Isolation and Identification of Inter-Correlated Genes from the Invasive Sun Corals Tubastraea Coccinea and Tubastraea Tagusensis (Scleractinia, Cnidaria)
Abstract
1. Introduction
2. Results and Discussion
Gene Network Analysis and Hub Genes
3. Materials and Methods
3.1. Sample Collection, Preservation, and RNA Extraction
3.2. Isolation and Identification of Genes
3.3. Real-Time qPCR Experiments
3.4. Interactomic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenner, D. Biogeography of three Caribbean corals (Scleractinia) and the invasion of Tubastraea coccinea into the Gulf of Mexico. Bull. Mar. Sci. 2001, 69, 1175–1189. [Google Scholar]
- Osman, R.; Shirley, T. (Eds.) The Gulf of Mexico and Caribbean Marine Invasive Species Workshop: Proceedings and Final Report; Harte Research Institute, Texas A&M University: Corpus Christi, TX, USA, 2007. [Google Scholar]
- de Paula, A.F.; Creed, J.C. Two species of the coral Tubastraea (Cnidaria, Scleractinia) in Brazil: A case of accidental introduction. Bull. Mar. Sci. 2004, 74, 175–183. [Google Scholar]
- Creed, J.C.; Fenner, D.; Sammarco, P.; Cairns, S.; Capel, K.; Junqueira, A.O.; Cruz, I.; Miranda, R.J.; Carlos-Junior, L.; Mantelatto, M.C.; et al. The invasion of the azooxanthellate coral Tubastraea (Scleractinia: Dendrophylliidae) throughout the world: History, pathways and vectors. Biol. Invasions 2017, 19, 283–305. [Google Scholar] [CrossRef]
- de Oliveira Soares, M.; Davis, M.; de Macêdo Carneiro, P.B. Northward range expansion of the invasive coral (Tubastraea tagusensis) in the southwestern Atlantic. Mar. Biodivers. 2018, 48, 1651–1654. [Google Scholar] [CrossRef]
- Yiu, S.K.F.; Qiu, J.W. Three new species of the sun coral genus Tubastraea (Scleractinia: Dendrophylliidae) from Hong Kong, China. Zool. Stud. 2022, 61, e45. [Google Scholar]
- Capel, K.C.C.; Creed, J.; Kitahara, M.V.; Chen, C.A.; Zilberberg, C. Multiple introductions and secondary dispersion of Tubastraea spp. in the Southwestern Atlantic. Sci. Rep. 2019, 9, 13978. [Google Scholar] [CrossRef]
- Creed, J.C. Two invasive alien azooxanthellate corals, Tubastraea coccinea and Tubastraea tagusensis, dominate the native zooxanthellate Mussismilia hispida in Brazil. Coral Reefs 2006, 25, 350. [Google Scholar] [CrossRef]
- Lages, B.G.; Fleury, B.G.; Mineola, C.; Creed, J.C. Change in tropical rocky shore communities due to an alien coral invasion. Mar. Ecol. Prog. Ser. 2011, 438, 85–96. [Google Scholar] [CrossRef]
- Lages, B.G.; Fleury, B.G.; Hovell, A.M.; Rezende, C.M.; Pinto, A.C.; Creed, J.C. Proximity to competitors changes secondary metabolites of non-indigenous cup corals, Tubastraea spp., in the southwest Atlantic. Mar. Biol. 2012, 159, 1551–1559. [Google Scholar] [CrossRef]
- Miranda, R.J.; Cruz, I.C.; Barros, F. Effects of the alien coral Tubastraea tagusensis on native coral assemblages in a southwestern Atlantic coral reef. Mar. Biol. 2016, 163, 45. [Google Scholar] [CrossRef]
