar] [CrossRef]
- Widerøe, M.; Vik, T.; Jacobsen, G.; Bakketeig, L.S. Does maternal smoking during pregnancy cause childhood overweight? Paediatr. Perinat. Epidemiol. 2003, 17, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, S.H.; Mommers, M.; Gubbels, J.S.; Kremers, S.P.; Stafleu, A.; Stehouwer, C.D.; Prins, M.H.; Penders, J.; Thijs, C. Maternal smoking during pregnancy and childhood overweight and fat distribution: The KOALA Birth Cohort Study. Pediatr. Obes. 2014, 9, e14–e25. [Google Scholar] [CrossRef] [PubMed]
- Magriplis, E.; Farajian, P.; Panagiotakos, D.B.; Risvas, G.; Zampelas, A. Maternal smoking and risk of obesity in school children: Investigating early life theory from the GRECO study. Prev. Med. Rep. 2017, 8, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Ino, T. Maternal smoking during pregnancy and offspring obesity: Meta-analysis. Pediatr. Int. 2010, 52, 94–99. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Reynolds, R.M.; Prescott, S.L.; Nyirenda, M.; Jaddoe, V.W.V.; Eriksson, J.G.; Broekman, B.F.P. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol. 2017, 5, 53–64. [Google Scholar] [CrossRef]
- Yu, Z.; Han, S.; Zhu, J.; Sun, X.; Ji, C.; Guo, X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: A systematic review and meta-analysis. PLoS ONE 2013, 8, e61627. [Google Scholar] [CrossRef]
- Patel, N.; Pasupathy, D.; Poston, L. Determining the consequences of maternal obesity for offspring health. Exp. Physiol. 2015, 100, 1421–1428. [Google Scholar] [CrossRef]
- Poston, L.; Harthoorn, L.F.; Van Der Beek, E.M.; Contributors to the ILSI Europe Workshop. Obesity in pregnancy: Implications for the mother and lifelong health of the child. A consensus statement. Pediatr. Res. 2011, 69, 175–180. [Google Scholar] [CrossRef]
- Vucetic, Z.; Reyes, T.M. Central dopaminergic circuitry controlling food intake and reward: Implications for the regulation of obesity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010, 2, 577–593. [Google Scholar] [CrossRef]
- Desai, M.; Han, G.; Ross, M.G. Programmed hyperphagia in offspring of obese dams: Altered expression of hypothalamic nutrient sensors, neurogenic factors and epigenetic modulators. Appetite 2016, 99, 193–199. [Google Scholar] [CrossRef]
- Yang, Q.-Y.; Liang, J.-F.; Rogers, C.J.; Zhao, J.-X.; Zhu, M.-J.; Du, M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes 2013, 62, 3727–3735. [Google Scholar] [CrossRef]
- Harmancıoğlu, B.; Kabaran, S. Maternal high fat diets: Impacts on offspring obesity and epigenetic hypothalamic programming. Front. Genet. 2023, 11, 1158089. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Wang, B.; Pan, H.; Zhu, M.-J.; Nathanielsz, P.W.; Du, M. Maternal obesity epigenetically alters visceral fat progenitor cell properties in male offspring mice. J. Physiol. 2016, 594, 4453–4466. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Hesketh, K.D.; Vuillermin, P.; Dodd, J.; Wen, L.M.; Baur, L.A.; Taylor, R.; Byrne, R.; Mihrshahi, S.; Burgner, D.; et al. Understanding the pathways between prenatal and postnatal factors and overweight outcomes in early childhood: A pooled analysis of seven cohorts. Int. J. Obes. 2023, 47, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G.; Sandboge, S.; Salonen, M.; Kajantie, E.; Osmond, C. Maternal weight in pregnancy and offspring body composition in late adulthood: Findings from the Helsinki Birth Cohort Study (HBCS). Ann. Med. 2015, 47, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Laura, H.; Santos, I.S.; Quadros, L.C.M.; Matijasevich, A. Maternal obesity and offspring body composition by indirect methods: A systematic review and meta-analysis. Cad. Saude Publica 2015, 31, 2073–2092. [Google Scholar] [CrossRef]
- Smith, J.; Cianflone, K.; Biron, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Lescelleur, O.; Biertho, L.; Simard, S.; Kral, J.G.; et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4275–4283. [Google Scholar] [CrossRef]
- Samuelsson, A.-M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.J.M.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C.; et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef]
- Korkmaz, L.; Baştuğ, O.; Kurtoğlu, S. Maternal Obesity and its Short- and Long-Term Maternal and Infantile Effects. J. Clin. Res. Pediatr. Endocrinol. 2016, 8, 114–124. [Google Scholar] [CrossRef]
- Stothard, K.J.; Tennant, P.W.G.; Bell, R.; Rankin, J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA 2009, 301, 636–650. [Google Scholar] [CrossRef]
- Institute of Medicine (US) and National Research Council (US). Committee to Reexamine IOM Pregnancy Weight Guidelines. In Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; The National Academies Collection: Reports funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2009. [Google Scholar]
- Mamun, A.A.; Mannan, M.; Doi, S.A.R. Gestational weight gain in relation to offspring obesity over the life course: A systematic review and bias-adjusted meta-analysis. Obes. Rev. 2014, 15, 338–347. [Google Scholar] [CrossRef]
- Tie, H.-T.; Xia, Y.-Y.; Zeng, Y.-S.; Zhang, Y.; Dai, C.-L.; Guo, J.J.; Zhao, Y. Risk of childhood overweight or obesity associated with excessive weight gain during pregnancy: A meta-analysis. Arch. Gynecol. Obstet. 2014, 289, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Nehring, I.; Lehmann, S.; von Kries, R. Gestational weight gain in accordance to the IOM/NRC criteria and the risk for childhood overweight: A meta-analysis. Pediatr. Obes. 2013, 8, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.Y.; Liu, J.; Archer, E.; McDonald, S.M.; Liu, J. Maternal weight gain in pregnancy and risk of obesity among offspring: A systematic review. J. Obes. 2014, 2014, 524939. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Steegers, E.A.P.; Franco, O.H.; Hofman, A.; Jaddoe, V.W.V. Maternal weight gain in different periods of pregnancy and childhood cardio-metabolic outcomes. The Generation R Study. Int. J. Obes. 2015, 39, 677–685. [Google Scholar] [CrossRef]
- Symonds, M.E.; Pope, M.; Sharkey, D.; Budge, H. Adipose tissue and fetal programming. Diabetologia 2012, 55, 1597–1606. [Google Scholar] [CrossRef]
- Philipps, L.H.; Santhakumaran, S.; Gale, C.; Prior, E.; Logan, K.M.; Hyde, M.J.; Modi, N. The diabetic pregnancy and offspring BMI in childhood: A systematic review and meta-analysis. Diabetologia 2011, 54, 1957–1966. [Google Scholar] [CrossRef]
- Kim, S.Y.; England, J.L.; Sharma, J.A.; Njoroge, T. Gestational diabetes mellitus and risk of childhood overweight and obesity in offspring: A systematic review. Exp. Diabetes Res. 2011, 2011, 541308. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sharma, A.J.; Callaghan, W.M. Gestational diabetes and childhood obesity:‘ what is the link? Curr. Opin. Obstet. Gynecol. 2012, 24, 376–381. [Google Scholar] [CrossRef]
- Al Bekai, E.; Beaini, C.E.; Kalout, K.; Safieddine, O.; Semaan, S.; Sahyoun, F.; Ghadieh, H.E.; Azar, S.; Kanaan, A.; Harb, F. The Hidden Impact of Gestational Diabetes: Unveiling Offspring Complications and Long-Term Effects. Life 2025, 11, 440. [Google Scholar] [CrossRef]
- Lowe, W.L.; Scholtens, D.M.; Lowe, L.P.; Kuang, A.; Nodzenski, M.; Talbot, O.; Catalano, P.M.; Linder, B.; Brickman, W.J.; Clayton, P.; et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 2018, 320, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Mitanchez, D.; Yzydorczyk, C.; Simeoni, U. What neonatal complications should the pediatrician be aware of in case of maternal gestational diabetes? World J. Diabetes 2015, 6, 734–743. [Google Scholar] [CrossRef]
- Franzago, M.; Borrelli, P.; Di Nicola, M.; Cavallo, P.; D’Adamo, E.; Di Tizio, L.; Gazzolo, D.; Stuppia, L.; Vitacolonna, E. From Mother to Child: Epigenetic Signatures of Hyperglycemia and Obesity during Pregnancy. Nutrients 2024, 16, 3502. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, E.L.; Grove, K.L. Metabolic imprinting in obesity. Forum Nutr. 2010, 63, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.H.; Ma, R.C.W.; Ozaki, R.; Li, A.M.; Chan, M.H.M.; Yuen, L.Y.; Lao, T.T.H.; Yang, X.; Ho, C.S.; Tutino, G.E.; et al. In utero exposure to maternal hyperglycemia increases childhood cardiometabolic risk in offspring. Diabetes Care 2017, 40, 679–686. [Google Scholar] [CrossRef]
- Luo, Z.-C.; Nuyt, A.-M.; Delvin, E.; Audibert, F.; Girard, I.; Shatenstein, B.; Cloutier, A.; Cousineau, J.; Djemli, A.; Deal, C.; et al. Maternal and fetal IGF-I and IGF-II levels, fetal growth, and gestational diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 1720–1728. [Google Scholar] [CrossRef]
- Kadakia, R.; Ma, M.; Josefson, J.L. Neonatal adiposity increases with rising cord blood IGF-1 levels. Clin. Endocrinol. 2016, 85, 70–75. [Google Scholar] [CrossRef]
- Soliman, A.; Ahmad, S.; Hamed, N.; Alyafei, F.; Alaaraj, N.; Soliman, N. Maternal, placental, and fetal Insulin-Like Growth Factor-I (IGF-1) and IGF Binding proteins (IGFBPs) in Diabetic pregnancies: Effects on fetal growth and birth size. World J. Adv. Res. Rev. 2023, 17, 287–295. [Google Scholar] [CrossRef]
- Scholtens, D.M.; Bain, J.R.; Reisetter, A.C.; Muehlbauer, M.J.; Nodzenski, M.; Stevens, R.D.; Ilkayeva, O.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. HAPO Study Cooperative Research Group Metabolic networks and metabolites underlie associations between maternal glucose during pregnancy and newborn size at birth. Diabetes 2016, 65, 2039–2050. [Google Scholar] [CrossRef]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef]
