Sequence Variation and In Silico Protein Characterization of γ-TMT Gene in Mutant Rodent Tuber (Typhonium flagelliforme Lodd.)
Abstract
1. Introduction
2. Results
2.1. Degenerate Primer Design for the γ-TMT Gene
2.2. γ-TMT Gene Amplification Using Degenerate Primers
2.3. Sequencing Verification
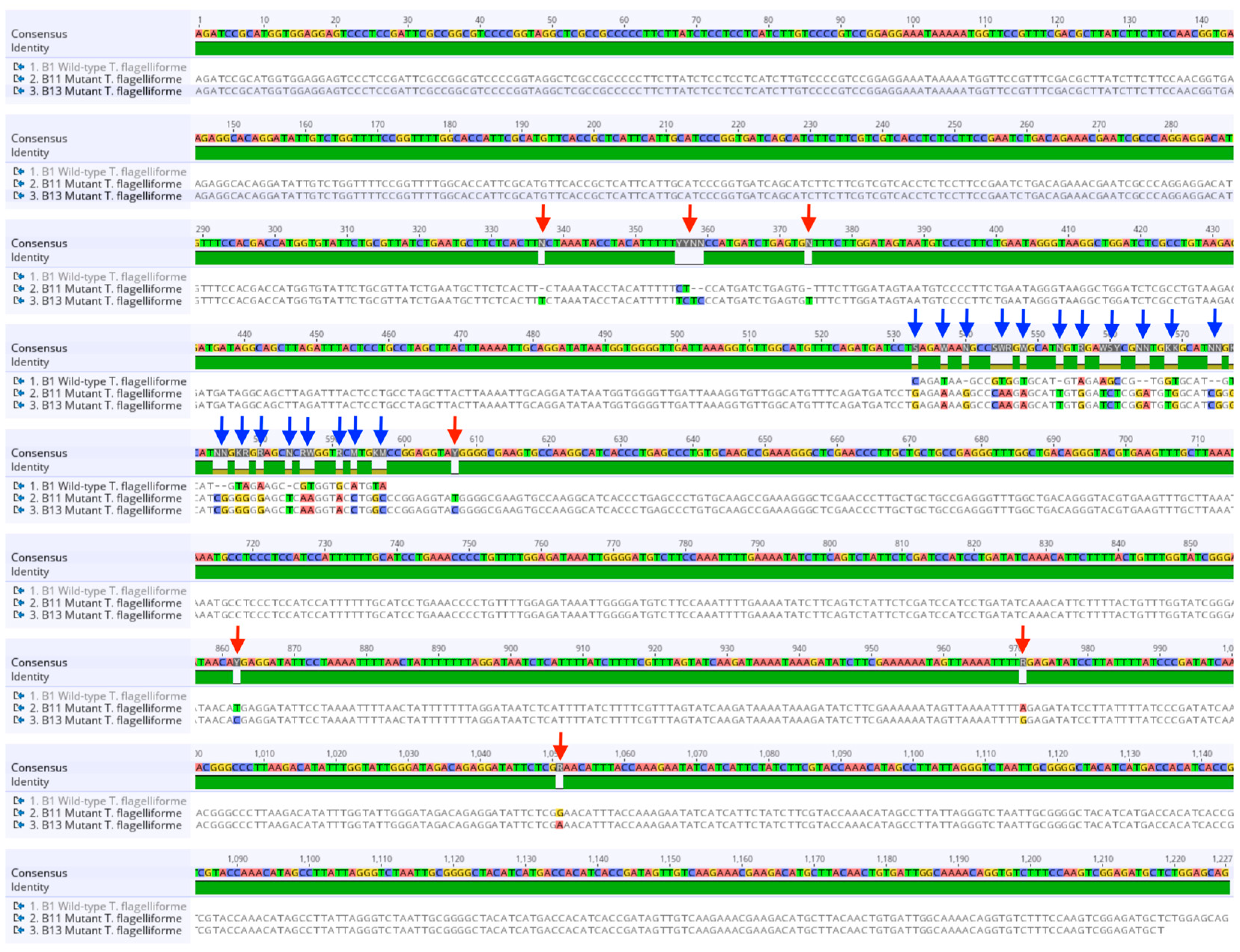
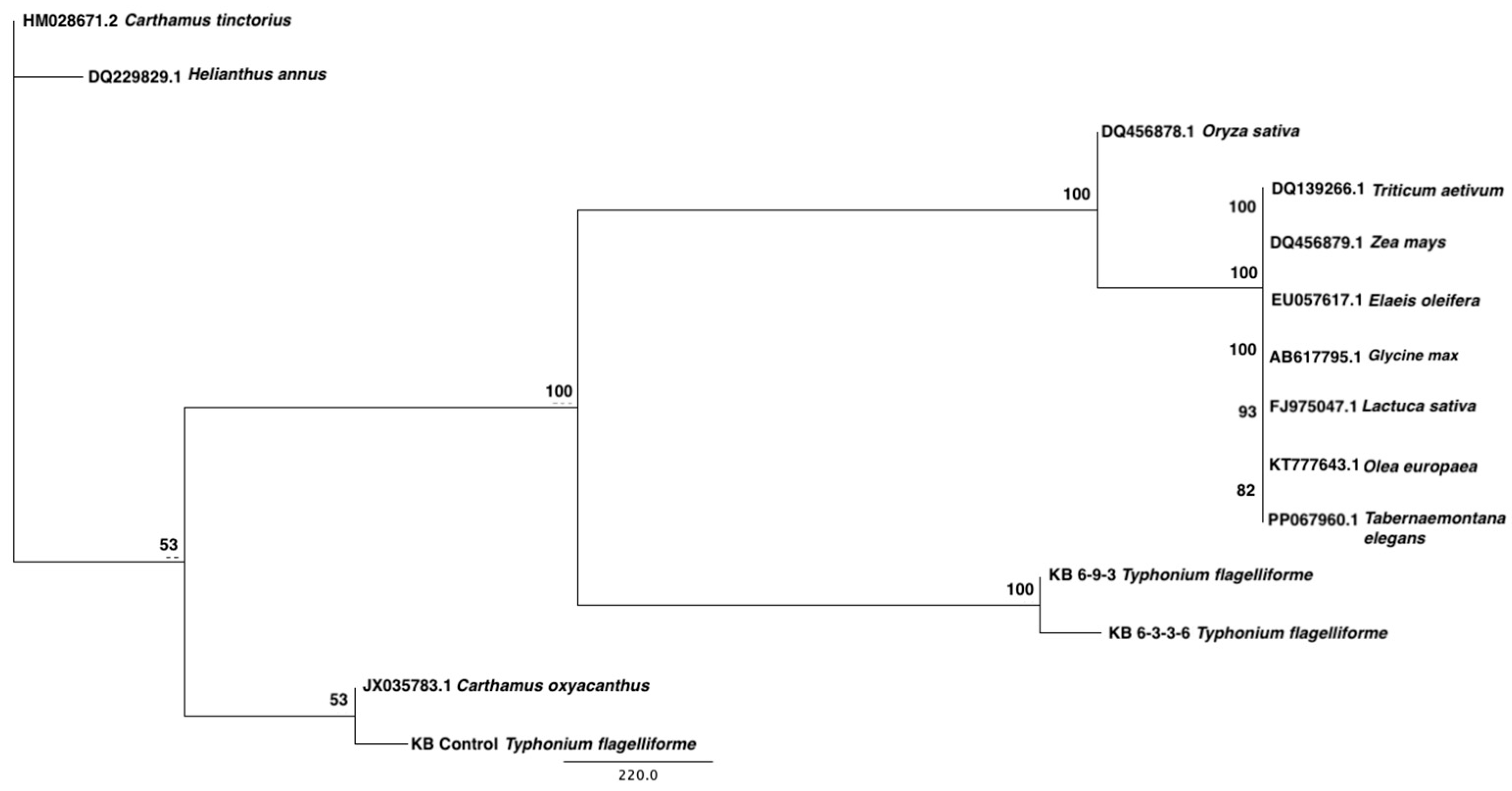
2.4. Sequence Alignment, Mutation Profiling, and Phylogenetic Analysis of γ-TMT Gene in Mutants
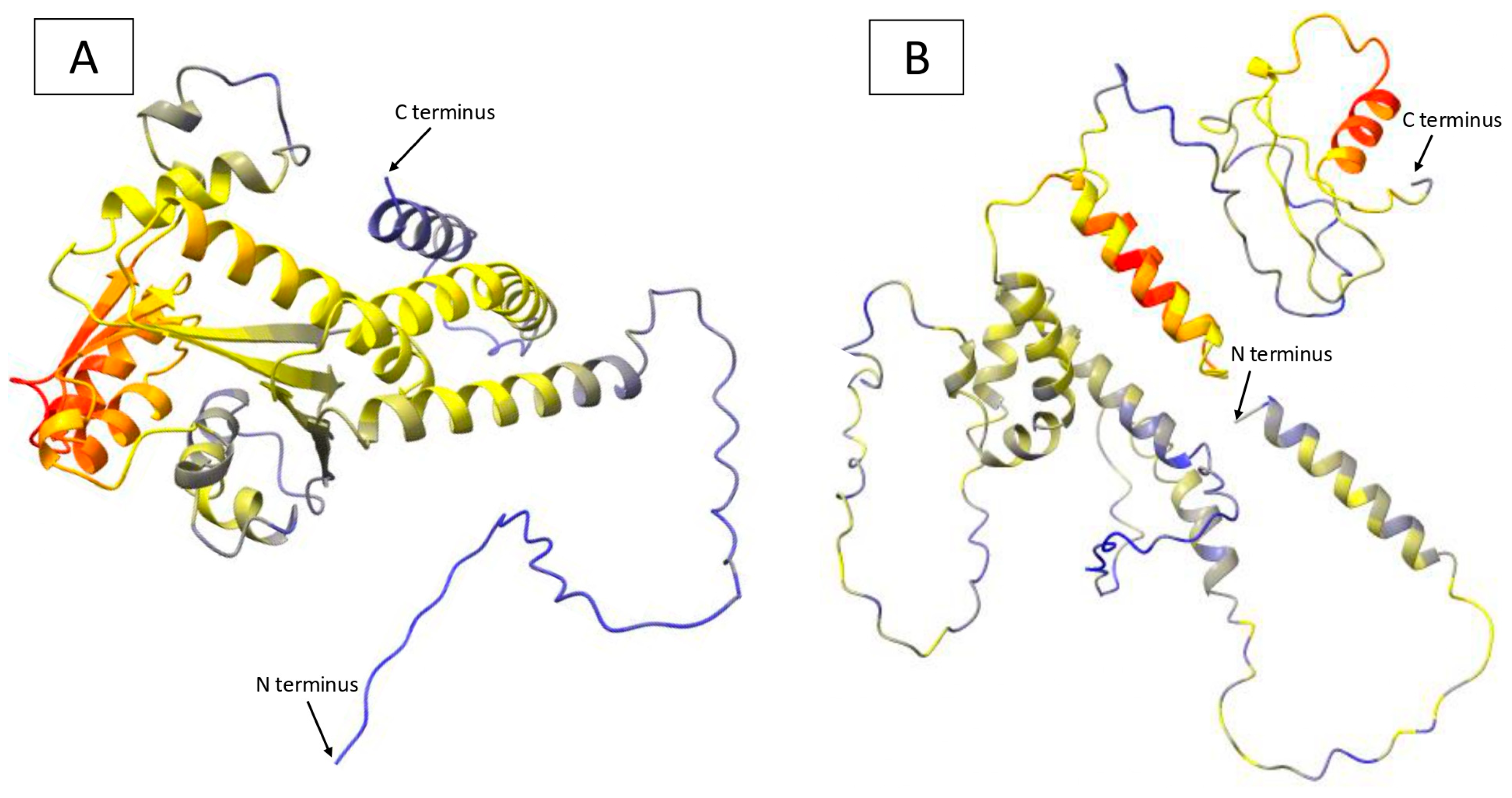
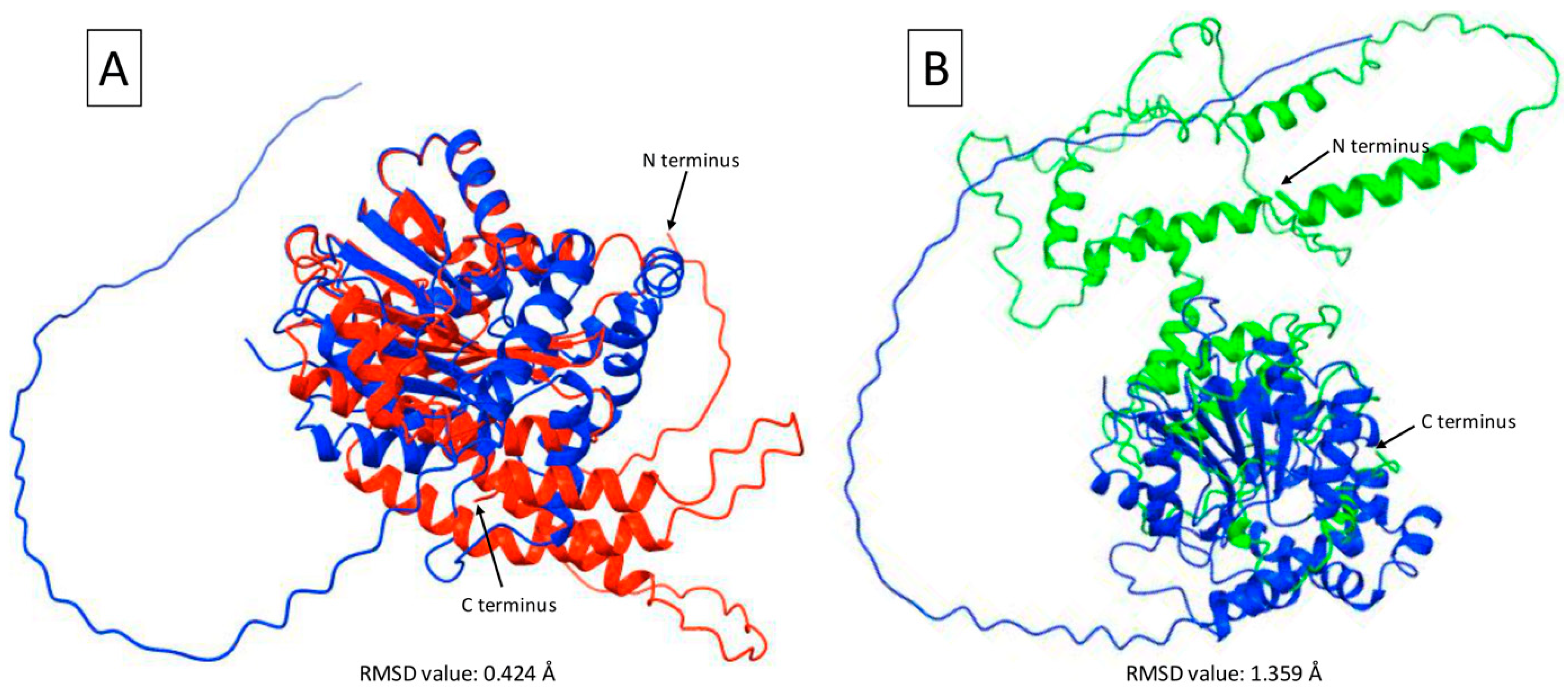
2.5. γ-TMT Protein Structure Prediction in Mutants
3. Discussion
3.1. Primer Efficiency and γ-TMT Gene Amplification
3.2. Sequence Variation and Bioinformatics Analysis
3.3. Protein Modeling and Structural Changes
4. Materials and Methods
4.1. Plant Genetic Materials
4.2. Genomic DNA Isolation and Quality Test
4.3. Primer Design and Amplification of γ-TMT
4.4. Bioinformatics Analysis
4.5. γ-TMT Protein Structure Prediction of Mutant Rodent Tuber
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sianipar, N.F.; Assidqi, K.; Hadisaputri, Y.E.; Salam, S. Mutant Plant Tipobio Variety of Rodent Tuber (Typhonium flagelliforme): Fatty Acids Compounds and in Vitro Anticancer Activity. E3S Web Conf. 2023, 01032, 4–8. [Google Scholar] [CrossRef]
- Maha, H.L.; Fidrianny, I.; Satrialdi; Suciati, T. An updated review of Typhonium flagelliforme: Phytochemical compound, pharmacological activities and the use of vitexin and isovitexin as flavonoid compound in cosmetics development. Pharmacia 2023, 70, 673–680. [Google Scholar] [CrossRef]
- Putra, A.; Riwanto, I.; Putra, S.T.; Wijaya, I. Typhonium flagelliforme extract induce apoptosis in breast cancer stem cells by suppressing survivin. J. Cancer Res. Ther. 2020, 16, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Sianipar, N.F.; Assidqi, K.; Hadisaputri, Y.E.; Salam, S.; Tarigan, R.; Purnamaningsih, R. Determination of Bioactive Compounds of Superior Mutant Rodent Tuber (Typhonium flagelliforme) in Various Fractions Using GC-MS. IOP Conf. Ser. Earth Environ. Sci. 2021, 794, 012144. [Google Scholar] [CrossRef]
