Accumulation Kinetics and Biological Action of Doxorubicin in Rabbit Intervertebral Discs
Abstract
1. Introduction
2. Results
2.1. Analytical Performance of Validated LC-HRMS Method
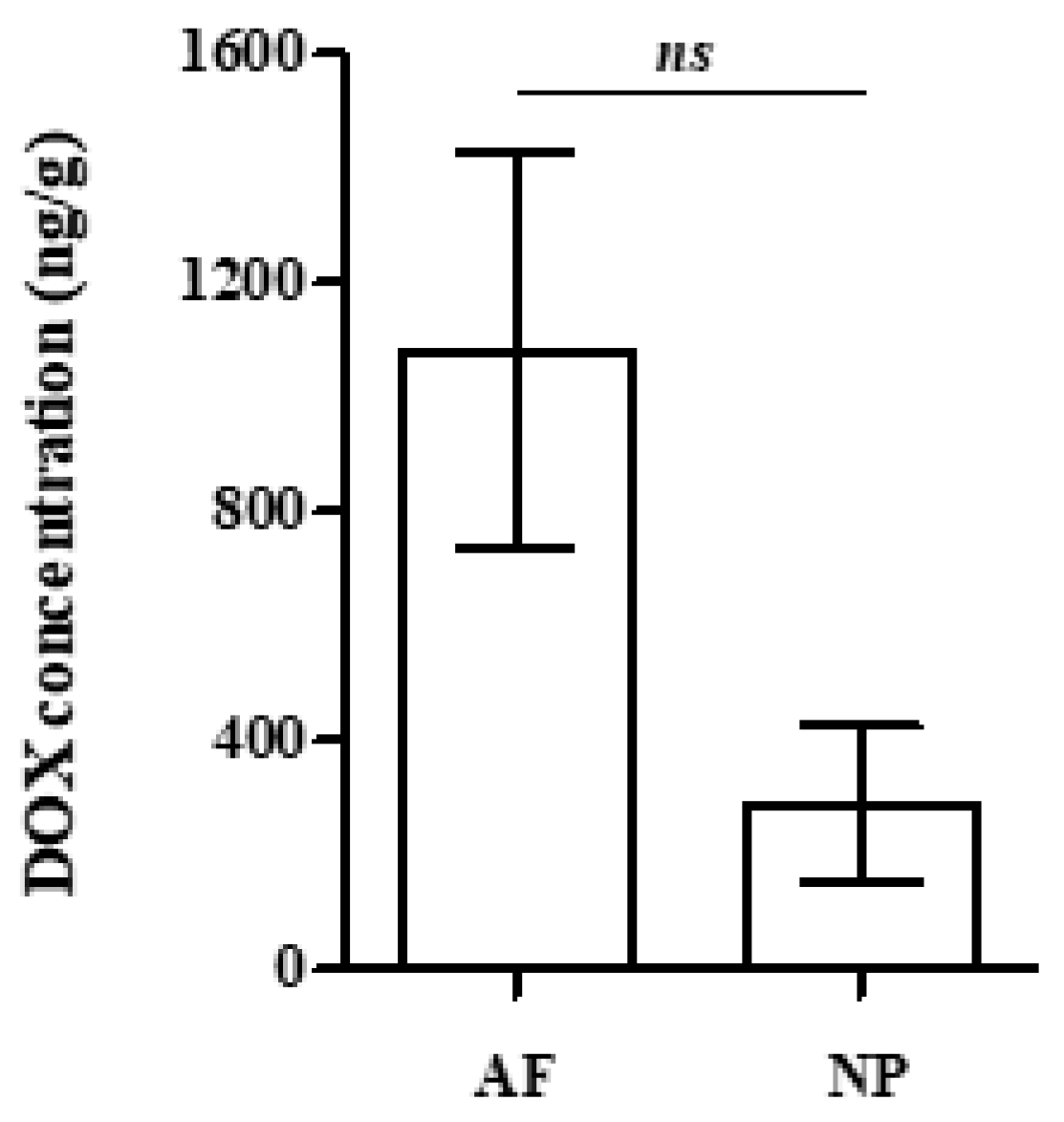
2.2. Analysis of Rabbit Blood Plasma, Skin and IVD Samples
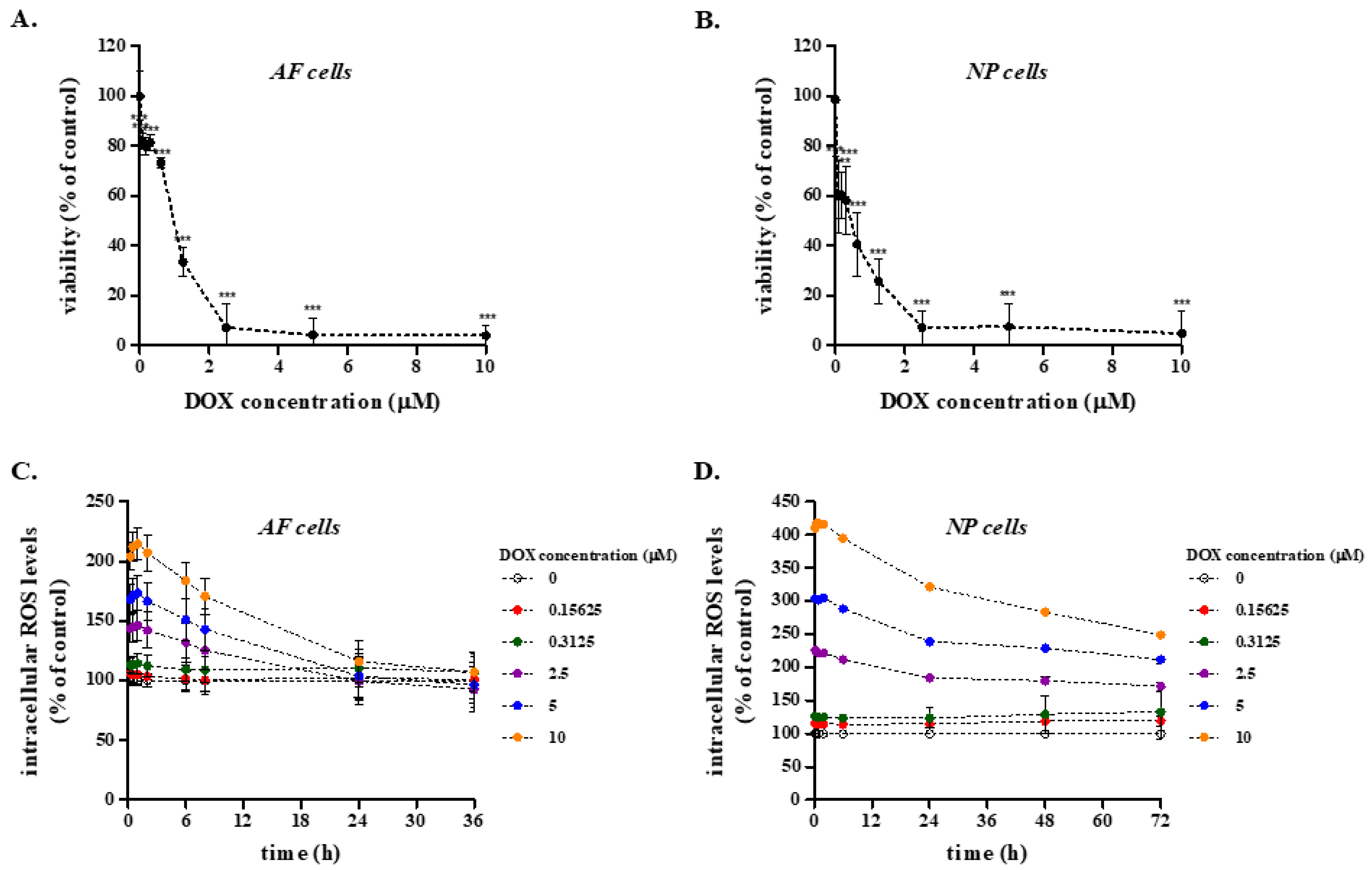
2.3. Effect of DOX on the Viability and Redox Status of Primary Rabbit AF and NP IVD Cells
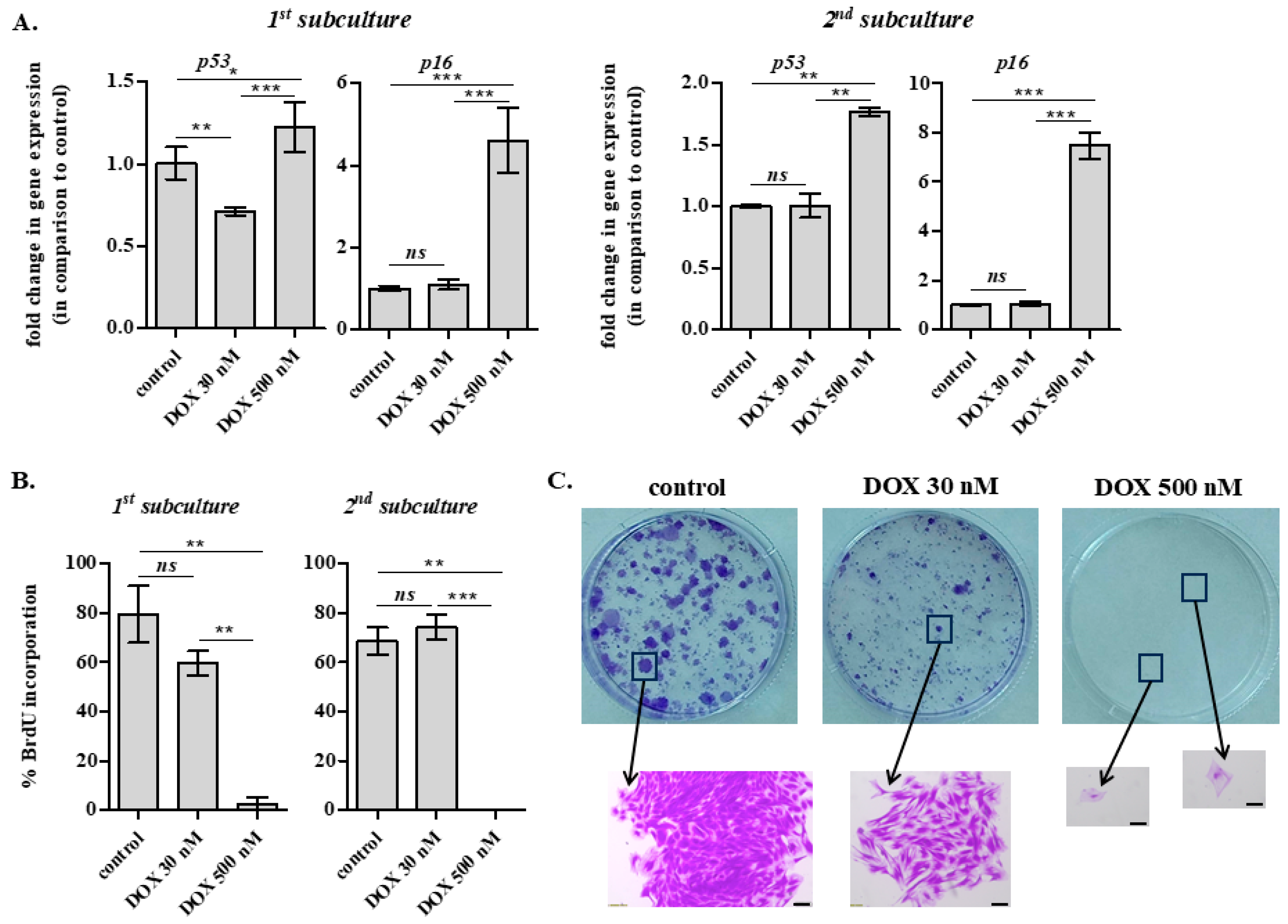
2.4. Effect of DOX on the Transcriptional Profile of Selected Genes in Rabbit AF and NP IVD Cells
2.5. Long-Term Effects of DOX Administration on IVD Cells
2.6. Effect of DOX Administration on ECM Quality of the Rabbit AF and NP In Vitro and In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Standards and Solutions
4.3. Animals and DOX Treatment
4.4. Plasma Isolation
4.5. Rabbit Skin and IVD Isolation
4.6. Preparation of Samples for LC-HRMS/MS Analysis
4.7. Instrumentation for the Determination of DOX
LC-HRMS/MS Analysis
4.8. Establishment of Primary Rabbit IVD Cells and Cell Culture Conditions
4.9. Estimation of Cell Viability
4.10. Measurement of Intracellular ROS Levels
4.11. Western Blot Analysis
4.12. RNA Extraction and Real-Time PCR Analysis
4.13. Immunofluorescence Experiments for the Estimation of Nuclear BrdU Incorporation
4.14. Staining with Crystal Violet
4.15. Sirious Red and Alcian Blue Staining
4.16. Histological Analysis
4.17. Statistical Analysis for Biological Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | acetonitrile |
| AF | annulus fibrosus |
| AGC | automatic gain control |
| ANOVA | analysis of variance |
| BrdU | bromodeoxyuridine |
| CEP | cartilaginous endplate |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DCFH-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| dd | double-distilled |
| DMM | destabilization of the medial meniscus |
| DOX | doxorubicin |
| ECM | extracellular matrix |
| ERKs | extracellular signal-regulated kinases |
| FA | formic acid |
| FBS | fetal bovine serum |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| IVD | intervertebral disc |
| JNKs | c-Jun N-terminal kinases |
| LC-HRMS | liquid chromatography-high resolution mass spectrometry |
| LD50 | median lethal dose |
| LOD | limit of detection |
| LOQ | limit of quantification |
| MAPK | MAP kinase |
| MMP | matrix metalloproteinase |
| MRM | multiple reaction monitoring |
| MTT | methylthiazolyldiphenyl-tetrazolium bromide |
| NP | nucleus pulposus |
| OA | osteoarthritis |
| PBS | phosphate buffered saline |
| QC | quality control |
| ROS | reactive oxygen species |
| SIPS | stress-induced premature senescence |
| SPE | solid phase extraction |
| TGF-β1 | transforming growth factor β1 |
| TIMP1 | tissue inhibitor of metalloproteinase 1 |
References
- Yun, U.J.; Lee, J.H.; Shim, J.; Yoon, K.; Goh, S.H.; Yi, E.H.; Ye, S.K.; Lee, J.S.; Lee, H.; Park, J.; et al. Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-Co A reductase via inhibition of EGFR/Src pathway. Lab. Investig. 2019, 99, 1157–1172. [Google Scholar] [CrossRef]
