Roles of Peripheral Nerves in Tumor Initiation and Progression
Abstract
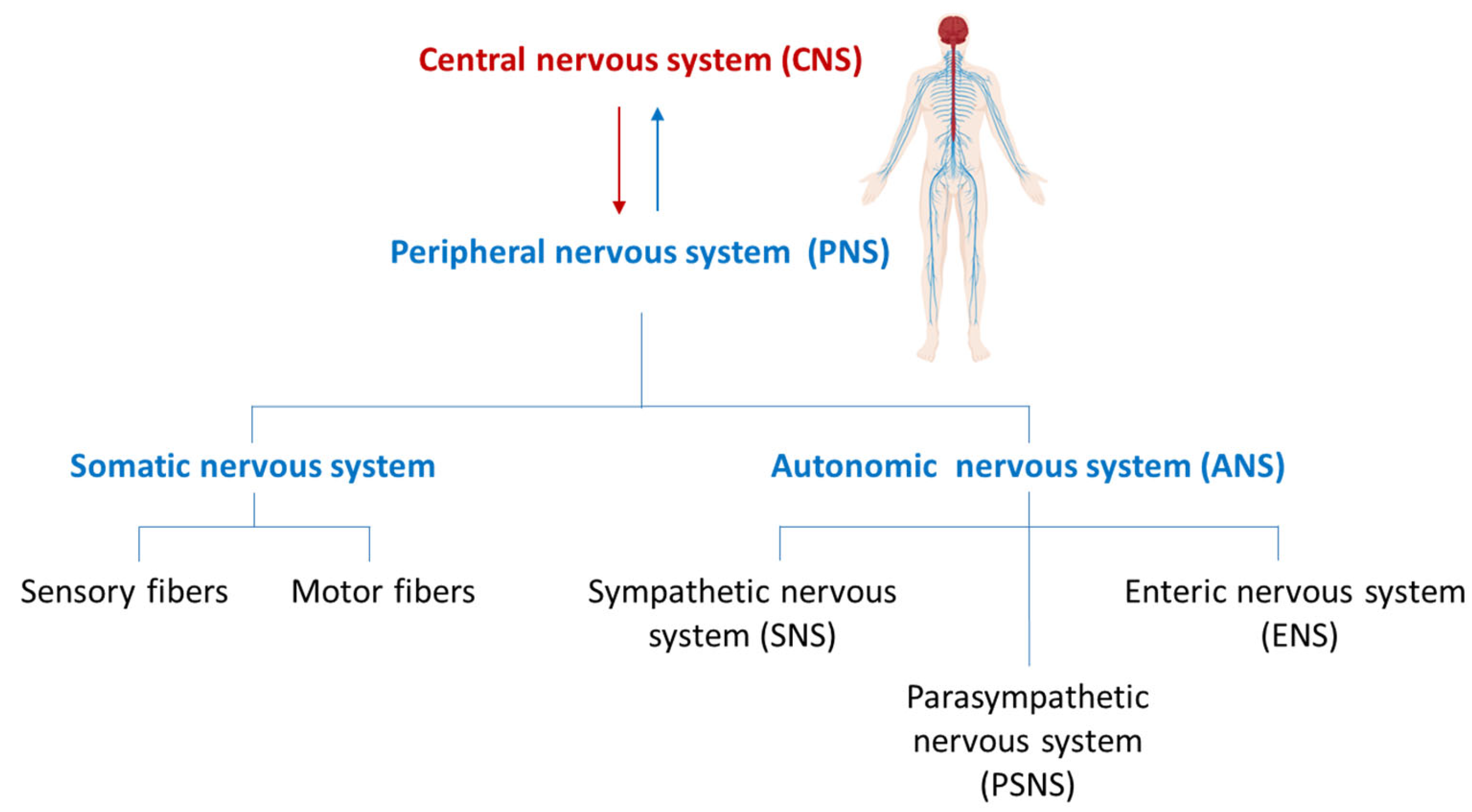
1. Introduction
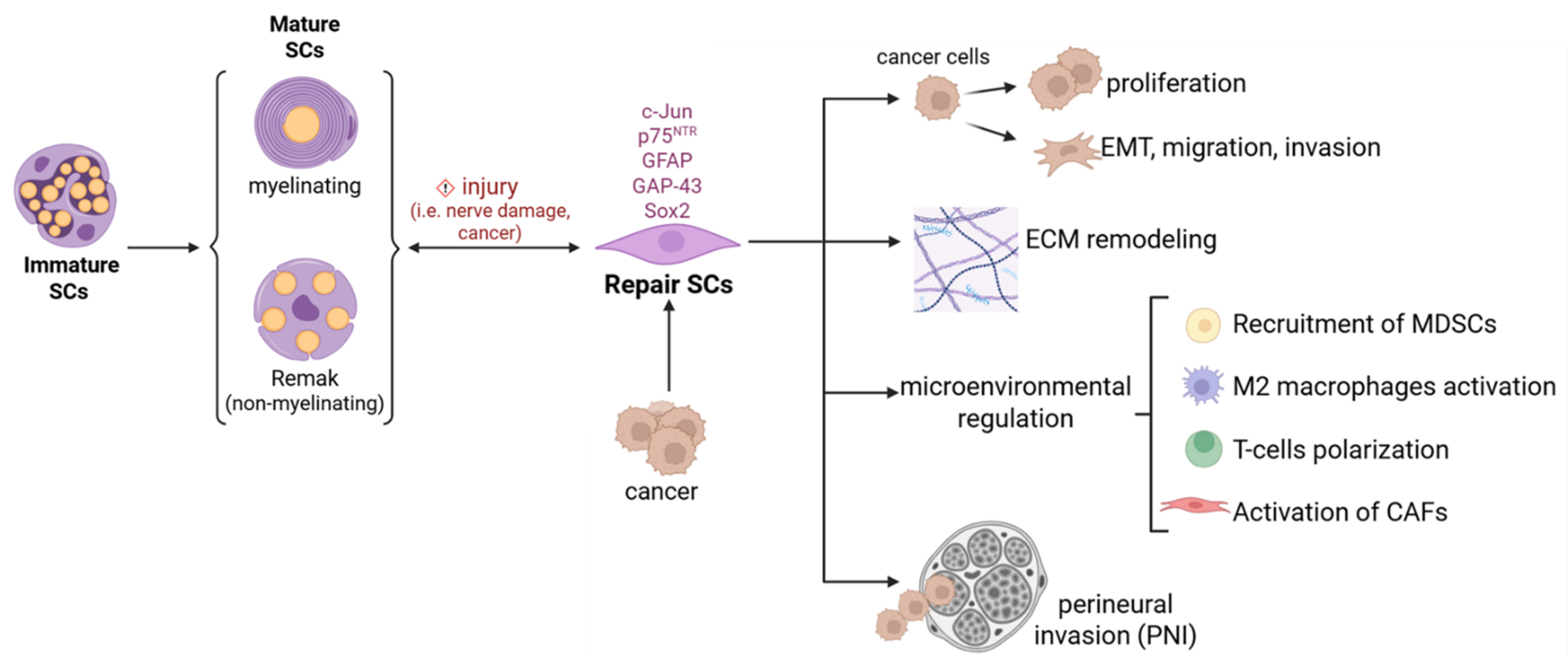
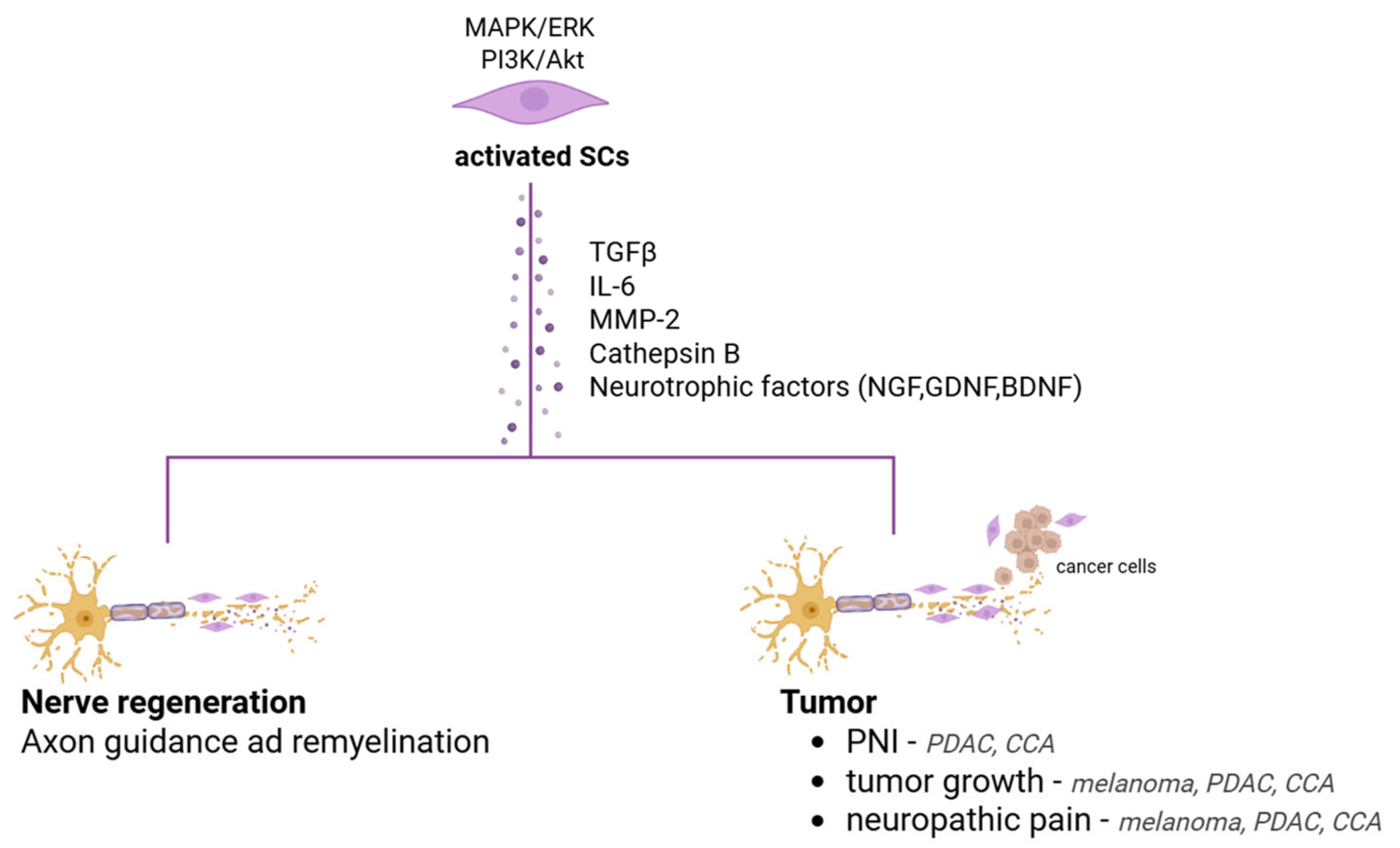
2. Schwann Cells: Master Architect of Peripheral Nerve Regeneration and Beyond
3. Innervation and Tumor Progression
4. Schwann Cells and Cancer
4.1. Melanoma and Innervation: The Role of Schwann Cells
4.1.1. Histopathological Characteristics of Melanoma–Nerves Interplay
Perineural Invasion
Neuropathic Changes
Neural Remodeling
Neurogenic Inflammation
Neurotrophic Factors and Receptors Expression
4.2. Innervation in Liver and Pancreas
4.2.1. Pancreatobiliary Tract Cancer: The Role of Nerves Fibres and Schwann Cells
Perineural Invasion
Neuropathic Changes
Neural Remodeling
Neurotrophic Factors and Receptors Expression
4.2.2. HCC and Innervation
Perineural Invasion and Neural Remodeling
PNS and Liver Chronic Inflammation
PNS and Tumor Growth
Neurotrophic Factors and Receptors Expression
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Virchow, R. Aetiologie der neoplastischen Geschwülste. In Die Krankhaften Geschwülste: Erster Band: Dreissig Vorlesungen, Gehalten Während des Wintersemesters 1862–1863 an der Universität zu Berlin; Virchow, R., Ed.; Springer: Berlin/Heidelberg, Germany, 1978; pp. 57–71. [Google Scholar]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315 (Suppl. 1), 1650–1659. [Google Scholar]
- Dupin, E.; Calloni, G.W.; Coelho-Aguiar, J.M.; Le Douarin, N.M. The issue of the multipotency of the neural crest cells. Dev. Biol. 2018, 444, S47–S59. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, A.D.; Sinanan, A.; Parmantier, E.; Zwart, R.; Broos, L.; Meijer, D.; Meier, C.; Jessen, K.R.; Mirsky, R. Oct-6 (SCIP Tst-1) is expressed in Schwann cell precursors, embryonic Schwann cells, and postnatal myelinating Schwann cells: Comparison with Oct-1, Krox-20, and Pax-3. J. Neurosci. Res. 1996, 46, 630–640. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. Schwann Cell Precursors; Multipotent Glial Cells in Embryonic Nerves. Front. Mol. Neurosci. 2019, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Woodhoo, A.; Dean, C.H.; Droggiti, A.; Mirsky, R.; Jessen, K.R. The trunk neural crest and its early glial derivatives: A study of survival responses, developmental schedules and autocrine mechanisms. Mol. Cell. Neurosci. 2004, 25, 30–41. [Google Scholar] [CrossRef]
- Feltri, M.L.; Poitelon, Y.; Previtali, S.C. How Schwann Cells Sort Axons: New Concepts. Neuroscientist 2016, 22, 252–265. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Belfiore, L.; Chu, T.-H.; Fleming, T.; Midha, R.; Biernaskie, J.; Schuurmans, C. Insights Into the Role and Potential of Schwann Cells for Peripheral Nerve Repair From Studies of Development and Injury. Front. Mol. Neurosci. 2020, 13, 608442. [Google Scholar] [CrossRef]
- Bosch-Queralt, M.; Fledrich, R.; Stassart, R.M. Schwann cell functions in peripheral nerve development and repair. Neurobiol. Dis. 2023, 176, 105952. [Google Scholar] [CrossRef]
- Jessen, K.R.; Mirsky, R. The Success and Failure of the Schwann Cell Response to Nerve Injury. Front. Cell. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef]
- Hromada, C.; Szwarc-Hofbauer, D.; Nguyen, M.Q.; Tomasch, J.; Purtscher, M.; Hercher, D.; Teuschl-Woller, A.H. Strain-induced bands of Büngner formation promotes axon growth in 3D tissue-engineered constructs. J. Tissue Eng. 2024, 15, 20417314231220396. [Google Scholar] [CrossRef]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef]
- Revazova, E.S.; Solov’Ev, I.N.; Litvinova, L.V.; Kochetkova, O.D. Human melanoma strains transplantable into athymic mice and rats. Biull. Eksp. Biol. Med. 1985, 99, 481–483. [Google Scholar] [PubMed]
