Brassinosteroids in Cucurbits: Modulators of Plant Growth Architecture and Stress Response
Abstract
1. Introduction
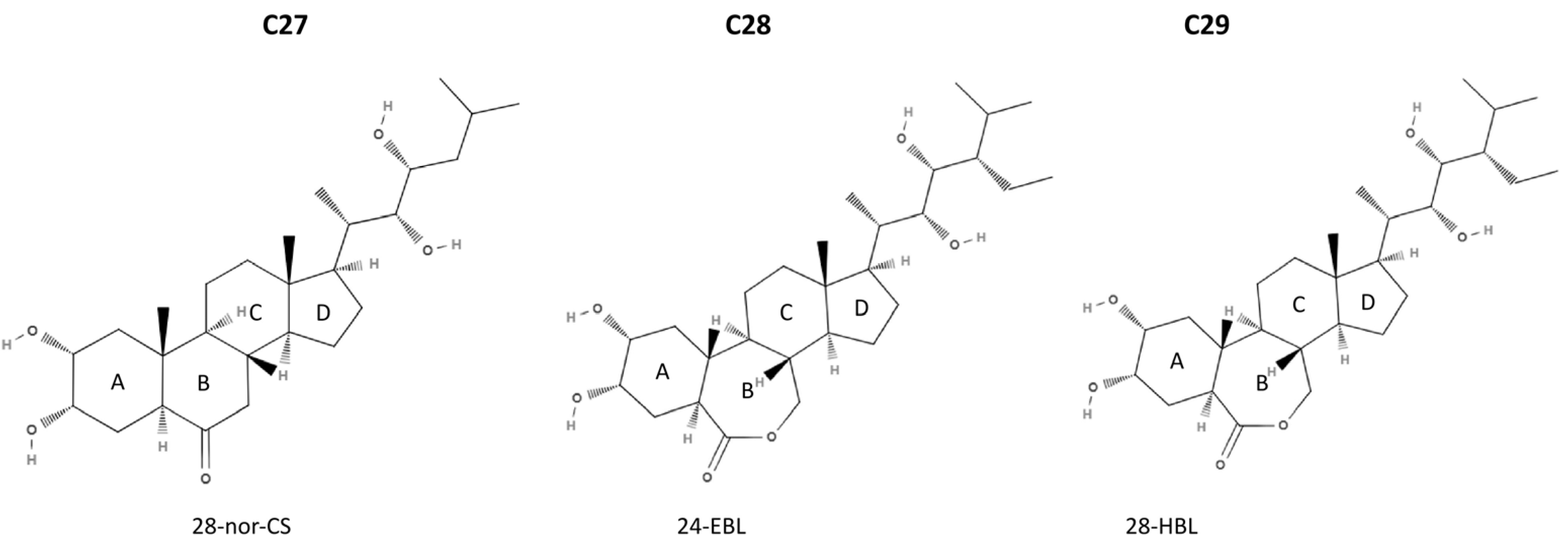
2. Chemical Structure and Occurrence of BRs
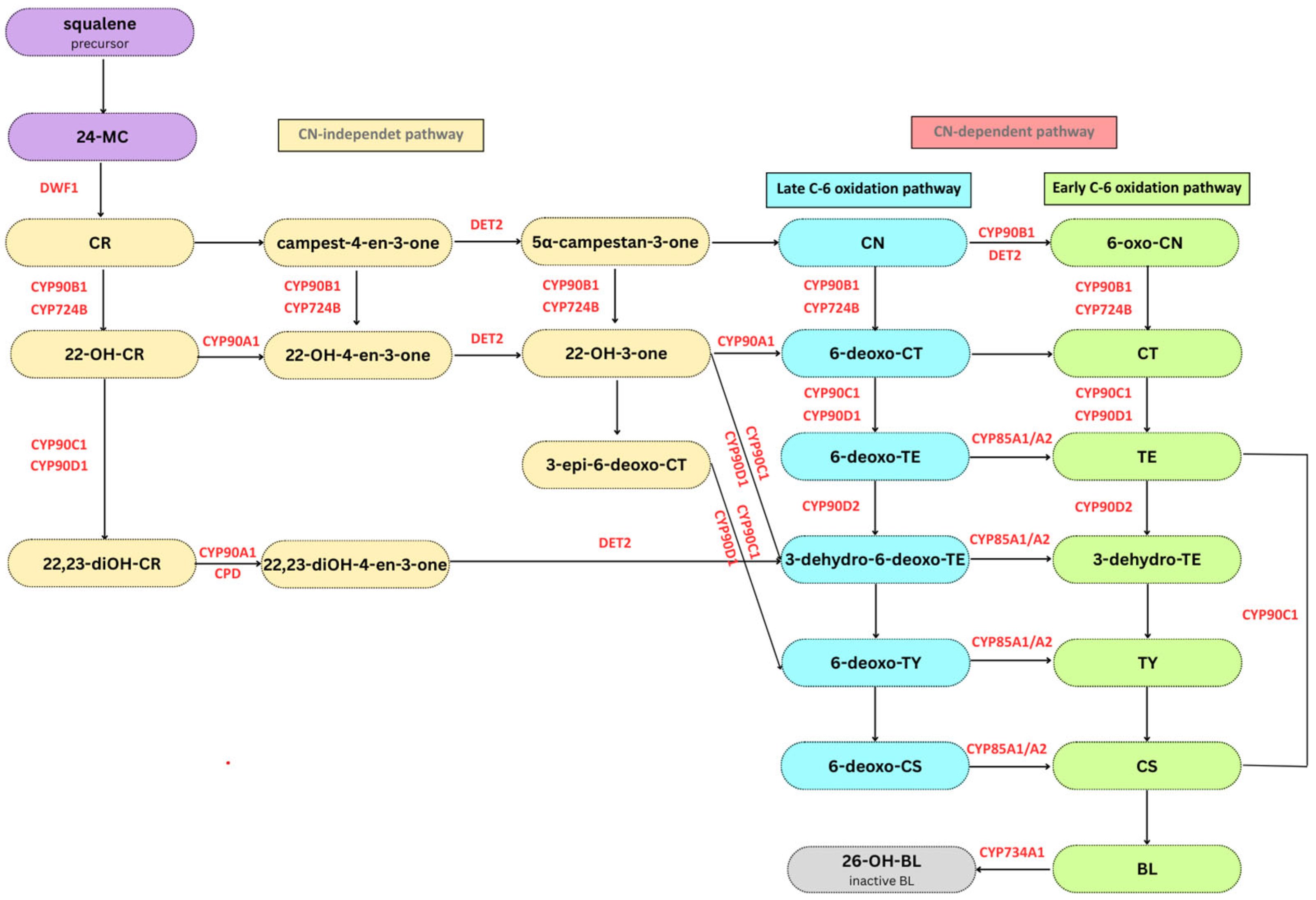
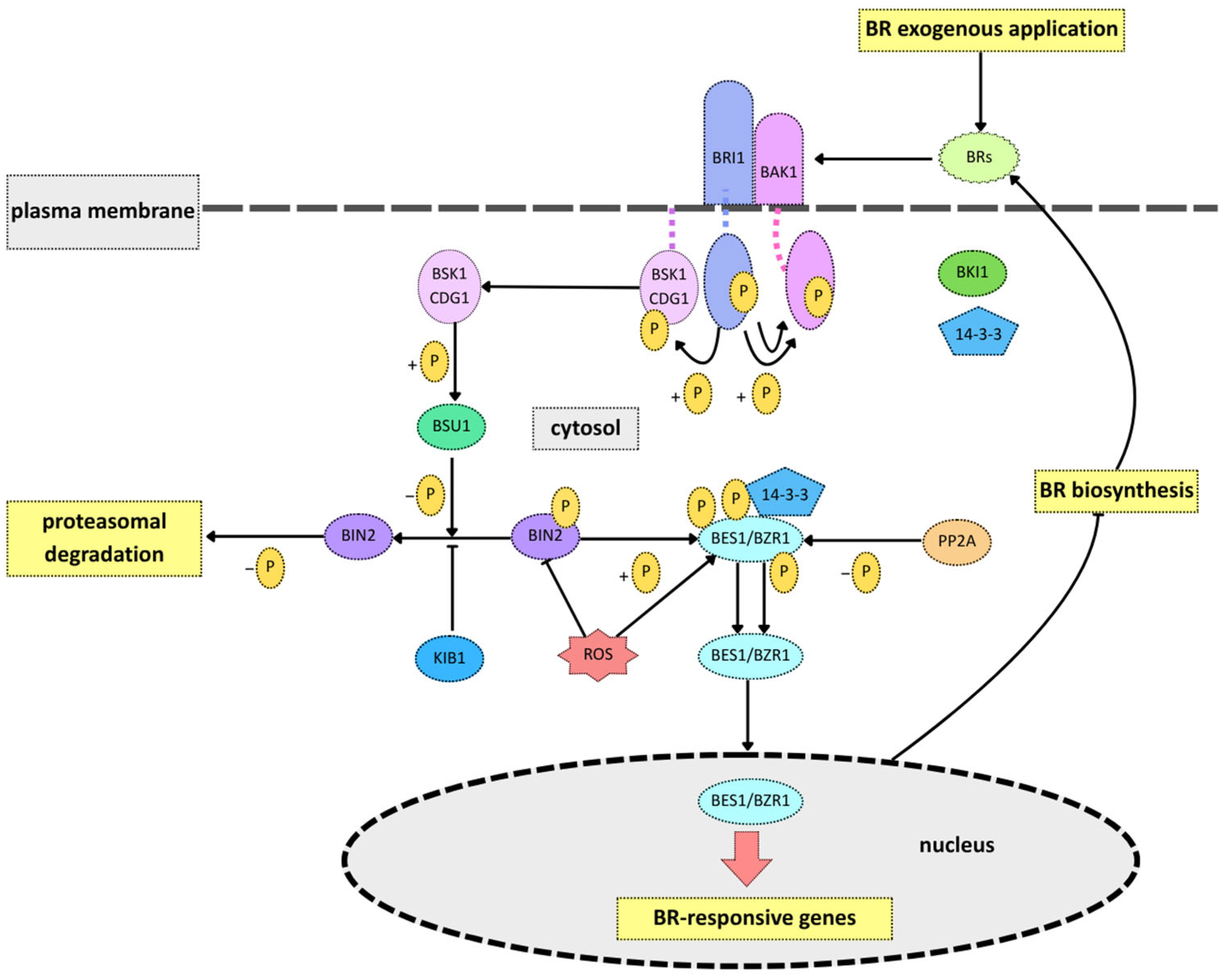
3. BR Biosynthesis and Signaling
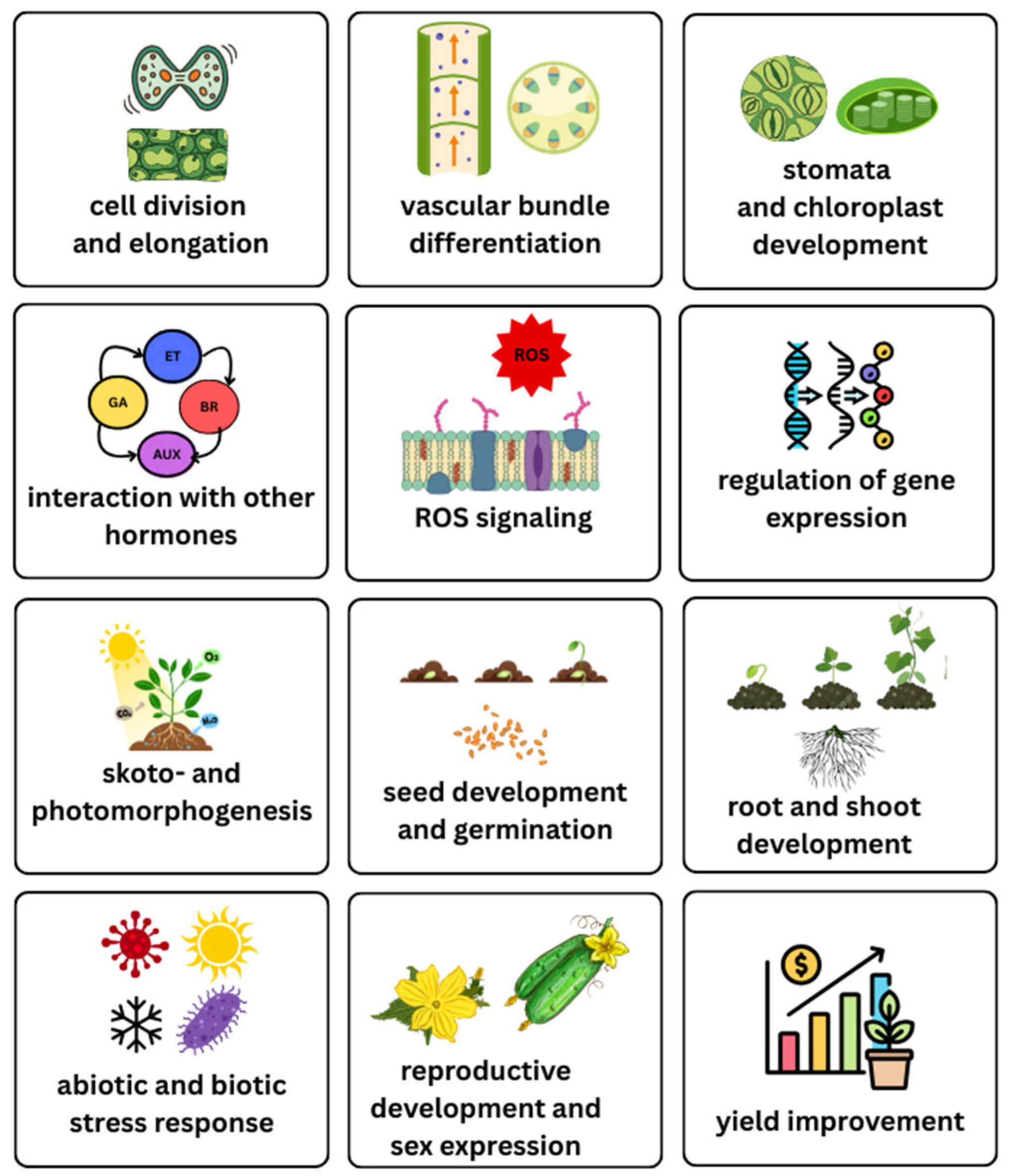
4. Functions of BRs in Plants
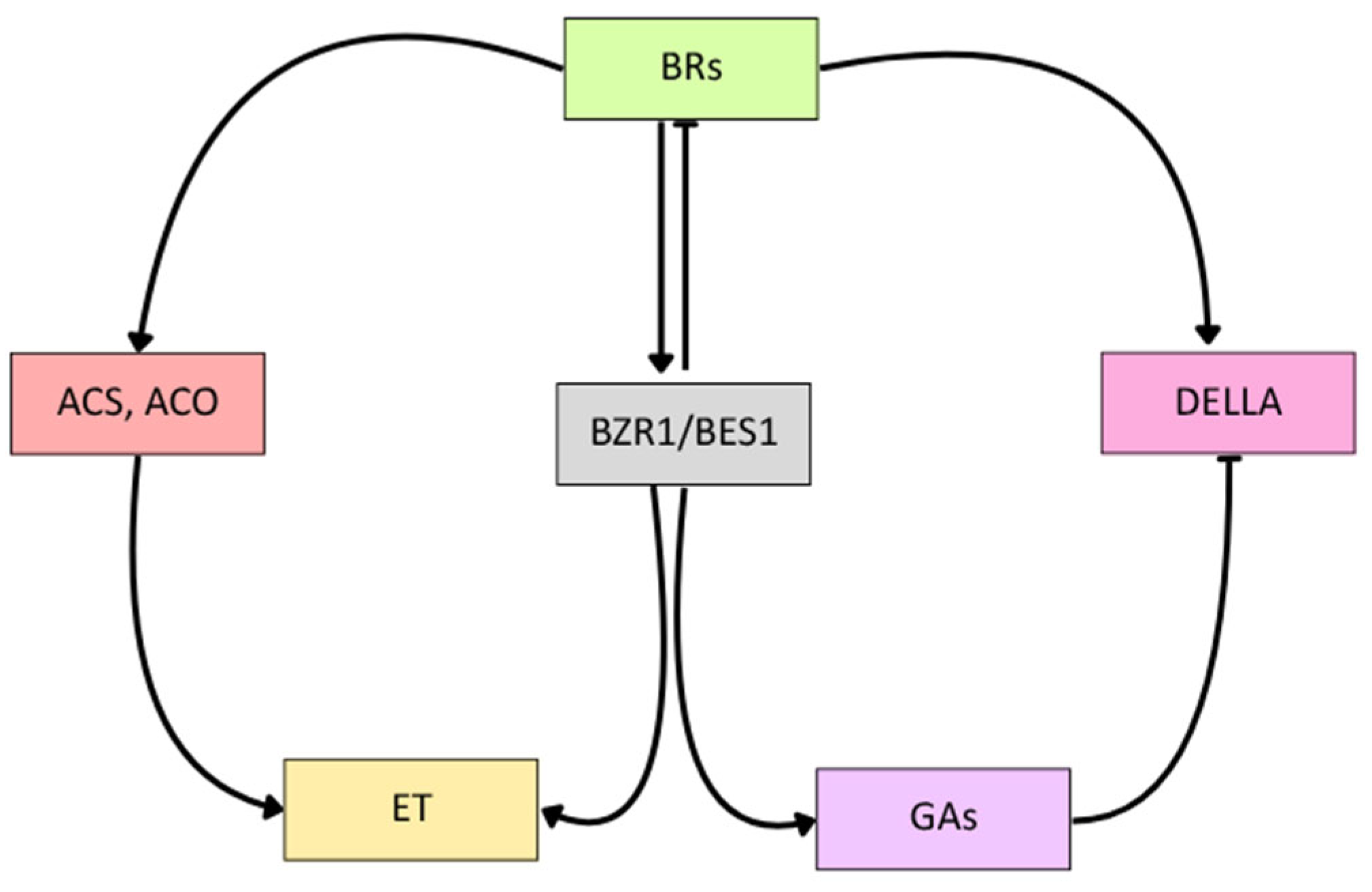
5. Crosstalk Between BRs and Other Phytohormones
5.1. Interaction Between BRs and Ethylene
5.2. Interaction Between BRs and Gibberellins
5.3. Interaction Between BRs and Auxins
6. Mutants in Cucurbits with Affected BR Biosynthesis
| Species | Mutant | Phenotype | Candidate Gene | Gene ID * | Gene Annotation | Reference |
|---|---|---|---|---|---|---|
| Cucumis sativus | cpa compact plant architecture | extreme dwarf phenotype shortened internodes and petioles dark green and wrinkled leaves | CsDWF5 | CsaV3_7G033720 | 7–dehydrocholesterol reductase | [92] |
| cpa–2 compact plant architecture 2 | compact phenotype short stem with few branches shortened internodes and petioles dark green and wrinkled leaves short hypocotyl abnormal stigma and ovary | CsDWF1 | CsaV3_7G030530 | sterol-C24-reductase | [93] | |
| scp–1 super compact–1 | extremely reduced internodes and mature vine length dark green and wrinkled leaves with a rounder shape no tendrils smaller root length and volume abnormal stigma and ovary de-etiolation in the dark | CsCYP85A1 | CsaV3_5G038650 | BR-6-oxidase | [90] | |
| scp–2 super compact–2 | extreme dwarf phenotype shortened petioles dark green and wrinkled leaves dark green cotyledons short and inflated hypocotyl de-etiolation in the dark defects in cell elongation and vascular development partially female sterile | CsDET2 | CsaV3_3G034190 | steroid-5-alpha reductase | [91] | |
| scp–3 super compact–3 | shortened internodes dark green and wrinkled leaves | CsDWF7 | CsaV3_4G028790 | delta7-sterol C5-reductase | [94] | |
| Cucurbita pepo | dwfcp | shortened internodes dark green and wrinkled leaves shorter and thicker roots greater root biomass reduced fertility | CpDWF5 | Cp4.1LG17 | 7–dehydrocholesterol-reductase | [97] |
| Citrullus lanatus | dwarf | dwarf phenotype shortened internodes smaller leaf area lacking clear lobulations | ClDUF21 ClDWF1 | Cla97C06G115300 Cla97C09G166970 | DUF21 domain protein sterol-C24-reductase | [99] |
7. Role of BRs in the Development and Growth of Cucurbits
| Species | Role of BRs | Reference |
|---|---|---|
| Cucumis sativus | promoting earlier and increased female flower production in monoecious genotypes | [79] |
| role in early fruit development and parthenocarpy | [104] | |
| promoting photosynthesis and growth by positive regulation of synthesis and activation of photosynthesis-related enzymes, including Rubisco | [107] | |
| promoting vegetative growth and yield | [102] | |
| role in the regulation of the antioxidant system and protection of the chloroplast under autotoxicity stress conditions | [105] | |
| increasing yield parameters | [103] | |
| Cucurbita pepo | role in sex expression and flower development | [100] |
