Luminescent Properties and Cytotoxic Activity of 2-phenylbenzoxazole Fluorosulfate Derivatives
Abstract
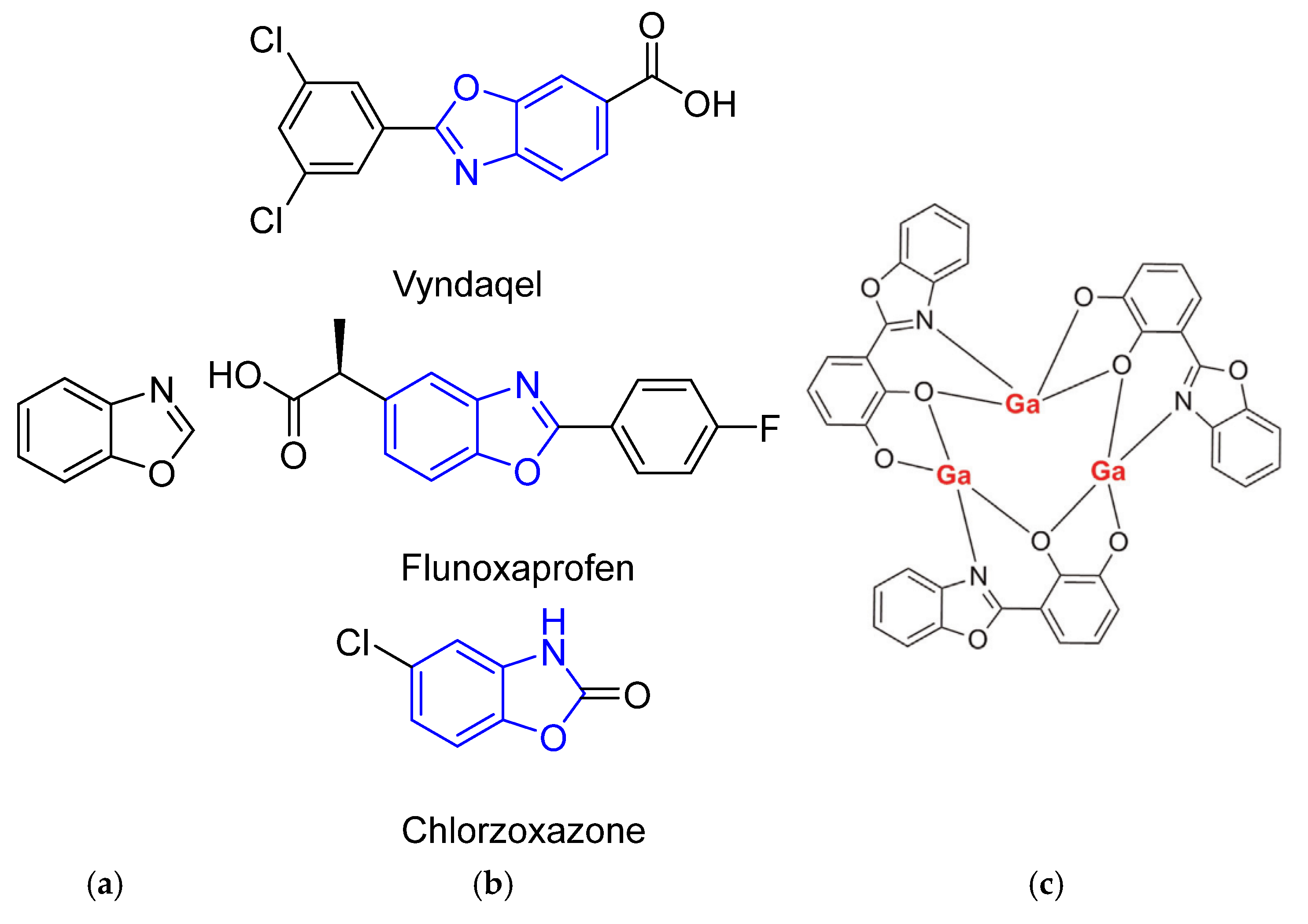
1. Introduction
2. Results and Discussion
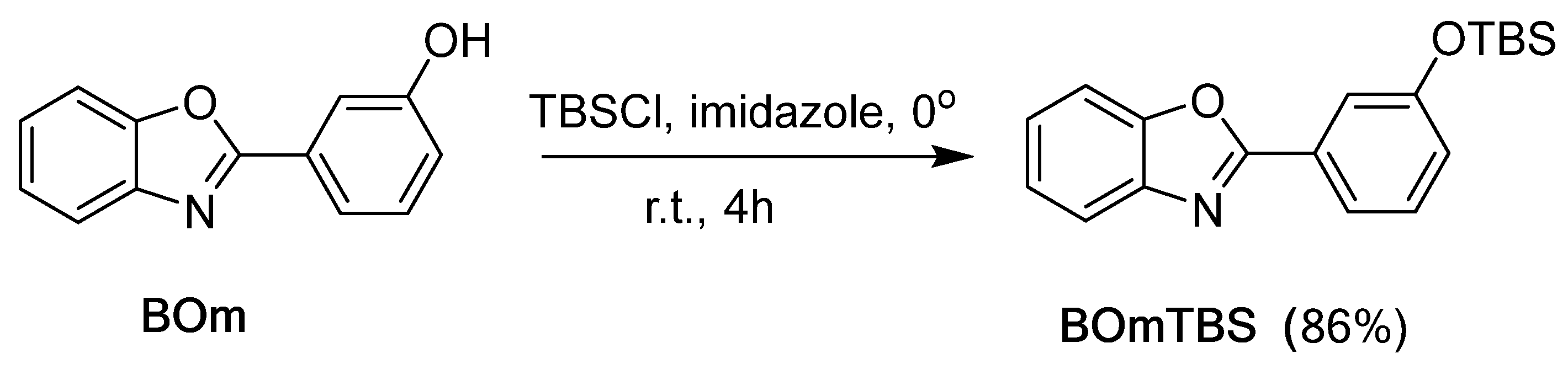
2.1. Synthesis of Benzoxazole Derivatives
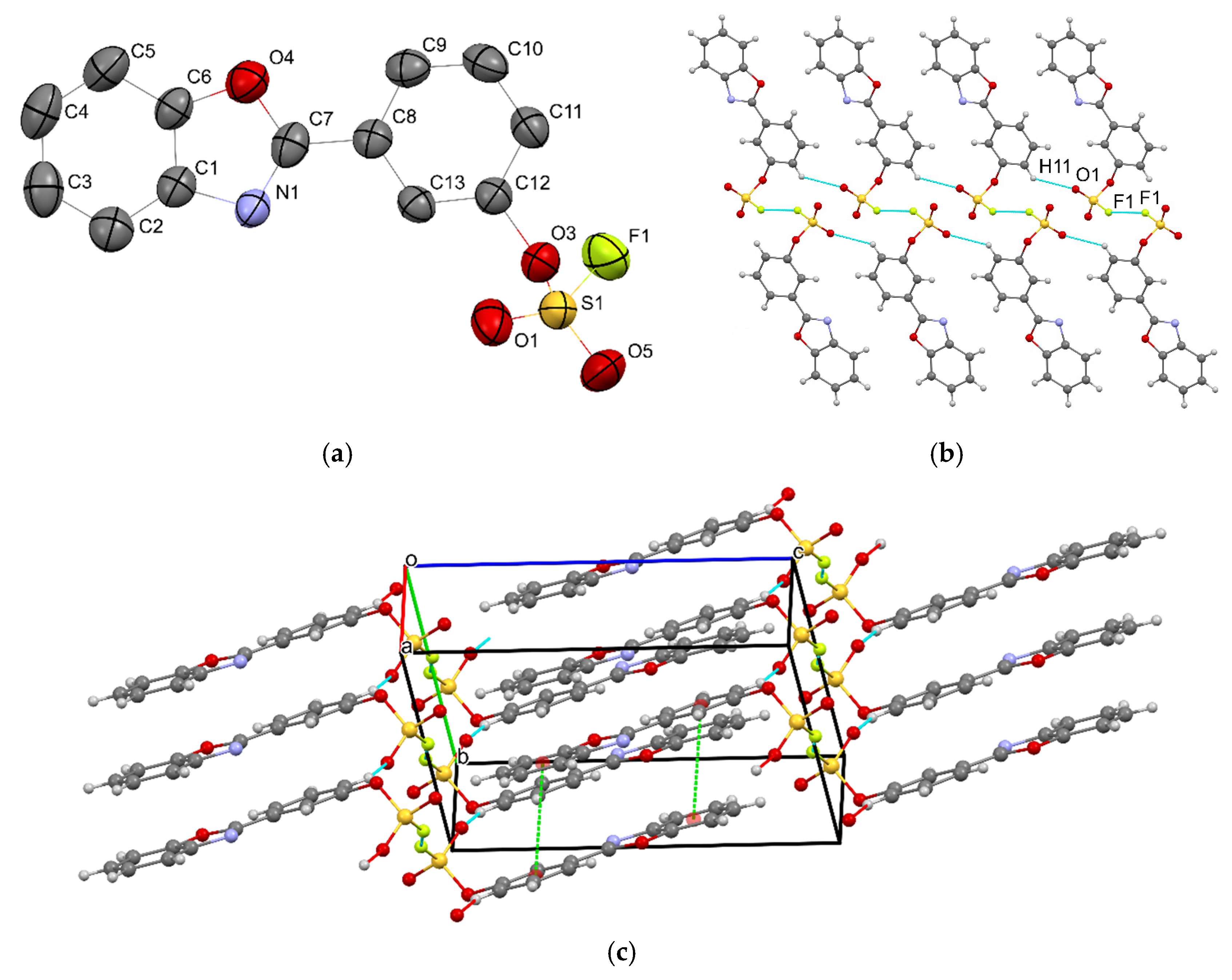
2.2. Single-Crystal X-Ray Analysis
2.3. Cytotoxic Activity
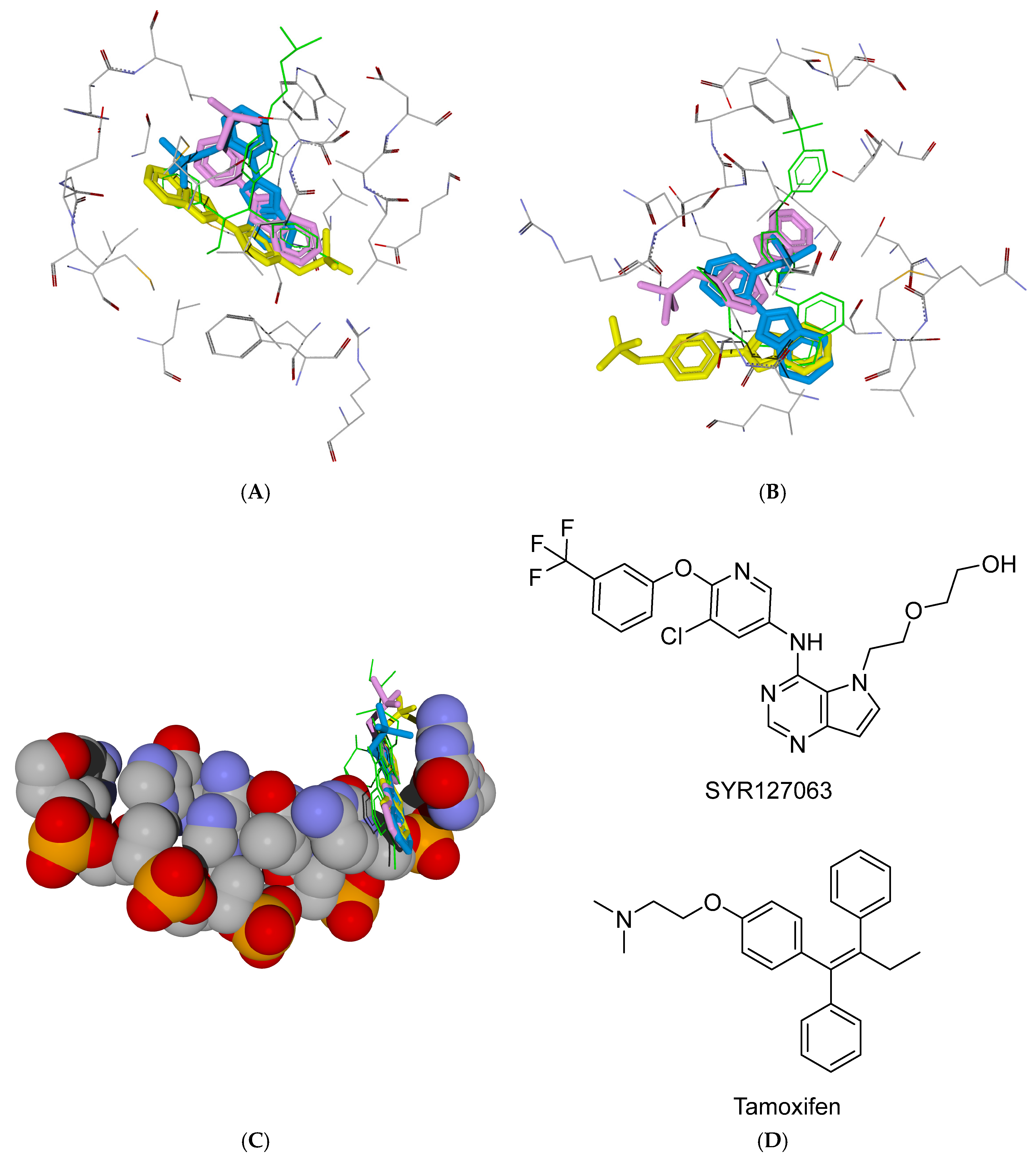
2.4. The Docking Study
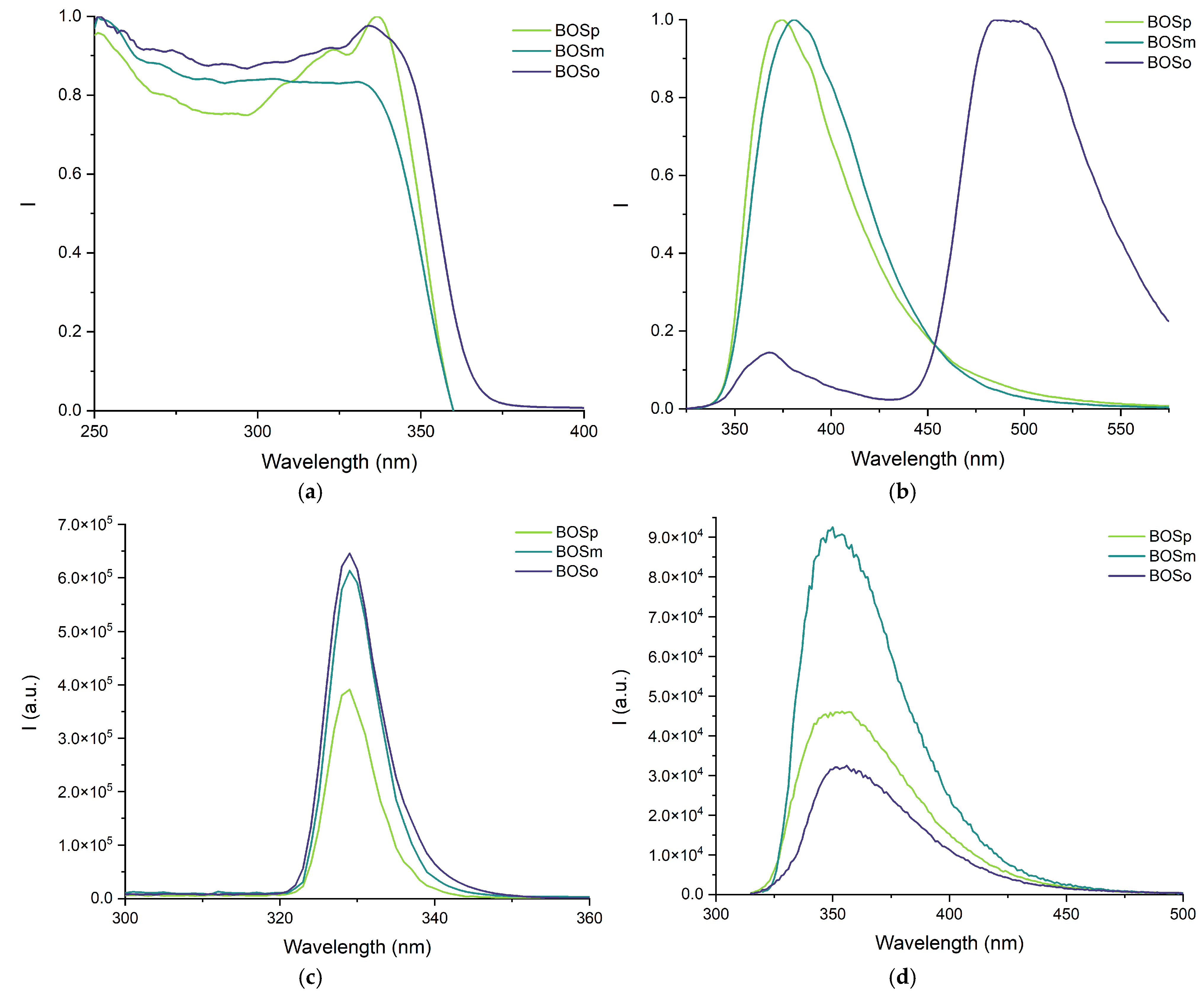
2.5. Luminescent Properties
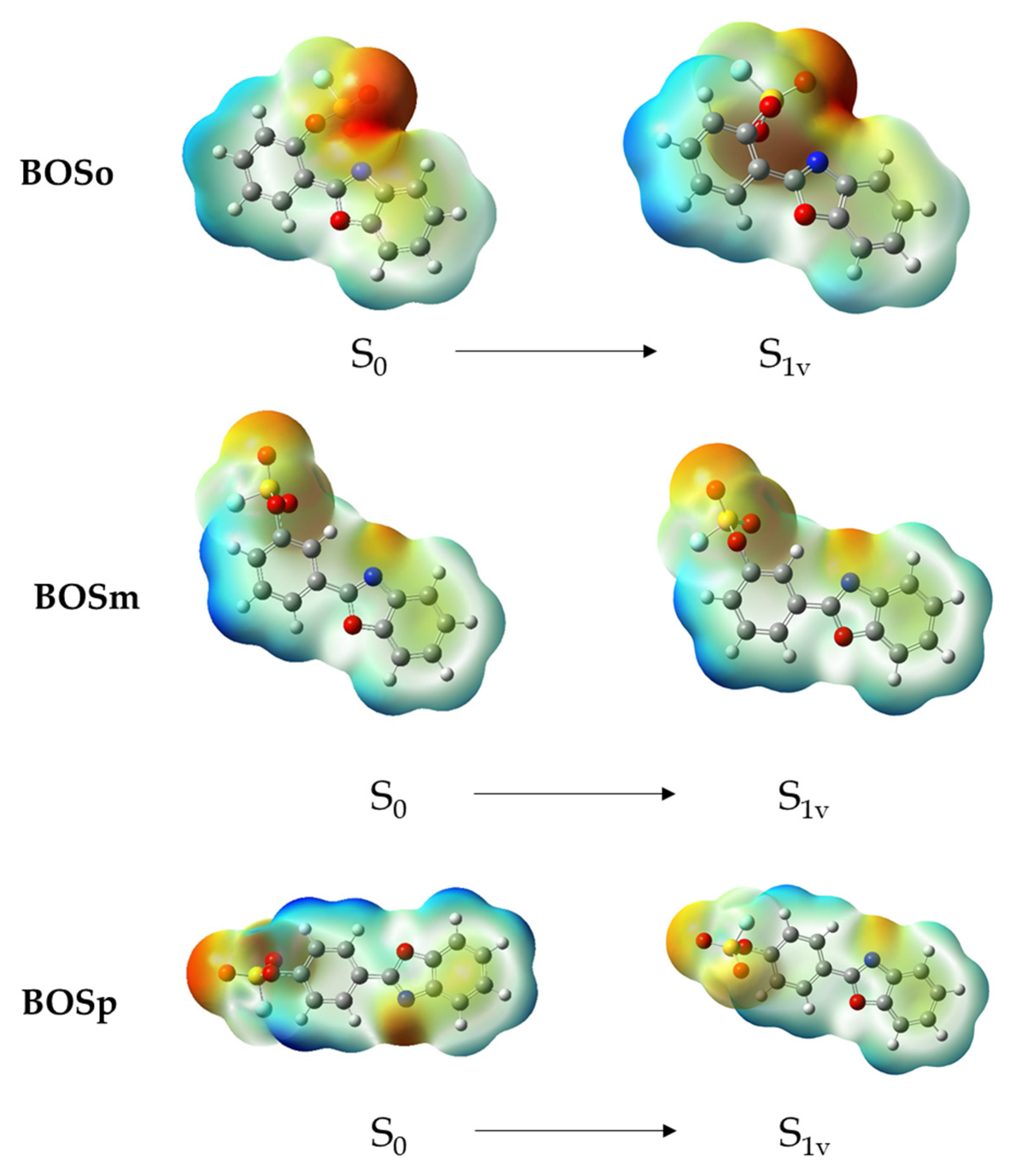
2.6. DFT Calculations of the Luminescent Properties
3. Materials and Methods
3.1. General Information and Synthesis of Compounds
3.2. X-Ray Crystal Structure Determination
3.3. Assessment of Cell Viability and Cytotoxicity
3.3.1. Cell Culture and Maintenance
3.3.2. Cell Treatment
3.3.3. MTT Viability Assay
3.3.4. Statistical Data Processing
3.4. Molecular Docking
3.5. Luminescence Measurements
3.6. DFT and TDDFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yildiz-Oren, I.; Yalcin, I.; Aki-Sener, E.; Ucarturk, N. Synthesis and Structure–Activity Relationships of New Antimicrobial Active Multisubstituted Benzazole Derivatives. Eur. J. Med. Chem. 2004, 39, 291–298. [Google Scholar] [CrossRef]
- Akbay, A.; Ören, İ.; Temiz-Arpacı, Ö.; Akı-Sener, E.; Yalcçın, I. Synthesis and HIV-1 Reverse Transcriptase Inhibitor Activity of Some 2,5,6-Substituted Benzoxazole, Benzimidazole, Benzothiazole and Oxazolo(4,5-b)Pyridine Derivatives. Arzneimittelforschung 2011, 53, 266–271. [Google Scholar] [CrossRef]
- Lage, H.; Aki-Sener, E.; Yalcin, I. High Antineoplastic Activity of New Heterocyclic Compounds in Cancer Cells with Resistance against Classical DNA Topoisomerase II-targeting Drugs. Int. J. Cancer 2006, 119, 213–220. [Google Scholar] [CrossRef]
- Pinar, A.; Yurdakul, P.; Yildiz, I.; Temiz-Arpaci, O.; Acan, N.L.; Aki-Sener, E.; Yalcin, I. Some Fused Heterocyclic Compounds as Eukaryotic Topoisomerase II Inhibitors. Biochem. Biophys. Res. Commun. 2004, 317, 670–674. [Google Scholar] [CrossRef]