- Sianipar, N.F.; Muflikhati, Z.; Mangindaan, D.; Assidqi, K. Anticancer Potential of Tocopherols-Containing Plants and Semi-Synthetic Tocopherols. Plants 2024, 13, 2994. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Njiki, S.; Mbemi, A.; Yedjou, C.G.; Tchounwou, P.B. Pharmacological Effects of Cisplatin Combination with Natural Products in Cancer Chemotherapy. Int. J. Mol. Sci. 2022, 23, 1532. [Google Scholar] [CrossRef]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.; Sousa, S.F. An Atomic-Level Perspective of HMG-CoA-Reductase: The Target Enzyme to Treat Hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef]
- Idriss, M.; Hodroj, M.H.; Fakhoury, R.; Rizk, S. Beta-Tocotrienol Exhibits More Cytotoxic Effects than Gamma-Tocotrienol on Breast Cancer Cells by Promoting Apoptosis via a P53-Independent PI3-Kinase Dependent Pathway. Biomolecules 2020, 10, 577. [Google Scholar] [CrossRef]
- Somsri, A.; Chu, S.H.; Nawade, B.; Lee, C.Y.; Park, Y.J. Harnessing gamma-TMT Genetic Variations and Haplotypes for Vitamin E Diversity in the Korean Rice Collection. Antioxidants 2024, 13, 234. [Google Scholar] [CrossRef]
- Savino, S.; Desmet, T.; Franceus, J. Insertions and deletions in protein evolution and engineering. Biotechnol. Adv. 2022, 60, 108010. [Google Scholar] [CrossRef]
- Kalendar, R.; Shevtsov, A.; Otarbay, Z.; Ismailova, A. In silico PCR analysis: A comprehensive bioinformatics tool for enhancing nucleic acid amplification assays. Front. Bioinform. 2024, 4, 1464197. [Google Scholar] [CrossRef]
- Sapkota, S.; Pillman, K.A.; Dredge, B.K.; Liu, D.; Bracken, J.M.; Kachooei, S.A.; Chereda, B.; Gregory, P.A.; Bracken, C.P.; Goodall, G.J. On the rules of engagement for microRNAs targeting protein coding regions. Nucleic Acids Res. 2023, 51, 9938–9951. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Kleine, J.; Schemmerer, M.; Kreibich, J.; Maier, W.; Battur, N.; Krannich, T.; Sedaghatjoo, S.; Jaki, L.; Maks, A.; et al. varVAMP: Degenerate primer design for tiled full genome sequencing and qPCR. Nat. Commun. 2025, 16, 5067. [Google Scholar] [CrossRef] [PubMed]
