Ibuprofen Does Not Prevent Inhibition of Fetal Breathing Movements Caused by Intrauterine Inflammation in Fetal Sheep
Abstract
1. Introduction
2. Results
2.1. Fetal Characteristics: Fetal Breathing Movement Characterisation
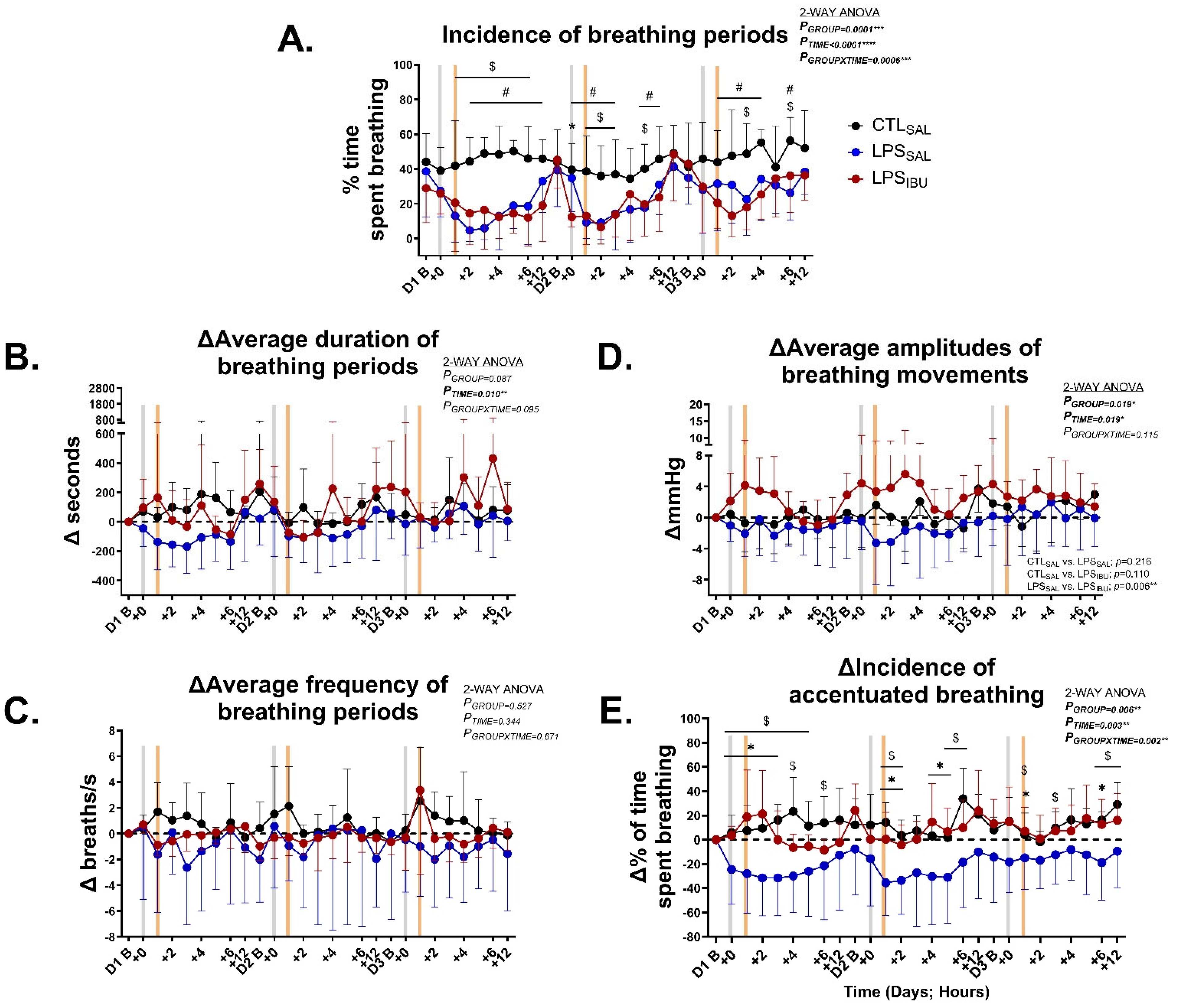
2.2. Fetal Breathing Movement Characterisation
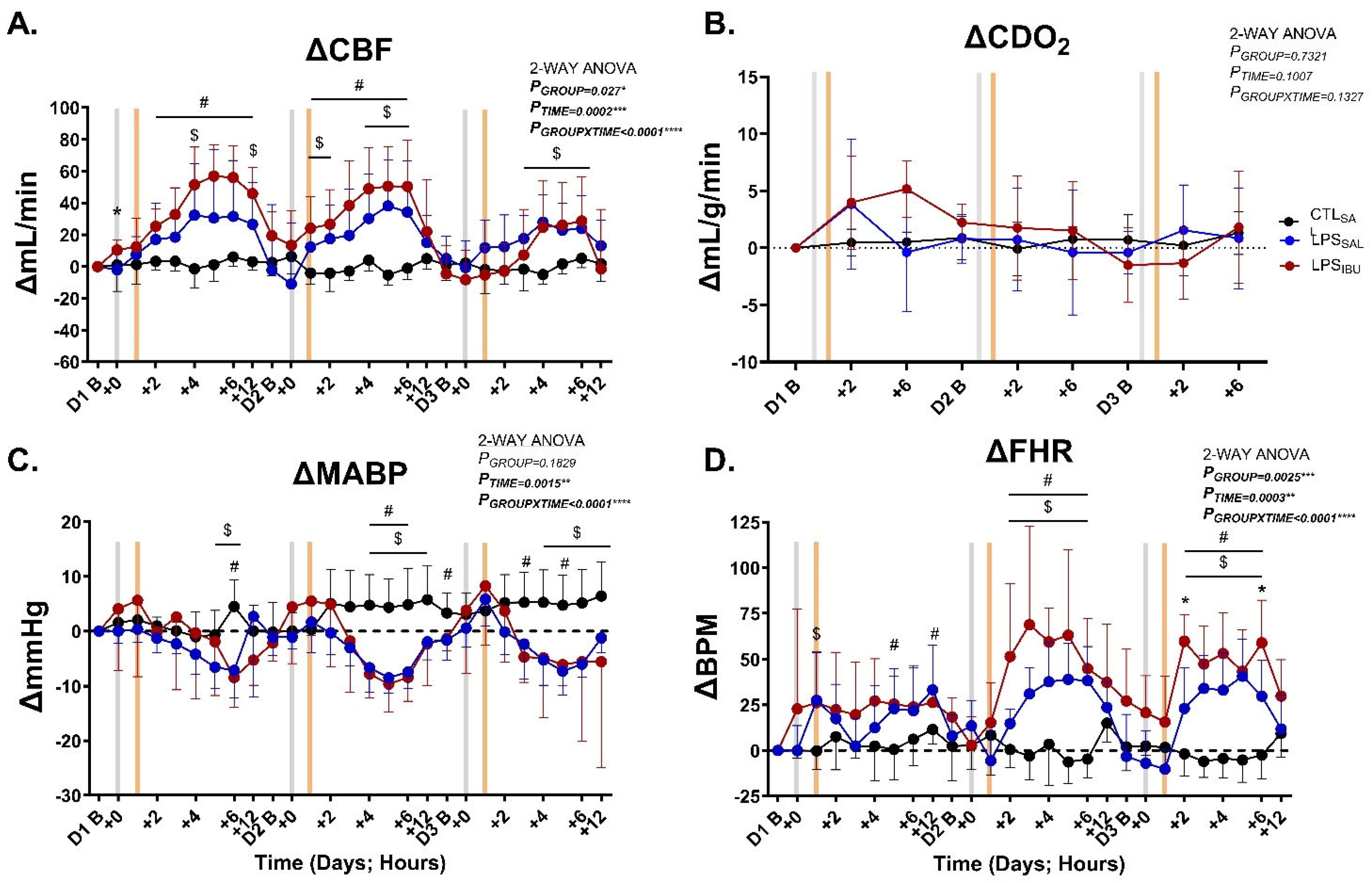
2.3. Systemic Hemodynamics
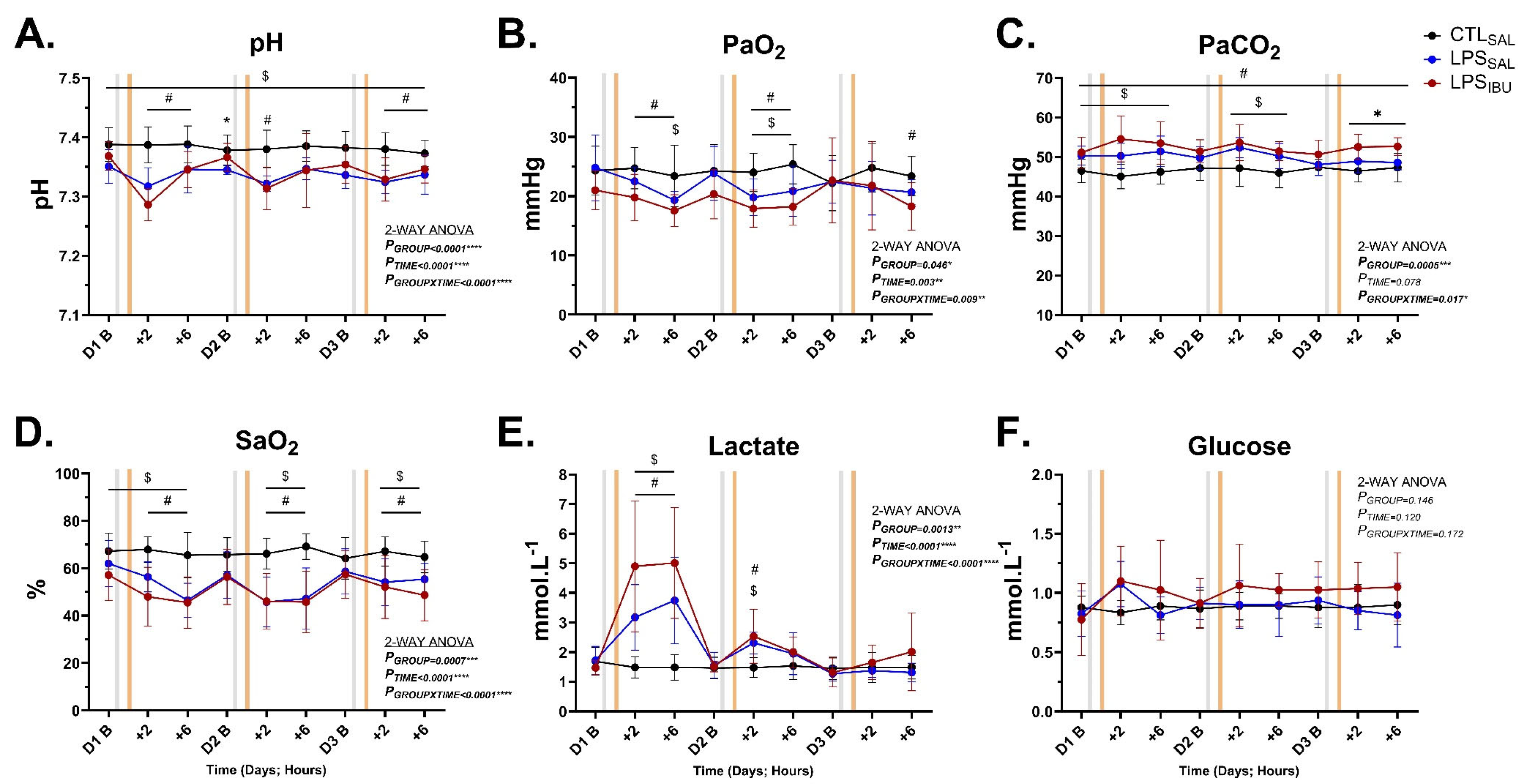
2.4. Fetal Blood Gases and Metabolites
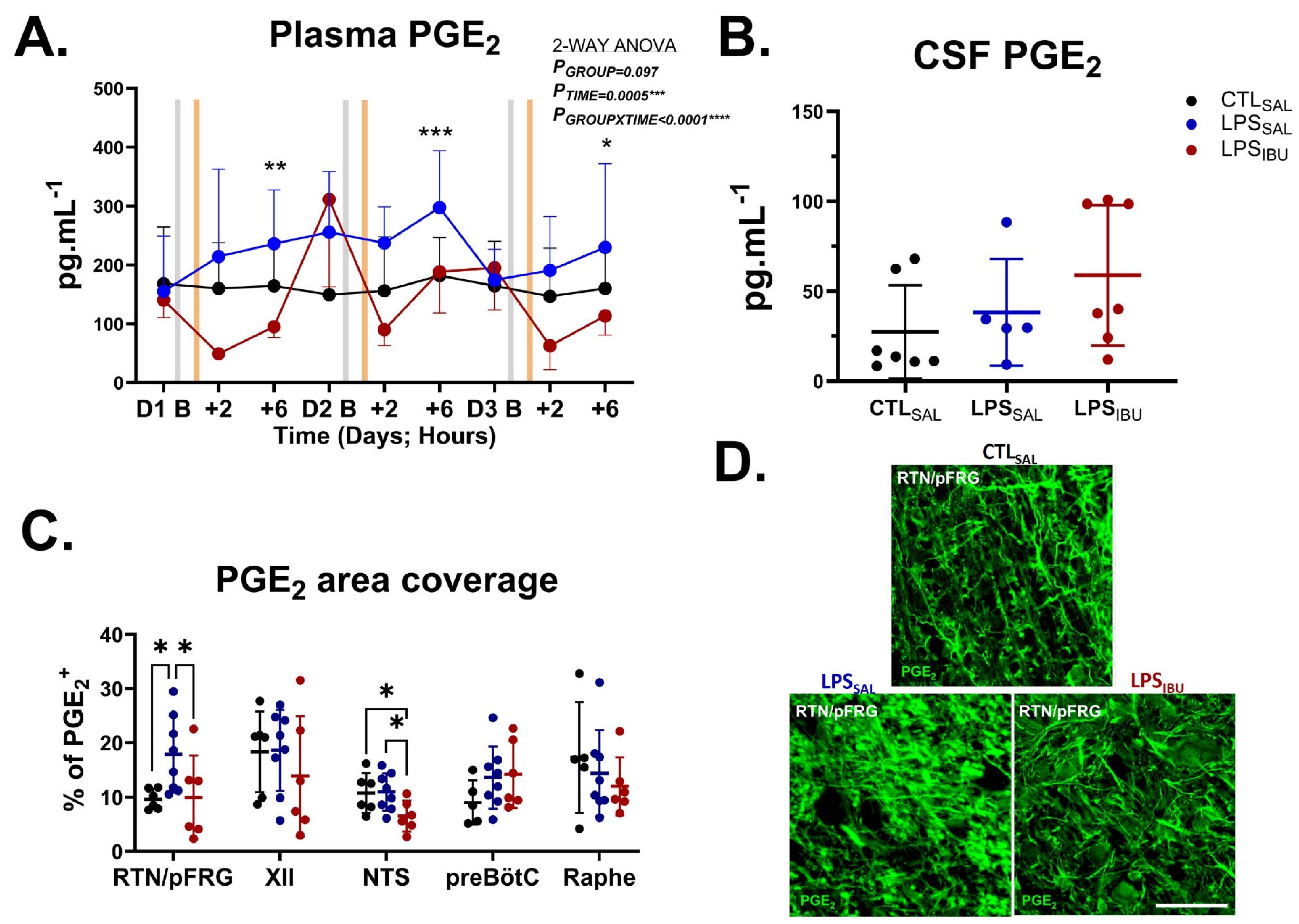
2.5. PGE2 Levels
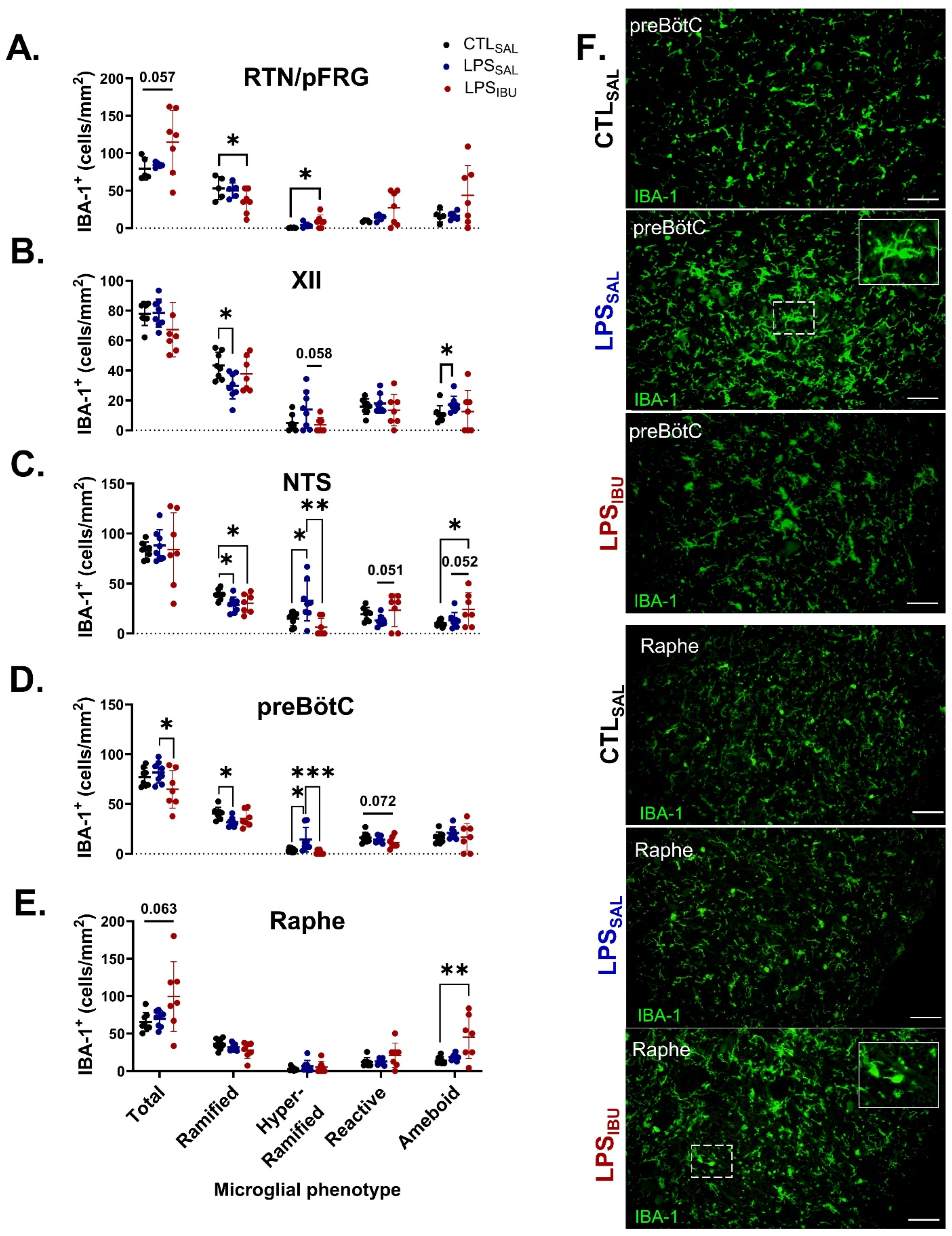
2.6. Microglia and Astrocyte Assessments
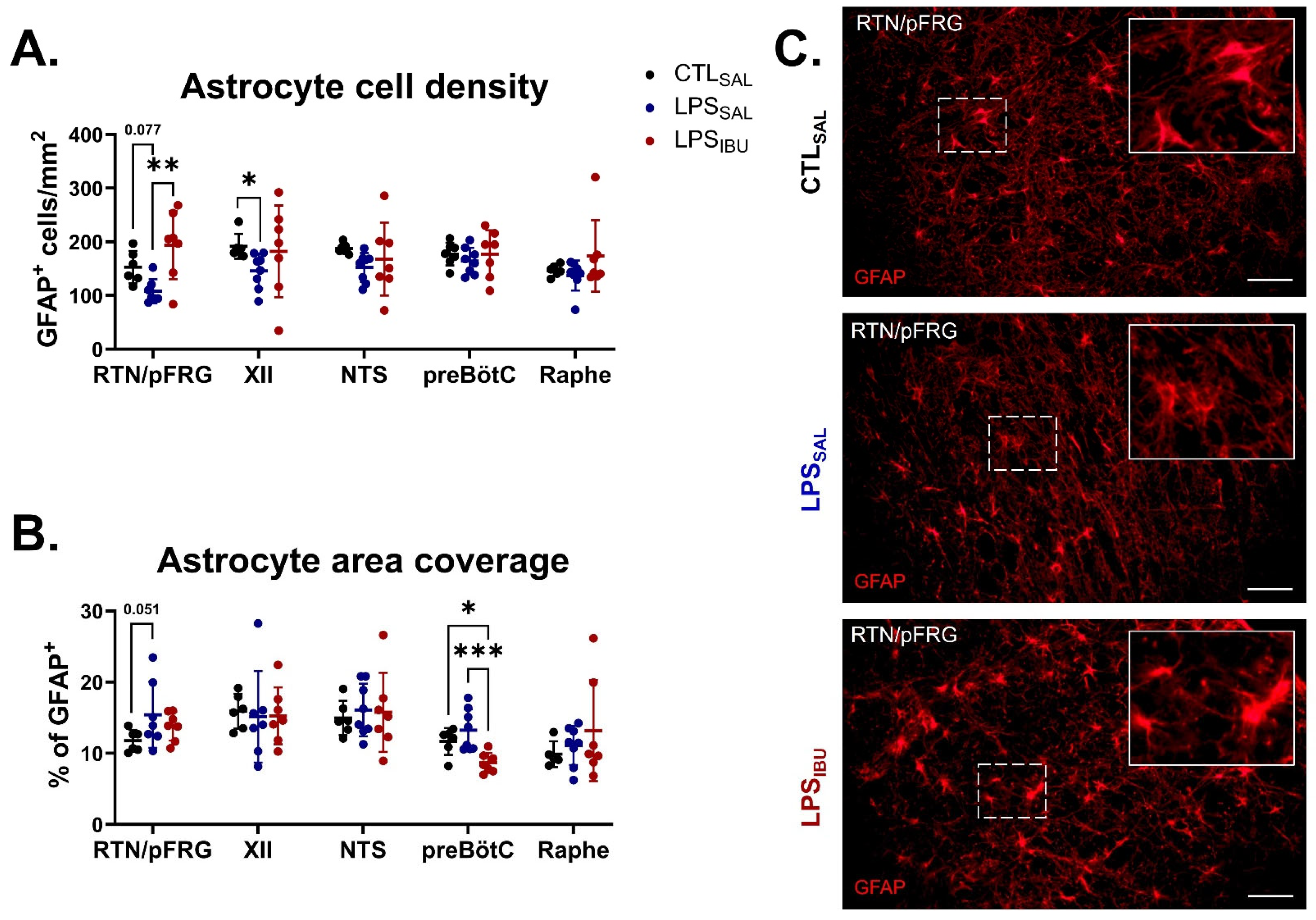
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Animal Experimental Procedures
- Tracheal catheter to measure fetal breathing movements (FBM);
- Right brachial arterial catheter to measure mean arterial blood pressure (MABP) and fetal heart rate (FHR), and for sampling arterial blood gases;
- Right axillary vein catheter to administer postoperative antibiotics, LPS/saline, and ibuprofen;
- Amniotic catheter for fetal MABP correction from maternal movement;
- A right carotid artery ultrasonic flow probe (3 mm) to measure carotid arterial blood flow (CBF; Transonic Systems, Ithaca, NY, USA) and fetal heart rate, which was derived from the carotid arterial beat-to-beat interval.
4.3. Physiology Measurements and Sample Collection
4.4. Immunohistochemistry
4.5. Fluorescent Imaging and Quantitative Analysis
4.6. Plasma and CSF PGE2
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| % | Percent |
| °C | Degrees Celsius |
| µm | Micrometre |
| ANOVA | Analysis of variance |
| CBF | Carotid blood flow |
| CDO2 | Cerebral oxygen delivery |
| COX | Cyclooxygenase |
| CSF | Cerebral spinal fluid |
| CTL | Control |
| CV | Coefficient of variability |
| d/D | Days |