- Chen, L.-N.; Kuo, C.-C.; Chiu, Y.-C.; Chen, W.-C. Ultra Metal Ions and PH Sensing Characteristics of Thermoresponsive Luminescent Electrospun Nanofibers Prepared from Poly(HPBO-Co-NIPAAm-Co-SA). RSC Adv. 2014, 4, 45345–45353. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Deng, G.; Liu, Z.; He, Y.; Fan, Q. Fluorescent Dendritic Organogels Based on 2-(2′-Hydroxyphenyl)Benzoxazole: Emission Enhancement and Multiple Stimuli-Responsive Properties. Chem.—A Eur. J. 2015, 21, 11018–11028. [Google Scholar] [CrossRef]
- Fuks-Janczarek, I.; Kityk, I.V.; Miedziński, R.; Gondek, E.; Ebothe, J.; Nzoghe-Mendome, L.; Danel, A. Push-Pull Benzoxazole Based Stilbenes as New Promising Electrooptics Materials. J. Mater. Sci. Mater. Electron. 2007, 18, 519–526. [Google Scholar] [CrossRef]
- Wang, J.; Chu, Q.; Liu, X.; Wesdemiotis, C.; Pang, Y. Large Fluorescence Response by Alcohol from a Bis(Benzoxazole)–Zinc(II) Complex: The Role of Excited State Intramolecular Proton Transfer. J. Phys. Chem. B 2013, 117, 4127–4133. [Google Scholar] [CrossRef]
- Xiao, L.; Sun, S.; Pei, Z.; Pei, Y.; Pang, Y.; Xu, Y. A Ga3+self-Assembled Fluorescent Probe for ATP Imaging in Vivo. Biosens. Bioelectron. 2015, 65, 166–170. [Google Scholar] [CrossRef]
- Burlov, A.S.; Vlasenko, V.G.; Garnovskii, D.A.; Uraev, A.I.; Koshchienko, Y.V.; Mal’tsev, E.I.; Lypenko, D.A.; Dmitriev, A.V. Luminescent Metal Complexes of 2-(2’-Phenyl-Substituted)Benzazoles. Russ. J. Coord. Chem. 2023, 49, S38–S48. [Google Scholar] [CrossRef]
- Carayon, C.; Fery-Forgues, S. 2-Phenylbenzoxazole Derivatives: A Family of Robust Emitters of Solid-State Fluorescence. Photochem. Photobiol. Sci. 2017, 16, 1020–1035. [Google Scholar] [CrossRef]
- Ghodbane, A.; D’Altério, S.; Saffon, N.; McClenaghan, N.D.; Scarpantonio, L.; Jolinat, P.; Fery-Forgues, S. Facile Access to Highly Fluorescent Nanofibers and Microcrystals via Reprecipitation of 2-Phenyl-Benzoxazole Derivatives. Langmuir 2012, 28, 855–863. [Google Scholar] [CrossRef]
