Chemosensitizer Effects of Cisplatin- and 5-Fluorouracil-Treated Hepatocellular Carcinomas by Lidocaine
Abstract
1. Introduction
2. Results
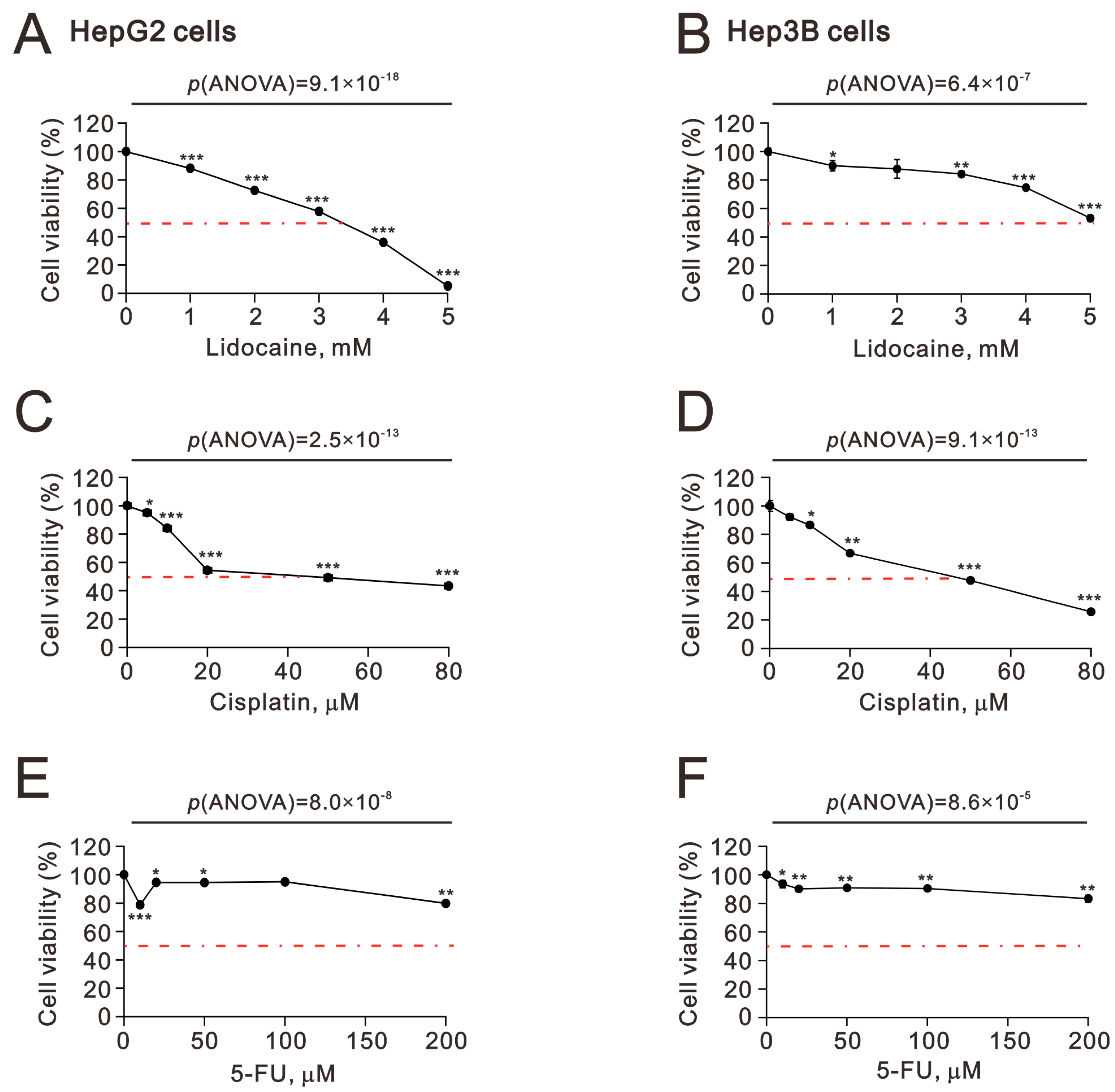
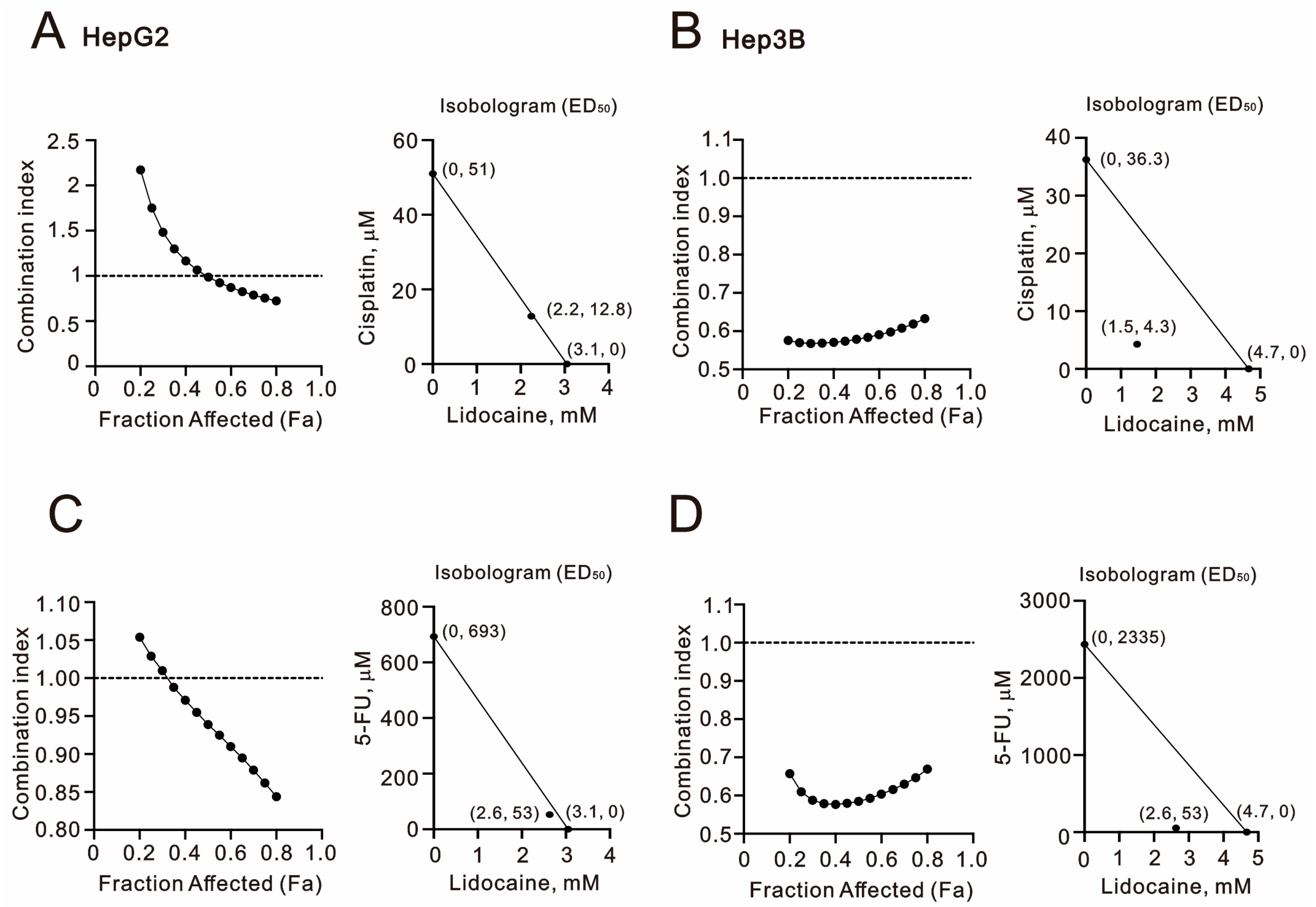
2.1. The Synergistic Effect of Lidocaine Combined with Cisplatin or 5-FU in Human Hepatocellular Carcinoma Cell Lines
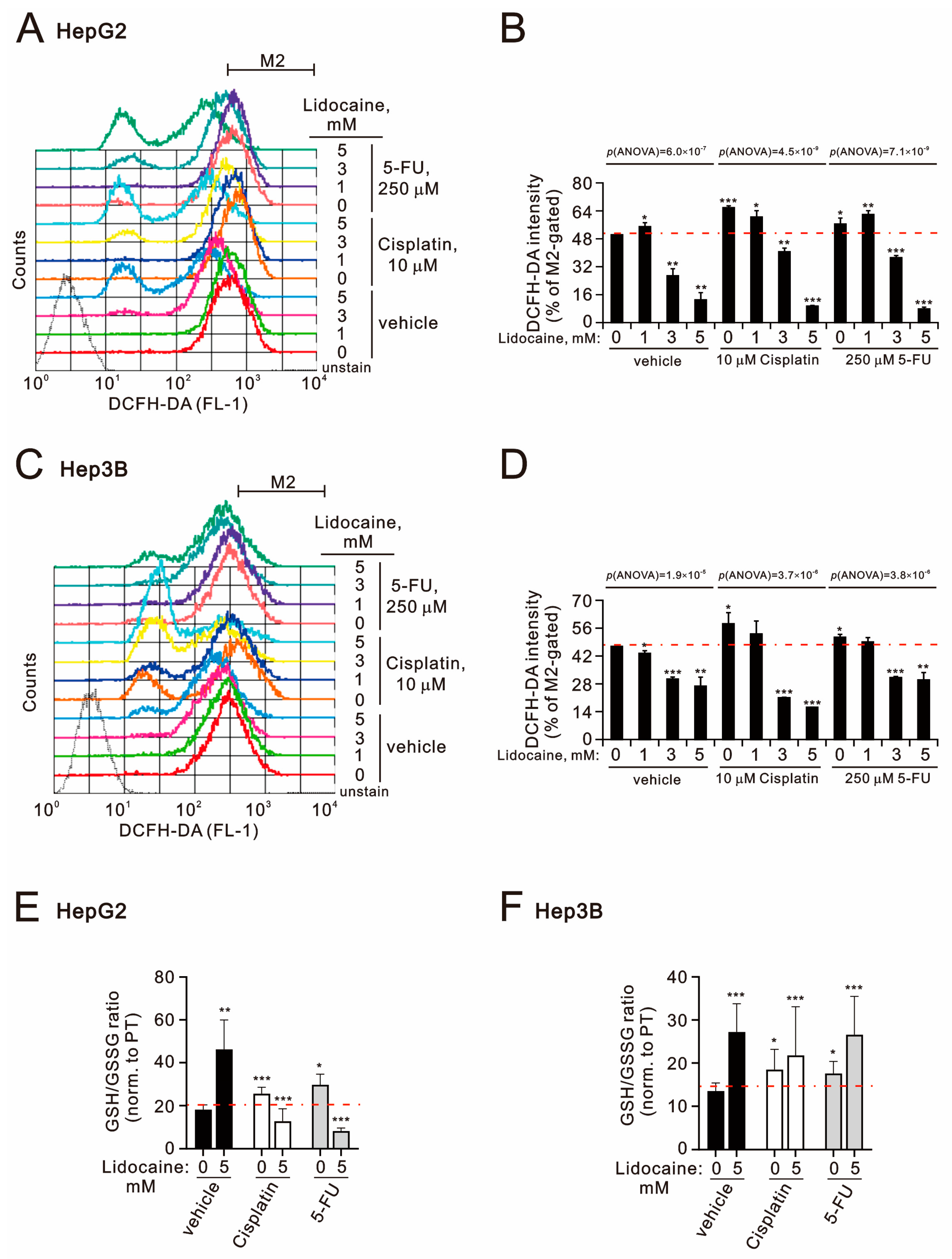
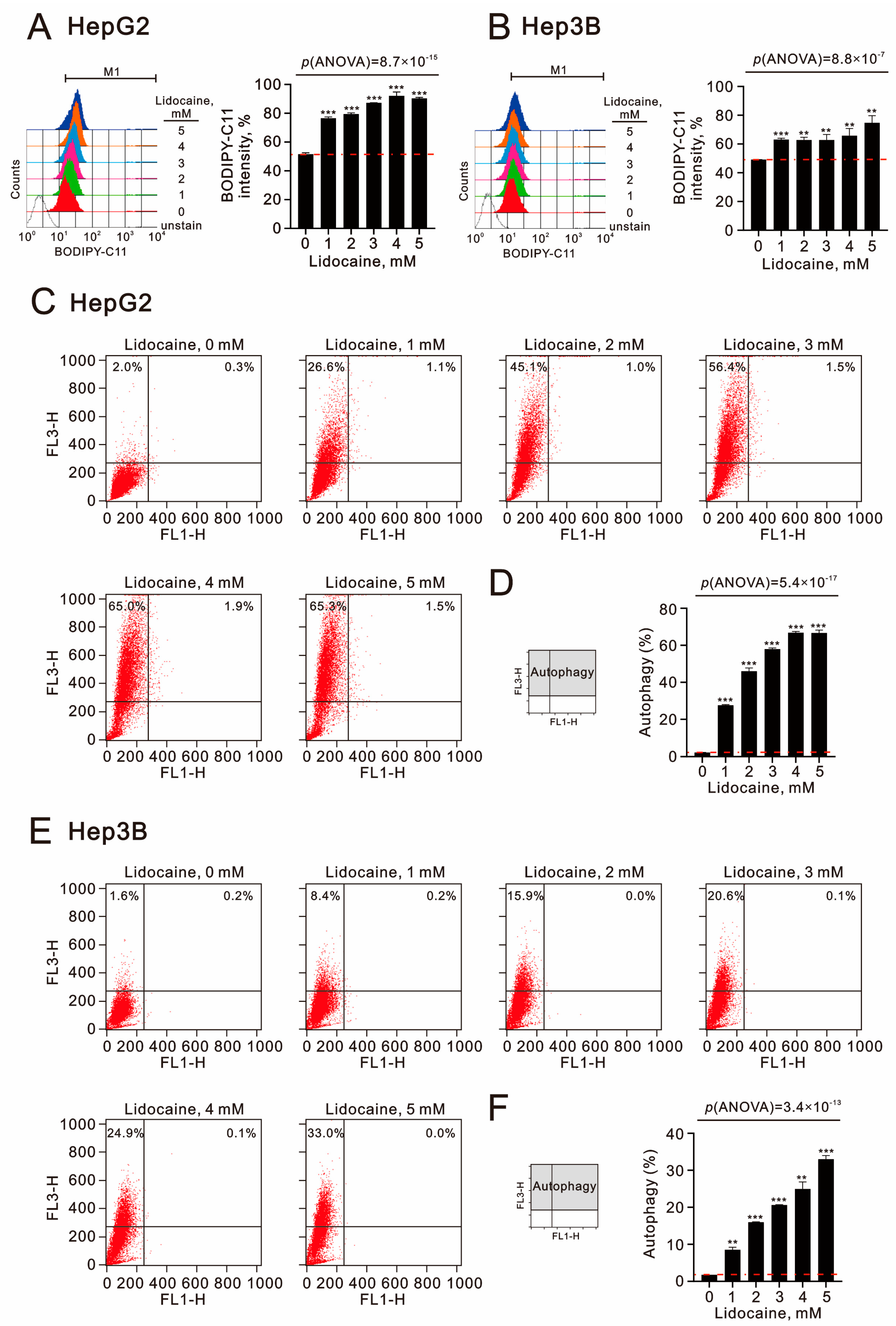
2.2. The Potential Mechanisms of Synergistic Effect of Lidocaine Combined with Cisplatin or 5-FU in Human Hepatocellular Carcinoma Cell Lines
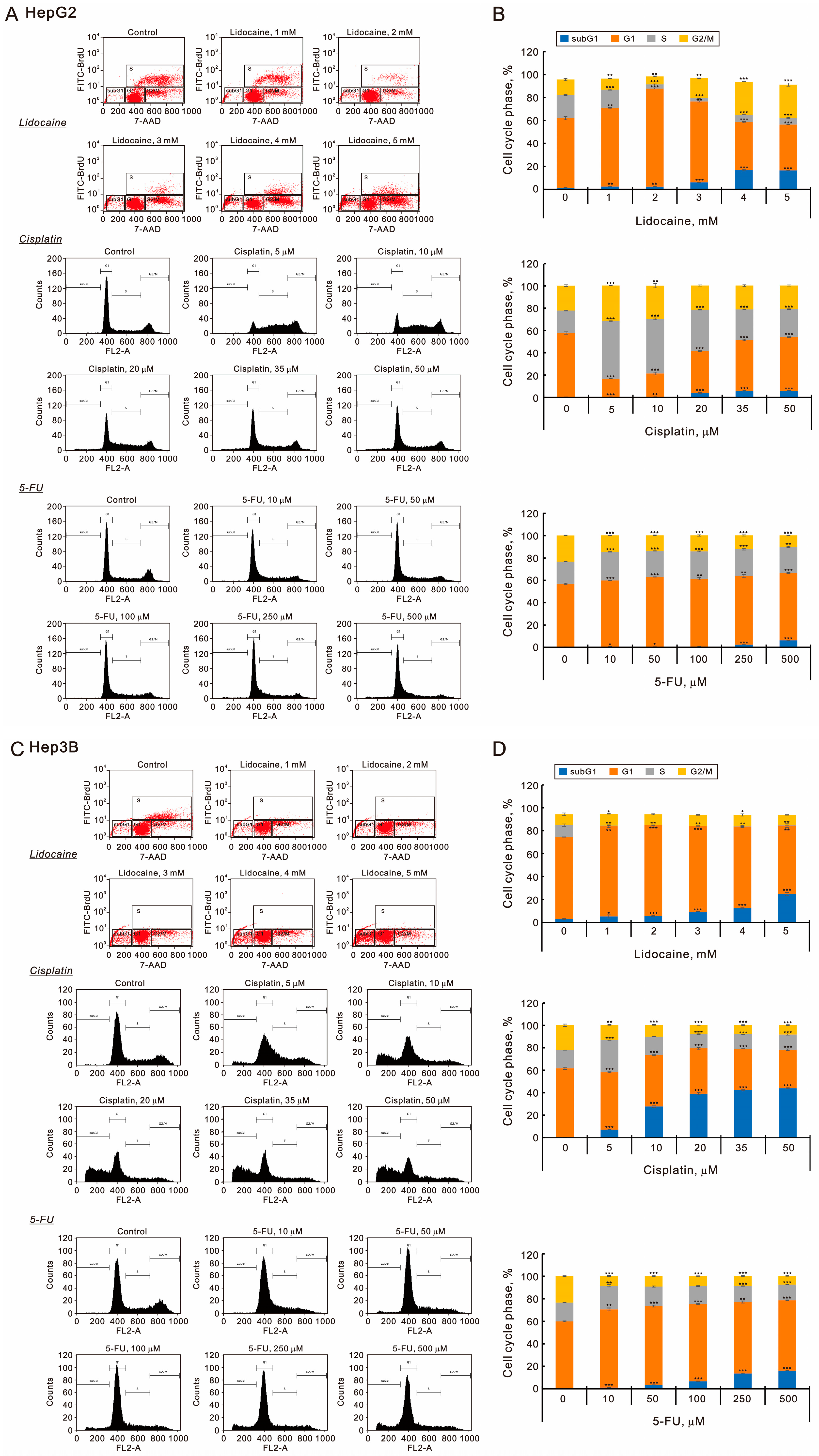
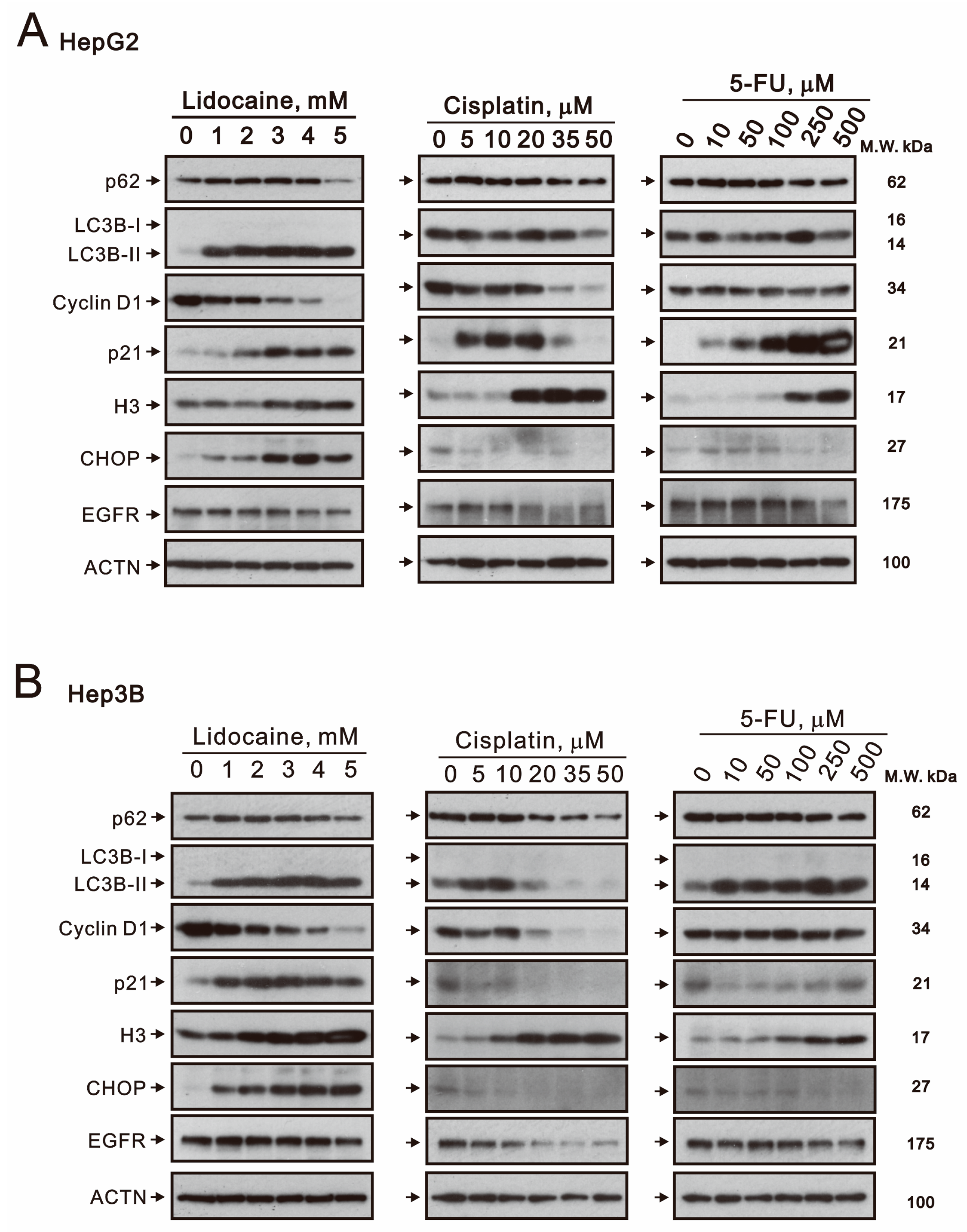
2.3. The Effects of Lidocaine, Cisplatin, and 5-FU on Autophagy, Cell Cycle Progression, Endoplasmic Reticulum Stress, and Signaling Proteins in Human Hepatocellular Carcinoma Cell Lines
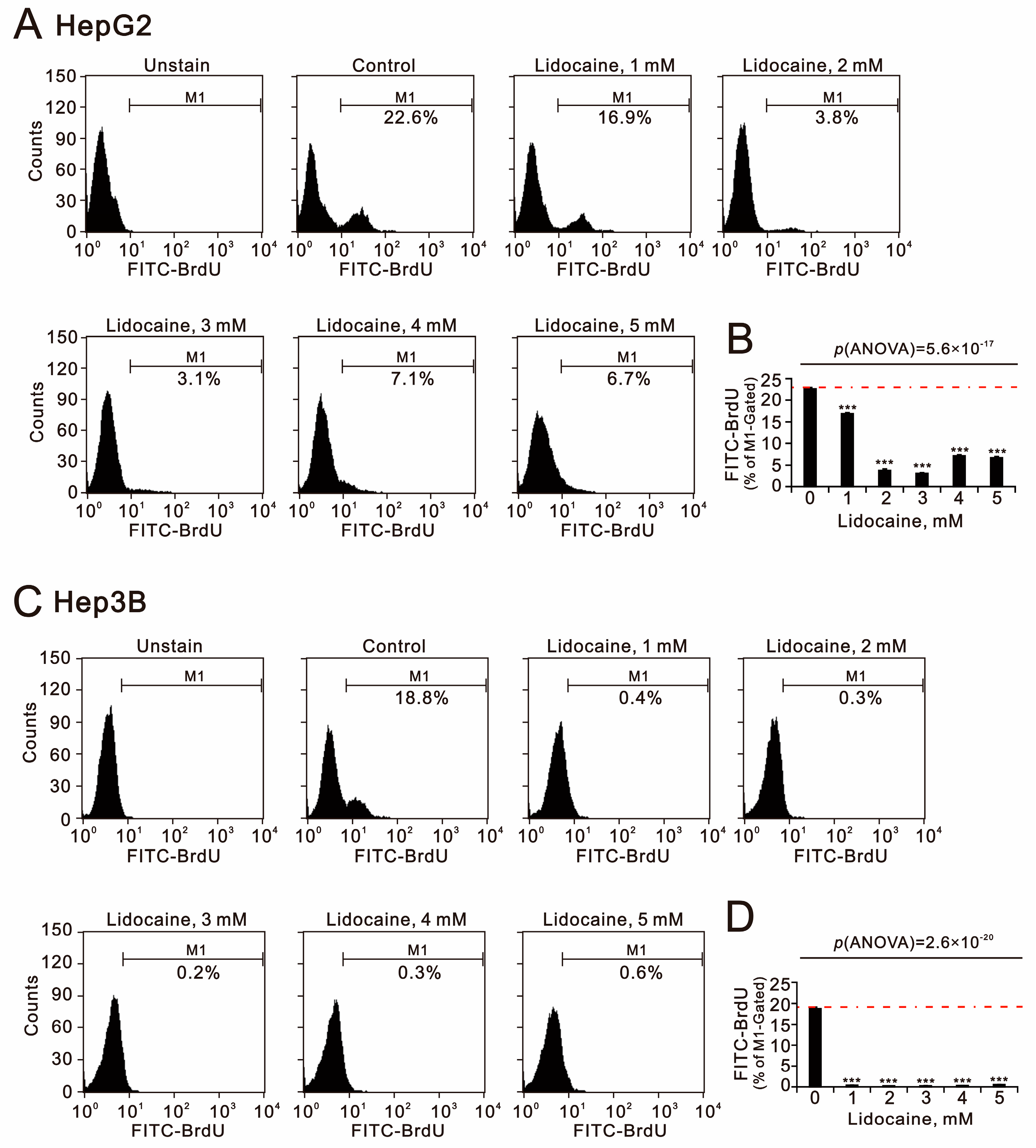
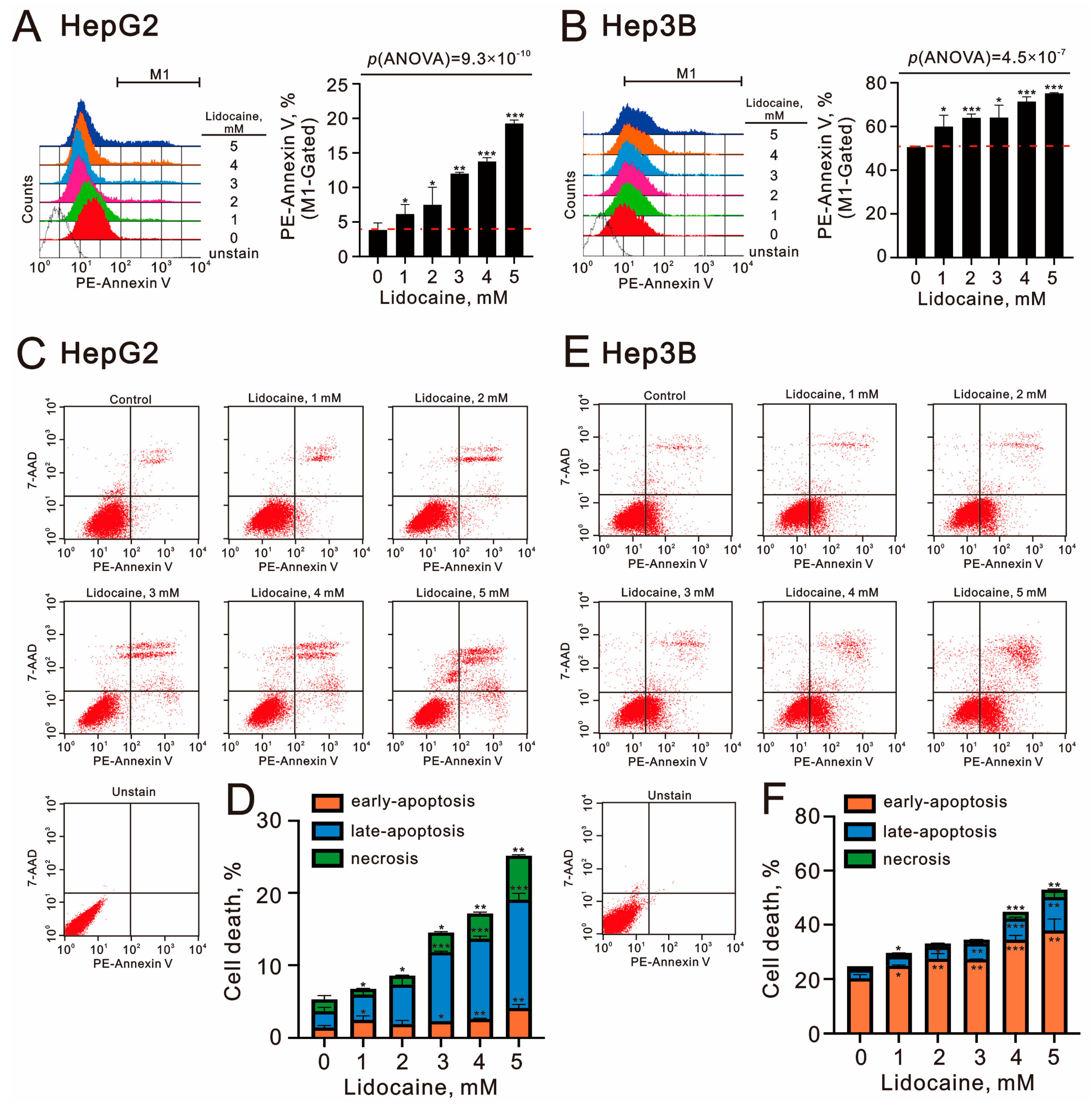
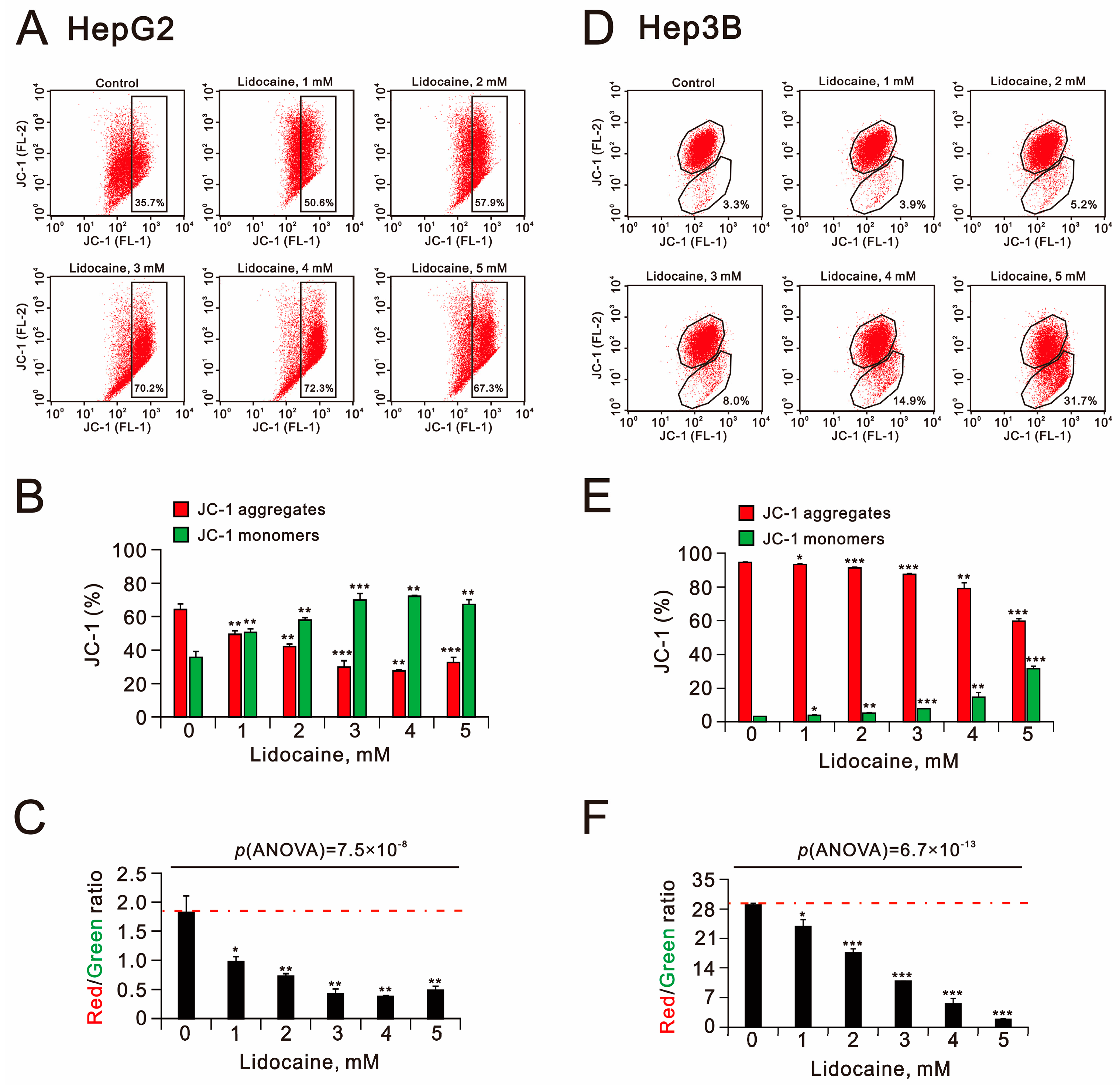
2.4. The Cytotoxic Effects of Lidocaine in Human Hepatocellular Carcinoma Cell Lines
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Viability Analysis and Combination Index Calculated
4.3. Fluorescence-Activated Cell Sorting (FACS), Cell Proliferation, Cell Cycle Profiling, Apoptosis, ROS, Mitochondrial Membrane Potential Analysis, Lipid Peroxidation, and Acidic Vesicular Organelles Detection
4.4. GSH/GSSG Measurement
4.5. Western Blotting Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; Castet, F.; Heikenwalder, M.; Maini, M.K.; Mazzaferro, V.; Pinato, D.J.; Pikarsky, E.; Zhu, A.X.; Finn, R.S. Immunotherapies for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2022, 19, 151–172. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yang, X.R.; Chung, W.Y.; Dennison, A.R.; Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target. Ther. 2020, 5, 146. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Letai, A.; de The, H. Conventional chemotherapy: Millions of cures, unresolved therapeutic index. Nat. Rev. Cancer 2025, 25, 209–218. [Google Scholar] [CrossRef]
- Tilsed, C.M.; Fisher, S.A.; Nowak, A.K.; Lake, R.A.; Lesterhuis, W.J. Cancer chemotherapy: Insights into cellular and tumor microenvironmental mechanisms of action. Front. Oncol. 2022, 12, 960317. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Plana, D.; Palmer, A.C.; Sorger, P.K. Independent Drug Action in Combination Therapy: Implications for Precision Oncology. Cancer Discov. 2022, 12, 606–624. [Google Scholar] [CrossRef]
- Datta, J.; Narayan, R.R.; Kemeny, N.E.; D’Angelica, M.I. Role of Hepatic Artery Infusion Chemotherapy in Treatment of Initially Unresectable Colorectal Liver Metastases: A Review. JAMA Surg. 2019, 154, 768–776. [Google Scholar] [CrossRef]
- Ganeshan, A.; Upponi, S.; Hon, L.Q.; Warakaulle, D.; Uberoi, R. Hepatic arterial infusion of chemotherapy: The role of diagnostic and interventional radiology. Ann. Oncol. 2008, 19, 847–851. [Google Scholar] [CrossRef]
- Laface, C.; Laforgia, M.; Molinari, P.; Ugenti, I.; Gadaleta, C.D.; Porta, C.; Ranieri, G. Hepatic Arterial Infusion of Chemotherapy for Advanced Hepatobiliary Cancers: State of the Art. Cancers 2021, 13, 3091. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Telleria, C.M. Drug Repurposing for Cancer Therapy. J. Cancer Sci. Ther. 2012, 4, ix–xi. [Google Scholar] [CrossRef] [PubMed]
