Real-World Evaluation of Microsatellite Instability Detection via Targeted NGS Panels in Routine Molecular Diagnostics
Abstract
1. Introduction
2. Results
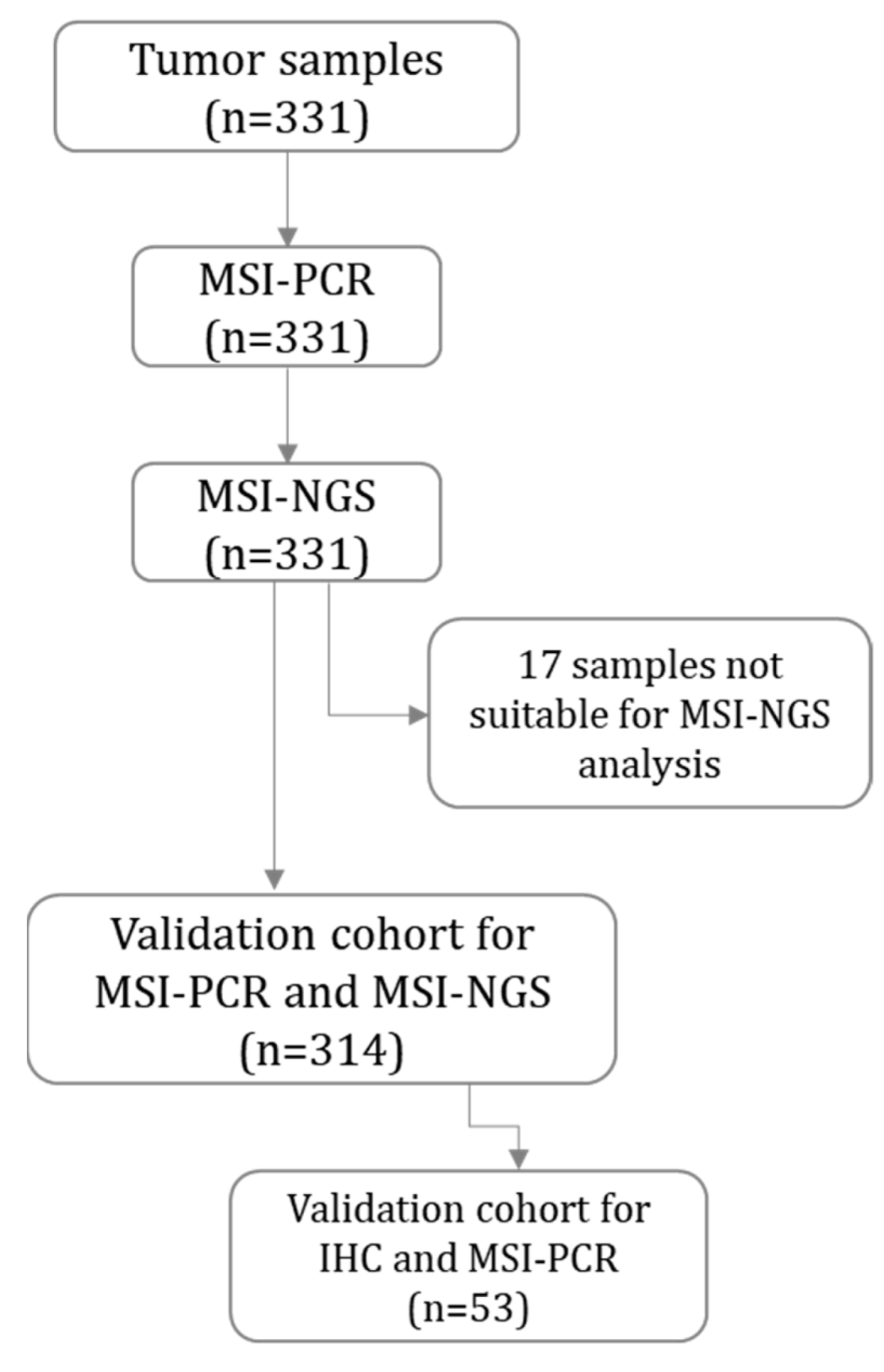
2.1. Validation Cohort
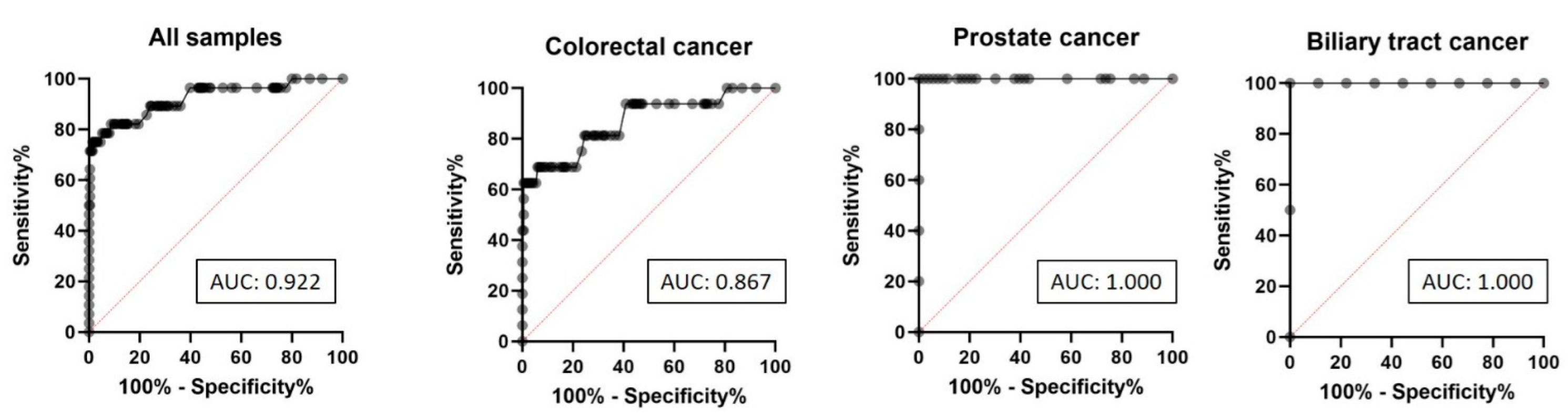
2.2. ROC Curve Analysis of MSI-NGS Diagnostic Performance
2.3. Determination of MSI Score Cut-Off Value and Evaluation of Diagnostic Performance Across Different MSI Score Cut-Offs
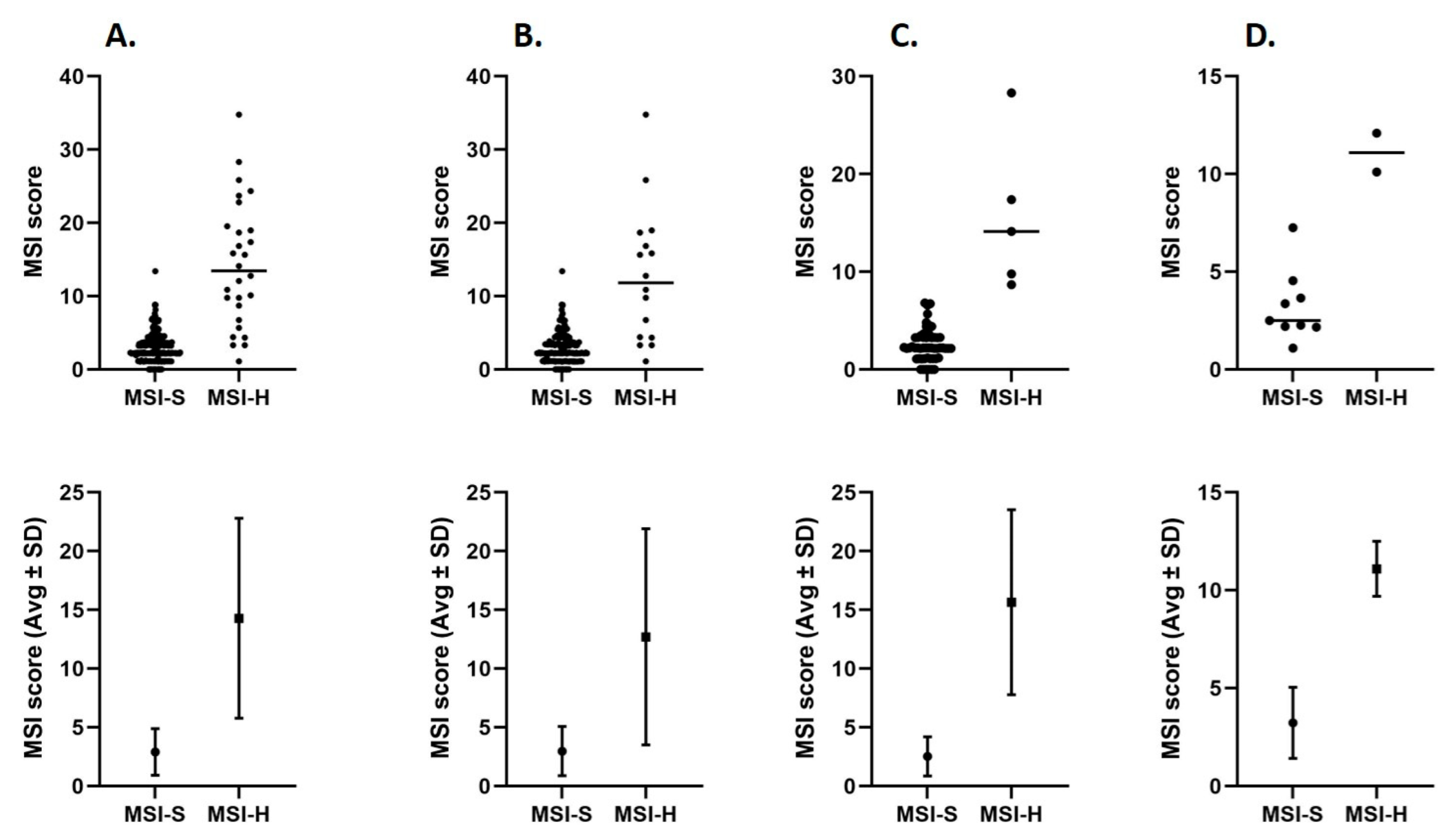
2.4. MSI Score Distributions in MSI-H and MSI-S Tumor Samples
2.5. Usable and Unstable Microsatellite Sites in MSI-H and MSI-S Tumor Samples
2.6. Association Between MSI Score, Tumor Cell Content, and Tumor Mutational Burden in MSI-H Tumor Samples
2.7. Tumor Mutational Burden in MSI-H and MSI-S Tumor Samples
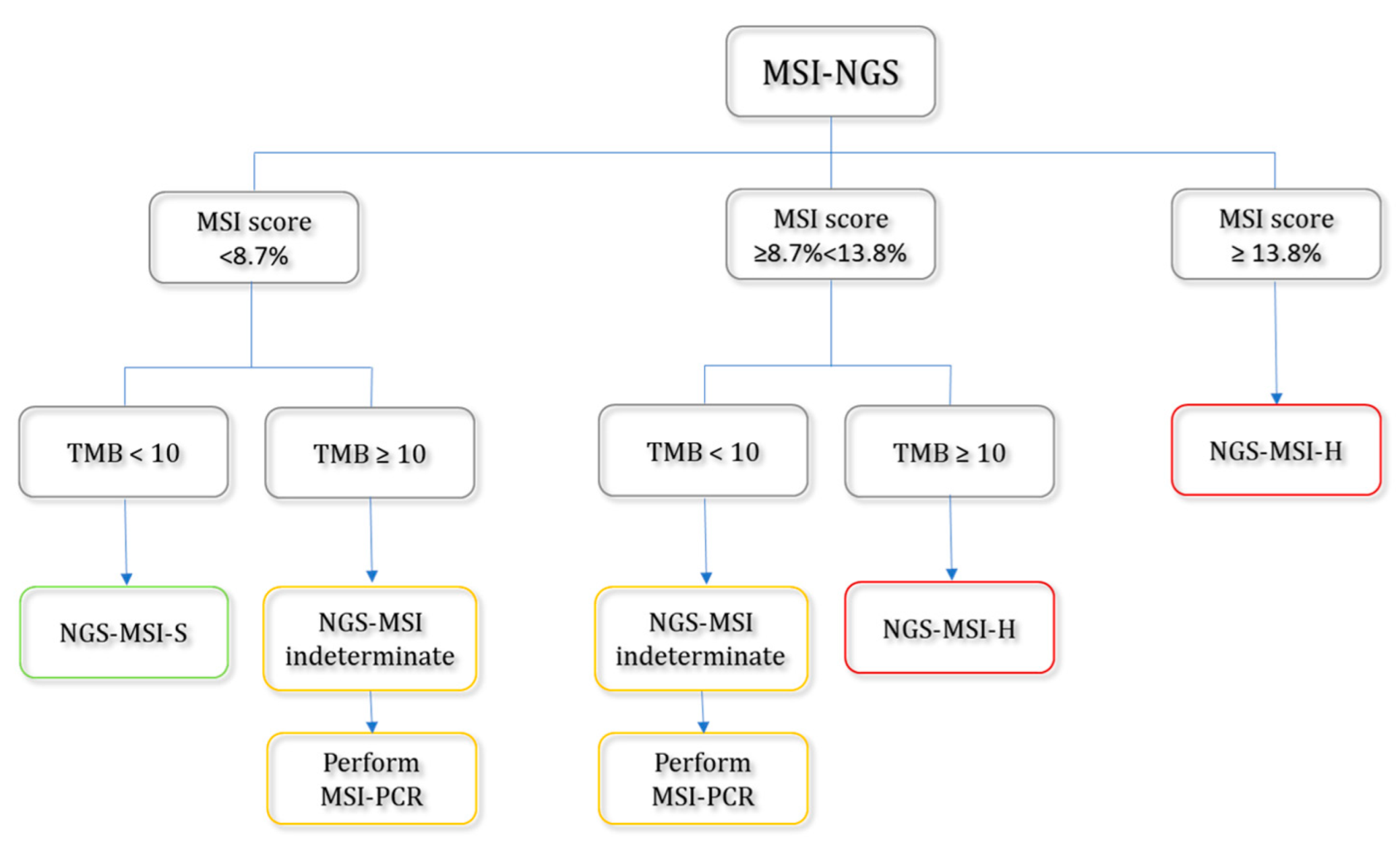
2.8. Suggested Workflow for the Detection of MSI-H Tumors Based on MSI Scores and Tumor Mutational Burden (TMB)
3. Discussion
4. Materials and Methods
4.1. Patients and Tumor Samples
4.2. DNA Isolation
4.3. MSI Detection Using PCR (MSI-PCR)
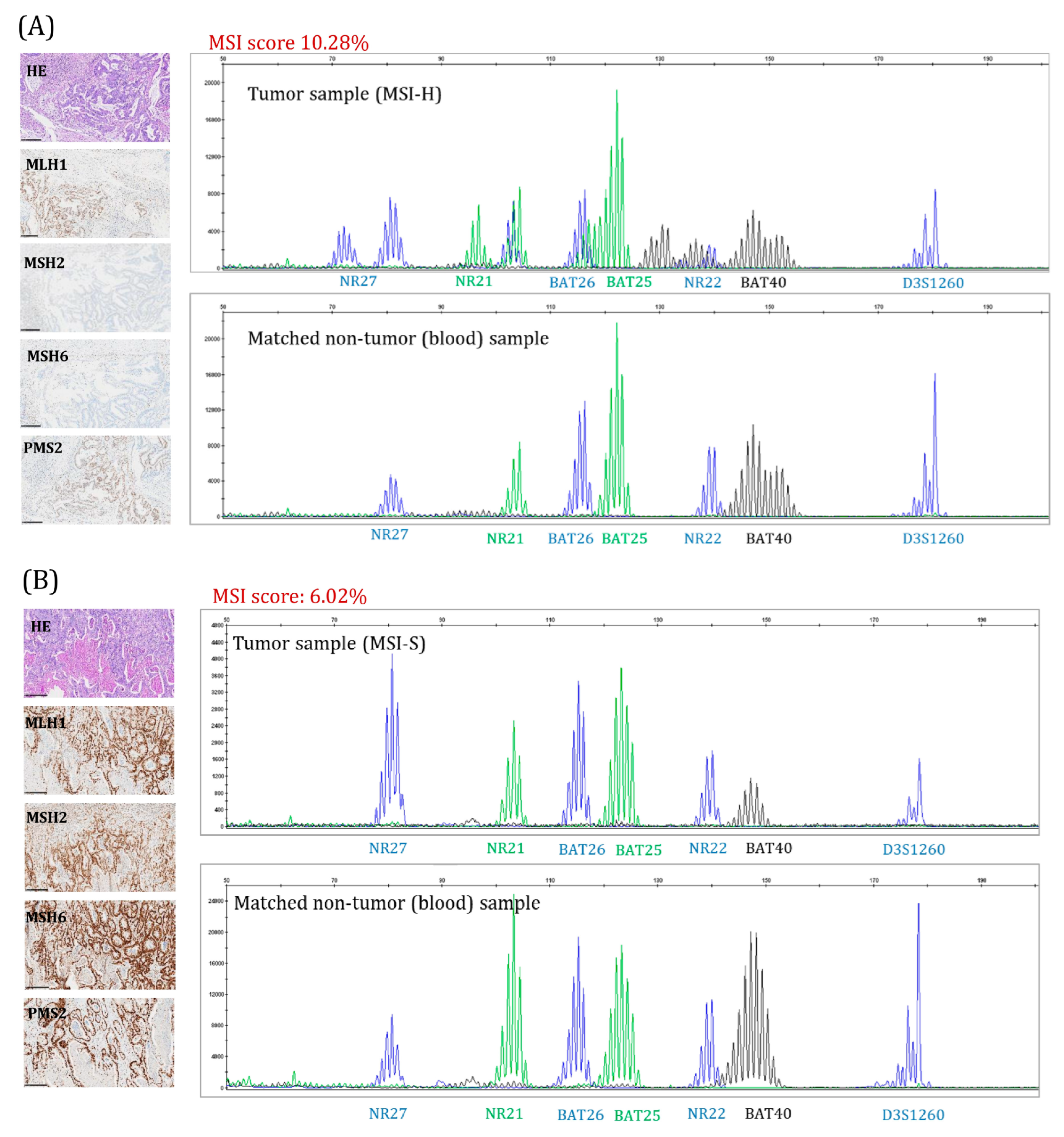
4.4. MSI Detection Using Next Generation Sequencing (MSI-NGS) and MSI Score Determination
4.5. Tumor Mutational Burden (TMB)
4.6. Immunohistochemical (IHC) Analysis
4.7. Methylation-Specific MLPA (MS-MLPA)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSI | Microsatellite instability |
| MSI-H | Microsatellite instability high |
| MSI-S | Microsatellite stable |
| MS loci | Microsatellite loci |
| IHC | Immunohistochemistry |
| PCR | Polymerase chain reaction |
| NGS | Next-generation sequencing |
| MMR | Mismatch repair |
| TMB | Tumor mutational burden |
| ROC | Receiver operating characteristic curve |
| AUC | Area under the curve |
| LR | Likelihood ratio |
