Docetaxel Resistance in Breast Cancer: Current Insights and Future Directions
Abstract
1. Introduction
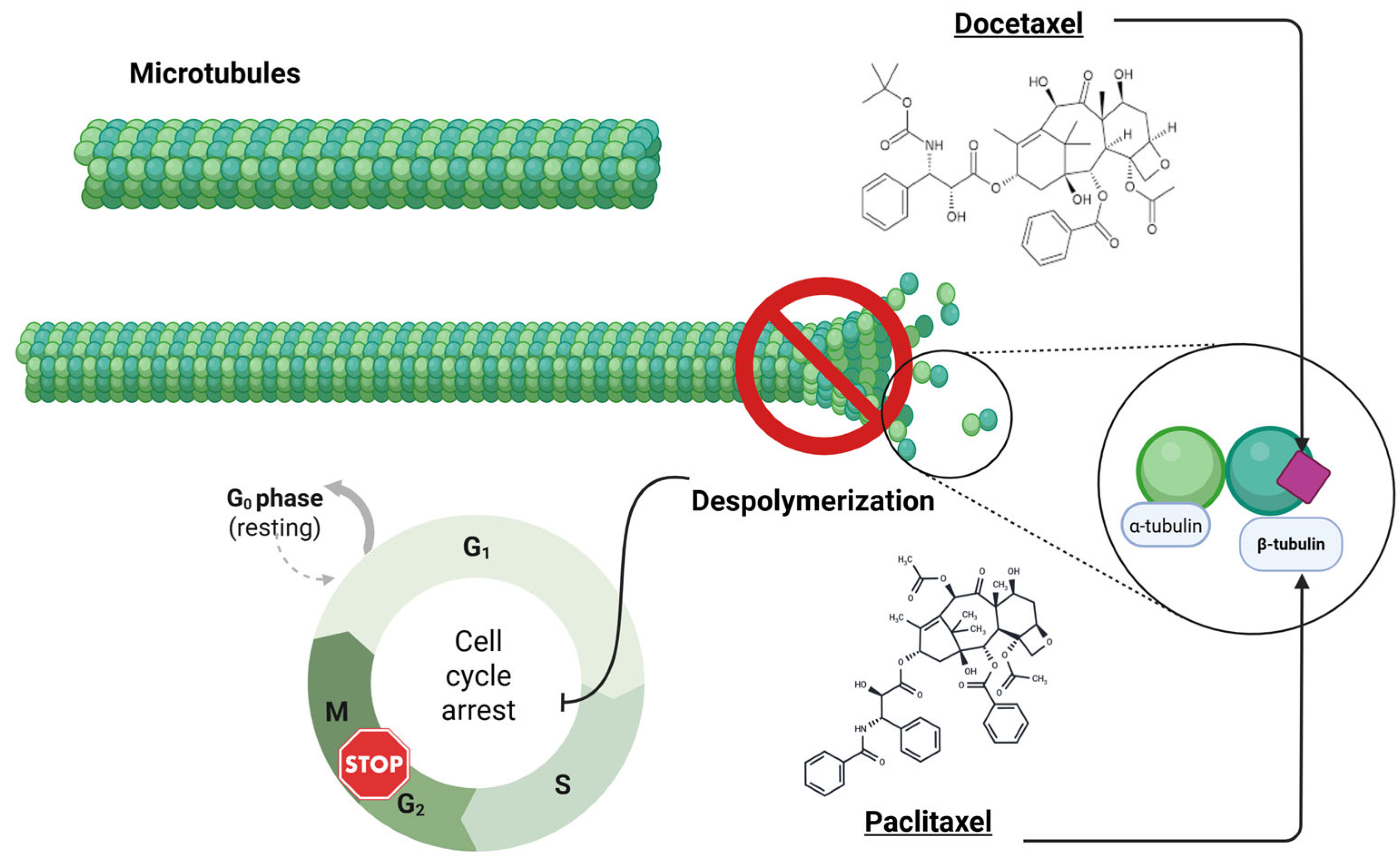
2. Molecular Mechanism of Action of Taxanes (Docetaxel)
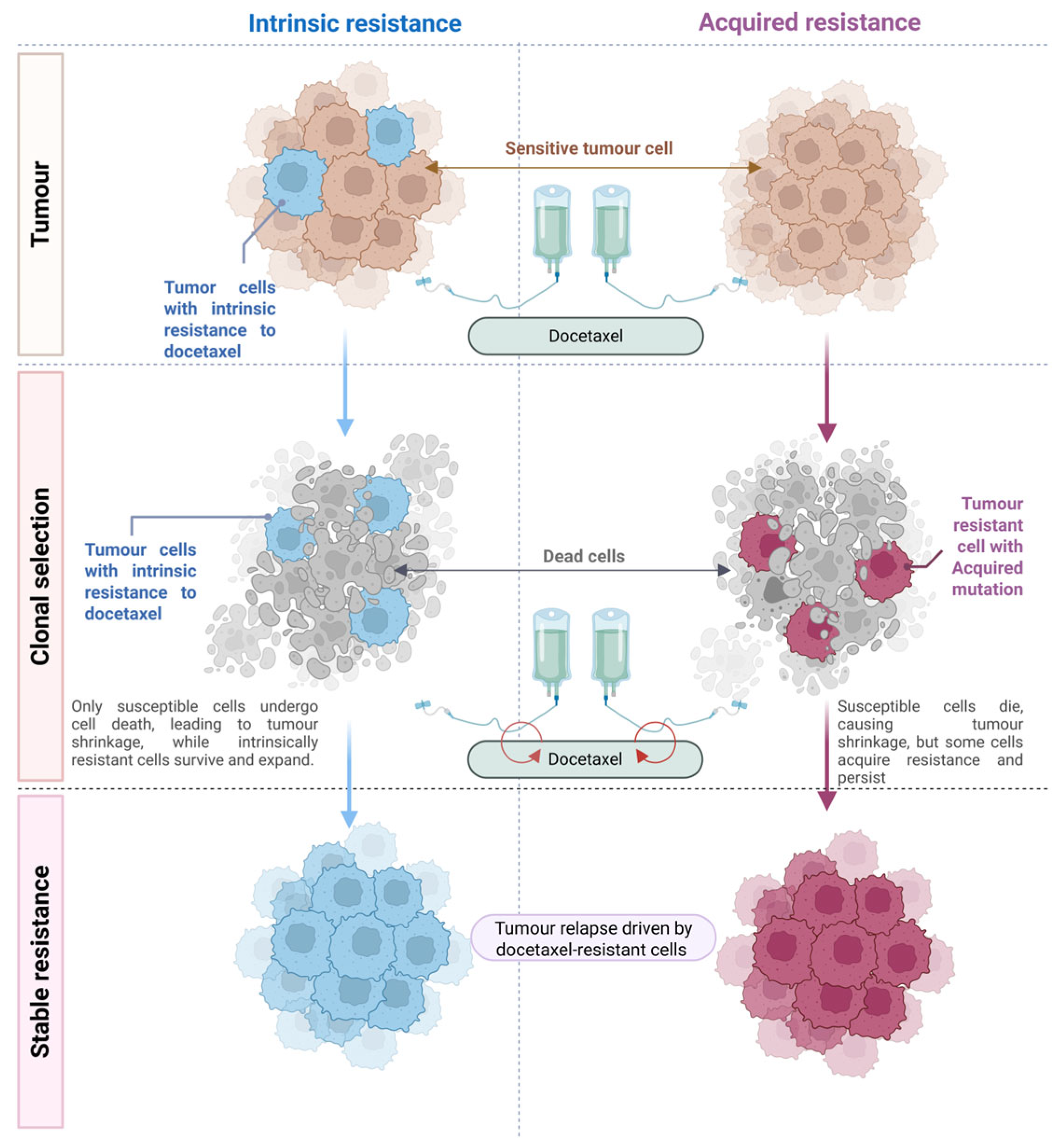
3. Evolutionary Perspective: Intrinsic vs. Acquired Resistance in Cancer
4. Mechanisms of Docetaxel Resistance in Breast Cancer
4.1. Alterations in Tubulin and Microtubule Dynamics
4.2. Increased Drug Efflux and Decreased Intracellular Drug Accumulation
4.3. Evasion of Apoptosis and Cell Death Programs
4.4. Activation of Survival Pathways and EMT
4.5. Cancer Stem Cells and Tumour Heterogeneity
5. Role of Fascin in Tumour Biology and Chemoresistance
6. Current Research Advances and Future Directions
6.1. Genomic and Transcriptomic Profiling of Resistant Tumours
6.2. Non-Coding RNAs (lncRNAs, miRNAs, and circRNAs)
6.3. Novel Therapeutic Agents and Combinations
- Next-generation taxanes: Analogues such as cabazitaxel (a semisynthetic taxane) have been developed to evade P-gp efflux and exhibit activity in docetaxel-resistant tumours (cabazitaxel is approved in prostate cancer after docetaxel failure). Trials on breast cancer are not ongoing, but cabazitaxel and other drugs (e.g., larotaxel) may offer options for taxane-resistant cases [139,140,141].
- Tubulin inhibitors with different mechanisms: Epothilones (such as ixabepilone) also stabilise microtubules but can retain activity in some taxane-resistant tumours, particularly those overexpressing TUBB3, because epothilones bind β-tubulin at different sites. Ixabepilone has been tested in breast cancer and approved for use in certain resistant metastatic cases, highlighting how drugs in the same functional class can sometimes overcome specific resistance, such as P-gp (ixabepilone is a poor P-gp substrate) or β-tubulin alterations [142,143].
- Targeted pathway inhibitors: Combining docetaxel with targeted inhibitors is a major research area. PI3K inhibitors (e.g., buparlisib and alpelisib) and AKT inhibitors have been used to overcome resistance mediated by the PI3K/Akt pathway [144]. mTOR inhibitors (everolimus) combined with taxanes have shown some synergy in preclinical models, and a clinical trial of everolimus with weekly paclitaxel showed improved pathological complete response in HER2-negative breast cancer, suggesting that a similar strategy might improve docetaxel efficacy [145]. Notch pathway inhibitors (gamma secretase inhibitors) and hedgehog inhibitors are being studied to deplete cancer stem cells and potentially improve the chemo response [146,147]. There is also interest in inhibiting anti-apoptotic proteins; for example, a phase I trial combining venetoclax (BCL-2 inhibitor) with pegylated liposomal doxorubicin in TNBC could be envisioned with docetaxel, if the preclinical rationale is strong.
- Immunotherapy combinations: While not directly reversing a resistance mechanism in the classical sense, the use of immunotherapy (such as checkpoint inhibitors) with chemotherapy can provide an alternative way to kill tumour cells by harnessing the immune system. In TNBC, the addition of atezolizumab (anti-PD-L1) to nab-paclitaxel improves outcomes in PD-L1+ patients. Trials have added checkpoint inhibitors to docetaxel. The idea is that even if some cells resist the direct effect of docetaxel, they may become more immunogenic (chemotherapy can cause immunogenic cell death in some cases), and immunotherapy can then eliminate those cells. In addition, chemotherapy may modulate the immune environment to make immunotherapy more effective. This multipronged kill approach may circumvent individual cellular resistance mechanisms.
