Choline—An Essential Nutrient with Health Benefits and a Signaling Molecule
Abstract
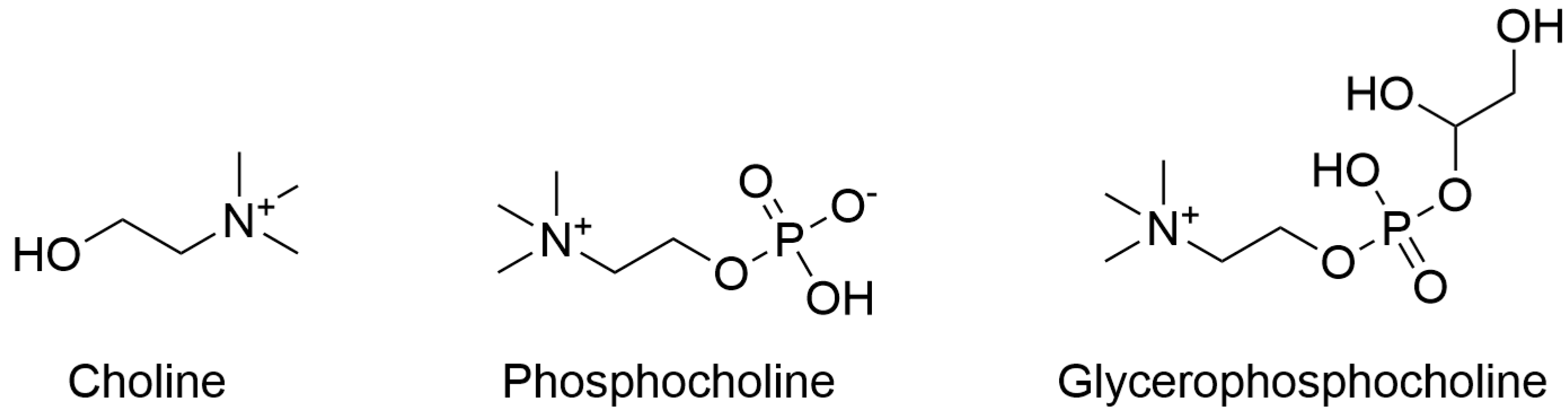
1. Introduction
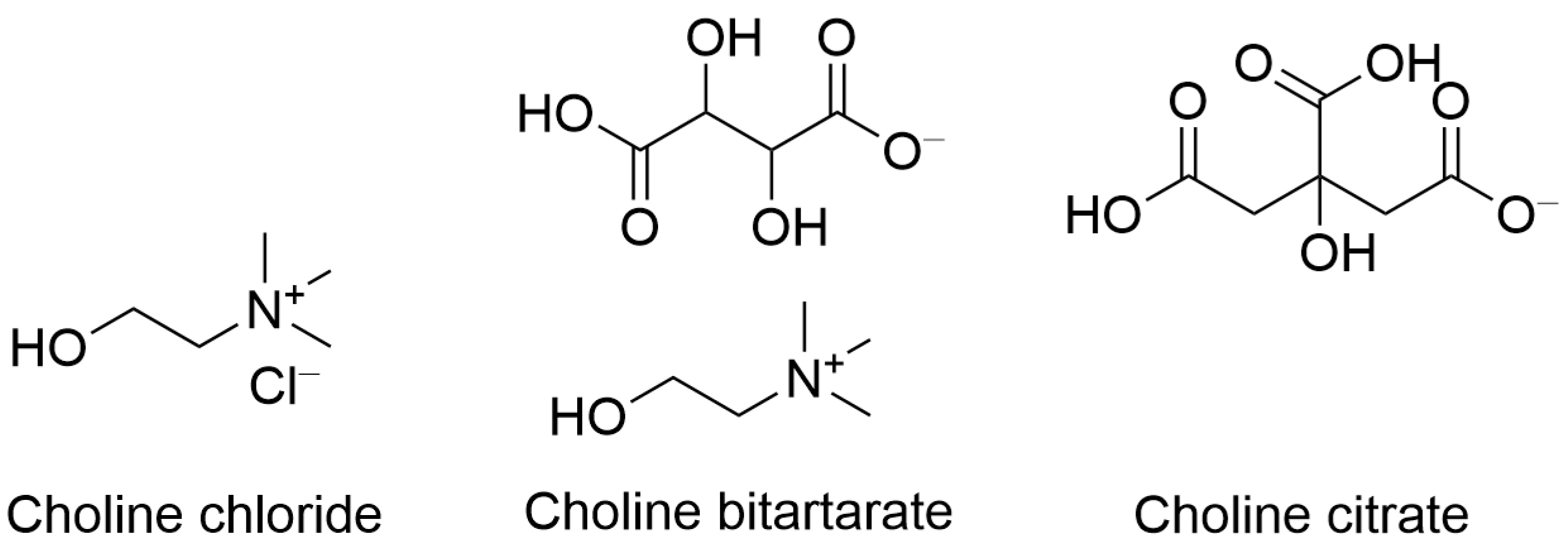
2. Choline Intake Recommendations and Dietary Sources
3. Mechanisms of Transport of Extracellular Choline
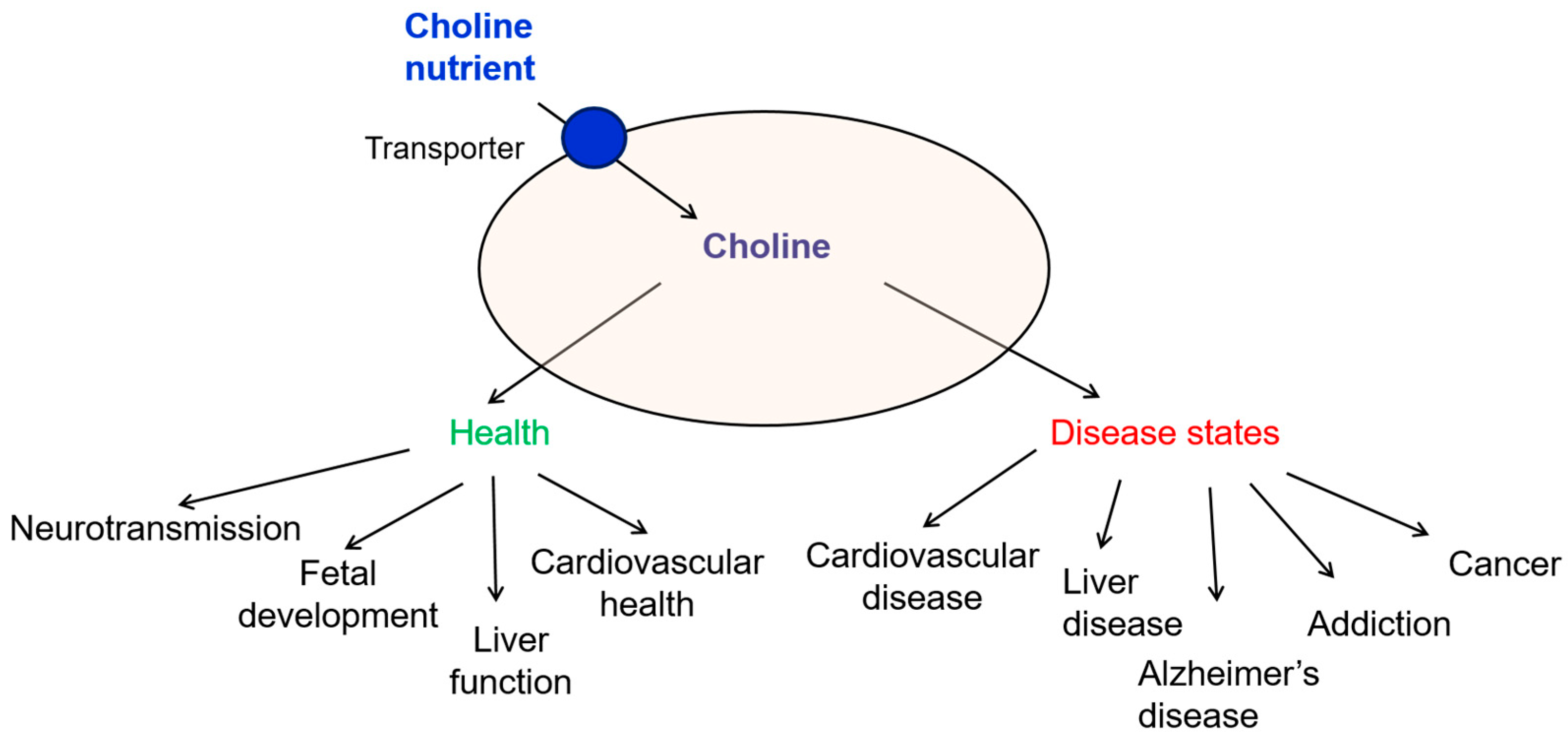
4. Biological Roles of Choline and Implications in Disease States
4.1. Choline and Fetal Development
4.2. Choline and Liver Function
4.3. Choline and Cardiovascular Health
4.4. Choline in Alzheimer’s Disease and Cognitive Decline
4.5. Choline and Addiction
4.6. Choline and Cancer
5. Detection of Choline in Biological Samples
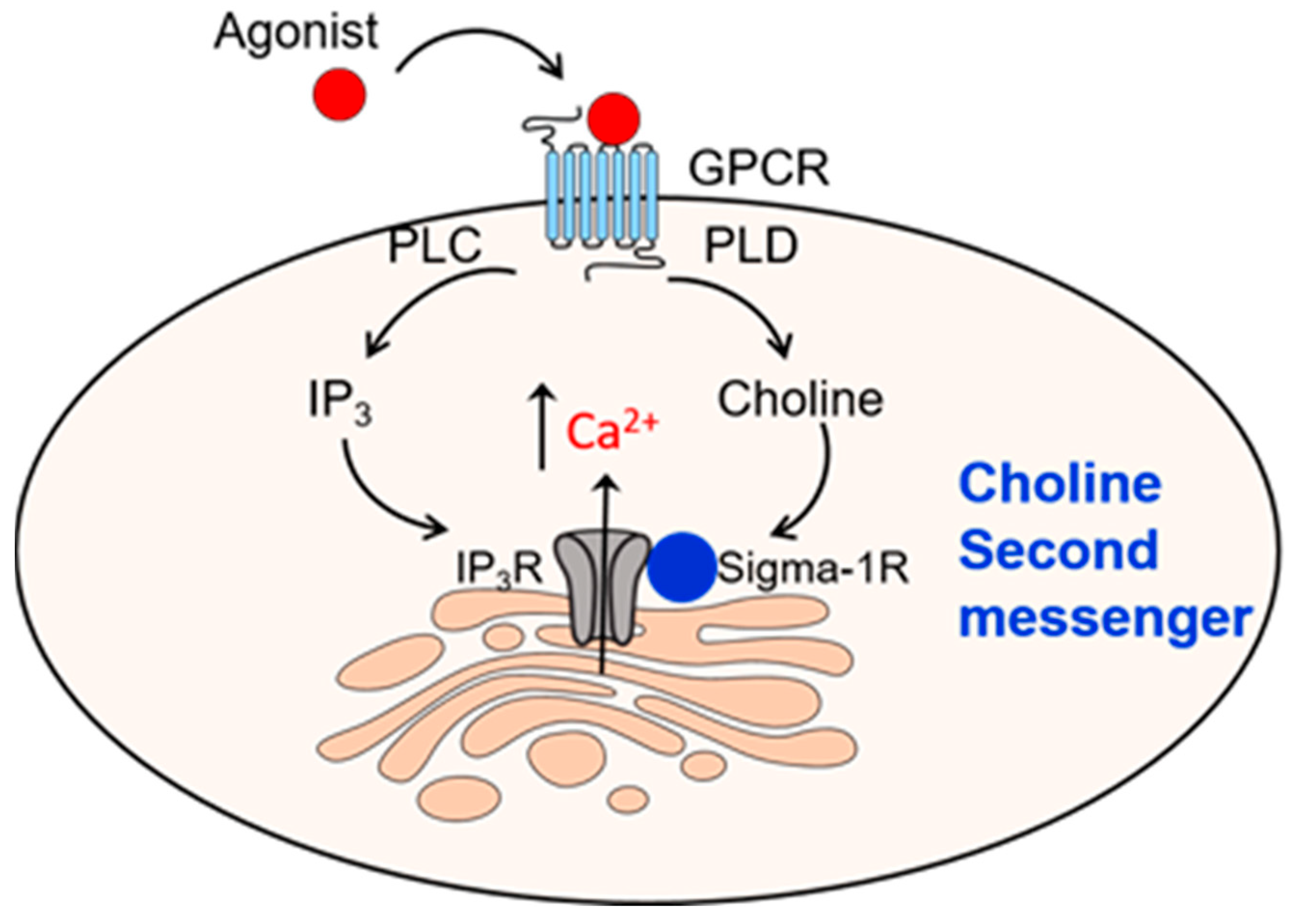
6. Second Messenger Role for Choline Acting on Sigma-1R
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AI | Adequate daily intake |
| CHT1 | High-affinity choline transporter |
| CTL | Choline transporter-like proteins |
| DAG | Diacyl glycerol |
| DHA | Docosahexaenoic acid |
| FASD | Fetal Alcohol Spectrum Disorder |
| GPCR | G protein-coupled receptor |
| IP3 | Inositol 1,4,5-trisphosphate |
| IP3R | Inositol 1,4,5-trisphosphate (IP3) receptor |
| PKC | Protein kinase C |
| PLC | Phospholipase C |
| PLD | Phospholipase D |
| VLDL | Very-low-density lipoprotein |
References
- InstituteMedicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Zeisel, S.H.; Da Costa, K.A.; Franklin, P.D.; Alexander, E.A.; Lamont, J.T.; Sheard, N.F.; Beiser, A. Choline, an essential nutrient for humans. FASEB J. 1991, 5, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Blusztajn, J.K. Choline and human nutrition. Annu. Rev. Nutr. 1994, 14, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Jieru, P.; Zhang, S.; Cai, L.; Long, W.; Wang, Y.; Zhang, L.; Dong, Y.; Zhang, W.; Liao, J.; Yang, C. Dietary choline intake and health outcomes in U.S. adults: Exploring the impact on cardiovascular disease, cancer prevalence, and all-cause mortality. J. Health Popul. Nutr. 2024, 43, 59. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Zeisel, S.H. Choline: The Neurocognitive Essential Nutrient of Interest to Obstetricians and Gynecologists. J. Diet. Suppl. 2020, 17, 733–752. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Klatt, K.C.; Caudill, M.A. Choline. Adv. Nutr. 2018, 9, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H.; Mar, M.H.; Howe, J.C.; Holden, J.M. Concentrations of choline-containing compounds and betaine in common foods. J. Nutr. 2003, 133, 1302–1307. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary Choline Intake: Current State of Knowledge Across the Life Cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Fulgoni, V.L., 3rd. Assessment of Total Choline Intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Bligh, J. The level of free choline in plasma. J. Physiol. 1952, 117, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Allen, D.D. The transport of choline. Drug Dev. Ind. Pharm. 2002, 28, 749–771. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.; Segrado, F.; Pasanisi, P.; Bruno, E.; Lopez, S.; Raspagliesi, F.; Bianchi, M.; Venturelli, E. Circulating choline and phosphocholine measurement by a hydrophilic interaction liquid chromatography-tandem mass spectrometry. Heliyon 2023, 9, e21921. [Google Scholar] [CrossRef] [PubMed]
- Haga, T. Molecular properties of the high-affinity choline transporter CHT1. J. Biochem. 2014, 156, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V. Choline transporters, cholinergic transmission and cognition. Nat. Rev. Neurosci. 2005, 6, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Black, S.A.; Prado, V.F.; Rylett, R.J.; Ferguson, S.S.; Prado, M.A. The “ins” and “outs” of the high-affinity choline transporter CHT1. J. Neurochem. 2006, 97, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ojiakor, O.A.; Rylett, R.J. Modulation of sodium-coupled choline transporter CHT function in health and disease. Neurochem. Int. 2020, 140, 104810. [Google Scholar] [CrossRef] [PubMed]
- Ri, K.; Weng, T.H.; Claveras Cabezudo, A.; Josting, W.; Zhang, Y.; Bazzone, A.; Leong, N.C.P.; Welsch, S.; Doty, R.T.; Gursu, G.; et al. Molecular mechanism of choline and ethanolamine transport in humans. Nature 2024, 630, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Cater, R.J.; Mukherjee, D.; Gil-Iturbe, E.; Erramilli, S.K.; Chen, T.; Koo, K.; Santander, N.; Reckers, A.; Kloss, B.; Gawda, T.; et al. Structural and molecular basis of choline uptake into the brain by FLVCR2. Nature 2024, 629, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.T.A.; Le, T.N.U.; Nguyen, T.Q.; Thi Thuy Ha, H.; Artati, A.; Leong, N.C.P.; Nguyen, D.T.; Lim, P.Y.; Susanto, A.V.; Huang, Q.; et al. MFSD7c functions as a transporter of choline at the blood-brain barrier. Cell Res. 2024, 34, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Scala, M.; Leong, N.C.P.; Le, T.N.U.; Zhang, Y.; Wu, Y.; Severino, M.; Madia, F.; Nosrati, M.S.S.; Dostmohammadi, A.; Capra, V.; et al. A hypomorphic FLVCR2 variant resulting in moderate transport deficiency causes hydranencephaly syndrome with brain calcifications. Eur. J. Hum. Genet. 2025. [Google Scholar] [CrossRef] [PubMed]