- Franke, K.; Harder, T.; Aerts, L.; Melchior, K.; Fahrenkrog, S.; Rodekamp, E.; Ziska, T.; Van Assche, F.A.; Dudenhausen, J.W.; Plagemann, A. Programming’ of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res. 2005, 1031, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Arata, N.; Ogawa, Y. Obesity and abnormal glucose tolerance in the offspring of mothers with diabetes. Curr Opin Obstet Gynecol. 2018, 30, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Chatzi, L.; Rifas-Shiman, S.L.; Georgiou, V.; Joung, K.E.; Koinaki, S.; Chalkiadaki, G.; Margioris, A.; Sarri, K.; Vassilaki, M.; Vafeiadi, M.; et al. Adherence to the Mediterranean diet during pregnancy and offspring adiposity and cardiometabolic traits in childhood. Pediatr. Obes. 2017, 12 (Suppl. S1), 47–56. [Google Scholar] [CrossRef] [PubMed]
- Grivell, R.M.; Yelland, L.N.; Deussen, A.; Crowther, C.A.; Dodd, J.M. Antenatal dietary and lifestyle advice for women who are overweight or obese and the effect on fetal growth and adiposity: The LIMIT randomised trial. BJOG 2016, 123, 233–243. [Google Scholar] [CrossRef]
- Farella, I.; Miselli, F.; Campanozzi, A.; Grosso, F.M.; Laforgia, N.; Baldassarre, M.E. Mediterranean Diet in Developmental Age: A Narrative Review of Current Evidences and Research Gaps. Children 2022, 9, 906. [Google Scholar] [CrossRef]
- Claesson, I.-M.; Josefsson, A.; Olhager, E.; Oldin, C.; Sydsjö, G. Effects of a gestational weight gain restriction program for obese women: Sibling pairs’ weight development during the first five years of life. Sex. Reprod. Healthc. 2018, 17, 65–74. [Google Scholar] [CrossRef]
- O’Brien, C.M.; Louise, J.; Deussen, A.; Dodd, J.M. In Overweight or Obese Pregnant Women, Maternal Dietary Factors are not Associated with Fetal Growth and Adiposity. Nutrients 2018, 10, 870. [Google Scholar] [CrossRef]
- Brei, C.; Stecher, L.; Meyer, D.M.; Young, V.; Much, D.; Brunner, S.; Hauner, H. Impact of Dietary Macronutrient Intake during Early and Late Gestation on Offspring Body Composition at Birth, 1, 3, and 5 Years of Age. Nutrients 2018, 10, 579. [Google Scholar] [CrossRef]
- Stratakis, N.; Gielen, M.; Chatzi, L.; Zeegers, M.P. Effect of maternal n-3 long-chain polyunsaturated fatty acid supplementation during pregnancy and/or lactation on adiposity in childhood: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2014, 68, 1277–1287. [Google Scholar] [CrossRef]
- Maslova, E.; Rytter, D.; Bech, B.H.; Henriksen, T.B.; Rasmussen, M.A.; Olsen, S.F.; Halldorsson, T.I. Maternal protein intake during pregnancy and offspring overweight 20 y later. Am. J. Clin. Nutr. 2014, 100, 1139–1148. [Google Scholar] [CrossRef]
- Goran, M.I.; Dumke, K.; Bouret, S.G.; Kayser, B.; Walker, R.W.; Blumberg, B. The obesogenic effect of high fructose exposure during early development. Nat. Rev. Endocrinol. 2013, 9, 494–500. [Google Scholar] [CrossRef]
- Walsh, J.M.; McGowan, C.A.; Mahony, R.; Foley, M.E.; McAuliffe, F.M. Low glycaemic index diet in pregnancy to prevent macrosomia (ROLO study): Randomised control trial. BMJ 2012, 345, e5605. [Google Scholar] [CrossRef]
- Godfrey, K.M.; Sheppard, A.; Gluckman, P.D.; Lillycrop, K.A.; Burdge, G.C.; McLean, C.; Rodford, J.; Slater-Jefferies, J.L.; Garratt, E.; Crozier, S.R.; et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes 2011, 60, 1528–1534. [Google Scholar] [CrossRef]
- Bianchi, M.; Alisi, A.; Fabrizi, M.; Vallone, C.; Ravà, L.; Giannico, R.; Vernocchi, P.; Signore, F.; Manco, M. Maternal Intake of n-3 Polyunsaturated Fatty Acids During Pregnancy Is Associated With Differential Methylation Profiles in Cord Blood White Cells. Front. Genet. 2019, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Foster, B.A.; Escaname, E.; Powell, T.L.; Larsen, B.; Siddiqui, S.K.; Menchaca, J.; Aquino, C.; Ramamurthy, R.; Hale, D.E. Randomized Controlled Trial of DHA Supplementation during Pregnancy: Child Adiposity Outcomes. Nutrients 2017, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Ferland-McCollough, D.; Fernandez-Twinn, D.S.; Cannell, I.G.; David, H.; Warner, M.; Vaag, A.A.; Bork-Jensen, J.; Brøns, C.; Gant, T.W.; Willis, A.E.; et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2012, 19, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Elshenawy, S.; Simmons, R. Maternal obesity and prenatal programming. Mol. Cell. Endocrinol. 2016, 435, 2–6. [Google Scholar] [CrossRef]
- Montalvo-Martínez, L.; Maldonado-Ruiz, R.; Cárdenas-Tueme, M.; Reséndez-Pérez, D.; Camacho, A. Maternal Overnutrition Programs Central Inflammation and Addiction-Like Behavior in Offspring. Biomed. Res. Int. 2018, 2018, 8061389. [Google Scholar] [CrossRef]
- Muhlhausler, B.S.; Ong, Z.Y. The fetal origins of obesity: Early origins of altered food intake. Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 189–197. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Gugusheff, J.R.; Muhlhausler, B.S. Perinatal overnutrition and the programming of food preferences: Pathways and mechanisms. J. Dev. Orig. Health Dis. 2012, 3, 299–308. [Google Scholar] [CrossRef]
- Tellechea, M.L.; Mensegue, M.F.; Pirola, C.J. The Association between High Fat Diet around Gestation and Metabolic Syndrome-related Phenotypes in Rats: A Systematic Review and Meta-Analysis. Sci. Rep. 2017, 7, 5086. [Google Scholar] [CrossRef] [PubMed]
- Heerwagen, M.J.R.; Miller, M.R.; Barbour, L.A.; Friedman, J.E. Maternal obesity and fetal metabolic programming: A fertile epigenetic soil. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R711–R722. [Google Scholar] [CrossRef] [PubMed]
- Brombach, C.; Tong, W.; Giussani, D.A. Maternal obesity: New placental paradigms unfolded. Trends Mol. Med. 2022, 28, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Larqué, E.; Labayen, I.; Flodmark, C.-E.; Lissau, I.; Czernin, S.; Moreno, L.A.; Pietrobelli, A.; Widhalm, K. From conception to infancy—Early risk factors for childhood obesity. Nat. Rev. Endocrinol. 2019, 15, 456–478. [Google Scholar] [CrossRef]
- Ronnenberg, A.G.; Wang, X.; Xing, H.; Chen, C.; Chen, D.; Guang, W.; Guang, A.; Wang, L.; Ryan, L.; Xu, X. Low preconception body mass index is associated with birth outcome in a prospective cohort of Chinese women. J. Nutr. 2003, 133, 3449–3455. [Google Scholar] [CrossRef]
- Zamojska, J.; Niewiadomska-Jarosik, K.; Kierzkowska, B.; Gruca, M.; Wosiak, A.; Smolewska, E. Lipid profile in children born small for gestational age. Nutrients 2023, 15, 4781. [Google Scholar] [CrossRef]
- Barker, D.J.P.; Eriksson, J.G.; Forsén, T.; Osmond, C. Fetal origins of adult disease: Strength of effects and biological basis. Int. J. Epidemiol. 2002, 31, 1235–1239. [Google Scholar] [CrossRef]
- Barker, D.J. Fetal origins of coronary heart disease. Br. Heart J. 1993, 69, 195–196. [Google Scholar] [CrossRef]
- Barker, D.J.; Fall, C.H. Fetal and infant origins of cardiovascular disease. Arch. Dis. Child. 1993, 68, 797–799. [Google Scholar] [CrossRef]
- Barker, D.J.P. Fetal origins of cardiovascular disease. Ann. Med. 1999, 31, 3–6. [Google Scholar] [CrossRef]
- Dietz, W.H. Periods of risk in childhood for the development of adult obesity--what do we need to learn? J. Nutr. 1997, 127, 1884S–1886S. [Google Scholar] [CrossRef]
- Kuhle, S.; Tong, O.S.; Woolcott, C.G. Association between caesarean section and childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Liu, J. The impact of cesarean section on offspring overweight and obesity: A systematic review and meta-analysis. Int. J. Obes. 2013, 37, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Słabuszewska-Jóźwiak, A.; Szymański, J.K.; Ciebiera, M.; Sarecka-Hujar, B.; Jakiel, G. Pediatrics Consequences of Caesarean Section-A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8031. [Google Scholar] [CrossRef] [PubMed]
- Chiavarini, M.; De Socio, B.; Giacchetta, I.; Fabiani, R. Overweight and Obesity in Adult Birth by Cesarean Section: A Systematic Review with Meta-analysis. J. Public Health Manag. Pract. 2023, 29, 128–141. [Google Scholar] [CrossRef]
- Mueller, N.T.; Rifas-Shiman, S.L.; Blaser, M.J.; Gillman, M.W.; Hivert, M.F. Association of prenatal antibiotics with foetal size and cord blood leptin and adiponectin. Pediatr. Obes. 2017, 12, 129–136. [Google Scholar] [CrossRef]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Mueller, N.T.; Hourigan, S.K.; Hoffmann, D.E.; Levy, L.; von Rosenvinge, E.C.; Chou, B.; Dominguez-Bello, M.-G. Bacterial Baptism: Scientific, Medical, and Regulatory Issues Raised by Vaginal Seeding of C-Section-Born Babies. J. Law Med. Ethics 2019, 47, 568–578. [Google Scholar] [CrossRef]
- Prior, E.; Santhakumaran, S.; Gale, C.; Philipps, L.H.; Modi, N.; Hyde, M.J. Breastfeeding after cesarean delivery: A systematic review and meta-analysis of world literature. Am. J. Clin. Nutr. 2012, 95, 1113–1135. [Google Scholar] [CrossRef]
- Horta, B.L.; Rollins, N.; Dias, M.S.; Garcez, V.; Pérez-Escamilla, R. Systematic review and meta-analysis of breastfeeding and later overweight or obesity expands on previous study for World Health Organization. Acta Paediatr. 2023, 112, 34–41. [Google Scholar] [CrossRef]
- Słabuszewska-Jóźwiak, A.; Malinowska, M.; Kloska, A.; Jakóbkiewicz-Banecka, J.; Gujski, M.; Bojar, I.; Raczkiewicz, D.; Jakiel, G. Global Changes of 5-mC/5h-mC Ratio and Methylation of Adiponectin and Leptin Gene in Placenta Depending on Mode of Delivery. Int. J. Mol. Sci. 2021, 22, 3195. [Google Scholar] [CrossRef] [PubMed]
- Oken, E.; Levitan, E.B.; Gillman, M.W. Maternal smoking during pregnancy and child overweight: Systematic review and meta-analysis. Int. J. Obes. 2008, 32, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Rayfield, S.; Plugge, E. Systematic review and meta-analysis of the association between maternal smoking in pregnancy and childhood overweight and obesity. J. Epidemiol. Community Health 2017, 71, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Di, H.-K.; Gan, Y.; Lu, K.; Wang, C.; Zhu, Y.; Meng, X.; Xia, W.-Q.; Xu, M.-Z.; Feng, J.; Tian, Q.-F.; et al. Maternal smoking status during pregnancy and low birth weight in offspring: Systematic review and meta-analysis of 55 cohort studies published from 1986 to 2020. World J. Pediatr. 2022, 18, 176–185. [Google Scholar] [CrossRef]