| NTC Registry | Study Title | Phase | Interventions | Design | Start Date | References |
|---|---|---|---|---|---|---|
| NCT05165225 | Phase II Neoadjuvant Pyrotinib Combined with Neoadjuvant Chemotherapy in HER2-Low-Expressing and HR Positive Early or Locally Advanced Breast Cancer: a Single-Arm, Non-Randomized, Single-Center, Open Label Trial | 2 | pyrotinib + epirubicin and cyclophosphamide followed by docetaxel | NA; single group | 13 July 2021 | [148] |
| NCT04293393 | Neoadjuvant Study Chemotherapy vs. Letrozole + Abemaciclib in HR+/HER2− High/Intermediate Risk Breast Cancer Patients | 2 | doxorubicin + cyclophosphamide + docetaxel vs. letrozole +abermaciclib +/− LHRH | RCT; open label | 1 October 2020 | [149] |
| NCT03201861 | Addition of Cisplatin to Adjuvant Chemotherapy for Early-Stage Breast Cancer in High-Risk Women | 3 | wpirubicin + cyclophospamide to docetaxel or paclitaxel vs. paclitaxel + cisplatin | RCT; open label | 27 July 2017 | [150] |
| NCT06107673 | Dalpiciclib Plus AI (Neoadjuvant Endocrine Therapy) Compared with Neoadjuvant Chemotherapy in Early Breast Cancer (EBC) | 2 | NACT-dalpiciclib vs. ciclophosphamide + docetaxel | RCT; masking triple | 30 September 2023 | [151] |
| NTC Registry | Study Title | Phase | Interventions | Design | Start Date | References |
|---|---|---|---|---|---|---|
| NCT06009627 | Study of Neoadjuvant Endocrine Therapy in HR Positive and HER2 Negative Premenopausal Breast Cancer Patients | 2/3 | darxil + exenestane + goserelin vs. docetaxel + doxorubicin + cyclophosphamide | R; open label | 11 April 2023 | [152] |
| NCT03701334 | A Trial to Evaluate Efficacy and Safety of Ribociclib with Endocrine Therapy as Adjuvant Treatment in Patients With HR+/HER2- Early Breast Cancer (NATALEE) | 3 | ribociclib + endocrine therapy (ET) vs. ET | RCT; open label | 10 October 2018 | [153] |
| NCT06375707 | Efficacy and Safety of Ribociclib in Combination with NSAI vs. Physician’s Choice of Chemotherapy Sequential Endocrine Therapy in HR+/HER2− Advanced Breast Cancer | 2 | docetaxel or paclitaxel + vinorelbine + capecitabine: sequential ribociclib: 600mg/d, 3 weeks continuous oral withdrawal for 1-week NSAI: anastrozole 1mg, 1 time/d, oral or letrozole: 2.5mg, 1 time/d, oral | RCT; open label | 9 January 2024 | N/A |
| NCT04872985 | Pyrotinib in Combination with Neoadjuvant Chemotherapy in HR+/HER2-, HER4 High Expression Breast Cancer Patients: a Phase II Trial | 2 | pyrotinib + doxorubicin/epirubicin + cyclophosphamide followed by docetaxel/nab-paclitaxel | RCT; DB | 20 April 2021 | [148] |
| NCT05296746 | Neoadjuvant and Adjuvant Ribociclib and ET for Clinically High-Risk ER+ and HER2- Breast Cancer | 2 | NACT–ACT ribociclib + letrozole (responder) vs. (non-responder NACT: ribociclib + letrozole ACT—Arm1: doxorubicin + cyclophosphamide + docetaxel; Arm2: docetaxel + cyclophosphamide; Arm3: paclitaxel + doxorubicin + cyclophosphamide. All patients receive ribociclib + letrozole or AI | NR; parallel assigned | 3 May 2022 | [154] |
| NTC Registry | Study Title | Phase | Interventions | Design | Start Date | References |
|---|---|---|---|---|---|---|
| NCT05638594 | Pyrotinib Combined with Trastuzumab, Dalpiciclib, Letrozole vs. TCbHP (Trastuzumab Plus Pertuzumab with Docetaxel and Carboplatin) as Neoadjuvant Treatment in HR +/HER2 + Breast Cancer | 2 | pyrotinib + trastuzumab + dalpiciclib + letrozole vs. trastuzumab + pertuzumab + docetaxel + carboplatin | RCT; open label | 20 December 2022 | [155] |
| NCT05346224 | A Study to Evaluate the Efficacy and Safety of HLX11 vs. EU-Perjeta® in the Neoadjuvant Therapy of HER2-Positive and HR-Negative Early-Stage or Locally Advanced Breast Cancer | 3 | NACT: HLX11 + trastuzumab docetaxel; ACT: doxorubicin + cyclophosphamide + trastuzumab +HLX11 vs. NACT: EU-Perjeta® + trastuzumab docetaxel; ACT: doxorubicin + cyclophosphamide + trastuzumab + EU-Perjeta® | RCT; open label | 17 April 2022 | [156] |
| NCT05319873 | Ribociclib, Tucatinib, and Trastuzumab for the Treatment of HER2 Positive Breast Cancer | 1/2 | Phase 1b; ribociclib + tucatinib + trastuzumab, if no progression diseases or unacceptable toxicity, allowed to Phase 2 Arm A: ribociclib + tucatinib + trastuzumab + fulvestrant; Arm B: docetaxel + carboplatin + trastuzumab; Arm C: ribociclib + tucatinib + trastuzumab | RCT; sequential assignment | 7 April 2022 | [157] |
| NCT05900206 | Trastuzumab Deruxtecan vs. Standard Neoadjuvant Treatment for HER2-Positive Breast Cancer | 2 | Arm 1: trastuzumab deruxtecan; Arm 2: docetaxel/paclitaxel + carboplatin + trastuzumab + pertuzumab; Arm 3: (ER + and luminal) ribociclib + letrozole; Arm 4: (ER- and luminal) epirubicin vs. cyclosporine Arm 5: trastuzumab deruxtecan or docetaxel/paclitaxel + carboplatin + trastuzumab + pertuzumab | RCT; parallel assigment; open label | 26 October 2023 | Link |
| NCT06770296 | The Dosing Regimen of Pyrotinib in HER2-Positive Advanced First-Line Breast Cancer: a Phase I Clinical Study | 1 | pyrotinib low dose + trastuzumab + docetaxel vs. pyrotinib normal dose + trastuzumab + docetaxel | RCT; sequential assignment; open label | 1 November 2024 | N/A |
| NCT05704829 | NeoAdjuvant Therapy With Trastuzumab-deruxtecan vs. Chemotherapy+Trastuzumab+Pertuzumab in HER2+ Early Breast Cancer | 2 | T-DXd iv NACT-ACT vs. pacli-/docetacel + carboplatin + trastuzumab + pertuzumab | RCT; crossover assignment; open label | 5 February 2024 | [158] |
| NCT05720026 | Study to Evaluate the Efficacy and Safety of SYSA1901 vs. Perjeta® of HER2-Positive Breast Cancer | 3 | SYSA1901 + trastuzumab + docetaxel vs. Perjeta® + trastuzumab + docetaxel | RCT; DB; parallel-controlled | 9 January 2023 | N/A |
| NCT06278870 | Disitamab Vedotin + Pyrotinib vs. THP in the First-Line Treatment for HER2+ Advanced Breast Cancer Clinical Trial | 3 | disitamab vedotin + pyrotinib + trastuzumab vs. trastuzumab + pertuzumab + docetaxel/paclitaxel/paclitaxel liposomal/paclitaxel alb. | RCT; quadruple; parallel assignment | 30 June 2023 | [159] |
| NCT06747338 | A Phase III Study of KN026 in Combination with HB1801 ± Carboplatin as Neoadjuvant Treatment for Early or Locally Advanced HER2-Positive Breast Cancer | 3 | KN026 + HB1801 + carboplatin vs. trastuzumab + pertuzumab + docetaxel + carboplatin | RCT; parallel assignment; open label | 16 December 2024 | [160] |
| NCT06038539 | Efficacy and Safety of the Proposed Biosimilar Pertuzumab (PERT-IJS) vs. EU-Perjeta® Along with Trastuzumab and Chemotherapy (Carboplatin and Docetaxel) as Neoadjuvant Treatment in Chemotherapy naïve Patients with Early Stage or Locally Advanced HR Negative and HER2 Positive Breast Cancer | 3 | PERT-IJS + trastuzumab + carboplatin and docetaxel vs. EU-Perjeta® + trastuzumab + carboplatin and docetaxel | RCT; DB; parallel assignment | 31 January 2025 | [161] |
| NTC Registry | Study Title | Phase | Interventions | Design | Start Date | References |
|---|---|---|---|---|---|---|
| NCT04760431 | TKIs vs. Pertuzumab in HER2+ Breast Cancer Patients with Active Brain Metastases (HER2BRAIN) | 2 | trastuzumab docetaxel pyrotinib vs. trastuzumab + docetaxel + pertuzumab | RCT; 1:1 | 25 January 2021 | N/A |
| NCT05621434 | A Study to Evaluate Inetetamab + Pyrotinib + Chemotherapy in Previously Untreated HER2-Positive Metastatic Breast Cancer | 2 | inetetamab + pyrotinib and (taxane, vinorelbine, capecitabine, eribulin, and other agents (physicians choices). | NR | 10 December 2022 | [162] |
| NCT05296798 | A Study to Evaluate the Efficacy and Safety of Giredestrant in Combination with Phesgo (Pertuzumab, Trastuzumab, and Hyaluronidase-zzxf) vs. Phesgo in Participants with Locally Advanced or Metastatic Breast Cancer (heredERA Breast Cancer) | 3 | Induction: giredestrant + pertuzumab + trastuzumab + taxane. Maintenance Arm A: giredestrant + pertuzumab + trastuzumab or Arm B: giredestrant + pertuzumab + trastuzumab + ET | RCT; open label | 18 July 2022 | [163] |
| NCT06057610 | A Phase III Study of SHR-A1811 Injection with or Without Pertuzumab in HER2-Positive Recurrent or Metastatic Breast Cancer | 3 | A: SHR-A1811; B: SHR-A1811 + pertuzumab; C: trastuzumab + pertuzumab + docetaxel | RCT; open label | 16 October 2023 | [164] |
| NCT05698186 | Thero2-01S22 in HER2-Positive Breast Cancer | 3 | thero2-01S22/placebo + docetaxel or vinorelbine + pertuzumab + trastuzumab | RCT; DB; placebo-controlled | 15 May 2023 | N/A |
| NCT06135714 | Metastasis-Directed Therapy for Oligometastases of Breast Cancer | 3 | Luminal BC: CDK4/6 inhibitors + ET; HER2+: trastuzumab + pertuzumab + docetaxel; TNBC: immune checkpoint inhibitors expressing PD-L1; Arm A continues systemic chemotherapy alone; Arm B followed the same treatment. | RCT; parallel assignment; open label | 11 August 2023 | [165] |
| NCT06439693 | The SAPPHO Study: Sequential Therapy with Curative Intent in de Novo HER2+ Metastatic Breast Cancer | 2 | taxane + trastuzumab + pertuzumab followed by trastuzumab deruxtecan, followed by tucatinib + ado-trastuzumab emtansine, followed by trastuzumab + pertuzumab + tucatinib | NA; single group sequential treatment | 8 August 2024 | N/A |
| NCT06445400 | A Study of BL-M07D1, BL-M07D1+Pertuzumab and BL-M07D1+Pertuzumab+Docetaxel in Patients with Unresectable Locally Advanced or Metastatic HER2-Positive Breast Cancer | 2 | Arm A: BL-M07D1 + pertuzumab; Arm B: BL-M07D1 + pertuzumab + docetaxel | NA; single group; open label | 19 June 2024 | N/A |
| NCT07003074 | A Clinical Study of TQB2102 vs. Docetaxel Plus Trastuzumab and Pertuzumab in the Treatment of HER2 Positive Recurrent or Metastatic Breast Cancer | 3 | Arm A: TQB2102; Arm B: docetaxel + trastuzumab + pertuzumab | RCT; open label; parallel-controlled | August 2025 | [166] |
| NTC Registry | Study Title | Phase | Interventions | Design | Start Date | References |
|---|---|---|---|---|---|---|
| NCT04836156 | Neoadjuvant Therapy Study Guided by Drug Screening in Vitro for Human Epidermal Growth Factor Receptor 2 (HER2) Negative Early Breast Cancer Patients | 1/2 | docetaxel + carboplatin docetaxel + epirubicin | NR; single group assignment; open label | 2 April 2021 | [167] |
| NCT05475678 | Clinical Study of Camrelizumab Combined with TCb vs. TCb in Neoadjuvant Treatment of Triple-Negative Breast Cancer | 2 | carrelizumab + docetaxel + carboplatin vs. docetaxel + carboplatin | RCT; parallel assignment; open label | 19 July 2022 | [168] |
| NCT05645380 | Neoadjuvant TIL- and Response-Adapted Chemoimmunotherapy for TNBC | 2 | Arm A: carboplatin + docetaxel + pembrolizumab; Arm B: carboplatin + docetaxel + doxorubicin + cyclophosphamide + pembrolizumab | NR; parallel assignment; open label | 5 December 2022 | [169] |
| NCT04947189 | Seviteronel in Combination with Chemotherapy in Androgen-receptor Positive Metastatic Triple-Negative Breast Cancer | 1/2 | seviteronel + dexamethasone + docetaxel | NR; single group; open label | 1 November 2021 | [170] |
| NCT05076760 | MEM-288 Oncolytic Virus Alone and in Combination with Standard of Care Therapy in Advanced Solid Tumours | 1 | MEM-288 vs. nivolumab + docetaxel | NR; single group; open label | 21 April 2022 | [171] |