| dGA | Days gestational age |
| ELISA | Enzyme-linked immunosorbent assay |
| FBM | Fetal breathing movement |
| FDA | Federal drug administration |
| FHR | Fetal heart rate |
| GFAP | Glial fibrillary acidic protein |
| g | Grams |
| h | Hour |
| IBA | Ionized calcium binding adaptor molecule |
| IBU | Ibuprofen |
| IL | Interleukin |
| i.v. | Intravenous |
| Kg | Kilogram |
| LPS | Lipopolysaccharide |
| MABP | Mean arterial blood pressure |
| mg | Milligram |
| Min | Minute |
| mm | Millimetre |
| mmHg | Millimetres of mercury |
| mPGES-1 | Microsomal prostaglandin E synthase-1 |
| n | Number of |
| NTS | Nucleus tractus solitarius |
| PGE2 | Prostaglandin E2 |
| PaCO2 | Arterial partial pressure of carbon dioxide |
| PaO2 | Arterial partial pressure of oxygen |
| PBS | Phosphate-buffered saline |
| PDA | Patent ductus arteriosus |
| pFRG | Parafacial respiratory group |
| preBötC | preBötzinger |
| RTN | Retrotrapezoid |
| s | Second |
| SD | Standard deviation |
| SAL | Saline |
| SO2 | Arterial oxygen saturation |
| TNF | Tumor necrosis factor |
| XII | Hypoglossal nucleus |
References
- Tita, A.T.N.; Andrews, W.W. Diagnosis and Management of Clinical Chorioamnionitis. Clin. Perinatol. 2010, 37, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Polglase, G.R.; Nitsos, I.; Baburamani, A.A.; Crossley, K.J.; Slater, M.K.; Gill, A.W.; Allison, B.J.; Moss, T.J.; Pillow, J.J.; Hooper, S.B.; et al. Inflammation in utero exacerbates ventilation-induced brain injury in preterm lambs. J. Appl. Physiol. 2012, 112, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Siljehav, V.; Hofstetter, A.M.; Leifsdottir, K.; Herlenius, E. Prostaglandin E2 Mediates Cardiorespiratory Disturbances During Infection in Neonates. J. Pediatr. 2015, 167, 1207–1213.e1203. [Google Scholar] [CrossRef]
