Molecular Mechanisms of the Endocannabinoid System with a Focus on Reproductive Physiology and the Cannabinoid Impact on Fertility
Abstract
1. Introduction
2. The Endocannabinoid System
2.1. Cannabinoid Receptors
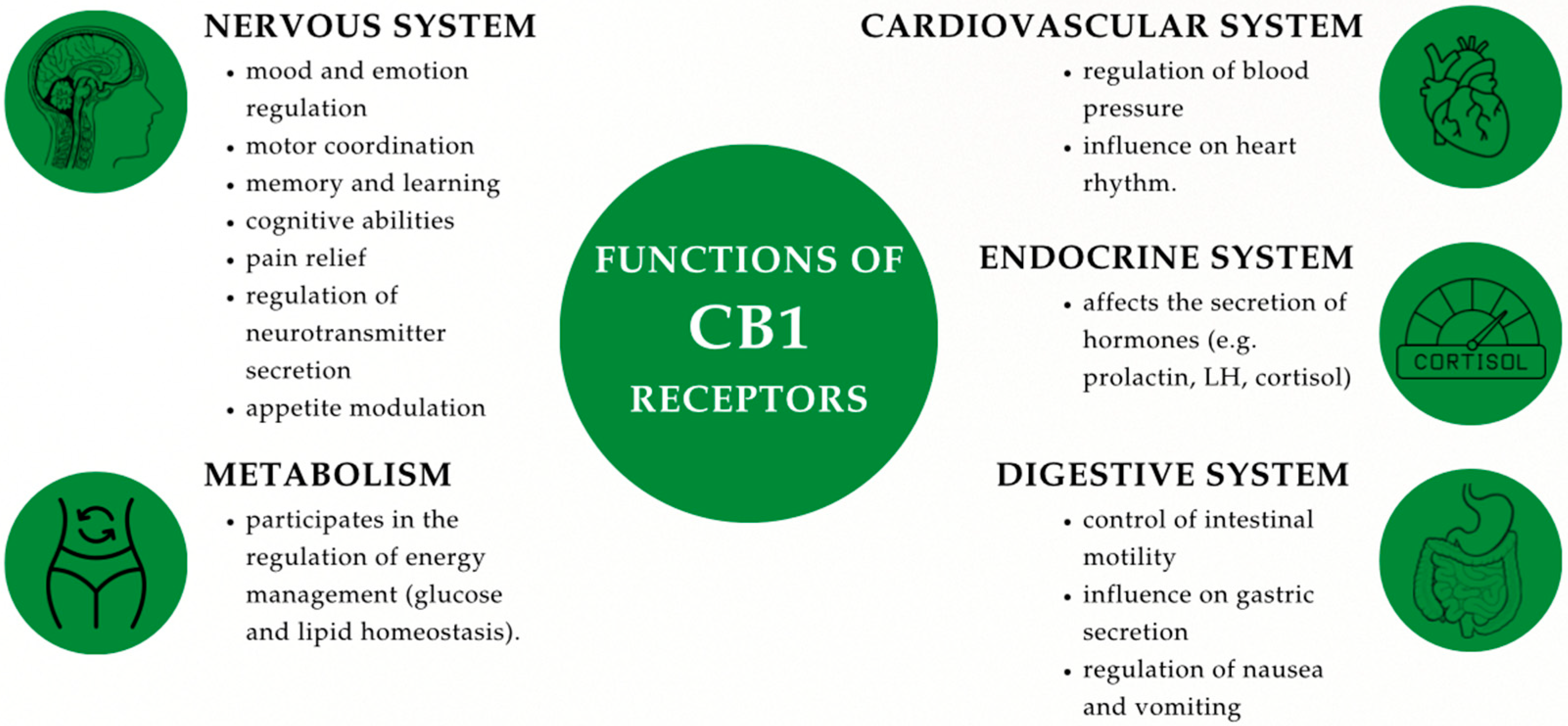
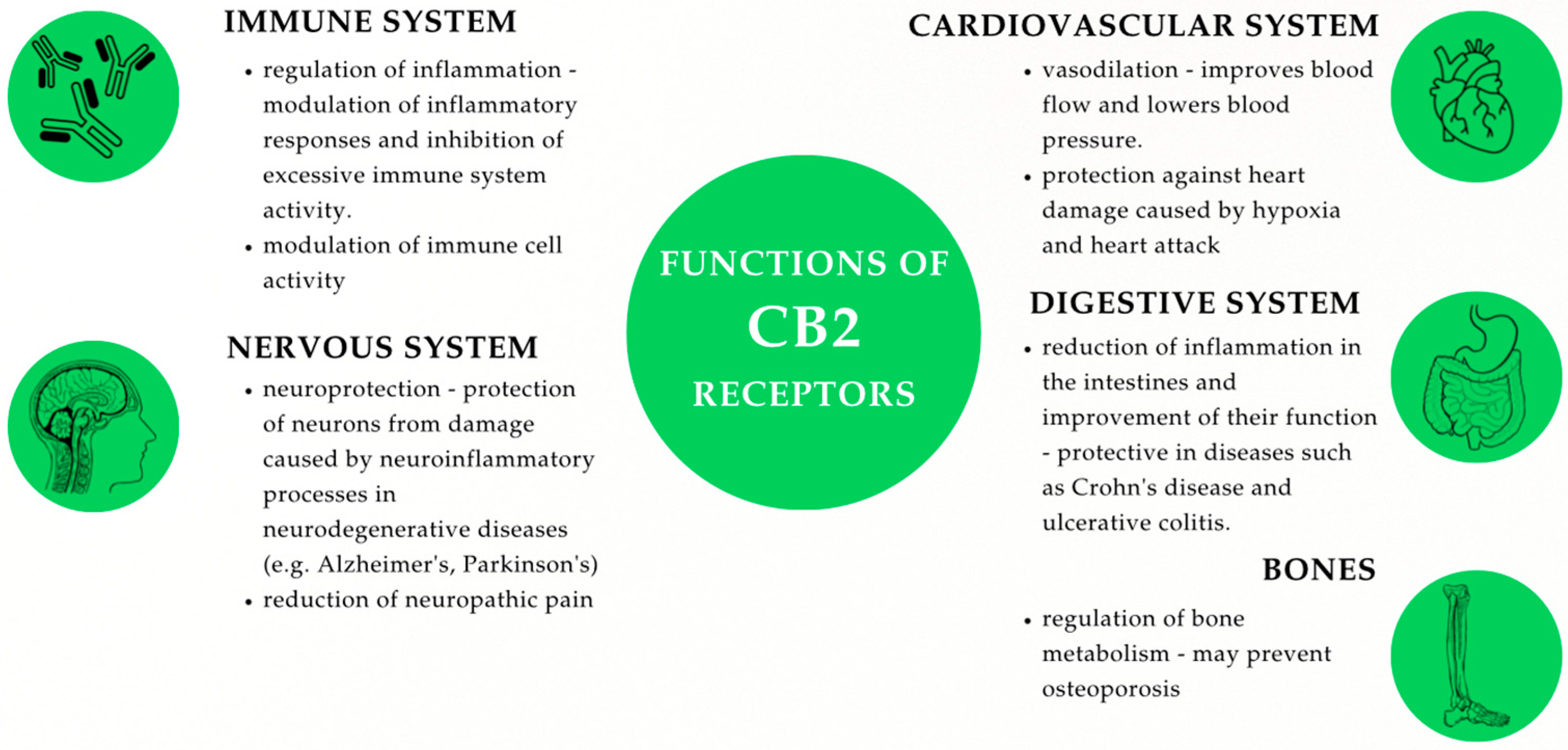
2.1.1. CB1 and CB2: Types, Structure, and Mechanisms of Action
2.1.2. Location of CB1 and CB2
2.2. Cannabinoid Ligands
2.2.1. Endogenous Cannabinoids (Endocannabinoids)
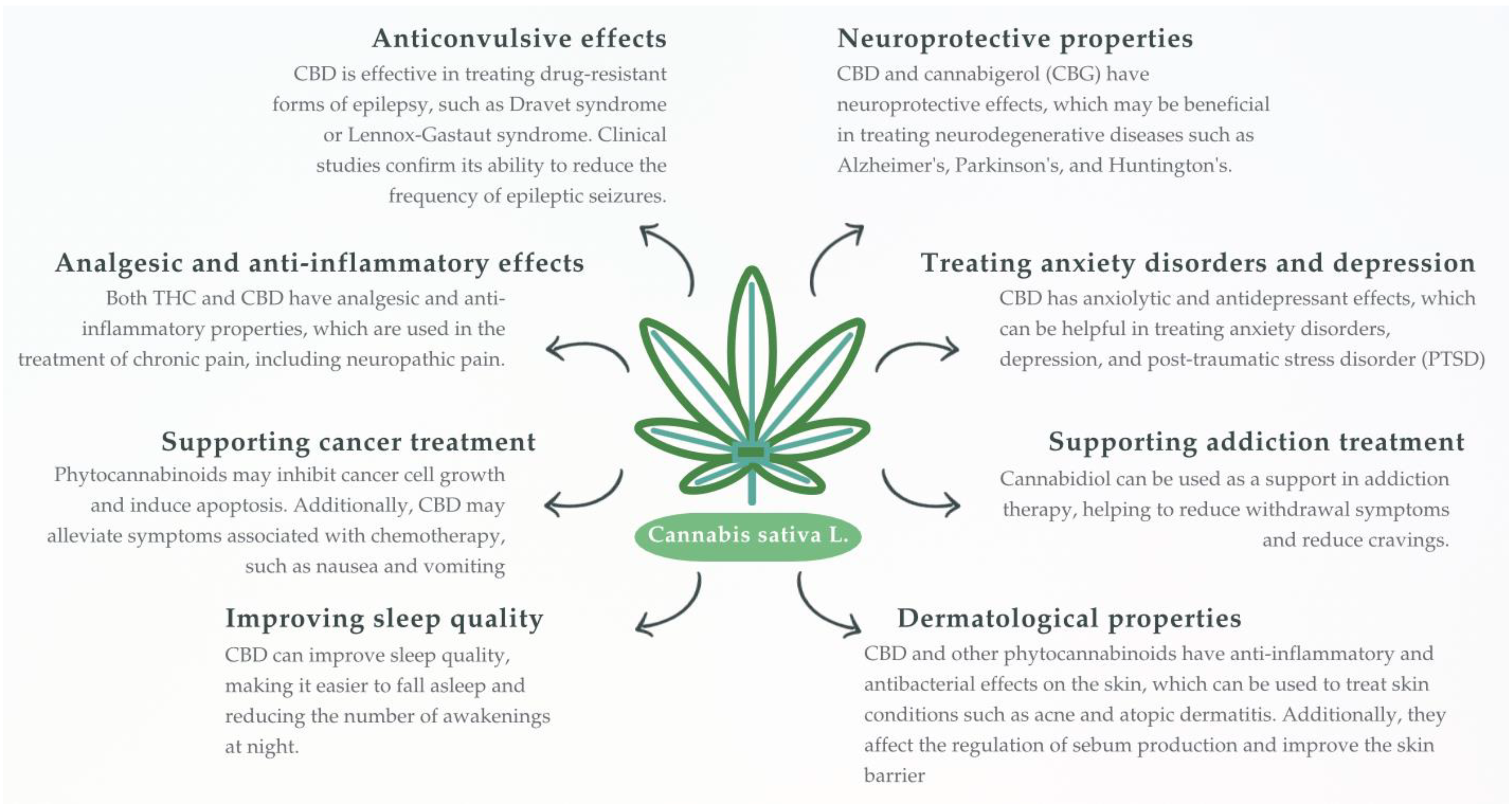
2.2.2. Plant-Derived Cannabinoids (Phytocannabinoids)
Δ9-Tetrahydrocannabinol
Cannabidiol
Other Minor Phytocannabinoids
2.2.3. Synthetic Cannabinoids
2.3. Non-Classical Cannabinoid Receptors
3. The Role of the Endocannabinoid System and Cannabinoids in Reproductive Processes
3.1. ECS and Cannabinoids in Female Reproductive Processes
3.1.1. ECS and Its Physiological Connections with the Hypothalamic–Pituitary–Gonadal Axis(HPG Axis): Influence on the Secretion of Sex Hormones and Menstrual Cycle Regulation
3.1.2. ECS in Female Reproductive Tissues and Gametes
3.1.3. ECS in Fertilization, Pregnancy, and Lactation
3.2. ECS and Cannabinoids in Male Reproductive Processes
3.2.1. ECS in Male Reproductive Tissues and Male Gametes
3.2.2. ECS in Spermatogenesis and Male Reproductive Health
3.3. ECS and Cannabinoids in Gonadal Development and Steroidogenesis
3.4. The Role of the ECS in Libido Regulation
3.5. Sex-Specific Differences in Reproductive ECS Activity
3.6. ECS Receptors and Endocrine-Disrupting Chemicals (EDCs)
3.7. Cannabinoids and Non-Steroidial Anti-Inflammatory Drugs (NSAIDs) in the Context of Reproduction
4. Meaning of the Endo- and Exocannabinoids for the Mammary Tumor (Development and Treatment)
4.1. The Role of the ECS in Cancer Biology
4.2. Interaction Between ECS and Hormonal Regulation in Breast Cancer
4.3. Cannabinoids in Triple-Negative Breast Cancer (TNBC)
4.4. Cannabinoids in HER2+ Breast Cancer
4.5. Cannabinoids in Luminal A (ER+) Breast Cancer
4.6. Cannabinoids in Combination with Hormonal Therapies
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| AEA | Anandamide |
| Act1 | NF-κB Activating Protein 1 |
| AI | Aromatase Inhibitor |
| AIDS | Acquired Immunodeficiency Syndrome |
| AKT | Protein Kinase B |
| BPA | Bisphenol-A |
| cAMP | Cyclic Adenosine Monophosphate |
| CBC | Cannabichromene |
| CBD | Cannabidiol |
| CBDA | Cannabidiolic Acid |
| CBG | Cannabigerol |
| CBN | Cannabinol |
| CBR | Cannabinoid Receptor |
| CB1 | Cannabinoid Receptor type 1 |
| CB2 | Cannabinoid Receptor type 2 |
| CDK1 | Cyclin-Dependent Kinase 1 |
| CL | Corpus Luteum |
| CNS | Central Nervous System |
| COX-1 | Cyclooxygenase type 1 |
| COX-2 | Cyclooxygenase type 2 |
| DINP | Diisononyl phthalate |
| E2 | Estradiol |
| ECS | Endocannabinoid System |
| EDC | Endocrine-disrupting chemical |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| ER- | Estrogen Receptor Negative |
| ER+ | Estrogen Receptor Positive |
| ERα | Estrogen Receptor Alpha |
| ERβ | Estrogen Receptor Beta |
| ERK | Extracellular Signal-Regulated Kinase |
| EVSA-T | Estrogen-Responsive Mammary Tumor Cell Line |
| FAAH | Fatty Acid Amide Hydrolase |
| FDA | Food and Drug Administration |
| FSH | Follicle-Stimulating Hormone |
| GCNIS | Germ cell neoplasia in situ |
| GnRH | Gonadotropin-Releasing Hormone |
| GPCR | G Protein-Coupled Receptor |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HER2+ | Human Epidermal Growth Factor Receptor 2 Positive |
| HIV | Human Immunodeficiency Virus |
| HPG axis | Hypothalamic–Pituitary–Gonadal Axis |
| Id-1 | Inhibitor of Differentiation Protein 1 |
| LH | Luteinizing Hormone |
| MAGL | Monoacylglycerol Lipase |
| MAPK | Mitogen-Activated Protein Kinase |
| MMTV-neu | Mouse Mammary Tumor Virus-neu |
| mRNA | Messenger Ribonucleic Acid |
| mTOR | Mechanistic Target of Rapamycin |
| NAM | Negative Allosteric Modulator |
| NGF | Nerve Growth Factor |
| NPS | New Psychoactive Substances |
| NSAID | Non-Steroidal Anti-Inflammatory Drug |
| OSAS | Obstructive Sleep Apnea Syndrome |
| P4 | Progesterone |
| PCOS | Polycystic Ovary Syndrome |
| PGE | Prostaglandin E |
| PGE1 | Prostaglandin E1 |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol-3-kinase |
| PKC | Protein Kinase C |
| PPAR | Peroxisome Proliferator-Activated Receptor |
| PR | Progesterone Receptor |
| PR- | Progesterone Receptor Negative |
| PR+ | Progesterone Receptor Positive |
| PRL | Prolactin |
| PTSD | Post-Traumatic Stress Disorder |
| ROS | Reactive Oxygen Species |
| SC | Synthetic Cannabinoid |
| SERM | Selective Estrogen Receptor Modulator |
| T | Testosterone |
| TDS | Testicular dysgenesis syndrome |
| THC | ∆9-Tetrahydrocannabinol |
| THCV | Tetrahydrocannabivarin |
| TNBC | Triple-Negative Breast Cancer |
| TRPV1 | Transient Receptor Potential Vanilloid 1 |
| VEGF | Vascular Endothelial Growth Factor |
References
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648. [Google Scholar] [CrossRef]
- Touw, M. The Religious and Medicinal Uses of Cannabisin China, India and Tibet. J. Psychoact. Drugs 1981, 13, 23–34. [Google Scholar] [CrossRef]
- Aldrich, M. History of Therapeutic cannabis. In Cannabis in Medical Practice; Mathre, M.L., Ed.; McFarland: Jefferson, NC, USA, 1997; pp. 35–55. [Google Scholar]
- Fankhauser, M. History of Cannabis in Western Medicine. In Cannabis and Cannabinoids; Grotenhermen, F., Russo, E., Eds.; The Haworth Integrative Healing Press: Philadelphia, PA, USA, 2002; pp. 37–51. [Google Scholar]
- Pinho, A.R. Social and Medical Aspects of the Use of Cannabis in Brazil. In Cannabis and Culture; Rubin, V., Ed.; De Gruyter Mouton: Berlin, NY, USA, 1975; pp. 293–302. [Google Scholar] [CrossRef]
- Du Toit, B.M. Cannabis in Africa; Balkema: Rotterdam, The Neatherlands, 1980. [Google Scholar]
- Brill, H.; Nahas, G.G. Cannabis Intoxication and Mental Illness. In Marihuana in Science and Medicine; Nahas, G.G., Ed.; Raven Press: New York, NY, USA, 1984; pp. 263–306. [Google Scholar]
- Long, E.L.; Malone, D.; Taylor, D. The Pharmacological Effects of cannabidiol. DrugsFuture 2005, 30, 747. [Google Scholar]
- Silver, R.J. The Endocannabinoid System of Animals. Animals 2019, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Hartsel, J.A.; Boyar, K.; Pham, A.; Silver, R.J. Cannabis in Veterinary medicine: Cannabinoid Therapies for Animals. In Nutraceuticals in Veterinary Medicine; Gupta, R.C., Srivastava, A., Lall, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 121–155. [Google Scholar]
- Yu, C.H.J.; Rupasinghe, V.H.P. Cannabidiol-based Natural Health Products for Companion animals: Recent Advances in the Management of anxiety, pain, and Inflammation. Res. Vet. Sci. 2021, 140, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Campolongo, P.; Trezza, V. The Endocannabinoid system: A Key Modulator of Emotions and Cognition. Front. Behav. Neurosci. 2012, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Corsato Alvarenga, I.; Panickar, K.S.; Hess, H.; McGrath, S. Scientific Validation of Cannabidiol for Management of Dog and Cat Diseases. Annu. Rev. Anim. Biosci. 2023, 11, 227–246. [Google Scholar] [CrossRef]
- Osei-Hyiaman, D.; Harvey-White, J.; Bátkai, S.; Kunos, G. The Role of the Endocannabinoid System in the Control of Energy Homeostasis. Int. J. Obes. 2006, 30, S33–S38. [Google Scholar] [CrossRef]
- Silvestri, C.; Di Marzo, V. The Endocannabinoid System in Energy Homeostasis and the Etiopathology of Metabolic Disorders. Cell Metab. 2013, 17, 475–490. [Google Scholar] [CrossRef]
- Tibiriça, E. The Multiple Functions of the Endocannabinoid system: A Focus on the Regulation of Food Intake. Diabetol. Metab. Syndr. 2010, 2, 5. [Google Scholar] [CrossRef]
- Woods, S.C. The Endocannabinoid System: Mechanisms behind Metabolic Homeostasis and Imbalance. Am. J. Med. 2007, 120, S9–S17. [Google Scholar] [CrossRef]
- Guindon, J.; Hohmann, A.G. The Endocannabinoid System and cancer: Therapeutic Implications. Br. J. Pharmacol. 2011, 163, 1447–1463. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscitelli, A.; Ligresti, A.; Bifulco, M.; Di Marzo, V.; et al. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Śledziński, P.; Zeyland, J.; Słomski, R.; Nowak, A. The Current State and Future Perspectives of Cannabinoids in Cancer Biology. Cancer Med. 2018, 7, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.; Amaral, C.; Teixeira, N.; Correia-da-Silva, G. Cannabis sativa: Much More beyond Δ9-tetrahydrocannabinol. Pharmacol. Res. 2020, 157, 104822. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Makriyannis, A. 2012 Division of Medicinal Chemistry Award Address. Trekking the Cannabinoid Road: A Personal Perspective. J. Med. Chem. 2014, 57, 3891–3911. [Google Scholar] [CrossRef]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Andrzejewska, A.; Staszak, K.; Hryhorowicz, M.; Korcz, A.; Słomski, R. Allosteric Modulation of Cannabinoid Receptor 1—Current Challenges and Future Opportunities. Int. J. Mol. Sci. 2019, 20, 5874. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Howlett, A.C. Inhibition of Neuroblastoma Adenylate Cyclase by Cannabinoid and Nantradol Compounds. Life Sci. 1984, 35, 1803–1810. [Google Scholar] [CrossRef]
- Latorraca, N.R.; Venkatakrishnan, A.J.; Dror, R.O. GPCR Dynamics: Structures in Motion. Chem. Rev. 2016, 117, 139–155. [Google Scholar] [CrossRef]
