Molecular Pathways Potentially Involved in Hallucinatory Experiences During Sleep Paralysis: The Emerging Role of β-Arrestin-2
Abstract
1. Introduction
2. Review
2.1. Characteristics of Serotonergic Hallucinations
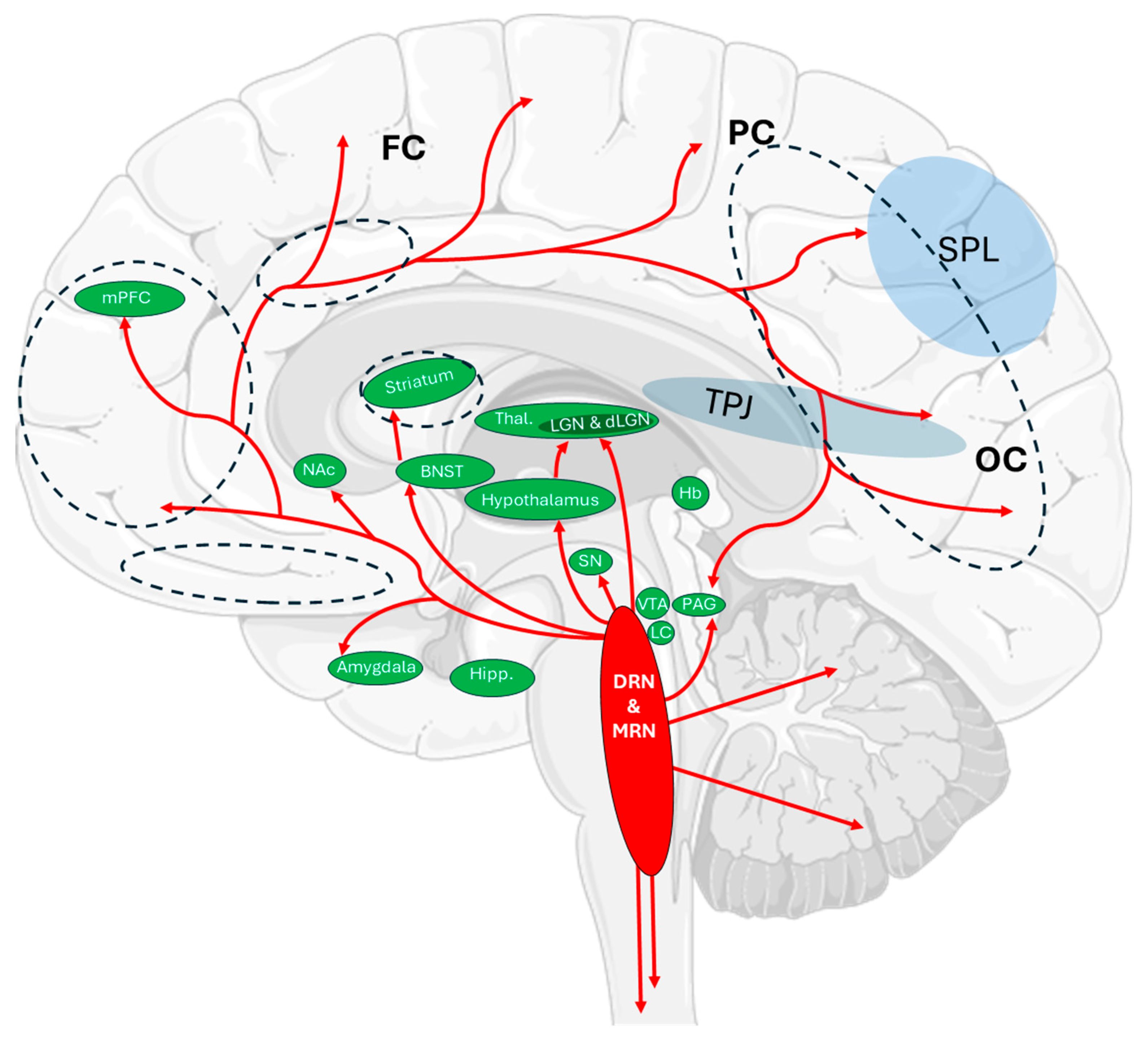
2.2. Aberrations of Serotonin Signaling During Sleep Paralysis
2.3. 5-HT Receptors
2.4. The Role of the 5-HT2A Receptor in Physiological Sensory Processing
2.5. The Role of 5-HT2A in Shaping Perception: Molecular Pathways and Cortical Rhythms
2.6. 5-HT2A Receptor Pathophysiology and Its Hallucinogenic Potential
2.7. 5-HT2A-Receptor-Dependent Molecular Signaling Pathways Promoting Hallucinogenic Effects
2.8. Serotonin
2.9. LSD
2.10. Psilocybin
2.11. Unresolved Questions and Working Hypothesis
3. Summary
4. Methodology
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | serotonin (5-hydroxytryptamine) |
| 5-HT2A | 5-hydroxytryptamine receptor 2A |
| AA | arachidonic acid |
| Akt | protein kinase B |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (receptor) |
| ARF | ADP-ribosylation factor |
| βarr | β-arrestin |
| βarr1-KO | β-arrestin-1 knockout |
| βarr2-KO | β-arrestin-2 knockout |
| BNST | bed nucleus of the stria terminalis |
| CaMKs | calcium/calmodulin-dependent kinases |
| CNS | central nervous system |
| DAG | diacylglycerol |
| dLGN | dorsal lateral geniculate nucleus |
| DOI | ,5-dimethoxy-4-iodoamphetamine |
| DRN | dorsal raphe nuclei |
| EEG | electro-encephalogram |
| E/I | excitation/inhibition |
| EPSCs | excitatory postsynaptic currents |
| ERK | extracellular signal-regulated kinase |
| FC | frontal cortex |
| GPCR | G protein-coupled receptor |
| Hb | habenula |
| Hipp | hippocampus |
| HTR | head-twitch response |
| IP3 | inositol 1,4,5-trisphosphate |
| ISP | isolated sleep paralysis |
| LC | locus coeruleus |
| LFP | local field potential |
| LGN | lateral geniculate nucleus |
| LSD | lysergic acid diethylamide |
| MAPK | mitogen-activated protein kinase |
| MDL | MDL100907 |
| mPFC | medial prefrontal cortex |
| MRN | median raphe nuclei |
| NAc | nucleus accumbens |
| OBE | out-of-body experience |
| OC | occipital cortex |
| PAG | periaqueductal gray |
| PC | parietal cortex |
| PKC | protein kinase C |
| PLA2 | phospholipase A2 |
| PLC | phospholipase C |
| PPI | prepulse inhibition |
| PSIL | psilocybin (4-PO-DMT) |
| PV | parvalbumin-positive (interneurons) |
| REM | rapid eye movement |
| SN | substantia nigra |
| SP | sleep paralysis |
| SPL | superior parietal lobe |
| Src | proto-oncogene tyrosine-protein kinase |
| SSRIs | selective serotonin reuptake inhibitors |
| Thal | thalamus |
| TPJ | temporoparietal junction |
| V1 | primary visual cortex |
| VTA | ventral tegmental area |
| WT | wild type |
References
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.A.; Hudspeth, A.J. Principles of Neural Science, 4th ed.; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Brooks, P.L.; Peever, J.H. Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis. J. Neurosci. 2012, 32, 9785–9795. [Google Scholar] [CrossRef]
- Jalal, B.; Hinton, D.E. Rates and characteristics of sleep paralysis in the general population of Denmark and Egypt. Cult. Med. Psychiatry 2013, 37, 534–548. [Google Scholar] [CrossRef]
- Studerus, E.; Kometer, M.; Hasler, F.; Vollenweider, F.X. Acute, subacute and long-term subjective effects of psilocybin in healthy humans: A pooled analysis of experimental studies. J. Psychopharm. 2011, 25, 1434–1452. [Google Scholar] [CrossRef]