- Doroshow, J.H. Doxorubicin-induced cardiac toxicity. N. Engl. J. Med. 1991, 324, 843–845. [Google Scholar] [CrossRef]
- Rana, T.; Chakrabarti, A.; Freeman, M.; Biswas, S. Doxorubicin-mediated bone loss in breast cancer bone metastases is driven by an interplay between oxidative stress and induction of TGFβ. PLoS ONE 2013, 8, e78043. [Google Scholar] [CrossRef]
- Shusterman, S.; Meadows, A.T. Long term survivors of childhood leukemia. Curr. Opin. Hematol. 2000, 7, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Hadji, P.; Ziller, M.; Maskow, C.; Albert, U.; Kalder, M. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur. J. Cancer 2009, 45, 3205–3212. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, B.L.; Kamps, W.A.; Hartel, R.M.; Veth, R.P.; Sluiter, W.J.; Hoekstra, H.J. Effect of single chemotherapeutic agents on the growing skeleton of the rat. Ann. Oncol. 2000, 11, 1121–1126. [Google Scholar] [CrossRef]
- Friedlaender, G.E.; Tross, R.B.; Doganis, A.C.; Kirkwood, J.M.; Baron, R. Effects of chemotherapeutic agents on bone. I. Short-term methotrexate and doxorubicin (adriamycin) treatment in a rat model. J. Bone Jt. Surg. Am. 1984, 66, 602–607. [Google Scholar] [CrossRef]
- Young, D.M.; Fioravanti, J.L.; Olson, H.M.; Prieur, D.J. Chemical and morphologic alterations of rabbit bone induced by adriamycin. Calcif. Tissue Res. 1975, 18, 47–63. [Google Scholar] [CrossRef]
- Mwale, F.; Marguier, G.; Ouellet, J.A.; Petit, A.; Epure, L.M.; Antoniou, J.; Chalifour, L.E. Effect of dexrazoxane and amifostine on the vertebral bone quality of Doxorubicin treated male rats. Open Orthop. J. 2008, 2, 115–120. [Google Scholar] [CrossRef]
- Mwale, F.; Antoniou, J.; Héon, S.; Servant, N.; Wang, C.; Kirby, G.M.; Demers, C.N.; Chalifour, L.E. Gender-dependent reductions in vertebrae length, bone mineral density and content by doxorubicin are not reduced by dexrazoxane in young rats: Effect on growth plate and intervertebral discs. Calcif. Tissue Int. 2005, 76, 214–221. [Google Scholar] [CrossRef]
- Tortolani, P.J.; Park, A.E.; Louis-Ugbo, J.; Attallah-Wasef, E.S.; Kraiwattanapong, C.; Heller, J.G.; Boden, S.D.; Yoon, S.T. The effects of doxorubicin (adriamycin) on spinal fusion: An experimental model of posterolateral lumbar spinal arthrodesis. Spine J. 2004, 4, 669–674. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, X.; Hu, Y.; Lin, M.; Zhang, R.; Chen, X.; Yu, D.; Yao, X.; Wang, P.; Zhou, H. New Hope for Treating Intervertebral Disc Degeneration: Microsphere-Based Delivery System. Front. Bioeng. Biotechnol. 2022, 10, 933901. [Google Scholar] [CrossRef]
- Christophoridis, C.; Kouroumalis, A.; Kletsas, D. Accumulation of zoledronic acid in rabbit intervertebral discs. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2022, 1197, 123229. [Google Scholar] [CrossRef]
- Li, C.; Bai, Q.; Lai, Y.; Tian, J.; Li, J.; Sun, X.; Zhao, Y. Advances and Prospects in Biomaterials for Intervertebral Disk Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 766087. [Google Scholar] [CrossRef]