| Citrullus lanatus | increasing female flower production and yield parameters | [101] |
8. BRs in the Stress Response of Cucurbits
8.1. Abiotic Stress
8.2. Biotic Stress
| Species | Investigated Accessions | Stress Factor | Exposure Time | Investigated Gene | Reference |
|---|---|---|---|---|---|
| Cucumis sativus | cv. Xintaimici | cold 6 °C | 6, 12, 24 h, 2, 3, 6, and 9 d | CsBES1 | [106] |
| NaCl 150 mM | 1, 3, 6, 9, 12, and 24 h | ||||
| PEG6000 10% | |||||
| cv. Changchunmici—wild type Csbpc2—knockout mutants | cold 4 °C | 6 h | CsBZR1 CsBZR2 CsCYP90A1 CsDET2 | [109] | |
| cv. Xinchun 4 | CdCl2 200 μM | 6, 12, and 24 h | CsBZR | [108] | |
| cold 12 °C/8 °C | |||||
| NaCl 200 mM | |||||
| PEG 6000 20% | |||||
| Gy14 R-line B10 S-line | Pseudomonas syringae pv. lachrymans | 1 dpi | CsBZR | [112] | |
| PI 183967 R-line 931 S-line | gummy stem blight | 12 hpi | CsBAK1 | [111] | |
| SSL508–28 R-line D8 S-line | powdery mildew | 48 hpi | |||
| PI 197088 R-line cv. Vlaspik S-line | downy mildew | 24 hpi | |||
| 9110 Gt R-line 9930 S-line | gray mold and fusarium wilt | 12, 48, and 96 hpi | |||
| Cucurbita moschata | cv. TianMiyihao | cold (4 °C) | 6 h | CmoBES1 | [110] |
| NaCl (150 mM) | |||||
| PEG6000 (20%) | |||||
| Cucurbita pepo | MUCU16—wild type dwfcp—mutant | NaCl (200 mM) | 16 h for seed germination | CpDWF5 | [97] |
| NaCl (100 mM) | 72 h for seedling elongation |
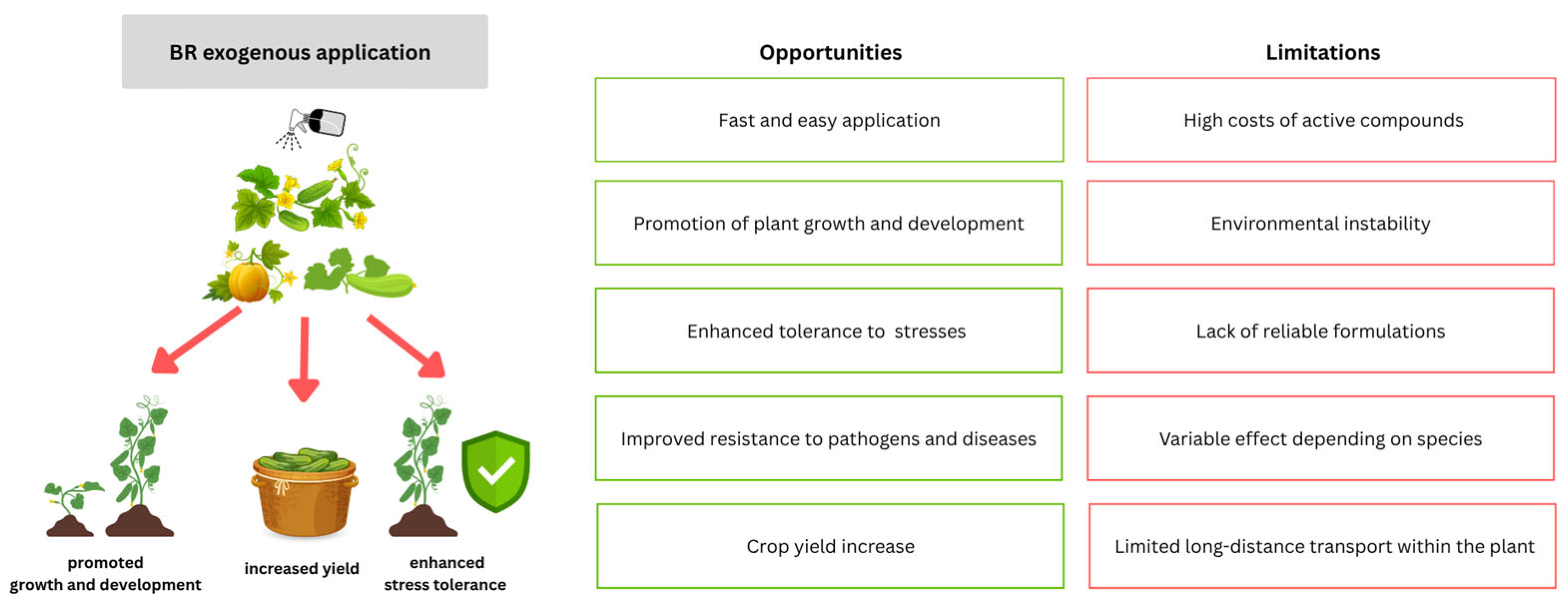
9. Application of BRs to Alleviate Stress During Cucurbit Production
| Species | Stress Factor | BRs Concentration and Type | Treatment Method | Reference |
|---|---|---|---|---|
| Cucumis sativus | Ca(NO3)2 80 mM | 0.1 μM EBL | foliar spraying | [128] |
| 1, 5, and 10 μM EBL | adding to the hydroponic medium | [131,132] | ||
| cold 4 °C | 1.0 μM BL | foliar spraying | [81] | |
| cold 10 °C/7 °C | 0.1 μM EBL | foliar spraying | [116] | |
| cold 12 °C/8 °C | 0.1 μM EBL | foliar spraying | [117] | |
| cold 14 °C | 0.1 μM EBL | foliar spraying | [118] | |
| copper 100 mg·kg−1 | 0.01 μM EBL | foliar spraying | [126] | |
| ferrum deficiency | 0.01, 0.1, and 0.5 μM EBL | adding to a solid medium | [134] | |
| hypoxia | 1 μg·L−1 EBL | adding to the hydroponic medium | [119] | |
| salinity 60 and 120 mM NaCl | 1, 3, and 5 μM HBL | foliar spraying | [127] | |
| salinity 150 mM NaCl | 0.01 μM EBL | foliar spraying | [126] | |
| salinity 200 mM NaCl | 1 μM BL | foliar spraying | [81] | |
| salinity 250 mM | 5 μM EBL | seed soaking | [80] | |
| NaHCO3 30 mM | 0.2 μM EBL | adding to the hydroponic medium | [130] | |
| PEG6000 16% | 1 μM BL | foliar spraying | [81] | |
| Fusarium oxysporum | 0.1 and 0.2 μM EBL | adding to the hydroponic medium/foliar spraying | [135] | |
| 0.2 μM EBL | foliar spraying | [136] | ||
| Phytophtora melonis | 100 μM EBL | foliar spraying | [87] | |
| Cucumis melo | drought | 0.05, 0.10, and 0.15 ppm BR | seed soaking | [124] |
| heat 42/32 °C | 0.05, 0.1, 0.5, 1, and 1.5 mg·L−1 EBL | foliar spraying | [123] | |
| heat 47 ± 3 °C | 0.1, 0.2, and 0.3 mg·L−1 EBL | foliar spraying | [121,122] | |
| postharvest fruit chilling | 0.1 mg∙L−1 EBL | preharvest foliar spraying | [121] | |