- Weis, W.I.; Kobilka, B.K. The Molecular Basis of G Protein–Coupled Receptor Activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef]
- Calandra, B.; Portier, M.; Kernéis, A.; Delpech, M.; Carillon, C.; Le Fur, G.; Ferrara, P.; Shire, D. Dual Intracellular Signaling Pathways Mediated by the Human Cannabinoid CB1 Receptor. Eur. J. Pharmacol. 1999, 374, 445–455. [Google Scholar] [CrossRef]
- Glass, M.; Felder, C.C. Concurrent Stimulation of Cannabinoid CB1 and Dopamine D2 Receptors Augments cAMP Accumulation in Striatal Neurons: Evidence for a GsLinkage to the CB1 Receptor. J. Neurosci. 1997, 17, 5327–5333. [Google Scholar] [CrossRef] [PubMed]
- Bonhaus, D.W.; Chang, L.K.; Kwan, J.; Martin, G.R. Dual Activation and Inhibition of Adenylyl Cyclase by Cannabinoid Receptor Agonists: Evidence for Agonist-Specific Trafficking of Intracellular Responses. J. Pharmacol. Exp. Ther. 1998, 287, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Childers, S.R.; Deadwyler, S.A. Role of Cyclic AMP in the Actions of Cannabinoid Receptors. Biochem. Pharmacol. 1996, 52, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Deadwyler, S.A.; Hampson, R.E.; Mu, J.; Whyte, A.; Childers, S. Cannabinoids Modulate Voltage Sensitive Potassium A-current in Hippocampal Neurons via a cAMP-dependent process. J. Pharmacol. Exp. Ther. 1995, 273, 734–743. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef]
- Mashabela, M.D.; Kappo, A.P. Anti-Cancer and Anti-Proliferative Potential of Cannabidiol: A Cellular and Molecular Perspective. Int. J. Mol. Sci. 2024, 25, 5659. [Google Scholar] [CrossRef]
- Gouldson, P.; Calandra, B.; Legoux, P.; Kernéis, A.; Rinaldi-Carmona, M.; Barth, F.; Broillet, M.C.; Le Fur, G.; Perret, C.; Pertwee, R.; et al. Mutational Analysis and Molecular Modelling of the Antagonist SR 144528 Binding Site on the Human Cannabinoid CB2 Receptor. Eur. J. Pharmacol. 2000, 401, 17–25. [Google Scholar] [CrossRef]
- Tao, Q.; McAllister, S.D.; Andreassi, J.; Nowell, K.W.; Cabral, G.A.; Hurst, D.P.; Griffin, G.; Abood, M.E.; Makriyannis, A.; Pertwee, R.; et al. Role of a Conserved Lysine Residue in the Peripheral Cannabinoid Receptor (CB2): Evidence for Subtype Specificity. Mol. Pharmacol. 1999, 55, 605–613. [Google Scholar] [CrossRef]
- Caulfield, M.P.; Brown, D.A. Cannabinoid Receptor Agonists Inhibit Ca Current in NG108-15 Neuroblastoma Cells via a Pertussis toxin-sensitive Mechanism. Br. J. Pharmacol. 1992, 106, 231–232. [Google Scholar] [CrossRef]
- Felder, C.C.; Joyce, K.E.; Briley, E.M.; Mansouri, J.; Mackie, K.; Blond, O.; Lai, Y.; Seltzman, H.H.; Makriyannis, A.; Martin, B.R.; et al. Comparison of the Pharmacology and Signal Transduction of the Human Cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 1995, 48, 443–450. [Google Scholar] [CrossRef]
- Henry, D.J.; Chavkin, C. Activation of Inwardly Rectifying Potassium Channels (GIRK1) by co-expressed Rat Brain Cannabinoid Receptors in Xenopus Oocytes. Neurosci. Lett. 1995, 186, 91–94. [Google Scholar] [CrossRef]
- Mackie, K.; Hille, B. Cannabinoids Inhibit N-type Calcium Channels in neuroblastoma-glioma Cells. Proc. Natl. Acad. Sci. USA 1992, 89, 3825–3829. [Google Scholar] [CrossRef]
- Mackie, K.; Lai, Y.; Westenbroek, R.E.; Mitchell, R.E. Cannabinoids Activate an Inwardly Rectifying Potassium Conductance and Inhibit Q-type Calcium Currents in AtT20 Cells Transfected with Rat Brain Cannabinoid Receptor. J. Neurosci. 1995, 15, 6552–6561. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Melck, D.; Bisogno, T.; De Petrocellis, L. Endocannabinoids: Endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1999, 22, 80. [Google Scholar] [CrossRef]
- Meschler, J.P.; Conley, T.J.; Howlett, A.C. Cannabinoid and Dopamine Interaction in Rodent brain: Effects on Locomotor Activity. Pharmacol. Biochem. Behav. 2000, 67, 567–573. [Google Scholar] [CrossRef]
- Nava, F.; Carta, G.; Battasi, A.M.; Gessa, G.L. D2 Dopamine Receptors Enable Δ9-tetrahydrocannabinol-induced Memory Impairment and Reduction of Hippocampal Extracellular Acetylcholine Concentration. Br. J. Pharmacol. 2000, 130, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M. Cellular and Molecular Mechanisms Underlying Learning and Memory Impairments Produced by Cannabinoids. Learn. Mem. 2000, 7, 132–139. [Google Scholar] [CrossRef]
- Tsou, K.; Mackie, K.; Sañudo-Peña, M.C.; Walker, J.M. Cannabinoid CB1 Receptors Are Localized Primarily on cholecystokinin-containing GABAergic Interneurons in the Rat Hippocampal Formation. Neuroscience 1999, 93, 969–975. [Google Scholar] [CrossRef]
- Hua, T.; Vemuri, K.; Nikas, S.P.; Laprairie, R.B.; Wu, Y.; Qu, L.; Pu, M.; Korde, A.; Jiang, S.; Ho, J.H.; et al. Crystal Structures of agonist-bound Human Cannabinoid Receptor CB 1. Nature 2017, 547, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Vemuri, K.; Pu, M.C.; Qu, L.; Han, G.W.; Wu, Y.R.; Zhao, S.; Shui, W.; Sun, H.J.; Wu, L.J.; et al. Crystal Structure of the Human Cannabinoid Receptor CB1. Cell 2016, 167, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; pp. 299–325. [Google Scholar]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a Cannabinoid Receptor and Functional Expression of the Cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Pryce, G.; Giovannoni, G.; Thompson, A.J. The Therapeutic Potential of Cannabis. Lancet Neurol. 2003, 2, 291–298. [Google Scholar] [CrossRef]
- Hryhorowicz, S.; Kaczmarek-Ryś, M.; Zielińska, A.; Scott, R.J.; Słomski, R.; Pławski, A. Endocannabinoid System as a Promising Therapeutic Target in Inflammatory Bowel Disease–a Systematic Review. Front. Immunol. 2021, 12, 790803. [Google Scholar] [CrossRef]
- Ahluwalia, J.; Urban, L.; Capogna, M.; Bevan, S.; Nagy, I. Cannabinoid 1 Receptors Are Expressed in Nociceptive Primary Sensory Neurons. Neuroscience 2000, 100, 685–688. [Google Scholar] [CrossRef]
- Buckley, N.E.; Hansson, S.; Harta, G.; Mezey, É. Expression of the CB1 and CB2 Receptor Messenger RNAs during Embryonic Development in the Rat. Neuroscience 1998, 82, 1131–1149. [Google Scholar] [CrossRef]
- Calignano, A.; Rana, G.L.; Giuffrida, A.; Piomelli, D. Control of Pain Initiation by Endogenous Cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef]
- Friedel, R.H.; Schnürch, H.; Stubbusch, J.; Barde, Y.A. Identification of Genes Differentially Expressed by Nerve Growth factor- and neurotrophin-3-dependent Sensory Neurons. Proc. Natl. Acad. Sci. USA 1997, 94, 12670–12675. [Google Scholar] [CrossRef]
- Howlett, A.C.; Barth, F.; Bonner, T.I.; Cabral, G.; Casellas, P.; Devane, W.A.; Felder, C.C.; Herkenham, M.; Martin, B.R.; Mechoulam, R.; et al. International Union of Pharmacology. XXVII Classif. Cannabinoid Recept. Pharmacol. Rev. 2002, 54, 161–202. [Google Scholar]
- Ishac, E.; Jiang, L.; Lake, K.D.; Varga, K.; Abood, M.E.; Kunos, G. Inhibition of Exocytotic Noradrenaline Release by Presynaptic Cannabinoid CB1 Receptors on Peripheral Sympathetic Nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.S.; Luong, L.A.; Welsh, N.J.; Eglen, R.M.; Martin, G.R.; MacLennan, S.J. Effects of Cannabinoid Receptor Agonists on neuronally-evoked Contractions of Urinary Bladder Tissues Isolated from rat, mouse, pig, dog, Monkey and Human. Br. J. Pharmacol. 2000, 129, 1707–1715. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Cannabinoids and the Gastrointestinal Tract. Gut 2001, 48, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Rice, W.; Shannon, J.M.; Burton, F.; Fiedeldey, D. Expression of a brain-type Cannabinoid Receptor (CB1) in Alveolar Type II Cells in the lung: Regulation by Hydrocortisone. Eur. J. Pharmacol. 1997, 327, 227–232. [Google Scholar] [CrossRef]
- Ross, R.A.; Coutts, A.A.; McFarlane, S.M.; Anavi-Goffer, S.; Irving, A.J.; Pertwee, R.G.; Makriyannis, A.; Seltzman, H.H.; Martin, B.R.; Di Marzo, V.; et al. Actions of Cannabinoid Receptor Ligands on Rat Cultured Sensory neurones: Implications for Antinociception. Neuropharmacology 2001, 40, 221–232. [Google Scholar] [CrossRef]
- Schatz, A.R.; Lee, M.; Condie, R.B.; Pulaski, J.T.; Kaminski, N.E. Cannabinoid Receptors CB1 and CB2: A Characterization of Expression and Adenylate Cyclase Modulation within the Immune System. Toxicol. Appl. Pharmacol. 1997, 142, 278–287. [Google Scholar] [CrossRef]
- Elphick, M.R.; Egertova, M. The Neurobiology and Evolution of Cannabinoid Signalling. Philos. Trans. R Soc. Lond. B Biol. Sci. 2001, 356, 381–408. [Google Scholar] [CrossRef]
- Piomelli, D.; Giuffrida, A.; Calignano, A.; Rodríguez de Fonseca, F. The Endocannabinoid System as a Target for Therapeutic Drugs. Trends. Pharmacol. Sci. 2000, 21, 218–224. [Google Scholar] [CrossRef]
- Rubino, T.; Viganò, D.; Massi, P.; Parolaro, D. Changes in the Cannabinoid Receptor Binding, G Protein Coupling, and Cyclic AMP Cascade in the CNS of Rats Tolerant to and Dependent on the Synthetic Cannabinoid Compound CP55,940. J. Neurochem. 2000, 75, 2080–2086. [Google Scholar] [CrossRef]
- Chen Djie Gao, M.; Gao Ffei Su, Q.; Wu, J. Brain Cannabinoid Receptor 2: Expression, Function and Modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef]