- Miranda, R.J.; José de Anchieta, C.C.; Mariano-Neto, E.; Sippo, J.Z.; Barros, F. Do invasive corals alter coral reef processes? An empirical approach evaluating reef fish trophic interactions. Mar. Environ. Res. 2018, 138, 19–27. [Google Scholar] [CrossRef]
- Neves da Rocha, L.S.; Nunes, J.A.C.; Miranda, R.J.; Kikuchi, R.K. Effects of invasive sun corals on habitat structural complexity mediate reef trophic pathways. Mar. Biol. 2024, 171, 76. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Atchison, A.; Boland, G.S. Expansion of coral communities within the northern Gulf of Mexico via offshore oil and gas platforms. Mar. Ecol. Prog. Ser. 2004, 280, 129–143. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Atchison, A.D.; Brazeau, D.A.; Boland, G.S.; Lirette, A. Expansion of Scleractinian Corals Across the N. Gulf of Mexico: A Bird’s Eye View of Large-Scale Patterns and Genetic Affinities. In Proceedings of the Australian Marine Sciences Association (AMSA), Melbourne, VIC, Australia, 9–13 July 2007. [Google Scholar]
- Sammarco, P.W.; Brazeau, D.A.; Atchison, A.D.; Boland, G.S.; Lirette, A. Coral distribution, abundance, and genetic affinities on oil/gas platforms in the N. In Gulf of Mexico: A Preliminary Look at the Big Picture, Proceedings of the United States Department of the Interior, Minerals Management Service Information Transfer Meeting, New Orleans, LA, USA, January 2007; OCS Study MMS: Washington, DC, USA, 2007. [Google Scholar]
- Sammarco, P.W.; Atchison, A.D.; Boland, G.S.; Sinclair, J.; Lirette, A. Geographic expansion of hermatypic and ahermatypic corals in the Gulf of Mexico, and implications for dispersal and recruitment. J. Exp. Mar. Biol. Ecol. 2012, 436–437, 36–49. [Google Scholar] [CrossRef]
- Schmahl, G.P. Biodiversity associated with topographic features in the northwestern Gulf of Mexico. In Proceedings of the United States Department of the Interior, Minerals Management Service Information Transfer Meeting, Gulf of Mexico, OCS Region, Kenner, LA, USA; 2003. [Google Scholar]
- Hickerson, E.L.; Schmahl, G.P.; Weaver, D.C. Patterns of deep coral communities on reefs and banks in the northwestern Gulf of Mexico. Eos Trans. Am. Geophys. Union 2006, 87, 36. [Google Scholar]
- Schmahl, G.P.; Hickerson, E.L. Ecosystem approaches to the identification and characterization of a network of reefs and banks in the northwestern Gulf of Mexico. Eos Trans. Am. Geophys. Union 2006, 87, 36 suppl. [Google Scholar]
- Fenner, D.; Banks, K. Orange cup coral Tubastraea coccinea invades Florida and the Flower Garden Banks, northwestern Gulf of Mexico. Coral Reefs 2004, 23, 505–507. [Google Scholar] [CrossRef]
