Elevated Plasma BDNF in Early Primary Biliary Cholangitis: Associations with Liver Fibrosis, IL-6, IL-18, Fatigue, and Cognitive Impairment
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Brain-Derived Neurotrophic Factor (BDNF) Level
2.3. Plasma BDNF Levels in Relation to Liver Stiffness
2.4. Association of Plasma BDNF Levels with Inflammatory and Hepatic Parameters in Multivariate Analysis
2.5. Association Between BDNF Levels, Cognitive Performance, and Fatigue
3. Discussion
4. Methods and Materials
4.1. Brain-Derived Neurotrophic Factor Measurement
4.2. Interleukin-6 (Il-6) Measurement
4.3. Interleukin-18 (IL-18) Measurement
4.4. Liver Ultrasound and Elastography
4.5. Non-Invasive Markers of Liver Fibrosis
4.6. Neuropsychological and Fatigue Assessment
4.7. Statistical Analysis
4.8. Ethical Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaplan, M.M.; Gershwin, M.E. Primary biliary cirrhosis. N. Engl. J. Med. 2005, 353, 1261–1273. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; Leighton, J.; Lee Wong, L.; Jones, D.E. Symptoms of PBC—Pathophysiology and management. Best Pract. Res. Clin. Gastroenterol. 2018, 34–35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Pelszyńska, M.; Prokopowicz, D.; Rogalska, M.; Grygoruk, U. High concentration of antimitochondrial antibodies predicts progressive primary biliary cirrhosis. World J. Gastroenterol. 2005, 11, 5706–5709. [Google Scholar] [CrossRef] [PubMed]
- Phaw, N.A.; Dyson, J.K.; Mells, G.; Jones, D. Understanding fatigue in primary biliary cholangitis. Dig. Dis. Sci. 2021, 66, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Jopson, L.; Dyson, J.K.; Jones, D.E. Understanding and treating fatigue in primary biliary cirrhosis and primary sclerosing cholangitis. Clin. Liver Dis. 2016, 20, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Kremer, A.E.; Swain, M.G.; Jones, D.; Bowlus, C.; Trauner, M.; Henry, L.; Gerber, L. Assessment of fatigue and its impact in chronic liver disease. J. Hepatol. 2024, 81, 726–742. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowska-Charkiewicz, D.; Rogalski, P.; Janica, J.; Rogalska, M.; Aleksiejuk, E.; Antonowicz, S.; Dabrowski, A.; Daniluk, J. Minimal hepatic encephalopathy may be present despite the absence of non-invasive and elastography evidence of cirrhosis in patients with primary biliary cholangitis. Adv. Med. Sci. 2021, 66, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, K.G.; Jones, D.E.; Taylor, R.; Frith, J.; Blamire, A.M.; Newton, J.L. Impaired