- Chow, S.S.W.; Creighton, P.; Chambers, G.M.; Lui, K. Report of the Australian and New Zealand Neonatal Network 2020; ANZNN: Sydney, Australia, 2022. [Google Scholar]
- Ohlsson, A.; Walia, R.; Shah, S.S. Ibuprofen for the treatment of patent ductus arteriosus in preterm or low birth weight (or both) infants. Cochrane Database Syst. Rev. 2020, 2, Cd003481. [Google Scholar] [CrossRef]
- Guerra, F.A.; Savich, R.D.; Wallen, L.D.; Lee, C.H.; Clyman, R.I.; Mauray, F.E.; Kitterman, J.A. Prostaglandin E2 causes hypoventilation and apnea in newborn lambs. J. Appl. Physiol. 1988, 64, 2160–2166. [Google Scholar] [CrossRef] [PubMed]
- Dawes, G.S. The central control of fetal breathing and skeletal muscle movements. J. Physiol. 1984, 346, 1–18. [Google Scholar] [CrossRef]
- Tai, T.C.; MacLusky, N.J.; Adamson, S.L. Ontogenesis of prostaglandin E2 binding sites in the brainstem of the sheep. Brain Res. 1994, 652, 28–39. [Google Scholar] [CrossRef]
- Wlodek, M.E.; Hooper, S.B.; Thorburn, G.D.; Tester, M.L.; Harding, R. Effects of prostaglandin E2 on renal function and lung liquid dynamics in foetal sheep. Clin. Exp. Pharmacol. Physiol. 1998, 25, 805–812. [Google Scholar] [CrossRef]
- Murai, D.T.; Clyman, R.I.; Mauray, F.E.; Lee, C.C.; Kitterman, J.A. Meclofenamate and prostaglandin E2 affect breathing movements independently of glucose concentrations in fetal sheep. Am. J. Obstet. Gynecol. 1984, 150, 758–764. [Google Scholar] [CrossRef]
- Savich, R.D.; Guerra, F.A.; Lee, C.C.; Kitterman, J.A. Prostaglandin E2 decreases fetal breathing movements, but not pulmonary blood flow, in fetal sheep. J. Appl. Physiol. 1995, 78, 1477–1484. [Google Scholar] [CrossRef]
- Hofstetter, A.O.; Saha, S.; Siljehav, V.; Jakobsson, P.J.; Herlenius, E. The induced prostaglandin E2 pathway is a key regulator of the respiratory response to infection and hypoxia in neonates. Proc. Natl. Acad. Sci. USA. 2007, 104, 9894–9899. [Google Scholar] [CrossRef] [PubMed]