- Ghodbane, A.; Saffon, N.; Blanc, S.; Fery-Forgues, S. Influence of the Halogen Atom on the Solid-State Fluorescence Properties of 2-Phenyl-Benzoxazole Derivatives. Dye. Pigment. 2015, 113, 219–226. [Google Scholar] [CrossRef]
- Colman, R.F. Chemical Arrows for Enzymatic Targets. FASEB J. 1997, 11, 217–226. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Li, S.; Li, G.; Sharpless, K.B.; Wu, P. SuFEx Click Chemistry Enabled Late-Stage Drug Functionalization. J. Am. Chem. Soc. 2018, 140, 2919–2925. [Google Scholar] [CrossRef]
- Wyatt, J.L.; Colman, R.F. Affinity Labeling of Rabbit Muscle Pyruvate Kinase by 5’-p-Fluorosulfonylbenzoyladenosine. Biochemistry 1977, 16, 1333–1342. [Google Scholar] [CrossRef]
- Randall, J.D.; Eyckens, D.J.; Stojcevski, F.; Francis, P.S.; Doeven, E.H.; Barlow, A.J.; Barrow, A.S.; Arnold, C.L.; Moses, J.E.; Henderson, L.C. Modification of Carbon Fibre Surfaces by Sulfur-Fluoride Exchange Click Chemistry. ChemPhysChem 2018, 19, 3176–3181. [Google Scholar] [CrossRef]
- Danilenko, N.; Shmalyuk, V.; Khlebnikov, A. 2-(2-(Fluorosulfonyloxy)Phenyl)Benzoxazole. Molbank 2021, 2021, M1242. [Google Scholar] [CrossRef]
- Danilenko, N.V.; Lutsuk, M.O.; Patlasova, S.E.; Korotkova, E.I.; Khlebnikov, A.I. 2-(4-(Fluorosulfonyloxy)Phenyl)Benzoxazole. Molbank 2023, 2023, M1746. [Google Scholar] [CrossRef]
- Yeh, V.; Iyengar, R. Oxazoles. In Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; pp. 487–543. [Google Scholar]
- Veryser, C.; Demaerel, J.; Bieliūnas, V.; Gilles, P.; De Borggraeve, W.M. Ex Situ Generation of Sulfuryl Fluoride for the Synthesis of Aryl Fluorosulfates. Org. Lett. 2017, 19, 5244–5247. [Google Scholar] [CrossRef]
- Dong, J.; Krasnova, L.; Finn, M.G.; Sharpless, K.B. Sulfur(VI) Fluoride Exchange (SuFEx): Another Good Reaction for Click Chemistry. Angew. Chemie Int. Ed. 2014, 53, 9430–9448. [Google Scholar] [CrossRef]
- Liang, D.; Streefkerk, D.E.; Jordaan, D.; Wagemakers, J.; Baggerman, J.; Zuilhof, H. Silicon-Free SuFEx Reactions of Sulfonimidoyl Fluorides: Scope, Enantioselectivity, and Mechanism. Angew. Chemie Int. Ed. 2020, 59, 7494–7500. [Google Scholar] [CrossRef]