cerebral autoregulation in primary biliary cirrhosis: Implications for the pathogenesis of cognitive decline. Liver Int. 2010, 30, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.L.; Hollingsworth, K.G.; Taylor, R.; El-Sharkawy, A.M.; Khan, Z.U.; Pearce, R.; Sutcliffe, K.; Okonkwo, O.; Davidson, A.; Burt, J.; et al. Cognitive impairment in primary biliary cirrhosis: Symptom impact and potential etiology. Hepatology 2008, 48, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Bufton, S.; Monaco, A.; Griffiths, L.; Jones, D.E.; Neuberger, J.M. The effect of liver transplantation on fatigue in patients with primary biliary cirrhosis: A prospective study. J. Hepatol. 2013, 59, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, K.G.; Newton, J.L.; Taylor, R.; McDonald, C.; Palmer, J.M.; Blamire, A.M.; Jones, D.E.J. Pilot study of peripheral muscle function in primary biliary cirrhosis: Potential implications for fatigue pathogenesis. Clin. Gastroenterol. Hepatol. 2008, 6, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Newton, J.; Lai, H.M.; Baker, S.N.; Jones, D.E. Central nervous system dysfunction in primary biliary cirrhosis and its relationship to symptoms. J. Hepatol. 2010, 53, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Mosher, V.A.L.; Swain, M.G.; Pang, J.X.Q.; Kaplan, G.G.; Sharkey, K.A.; MacQueen, G.M.; Goodyea, B.G. Primary biliary cholangitis alters functional connections of the brain’s deep gray matter. Clin. Transl. Gastroenterol. 2017, 8, e107. [Google Scholar] [CrossRef] [PubMed]
- Zenouzi, R.; von der Gablentz, J.; Heldmann, M.; Göttlich, M.; Weiler-Normann, C.; Sebode, M.; Ehlken, H.; Hartl, J.; Fellbrich, A.; Siemonsen, S.; et al. Patients with primary biliary cholangitis and fatigue present with depressive symptoms and selected cognitive deficits, but with normal attention performance and brain structure. PLoS ONE 2018, 13, e0190005. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.C.; Zimbone, S.; Saab, M.W.; Tomasello, M.F. The pleiotropic potential of BDNF beyond neurons: Implication for a healthy mind in a healthy body. Life 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Podyma, B.; Parekh, K.; Güler, A.D.; Deppmann, C.D. Metabolic homeostasis via BDNF and its receptors. Trends Endocrinol. Metab. 2021, 32, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, P.; Ng, C.F.; Pang, B.P.S.; Chan, W.S.; Tse, M.C.L.; Bi, X.; Kwan, H.L.R.; Brobst, D.; Herlea-Pana, O.; Yang, X.; et al. Muscle-generated BDNF (brain derived neurotrophic factor) maintains mitochondrial quality control in female mice. Autophagy 2022, 18, 1367–1384. [Google Scholar] [CrossRef] [PubMed]
