Parishin C Attenuates Oxidative Stress and Inflammation in HT22 Hippocampal Neurons and BV2 Microglia Through Nrf2 Signaling Pathway
Abstract
1. Introduction
2. Results
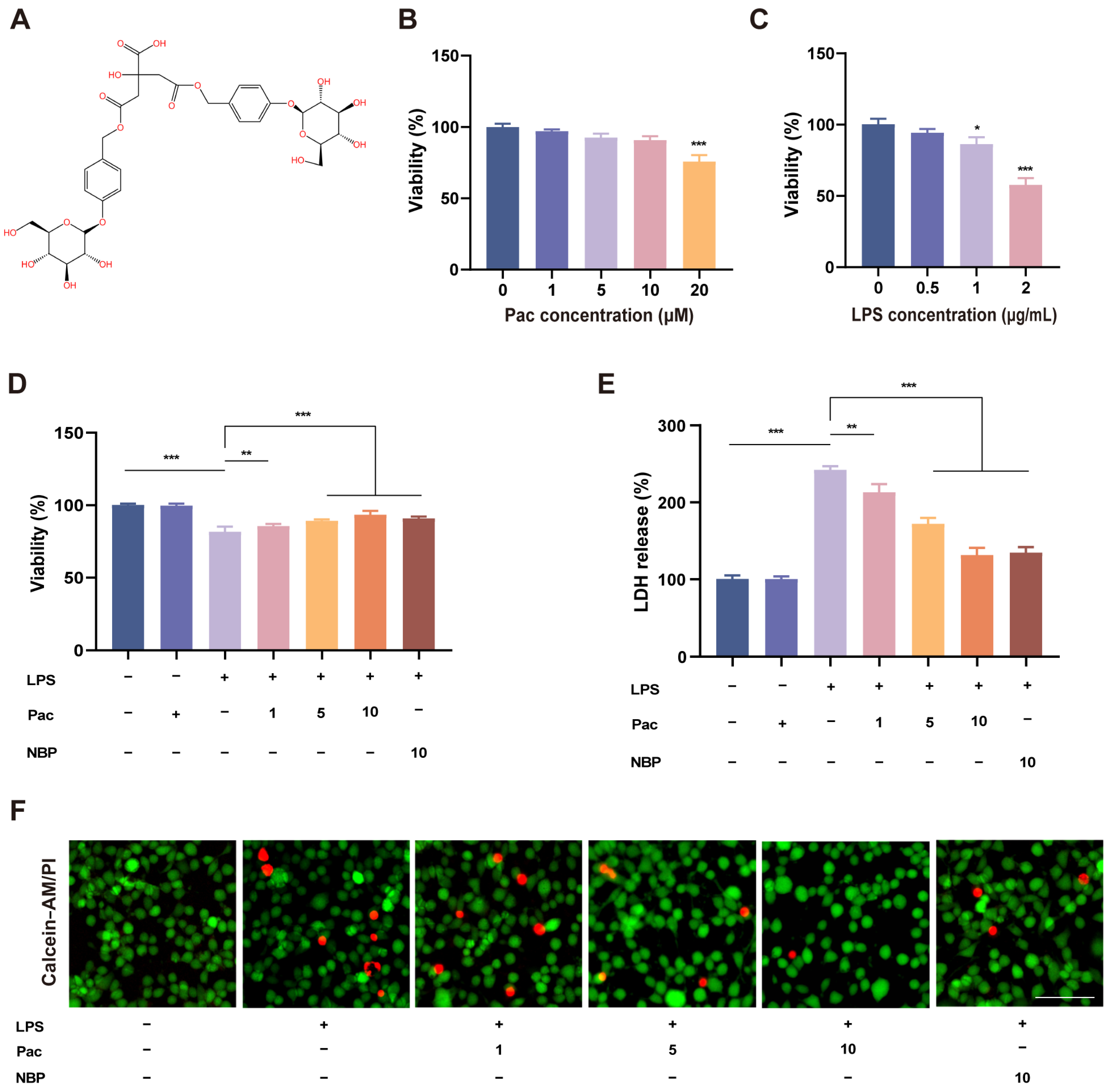
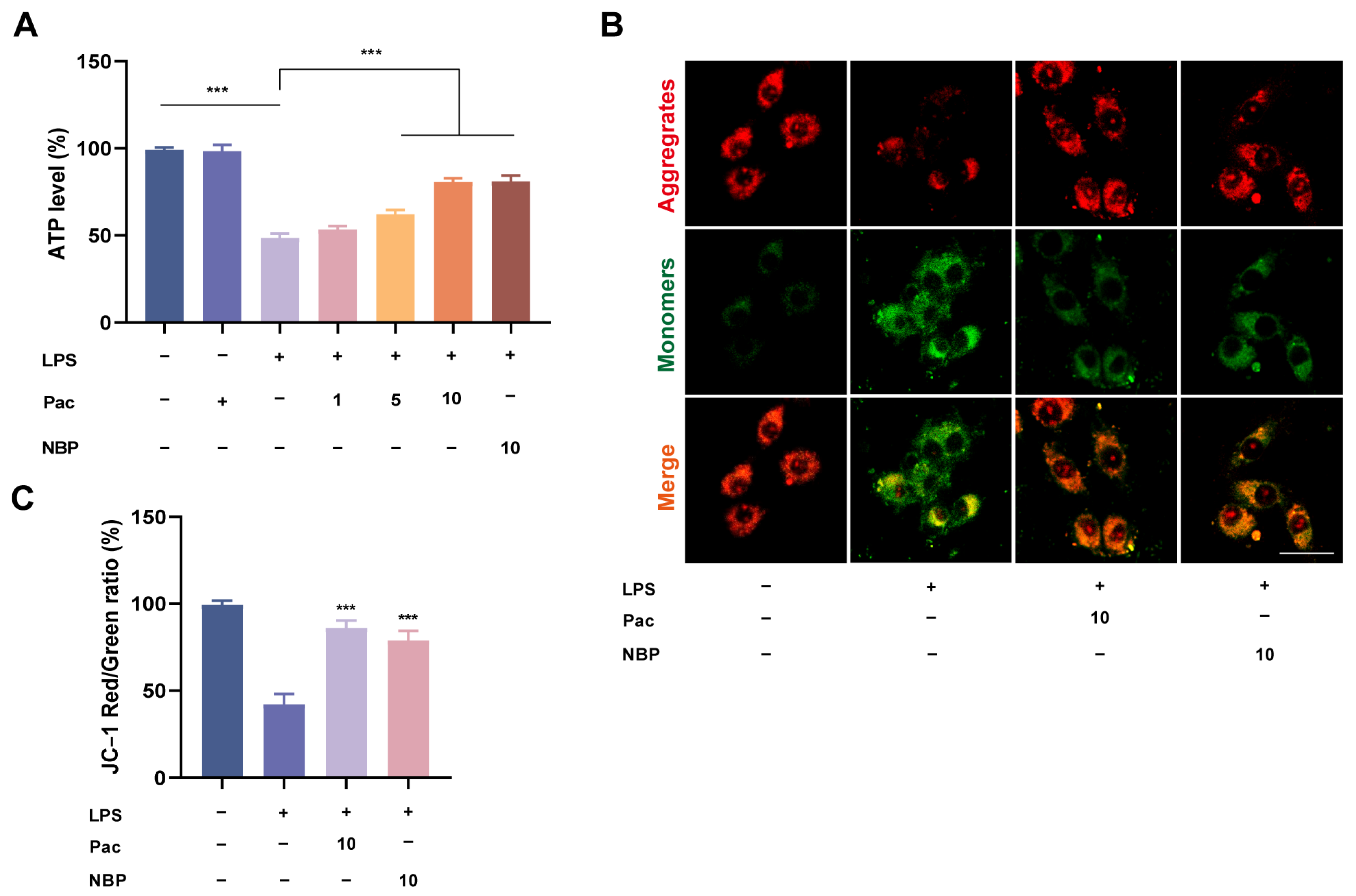
2.1. The Effect of PaC on LPS–Stimulated HT22 Cell Viability
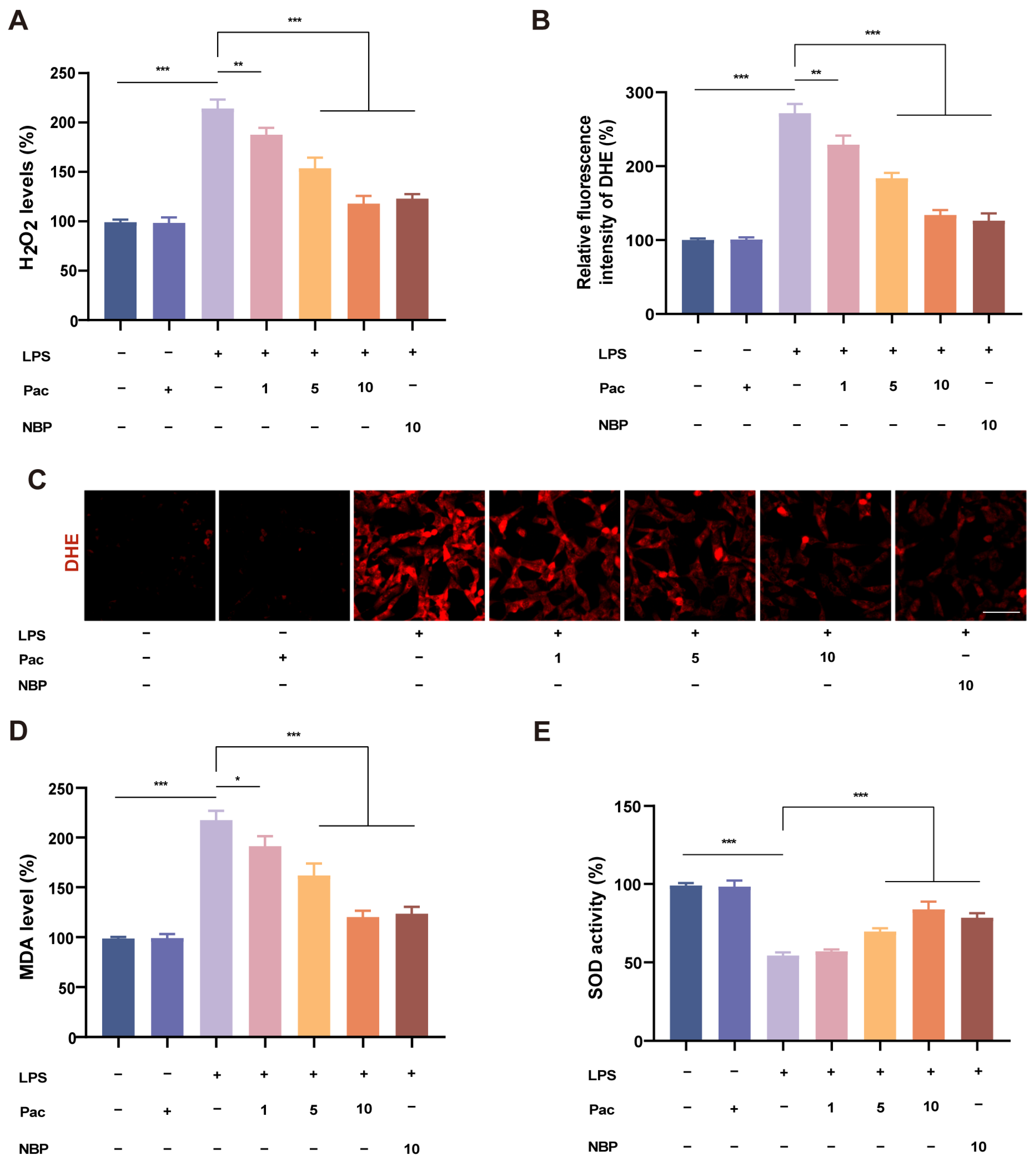
2.2. PaC Can Regulate Oxidative Stress
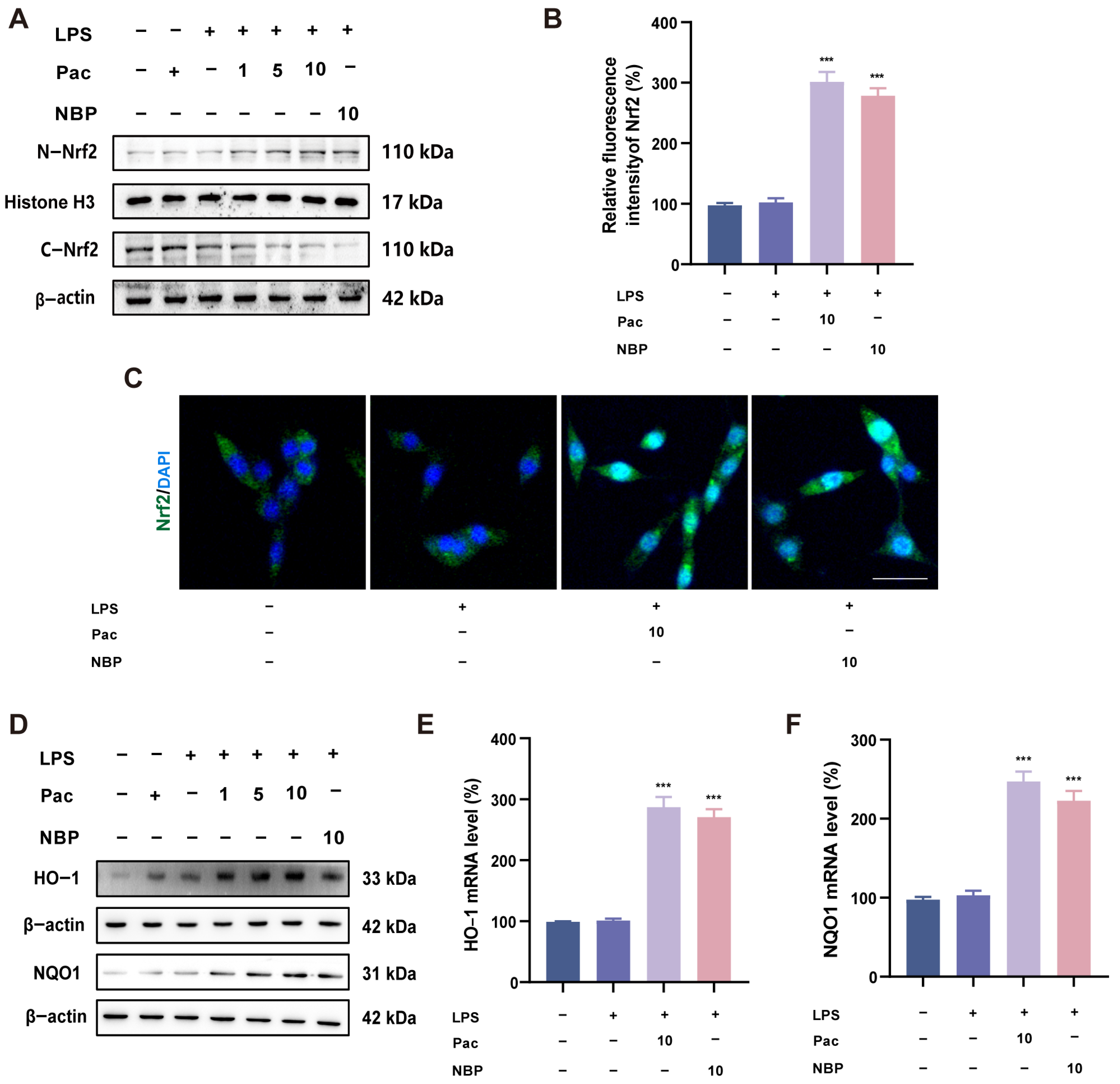
2.3. PaC Can Activate the Nrf2 Signaling Pathway
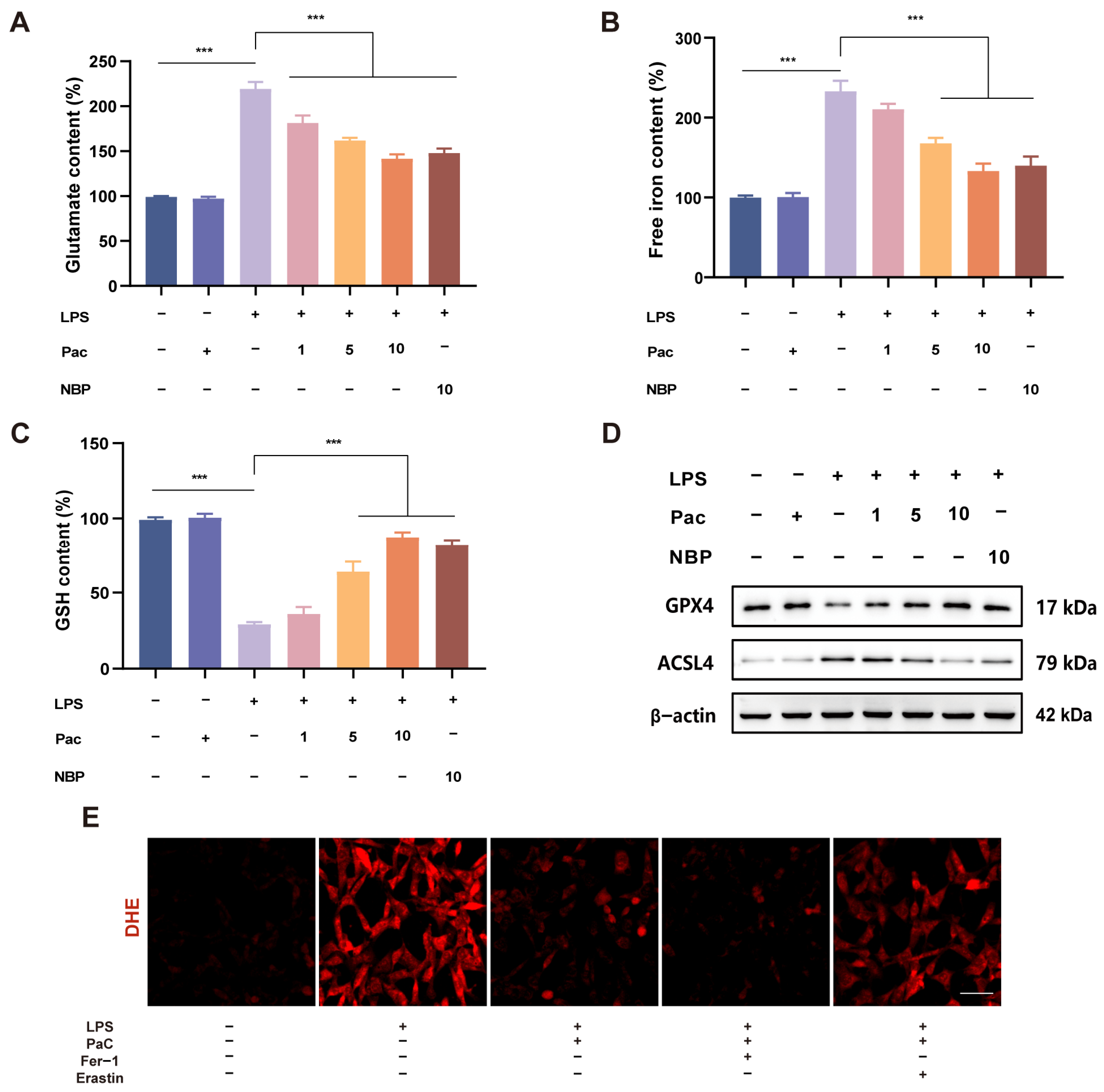
2.4. PaC Inhibited HT22 Cells Ferroptosis
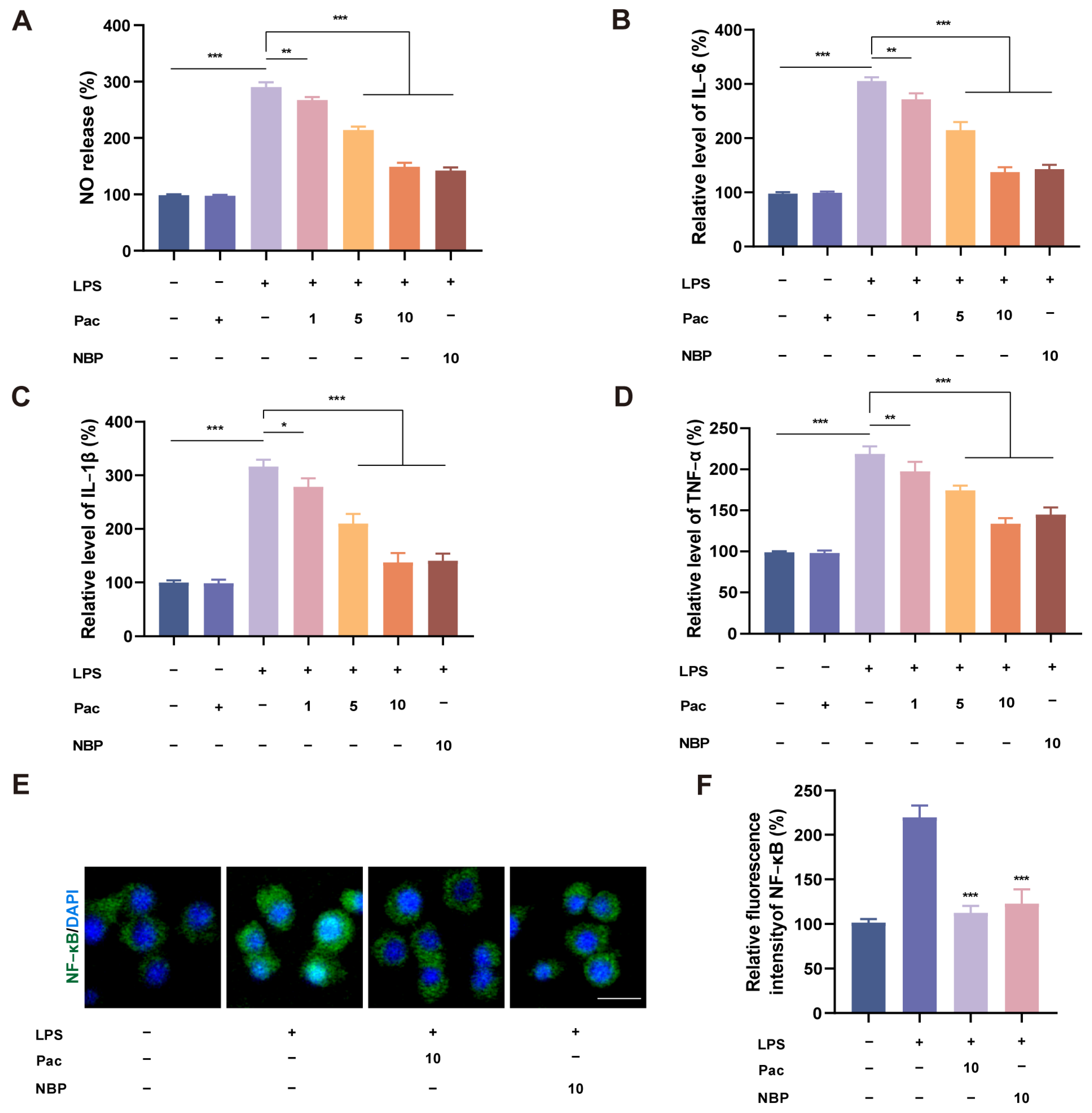
2.5. PaC Can Inhibit Neuroinflammation in BV2 Cells
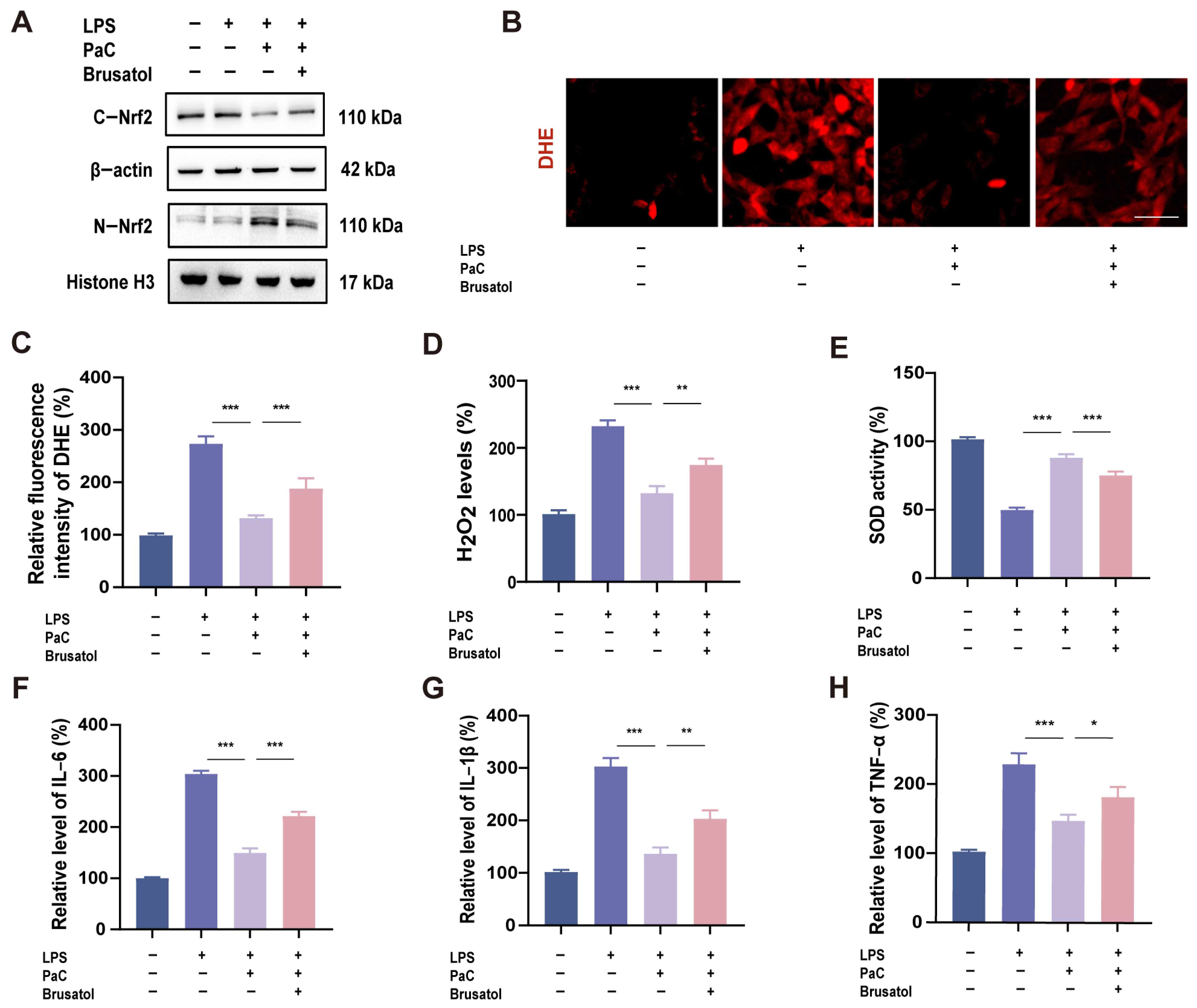
2.6. PaC Regulated Oxidative Stress and Inflammation by Targeting Nrf2
2.7. PaC Inhibited Ferroptosis by Targeting Nrf2
2.8. Knocking Down Nrf2 Weakened the Neuroprotective Effect of PaC
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Antibodies
4.2. Cell Culture
4.3. Cell Survival Assessment
4.4. Oxidative Stress Assessment
4.5. Ferroptosis Assessment
4.6. Mitochondrial Membrane Potential
4.7. Western Blot
4.8. Quantitative RT–PCR
4.9. Immunofluorescence (IF)
4.10. HT22 and BV2 Co–Culture System
4.11. NO Release
4.12. Knock Down Nrf2