| PPV | Positive predictive value |
| NPV | Negative predictive value |
References
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite Instability: A Review of What the Oncologist Should Know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Stjepanovic, N.; Moreira, L.; Carneiro, F.; Balaguer, F.; Cervantes, A.; Balmaña, J.; Martinelli, E. Hereditary Gastrointestinal Cancers: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1558–1571. [Google Scholar] [CrossRef] [PubMed]
- Awosika, J.A.; Gulley, J.L.; Pastor, D.M. Deficient Mismatch Repair and Microsatellite Instability in Solid Tumors. Int. J. Mol. Sci. 2025, 26, 4394. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, M.; Mehnert, J.; Silk, A.W.; Jabbour, S.K.; Ganesan, S.; Popli, P.; Riedlinger, G.; Stephenson, R.; de Meritens, A.B.; Leiser, A.; et al. Real-World Application of Tumor Mutational Burden-High (TMB-High) and Microsatellite Instability (MSI) Confirms Their Utility as Immunotherapy Biomarkers. ESMO Open 2022, 7, 100336. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.Y.; Andre, F.; et al. ESMO Recommendations on Microsatellite Instability Testing for Immunotherapy in Cancer, and Its Relationship with PD-1/PD-L1 Expression and Tumour Mutational Burden: A Systematic Review-Based Approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Vikas, P.; Messersmith, H.; Compton, C.; Sholl, L.; Broaddus, R.R.; Davis, A.; Estevez-Diz, M.; Garje, R.; Konstantinopoulos, P.A.; Leiser, A.; et al. Mismatch Repair and Microsatellite Instability Testing for Immune Checkpoint Inhibitor therapy: ASCO Endorsement of College of American Pathologists Guideline. J. Clin. Oncol. 