- Taesuwan, S.; McDougall, M.Q.; Malysheva, O.V.; Bender, E.; Nevins, J.E.H.; Devapatla, S.; Vidavalur, R.; Caudill, M.A.; Klatt, K.C. Choline metabolome response to prenatal choline supplementation across pregnancy: A randomized controlled trial. FASEB J. 2021, 35, e22063. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef] [PubMed]
- Bahnfleth, C.L.; Strupp, B.J.; Caudill, M.A.; Canfield, R.L. Prenatal choline supplementation improves child sustained attention: A 7-year follow-up of a randomized controlled feeding trial. FASEB J. 2022, 36, e22054. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Slack, B.E.; Mellott, T.J. Neuroprotective Actions of Dietary Choline. Nutrients 2017, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Cuenca, M.; Yepez Garcia, M.C.; Cortes Sanabria, L.Y.; Hernandez, P.; Ramirez, G.; Vasquez, M.; Sifontes, Y.; Gomez, G.; Liria-Dominguez, M.R.; Rigotti, A.; et al. Inadequate Intake of Choline and Essential Fatty Acids in Latin American Childbearing-Age Women as a Regional Pre-Conceptional Disadvantage: ELANS Results. Nutrients 2024, 16, 3150. [Google Scholar] [CrossRef] [PubMed]
- Obeid, R.; Schon, C.; Derbyshire, E.; Jiang, X.; Mellott, T.J.; Blusztajn, J.K.; Zeisel, S.H. A Narrative Review on Maternal Choline Intake and Liver Function of the Fetus and the Infant; Implications for Research, Policy, and Practice. Nutrients 2024, 16, 260. [Google Scholar] [CrossRef] [PubMed]
- Klatt, K.C.; McDougall, M.Q.; Malysheva, O.V.; Taesuwan, S.; Loinard-Gonzalez, A.A.P.; Nevins, J.E.H.; Beckman, K.; Bhawal, R.; Anderson, E.; Zhang, S.; et al. Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid (DHA) status among pregnant participants consuming supplemental DHA: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W.; Bockmann, K.; Maas, C.; Mathes, M.; Hovelmann, J.; Shunova, A.; Hund, V.; Schleicher, E.; Poets, C.F.; Franz, A.R. Combined choline and DHA supplementation: A randomized controlled trial. Eur. J. Nutr. 2020, 59, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Lo Van, A.; Bernoud-Hubac, N.; Lagarde, M. Esterification of Docosahexaenoic Acid Enhances Its Transport to the Brain and Its Potential Therapeutic Use in Brain Diseases. Nutrients 2022, 14, 4550. [Google Scholar] [CrossRef] [PubMed]
- Blusztajn, J.K.; Mellott, T.J. Choline nutrition programs brain development via DNA and histone methylation. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Paules, E.M.; Silva-Gomez, J.A.; Friday, W.B.; Zeisel, S.H.; Trujillo-Gonzalez, I. Choline Regulates SOX4 through miR-129-5p and Modifies H3K27me3 in the Developing Cortex. Nutrients 2023, 15, 2774. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, H.M.; Jiang, Y.N.; Wang, C.; Song, H.X.; Pan, Z.Y.; Fan, K.; Du, J.; Fan, Y.H.; Du, Z.M.; et al. Activation of cardiac M3 muscarinic acetylcholine receptors has cardioprotective effects against ischaemia-induced arrhythmias. Clin. Exp. Pharmacol. Physiol. 2012, 39, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, H.L.; Li, D.L.; Wang, L.Y.; Gao, Y.; Wang, Y.P.; Du, Z.M.; Lu, Y.J.; Yang, B.F. Choline produces antiarrhythmic actions in animal models by cardiac M3 receptors: Improvement of intracellular Ca2+ handling as a common mechanism. Can. J. Physiol. Pharmacol. 2008, 86, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, H.M.; Pan, Z.W.; Hang, P.Z.; Sun, L.H.; Jiang, Y.N.; Song, H.X.; Du, Z.M.; Liu, Y. Choline inhibits angiotensin II-induced cardiac hypertrophy by intracellular calcium signal and p38 MAPK pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Wu, J.; Wang, Y.; Zhu, W.; Zhang, Y.; Du, Z. Choline protects against cardiac hypertrophy induced by increased after-load. Int. J. Biol. Sci. 2013, 9, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, T.; Hang, P.; Li, W.; Guo, J.; Pan, Y.; Du, J.; Zheng, Y.; Du, Z. Choline Attenuates Cardiac Fibrosis by Inhibiting p38MAPK Signaling Possibly by Acting on M(3) Muscarinic Acetylcholine Receptor. Front. Pharmacol. 2019, 10, 1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lu, Y.; Bi, X.; Xu, M.; Yu, X.; Xue, R.; He, X.; Zang, W. Choline ameliorates cardiovascular damage by improving vagal activity and inhibiting the inflammatory response in spontaneously hypertensive rats. Sci. Rep. 2017, 7, 42553. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, S.; Meng, X.; Liu, H. A nutrient wide association study of cardiovascular disease prevalence in older adults from NHANES 2007 to 2018. Sci. Rep. 2025, 15, 12710. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J. How Anticholinergic Drugs Might Promote Alzheimer’s Disease: More Amyloid-beta and Less Phosphatidylcholine. J. Alzheimer’s Dis. 2015, 46, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Wurtman, R.J. Synapse formation in the brain can be enhanced by co-administering three specific nutrients. Eur. J. Pharmacol. 2017, 817, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Khachaturian, A.S.; Vergallo, A.; Farlow, M.R.; Snyder, P.J.; Giacobini, E.; Khachaturian, Z.S. Revisiting the Cholinergic Hypothesis in Alzheimer’s Disease: Emerging Evidence from Translational and Clinical Research. J. Prev. Alzheimer’s Dis. 2019, 6, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Ferreira, E.; Knowles, S.; Fux, C.; Rodin, A.; Winslow, W.; Oddo, S. Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell 2019, 18, e13037. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Ferreira, E.; Winslow, W.; Dave, N.; Piras, I.S.; Naymik, M.; Huentelman, M.J.; Tran, A.; Caccamo, A.; Oddo, S. Maternal choline supplementation ameliorates Alzheimer’s disease pathology by reducing brain homocysteine levels across multiple generations. Mol. Psychiatry 2019, 25, 2620–2629. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, R.; Ash, J.A.; Powers, B.E.; Kelley, C.M.; Strawderman, M.; Luscher, Z.I.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation improves spatial learning and adult hippocampal neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis. 2013, 58, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Powers, B.E.; Kelley, C.M.; Velazquez, R.; Ash, J.A.; Strawderman, M.S.; Alldred, M.J.; Ginsberg, S.D.; Mufson, E.J.; Strupp, B.J. Maternal choline supplementation in a mouse model of Down syndrome: Effects on attention and nucleus basalis/substantia innominata neuron morphology in adult offspring. Neuroscience 2017, 340, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Rahmanian, S.; Shapouri, M.; Mohammadian, M.K.; Mahmoudi, Z.; Saeedirad, Z.; Mobarakeh, K.A.; Parhiz, A.; Shekari, S.; Harsini, A.R.; Valisoltani, N.; et al. Does choline have an effect on Transient Global Amnesia (TGA)? BMC Neurosci. 2024, 25, 72. [Google Scholar] [CrossRef] [PubMed]
- Johns, B.E.; Ficken, M.; Engberg, M.E.; Wecker, L.; Philpot, R.M. Increasing dietary choline attenuates spatial memory deficits resulting from exposure to the chemotherapeutic agents cyclophosphamide and doxorubicin. J. Psychopharmacol. 2021, 35, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Baumel, B.S.; Doraiswamy, P.M.; Sabbagh, M.; Wurtman, R. Potential Neuroregenerative and Neuroprotective Effects of Uridine/Choline-Enriched Multinutrient Dietary Intervention for Mild Cognitive Impairment: A Narrative Review. Neurol. Ther. 2021, 10, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Virdee, M.S.; Eckerle, J.K.; Sandness, K.E.; Georgieff, M.K.; Boys, C.J.; Zeisel, S.H.; Wozniak, J.R. Polymorphisms in SLC44A1 are associated with cognitive improvement in children diagnosed with fetal alcohol spectrum disorder: An exploratory study of oral choline supplementation. Am. J. Clin. Nutr. 2021, 114, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Barr, J.L.; Zhao, P.; Brailoiu, G.C.; Brailoiu, E. Choline-Sigma-1R as an Additional Mechanism for Potentiation of Orexin by Cocaine. Int. J. Mol. Sci. 2021, 22, 5160. [Google Scholar] [CrossRef] [PubMed]
- de Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.; Gautvik, V.T.; Bartlett, F.S., 2nd; et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Mahler, S.V.; Smith, R.J.; Moorman, D.E.; Sartor, G.C.; Aston-Jones, G. Multiple roles for orexin/hypocretin in addiction. Prog. Brain Res. 2012, 198, 79–121. [Google Scholar] [CrossRef] [PubMed]
- Cheung, O.; Sanyal, A.J. Recent advances in nonalcoholic fatty liver disease. Curr. Opin. Gastroenterol. 2010, 26, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Li, X.; Ren, A.; Du, M.; Du, H.; Shu, Y.; Zhu, L.; Wang, W. Choline and betaine consumption lowers cancer risk: A meta-analysis of epidemiologic studies. Sci. Rep. 2016, 6, 35547. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gammon, M.D.; Zeisel, S.H.; Bradshaw, P.T.; Wetmur, J.G.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M.; Chen, J. High intakes of choline and betaine reduce breast cancer mortality in a population-based study. FASEB J. 2009, 23, 4022–4028. [Google Scholar] [CrossRef] [PubMed]
