BDNF Overexpression Enhances Neuronal Activity and Axonal Growth in Human iPSC-Derived Neural Cultures
Abstract
1. Introduction
2. Results
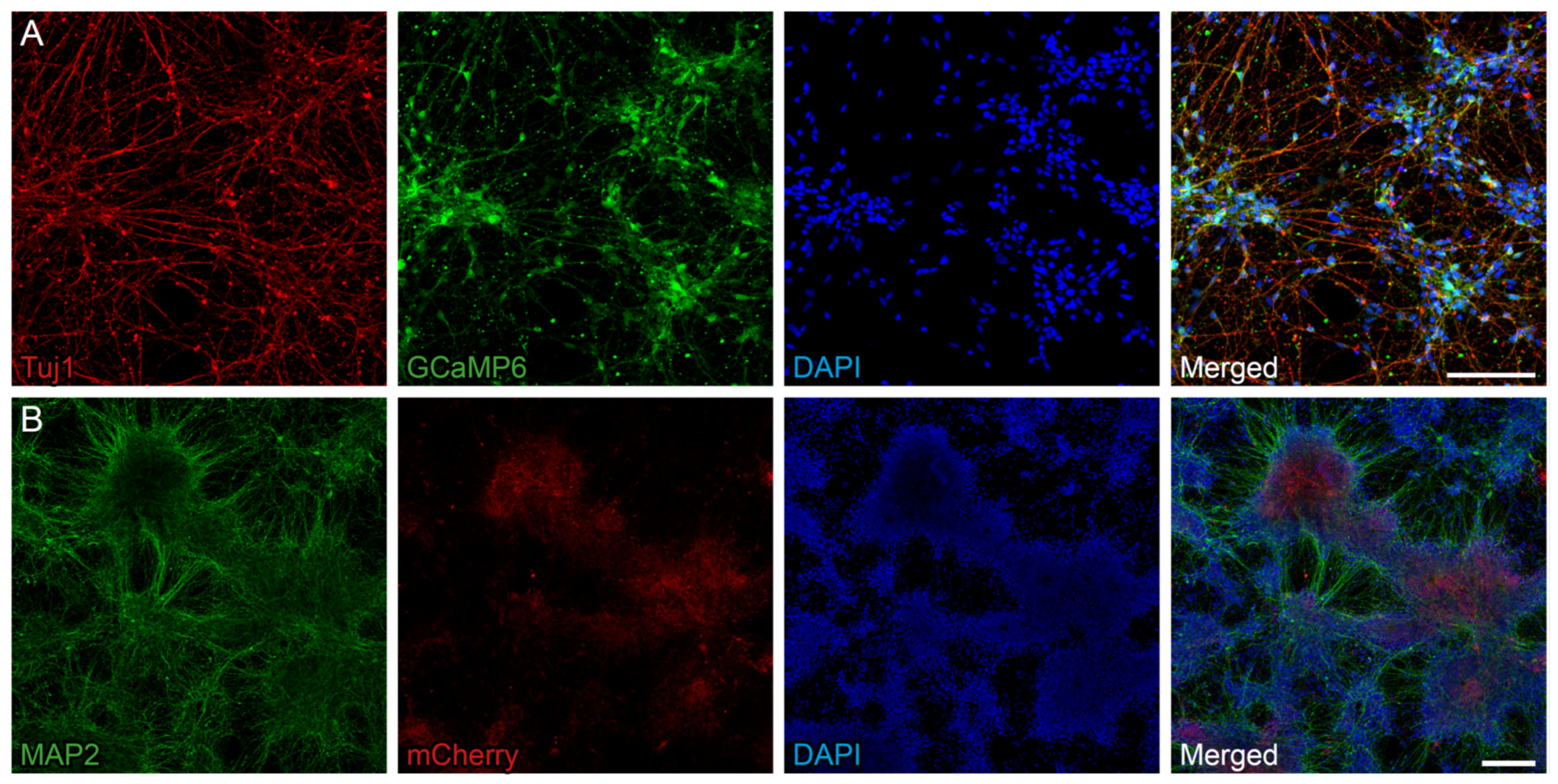
2.1. BDNF Can Be Overexpressed in NPCs with a Fluorescent Calcium Indicator
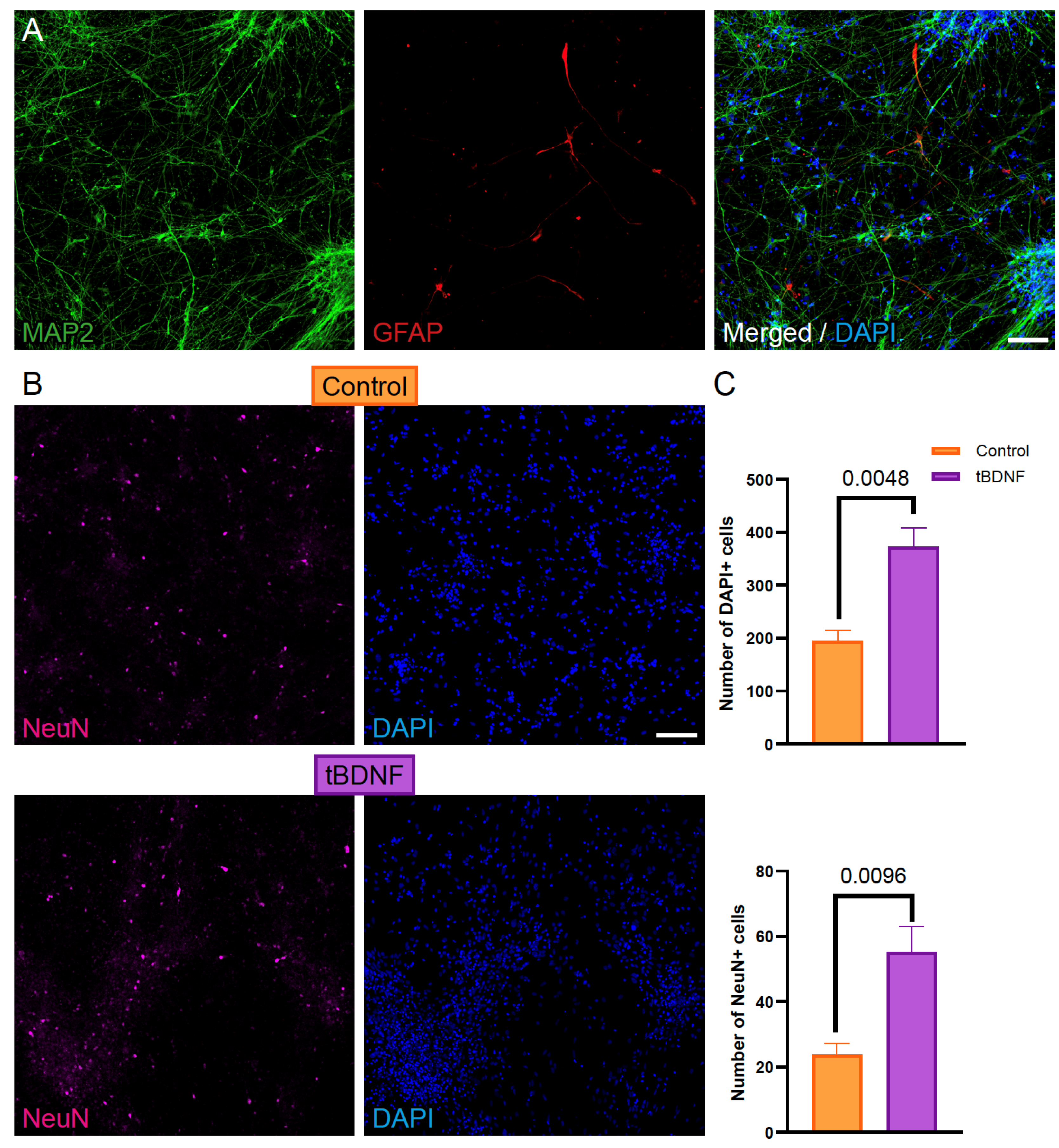
2.2. BDNF Overexpression Enhances Neuronal Differentiation of Human iPSC-Derived NPCs
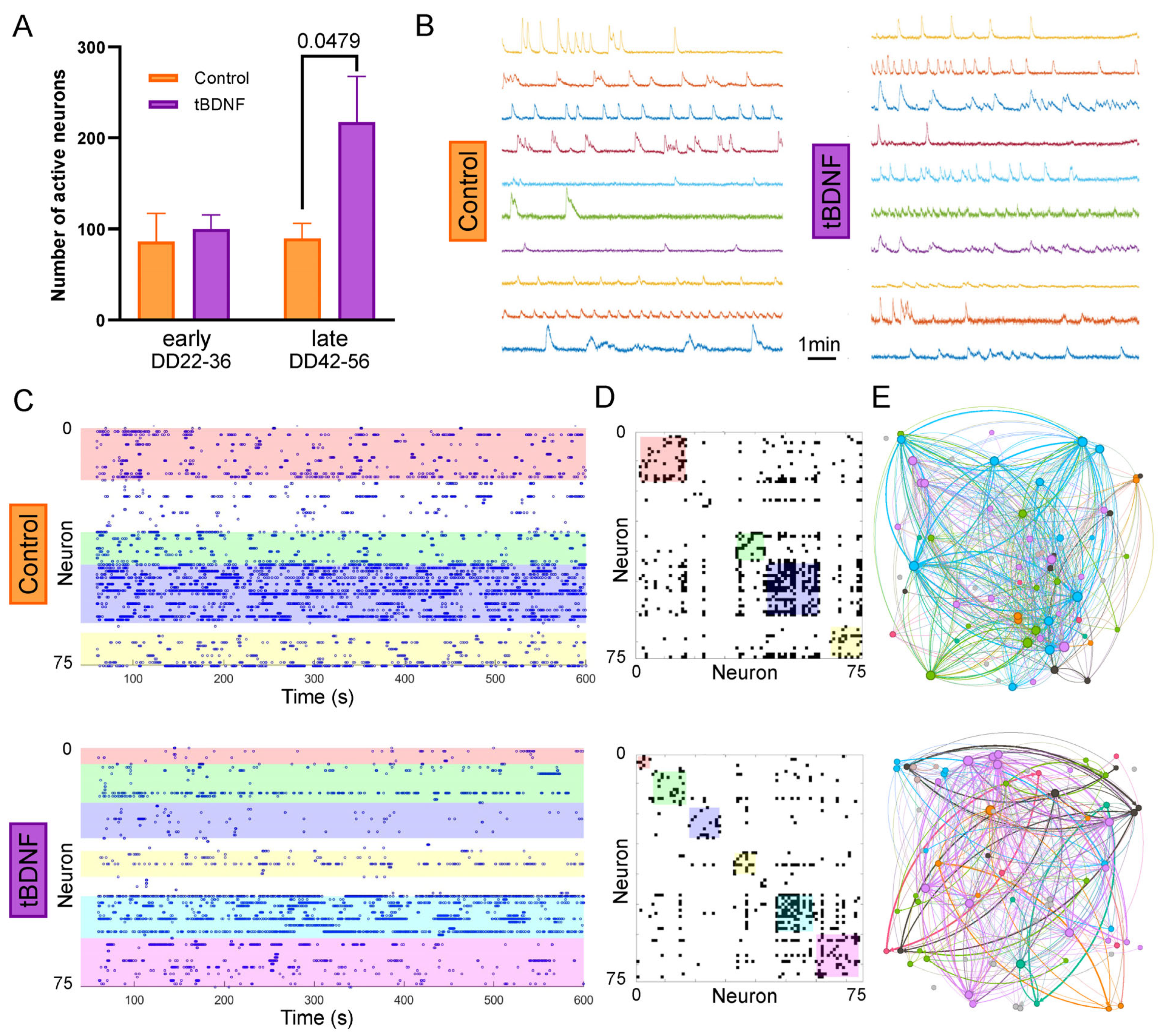
2.3. BDNF Overexpression Increases the Number of Active Neurons Without Altering Network Topology
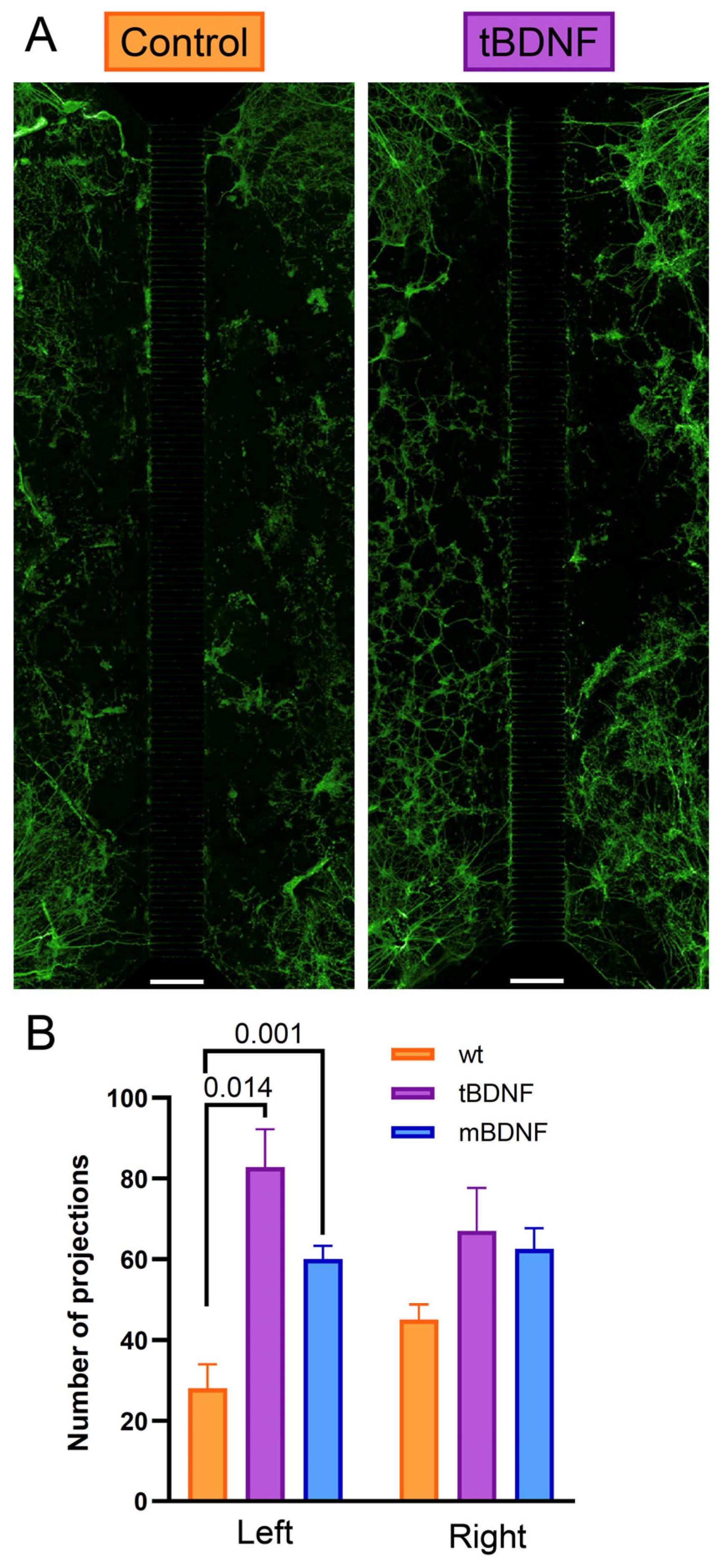
2.4. BDNF Promotes Directional Axonal Outgrowth in a Microfluidic System
3. Discussion
4. Materials and Methods
4.1. Cell Line and Cortical Priming
4.2. Lentiviral Transfection
4.3. Quantitative PCR
4.4. Immunocytochemistry
4.5. Quantifications
4.6. Calcium Fluorescence Imaging
4.7. Calcium Imaging Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain-derived neurotrophic factor |
| NPCs | Neural progenitor cells |
| iPSC | Induced pluripotent stem cells |
| lt-NES | Long-term neuroepithelial-like stem |
| NSCs | Neural stem cells |
| MSCs | Mesenchymal stem cells |
References
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The Global Burden of Neurological Disorders: Translating Evidence into Policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Wilson, L.; Stewart, W.; Dams-O’Connor, K.; Diaz-Arrastia, R.; Horton, L.; Menon, D.K.; Polinder, S. The Chronic and Evolving Neurological Consequences of Traumatic Brain Injury. Lancet Neurol. 2017, 16, 813–825. [Google Scholar] [CrossRef]
- Lindvall, O.; Kokaia, Z. Stem Cells in Human Neurodegenerative Disorders—Time for Clinical Translation? J. Clin. Investig. 2010, 120, 29–40. [Google Scholar] [CrossRef]