- Galiazzo, G.; Giancola, F.; Stanzani, A.; Fracassi, F.; Bernardini, C.; Forni, M.; Olivieri, M.; Capucchio, M.T.; Pietra, M.; Abramo, F.; et al. Localization of Cannabinoid Receptors CB1, CB2, GPR55, and PPARα in the Canine Gastrointestinal Tract. Histochem. Cell. Biol. 2018, 150, 187–205. [Google Scholar] [CrossRef]
- Li, X.T.; Hua, T.; Vemuri, K.; Ho, J.H.; Wu, Y.R.; Wu, L.J.; Zhuang, Y.; Korde, A.; Zhao, Q.; Shen, L.; et al. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 2019, 176, 459–467. [Google Scholar] [CrossRef]
- Pertwee, R. Cannabinoid Receptors and Their Ligands. Eur. Neuropsychopharmacol. 2002, 12, 127–128. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Ross, R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2002, 66, 101–121. [Google Scholar] [CrossRef]
- Mackie, K. Cannabinoid receptors: Where They Are and What They Do. J. Neuroendocrinol. 2008, 20, 10–14. [Google Scholar] [CrossRef]
- Bouaboula, M.; Poinot-Chazel, C.; Marchand, J.; Canat, X.; Bourrie, B.; Rinaldi-Carmona, M.; Shire, D.; Fur, G.L.; Kunos, G.; Pertwee, R.G.; et al. Signaling Pathway Associated with Stimulation of CB2 Peripheral Cannabinoid Receptor. Involvement of Both Mitogen-Activated Protein Kinase and Induction of Krox-24 Expression. Eur. J. Biochem. 1996, 237, 704–711. [Google Scholar] [CrossRef]
- Saroz, Y.; Kho, D.T.; Glass, M.; Graham, E.S.; Grimsey, N.L. Cannabinoid Receptor 2 (CB2) Signals via Gαs and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes. ACS Pharmacol. Transl. Sci. 2019, 2, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Slipetz, D.M.; O’Neill, G.P.; Favreau, L.; Dufresne, C.; Gallant, M.; Gareau, Y.; Jamali, K.; Makriyannis, A.; Young, A.; Pertwee, R.G.; et al. Activation of the Human Peripheral Cannabinoid Receptor Results in Inhibition of Adenylyl cyclase. Mol. Pharmacol. 1995, 48, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Soethoudt, M.; Grether, U.; Fingerle, J.; Grim, T.W.; Fezza, F.; De Petrocellis, L.; Ligresti, A.; Ross, R.A.; Di Marzo, V.; Pertwee, R.G.; et al. Cannabinoid CB2 Receptor Ligand Profiling Reveals Biased Signalling and off-target Activity. Nat. Commun. 2017, 8, 13958. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, F.; Grandi, V.; Banerjee, A.; Trant, J.F. Cannabinoids and Cannabinoid Receptors: The Story so Far. iScience 2020, 23, 101301. [Google Scholar] [CrossRef] [PubMed]
- Tsutahara, N.M.; Weems, Y.S.; Arreguin-Arevalo, J.A.; Nett, T.M.; LaPorte, M.E.; Uchida, J.; Taylor, A.A.; Pertwee, R.G.; Kunos, G.; Makriyannis, A.; et al. Effects of Endocannabinoid 1 and 2 (CB1; CB2) Receptor Agonists on Luteal weight, Circulating progesterone, Luteal mRNA for Luteinizing Hormone (LH) receptors, and Luteal Unoccupied and Occupied Receptors for LH in Vivo in Ewes. Prostaglandins Other Lipid Mediat. 2011, 94, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Maccarrone, M.; Battista, N.; Centonze, D. The Endocannabinoid Pathway in Huntington’s disease: A Comparison with Other Neurodegenerative Diseases. Prog. Neurobiol. 2007, 81, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Bouaboula, M.; Rinaldi, M.; Carayon, P.; Carillon, C.; Delpech, B.; Shire, D.; Kunos, G.; Pertwee, R.G.; Martin, B.R.; Makriyannis, A.; et al. Cannabinoid-receptor Expression in Human Leukocytes. Eur. J. Biochem. 1993, 214, 173–180. [Google Scholar] [CrossRef]
- Cacciola, G.; Chioccarelli, T.; Altucci, L.; Fasano, S.; Pierantoni, R.; Cobellis, G. The Endocannabinoid System in Vertebrate Male reproduction: A Comparative Overview. Mol. Cell. Endocrinol. 2008, 286, S17–S23. [Google Scholar] [CrossRef]
- Christopoulos, A.; Coles, P.; Lay, L.; Lew, M.J.; Angus, J.A. Pharmacological Analysis of Cannabinoid Receptor Activity in the Rat Vas Deferens. Br. J. Pharmacol. 2001, 132, 1281–1291. [Google Scholar] [CrossRef]
- Das, S.K.; Paria, B.C.; Chakraborty, I.; Dey, S.K. Cannabinoid ligand-receptor Signaling in the Mouse uterus. Proc. Natl. Acad. Sci. USA 1995, 92, 4332–4336. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. The Role of Endocannabinoids in Pregnancy. BJOG 2009, 116, 1249–1257. [Google Scholar]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. Localisation and Function of the Endocannabinoid System in the Human Ovary. Dey SK, editor. PLoS ONE 2009, 4, e4579. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Elson, J.C.; Brown, L.; Davidson, A.C.; Konje, J.C. The Relationship between Plasma Anandamide Levels and the Duration of the Follicular Phase of the Menstrual Cycle. Fertil. Steril. 2009, 92, 802–807. [Google Scholar]
- Fonseca, B.M.; Teixeira, N.A.; Almada, M.; Taylor, A.H.; Konje, J.C.; Correia-da-Silva, G. Modulation of the Novel Cannabinoid Receptor-GPR55-during Rat Fetoplacental Development. Placenta 2011, 32, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Gurm, H.; Hirota, J.A.; Raha, S. Cannabinoid Signalling in Immune–reproductive Crosstalk during Human Pregnancy. Biomedicines 2021, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Mollereau, C.; Vassart, G.; Parmentier, M. Molecular Cloning of a Human Cannabinoid Receptor Which Is Also Expressed in Testis. Biochem. J. 1991, 279, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.G.; Visconti, P.E. Molecular Changes and Signaling Events Occurring in Sperm during Epididymal Transit. Andrology 2016, 4, 832–845. [Google Scholar]
- Grimaldi, P.; Pucci, M.; Siena, D.; Giacomo, D.; Pirazzi, V.; Geremia, R. The Endocannabinoid System and Pivotal Role of the CB2 Receptor in Mouse Spermatogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 11131–11136. [Google Scholar] [CrossRef]
- Maccarrone, M.; Finazzi-Agrò, A. Endocannabinoid Signaling at the periphery: Role in Female Reproduction. Trends Pharmacol. Sci. 2015, 36, 524–534. [Google Scholar] [CrossRef]
- Maccarrone, M.; Falciglia, K.; Rienzo, D.; Finazzi-Agrò, A. Endocannabinoids, hormone-cytokine Networks and Human Fertility. J. Neuroendocrinol. 2005, 17, 509–510. [Google Scholar] [CrossRef]
- Mechoulam, C.T.; Ross, R.A. Inhibition of Human Tumor Cell Line Proliferation by endocannabinoids: Association with Cannabinoid Receptors and Other Targets. Br. J. Pharmacol. 2012, 165, 2414–2427. [Google Scholar]
- Park, B.; Gibbons, H.M.; Mitchell, M.D.; Glassa, M. Identification of the CB1 Cannabinoid Receptor and Fatty Acid Amide Hydrolase (FAAH) in the Human Placenta. Placenta 2003, 24, 473–478. [Google Scholar] [CrossRef]
- Rossato, M.; Popa, I.; Ferigo, M.; Clari, G.; Foresta, C. Human Sperm Express Cannabinoid Receptor Cb1, the Activation of Which Inhibits motility, Acrosome reaction, and Mitochondrial Function. J. Clin. Endocrinol. Metab. 2005, 90, 984–991. [Google Scholar] [CrossRef]
- Sampaio, C.; Watanabe, M.; Contarini, M.S.; Gonzalez-Martinez, S.; Oliveira, A.R.; González-Calvo, L.; Dalmaz, C.; De Felício, C.; Pertwee, R.G.; Makriyannis, A.; et al. Expression of Cannabinoid Receptors and Their Role in the Regulation of Mammary Gland Development. Eur. J. Pharmacol. 2010, 648, 1–7. [Google Scholar]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Pertwee, R.G.; Makriyannis, A.; Martin, B.R.; Di Marzo, V.; et al. Evidence That anandamide-signaling Regulates Human Sperm Functions Required for Fertilization. Mol. Hum. Reprod. 2002, 8, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Xie, H.; Yang, J.; Wang, H.; Bradshaw, H.B.; Dey, S.K. Endocannabinoid Signaling Directs Differentiation of Trophoblast Cell Lineages and Blastocyst Implantation. Proc. Natl. Acad. Sci. USA 2004, 101, 3897–3902. [Google Scholar]
- Wang, H.; Guo, Y.; Wang, D.; Kingsley, P.J.; Marnett, L.J.; Das, S.K.; Makriyannis, A.; Martin, B.R.; Di Marzo, V.; Pertwee, R.G.; et al. Aberrant Cannabinoid Signaling Impairs Oviductal Transport of Embryos. Nat. Med. 2004, 10, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, H.; Dey, S.K. Endocannabinoid Signaling Directs Periimplantation Events. AJP-Endocrinol. Metab. 2006, 290, E21–E26. [Google Scholar] [CrossRef]
- Weems, Y.S.; Lewis, A.W.; Neuendorff, D.A.; Randel, R.D.; Weems, C.W. Endocannabinoid (ECN) Receptor Agonists Affect Cow Luteal Function. Prostaglandins Other Lipid Mediat. 2009, 90, 89–93. [Google Scholar] [CrossRef]
- Maccarrone, M.; Bab, I.; Bíró, T.; Cabral, G.A.; Dey, S.K.; Di Marzo, V.; Pertwee, R.G.; Makriyannis, A.; Martin, B.R.; Mechoulam, R.; et al. Endocannabinoid Signaling at the periphery: 50 Years after THC. Trends Pharmacol. Sci. 2009, 30, 601–610. [Google Scholar] [CrossRef]
- Devane, W.; Hanus, L.; Breuer, A.; Pertwee, R.; Stevenson, L.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Sulton-Mekkes, J.; Meiri, H.; et al. Isolation and Structure of a Brain Constituent That Binds to the Cannabinoid Receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S.; Hanus, L.; Ligumsky, M.; Kaminski, N.E.; Schatz, A.R.; Sheskin, T.; Gopher, A.; Almog, S.; Martin, B.R.; et al. Identification of an Endogenous 2-monoglyceride, Present in Canine gut, That Binds to Cannabinoid Receptors. Biochem. Pharmacol. 1995, 50, 83–90. [Google Scholar] [CrossRef]
- Picone, R.P.; Khanolkar, A.D.; Xu, W.; Ayotte, L.A.; Thakur, G.A.; Hurst, D.P.; Makriyannis, A.; Griffin, G.; Martin, B.R.; Pauli, G.F.; et al. (-)-7′-Isothiocyanato-11-hydroxy-1′,1′-dimethylheptylhexahydrocannabinol (AM841), a High-Affinity Electrophilic Ligand, Interacts Covalently with a Cysteine in Helix Six and Activates the CB1 Cannabinoid Receptor. Mol. Pharmacol. 2005, 68, 1623–1635. [Google Scholar] [CrossRef]
