Food-Derived Phytochemicals: Multicultural Approaches to Oxidative Stress and Immune Response
Abstract
1. Introduction
2. Lentinan
2.1. Lentinan Mechanism of Action
2.2. Lentinan Administration and Contradcitions
3. Panax Ginseng
3.1. Ginseng Mechanism of Action
3.2. Panax Ginseng Administration and Contraindications
4. Turmeric
4.1. Chemical, In Vitro, and In Vivo Antioxidative Characteristics
4.2. Conclusions on Curcumin
5. Black Cumin
5.1. Chemical, In Vitro, and In Vivo Antioxidative Characteristics
5.2. Conclusions on Black Seed
6. Berries and Moringa Oleifera
6.1. Quercetin
6.2. Kaempferol
6.3. Chlorogenic Acid
6.4. Combination of Quercetin, Kaempferol, and Pterostilbene
7. Holy Basil
Holy Basil Mechanism of Action
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| STZ | Streptozotocin |
| APC | Antigen-presenting cells |
| IFN-γ | Interferon gamma |
| NSCLC | Non-small-cell lung cancer |
| Nrf2 | Nuclear factor E2-related factor 2 |
| CMI | Cell-mediated immune response |
| MPE | Malignant pleural effusion |
| IBD | Irritable bowel disease |
| DSS | Dextran sodium sulfate |
| LDL | Low-density lipoprotein |
| HDL | High-density lipoprotein |
| PKC | Protein kinase C |
| SOD | Superoxide dismutase |
| GPX | Glutathione peroxidase |
| HbA1c | Glycated hemoglobin |
| iNOS | Inducible nitric oxide synthase |
| HO-1 | Heme oxygenase 1 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| PT | Prothrombin time |
| INR | International normalized ratio |
| AGE | Advanced glycation end-products |
References
- Cooper, G.M. The development and causes of cancer. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Shang, Q.; Yu, X.; Sun, Q.; Li, H.; Sun, C.; Liu, L. Polysaccharides regulate th1/th2 balance: A new strategy for tumor immunotherapy. Biomed. Pharmacother. 2024, 170, 115976. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, Y.; Jiang, J.; Chen, Y.; Wei, C. Identification of the prognostic value of th1/th2 ratio and a novel prognostic signature in basal-like breast cancer. Hereditas 2023, 160, 2. [Google Scholar] [CrossRef]
- Geindreau, M.; Bruchard, M.; Vegran, F. Role of cytokines and chemokines in angiogenesis in a tumor context. Cancers 2022, 14, 2446. [Google Scholar] [CrossRef]
- Liu, W.; Gong, X.; Luo, J.; Jiang, L.; Lu, W.; Pan, C.; Yao, W.; Gao, X.; Tian, H. A purified acidic polysaccharide from sarcandra glabra as vaccine adjuvant to enhance anti-tumor effect of cancer vaccine. Carbohydr. Polym. 2021, 263, 117967. [Google Scholar] [CrossRef]
- Zhou, G.; Liu, H.; Yuan, Y.; Wang, Q.; Wang, L.; Wu, J. Lentinan progress in inflammatory diseases and tumor diseases. Eur. J. Med. Res. 2024, 29, 8. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Zhang, G.; Kuai, J.; Fan, P.; Wang, X.; Zhou, P.; Yang, D.; Zheng, X.; Liu, X.; Wu, Q.; et al. Lentinan inhibits tumor angiogenesis via interferon gamma and in a t cell independent manner. J. Exp. Clin. Cancer Res. 2018, 37, 260, Correction in J. Exp. Clin. Cancer Res. 2021, 40, 366. [Google Scholar] [CrossRef]
- Song, Z.; Luo, W.; Zheng, H.; Zeng, Y.; Wang, J.; Chen, T. Translational nanotherapeutics reprograms immune microenvironment in malignant pleural effusion of lung adenocarcinoma. Adv. Healthc. Mater. 2021, 10, e2100149. [Google Scholar] [CrossRef]