| Pseudoperonospora cubensis | 0.5, 1, and 2 mg·L−1 BL | foliar spraying | [133] | |
| Cucurbita pepo | NaCl 40 and 80 mM | 0.01 and 0.1 μM EBL | adding to the hydroponic medium | [125] |
| postharvest fruit chilling | 0.1 μM EBL | fruit spraying | [120] | |
| cucumber mosaic virus (CMV) | 0.2 μM EBL | foliar spraying | [137] | |
| Citrullus lanatus | Zn 2.5, 5, and 10 mM | 0.025, 0.05, 0.1, 0.2, and 0.5 μM EBL | foliar spraying | [133] |
| Momordica charantia | cold 8 °C | 0.0001, 0.001. 0.01, 0.1, and 10 mg·L−1 EBL | foliar spraying | [115] |
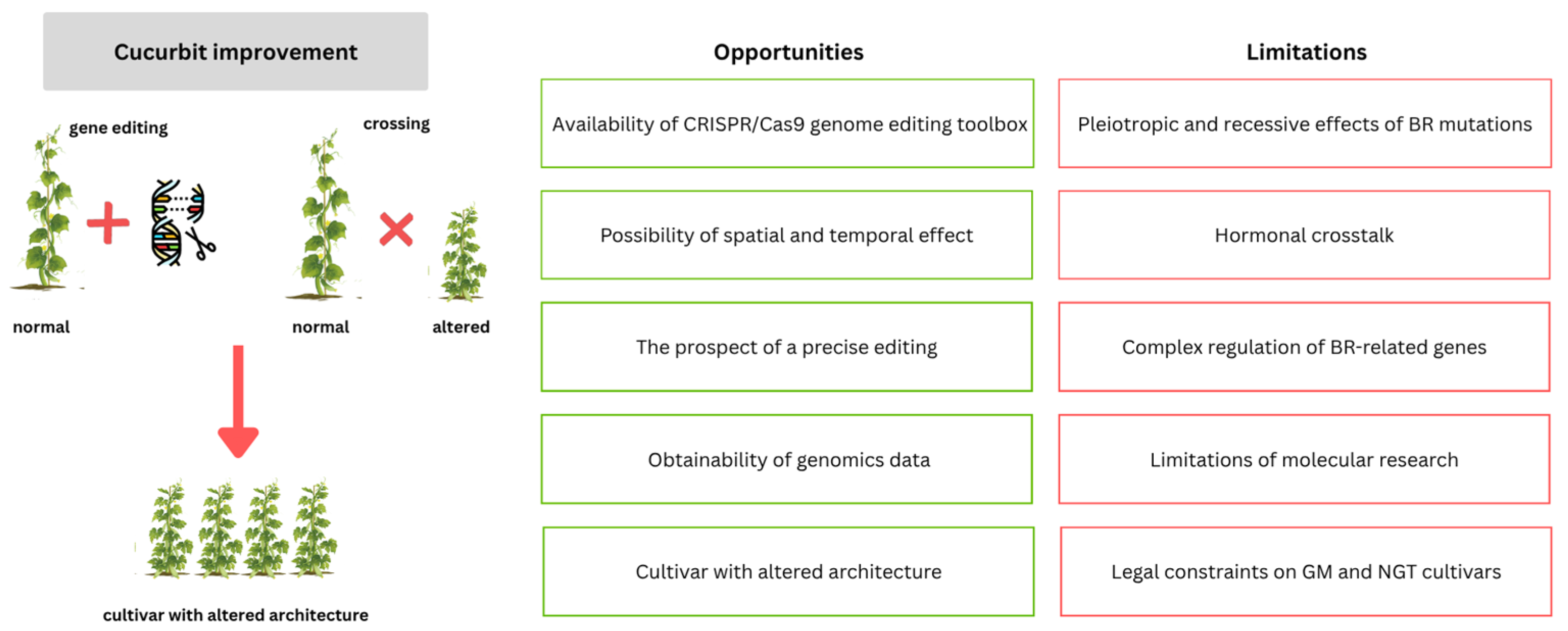
10. Perspectives and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 14-3-3 | 14-3-3 proteins |
| 22-OH-CR | 22-alpha-hydroxycampesterol |
| 22,23-diOH-CR | 22,23-dihydroxycampesterol |
| 22-OH-3-one | 22-alpha-hydroxy-5-alpha-campestan-3-one |
| 22-OH-4-en-3-one | 22-alpha-hydroxy-campest-4-en-3-one |
| 24-MC | 24-methylene cholesterol |
| 26-OH-BL | 26-hydroxybrassinolide |
| 28-norCS | 28-norcastasterone |
| 3-dehydro-TE | 3-dehydroteasterone |
| 3-epi-6-deoxo-CT | 3-epi-6-deoxocathasterone |
| campest-4-en-3-one | campest-4-en-3-one |
| 3-dehydro-6-deoxo-TE | 3-dehydro-6-deoxoteasteroneteasterone |
| 6-oxo-CN | 6-oxocampestanol |
| 6-deoxo-CS | 6-deoxocastasterone |
| 6-deoxo-CT | 6-deoxocathasterone |
| 6-deoxo-TE | 6-deoxoteasterone |
| 6-deoxo-TY | 6-deoxotyphasterol |
| ABA | abscisic acid |
| ACC | 1-aminocyclopropane-1-carboxylic acid |
| ACO | ACC oxidase |
| ACS | ACC synthase |
| APX | ascorbate peroxidase |
| ARF | auxin response factor |
| AsA | ascorbate |
| AOA | aminooxyacetic acid |
| AUX | auxin |
| AVG | aminoethoxyvinylglycine |
| BAK1 | BRI1-ASSOCIATED KINASE 1 |
| BAS1 | PHYB ACTIVATION TAGGED SUPPRESSOR 1 (also known as CYP734A1) |
| BES1 | BRI1-EMS-SUPPRESSOR 1 |
| BIN2 | BRASSINOSTEROID INSENSITIVE 2 |
| BKI1 | BRI1 KINASE INHIBITOR 1 |
| BL | brassinolide |
| BR | brassinosteroid |
| BR6OX1 | BRASSINOSTEROID-6-OXIDASE 1 (also known as CYP85A1) |
| BR6OX2 | BRASSINOSTEROID-6-OXIDASE 2 (also known as CYP85A2) |
| BRC1 | BRANCHED1 |
| BRI1 | BRASSINOSTEROID INSENSITIVE 1 |
| BRZ | brassinazole |
| BSK1 | BR-SIGNALING KINASE 1 |
| BSU1 | BRI1 SUPPRESSOR 1 |
| BZR1 | BRASSINOZOLE-RESISTANT 1 |
| CAT | catalase |
| CDG1 | CONSTITUTIVE DIFFERENTIAL GROWTH 1 |
| CDK | cyclin-dependent kinase |
| ClDUF21 | Citrulus lanatus protein with domain of unknown function 21 |
| ClDWF1 | Citrulus lanatus C24 reductase |
| CMV | cucumber mosaic virus |
| CN | campestanol |
| CpDWF5 | Cucurbita pepo 7-dehydrocholesterol reductase |
| CpTINY4 | Cucurbita pepo TINY4 |
| CPD | CONSTITUTIVE PHOTOMORPHOGENIC DWARF (also known as CYP90A1) |
| cpa | compact |
| cpa-2 | compact-2 |
| CR | campesterol |
| CSA | CARBON STARVED ANTHER |
| CS | castasterone |
| CsAOX | cucumber alternative oxidase |
| CsACS1 | cucumber ACC synthase 1 |
| CsACS2 | cucumber ACC synthase 2 |
| CsACS3 | cucumber ACC synthase 3 |
| CsACO1 | cucumber ACC oxidase 1 |
| CsACO2 | cucumber ACC oxidase 2 |
| CsBAK1 | cucumber BRI1-ASSOCIATED KINASE 1 |
| CsBES1 | cucumber BRI1-EMS-SUPPRESSOR 1 |