- Sugiura, T.; Kondo, S.; Sukagawa, A.; Nakane, S.; Shinoda, A.; Itoh, K.; Yamamoto, I.; Waku, K.; Satoh, Y.; Matsuda, S.; et al. 2-Arachidonoylglycerol: A Possible Endogenous Cannabinoid Receptor Ligand in Brain. Biochem. Biophys. Res. Commun. 1995, 215, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Mackie, K. Cannabinoid Receptors as Therapeutic Targets. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Bouaboula, M.; Poinot-Chazel, C.; Bourrié, B.; Canat, X.; Calandra, B.; Rinaldi-Carmona, M.; Shire, D.; Kunos, G.; Pertwee, R.G.; Makriyannis, A.; et al. Activation of mitogen-activated Protein Kinase by Stimulation of the Central Cannabinoid Receptor CB1. Biochem. J. 1995, 312, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Habayeb, O.M.; Bell, S.C.; Konje, J.C. Endogenous cannabinoids: Metabolism and Their Role in Reproduction. Life Sci. 2002, 70, 1963–1977. [Google Scholar] [CrossRef]
- Jhaveri, M.D.; Richardson, D.; Chapman, V. Endocannabinoid Metabolism and uptake: Novel Targets for Neuropathic and Inflammatory Pain. Br. J. Pharmacol. 2007, 152, 624–632. [Google Scholar] [CrossRef]
- McAllister, S.D.; Glass, M. CB1 and CB2 receptor-mediated signalling: A Focus on Endocannabinoids. Prostaglandins Leukot. Essent. Fat. Acids (PLEFA) 2002, 66, 161–171. [Google Scholar] [CrossRef]
- Stella, N.; Schweitzer, P.; Piomelli, D. A Second Endogenous Cannabinoid That Modulates long-term Potentiation. Nature 1997, 388, 773–778. [Google Scholar] [CrossRef]
- Matias, I.; Gonthier, M.P.; Orlando, P.; Martiadis, V.; De Petrocellis, L.; Cervino, C.; Di Marzo, V.; Makriyannis, A.; Pertwee, R.G.; Martin, B.R.; et al. Regulation, Function, and Dysregulation of Endocannabinoids in Models of Adipose and β-Pancreatic Cells and in Obesity and Hyperglycemia. J. Clin Endocrinol. Metab. 2006, 91, 3171–3180. [Google Scholar] [CrossRef]
- Sigel, E.; Baur, R.; Racz, I.; Marazzi, J.; Smart, T.G.; Zimmer, A.; Mechoulam, R.; Pertwee, R.G.; Makriyannis, A.; Martin, B.R.; et al. The Major Central Endocannabinoid Directly Acts at GABA (A) Receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 18150–18155. [Google Scholar] [CrossRef]
- Spivak, C.E.; Kim, W.; Liu, Q.R.; Lupica, C.R.; Doyle, M.E. Blockade of beta-cell K(ATP) Channels by the endocannabinoid, 2-arachidonoylglycerol. Biochem. Biophys. Res. Commun. 2012, 423, 13–18. [Google Scholar] [CrossRef]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid Receptors on Sensory Nerves Mediate the Vasodilator Action of Anandamide. Nature 1999, 400, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Blebea, N.M.; Pricopie, A.I.; Vlad, R.A.; Hancu, G. Phytocannabinoids: Exploring Pharmacological Profiles and Their Impact on Therapeutical Use. Int. J. Mol. Sci. 2024, 25, 4204. [Google Scholar] [CrossRef] [PubMed]
- Croxford, J.L.; Yamamura, T. Cannabinoids and the Immune system: Potential for the Treatment of Inflammatory diseases? J. Neuroimmunol. 2005, 166, 3–18. [Google Scholar] [CrossRef]
- Darmani, N.A. Delta(9)-tetrahydrocannabinol and Synthetic Cannabinoids Prevent Emesis Produced by the Cannabinoid CB1 Receptor antagonist/inverse Agonist SR141716A. Neuropsychopharmacology 2001, 24, 198–203. [Google Scholar] [CrossRef]
- Mattes, R.D.; Engelman, K.; Shaw, L.M.; Elsohly, M.A. Cannabinoids and Appetite Stimulation. Pharmacol. Biochem. Behav. 1994, 49, 187–195. [Google Scholar] [CrossRef]
- Smith, F.L.; Fujimori, K.; Lowe, J.; Welch, S.P. Characterization of Δ9-Tetrahydrocannabinol and Anandamide Antinociception in Nonarthritic and Arthritic Rats. Pharmacol. Biochem. Behav. 1998, 60, 183–191. [Google Scholar] [CrossRef]
- Sofia, R.D.; Solomon, T.A.; Barry, H. Anticonvulsant Activity of delta9-tetrahydrocannabinol Compared with Three Other Drugs. Eur. J. Pharmacol. 1976, 35, 7–16. [Google Scholar] [CrossRef]
- Van Klingeren, B.; Ten Ham, M. Antibacterial Activity of delta9-tetrahydrocannabinol and Cannabidiol. Antonie Van Leeuwenhoek 1976, 42, 9–12. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-Da-Silva, G.; Teixeira, N.A. Cannabinoid-induced Cell Death in Endometrial Cancer cells: Involvement of TRPV1 Receptors in Apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef]
- Blázquez, C.; González-Feria, L.; Álvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids Inhibit the Vascular Endothelial Growth Factor Pathway in Gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Palomo-Garo, C.; García-Arencibia, M.; Ramos, J.A.; Pertwee, R.G.; Fernández-Ruiz, J. Symptom-relieving and Neuroprotective Effects of the Phytocannabinoid Δ9-THCV in Animal Models of Parkinson’s Disease. Br. J. Pharmacol. 2011, 163, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Noonan, J.; Tanveer, R.; Klompas, A.; Gowran, A.; McKiernan, J.; Campbell, V.A. Endocannabinoids Prevent beta-amyloid-mediated Lysosomal Destabilization in Cultured Neurons. J. Biol. Chem. 2010, 285, 38543–38554. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Rebelo, I. Cannabis and Cannabinoids in Reproduction and Fertility: Where We Stand. Reprod. Sci. 2022, 29, 2429–2439. [Google Scholar] [CrossRef]
- De Briyne, N.; Holmes, D.; Sandler, I.; Stiles, E.; Szymanski, D.; Moody, S.; Breen, J.; Martin, B.R.; Pertwee, R.G.; Makriyannis, A.; et al. Cannabis, Cannabidiol Oils and Tetrahydrocannabinol—What Do Veterinarians Need to Know? Animals 2021, 11, 892. [Google Scholar] [CrossRef]
- Krishna Kumar, K.; Shalev-Benami, M.; Robertson, M.J.; Hu, H.; Banister, S.D.; Hollingsworth, S.A.; Gumpper, R.H.; Carpenter, B.; Skripnikova, E.; Makriyannis, A.; et al. Structure of a Signaling Cannabinoid Receptor 1-G Protein Complex. Cell 2019, 176, 448–458.e12. [Google Scholar] [CrossRef]
- Burstein, S.; Levin, E.; Varanelli, C. Prostaglandins and cannabis—II Inhibition of Biosynthesis by the Naturally Occurring Cannabinoids. Biochem. Pharmacol. 1973, 22, 2905–2910. [Google Scholar] [CrossRef]
- Rabin, R.A.; George, T.P. Cannabis and psychosis: Understanding the Smoke Signals. Lancet Psychiatry 2016, 3, 909–910. [Google Scholar] [CrossRef]
- Volkow, N.; Baler, R.; Compton, W.; Weiss, S. Adverse Health Effects of Marijuana Use. N. Engl. J. Med. 2014, 371, 878–879. [Google Scholar] [CrossRef]
- Blest-Hopley, G.; Colizzi, M.; Giampietro, V.; Bhattacharyya, S. Is the Adolescent Brain at Greater Vulnerability to the Effects of Cannabis? A Narrative Review of the Evidence. Front Psychiatry 2020, 11, 859. [Google Scholar] [CrossRef]
- Blest-Hopley, G.; O’Neill, A.; Wilson, R.; Giampietro, V.; Bhattacharyya, S. Disrupted parahippocampal and midbrain function underlie slower verbal learning in adolescent-onset regular cannabis use. Psychopharmacology 2021, 238, 1315–1331. [Google Scholar] [CrossRef]
- Tomiyama, K.; Funada, M. Cytotoxicity of Synthetic Cannabinoids on Primary Neuronal Cells of the forebrain: The Involvement of Cannabinoid CB1 Receptors and Apoptotic Cell Death. Toxicol. Appl. Pharmacol. 2014, 274, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Peters, M.; Murillo-Rodriguez, E.; Hanuš, L.O. Cannabidiol–Recent Advances. Chem. Biodivers. 2007, 4, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Hacke, A.C.M.; Lima, D.; Costa, D.; Deshmukh, K.; Li, N.; Chow, A.M.; Zhang, Y.; Kim, Y.; Huang, X.; Wang, L.; et al. Probing the Antioxidant Activity of delta(9)-tetrahydrocannabinol and Cannabidiol in Cannabis Sativa Extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef]
- Ranganarayanan, P.; Thanigesan, N.; Ananth, V.; Jayaraman, V.K.; Ramakrishnan, V. Identification of Glucose-Binding Pockets in Human Serum Albumin Using Support Vector Machine and Molecular Dynamics Simulations. IEEE/ACM Trans. Comput. Biol. Bioinform. 2016, 13, 148–157. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol Is a Negative Allosteric Modulator of the Cannabinoid CB1 Receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G. Endocannabinoid Biosynthesis and inactivation, from Simple to Complex. Drug Discov. Today 2010, 15, 474–483. [Google Scholar] [CrossRef]
- Del Giudice, E.; Rinaldi, L.; Passarotto, M.; Facchinetti, F.; D’Arrigo, A.; Guiotto, A.; Leon, A.; Baraldi, P.G.; Peri, F.; Buriani, A.; et al. Cannabidiol, unlike Synthetic cannabinoids, Triggers Activation of RBL-2H3 Mast Cells. J. Leukoc. Biol. 2007, 81, 1512–1522. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Bisogno, T.; Maccarrone, M.; Davis, J.B.; Finazzi-Agrò, A.; Di Marzo, V. The Activity of Anandamide at Vanilloid VR1 Receptors Requires Facilitated Transport across the Cell Membrane and Is Limited by Intracellular Metabolism. J. Biol. Chem. 2001, 276, 12856–12863. [Google Scholar]
- Bow, E.W.; Rimoldi, J.M. The Structure–Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect Med. Chem. 2016, 8, 17–39. [Google Scholar] [CrossRef]
- Kupczyk, P.; Rykała, M.; Serek, P.; Pawlak, A.; Słowikowski, B.; Hołysz, M.; Małopolska, M.; Dzięgiel, P.; Piasecki, T.; Gajewska, M.; et al. The Cannabinoid Receptors System in horses: Tissue Distribution and Cellular Identification in Skin. J. Vet. Intern. Med. 2022, 36, 1508–1524. [Google Scholar] [CrossRef]