- Araujo, C.L.; Blanco, I.; Souza, L.; Tiwari, S.; Pereira, L.C.; Ghosh, P.; Azevedo, V.; Silva, A.; Folador, A. In silico functional prediction of hypothetical proteins from the core genome of Corynebacterium pseudotuberculosis biovar ovis. PeerJ 2020, 8, e9643. [Google Scholar] [CrossRef] [PubMed]
- Topolska, M.; Beltran, A.; Lehner, B. Deep indel mutagenesis reveals the impact of amino acid insertions and deletions on protein stability and function. Nat. Commun. 2025, 16, 2617. [Google Scholar] [CrossRef]
- Es-Sai, B.; Wahnou, H.; Benayad, S.; Rabbaa, S.; Laaziouez, Y.; El Kebbaj, R.; Limami, Y.; Duval, R.E. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules 2025, 30, 653. [Google Scholar] [CrossRef]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Gultom, T.; Simbolon, D.L.; Nainggolan, W.S. Effect of gamma rays on phenotypic of garlic cultivar doulu. IOP Conf. Ser. Mater. Sci. Eng. 2020, 725, 012081. [Google Scholar] [CrossRef]
- Sianipar, N.F.; Assidqi, K.; Abbas, B.S. The Effects of Subculture on the Mutant Plant Regeneration of Rodent Tuber (Typhonium flagelliforme) in Vitro Mutagenesis Using Gamma-Ray Irradiation. IOP Conf. Ser. Earth Environ. Sci. 2020, 426, 012180. [Google Scholar] [CrossRef]
- Bulot, B.; Isabelle, S.; Montoya, R.; Nadeau, L.F.; Tremblay, J.; Goulet, C. Structural variations in the phytoene synthase 1 gene affect carotenoid accumulation in tomato fruits and result in bicolor and yellow phenotypes. bioRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.; Liu, B.; Zhang, L.; Zhang, W.; Chen, R.; Wang, L. Overexpression of the maize gamma-tocopherol methyltransferase gene (ZmTMT) increases alpha-tocopherol content in transgenic Arabidopsis and maize seeds. Transgenic Res. 2020, 29, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Stern, D.L.; Han, C. Gene Structure-Based Homology Search Identifies Highly Divergent Putative Effector Gene Family. Genome Biol. Evol. 2022, 14, evac069. [Google Scholar] [CrossRef] [PubMed]