| CsBPC2 | cucumber BASIC PENTACYSTEINE 2 |
| CsBZR | cucumber BRASSINOZOLE-RESISTANT |
| CsBZR1 | cucumber BRASSINOZOLE-RESISTANT 1 |
| CsBZR2 | cucumber BRASSINOZOLE-RESISTANT 2 |
| CsBZR3 | cucumber BRASSINOZOLE-RESISTANT 3 |
| CsBZR4 | cucumber BRASSINOZOLE-RESISTANT 4 |
| CsBZR5 | cucumber BRASSINOZOLE-RESISTANT 5 |
| CsBZR6 | cucumber BRASSINOZOLE-RESISTANT 6 |
| CsCYP85A1 | cucumber BR C6 oxidase |
| CsDET2 | cucumber steroid 5-alpha reductase |
| CsDWF1 | cucumber sterol C24 reductase |
| CsDWF5 | cucumber 7-dehydrocholesterol reductase |
| CsDWF7 | cucumber delta7-sterol C5 reductase |
| CsIAGLU | cucumber IAA glucosyltransferase |
| CT | cathasterone |
| CYP724B1 | BR C22 hydroxylase (also known as DWF11) |
| CYP734A1 | BR C26 hydroxylase (also known as BAS1) |
| CYP85A1 | BR C6 oxidase (also known as BR6OX1) |
| CYP85A2 | BR C6 oxidase (also known as BR6OX2) |
| CYP90A1 | BR C3 oxidase (also known as CPD) |
| CYP90B1 | BR C23 hydroxylase (also known as DWARF4) |
| CYP90C1 | BR C23 hydroxylase (also known as ROT3) |
| CYP90Ds | BR C23 hydroxylases |
| DET2 | steroid-5-alpha reductase (also known as DWARF6) |
| DWF1 | DWARF1 sterol C24 reductase |
| DWF4 | DWARF4 (also known as CYP90B1) |
| DWF5 | DWARF5 5,7-sterol-7-reductase |
| DWF6 | DWARF6 (also known as DET2) |
| DWF7 | DWARF7,7-sterol-C5-desaturase |
| DWF11 | DWARF11 (also known as CYP724B1) |
| dwfcp | DWARF in Cucurbita pepo |
| EBL | 24-epibrassinolide |
| ERECTA | receptor-like kinase (RLK) |
| ET | ethylene |
| GA | gibberellin |
| GA20ox1 | GA20 oxidase 1 |
| GABA | gamma-aminobutyric acid |
| GPOX | guaiacol peroxidase |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| GSK3 | glycogen synthase kinase 3 |
| GST | glutathione-S-transferase |
| HBL | 28-homobrassinolide |
| HG | homogalacturonan |
| IAA | indole-3-acetic acid |
| KAO | kaurenoic acid oxidase |
| KIB1 | KINK SUPPRESSED IN BZR1-1D |
| LRR-RLK | protein kinase with leucine-rich repeats |
| MDA | malondialdehyde |
| MAPK | mitogen-activated protein kinase |
| NaCl | sodium chloride |
| PEG | polyethylene glycol |
| POD | peroxidase |
| PP2A | PROTEIN PHOSPHATASE 2A |
| ROS | reactive oxygen species |
| ROT3 | ROTUNDIFOLIA 3 (also known as CYP90B1) |
| scp-1 | super compact-1 |
| scp-2 | super compact-2 |
| scp-3 | super compact-3 |
| SERK | SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE |
| SHAM | salicylhydroxamic acid |
| SOD | superoxide dismutase |
| TCP | TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTORS |
| TE | teasterone |
| TY | typhasterol |
| YABBY1 | transcription factor YABBY1 |
References
- Wehner, T.C.; Naegele, R.P.; Myers, J.R.; Dhillon, N.P.S.; Crosby, K. Cucurbits, 2nd ed.; CABI: Wallingford, UK, 2020. [Google Scholar]
- Chomicki, G.; Schaefer, H.; Renner, S.S. Origin and domestication of Cucurbitaceae crops: Insights from phylogenies, genomics and archaeology. New Phytol. 2019, 226, 1240–1255. [Google Scholar] [CrossRef]
- FAOSTAT. Statistics Database of Food and Agriculture Organization of the United Nations. Available online: https://fao.org/faostat/en/#compare (accessed on 25 March 2025).