- van der Zwaard, R.; Polak, M.A. Pseudohallucinations: A pseudoconcept? A review of the validity of the concept, related to associate symptomatology. Comp. Psychiatry 2001, 42, 42–50. [Google Scholar] [CrossRef]
- Jalal, B. The neuropharmacology of sleep paralysis hallucinations: Serotonin 2A activation and a novel therapeutic drug. Psychopharmacology 2018, 235, 3083–3091. [Google Scholar] [CrossRef]
- Gaebel, W.; Zielasek, J. Future classification of psychotic disorders. Eur. Arch. Psychiatry Clin. Neurosci. 2009, 259, 213–218. [Google Scholar] [CrossRef]
- Pompeiano, M.; Palacios, J.M.; Mengod, G. Distribution of the serotonin 5-HT2 receptor family mRNAs: Comparison between 5-HT2A and 5-HT2C receptors. Brain Res. Mol. Brain Res. 1994, 23, 163–178. [Google Scholar] [CrossRef]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT2A receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Fiorella, D.; Rabin, R.A.; Winter, J.C. The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs. Psychopharmacology 1995, 121, 347–356. [Google Scholar] [CrossRef]
- Sadzot, B.; Baraban, J.M.; Glennon, R.A.; Lyon, R.A.; Leonhardt, S.; Janowsky, A.; Paul, S.M. Hallucinogenic drug interactions at human brain 5-HT2 receptors: [3H]ketanserin binding studies. Neurosci. Let. 1989, 98, 41–46. [Google Scholar]
- Aghajanian, G.K.; Marek, G.J. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999, 825, 161–171. [Google Scholar] [CrossRef]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 2003, 452, 93–97. [Google Scholar] [CrossRef]
- Preller, K.H.; Razi, A.; Zeidman, P.; Stämpfli, P.; Friston, K.J.; Vollenweider, F.X. Effective connectivity changes in LSD-induced altered states of consciousness in humans. Proc. Nat. Acad. Sci. USA 2018, 116, 2743–2748. [Google Scholar] [CrossRef]
- McLean, S.; Weber, E.; Nichols, D.E. Pharmacology and behavioral effects of the hallucinogenic phenethylamine 2,5-dimethoxy-4-iodoamphetamine (DOI) in rats. Psychopharmacology 2006, 186, 581–588. [Google Scholar]
- Jalal, B.; Ramachandran, V.S. Sleep paralysis and the shadowy bedroom intruder: The role of the right superior parietal, phantom pain and body image projection. Med. Hypotheses 2014, 83, 755–757. [Google Scholar] [CrossRef]
- Jalal, B.; Romanelli, A.; Hinton, D.E. Sleep paralysis in Italy: Frequency, hallucinatory experiences, and other features. Transcult. Psychiatry 2021, 58, 427–439. [Google Scholar] [CrossRef]
- Jalal, B.; Simons-Rudolph, J.; Jalal, B.; Hinton, D.E. Explanations of sleep paralysis among Egyptian college students and the general population in Egypt and Denmark. Transcult. Psychiatry 2015, 52, 560–575. [Google Scholar] [CrossRef]
- Cheyne, J.A.; Rueffer, S.D.; Newby-Clark, I.R. Hypnagogic and hypnopompic hallucinations during sleep paralysis: Neurological and cultural construction of the nightmare. Conscious. Cogn. 1999, 8, 319–337. [Google Scholar] [CrossRef]
- Cheyne, J.A. Sleep paralysis and the structure of waking-nightmare hallucinations. Dreaming 2003, 13, 163–179. [Google Scholar] [CrossRef]
- Peever, J.; Fuller, P.M. The biology of REM sleep. Curr. Biol. 2016, 26, R1070–R1078. [Google Scholar] [CrossRef]