- Zhao, C.-M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W.; et al. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef] [PubMed]
- Coarfa, C.; Florentin, D.; Putluri, N.; Ding, Y.; Au, J.; He, D.; Ragheb, A.; Frolov, A.; Michailidis, G.; Lee, M.; et al. Influence of the neural microenvironment on prostate cancer. Prostate 2017, 78, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-W.; Xie, J.-D.; Ling, Y.-H.; Li, P.; Yan, S.-M.; Xi, S.-Y.; Luo, R.-Z.; Yun, J.-P.; Xie, D.; Cai, M.-Y. The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer 2014, 14, 313. [Google Scholar] [CrossRef]
- Duchalais, E.; Guilluy, C.; Nedellec, S.; Touvron, M.; Bessard, A.; Touchefeu, Y.; Bossard, C.; Boudin, H.; Louarn, G.; Neunlist, M.; et al. Colorectal Cancer Cells Adhere to and Migrate Along the Neurons of the Enteric Nervous System. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 31–49. [Google Scholar] [CrossRef]
- Vats, K.; Kruglov, O.; Sahoo, B.; Soman, V.; Zhang, J.; Shurin, G.V.; Chandran, U.R.; Skums, P.; Shurin, M.R.; Zelikovsky, A.; et al. Sensory Nerves Impede the Formation of Tertiary Lymphoid Structures and Development of Protective Antimelanoma Immune Responses. Cancer Immunol. Res. 2022, 10, 1141–1154. [Google Scholar] [CrossRef]
- Bunimovich, Y.L.; Keskinov, A.A.; Shurin, G.V.; Shurin, M.R. Schwann cells: A new player in the tumor microenvironment. Cancer Immunol. Immunother. 2017, 66, 959–968. [Google Scholar] [CrossRef]
- Hernandez, S.; Serrano, A.G.; Soto, L.M.S. The Role of Nerve Fibers in the Tumor Immune Microenvironment of Solid Tumors. Adv. Biol. 2022, 6, e2200046. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the nervous system in cancers: A review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef]