- Urban, J.P.; Roberts, S. Degeneration of the intervertebral disc. Arthritis Res. Ther. 2003, 5, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.P.; Holm, S.; Maroudas, A.; Nachemson, A. Nutrition of the intervertebral disk. An in vivo study of solute transport. Clin. Orthop. Relat. Res. 1977, 101–114. [Google Scholar] [CrossRef]
- Bibby, S.R.; Urban, J.P. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur. Spine J. 2004, 13, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Bibby, S.R.; Jones, D.A.; Lee, R.B.; Yu, J.; Urban, J.P.G. The pathophysiology of the intervertebral disc. Jt. Bone Spine 2001, 68, 537–542. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Z.; Wang, Y.; Yang, J. Multiple nano-drug delivery systems for intervertebral disc degeneration: Current status and future perspectives. Bioact. Mater. 2023, 23, 274–299. [Google Scholar] [CrossRef]
- Blanquer, S.B.; Grijpma, D.W.; Poot, A.A. Delivery systems for the treatment of degenerated intervertebral discs. Adv. Drug Deliv. Rev. 2015, 84, 172–187. [Google Scholar] [CrossRef]
- Urban, J.P. The role of the physicochemical environment in determining disc cell behaviour. Biochem. Soc. Trans. 2002, 30 Pt 6, 858–864. [Google Scholar] [CrossRef]
- Maliszewska, O.; Plenis, A.; Olędzka, I.; Kowalski, P.; Miękus, N.; Bień, E.; Krawczyk, M.A.; Adamkiewicz-Drożynska, E.; Bączek, T. Optimization of LC method for the quantification of doxorubicin in plasma and urine samples in view of pharmacokinetic, biomedical and drug monitoring therapy studies. J. Pharm. Biomed. Anal. 2018, 158, 376–385. [Google Scholar] [CrossRef]
- Ma, W.; Wang, J.; Guo, Q.; Tu, P. Simultaneous determination of doxorubicin and curcumin in rat plasma by LC-MS/MS and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2015, 111, 215–221. [Google Scholar] [CrossRef]
- Mazzucchelli, S.; Ravelli, A.; Gigli, F.; Minoli, M.; Corsi, F.; Ciuffreda, P.; Ottria, R. LC-MS/MS method development for quantification of doxorubicin and its metabolite 13-hydroxy doxorubicin in mice biological matrices: Application to a pharmaco-delivery study. Biomed. Chromatogr. 2017, 31, e3863. [Google Scholar] [CrossRef]
- Lucas, A.T.; O’Neal, S.K.; Santos, C.M.; White, T.F.; Zamboni, W.C. A sensitive high performance liquid chromatography assay for the quantification of doxorubicin associated with DNA in tumor and tissues. J. Pharm. Biomed. Anal. 2016, 119, 122–129. [Google Scholar] [CrossRef]
- Ibsen, S.; Su, Y.; Norton, J.; Zahavy, E.; Hayashi, T.; Adams, S.; Wrasidlo, W.; Esener, S. Extraction protocol and mass spectrometry method for quantification of doxorubicin released locally from prodrugs in tumor tissue. J. Mass. Spectrom. 2013, 48, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.D.; Slack, J.E.; Straubinger, R.M. Quantification of Doxorubicin and metabolites in rat plasma and small volume tissue samples by liquid chromatography/electrospray tandem mass spectroscopy. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 808, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Nebigil, C.G.; Désaubry, L. Updates in Anthracycline-Mediated Cardiotoxicity. Front. Pharmacol. 2018, 9, 1262. [Google Scholar] [CrossRef] [PubMed]