- Vaklavas, C.; Chatzizisis, Y.S.; Tsimberidou, A.M. Common cardiovascular medications in cancer therapeutics. Pharmacol. Ther. 2011, 130, 177–190. [Google Scholar] [CrossRef]
- Lu, G.Y.; Liu, S.T.; Huang, S.M.; Chang, Y.L.; Lin, W.S. Multiple effects of digoxin on subsets of cancer-associated genes through the alternative splicing pathway. Biochimie 2014, 106, 131–139. [Google Scholar] [CrossRef]
- Chang, Y.L.; Huang, L.C.; Chen, Y.C.; Wang, Y.W.; Hueng, D.Y.; Huang, S.M. The synergistic effects of valproic acid and fluvastatin on apoptosis induction in glioblastoma multiforme cell lines. Int. J. Biochem. Cell Biol. 2017, 92, 155–163. [Google Scholar] [CrossRef]
- Hudson, T.L.; Dukes, S.F.; Reilly, K. Use of local anesthesia for arterial punctures. Am. J. Crit. Care 2006, 15, 595–599. [Google Scholar] [CrossRef]
- Evans, D.R.; Fowler-Williams, C.; Ma, D. Is Volatile Anesthesia During Cancer Surgery Likely to Increase the Metastatic Risk? Int. Anesth. Clin. 2016, 54, 92–107. [Google Scholar] [CrossRef]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for Cancer: A Trigger for Metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Jin, W.; Cao, J.; Fu, T.; Ma, D.; Zhang, Y. Lidocaine inhibits the proliferation and invasion of hepatocellular carcinoma by downregulating USP14 induced PI3K/Akt pathway. Pathol. Res. Pr. 2020, 216, 152963. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, R.; Cheng, Y.; Wu, X.; Xi, S.; Sun, Y.; Jiang, H. Lidocaine inhibits the proliferation of lung cancer by regulating the expression of GOLT1A. Cell Prolif. 