- Matthews, V.B.; Aström, M.B.; Chan, M.H.; Bruce, C.R.; Krabbe, K.S.; Prelovsek, O.; Åkerström, T.; Yfanti, C.; Broholm, C.; Mortensen, O.H.; et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009, 52, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Yamada, H.; Munetsuna, E.; Ando, Y.; Mizuno, G.; Fujii, R.; Tsuboi, Y.; Ichino, N.; Osakabe, K.; Sugimoto, K.; et al. Increased brain-derived neurotrophic factor in the serum of persons with nonalcoholic fatty liver disease. Endocr. J. 2022, 69, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.T.; Liu, X.L.; Yang, G.Y.; Zhang, W.; Tao, L.; Ma, W.T.; Wu, L.; Liu, C. Neurotrophic factors stimulate the activation of hepatic stellate cells in liver fibrosis. Biochem. Biophys. Res. Commun. 2022, 630, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Stawicka, A.; Świderska, M.; Zbrzeźniak, J.; Sołowianowicz, N.; Woszczenko, A.; Flisiak, R.; Jaroszewicz, J. Brain-derived neurotrophic factor as a potential diagnostic marker in minimal hepatic encephalopathy. Clin. Exp. Hepatol. 2021, 7, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Montoliu, C.; Piedrafita, B.; Serra, M.A.; del Olmo, J.A.; Urios, A.; Rodrigo, J.M.; Felipo, V. IL-6 and IL-18 in blood may discriminate cirrhotic patients with and without minimal hepatic encephalopathy. J. Clin. Gastroenterol. 2009, 43, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kermani, P.; Rafii, D.; Jin, D.K.; Whitlock, P.; Schaffer, W.; Chiang, A.; Vincent, L.; Friedrich, M.; Shido, K.; Hackett, N.R.; et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. J. Clin. Investig. 2005, 115, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Harvey, T.; Rios, M. The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update. Biomolecules 2024, 14, 424. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakahashi, T.; Kambayashi, J.I.; Sun, B.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Bouhaddou, N.; Mabrouk, M.; Atifi, F.; Bouyahya, A.; Zaid, Y. The link between BDNF and platelets in neurological disorders. Heliyon 2024, 10, e39278. [Google Scholar] [CrossRef] [PubMed]