- Iliadou, A.N.; Koupil, I.; Villamor, E.; Altman, D.; Hultman, C.; Långström, N.; Cnattingius, S. Familial factors confound the association between maternal smoking during pregnancy and young adult offspring overweight. Int. J. Epidemiol. 2010, 39, 1193–1202. [Google Scholar] [CrossRef]
- Riedel, C.; Schönberger, K.; Yang, S.; Koshy, G.; Chen, Y.-C.; Gopinath, B.; Ziebarth, S.; von Kries, R. Parental smoking and childhood obesity: Higher effect estimates for maternal smoking in pregnancy compared with paternal smoking—A meta-analysis. Int. J. Epidemiol. 2014, 43, 1593–1606. [Google Scholar] [CrossRef]
- Chen, W.-J.A.; Kelly, R.B. Effect of prenatal or perinatal nicotine exposure on neonatal thyroid status and offspring growth in rats. Life Sci. 2005, 76, 1249–1258. [Google Scholar] [CrossRef]
- Holloway, A.C.; Lim, G.E.; Petrik, J.J.; Foster, W.G.; Morrison, K.M.; Gerstein, H.C. Fetal and neonatal exposure to nicotine in Wistar rats results in increased beta cell apoptosis at birth and postnatal endocrine and metabolic changes associated with type 2 diabetes. Diabetologia 2005, 48, 2661–2666. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Holloway, A.C.; Zeng, Z.; Lim, G.E.; Petrik, J.J.; Foster, W.G.; Lee, R.M.K.W. Prenatal exposure to nicotine causes postnatal obesity and altered perivascular adipose tissue function. Obes. Res. 2005, 13, 687–692. [Google Scholar] [CrossRef]
- Nielsen, C.H.; Larsen, A.; Nielsen, A.L. DNA methylation alterations in response to prenatal exposure of maternal cigarette smoking: A persistent epigenetic impact on health from maternal lifestyle? Arch. Toxicol. 2016, 90, 231–245. [Google Scholar] [CrossRef]
- Braun, J.M. Early-life exposure to EDCs: Role in childhood obesity and neurodevelopment. Nat. Rev. Endocrinol. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Street, M.E.; Bernasconi, S. Endocrine-Disrupting Chemicals in Human Fetal Growth. Int. J. Mol. Sci. 2020, 21, 1430. [Google Scholar] [CrossRef]
- Heindel, J.J.; Newbold, R.; Schug, T.T. Endocrine disruptors and obesity. Nat. Rev. Endocrinol. 2015, 11, 653–661. [Google Scholar] [CrossRef]
- Núñez-Sánchez, M.Á.; Jiménez-Méndez, A.; Suárez-Cortés, M.; Martínez-Sánchez, M.A.; Sánchez-Solís, M.; Blanco-Carnero, J.E.; Ruiz-Alcaraz, A.J.; Ramos-Molina, B. Inherited Epigenetic Hallmarks of Childhood Obesity Derived from Prenatal Exposure to Obesogens. Int. J. Environ. Res. Public Health 2023, 20, 4711. [Google Scholar] [CrossRef] [PubMed]
- Predieri, B.; Iughetti, L.; Bernasconi, S.; Street, M.E. Endocrine disrupting chemicals’ effects in children: What we know and what we need to learn? Int. J. Mol. Sci. 2022, 23, 11899. [Google Scholar] [CrossRef] [PubMed]
- Hoepner, L.A. Bisphenol a: A narrative review of prenatal exposure effects on adipogenesis and childhood obesity via peroxisome proliferator-activated receptor gamma. Environ. Res. 2019, 173, 54–68. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Kounatidis, D.; Tsilingiris, D.; Vallianou, N.G.; Karampela, I.; Psallida, S.; Papavassiliou, A.G. The role of endocrine disruptors bisphenols and phthalates in obesity: Current evidence, perspectives and controversies. Int. J. Mol. Sci. 2024, 25, 675. [Google Scholar] [CrossRef]
- Hao, C.; Cheng, X.; Xia, H.; Ma, X. The endocrine disruptor mono-(2-ethylhexyl) phthalate promotes adipocyte differentiation and induces obesity in mice. Biosci. Rep. 2012, 32, 619–629. [Google Scholar] [CrossRef]
- Jerman, I.; Ogrizek, L.; Krapež, V.P.; Jan, L. Molecular Signal Transfer of Highly Diluted Antibodies to Interferon-Gamma Regarding Kind, Time, and Distance of Exposition. Int. J. Mol. Sci. 2024, 25, 656. [Google Scholar] [CrossRef]
- Simkova, S.; Veleminsky, M.; Sram, R.J. The impact of air pollution to obesity. Neuro Endocrinol. Lett. 2020, 41, 146–153. [Google Scholar]
- Bloemsma, L.D.; Dabelea, D.; Thomas, D.S.K.; Peel, J.L.; Adgate, J.L.; Allshouse, W.B.; Martenies, S.E.; Magzamen, S.; Starling, A.P. Prenatal exposure to ambient air pollution and traffic and indicators of adiposity in early childhood: The Healthy Start study. Int. J. Obes. 2022, 46, 494–501. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, W.; Chen, M.; Zhou, J.; Huang, X.; Tao, S.; Pan, B.; Li, Z.; Xie, X.; Li, W.; et al. Developmental programming of obesity by maternal exposure to concentrated ambient PM2.5 is maternally transmitted into the third generation in a mouse model. Part. Fibre Toxicol. 2019, 16, 27. [Google Scholar] [CrossRef]
- Street, M.E.; Bernasconi, S. Microplastics, environment and child health. Ital. J. Pediatr. 2021, 47, 75. [Google Scholar] [CrossRef] [PubMed]
- Durkin, A.M.; Zou, R.; Boucher, J.M.; Boyles, M.S.; van Boxel, J.; Bustamante, M.; Christopher, E.A.; Dadvand, P.; Dusza, H.M.; van Duursen, M.; et al. Investigating Exposure and Hazards of Micro- and Nanoplastics During Pregnancy and Early Life (AURORA Project): Protocol for an Interdisciplinary Study. JMIR Res. Protoc. 2024, 13, e63176. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Han, R.; Yao, Z.; Yan, H.; Liu, Z.; Liu, X.; Yue, T.; Zhao, J.; Wang, Z.; Xing, B. Intergenerational transfer of micro(nano)plastics in different organisms. J. Hazard. Mater. 2025, 488, 137404. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, L.-G.; Wu, X.-F.; Zhao, Z.-G.; Lu, Y.; Sun, H.-M. Impact of micro-nano plastics in daily life on human health: Toxicological evaluation from the perspective of normal tissue cells and organoids. Toxicol. Res. 2024, 13, tfae205. [Google Scholar] [CrossRef]
- Entringer, S.; Buss, C.; Wadhwa, P.D. Prenatal stress and developmental programming of human health and disease risk: Concepts and integration of empirical findings. Curr. Opin. Endocrinol. Diabetes Obes. 2010, 17, 507–516. [Google Scholar] [CrossRef]
- Li, J.; Olsen, J.; Vestergaard, M.; Obel, C.; Baker, J.L.; Sørensen, T.I.A. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS ONE 2010, 5, e11896. [Google Scholar] [CrossRef]
- Stout, S.A.; Espel, E.V.; Sandman, C.A.; Glynn, L.M.; Davis, E.P. Fetal programming of children’s obesity risk. Psychoneuroendocrinology 2015, 53, 29–39. [Google Scholar] [CrossRef]
- Street, M.E.; Smerieri, A.; Petraroli, A.; Cesari, S.; Viani, I.; Garrubba, M.; Rossi, M.; Bernasconi, S. Placental cortisol and cord serum IGFBP-2 concentrations are important determinants of postnatal weight gain. J. Biol. Regul. Homeost. Agents 2012, 26, 721–731. [Google Scholar]
- Tate, E.B.; Wood, W.; Liao, Y.; Dunton, G.F. Do stressed mothers have heavier children? A meta-analysis on the relationship between maternal stress and child body mass index. Obes. Rev. 2015, 16, 351–361. [Google Scholar] [CrossRef]
- Darendeliler, F. IUGR: Genetic influences, metabolic problems, environmental associations/triggers, current and future management. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101260. [Google Scholar] [CrossRef]
- Arya, S.; Uzoma, A.; Robinson, A.; Moreira, A.G.; Jain, S.K. Impact of intrauterine growth restriction and placental insufficiency on nutritional outcomes of extremely low birth weight infants. Cureus 2022, 14, e31611. [Google Scholar] [CrossRef]
- D’Agostin, M.; Di Sipio Morgia, C.; Vento, G.; Nobile, S. Long-term implications of fetal growth restriction. World J. Clin. Cases 2023, 11, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Mericq, V.; Martinez-Aguayo, A.; Uauy, R.; Iñiguez, G.; Van der Steen, M.; Hokken-Koelega, A. Long-term metabolic risk among children born premature or small for gestational age. Nat. Rev. Endocrinol. 2017, 13, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, K.; Devaskar, S.U. Intrauterine growth restriction: Postnatal monitoring and outcomes. Pediatr. Clin. N. Am. 2019, 66, 403–423. [Google Scholar] [CrossRef] [PubMed]
- von Beckerath, A.-K.; Kollmann, M.; Rotky-Fast, C.; Karpf, E.; Lang, U.; Klaritsch, P. Perinatal complications and long-term neurodevelopmental outcome of infants with intrauterine growth restriction. Am. J. Obstet. Gynecol. 2013, 208, 130.e1–130.e6. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Gumina, D.L.; Su, E.J. Mechanistic insights into the development of severe fetal growth restriction. Clin. Sci. 2023, 137, 679–695. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, C.; Wang, P.; Yang, W.; Zhu, H.; Zhang, S. Regulators involved in trophoblast syncytialization in the placenta of intrauterine growth restriction. Front. Endocrinol. 2023, 14, 1107182. [Google Scholar] [CrossRef]
- Nardozza, L.M.M.; Caetano, A.C.R.; Zamarian, A.C.P.; Mazzola, J.B.; Silva, C.P.; Marçal, V.M.G.; Lobo, T.F.; Peixoto, A.B.; Araujo Júnior, E. Fetal growth restriction: Current knowledge. Arch. Gynecol. Obstet. 2017, 295, 1061–1077. [Google Scholar] [CrossRef]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Highlighting the trajectory from intrauterine growth restriction to future obesity. Front. Endocrinol. 2022, 13, 1041718. [Google Scholar] [CrossRef] [PubMed]
- Jee, Y.H.; Jumani, S.; Mericq, V. The association of accelerated early growth, timing of puberty, and metabolic consequences in children. J. Clin. Endocrinol. Metab. 2023, 108, e663–e670. [Google Scholar] [CrossRef] [PubMed]
- Wesolowski, S.R.; Hay, W.W. Role of placental insufficiency and intrauterine growth restriction on the activation of fetal hepatic glucose production. Mol. Cell. Endocrinol. 2016, 435, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Nordman, H.; Jääskeläinen, J.; Voutilainen, R. Birth size as a determinant of cardiometabolic risk factors in children. Horm. Res. Paediatr. 2020, 93, 144–153. [Google Scholar] [CrossRef]
- Maguolo, A.; Olivieri, F.; Zusi, C.; Miraglia Del Giudice, E.; Morandi, A.; Maffeis, C. The risk of metabolic derangements is higher in children and adolescents with overweight or obesity born small for gestational age. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1903–1910. [Google Scholar] [CrossRef]