- Hickerson, E.L.; Schmahl, G.P.; Robbart, M.; Precht, W.F.; Caldow, C. The State of Coral Reef Ecosystems of the Flower Garden Banks, Stetson Bank, and Other Banks in the Northwestern Gulf of Mexico; National Oceanic and Atmospheric Administration (NOAA): Galveston, TX, USA, 2008. [Google Scholar]
- Ayre, D.J.; Resing, J.M. Sexual and asexual production of planulae in reef corals. Mar. Biol. 1986, 90, 187–190. [Google Scholar] [CrossRef]
- Shearer, T.L. Range expansion of an introduced coral: Investigating the source and ecological impact of the invasion. In Proceedings of the Ocean Sciences Meeting: From the Watershed to the Global Ocean, Orlando, FL, USA, 2–7 March 2008. [Google Scholar]
- Sammarco, P.W.; Brazeau, D.A.; Sinclair, J. Genetic connectivity in scleractinian corals across the northern Gulf of Mexico: Oil/gas platforms, and relationship to the Flower Garden Banks. PLoS ONE 2012, 7, e30144. [Google Scholar] [CrossRef]
- Glynn, P.W.; Colley, S.B.; Mate, J.L.; Cortes, J.; Guzman, H.M.; Bailey, R.L.; Feingold, J.S.; Enochs, I.C. Reproductive ecology of the azooxanthellate coral Tubastraea coccinea in the equatorial eastern Pacific: Part V. Dendrophylliidae. Mar. Biol. 2008, 153, 529–544. [Google Scholar] [CrossRef]
- de Paula, A.F.; de Oliveira Pires, D.; Creed, J.C. Reproductive strategies of two invasive sun corals (Tubastraea spp.) in the southwestern Atlantic. J. Mar. Biol. Assoc. United Kingd. 2014, 94, 481–492. [Google Scholar] [CrossRef]
- Cairns, S.D.; Zibrowius, H. Cnidaria Anthozoa: Azooxanthellate Scleractinia from the Philippine and Indonesian regions. Mem. Mus. Hist. Nat. 1997, 172, 27–243. [Google Scholar]
- Cairns, S.D. Revision of the shallow-water azooxanthellate Scleractinia of the western Atlantic. Stud. Nat. Hist. Caribb. Reg. 2000, 75, 1–240. [Google Scholar]
- Fenner, D. New observations on the stony coral (Scleractinia, Milleporidae, and Stylasteridae) species of Belize (Central America) and Cozumel (Mexico). Bull. Mar. Sci. 1999, 64, 143–154. [Google Scholar]
- Humann, P.; DeLoach, N. Reef Coral Identification: Florida, Caribbean, Bahamas, Including Marine Plants; New World: Jacksonville, FL, USA, 2002. [Google Scholar]
- Elton, C.S. The Ecology of Invasions by Animals and Plants; The University of Chicago Press: Chicago, MI, USA, 2000; 196p. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Qasem, J.R.; Foy, C.L. Weed allelopathy, its ecological impacts and future prospects: A review. J. Crop. Prod. 2001, 4, 43–119. [Google Scholar] [CrossRef]
- Sammarco, P.W.; Coll, J.C.; LaBarre, S.; Willis, B. Competitive strategies of soft corals: Allelochemical effects on selected scleractinian corals. Coral Reefs 1983, 1, 173–178. [Google Scholar] [CrossRef]
- Coll, J.C.; Bowden, B.F.; Alino, P.M.; Heaton, A.; Kong, G.M.; DeNys, R.; Willis, R.H.; Sammarco, P.W.; Clayton, M. Chemically mediated interactions between marine organisms. Chem. Scr. 1990, 29, 383–388. [Google Scholar]