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, C.; Tian, Z.; Ruan, W.; Wu, M.; Wang, W.; Liu, Z. Erianin Isolated from Dendrobium Huoshanense Alleviated Neuroinflammation in MPTP-Induced Parkinson’s Disease Model via NF-κB/NLRP3 Pathway. J. Ethnopharmacol. 2025, 345, 119620. [Google Scholar] [CrossRef]
- Du, X.; Zhang, M.; Wang, S.; Li, J.; Zhang, J.; Liu, D. Ethnopharmacology, chemical composition and functions of Cymbopogon citratus. Chin. Herb. Med. 2023, 16, 358–374. [Google Scholar] [CrossRef]
- Li, Z.; Lu, Y.; Zhen, Y.; Jin, W.; Ma, X.; Yuan, Z.; Liu, B.; Zhou, X.-L.; Zhang, L. Avicularin Inhibits Ferroptosis and Improves Cognitive Impairments in Alzheimer’s Disease by Modulating the NOX4/Nrf2 Axis. Phytomedicine 2024, 135, 156209. [Google Scholar] [CrossRef]
- Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New Insights into Oxidative Stress and Inflammatory Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef]
- Alqahtani, T.; Deore, S.L.; Kide, A.A.; Shende, B.A.; Sharma, R.; Dadarao Chakole, R.; Nemade, L.S.; Kishor Kale, N.; Borah, S.; Shrikant Deokar, S.; et al. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease, and Parkinson’s Disease, Huntington’s Disease and Amyotrophic Lateral Sclerosis—An Updated Review. Mitochondrion 2023, 71, 83–92. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, D.; Chen, X.; Li, X.