- Varvarigou, A.A. Intrauterine growth restriction as a potential risk factor for disease onset in adulthood. J. Pediatr. Endocrinol. Metab. 2010, 23, 215–224. [Google Scholar] [CrossRef]
- Eriksson, J.G. Early growth and coronary heart disease in later life: Longitudinal study. BMJ 2001, 322, 949–953. [Google Scholar] [CrossRef]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Panera, N.; Agostoni, C. Low birth weight and catch-up-growth associated with metabolic syndrome: A ten year systematic review. Pediatr. Endocrinol. Rev. 2008, 6, 241–247. [Google Scholar]
- Leunissen, R.W.J.; Kerkhof, G.F.; Stijnen, T.; Hokken-Koelega, A.C.S. Effect of birth size and catch-up growth on adult blood pressure and carotid intima-media thickness. Horm. Res. Paediatr. 2012, 77, 394–401. [Google Scholar] [CrossRef]
- Leunissen, R.W.J.; Kerkhof, G.F.; Stijnen, T.; Hokken-Koelega, A. Timing and tempo of first-year rapid growth in relation to cardiovascular and metabolic risk profile in early adulthood. JAMA 2009, 301, 2234–2242. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Urquidi, C.; Pittaluga, E.; Iñiguez, G.; Avila, A.; Carrasco, F.; Mericq, V. Differences in body composition and resting energy expenditure in childhood in preterm children born with very low birth weight. Horm. Res. Paediatr. 2013, 79, 347–355. [Google Scholar] [CrossRef]
- Calek, E.; Binder, J.; Palmrich, P.; Eibensteiner, F.; Thajer, A.; Kainz, T.; Harreiter, K.; Berger, A.; Binder, C. Effects of intrauterine growth restriction (IUGR) on growth and body composition compared to constitutionally small infants. Nutrients 2023, 15, 4158. [Google Scholar] [CrossRef] [PubMed]
- Manapurath, R.; Gadapani, B.; Pereira-da-Silva, L. Body Composition of Infants Born with Intrauterine Growth Restriction: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1085. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, Z.; Wang, Y.; Yu, X.; Xin, Q.; Chen, Y. Effects of rapid growth on fasting insulin and insulin resistance: A system review and meta-analysis. Eur. J. Clin. Nutr. 2021, 75, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; García-Beltran, C.; Pie, S.; Guerra, A.; López-Bermejo, A.; de Toledo, J.S.; de Zegher, F.; Rosés, F.; Ibáñez, L. The sequence of prenatal growth restraint and postnatal catch-up growth: Normal heart but thicker intima-media and more pre-peritoneal fat in late infancy. Pediatr. Obes. 2019, 14, e12476. [Google Scholar] [CrossRef]
- Vohr, B.R.; Allan, W.; Katz, K.H.; Schneider, K.C.; Ment, L.R. Early predictors of hypertension in prematurely born adolescents. Acta Paediatr. 2010, 99, 1812–1818. [Google Scholar] [CrossRef]
- Uthaya, S.; Thomas, E.L.; Hamilton, G.; Doré, C.J.; Bell, J.; Modi, N. Altered adiposity after extremely preterm birth. Pediatr. Res. 2005, 57, 211–215. [Google Scholar] [CrossRef]
- Giannì, M.L.; Roggero, P.; Liotto, N.; Amato, O.; Piemontese, P.; Morniroli, D.; Bracco, B.; Mosca, F. Postnatal catch-up fat after late preterm birth. Pediatr. Res. 2012, 72, 637–640. [Google Scholar] [CrossRef]
- Okada, T.; Takahashi, S.; Nagano, N.; Yoshikawa, K.; Usukura, Y.; Hosono, S. Early postnatal alteration of body composition in preterm and small-for-gestational-age infants: Implications of catch-up fat. Pediatr. Res. 2015, 77, 136–142. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Gorman, S.; Watson, L.P.E.; Dunger, D.B.; Beardsall, K. Catch-Up Growth in Children Born Small for Gestational Age Related to Body Composition and Metabolic Risk at Six Years of Age in the UK. Horm. Res. Paediatr. 2020, 93, 119–127. [Google Scholar] [CrossRef]
- Iñiguez, G.; Ong, K.; Peña, V.; Avila, A.; Dunger, D.; Mericq, V. Fasting and post-glucose ghrelin levels in SGA infants: Relationships with size and weight gain at one year of age. J. Clin. Endocrinol. Metab. 2002, 87, 5830–5833. [Google Scholar] [CrossRef][Green Version]
- Ikenasio-Thorpe, B.A.; Breier, B.H.; Vickers, M.H.; Fraser, M. Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J. Endocrinol. 2007, 193, 31–37. [Google Scholar] [CrossRef]
- Schultz, N.S.; Broholm, C.; Gillberg, L.; Mortensen, B.; Jørgensen, S.W.; Schultz, H.S.; Scheele, C.; Wojtaszewski, J.F.P.; Pedersen, B.K.; Vaag, A. Impaired leptin gene expression and release in cultured preadipocytes isolated from individuals born with low birth weight. Diabetes 2014, 63, 111–121. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T.; Low, F.M.; Beedle, A.S. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat. Rev. Endocrinol. 2009, 5, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, Z.; Lin, M.; Zhu, L.; Xu, L.; Wang, X. Deciphering the epigenetic landscape: Placental development and its role in pregnancy outcomes. Stem Cell Rev. Rep. 2024, 20, 996–1014. [Google Scholar] [CrossRef] [PubMed]
- Joseph, R.; Poschmann, J.; Sukarieh, R.; Too, P.G.; Julien, S.G.; Xu, F.; Teh, A.L.; Holbrook, J.D.; Ng, K.L.; Chong, Y.S.; et al. ACSL1 is associated with fetal programming of insulin sensitivity and cellular lipid content. Mol. Endocrinol. 2015, 29, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Crump, C. An overview of adult health outcomes after preterm birth. Early Hum. Dev. 2020, 150, 105187. [Google Scholar] [CrossRef]
- Eichner-Seitz, N.; Pate, R.R.; Paul, I.M. Physical activity in infancy and early childhood: A narrative review of interventions for prevention of obesity and associated health outcomes. Front. Endocrinol. 2023, 14, 1155925. [Google Scholar] [CrossRef]
- Woo, J.G.; Martin, L.J. Does breastfeeding protect against childhood obesity? moving beyond observational evidence. Curr. Obes. Rep. 2015, 4, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.C.; Stewart, C.J. Role of breastfeeding in disease prevention. Microb. Biotechnol. 2024, 17, e14520. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garay, A.G.; Serralde-Zúñiga, A.E.; Medina Vera, I.; Velasco Hidalgo, L.; Alonso Ocaña, M.V. Higher versus lower protein intake in formula-fed term infants. Cochrane Database Syst. Rev. 2023, 11, CD013758. [Google Scholar] [CrossRef] [PubMed]
- Scorecard, G.B. Increasing Commitment to Breastfeeding Through Funding and Improved Policies and Programmes; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Qiao, J.; Dai, L.-J.; Zhang, Q.; Ouyang, Y.-Q. A Meta-Analysis of the Association Between Breastfeeding and Early Childhood Obesity. J. Pediatr. Nurs. 2020, 53, 57–66. [Google Scholar] [CrossRef]
- Yan, J.; Liu, L.; Zhu, Y.; Huang, G.; Wang, P.P. The association between breastfeeding and childhood obesity: A meta-analysis. BMC Public Health 2014, 14, 1267. [Google Scholar] [CrossRef]
- Brion, M.-J.A.; Lawlor, D.A.; Matijasevich, A.; Horta, B.; Anselmi, L.; Araújo, C.L.; Menezes, A.M.B.; Victora, C.G.; Smith, G.D. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int. J. Epidemiol. 2011, 40, 670–680. [Google Scholar] [CrossRef]
- Zheng, M.; D’Souza, N.J.; Atkins, L.; Ghobadi, S.; Laws, R.; Szymlek-Gay, E.A.; Grimes, C.; Baker, P.; He, Q.-Q.; Campbell, K.J. Breastfeeding and the longitudinal changes of body mass index in childhood and adulthood: A systematic review. Adv. Nutr. 2024, 15, 100152. [Google Scholar] [CrossRef]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Cuppari, C.; Salpietro, V.; Filippelli, M.; Trovato, A.; Gitto, E.; Salpietro, C.; Arrigo, T. Obesity and breastfeeding: The strength of association. Women Birth 2015, 28, 81–86. [Google Scholar] [CrossRef]
- Savino, F.; Costamagna, M.; Prino, A.; Oggero, R.; Silvestro, L. Leptin levels in breast-fed and formula-fed infants. Acta Paediatr. 2002, 91, 897–902. [Google Scholar] [CrossRef]
- Sinkiewicz-Darol, E.; Adamczyk, I.; Łubiech, K.; Pilarska, G.; Twarużek, M. Leptin in Human Milk-One of the Key Regulators of Nutritional Programming. Molecules 2022, 27, 3581. [Google Scholar] [CrossRef]
- Briollais, L.; Rustand, D.; Allard, C.; Wu, Y.; Xu, J.; Rajan, S.G.; Hivert, M.-F.; Doyon, M.; Bouchard, L.; McGowan, P.O.; et al. DNA methylation mediates the association between breastfeeding and early-life growth trajectories. Clin. Epigenetics 2021, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Weiskirchen, R.; Stremmel, W.; John, S.M.; Schmitz, G. Risk of Fat Mass- and Obesity-Associated Gene-Dependent Obesogenic Programming by Formula Feeding Compared to Breastfeeding. Nutrients 2024, 16, 2451. [Google Scholar] [CrossRef] [PubMed]
- Doñate Carramiñana, L.; Guillén Sebastián, C.; Iglesia Altaba, I.; Nagore Gonzalez, C.; Alvarez Sauras, M.L.; García Enguita, S.; Rodriguez Martinez, G. Rapid Growth between 0 and 2 Years Old in Healthy Infants Born at Term and Its Relationship with Later Obesity: A Systematic Review and Meta-Analysis of Evidence. Nutrients 2024, 16, 2939. [Google Scholar] [CrossRef] [PubMed]
- Patro-Gołąb, B.; Zalewski, B.M.; Kołodziej, M.; Kouwenhoven, S.; Poston, L.; Godfrey, K.M.; Koletzko, B.; van Goudoever, J.B.; Szajewska, H. Nutritional interventions or exposures in infants and children aged up to 3 years and their effects on subsequent risk of overweight, obesity and body fat: A systematic review of systematic reviews. Obes. Rev. 2016, 17, 1245–1257. [Google Scholar] [CrossRef]
- Kouwenhoven, S.M.P.; Muts, J.; Finken, M.J.J.; Goudoever, J.B. van Low-Protein Infant Formula and Obesity Risk. Nutrients 2022, 14, 2728. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Scientific Opinion on the safety and suitability for use by infants of follow-on formulae with a protein content of at least 1.6 g/100 kcal. EFSA J. 2017, 15, e04781. [Google Scholar] [CrossRef]
- Nuti, F.; Fernández, F.R.; Severi, M.; Traversi, R.; Fanos, V.; Street, M.E.; Palanza, P.; Rovero, P.; Papini, A.M. Study of Endocrine-Disrupting Chemicals in Infant Formulas and Baby Bottles: Data from the European LIFE-MILCH PROJECT. Molecules 2024, 29, 5434. [Google Scholar] [CrossRef]