| NCT05929768 | Shorter Anthracycline-Free Chemo Immunotherapy Adapted to Pathological Response in Early Triple Negative Breast Cancer (SCARLET), A Randomized Phase III Study | 3 | paclitaxel + carboplatin + pembrolizumab, followed by doxorubicin + cyclophosphamide + pembrolizumab; ACT: pembrolizumab vs. docetaxel + carboplatin + pembrolizumab; ACT: pembrolizumab | RCT; parallel assignment; open label | 15 September 2023 | N/A |
| NCT05978648 | Trilaciclib in Patients with Early-Stage HR-Negative Breast Cancer Receiving Adjuvant Chemotherapy | 2 | trilaciclib + epirubicin + cyclophosphamide + paclitaxel | NR; single group; open label | 20 September 2023 | N/A |
| NCT06225284 | Neoadjuvant Chemotherapy with or Without GnRH Agonist for Premenopausal Triple-negative Early Breast Cancer Patients | 2 | GnRH: goserelin or leuprolide or triptorelin + anthracycline + cyclophosphamide, followed by taxane and optional pembrolizumab vs. anthracycline + cyclophosphamide, followed by taxane and optional pembrolizumab | RCT; parallel assignment; open label | 22 August 2024 | N/A |
| NCT06795503 | Non-Inferiority Study on MRNA-lncRNA Model in Low-Risk Triple-Negative Breast Cancer Patients | 3 | docetaxel + cyclophosphamide vs. epirubicin + cyclophosphamide, followed by paclitaxel | RCT; parallel assignment; open label | 27 January 2025 | N/A |
6.4. Adaptive Therapy and Dosing Strategies
6.5. Biomarker-Guided Therapy
6.6. Targeting the Tumour Microenvironment
6.7. Emerging Drug Targets from Omics
6.8. Clinical Rechallenge and Sequencing
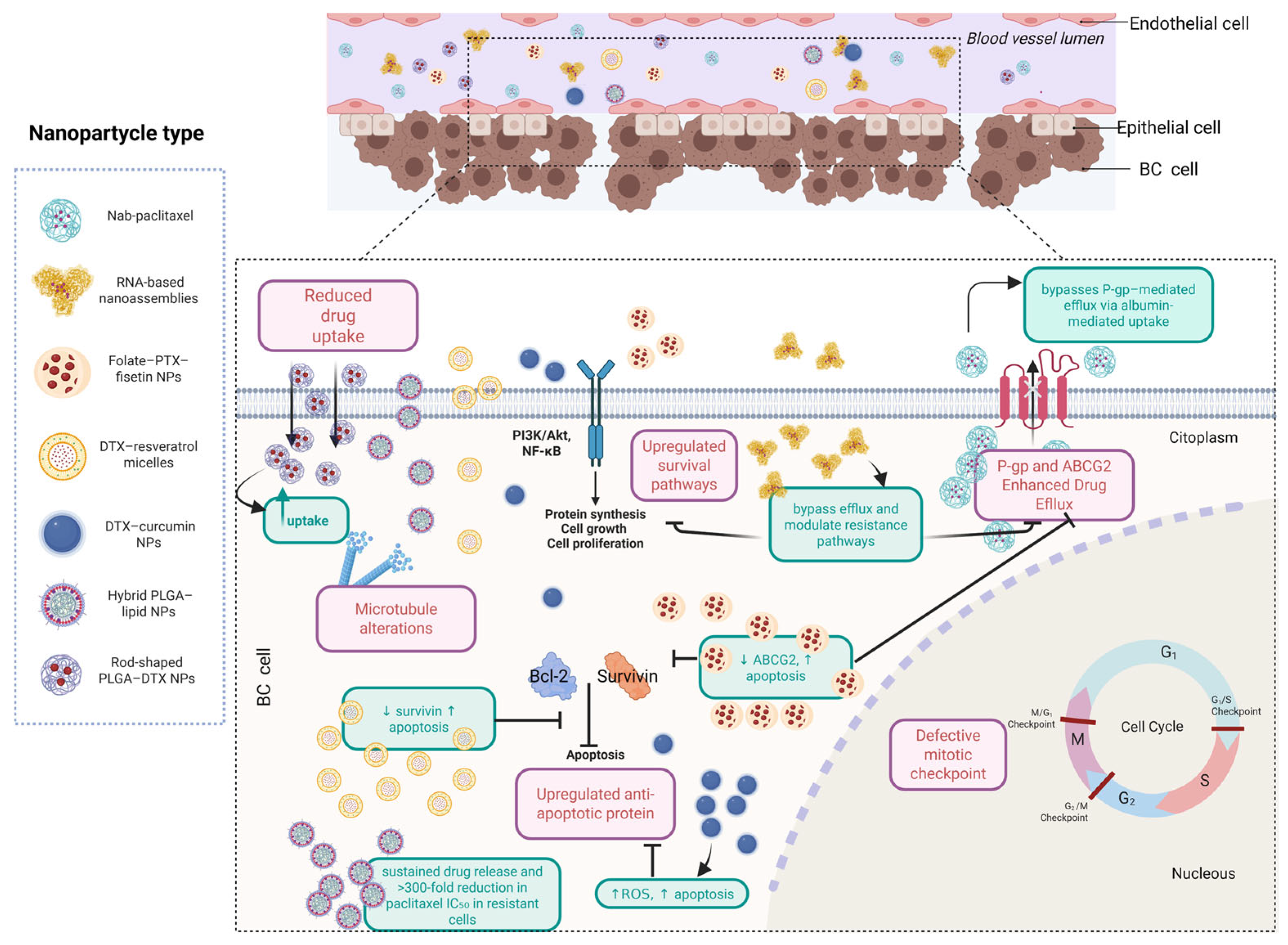
6.9. Nanoparticle-Based Strategies to Overcome Taxane Resistance: Advances, Benefits, and Current Challenges
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKT | Protein Kinase B |
| ALDH | Aldehyde Dehydrogenase |
| ATP | Adenosine Triphosphate |
| BAD | BCL2 Associated Agonist of Cell Death |
| BAK | BCL2 Antagonist/Killer |
| BAX | BCL2 Associated X Protein |
| BCL | B-Cell Lymphoma |
| BCRP | Breast Cancer Resistance Protein |
| CR | Complete Response |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CSC | Cancer Stem Cell |
| DNA | Deoxyribonucleic Acid |
| DTX | Docetaxel |
| EGF | Epidermal Growth Factor |
| ER | Estrogen Receptor |
| ERK | Extracellular Signal-Regulated Kinase |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HIF | Hypoxia-Inducible Factor |
| IGF | Insulin-like Growth Factor |
| IKK | IκB Kinase |
| JAK | Janus Kinase |
| MAP | Microtubule-Associated Protein |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-Activated Protein Kinase |
| MMP | Matrix Metalloproteinase |
| MYC | Myelocytomatosis Viral Oncogene |
| NF | Nuclear Factor |
| OXPHOS | Oxidative Phosphorylation |
| PARP | Poly (ADP-Ribose) Polymerase |
| PD | Progressive Disease |
| PF | Progression-Free |
| PR | Progesterone Receptor |
| PTEN | Phosphatase and Tensin Homolog |
| RAF | Rapidly Accelerated Fibrosarcoma |
| RAS | Rat Sarcoma Viral Oncogene Homolog |
| RNA | Ribonucleic Acid |
| ROR | Regulator of Reprogramming |
| SMAC | Second Mitochondria-Derived Activator of Caspases |
| TGF | Transforming Growth Factor |
| VEGF | Vascular Endothelial Growth Factor |
| XIAP | X-linked Inhibitor of Apoptosis Protein |
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2024, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Moja, L.; Tagliabue, L.; Balduzzi, S.; Parmelli, E.; Pistotti, V.; Guarneri, V.; D’Amico, R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. 2012, 2021, CD006243. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2023, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Bui, K.T.; Willson, M.L.; Goel, S.; Beith, J.; Goodwin, A. Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database Syst. Rev. 2020, 2020, CD013538. [Google Scholar] [CrossRef] [PubMed]
- Willson, M.L.; Burke, L.; Ferguson, T.; Ghersi, D.; Nowak, A.K.; Wilcken, N. Taxanes for adjuvant treatment of early breast cancer. Cochrane Database Syst. Rev. 2019, 2019, CD013538. [Google Scholar] [CrossRef] [PubMed]
- Aranda, E.; Teruel, J.A.; Ortiz, A.; Pérez-Cárceles, M.D.; Aranda, F.J. Interaction of Docetaxel with Phosphatidylcholine Membranes: A Combined Experimental and Computational Study. J. Membr. Biol. 2022, 255, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Braybrooke, J.; Bradley, R.; Gray, R.; Hills, R.K.; Pan, H.; Peto, R.; Dodwell, D.; McGale, P.; Taylor, C.; Aihara, T.; et al. Anthracycline-containing and taxane-containing chemotherapy for early-stage operable breast cancer: A patient-level meta-analysis of 100,000 women from 86 randomised trials. Lancet 2023, 401, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.; Friedrichs, K.; Noel, D.; Pintér, T.; Van Belle, S.; Vorobiof, D.; Duarte, R.; Gil, M.G.; Bodrogi, I.; Murray, E.; et al. Prospective randomized trial of docetaxel versus doxorubicin in patients with metastatic breast cancer. J. Clin. Oncol. 1999, 17, 2341–2354. [Google Scholar] [CrossRef] [PubMed]
- Debien, V.; Agostinetto, E.; Bruzzone, M.; Ceppi, M.; Martins-Branco, D.; Molinelli, C.; Jacobs, F.; Nader-Marta, G.; Lambertini, M.; de Azambuja, E. The Impact of Initial Tumor Response on Survival Outcomes of Patients with HER2-Positive Advanced Breast Cancer Treated With Docetaxel, Trastuzumab, and Pertuzumab: An Exploratory Analysis of the CLEOPATRA Trial. Clin. Breast Cancer 2024, 24, 421–430.e3. [Google Scholar] [CrossRef] [PubMed]
- Nabholtz, J.M.; Senn, H.J.; Bezwoda, W.R.; Melnychuk, D.; Deschênes, L.; Douma, J.; Vandenberg, T.A.; Rapoport, B.; Rosso, R.; Trillet-Lenoir, V.; et al. Prospective randomized trial of docetaxel versus mitomycin plus vinblastine in patients with metastatic breast cancer progressing despite previous anthracycline-containing chemotherapy. J. Clin. Oncol. 1999, 17, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Ohtani, S.; Nagai, S.E.; Takashima, S.; Yamaguchi, M.; Tsuneizumi, M.; Komoike, Y.; Osako, T.; Ito, Y.; Ikeda, M.; et al. The efficacy and safety of pertuzumab plus trastuzumab and docetaxel as a first-line therapy in Japanese patients with inoperable or recurrent HER2-positive breast cancer: The COMACHI study. Breast Cancer Res. Treat. 2021, 185, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Wooten, E.C.; Tsimelzon, A.; Hilsenbeck, S.G.; Gutierrez, M.C.; Tham, Y.L.; Kalidas, M.; Elledge, R.; Mohsin, S.; Osborne, C.K.; et al. Patterns of resistance and incomplete response to docetaxel by gene expression profiling in breast cancer patients. J. Clin. Oncol. 2005, 23, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Wooten, E.C.; Tsimelzon, A.; Hilsenbeck, S.G.; Gutierrez, M.C.; Elledge, R.; Mohsin, S.; Osborne, C.K.; Chamness, G.C.; Allred, D.C.; et al. Gene expression profiling for the prediction of therapeutic response to docetaxel in patients with breast cancer. Lancet 2003, 362, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.N.; Westergaard, D.; Thomsen, M.B.H.; Vistesen, M.; Do, K.N.; Fogh, L.; Belling, K.C.; Wang, J.; Yang, H.; Gupta, R.; et al. Acquisition of docetaxel resistance in breast cancer cells reveals upregulation of ABCB1 expression as a key mediator of resistance accompanied by discrete upregulation of other specific genes and pathways. Tumor Biol. 2015, 36, 4327–4338. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.N.; Ehlers, N.S.; Zhu, S.; Thomsen, M.B.H.; Nielsen, R.L.; Liu, D.; Wang, G.; Hou, Y.; Zhang, X.; Xu, X.; et al. The stepwise evolution of the exome during acquisition of docetaxel resistance in breast cancer cells. BMC Genom. 2016, 17, 442. [Google Scholar] [CrossRef] [PubMed]
- Ingham, J.; Ruan, J.-L.; Coelho, M.A. Breaking barriers: We need a multidisciplinary approach to tackle cancer drug resistance. BJC Rep. 2025, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Fatima, K.; Aisha, S.; Malik, F. Unveiling the mechanisms and challenges of cancer drug resistance. Cell Commun. Signal. 2024, 22, 109. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.; Briasoulis, E.; Linardou, H.; Bafaloukos, D.; Papadimitriou, C. Taxane resistance in breast cancer: Mechanisms, predictive biomarkers and circumvention strategies. Cancer Treat. Rev. 2012, 38, 890–903. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, H.; Zhang, M.; Zhang, C.; Huang, M.; Yan, C.; Wang, Z.; Hou, L.; Yang, L.; Ling, R. Regulation of docetaxel chemosensitivity by NR2F6 in breast cancer. Endocr. Relat. Cancer 2020, 27, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, J.; Xu, T.; Ouyang, Q.; Wang, X.; Wang, J.; Gan, L.; Ouyang, Z.; Lin, D.; Sun, T.; et al. Efficacy and safety of KN026 and docetaxel for HER2-positive breast cancer: A phase II clinical trial. Cancer Commun. 2025, 45, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, A.M.; Basharina, A.A.; Sorokin, D.V.; Mikhaevich, E.I.; Mizaeva, I.E.; Mikhaylova, A.L.; Bogush, T.A.; Krasil’nikov, M.A. Targeting hormone-resistant breast cancer cells with docetaxel: A look inside the resistance. Cancer Drug Resist. 2023, 6, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.; Hwang, I.; Zhang, J.; Chen, Z.; Han, J.; Jeon, J.; Koo, B.K.; Kim, S.; Lee, J.E.; Kim, Y.; et al. Exploration of drug resistance mechanisms in triple negative breast cancer cells using a microfluidic device and patient tissues. Elife 2024, 12, RP88830. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saleem, S.; Chaudhuri, A.; Ali, J.; Baboota, S. Docetaxel: An update on its molecular mechanisms, therapeutic trajectory and nanotechnology in the treatment of breast, lung and prostate cancer. J. Drug Deliv. Sci. Technol. 2020, 60, 101959. [Google Scholar] [CrossRef]
- Bin Emran, T.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef] [PubMed]
- Kinnel, B.; Singh, S.K.; Oprea-Ilies, G.; Singh, R. Targeted Therapy and Mechanisms of Drug Resistance in Breast Cancer. Cancers 2023, 15, 1320. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Miragaya, J.; Díaz-Navarro, A.; Tonda, R.; Beltran, S.; Palomero, L.; Palafox, M.; Dobrolecki, L.E.; Huang, C.; Vasaikar, S.; Zhang, B.; et al. Chromosome 12p amplification in triple-negative/BRCA1-mutated breast cancer associates with emergence of docetaxel resistance and carboplatin sensitivity. Cancer Res. 2019, 79, 4258–4270. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Roy, D.; Sharma, S.; Vishnoi, J.R.; Pareek, P.; Elhence, P.; Sharma, P.; Purohit, P. ABC transporters in breast cancer: Their roles in multidrug resistance and beyond. J. Drug Target. 2022, 30, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.V.L.; Ruginsk, B.E.; Prado, L.d.O.; de Lima, D.E.; Daniel, I.W.; Moure, V.R.; Valdameri, G. The association of ABC proteins with multidrug resistance in cancer. Biochim. Biophys. Acta-Mol. Cell Res. 2025, 1872, 119878. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Aziz, Y.S.; Spillane, A.J.; Jansson, P.J.; Sahni, S. Role of ABCB1 in mediating chemoresistance of triple-negative breast cancers. Biosci. Rep. 2021, 41, BSR20204092. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Li, F.; Li, L.; You, Y.; Luo, S.; Dong, Z.; Gao, Q.; Wu, S.; Brünner, N.; Stenvang, J. lncRNA profile study reveals the mRNAs and lncRNAs associated with docetaxel resistance in breast cancer cells. Sci. Rep. 2018, 8, 17970. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kang, J.; Liu, W.; Liu, J.; Pan, G.; Mao, A.; Zhang, Q.; Lu, J.; Ding, J.; Li, H. Docetaxel-resistant triple-negative breast cancer cell-derived exosomal lncrna linc00667 reduces the chemosensitivity of breast cancer cells to docetaxel via targeting mir-200b-3p/bcl-2 axis. Eur. J. Histochem. 2022, 66, 3529. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kong, L.; Bian, W.; Lin, X.; Wei, F.; Chu, J. CircRNA CircABCB1 Diminishes the Sensitivity of Breast Cancer Cells to Docetaxel by Sponging MiR-153-3p. Tohoku J. Exp. Med. 2023, 261, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.; Gu, J.; Wei, Y.; Wang, Y. circUBR5 promotes ribosome biogenesis and induces docetaxel resistance in triple-negative breast cancer cell lines via the miR-340-5p/CMTM6/c-MYC axis. Neoplasia 2025, 59, 101062. [Google Scholar] [CrossRef] [PubMed]