- Danesi, R.; Fogli, S.; Gennari, A.; Conte, P.; Del Tacca, M. Pharmacokinetic-pharmacodynamic relationships of the anthracycline anticancer drugs. Clin. Pharmacokinet. 2002, 41, 431–444. [Google Scholar] [CrossRef]
- Patel, K.J.; Trédan, O.; Tannock, I.F. Distribution of the anticancer drugs doxorubicin, mitoxantrone and topotecan in tumors and normal tissues. Cancer Chemother. Pharmacol. 2013, 72, 127–138. [Google Scholar] [CrossRef]
- Sartiano, G.P.; Lynch, W.E.; Bullington, W.D. Mechanism of action of the anthracycline anti-tumor antibiotics, doxorubicin, daunomycin and rubidazone: Preferential inhibition of DNA polymerase alpha. J. Antibiot. 1979, 32, 1038–1045. [Google Scholar] [CrossRef]
- Greene, R.F.; Collins, J.M.; Jenkins, J.F.; Speyer, J.L.; Myers, C.E. Plasma pharmacokinetics of adriamycin and adriamycinol: Implications for the design of in vitro experiments and treatment protocols. Cancer Res. 1983, 43, 3417–3421. [Google Scholar] [PubMed]
- Ottewell, P.D.; Woodward, J.K.; Lefley, D.V.; Evans, C.A.; Coleman, R.E.; Holen, I. Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Mol. Cancer Ther. 2009, 8, 2821–2832. [Google Scholar] [CrossRef]
- Siebel, C.; Lanvers-Kaminsky, C.; Würthwein, G.; Hempel, G.; Boos, J. Bioanalysis of doxorubicin aglycone metabolites in human plasma samples-implications for doxorubicin drug monitoring. Sci. Rep. 2020, 10, 18562. [Google Scholar] [CrossRef] [PubMed]
- Colombo, T.; Gonzalez Paz, O.; D’Incalci, M. Distribution and activity of doxorubicin combined with SDZ PSC 833 in mice with P388 and P388/DOX leukaemia. Br. J. Cancer 1996, 73, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.A.; Dogramatzis, D.; Benvenuto, J.A.; Trevino, M.; Stephens, L.C.; Wondergem, J.; Strebel, R.; Baba, H.; Bull, J.M. Effect of whole-body hyperthermia on pharmacokinetics and tissue distribution of doxorubicin. Int. J. Hyperth. 1992, 8, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Averbach, A.; Stuart, O.A.; Chang, D.; Sugarbaker, P.H. Hyperthermic intraperitoneal doxorubicin: Pharmacokinetics, metabolism, and tissue distribution in a rat model. Cancer Chemother. Pharmacol. 1998, 41, 147–154. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, F.; Guo, L.; Xu, Y.; Yu, X.; Zhang, Z.; Zhang, Y. Plasma Pharmacokinetics and Tissue Distribution of Doxorubicin in Rats following Treatment with Astragali Radix. Pharmaceuticals 2022, 15, 1104. [Google Scholar] [CrossRef]
- Terasaki, T.; Iga, T.; Sugiyama, Y.; Hanano, M. Pharmacokinetic study on the mechanism of tissue distribution of doxorubicin: Interorgan and interspecies variation of tissue-to-plasma partition coefficients in rats, rabbits, and guinea pigs. J. Pharm. Sci. 1984, 73, 1359–1363. [Google Scholar] [CrossRef]
- Jacquet, P.; Stuart, O.A.; Chang, D.; Sugarbaker, P.H. Effects of intra-abdominal pressure on pharmacokinetics and tissue distribution of doxorubicin after intraperitoneal administration. Anti-Cancer Drugs 1996, 7, 596–603. [Google Scholar] [CrossRef]
- Blueschke, G.; Boico, A.; Negussie, A.H.; Yarmolenko, P.; Wood, B.J.; Spasojevic, I.; Fan, P.; Erdmann, D.; Schroeder, T.; Sauerbier, M.; et al. Enhanced Drug Delivery to the Skin Using Liposomes. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1739. [Google Scholar] [CrossRef] [PubMed]