- Rogalski, P.; Bogdanowska-Charkiewicz, D.; Rogalska-Plonska, M.; Lukaszewicz-Zajac, M.; Kostecka-Roslen, I.; Mroczko, B.; Dabrowska, M.; Wasielica-Berger, J.; Aleksiejuk, E.; Antonowicz, S.; et al. Elevated levels of soluble glycoprotein V—The plasma marker of platelet activation by thrombin in patients with early stage primary biliary cholangitis (PBC). Adv. Med. Sci. 2023, 68, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, E.; Dorofeikova, M.; Tsyrenova, K.; Petrova, N. A Cross-Sectional Study on Associations Between BDNF, CRP, IL-6 and Clinical Symptoms, Cognitive and Personal Performance in Patients With Paranoid Schizophrenia. Front. Psychiatry 2022, 13, 943869. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Kang, H.J.; Kim, J.W.; Kim, H.K.; Kang, H.C.; Kim, S.W.; Kim, J.C.; Ahn, Y.; Jeong, M.H.; Kim, J.M. Modifying Effect of the Interleukin-18 Level on the Association between BDNF Methylation and Long-Term Cardiovascular Outcomes in Patients with Acute Coronary Syndrome. Int. J. Mol. Sci. 2022, 23, 15270. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Koyama, Y.; Shimada, S. Inflammation From Peripheral Organs to the Brain: How Does Systemic Inflammation Cause Neuroinflammation? Front. Aging Neurosci. 2022, 14, 903455. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Altarejos, P.; Martínez-García, M.; Felipo, V. Extracellular Vesicles From Hyperammonemic Rats Induce Neuroinflammation in Cerebellum of Normal Rats: Role of Increased TNFα Content. Front. Immunol. 2022, 13, 921947. [Google Scholar] [CrossRef] [PubMed]
- Arenas, Y.M.; López-Gramaje, A.; Montoliu, C.; Llansola, M.; Felipo, V. Increased levels and activation of the IL-17 receptor in microglia contribute to enhanced neuroinflammation in cerebellum of hyperammonemic rats. Biol. Res. 2024, 57, 18. [Google Scholar] [CrossRef] [PubMed]
- Arenas, Y.M.; Martínez-García, M.; Llansola, M.; Felipo, V. Enhanced BDNF and TrkB Activation Enhance GABA Neurotransmission in Cerebellum in Hyperammonemia. Int. J. Mol. Sci. 2022, 23, 11770. [Google Scholar] [CrossRef] [PubMed]
- Arenas, Y.M.; Balzano, T.; Ivaylova, G.; Llansola, M.; Felipo, V. The S1PR2-CCL2-BDNF-TrkB pathway mediates neuroinflammation and motor incoordination in hyperammonaemia. Neuropathol. Appl. Neurobiol. 2022, 48, e12799. [Google Scholar] [CrossRef] [PubMed]