- Magnon, C.; Hondermarck, H. The neural addiction of cancer. Nat. Rev. Cancer 2023, 23, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Gysler, S.M.; Drapkin, R. Tumor innervation: Peripheral nerves take control of the tumor microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef] [PubMed]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.-P.; Firlej, V.; Allory, Y.; Roméo, P.-H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678. [Google Scholar] [CrossRef]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef]
- Costa, P.A.C.; Silva, W.N.; Prazeres, P.H.D.M.; Picoli, C.C.; Guardia, G.D.A.; Costa, A.C.; Oliveira, M.A.; Guimarães, P.P.G.; Gonçalves, R.; Pinto, M.C.X.; et al. Chemogenetic modulation of sensory neurons reveals their regulating role in melanoma progression. Acta Neuropathol. Commun. 2021, 9, 183. [Google Scholar] [CrossRef]
- Itami, T.; Kurokawa, Y.; Hagi, T.; Nagano, S.; Nakamoto, R.; Kamakura, Y.; Takahashi, T.; Saito, T.; Yamamoto, K.; Momose, K.; et al. Sympathetic innervation induced by nerve growth factor promotes malignant transformation in gastric cancer. Sci. Rep. 2025, 15, 3824. [Google Scholar] [CrossRef]
- Petrescu, M.; Târtea, G.; Udriștoiu, I.; Militaru, F.; Petrescu, A.-R.; Ciurea, A.-M.; Petrescu, A.-M.; Obleagă, C.; Vere, C.C. Sympathetic Nervous Influences Are Negative Prognostic Factors in Stomach Cancer. Life 2024, 14, 368. [Google Scholar] [CrossRef]
- Târtea, E.-A.; Petrescu, M.; Udriștoiu, I.; Gheorman, V.; Biciușcă, V.; Petrescu, A.-R.; Ciurea, A.-M.; Vere, C.C. Clinical Outcomes Depending on Sympathetic Innervation in Pancreatic Cancer. Cancers 2023, 15, 3040. [Google Scholar] [CrossRef]
- Erin, N.; Barkan, G.A.; Harms, J.F.; Clawson, G.A. Vagotomy enhances experimental metastases of 4THMpc breast cancer cells and alters Substance P level. Regul. Pept. 2008, 151, 35–42. [Google Scholar] [CrossRef]