- Luecke, R.H.; Ryan, M.P.; Wosilait, W.D. A mathematical model and computer program for adriamycin distribution and elimination. Comput. Methods Programs Biomed. 1985, 20, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Edison, T.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, B.L.; Hartel, R.M.; Jansen, H.W.; Kamps, W.A.; Hoekstra, H.J. The effect of chemotherapy on the morphology of the growth plate and metaphysis of the growing skeleton. Eur. J. Surg. Oncol. 2003, 29, 49–58. [Google Scholar] [CrossRef]
- Kumagai, K.; Imai, S.; Toyoda, F.; Okumura, N.; Isoya, E.; Matsuura, H.; Matsusue, Y. 17β-Oestradiol inhibits doxorubicin-induced apoptosis via block of the volume-sensitive Cl(-) current in rabbit articular chondrocytes. Br. J. Pharmacol. 2012, 166, 702–720. [Google Scholar] [CrossRef]
- Wu, C.; Luo, J.; Liu, Y.; Fan, J.; Shang, X.; Liu, R.; Ye, C.; Yang, J.; Cao, H. Doxorubicin suppresses chondrocyte differentiation by stimulating ROS production. Eur. J. Pharm. Sci. 2021, 167, 106013. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Mao, W.; Shen, C. Platelet-rich plasma inhibits Adriamycin-induced inflammation via blocking the NF-κB pathway in articular chondrocytes. Mol. Med. 2021, 27, 66. [Google Scholar] [CrossRef]
- Creasey, W.A.; McIntosh, L.S.; Brescia, T.; Odujinrin, O.; Aspnes, G.T.; Murray, E.; Marsh, J.C. Clinical effects and pharmacokinetics of different dosage schedules of adriamycin. Cancer Res. 1976, 36, 216–221. [Google Scholar]
- Van Tine, B.A.; Krarup-Hansen, A.; Hess, L.M.; Abdul Razak, A.R.; Soldatenkova, V.; Wright, J.; Park, S.H. Quality of life of patients with soft tissue sarcoma treated with doxorubicin in the ANNOUNCE phase III clinical trial. Rare Tumors 2022, 14, 1–9. [Google Scholar] [CrossRef]
- Calvo-Echenique, A.; Cegoñino, J.; Correa-Martín, L.; Bances, L.; Palomar, A.P. Intervertebral disc degeneration: An experimental and numerical study using a rabbit model. Med. Biol. Eng. Comput. 2018, 56, 865–877. [Google Scholar] [CrossRef]
- Mwale, F.; Masuda, K.; Grant, M.P.; Epure, L.M.; Kato, K.; Miyazaki, S.; Cheng, K.; Yamada, J.; Bae, W.C.; Muehleman, C.; et al. Short Link N promotes disc repair in a rabbit model of disc degeneration. Arthritis Res. Ther. 2018, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, K.; Chamoli, U.; Masuda, K.; Miyazaki, S.; Kato, K.; Diwan, A.D. A novel magnetic resonance imaging postprocessing technique for the assessment of intervertebral disc degeneration-Correlation with histological grading in a rabbit disc degeneration model. JOR Spine 2019, 2, e1060. [Google Scholar] [CrossRef] [PubMed]
- Chujo, T.; An, H.S.; Akeda, K.; Miyamoto, K.; Muehleman, C.; Attawia, M.; Andersson, G.; Masuda, K. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine 2006, 31, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Boucek, R.J., Jr.; Miracle, A.; Anderson, M.; Engelman, R.; Atkinson, J.; Dodd, D.A. Persistent effects of doxorubicin on cardiac gene expression. J. Mol. Cell. Cardiol. 1999, 31, 1435–1446. [Google Scholar] [CrossRef]