- Street, M.E.; Shulhai, A.-M.; Rotondo, R.; Giannì, G.; Caffarelli, C. Current knowledge on the effects of environmental contaminants in early life nutrition. Front. Nutr. 2023, 10, 1120293. [Google Scholar] [CrossRef]
- Mameli, C.; Mazzantini, S.; Zuccotti, G.V. Nutrition in the first 1000 days: The origin of childhood obesity. Int. J. Environ. Res. Public Health 2016, 13, 838. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Y.; Xiong, G.; Chao, T.; Jin, Q.; Liu, R.; Hao, L.; Wei, S.; Yang, N.; Yang, X. Introduction of complementary feeding before 4months of age increases the risk of childhood overweight or obesity: A meta-analysis of prospective cohort studies. Nutr. Res. 2016, 36, 759–770. [Google Scholar] [CrossRef]
- Clayton, P.K.; Putnick, D.L.; Trees, I.R.; Ghassabian, A.; Tyris, J.N.; Lin, T.-C.; Yeung, E.H. Early Infant Feeding Practices and Associations with Growth in Childhood. Nutrients 2024, 16, 714. [Google Scholar] [CrossRef] [PubMed]
- Kittisakmontri, K.; Fewtrell, M. Impact of complementary feeding on obesity risk. Curr. Opin. Clin. Nutr. Metab. Care 2023, 26, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Das, J.K.; Salam, R.A.; Hadi, Y.B.; Sadiq Sheikh, S.; Bhutta, A.Z.; Weise Prinzo, Z.; Bhutta, Z.A. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst. Rev. 2019, 5, CD012611. [Google Scholar] [CrossRef] [PubMed]
- Arnesen, E.K.; Thorisdottir, B.; Lamberg-Allardt, C.; Bärebring, L.; Nwaru, B.; Dierkes, J.; Ramel, A.; Åkesson, A. Protein intake in children and growth and risk of overweight or obesity: A systematic review and meta-analysis. Food Nutr. Res. 2022, 66. [Google Scholar] [CrossRef]
- Bergamini, M.; Simeone, G.; Verga, M.C.; Doria, M.; Cuomo, B.; D’Antonio, G.; Dello Iacono, I.; Di Mauro, G.; Leonardi, L.; Miniello, V.L.; et al. Complementary Feeding Caregivers’ Practices and Growth, Risk of Overweight/Obesity, and Other Non-Communicable Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2646. [Google Scholar] [CrossRef]
- Hildebrand, M.; Øglund, G.P.; Wells, J.C.; Ekelund, U. Prenatal, birth and early life predictors of sedentary behavior in young people: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 63. [Google Scholar] [CrossRef][Green Version]
- Reilly, J.J.; Hughes, A.R.; Gillespie, J.; Malden, S.; Martin, A. Physical activity interventions in early life aimed at reducing later risk of obesity and related non-communicable diseases: A rapid review of systematic reviews. Obes. Rev. 2019, 20 (Suppl. S1), 61–73. [Google Scholar] [CrossRef]
- Wyszyńska, J.; Ring-Dimitriou, S.; Thivel, D.; Weghuber, D.; Hadjipanayis, A.; Grossman, Z.; Ross-Russell, R.; Dereń, K.; Mazur, A. Physical activity in the prevention of childhood obesity: The position of the european childhood obesity group and the european academy of pediatrics. Front. Pediatr. 2020, 8, 535705. [Google Scholar] [CrossRef]
- Poitras, V.J.; Gray, C.E.; Janssen, X.; Aubert, S.; Carson, V.; Faulkner, G.; Goldfield, G.S.; Reilly, J.J.; Sampson, M.; Tremblay, M.S. Systematic review of the relationships between sedentary behaviour and health indicators in the early years (0-4 years). BMC Public Health 2017, 17, 868. [Google Scholar] [CrossRef]
- Hewitt, L.; Kerr, E.; Stanley, R.M.; Okely, A.D. Tummy time and infant health outcomes: A systematic review. Pediatrics 2020, 145, e20192168. [Google Scholar] [CrossRef]
- Swider-Cios, E.; Vermeij, A.; Sitskoorn, M.M. Young children and screen-based media: The impact on cognitive and socioemotional development and the importance of parental mediation. Cogn. Dev. 2023, 66, 101319. [Google Scholar] [CrossRef]
- Guram, S.; Heinz, P. Media use in children: American Academy of Pediatrics recommendations 2016. Arch. Dis. Child. Educ. Pract. Ed. 2018, 103, 99–101. [Google Scholar] [CrossRef]
- Lobelo, F.; Muth, N.D.; Hanson, S.; Nemeth, B.A. Council on sports medicine and fitness; section on obesity physical activity assessment and counseling in pediatric clinical settings. Pediatrics 2020, 145, e20193992. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Risica, P.M.; Tovar, A.; Stowers, K.C.; Schwartz, M.B.; Lombardi, C.; Alhassan, S.; Gans, K.M. Effect of applying best practices for physical activity and screen time to family childcare homes. Prev. Chronic Dis. 2023, 20, E60. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.L.; Del Pozo Cruz, B.; Sanders, T.; Zheng, M.; Hnatiuk, J.A.; Salmon, J.; Hesketh, K.D. Outdoor time, screen time and sleep reported across early childhood: Concurrent trajectories and maternal predictors. Int. J. Behav. Nutr. Phys. Act. 2022, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Matarma, T.; Koski, P.; Löyttyniemi, E.; Lagström, H. The factors associated with toddlers’ screen time change in the STEPS Study: A two-year follow-up. Prev. Med. 2016, 84, 27–33. [Google Scholar] [CrossRef]
- Pampel, F.C.; Denney, J.T.; Krueger, P.M. Obesity, SES, and economic development: A test of the reversal hypothesis. Soc. Sci. Med. 2012, 74, 1073–1081. [Google Scholar] [CrossRef]
- Anekwe, C.V.; Jarrell, A.R.; Townsend, M.J.; Gaudier, G.I.; Hiserodt, J.M.; Stanford, F.C. Socioeconomics of Obesity. Curr. Obes. Rep. 2020, 9, 272–279. [Google Scholar] [CrossRef]
- Vilar-Compte, M.; Burrola-Méndez, S.; Lozano-Marrufo, A.; Ferré-Eguiluz, I.; Flores, D.; Gaitán-Rossi, P.; Teruel, G.; Pérez-Escamilla, R. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: A global systematic literature review. Int. J. Equity Health 2021, 20, 40. [Google Scholar] [CrossRef]
- Gato-Moreno, M.; Martos-Lirio, M.F.; Leiva-Gea, I.; Bernal-López, M.R.; Vegas-Toro, F.; Fernández-Tenreiro, M.C.; López-Siguero, J.P. Early nutritional education in the prevention of childhood obesity. Int. J. Environ. Res. Public Health 2021, 18, 6569. [Google Scholar] [CrossRef]
- Anzman, S.L.; Rollins, B.Y.; Birch, L.L. Parental influence on children’s early eating environments and obesity risk: Implications for prevention. Int. J. Obes. 2010, 34, 1116–1124. [Google Scholar] [CrossRef]
- Holt, N.L.; Cunningham, C.-T.; Sehn, Z.L.; Spence, J.C.; Newton, A.S.; Ball, G.D.C. Neighborhood physical activity opportunities for inner-city children and youth. Health Place 2009, 15, 1022–1028. [Google Scholar] [CrossRef]
- Fasano, A.; Chassaing, B.; Haller, D.; Flores Ventura, E.; Carmen-Collado, M.; Pastor, N.; Koren, O.; Berni Canani, R. Microbiota during pregnancy and early life: Role in maternal-neonatal outcomes based on human evidence. Gut Microbes 2024, 16, 2392009. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Wagner, B.D.; Iszatt, N.; Dahl, C.; Sontag, M.K.; Knight, R.; Lozupone, C.A.; Eggesbø, M. Gut Microbiota in the First 2 Years of Life and the Association with Body Mass Index at Age 12 in a Norwegian Birth Cohort. mBio 2018, 23, e01751-18. [Google Scholar] [CrossRef]
- Gore, A.C.; Merrill, M.L.; Patisaul, H. Endocrine Disrupting Chemicals: Threats to Human Health. The Endocrine Society and IPEN. February 2024. Available online: https://ipen.org/sites/default/files/documents/edc_report-2024-final-compressed.pdf (accessed on 7 May 2025).
- Bridgeman, S.C.; Northrop, W.; Melton, P.E.; Ellison, G.C.; Newsholme, P.; Mamotte, C.D.S. Butyrate generated by gut microbiota and its therapeutic role in metabolic syndrome. Pharmacol. Res. 2020, 16, 105174. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef] [PubMed]
- Perez-Muñoz, M.E.; Arrieta, M.C.; Ramer-Tait, A.E.; Walter, J. A critical assessment of the “sterile womb” and “in utero colonization” hypotheses: Implications for research on the pioneer infant microbiome. Microbiome 2017, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Banchi, P.; Colitti, B.; Opsomer, G.; Rota, A.; Van Soom, A. The dogma of the sterile uterus revisited: Does microbial seeding occur during fetal life in humans and animals? Reproduction 2023, 167, e230078. [Google Scholar] [CrossRef]
- Catassi, G.; Aloi, M.; Giorgio, V.; Gasbarrini, A.; Cammarota, G.; Ianiro, G. The Role of Diet and Nutritional Interventions for the Infant Gut Microbiome. Nutrients 2024, 6, 400. [Google Scholar] [CrossRef]
- Zhou, M.; Hu, L.; Li, F.; Wen, J.; Liang, Z.; Chen, D. Beneficial effects of short-term breastfeeding versus non-breastfeeding in early life against childhood obesity: Findings from the US-based population study NHANES. Int. Breastfeed. J. 2024, 19, 56. [Google Scholar] [CrossRef]
- Chen, L.-W.; Xu, J.; Soh, S.E.; Aris, I.M.; Tint, M.-T.; Gluckman, P.D.; Tan, K.H.; Shek, L.P.-C.; Chong, Y.-S.; Yap, F.; et al. Implication of gut microbiota in the association between infant antibiotic exposure and childhood obesity and adiposity accumulation. Int. J. Obes. 2020, 44, 1508–1520. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Ding, X.; Wang, B.; Li, L.; An, X.; Yao, Q.; Song, R.; Zhang, J.-A. Antibiotic Exposure in Early Life Increases Risk of Childhood Obesity: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2017, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Aversa, Z.; Atkinson, E.J.; Schafer, M.J.; Theiler, R.N.; Rocca, W.A.; Blaser, M.J.; LeBrasseur, N.K. Association of infant antibiotic exposure with childhood health outcomes. Mayo Clin. Proc. 2021, 96, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Di Pietro, G.; Forcucci, F.; Chiarelli, F. Endocrine disruptor chemicals and children’s health. Int. J. Mol. Sci. 2023, 24, 2671. [Google Scholar] [CrossRef]
- Calcaterra, V.; Cena, H.; Loperfido, F.; Rossi, V.; Grazi, R.; Quatrale, A.; De Giuseppe, R.; Manuelli, M.; Zuccotti, G. Evaluating Phthalates and Bisphenol in Foods: Risks for Precocious Puberty and Early-Onset Obesity. Nutrients 2024, 16, 2732. [Google Scholar] [CrossRef]
- I Who Report of the Commission on Ending Childhood Obesity. Available online: https://www.who.int/publications/i/item/9789241510066 (accessed on 1 May 2025).