- Sulaiman, S.A.J.; Al-Rasbi, G.S.; Abou-Zied, O.K. Photophysical Properties of Hydroxyphenyl Benzazoles and Their Applications as Fluorescent Probes to Study Local Environment in DNA, Protein and Lipid. Luminescence 2016, 31, 614–625. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the Halogen Bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Lazennec, G.; Kern, L.; Salbert, G.; Saligaut, D.; Valotaire, Y. Cooperation between the Human Estrogen Receptor (ER) and MCF-7 Cell- Specific Transcription Factors Elicits High Activity of an Estrogen-Inducible Enhancer from the Trout ER Gene Promoter. Mol. Endocrinol. 1996, 10, 1116–1126. [Google Scholar] [CrossRef]
- Shiau, A.K.; Barstad, D.; Loria, P.M.; Cheng, L.; Kushner, P.J.; Agard, D.A.; Greene, G.L. The Structural Basis of Estrogen Receptor/Coactivator Recognition and the Antagonism of This Interaction by Tamoxifen. Cell 1998, 95, 927–937. [Google Scholar] [CrossRef]
- Iqbal, N.; Iqbal, N. Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications. Mol. Biol. Int. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Mitri, Z.; Constantine, T.; O’Regan, R. The HER2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Lyskov, S.; Chou, F.-C.; Conchúir, S.Ó.; Der, B.S.; Drew, K.; Kuroda, D.; Xu, J.; Weitzner, B.D.; Renfrew, P.D.; Sripakdeevong, P.; et al. Serverification of Molecular Modeling Applications: The Rosetta Online Server That Includes Everyone (ROSIE). PLoS ONE 2013, 8, e63906. [Google Scholar] [CrossRef]
- Tang, Z.; Han, H.; Ding, J.; Zhou, P. Dual Fluorescence of 2-(2′-Hydroxyphenyl) Benzoxazole Derivatives via the Branched Decays from the Upper Excited-State. Phys. Chem. Chem. Phys. 2021, 23, 27304–27311. [Google Scholar] [CrossRef]
- Heller, A.; Williams, D.L. Intramolecular Proton Transfer Reactions in Excited Fluorescent Compounds. J. Phys. Chem. 1970, 74, 4473–4480. [Google Scholar] [CrossRef]