- Kawai, H.; Sato, K.; Kato, T.; Kamiya, H. Correction of substitution, deletion, and insertion mutations by 5′-tailed duplexes. J. Biosci. Bioeng. 2024, 137, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhu, Y.; Wang, Y.; Wang, T.; Zhao, S.; Feng, K.; Li, L.; Wu, P. Molecular identification of phenylalanine ammonia lyase-encoding genes EfPALs and EfPAL2-interacting transcription factors in Euryale ferox. Front. Plant Sci. 2023, 14, 1114345. [Google Scholar] [CrossRef]
- Muflikhati, Z.; Sianipar, N.F.; Reflinur, R.; Anas, A. Development of lectin gene-based SNAP and ARMS markers as anticancer biomarkers in mutant rodent tuber (Typhonium flagelliforme) of Pekalongan accession, Indonesia. Biodiversitas J. Biol. Divers. 2025, 695–707. [Google Scholar] [CrossRef]
- Jiang, L.; Strobbe, S.; Van Der Straeten, D.; Zhang, C. Regulation of Plant Vitamin Metabolism: Backbone of Biofortification for the Alleviation of Hidden Hunger. Mol. Plant 2021, 14, 40–60. [Google Scholar] [CrossRef]
- Yang, Z.; Zeng, X.; Zhao, Y.; Chen, R. AlphaFold2 and its applications in the fields of biology and medicine. Signal Transduct. Target. Ther. 2023, 8, 115. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Núñez-Villanueva, D. Revisiting 310-helices: Biological relevance, mimetics and applications. Explor. Drug Sci. 2024, 2, 6–37. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef]
- Prabantu, V.M.; Gadiyaram, V.; Vishveshwara, S.; Srinivasan, N. Understanding structural variability in proteins using protein structural networks. Curr. Res. Struct. Biol. 2022, 4, 134–145. [Google Scholar] [CrossRef]
- Saha, S.; Sotomayor-Vivas, C.; Hernández-Lemus, E.; Dorantes-Gilardi, R. Linking protein structural and functional change to mutation using amino acid networks. PLoS ONE 2022, 17, e0261829. [Google Scholar] [CrossRef]
- Quintal-Bojórquez, N.d.C.; Carrillo-Cocom, L.M.; Hernández-Álvarez, A.J.; Segura-Campos, M.R. Anticancer activity of protein fractions from chia (Salvia hispanica L.). J. Food Sci. 2021, 86, 2861–2871. [Google Scholar] [CrossRef] [PubMed]