2017, 50, e12364. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Yi, X.; Jin, C. Lidocaine inhibits the lung cancer progression through decreasing the HIST1H2BL levels via SIRT5 mediated succinylation. Sci. Rep. 2024, 14, 23310. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Liu, S.T.; Huang, S.M.; Wu, Z.F. Apoptosis, Proliferation, and Autophagy Are Involved in Local Anesthetic-Induced Cytotoxicity of Human Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 15455. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. Evaluation of synergism in drug combinations and reference models for future orientations in oncology. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100110. [Google Scholar] [CrossRef]
- Foucquier, J.; Guedj, M. Analysis of drug combinations: Current methodological landscape. Pharmacol. Res. Perspect. 2015, 3, e00149. [Google Scholar] [CrossRef]
- Ma, J.; Motsinger-Reif, A. Current Methods for Quantifying Drug Synergism. Proteom. Bioinform. 2019, 1, 43–48. [Google Scholar]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Greco, W.R.; Bravo, G.; Parsons, J.C. The search for synergy: A critical review from a response surface perspective. Pharmacol. Rev. 1995, 47, 331–385. [Google Scholar] [CrossRef]
- Qiu, G.H.; Xie, X.; Xu, F.; Shi, X.; Wang, Y.; Deng, L. Distinctive pharmacological differences between liver cancer cell lines HepG2 and Hep3B. Cytotechnology 2015, 67, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Slany, A.; Haudek, V.J.; Zwickl, H.; Gundacker, N.C.; Grusch, M.; Weiss, T.S.; Seir, K.; Rodgarkia-Dara, C.; Hellerbrand, C.; Gerner, C. Cell characterization by