- Stojanovska, V.; Atta, J.; Kelly, S.B.; Zahra, V.A.; Matthews-Staindl, E.; Nitsos, I.; Moxham, A.; Pham, Y.; Hooper, S.B.; Herlenius, E.; et al. Increased Prostaglandin E2 in Brainstem Respiratory Centers Is Associated with Inhibition of Breathing Movements in Fetal Sheep Exposed to Progressive Systemic Inflammation. Front. Physiol. 2022, 13, 841229. [Google Scholar] [CrossRef] [PubMed]
- Sherer, D.M.; Spong, C.Y.; Salafia, C.M. Fetal breathing movements within 24 hours of delivery in prematurity are related to histologic and clinical evidence of amnionitis. Am. J. Perinatol. 1997, 14, 337–340. [Google Scholar] [CrossRef]
- Vintzileos, A.M.; Campbell, W.A.; Nochimson, D.J.; Weinbaum, P.J. Fetal breathing as a predictor of infection in premature rupture of the membranes. Obs. Gynecol. 1986, 67, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Panneflek, T.J.R.; Kuypers, K.; Polglase, G.R.; Hooper, S.B.; van den Akker, T.; Te Pas, A.B. Effect of clinical chorioamnionitis on breathing effort in premature infants at birth: A retrospective case-control study. Arch. Dis. Child. Fetal Neonatal Ed. 2023, 108, 280–285. [Google Scholar] [CrossRef]
- Panneflek, T.J.R.; Kuypers, K.; Polglase, G.R.; Derleth, D.P.; Dekker, J.; Hooper, S.B.; van den Akker, T.; Pas, A.B.T. The influence of chorioamnionitis on respiratory drive and spontaneous breathing of premature infants at birth: A narrative review. Eur. J. Pediatr. 2024, 183, 2539–2547. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [CrossRef]
- Parepally, J.M.; Mandula, H.; Smith, Q.R. Brain uptake of nonsteroidal anti-inflammatory drugs: Ibuprofen, flurbiprofen, and indomethacin. Pharm. Res. 2006, 23, 873–881. [Google Scholar] [CrossRef]