- Zong, B.; Sun, L.; Peng, Y.; Wang, Y.; Yu, Y.; Lei, J.; Zhang, Y.; Guo, S.; Li, K.; Liu, S. HORMAD1 promotes docetaxel resistance in triple negative breast cancer by enhancing DNA damage tolerance. Oncol. Rep. 2021, 46, 138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, P.; Huang, T.; He, S.; Li, L.; Xue, G. Individualized chemotherapy guided by the expression of ERCC1, RRM1, TUBB3, TYMS and TOP2A genes versus classic chemotherapy in the treatment of breast cancer: A comparative effectiveness study. Oncol. Lett. 2020, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Lan, L.; Zhang, M.; Zhang, Z. METTL3/LINC00662/miR-186-5p feedback loop regulates docetaxel resistance in triple negative breast cancer. Sci. Rep. 2022, 12, 16715. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Tan, Y.; Xie, J.; Yuan, J.; Deng, X.; Shao, R.; Song, C.; Cao, X.; Xie, X.; He, R.; et al. Methylation of GPRC5A promotes liver metastasis and docetaxel resistance through activating mTOR signaling pathway in triple negative breast cancer. Drug Resist. Updat. 2024, 73, 101063. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.; Yang, N.; Montpetit, R.; Kirschenman, R.; Lemieux, H.; Goping, I.S. BAD sensitizes breast cancer cells to docetaxel with increased mitotic arrest and necroptosis. Sci. Rep. 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Xiong, H.; Jiang, X.; Huang, Z.; Guo, Y.; Cao, Y.; Ding, J. Factors influencing pathological complete response to neoadjuvant chemotherapy in resectable breast cancer: A retrospective study. Oncol. Lett. 2025, 30, 366. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Papakotoulas, P.; Ardavanis, A.; Syrigos, K.; Kakolyris, S.; Ziras, N.; Kouroussis, C.; Malamos, N.; Polyzos, A.; Christophyllakis, C.; et al. Randomized phase III trial comparing docetaxel plus epirubicin versus docetaxel plus capecitabine as first-line treatment in women with advanced breast cancer. Ann. Oncol. 2009, 21, 48–54. [Google Scholar] [CrossRef] [PubMed]
- de Hoon, J.P.J.; Veeck, J.; Vriens, B.E.P.J.; Calon, T.G.A.; van Engeland, M.; Tjan-Heijnen, V.C.G. Taxane resistance in breast cancer: A closed HER2 circuit? Biochim. Biophys. Acta-Rev. Cancer 2012, 1825, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Field, J.J.; Kanakkanthara, A.; Miller, J.H. Microtubule-targeting agents are clinically successful due to both mitotic and interphase impairment of microtubule function. Bioorganic Med. Chem. 2015, 22, 5050–5059. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Deng, Y. Long Noncoding RNAs in Taxane Resistance of Breast Cancer. Int. J. Mol. Sci. 2023, 24, 12253. [Google Scholar] [CrossRef] [PubMed]
- Fromes, Y.; Gounon, P.; Veitia, R.; Bissery, M.C.; Fellous, A. Influence of microtubule-associated proteins on the differential effects of paclitaxel and docetaxel. J. Protein Chem. 1996, 15, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.; Clavel, M.; Chevalier, B. Paclitaxel (TaxolTM) and docetaxel (TaxotereTM): Not simply two of a kind. Ann. Oncol. 1994, 5, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.L.; Gray, H.; Payne, C.M.; Gillies, R.J. Docetaxel induces cell death through mitotic catastrophe in human breast cancer cells. Mol. Cancer Ther. 2005, 4, 1495–1504. [Google Scholar] [CrossRef] [PubMed]
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, microtubules and chemoresistant breast cancer. Biochim. Biophys. Acta-Rev. Cancer 2008, 1785, 96–132. [Google Scholar] [CrossRef] [PubMed]
- Han, T.L.; Sha, H.; Ji, J.; Li, Y.T.; Wu, D.S.; Lin, H.; Hu, B.; Jiang, Z.X. Depletion of Survivin suppresses docetaxel-induced apoptosis in HeLa cells by facilitating mitotic slippage. Sci. Rep. 2021, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.P.; Xu, L.B.; Smith, E.R.; Fleishman, J.S.; Chen, Z.S.; Xu, X.X. Cell death in cancer chemotherapy using taxanes. Front. Pharmacol. 2023, 14, 1338633. [Google Scholar] [CrossRef] [PubMed]
- Alalawy, A.I. Key genes and molecular mechanisms related to Paclitaxel Resistance. Cancer Cell Int. 2024, 24, 244. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.W.; Kim, O.H.; Shin, J.S.; Hong, H.E.; Kim, C.H.; Kim, S.J. Potentiation of the Anticancer Effects by Combining Docetaxel with Ku-0063794 against Triple-Negative Breast Cancer Cells. Cancer Res. Treat. 2022, 54, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Abal, M.; Andreu, J.; Barasoain, I. Taxanes: Microtubule and Centrosome Targets, and Cell Cycle Dependent Mechanisms of Action. Curr. Cancer Drug Targets 2005, 3, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.; Ocean, A.J. Peripheral neuropathy with microtubule-targeting agents: Occurrence and management approach. Clin. Breast Cancer 2011, 11, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Kudlowitz, D.; Muggia, F. Clinical features of taxane neuropathy. Anticancer. Drugs 2014, 25, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Assaraf, Y.G.; Brozovic, A.; Gonçalves, A.C.; Jurkovicova, D.; Linē, A.; Machuqueiro, M.; Saponara, S.; Sarmento-Ribeiro, A.B.; Xavier, C.P.R.; Vasconcelos, M.H. The multi-factorial nature of clinical multidrug resistance in cancer. Drug Resist. Updat. 2019, 46, 100645. [Google Scholar] [CrossRef] [PubMed]
- Savy, T.; Flanders, L.; Karpanasamy, T.; Sun, M.; Gerlinger, M. Cancer evolution: From Darwin to the Extended Evolutionary Synthesis. Trends Cancer 2025, 11, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Brown, J.S. The evolution and ecology of resistance in cancer therapy. Cold Spring Harb. Perspect. Med. 2020, 10, a040972. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Poskus, M.D.; Stylianopoulos, T.; Zervantonakis, I.K. Beyond matrix stiffness: Targeting force-induced cancer drug resistance. Trends Cancer 2023, 9, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Mansur, M.B.; deSouza, N.M.; Natrajan, R.; Abegglen, L.M.; Schiffman, J.D.; Greaves, M. Evolutionary determinants of curability in cancer. Nat. Ecol. Evol. 2023, 7, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Ghebeh, H.; Al-Khaldi, S.; Olabi, S.; Al-Dhfyan, A.; Al-Mohanna, F.; Barnawi, R.; Tulbah, A.; Al-Tweigeri, T.; Ajarim, D.; Al-Alwan, M. Fascin is involved in the chemotherapeutic resistance of breast cancer cells predominantly via the PI3K/Akt pathway. Br. J. Cancer 2014, 111, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, N.M.; Roady, T.J.; Schwermann, M.P.; Eteshola, E.O.; MacDonald, W.J.; Purcell, C.; Ryspayeva, D.; Verovkina, N.; Tajiknia, V.; Ghandali, M.; et al. Acquired resistance to molecularly targeted therapies for cancer. Cancer Drug Resist. 2025, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Vander Velde, R.; Yoon, N.; Marusyk, V.; Durmaz, A.; Dhawan, A.; Miroshnychenko, D.; Lozano-Peral, D.; Desai, B.; Balynska, O.; Poleszhuk, J.; et al. Resistance to targeted therapies as a multifactorial, gradual adaptation to inhibitor specific selective pressures. Nat. Commun. 2020, 11, 2393. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R. Drug resistance in cancer: Molecular evolution and compensatory proliferation. Oncotarget 2016, 7, 11746–11755. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.; Scatena, C.; Ghilli, M.; Bargagna, I.; Lorenzini, G.; Nicolini, A. Molecular Mechanisms, Biomarkers and Emerging Therapies for Chemotherapy Resistant TNBC. Int. J. Mol. Sci. 2022, 23, 1665. [Google Scholar] [CrossRef] [PubMed]
- Seidel, E.; von Karstedt, S. Extrinsic cell death pathway plasticity: A driver of clonal evolution in cancer? Cell Death Discov. 2022, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger, M.; Swanton, C. How Darwinian models inform therapeutic failure initiated by clonal heterogeneity in cancer medicine. Br. J. Cancer 2010, 103, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, S. Rationale for the design of an oncology trial using a generic targeted therapy multi-drug regimen for NSCLC patients without treatment options (Review). Oncol. Rep. 2013, 30, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Engle, K.; Kumar, G. Cancer multidrug-resistance reversal by ABCB1 inhibition: A recent update. Eur. J. Med. Chem. 2022, 239, 114542. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Ilari, A.; Fazi, F.; Assaraf, Y.G.; Colotti, G. Taxanes in cancer treatment: Activity, chemoresistance and its overcoming. Drug Resist. Updat. 2021, 54, 100742. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Bhattacharya, R.; Cabral, F. Human mutations that confer paclitaxel resistance. Mol. Cancer Ther. 2010, 9, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.F.; Zhu, L.J.; Zheng, W.E.; Chen, H.; Wu, L.L.; Zhang, W.; Sun, H.Y.; Chen, W.J. Expression of β-tubulin III and survivin in advance stage breast cancer correlates with chemotheraputic effects of docetaxel. Asian Pac. J. Cancer Prev. 2012, 13, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Lebok, P.; Öztürk, M.; Heilenkötter, U.; Jaenicke, F.; Müller, V.; Paluchowski, P.; Geist, S.; Wilke, C.; Burandt, E.; Lebeau, A.; et al. High levels of class III β-tubulin expression are associated with aggressive tumor features in breast cancer. Oncol. Lett. 2016, 11, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Rouzier, R.; Rajan, R.; Wagner, P.; Hess, K.R.; Gold, D.L.; Stec, J.; Ayers, M.; Ross, J.S.; Zhang, P.; Buchholz, T.A.; et al. Microtubule-associated protein tau: A marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 8315–8320. [Google Scholar] [CrossRef] [PubMed]
- Mimori, K.; Sadanaga, N.; Yoshikawa, Y.; Ishikawa, K.; Hashimoto, M.; Tanaka, F.; Sasaki, A.; Inoue, H.; Sugimachi, K.; Mori, M. Reduced tau expression in gastric cancer can identify candidates for successful Paclitaxel treatment. Br. J. Cancer 2006, 94, 1894–1897. [Google Scholar] [CrossRef] [PubMed]