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef]
- Gillman, M.W.; Ludwig, D.S. How early should obesity prevention start? N. Engl. J. Med. 2013, 369, 2173–2175. [Google Scholar] [CrossRef]
- Weng, S.F.; Redsell, S.A.; Swift, J.A.; Yang, M.; Glazebrook, C.P. Systematic review and meta-analyses of risk factors for childhood overweight identifiable during infancy. Arch. Dis. Child. 2012, 97, 1019–1026. [Google Scholar] [CrossRef]
- Gaillard, R. Maternal obesity during pregnancy and cardiovascular development and disease in the offspring. Eur. J. Epidemiol. 2015, 30, 1141–1152. [Google Scholar] [CrossRef]
- Jacob, C.M.; Newell, M.-L.; Hanson, M. Narrative review of reviews of preconception interventions to prevent an increased risk of obesity and non-communicable diseases in children. Obes. Rev. 2019, 20 (Suppl. S1), 5–17. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26 (Suppl. S1), 285–301. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Dudás, I.; Métneki, J. Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Final report. Arch. Gynecol. Obstet. 1994, 255, 131–139. [Google Scholar] [CrossRef]
- Steel, A.; Lucke, J.; Adams, J. The prevalence and nature of the use of preconception services by women with chronic health conditions: An integrative review. BMC Womens Health 2015, 15, 14. [Google Scholar] [CrossRef]
- Agha, M.; Agha, R.A.; Sandall, J. Interventions to reduce and prevent obesity in pre-conceptual and pregnant women: A systematic review and meta-analysis. PLoS ONE 2014, 9, e95132. [Google Scholar] [CrossRef]
- Grobler, L.; Visser, M.; Siegfried, N. Healthy Life Trajectories Initiative: Summary of the evidence base for pregnancy-related interventions to prevent overweight and obesity in children. Obes. Rev. 2019, 20 (Suppl. S1), 18–30. [Google Scholar] [CrossRef] [PubMed]
- Horne, B.; Kabir, E.; Alam, K. Impact of prenatal maternal dietary exclusion on childhood obesity and overweight risk. PLoS ONE 2024, 19, e0297614. [Google Scholar] [CrossRef] [PubMed]
- Kusinski, L.C.; Jones, D.; Atta, N.; Turner, E.; Smith, S.; Oude Griep, L.M.; Rennie, K.; De Lucia Rolfe, E.; Sharp, S.J.; Farewell, V.; et al. Reduced-energy diet in women with gestational diabetes: The dietary intervention in gestational diabetes DiGest randomized clinical trial. Nat. Med. 2025, 31, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Venditti, N.; Merolla, F.; Fusco, S.; Guerra, G.; Zoroddu, S.; De Luca, A.; Bagella, L. Nutraceuticals in pregnancy: A special focus on probiotics. Int. J. Mol. Sci. 2024, 25, 9688. [Google Scholar] [CrossRef]
- Krauss-Silva, L.; Moreira, M.E.L.; Alves, M.B.; Braga, A.; Camacho, K.G.; Batista, M.R.R.; Almada-Horta, A.; Rebello, M.R.; Guerra, F. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: Preliminary results. Trials 2011, 12, 239. [Google Scholar] [CrossRef]
- Pellonperä, O.; Mokkala, K.; Houttu, N.; Vahlberg, T.; Koivuniemi, E.; Tertti, K.; Rönnemaa, T.; Laitinen, K. Efficacy of Fish Oil and/or Probiotic Intervention on the Incidence of Gestational Diabetes Mellitus in an At-Risk Group of Overweight and Obese Women: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabetes Care 2019, 42, 1009–1017. [Google Scholar] [CrossRef]
- Shahriari, A.; Karimi, E.; Shahriari, M.; Aslani, N.; Khooshideh, M.; Arab, A. The effect of probiotic supplementation on the risk of gestational diabetes mellitus among high-risk pregnant women: A parallel double-blind, randomized, placebo-controlled clinical trial. Biomed. Pharmacother. 2021, 141, 111915. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Kostiuk, L.L.; Cuthbert, A.; Weeks, J. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2024, 7, CD008873. [Google Scholar] [CrossRef]
- Sheyholislami, H.; Connor, K.L. Are Probiotics and Prebiotics Safe for Use during Pregnancy and Lactation? A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2382. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sheng, J.; Zhang, D.; Chen, L.; Jiang, Y.; Cheng, D.; Su, Y.; Yu, Y.; Jia, H.; He, P.; et al. The role of dietary fiber on preventing gestational diabetes mellitus in an at-risk group of high triglyceride-glucose index women: A randomized controlled trial. Endocrine 2023, 82, 542–549. [Google Scholar] [CrossRef]
- Hull, H.R.; Brown, A.; Gajewski, B.; Sullivan, D.K.; Carlson, S.E. The effect of prenatal docosahexaenoic acid supplementation on offspring fat mass and distribution at 24 months old. Curr. Dev. Nutr. 2024, 8, 103771. [Google Scholar] [CrossRef]
- Carlson, S.E.; Gajewski, B.J.; Valentine, C.J.; Rogers, L.K.; Weiner, C.P.; DeFranco, E.A.; Buhimschi, C.S. Assessment of DHA on reducing early preterm birth: The ADORE randomized controlled trial protocol. BMC Pregnancy Childbirth 2017, 17, 62. [Google Scholar] [CrossRef]
- Bisgaard, H.; Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Schoos, A.M.; Wolsk, H.M.; Pedersen, T.M.; Vinding, R.K.; Thorsteinsdóttir, S.; et al. Fish Oil-Derived Fatty Acids in Pregnancy and Wheeze and Asthma in Offspring. N. Engl. J. Med. 2016, 375, 2530–2539. [Google Scholar] [CrossRef]
- Keski-Nisula, L.; Kyynäräinen, H.-R.; Kärkkäinen, U.; Karhukorpi, J.; Heinonen, S.; Pekkanen, J. Maternal intrapartum antibiotics and decreased vertical transmission of Lactobacillus to neonates during birth. Acta Paediatr. 2013, 102, 480–485. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Burmeister, C.; Havstad, S.; Levin, A.M.; Lynch, S.V.; Ownby, D.R.; Rundle, A.G.; Woodcroft, K.J.; Zoratti, E.M.; Johnson, C.C.; et al. Prenatal antimicrobial use and early-childhood body mass index. Int. J. Obes. 2018, 42, 1–7. [Google Scholar] [CrossRef]
- Mueller, N.T.; Whyatt, R.; Hoepner, L.; Oberfield, S.; Dominguez-Bello, M.G.; Widen, E.M.; Hassoun, A.; Perera, F.; Rundle, A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int. J. Obes. 2015, 39, 665–670. [Google Scholar] [CrossRef]
- Salomäki, H.; Heinäniemi, M.; Vähätalo, L.H.; Ailanen, L.; Eerola, K.; Ruohonen, S.T.; Pesonen, U.; Koulu, M. Prenatal metformin exposure in a maternal high fat diet mouse model alters the transcriptome and modifies the metabolic responses of the offspring. PLoS ONE 2014, 9, e115778. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Zahlsen, K.; Spigset, O.; Carlsen, S.M. Placental passage of metformin in women with polycystic ovary syndrome. Fertil. Steril. 2005, 83, 1575–1578. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Kerr, J.A.; Lodge, C.; Garner, C.; Dharmage, S.C.; Wake, M.; Allen, K.J. Infant and young child feeding interventions targeting overweight and obesity: A narrative review. Obes. Rev. 2019, 20 (Suppl. S1), 31–44. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; van Jaarsveld, C.H.M.; Llewellyn, C.H.; Cole, T.J.; Wardle, J. Associations between infant feeding and the size, tempo and velocity of infant weight gain: SITAR analysis of the Gemini twin birth cohort. Int. J. Obes. 2014, 38, 980–987. [Google Scholar] [CrossRef]