- Sammarco, P.W.; Coll, J.C. Chemical adaptations in the Octocorallia: Evolutionary considerations. Mar. Ecol. Prog. Ser. 1992, 88, 93–104. [Google Scholar] [CrossRef]
- Maida, M.; Sammarco, P.W.; Coll, J.C. Effects of soft corals on scleractinian coral recruitment II: Allelopathy, coral spat survivorship, and reef community structure. Mar. Ecol. 2001, 22, 397–414. [Google Scholar] [CrossRef]
- Fleury, B.G.; Coll, J.C.; Sammarco, P.W.; Tentori, E.; Duquesne, S.W. Variability in complementary (secondary) metabolites related to interspecific competition between a soft and hard coral on the Great Barrier Reef. J. Exp. Mar. Biol. Ecol. 2004, 303, 115–131. [Google Scholar] [CrossRef]
- Ritson-Williams, R.; Arnold, S.N.; Paul, V.J.; Steneck, R.S. Larval settlement preferences of Acropora palmata and Montastraea faveolata in response to diverse red algae. Coral Reefs 2013, 33, 59–66. [Google Scholar] [CrossRef]
- Figueroa, D.F.; McClure, A.; Figueroa, N.J.; Hicks, D.W. Hiding in plain sight: Invasive coral Tubastraea tagusensis (Scleractinia: Hexacorallia) in the Gulf of Mexico. Coral Reefs 2019, 38, 395–403. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Chang, X.; Tang, C.; Chen, K.; Bao, L.; Zhang, L.; Hu, J.; Wang, S.; Bao, Z. High-quality genome assembly of the azooxanthellate coral Tubastraea coccinea (Lesson, 1829). Sci. Data 2025, 12, 507. [Google Scholar] [CrossRef]
- Vinci, M.; Greco, D.; Figura, M.G.; Treccarichi, S.; Musumeci, A.; Greco, V.; Pettinato, R.; Gloria, A.; Papa, C.; Saccone, S.; et al. Exploring the Role of FICD, a New Potential Gene Involved in Borderline Intellectual Functioning, Psychological and Metabolic Disorders. Genes 2024, 15, 1655. [Google Scholar] [CrossRef]
- Zech, M.; Kopajtich, R.; Steinbrücker, K.; Bris, C.; Gueguen, N.; Feichtinger, R.G.; Achleitner, M.T.; Duzkale, N.; Périvier, M.; Koch, J.; et al. Variants in Mitochondrial ATP Synthase Cause Variable Neurologic Phenotypes. Ann Neurol. 2022, 91, 225–237. [Google Scholar] [CrossRef]
- Cuvertino, S.; Stuart, H.M.; Chandler, K.E.; Roberts, N.A.; Armstrong, R.; Bernardini, L.; Bhaskar, S.; Callewaert, B.; Clayton-Smith, J.; Davalillo, C.H.; et al. ACTB Loss-of-Function Mutations Result in a Pleiotropic Developmental Disorder. Am. J. Hum. Genet. 2017, 101, 1021–1033. [Google Scholar] [CrossRef]
- Kalo, A.; Kanter, I.; Shraga, A.; Sheinberger, J.; Tzemach, H.; Kinor, N.; Singer, R.H.; Lionnet, T.; Shav-Tal, Y. Cellular Levels of Signaling Factors Are Sensed by β-actin Alleles to Modulate Transcriptional Pulse Intensity. Cell Rep. 2015, 11, 419–432. [Google Scholar] [CrossRef]
- Singh, R.K.; Saini, S.K.; Prakasam, G.; Kalairasan, P.; Bamezai, R.N.K. Role of ectopically expressed mtDNA encoded cytochrome c oxidase subunit I (MT-COI) in tumorigenesis. Mitochondrion 2019, 49, 56–65. [Google Scholar] [CrossRef]