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Chen, X.; Tang, D.; Liu, J. Copper-Dependent Autophagic Degradation of GPX4 Drives Ferroptosis. Autophagy 2023, 19, 1982–1996. [Google Scholar] [CrossRef]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.d.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8765. [Google Scholar] [CrossRef]
- Meng, Z.; Li, M.; Wang, X.; Zhang, K.; Wu, C.; Zhang, X. Inula Britannica Ameliorates Alcohol-Induced Liver Injury by Modulating SIRT1-AMPK/Nrf2/NF-κB Signaling Pathway. Chin. Herb. Med. 2024, 16, 667–678. [Google Scholar] [CrossRef]
- Pandey, P.; Singh, A.K.; Singh, M.; Tewari, M.; Shukla, H.S.; Gambhir, I.S. The See-Saw of Keap1-Nrf2 Pathway in Cancer. Crit. Rev. Oncol. Hematol. 2017, 116, 89–98. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Zhu, X.; Chen, Y.; Xu, B.; Bai, X.; Weng, X.; Xu, J.; Tao, Y.; Yang, D.; et al. MCL Attenuates Atherosclerosis by Suppressing Macrophage Ferroptosis via Targeting KEAP1/NRF2 Interaction. Redox Biol. 2024, 69, 102987. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 1759091419899782. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, M.; Ding, X.; Tian, J.; Sun, D.; Gao, X.; Jin, C.; Peng, D.; Gui, S.; Wang, X. Exploration the Effective Components of Gastrodia Elata in Improving Cerebral Ischemia Reperfusion Injury Based on “Spectrum-Effect” Correlation and Zebrafish Verification Experiment. Phytomedicine 2024, 135, 156211. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, H.; Yao, C.; Sun, X.; He, Q.; Choudharyc, M.I.; Chen, S.; Liu, X.; Jiang, N. Fresh Gastrodia Elata Blume Alleviates Simulated Weightlessness-Induced Cognitive Impairment by Regulating Inflammatory and Apoptosis-Related Pathways. Front. Pharmacol. 2023, 14, 1173920. [Google Scholar] [CrossRef]
- Sun, X.; Jia, B.; Sun, J.; Lin, J.; Lu, B.; Duan, J.; Li, C.; Wang, Q.; Zhang, X.; Tan, M.; et al. Gastrodia Elata Blume: A Review of Its Mechanisms and Functions on Cardiovascular Systems. Fitoterapia 2023, 167, 105511. [Google Scholar] [CrossRef]
- Tang, C.; Wang, L.; Liu, X.; Cheng, M.; Qu, Y.; Xiao, H. Comparative Pharmacokinetics of Gastrodin in Rats after Intragastric Administration of Free Gastrodin, Parishin and Gastrodia Elata Extract. J. Ethnopharmacol. 2015, 176, 49–54. [Google Scholar] [CrossRef]
- Jiang, N.; Yao, C.; Zhang, Y.; Chen, Y.; Chen, F.; Luo, Y.; Choudhary, M.I.; Pan, R.; Liu, X. Antidepressant Effects of Parishin C in Chronic Social Defeat Stress-Induced Depressive Mice. J. Ethnopharmacol. 2024, 325, 117891. [Google Scholar] [CrossRef]