- Vetrova, E.V.; Tupaeva, I.O.; Demidov, O.P.; Sayapin, Y.A.; Gusakov, E.A.; Minkin, V.I.; Metelitsa, A.V. Dual-State Emission Properties of 2-(2-Carboalkoxy-3,4-Dichloro-6-Hydroxyphenyl)Benzoxazoles and Its Zn(II) and Cd(II) Complexes. J. Lumin. 2023, 263, 120111. [Google Scholar] [CrossRef]
- Hong, N.T.T.; Trusova, M.E. The Synthesis of Iodbenzimidazoles and Iodbenzoxazoles via Iodination of Arenediazonium Tosylates. Adv. Mater. Res. 2014, 1040, 423–428. [Google Scholar] [CrossRef]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Aertgeerts, K.; Skene, R.; Yano, J.; Sang, B.-C.; Zou, H.; Snell, G.; Jennings, A.; Iwamoto, K.; Habuka, N.; Hirokawa, A.; et al. Structural Analysis of the Mechanism of Inhibition and Allosteric Activation of the Kinase Domain of HER2 Protein. J. Biol. Chem. 2011, 286, 18756–18765. [Google Scholar] [CrossRef]
- Lipscomb, L.A.; Peek, M.E.; Zhou, F.X.; Bertrand, J.A.; VanDerveer, D.; Williams, L.D. Water Ring Structure at DNA Interfaces: Hydration and Dynamics of DNA-Anthracycline Complexes. Biochemistry 1994, 33, 3649–3659. [Google Scholar] [CrossRef]
- DeLuca, S.; Khar, K.; Meiler, J. Fully Flexible Docking of Medium Sized Ligand Libraries with RosettaLigand. PLoS ONE 2015, 10, e0132508. [Google Scholar] [CrossRef]
- Combs, S.A.; DeLuca, S.L.; DeLuca, S.H.; Lemmon, G.H.; Nannemann, D.P.; Nguyen, E.D.; Willis, J.R.; Sheehan, J.H.; Meiler, J. Small-Molecule Ligand Docking into Comparative Models with Rosetta. Nat. Protoc. 2013, 8, 1277–1298. [Google Scholar] [CrossRef]
- Kothiwale, S.; Mendenhall, J.L.; Meiler, J. BCL::Conf: Small Molecule Conformational Sampling Using a Knowledge Based Rotamer Library. J. Cheminform. 2015, 7, 47. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Function. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Papajak, E.; Zheng, J.; Xu, X.; Leverentz, H.R.; Truhlar, D.G. Perspectives on Basis Sets Beautiful: Seasonal Plantings of Diffuse Basis Functions. J. Chem. Theory Comput. 2011, 7, 3027–3034. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 vers. A03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]