- Smoter, M.; Bodnar, L.; Duchnowska, R.; Stec, R.; Grala, B.; Szczylik, C. The role of Tau protein in resistance to paclitaxel. Cancer Chemother. Pharmacol. 2011, 68, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Spicakova, T.; O’Brien, M.M.; Duran, G.E.; Sweet-Cordero, A.; Sikic, B.I. Expression and silencing of the microtubule-associated protein Tau in breast cancer cells. Mol. Cancer Ther. 2010, 9, 2970–2981. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Baquero, M.T.; Hanna, J.A.; Neumeister, V.; Cheng, H.; Molinaro, A.M.; Harris, L.N.; Rimm, D.L. Stathmin expression and its relationship to microtubule-associated protein tau and outcome in breast cancer. Cancer 2012, 118, 4660–4669. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updat. 2021, 55, 100754. [Google Scholar] [CrossRef] [PubMed]
- Galletti, G.; Zhang, C.; Gjyrezi, A.; Cleveland, K.; Zhang, J.; Powell, S.; Thakkar, P.V.; Betel, D.; Shah, M.A.; Giannakakou, P. Microtubule Engagement with Taxane Is Altered in Taxane-Resistant Gastric Cancer. Clin. Cancer Res. 2020, 26, 3771–3783. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yu, A.-M. ABC Transporters in Multidrug Resistance and Pharmacokinetics, and Strategies for Drug Development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jia, T.; Chen, X.; Jiang, H.; Guo, T.; Dong, J.; Zeng, H.; Wang, Y.; Yuan, Y. Bufalin reverses ABCB1-mediated resistance to docetaxel in breast cancer. Heliyon 2023, 9, e13840. [Google Scholar] [CrossRef] [PubMed]
- Domanitskaya, N.; Wangari-Talbot, J.; Jacobs, J.; Peiffer, E.; Mahdaviyeh, Y.; Paulose, C.; Malofeeva, E.; Foster, K.; Cai, K.Q.; Zhou, Y.; et al. Abcc10 status affects mammary tumour growth, metastasis, and docetaxel treatment response. Br. J. Cancer 2014, 111, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Fatayer, H.; Hanby, A.M.; Horgan, K.; Perry, S.L.; Valleley, E.M.A.; Verghese, E.T.; Williams, B.J.; Thorne, J.L.; Hughes, T.A. Neoadjuvant Chemotherapy Induces Expression Levels of Breast Cancer Resistance Protein That Predict Disease-Free Survival in Breast Cancer. PLoS ONE 2013, 8, e62766. [Google Scholar] [CrossRef] [PubMed]
- Hirth, J.A.; Watkins, P.B.; Strawderman, M.; Schott, A.; Bruno, R.; Baker, L.H. The effect of an individual’s cytochrome CYP3A4 activity on docetaxel clearance. Clin. Cancer Res. 2000, 6, 1255–1258. [Google Scholar] [PubMed]
- Guo, Y.; He, W.; Yang, S.; Zhao, D.; Li, Z.; Luan, Y. Co-delivery of docetaxel and verapamil by reduction-sensitive PEG-PLGA-SS-DTX conjugate micelles to reverse the multi-drug resistance of breast cancer. Colloids Surf. B Biointerfaces 2017, 151, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Lou, L. ZD6474 reverses multidrug resistance by directly inhibiting the function of P-glycoprotein. Br. J. Cancer 2007, 97, 934–940. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pusztai, L.; Wagner, P.; Ibrahim, N.; Rivera, E.; Theriault, R.; Booser, D.; Symmans, F.W.; Wong, F.; Blumenschein, G.; Fleming, D.R.; et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 2005, 104, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hung, N.; Hung, T.; Eden, K.; Chan, W.K.; Kwan, R.; Qin, A.; Chang, C.; Duffull, S.; Glue, P.; et al. Oral docetaxel plus encequidar—A phase 1 clinical trial. Cancer Chemother. Pharmacol. 2024, 94, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, C.; Cai, Z.; Li, Y.; Liu, W.; Luan, Y.; Wang, C. Effective therapy of advanced breast cancer through synergistic anticancer by paclitaxel and P-glycoprotein inhibitor. Mater. Today Bio 2024, 26, 101029. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lei, C.; Yang, Y.; Bu, X.; Ma, H.; Gong, H.; Liu, J.; Fang, X.; Hu, Z.; Fang, Q. Abraxane, the nanoparticle formulation of paclitaxel can induce drug resistance by up-regulation of P-gp. PLoS ONE 2015, 10, e0131429. [Google Scholar] [CrossRef] [PubMed]
- Pluta, P.; Jesionek-Kupnicka, D.; Pluta, A.; Brzozowski, K.; Braun, M.; Kubicka-Wołkowska, J.; Piekarski, J. Prognostic value of XIAP and survivin expression in locally advanced breast cancer patients treated with anthracycline-based neoadjuvant chemotherapy. Arch. Med. Sci. 2023, 19, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, M.B.; Wang, L.Q.; Yang, L.; Liu, C.Y.; Lu, P.H. Bcl-2 expression predicts sensitivity to chemotherapy in breast cancer: A systematic review and meta-analysis. J. Exp. Clin. Cancer Res. 2013, 32, 105. [Google Scholar] [CrossRef] [PubMed]
- Tewari, M.; Pradhan, S.; Singh, U.; Singh, T.B.; Shukla, H.S. Assessment of predictive markers of response to neoadjuvant chemotherapy in breast cancer. Asian J. Surg. 2010, 33, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Roberts, A.W.; Colman, P.M.; Adams, J.M. Targeting BCL-2-like Proteins to Kill Cancer Cells. Trends Cancer 2016, 2, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, H.; Han, D.; Moon, W.K.; Kim, K.; Oh, H.J.; Choi, J.; Hwang, E.H.; Kang, S.E.; Im, S.A.; et al. Combined the SMAC mimetic and BCL2 inhibitor sensitizes neoadjuvant chemotherapy by targeting necrosome complexes in tyrosine aminoacyl-tRNA synthase-positive breast cancer. Breast Cancer Res. 2020, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Bonaiuti, P.; Chiroli, E.; Gross, F.; Corno, A.; Vernieri, C.; Štefl, M.; Cosentino Lagomarsino, M.; Knop, M.; Ciliberto, A. Cells Escape an Operational Mitotic Checkpoint through a Stochastic Process. Curr. Biol. 2018, 28, 28–37.e7. [Google Scholar] [CrossRef] [PubMed]
- Lok, T.M.; Wang, Y.; Xu, W.K.; Xie, S.; Ma, H.T.; Poon, R.Y.C. Mitotic slippage is determined by p31comet and the weakening of the spindle-assembly checkpoint. Oncogene 2020, 39, 2819–2834. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Bharti, R.; Das, A.K.; Sen, R.; Mandal, M. Resensitization of Akt Induced Docetaxel Resistance in Breast Cancer by ‘Iturin A’ a Lipopeptide Molecule from Marine Bacteria Bacillus megaterium. Sci. Rep. 2017, 7, 17324. [Google Scholar] [CrossRef] [PubMed]
- Kaboli, P.J.; Imani, S.; Jomhori, M.; Ling, K.-H. Chemoresistance in breast cancer: PI3K/Akt pathway inhibitors vs. the current chemotherapy. Am. J. Cancer Res. 2021, 11, 5155–5183. [Google Scholar] [PubMed]
- Dong, M.; Shan, B.; Han, X.; Zhao, X.; Wang, F.; Zhu, L.; Ou, Q.; Ma, X.; Pan, Y. Baseline Mutations and Up-Regulation of PI3K-AKT Pathway Serve as Potential Indicators of Lack of Response to Neoadjuvant Chemotherapy in Stage II/III Breast Cancer. Front. Oncol. 2022, 11, 784985. [Google Scholar] [CrossRef] [PubMed]
- Al-Alwan, M.; Olabi, S.; Ghebeh, H.; Barhoush, E.; Tulbah, A.; Al-Tweigeri, T.; Ajarim, D.; Adra, C. Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules. PLoS ONE 2011, 6, e27339. [Google Scholar] [CrossRef] [PubMed]
- Safi, A.; Heidarian, E.; Ahmadi, R. Quercetin Synergistically Enhances the Anticancer Efficacy of Docetaxel through Induction of Apoptosis and Modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 Signaling Pathways in MDA-MB-231 Breast Cancer Cell Line. Int. J. Mol. Cell. Med. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.; Huang, J.; Huang, X.Y.; Zhang, J.J. A signal transducer and activator of transcription 3·nuclear factor κB (Stat3·NFκB) complex is necessary for the expression of fascin in metastatic breast cancer cells in response to interleukin (IL)-6 and tumor necrosis factor (TNF)-α. J. Biol. Chem. 2014, 289, 30082–30089. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Afolabi, H.A.; Syed, N.H.; Talib, M.; Murtadha, A.H.; Hajissa, K.; Mokhtar, N.F.; Mohamud, R.; Hassan, R. The NF-κB Transcriptional Network Is a High-Dose Vitamin C-Targetable Vulnerability in Breast Cancer. Biomedicines 2023, 11, 1060. [Google Scholar] [CrossRef] [PubMed]
- Sprowl, J.A.; Reed, K.; Armstrong, S.R.; Lanner, C.; Guo, B.; Kalatskaya, I.; Stein, L.; Hembruff, S.L.; Tam, A.; Parissenti, A.M. Alterations in tumor necrosis factor signaling pathways are associated with cytotoxicity and resistance to taxanes: A study in isogenic resistant tumor cells. Breast Cancer Res. 2012, 14, R2. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Luo, X.; Zhang, Q.; Song, L. Baicalin, a Potent Inhibitor of NF-κB Signaling Pathway, Enhances Chemosensitivity of Breast Cancer Cells to Docetaxel and Inhibits Tumor Growth and Metastasis Both In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.C.; Yan, Z.; Zong, Q.; Fang, D.D.; Painter, C.; Zhang, Q.; Chen, E.; Lira, M.E.; John-Baptiste, A.; Christensen, J.G. Synergistic Effect of the γ-Secretase Inhibitor PF-03084014 and Docetaxel in Breast Cancer Models. Stem Cells Transl. Med. 2013, 2, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, K.; O’Neill, A.; Prencipe, M.; Bugler, J.; Murphy, L.; Fabre, A.; Puhr, M.; Culig, Z.; Murphy, K.; Watson, R.W. The role of epithelial-mesenchymal transition drivers ZEB1 and ZEB2 in mediating docetaxel-resistant prostate cancer. Mol. Oncol. 2017, 11, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Chen, Y.; Song, H.; Chen, L.; Wang, R. Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line. J. Cell. Biochem. 2013, 114, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Sun, Z.; Liu, Z. Silencing of TGM2 reverses epithelial to mesenchymal transition and modulates the chemosensitivity of breast cancer to docetaxel. Exp. Ther. Med. 2015, 10, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Chen, J.; Tao, L.; Zhang, K.; Wang, R.; Chu, X.; Chen, L. Long noncoding RNA ROR regulates chemoresistance in docetaxelresistant lung adenocarcinoma cells via epithelial mesenchymal transition pathway. Oncotarget 2017, 8, 33144–33158. [Google Scholar] [CrossRef] [PubMed]
- Damiano, V.; Brisotto, G.; Borgna, S.; di Gennaro, A.; Armellin, M.; Perin, T.; Guardascione, M.; Maestro, R.; Santarosa, M. Epigenetic silencing of miR-200c in breast cancer is associated with aggressiveness and is modulated by ZEB1. Genes Chromosom. Cancer 2017, 56, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.A.; Al-Ateyah, A.; Luqmani, Y.A. MicroRNA expression profiling of endocrine sensitive and resistant breast cancer cell lines. Biochem. Biophys. Rep. 2022, 31, 101316. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.S.; Qiu, W.S.; Yao, R.Y.; Zhang, Q.; Zhuang, L.K.; Zhou, F.; Sun, L.B.; Yue, L. MiR-141 confers docetaxel chemoresistance of breast cancer cells via regulation of EIF4E expression. Oncol. Rep. 2015, 33, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Brennan, K. Notch Signalling in Breast Development and Cancer. Front. Cell Dev. Biol. 2021, 9, 692173. [Google Scholar] [CrossRef] [PubMed]
- Barnawi, R.; Al-Khaldi, S.; Majed Sleiman, G.; Sarkar, A.; Al-Dhfyan, A.; Al-Mohanna, F.; Ghebeh, H.; Al-Alwan, M. Fascin Is Critical for the Maintenance of Breast Cancer Stem Cell Pool Predominantly via the Activation of the Notch Self-Renewal Pathway. Stem Cells 2016, 34, 2799–2813. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Espinosa, A.; García-Rodríguez, J.; Alonso-Aguirre, V.; Acosta-Ortega, J.M.; Conesa-Zamora, P.; García-Solano, J.; Luengo-Gil, G. Expression of Fascin and DNA Topoisomerase 2-Alpha in Breast Carcinoma: Correlation with Histological Subtypes and Other Prognostic Markers. Int. J. Mol. Sci. 2025, 26, 3076. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Li, L.; Cao, J.; Guo, Y.; Wu, Y.; Gao, W. Fascin actin-bundling protein 1 in human cancer: Promising biomarker or therapeutic target? Mol. Ther.-Oncolytics 2021, 20, 240–264. [Google Scholar] [CrossRef] [PubMed]