- Wixey, J.A.; Sukumar, K.R.; Pretorius, R.; Lee, K.M.; Colditz, P.B.; Bjorkman, S.T.; Chand, K.K. Ibuprofen Treatment Reduces the Neuroinflammatory Response and Associated Neuronal and White Matter Impairment in the Growth Restricted Newborn. Front. Physiol. 2019, 10, 541. [Google Scholar] [CrossRef]
- Chand, K.K.; Miller, S.M.; Cowin, G.J.; Mohanty, L.; Pienaar, J.; Colditz, P.B.; Bjorkman, S.T.; Wixey, J.A. Neurovascular Unit Alterations in the Growth-Restricted Newborn Are Improved Following Ibuprofen Treatment. Mol. Neurobiol. 2022, 59, 1018–1040. [Google Scholar] [CrossRef]
- Richardson, R.L.; Kim, E.M.; Shephard, R.A.; Gardiner, T.; Cleary, J.; O’Hare, E. Behavioural and histopathological analyses of ibuprofen treatment on the effect of aggregated Abeta(1–42) injections in the rat. Brain Res. 2002, 954, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, S.; Guo, C.; Hu, Z.; Peng, J.; Guo, K.; Zhang, X.; Li, J. Ibuprofen Exerts Antiepileptic and Neuroprotective Effects in the Rat Model of Pentylenetetrazol-Induced Epilepsy via the COX-2/NLRP3/IL-18 Pathway. Neurochem. Res. 2020, 45, 2516–2526. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.B.; Stojanovska, V.; Zahra, V.A.; Moxham, A.; Miller, S.L.; Moss, T.J.M.; Hooper, S.B.; Nold, M.F.; Nold-Petry, C.A.; Dean, J.M.; et al. Interleukin-1 blockade attenuates white matter inflammation and oligodendrocyte loss after progressive systemic lipopolysaccharide exposure in near-term fetal sheep. J. Neuroinflammation 2021, 18, 189. [Google Scholar] [CrossRef]
- Kelly, S.B.; Dean, J.M.; Zahra, V.A.; Dudink, I.; Thiel, A.; Polglase, G.R.; Miller, S.L.; Hooper, S.B.; Bennet, L.; Gunn, A.J.; et al. Progressive inflammation reduces high-frequency EEG activity and cortical dendritic arborisation in late gestation fetal sheep. J. Neuroinflammation 2023, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Hofstetter, A.O.; Herlenius, E. Interleukin-1beta depresses hypoxic gasping and autoresuscitation in neonatal DBA/1lacJ mice. Respir. Physiol. Neurobiol. 2005, 146, 135–146. [Google Scholar] [CrossRef]