- Wang, X.E.; Wang, Y.H.; Zhou, Q.; Peng, M.; Zhang, J.; Chen, M.; Ma, L.J.; Xie, G.M. Immunomodulatory effect of lentinan on aberrant t subsets and cytokines profile in non-small cell lung cancer patients. Pathol. Oncol. Res. 2020, 26, 499–505. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Zhao, Y.; Zong, S.; Tian, Y.; Chen, S.; Li, M.; Liu, H.; Zhang, Q.; Jing, X.; et al. Therapeutic effects of lentinan on inflammatory bowel disease and colitis-associated cancer. J. Cell. Mol. Med. 2019, 23, 750–760, Correction in J. Cell. Mol. Med. 2023, 27, 163. [Google Scholar] [CrossRef]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in inflammatory bowel disease: Pathogenic role and potential therapeutic targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A common factor in human diseases. Biomed. Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zheng, M.; Zhou, M.; Zhou, L.; Ge, X.; Pang, N.; Li, H.; Li, X.; Li, M.; Zhang, J.; et al. Lentinan supplementation protects the gut-liver axis and prevents steatohepatitis: The role of gut microbiota involved. Front. Nutr. 2021, 8, 803691. [Google Scholar]
- Chong, W.P.; Mattapallil, M.J.; Raychaudhuri, K.; Bing, S.J.; Wu, S.; Zhong, Y.; Wang, W.; Chen, Z.; Silver, P.B.; Jittayasothorn, Y.; et al. The cytokine il-17a limits th17 pathogenicity via a negative feedback loop driven by autocrine induction of il-24. Immunity 2020, 53, 384–397.e5. [Google Scholar] [CrossRef]
- Ross, D.; Siegel, D. The diverse functionality of nqo1 and its roles in redox control. Redox Biol. 2021, 41, 101950. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, S.; Nie, Y.; Chen, R.; Chen, P. Lentinan alleviates arsenic-induced hepatotoxicity in mice via downregulation of ox40/il-17a and activation of nrf2 signaling. BMC Pharmacol. Toxicol. 2022, 23, 16. [Google Scholar] [CrossRef]
- Gordon, M.; Bihari, B.; Goosby, E.; Gorter, R.; Greco, M.; Guralnik, M.; Mimura, T.; Rudinicki, V.; Wong, R.; Kaneko, Y. A placebo-controlled trial of the immune modulator, lentinan, in hiv-positive patients: A phase i/ii trial. J. Med. 1998, 29, 305–330. [Google Scholar] [PubMed]
- deVere White, R.W.; Hackman, R.M.; Soares, S.E.; Beckett, L.A.; Sun, B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology 2002, 60, 640–644. [Google Scholar] [CrossRef]
- Gabriel, M.F.; Gonzalez-Delgado, P.; Postigo, I.; Fernandez, J.; Soriano, V.; Cueva, B.; Martinez, J. From respiratory sensitization to food allergy: Anaphylactic reaction after ingestion of mushrooms (agaricus bisporus). Med. Mycol. Case Rep. 2015, 8, 14–16. [Google Scholar] [CrossRef]
- Lin, Y.; Wei, Y.; Hu, X.; Wu, M.; Yao, J.; Ying, X.; Fu, X.; Ding, M.; Qiao, L. Evaluation of lentinan effects on cytochrome p450 activity in rats by a cocktail method. Iran. J. Basic. Med. Sci. 2019, 22, 296–301. [Google Scholar]
- Clark, N.P.; Hoang, K.; Delate, T.; Horn, J.R.; Witt, D.M. Warfarin interaction with hepatic cytochrome p-450 enzyme-inducing anticonvulsants. Clin. Appl. Thromb. Hemost. 2018, 24, 172–178. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Bohm, M. Advanced glycation end products: Key players in skin aging? Dermatoendocrinol 2012, 4, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef]
- Hsu, R.K.; Hsu, C.Y. Proteinuria and reduced glomerular filtration rate as risk factors for acute kidney injury. Curr. Opin. Nephrol. Hypertens. 2011, 20, 211–217. [Google Scholar] [CrossRef]
- Yun, T.K. Brief introduction of panax ginseng c.A. Meyer. J. Korean Med. Sci. 2001, 16, S3-5. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.M.; Han, S.B.; Kim, N.Y.; Surh, Y.J.; Lee, S.K.; Kim, N.D.; Park, M.K. A new processed ginseng with fortified activity. In Proceedings of the Ginseng Society Conference; The Korean Society of Ginseng: Seoul, Republic of Korea, 1975; pp. 146–159. [Google Scholar]
- Chen, H.; Yin, J.; Deng, Y.; Yang, M.; Xu, L.; Teng, F.; Li, D.; Cheng, Y.; Liu, S.; Wang, D.; et al. The protective effects of ginsenoside rg1 against hypertension target-organ damage in spontaneously hypertensive rats. BMC Complement. Altern. Med. 2012, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Chung, W.S.; Lee, S.K.; Leung, A.W.; Cheng, C.H.; Yue, K.K. Ginsenoside re of panax ginseng possesses significant antioxidant and antihyperlipidemic efficacies in streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2006, 550, 173–179. [Google Scholar] [CrossRef]