- Barnawi, R.; Al-Khaldi, S.; Bakheet, T.; Fallatah, M.; Alaiya, A.; Ghebeh, H.; Al-Alwan, M. Fascin Activates β-Catenin Signaling and Promotes Breast Cancer Stem Cell Function Mainly Through Focal Adhesion Kinase (FAK): Relation With Disease Progression. Front. Oncol. 2020, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gao, S.; Sun, W.; Zhang, H.; Sun, J.; Yang, S.; Hao, J. Hypoxia-Inducible factor-1 promotes pancreatic ductal adenocarcinoma invasion and metastasis by activating transcription of the actin-Bundling protein fascin. Cancer Res. 2014, 74, 2455–2464. [Google Scholar] [CrossRef] [PubMed]
- Bu, M.; Liu, X.; Liu, X.; Xu, W. Upregulation of fascin-1 is involved in HIF-1α-dependent invasion and migration of hypopharyngeal squamous cell carcinoma. Int. J. Oncol. 2019, 55, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wei, X.; Jiao, Y.; Bai, Y.; Sam, W.N.; Yan, Q.; Sun, X.; Li, G.; Ma, J.; Wei, W.; et al. STAT3/HIF-1α/fascin-1 axis promotes RA FLSs migration and invasion ability under hypoxia. Mol. Immunol. 2022, 142, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.; Huang, X.Y.; Zhang, J.J. Signal Transducers and Activators of Transcription 3 (STAT3) directly regulates cytokine-induced fascin expression and is required for breast cancer cell migration. J. Biol. Chem. 2011, 286, 38886–38893. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Dawson, J.C.; Forero-Vargas, M.; Spence, H.J.; Yu, X.; König, I.; Anderson, K.; Machesky, L.M. The Actin-Bundling Protein Fascin Stabilizes Actin in Invadopodia and Potentiates Protrusive Invasion. Curr. Biol. 2010, 20, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.S.; Zhao, L.; Zhang, X.N.; Li, H.X.; Yin, Z.H. Effect of rs67085638 in long non-coding RNA (CCAT1) on colon cancer chemoresistance to paclitaxel through modulating the microRNA-24-3p and FSCN1. J. Cell. Mol. Med. 2021, 25, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.R.; Gamal El-Din, A.M.; El-Mahdy, H.A.; Ismail, Y.; El-Husseiny, A.A. The crucial role of fascin-1 in the pathogenesis, metastasis, and chemotherapeutic resistance of breast cancer. Pathol. Res. Pract. 2024, 254, 155079. [Google Scholar] [CrossRef] [PubMed]
- Min, K.W.; Chae, S.W.; Kim, D.H.; Do, S.I.; Kim, K.; Lee, H.J.; Sohn, J.H.; Pyo, J.S.; Kim, D.H.; Oh, S.; et al. Fascin expression predicts an aggressive clinical course in patients with advanced breast cancer. Oncol. Lett. 2015, 10, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Chen, X.; Zhang, Y.; Li, B.; Sun, J.; Shen, H.; Kong, C. Fascin is a predictor for invasiveness and recurrence of urothelial carcinoma of bladder. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Belnap, C.; Divis, T.; Kingsley, K.; Howard, K.M. Differential Expression of MicroRNA MiR-145 and MiR-155 Downstream Targets in Oral Cancers Exhibiting Limited Chemotherapy Resistance. Int. J. Mol. Sci. 2024, 25, 2167. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Dey, R.; Wang, Y.; Jakoncic, J.; Kurinov, I.; Huang, X.Y. Structural Insights into the Induced-fit Inhibition of Fascin by a Small-Molecule Inhibitor. J. Mol. Biol. 2018, 430, 1324–1335. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Cantó, A.; Rodríguez-Braun, E.; Beltrán-Videla, A.; Hurtado, A.M.; Conesa-Zamora, P. Effects of imipramine on cancer patients over-expressing Fascin1; description of the HITCLIF clinical trial. Front. Oncol. 2023, 13, 1238464. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jillian Zhang, J.; Huang, X.Y. Anti-metastasis fascin inhibitors decrease the growth of specific subtypes of cancers. Cancers 2020, 12, 2287. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.H.; Lee, H.J.; Ahn, J.H.; Yoon, D.H.; Kim, S.B.; Gong, G.; Son, B.H.; Ahn, S.H.; Jung, K.H. Tau and PTEN status as predictive markers for response to trastuzumab and paclitaxel in patients with HER2-positive breast cancer. Tumor Biol. 2015, 36, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Alkhathami, A.G.; Pallathadka, H.; Shah, S.; Ganesan, S.; Sharma, A.; Devi, S.; Mustafa, Y.F.; Alasheqi, M.Q.; Kadhim, A.J.; Zwamel, A.H. Mechanisms behind the LncRNAs-mediated regulation of paclitaxel (PTX) resistance in human malignancies. Exp. Cell Res. 2025, 445, 114434. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, Y.; Wang, J.; Gao, D.; Zhang, M.; Ding, N.; Li, Y. Synthesis and biological evaluation of novel larotaxel analogues. Eur. J. Med. Chem. 2018, 156, 692–710. [Google Scholar] [CrossRef] [PubMed]
- Valsalakumari, R.; Feith, M.; Pettersen, S.; Åslund, A.K.O.; Mørch, Ý.; Skotland, T.; Sandvig, K.; Mælandsmo, G.M.; Iversen, T.G. Combinatorial Effects of Free and Nanoencapsulated Forms of Cabazitaxel and RAS-Selective Lethal 3 in Breast Cancer Cells. Pharmaceutics 2025, 17, 657. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Wilmes, P.; Huober, J.; Untch, M.; Blohmer, J.U.; Janni, W.; Denkert, C.; Klare, P.; Link, T.; Rhiem, K.; Bayer, C.; et al. Long-term outcomes of a randomized, open-label, phase II study comparing cabazitaxel versus paclitaxel as neoadjuvant treatment in patients with triple-negative or luminal B/HER2-negative breast cancer (GENEVIEVE). ESMO Open 2024, 9, 103009. [Google Scholar] [CrossRef] [PubMed]
- Erciyestepe, M.; Ekinci, Ö.B.; Seçmeler, Ş.; Selvi, O.; Öztürk, A.E.; Aydin, O.; Büyükkuşcu, A.; Atasever, T.; Çelik, E.; Ertürk, K.; et al. Evaluation of ixabepilone efficacy and tolerability in metastatic breast cancer. Medicine 2024, 103, e40649. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Targeted and cytotoxic inhibitors used in the treatment of lung cancers. Pharmacol. Res. 2024, 209, 107465. [Google Scholar] [CrossRef] [PubMed]
- James, N.; Owusu, E.; Rivera, G.; Bandyopadhyay, D. Small Molecule Therapeutics in the Pipeline Targeting for Triple-Negative Breast Cancer: Origin, Challenges, Opportunities, and Mechanisms of Action. Int. J. Mol. Sci. 2024, 25, 6285. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Dalenc, F.; Campone, M.; O’Regan, R.M.; Tjan-Heijnen, V.C.; Gligorov, J.; Llombart, A.; Jhangiani, H.; Mirshahidi, H.R.; Tan-Chiu, E.; et al. A phase 2 study of everolimus combined with trastuzumab and paclitaxel in patients with HER2-overexpressing advanced breast cancer that progressed during prior trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2013, 141, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Santhanam, R.K.; Xing, H.; Zhou, M.; Jia, H. Inhibition of γ-secretase/Notch pathway as a potential therapy for reversing cancer drug resistance. Biochem. Pharmacol. 2024, 220, 115991. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, T.; Song, X.; Gao, J.; Xu, G.; Ma, Y.; Song, G. Current Status of Hedgehog Signaling Inhibitors. Curr. Top. Med. Chem. 2024, 24, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Xia, Y.; Zhu, Y.; Yang, Y.; Lin, Q.; Liu, Q.; Yang, W.; Ling, L.; Zhong, J.; Duan, Z.; et al. Preclinical study and phase 2 trial of neoadjuvant pyrotinib combined with chemotherapy in luminal/HER2-low breast cancer: PILHLE-001 study. Cell Rep. Med. 2024, 5, 101807. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.M.; Zotano, A.L.G.; Perez, M.E.; Borrego, M.R.; Martinez, N.; Lopez-Muniz, J.I.C.; Gil, M.J.; Conejero, R.A.; Bermejo, B.; Sanchez-Rovira, P.; et al. 112MO Neoadjuvant study of 12 months of abemaciclib plus letrozole vs. chemotherapy in HR+/HER2– highly proliferative (Ki67≥20%) breast cancer: CARABELA (GEICAM/2019-01) trial. ESMO Open 2024, 9, 103100. [Google Scholar] [CrossRef]
- Sparano, J.A.; Zhao, F.; Martino, S.; Ligibel, J.A.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W.; Wood, W.C.; Davidson, N.E. Long-term follow-up of the E1199 phase III trial evaluating the role of taxane and schedule in operable breast cancer. J. Clin. Oncol. 2015, 33, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Geng, C.; Liu, Y.; Han, J.; Han, M.; Huang, R.; Li, X.; Luo, R.; Li, Y.; Ma, J.; et al. A phase II trial comparing dalpiciclib in combination with letrozole versus standard chemotherapy as neoadjuvant therapy in patients with high-risk HR- positive HER-2 negative breast cancer: DARLING-02. J. Clin. Oncol. 2024, 42, TPS626. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, X.; Chen, A.; Meng, R.; Zhao, Y.; Liu, H.; Ge, J.; Huang, R.; Bai, J.; Cao, X. Neoadjuvant dalpiciclib, exemestane, and goserelin in premenopausal women with HR-positive, HER2-negative breast cancer: A prospective, multicenter, randomized two-group, non-controlled phase 2 clinical trial. J. Clin. Oncol. 2024, 42, TPS625. [Google Scholar] [CrossRef]
- Slamon, D.J.; Fasching, P.A.; Hurvitz, S.; Chia, S.; Crown, J.; Martín, M.; Barrios, C.H.; Bardia, A.; Im, S.A.; Yardley, D.A.; et al. Rationale and trial design of NATALEE: A Phase III trial of adjuvant ribociclib + endocrine therapy versus endocrine therapy alone in patients with HR+/HER2− early breast cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231178125. [Google Scholar] [CrossRef] [PubMed]
- Pascual, T.; Chic, N.; Fernandez-Martinez, A.; González-Farré, B.; Paré, L.; Saura, C.; Hernando, C.; Muñoz, M.; Arumí, M.; Galván, P.; et al. Abstract PD17-07: Cell-Cycle Inhibition and Immune Microenvironment in HR+/HER2- Breast Cancer During and After preoperative ribociclib and letrozole vesus chemotherapy: A correlative analysis of the 1402-SOLTI/CORALLEEN phase 2 trial. Cancer Res. 2023, 83, PD17-07. [Google Scholar] [CrossRef]
- Huo, S.; Xue, J.; Wang, S.; Shan, H.; Chen, G.; Niu, N.; Wang, Y.; Qiu, F.; Zhao, Y.; Xing, F.; et al. A pilot trial of neoadjuvant pyrotinib plus trastuzumab, dalpiciclib, and letrozole for triple-positive breast cancer. MedComm 2024, 5, e505. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, L.; Long, Q.; Zhang, Q.; Sun, G.; Zhou, L.; Wang, Q.; Zhu, J.; Li, F.; Hu, W. HLX11, a Proposed Pertuzumab Biosimilar: Pharmacokinetics, Immunogenicity, and Safety Profiles Compared to Three Reference Biologic Products (US-, EU-, and CN-Approved Pertuzumab) Administered to Healthy Male Subjects. BioDrugs 2022, 36, 393–409. [Google Scholar] [CrossRef] [PubMed]
- McAndrew, N.P.; Hurvitz, S.A.; Tetef, M.L.; Kivork, C.; Ikenouye, L.; Slamon, D.J. UCLA B-13: A phase 1b trial evaluating the safety of ribociclib, tucatinib, and trastuzumab in patients with metastatic, HER2+ breast cancer and a multicenter, randomized, open-label, phase 2 study of preoperative treatment with ribociclib, trastuzumab, t. J. Clin. Oncol. 2023, 41, TPS1116. [Google Scholar] [CrossRef]