- Jalal, B.; Ramachandran, V.S. The neuropharmacology of sleep paralysis hallucinations: Serotonin 2A activation and the induction of “felt presence” and out-of-body experiences. Psychopharmacology 2017, 234, 1299–1307. [Google Scholar]
- Arzy, S.; Thut, G.; Mohr, C.; Michel, C.M.; Blanke, O. Neural basis of embodiment: Distinct contributions of temporoparietal junction and extrastriate body area. J. Neurosci. 2006, 26, 8074–8081. [Google Scholar] [CrossRef]
- Farrer, C.; Franck, N.; Georgieff, N.; Frith, C.D.; Decety, J.; Jeannerod, M. Modulating the experience of agency: A positron emission tomography study. NeuroImage 2003, 18, 324–333. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Beliveau, V.; Ganz, M.; Feng, L.; Ozenne, B.; Højgaard, L.; Fisher, P.M.; Svarer, C.; Greve, D.N.; Knudsen, G.M. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. J. Neurosci. 2017, 37, 120–128. [Google Scholar] [CrossRef]
- Vogelsang, D.A.; D’Esposito, M. Is There Evidence for a Rostral-Caudal Gradient in Fronto-Striatal Loops and What Role Does Dopamine Play? Front. Neurosci. 2018, 12, 242. [Google Scholar] [CrossRef]
- Molendijk, M.; Molero, P.; Ortuno Sánchez-Pedreño, F.; van der Does, W.; Angel Martínez-González, M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef]
- Savage, C. Lysergic acid diethylamide; a clinical-psychological study. Am. J. Psychiatry 1952, 108, 896–900. [Google Scholar] [CrossRef]
- Pahnke, W.N.; Richards, W.A. Implications of LSD and experimental mysticism. J. Relig. Health 1966, 5, 175–208. [Google Scholar] [CrossRef]
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The pharmacology of lysergic acid diethylamide: A review. CNS Neurosci. Ther. 2008, 14, 295–314. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- Hornung, J.-P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [CrossRef]
- Kosofsky, B.E.; Molliver, M.E. The serotoninergic innervation of cerebral cortex: Different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse 1987, 1, 153–168. [Google Scholar] [CrossRef]
- Moreau, A.W.; Amar, M.; Le Roux, N.; Morel, N.; Fossier, P. Serotoninergic fine-tuning of the excitation-inhibition balance in rat visual cortical networks. Cereb. Cortex 2010, 20, 456–467. [Google Scholar] [CrossRef]
- Zhang, G.; Stackman, R.W., Jr. The role of serotonin 5-HT2A receptors in memory and cognition. Front. Pharmacol. 2015, 6, 225. [Google Scholar] [CrossRef]
- Tork, I. Anatomy of the serotonergic system. Ann. N. Y. Acad. Sci. 1990, 600, 9–34. [Google Scholar] [CrossRef]
- Bayer, L.; Serafin, M.; Eggermann, E.; Saint-Mleux, B.; Machard, D.; Jones, B.E.; Mühlethaler, M. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J. Neurosci. 2004, 24, 6760–6764. [Google Scholar] [CrossRef]
- Porter, R.H.; Benwell, K.R.; Lamb, H.; Malcolm, C.S.; Allen, N.H.; Revell, D.F.; Adams, D.R.; Sheardown, M.J. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Brit. J. Pharmacol. 1999, 128, 13–20. [Google Scholar] [CrossRef]
- Zifa, E.; Fillion, G. 5-Hydroxytryptamine receptors. Pharmacol. Rev. 1992, 44, 401–458. [Google Scholar] [CrossRef]
- Boess, F.G.; Martin, I.L. Molecular biology of 5-HT receptors. Neuropharmacology 1994, 33, 275–317. [Google Scholar] [CrossRef]
- Hoyer, D.; Clarke, D.E.; Fozard, J.R.; Hartig, P.R.; Martin, G.R.; Mylecharane, E.J.; Saxena, P.R.; Humphrey, P.P.A. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994, 46, 157–203. [Google Scholar] [CrossRef]