- Jafri, A.; Belkadi, A.; Zaidi, S.I.; Getsy, P.; Wilson, C.G.; Martin, R.J. Lung inflammation induces IL-1β expression in hypoglossal neurons in rat brainstem. Respir. Physiol. Neurobiol. 2013, 188, 21–28. [Google Scholar] [CrossRef]
- Olsson, A.; Kayhan, G.; Lagercrantz, H.; Herlenius, E. IL-1β Depresses Respiration and Anoxic Survival via a Prostaglandin-Dependent Pathway in Neonatal Rats. Pediatr. Res. 2003, 54, 326–331. [Google Scholar] [CrossRef]
- Ramirez, S.C.; Koschnitzky, J.E.; Baertsch, N.A.; Anderson, T.M.; Smith, C.V.; Ramirez, J.-M. Disturbances in central respiratory rhythm generation may contribute to breathing disturbances in prematurely born mice. FASEB J. 2016, 30, 987.984. [Google Scholar] [CrossRef]
- Siljehav, V.; Shvarev, Y.; Herlenius, E. Il-1beta and prostaglandin E2 attenuate the hypercapnic as well as the hypoxic respiratory response via prostaglandin E receptor type 3 in neonatal mice. J. Appl. Physiol. 2014, 117, 1027–1036. [Google Scholar] [CrossRef]
- Balan, K.V.; Kc, P.; Mayer, C.A.; Wilson, C.G.; Belkadi, A.; Martin, R.J. Intrapulmonary lipopolysaccharide exposure upregulates cytokine expression in the neonatal brainstem. Acta Paediatr. 2012, 101, 466–471. [Google Scholar] [CrossRef]
- Galic, M.A.; Riazi, K.; Pittman, Q.J. Cytokines and brain excitability. Front. Neuroendocrinol. 2012, 33, 116–125. [Google Scholar] [CrossRef]