proteome profiling applied to primary hepatocytes and hepatocyte cell lines Hep-G2 and Hep-3B. J. Proteome Res. 2010, 9, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wen, X.; Li, H.; He, J.; Wang, Y.; Wu, H.; Wang, H.; Wang, X. Up-regulation of Cav3.1 expression in SH-SY5Y cells induced by lidocaine hydrochloride. Artif. Cells Nanomed. Biotechnol. 2018, 46, 372–379. [Google Scholar] [CrossRef]
- Debnath, J. The multifaceted roles of autophagy in tumors-implications for breast cancer. J. Mammary Gland. Biol. Neoplasia 2011, 16, 173–187. [Google Scholar] [CrossRef]
- Gao, P.; Peng, F.; Liu, J.; Wu, W.; Zhao, G.; Liu, C.; Cao, H.; Li, Y.; Qiu, F.; Zhang, W. Lidocaine Enhanced Antitumor Efficacy and Relieved Chemotherapy-Induced Hyperalgesia in Mice with Metastatic Gastric Cancer. Int. J. Mol. Sci. 2025, 26, 828. [Google Scholar] [CrossRef]
- Chen, T.H.; Lin, S.H.; Lee, M.Y.; Wang, H.C.; Tsai, K.F.; Chou, C.K. Mitochondrial alterations and signatures in hepatocellular carcinoma. Cancer Metastasis Rev. 2025, 44, 34. [Google Scholar] [CrossRef]
- Ciccarone, F.; Castelli, S.; Ciriolo, M.R. Oxidative Stress-Driven Autophagy acROSs Onset and Therapeutic Outcome in Hepatocellular Carcinoma. Oxid. Med. Cell Longev. 2019, 2019, 6050123. [Google Scholar] [CrossRef]
- van Mierlo, K.M.; Schaap, F.G.; Dejong, C.H.; Olde Damink, S.W. Liver resection for cancer: New developments in prediction, prevention and management of postresectional liver failure. J. Hepatol. 2016, 65, 1217–1231. [Google Scholar] [CrossRef]