2023, 41, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Latham, A.; Srinivasan, P.; Kemel, Y.; Shia, J.; Bandlamudi, C.; Mandelker, D.; Middha, S.; Hechtman, J.; Zehir, A.; Dubard-Gault, M.; et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J. Clin. Oncol. 2018, 37, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Gilson, P.; Merlin, J.L.; Harlé, A. Detection of Microsatellite Instability: State of the Art and Future Applications in Circulating Tumour Dna (Ctdna). Cancers 2021, 13, 1491. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Luthra, R.; Saraiya, D.S.; Urbauer, D.L.; Broaddus, R.R. Identification of Cancer Patients with Lynch Syndrome: Clinically Significant Discordances and Problems in Tissue-Based Mismatch Repair Testing. Cancer Prev. Res. 2012, 5, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Bartels, S.; Grote, I.; Wagner, M.; Boog, J.; Schipper, E.; Reineke-Plaass, T.; Kreipe, H.; Lehmann, U. Concordance in Detection of Microsatellite Instability by PCR and NGS in Routinely Processed Tumor Specimens of Several Cancer Types. Cancer Med. 2023, 12, 16707–16715. [Google Scholar] [CrossRef] [PubMed]
- Sfakianaki, M.; Tzardi, M.; Tsantaki, K.; Koutoulaki, C.; Messaritakis, I.; Datseri, G.; Moustou, E.; Mavroudis, D.; Souglakos, J. Evaluation of Microsatellite Instability Molecular Analysis versus Immuno-Histochemical Interpretation in Malignant Neoplasms with Different Localizations. Cancers 2023, 15, 353. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Huang, Y.; Fang, X.; Liu, C.; Deng, W.; Zhong, C.; Xu, J.; Xu, D.; Yuan, Y. A Novel and Reliable Method to Detect Microsatellite Instability in Colorectal Cancer by Next-Generation Sequencing. J. Mol. Diagn. 