- Raymond, J.R.; Mukhin, Y.V.; Gelasco, A.; Turner, J.; Collinsworth, G.; Gettys, T.W.; Grewal, J.S.; Garnovskaya, M.N. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol. Ther. 2001, 92, 179–212. [Google Scholar] [CrossRef]
- Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behaviour. Brain. Res. 2008, 195, 198–213. [Google Scholar] [CrossRef]
- Gether, U. Uncovering molecular mechanisms involved in activation of G protein-coupled receptors. Endocr. Rev. 2000, 21, 90–113. [Google Scholar] [CrossRef]
- Gether, U.; Lin, S.; Kobilka, B.K. G protein-coupled receptors II. Mechanism of agonist activation. J. Biol. Chem. 2002, 277, 3125–3128. [Google Scholar]
- Pierce, K.L.; Premont, R.T.; Lefkowitz, R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell. Biol. 2002, 3, 639–650. [Google Scholar] [CrossRef]
- Schöneberg, T.; Schulz, A.; Biebermann, H.; Hermsdorf, T.; Römpler, H.; Sangkuhl, K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol. Ther. 2002, 104, 173–206. [Google Scholar] [CrossRef]
- Kristiansen, K. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: From the biophysics of receptor activation to drug design. Pharmacol. Ther. 2004, 103, 21–80. [Google Scholar] [CrossRef]
- Pazos, A.; Cortés, R.; Palacios, J.M. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. II. Serotonin-2 receptors. Brain Res. 1985, 346, 231–249. [Google Scholar] [CrossRef]
- López-Giménez, J.F.; Mengod, G.; Palacios, J.M.; Vilaró, M.T. Regional distribution and cellular localization of 5-HT2A receptor mRNA in monkey brain: Comparison with receptor binding distribution. J. Comp. Neurol. 2001, 429, 571–589. [Google Scholar] [CrossRef]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef]
- Kometer, M.; Schmidt, A.; Jäncke, L.; Vollenweider, F.X. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on α oscillations, N170 visual-evoked potentials, and visual hallucinations. J. Neurosci. 2013, 33, 10544–10551. [Google Scholar] [CrossRef]
- Preller, K.H.; Herdener, M.; Pokorny, T.; Planzer, A.; Kraehenmann, R.; Stämpfli, P.; Liechti, M.E.; Seifritz, E.; Vollenweider, F.X. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr. Biol. 2017, 27, 451–457. [Google Scholar] [CrossRef]
- Vollenweider, F.; Kometer, M. The neurobiology of psychedelic drugs: Implications for the treatment of mood disorders. Nat. Rev. Neurosci. 2010, 11, 642–651. [Google Scholar] [CrossRef]
- Carhart-Harris, R.L.; Erritzoe, D.; Williams, T.; Stone, J.M.; Reed, L.J.; Colasanti, A.; Tyacke, R.J.; Leech, R.; Malizia, A.L.; Murphy, K.; et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proc. Natl. Acad. Sci. USA 2012, 109, 2138–2143. [Google Scholar] [CrossRef]
- Jakab, R.L.; Goldman-Rakic, P.S. 5-Hydroxytryptamine2A serotonin receptors in the primate cerebral cortex: Possible site of action of hallucinogenic and antipsychotic drugs in pyramidal cell apical dendrites. Proc. Natl. Acad. Sci. USA 1998, 95, 735–740. [Google Scholar] [CrossRef]
- Duerler, P.; Brem, S.; Fraga-González, G.; Neef, T.; Allen, M.; Zeidman, P.; Stämpfli, P.; Vollenweider, F.X.; Preller, K.H. Psilocybin Induces Aberrant Prediction Error Processing of Tactile Mismatch Responses—A Simultaneous EEG–FMRI Study. Cerebral. Cortex 2022, 32, 186–196. [Google Scholar] [CrossRef]
- Barzan, R.; Bozkurt, B.; Nejad, M.M.; Süß, S.T.; Surdin, T.; Böke, H.; Spoida, K.; Azimi, Z.; Grömmke, M.; Eickelbeck, D.; et al. Gain control of sensory input across polysynaptic circuitries in mouse visual cortex by a single G protein-coupled receptor type (5-HT2A). Nat. Commun. 2024, 15, 8078. [Google Scholar] [CrossRef]