- Pantea, R.; Bednarsch, J.; Schmitz, S.; Meister, P.; Heise, D.; Ulmer, F.; Neumann, U.P.; Lang, S.A. The assessment of impaired liver function and prognosis in hepatocellular carcinoma. Expert. Rev. Gastroenterol. Hepatol. 2024, 18, 779–794. [Google Scholar] [CrossRef]
- Liu, H.; Dilger, J.P.; Lin, J. Effects of local anesthetics on cancer cells. Pharmacol. Ther. 2020, 212, 107558. [Google Scholar] [CrossRef]
- Cata, J.P.; Ramirez, M.F.; Perez-Gonzalez, O. Local Anesthetics: Hunting for the Holy Grail of Onco-anesthesia. Pain. Med. 2020, 21, 219–220. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, L.; Cui, Q.; Iftikhar, R.; Xia, Y.; Xu, P. Repositioning Lidocaine as an Anticancer Drug: The Role Beyond Anesthesia. Front. Cell Dev. Biol. 2020, 8, 565. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Baehrecke, E.H.; Brumell, J.H.; Chu, C.T.; Codogno, P.; Cuervo, A.M.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.L.; et al. A comprehensive glossary of autophagy-related molecules and processes (2nd edition). Autophagy 2011, 7, 1273–1294. [Google Scholar] [CrossRef] [PubMed]
- Thorburn, A.; Debnath, J. Targeting chaperone-mediated autophagy in cancer. Sci. Transl. Med. 2011, 3, 109ps45. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Tsukimoto, S.; Kosai, A.; Komatsu, N.; Ouchi, T.; Kimura, M.; Sato-Boku, A.; Yoshida, A.; Yoshino, F.; Abe, T.; et al. Effect of Dental Local Anesthetics on Reactive Oxygen Species: An In Vitro Study. Cureus 2024, 16, e63479. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; He, B.; Wan, T.; Zhu, W.; Han, J.; Zha, B.; Chen, H.; Yang, F.; Li, Q.; Wang, W.; et al. 5-Fluorouracil nanoparticles inhibit hepatocellular carcinoma via activation of the p53 pathway in the orthotopic transplant mouse model. PLoS ONE 2012, 7, e47115. [Google Scholar] [CrossRef]