- Palma-Tortosa, S.; Coll-San Martin, B.; Kokaia, Z.; Tornero, D. Neuronal Replacement in Stem Cell Therapy for Stroke: Filling the Gap. Front. Cell Dev. Biol. 2021, 9, 662636. [Google Scholar] [CrossRef]
- Kirkeby, A.; Nelander, J.; Hoban, D.B.; Rogelius, N.; Bjartmarz, H.; Storm, P.; Fiorenzano, A.; Adler, A.F.; Vale, S.; Mudannayake, J.; et al. Preclinical Quality, Safety, and Efficacy of a Human Embryonic Stem Cell-Derived Product for the Treatment of Parkinson’s Disease, STEM-PD. Cell Stem Cell 2023, 30, 1299–1314.e9. [Google Scholar] [CrossRef]
- Barker, R.A.; Parmar, M.; Studer, L.; Takahashi, J. Human Trials of Stem Cell-Derived Dopamine Neurons for Parkinson’s Disease: Dawn of a New Era. Cell Stem Cell 2017, 21, 569–573. [Google Scholar] [CrossRef]
- Sawamoto, N.; Doi, D.; Nakanishi, E.; Sawamura, M.; Kikuchi, T.; Yamakado, H.; Taruno, Y.; Shima, A.; Fushimi, Y.; Okada, T.; et al. Phase I/II Trial of IPS-Cell-Derived Dopaminergic Cells for Parkinson’s Disease. Nature 2025, 641, 971–977. [Google Scholar] [CrossRef]
- Tabar, V.; Sarva, H.; Lozano, A.M.; Fasano, A.; Kalia, S.K.; Yu, K.K.H.; Brennan, C.; Ma, Y.; Peng, S.; Eidelberg, D.; et al. Phase I Trial of HES Cell-Derived Dopaminergic Neurons for Parkinson’s Disease. Nature 2025, 641, 978–983. [Google Scholar] [CrossRef]
- Deuse, T.; Hu, X.; Gravina, A.; Wang, D.; Tediashvili, G.; De, C.; Thayer, W.O.; Wahl, A.; Garcia, J.V.; Reichenspurner, H.; et al. Hypoimmunogenic Derivatives of Induced Pluripotent Stem Cells Evade Immune Rejection in Fully Immunocompetent Allogeneic Recipients. Nat. Biotechnol. 2019, 37, 252–258. [Google Scholar] [CrossRef]