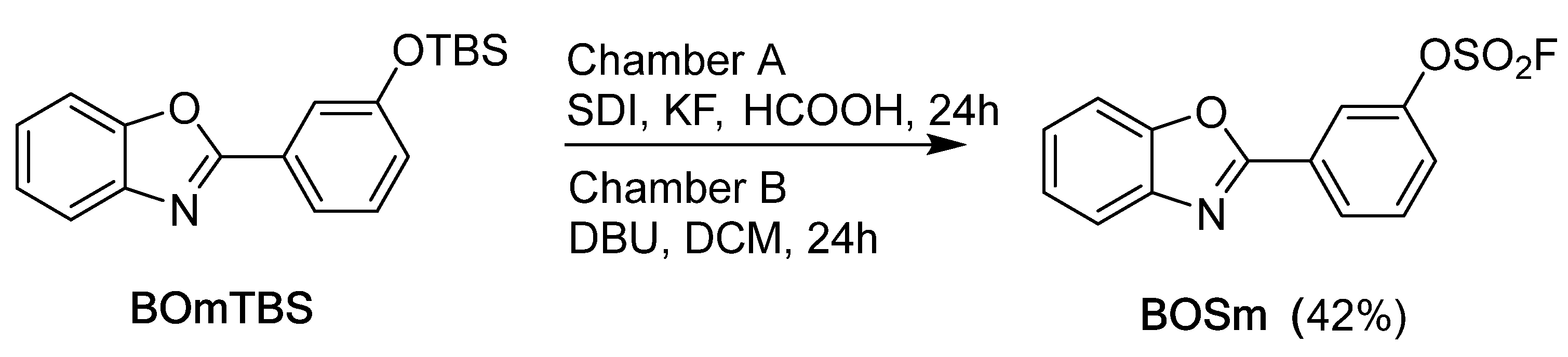

| Acid | 2-(3-(fluorosulfato)phenyl)benzoxazole | 2-(4-(fluorosulfato)phenyl)benzoxazole |
|---|---|---|
| TFA | 24% | 19% |
| Formic acid | 53% | 59% [19] |
| Bond | Length, Å | Bond | Length, Å |
|---|---|---|---|
| S1–F1 | 1.5098(15) | C7–C8 | 1.463(3) |
| S1–O1 | 1.4039(18) | O4–C6 | 1.382(3) |
| S1–O3 | 1.5389(17) | O4–C7 | 1.347(3) |
| S1–O5 | 1.4001(18) | N1–C1 | 1.401(3) |
| O3–C12 | 1.442(2) | N1–C7 | 1.301(3) |
| Compounds | BOo | BOm | BOp | BOSo | BOSm | BOSp | Cisplatin |
|---|---|---|---|---|---|---|---|
| Cell Line | |||||||
| BT-474 | 209.3 (190.1–231.7) | 256.8 (229.1–291.3) | 284.2 (245.6–336.4) | 123.3 (119.9–126.8) | 426.8 (363.8–527.5) | 542.5 (414.5–840.9) | 38.5 (36.4–40.7) |
| MCF-7 | 122.9 (113.0–133.8) | 186.9 (166.9–210.7) | 187.1 (169.5–207.6) | 87.18 (83.0–91.5) | 263.9 (225.4–317.6) | 343.1 (302.8–398.6) | 33.9 (31.9–36.1) |
| Ligand | Residues (Ligand Groups) Participating in Hydrogen Bonding Interactions and π,π-Stacking with DNA Bases | Interface Energy Score |
|---|---|---|
| hER receptor | ||
| BOo | Glu353 (OH) | −12.802 |
| BOm | Arg394 (OH), Glu353 (OH, weak) | −13.743 |
| BOp | Arg394 (OH), Glu353 (OH, weak) | −14.975 |
| BOSo | His524 (SO2F oxygen atom) | −11.828 |
| BOSm | Thr347 (SO2F oxygen atom) | −12.135 |
| BOSp | Arg394 (SO2F oxygen atom) | −11.979 |
| HER2 receptor | ||
| BOo | Ser783 (OH, NHet), Asp863 (OHet, weak) | −16.151 |
| BOm | Arg849 (OH), Asn850 (OH), Asp863 (OH, weak) | −14.537 |
| BOp | Ser728 (OH), Arg849 (OH), Thr862 (NHet) | −16.384 |
| BOSo | Thr798 (SO2F oxygen atom), Thr862 (PhOSO2F oxygen atom) | −12.909 |
| BOSm | Arg849 (SO2F oxygen atom), Asn850 (SO2F oxygen atom, weak; PhOSO2F oxygen atom) | −13.853 |
| BOSp | Arg849 (SO2F oxygen atom), Ser728 (PhOSO2F oxygen atom) | −14.863 |
| DNA | ||
| BOo | π,π-stacking with G and C bases | −9.505 |
| BOm | π,π-stacking with G and C bases | −9.532 |
| BOp | π,π-stacking with G and C bases | −9.514 |