2018, 20, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Trabucco, S.E.; Gowen, K.; Maund, S.L.; Sanford, E.; Fabrizio, D.A.; Hall, M.J.; Yakirevich, E.; Gregg, J.P.; Stephens, P.J.; Frampton, G.M.; et al. A Novel Next-Generation Sequencing Approach to Detecting Microsatellite Instability and Pan-Tumor Characterization of 1000 Microsatellite Instability–High Cases in 67,000 Patient Samples. J. Mol. Diagn. 2019, 21, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Chen, H.Z.; Smith, A.; Samorodnitsky, E.; Wing, M.R.; Reeser, J.W.; Roychowdhury, S. Detection of Microsatellite Instability Biomarkers via Next-Generation Sequencing. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2020; Volume 2055, pp. 119–132. [Google Scholar]
- Boyarskikh, U.; Kechin, A.; Khrapov, E.; Fedyanin, M.; Raskin, G.; Mukhina, M.; Kravtsova, E.; Tsukanov, A.; Achkasov, S.; Filipenko, M. Detecting Microsatellite Instability in Endometrial, Colon, and Stomach Cancers Using Targeted NGS. Cancers 2023, 15, 5065. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.Y.; Kim, D.G.; Ahn, S.; Ha, S.Y.; Jang, K.T.; Kim, K.M. Comparative Analysis of Microsatellite Instability by Next-Generation Sequencing, MSI PCR and MMR Immunohistochemistry in 1942 Solid Cancers. Pathol. Res. Pract. 2022, 233, 153874. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.I.; Quintanilha, J.C.F.; Danziger, N.; Lang, L.; Levitan, D.; Hayne, C.; Hiemenz, M.C.; Smith, D.L.; Albacker, L.A.; Leibowitz, J.; et al. Pan-Tumor Validation of a NGS Fraction-Based MSI Analysis as a Predictor of Response to Pembrolizumab. NPJ Precis. Oncol. 2024, 8, 204. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Ferraro-Peyret, C.; Michaud, F.; Song, L.; Smith, E.; Fabre, G.; Willig, A.; Wong, M.M.L.; Xing, X.; Chong, C.; et al. Improved NGS-Based Detection of Microsatellite Instability Using Tumor-Only Data. Front. Oncol. 2022, 12, 969238. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Nagakubo, Y.; Amemiya, K.; Oyama, T.; Mochizuki, H.; Omata, M. Microsatellite Instability Status Is Determined by Targeted Sequencing with MSIcall in 25 Cancer Types. Clin. Chim. Acta 2020, 502, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Al-Kateb, H.; Knight, S.M.; Sivasankaran, G.; Voss, J.S.; Pitel, B.A.; Blommel, J.H.; Jerde, C.R.; Rumilla, K.M.; Lee, J.L.; Mattson, N.R.; et al. Clinical Validation of the TruSight Oncology 500 Assay for the Detection and Reporting of Pan-Cancer Biomarkers. J. Mol. Diagn. 2025, 27, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Froyen, G.; Geerdens, E.; Berden, S.; Cruys, B.; Maes, B. Diagnostic Validation of a Comprehensive Targeted Panel for Broad Mutational and Biomarker Analysis in Solid Tumors. Cancers 2022, 14, 2457. [Google Scholar] [CrossRef] [PubMed]
- Dedeurwaerdere, F.; Claes, K.B.; Van Dorpe, J.; Rottiers, I.; Van der Meulen, J.; Breyne, J.; Swaerts, K.; Martens, G. Comparison of Microsatellite Instability Detection by Immunohistochemistry and Molecular Techniques in Colorectal and Endometrial Cancer. Sci. Rep. 2021, 11, 12880. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Li, W.; Huang, Y.; Huang, M.; Li, S.; Zhai, X.; Zhao, J.; Gao, C.; Xie, W.; Qin, H.; et al. A Next-Generation Sequencing-Based Strategy Combining Microsatellite Instability and Tumor Mutation Burden for Comprehensive Molecular Diagnosis of Advanced Colorectal Cancer. BMC Cancer 2021, 21, 282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Chun, S.M.; Hong, Y.S.; Kim, K.P.; Kim, S.Y.; Kim, J.; Sung, C.O.; Cho, E.J.; Kim, T.W.; Jang, S.J. Mutation Burden and I Index for Detection of Microsatellite Instability in Colorectal Cancer by Targeted Next-Generation Sequencing. J. Mol. Diagn. 