- Huang, X.; Yan, P.; Song, X.; Zhang, S.; Deng, Y.; Huang, C.; Zhao, X.; Liu, S.; Cheng, X.; Liao, D. MT-CO1 expression in nine organs and tissues of different-aged MRL/lpr mice: Investigation of mitochondrial respiratory chain dysfunction at organ level in systemic lupus erythematosus pathogenesis. Arch. Rheumatol. 2022, 37, 504–516. [Google Scholar] [CrossRef]
- Ng, Y.S.; Lax, N.Z.; Maddison, P.; Alston, C.L.; Blakely, E.L.; Hepplewhite, P.D.; Riordan, G.; Meldau, S.; Chineery, P.F.; Pierre, G.; et al. MT-ND5 mutation exhibits highly variable neurological manifestations at low mutant load. EBioMedicine 2018, 30, 86–93. [Google Scholar] [CrossRef]
- Chaudhry, M.A.; Omaruddin, R.A. Mitochondrial gene expression in directly irradiated and nonirradiated bystander cells. Cancer Biother. Radiopharm. 2011, 26, 657–663. [Google Scholar] [CrossRef]
- Alkhaldi, H.A.; Phan, D.H.; Vik, S.B. Analysis of human clinical mutations of mitochondrial ND1 in a bacterial model system for complex I. Life 2022, 12, 1934. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, Y.; Xue, L. Mitochondrial complex I subunit MT-ND1 mutations affect disease progression. Heliyon 2024, 10, e28808. [Google Scholar] [CrossRef]
- Torres-Benito, L.; Schneider, S.; Rombo, R.; Ling, K.K.; Grysko, V.; Upadhyay, A.; Kononenko, N.L.; Rigo, F.; Bennett, C.F.; Wirth, B. NCALD antisense oligonucleotide therapy in addition to nusinersen further ameliorates spinal muscular atrophy in mice. Am. J. Hum. Genet. 2019, 105, 221–230. [Google Scholar] [CrossRef]
- Windhorst, S.; Song, K.; Gazdar, A.F. Inositol-1,4,5-trisphosphate 3-kinase-A (ITPKA) is frequently over-expressed and functions as an oncogene in several tumor types. Biochem. Pharmacol. 2017, 137, 1–9. [Google Scholar] [CrossRef]
- Figueiredo, T.; Melo, U.S.; Pessoa, A.L.S.; Nobrega, P.R.; Kitajima, J.P.; Rusch, H.; Vaz, F.; Lucato, L.T.; Zatz, M.; Kok, F.; et al. A homozygous loss-of-function mutation in inositol monophosphatase 1 (IMPA1) causes severe intellectual disability. Mol. Psychiatry 2016, 21, 1125–1129. [Google Scholar] [CrossRef]
- Lu, Y.F.; Chen, J.B.; Zhang, B.; Li, Q.G.; Wang, Z.X.; Zhang, H.; Wu, K.L. Cloning, expression, and polymorphism of the ECI1 gene in various pig breeds. J. Integr. Agric. 2017, 16, 1789–1799. [Google Scholar] [CrossRef]
- Trompier, D.; Gondcaille, C.; Lizard, G.; Savary, S. Regulation of the adrenoleukodystrophy-related gene (ABCD2): Focus on oxysterols and LXR antagonists. Biochem. Biophys. Res. Commun. 2014, 446, 651–655. [Google Scholar] [CrossRef]
- Moffat, C.; Bhatia, L.; Nguyen, T.; Lynch, P.; Wang, M.; Wang, D.; Ilkayeva, O.R.; Han, X.; Hirschey, M.D.; Claypool, S.M.; et al. Acyl-CoA thioesterase-2 facilitates mitochondrial fatty acid oxidation in the liver. J. Lipid Res. 2014, 55, 2458–2470. [Google Scholar] [CrossRef]
- Li, J.; Feng, S.; Ma, X.; Yuan, S.; Wang, X. METTL21A, a Non-Histone Methyltransferase, Is Dispensable for Spermatogenesis and Male Fertility in Mice. Int. J. Mol. Sci. 2022, 23, 1942. [Google Scholar] [CrossRef]