- Grumet, R.; Colle, M. Genomic analysis of cucurbit fruit growth. In Genetics and Genomics of Cucurbitaceae; Grumet, R., Katzir, N., Garcia-Mas, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 321–344. [Google Scholar]
- Ajuru, M.; Nmom, F. A review on the economic uses of species of Cucurbitaceae and their sustainability in Nigeria. Am. J. Plant Biol. 2017, 2, 17–24. [Google Scholar]
- Teichmann, T.; Muhr, M. Shaping plant architecture. Front. Plant Sci. 2015, 6, 233. [Google Scholar] [CrossRef]
- Guo, W.; Chen, L.; Herrera-Estrella, L.; Cao, D.; Tran, L.S.P. Altering plant architecture to improve performance and resistance. Trends Plant Sci. 2020, 25, 1154–1170. [Google Scholar] [CrossRef]
- Rahmati Ishka, M.; Julkowska, M. Tapping into the plasticity of plant architecture for increased stress resilience. F1000Research 2023, 12, 1257. [Google Scholar] [CrossRef]
- Song, L.; Liu, J.; Cao, B.; Liu, B.; Zhang, X.; Chen, Z.; Dong, C.; Liu, X.; Zhang, Z.; Wang, W.; et al. Reducing brassinosteroid signalling enhances grain yield in semi-dwarf wheat. Nature 2023, 617, 118–124. [Google Scholar] [CrossRef]
- Bhujbal, S.K.; Rai, A.N.; Joshi-Saha, A. Dwarfs standing tall: Breeding towards the ‘Yellow revolution’ through insights into plant height regulation. Plant Mol. Biol. 2025, 115, 34. [Google Scholar] [CrossRef]
- Li, Y.; Xu, A.; Dong, W.; Li, Z.; Li, G. Genetic analysis of a dwarf vine and small fruit watermelon mutant. Hortic. Plant J. 2016, 2, 224–228. [Google Scholar] [CrossRef]
- Liu, X.F.; Chen, J.C.; Zhang, X.L. Genetic regulation of shoot architecture in cucumber. Hortic. Res. 2021, 8, 143. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroids—occurrence and chemical structures in plants. In Brassinosteroids: A Class of Plant Hormone; Hayat, S., Ahmad, A., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 1–27. [Google Scholar]
- Ohnishi, T. Recent advances in brassinosteroid biosynthetic pathway: Insight into novel brassinosteroid shortcut pathway. J. Pestic. Sci. 2018, 43, 159–167. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, A.; Ahmad, A. Brassinosteroids: Bioactivity and Crop Productivity; Springer: Cham, Switzerland, 2003. [Google Scholar]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D.; Steffens, G.L.; Flippen-Anderson, J.L.; Cook, J.C. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Yokota, T.; Ohnishi, T.; Shibata, K.; Asahina, M.; Nomura, T.; Fujita, T.; Ishizaki, K.; Kohchi, T. Occurrence of brassinosteroids in non-flowering land plants, liverwort, moss, Lycophyte and Fern. Phytochemistry 2017, 136, 46–55. [Google Scholar] [CrossRef]
- Seo, C.; Moon, J.; Yeom, H.S.; Bae, Y.; Kim, E.; Roh, J.; Kim, S.K. Cytochrome P450 710A1/A2 as brassinosteroid C24-desaturases to connect C27- and C28-brassinosteroids biosynthesis in Arabidopsis thaliana. J. Plant Biol. 2024, 67, 59–70. [Google Scholar] [CrossRef]
- Bajguz, A.; Chmur, M.; Gruszka, D. Comprehensive overview of the brassinosteroid biosynthesis pathways: Substrates, products, inhibitors, and connections. Front. Plant Sci. 2020, 11, 1034. [Google Scholar] [CrossRef]
- Joo, S.H.; Jang, M.S.; Kim, M.K.; Lee, J.E.; Kim, S.K. Biosynthetic relationship between C28-brassinosteroids and C29-brassinosteroids in rice (Oryza sativa) seedlings. Phytochemistry 2015, 111, 84–90. [Google Scholar] [CrossRef]
- Fujioka, S.; Yokota, T. Biosynthesis and metabolism of brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef]
- Bajguz, A.; Piotrowska-Niczyporuk, A. Brassinosteroids implicated in growth and stress responses. In Phytohormones: A Window to Metabolism, Signaling and Biotechnological Applications; Tran, L.-S.P., Pal, S., Eds.; Springer: New York, NY, USA, 2014; pp. 163–190. [Google Scholar]
- Wendeborn, S.; Lachia, M.; Jung, P.M.J.; Leipner, J.; Brocklehurst, D.; De Mesmaeker, A.; Gaus, K.; Mondière, R. Biological activity of brassinosteroids—Direct comparison of known and new analogs in planta. Helv. Chim. Acta 2017, 100, e1600305. [Google Scholar] [CrossRef]
- Zullo, M.A.T.; Bajguz, A. Brassinosteroids: Plant Growth and Development. In Brassinosteroids: Plant Growth and Development; Hayat, S., Yusuf, M., Bhardwaj, R., Bajguz, A., Eds.; Springer: Singapore, 2019; pp. 1–44. [Google Scholar]
- Kanwar, M.K.; Bajguz, A.; Zhou, J.; Bhardway, R. Analysis of brassonosteroids in plants. J. Plant Growth Regul. 2017, 36, 1002–1030. [Google Scholar] [CrossRef]
- Hou, Y.; Qiu, J.; Wang, Y.; Li, Z.; Zhao, J.; Tong, X.; Lin, H.; Zhang, J. A quantitative proteomic analysis of brassinosteroid-induced protein phosphorylation in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 514. [Google Scholar] [CrossRef]
- Wada, K.; Marumo, S. Synthesis and plant growth-promoting activity of brassinolide analogues. Agric. Biol. Chem. 1981, 45, 2579–2585. [Google Scholar]
- Clouse, S.D. Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. Plant Cell 2011, 23, 1219–1230. [Google Scholar] [CrossRef]
- Zebosi, B.; Vollbrecht, E.; Best, N.B. Brassinosteroid biosynthesis and signaling: Conserved and diversified functions of core genes across multiple plant species. Plant Commun. 2024, 5, 100982. [Google Scholar] [CrossRef]
- Kim, T.W.; Hwang, J.Y.; Kim, Y.S.; Joo, S.H.; Chang, S.C.; Lee, J.S.; Takatsuto, S.; Kim, S.K. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar] [CrossRef]
- Nomura, T.; Kushiro, T.; Yokota, T.; Kamiya, Y.; Bishop, G.J.; Yamaguchi, S. The last reaction producing brassinolide is catalyzed by cytochrome P-450s, CYP85A3 in tomato and CYP85A2 in Arabidopsis. J. Biol. Chem. 2005, 280, 17873–17879. [Google Scholar] [CrossRef]
- Kim, B.K.; Fujioka, S.; Takatsuto, S.; Tsujimoto, M.; Choe, S. Castasterone is a likely end product of brassinosteroid biosynthetic pathway in rice. Biochem. Biophys. Res. Commun. 2008, 374, 614–619. [Google Scholar] [CrossRef]
- Bhanu, A.N. Brassinosteroids: Relevance in biological activities of plants and agriculture. J. Plant Sci. Res. 2019, 35, 1–15. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J. Regulation of brassinosteroid homeostasis in higher plants. Front. Plant Sci. 2020, 11, 583622. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid signaling, crosstalk and physiological functions in plants under heavy metal stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Tanabe, S.; Ashikari, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Yano, M.; Yoshimura, A.; Kitano, H.; Matsuoka, M.; Fujisawa, Y.; et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 2005, 17, 776–790. [Google Scholar] [CrossRef]
- Tanaka, K.; Asami, T.; Yoshida, S.; Nakamura, Y.; Matsuo, T.; Okamoto, S. Brassinosteroid homeostasis in Arabidopsis is ensured by feedback expressions of multiple genes involved in its metabolism. Plant Physiol. 2005, 138, 1117–1125. [Google Scholar] [CrossRef]
- Wang, X.; Chory, J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling from the plasma membrane. Science 2006, 313, 1118–1122. [Google Scholar] [CrossRef]
- Wang, X.; Kota, U.; He, K.; Blackburn, K.; Li, J.; Goshe, M.B.; Huber, S.C.; Clouse, S.D. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell 2008, 15, 220–235. [Google Scholar] [CrossRef]
- Peres, A.L.G.L.; Soares, J.S.; Tavares, R.G.; Righetto, G.; Zullo, M.A.T.; Mandava, N.B.; Menossi, M. Brassinosteroids, the sixth class of phytohormones: A molecular view from the discovery to hormonal interactions in plant development and stress adaptation. Int. J. Mol. Sci. 2019, 20, 331. [Google Scholar] [CrossRef]
- Tang, W.; Kim, T.W.; Oses-Prieto, J.A.; Sun, Y.; Deng, Z.; Zhu, S.; Wang, R.; Burlingame, A.L.; Wang, Z.Y. Brassinosteroid-signaling kinases (BSKs) mediate signal transduction from the receptor kinase BRI1 in Arabidopsis. Science 2008, 321, 557–560. [Google Scholar] [CrossRef]
- Kim, T.W.; Guan, S.; Burlingame, A.L.; Wang, Z.Y. The CDG1 kinase mediates brassinosteroid signal transduction from BRI1 receptor kinase to BSU1 phosphatase and GSK3-like kinase BIN2. Mol. Cell 2011, 43, 561–571. [Google Scholar] [CrossRef]
- Ryu, H.; Kim, K.; Cho, H.; Hwang, I. Predominant actions of cytosolic BSU1 and nuclear BIN2 regulate subcellular localization of BES1 in brassinosteroid signaling. Mol. Cells 2010, 29, 291–296. [Google Scholar] [CrossRef]
- Belkhadir, Y.; Jaillais, Y. The molecular circuitry of brassinosteroid signaling. New Phytol. 2015, 206, 522–540. [Google Scholar] [CrossRef]
- Jaillais, Y.; Hothorn, M.; Belkhadir, Y.; Dabi, T.; Nimchuk, Z.L.; Meyerowitz, E.M.; Chory, J. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev. 2011, 25, 232–237. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Ali, Q.; Liu, F. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int. J. Mol. Sci. 2022, 23, 1012. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Zahra, N.; Zahra, K.; Raza, A.; Khan, A.; Shaukat, K.; Khan, S. Brassinosteroids: Molecular and physiological responses in plant growth and abiotic stresses. Plant Stress 2021, 2, 100029. [Google Scholar] [CrossRef]
- Singh, A.P.; Savaldi-Goldstein, S. Growth control: Brassinosteroid activity gets context. J. Exp. Bot. 2015, 66, 1123–1132. [Google Scholar] [CrossRef]