| BOSo | Guanine (SO2F oxygen atom), π,π-stacking with G and C bases | −10.237 |
| BOSm | Guanine (SO2F oxygen atom), π,π-stacking with G and C bases | −10.202 |
| BOSp | Guanine (SO2F oxygen atom), π,π-stacking with G and C bases | −9.973 |
| Compound | Formula | State | Excitation λmax, nm | Emission λmax, nm | Θ, % | τ, ns |
|---|---|---|---|---|---|---|
| BOo | 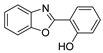 | solid | br. 250–350 nm; 428 sh.; 457; 489; 526 | 480; 503; 537; 571 | 5 | 2.2 |
| MeCN | 353 sh.; 435 sh.; 464; 497 | 427; 517; 544 | 45 | 3.4 | ||
| PBS | br. 250–350 nm | 401; 482; 535; 573 | <1% | n.a. | ||
| BOm | 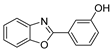 | solid | 366; 378; 455; 486; 522 | 410; 430 sh.; 525; 565 | <1% | – |
| MeCN | 334 sh.; 436 sh.; 464; 497 | 427; 522; 545 | 28 | 3.5 | ||
| PBS | 293 | 359 | <1% | n.a. | ||
| BOp | 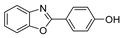 | solid | 340; 357; 462; 487 sh.; 494; 526 | 420; 440 | <1% | – |
| MeCN | 334 sh.; 437 sh.; 464; 497 | 431; 514; 543 | 23 | 3.4 | ||
| PBS | 300 | 363 | 24 | 1.4 | ||
| BOSo | 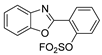 | solid | broad 250–350 nm; 334 | 368, 494 | 16 | 6.6 |
| MeCN | 329 | 354 | 40 | 1.4 | ||
| PBS | 331 | 363; 495 | 32 | 6.2 | ||
| BOSm | 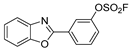 | solid | broad 250–350 nm; 334 | 381 | 15 | 1.9 |
| MeCN | 329 | 349 | 27 | 2.0 | ||
| PBS | 295 | 361 | 27 | 1.7 | ||
| BOSp |  | solid | broad 250–350 nm; 337 | 375 | 33 | 8.2 (7%); 5.3 (93%) |
| MeCN | 329 | 353 | 64 | 1.9 | ||
| PBS | 298 | 358 | 24 | 1.6 |
| Compound | Calculated λmax (nm) | Experimental λmax (nm) | Transition |
|---|---|---|---|
| BOSo | 343 | 354 | LUMO-HOMO |
| BOSm | 346 | 349 | LUMO-HOMO |
| BOSp | 350 | 353 | LUMO-HOMO |
| Parameter | Value |
|---|---|
| Empirical formula | C13NO4SFH8 |
| Formula weight | 293.26 |
| Temperature, K | 290(2) |
| Crystal system | triclinic |
| Space group | P-1 |
| a, Å | 6.7044(8) |
| b, Å | 7.2299(13) |
| c, Å | 13.1344(17) |
| α, ° | 82.644(12) |
| β, ° | 79.806(10) |
| γ, ° | 86.937(12) |
| Volume, Å3 | 621.14(16) |