- Guo, J.; Bian, Y.; Wang, Y.; Chen, L.; Yu, A.; Sun, X. FAM107B is regulated by S100A4 and mediates the effect of S100A4 on the proliferation and migration of MGC803 gastric cancer cells. Cell Biol. Int. 2017, 41, 1103–1109. [Google Scholar] [CrossRef]
- De Jesus, D.F.; Kimura, T.; Gupta, M.K.; Kulkarni, R.N. NREP contributes to development of NAFLD by regulating one-carbon metabolism in primary human hepatocytes. Cell Chem. Biol. 2023, 30, 1144–1155. [Google Scholar] [CrossRef]
- Ruan, Y.; Qiao, J.; Wang, J.; Liu, Z. NREP, transcriptionally upregulated by HIF-1α, aggravates breast cancer cell growth and metastasis by promoting glycolysis. Cell Death Discov. 2024, 10, 210. [Google Scholar] [CrossRef]
- Koh, J.Y.; Lee, S.J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain 2020, 13, 116. [Google Scholar] [CrossRef]
- Xu, H.; Robinson, G.W.; Huang, J.; Lim, J.Y.; Zhang, H.; Bass, J.K.; Broniscer, A.; Chintagumpala, M.; Bartels, U.; Gururangan, S.; et al. Common variants in ACYP2 influence susceptibility to cisplatin-induced hearing loss. Nat. Genet. 2015, 47, 263–266. [Google Scholar] [CrossRef]
- Li, M.; Ruan, B.; Wei, J.; Yang, Q.; Chen, M.; Ji, M.; Hou, P. ACYP2 contributes to malignant progression of glioma through promoting Ca2+ efflux and subsequently activating c-Myc and STAT3 signals. J. Exp. Clin. Cancer Res. 2020, 39, 106. [Google Scholar] [CrossRef]
- Capel, K.C.C.; Migotto, A.E.; Zilberberg, C.; Lin, M.F.; Forsman, Z.; Miller, D.J.; Kitahara, M.V. Complete mitochondrial genome sequences of Atlantic representatives of the invasive Pacific coral species Tubastraea coccinea and T. tagusensis (Scleractinia, Dendrophylliidae): Implications for species identification. Gene 2016, 590, 270–277. [Google Scholar] [CrossRef]
- Silva, A.G.; Lima, R.P.; Gomes, A.N.; Fleury, B.G.; Creed, J.C. Expansion of the invasive corals Tubastrea coccinea and Tubastrea tagusensis into tamois ecological station marine protected area, Brazil. Aquat. Invasions 2011, 6, S105–S110. [Google Scholar] [CrossRef]
- Chang, H.C.; Chu, C.P.; Lin, S.J.; Hsiao, C.K. Network hub-node prioritization of gene regulation with intra-network association. BMC Bioinform. 2020, 21, 10. [Google Scholar] [CrossRef]
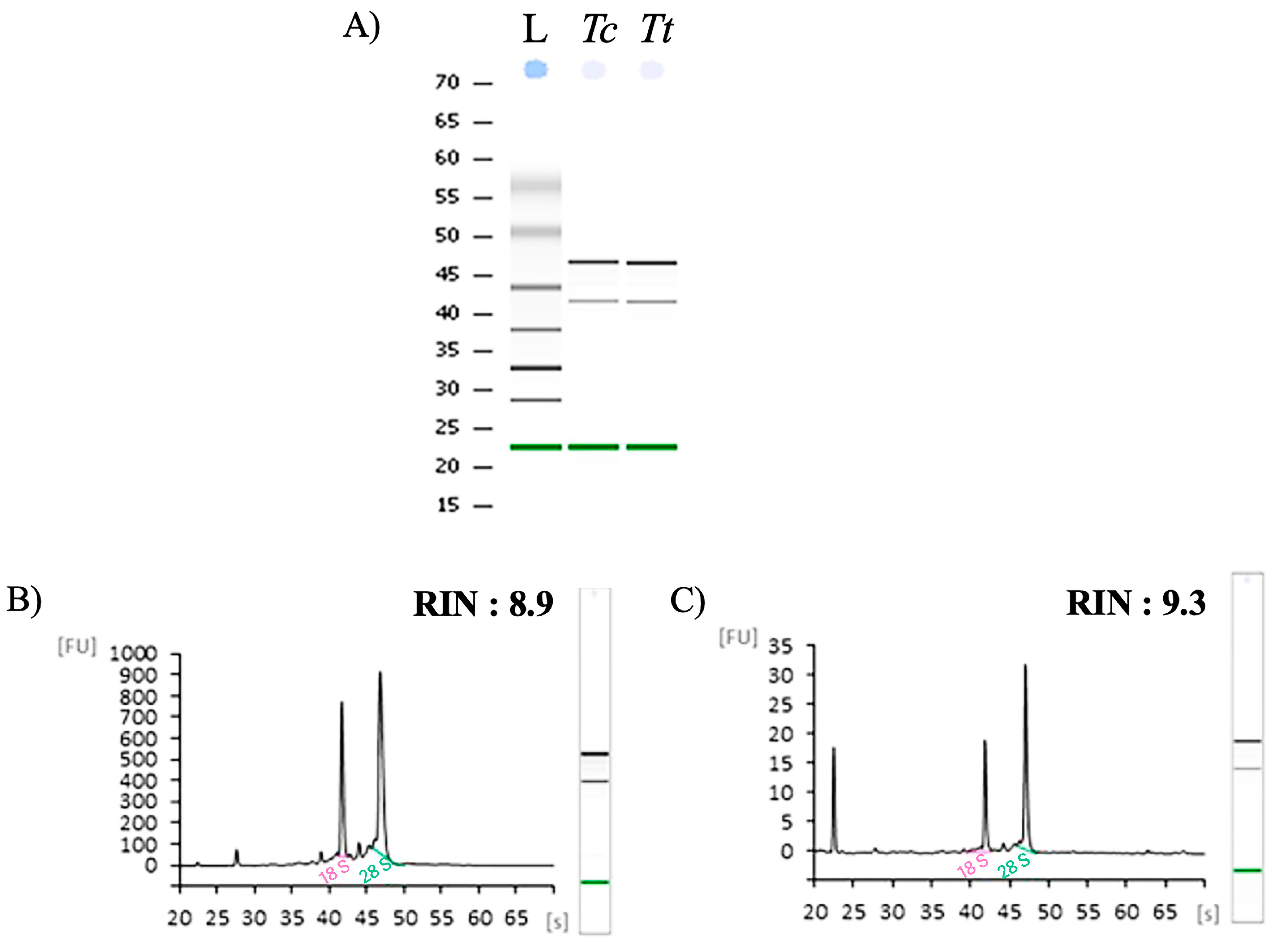


| Sample | RNA Quantity (µg) | A260/A230 | A260/A280 | RIN |
|---|---|---|---|---|
| RNA_Tc | 14.8 | 2.25 | 1.98 | 9.3 |
| RNA_Tt | 7.5 | 2.15 | 1.97 | 10.0 |
| Gene Type | Acronym | Acc. Number | Gene Name | Primer | Sequence 5′>3′ | Fragment Lenght (bp) |
|---|---|---|---|---|---|---|
| Housekeeping | 18S RNA | LT630999 | 18S ribosomal RNA | 18S_Tc_F1 | CATAGTAACTGATCGAATCGC | 185 |
| 18S_Tc_R1 | CGCGCCTGCTGCCTTCCTTG | |||||
| 28S RNA | AF265625 | 28S ribosomal RNA | 28S_Tc_F1 | GCGGAGGAAAAGAAACTAAC | 195 | |