- Lin, W.H. Designed manipulation of the brassinosteroid signal to enhance crop yield. Front. Plant Sci. 2020, 11, 854. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, M.P.; Vilarrasa-Blasi, J.; Zhiponova, M.; Divol, F.; Mora-Garcia, S.; Russinova, E.; Cano-Delgado, A.I. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 2011, 138, 849–859. [Google Scholar] [CrossRef]
- Pavelescu, I.; Vilarrasa-Blasi, J.; Planas-Riverola, A.; González-García, M.P.; Caño-Delgado, A.I.; Ibañes, M. A Sizer model for cell differentiation in Arabidopsis thaliana root growth. Mol. Syst. Biol. 2018, 14, e7687. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Wang, Y.; Wang, L.; Fu, Y.; Wang, X. Brassinosteroids regulate pavement cell growth by mediating BIN2-induced microtubule stabilization. J. Exp. Bot. 2018, 69, 1037–1049. [Google Scholar] [CrossRef]
- Lampard, G.R.; Macalister, C.A.; Bergmann, D.C. Arabidopsis stomatal initiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 2008, 322, 1113–1116. [Google Scholar] [CrossRef]
- Kelly-Bellow, R.; Lee, K.; Kennaway, R.; Barclay, J.E.; Whibley, A.; Bushell, C.; Spooner, J.; Yu, M.; Brett, P.; Kular, B.; et al. Brassinosteroid coordinates cell layer interactions in plants via cell wall and tissue mechanics. Science 2023, 380, 1275–1281. [Google Scholar] [CrossRef]
- Kondo, Y.; Ito, T.; Nakagami, H.; Hirakawa, Y.; Saito, M.; Tamaki, T.; Shirasu, K.; Fukuda, H. Plant GSK3 proteins regulate xylem cell differentiation downstream of TDIF-TDR signalling. Nat. Commun. 2014, 5, 3504. [Google Scholar] [CrossRef]
- Saito, M.; Kondo, Y.; Fukuda, H. BES1 and BZR1 redundantly promote phloem and xylem differentiation. Plant Cell Physiol. 2018, 59, 590–600. [Google Scholar] [CrossRef]
- Anne, P.; Azzopardi, M.; Gissot, L.; Beaubiat, S.; Hématy, K.; Palauqui, J.C. OCTOPUS negatively regulates BIN2 to control phloem differentiation in Arabidopsis thaliana. Curr. Biol. 2015, 25, 2584–2590. [Google Scholar] [CrossRef]
- Hu, J.; Hu, X.; Yang, Y.; He, C.; Hu, J.; Wang, X. Strigolactone signaling regulates cambial activity through repression of WOX4 by transcription factor BES1. Plant Physiol. 2021, 188, 255–267. [Google Scholar] [CrossRef]
- Hartwig, T.; Chuck, G.S.; Fujioka, S.; Klempien, A.; Weizbauer, R.; Potluri, D.P.; Choe, S.; Johal, G.S.; Schulz, B. Brassinosteroid control of sex determination in maize. Proc. Natl. Acad. Sci. USA 2011, 108, 19814–19819. [Google Scholar] [CrossRef]
- Makarevitch, I.; Thompson, A.; Muehlbauer, G.J.; Springer, N.M. Brd1 gene in maize encodes a brassinosteroid C-6 oxidase. PLoS ONE 2012, 7, e30798. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lv, M.H.; Wang, Y.Z.; Wang, P.A.; Cui, Y.W.; Li, M.Z.; Wang, R.S.; Gou, X.P.; Li, J. BES1 is activated by EMS1-TPD1-SERK1/2-mediated signaling to control tapetum development in Arabidopsis thaliana. Nat. Commun. 2019, 10, 4164. [Google Scholar] [CrossRef]
- Zhu, X.; Liang, W.; Cui, X.; Chen, M.; Yin, C.; Luo, Z.; Zhu, J.; Lucas, W.J.; Wang, Z.; Zhang, D. Brassinosteroids promote development of rice pollen grains and seeds by triggering expression of Carbon Starved Anther, a MYB domain protein. Plant J. 2015, 82, 570–581. [Google Scholar] [CrossRef]
- Li, Z.; He, Y. Roles of brassinosteroids in plant reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef]
- Clouse, S.D.; Langford, M.; McMorris, T.C. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiol. 1996, 111, 671–678. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Z.Y.; Mora-Garcia, S.; Li, J.; Yoshida, S.; Asami, T.; Chory, J. BES1 accumulates in the nucleus in response to brassinosteroids to regulate gene expression and promote stem elongation. Cell 2002, 109, 181–191. [Google Scholar] [CrossRef]
- Gendron, J.M.; Liu, J.S.; Fan, M.; Bai, M.Y.; Wenkel, S.; Springer, P.S.; Barton, M.K.; Wang, Z.Y. Brassinosteroids regulate organ boundary formation in the shoot apical meristem of Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 21152–21157. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, B.; Du, F.; Guo, X.; Tian, C.; Hu, J.; Lü, S.; Long, M.; Zhang, L.; Wang, Y.; et al. A crosstalk between auxin and brassinosteroid regulates leaf shape by modulating growth anisotropy. Mol. Plant 2021, 14, 949–962. [Google Scholar] [CrossRef]
- Xia, X.; Dong, H.; Yin, Y.; Hu, J. Brassinosteroid signaling integrates multiple pathways to release apical dominance in tomato. Proc. Natl. Acad. Sci. USA 2021, 118, e2004384118. [Google Scholar] [CrossRef]
- Zolkiewicz, K.; Gruszka, D. Take a deep breath: Manipulating brassinosteroid homeostasis helps cereals adapt to environmental stress. Plant Physiol. 2025, 197, kiaf003. [Google Scholar] [CrossRef]
- Nolan, T.M.; Vukašinović, N.; Liu, D.; Russinova, E.; Yin, Y. Brassinosteroids: Multidimensional regulators of plant growth, development, and stress responses. Plant Cell 2019, 32, 295–318. [Google Scholar] [CrossRef]
- Li, H.; Ye, K.; Shi, Y.; Cheng, J.; Zhang, X.; Yang, S. BZR1 positively regulates freezing tolerance via CBF-dependent and CBF-independent pathways in Arabidopsis. Mol. Plant 2017, 10, 545–559. [Google Scholar] [CrossRef]
- Albertos, P.; Dündar, G.; Schenk, P.; Carrera, S.; Cavelius, P.; Sieberer, T.; Poppenberger, B. Transcription factor BES1 interacts with HSFA1 to promote heat stress resistance of plants. EMBO J. 2022, 41, e108664. [Google Scholar] [CrossRef]
- Chen, J.; Nolan, T.M.; Ye, H.; Zhang, M.; Tong, H.; Xin, P.; Chu, J.; Chu, C.; Li, Z.; Yin, Y. Arabidopsis WRKY46, WRKY54, and WRKY70 transcription factors are involved in brassinosteroid-regulated plant growth and drought esponses. Plant Cell 2017, 29, 1425–1439. [Google Scholar]
- Ye, H.; Liu, S.; Tang, B.; Chen, J.; Xie, Z.; Nolan, T.M.; Jiang, H.; Guo, H.; Lin, H.Y.; Li, L.; et al. RD26 mediates crosstalk between drought and brassinosteroid signalling pathways. Nat Commun. 2017, 8, 14573. [Google Scholar] [CrossRef]
- Cui, X.Y.; Gao, Y.; Guo, J.; Yu, T.F.; Zheng, W.J.; Liu, Y.W.; Chen, J.; Xu, Z.S.; Ma, Y.Z. BES/BZR transcription factor TaBZR2 positively regulates drought responses by activation of TaGST1. Plant Physiol. 2019, 180, 605–620. [Google Scholar] [CrossRef]
- Sun, F.; Ding, L.; Feng, W.; Cao, Y.; Lu, F.; Yang, Q.; Li, W.; Lu, Y.; Shabek, N.; Fu, F.; et al. Maize transcription factor ZmBES1/BZR1-5 positively regulates kernel size. J. Exp. Bot. 2021, 72, 1714–1726. [Google Scholar] [CrossRef]
- Guo, D.; Gao, X.; Li, H.; Zhang, T.; Chen, G.; Huang, P.; An, L.; Li, N. EGY1 plays a role in regulation of endodermal plastid size and number that are involved in ethylene-dependent gravitropism of light-grown Arabidopsis hypocotyls. Plant Mol. Biol. 2008, 66, 345–360. [Google Scholar] [CrossRef]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS protein stability by cytokinin and brassinosteroid. Plant J. 2009, 57, 606–614. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Grumet, R. Brassinosteriod-induced femaleness in cucumber and relationship to ethylene production. HortScience 2005, 40, 1763–1767. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, J.; Xia, X.; Zhang, W.H. Ameliorative effect of brassinosteroid and ethylene on germination of cucumber seeds in the presence of sodium chloride. Plant Growth Regul. 2011, 65, 407–413. [Google Scholar] [CrossRef]
- Wei, L.J.; Deng, X.G.; Zhu, T.; Zheng, T.; Li, P.X.; Wu, J.Q.; Zhang, D.W.; Lin, H.H. Ethylene is involved in brassinosteroids induced alternative respiratory pathway in cucumber (Cucumis sativus L.) seedlings response to abiotic stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef]
- Bai, M.Y.; Shang, J.X.; Oh, E.; Fan, M.; Bai, Y.; Zentella, R.; Sun, T.P.; Wang, Z.Y. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat. Cell Biol. 2012, 14, 810–817. [Google Scholar] [CrossRef]
- Gallego-Bartolomé, J.; Minguet, E.G.; Grau-Enguix, F.; Abbas, M.; Locascio, A.; Thomas, S.G.; Alabadí, D.; Blázquez, M.A. Molecular mechanism for the interaction between gibberellin and brassinosteroid signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 13446–13451. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, C.; Jiang, L.; Li, S.; Sun, S.S.; He, J.X. An interaction between BZR1 and DELLAs mediates direct signaling crosstalk between brassinosteroids and gibberellins in Arabidopsis. Sci. Signal. 2012, 5, ra72. [Google Scholar] [CrossRef]
- Stewart Lilley, J.L.; Gan, Y.; Graham, I.A.; Nemhauser, J.L. The effects of DELLAs on growth change with developmental stage and brassinosteroid levels. Plant J. 2013, 76, 165–173. [Google Scholar] [CrossRef]
- Ross, J.J.; Quittenden, L.J. Interactions between brassinosteroids and gibberellins: Synthesis or signaling? Plant Cell 2016, 28, 829–832. [Google Scholar] [CrossRef]
- Kang, Y.; Jiang, Z.; Meng, C.; Ning, X.; Pan, G.; Yang, X.; Zhong, M. A multifaceted crosstalk between brassinosteroid and gibberellin regulates the resistance of cucumber to Phytophthora melonis. Plant J. 2024, 119, 1353–1368. [Google Scholar] [CrossRef]