- Harbeck, N.; Braun, M.; Gluz, O.; Schmid, P.; Graeser, M.K.; Hartkopf, A.D.; Hoffmann, O.; Kostara, A.; Polata, S.; Volmer, L.L.; et al. Neoadjuvant dynamic marker-adjusted personalized therapy comparing trastuzumab-deruxtecan versus pacli-/docetaxel + carboplatin + trastuzumab + pertuzumab in HER2+ early breast cancer: WSG-ADAPT-HER2-IV. J. Clin. Oncol. 2024, 42, TPS631. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, N.; Zhou, X. Abstract P5-05-24: RC48-ADC (Disitamab vedotin, a HER2-directed antibody-drug conjugate) in Combination with Pyrotinib for Trastuzumab-treated HER2-Positive Recurrent or Metastatic Breast Cancer: A Phase Ib/II Study. Clin. Cancer Res. 2025, 31, P5-05-24. [Google Scholar] [CrossRef]
- Liu, J.; Song, C.; Yang, Y.; Wang, X.; Ni, M.; Wang, X.; Chen, L.; Yang, H.; Zhao, R.; Xu, T.; et al. Safety and Efficacy of KN046 in Combination with KN026 in Patients with Advanced HER2-Positive Breast Cancer: A Phase II Trial. Clin. Cancer Res. 2025, 31, 2379–2385. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, S.; Klein, J.; Haggag, A.Z.; Shaw, A.A.; Lawrence, T.E.; Liu, M.S.; Rao Betha, M. A randomized, double-blind, three-arm, parallel group, phase 1 study to assess the pharmacokinetics, safety, and tolerability of PERT-IJS following a single dose of 420 mg intravenous infusion compared to the EU-and US-marketed drug product (pertuzumab; PERT) in healthy male volunteers. J. Clin. Oncol. 2025, 43, e13009. [Google Scholar] [CrossRef]
- Liu, B.; Xie, N.; Tian, C.; Feng, R.; Hu, Z.Y.; Li, J.; Liu, L.; Xiao, H.; Yang, X.; Zeng, M.; et al. Exploring the clinical outcomes and safety profile of inetetamab treatment in metastatic breast cancer patients: A multicenter assessment of a Chinese-origin recombinant Anti-HER2 monoclonal antibody. Breast 2023, 72, 103597. [Google Scholar] [CrossRef] [PubMed]
- Kuemmel, S.; Harper-Wynne, C.; Park, Y.H.; Franke, F.; de Laurentiis, M.; Schumacher-Wulf, E.; Eiger, D.; Heeson, S.; Cardona, A.; Özyilkan, Ö.; et al. heredERA Breast Cancer: A phase III, randomized, open-label study evaluating the efficacy and safety of giredestrant plus the fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection in patients with previously untreated HER2-positi. BMC Cancer 2024, 24, 641. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Z.H.; Chen, L.; Zhang, W.J.; Ma, L.X.X.; Wu, J.; Liu, G.Y.; Hou, Y.F.; Yu, K.D.; Di, G.H.; et al. Efficacy and safety of neoadjuvant SHR-A1811 with or without pyrotinib in women with locally advanced or early HER2-positive breast cancer: A randomized, open-label, phase II trial. Ann. Oncol. 2025, 36, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Ishiba, T.; Nishibuchi, I.; Hara, F.; Sasaki, K.; Sadate, R.; Sekino, Y.; Machida, R.; Fukuda, H.; Kogawa, T.; Fujisawa, T.; et al. Abstract PO3-19-01: A Randomized Controlled Trial of Metastasis-directed Therapy for Oligometastases in Breast Cancer (OLIGAMI trial; JCOG2110). Cancer Res. 2024, 84, PO3-19-01. [Google Scholar] [CrossRef]
- Xu, R.-H.; Wang, S.; Ruan, D.; Lin, S.; Liu, F.-R.; Wu, H.-X.; Huang, J.J.; Zheng, Q.; Jiang, K.; Du, X.; et al. Safety and efficacy of TQB2102, a novel bispecific anti-HER2 antibody–drug conjugate, in patients with advanced solid tumors: Preliminary data from the first-in-human phase 1 trial. J. Clin. Oncol. 2025, 43, 3003. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Yin, S.; Liu, M.; Yang, H.; Xie, F.; Peng, Y.; Yang, Y.; Wang, S.; Du, W.; et al. A prospective phase II study of neoadjuvant therapy guided by personalized drug-testing on patient-derived tumor-like cell clusters for early breast cancer. J. Clin. Oncol. 2024, 42, e12625. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, Y.; Wang, X.; Bi, Z.; Qiu, P.; Qiao, G.; Bi, X.; Shi, Z.; Zhang, Z.; Chen, P.; et al. Clinical efficacy and biomarker analysis of neoadjuvant camrelizumab plus chemotherapy for early-stage triple-negative breast cancer: A experimental single-arm phase II clinical trial pilot study. Int. J. Surg. 2024, 110, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Stecklein, S.R.; Aripoli, A.; Salgado, R.; O’Dea, A.; Nye, L.E.; Larson, K.E.; Satelli, D.; Rader, R.; Madan, R.; Yoder, R.; et al. NeoTRACT: Phase II trial of neoadjuvant tumor infiltrating lymphocyte- and response-adapted chemoimmunotherapy for triple-negative breast cancer (TNBC). J. Clin. Oncol. 2024, 42, TPS629. [Google Scholar] [CrossRef]
- Bardia, A.; Gucalp, A.; DaCosta, N.; Gabrail, N.; Danso, M.; Ali, H.; Blackwell, K.L.; Carey, L.A.; Eisner, J.R.; Baskin-Bey, E.S.; et al. Phase 1 study of seviteronel, a selective CYP17 lyase and androgen receptor inhibitor, in women with estrogen receptor-positive or triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Saltos, A.N.; Arrowood, C.; Beasley, G.; Ronald, J.; El-Haddad, G.; Khan, U.; Guerra-Guevara, L.; Wolf, S.; Gu, L.; Wang, X.F.; et al. Abstract CT103: A phase 1 first-in-human study of MEM-288 oncolytic virus in solid tumors including non-small cell lung cancer (NSCLC): Impact on tumor and systemic T cell immunity. Cancer Res. 2023, 83, CT103. [Google Scholar] [CrossRef]
- Zeng, H.A.; Lv, H.M.; Zhang, M.W.; Niu, L.M.; Wang, J.; Sun, H.H.; Liu, Z.Z.; Yan, M. Docetaxel rechallenge in HER2-negative metastatic breast cancer: A real-world study of previously discontinued patients for non-progression reasons. J. Cancer Res. Clin. Oncol. 2025, 151, 89. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liu, J.; Shen, X.; Wang, Z.; Li, Q.; Li, G. Adverse Event Profile for Nanoparticle Albumin-Bound Paclitaxel Compared With Solvent-Based Taxanes in Solid-Organ Tumors: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Ann. Pharmacother. 2022, 56, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vieweger, M.; Zhang, K.; Yin, H.; Wang, H.; Li, X.; Li, S.; Hu, S.; Sparreboom, A.; Evers, B.M.; et al. Ultra-thermostable RNA nanoparticles for solubilizing and high-yield loading of paclitaxel for breast cancer therapy. Nat. Commun. 2020, 11, 972. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.; Carvalho, M.A.; Castanheira, E.M.S. Functionalized Liposome and Albumin-Based Systems as Carriers for Poorly Water-Soluble Anticancer Drugs: An Updated Review. Biomedicines 2022, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Niza, E.; Ocaña, A.; Castro-Osma, J.A.; Bravo, I.; Alonso-Moreno, C. Polyester polymeric nanoparticles as platforms in the development of novel nanomedicines for cancer treatment. Cancers 2021, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. [Google Scholar] [CrossRef] [PubMed]
- McFadden, M.; Singh, S.K.; Kinnel, B.; Varambally, S.; Singh, R. The effect of paclitaxel- and fisetin-loaded PBM nanoparticles on apoptosis and reversal of drug resistance gene ABCG2 in ovarian cancer. J. Ovarian Res. 2023, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Souchek, J.J.; Davis, A.L.; Hill, T.K.; Holmes, M.B.; Qi, B.; Singh, P.K.; Kridel, S.J.; Mohs, A.M. Combination treatment with orlistat-containing nanoparticles and taxanes is synergistic and enhances microtubule stability in taxane-resistant prostate cancer cells. Mol. Cancer Ther. 2017, 16, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhao, Z.; Chen, D.; Qiao, M.; Wan, F.; Cun, D.; Sun, Y.; Yang, M. Co-delivery of resveratrol and docetaxel via polymeric micelles to improve the treatment of drug-resistant tumors. Asian J. Pharm. Sci. 2019, 14, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines 2022, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Dian, C.; Qian, Z.; Ran, M.; Yan, X.; Dian, L. Co-Delivery of Docetaxel and Curcumin Functionalized Mixed Micelles for the Treatment of Drug-Resistant Breast Cancer by Oral Administration. Int. J. Nanomed. 2024, 19, 8603–8620. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Lirdprapamongkol, K.; Atjanasuppat, K.; Chaisuriya, P.; Niamsiri, N.; Svasti, J. PLGA-Lipid Hybrid Nanoparticles for Overcoming Paclitaxel Tolerance in Anoikis-Resistant Lung Cancer Cells. Molecules 2022, 27, 8295. [Google Scholar] [CrossRef] [PubMed]
- Bowerman, C.J.; Byrne, J.D.; Chu, K.S.; Schorzman, A.N.; Keeler, A.W.; Sherwood, C.A.; Perry, J.L.; Luft, J.C.; Darr, D.B.; Deal, A.M.; et al. Docetaxel-loaded PLGA nanoparticles improve efficacy in taxane-resistant triple-negative breast cancer. Nano Lett. 2017, 17, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.R.; Pattni, B.S.; Abouzeid, A.H.; Torchilin, V.P. Nanopreparations to overcome multidrug resistance in cancer. Adv. Drug Deliv. Rev. 2013, 65, 1748–1762. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Mumper, R.J. A critical review of lipid-based nanoparticles for taxane delivery. Cancer Lett. 2013, 334, 157–175. [Google Scholar] [CrossRef] [PubMed]
- Swetha, K.L.; Paul, M.; Maravajjala, K.S.; Kumbham, S.; Biswas, S.; Roy, A. Overcoming drug resistance with a docetaxel and disulfiram loaded pH-sensitive nanoparticle. J. Control. Release 2023, 356, 93–114. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Chen, X.; He, R.; Meng, W.; Chen, W.; Wang, F.; Meng, X. Enhanced anti-breast cancer efficacy of co-delivery liposomes of docetaxel and curcumin. Front. Pharmacol. 2022, 13, 969611. [Google Scholar] [CrossRef] [PubMed]
- Di Gregorio, E.; Romiti, C.; Di Lorenzo, A.; Cavallo, F.; Ferrauto, G.; Conti, L. RGD_PLGA Nanoparticles with Docetaxel: A Route for Improving Drug Efficiency and Reducing Toxicity in Breast Cancer Treatment. Cancers 2023, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Nguyen, T.N.; Lee, Y.; Tran, P.N.; Park, J.S. Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells. Korean J. Physiol. Pharmacol. 2021, 25, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, M.C.O.; Da Silva, P.B.; Radicchi, M.A.; Andrade, B.Y.G.; De Oliveira, J.V.; Venus, T.; Merker, C.; Estrela-Lopis, I.; Longo, J.P.F.; Báo, S.N. Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J. Nanobiotechnol. 2020, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Wang, X.; Yao, P.; Dai, Q.; Qi, X.; Yang, M.; Zhang, X.; Huang, R.; Yang, J.; et al. Docetaxel-loaded pH/ROS dual-responsive nanoparticles with self-supplied ROS for inhibiting metastasis and enhancing immunotherapy of breast cancer. J. Nanobiotechnol. 2023, 21, 286. [Google Scholar] [CrossRef] [PubMed]
- Basak, M.; Kulkarni, M.; Narisepalli, S.; Chitkara, D.; Mittal, A. Exosomal fragment enclosed polyamine-salt nano-complex for co-delivery of docetaxel and mir-34a exhibits higher cytotoxicity and apoptosis in breast cancer cells. Sci. Rep. 2024, 14, 21669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cheng, W.Y.; Zhu, J.; He, L.; Ren, W.Y.; Bao, D.; Piao, J.G. Programmed Co-delivery of tamoxifen and docetaxel using lipid-coated mesoporous silica nanoparticles for overcoming CYP3A4-mediated resistance in triple-negative breast cancer treatment. Biomed. Pharmacother. 2024, 170, 116084. [Google Scholar] [CrossRef] [PubMed]

| Biomarker (Type) | Mechanism/Rationale | Evidence Type | Key Findings (Study Details) | References |
|---|---|---|---|---|
| ABCB1 (P-glycoprotein efflux pump) | Drug efflux transporter; overexpression reduces intracellular docetaxel response. | Preclinical | Overexpressed in resistant cells; inhibition restores sensitivity and drug accumulation; validated in mouse models. | [28,29,30] |