- Wang, G.; Lei, C.; Tian, Y.; Wang, Y.; Zhang, L.; Zhang, R. Rb1, the primary active ingredient in panax ginseng c.A. Meyer, exerts antidepressant-like effects via the bdnf-trkb-creb pathway. Front. Pharmacol. 2019, 10, 1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.A.; Wang, M.; Zhou, J.; Yao, Q.Y.; Ma, J.M.; Jiang, C.L. Protective effect of ginsenoside against acute renal failure and expression of tyrosine hydroxylase in the locus coeruleus. Physiol. Res. 2010, 59, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Meng, Q.T.; Jiang, Y.; Xia, Z.Y. Ginsenoside rb1 attenuates intestinal ischemia reperfusion induced renal injury by activating nrf2/are pathway. Molecules 2012, 17, 7195–7205. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Singh, D.; Kim, S.H.; Lee, S.J.; Lee, C.H. Ultrahigh pressure processing produces alterations in the metabolite profiles of panax ginseng. Molecules 2016, 21, 816. [Google Scholar] [CrossRef]
- Kang, K.S.; Ham, J.; Kim, Y.J.; Park, J.H.; Cho, E.J.; Yamabe, N. Heat-processed panax ginseng and diabetic renal damage: Active components and action mechanism. J. Ginseng Res. 2013, 37, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Owada, S. Effect of ginsenoside-rd in cephaloridine-induced renal disorder. Nephron 1999, 81, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Liu, Z.W.; Dong, E. A study of ginsenoside-rd in a renal ischemia-reperfusion model. Nephron 1998, 78, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.S.; Kang, K.S.; Kim, S.Y. Panax ginseng pharmacopuncture: Current status of the research and future challenges. Biomolecules 2019, 10, 33. [Google Scholar] [CrossRef]
- Yuan, C.S.; Wei, G.; Dey, L.; Karrison, T.; Nahlik, L.; Maleckar, S.; Kasza, K.; Ang-Lee, M.; Moss, J. Brief communication: American ginseng reduces warfarin’s effect in healthy patients: A randomized, controlled trial. Ann. Intern. Med. 2004, 141, 23–27. [Google Scholar] [CrossRef]
- Malati, C.Y.; Robertson, S.M.; Hunt, J.D.; Chairez, C.; Alfaro, R.M.; Kovacs, J.A.; Penzak, S.R. Influence of panax ginseng on cytochrome p450 (cyp)3a and p-glycoprotein (p-gp) activity in healthy participants. J. Clin. Pharmacol. 2012, 52, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Paik, D.J.; Lee, C.H. Review of cases of patient risk associated with ginseng abuse and misuse. J. Ginseng Res. 2015, 39, 89–93. [Google Scholar] [CrossRef]
- Qin, S.; Huang, L.; Gong, J.; Shen, S.; Huang, J.; Ren, H.; Hu, H. Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutr. J. 2017, 16, 68. [Google Scholar] [CrossRef]
- Kocaadam, B.; Sanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative stress in human pathology and aging: Molecular mechanisms and perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gulcin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Keramat, M.; Golmakani, M.T. Antioxidant potency and inhibitory mechanism of curcumin and its derivatives in oleogel and emulgel produced by linseed oil. Food Chem. 2024, 445, 138754. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The molecular mechanisms regulating the keap1-nrf2 pathway. Mol. Cell Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bai, D.; Wei, Z.; Zhang, Y.; Huang, Y.; Deng, H.; Huang, X. Curcumin attenuates oxidative stress in raw264.7 cells by increasing the activity of antioxidant enzymes and activating the nrf2-keap1 pathway. PLoS ONE 2019, 14, e0216711. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Rokavec, M.; Huang, Z.; Hermeking, H. Curcumin activates a ros/keap1/nrf2/mir-34a/b/c cascade to suppress colorectal cancer metastasis. Cell Death Differ. 2023, 30, 1771–1785. [Google Scholar] [CrossRef]