2019, 21, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Stegel, V.; Blatnik, A.; Škof, E.; Dragoš, V.Š.; Krajc, M.; Gregorič, B.; Škerl, P.; Strojnik, K.; Klančar, G.; Banjac, M.; et al. Real-World Data on Detection of Germline and Somatic Pathogenic/Likely Pathogenic Variants in BRCA1/2 and Other Susceptibility Genes in Ovarian Cancer Patients Using Next Generation Sequencing. Cancers 2022, 14, 1434. [Google Scholar] [CrossRef] [PubMed]
- Klančar, G.; Blatnik, A.; Dragoš, V.Š.; Vogrič, V.; Stegel, V.; Blatnik, O.; Drev, P.; Gazič, B.; Krajc, M.; Novaković, S. A Novel Germline MLH1 In-Frame Deletion in a Slovenian Lynch Syndrome Family Associated with Uncommon Isolated PMS2 Loss in Tumor Tissue. Genes 2020, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Pagin, A.; Zerimech, F.; Leclerc, J.; Wacrenier, A.; Lejeune, S.; Descarpentries, C.; Escande, F.; Porchet, N.; Buisine, M.P. Evaluation of a New Panel of Six Mononucleotide Repeat Markers for the Detection of DNA Mismatch Repair-Deficient Tumours. Br. J. Cancer 2013, 108, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Unk, M.; Bombač, A.; Novaković, B.J.; Stegel, V.; Dragoš, V.Š.; Blatnik, O.; Klančar, G.; Novaković, S. Correlation of Treatment Outcome in Sanger/RT-qPCR KIT/PDGFRA Wild-type Metastatic Gastrointestinal Stromal Tumors with Next-generation Sequencing Results: A Single-center Report. Oncol. Rep. 2022, 48, 167. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.; et al. Association of Tumour Mutational Burden with Outcomes in Patients with Advanced Solid Tumours Treated with Pembrolizumab: Prospective Biomarker Analysis of the Multicohort, Open-Label, Phase 2 KEYNOTE-158 Study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]

| Tumor Type | Lynch Syndrome-Associated Cancer | Initial Tumor Samples Group (n) | Validation Cohort (n) |