- Kramer, M.S.; Chalmers, B.; Hodnett, E.D.; Sevkovskaya, Z.; Dzikovich, I.; Shapiro, S.; Collet, J.P.; Vanilovich, I.; Mezen, I.; Ducruet, T.; et al. PROBIT Study Group (Promotion of Breastfeeding Intervention Trial) Promotion of Breastfeeding Intervention Trial (PROBIT): A randomized trial in the Republic of Belarus. JAMA 2001, 285, 413–420. [Google Scholar] [CrossRef]
- Kramer, M.S.; Matush, L.; Vanilovich, I.; Platt, R.W.; Bogdanovich, N.; Sevkovskaya, Z.; Dzikovich, I.; Shishko, G.; Collet, J.-P.; Martin, R.M.; et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: Evidence from a large randomized trial. Am. J. Clin. Nutr. 2007, 86, 1717–1721. [Google Scholar] [CrossRef]
- Albernaz, E.; Victora, C.G.; Haisma, H.; Wright, A.; Coward, W.A. Lactation counseling increases breast-feeding duration but not breast milk intake as measured by isotopic methods. J. Nutr. 2003, 133, 205–210. [Google Scholar] [CrossRef]
- Cohen, R.J.; Brown, K.H.; Canahuati, J.; Rivera, L.L.; Dewey, K.G. Effects of age of introduction of complementary foods on infant breast milk intake, total energy intake, and growth: A randomised intervention study in Honduras. Lancet 1994, 344, 288–293. [Google Scholar] [CrossRef]
- Jonsdottir, O.H.; Thorsdottir, I.; Hibberd, P.L.; Fewtrell, M.S.; Wells, J.C.; Palsson, G.I.; Lucas, A.; Gunnlaugsson, G.; Kleinman, R.E. Timing of the introduction of complementary foods in infancy: A randomized controlled trial. Pediatrics 2012, 130, 1038–1045. [Google Scholar] [CrossRef]
- Koletzko, B.; Boey, C.C.M.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current information and Asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef]
- Rodríguez, G.; Iglesia, I.; Bel-Serrat, S.; Moreno, L.A. Effect of n-3 long chain polyunsaturated fatty acids during the perinatal period on later body composition. Br. J. Nutr. 2012, 107 (Suppl. S2), S117–S128. [Google Scholar] [CrossRef]
- Koletzko, B.; von Kries, R.; Closa, R.; Escribano, J.; Scaglioni, S.; Giovannini, M.; Beyer, J.; Demmelmair, H.; Gruszfeld, D.; Dobrzanska, A.; et al. Lower protein in infant formula is associated with lower weight up to age 2 y: A randomized clinical trial. Am. J. Clin. Nutr. 2009, 89, 1836–1845. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Grote, V.; Closa-Monasterolo, R.; Escribano, J.; Langhendries, J.-P.; Dain, E.; Giovannini, M.; Verduci, E.; Gruszfeld, D.; Socha, P.; et al. Lower protein content in infant formula reduces BMI, obesity risk at school age: Follow-up of a randomized trial. Am. J. Clin. Nutr. 2014, 99, 1041–1051. [Google Scholar] [CrossRef] [PubMed]
- Zehakk, P.; Sausenthaler, S.; Koletzko, S.; Reinhardt, D.; von Berg, A.; Krämer, U.; Berdel, D.; Bollrath, C.; Grübl, A.; Bauer, C.P.; et al. Long-term effects of hydrolyzed protein infant formulas on growth--extended follow-up to 10 y of age: Results from the German Infant Nutritional Intervention (GINI) study. Am. J. Clin. Nutr. 2011, 94, 1803S–1807S. [Google Scholar] [CrossRef]
- Mennella, J.A.; Ventura, A.K.; Beauchamp, G.K. Differential growth patterns among healthy infants fed protein hydrolysate or cow-milk formulas. Pediatrics 2011, 127, 110–118. [Google Scholar] [CrossRef]
- Redsell, S.A.; Edmonds, B.; Swift, J.A.; Siriwardena, A.N.; Weng, S.; Nathan, D.; Glazebrook, C. Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Matern. Child. Nutr. 2016, 12, 24–38. [Google Scholar] [CrossRef]
- Damianidi, L.; Gruszfeld, D.; Verduci, E.; Vecchi, F.; Xhonneux, A.; Langhendries, J.P.; Luque, V.; Theurich, M.A.; Zaragoza-Jordana, M.; Koletzko, B.; et al. Protein intakes and their nutritional sources during the first 2 years of life: Secondary data evaluation from the European Childhood Obesity Project. Eur. J. Clin. Nutr. 2016, 70, 1291–1297. [Google Scholar] [CrossRef]
- Niinikoski, H.; Lagström, H.; Jokinen, E.; Siltala, M.; Rönnemaa, T.; Viikari, J.; Raitakari, O.T.; Jula, A.; Marniemi, J.; Näntö-Salonen, K.; et al. Impact of repeated dietary counseling between infancy and 14 years of age on dietary intakes and serum lipids and lipoproteins: The STRIP study. Circulation 2007, 116, 1032–1040. [Google Scholar] [CrossRef]
- Brown, T.; Moore, T.H.; Hooper, L.; Gao, Y.; Zayegh, A.; Ijaz, S.; Elwenspoek, M.; Foxen, S.C.; Magee, L.; O’Malley, C.; et al. Interventions for preventing obesity in children. Cochrane Database Syst. Rev. 2019, 7, CD001871. [Google Scholar] [CrossRef]
- Hokken-Koelega, A.C.S.; van der Steen, M.; Boguszewski, M.C.S.; Cianfarani, S.; Dahlgren, J.; Horikawa, R.; Mericq, V.; Robert Rapaport, R.; Alherbish, A.; Braslavsky, D.; et al. International Consensus Guideline on Small for Gestational Age: Etiology and Management From Infancy to Early Adulthood. Endocr. Rev. 2023, 44, 539–565. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.A.; Mallan, K.M.; Nicholson, J.M.; Thorpe, K.; Nambiar, S.; Mauch, C.E.; Magarey, A. An early feeding practices intervention for obesity prevention. Pediatrics 2015, 136, e40–e49. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.M.; Baur, L.A.; Simpson, J.M.; Xu, H.; Hayes, A.J.; Hardy, L.L.; Williams, M.; Rissel, C. Sustainability of Effects of an Early Childhood Obesity Prevention Trial Over Time: A Further 3-Year Follow-up of the Healthy Beginnings Trial. JAMA Pediatr. 2015, 169, 543–551. [Google Scholar] [CrossRef]
- Wen, L.M.; Baur, L.A.; Simpson, J.M.; Rissel, C.; Wardle, K.; Flood, V.M. Effectiveness of home based early intervention on children’s BMI at age 2: Randomised controlled trial. BMJ 2012, 344, e3732. [Google Scholar] [CrossRef] [PubMed]
- Verbestel, V.; De Coen, V.; Van Winckel, M.; Huybrechts, I.; Maes, L.; De Bourdeaudhuij, I. Prevention of overweight in children younger than 2 years old: A pilot cluster-randomized controlled trial. Public Health Nutr. 2014, 17, 1384–1392. [Google Scholar] [CrossRef]
- Paul, I.M.; Savage, J.S.; Anzman, S.L.; Beiler, J.S.; Marini, M.E.; Stokes, J.L.; Birch, L.L. Preventing obesity during infancy: A pilot study. Obesity 2011, 19, 353–361. [Google Scholar] [CrossRef]
- Kling, S.M.R.; Roe, L.S.; Keller, K.L.; Rolls, B.J. Double trouble: Portion size and energy density combine to increase preschool children’s lunch intake. Physiol. Behav. 2016, 162, 18–26. [Google Scholar] [CrossRef]
- Powell, E.M.; Frankel, L.A.; Hernandez, D.C. The mediating role of child self-regulation of eating in the relationship between parental use of food as a reward and child emotional overeating. Appetite 2017, 113, 78–83. [Google Scholar] [CrossRef]
- Heerman, W.J.; Rothman, R.L.; Sanders, L.M.; Schildcrout, J.S.; Flower, K.B.; Delamater, A.M.; Kay, M.C.; Wood, C.T.; Gross, R.S.; Bian, A.; et al. A digital health behavior intervention to prevent childhood obesity: The greenlight plus randomized clinical trial. JAMA 2024, 332, 2068–2080. [Google Scholar] [CrossRef]