- Al-Rubaei, Z.M.; Mohammad, T.U.; Ali, L.K. Effects of local curcumin on oxidative stress and total antioxidant capacity in vivo study. Pak. J. Biol. Sci. 2014, 17, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Pullarkat, V.; Meng, Z.; Tahara, S.M.; Johnson, C.S.; Kalra, V.K. Proteasome inhibition induces both antioxidant and hb f responses in sickle cell disease via the nrf2 pathway. Hemoglobin 2014, 38, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Druzga, A.; Katarzyna, J.; Skonieczna-Zydecka, K. Antioxidant potential of curcumin-a meta-analysis of randomized clinical trials. Antioxidants 2020, 9, 1092. [Google Scholar] [CrossRef]
- Boshagh, K.; Khorvash, F.; Sahebkar, A.; Majeed, M.; Bahreini, N.; Askari, G.; Bagherniya, M. The effects of curcumin-piperine supplementation on inflammatory, oxidative stress and metabolic indices in patients with ischemic stroke in the rehabilitation phase: A randomized controlled trial. Nutr. J. 2023, 22, 69. [Google Scholar] [CrossRef]
- Sharifi, S.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Atkin, S.L.; Jamialahmadi, T.; Sahebkar, A.; Askari, G. Efficacy of curcumin plus piperine co-supplementation in moderate-to-high hepatic steatosis: A double-blind, randomized, placebo-controlled clinical trial. Phytother. Res. 2023, 37, 2217–2229. [Google Scholar] [CrossRef]
- Li, G.; Fang, S.; Shao, X.; Li, Y.; Tong, Q.; Kong, B.; Chen, L.; Wang, Y.; Yang, J.; Yu, H.; et al. Curcumin reverses nnmt-induced 5-fluorouracil resistance via increasing ros and cell cycle arrest in colorectal cancer cells. Biomolecules 2021, 11, 1295. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, M.; Zandieh Doulabi, B.; Sun, Y.; Liu, Y. Paradox: Curcumin, a natural antioxidant, suppresses osteosarcoma cells via excessive reactive oxygen species. Int. J. Mol. Sci. 2023, 24, 11975. [Google Scholar] [CrossRef] [PubMed]
- El Assar, M.; Alvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodriguez-Manas, L. Effect of physical activity/exercise on oxidative stress and inflammation in muscle and vascular aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of curcumin in skin disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef]
- Kooti, W.; Hasanzadeh-Noohi, Z.; Sharafi-Ahvazi, N.; Asadi-Samani, M.; Ashtary-Larky, D. Phytochemistry, pharmacology, and therapeutic uses of black seed (Nigella sativa). Chin. J. Nat. Med. 2016, 14, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Sakib, R.; Caruso, F.; Aktar, S.; Belli, S.; Kaur, S.; Hernandez, M.; Rossi, M. Antioxidant properties of thymoquinone, thymohydroquinone and black cumin (Nigella sativa L.) seed oil: Scavenging of superoxide radical studied using cyclic voltammetry, dft and single crystal x-ray diffraction. Antioxidants 2023, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Velagapudi, R.; Kumar, A.; Bhatia, H.S.; El-Bakoush, A.; Lepiarz, I.; Fiebich, B.L.; Olajide, O.A. Inhibition of neuroinflammation by thymoquinone requires activation of nrf2/are signalling. Int. Immunopharmacol. 2017, 48, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, X.; Wang, S.; Xu, C.; Gao, M.; Liu, S.; Li, X.; Cheng, N.; Han, Y.; Wang, X.; et al. Thymoquinone prevents dopaminergic neurodegeneration by attenuating oxidative stress via the nrf2/are pathway. Front. Pharmacol. 2020, 11, 615598. [Google Scholar] [CrossRef] [PubMed]