- Katsumi, M. Interaction of a brassinosteroid with IAA and GA3 in the elongation of cucumber hypocotyl sections. Plant Cell Physiol. 1985, 26, 615–625. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, P.; Yao, X.; Meng, Y.; Lou, L.; Zhang, M.; Liu, G.; Yang, X.; Liu, J.; Zhu, L.; et al. Cucumber Auxin Response Factor CsARF10a regulates leaf morphogenesis and parthenocarpic fruit set in tomato. Horticulturae 2024, 10, 79. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Qin, Y.; Pan, Y.; Wang, X.; Weng, Y.; Chen, P.; Li, Y. The cytochrome P450 gene CsCYP85A1 is a putative candidate Super compact-1 (Scp-1) plant architecture mutation in cucumber (Cucumis sativus L.). Front. Plant Sci. 2017, 8, 266. [Google Scholar] [CrossRef]
- Hou, S.; Niu, H.; Tao, Q.; Wang, S.; Gong, Z.; Li, S.; Weng, Y.; Li, Z. A mutant in the CsDET2 gene leads to a systemic brassinosteroid deficiency and super compact phenotype in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2017, 130, 1693–1703. [Google Scholar] [CrossRef]
- Zhang, M.; Song, M.; Cheng, F.; Yang, Z.; Davoudi, M.; Chen, J.; Lou, Q. Identification of a putative candidate gene encoding 7-dehydrocholesterol reductase involved in brassinosteroids biosynthesis for compact plant architecture in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2021, 134, 2023–2034. [Google Scholar] [CrossRef]
- Zhang, M.; Song, M.; Davoudi, M.; Cheng, F.; Yin, J.; Zha, G.; Yang, Z.; Chen, J.; Lou, Q. The mutation of C-24 reductase, a key enzyme involved in brassinolide biosynthesis, confers a novel compact plant architecture phenotype to cucumber. Theor. Appl. Genet. 2022, 135, 2711–2723. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Wang, Y.; Mu, S.; Yue, H.; Luo, Q.; Zhang, Z.; Li, Y.; Chen, P. A mutation in CsDWF7 gene encoding a delta7 sterol C-5(6) desaturase leads to the phenotype of super compact in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2024, 137, 20. [Google Scholar] [CrossRef]
- Choe, S.; Tanaka, A.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Ross, A.S.; Tax, F.E.; Yoshida, S.; Feldmann, K.A. Lesions in the sterol delta reductase gene of Arabidopsis cause dwarfism due to a block in brassinosteroid biosynthesis. Plant J. 2000, 21, 431–443. [Google Scholar] [CrossRef]
- Xiang, X.; Yang, H.; Yuan, X.; Dong, X.; Mai, S.; Zhang, Q.; Chen, L.; Cao, D.; Chen, H.; Guo, W.; et al. CRISPR/Cas9-mediated editing of GmDWF1 brassinosteroid biosynthetic gene induces dwarfism in soybean. Plant Cell Rep. 2024, 43, 116. [Google Scholar] [CrossRef]
- Alonso, S.; Cebrián, G.; Gautam, K.; Iglesias-Moya, J.; Martínez, C.; Jamilena, M. A mutation in the brassinosteroid biosynthesis gene CpDWF5 disrupts vegetative and reproductive development and the salt stress response in squash (Cucurbita pepo). Hortic. Res. 2024, 11, uhae050. [Google Scholar] [CrossRef]
- Asensio, L.; Capel, C.; Lebrón, R.; Suárez-Alcaraz, A.; Lozano, R.; Capel, J. Zucchini TINY4 gene regulates plant development through brassinosteroid signalling pathway. In Proceedings of the 3rd International Electronic Conference on Plant Sciences, Online, 15–17 January 2024; MDPI: Basel, Switzerland, 2024. [Google Scholar]
- Sun, P.; Zhao, H.; Cao, L.; Zhang, T.; Zhang, H.; Yang, T.; Zhao, B.; Jiang, Y.; Dong, J.; Chen, T.; et al. A DUF21 domain–containing protein regulates plant dwarfing in watermelon. Plant Physiol. 2024, 196, 3091–3104. [Google Scholar] [CrossRef]
- Manzano, S.; Martínez, C.; Megías, Z.; Gómez, P.; Garrido, D.; Jamilena, M. The role of ethylene and brassinosteroids in the control of sex expression and flower development in Cucurbita pepo. Plant Growth Regul. 2011, 65, 213–221. [Google Scholar] [CrossRef]
- Susila, T.; Amarender Reddy, S.; Rajkumar, M.; Padmaja, A.; Rao, P.V. Effect of plant growth regulators on flowering and yield of watermelon (Citrullus lanatus (Thunb.) Matsum. & Nakai). JHSOP 2010, 2, 19–23. [Google Scholar]
- Dhall, R.K.; Singh, K. Alleviation effects of brassinolides on growth and yield of cucumber (Cucumis sativus L.). Veg. Sci. 2016, 43, 83–86. [Google Scholar]
- Yadav, N.P.; Bahadur, V.; Singh, N.V.; Singh, G. Effect of foliar spray of brassinosteroids, salicylic acid and gibberellic acid on the fruit yield and yield traits of cucumber (Cucumis sativus L.) cv. Arpit. J. Pharm. Innov. 2022, 11, 1330–1333. [Google Scholar]
- Fu, F.Q.; Mao, W.H.; Shi, K.; Zhou, Y.H.; Asami, T.; Yu, J.Q. A role of brassinosteroids in early fruit development in cucumber. J. Exp. Bot. 2008, 59, 2299–2308. [Google Scholar] [CrossRef]
- Yang, P.; Azher Nawaz, M.; Li, F.; Bai, L.; Li, J. Brassinosteroids regulate antioxidant system and protect chloroplast ultrastructure of autotoxicity-stressed cucumber (Cucumis sativus L.) seedlings. Agronomy 2019, 9, 265. [Google Scholar] [CrossRef]
- Ma, S.; Ji, T.; Liang, M.; Li, S.; Tian, Y.; Gao, L. Genome-wide identification, structural, and gene expression analysis of BRI1-EMS-Suppressor 1 transcription factor family in Cucumis sativus. Front. Genet. 2020, 11, 583996. [Google Scholar] [CrossRef]
- Xia, X.J.; Huang, L.F.; Zhou, Y.H.; Mao, W.H.; Shi, K.; Wu, J.X.; Asami, T.; Chen, Z.; Yu, J.Q. Brassinosteroids promote photosynthesis and growth by enhancing activation of Rubisco and expression of photosynthetic genes in Cucumis sativus. Planta 2009, 230, 1185–1196. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, G.; Zhang, Z.; Wan, Z.; Liu, Z.; Lv, J.; Yu, J. Genome-wide identification and expression analysis of BZR gene family and associated responses to abiotic stresses in cucumber (Cucumis sativus L.). BMC Plant Biol. 2023, 23, 214. [Google Scholar] [CrossRef]
- Meng, D.; Li, S.; Feng, X.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M.; et al. CsBPC2 is essential for cucumber survival under cold stress. BMC Plant Biol. 2023, 23, 566. [Google Scholar] [CrossRef]
- Xu, L.; Wang, C.; Cao, W.; Zhou, S.; Wu, T. CLAVATA1-type receptor-like kinase CsCLAVATA1 is a putative candidate gene for dwarf mutation in cucumber. Mol. Genet. Genomics 2018, 293, 1393–1405. [Google Scholar] [CrossRef]
- Han, J.; Dong, S.; Guan, J.; Liu, X.; Gu, X.; Miao, H.; Zhang, S. Genome-wide identification of Brassinosteroid insensitive 1-associated receptor kinase 1 genes and expression analysis in response to pathogen infection in cucumber (Cucumis sativus L.). BMC Plant Biol. 2024, 24, 737. [Google Scholar] [CrossRef]
- Słomnicka, R.; Olczak-Woltman, H.; Sobczak, M.; Bartoszewski, G. Transcriptome profiling of cucumber (Cucumis sativus L.) early response to Pseudomonas syringae pv. lachrymans. Int. J. Mol. Sci. 2021, 22, 4192. [Google Scholar] [CrossRef]
- Li, S.; Zheng, H.; Lin, L.; Wang, F.; Sui, N. Roles of brassinosteroids in plant growth and abiotic stress response. Plant Growth Regul. 2021, 93, 29–38. [Google Scholar] [CrossRef]
- Silva, A.P.S.d.; Alencar, A.A.d.S.; Sudré, C.P.; Araújo, M.d.S.B.d.; Lobato, A.K.d.S. Brassinosteroids: Relevant evidence related to mitigation of abiotic and biotic stresses in plants. Agronomy 2024, 14, 840. [Google Scholar] [CrossRef]
- Huang, Y.H.; Luo, H.L.; Chen, X.F. Effect of brassinosteroid on improving chilling resistance of bitter gourd seedlings. J. South. Agric. 2011, 42, 488–491. [Google Scholar]
- Jiang, Y.P.; Huang, L.F.; Cheng, F.; Zhou, Y.H.; Xia, X.J.; Mao, W.H.; Shi, K.; Yu, J.Q. Brassinosteroids accelerate recovery of photosynthetic apparatus from cold stress by balancing the electron partitioning, carboxylation and redox homeostasis in cucumber. Physiol. Plant. 2013, 148, 133–145. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-epibrassinolide ameliorates endogenous hormone levels to enhance low-temperature stress tolerance in cucumber seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef]
- Anwar, A.; Di, Q.; Yan, Y.; He, C.; Li, Y.; Yu, X. Exogenous 24-epibrassinolide alleviates the detrimental effects of suboptimal root zone temperature in cucumber seedlings. Arch. Agron. Soil Sci. 2019, 65, 1927–1940. [Google Scholar] [CrossRef]
- Kang, Y.Y.; Guo, S.R.; Li, J.; Duan, J. Effect of root applied 24-epibrassinolide on carbohydrate status and fermentative enzyme activities in cucumber (Cucumis sativus L.) seedlings under hypoxia. Plant Growth Regul. 2009, 57, 259–269. [Google Scholar] [CrossRef]
- Massolo, J.F.; Sánchez, R.; Zaro, M.J.; Concellón, A.; Vicente, A.R. Low-dose prestorage 24-epibrassinolide spray enhances postharvest chilling tolerance in zucchini squash (Cucurbita pepo L.) by eliciting peroxidase and phenolic antioxidants. J. Food Process. Preserv. 2022, 46, e16576. [Google Scholar] [CrossRef]
- Amarasinghe, R.; Zaharah, S.S.; Megat Wahab, P.E.M.; Ramlee, S.I.; Nakasha, J.J. Frequency of application of 24-epibrassinolide on plant growth, physiology and postharvest fruit quality of Cantaloupe grown at elevated temperature. Int. J. Veg. Sci. 2023, 29, 358–374. [Google Scholar] [CrossRef]
- Amarasinghe, R.; Zaharah, S.; Wahab, P.E.M.; Ramlee, S.I.; Nakasha, J.J. Influence of brassinolides on plant physiology and yield of cantaloupe under high temperature stress. Iraqi J. Agric. Sci. 2022, 53, 1377–1387. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhu, X.H.; Ding, H.D.; Yang, S.J.; Chen, Y.Y. Foliar application of 24-epibrassinolide alleviates high-temperature-induced inhibition of photosynthesis in seedlings of two melon cultivars. Photosynthetica 2013, 51, 341–349. [Google Scholar] [CrossRef]