- Bidram, M.; Ganjalikhany, M.R. Bioactive peptides from food science to pharmaceutical industries: Their mechanism of action, potential role in cancer treatment and available resources. Heliyon 2024, 10, e40563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yuan, Y.; Zeng, Q.; Xiong, J.; Gan, Y.; Jiang, K.; Xie, N. Plant protein-derived anti-breast cancer peptides: Sources, therapeutic approaches, mechanisms, and nanoparticle design. Front. Pharmacol. 2025, 15, 1468977. [Google Scholar] [CrossRef] [PubMed]
- Alhadrami, H.A.; Hamed, A.A.; Hassan, H.M.; Belbahri, L.; Rateb, M.E.; Sayed, A.M. Flavonoids as Potential anti-MRSA Agents through Modulation of PBP2a: A Computational and Experimental Study. Antibiotics 2020, 9, 562. [Google Scholar] [CrossRef]
- Nanjundaswamy, S.; Bindhu, S.; Arun Renganathan, R.R.; Nagashree, S.; Karthik, C.S.; Mallu, P.; Ravishankar Rai, V. Design, synthesis of pyridine coupled pyrimidinone/pyrimidinthione as anti-MRSA agent: Validation by molecular docking and dynamics simulation. J. Biomol. Struct. Dyn. 2021, 40, 12106–12117. [Google Scholar] [CrossRef]
- Ma, J.; Qiu, D.; Pang, Y.; Gao, H.; Wang, X.; Qin, Y. Diverse roles of tocopherols in response to abiotic and biotic stresses and strategies for genetic biofortification in plants. Mol. Breed. 2020, 40, 18. [Google Scholar] [CrossRef]
- Gerasimavicius, L.; Livesey, B.J.; Marsh, J.A. Loss-of-function, gain-of-function and dominant-negative mutations have profoundly different effects on protein structure. Nat. Commun. 2022, 13, 3895. [Google Scholar] [CrossRef]
- Kosova, K.; Vitamvas, P.; Prasil, I.T.; Klima, M.; Renaut, J. Plant Proteoforms Under Environmental Stress: Functional Proteins Arising From a Single Gene. Front. Plant Sci. 2021, 12, 793113. [Google Scholar] [CrossRef]
- Dorani, E.; Dehghanian, Z.; Gougerdchi, V.; Hamedpour-Darabi, M. Application of Somaclonal Variation in Crop Improvements. In Plant Mutagenesis: Sustainable Agriculture and Rural Landscapes; Springer: Berlin/Heidelberg, Germany, 2024; pp. 93–109. [Google Scholar]
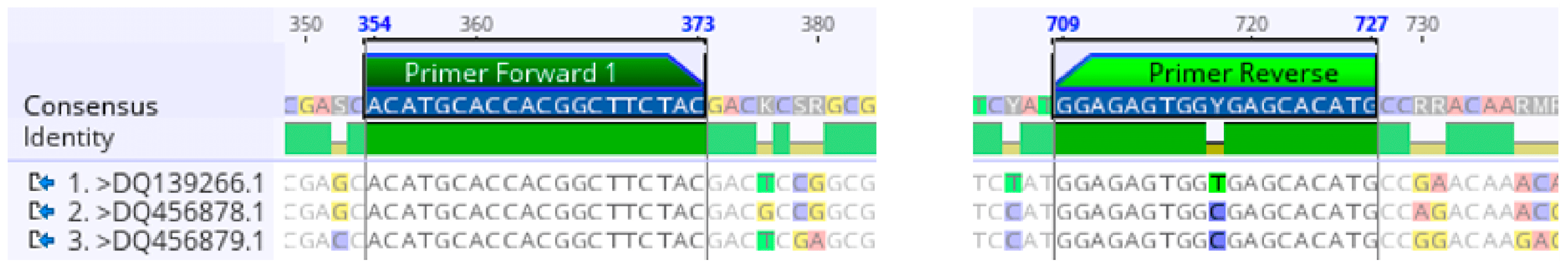



| Gene | Primer Id | Sequences Primer (5′→3′) | Tm (°C) |
|---|---|---|---|
| γ-TMT | TMT_F_1 | ACATGCACCACGGCTTCTAC | 64.6 |