- Ibiebele, T.I.; Hughes, M.C.; Pandeya, N.; Zhao, Z.; Montgomery, G.; Hayward, N.; Green, A.C.; Whiteman, D.C.; Webb, P.M.; Study of Digestive, H.; et al. High intake of folate from food sources is associated with reduced risk of esophageal cancer in an Australian population. J. Nutr. 2011, 141, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.; Rahbar, M.H.; Hallman, D.M.; Hernandez, L.M.; Spitz, M.R.; Forman, M.R.; Gorlova, O.Y. Associations between dietary intake of choline and betaine and lung cancer risk. PLoS ONE 2013, 8, e54561. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.F.; Xu, C.H.; Liu, Y.T.; Fan, Y.Y.; Lin, X.L.; Lu, Y.K.; Zhang, C.X.; Chen, Y.M. Choline and betaine intakes are associated with reduced risk of nasopharyngeal carcinoma in adults: A case-control study. Br. J. Cancer 2014, 110, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Giovannucci, E.L.; Joh, H.K. Nutrients related to one-carbon metabolism and risk of renal cell cancer. Cancer Causes Control 2013, 24, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Hankinson, S.E.; Tworoger, S.S. Dietary betaine and choline intake are not associated with risk of epithelial ovarian cancer. Eur. J. Clin. Nutr. 2010, 64, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Li, W.; Xu, G.; Duan, N.; Yu, G.; Qu, J. Choline metabolism and its implications in cancer. Front. Oncol. 2023, 13, 1234887. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Bidulescu, A.; Barber, J.R.; Zeisel, S.H.; Joshu, C.E.; Prizment, A.E.; Vitolins, M.Z.; Platz, E.A. Dietary choline and betaine intakes and risk of total and lethal prostate cancer in the Atherosclerosis Risk in Communities (ARIC) Study. Cancer Causes Control 2019, 30, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Richman, E.L.; Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.L.; Zeisel, S.H.; Willett, W.C.; Chan, J.M. Choline intake and risk of lethal prostate cancer: Incidence and survival. Am. J. Clin. Nutr. 2012, 96, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Oyer, H.M.; Sanders, C.M.; Kim, F.J. Small-Molecule Modulators of Sigma1 and Sigma2/TMEM97 in the Context of Cancer: Foundational Concepts and Emerging Themes. Front. Pharmacol. 2019, 10, 1141. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Matich, E.K.; Laryea, J.; Hsu, P.C.; Su, L.J. A Case-Control Study of Dietary Choline Intake and Risk of Colorectal Cancer Modified by Dietary B-Vitamin Intake. Nutrients 2024, 16, 4200. [Google Scholar] [CrossRef] [PubMed]
- Potter, P.E.; Meek, J.L.; Neff, N.H. Acetylcholine and choline in neuronal tissue measured by HPLC with electrochemical detection. J. Neurochem. 1983, 41, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Webb, L.E.; Johnson, R.C. Choline in plasma measured by liquid-chromatography with electrochemical detection. Clin. Biochem. 1986, 19, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Koc, H.; Mar, M.H.; Ranasinghe, A.; Swenberg, J.A.; Zeisel, S.H. Quantitation of choline and its metabolites in tissues and foods by liquid chromatography/electrospray ionization-isotope dilution mass spectrometry. Anal. Chem. 2002, 74, 4734–4740. [Google Scholar] [CrossRef] [PubMed]
- Mimmi, M.C.; Picotti, P.; Corazza, A.; Betto, E.; Pucillo, C.E.; Cesaratto, L.; Cedolini, C.; Londero, V.; Zuiani, C.; Bazzocchi, M.; et al. High-performance metabolic marker assessment in breast cancer tissue by mass spectrometry. Clin. Chem. Lab. Med. 2011, 49, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhao, Y.Y.; Goruk, S.; Oilund, K.; Field, C.J.; Jacobs, R.L.; Curtis, J.M. Validation of an LC-MS/MS method for the quantification of choline-related compounds and phospholipids in foods and tissues. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 911, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Lamy, E.; Pilyser, L.; Paquet, C.; Bouaziz-Amar, E.; Grassin-Delyle, S. High-sensitivity quantification of acetylcholine and choline in human cerebrospinal fluid with a validated LC-MS/MS method. Talanta 2021, 224, 121881. [Google Scholar] [CrossRef] [PubMed]
- Hefni, M.E.; Bergstrom, M.; Lennqvist, T.; Fagerstrom, C.; Witthoft, C.M. Simultaneous quantification of trimethylamine N-oxide, trimethylamine, choline, betaine, creatinine, and propionyl-, acetyl-, and L-carnitine in clinical and food samples using HILIC-LC-MS. Anal. Bioanal. Chem. 2021, 413, 5349–5360. [Google Scholar] [CrossRef] [PubMed]