- Seillier, A.; Martinez, A.A.; Giuffrida, A. Differential effects of Δ9-tetrahydrocannabinol dosing on correlates of schizophrenia in the sub-chronic PCP rat model. PLoS ONE 2020, 15, e0230238. [Google Scholar] [CrossRef]
- Azimi, Z.; Barzan, R.; Spoida, K.; Surdin, T.; Wollenweber, P.; Mark, M.D.; Herlitze, S.; Jancke, D. Separable gain control of ongoing and evoked activity in the visual cortex by serotonergic input. eLife 2020, 9, e53552. [Google Scholar] [CrossRef]
- Béïque, J.C.; Imad, M.; Mladenovic, L.; Gingrich, J.A.; Andrade, R. Mechanism of the 5-hydroxytryptamine 2A receptor-mediated facilitation of synaptic activity in prefrontal cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 9870–9875. [Google Scholar] [CrossRef]
- Villalobos, C.; Beique, J.C.; Gingrich, J.A.; Andrade, R. Serotonergic regulation of calcium-activated potassium currents in rodent prefrontal cortex. Eur. J. Neurosci. 2005, 22, 1120–1126. [Google Scholar] [CrossRef]
- Andrade, R. Serotonergic regulation of neuronal excitability in the prefrontal cortex. Neuropharmacology 2011, 61, 382–386. [Google Scholar] [CrossRef]
- Araneda, R.; Andrade, R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 1991, 40, 399–412. [Google Scholar] [CrossRef]
- Stephens, E.K.; Baker, A.L.; Gulledge, A.T. Mechanisms Underlying Serotonergic Excitation of Callosal Projection Neurons in the Mouse Medial Prefrontal Cortex. Front. Neural Circuits 2018, 12, 2. [Google Scholar] [CrossRef]
- Weber, E.T.; Andrade, R. Htr2a Gene and 5-HT2A Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front. Neurosci. 2010, 4, 36. [Google Scholar] [CrossRef]
- Kometer, M.; Vollenweider, F.X. Serotonergic Hallucinogen-Induced Visual Perceptual Alterations. Curr. Top. Behav. Neurosci. 2018, 36, 257–282. [Google Scholar]
- Vejmola, Č.; Tylš, F.; Piorecká, V.; Koudelka, V.; Kadeřábek, L.; Novák, T.; Páleníček, T. Psilocin, LSD, mescaline, and DOB all induce broadband desynchronization of EEG and disconnection in rats with robust translational validity. Transl. Psychiatry 2021, 11, 506. [Google Scholar] [CrossRef]
- Sheffler, D.J.; Kroeze, W.K.; Garcia, B.G.; Deutch, A.Y.; Hufeisen, S.J.; Leahy, P.; Brüning, J.C.; Roth, B.L. p90 ribosomal S6 kinase 2 exerts a tonic brake on G proteincoupled receptor signaling. Proc. Natl. Acad. Sci. USA 2006, 103, 4717–4722. [Google Scholar] [CrossRef]
- Zhang, G.; Ásgeirsdóttir, H.N.; Cohen, S.J.; Munchow, A.H.; Barrera, M.P.; Stackman, R.W., Jr. Stimulation of serotonin 2A receptors facilitates consolidation and extinction of fear memory in C57BL/6J mice. Neuropharmacology 2013, 64, 403–413. [Google Scholar] [CrossRef]
- Jean-Charles, P.Y.; Kaur, S.; Shenoy, S.K. G Protein-Coupled Receptor Signaling Through β-Arrestin-Dependent Mechanisms. J. Cardiovasc. Pharmacol. 2017, 70, 142–158. [Google Scholar] [CrossRef]
- Kahsai, A.W.; Shah, K.S.; Shim, P.J.; Lee, M.A.; Shreiber, B.N.; Schwalb, A.M.; Zhang, X.; Kwon, H.Y.; Huang, L.Y.; Soderblom, E.J.; et al. Signal transduction at GPCRs: Allosteric activation of the ERK MAPK by β-arrestin. Proc. Natl. Acad. Sci. USA 2023, 120, e2303794120. [Google Scholar] [CrossRef]