- Miagkoff, L.; Girard, C.A.; St-Jean, G.; Richard, H.; Beauchamp, G. Cannabinoid Receptors Are Expressed in Equine Synovium and Upregulated with Synovitis. Equine Vet. J. 2022, 55, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The Therapeutic Potential of Drugs That Target Cannabinoid Receptors or Modulate the Tissue Levels or Actions of endocannabinoids. AAPS J. 2005, 7, E625–E654. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, B.; Meissner, H.; Gupta, V. Dronabinol; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Berlach, D.M.; Shir, Y.; Ware, M.A. Experience with the Synthetic Cannabinoid Nabilone in Chronic Noncancer Pain. Pain Med. 2006, 7, 25–29. [Google Scholar] [CrossRef]
- Peball, M.; Heim, B.; Carbone, F.; Schorr, O.; Werkmann, M.; Ellmerer, P.; Mahlknecht, P.; Knaus, H.G.; Goebel, G.; Seppi, K.; et al. Long-term Safety and Efficacy of open-label Nabilone on Sleep and Pain in Parkinson’s Disease. NPJ Parkinson’s Dis. 2024, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The Effects of Nabilone on Sleep in Fibromyalgia: Results of a Randomized Controlled Trial. Anesth Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef]
- Capozzi, A.; Caissutti, D.; Mattei, V.; Gado, F.; Martellucci, S.; Longo, A.; Lattanzi, W.; Ortona, E.; Aloisi, A.M.; Maccarrone, M.; et al. Anti-Inflammatory Activity of a CB2 Selective Cannabinoid Receptor Agonist: Signaling and Cytokines Release in Blood Mononuclear Cells. Molecules 2021, 27, 64. [Google Scholar] [CrossRef]
- Bukke, V.N.; Archana, M.; Villani, R.; Serviddio, G.; Cassano, T. Pharmacological and Toxicological Effects of Phytocannabinoids and Recreational Synthetic cannabinoids: Increasing Risk of Public health. Pharmaceuticals 2021, 14, 965. [Google Scholar] [CrossRef]
- De Oliveira, M.C.; Vides, M.C.; Lassi, D.L.S.; Torales, J.; Ventriglio, A.; Bombana, H.S.; Tavares, H.; Souza, D.; Molina, V.A.; Fabrazzo, M.; et al. Toxicity of Synthetic Cannabinoids in K2/Spice: A Systematic Review. Brain Sci. 2023, 13, 990. [Google Scholar] [CrossRef]
- Gunderson, E.W.; Haughey, H.M.; Ait-Daoud, N.; Joshi, A.S.; Hart, C.L. “Spice” and “K2” Herbal Highs: A Case Series and Systematic Review of the Clinical Effects and Biopsychosocial Implications of Synthetic Cannabinoid Use in Humans. Am. J. Addict. 2012, 21, 320–326. [Google Scholar] [CrossRef]
- Day, I.N.M.; Thompson, R.J. UCHL1 (PGP 9.5): Neuronal Biomarker and Ubiquitin System Protein. Prog. Neurobiol. 2010, 90, 327–362. [Google Scholar] [CrossRef]
- Andrews, R.; Jorge, R.; Christie, R.; Gallegos, A. From JWH-018 to OXIZIDS: Structural Evolution of Synthetic Cannabinoids in the European Union from 2008 to Present Day. Drug Test Anal. 2022, 15, 378–387. [Google Scholar] [CrossRef]
- Pařízek, A.; Suchopár, J.; Laštůvka, Z.; Alblová, M.; Hill, M.; Dušková, M. The Endocannabinoid System and Its Relationship to Human Reproduction. Physiol. Res. 2023, 72, S365–S380. [Google Scholar] [CrossRef] [PubMed]
- Wenger, T.; Ledent, C.; Csernus, V.; Gerendai, I. The Central Cannabinoid Receptor Inactivation Suppresses Endocrine Reproductive Functions. Biochem. Biophys. Res. Commun. 2001, 284, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, S.; Rossi, G.; Castellucci, A.; D’Andrea, G.; Maccarrone, M. Endocannabinoid Signaling in Mammalian Ovary. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 178, 6–11. [Google Scholar] [CrossRef]
- Resuehr, D.; Glore, D.R.; Taylor, H.S.; Bruner-Tran, K.L.; Osteen, K.G. Progesterone-dependent Regulation of Endometrial Cannabinoid Receptor Type 1 (CB1-R) Expression Is Disrupted in Women with Endometriosis and in Isolated Stromal Cells Exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Fertil Steril. 2012, 98, 948–956.e1. [Google Scholar] [CrossRef]
- Ayalon, D.; Nir, I.; Cordova, T. Acute Effect of delta1-tetrahydrocannabinol on the hypothalamo-pituitary-ovarian Axis in the Rat. Neuroendocrinology 1977, 23, 31–42. [Google Scholar] [CrossRef]
- Brents, L.K. Marijuana, the Endocannabinoid System and the Female Reproductive System. Yale J. Biol. Med. 2016, 89, 175–191. [Google Scholar]
- Chakravarty, I.; Shah, P.G.; Sheth, A.R.; Ghosh, J.J. Mode of Action of delta-9-tetrahydrocannabinol on hypothalamo—pituitary Function in Adult Female Rats. Reproduction 1979, 57, 113–115. [Google Scholar] [CrossRef]
- Dalterio, S.; Mayfield, D.; Bartke, A. Effects of Delta 9-THC on Plasma Hormone Levels in Female Mice. Subst. Alcohol Actions Misuse 1983, 4, 339–345. [Google Scholar]
- Liu, X.; Herbison, A.E. Dopamine Regulation of gonadotropin-releasing Hormone Neuron Excitability in Male and Female Mice. Endocrinology 2013, 154, 340–350. [Google Scholar] [CrossRef]
- Mendelson, J.H.; Mello, N.K.; Ellingboe, J. Acute Effects of Marihuana Smoking on Prolactin Levels in Human females. J. Pharmacol. Exp. Ther. 1985, 232, 220–222. [Google Scholar] [CrossRef]
- Mendelson, J.H.; Mello, N.K.; Ellingboe, J.; Skupny, A.S.; Lex, B.W.; Griffin, M. Marihuana Smoking Suppresses Luteinizing Hormone in women. J. Pharmacol. Exp. Ther. 1986, 237, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Field, E.; Tyrey, L. Delayed Sexual Maturation during Prepubertal Cannabinoid treatment: Importance of the Timing of treatment. J. Pharmacol. Exp. Ther. 1990, 254, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Neradugomma, N.K.; Drafton, K.; Mor, G.G.; Mao, Q.C. Marijuana-derived Cannabinoids Inhibit Uterine Endometrial Stromal Cell Decidualization and Compromise trophoblast-endometrium Crosstalk. Reprod. Toxicol. 2019, 87, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Gammon, C.M.; Freeman, G.M.; Xie, W.; Petersen, S.L.; Wetsel, W.C. Regulation of Gonadotropin-Releasing Hormone Secretion by Cannabinoids. Endocrinology 2005, 146, 4491–4499. [Google Scholar] [CrossRef]
- Maia, J.; Almada, M.; Silva, A.; Correia-da-Silva, G.; Teixeira, N.; Sá, S.I.; Amaral, C.; Fonseca, B.M.; Oliveira, P.; Moreira, J.A.; et al. The Endocannabinoid System Expression in the Female Reproductive Tract Is Modulated by Estrogen. J. Steroid Biochem. Mol. Biol. 2017, 174, 40–47. [Google Scholar] [CrossRef]
- Scotchie, J.G.; Savaris, R.F.; Martin, C.E.; Young, S.L. Endocannabinoid Regulation in Human Endometrium across the Menstrual Cycle. Reprod. Sci. 2014, 22, 113–123. [Google Scholar] [CrossRef]
- Maccarrone, M.; Valensise, H.; Bari, M.; Lazzarin, N.; Romanini, C.; Finazzi-Agrò, A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. Lancet 2000, 355, 1326–1329. [Google Scholar] [CrossRef]
- Juan, C.C.; Chen, K.H.; Wang, P.H.; Hwang, J.L.; Seow, K.M. Endocannabinoid System Activation May Be Associated with Insulin Resistance in Women with Polycystic Ovary Syndrome. Fertil Steril. 2015, 104, 200–206. [Google Scholar] [CrossRef]
- Shen, X.; Duan, H.; Wang, S.; Gan, L.; Xu, Q.; Li, J.J. Decreased Expression of Cannabinoid Receptors in the Eutopic and Ectopic Endometrium of Patients with Adenomyosis. Biomed. Res. Int. 2019, 2019, 5468954. [Google Scholar] [CrossRef]
- Tanaka, K.; Mayne, L.; Khalil, A.; Baartz, D.; Eriksson, L.; Mortlock, S.A.; Cooper, D.; Ewing, A.; Lee, A.; Sgro, M.; et al. The Role of the Endocannabinoid System in Aetiopathogenesis of endometriosis: A Potential Therapeutic Target. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 244, 87–94. [Google Scholar] [CrossRef]
- Pirone, A.; Lenzi, C.; Briganti, A.; Abbate, F.; Levanti, M.; Abramo, F.; Castaldo, L.; Miragliotta, V.; Mariotti, V.; Casini, L.; et al. Spatial Distribution of Cannabinoid Receptor 1 and Fatty Acid Amide Hydrolase in the Cat Ovary and Oviduct. Acta Histochem. 2017, 119, 417–422. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Anandamide-Induced Cell Death: Dual Effects in Primary Rat Decidual Cell Cultures. Placenta 2009, 30, 686–692. [Google Scholar] [CrossRef]
- Battista, N.; Bari, M.; Rapino, C.; Trasatti, F.; D’Agostino, A.; Maccarrone, M. Regulation of Female Fertility by the Endocannabinoid System. Hum. Fertil. 2007, 10, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Battista, N.; Pasquariello, N.; Di Thomaso, M.; Maccarrone, M. Interplay between endocannabinoids, Steroids and Cytokines in the Control of Human Reproduction. J. Neuroendocrinol. 2008, 20, 82–89. [Google Scholar] [CrossRef]
- Fride, E. Multiple Roles for the Endocannabinoid System during the Earliest Stages of Life: Pre- and Postnatal Development. J. Neuroendocrinol. 2008, 20, 75–81. [Google Scholar] [CrossRef]
- Fride, E.; Gobshtis, N.; Dahan, H.; Weller, A.; Giuffrida, A.; Ben-Shabat, S. The Endocannabinoid System during development: Emphasis on Perinatal Events and Delayed Effects. Vitam. Horm. 2009, 81, 139–158. [Google Scholar] [PubMed]
- Habayeb, O.M.; Taylor, A.H.; Bell, S.C.; Taylor, D.J.; Konje, J.C. Expression of the Endocannabinoid System in Human First Trimester Placenta and Its Role in Trophoblast Proliferation. Endocrinology 2008, 149, 5052–5060. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Cota, D.; Lutz, B.; Pasquali, R. The Emerging Role of the Endocannabinoid System in Endocrine Regulation and Energy Balance. Endocr. Rev. 2006, 27, 73–100. [Google Scholar] [CrossRef]
- Trabucco, E.; Acone, G.; Marenna, A.; Pierantoni, R.; Cacciola, G.; Chioccarelli, T.; Cobellis, G.; Manna, C.; Fasano, S.; Ferraro, B.; et al. Endocannabinoid System in First Trimester Placenta: Low FAAH and High CB1 Expression Characterize Spontaneous Miscarriage. Placenta 2009, 30, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Niswender, G.D.; Schwall, R.H.; Fitz, T.A.; Farin, C.E.; Sawyer, H.R. Regulation of Luteal Function in Domestic ruminants: New Concepts. Recent Prog. Horm. Res. 1985, 41, 101–151. [Google Scholar] [PubMed]