- Giorgi Rossi, P.; Ferrari, F.; Amarri, S.; Bassi, A.; Bonvicini, L.; Dall’Aglio, L.; Della Giustina, C.; Fabbri, A.; Ferrari, A.M.; Ferrari, E.; et al. Describing the Process and Tools Adopted to Cocreate a Smartphone App for Obesity Prevention in Childhood: Mixed Method Study. JMIR Mhealth Uhealth 2020, 8, e16165. [Google Scholar] [CrossRef]
- Robbins, R.; Niederdeppe, J.; Lundell, H.; Meyerson, J. Views of city, county, and state policy makers about childhood obesity in New York State, 2010-2011. Prev. Chronic Dis. 2013, 10, E195. [Google Scholar] [CrossRef][Green Version]
- Williamson, A.; Tait, H.; El Jardali, F.; Wolfenden, L.; Thackway, S.; Stewart, J.; O’Leary, L.; Dixon, J. How are evidence generation partnerships between researchers and policy-makers enacted in practice? A qualitative interview study. Health Res. Policy Syst. 2019, 17, 41. [Google Scholar] [CrossRef]
- Willumsen, J.; Bull, F. Development of WHO guidelines on physical activity, sedentary behavior, and sleep for children less than 5 years of age. J. Phys. Act. Health 2020, 17, 96–100. [Google Scholar] [CrossRef]
- Bianconi, A.; Fiore, M.; Zauli, E.; Acuti Martellucci, C.; Rosso, A.; Dallolio, L.; Flacco, M.E.; Manzoli, L. How strong is the evidence supporting the WHO guidelines on physical activity, sedentary behaviour and sleep in early childhood? Eur. J. Clin. Invest. 2024, 54, e14294. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, A.E.; Gericke, C.; du Plessis, W. Longitudinal pathways of associations between motor proficiency and physical fitness during earlier and later childhood: The NW-CHILD study. Sci. Prog. 2024, 107, 368504241232515. [Google Scholar] [CrossRef] [PubMed]
- Engel, A.C.; Broderick, C.R.; van Doorn, N.; Hardy, L.L.; Parmenter, B.J. Exploring the Relationship Between Fundamental Motor Skill Interventions and Physical Activity Levels in Children: A Systematic Review and Meta-analysis. Sports Med. 2018, 48, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Downing, K.L.; Hnatiuk, J.A.; Hinkley, T.; Salmon, J.; Hesketh, K.D. Interventions to reduce sedentary behaviour in 0-5-year-olds: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2018, 52, 314–321. [Google Scholar] [CrossRef]
- Bozzola, E.; Spina, G.; Ruggiero, M.; Vecchio, D.; Caruso, C.; Bozzola, M.; Staiano, A.M.; Agostiniani, R.; Del Vecchio, A.; Banderali, G.; et al. Media use during adolescence: The recommendations of the Italian Pediatric Society. Ital. J. Pediatr. 2019, 45, 149. [Google Scholar] [CrossRef]
- Moir, C.; Meredith-Jones, K.; Taylor, B.J.; Gray, A.; Heath, A.-L.M.; Dale, K.; Galland, B.; Lawrence, J.; Sayers, R.M.; Taylor, R.W. Early intervention to encourage physical activity in infants and toddlers: A randomized controlled trial. Med. Sci. Sports Exerc. 2016, 48, 2446–2453. [Google Scholar] [CrossRef]
- Mallawaarachchi, S.R.; Anglim, J.; Hooley, M.; Horwood, S. Associations of smartphone and tablet use in early childhood with psychosocial, cognitive and sleep factors: A systematic review and meta-analysis. Early Child. Res. Q. 2022, 60, 13–33. [Google Scholar] [CrossRef]
- Porri, D.; Luppino, G.; Aversa, T.; Corica, D.; Valenzise, M.; Messina, M.F.; Pepe, G.; Morabito, L.A.; La Rosa, E.; Lugarà, C.; et al. Preventing and treating childhood obesity by sleeping better: A systematic review. Front. Endocrinol. 2024, 15, 1426021. [Google Scholar] [CrossRef]
- Killedar, A.; Lung, T.; Taylor, R.W.; Taylor, B.J.; Hayes, A. Is the cost-effectiveness of an early-childhood sleep intervention to prevent obesity affected by socioeconomic position? Obesity 2023, 31, 192–202. [Google Scholar] [CrossRef]
- Bouter, K.E.; van Raalte, D.H.; Groen, A.K.; Nieuwdorp, M. Role of the Gut Microbiome in the Pathogenesis of Obesity and Obesity-Related Metabolic Dysfunction. Gastroenterology 2017, 152, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, M.; Deehan, E.C.; Cai, C.; Madsen, K.L.; Wine, E.; Li, G.; Li, J.; Liu, J.; Zhang, Z. Dietary fiber for the prevention of childhood obesity: A focus on the involvement of the gut microbiota. Gut Microbes 2024, 16, 2387796. [Google Scholar] [CrossRef] [PubMed]
- Bellenger, J.; Bellenger, S.; Escoula, Q.; Bidu, C.; Narce, M. N-3 polyunsaturated fatty acids: An innovative strategy against obesity and related metabolic disorders, intestinal alteration and gut microbiota dysbiosis. Biochimie 2019, 159, 66–71. [Google Scholar] [CrossRef]
- Vallianou, N.; Dalamaga, M.; Stratigou, T.; Karampela, I.; Tsigalou, C. Do Antibiotics Cause Obesity Through Long-term Alterations in the Gut Microbiome? A Review of Current Evidence. Curr. Obes. Rep. 2021, 10, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.S.; Narain, N.P.; Marathe, S.M.; Sonawale, S.B.; Veligandla, K.C. Early-Life Antibiotics and Childhood Obesity: Yeast Probiotics as a Strategy to Modulate Gut Microbiota. Cureus 2023, 15, e36795. [Google Scholar] [CrossRef]
- Lobstein, T.; Brownell, K.D. Endocrine-disrupting chemicals and obesity risk: A review of recommendations for obesity prevention policies. Obes. Rev. 2021, 22, e13332. [Google Scholar] [CrossRef]
- Encarnação, T.; Pais, A.A.; Campos, M.G.; Burrows, H.D. Endocrine disrupting chemicals: Impact on human health, wildlife and the environment. Sci. Prog. 2019, 102, 3–42. [Google Scholar] [CrossRef]
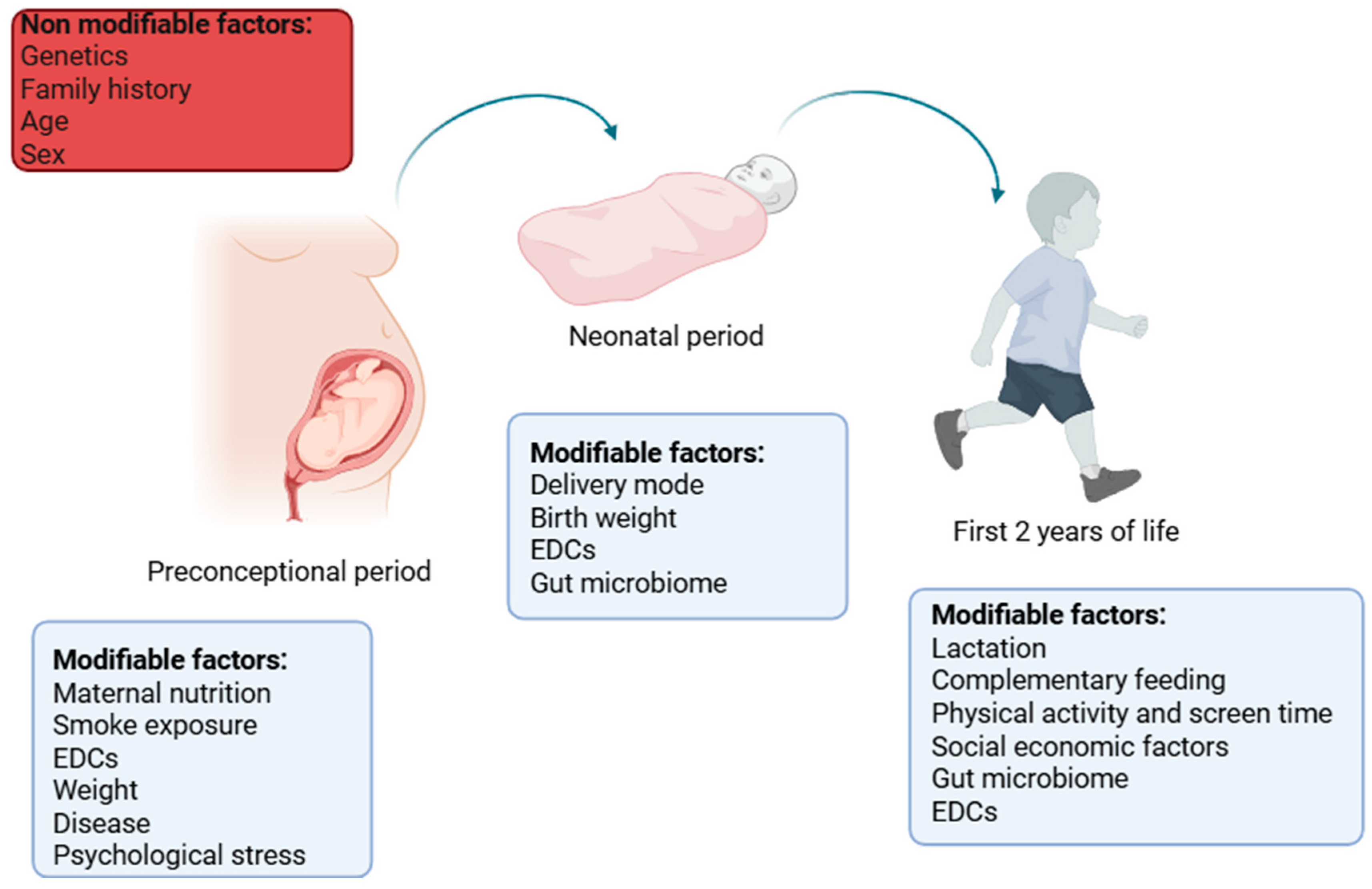
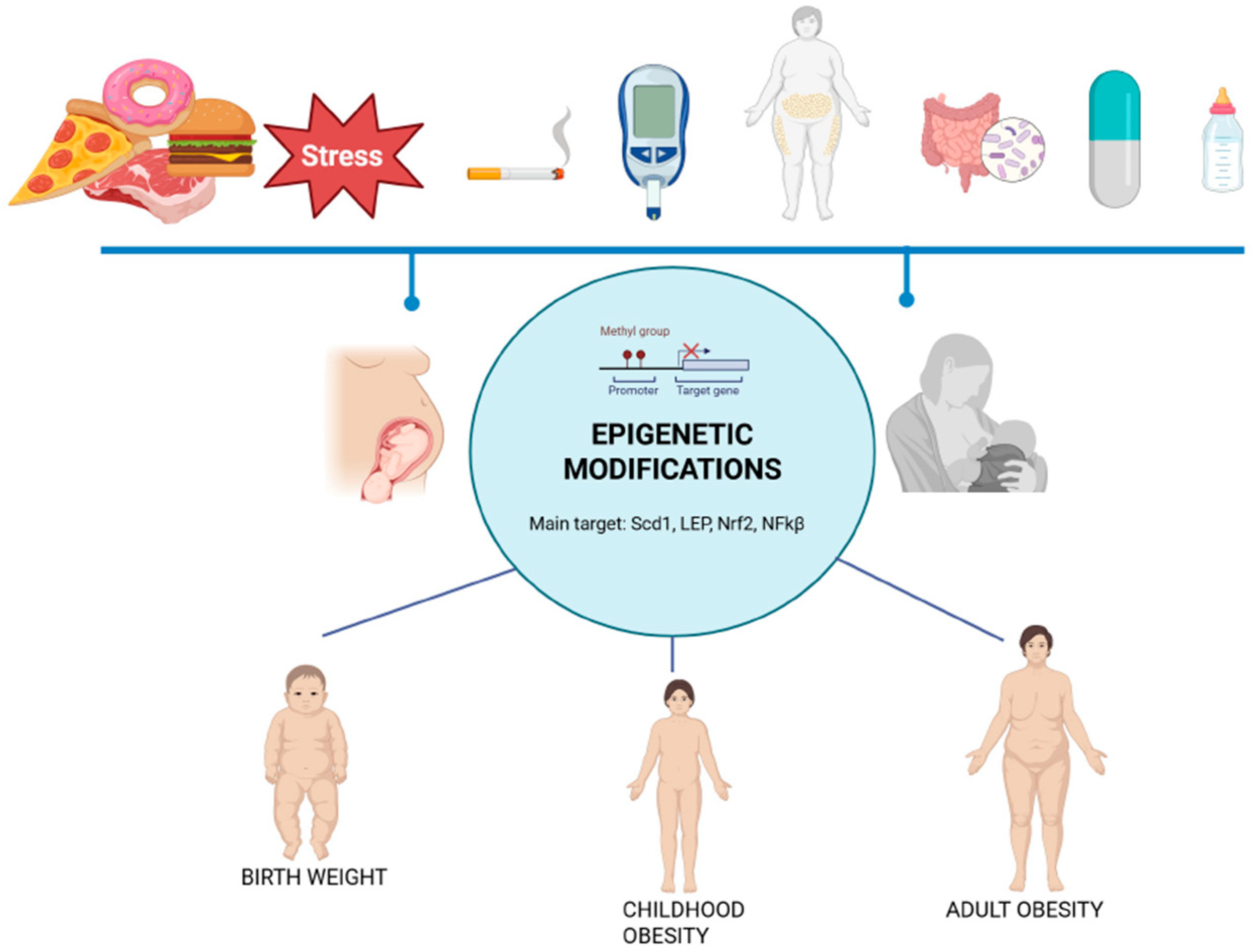
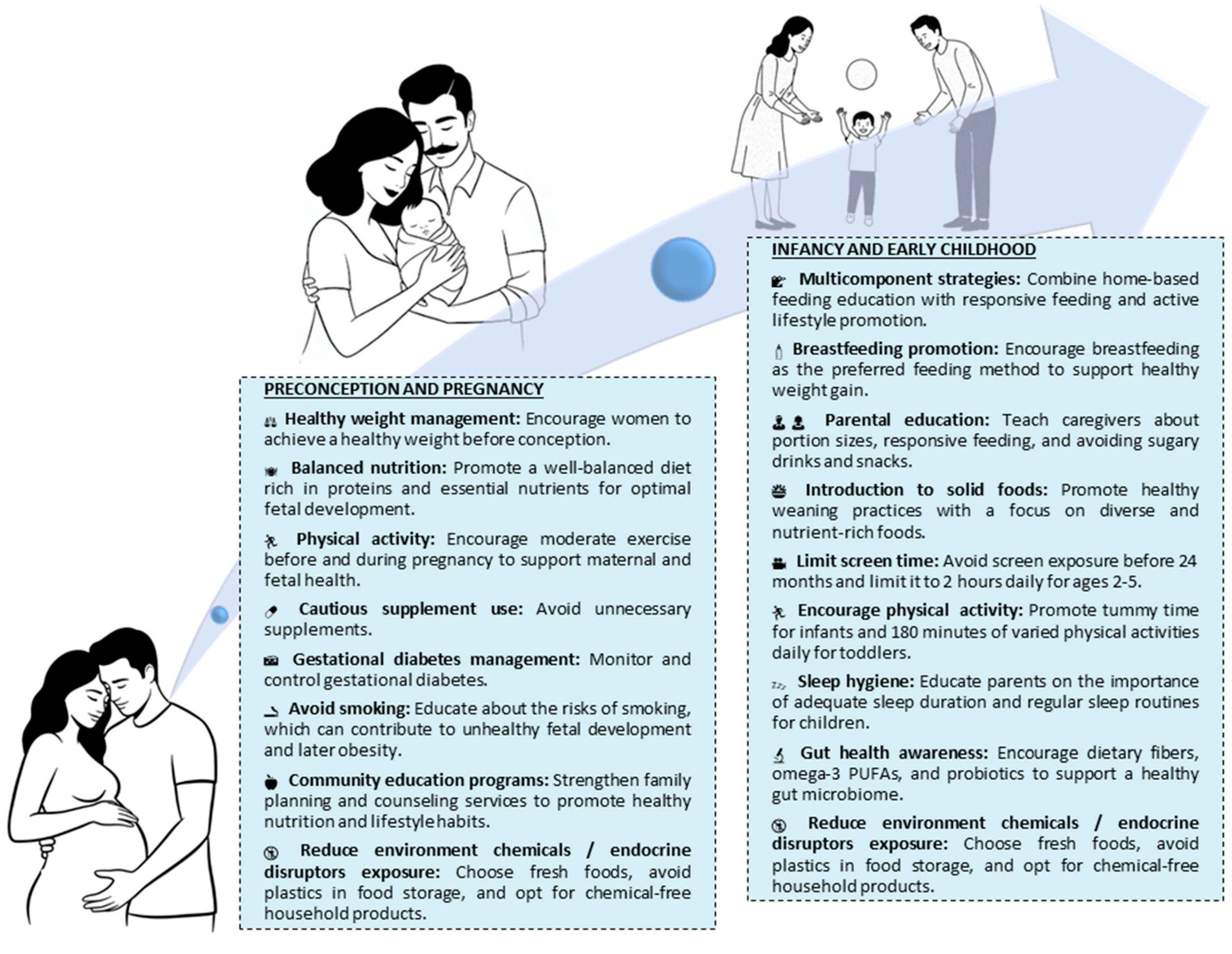
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umano, G.R.; Bellone, S.; Buganza, R.; Calcaterra, V.; Corica, D.; De Sanctis, L.; Di Sessa, A.; Faienza, M.F.; Improda, N.; Licenziati, M.R.; et al. Early Roots of Childhood Obesity: Risk Factors, Mechanisms, and Prevention Strategies. Int. J. Mol. Sci. 2025, 26, 7388. https://doi.org/10.3390/ijms26157388
Umano GR, Bellone S, Buganza R, Calcaterra V, Corica D, De Sanctis L, Di Sessa A, Faienza MF, Improda N, Licenziati MR, et al. Early Roots of Childhood Obesity: Risk Factors, Mechanisms, and Prevention Strategies. International Journal of Molecular Sciences. 2025; 26(15):7388. https://doi.org/10.3390/ijms26157388
Chicago/Turabian StyleUmano, Giuseppina Rosaria, Simonetta Bellone, Raffaele Buganza, Valeria Calcaterra, Domenico Corica, Luisa De Sanctis, Anna Di Sessa, Maria Felicia Faienza, Nicola Improda, Maria Rosaria Licenziati, and et al. 2025. "Early Roots of Childhood Obesity: Risk Factors, Mechanisms, and Prevention Strategies" International Journal of Molecular Sciences 26, no. 15: 7388. https://doi.org/10.3390/ijms26157388
APA StyleUmano, G. R., Bellone, S., Buganza, R., Calcaterra, V., Corica, D., De Sanctis, L., Di Sessa, A., Faienza, M. F., Improda, N., Licenziati, M. R., Manco, M., Ungaro, C., Urbano, F., Valerio, G., Wasniewska, M., & Street, M. E. (2025). Early Roots of Childhood Obesity: Risk Factors, Mechanisms, and Prevention Strategies. International Journal of Molecular Sciences, 26(15), 7388. https://doi.org/10.3390/ijms26157388