- Connors, G.; Hunse, C.; Carmichael, L.; Natale, R.; Richardson, B. The role of carbon dioxide in the generation of human fetal breathing movements. Am. J. Obstet. Gynecol. 1988, 158, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, D.; Horn, Z.; Tserga, E.; Smedler, E.; Silberberg, G.; Shvarev, Y.; Kaila, K.; Uhlén, P.; Herlenius, E. CO2-evoked release of PGE2 modulates sighs and inspiration as demonstrated in brainstem organotypic culture. eLife 2016, 5, e14170. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Caughie, C.; Elsen, F.P.; Doi, A.; Garcia, A.J., 3rd; Zanella, S.; Ramirez, J.M. Prostaglandin E2 differentially modulates the central control of eupnoea, sighs and gasping in mice. J. Physiol. 2015, 593, 305–319. [Google Scholar] [CrossRef]
- Stojanovska, V.; Miller, S.L.; Hooper, S.B.; Polglase, G.R. The Consequences of Preterm Birth and Chorioamnionitis on Brainstem Respiratory Centers: Implications for Neurochemical Development and Altered Functions by Inflammation and Prostaglandins. Front. Cell Neurosci. 2018, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Cimino, P.J.; Keene, C.D.; Breyer, R.M.; Montine, K.S.; Montine, T.J. Therapeutic targets in prostaglandin E2 signaling for neurologic disease. Curr. Med. Chem. 2008, 15, 1863–1869. [Google Scholar] [CrossRef]
- Polglase, G.R.; Wallace, M.J.; Grant, D.A.; Hooper, S.B. Influence of Fetal Breathing Movements on Pulmonary Hemodynamics in Fetal Sheep. Pediatr. Res. 2004, 56, 932–938. [Google Scholar] [CrossRef]
- Badurdeen, S.; Gill, A.W.; Kluckow, M.; Roberts, C.T.; Galinsky, R.; Klink, S.; Miller, S.L.; Davis, P.G.; Schmölzer, G.M.; Hooper, S.B.; et al. Excess cerebral oxygen delivery follows return of spontaneous circulation in near-term asphyxiated lambs. Sci. Rep. 2020, 10, 16443. [Google Scholar] [CrossRef]
- Johnson, J.K.; Sudheimer, K.D.; Davis, K.K.; Kerndt, G.M.; Winn, B.M. The Sheep Brain Atlas. Available online: https://brains.anatomy.msu.edu/brains/sheep/index.html (accessed on 31 August 2021).