- Niswender, G.D.; Davis, T.L.; Griffith, R.J.; Bogan, R.L.; Monser, K.; Bott, R.C.; Wiltbank, M.C.; Stormshak, F.; Nett, T.M.; Juengel, J.L.; et al. Judge, Jury and executioner: The auto-regulation of Luteal Function. Soc. Reprod. Fertil. 2007, 64, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Weems, C.W.; Weems, Y.S.; Randel, R.D. Prostaglandins and Reproduction in Female Farm Animals. Vet. J. 2006, 172, 206–228. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Diskin, M.G.; Niswender, G.D. Differential Actions of Second Messenger Systems in the Corpus luteum. J. Reprod. Fertil. 1991, 43, 65–75. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Belfiore, C.J.; Niswender, G.D. Steroidogenic Enzyme Activity after Acute Activation of Protein Kinase (PK) a and PKC in Ovine Small and Large Luteal Cells. Mol. Cell. Endocrinol. 1993, 97, 1–7. [Google Scholar] [CrossRef]
- McGuire, W.J.; Juengel, J.L.; Niswender, G.D. Protein Kinase C Second Messenger System Mediates the Antisteroidogenic Effects of Prostaglandin F2α in the Ovine Corpus Luteum in Vivo. Biol. Reprod. 1994, 51, 800–806. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Guthrie, P.B.; Matfson, M.P.; Kater, S.B.; Niswender, G.D. Hormonal Regulation of Free Intracellular Calcium Concentrations in Small and Large Ovine Luteal Cells1. Biol. Reprod. 1989, 41, 771–778. [Google Scholar] [CrossRef]
- Lo, J.O.; Hedges, J.C.; Girardi, G. Impact of cannabinoids on pregnancy, reproductive health, and offspring out-comes. Am. J. Obstet. Gynecol. 2022, 227, 571–581. [Google Scholar] [CrossRef]
- Liu, W.M.; Duan, E.K.; Cao, Y.J. Effects of Anandamide on Embryo Implantation in the Mouse. Life Sci. 2002, 71, 1623–1632. [Google Scholar] [CrossRef]
- Horne, A.W.; Phillips, J.A.; Kane, N.; Lourenco, P.C.; McDonald, S.E.; Williams, A.R.W.; Milne, S.A.; Shaw, J.L.V.; Critchley, H.O.D.; Saunders, P.T.K.; et al. CB1 Expression Is Attenuated in Fallopian Tube and Decidua of Women with Ectopic Pregnancy. Aziz SA, editor. PLoS ONE 2008, 3, e3969. [Google Scholar] [CrossRef] [PubMed]
- Shao, R. Understanding the Mechanisms of Human Tubal Ectopic pregnancies: New Evidence from Knockout Mouse Models. Hum. Reprod. 2009, 25, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dey, S.K. Endocannabinoid Signaling in Female Reproduction. ACS Chem. Neurosci. 2012, 3, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Leemaqz, S.Y.; Dekker, G.A.; McCowan, L.M.; Kenny, L.C.; Myers, J.E.; Simpson, N.A.B.; Roberts, C.T.; Giannoulatou, E.; Denny, K.J.; Clifton, V.L.; et al. Maternal Marijuana Use Has Independent Effects on Risk for Spontaneous Preterm Birth but Not Other Common Late Pregnancy Complications. Reprod. Toxicol. 2016, 62, 77–86. [Google Scholar] [CrossRef]
- Kasman, A.M.; Thoma, M.E.; McLain, A.C.; Eisenberg, M.L. Association between Use of Marijuana and Time to Pregnancy in Men and women: Findings from the National Survey of Family Growth. Fertil. Steril. 2018, 109, 866–871. [Google Scholar] [CrossRef]
- Wise, L.A.; Wesselink, A.K.; Hatch, E.E.; Rothman, K.J.; Mikkelsen, E.M.; Sørensen, H.T.; Hahn, K.A.; McKinnon, C.; Mahalingaiah, S.; Yeung, E.H.; et al. Marijuana Use and Fecundability in a North American Preconception Cohort Study. J. Epidemiol. Community Health 2017, 72, 208–215. [Google Scholar] [CrossRef]
- Bertrand, K.A.; Hanan, N.J.; Honerkamp-Smith, G.; Best, B.M.; Chambers, C.D. Marijuana Use by Breastfeeding Mothers and Cannabinoid Concentrations in Breast Milk. Pediatrics 2018, 142, e20181076. [Google Scholar] [CrossRef]
- Blackard, C.; Tennes, K. Human Placental Transfer of Cannabinoids. N. Engl. J. Med. 1984, 311, 797. [Google Scholar]
- Amoako, A.A.; Marczylo, T.H.; Lam, P.M.W.; Willets, J.M.; Derry, A.; Elson, J.; Taylor, A.H.; Konje, J.C.; Cooke, M.S.; Jabbour, H.N.; et al. Quantitative Analysis of Anandamide and Related Acylethanolamides in Human Seminal Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry. J. Chromatogr. B 2010, 878, 3231–3237. [Google Scholar] [CrossRef]
- Aquila, S.; Guido, C.; Santoro, A.; Perrotta, I.; Laezza, C.; Bifulco, M.; Fasano, S.; Maradonna, F.; Sciarra, F.; Jannini, E.A.; et al. Human Sperm Anatomy: Ultrastructural Localization of the Cannabinoid1 Receptor and a Potential Role of Anandamide in Sperm Survival and Acrosome Reaction. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2009, 293, 298–309. [Google Scholar] [CrossRef]
- Bari, M.; Battista, N.; Pirazzi, V.; Maccarrone, M. The Manifold Actions of Endocannabinoids on Female and Male Reproductive Events. Front. Biosci. 2011, 16, 498. [Google Scholar] [CrossRef]
- Agirregoitia, E.; Carracedo, A.; Subirán, N.; Valdivia, A.; Agirregoitia, N.; Peralta, L.; Gómez, M.; Rodriguez, J.; López, R.; Fernández, L.; et al. The CB(2) Cannabinoid Receptor Regulates Human Sperm Cell Motility. Fertil Steril. 2010, 93, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Amoako, A.A.; Marczylo, T.H.; Marczylo, E.L.; Elson, J.; Willets, J.M.; Taylor, A.H.; Konje, J.C.; Cooke, M.S.; Jabbour, H.N.; Macdonald, I.A.; et al. Anandamide Modulates Human Sperm motility: Implications for Men with Asthenozoospermia and Oligoasthenoteratozoospermia. Hum. Reprod. 2013, 28, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Gasperi, V.; Paro, R.; Barsacchi, D.; Cecconi, S.; Maccarrone, M. Follicle-Stimulating Hormone Activates Fatty Acid Amide Hydrolase by Protein Kinase a and Aromatase-Dependent Pathways in Mouse Primary Sertoli Cells. Endocrinology 2007, 148, 1431–1439. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, F.; Donzé, N.; Rahban, R.; Senn, A.; Stettler, E.; Rudaz, S.; Nef, S.; Rossier, M.F. Semen Endocannabinoids Are Correlated to Sperm Quality in a Cohort of 200 Young Swiss Men. Andrology 2020, 8, 1126–1135. [Google Scholar] [CrossRef]
- Lee, M.S.; Lanes, A.; Ginsburg, E.S.; Fox, J.H. Delta-9 THC Can Be Detected and Quantified in the Semen of Men Who Are Chronic Users of Inhaled Cannabis. J. Assist. Reprod. Genet. 2020, 37, 1497–1504. [Google Scholar] [CrossRef]
- Fantus, R.J.; Lokeshwar, S.D.; Kohn, T.P.; Ramasamy, R. The Effect of Tetrahydrocannabinol on Testosterone among Men in the United States: Results from the National Health and Nutrition Examination Survey. World J. Urol. 2020, 38, 3275–3282. [Google Scholar] [CrossRef]
- Kolodny, R.C.; Masters, W.H.; Kolodner, R.M.; Toro, G. Depression of Plasma Testosterone Levels after Chronic Intensive Marihuana Use. N. Engl. J. Med. 1974, 290, 872–874. [Google Scholar] [CrossRef]
- Carroll, K.; Pottinger, A.M.; Wynter, S.; DaCosta, V. Marijuana Use and Its Influence on Sperm Morphology and motility: Identified Risk for Fertility among Jamaican Men. Andrology 2019, 8, 136–142. [Google Scholar] [CrossRef]
- Murphy, S.K.; Itchon-Ramos, N.; Visco, Z.; Huang, Z.; Grenier, C.; Schrott, R.; Smith, K.; Kirby, E.; Tsuruda, L.; Fiedler, K.; et al. Cannabinoid Exposure and Altered DNA Methylation in Rat and Human Sperm. Epigenetics 2018, 13, 1208–1221. [Google Scholar] [CrossRef]
- Pizzol, D.; Demurtas, J.; Stubbs, B.; Soysal, P.; Mason, C.; Isik, A.T.; Corsonello, A.; Maggi, G.; Pilotto, A.; Onder, G.; et al. Relationship between Cannabis Use and Erectile Dysfunction: A Systematic Review and Meta-Analysis. Am. J. Mens. Health 2019, 13, 1557988319892464. [Google Scholar] [CrossRef]
- de Araújo-Ramos, A.T.; Martino-Andrade, A.J. Role of the endocannabinoid system in gonadal development: Implications for endocrine disruption and reproductive toxicity. Reprod. Toxicol. 2025, 132, 108822. [Google Scholar] [CrossRef]
- Rodríguez-Manzo, G.; Canseco-Alba, A. The endogenous cannabinoid system modulates male sexual behavior expression. Front. Behav. Neurosci. 2023, 17, 1198077. [Google Scholar] [CrossRef]
- Lynn, B.; Gee, A.; Zhang, L.; Pfaus, J.G. Effects of Cannabinoids on Female Sexual Function. Sex Med. Rev. 2020, 8, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Rastrelli, G.; Sparano, C.; Castellini, C.; Vignozzi, L.; Maggi, M.; Lenzi, A.; Isidori, A.M.; Corona, G.; Forti, G.; et al. Is marijuana a foe of male sexuality? Data from a large cohort of men with sexual dysfunction. Andrology 2024, 12, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Barchi, M.; Innocenzi, E.; Giannattasio, T.; Dolci, S.; Rossi, P.; Grimaldi, P. Cannabinoid Receptors Signaling in the Development, Epigenetics, and Tumours of Male Germ Cells. Int. J. Mol. Sci. 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Misawa, K.; Yamamoto, I.; Watanabe, K. Cannabidiolic Acid as a Selective cyclooxygenase-2 Inhibitory Component in Cannabis. Drug Metab. Dispos. 2008, 36, 1917–1921. [Google Scholar] [CrossRef]
- Hayes, E.C.; Rock, J.A. COX-2 Inhibitors and Their Role in Gynecology. Obstet. Gynecol. Surv. 2002, 57, 768–780. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Park, J.Y.; Smith, K.J.; Hamberg, M.; Roberts, L.J.; Seibert, K.; et al. Structural Basis for Selective Inhibition of cyclooxygenase-2 by anti-inflammatory Agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Algire, J.E.; Srikandakumar, A.; Guilbault, L.A.; Downey, B.R. Preovulatory Changes in Follicular Prostaglandins and Their Role in Ovulation in cattle. Can. J. Vet. Res. 1992, 56, 67–69. [Google Scholar]