- Sabir, S.; Saleem, U.; Akash, M.S.H.; Qasim, M.; Chauhdary, Z. Thymoquinone induces nrf2 mediated adaptive homeostasis: Implication for mercuric chloride-induced nephrotoxicity. ACS Omega 2022, 7, 7370–7379. [Google Scholar] [CrossRef]
- Dogru, S.; Taysi, S.; Yucel, A. Effects of thymoquinone in the lungs of rats against radiation-induced oxidative stress. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 191–198. [Google Scholar]
- Ardiana, M.; Pikir, B.S.; Santoso, A.; Hermawan, H.O.; Al-Farabi, M.J. Effect of Nigella sativa supplementation on oxidative stress and antioxidant parameters: A meta-analysis of randomized controlled trials. Sci. World J. 2020, 2020, 2390706. [Google Scholar] [CrossRef] [PubMed]
- Shoaei-Hagh, P.; Kamelan Kafi, F.; Najafi, S.; Zamanzadeh, M.; Heidari Bakavoli, A.; Ramezani, J.; Soltanian, S.; Asili, J.; Hosseinzadeh, H.; Eslami, S.; et al. A randomized, double-blind, placebo-controlled, clinical trial to evaluate the benefits of Nigella sativa seeds oil in reducing cardiovascular risks in hypertensive patients. Phytother. Res. 2021, 35, 4388–4400. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Chen, P.; Sheikh, S.; Ahmad, A.; Ahmad, M.; Paithankar, M.; Desai, B.; Patel, P.; Khan, M.; Chaturvedi, A.; et al. Thymoquinone with metformin decreases fasting, post prandial glucose, and hba1c in type 2 diabetic patients. Drug Res. 2021, 71, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the nrf2-are signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, L.Y.; Tang, F.; Liu, D.; Zhao, X.L.; Zhang, J.N.; Xia, J.; Wu, J.J.; Yang, Y.; Peng, C.; et al. New perspectives on the therapeutic potential of quercetin in non-communicable diseases: Targeting nrf2 to counteract oxidative stress and inflammation. J. Pharm. Anal. 2024, 14, 100930. [Google Scholar] [CrossRef]
- Patil, S.V.; Mohite, B.V.; Marathe, K.R.; Salunkhe, N.S.; Marathe, V.; Patil, V.S. Moringa tree, gift of nature: A review on nutritional and industrial potential. Curr. Pharmacol. Rep. 2022, 8, 262–280. [Google Scholar] [CrossRef]
- Fahey, J.W.; Wade, K.L.; Stephenson, K.K.; Shi, Y.; Liu, H.; Panjwani, A.A.; Warrick, C.R.; Olson, M.E. A strategy to deliver precise oral doses of the glucosinolates or isothiocyanates from moringa oleifera leaves for use in clinical studies. Nutrients 2019, 11, 1547. [Google Scholar] [CrossRef]
- Jaja-Chimedza, A.; Graf, B.L.; Simmler, C.; Kim, Y.; Kuhn, P.; Pauli, G.F.; Raskin, I. Biochemical characterization and anti-inflammatory properties of an isothiocyanate-enriched moringa (Moringa oleifera) seed extract. PLoS ONE 2017, 12, e0182658. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J.; Hartman, M.J. Review of the safety and efficacy of moringa oleifera. Phytother. Res. 2015, 29, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.; Pearce, K. Mitochondrial dysfunction and diabetes in south africa: A review. Endocr. Metab. Sci. 2024, 14, 100157. [Google Scholar] [CrossRef]
- Wang, F.; Bao, Y.; Shen, X.; Zengin, G.; Lyu, Y.; Xiao, J.; Weng, Z. Niazirin from moringa oleifera lam. Attenuates high glucose-induced oxidative stress through pkczeta/nox4 pathway. Phytomedicine 2021, 86, 153066. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Abad-Jimenez, Z.; Maranon, A.M.; Iannantuoni, F.; Escribano-Lopez, I.; Lopez-Domenech, S.; Salom, C.; Jover, A.; Mora, V.; Roldan, I.; et al. Relationship between oxidative stress, er stress, and inflammation in type 2 diabetes: The battle continues. J. Clin. Med. 2019, 8, 1385. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent advances in potential health benefits of quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yu, H.; Yang, Y.; Li, M.; Hang, L.; Xu, X. A solid dispersion of quercetin shows enhanced nrf2 activation and protective effects against oxidative injury in a mouse model of dry age-related macular degeneration. Oxid. Med. Cell. Longev. 