- Paxinos, G. The Rat Brain in Stereotaxic Coordinates, 2nd ed.; Academic Press: Sydney, Australia, 1986. [Google Scholar]

| CTLSAL | LPSSAL | LPSIBU | ||
|---|---|---|---|---|
| Number (n) | 9 | 8 | 8 | |
| Male/Female | 7:2 | 7:1 | 5:3 | |
| Single/Twin | 6:3 | 5:3 | 8:0 | p-Value |
| Body weight (kg) | 4.55 ± 0.60 | 4.56 ± 0.49 | 4.84 ± 0.38 | 0.196 |
| Brain weight (g) | 51.47 ± 3.70 | 48.77 ± 3.83 | 47.73 ± 3.39 | 0.131 |
| Brain/Body ratio | 11.40 ± 1.77 | 10.75 ± 0.77 | 9.88 ± 0.44 | 0.088 |
| Heart weight (g) | 31.98 ± 5.75 | 32.94 ± 4.97 | 40.20 ± 3.07 **## | 0.004 |
| Heart/Body ratio | 7.06 ± 1.07 | 7.26 ± 1.03 | 8.37 ± 1.02 # | 0.046 |
| Lungs (g) | 180.00 ± 27.17 | 156.00 ± 17.00 | 178.83 ± 41.85 | 0.219 |
| Lungs/Body Ratio | 39.80 ± 5.63 | 34.34 ± 3.05 | 37.02 ± 8.68 | 0.104 |
| Liver (g) | 131.10 ± 30.06 | 134.40 ± 21.41 | 142.38 ± 23.48 | 0.433 |
| Right Kidney (g) | 12.39 ± 3.03 | 12.66 ± 1.50 | 13.60 ± 1.94 | 0.537 |
| Blood gases at end of experiment | ||||
| pH | 7.4 ± 0.0 | 7.3 ± 0.0 | 7.3 ± 0.0 | 0.136 |
| PaO2 (mmHg) | 24.1 ± 2.6 | 23.4 ± 1.7 | 21.3 ± 4.3 | 0.192 |
| PaCO2 (mmHg) | 47.2 ± 3.5 | 48.2 ± 1.9 | 51.2 ± 3.3 # | 0.039 |
| SaO2 (%) | 64.6 ± 7.4 | 61.7 ± 5.7 | 60.6 ± 14.8 | 0.716 |
| Lactate (mmol.L−1) | 1.5 ± 0.4 | 1.0 ± 0.2 | 1.5 ± 0.8 | 0.161 |
| Glucose (mmol.L−1) | 0.9 ± 0.1 | 0.9 ± 0.2 | 1.2 ± 0.3 | 0.056 |
| Group | ||||
|---|---|---|---|---|
| CTLSAL | LPSSAL | LPSIBU | p-Value | |
| Breathing incidence (%) | 44.0 ± 16.2 | 38.6 ± 26.4 | 28.9 ± 19.9 | 0.346 |
| Breathing duration (s) | 75.5 ± 70.2 | 192.8 ± 171.7 | 145.6 ± 85.0 | 0.152 |
| Breathing frequency (breaths/s) | 2.5 ± 0.2 | 4.0 ± 3.4 | 2.7 ± 1.2 | 0.967 |
| Breathing amplitude (mmHg) | 6.6 ± 2.9 | 5.8 ± 2.8 | 4.2 ± 1.5 | 0.230 |
| Accentuated breathing (% of time spent breathing) | 7.5 ± 4.2 | 34.4 ± 30.6 | 10.3 ± 10.0 | 0.233 |
| CBF (mL/min) | 72.3 ± 9.8 | 90.7 ± 26.2 | 64.0 ± 16.1 | 0.085 |
| CDO2 (mL/g/min) | 11.6 ± 1.9 | 16.5 ± 5.6 | 12.4 ± 2.5 | 0.099 |
| MABP (mmHg) | 39.6 ± 7.0 | 44.2 ± 2.9 | 43.5 ± 6.3 | 0.230 |
| FHR (bpm) | 155.0 ± 14.4 | 146.2 ± 31.5 | 158.1 ± 12.6 | 0.624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.T.; Stojanovska, V.; Kelly, S.B.; Vidinopoulos, K.; Atta, J.; Matthews-Staindl, E.; Zahra, V.A.; Pham, Y.; Herlenius, E.A.P.; Hooper, S.B.; et al. Ibuprofen Does Not Prevent Inhibition of Fetal Breathing Movements Caused by Intrauterine Inflammation in Fetal Sheep. Int. J. Mol. Sci. 2025, 26, 5591. https://doi.org/10.3390/ijms26125591
Tran NT, Stojanovska V, Kelly SB, Vidinopoulos K, Atta J, Matthews-Staindl E, Zahra VA, Pham Y, Herlenius EAP, Hooper SB, et al. Ibuprofen Does Not Prevent Inhibition of Fetal Breathing Movements Caused by Intrauterine Inflammation in Fetal Sheep. International Journal of Molecular Sciences. 2025; 26(12):5591. https://doi.org/10.3390/ijms26125591
Chicago/Turabian StyleTran, Nhi T., Vanesa Stojanovska, Sharmony B. Kelly, Kayla Vidinopoulos, John Atta, Eva Matthews-Staindl, Valerie A. Zahra, Yen Pham, Eric A. P. Herlenius, Stuart B. Hooper, and et al. 2025. "Ibuprofen Does Not Prevent Inhibition of Fetal Breathing Movements Caused by Intrauterine Inflammation in Fetal Sheep" International Journal of Molecular Sciences 26, no. 12: 5591. https://doi.org/10.3390/ijms26125591
APA StyleTran, N. T., Stojanovska, V., Kelly, S. B., Vidinopoulos, K., Atta, J., Matthews-Staindl, E., Zahra, V. A., Pham, Y., Herlenius, E. A. P., Hooper, S. B., Allison, B. J., Galinsky, R., & Polglase, G. R. (2025). Ibuprofen Does Not Prevent Inhibition of Fetal Breathing Movements Caused by Intrauterine Inflammation in Fetal Sheep. International Journal of Molecular Sciences, 26(12), 5591. https://doi.org/10.3390/ijms26125591