- Hurlemann, R.; Hawellek, B.; Matusch, A.; Kolsch, H.; Wollersen, H.; Madea, B.; Vogeley, K.; Maier, W.; Dolan, R.J. Noradrenergic modulation of emotion-induced forgetting and remembering. J. Neurosci. 2008, 25, 6343–6349. [Google Scholar] [CrossRef]
- Mokler, D.J.; Stoll, D.R.; Geyer, M.A. Effects of serotonergic agonists on locomotor activity and startle response in rats. Pharmacol Biochem. Behav. 1983, 19, 1–6. [Google Scholar]
- Adams, L.M.; Geyer, M.A. A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav. Neurosci. 1985, 99, 881–900. [Google Scholar] [CrossRef]
- Silbergeld, E.K.; Hruska, R.E. Lisuride and LSD: Dopaminergic and serotonergic interactions in the “serotonin syndrome”. Psychopharmacology 1979, 65, 233–237. [Google Scholar] [CrossRef]
- Marona-Lewicka, D.; Thisted, R.A.; Nichols, D.E. Distinct temporal phases in the behavioral pharmacology of LSD: Dopamine D2 receptor-mediated effects in the rat and implications for psychosis. Psychopharmacology 2002, 164, 353–361. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Nichols, C.D.; Gainetdinov, R.R.; Nichols, D.E.; Kalueff, A.V. Psychedelic drugs in biomedicine. Trends Pharmacol. Sci. 2017, 38, 992–1005. [Google Scholar] [CrossRef]
- Ameen, S.; Praharaj, S.K. Functional auditory hallucinations in a case of serotonin syndrome. J. Neuropsych. Clin. Neurosci. 2013, 25, E60–E61. [Google Scholar] [CrossRef]
- Roth, B.L.; Driscol, J.; Meltzer, H.Y. Atypical antipsychotic drug actions: Insights into mechanisms of action. Schizophr. Res. 1998, 31, 107–119. [Google Scholar]
- Millan, M.J.; Marin, P.; Bockaert, J.; Mannoury la Cour, C.; Corradetti, R. Signaling at G-protein-coupled serotonin receptors: Recent advances and future research directions. Trends Pharmacol. Sci. 2008, 29, 454–464. [Google Scholar] [CrossRef]
- Raote, I.; Bhattacharyya, S.; Panicker, M.M.; Miledi, R. Functional selectivity in serotonin receptor signaling. Trends Pharmacol. Sci. 2007, 28, 377–384. [Google Scholar]
- Urban, J.D.; Clarke, W.P.; von Zastrow, M.; Nichols, D.E.; Kobilka, B.; Weinstein, H.; Javitch, J.A.; Roth, B.L.; Christopoulos, A.; Sexton, P.M.; et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 2007, 320, 1–13. [Google Scholar] [CrossRef]
- Kenakin, T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol. Sci. 1995, 16, 232–238. [Google Scholar] [CrossRef]
- Kenakin, T. Functional selectivity through protean agonism: A new concept in receptor theory. Trends Pharmacol. Sci. 1997, 18, 416–417. [Google Scholar] [CrossRef]
- Berg, K.A.; Clarke, W.P.; Sailstad, C.; Saltzman, A.; Maayani, S. Signal transduction differences between 5-hydroxytryptamine type 2A and type 2C receptor systems. Mol. Pharmacol. 1998, 54, 94–104. [Google Scholar] [CrossRef]
- López-Giménez, J.F.; González-Maeso, J. Hallucinogens and serotonin 5-HT2A receptor-mediated signaling pathways. Curr. Top. Behav. Neurosci. 2018, 36, 45–73. [Google Scholar]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Pierce, K.L.; Lefkowitz, R.J. Classical and new roles of beta-arrestins in the regulation of G-PROTEIN-COUPLED receptors. Nat. Rev. Neurosci. 2001, 2, 727–733. [Google Scholar] [CrossRef]
- Shenoy, S.K.; Lefkowitz, R.J. Transduction of receptor signals by beta-arrestins. Science 2005, 308, 512–517. [Google Scholar]
- Schmid, C.L.; Raehal, K.M.; Bohn, L.M. Agonist-directed signaling of the serotonin 2A receptor depends on beta-arrestin-2 interactions in vivo. Proc. Nat. Acad. Sci. USA 2008, 105, 1079–1084. [Google Scholar] [CrossRef]