2019, 2019, 1479571. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological potential of kaempferol, a flavonoid in the management of pathogenesis via modulation of inflammation and other biological activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alsharif, K.F.; Hayat Khan, A.; Aschner, M.; Saso, L. The therapeutic potential of kaemferol and other naturally occurring polyphenols might be modulated by nrf2-are signaling pathway: Current status and future direction. Molecules 2022, 27, 4145. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Ma, J.Q.; Xie, W.R.; Liu, S.S.; Feng, Z.J.; Zheng, G.H.; Wang, A.M. Quercetin protects mouse liver against nickel-induced DNA methylation and inflammation associated with the nrf2/ho-1 and p38/stat1/nf-kappab pathway. Food Chem. Toxicol. 2015, 82, 19–26. [Google Scholar] [CrossRef]
- Alshehri, A.S. Kaempferol attenuates diabetic nephropathy in streptozotocin-induced diabetic rats by a hypoglycaemic effect and concomitant activation of the nrf-2/ho-1/antioxidants axis. Arch. Physiol. Biochem. 2023, 129, 984–997. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Arias-Sante, M.F.; Fuentes, J. Oxidation of quercetin and kaempferol markedly amplifies their antioxidant, cytoprotective, and anti-inflammatory properties. Antioxidants 2023, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Lapshina, E.A.; Zavodnik, I.B. Comparative analysis of molecular properties and reactions with oxidants for quercetin, catechin, and naringenin. Mol. Cell Biochem. 2021, 476, 4287–4299. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Yang, C.C.; Hsiao, L.D.; Wang, C.Y.; Lin, W.N.; Shih, Y.F.; Chen, Y.W.; Cho, R.L.; Tseng, H.C.; Yang, C.M. Ho-1 upregulation by kaempferol via ros-dependent nrf2-are cascade attenuates lipopolysaccharide-mediated intercellular cell adhesion molecule-1 expression in human pulmonary alveolar epithelial cells. Antioxidants 2022, 11, 782. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Salerno, N.; Scalise, M.; Salerno, L.; Torella, A.; Molinaro, C.; Chiefalo, A.; Filardo, A.; Siracusa, C.; Panuccio, G.; et al. Streptozotocin-induced type 1 and 2 diabetes mellitus mouse models show different functional, cellular and molecular patterns of diabetic cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 1132. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ros) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K. Chlorogenic acid stimulates glucose transport in skeletal muscle via ampk activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef]
- Bender, O.; Atalay, A. Chapter 28—Polyphenol chlorogenic acid, antioxidant profile, and breast cancer. In Cancer: Oxidative Stress and Dietary Antioxidants, 2nd ed.; Preedy, V.R., Patel, V.B., Eds.; Biotechnology Institute, Ankara University: Ankara, Turkey, 2021; pp. 311–321. [Google Scholar]
- Tomiyama, H.; Kushiro, T.; Okazaki, R.; Yoshida, H.; Doba, N.; Yamashina, A. Influences of increased oxidative stress on endothelial function, platelets function, and fibrinolysis in hypertension associated with glucose intolerance. Hypertens. Res. 2003, 26, 295–300. [Google Scholar] [CrossRef]
- Wang, D.; Hou, J.; Wan, J.; Yang, Y.; Liu, S.; Li, X.; Li, W.; Dai, X.; Zhou, P.; Liu, W.; et al. Dietary chlorogenic acid ameliorates oxidative stress and improves endothelial function in diabetic mice via nrf2 activation. J. Int. Med. Res. 2021, 49, 300060520985363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, L.; Ruan, Z.; Mi, S.; Jiang, M.; Li, X.; Wu, X.; Deng, Z.; Yin, Y. Chlorogenic acid ameliorates intestinal mitochondrial injury by increasing antioxidant effects and activity of respiratory complexes. Biosci. Biotechnol. Biochem. 2016, 80, 962–971. [Google Scholar] [CrossRef]