- Morizane, A.; Takahashi, J. Evading the Immune System: Immune Modulation and Immune Matching in Cell Replacement Therapies for Parkinson’s Disease. J. Park. Dis. 2021, 11, S167–S172. [Google Scholar] [CrossRef]
- Mishchenko, T.A.; Klimenko, M.O.; Kuznetsova, A.I.; Yarkov, R.S.; Savelyev, A.G.; Sochilina, A.V.; Mariyanats, A.O.; Popov, V.K.; Khaydukov, E.V.; Zvyagin, A.V.; et al. 3D-Printed Hyaluronic Acid Hydrogel Scaffolds Impregnated with Neurotrophic Factors (BDNF, GDNF) for Post-Traumatic Brain Tissue Reconstruction. Front. Bioeng. Biotechnol. 2022, 10, 895406. [Google Scholar] [CrossRef]
- Kurozumi, K.; Nakamura, K.; Tamiya, T.; Kawano, Y.; Kobune, M.; Hirai, S.; Uchida, H.; Sasaki, K.; Ito, Y.; Kato, K.; et al. BDNF Gene-Modified Mesenchymal Stem Cells Promote Functional Recovery and Reduce Infarct Size in the Rat Middle Cerebral Artery Occlusion Model. Mol. Ther. 2004, 9, 189–197. [Google Scholar] [CrossRef]
- Habashy, K.J.; Omais, S.; Haupt, B.; Sonabend, A.M.; Ahuja, C.S. Cell-Based Therapies for the Treatment of Traumatic Brain Injury: Promises and Trajectories. Biologics 2024, 4, 161–176. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Mothe, A.; Khazaei, M.; Badhiwala, J.H.; Gilbert, E.A.; Kooy, D.; Morshead, C.M.; Tator, C.; Fehlings, M.G. The Leading Edge: Emerging Neuroprotective and Neuroregenerative Cell-Based Therapies for Spinal Cord Injury. Stem Cells Transl. Med. 2020, 9, 1509–1530. [Google Scholar] [CrossRef]
- Canals, J.M.; Checa, N.; Marco, S.; Åkerud, P.; Michels, A.; Pérez-Navarro, E.; Tolosa, E.; Arenas, E.; Alberch, J. Expression of Brain-Derived Neurotrophic Factor in Cortical Neurons Is Regulated by Striatal Target Area. J. Neurosci. 2001, 21, 117–124. [Google Scholar] [CrossRef]