- Peters, G.J.; Backus, H.H.; Freemantle, S.; van Triest, B.; Codacci-Pisanelli, G.; van der Wilt, C.L.; Smid, K.; Lunec, J.; Calvert, A.H.; Marsh, S.; et al. Induction of thymidylate synthase as a 5-fluorouracil resistance mechanism. Biochim. et Biophys. Acta 2002, 1587, 194–205. [Google Scholar] [CrossRef]
- Song, M.J.; Bae, S.H.; Chun, H.J.; Choi, J.Y.; Yoon, S.K.; Park, J.Y.; Han, K.H.; Kim, Y.S.; Yim, H.J.; Um, S.H.; et al. A randomized study of cisplatin and 5-FU hepatic arterial infusion chemotherapy with or without adriamycin for advanced hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2015, 75, 739–746. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Abdelmalek, M.; Khan, S.; Moylan, C.A.; Rodriquez, L.; Villanueva, A.; Yang, J.D. Current and emerging strategies for the prevention of hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 173–190. [Google Scholar] [CrossRef]
- Fan, H.L.; Liu, S.T.; Chang, Y.L.; Chiu, Y.L.; Huang, S.M.; Chen, T.W. In Vitro Cell Density Determines the Sensitivity of Hepatocarcinoma Cells to Ascorbate. Front. Oncol. 2022, 12, 843742. [Google Scholar] [CrossRef]
- Wu, T.M.; Liu, S.T.; Chen, S.Y.; Chen, G.S.; Wu, C.C.; Huang, S.M. Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells. Front. Oncol. 2020, 10, 1483. [Google Scholar] [CrossRef]
- Hsu, Y.P.; Huang, T.H.; Liu, S.T.; Huang, S.M.; Chen, Y.C.; Wu, C.C. Glucosamine and Silibinin Alter Cartilage Homeostasis through Glycosylation and Cellular Stresses in Human Chondrocyte Cells. Int. J. Mol. Sci. 2024, 25, 4905. [Google Scholar] [CrossRef]