- Mörschbächer, P.D.; Alves Garcez, T.N.; Paz, A.H.; Magrisso, A.B.; Mello, H.F.; Rolim, V.M.; Neuwald, E.B.; Driemeier, D.; Contesini, E.A.; Cirne-Lima, E. Treatment of dilated cardiomyopathy in rabbits with mesenchymal stem cell transplantation and platelet-rich plasma. Vet. J. 2016, 209, 180–185. [Google Scholar] [CrossRef]
- Talavera, J.; Giraldo, A.; Fernández-Del-Palacio, M.J.; García-Nicolás, O.; Seva, J.; Brooks, G.; Moraleda, J.M. An Upgrade on the Rabbit Model of Anthracycline-Induced Cardiomyopathy: Shorter Protocol, Reduced Mortality, and Higher Incidence of Overt Dilated Cardiomyopathy. BioMed Res. Int. 2015, 2015, 465342. [Google Scholar] [CrossRef]
- Lee, J.B.; Zhou, S.; Chiang, M.; Zang, X.; Kim, T.H.; Kagan, L. Interspecies prediction of pharmacokinetics and tissue distribution of doxorubicin by physiologically-based pharmacokinetic modeling. Biopharm. Drug Dispos. 2020, 41, 192–205. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, H.; Yang, J.; Qiu, H.; Cheng, Y.; Liu, M. Pharmacokinetics and cardiotoxicity of doxorubicin and its secondary alcohol metabolite in rats. Biomed. Pharmacother. 2019, 116, 108964. [Google Scholar] [CrossRef]
- Devalapally, H.; Rajan, K.S.; Akkinepally, R.R.; Devarakonda, R.K. Safety, pharmacokinetics and biodistribution studies of a beta-galactoside prodrug of doxorubicin for improvement of tumor selective chemotherapy. Drug Dev. Ind. Pharm. 2008, 34, 789–795. [Google Scholar] [CrossRef]
- Fournier, D.E.; Kiser, P.K.; Shoemaker, J.K.; Battié, M.C.; Séguin, C.A. Vascularization of the human intervertebral disc: A scoping review. JOR Spine 2020, 3, e1123. [Google Scholar] [CrossRef]
- Urban, J.P.; Winlove, C.P. Pathophysiology of the intervertebral disc and the challenges for MRI. J. Magn. Reson. Imaging 2007, 25, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Doxorubicin is a potent inhibitor of interleukin 1 induced cartilage proteoglycan resorption in-vitro. J. Pharm. Pharmacol. 1989, 41, 60–63. [Google Scholar] [CrossRef]
- Maroudas, A.; Stockwell, R.A.; Nachemson, A.; Urban, J. Factors involved in the nutrition of the human lumbar intervertebral disc: Cellularity and diffusion of glucose in vitro. J. Anat. 1975, 120 Pt 1, 113–130. [Google Scholar]
- Shi, S.; Chen, Y.; Luo, Z.; Nie, G.; Dai, Y. Role of oxidative stress and inflammation-related signaling pathways in doxorubicin-induced cardiomyopathy. Cell Commun. Signal. 2023, 21, 61. [Google Scholar] [CrossRef]
- Bientinesi, E.; Lulli, M.; Becatti, M.; Ristori, S.; Margheri, F.; Monti, D. Doxorubicin-induced senescence in normal fibroblasts promotes in vitro tumour cell growth and invasiveness: The role of Quercetin in modulating these processes. Mech. Ageing Dev. 2022, 206, 111689. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, L.; Feng, J.; Bao, L.; Wang, J.; Song, Z.; Mao, Z.; Li, J.; Hu, Z. Characterization of cellular senescence in doxorubicin-induced aging mice. Exp. Gerontol. 2022, 163, 111800. [Google Scholar] [CrossRef]