- Ma, T.; Chen, P.; Dong, H.; Wang, X. Identification of Key Anti-Neuroinflammatory Components in Gastrodiae Rhizoma Based on Spectrum-Effect Relationships and Its Mechanism Exploration. J. Pharm. Biomed. Anal. 2024, 248, 116266. [Google Scholar] [CrossRef]
- Wang, T.; Chen, H.; Xia, S.; Chen, X.; Sun, H.; Xu, Z. Ameliorative Effect of Parishin C Against Cerebral Ischemia-Induced Brain Tissue Injury by Reducing Oxidative Stress and Inflammatory Responses in Rat Model. Neuropsychiatr. Dis. Treat. 2021, 17, 1811–1823. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, Z.; Zhai, D.; Yang, C.; Lu, G.; Wang, S.; Xiao, S.; Li, C.; Chen, L.; Lin, X.; et al. Unveiling the Therapeutic Potential of Dl-3-n-Butylphthalide in NTG-Induced Migraine Mouse: Activating the Nrf2 Pathway to Alleviate Oxidative Stress and Neuroinflammation. J. Headache Pain 2024, 25, 50. [Google Scholar] [CrossRef]
- Pan, G.-P.; Liu, Y.-H.; Qi, M.-X.; Guo, Y.-Q.; Shao, Z.-L.; Liu, H.-T.; Qian, Y.-W.; Guo, S.; Yin, Y.-L.; Li, P. Alizarin Attenuates Oxidative Stress-Induced Mitochondrial Damage in Vascular Dementia Rats by Promoting TRPM2 Ubiquitination and Proteasomal Degradation via Smurf2. Phytomedicine 2024, 135, 156119. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhai, Y.; Chen, J.; Xu, X.; Wang, H. Kaempferol Ameliorates Oxygen-Glucose Deprivation/Reoxygenation-Induced Neuronal Ferroptosis by Activating Nrf2/SLC7A11/GPX4 Axis. Biomolecules 2021, 11, 923. [Google Scholar] [CrossRef]