- Ringstedt, T.; Linnarsson, S.; Wagner, J.; Lendahl, U.; Kokaia, Z.; Arenas, E.; Ernfors, P.; Ibáñez, C.F. BDNF Regulates Reelin Expression and Cajal-Retzius Cell Development in the Cerebral Cortex. Neuron 1998, 21, 305–315. [Google Scholar] [CrossRef]
- Wang, C.S.; Kavalali, E.T.; Monteggia, L.M. BDNF Signaling in Context: From Synaptic Regulation to Psychiatric Disorders. Cell 2022, 185, 62–76. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liang, J.; Chen, D.C.; Xiu, M.H.; De Yang, F.; Kosten, T.A.; Kosten, T.R. Low BDNF Is Associated with Cognitive Impairment in Chronic Patients with Schizophrenia. Psychopharmacology 2012, 222, 277–284. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; de Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Toader, C.; Serban, M.; Munteanu, O.; Covache-Busuioc, R.-A.; Enyedi, M.; Ciurea, A.V.; Tataru, C.P. From Synaptic Plasticity to Neurodegeneration: BDNF as a Transformative Target in Medicine. Int. J. Mol. Sci. 2025, 26, 4271. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lien, C.-C.; Hou, W.-H.; Chiang, P.-M.; Tsai, K.-J. Gain of BDNF Function in Engrafted Neural Stem Cells Promotes the Therapeutic Potential for Alzheimer’s Disease. Sci. Rep. 2016, 6, 27358. [Google Scholar] [CrossRef]
- Lu, P.; Jones, L.; Tuszynski, M.H. BDNF-Expressing Marrow Stromal Cells Support Extensive Axonal Growth at Sites of Spinal Cord Injury. Exp. Neurol. 2005, 191, 344–360. [Google Scholar] [CrossRef]