| TMT_R | CATGTGCTCRCCACTCTCC | 65.2–67.6 |
| No | Accessions | Monocots Species | Descriptions | Product Size |
|---|---|---|---|---|
| 1. | CP137583.1 | Eragrostis tef cultivar Dabbi | Chromosome 1B | 1273 bp |
| 2. | CP137582.1 | Eragrostis tef cultivar Dabbi | Chromosome 1A | 3377 bp |
| 3. | XM_052289477.1 | Oryza glaberrima | Probable tocopherol O-methyltransferase, chloroplastic (LOC127764584), mRNA | 368 bp |
| 4. | OK032047.1 | Oryza punctata | Isolate RWG-480 gamma-tocopherol methyl transferase (gammaTMT) gene, complete cds | 1688 bp |
| 5. | OK032037.1 | Oryza longistaminata | Isolate RWG-470 gamma-tocopherol methyl transferase (gammaTMT) gene, complete cds | 1690 bp |
| 6. | OK031972.1 | Oryza sativa Indica Group | Isolate RWG-405 gamma-tocopherol methyl transferase (gammaTMT) gene, complete cds | 1690 bp |
| 7. | XM_002454384.2 | Sorghum bicolor | Probable tocopherol O-methyltransferase, chloroplastic (LOC8073167), mRNA | 374 bp |
| 8. | KF184802.1 | Saccharum hybrid cultivar R570 | Clone BAC 258H24 complete sequence | 2092 bp |
| 9. | JQ246243.1 | Zea mays subsp. mays cultivar By804 | Gamma-tocopherol methyltransferase gene, complete cds | 1699 bp |
| 10. | XM_062364039.1 | Phragmites australis | Probable tocopherol O-methyltransferase, chloroplastic (LOC133919600), mRNA | 368 bp |
| 11. | AJ920394.2 | Triticum aestivum | mRNA for g-TMT protein | 309 bp |
| Species Name | Description | E-Value | Per. Identity | Acc. len | Accession | ||
|---|---|---|---|---|---|---|---|
| KB 6-9-3 | KB 6-3-3-6 | KB 6-9-3 | KB 6-3-3-6 | ||||
| Carthamus tinctorius (family: Asteraceae) | Gamma-tocopherol methyltransferase (γ-TMT) gene, complete cds | 8 × 10−27 | 1 × 10−25 | 77.90% | 77.35% | 3393 bp | HM028671.2 |
| Carthamus oxyacanthus (family: Asteraceae) | Gamma-tocopherol methyltransferase (γ-TMT) gene, complete cds | 8 × 10−27 | 3 × 10−25 | 76.97% | 76.11% | 3371 bp | JX035783.1 |
| Lactuca sativa (family: Asteraceae) | Gamma-tocopherol methyltransferase mRNA, complete cds | 8 × 10−27 | 3 × 10−25 | 76.67% | 76.11% | 1131 bp | FJ975047.1 |