- Li, J.; Jia, Y.-C.; Ding, Y.-X.; Bai, J.; Cao, F.; Li, F. The Crosstalk between Ferroptosis and Mitochondrial Dynamic Regulatory Networks. Int. J. Biol. Sci. 2023, 19, 2756–2771. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Q.; Wang, Y.; Peng, J.; Shao, L.; Li, X. Irisin Protects against Sepsis-Associated Encephalopathy by Suppressing Ferroptosis via Activation of the Nrf2/GPX4 Signal Axis. Free Radic. Biol. Med. 2022, 187, 171–184. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Sha, S.; Wang, S.; Shan, Y.; Li, L.; Xing, C.; Guan, H.; Du, H. Berberine Alleviates Alzheimer’s Disease by Activating Autophagy and Inhibiting Ferroptosis through the JNK-p38MAPK Signaling Pathway. Int. Immunopharmacol. 2025, 155, 114550. [Google Scholar] [CrossRef]
- Li, X.-N.; Shang, N.-Y.; Kang, Y.-Y.; Sheng, N.; Lan, J.-Q.; Tang, J.-S.; Wu, L.; Zhang, J.-L.; Peng, Y. Caffeic Acid Alleviates Cerebral Ischemic Injury in Rats by Resisting Ferroptosis via Nrf2 Signaling Pathway. Acta Pharmacol. Sin. 2024, 45, 248–267. [Google Scholar] [CrossRef]