- Loening, N.M.; Chamberlin, A.M.; Zepeda, A.G.; Gonzalez, R.G.; Cheng, L.L. Quantification of phosphocholine and glycerophosphocholine with 31P edited 1H NMR spectroscopy. NMR Biomed. 2005, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Muelle, A.B.; Moreno, P.G.; Fernandez, I. Quantitative quadrupolar NMR (qQNMR) using nitrogen-14 for the determination of choline in complex matrixes. Talanta 2021, 230, 122344. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Shalaurova, I.; Matyus, S.P.; Wolak-Dinsmore, J.; Oskardmay, D.N.; Connelly, M.A. Quantification of choline in serum and plasma using a clinical nuclear magnetic resonance analyzer. Clin. Chim. Acta 2022, 524, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Achanta, P.S.; Niemitz, M.; Friesen, J.B.; Tadjimukhamedov, F.K.; Bzhelyansky, A.; Giancaspro, G.I.; Chen, S.N.; Pauli, G.F. Pharmaceutical analysis by NMR can accommodate strict impurity thresholds: The case of choline. J. Pharm. Biomed. Anal. 2022, 214, 114709. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, R.A.; Thomas, M.A.; De Feyter, H.M. Metabolism of Choline and Deuterated Choline Detected by (1)H-(14)N 2D Heteronuclear Single-Quantum Coherence (HSQC) NMR. Anal. Chem. 2025, 97, 6586–6593. [Google Scholar] [CrossRef] [PubMed]
- Exton, J.H. Phospholipase D. Ann. N. Y. Acad. Sci. 2000, 905, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Onono, F.O.; Morris, A.J. Phospholipase D and Choline Metabolism. Handb. Exp. Pharmacol. 2020, 259, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Chakraborty, S.; Brailoiu, G.C.; Zhao, P.; Barr, J.L.; Ilies, M.A.; Unterwald, E.M.; Abood, M.E.; Taylor, C.W. Choline Is an Intracellular Messenger Linking Extracellular Stimuli to IP3-Evoked Ca2+ Signals through Sigma-1 Receptors. Cell Rep. 2019, 26, 330–337.e4. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Lachance, V.; Belanger, S.M.; Hay, C.; Le Corvec, V.; Banouvong, V.; Lapalme, M.; Tarmoun, K.; Beaucaire, G.; Lussier, M.P.; Kourrich, S. Overview of Sigma-1R Subcellular Specific Biological Functions and Role in Neuroprotection. Int. J. Mol. Sci. 2023, 24, 1971. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Patel, C.; Shenkman, M.; Kessel, A.; Ben-Tal, N.; Lederkremer, G.Z. The Sigma-1 receptor is an ER-localized type II membrane protein. J. Biol. Chem. 2021, 297, 101299. [Google Scholar] [CrossRef] [PubMed]
- Zhemkov, V.; Ditlev, J.A.; Lee, W.R.; Wilson, M.; Liou, J.; Rosen, M.K.; Bezprozvanny, I. The role of sigma 1 receptor in organization of endoplasmic reticulum signaling microdomains. eLife 2021, 10, e65192. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.; Varrassi, G.; Coleman, M.; Breve, F.; Christo, D.K.; Christo, P.J.; Moussa, C. The Sigma Enigma: A Narrative Review of Sigma Receptors. Cureus 2023, 15, e35756. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.R.; Kruse, A.C. The Molecular Function of sigma Receptors: Past, Present, and Future. Trends Pharmacol. Sci. 2019, 40, 636–654. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Bowen, W.D.; Walker, F.O.; Matsumoto, R.R.; De Costa, B.; Rice, K.C. Sigma receptors: Biology and function. Pharmacol. Rev. 1990, 42, 355–402. [Google Scholar] [CrossRef] [PubMed]
- Maurice, T.; Su, T.P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, L.; Mirabile, S.; Gitto, R.; Cosentino, G.; Alcaro, S.; Dichiara, M.; Marrazzo, A.; Amata, E.; Ortuso, F.; De Luca, L. Exploring Structural Requirements for Sigma-1 Receptor Linear Ligands: Experimental and Computational Approaches. J. Chem. Inf. Model. 2024, 64, 5701–5711. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Satyshur, K.A.; Guo, L.W.; Ruoho, A.E. Sphingoid Bases Regulate the Sigma-1 Receptor-Sphingosine and N,N’-Dimethylsphingosine Are Endogenous Agonists. Int. J. Mol. Sci. 2023, 24, 3013. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Fasselt, B.; Rumenapp, U.; Bienek, C.; Wieland, T.; van Koppen, C.J.; Jakobs, K.H. Rapid and persistent desensitization of m3 muscarinic acetylcholine receptor-stimulated phospholipase D. Concomitant sensitization of phospholipase C. J. Biol. Chem. 1995, 270, 19949–19956. [Google Scholar] [CrossRef] [PubMed]
- Cockcroft, S. Signalling roles of mammalian phospholipase D1 and D2. Cell Mol. Life Sci. 2001, 58, 1674–1687. [Google Scholar] [CrossRef] [PubMed]
- Jantti, M.H.; Putula, J.; Somerharju, P.; Frohman, M.A.; Kukkonen, J.P. OX1 orexin/hypocretin receptor activation of phospholipase D. Br. J. Pharmacol. 2012, 165, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Meacci, E.; Nuti, F.; Catarzi, S.; Vasta, V.; Donati, C.; Bourgoin, S.; Bruni, P.; Moss, J.; Vaughan, M. Activation of phospholipase D by bradykinin and sphingosine 1-phosphate in A549 human lung adenocarcinoma cells via different GTP-binding proteins and protein kinase C delta signaling pathways. Biochemistry 2003, 42, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Oude Weernink, P.A.; Han, L.; Jakobs, K.H.; Schmidt, M. Dynamic phospholipid signaling by G protein-coupled receptors. Biochim. Biophys. Acta 2007, 1768, 888–900. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Altshuller, Y.M.; Kim, Y.; Han, J.M.; Ryu, S.H.; Morris, A.J.; Frohman, M.A. Dual requirement for rho and protein kinase C in direct activation of phospholipase D1 through G protein-coupled receptor signaling. Mol. Biol. Cell 2000, 11, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Balboa, M.A.; Insel, P.A. Stimulation of phospholipase D via alpha1-adrenergic receptors in Madin-Darby canine kidney cells is independent of PKCalpha and -epsilon activation. Mol. Pharmacol. 1998, 53, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, G.M.; Frohman, M.A. Phospholipase D: A lipid centric review. Cell Mol. Life Sci. 2005, 62, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.D.; Thompson, N.; Saqib, K.M.; Clark, J.M.; Powner, D.; Thompson, N.T.; Solari, R.; Wakelam, M.J. Phospholipase D1 localises to secretory granules and lysosomes and is plasma-membrane translocated on cellular stimulation. Curr. Biol. 1998, 8, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Huang, P.; Liang, B.T.; Frohman, M.A. Phospholipase D2 localizes to the plasma membrane and regulates angiotensin II receptor endocytosis. Mol. Biol. Cell 2004, 15, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.C.; Schweizer, M.; Jagdmann, S.; Bernreuther, C.; Reinheckel, T.; Saftig, P.; Damme, M. Unconventional Trafficking of Mammalian Phospholipase D3 to Lysosomes. Cell Rep. 2018, 22, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Dransfeld, U.E.; Ambaw, Y.A.; Lopez-Scarim, J.; Farese, R.V., Jr.; Walther, T.C. PLD3 and PLD4 synthesize S,S-BMP, a key phospholipid enabling lipid degradation in lysosomes. Cell 2024, 187, 6820–6834.e24. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, E.W. On the biological role of cyclic AMP. JAMA 1970, 214, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, E.W.; Robison, G.A. The role of cyclic-3′,5′-AMP in responses to catecholamines and other hormones. Pharmacol. Rev. 1966, 18, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Aley, P.K.; Singh, N.; Brailoiu, G.C.; Brailoiu, E.; Churchill, G.C. Nicotinic acid adenine dinucleotide phosphate (NAADP) is a second messenger in muscarinic receptor-induced contraction of guinea pig trachea. J. Biol. Chem. 2013, 288, 10986–10993. [Google Scholar] [CrossRef] [PubMed]
- Son, J.S.; Kwon, Y.B. Sigma-1 Receptor Antagonist BD1047 Reduces Allodynia and Spinal ERK Phosphorylation Following Chronic Compression of Dorsal Root Ganglion in Rats. Korean J. Physiol. Pharmacol. 2010, 14, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Sak, K.; Samuel, K.; Kelve, M.; Webb, T.E. Pharmacological characterisation of pyrimidinoceptor responses in NG108-15 cells. Eur. J. Pharmacol. 2001, 415, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Maurice, T.; Su, T.P. Ca2+ signaling via sigma(1)-receptors: Novel regulatory mechanism affecting intracellular Ca2+ concentration. J. Pharmacol. Exp. Ther. 2000, 293, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Nuwayhid, S.J.; Werling, L.L. Modulation of bradykinin-induced calcium changes in SH-SY5Y cells by neurosteroids and sigma receptor ligands via a shared mechanism. Synapse 2004, 54, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bowen, W.D. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: Constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 2008, 283, 28198–28215. [Google Scholar] [CrossRef] [PubMed]
- Stricker, H.M.; Rommerswinkel, N.; Keil, S.; Gnoth, S.A.; Niggemann, B.; Dittmar, T. The phospholipase D inhibitor FIPI potently blocks EGF-induced calcium signaling in human breast cancer cells. Cell Commun. Signal 2021, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Vance, D.E. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- de Costa, B.R.; Bowen, W.D.; Hellewell, S.B.; Walker, J.M.; Thurkauf, A.; Jacobson, A.E.; Rice, K.C. Synthesis and evaluation of optically pure [3H]-(+)-pentazocine, a highly potent and selective radioligand for sigma receptors. FEBS Lett. 1989, 251, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xiao, Y.; Zhou, X.; Sun, Z. Insight into binding of endogenous neurosteroid ligands to the sigma-1 receptor. Nat. Commun. 2024, 15, 5619. [Google Scholar] [CrossRef] [PubMed]