| Helianthus annuus (family: Asteraceae) | Gamma-tocopherol methyltransferase (γ-TMT) gene, complete cds | 3 × 10−20 | 6 × 10−22 | 74.03% | 74.59% | 4029 bp | DQ229829.1 |
| Tabernaemontana elegans (family: Apocynaceae) | Gamma-tocopherol C-methyltransferase (TMT) mRNA, partial cds | 2 × 10−22 | 2 × 10−21 | 77.99% | 77.36% | 807 bp | PP067960.1 |
| Glycine max (family: Fabaceae) | g-tmt mRNA for gamma-tocopherol methyltransferase, complete cds | 3 × 10−20 | 1 × 10−18 | 74.16% | 73.60% | 1097 bp | AB617795.1 |
| Elaeis oleifera (family: Arecaceae) | Gamma-tocopherol methyltransferase (g-TMT) mRNA, partial cds | 3 × 10−19 | 4 × 10−18 | 75.00% | 74.39% | 1284 bp | EU057617.1 |
| Olea europaea (family: Oleaceae) | Gamma-tocopherol methyltransferase (GTMT) mRNA, complete cds | 3 × 10−19 | 1 × 10−17 | 76.71% | 76.03% | 1277 bp | KT777643.1 |
| Position of Mutations in Mutants (bp) | Variation in Mutations Between Mutants and Wild-Type | Type of Mutations |
|---|---|---|
| 533 | C to G | Substitution |
| 537 | T to A | Substitution |
| 544 | GTG to CAA | Substitution |
| 546 | T to A | Substitution |
| 556 | A to G | Substitution |
| 559 | AGC to TCT | Substitution |
| 568 | GT to TG | Substitution |
| 577 | TA to GG | Substitution |
| 580 | A to G | Substitution |
| 586 | GT to AA | Substitution |
| 591 | G to A | Substitution |
| 593 | A to C | Substitution |
| 596 | TA to GC | Substitution |
| 540 | G | Deletion in wild-type |
| 553 | T | Deletion in wild-type |
| 564 | GA | Deletion in wild-type |
| 574 | CG | Deletion in wild-type |
| Position of Mutations in Mutants (bp) | Variation of Mutations | Type of Mutations |
|---|---|---|
| 337 | T | Deletion in KB 6-9-3 |
| 374 | T | Deletion in KB 6-9-3 |
| 356–357 | CT to TC | Substitution |
| 358–359 | TC | Deletion in KB 6-9-3 |
| 607 | T to C | Substitution |
| 862 | T to C | Substitution |
| 971 | A to G | Substitution |
| 1051 | G to A | Substitution |
| No | Accession | Organism | Protein Name | Identity | E-Value | Query Cover |
|---|---|---|---|---|---|---|
| 1. | GAB2221792.1 | Drosera rotundifolia | Hypothetical protein | 73.61% | 3 × 10−24 | 21% |