- Arenas, Y.M.; Felipo, V. Sustained Hyperammonemia Activates NF-κB in Purkinje Neurons Through Activation of the TrkB-PI3K-AKT Pathway by Microglia-Derived BDNF in a Rat Model of Minimal Hepatic Encephalopathy. Mol. Neurobiol. 2023, 60, 3071–3085. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Mare, R.; Sporea, I.; Lupuşoru, R.; Şirli, R.; Popescu, A.; Danila, M.; Pienar, C. The value of ElastPQ for the evaluation of liver stiffness in patients with B and C chronic hepatopathies. Ultrasonics 2017, 77, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Wai, C.T.; Greenson, J.K.; Fontana, R.J.; Kalbfleisch, J.D.; Marrero, J.A.; Conjeevaram, H.S.; Lok, A.S.F. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003, 38, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Weissenborn, K. Diagnosis of minimal hepatic encephalopathy. J. Clin. Exp. Hepatol. 2015, 5, S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Fisk, J.D.; Ritvo, P.G.; Ross, L.; Haase, D.A.; Marrie, T.J.; Schlech, W.F. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin. Infect. Dis. 1994, 18, S79–S83. [Google Scholar] [CrossRef] [PubMed]
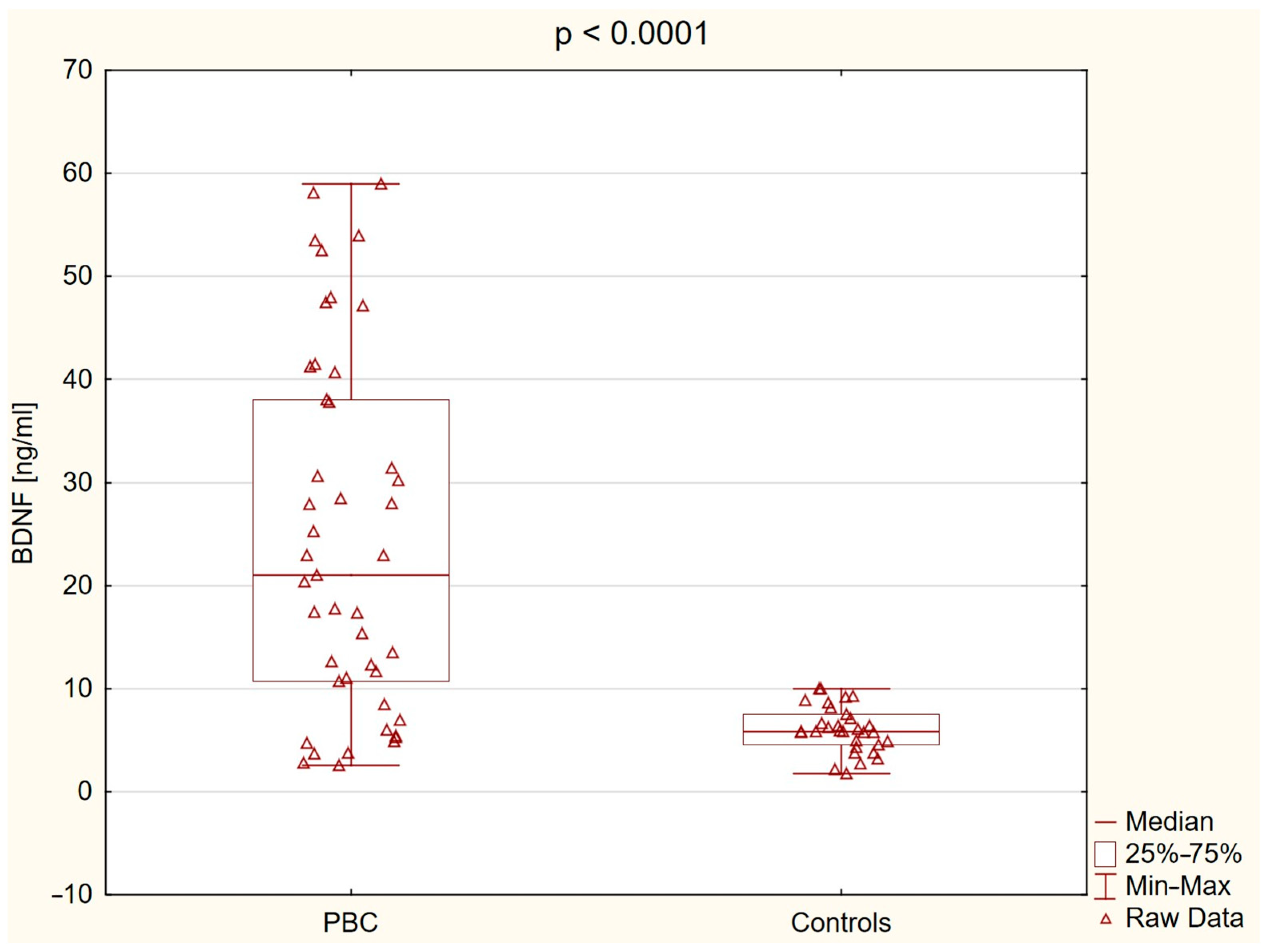
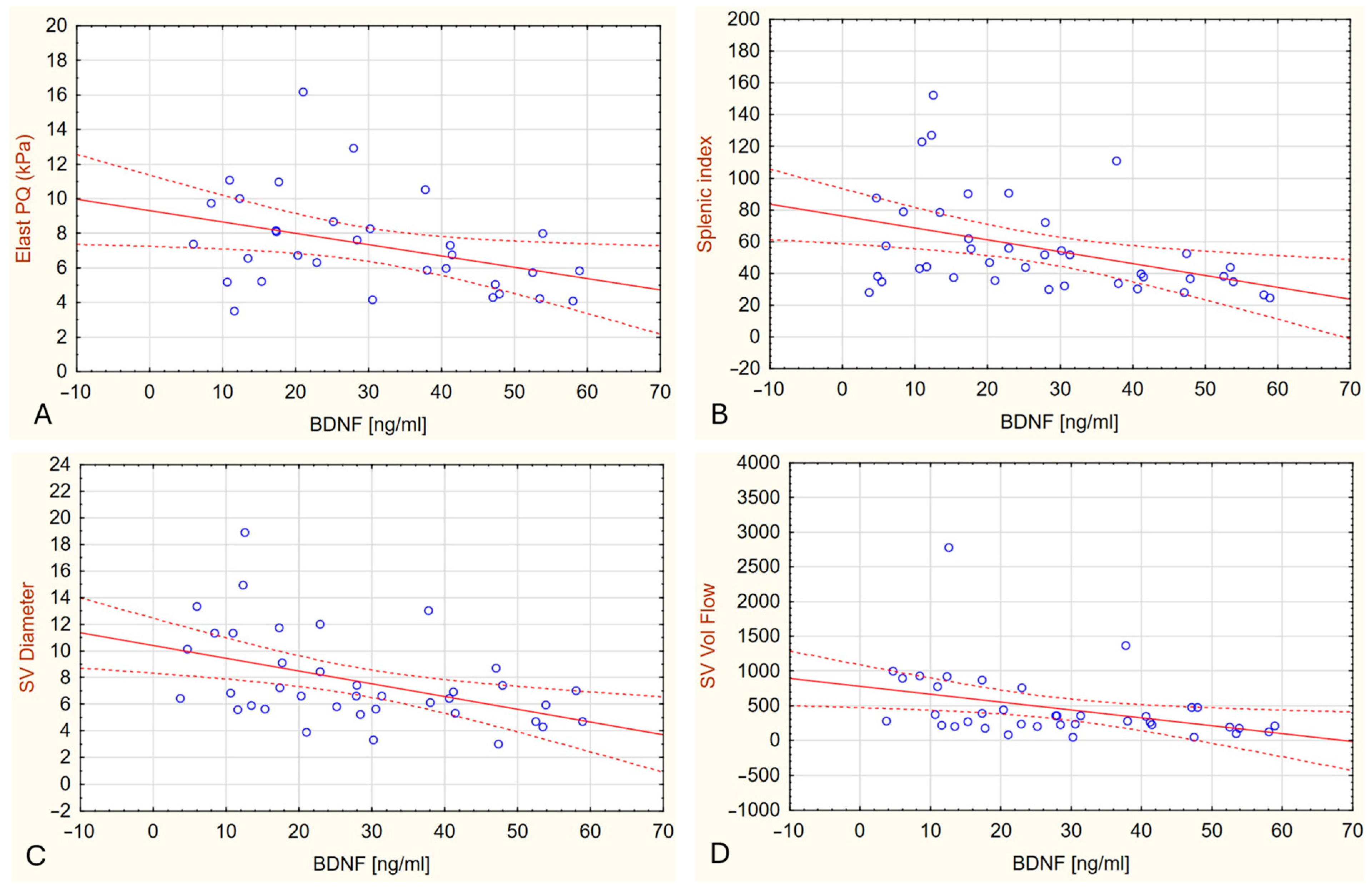


| Variable | Controls (n = 31) | PBC Group (n = 45) | p |
|---|---|---|---|
| Age (years) | 59.06 ± 5.42 | 61.51 ± 7.41 | 0.120 |
| Gender (F/%) | 30 (96.8%) | 43 (95.6%) | 1.000 |
| WBC (×103/µL) | 6.22 ± 1.65 | 5.55 ± 1.88 | 0.137 |
| RBC (×106/µL) | 4.49 (4.27–4.82) | 4.37 (4.04–4.57) | 0.104 |
| HGB (g/dL) | 13.55 (12.90–14.20) | 12.9 (12.10–14.00) | 0.080 |
| PLT (×103/µL) | 243.92 ± 61.96 | 185.42 ± 82.22 | 0.003 |
| ALT (IU/L) | 17.0 (15.00–21.00) | 28.0 (22.00–45.00) | <0.0001 |
| AST (IU/L) | 19.0 (16.00–24.00) | 32.5 (26.00–43.00) | <0.0001 |
| GGTP (IU/L) | 19.0 (16.5–23.0) | 68.0 (43.0–96.0) | 0.0001 |
| ALP (IU/L) | 62.0 (53.0–71.0) | 123.5 (93–173) | <0.0001 |
| Ammonia (µmol/L) | 30.2 (23.8–33.9) | 36.25 (29.6–47.8) | 0.045 |
| Bilirubin (mg/dL) | 0.63 (0.53–0.72) | 0.81 (0.59–1.31) | 0.024 |
| Creatinine (mg/dL) | 0.71 (0.63–0.82) | 0.76 (0.69–0.92) | 0.175 |
| PT (s) | 13.35 (12.70–13.75) | 12.55 (11.90–13.10) | 0.015 |
| Fibrinogen (mg/dL) | 341.46 ± 52.46 | 401.67 ± 75.65 | 0.001 |
| CRP (mg/L) | 1.1 (1.00–2.10) | 4.92 (2.30–7.80) | <0.0001 |
| IL-6 (pg/mL) | 233.6 (152.2–315.2) | 344.9 (252.0–573.4) | 0.0003 |
| IL-18 (pg/mL) | 213.60 (190.0–270.8) | 283.60 (236.0–358.6) | 0.0052 |
| APRI | 0.22 (0.17–0.24) | 0.45 (0.27–0.87) | <0.0001 |
| FIB-4 | 1.23 (1.02–1.30) | 1.79 (1.40–3.57) | <0.0001 |
| ElastPQ (kPa) | 3.26 (2.89–3.85) | 6.74 (5.18–8.46) | <0.0001 |
| Variable | Controls | PBC Group | p |
|---|---|---|---|