- Morato, X.; Fernandez-Duenas, V.; Perez-Villamor, P.; Valle-Leon, M.; Vela, J.M.; Merlos, M.; Burgueno, J.; Ciruela, F. Development of a Novel sigma(1) Receptor Biosensor Based on Its Heterodimerization with Binding Immunoglobulin Protein in Living Cells. ACS Chem. Neurosci. 2023, 14, 2201–2207. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.P.; Navon-Perry, L.; Cruz-Herranz, A.; Chen, K.; Hecker-Barth, G.; Spiegel, K.; Cohen, Y.; Niethammer, M.; Tan, A.M.; Schuring, H.; et al. The Safety Profile of Pridopidine, a Novel Sigma-1 Receptor Agonist for the Treatment of Huntington’s Disease. CNS Drugs 2025, 39, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Djebari, S.; Jimenez-Herrera, R.; Iborra-Lazaro, G.; Jimenez-Diaz, L.; Navarro-Lopez, J.D. Social and contextual memory impairments induced by Amyloid-beta oligomers are rescued by Sigma-1 receptor activation. Biomed. Pharmacother. 2025, 184, 117914. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Orozco, H.; Bencomo-Martinez, A.; Maya-Arteaga, J.P.; Rubio-De Anda, P.F.; Sanabria-Romero, F.; Casas, Z.G.M.; Rodriguez-Vargas, I.; Hernandez-Puga, A.G.; Sablon-Carrazana, M.; Menendez-Soto Del Valle, R.; et al. CNEURO-201, an Anti-amyloidogenic Agent and sigma1-Receptor Agonist, Improves Cognition in the 3xTg Mouse Model of Alzheimer’s Disease by Multiple Actions in the Pathology. Int. J. Mol. Sci. 2025, 26, 1301. [Google Scholar] [CrossRef] [PubMed]
- Sweed, E.; Khodir, S.A.; Motawea, S.M.; El-Haron, H.; Mostafa, B.A.; Elkholy, M.S.; Salim, M.; Shebl, D.Z.M. Targeting the sigma-1 receptor with pridopidine induces functional neurorestoration in spinal cord ischemia-reperfusion injury. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 9307–9321. [Google Scholar] [CrossRef] [PubMed]
- Shokr, M.M.; Badawi, G.A.; Elshazly, S.M.; Zaki, H.F.; Mohamed, A.F. Sigma 1 Receptor and Its Pivotal Role in Neurological Disorders. ACS Pharmacol. Transl. Sci. 2025, 8, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.; Fattakhov, N.; Toborek, M. Sigma-1 receptor signaling: A potential therapeutic approach for ischemic stroke. J. Cereb. Blood Flow. Metab. 2024, 44, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Lei, Z.G.; Chu, K.; Lam, O.J.H.; Chiang, C.Y.; Zhang, Z.J. N, N-Dimethyltryptamine, a natural hallucinogen, ameliorates Alzheimer’s disease by restoring neuronal Sigma-1 receptor-mediated endoplasmic reticulum-mitochondria crosstalk. Alzheimers Res. Ther. 2024, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Piechal, A.; Jakimiuk, A.; Mirowska-Guzel, D. Sigma receptors and neurological disorders. Pharmacol. Rep. 2021, 73, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jia, H. The Sigma Receptors in Alzheimer’s Disease: New Potential Targets for Diagnosis and Therapy. Int. J. Mol. Sci. 2023, 24, 2025. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.; Bhatt, L.K. Targeting Sigma-1 Receptor: A Promising Strategy in the Treatment of Parkinson’s Disease. Neurochem. Res. 2023, 48, 2925–2935. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.H.; Ye, Y.; Wan, B.B.; Yu, Y.D.; Liu, C.; Chen, Q.J. Emerging Benefits: Pathophysiological Functions and Target Drugs of the Sigma-1 Receptor in Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 5649–5666. [Google Scholar] [CrossRef] [PubMed]
- Zhemkov, V.; Geva, M.; Hayden, M.R.; Bezprozvanny, I. Sigma-1 Receptor (S1R) Interaction with Cholesterol: Mechanisms of S1R Activation and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 4082. [Google Scholar] [CrossRef] [PubMed]
- Salaciak, K.; Pytka, K. Revisiting the sigma-1 receptor as a biological target to treat affective and cognitive disorders. Neurosci. Biobehav. Rev. 2022, 132, 1114–1136. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Wang, J.Y.; Xu, M.J.; Chen, H.L.; Duan, J.Y.; Li, Y.F. Sigma-1 receptor activation produces faster antidepressant-like effect through enhancement of hippocampal neuroplasticity: Focus on sigma-1-5-HT1A heteroreceptor complex. Neurochem. Int. 2025, 184, 105937. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Cao, S.; Shi, L.; Zhang, Y.; Wang, X.; Yuan, S.; Shi, Y.; Wang, B.; Liu, J.; Han, C.J. Pharmacological modulation of Sigma-1 receptor ameliorates pathological neuroinflammation in rats with diabetic neuropathic pain via the AKT/GSK-3beta/NF-kappaB pathway. Brain Res. Bull. 2025, 221, 111226. [Google Scholar] [CrossRef] [PubMed]
- Galvez, R.; Mayoral, V.; Cebrecos, J.; Medel, F.J.; Morte, A.; Sust, M.; Vaque, A.; Montes-Perez, A.; Neira-Reina, F.; Canovas, L.; et al. E-52862-A selective sigma-1 receptor antagonist, in peripheral neuropathic pain: Two randomized, double-blind, phase 2 studies in patients with chronic postsurgical pain and painful diabetic neuropathy. Eur. J. Pain. 2025, 29, e4755. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, A.H.; Wei, L.; Welsh, W.J. Preclinical Evaluation of Sigma 1 Receptor Antagonists as a Novel Treatment for Painful Diabetic Neuropathy. ACS Pharmacol. Transl. Sci. 2024, 7, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Almaamari, A.; Sultan, M.; Zhang, T.; Qaed, E.; Wu, S.; Qiao, R.; Duan, Y.; Ding, S.; Liu, G.; Su, S. Sigma-1 Receptor Specific Biological Functions, Protective Role, and Therapeutic Potential in Cardiovascular Diseases. Cardiovasc. Toxicol. 2025, 25, 614–630. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Q.; Yang, J.Z.; Li, X.W.; Chen, L.J.; Zhang, K.K.; Liu, J.L.; Li, J.H.; Hsu, C.; Chen, L.; et al. Multi-Omics Analysis Reveals the Role of Sigma-1 Receptor in a Takotsubo-like Cardiomyopathy Model. Biomedicines 2023, 11, 2766. [Google Scholar] [CrossRef] [PubMed]