- Kowalewski, M.P.; Fox, B.; Gram, A.; Boos, A.; Reichler, I. Prostaglandin E2 Functions as a Luteotrophic Factor in the Dog. Reproduction 2013, 145, 213–226. [Google Scholar] [CrossRef]
- Murdoch, W.J. Effect of a Steriodal (prednisolone) and Nonsteroidal (indomethacin) Antiinflammatory Agent on Ovulation and Follicular Accumulation of Prostaglandin F2α in Sheep. Prostaglandins 1989, 37, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.; Khamashta, M.A.; Nelson-Piercy, C. Nonsteroidal Anti-Inflammatory Drugs and Reversible Female Infertility. Drug Saf. 2002, 25, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Concannon, P.W. Reproductive Cycles of the Domestic Bitch. Anim. Reprod. Sci. 2011, 124, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Kowalewski, M.P. Endocrine and Molecular Control of Luteal and Placental Function in Dogs. Reprod. Domest. Anim. 2012, 47, 19–24. [Google Scholar] [CrossRef]
- Papa, P.C.; Kowalewski, M.P. Factors Affecting the Fate of the Canine Corpus luteum: Potential Contributors to Pregnancy and non-pregnancy. Theriogenology 2020, 150, 339–346. [Google Scholar] [CrossRef]
- Chakraborty, I.; Dąs, S.K.; Wang, J.; Dey, S.K. Developmental Expression of the cyclooxygenase-1 and cyclooxygenase-2 Genes in the Periimplantation Mouse Uterus and Their Differential Regulation by the Blastocyst and Ovarian Steroids. J. Mol. Endocrinol. 1996, 16, 107–122. [Google Scholar] [CrossRef]
- Lim, H.; Paria, B.C.; Das, S.K.; Dinchuk, J.E.; Langenbach, R.; Trzaskos, J.M.; Meyer, K.; Smith, R.V.; Dubois, R.N.; Rodgers, J.; et al. Multiple Female Reproductive Failures in Cyclooxygenase 2–Deficient Mice. Cell 1997, 91, 197–208. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ma, W.; Smalley, W.; Trzaskos, J.M.; Breyer, R.M.; Dey, S.K. Diversification of Cyclooxygenase-2-Derived Prostaglandins in Ovulation and Implantation1. Biol. Reprod. 2001, 64, 1557–1565. [Google Scholar] [CrossRef]
- Sirois, J.; Doré, M. The Late Induction of Prostaglandin G/H Synthase-2 in Equine Preovulatory Follicles Supports Its Role as a Determinant of the Ovulatory Process. Endocrinology 1997, 138, 4427–4434. [Google Scholar] [CrossRef]
- Wang, H.; Dey, S.K. Lipid Signaling in Embryo Implantation. Prostaglandins Other Lipid Mediat. 2005, 77, 84–102. [Google Scholar] [CrossRef]
- Hester, K.E.; Harper, M.J.K.; Duffy, D.M. Oral Administration of the cyclooxygenase-2 (COX-2) Inhibitor Meloxicam Blocks Ovulation in non-human Primates When Administered to Simulate Emergency Contraception. Hum. Reprod. 2010, 25, 360–367. [Google Scholar] [CrossRef]
- Salhab, A.S.; Amro, B.I.; Shomaf, M.S. Further Investigation on Meloxicam Contraceptivity in Female rabbits: Luteinizing Unruptured follicles, a Microscopic Evidence. Contraception 2003, 67, 485–489. [Google Scholar] [CrossRef]
- Weiss, E.A.; Gandhi, M. Preferential Cyclooxygenase 2 Inhibitors as a Nonhormonal Method of Emergency contraception: A Look at the Evidence. J. Pharm. Pract. 2016, 29, 160–164. [Google Scholar] [CrossRef]
- Smith, G.C.; Roberts, R.M.; Hall, C.; Nuki, G. Reversible Ovulatory Failure Associated with the Development of Luteinized Unruptured Follicles in Women with Inflammatory Arthritis Taking non-steroidal anti-inflammatory drugs. Rheumatology 1996, 35, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Dzięcioł, M.; Szpaczek, A.; Uchańska, O.; Niżański, W. Influence of a Single Dose of Meloxicam Administrated during Canine Estrus on Progesterone Concentration and Fertility—A Clinical Case Study. Animals 2022, 12, 655. [Google Scholar] [CrossRef] [PubMed]
- Koblischke, P.; Kindahl, H.; Budik, S.; Aurich, J.; Palm, F.; Walter, I.; Aurich, C.; Hölker, M.; Lefebvre, R.; Troedsson, M.; et al. Embryo Transfer Induces a Subclinical Endometritis in Recipient Mares Which Can Be Prevented by Treatment with non-steroid anti-inflammatory Drugs. Theriogenology 2008, 70, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- Paksoy, Z.; Daş, H. Nonsteroid anti-inflammatory Drugs to Improve Fertility in Cows. In Success in Artificial Insemination—Quality of Semen and Diagnostics Employed; Lemma, A., Ed.; Intech: Houston, TX, USA, 2012. [Google Scholar]
- Singh, S.P.; Kumar, A.; Bhavsar, P.; Bhavsar, M.; Sourya, N.; Singh, A.K.; Patel, R.; Shah, N.; Desai, P.; Chauhan, J.; et al. Application of non-steroidal anti-inflammatory Drugs (NSAIDs) for Improvement of Cattle Fertility. Deleted J. 2021, 10, 404–407. [Google Scholar]
- McNaughtan, J.W. The Effect of Prostaglandin Inhibitor on Pregnancy Rates of Heifer Embryo Transfer Recipients; Brigham Young University Utah: Provo, UT, USA, 2004. [Google Scholar]
- Erdem, H.; Guzeloglu, A. Effect of Meloxicam Treatment during Early Pregnancy in Holstein Heifers. Reprod. Domest. Animals 2009, 45, 625–628. [Google Scholar] [CrossRef]
- Espey, L.L.; Kohda, H.; Mori, T.; Okamura, H. Rat Ovarian Prostaglandin Levels and Ovulation as Indicators of the Strength of non-steroidal anti-inflammatory Drugs. Prostaglandins 1988, 36, 875–879. [Google Scholar] [CrossRef]
- Cosentino, M.; Legnaro, M.; Luini, A.; Ferrari, M.; Sodergren, M.; Pacchetti, B.; Bianchi, F.; Castiglione, F.; Mazzei, M.; Rossi, A.; et al. Effect of Cannabidiol on Cyclooxygenase Type 1 and 2 Expression and Function in Human Neutrophils. Cannabis Cannabinoid Res. 2022, 8, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, C.; Capasso, A. The Endocannabinoid System in the Cancer therapy: An Overview. Curr. Med. Chem. 2011, 18, 1575–1583. [Google Scholar] [CrossRef] [PubMed]
- Malfitano, A.M.; Ciaglia, E.; Gangemi, G.; Gazzerro, P.; Laezza, C.; Bifulco, M. Update on the Endocannabinoid System as an Anticancer Target. Expert Opin. Ther. Targets 2011, 15, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.M.; Teixeira, N.A.; Correia-da-Silva, G. Cannabinoids as Modulators of Cell Death: Clinical Applications and Future Directions. Rev. Physiol. Biochem. Pharmacol. 2017, 173, 63–88. [Google Scholar]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the Use of Cannabinoids as Antitumour Agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Pérez-Gómez, E.; Guzmán, M.; Sánchez, C. Cannabinoids: A New Hope for Breast Cancer therapy? Cancer Treat Rev. 2012, 38, 911–918. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular Targets for Cannabidiol and Its Synthetic analogues: Effect on Vanilloid VR1 Receptors and on the Cellular Uptake and Enzymatic Hydrolysis of Anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Martín-Sabroso, C.; Torres-Suárez, A.I. Insights into the Effects of the Endocannabinoid System in cancer: A Review. Br. J. Pharmacol. 2018, 175, 2566–2580. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Petrosino, S.; Bifulco, M.; Di Marzo, V.; Portella, G.; et al. Antitumor Activity of Plant Cannabinoids with Emphasis on the Effect of Cannabidiol on Human Breast Carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol Induces Programmed Cell Death in Breast Cancer Cells by Coordinating the cross-talk between Apoptosis and Autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- Sultan, A.S.; Marie, M.A.; Sheweita, S.A. Novel Mechanism of cannabidiol-induced Apoptosis in Breast Cancer Cell Lines. Breast 2018, 41, 34–41. [Google Scholar] [CrossRef]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Delta-9-tetrahydrocannabinol Enhances Breast Cancer Growth and Metastasis by Suppression of the Antitumor Immune Response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Qamri, Z.; Nasser, M.; Sharma, S.; Gupta, P.; et al. Modulation of the Tumor Microenvironment and Inhibition of EGF/EGFR pathway: Novel Anti-tumor Mechanisms of Cannabidiol in Breast Cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.D.; Murase, R.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Allison, J.; Graham, N.; Patel, R.; Brundige, D.; Zhang, B.; et al. Pathways Mediating the Effects of Cannabidiol on the Reduction of Breast Cancer Cell proliferation, invasion, and Metastasis. Breast Cancer Res. Treat. 2011, 129, 37–47. [Google Scholar] [CrossRef] [PubMed]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.Y. Cannabidiol as a Novel Inhibitor of Id-1 Gene Expression in Aggressive Breast Cancer Cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [CrossRef]
- Sarmiento-Salinas, F.L.; Delgado-Magallón, A.; Montes-Alvarado, J.B.; Ramírez-Ramírez, D.; Flores-Alonso, J.C.; Cortés-Hernández, P.; Martínez-López, D.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; et al. Breast Cancer Subtypes Present a Differential Production of Reactive Oxygen Species (ROS) and Susceptibility to Antioxidant Treatment. Front Oncol. 2019, 9, 480. [Google Scholar] [CrossRef]
- Qamri, Z.; Preet, A.; Nasser, M.W.; Bass, C.E.; Leone, G.; Barsky, S.H.; Wani, N.A.; Elbaz, M.; Sharma, S.; Gupta, P.; et al. Synthetic Cannabinoid Receptor Agonists Inhibit Tumor Growth and Metastasis of Breast Cancer. Mol. Cancer Ther. 2009, 8, 3117–3129. [Google Scholar] [CrossRef]
- Grimaldi, C.; Pisanti, S.; Laezza, C.; Malfitano, A.M.; Santoro, A.; Vitale, M.; Di Francia, R.; Iacuzzo, I.; Borrelli, F.; Scuderi, M.; et al. Anandamide Inhibits Adhesion and Migration of Breast Cancer Cells. Exp. Cell. Res. 2006, 312, 363–373. [Google Scholar] [CrossRef]