|---|---|---|---|
| Colorectal cancer | yes | 209 | 201 |
| Prostate cancer | yes | 64 | 58 |
| Gastric cancer | yes | 16 | 16 |
| Biliary tract cancers | yes | 12 | 11 |
| Pancreatic cancer | yes | 13 | 12 |
| Endometrial cancer | yes | 4 | 3 |
| Urothelial cancer | yes | 3 | 3 |
| Esophageal cancer | no | 3 | 3 |
| Origo ignota | no | 2 | 2 |
| Malignant melanoma | no | 1 | 1 |
| Bone cancer | no | 1 | 1 |
| Penile cancer | no | 1 | 1 |
| Adrenal gland tumor | no | 1 | 1 |
| Breast cancer | no | 1 | 1 |
| All tumor samples | 331 | 314 |
| Analytical Parameter | MSI Score Cut-Off Value (%) | |||
|---|---|---|---|---|
| ≥5.7 | ≥8.7 | ≥9.7 | ≥13.8 | |
| Specificity % (95% CI) | 91.9 (88.2–94.6) | 98.6 (96.5–99.5) | 99.7 (98.1–100.0) | 100.0 (98.7–100.0) |
| Sensitivity % (95% CI) | 78.5 (60.4–90.0) | 75.0 (56.6–87.3) | 71.4 (52.9–84.8) | 50.0 (32.6–67.4) |
| Positive predictive value % | 48.9 | 84.0 | 95.2 | 100.0 |
| Negative predictive value % | 97.8 | 97.6 | 97.2 | 95.3 |
| Accuracy | 90.8 | 96.5 | 97.2 | 95.5 |
| TPR + TNR | 170.5 | 173.6 | 171.1 | 150.0 |
| Likelihood Ratio | 10 | 53 | 204 | / |
| MSI Score (%) | MSI-H (n = 28) | MSI-S (n = 286) |
|---|---|---|
| Mean ± SD | 14.28 ± 8.51 | 2.90 ± 1.97 |
| Minimum | 1.12 | 0 |
| Maximum | 34.78 | 13.41 |
| Range | 33.66 | 13.41 |
| Lower 95% CI of mean | 10.99 | 2.67 |
| Upper 95% CI of mean | 17.58 | 3.13 |
| MSI Status | MSI-S | MSI-H | All Samples |
|---|---|---|---|
| No. of usable MSI sites (mean ± SD) | 88.92 ± 5.82 | 91.04 ± 8.67 | 89.11 ± 6.15 |
| No. of unstable MSI sites (mean ± SD) | 2.56 ± 1.70 | 13.18 ± 8.57 |
| MSI Score Cut-Off Value in MSI-H Tumor Samples | MSI Score Cut-Off Value ≥8.7% (n = 21) | MSI Score Cut-Off Value <8.7% (n = 7) |
|---|---|---|