- Munguia-Galaviz, F.J.; Miranda-Diaz, A.G.; Cardenas-Sosa, M.A.; Echavarria, R. Sigma-1 Receptor Signaling: In Search of New Therapeutic Alternatives for Cardiovascular and Renal Diseases. Int. J. Mol. Sci. 2023, 24, 1997. [Google Scholar] [CrossRef] [PubMed]
- Balogh, D.B.; Hodrea, J.; Saeed, A.; Cserhalmi, M.; Rozsahegyi, A.; Lakat, T.; Lenart, L.; Szabo, A.J.; Wagner, L.J.; Fekete, A. Sigma-1 Receptor as a Novel Therapeutic Target in Diabetic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 3327. [Google Scholar] [CrossRef] [PubMed]
- Leotta, C.G.; Barbaraci, C.; Fiorito, J.; Coco, A.; di Giacomo, V.; Amata, E.; Marrazzo, A.; Pitari, G.M. HDAC/sigma1R Dual-Ligand as a Targeted Melanoma Therapeutic. Pharmaceuticals 2025, 18, 179. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.D.; Longen, C.G.; Oyer, H.M.; Chen, N.; Maher, C.M.; Salvino, J.M.; Kania, B.; Anderson, K.N.; Ostrander, W.F.; Knudsen, K.E.; et al. Sigma1 Targeting to Suppress Aberrant Androgen Receptor Signaling in Prostate Cancer. Cancer Res. 2017, 77, 2439–2452. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.S.; Osman, M.A. An Emerging Role for Sigma Receptor 1 in Personalized Treatment of Breast Cancer. Cancers 2023, 15, 3464. [Google Scholar] [CrossRef] [PubMed]
- Knowles, L.G.; Armanious, A.J.; Peng, Y.; Welsh, W.J.; James, M.H. Recent advances in drug discovery efforts targeting the sigma 1 receptor system: Implications for novel medications designed to reduce excessive drug and food seeking. Addict. Neurosci. 2023, 8, 100126. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Dryanovski, D.I.; Kimura, Y.; Jackson, S.N.; Woods, A.S.; Yasui, Y.; Tsai, S.Y.; Patel, S.; Covey, D.P.; Su, T.P.; et al. Cocaine-induced endocannabinoid signaling mediated by sigma-1 receptors and extracellular vesicle secretion. eLife 2019, 8, e47209. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Barr, J.L.; Wittorf, H.N.; Inan, S.; Unterwald, E.M.; Brailoiu, G.C. Modulation of the Blood-Brain Barrier by Sigma-1R Activation. Int. J. Mol. Sci. 2024, 25, 5147. [Google Scholar] [CrossRef] [PubMed]
- Nair, I.M.; Condon, E.; Prestwich, B.D.; Mackrill, J.J. Myo-D-inositol Trisphosphate Signalling in Oomycetes. Microorganisms 2022, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Damborsky, J.C.; Wilson, B.C.; Fannin, R.D.; Ward, J.M.; Gerrish, K.E.; He, B.; Martin, N.P.; Yakel, J.L. alpha7 nicotinic receptor activation mitigates herpes simplex virus type 1 infection in microglia cells. Antivir. Res. 2024, 228, 105934. [Google Scholar] [CrossRef] [PubMed]
- Bertino, F.; Mukherjee, D.; Bonora, M.; Bagowski, C.; Nardelli, J.; Metani, L.; Zanin Venturini, D.I.; Chianese, D.; Santander, N.; Salaroglio, I.C.; et al. Dysregulation of FLVCR1a-dependent mitochondrial calcium handling in neural progenitors causes congenital hydrocephalus. Cell Rep. Med. 2024, 5, 101647. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Lee, H.; Lee, H.J.; Kim, K.; Choi, J.; Han, J.M.; Min, D.S. PLD1 is a key player in cancer stemness and chemoresistance: Therapeutic targeting of cross-talk between the PI3K/Akt and Wnt/beta-catenin pathways. Exp. Mol. Med. 2024, 56, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burns, B.C.; Belani, J.D.; Wittorf, H.N.; Brailoiu, E.; Brailoiu, G.C. Choline—An Essential Nutrient with Health Benefits and a Signaling Molecule. Int. J. Mol. Sci. 2025, 26, 7159. https://doi.org/10.3390/ijms26157159
Burns BC, Belani JD, Wittorf HN, Brailoiu E, Brailoiu GC. Choline—An Essential Nutrient with Health Benefits and a Signaling Molecule. International Journal of Molecular Sciences. 2025; 26(15):7159. https://doi.org/10.3390/ijms26157159
Chicago/Turabian StyleBurns, Brianne C., Jitendra D. Belani, Hailey N. Wittorf, Eugen Brailoiu, and Gabriela C. Brailoiu. 2025. "Choline—An Essential Nutrient with Health Benefits and a Signaling Molecule" International Journal of Molecular Sciences 26, no. 15: 7159. https://doi.org/10.3390/ijms26157159
APA StyleBurns, B. C., Belani, J. D., Wittorf, H. N., Brailoiu, E., & Brailoiu, G. C. (2025). Choline—An Essential Nutrient with Health Benefits and a Signaling Molecule. International Journal of Molecular Sciences, 26(15), 7159. https://doi.org/10.3390/ijms26157159