- Schmid, C.L.; Bohn, L.M. Serotonin, but not N-methyltryptamines, activates the serotonin 2A receptor via a β-arrestin2/Src/Akt signaling complex in vivo. J. Neurosci. 2010, 30, 13513–13524. [Google Scholar] [CrossRef]
- van Gastel, J.; Hendrickx, J.O.; Leysen, H.; Santos-Otte, P.; Luttrell, L.M.; Martin, B.; Maudsley, S.; Martens, E. β-Arrestin based receptor signaling paradigms: Potential therapeutic targets for complex age-related disorders. Front. Pharmacol. 2018, 9, 1369. [Google Scholar] [CrossRef]
- Sanders-Bush, E.; Burris, K.D.; Knoth, K. Lysergic acid diethylamide and 2,5-dimethoxy-4-methylamphetamine are partial agonists at serotonin receptors linked to phosphoinositide hydrolysis. J. Pharmacol. Exp. Ther. 1988, 246, 924–928. [Google Scholar] [CrossRef]
- Egan, C.T.; Herrick-Davis, K.; Miller, K.; Glennon, R.A.; Teitler, M. Agonist high affinity binding to 5-HT2A receptors: Evidence for a G protein-independent state. Mol. Pharmacol. 1998, 54, 933–943. [Google Scholar]
- Kurrasch-Orbaugh, D.M.; Watts, V.J.; Barker, E.L.; Nichols, D.E. Serotonin 5-hydroxytryptamine2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J. Pharmacol. Exp. Ther. 2003, 304, 229–237. [Google Scholar] [CrossRef]
- Rodriguiz, R.M.; Nadler, M.J.S.; Wetsel, W.C. Behavioral phenotyping of genetically modified mice. Curr. Prot. Mouse Biol. 2021, 11, e72. [Google Scholar]
- Fantegrossi, W.E.; Harrington, A.W.; Kiessel, C.L.; Eckler, J.R.; Rabin, R.A.; Winter, J.C.; Coop, A.; Rice, K.C.; Woods, J.H. Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology 2008, 195, 517–525. [Google Scholar] [CrossRef]
- Kennett, G.A.; Lightowler, S.; Trail, B. 5-HT2C receptor agonists inhibit feeding behaviour in the rat. Brit. J. Pharmacol. 1994, 113, 816–820. [Google Scholar]
- Wallach, J.; Cao, A.B.; Calkins, M.M.; Heim, A.J.; Lanham, J.K.; Bonniwell, E.M.; Hennessey, J.J.; Bock, H.A.; Anderson, E.I.; Sherwood, A.M.; et al. Identification of 5-HT2A receptor signaling pathways associated with psychedelic potential. Nat. Comm. 2023, 14, 8221. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vontobel, P.; Hell, D.; Leenders, K.L. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man—A PET study with [11C]raclopride. Neuropsychopharmacology 1999, 20, 424–433. [Google Scholar] [CrossRef]
- Thut, G.; Nietzel, A.; Brandt, S.A.; Pascual-Leone, A. α-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 2006, 26, 9494–9502. [Google Scholar] [CrossRef]
- Romei, V.; Brodbeck, V.; Michel, C.; Amedi, A.; Pascual-Leone, A.; Thut, G. Spontaneous fluctuations in posterior α-band EEG activity reflect variability in excitability of human visual areas. Cereb. Cortex 2008, 18, 2010–2018. [Google Scholar] [CrossRef]
- Hanslmayr, S.; Gross, J.; Klimesch, W.; Shapiro, K.L. The role of alpha oscillations in temporal attention. Brain Res. Rev. 2011, 67, 331–343. [Google Scholar] [CrossRef]
- van den Berg, H.; Shin, W.C.; Chou, R.; George, J.S.; Saalmann, Y.B. EEG decoding reveals task-dependent recoding of sensory information during visual attention. NeuroImage 2023, 269, 119925. [Google Scholar]
- Swanson, O.K.; Maffei, A. From Hiring to Firing: Activation of Inhibitory Neurons and Their Recruitment in Behavior. Front. Mol. Neurosci. 2019, 12, 168. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, M.; Wang, L.; Lin, L.; Xu, J. Phase Coupled Firing of Prefrontal Parvalbumin Interneuron With High Frequency Oscillations. Front. Cell. Neurosci. 2020, 14, 610741. [Google Scholar] [CrossRef]