| PHES | 2.00 (1.00–3.00) | –1.00 (–3.00–0.50) | <0.0001 |
| NCT-A | 1.00 (0.00–1.00) | 0.00 (–1.00–0.00) | <0.0001 |
| NCT-B | 1.00 (0.00–1.00) | 0.00 (–1.00–0.00) | <0.0001 |
| SDT | 0.00 (0.00–1.00) | 0.00 (–1.50–0.00) | <0.0001 |
| DST | 0.00 (0.00–0.00) | 0.00 (–1.00–0.00) | 0.02 |
| LTT mistakes | 0.00 (0.00–1.00) | 0.00 (0.00–0.50) | 0.28 |
| LTT time | 0.00 (0.00–1.00) | 0.00 (0.00–0.00) | 0.39 |
| Variable | Controls (n = 28) | PBC Group (n = 27) | p |
|---|---|---|---|
| MFIS—total | 17.5 (14.5–22.5) | 31.0 (15.0–45.0) | 0.04 |
| MFIS_f | 8.5 (7.0–12.5) | 13.0 (7.0–19.0) | 0.05 |
| MFIS_p | 6.0 (4.5–12.0) | 12.0 (4.0–18.0) | 0.08 |
| MFIS_s | 2.32 ± 1.66 | 3.41 ± 2.63 | 0.075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogalska, M.; Ławicki, S.; Błachnio-Zabielska, A.; Zabielski, P.; Roszczyc-Owsiejczuk, K.; Janica, J.; Bogdanowska-Charkiewicz, D.; Andrzejuk, A.; Dąbrowski, A.; Flisiak, R.; et al. Elevated Plasma BDNF in Early Primary Biliary Cholangitis: Associations with Liver Fibrosis, IL-6, IL-18, Fatigue, and Cognitive Impairment. Int. J. Mol. Sci. 2025, 26, 7142. https://doi.org/10.3390/ijms26157142
Rogalska M, Ławicki S, Błachnio-Zabielska A, Zabielski P, Roszczyc-Owsiejczuk K, Janica J, Bogdanowska-Charkiewicz D, Andrzejuk A, Dąbrowski A, Flisiak R, et al. Elevated Plasma BDNF in Early Primary Biliary Cholangitis: Associations with Liver Fibrosis, IL-6, IL-18, Fatigue, and Cognitive Impairment. International Journal of Molecular Sciences. 2025; 26(15):7142. https://doi.org/10.3390/ijms26157142
Chicago/Turabian StyleRogalska, Magdalena, Sławomir Ławicki, Agnieszka Błachnio-Zabielska, Piotr Zabielski, Kamila Roszczyc-Owsiejczuk, Jacek Janica, Dagmara Bogdanowska-Charkiewicz, Aleksandra Andrzejuk, Andrzej Dąbrowski, Robert Flisiak, and et al. 2025. "Elevated Plasma BDNF in Early Primary Biliary Cholangitis: Associations with Liver Fibrosis, IL-6, IL-18, Fatigue, and Cognitive Impairment" International Journal of Molecular Sciences 26, no. 15: 7142. https://doi.org/10.3390/ijms26157142
APA StyleRogalska, M., Ławicki, S., Błachnio-Zabielska, A., Zabielski, P., Roszczyc-Owsiejczuk, K., Janica, J., Bogdanowska-Charkiewicz, D., Andrzejuk, A., Dąbrowski, A., Flisiak, R., & Rogalski, P. (2025). Elevated Plasma BDNF in Early Primary Biliary Cholangitis: Associations with Liver Fibrosis, IL-6, IL-18, Fatigue, and Cognitive Impairment. International Journal of Molecular Sciences, 26(15), 7142. https://doi.org/10.3390/ijms26157142