- Laezza, C.; Pisanti, S.; Malfitano, A.M.; Bifulco, M. The Anandamide analog, Met-F-AEA, Controls Human Breast Cancer Cell Migration via the RHOA/RHO Kinase Signaling Pathway. Endocr. Relat. Cancer 2008, 15, 965–974. [Google Scholar] [CrossRef]
- Nasser, M.W.; Qamri, Z.; Deol, Y.S.; Smith, D.; Shilo, K.; Zou, X.; Sharma, S.; Gupta, P.; Elbaz, M.; Leone, G.; et al. Crosstalk between Chemokine Receptor CXCR4 and Cannabinoid Receptor CB2 in Modulating Breast Cancer Growth and Invasion. Batra SK, editor. PLoS ONE 2011, 6, e23901. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Elbaz, M.; Nasser, M.; Sharma, S.; Gupta, P.; et al. Cannabinoids Reduce ErbB2-driven Breast Cancer Progression through Akt Inhibition. Mol. Cancer 2010, 9, 196. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V.; Petrosino, S.; Starowicz, K.; Matias, I.; et al. The Endogenous Cannabinoid Anandamide Inhibits Human Breast Cancer Cell Proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Sarrió, D.; Palacios, J.; Guzmán, M.; Sánchez, C. Delta9-tetrahydrocannabinol Inhibits Cell Cycle Progression in Human Breast Cancer Cells through Cdc2 Regulation. Cancer Res. 2006, 66, 6615–6621. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Moreno-Bueno, G.; Cerutti, C.; Palacios, J.; Guzman, M.; Mechta-Grigoriou, F.; Perez, E.; Mira, E.; Andradas, C.; Eskell, L.; et al. JunD Is Involved in the Antiproliferative Effect of Δ9-tetrahydrocannabinol on Human Breast Cancer Cells. Oncogene 2008, 27, 5033–5044. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Yamamoto, I.; Watanabe, K. Modulation of Δ9-tetrahydrocannabinol-induced MCF-7 Breast Cancer Cell Growth by Cyclooxygenase and Aromatase. Toxicology 2009, 259, 25–32. [Google Scholar] [CrossRef]
- Takeda, S.; Yamaori, S.; Motoya, E.; Matsunaga, T.; Kimura, T.; Yamamoto, I.; Hashimoto, K.; Kondo, S.; Sugiura, M.; Watanabe, K.; et al. Delta(9)-Tetrahydrocannabinol Enhances MCF-7 Cell Proliferation via Cannabinoid receptor-independent Signaling. Toxicology 2008, 245, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Motoya, E.; Matsuzawa, N.; Funahashi, T.; Kimura, T.; Matsunaga, T.; Tanaka, Y.; Yamaori, S.; Takeda, S.; Yamamoto, I.; et al. Marijuana Extracts Possess the Effects like the Endocrine Disrupting Chemicals. Toxicology 2005, 206, 471–478. [Google Scholar] [CrossRef] [PubMed]
- De La Harpe, A.; Beukes, N.; Frost, C.L. CBD Activation of TRPV1 Induces Oxidative Signaling and Subsequent ER Stress in Breast Cancer Cell Lines. Biotechnol. Appl. Biochem. 2022, 69, 420–430. [Google Scholar] [CrossRef]
- Von Bueren, A.O.; Schlumpf, M.; Lichtensteiger, W. Delta(9)-tetrahydrocannabinol Inhibits 17beta-estradiol-induced Proliferation and Fails to Activate Androgen and Estrogen Receptors in MCF7 Human Breast Cancer cells. Anticancer. Res. 2008, 28, 85–89. [Google Scholar]
- Almada, M.; Oliveira, A.; Amaral, C.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.; Silva, A.S.; Correia-da-Silva, G.; Teixeira, N.; Maretti-Costa, M.C.; et al. Anandamide Targets aromatase: A Breakthrough on Human Decidualization. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 158512. [Google Scholar] [CrossRef]
- Almada, M.; Amaral, C.; Oliveira, A.; Fernandes, P.A.; Ramos, M.J.; Fonseca, B.M.; Silva, A.S.; Correia-da-Silva, G.; Teixeira, N.; Maretti-Costa, M.C.; et al. Cannabidiol (CBD) but Not Tetrahydrocannabinol (THC) Dysregulate in Vitro Decidualization of Human Endometrial Stromal Cells by Disruption of Estrogen Signaling. Reprod. Toxicol. 2020, 93, 75–82. [Google Scholar] [CrossRef]
- Amaral, C.; Trouille, F.M.; Almeida, C.F.; Correia-da-Silva, G.; Teixeira, N. Unveiling the Mechanism of Action behind the anti-cancer Properties of Cannabinoids in ER+ Breast Cancer cells: Impact on Aromatase and Steroid Receptors. J. Steroid Biochem. Mol. Biol. 2021, 210, 105876. [Google Scholar] [CrossRef]
- Takeda, S.; Yoshida, K.; Nishimura, H.; Harada, M.; Okajima, S.; Miyoshi, H.; Sugiura, M.; Watanabe, K.; Motoya, E.; Yamamoto, I.; et al. Δ(9)-Tetrahydrocannabinol Disrupts estrogen-signaling through up-regulation of Estrogen Receptor Β (ERβ). Chem. Res. Toxicol. 2013, 26, 1073–1079. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Caffarel, M.M.; et al. Appraising the “entourage effect”: Antitumor Action of a Pure Cannabinoid versus a Botanical Drug Preparation in Preclinical Models of Breast Cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef]
- Prather, P.L.; FrancisDevaraj, F.; Dates, C.R.; Greer, A.K.; Bratton, S.M.; Ford, B.M.; Bolognese, R.J.; Schaus, J.M.; Kaplan, H.F.; Oldham, W.M.; et al. CB1 and CB2 Receptors Are Novel Molecular Targets for Tamoxifen and 4OH-Tamoxifen. Biochem. Biophys. Res. Commun. 2013, 441, 339–343. [Google Scholar] [CrossRef]
| Species | Receptor Subtype | Localization of Cannabinoid Receptors | Known/Potential Effects on |
|---|---|---|---|
| Humans | CB1 | HPG axis *, Uterus, Testes, Spermatozoa, Mammary Gland |
|
| CB2 | HPG axis *, Endometrium, Placenta |
| |
| Non-human primates | CB1 | HPG axis *, Endometrium, Myometrium, Testes, Spermatozoa |
|
| CB2 | HPG axis *, Endometrium, Testes, Spermatozoa |
| |
| Cattle | CB1 | Corpus Luteum, Uterus |
|
| CB2 | Corpus Luteum, Uterine Immune Cells |
| |
| Sheep | CB1 | Ovaries, Uterus |
|
| CB2 | Uterus, Uterine Immune Cells |
| |
| Pigs | CB1 | Uterus |
|
| CB2 | Placenta |
| |
| Rodents | CB1 | HPG axis *, Uterus, Ovarian Follicles, Testes, Epididymis, Vas Deferens |
|
| CB2 | HPG axis *, Uterus, Endometrium, Ovarian Follicles, Placenta, Testes, Epididymis, Vas Deferens | ||
| Cats | CB1 | Ovaries, Ovarian Follicles |
|
| CB2 | Ovarian Follicles, Luteal Cells |
|
| Female Reproductive Function | ECS Component | Effects/Role |
|---|---|---|
| Ovulation | CB1, CB2, AEA, 2-AG | Modulation of GnRH, LH, and FSH secretion; involvement in follicular development and ovulatory peak |
| Ovarian function | CB1, CB2, AEA, 2-AG | Regulation of folliculogenesis, steroidogenesis, and oocyte maturation |
| Oviductal transport | CB1, AEA | CB1 modulates embryo transport; high AEA disrupts oviductal motility |
| Uterine receptivity and implantation | CB1, CB2, AEA | AEA gradient crucial for implantation; dysregulation linked to miscarriage |
| Pregnancy and placenta | CB1, CB2, AEA, 2-AG | Modulates trophoblast proliferation, vascularization, and immune tolerance; altered ECS linked to complications |
| Lactation | CB1, CB2, AEA | Inhibition of prolactin secretion via CB1; involvement in hypothalamic regulation |
| Menstrual/cycle regulation | CB1, CB2, AEA, 2-AG | Regulation of HPG axis, endometrial remodeling, and steroid secretion |
| Pathological states (e.g., endometriosis, PCOS) | CB1, CB2, AEA, 2-AG | Dysregulated ECS expression; potential target for modulation |
| Male Reproductive Function | ECS Component | Effects/Role |
|---|---|---|
| Spermatogenesis and testicular function | CB1, CB2, AEA, 2-AG | Regulation of Sertoli cell activity, spermatid maturation, and testosterone secretion |
| Epididymal maturation | CB1, CB2, AEA | Modulation of sperm motility and maturation; protective role against oxidative stress |
| Sperm capacitation and fertilization | CB1, CB2, AEA | High AEA impairs capacitation; CB1 controls acrosome reaction |
| Libido/sexual behavior | CB1, AEA | Inhibitory effects on hypothalamic-pituitary-gonadal axis; suppression of sexual drive |
| Hormonal regulation | CB1, AEA | Decreased LH, FSH, and testosterone secretion via hypothalamic-pituitary modulation |
| Seminal plasma ECS content | AEA, 2-AG | Detected in semen; possible role in sperm activation and immune tolerance |
| Pathological states (e.g., infertility, varicocele) | CB1, CB2, AEA, 2-AG | ECS imbalance linked to poor sperm quality and reproductive disorders |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalak, P.; Kupczyk, P.; Szumny, A.; Gębarowski, T.; Jasiak, M.; Niedźwiedź, A.; Niżański, W.; Dzięcioł, M. Molecular Mechanisms of the Endocannabinoid System with a Focus on Reproductive Physiology and the Cannabinoid Impact on Fertility. Int. J. Mol. Sci. 2025, 26, 7095. https://doi.org/10.3390/ijms26157095
Kalak P, Kupczyk P, Szumny A, Gębarowski T, Jasiak M, Niedźwiedź A, Niżański W, Dzięcioł M. Molecular Mechanisms of the Endocannabinoid System with a Focus on Reproductive Physiology and the Cannabinoid Impact on Fertility. International Journal of Molecular Sciences. 2025; 26(15):7095. https://doi.org/10.3390/ijms26157095
Chicago/Turabian StyleKalak, Patrycja, Piotr Kupczyk, Antoni Szumny, Tomasz Gębarowski, Marcin Jasiak, Artur Niedźwiedź, Wojciech Niżański, and Michał Dzięcioł. 2025. "Molecular Mechanisms of the Endocannabinoid System with a Focus on Reproductive Physiology and the Cannabinoid Impact on Fertility" International Journal of Molecular Sciences 26, no. 15: 7095. https://doi.org/10.3390/ijms26157095
APA StyleKalak, P., Kupczyk, P., Szumny, A., Gębarowski, T., Jasiak, M., Niedźwiedź, A., Niżański, W., & Dzięcioł, M. (2025). Molecular Mechanisms of the Endocannabinoid System with a Focus on Reproductive Physiology and the Cannabinoid Impact on Fertility. International Journal of Molecular Sciences, 26(15), 7095. https://doi.org/10.3390/ijms26157095