- Fan, H.L.; Chen, J.L.; Liu, S.T.; Lee, J.T.; Huang, S.M.; Wu, Z.F.; Lai, H.C. Remimazolam induced cytotoxicity mediated through multiple stress pathways and acted synergistically with tyrosine kinase inhibitors in hepatocellular carcinoma. Redox Rep. 2025, 30, 2475696. [Google Scholar] [CrossRef]
- Wu, Z.S.; Huang, Y.H.; Huang, S.M. O-Desmethyltramadol Enhanced Anti-Cancer Efficacy over Tramadol Through Non-mu-Opioid Receptor and Differential Cellular Contexts of Human Breast Cancer Cells. Int. J. Mol. Sci. 2025, 26, 4139. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.-W.; Fan, H.-L.; Liu, S.-T.; Huang, S.-M. Chemosensitizer Effects of Cisplatin- and 5-Fluorouracil-Treated Hepatocellular Carcinomas by Lidocaine. Int. J. Mol. Sci. 2025, 26, 7137. https://doi.org/10.3390/ijms26157137
Chen T-W, Fan H-L, Liu S-T, Huang S-M. Chemosensitizer Effects of Cisplatin- and 5-Fluorouracil-Treated Hepatocellular Carcinomas by Lidocaine. International Journal of Molecular Sciences. 2025; 26(15):7137. https://doi.org/10.3390/ijms26157137
Chicago/Turabian StyleChen, Teng-Wei, Hsiu-Lung Fan, Shu-Ting Liu, and Shih-Ming Huang. 2025. "Chemosensitizer Effects of Cisplatin- and 5-Fluorouracil-Treated Hepatocellular Carcinomas by Lidocaine" International Journal of Molecular Sciences 26, no. 15: 7137. https://doi.org/10.3390/ijms26157137
APA StyleChen, T.-W., Fan, H.-L., Liu, S.-T., & Huang, S.-M. (2025). Chemosensitizer Effects of Cisplatin- and 5-Fluorouracil-Treated Hepatocellular Carcinomas by Lidocaine. International Journal of Molecular Sciences, 26(15), 7137. https://doi.org/10.3390/ijms26157137