- Sasaki, M.; Radtke, C.; Tan, A.M.; Zhao, P.; Hamada, H.; Houkin, K.; Honmou, O.; Kocsis, J.D. BDNF-Hypersecreting Human Mesenchymal Stem Cells Promote Functional Recovery, Axonal Sprouting, and Protection of Corticospinal Neurons after Spinal Cord Injury. J. Neurosci. 2009, 29, 14932–14941. [Google Scholar] [CrossRef]
- Wang, C.; Tian, C.; Cai, D.; Jiang, H.; Zhang, W.; Liu, S.; Peng, L.; Hu, X. BDNF-Overexpressing MSCs Delivered by Hydrogel in Acute Ischemic Stroke Treatment. Ann. Transl. Med. 2022, 10, 1393. [Google Scholar] [CrossRef]
- Palma-Tortosa, S.; Tornero, D.; Hansen, M.G.; Monni, E.; Hajy, M.; Kartsivadze, S.; Aktay, S.; Tsupykov, O.; Parmar, M.; Deisseroth, K.; et al. Activity in Grafted Human IPS Cell–Derived Cortical Neurons Integrated in Stroke-Injured Rat Brain Regulates Motor Behavior. Proc. Natl. Acad. Sci. USA 2020, 117, 9094–9100. [Google Scholar] [CrossRef]
- Tornero, D.; Tsupykov, O.; Granmo, M.; Rodriguez, C.; Grønning-Hansen, M.; Thelin, J.; Smozhanik, E.; Laterza, C.; Wattananit, S.; Ge, R.; et al. Synaptic Inputs from Stroke-Injured Brain to Grafted Human Stem Cell-Derived Neurons Activated by Sensory Stimuli. Brain 2017, 140, 692–706. [Google Scholar] [CrossRef]
- Martinez-Curiel, R.; Jansson, L.; Tsupykov, O.; Avaliani, N.; Aretio-Medina, C.; Hidalgo, I.; Monni, E.; Bengzon, J.; Skibo, G.; Lindvall, O.; et al. Oligodendrocytes in Human Induced Pluripotent Stem Cell-Derived Cortical Grafts Remyelinate Adult Rat and Human Cortical Neurons. Stem Cell Rep. 2023, 18, 1643–1656. [Google Scholar] [CrossRef]
- Tornero, D.; Wattananit, S.; Madsen, M.G.; Koch, P.; Wood, J.; Tatarishvili, J.; Mine, Y.; Ge, R.; Monni, E.; Devaraju, K.; et al. Human Induced Pluripotent Stem Cell-Derived Cortical Neurons Integrate in Stroke-Injured Cortex and Improve Functional Recovery. Brain 2013, 136, 3561–3577. [Google Scholar] [CrossRef]