- Xue, M.; Atallah, B.V.; Scanziani, M. Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 2014, 511, 596–600. [Google Scholar] [CrossRef]
- Ter Wal, M.; Tiesinga, P.H.E. Comprehensive characterization of oscillatory signatures in a model circuit with PV- and SOM-expressing interneurons. Biol. Cybern. 2021, 115, 487–517. [Google Scholar] [CrossRef]
- Scheuer, K.S.; Jansson, A.M.; Zhao, X.; Jackson, M.B. Inter and intralaminar excitation of parvalbumin interneurons in mouse barrel cortex. PLoS ONE 2024, 19, e0289901. [Google Scholar] [CrossRef]
- Maquet, P.; Ruby, P.; Maudoux, A.; Albouy, G.; Sterpenich, V.; Dang-Vu, T.; Desseilles, M.; Boly, M.; Perrin, F.; Peigneux, P.; et al. Human cognition during REM sleep and the activity profile within frontal and parietal cortices: A reappraisal of functional neuroimaging data. Prog. Brain. Res. 2005, 150, 219–227. [Google Scholar]
- Siclari, F.; Baird, B.; Perogamvros, L.; Bernardi, G.; LaRocque, J.J.; Riedner, B.; Boly, M.; Postle, B.R.; Tononi, G. The neural correlates of dreaming. Nat. Neurosci. 2017, 20, 872–878. [Google Scholar] [CrossRef]

| Compound (Common Name) | Receptor Interactions | Activated 5-HT2AR-Dependent Intracellular Pathways | Potential Behavioral Effects |
|---|---|---|---|
| 5-hydroxytryptamine—5-HT (serotonin) | 5-HT1–5-HT7 | PLC-IP3 (predominantly activated), βarr2 | No hallucinations (via PLC-IP3)/potential hallucinations (via βarr2) |
| lysergic acid diethylamide (LSD) | 5-HT2A, 5-HT1A, 5-HT2C, 5-HT1B, 5-HT1D, 5-HT5A, 5-HT6, 5-HT7, D1, D2, D4, α2A, α2B, α2C, α1A, α1B (predominantly 5-HT2A; additional binding sites reported) | PLA2-AA (preferentially engaged by LSD over PLC-IP3), PLC-IP3, βarr2 | Hallucinations |
| 2,5-dimethoxy-4-iodoamphetamine (DOI) | 5-HT2A, 5-HT2C, 5-HT2B (predominantly 5-HT2A; additional binding sites reported) | PLC-IP3 (predominantly activated) | Hallucinations |
| 4-phosphoryloxy-N,N-dimethyltryptamine—4-PO-DMT (psilocybin) | 5-HT2A, 5-HT1A, 5-HT2C, 5-HT1B, 5-HT7 (predominantly 5-HT2A; additional binding sites reported) | PLC-IP3 (predominantly activated) | Hallucinations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudy, L.M.; Godlewski, M.M. Molecular Pathways Potentially Involved in Hallucinatory Experiences During Sleep Paralysis: The Emerging Role of β-Arrestin-2. Int. J. Mol. Sci. 2025, 26, 7233. https://doi.org/10.3390/ijms26157233
Rudy LM, Godlewski MM. Molecular Pathways Potentially Involved in Hallucinatory Experiences During Sleep Paralysis: The Emerging Role of β-Arrestin-2. International Journal of Molecular Sciences. 2025; 26(15):7233. https://doi.org/10.3390/ijms26157233
Chicago/Turabian StyleRudy, Lena M., and Michał M. Godlewski. 2025. "Molecular Pathways Potentially Involved in Hallucinatory Experiences During Sleep Paralysis: The Emerging Role of β-Arrestin-2" International Journal of Molecular Sciences 26, no. 15: 7233. https://doi.org/10.3390/ijms26157233
APA StyleRudy, L. M., & Godlewski, M. M. (2025). Molecular Pathways Potentially Involved in Hallucinatory Experiences During Sleep Paralysis: The Emerging Role of β-Arrestin-2. International Journal of Molecular Sciences, 26(15), 7233. https://doi.org/10.3390/ijms26157233