- Linders, A.N.; Dias, I.B.; Ovchinnikova, E.S.; Vermeer, M.; Hoes, M.F.; Markousis Mavrogenis, G.; Deiman, F.E.; Arevalo Gomez, K.F.; Bliley, J.M.; Nehme, J.; et al. Evaluation of Senescence and Its Prevention in Doxorubicin-Induced Cardiotoxicity Using Dynamic Engineered Heart Tissues. JACC. CardioOncology 2023, 5, 298–315. [Google Scholar] [CrossRef]
- Gadeholt-Gothlin, G.; Gothlin, J.H. Comparison of nephrectomy and/or doxorubicin treatment in rabbit renal VX-2 carcinoma. J. Surg. Oncol. 1995, 58, 134–145. [Google Scholar] [CrossRef]
- Liang, B.; Zhao, D.; Liu, Y.; Guo, X.; Zhang, H.; Zhang, L.; Zheng, C. Chemoembolization of liver cancer with doxorubicin-loaded CalliSpheres microspheres: Plasma pharmacokinetics, intratumoral drug concentration, and tumor necrosis in a rabbit model. Drug Deliv. Transl. Res. 2020, 10, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Timm, K.N.; Ball, V.; Miller, J.J.; Savic, D.; West, J.A.; Griffin, J.L.; Tyler, D.J. Metabolic Effects of Doxorubicin on the Rat Liver Assessed with Hyperpolarized MRI and Metabolomics. Front. Physiol. 2021, 12, 782745. [Google Scholar] [CrossRef] [PubMed]
- Al-Shahari, E.A.; El Barky, A.R.; Mohamed, T.M.; Alm-Eldeen, A.A. Doxorubicin, L-arginine, or their combination as a prophylactic agent against hepatic carcinoma in mice. Environ. Sci. Pollut. Res. Int. 2021, 28, 37661–37671. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, M.; Zhang, Y. An animal model to create intervertebral disc degeneration characterized by radiography and molecular biology. J. Nanjing Med. Univ. 2008, 22, 308–312. [Google Scholar] [CrossRef]
- Pratsinis, H.; Kletsas, D. Organotypic Cultures of Intervertebral Disc Cells: Responses to Growth Factors and Signaling Pathways Involved. BioMed Res. Int. 2015, 2015, 427138. [Google Scholar] [CrossRef]
- Sklirou, A.D.; Angelopoulou, M.T.; Argyropoulou, A.; Chaita, E.; Boka, V.I.; Cheimonidi, C.; Niforou, K.; Mavrogonatou, E.; Pratsinis, H.; Kalpoutzakis, E.; et al. Phytochemical Study and In Vitro Screening Focusing on the Anti-Aging Features of Various Plants of the Greek Flora. Antioxidants 2021, 10, 1206. [Google Scholar] [CrossRef] [PubMed]
- Mavrogonatou, E.; Angelopoulou, M.; Rizou, S.V.; Pratsinis, H.; Gorgoulis, V.G.; Kletsas, D. Activation of the JNKs/ATM-p53 axis is indispensable for the cytoprotection of dermal fibroblasts exposed to UVB radiation. Cell Death Dis. 2022, 13, 647. [Google Scholar] [CrossRef] [PubMed]
- Dimozi, A.; Mavrogonatou, E.; Sklirou, A.; Kletsas, D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur. Cells Mater. 2015, 30, 89–102; discussion 103. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Fotopoulou, A.; Angelopoulou, M.T.; Pratsinis, H.; Mavrogonatou, E.; Kletsas, D. A subset of human dermal fibroblasts overexpressing Cockayne syndrome group B protein resist UVB radiation-mediated premature senescence. Aging Cell 2025, 24, e14422. [Google Scholar] [CrossRef]
- Younesi Soltani, F.; Javanshir, S.; Dowlati, G.; Parham, A.; Naderi-Meshkin, H. Differentiation of human adipose-derived mesenchymal stem cells toward tenocyte by platelet-derived growth factor-BB and growth differentiation factor-6. Cell Tissue Bank. 2022, 23, 237–246. [Google Scholar] [CrossRef]