- Estévez-Priego, E.; Moreno-Fina, M.; Monni, E.; Kokaia, Z.; Soriano, J.; Tornero, D. Long-Term Calcium Imaging Reveals Functional Development in HiPSC-Derived Cultures Comparable to Human but Not Rat Primary Cultures. Stem Cell Rep. 2023, 18, 205–219. [Google Scholar] [CrossRef]
- Soriano, J. Neuronal Cultures: Exploring Biophysics, Complex Systems, and Medicine in a Dish. Biophysica 2023, 3, 181–202. [Google Scholar] [CrossRef]
- Castrén, E.; Antila, H. Neuronal Plasticity and Neurotrophic Factors in Drug Responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- Hoshino, N.; Vatterott, P.; Egwiekhor, A.; Rochlin, M.W. Brain-Derived Neurotrophic Factor Attracts Geniculate Ganglion Neurites during Embryonic Targeting. Dev. Neurosci. 2010, 32, 184–196. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, A.; Puigsasllosas-Pastor, C.; Arcas-Marquez, A.; Tornero, D. Updated Toolbox for Assessing Neuronal Network Reconstruction after Cell Therapy. Bioengineering 2024, 11, 487. [Google Scholar] [CrossRef]
- Tornero, D. Neuronal Circuitry Reconstruction after Stem Cell Therapy in Damaged Brain. Neural Regen. Res. 2022, 17, 1959–1960. [Google Scholar] [CrossRef]
- Grønning Hansen, M.; Laterza, C.; Palma-Tortosa, S.; Kvist, G.; Monni, E.; Tsupykov, O.; Tornero, D.; Uoshima, N.; Soriano, J.; Bengzon, J.; et al. Grafted Human Pluripotent Stem Cell-Derived Cortical Neurons Integrate into Adult Human Cortical Neural Circuitry. Stem Cells Transl. Med. 2020, 9, 1365–1377. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Gasco, A.; Percopo, F.; Font-Guixe, A.; Ramos-Bartolome, S.; Cami-Bonet, A.; Magem-Planas, M.; Fabrellas-Monsech, M.; Esquirol-Albala, E.; Goulet, L.; Fornos-Zapater, S.; et al. BDNF Overexpression Enhances Neuronal Activity and Axonal Growth in Human iPSC-Derived Neural Cultures. Int. J. Mol. Sci. 2025, 26, 7262. https://doi.org/10.3390/ijms26157262
Ortega-Gasco A, Percopo F, Font-Guixe A, Ramos-Bartolome S, Cami-Bonet A, Magem-Planas M, Fabrellas-Monsech M, Esquirol-Albala E, Goulet L, Fornos-Zapater S, et al. BDNF Overexpression Enhances Neuronal Activity and Axonal Growth in Human iPSC-Derived Neural Cultures. International Journal of Molecular Sciences. 2025; 26(15):7262. https://doi.org/10.3390/ijms26157262
Chicago/Turabian StyleOrtega-Gasco, Alba, Francesca Percopo, Ares Font-Guixe, Santiago Ramos-Bartolome, Andrea Cami-Bonet, Marc Magem-Planas, Marc Fabrellas-Monsech, Emma Esquirol-Albala, Luna Goulet, Sergi Fornos-Zapater, and et al. 2025. "BDNF Overexpression Enhances Neuronal Activity and Axonal Growth in Human iPSC-Derived Neural Cultures" International Journal of Molecular Sciences 26, no. 15: 7262. https://doi.org/10.3390/ijms26157262
APA StyleOrtega-Gasco, A., Percopo, F., Font-Guixe, A., Ramos-Bartolome, S., Cami-Bonet, A., Magem-Planas, M., Fabrellas-Monsech, M., Esquirol-Albala, E., Goulet, L., Fornos-Zapater, S., Arcas-Marquez, A., Haeb, A.-C., Gomez-Bravo, C., Introna, C., Canals, J. M., & Tornero, D. (2025). BDNF Overexpression Enhances Neuronal Activity and Axonal Growth in Human iPSC-Derived Neural Cultures. International Journal of Molecular Sciences, 26(15), 7262. https://doi.org/10.3390/ijms26157262