- Pearson, R.A.; Wicha, S.G.; Okour, M. Drug combination modeling: Methods and applications in drug development. J. Clin. Pharmacol. 2023, 63, 151–165. [Google Scholar] [CrossRef]
- Cohen, M.M. Tulsi—Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014, 5, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sarkar, B. In silico approach to assessing the polyphenols from krishna tulsi (Ocimum tenuiflorum L.) as a keap1/nrf2 receptor for the treatment of inflammatory bowel disease. Med. Sci. Forum 2023, 21, 13. [Google Scholar]
- Ponnusam, Y.; Louis, T.; Madhavachandran, V.; Kumar, S.; Thoprani, N.; Hamblin, M.R.; Lakshmanan, M. Antioxidant activity of the ancient herb, holy basil in ccl4-induced liver injury in rats. Ayurvedic 2015, 2, 178. [Google Scholar] [CrossRef]
- De Rango, P.; Estrera, A.L.; Miller, C., III; Lee, T.Y.; Keyhani, K.; Abdullah, S.; Safi, H. Operative outcomes using a side-branched thoracoabdominal aortic graft (stag) for thoraco-abdominal aortic repair. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 41–47. [Google Scholar] [CrossRef]
- Halim, E.M.; Mukhopadhyay, A.K. Effect ofocimum sanctum (tulsi) and vitamin e on biochemical parameters and retinopathy in streptozotocin induced diabetic rats. Indian. J. Clin. Biochem. 2006, 21, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, T.; Ye, H.; Hao, H.; Zhang, H.; Zhao, C. In vitro probiotic screening and evaluation of space-induced mutant lactobacillus plantarum. Food Sci. Nutr. 2020, 8, 6031–6036. [Google Scholar] [CrossRef] [PubMed]
- Sohilait, H.J.; Kainama, H. Free radical scavenging activity of essential oil of eugenia caryophylata from amboina island and derivatives of eugenol. Open Chem. 2019, 17, 422–428. [Google Scholar] [CrossRef]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Kepka, S.; Lemaitre, L.; Marx, T.; Bilbault, P.; Desmettre, T. A common gesture with a rare but potentially severe complication: Re-expansion pulmonary edema following chest tube drainage. Respir. Med. Case Rep. 2019, 27, 100838. [Google Scholar] [CrossRef]
- Jilani, A.; Hussain, S.Z.; Melaibari, A.A.; Abu-Hamdeh, N.H. Development and mechanistic studies of ternary nanocomposites for hydrogen production from water splitting to yield sustainable/green energy and environmental remediation. Polymers 2022, 14, 1290. [Google Scholar] [CrossRef]
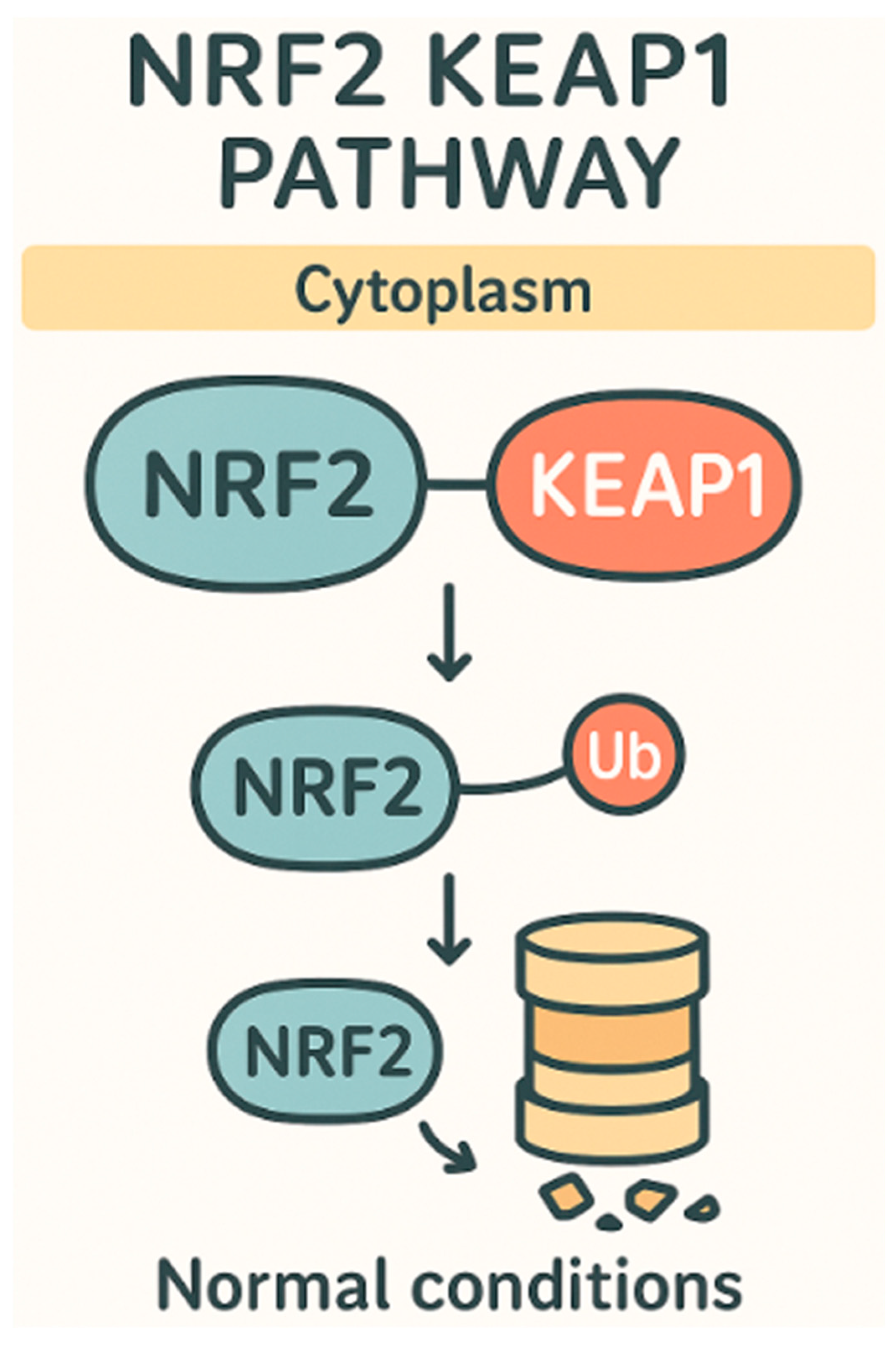
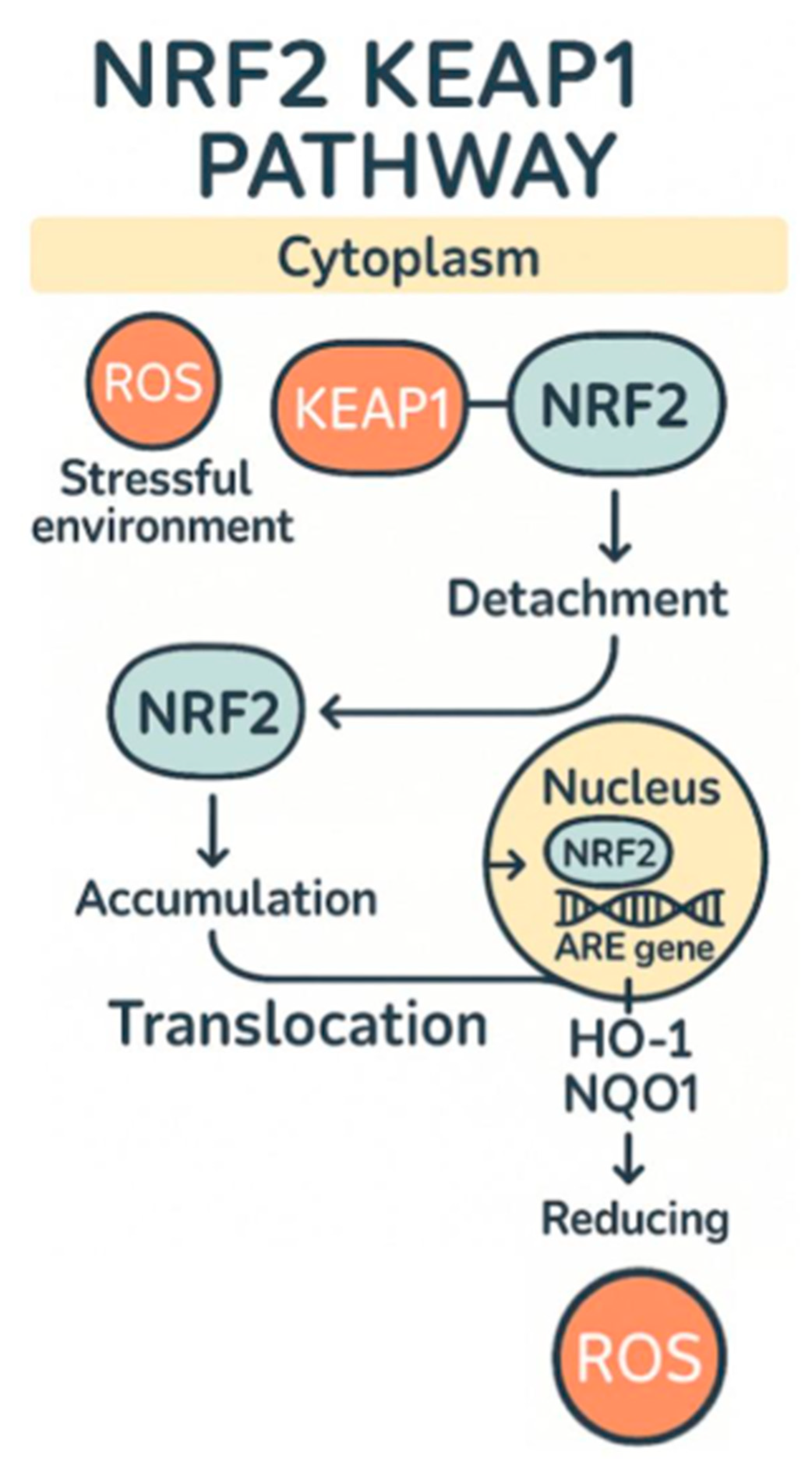
| Plant | Effective Compound | Pathways Effected |
|---|---|---|
| Shitake Mushroom | Lentinan | Modulation of Th1/Th2 immune balance, TLR activation, increased APC signaling, improved CMI response |
| Panax ginseng | Ginsenosides (Rg1, Re) | Enhanced Nrf2 activation, decreased oxidative stress, improved glucose metabolism, reduced AGE formation |
| Turmeric (Curcuma longa) | Curcumin | Nrf2 pathway activation, ROS scavenging, increased antioxidant enzyme expression (SOD, catalase, GPx) |
| Black Seed (Nigella sativa) | Thymoquinone | Nrf2 pathway activation, direct ROS scavenging, reduced neuroinflammation and oxidative damage |
| Berries (e.g., blueberries, strawberries) | Quercetin, Kaempferol, Pterostilbene | Nrf2 pathway activation, antioxidant enzyme upregulation, ROS reduction |
| Moringa Oleifera | Quercetin, Kaempferol, Nazarin | Downregulation of PKC/Nox4, Nrf2 pathway activation, improved antioxidant capacity |
| Holy Basil (Ocimum tenuiflorum) | Eugenol | Nrf2 activation, increased SOD, GPx, CAT activity, reduced oxidative stress in diabetic and cancer models |