- Guan, Y.; Li, L.; Yang, R.; Lu, Y.; Tang, J. Targeting Mitochondria with Natural Polyphenols for Treating Neurodegenerative Diseases: A Comprehensive Scoping Review from Oxidative Stress Perspective. J. Transl. Med. 2025, 23, 572. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Zhang, X.; Fang, Q.; Xu, Y.; Wang, H. Rutin Alleviates Pb-Induced Oxidative Stress, Inflammation and Cell Death via Activating Nrf2/ARE System in SH-SY5Y Cells. Neurotoxicology 2024, 104, 1–10. [Google Scholar] [CrossRef]
- Wu, W.; Wang, D.; Shi, Y.; Wang, Y.; Wu, Y.; Wu, X.; Shah, B.A.; Ye, G. 1,8-Cineole Alleviates Hippocampal Oxidative Stress in CUMS Mice via the PI3K/Akt/Nrf2 Pathway. Nutrients 2025, 17, 1027. [Google Scholar] [CrossRef]
- Su, D.; Xu, S.; Ji, K.; Xu, H.; Li, Y.; Zhang, Z.; Shen, Y.; Chen, G. Pterostilbene Supresses Inflammation-Induced Melanoma Metastasis by Impeding Neutrophil Elastase-Mediated Thrombospondin-1 Degradation. Chin. Herb. Med. 2023, 15, 94–101. [Google Scholar] [CrossRef]
- Jain, S.; Tripathi, S.; Tripathi, P.K. Antioxidant and Antiarthritic Potential of Berberine: In Vitro and in Vivo Studies. Chin. Herb. Med. 2023, 15, 549–555. [Google Scholar] [CrossRef]


| Gene | Primer Sequence |
|---|---|
| HO–1 | F: 5′–ACCATATCTACACGGCCCTG–3′ R: 5′–CAGTGAGGCCCATACCAGAA–3′ |
| IL–6 | F: 5′–TAGTCCTTCCTACCCCAATTTCC–3′ R: 5′–TTGGTCCTTAGCCACTCCTTC–3′ |
| IL–1β | F: 5′–TGACGGACCCCAAAAGATGA–3′ R: 5′–TCTCCACAGCCACAATGAGT–3′ |
| TNF–α | F: 5′–CCCTCACACTCAGATCATCTTCT–3′ R: 5′–GCTACGACGTGGGCTACAG–3′ |