- Rifki, A.N.R.; Solichatun; Pitoyo, A. Induction of drought resistance in melon (Cucumis melo L.) M15 with hormopriming brassinosteroid based on morfology, anatomy, and physiology aspects. BIOMA: J. Ilm. Biol. 2024, 13, 110–125. [Google Scholar] [CrossRef]
- Nejad-Alimoradi, F.; Nasibi, F.; Kalantari, K.M. 24-epibrassinolide pre-treatment alleviates the salt-induced deleterious effects in medicinal pumpkin (Cucurbita pepo) by enhancement of GABA content and enzymatic antioxidants. S. Afr. J. Bot. 2019, 124, 111–117. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khalil, R.R.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide regulates photosynthesis, antioxidant enzyme activities and proline content of Cucumis sativus under salt and/or copper stress. Environ. Monit. Assess. 2013, 185, 7845–7856. [Google Scholar] [CrossRef]
- Ahmad, H.; Hayat, S.; Ali, M.; Ghani, M.I.; Zhihui, Z. Regulation of growth and physiological traits of cucumber (Cucumis sativus L.) through various levels of 28-homobrassinolide under salt stress conditions. Can. J. Plant Sci. 2017, 98, 132–140. [Google Scholar] [CrossRef]
- Yuan, L.; Zhu, S.; Shu, S.; Sun, J.; Guo, S. Regulation of 2,4-epibrassinolide on mineral nutrient uptake and ion distribution in Ca(NO3)2 stressed cucumber plants. J. Plant Physiol. 2015, 188, 29–36. [Google Scholar] [CrossRef]
- An, Y.; Zhou, H.; Zhong, M.; Sun, J.; Shu, S.; Shao, Q.; Guo, S. Root proteomics reveals cucumber 24-epibrassinolide responses under Ca(NO3)2 stress. Plant Cell Rep. 2016, 35, 1081–1101. [Google Scholar] [CrossRef]
- Nie, W.; Gong, B.; Wen, D.; Qiao, P.; Guo, H.; Shi, Q. Brassinosteroid enhances cucumber stress tolerance to NaHCO3 by modulating nitrogen metabolism, ionic balance and phytohormonal response. Plants 2024, 13, 80. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Yasin, N.A. 24-epibrassinolide triggers cadmium stress mitigation in Cucumis sativus through intonation of antioxidant system. S. Afr. J. Bot. 2019, 127, 349–360. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Abbas, M.; Yasin, N.A. Seed priming with 3-epibrassinolide alleviates cadmium stress in Cucumis sativus through modulation of antioxidative system and gene expression. Sci. Hortic. 2020, 265, 109203. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, Q.; Liu, W.; Zhang, J. 24-Epibrassinolide confers zinc stress tolerance in watermelon seedlings through modulating antioxidative capacities and lignin accumulation. PeerJ 2023, 11, e15330. [Google Scholar] [CrossRef]
- Wang, B.; Li, Y.; Zhang, W.H. Brassinosteroids are involved in response of cucumber (Cucumis sativus) to iron deficiency. Ann. Bot. 2012, 110, 681–688. [Google Scholar] [CrossRef]
- Ding, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of root and foliar applications of 24-epibrassinolide on fusarium wilt and antioxidant metabolism in cucumber roots. HortScience 2009, 44, 1340–1345. [Google Scholar] [CrossRef]
- Xia, X.J.; Zhou, Y.H.; Ding, J.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.Q. Induction of systemic stress tolerance by brassinosteroid in Cucumis sativus. New Phytol. 2011, 191, 706–720. [Google Scholar] [CrossRef]
- Tao, Y.; Yu, Q.X.; Zhou, Y.H.; Shi, K.; Zhou, J.; Yu, J.Q.; Xia, X.J. Application of 24-epibrassinolide decreases the susceptibility to cucumber mosaic virus in zucchini (Cucurbita pepo L.). Sci. Hortic. 2015, 195, 116–123. [Google Scholar] [CrossRef]
- Liu, T.; Xu, H.; Amanullah, S.; Du, Z.; Hu, X.; Che, Y.; Zhang, L.; Jiang, Z.; Zhu, L.; Wang, D. Deciphering the enhancing impact of exogenous brassinolide on physiological indices of melon plants under downy mildew induced stress. Plants 2024, 13, 779. [Google Scholar] [CrossRef]
- Wang, S.; Wang, K.; Li, Z.; Li, Y.; He, J.; Li, H.; Wang, B.; Xin, T.; Tian, H.; Tian, J.; et al. Architecture design of cucurbit crops for enhanced productivity by a natural allele. Nat. Plants 2022, 8, 1394–1407. [Google Scholar] [CrossRef]
- Ferrero-Serrano, A.; Cantos, C.; Assmann, S.M. The role of dwarfing traits in historical and modern agriculture with a focus on rice. Cold Spring Harb. Perspect. Biol. 2019, 11, a034645. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, C.; Qian, Q.; Tong, H. Leveraging brassinosteroids towards the next Green Revolution. Trends Plant Sci. 2024, 29, 86–98. [Google Scholar] [CrossRef]
- Wang, X.; Bao, K.; Reddy, U.K.; Bai, Y.; Hammar, S.A.; Jiao, C.; Wehner, T.C.; Ramírez-Madera, A.O.; Weng, Y.; Grumet, R.; et al. The USDA cucumber (Cucumis sativus L.) collection: Genetic diversity, population structure, genome-wide association studies, and core collection development. Hortic. Res. 2018, 5, 64. [Google Scholar] [CrossRef]
- Levi, A.; Jarret, R.; Kousik, S.; Wechter, W.P.; Nimmakayala, P.; Reddy, U.K. Genetic resources of watermelon. In Genetics and Genomics of Cucurbitaceae; Grumet, R., Katzir, N., Garcia-Mas, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 87–110. [Google Scholar]
- Shimada, Y.; Goda, H.; Nakamura, A.; Takatsuto, S.; Fujioka, S.; Yoshida, S. Organ-specific expression of brassinosteroid-biosynthetic genes and distribution of endogenous brassinosteroids in Arabidopsis. Plant Physiol. 2003, 131, 287–297. [Google Scholar] [CrossRef]
- Symons, G.M.; Reid, J.B. Brassinosteroids do not undergo long-distance transport in pea. Implications for the regulation of endogenous brassinosteroid levels. Plant Physiol. 2004, 135, 2196–2206. [Google Scholar] [CrossRef]
- Zhou, B.; Luo, Q.; Shen, Y.; Wei, L.; Song, X.; Liao, H.; Ni, L.; Shen, T.; Du, X.; Han, J.; et al. Coordinated regulation of vegetative phase change by brassinosteroids and the age pathway in Arabidopsis. Nat. Commun. 2023, 14, 2608. [Google Scholar] [CrossRef]
- Miao, R.; Li, C.; Liu, Z.; Zhou, X.; Chen, S.; Zhang, D.; Luo, J.; Tang, W.; Wang, C.; Wu, J.; et al. The role of endogenous brassinosteroids in the mechanisms regulating plant reactions to various abiotic stresses. Agronomy 2024, 14, 356. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, C.; Wei, Z.; Chai, S.; Jia, H.; Gao, M.; Allison, J.; Li, Z.; Song, C.; Wang, X. Analysis of grapevine’s somatic embryogenesis receptor kinase (SERK) gene family: VqSERK3/BAK1 overexpression enhances disease resistance. Phytopathology 2022, 112, 1081–1092. [Google Scholar] [CrossRef]
- Xin, T.; Tian, H.; Ma, Y.; Wang, S.; Yang, L.; Li, X.; Zhang, M.; Chen, C.; Wang, H.; Li, H.; et al. Targeted creating new mutants with compact plant architecture using CRISPR/Cas9 genome editing by an optimized genetic transformation procedure in cucurbit plants. Hortic. Res. 2022, 9, uhab086. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Liu, X.; Chen, G.; Liu, L.; Cheng, Z.; Song, W.; Han, L.; Wang, S.; Wang, L.; et al. CsIAGLU regulates the angle of leaf petiole by affecting endogenous content of auxin in cucumber (Cucumis sativus L.). Genes 2022, 13, 2216. [Google Scholar] [CrossRef]
- Rahman, F.; Mishra, A.; Gupta, A.; Sharma, R. Spatiotemporal regulation of CRISPR/Cas9 enables efficient, precise, and heritable edits in plant genomes. Front. Genome Ed. 2022, 4, 870108. [Google Scholar] [CrossRef]
- Barro-Trastoy, D.; Carrera, E.; Baños, J.; Palau-Rodríguez, J.; Ruiz-Rivero, O.; Tornero, P.; Alonso, J.M.; López-Díaz, I.; Gómez, M.D.; Pérez-Amador, M.A. Regulation of ovule initiation by gibberellins and brassinosteroids in tomato and Arabidopsis: Two plant species, two molecular mechanisms. Plant J. 2020, 102, 1026–1041. [Google Scholar] [CrossRef]
- Zhu, T.; Tan, W.R.; Deng, X.G.; Zheng, T.; Zhang, D.W.; Lin, H.H. Effects of brassinosteroids on quality attributes and ethylene synthesis in postharvest tomato fruit. Postharvest Biol. Technol. 2015, 100, 196–204. [Google Scholar] [CrossRef]
- Chakraborty, N.; Ganguly, R.; Sarkar, A.; Dasgupta, D.; Sarkar, J.; Acharya, K.; Burachevskaya, M.; Minkina, T.; Keswani, C. Multifunctional role of brassinosteroids in plant growth, development, and defense. J. Plant Growth Regul. 2025, 44, 2627–2640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słomnicka, R.; Cieplak, M.; Martín-Hernández, A.M.; Bartoszewski, G. Brassinosteroids in Cucurbits: Modulators of Plant Growth Architecture and Stress Response. Int. J. Mol. Sci. 2025, 26, 7234. https://doi.org/10.3390/ijms26157234
Słomnicka R, Cieplak M, Martín-Hernández AM, Bartoszewski G. Brassinosteroids in Cucurbits: Modulators of Plant Growth Architecture and Stress Response. International Journal of Molecular Sciences. 2025; 26(15):7234. https://doi.org/10.3390/ijms26157234
Chicago/Turabian StyleSłomnicka, Renata, Magdalena Cieplak, Ana Montserrat Martín-Hernández, and Grzegorz Bartoszewski. 2025. "Brassinosteroids in Cucurbits: Modulators of Plant Growth Architecture and Stress Response" International Journal of Molecular Sciences 26, no. 15: 7234. https://doi.org/10.3390/ijms26157234
APA StyleSłomnicka, R., Cieplak, M., Martín-Hernández, A. M., & Bartoszewski, G. (2025). Brassinosteroids in Cucurbits: Modulators of Plant Growth Architecture and Stress Response. International Journal of Molecular Sciences, 26(15), 7234. https://doi.org/10.3390/ijms26157234