| Z | 2 |
| ρcalc, g/cm3 | 1.568 |
| μ, mm−1 | 0.286 |
| F(000) | 300.0 |
| Crystal size, mm3 | 0.3 × 0.25 × 0.2 |
| 2Θ range for data collection, ° | 5.684 to 58.068 |
| Index ranges | −9 ≤ h ≤ 8, −7 ≤ k ≤ 9, −17 ≤ l ≤ 17 |
| Reflections collected | 5147 |
| Independent reflections | 2747 [Rint = 0.0192, Rsigma = 0.0335] |
| Data/Restraints/Parameters | 2747/0/181 |
| Goodness-of-fit on F2 | 1.052 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0484, wR2 = 0.1257 |
| Final R indexes [all data] | R1 = 0.0650, wR2 = 0.1357 |
| Largest diff. peak/hole, e·Å−3 | 0.24/−0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danilenko, N.V.; Lutsuk, M.O.; Ryadun, A.A.; Pavlov, D.I.; Plotnikov, E.V.; Eskova, D.D.; Klimenko, Y.D.; Potapov, A.S.; Khlebnikov, A.I. Luminescent Properties and Cytotoxic Activity of 2-phenylbenzoxazole Fluorosulfate Derivatives. Int. J. Mol. Sci. 2025, 26, 7261. https://doi.org/10.3390/ijms26157261
Danilenko NV, Lutsuk MO, Ryadun AA, Pavlov DI, Plotnikov EV, Eskova DD, Klimenko YD, Potapov AS, Khlebnikov AI. Luminescent Properties and Cytotoxic Activity of 2-phenylbenzoxazole Fluorosulfate Derivatives. International Journal of Molecular Sciences. 2025; 26(15):7261. https://doi.org/10.3390/ijms26157261
Chicago/Turabian StyleDanilenko, Nadezhda V., Mariia O. Lutsuk, Alexey A. Ryadun, Dmitry I. Pavlov, Evgenii V. Plotnikov, Daria D. Eskova, Yulia D. Klimenko, Andrei S. Potapov, and Andrei I. Khlebnikov. 2025. "Luminescent Properties and Cytotoxic Activity of 2-phenylbenzoxazole Fluorosulfate Derivatives" International Journal of Molecular Sciences 26, no. 15: 7261. https://doi.org/10.3390/ijms26157261
APA StyleDanilenko, N. V., Lutsuk, M. O., Ryadun, A. A., Pavlov, D. I., Plotnikov, E. V., Eskova, D. D., Klimenko, Y. D., Potapov, A. S., & Khlebnikov, A. I. (2025). Luminescent Properties and Cytotoxic Activity of 2-phenylbenzoxazole Fluorosulfate Derivatives. International Journal of Molecular Sciences, 26(15), 7261. https://doi.org/10.3390/ijms26157261


_Kim.png)