| GAPDH | F: 5′–AGGTCGGTGTGAACGGATTTG–3′ R: 5′–TGTAGACCATGTAGTTGAGGTCA–3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, W.; Wu, X.; Shah, B.A.; Lombardo, M.; Ye, G. Parishin C Attenuates Oxidative Stress and Inflammation in HT22 Hippocampal Neurons and BV2 Microglia Through Nrf2 Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 7263. https://doi.org/10.3390/ijms26157263
Wang Y, Wu W, Wu X, Shah BA, Lombardo M, Ye G. Parishin C Attenuates Oxidative Stress and Inflammation in HT22 Hippocampal Neurons and BV2 Microglia Through Nrf2 Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(15):7263. https://doi.org/10.3390/ijms26157263
Chicago/Turabian StyleWang, Yichen, Wenze Wu, Xinyan Wu, Basit Ali Shah, Mauro Lombardo, and Gang Ye. 2025. "Parishin C Attenuates Oxidative Stress and Inflammation in HT22 Hippocampal Neurons and BV2 Microglia Through Nrf2 Signaling Pathway" International Journal of Molecular Sciences 26, no. 15: 7263. https://doi.org/10.3390/ijms26157263
APA StyleWang, Y., Wu, W., Wu, X., Shah, B. A., Lombardo, M., & Ye, G. (2025). Parishin C Attenuates Oxidative Stress and Inflammation in HT22 Hippocampal Neurons and BV2 Microglia Through Nrf2 Signaling Pathway. International Journal of Molecular Sciences, 26(15), 7263. https://doi.org/10.3390/ijms26157263