| 28S_Tc_R1 | GTCGGCCGTGCCACAAACGG | |||||
| Stress | NADHox | MW139629 | NADH-ubiquinone oxidoreductase | NADHox_Tc_F1 | GGGTTGGTTTATGTTCTTATC | 200 |
| NADHox_Tc_R1 | GCTAGATGGGGCAGAAACAAC | |||||
| Beta-act | MW139511 | Beta-actin | Beta-act_Tc_F1 | CACCAGCATTTTATGTCGCC | 178 | |
| Beta-act_Tc_R1 | CTTCATGAGGTAGTCGGTC | |||||
| AMPt | MW139419 | Adenosine-monophosphate-protein-transferase | AMPt_Tc_F1 | CACTGTGAGTGATGTTCTTG | 170 | |
| AMPt_Tc_R1 | CTCTGGATAACAGCCAGTC | |||||
| NC | MW110554 | Neurocalcin-like protein gene | NC_Tc_F1 | CAGAGCTCAAAGAATGGTAC | 175 | |
| NC_Tc_R1 | GAAATCAATAGTGCCATCGTC | |||||
| NADH5 | OQ697663 | NADH dehydrogenase subunit 5 | NADH5_Tc_F1 | CTCATATTCCTCGCTTTATGTC | 188 | |
| NADH5_Tc_R1 | GACTAACATGGCTTTTATGGC | |||||
| ATPs | OQ697278 | Adenosine triphosphate synthase | ATPs_Tc_F1 | GTGGCTCTGATCGCCTTGAC | 204 | |
| ATPs_Tc_R1 | GAAGAGAGAGACAATAAAAGG | |||||
| Cytb | OQ696950 | Cytochrome b | Cb_Tc_F1 | GCCACTGCGCAAAGAGAATC | 167 | |
| Cb_Tc_R1 | CTGCACAATAATGCATGGAC |
| Gene Name | T. coccinea | Human |
|---|---|---|
| adenosine-monophosphate-protein-transferase | AMPt | FICD |
| ATP synthase | ATPs | ATP5F1A |
| beta-actin | Beta-act | ACTB |
| cytochrome b | Cytb | MT-CYB |
| NADH dehydrogenase subunit 5 | NADH5 | MT-ND5 |
| NADH-ubiquinone oxidoreductase | NADHox | ND1 |
| neurocalcin-like protein | NC | NCALD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, M.; Guida, F.; Amorim, C.G.; da Nóbrega, L.B.; Esposito, R.; Zupo, V.; Fleury, B.G. Isolation and Identification of Inter-Correlated Genes from the Invasive Sun Corals Tubastraea Coccinea and Tubastraea Tagusensis (Scleractinia, Cnidaria). Int. J. Mol. Sci. 2025, 26, 7235. https://doi.org/10.3390/ijms26157235
Costantini M, Guida F, Amorim CG, da Nóbrega LB, Esposito R, Zupo V, Fleury BG. Isolation and Identification of Inter-Correlated Genes from the Invasive Sun Corals Tubastraea Coccinea and Tubastraea Tagusensis (Scleractinia, Cnidaria). International Journal of Molecular Sciences. 2025; 26(15):7235. https://doi.org/10.3390/ijms26157235
Chicago/Turabian StyleCostantini, Maria, Fulvia Guida, Carolina G. Amorim, Lucas B. da Nóbrega, Roberta Esposito, Valerio Zupo, and Beatriz G. Fleury. 2025. "Isolation and Identification of Inter-Correlated Genes from the Invasive Sun Corals Tubastraea Coccinea and Tubastraea Tagusensis (Scleractinia, Cnidaria)" International Journal of Molecular Sciences 26, no. 15: 7235. https://doi.org/10.3390/ijms26157235
APA StyleCostantini, M., Guida, F., Amorim, C. G., da Nóbrega, L. B., Esposito, R., Zupo, V., & Fleury, B. G. (2025). Isolation and Identification of Inter-Correlated Genes from the Invasive Sun Corals Tubastraea Coccinea and Tubastraea Tagusensis (Scleractinia, Cnidaria). International Journal of Molecular Sciences, 26(15), 7235. https://doi.org/10.3390/ijms26157235