| MSI score (%) (mean ± SD) | 17.67 ± 6.80 | 4.13 ± 1.68 |
| % of tumor cells in tumor sample (mean ± SD) | 73.53 ± 33.84 | 56.43 ± 24.31 |
| TMB (mean ± SD) | 38.15 ± 17.91 | 27.14 ± 13.47 |
| TMB | MSI-H (n = 28) | MSI-S * (n = 269) |
|---|---|---|
| Mean ± SD | 33.77 ± 18.75 | 6.90 ± 4.00 |
| Minimum | 4 | 0 |
| Maximum | 82 | 22 |
| Range | 78 | 22 |
| Lower 95% CI of mean | 25.46 | 6.4 |
| Upper 95% CI of mean | 42.08 | 7.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škerl, P.; Vogrič, V.; Stegel, V.; Šetrajčič Dragoš, V.; Blatnik, O.; Klančar, G.; Novaković, S. Real-World Evaluation of Microsatellite Instability Detection via Targeted NGS Panels in Routine Molecular Diagnostics. Int. J. Mol. Sci. 2025, 26, 7138. https://doi.org/10.3390/ijms26157138
Škerl P, Vogrič V, Stegel V, Šetrajčič Dragoš V, Blatnik O, Klančar G, Novaković S. Real-World Evaluation of Microsatellite Instability Detection via Targeted NGS Panels in Routine Molecular Diagnostics. International Journal of Molecular Sciences. 2025; 26(15):7138. https://doi.org/10.3390/ijms26157138
Chicago/Turabian StyleŠkerl, Petra, Vesna Vogrič, Vida Stegel, Vita Šetrajčič Dragoš, Olga Blatnik, Gašper Klančar, and Srdjan Novaković. 2025. "Real-World Evaluation of Microsatellite Instability Detection via Targeted NGS Panels in Routine Molecular Diagnostics" International Journal of Molecular Sciences 26, no. 15: 7138. https://doi.org/10.3390/ijms26157138
APA StyleŠkerl, P., Vogrič, V., Stegel, V., Šetrajčič Dragoš, V., Blatnik, O., Klančar, G., & Novaković, S. (2025). Real-World Evaluation of Microsatellite Instability Detection via Targeted NGS Panels in Routine Molecular Diagnostics. International Journal of Molecular Sciences, 26(15), 7138. https://doi.org/10.3390/ijms26157138