| EPB41L4A-AS2 (lncRNA) | Loss of tumour-suppressor lncRNA increases ABCB1 expression. | Preclinical | Absent in resistant cells; low expression associated with poor response to docetaxel. | [31] |
| LINC00667 (exosomal lncRNA) | Sponges miR-200b-3p to upregulate BCL2, reducing apoptosis. | Preclinical | Found in resistant TNBC exosomes; downregulation sensitises cells to docetaxel. | [32] |
| circABCB1 (circular RNA) | CircABCB1 contributed to the docetaxel resistance of breast cancer, possibly via miR-153-3p sponging. | Preclinical | The overexpression of circABCB1 contributed to cell viability, docetaxel-resistance, and migration/invasion. | [33] |
| circUBR5 (circular RNA) | circUBR5 sponges miR-340-5p, releasing suppression of CMTM6 and promoting c-MYC-driven ribosome biogenesis, contributing to docetaxel resistance. | Preclinical | Knocking down circUBR5 increased miR-340-5p activity, decreased CMTM6 levels, suppressed c-MYC activity, and sensitized cells to docetaxel—inducing apoptosis and reducing colony formation. | [34] |
| HORMAD1 (protein) | Promotes DNA damage tolerance via enhanced homologous recombination repair and protective autophagy, reducing docetaxel-induced apoptosis. | Preclinical | HORMAD1 overexpression protects TNBC cells from docetaxel-induced DNA damage and apoptosis; its knockdown restores chemosensitivity via impaired DNA repair and enhanced apoptosis. | [35] |
| TUBB3 (βIII-tubulin) | Alters microtubule dynamics, reducing docetaxel binding. | Preclinical + Clinical | High expression correlates with poor docetaxel response; inversely related to sensitivity. | [22,36] |
| METTL3 (RNA methyltransferase), LINC00662 (lncRNA) | Promotes docetaxel resistance by m6A-dependent stabilisation of LINC00662, forming a feedback loop with miR-186-5p that sustains METTL3 expression. | Preclinical | High METTL3 and LINC00662 levels were observed in docetaxel-resistant TNBC cells and patient samples; disrupting the METTL3/LINC00662/miR-186-5p axis restored chemosensitivity and increased apoptosis. | [37,38] |
| BAD (pro-apoptotic protein) | Facilitates necroptosis during prolonged mitotic arrest induced by docetaxel, preventing mitotic slippage and survival of resistant cells. | Preclinical | BAD expression promotes mitotic arrest and necroptotic death upon docetaxel treatment; its loss enables mitotic slippage and survival of chemoresistant polyploid cells. Tumours with high BAD show better response to taxanes. | [39] |
| ER/PR-positive status | Hormone-driven, low-proliferation tumours less responsive to taxanes. | Clinical | Associated with lower pCR rates compared to ER-negative tumours. | [40] and others |
| HER2-positive status | High proliferation and HER2-targetability improve taxane response. | Clinical | HER2+ tumours respond well to docetaxel-based regimens with HER2 inhibition. | [40] and others |
| Triple-negative subtype | High initial sensitivity; prone to relapse if pCR not achieved. | Clinical | Higher pCR rates but vulnerable to resistance upon incomplete response. | [40] and others |
| Delivery System | Formulation Type | Mechanism | Pros | Cons | Reference |
|---|---|---|---|---|---|
| Liposomes | Lipid bilayer vesicles (~50–200 nm) encapsulating drugs (e.g., PEGylated liposomal doxorubicin). | Passive tumour targeting via EPR; PEGylation (“stealth”) extends circulation; can be functionalized with ligands for active targeting. | Biodegradable, biocompatible; carry both hydrophilic and hydrophobic drugs; protect drug, improve pharmacokinetics (prolonged half-life, stability) and reduce systemic toxicity. | Rapid clearance by mononuclear phagocyte system without PEGylation; potential premature drug leakage and short circulation half-life; high manufacturing cost. | [175] |
| Polymeric NPs | Biodegradable polymer nanoparticles (e.g., PLA/PLGA nanospheres or nanocapsules loaded with paclitaxel—PICN® is a polymeric PTX NP approved in India for metastatic breast cancer). | Polymer matrix entraps drug and releases it via controlled degradation; passive EPR targeting (with possible ligand-mediated active targeting). | Highly versatile (wide choice of polymers); stable during storage and scalable manufacturing; tunable surface properties and drug release; high drug payload capacity; improve bioavailability and circulation time. | Possible stability issues (tendency to aggregate); require precise manufacturing conditions; some formulations need PEGylation for prolonged circulation; potential toxicity of residual monomers or solvents. | [176] |
| Polymeric Micelles | Self-assembled amphiphilic copolymer micelles (10–100 nm) solubilizing hydrophobic drugs in a core (e.g., PEG-PLA micelle paclitaxel, Genexol-PM®). | Spontaneous micelle formation above a critical micelle concentration; drugs carried in core are released upon micelle dissociation or stimulus in tumour microenvironment. | Easy to prepare; improve water solubility of hydrophobic drugs; prolong circulation and enhance tumour accumulation via EPR; increase drug efficacy and reduce toxicity (no need for harsh solubilizers like Cremophor) | Limited stability in bloodstream—dilution below critical micelle concentration causes disassembly and rapid drug clearance (short half-life in circulation). | [177] |
| Albumin-Bound NPs | Albumin-based nanoparticles or albumin–drug complexes (e.g., nab-Paclitaxel, Abraxane® ~130 nm, an albumin-bound paclitaxel approved for metastatic breast cancer). | Exploit albumin’s natural pathways: passive tumour accumulation via EPR and active transcytosis (gp60 receptor) and binding to SPARC in tumour stroma, enhancing drug delivery to tumour sites. | Biocompatible, non-immunogenic carrier; avoids toxic solvents (Abraxane is Cremophor-free); long circulation and tumour uptake via albumin receptors; improves drug solubility and bioavailability | Require cross-linking for nanoparticle stability (e.g., glutaraldehyde crosslinker, which can leave toxic residues); net negative charge of albumin can limit drug loading unless chemically modified. | [175] |
| Nanoparticle Type | Cargo (Co-Delivered Agents) | Targeting Strategy | Resistance Mechanism Addressed | Key Findings | Stage | Reference |
|---|---|---|---|---|---|---|
| pH-sensitive PLGA nanoparticle | Docetaxel + Disulfiram (DSF) | pH-triggered release, TPGS-mediated P-gp inhibition | P-gp efflux, CSC survival, tumour stroma barrier | Restored sensitivity in resistant cells, enhanced tumour accumulation, inhibited metastasis, superior efficacy in vivo | Preclinical | [187] |
| Liposome (CUR-DTX-L) | Docetaxel + Curcumin | Passive targeting (EPR) | MDR via efflux transporters, survival signalling | Synergistic cytotoxicity, prolonged half-life, tumour growth inhibition in xenograft model | Preclinical | [188] |
| RGD-decorated PLGA nanoparticle | Docetaxel (+ MRI/fluorescent tracers) | αvβ3 integrin targeting | Limited tumour uptake, systemic toxicity | Higher tumour localization, reduced cardiotoxicity, improved efficacy in TNBC and HER2+ models | Preclinical | [189] |
| PLGA–TPGS polymeric nanoparticle | Docetaxel | Passive targeting, TPGS-mediated P-gp inhibition | General MDR, poor intracellular accumulation | Increased potency, reduced IC50, sustained release, improved anti-proliferative effect | Preclinical | [190] |
| Solid lipid nanoparticle (SLN) | Docetaxel | Passive targeting, controlled release | EMT, IL-6/BCL-2 survival signalling | High cytotoxicity, G2/M arrest, prevented metastasis, suppressed IL-6 and BCL-2 | Preclinical | [191] |
| Folate-targeted pH/ROS-dual responsive nanoparticle | Docetaxel + Cinnamaldehyde | Folate receptor targeting, stimuli-responsive release | TNBC metastasis, immune evasion | Immunogenic cell death, blocked invasion, halted metastasis, enhanced anti-PD-1 response | Preclinical | [192] |
| Exosome-coated polyamine nanocomplex | Docetaxel + miR-34a | Biomimetic targeting (exosomal membrane) | miR-34a loss, anti-apoptotic signalling | High cytotoxicity, BCL-2 downregulation, potent apoptosis induction | Preclinical | [193] |
| Lipid-coated mesoporous silica nanoparticle (LP-MSN) | Docetaxel + Tamoxifen | Sequential release (Tamoxifen then DTX) | CYP3A4-mediated metabolic resistance | Enhanced cytotoxicity via CYP3A4 inhibition, selective toxicity to TNBC cells | Preclinical | [194] |
| Trial Identifier | Nanoparticle Formulation | Combination Therapy | Targeting Strategy/Delivery Type | Phase | Status | Objective Summary |
|---|---|---|---|---|---|---|
| NCT03671044 | Nanosomal Docetaxel Lipid Suspension (NDLS) | None (monotherapy) | Lipid-based, polysorbate-free formulation to improve solubility and tumour delivery | Phase 3 | Recruiting | Compare efficacy and safety of NDLS vs. conventional docetaxel in TNBC patients resistant to prior chemotherapy. |
| NCT04931823 | CPO-100 (Albumin-bound Docetaxel) | None | Albumin nanoparticle, solvent-free to enhance safety and tumour targeting | Phase 1 | Active | Evaluate MTD, safety, PK, and preliminary efficacy in advanced solid tumours refractory to standard treatment, including breast cancer. |
| NCT05114915 | Albumin-bound Docetaxel (HB1801) | None | Albumin-stabilized nanoparticle, solvent-free for safer delivery | Phase 1 | Recruiting | Assess safety, tolerability, PK, and preliminary efficacy in advanced solid tumours unresponsive to standard therapies (includes breast cancer). |
| NCT05254665 | Polymeric Micellar Docetaxel | None | Polymeric micelle nanoparticle for improved tumour-specific delivery | Phase 2 | Not yet recruiting | Confirm dose, assess safety and anti-tumour efficacy in taxane-resistant advanced solid tumours, including breast cancer. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Postigo-Corrales, F.; Beltrán-Videla, A.; Lázaro-Sánchez, A.D.; Hurtado, A.M.; Conesa-Zamora, P.; Arroyo, A.B.; Luengo-Gil, G. Docetaxel Resistance in Breast Cancer: Current Insights and Future Directions. Int. J. Mol. Sci. 2025, 26, 7119. https://doi.org/10.3390/ijms26157119
Postigo-Corrales F, Beltrán-Videla A, Lázaro-Sánchez AD, Hurtado AM, Conesa-Zamora P, Arroyo AB, Luengo-Gil G. Docetaxel Resistance in Breast Cancer: Current Insights and Future Directions. International Journal of Molecular Sciences. 2025; 26(15):7119. https://doi.org/10.3390/ijms26157119
Chicago/Turabian StylePostigo-Corrales, Fátima, Asunción Beltrán-Videla, Antonio David Lázaro-Sánchez, Ana María Hurtado, Pablo Conesa-Zamora, Ana Belén Arroyo, and Ginés Luengo-Gil. 2025. "Docetaxel Resistance in Breast Cancer: Current Insights and Future Directions" International Journal of Molecular Sciences 26, no. 15: 7119. https://doi.org/10.3390/ijms26157119
APA StylePostigo-Corrales, F., Beltrán-Videla, A., Lázaro-Sánchez, A. D., Hurtado, A. M., Conesa-Zamora, P., Arroyo, A. B., & Luengo-Gil, G. (2025). Docetaxel Resistance in Breast Cancer: Current Insights and Future Directions. International Journal of Molecular Sciences, 26(15), 7119. https://doi.org/10.3390/ijms26157119