- Papadopoulou, A.; Kalodimou, V.E.; Mavrogonatou, E.; Karamanou, K.; Yiacoumettis, A.M.; Panagiotou, P.N.; Pratsinis, H.; Kletsas, D. Decreased differentiation capacity and altered expression of extracellular matrix components in irradiation-mediated senescent human breast adipose-derived stem cells. IUBMB Life 2022, 74, 969–981. [Google Scholar] [CrossRef]





| Blood Plasma | Skin | AF | NP | |
|---|---|---|---|---|
| linear range | 5–200 nM | 5–800 ng/g | 5–200 ng/g | 5–200 ng/g |
| linearity R2 | 0.977 | 0.975 | 0.944 | 0.982 |
| % mean rec/% rsd | 78.9/6.6 | 79.9/5.5 | 69.8/4.6 | 71.6/10.8 |
| single day % rsd | 3.0–8.6 | 6.5–7.9 | 5.5–6.8 | 7.9–9.9 |
| two day % rsd | 7.6–11.9 | 11.2–16.7 | 9.9–11.4 | 17.2–19.0 |
| Q3/Q1 * (% rsd) at Qc level | 0.890 (22.6) | 0.780 (11.7) | 0.750 (17.9) | 0.755 (11.4) |
| Q2/Q1 * (% rsd) at Qc level | 0.612 (18.3) | 0.590 (9.1) | 0.550 (20.2) | 0.558 (19.1) |
| LOD | 0.2 nM | 3 ng/g | 4 ng/g | 4 ng/g |
| LOQ | 0.6 nM | 9 ng/g | 12 ng/g | 12 ng/g |
| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| MMP1 | ATA-AAT-AAT-GGC-TAA-GGA-AGG-C | CAG-GAT-GAT-GTG-AGT-GAC-T |
| MMP3 | AAT-TGT-TCA-ACA-CTT-AGG-ACT-T | TCC-AGT-TAG-ATA-CAC-AGT-TCA |
| MMP13 | GTT-GGA-CCT-GTA-GGC-TAT-T | TCT-GGT-AGA-TGG-TTT-CCT-TTA |
| TIMP1 | AGC-AGA-GCC-TGC-ACC-TGT-GT | CCA-CAA-ACT-TGG-CCC-TGA-TG |
| TGFB1 | AAG-GGC-TAC-CAC-GCC-AAC-TT | CCG-GGT-TGT-GCT-GGT-TGT-AC |
| COL1A | ATG-GAT-GAG-GAA-ACT-GGC-AAC-T | GCC-ATC-GAC-AAG-AAC-AGT-GTA-AGT |
| COL2A1 | AAG-AAC-TGG-TGG-AGC-AGC-AAG-AG | ATG-GAA-GCC-GCC-GTT-GAT-GG |
| ACAN | GGT-CGT-GGT-GAA-AGG-TGT-TGT-G | CTG-GTG-GAA-GCC-ATC-CTC-GTA-G |
| p53 | CCA-GCC-TCT-TAG-TGA-CAA | AAA-TTG-ACC-CTG-AGC-ATT-G |
| p16INK4a | CAG-ACA-CTC-CGA-ACT-CAA | CTA-AGA-AAG-CAG-GGA-AGA-AC |
| GAPDH | TCC-TGG-TAT-GAC-AAC-GAA-T | GGT-TTG-AGG-GCT-CTT-ACT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrogonatou, E.; Kouroumalis, A.; Khaldi, L.; Christophoridis, C.; Kletsas, D. Accumulation Kinetics and Biological Action of Doxorubicin in Rabbit Intervertebral Discs. Int. J. Mol. Sci. 2025, 26, 7386. https://doi.org/10.3390/ijms26157386
Mavrogonatou E, Kouroumalis A, Khaldi L, Christophoridis C, Kletsas D. Accumulation Kinetics and Biological Action of Doxorubicin in Rabbit Intervertebral Discs. International Journal of Molecular Sciences. 2025; 26(15):7386. https://doi.org/10.3390/ijms26157386
Chicago/Turabian StyleMavrogonatou, Eleni, Anastasios Kouroumalis, Lubna Khaldi, Christophoros Christophoridis, and Dimitris Kletsas. 2025. "Accumulation Kinetics and Biological Action of Doxorubicin in Rabbit Intervertebral Discs" International Journal of Molecular Sciences 26, no. 15: 7386. https://doi.org/10.3390/ijms26157386
APA StyleMavrogonatou, E., Kouroumalis, A., Khaldi, L., Christophoridis, C., & Kletsas, D. (2025). Accumulation Kinetics and Biological Action of Doxorubicin in Rabbit Intervertebral Discs. International Journal of Molecular Sciences, 26(15), 7386. https://doi.org/10.3390/ijms26157386