| 2. | XP_018837640.1 | Juglans regia | probable tocopherol O-methyltransferase | 70.83% | 1 × 10−23 | 21% |
| 3. | KAJ3707098.1 | Rhynchospora tenuis | Hypothetical protein | 70.83% | 1 × 10−23 | 21% |
| 4. | EOY10208.1 | Theobroma cacao | Gamma-tocopherol methyltransferase | 73.61% | 3 × 10−23 | 21% |
| 6. | KAJ4799986.1 | Rhynchospora pubera | Gamma-tocopherol methyltransferase | 70.83% | 4 × 10−23 | 21% |
| 7. | KAJ4720949.1 | Melia azedarach | Gamma-tocopherol methyltransferase | 72.22% | 5 × 10−23 | 21% |
| 8. | XP_071685828.1 | Rutidosis leptorrhynchoides | Gamma-tocopherol methyltransferase | 72.22% | 6 × 10−23 | 21% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sianipar, N.F.; Muflikhati, Z.; Reflinur; Lawrie, M.D.; Mangindaan, D.; Assidqi, K.; Enyi, C.U.; Widyaningrum, D. Sequence Variation and In Silico Protein Characterization of γ-TMT Gene in Mutant Rodent Tuber (Typhonium flagelliforme Lodd.). Int. J. Mol. Sci. 2025, 26, 7148. https://doi.org/10.3390/ijms26157148
Sianipar NF, Muflikhati Z, Reflinur, Lawrie MD, Mangindaan D, Assidqi K, Enyi CU, Widyaningrum D. Sequence Variation and In Silico Protein Characterization of γ-TMT Gene in Mutant Rodent Tuber (Typhonium flagelliforme Lodd.). International Journal of Molecular Sciences. 2025; 26(15):7148. https://doi.org/10.3390/ijms26157148
Chicago/Turabian StyleSianipar, Nesti Fronika, Zidni Muflikhati, Reflinur, Muhammad Dylan Lawrie, Dave Mangindaan, Khoirunnisa Assidqi, Chukwunwike Uchenna Enyi, and Dwiyantari Widyaningrum. 2025. "Sequence Variation and In Silico Protein Characterization of γ-TMT Gene in Mutant Rodent Tuber (Typhonium flagelliforme Lodd.)" International Journal of Molecular Sciences 26, no. 15: 7148. https://doi.org/10.3390/ijms26157148
APA StyleSianipar, N. F., Muflikhati, Z., Reflinur, Lawrie, M. D., Mangindaan, D., Assidqi, K., Enyi, C. U., & Widyaningrum, D. (2025). Sequence Variation and In Silico Protein Characterization of γ-TMT Gene in Mutant Rodent Tuber (Typhonium flagelliforme Lodd.). International Journal of Molecular Sciences, 26(15), 7148. https://doi.org/10.3390/ijms26157148