| Plant/Compound | Dosage | Route | Notes | References |
|---|---|---|---|---|
| Lentinan (Shiitake Mushroom) | IV: 2–10 mg/week over 30 min; oral: 8 g/day | Intravenous or oral | IV more effective; oral has no side effects but lower efficacy | [9,31] |
| Panax ginseng (Rg1, Re, Rb1, Rd) | Oral: 1 g twice daily; IV: 1–2 mL/session, 1–3x per week | Oral or intravenous | Used in pharmacopuncture; oral better established | [27,28,29] |
| Curcumin (Turmeric) | Oral: 500 mg curcumin + 5–50 mg piperine/day | Oral | Piperine improves curcumin absorption | [43,44] |
| Thymoquinone (Black Seed) | Oral: 5–15 mg/kg/day in rats; 33 mg/day in humans | Oral | Derived from black seed oil studies and chromatography | [61,62] |
| Quercetin, Kaempferol, Pterostilbene (Berries) | Oral: quercetin 40–80 mg/day; kaempferol 200 mg/kg in mice | Oral | Shown to be effective in synergy in animal models | [68,69] |
| Moringa Oleifera (Quercetin, Kaempferol, Nazarin) | Quercetin 40–80 mg/day in mice; kaempferol 200 mg/kg | Oral | Nrf2 modulation and ROS inhibition observed | [11,75] |
| Eugenol (Holy Basil) | In vitro: 100 µg/mL; oral: 60 mg/kg/day in rats | In vitro and oral (rat model) | Antioxidant and antidiabetic effects in animal studies | [90,91] |
| Morbidity/Disease | Effective Compound | Physiological Response | References |
|---|---|---|---|
| Diabetes | Red ginseng (Panax ginseng) metabolites Rg1 and Re | Lowered blood glucose levels, reduced AGE formation, enhanced antioxidant enzymes (e.g., increased glutathione), improved kidney function, and reduced oxidative stress | [24,28] |
| Cancer | Lentinan (from shiitake mushroom) | Modulation of Th1/Th2 ratio, increased cytotoxic T-cell (CD8+) and NK cell activity, promotion of apoptosis in cancer cells, reduction in tumor angiogenesis and ROS | [2,3,9] |
| Obesity | Quercetin, kaempferol, pterostilbene (from berries) | Enhanced Nrf2 activation leading to increased antioxidant enzyme activity, reduced oxidative stress and inflammation associated with metabolic dysfunction | [69,70] |
| Cardiovascular disease | Curcumin (from turmeric) + piperine | Improved antioxidant capacity, reduced carotid intima–media thickness, enhanced Nrf2 pathway activation, reduced oxidative stress markers | [52,53] |
| Hypertension | Thymoquinone (from black seed) | Reduced blood pressure, decreased oxidative stress, improved lipid profiles, enhanced antioxidant enzyme activity through Nrf2 pathway | [66,67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gliozheni, E.; Salem, Y.; Cho, E.; Wahlstrom, S.; Olbrich, D.; Shams, B.; Alexander, M.; Ichii, H. Food-Derived Phytochemicals: Multicultural Approaches to Oxidative Stress and Immune Response. Int. J. Mol. Sci. 2025, 26, 7316. https://doi.org/10.3390/ijms26157316
Gliozheni E, Salem Y, Cho E, Wahlstrom S, Olbrich D, Shams B, Alexander M, Ichii H. Food-Derived Phytochemicals: Multicultural Approaches to Oxidative Stress and Immune Response. International Journal of Molecular Sciences. 2025; 26(15):7316. https://doi.org/10.3390/ijms26157316
Chicago/Turabian StyleGliozheni, Eiger, Yusuf Salem, Eric Cho, Samuel Wahlstrom, Dane Olbrich, Brandon Shams, Michael Alexander, and Hirohito Ichii. 2025. "Food-Derived Phytochemicals: Multicultural Approaches to Oxidative Stress and Immune Response" International Journal of Molecular Sciences 26, no. 15: 7316. https://doi.org/10.3390/ijms26157316
APA StyleGliozheni, E., Salem, Y., Cho, E., Wahlstrom, S., Olbrich, D., Shams, B., Alexander, M., & Ichii, H. (2025). Food-Derived Phytochemicals: Multicultural Approaches to Oxidative Stress and Immune Response. International Journal of Molecular Sciences, 26(15), 7316. https://doi.org/10.3390/ijms26157316