- Erin, N. Role of sensory neurons, neuroimmune pathways, and transient receptor potential vanilloid 1 (TRPV1) channels in a murine model of breast cancer metastasis. Cancer Immunol. Immunother. 2020, 69, 307–314. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef]
- Jerard, C.; Madhusudanan, P.; Swamy, A.; Ravikumar, K.; Shankarappa, S.A. Secretome mediated interactions between sensory neurons and breast cancer cells. Int. J. Cancer 2023, 153, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Garajová, I.; Trentini, F.; Leonardi, F.; Giovannetti, E. Unraveling the Connection: Pancreatic Cancer Cells and Schwann Cells. J. Clin. Med. 2024, 13, 1785. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Andrade, J.M.; Ghilardi, J.R.; Castañeda-Corral, G.; Kuskowski, M.A.; Mantyh, P.W. Preventive or late administration of anti-NGF therapy attenuates tumor-induced nerve sprouting, neuroma formation, and cancer pain. Pain 2011, 152, 2564–2574. [Google Scholar] [CrossRef]
- Prazeres, P.H.D.M.; Leonel, C.; Silva, W.N.; Rocha, B.G.S.; Santos, G.S.P.; Costa, A.C.; Picoli, C.C.; Sena, I.F.G.; Gonçalves, W.A.; Vieira, M.S.; et al. Ablation of sensory nerves favours melanoma progression. J. Cell. Mol. Med. 2020, 24, 9574–9589. [Google Scholar] [CrossRef] [PubMed]
- Martyn, G.V.; Shurin, G.V.; Keskinov, A.A.; Bunimovich, Y.L.; Shurin, M.R. Schwann cells shape the neuro-immune environs and control cancer progression. Cancer Immunol. Immunother. 2019, 68, 1819–1829. [Google Scholar] [CrossRef]
- Keskinov, A.A.; Tapias, V.; Watkins, S.C.; Ma, Y.; Shurin, M.R.; Shurin, G.V. Impact of the Sensory Neurons on Melanoma Growth In Vivo. PLoS ONE 2016, 11, e0156095. [Google Scholar] [CrossRef]
- Deborde, S.; Omelchenko, T.; Lyubchik, A.; Zhou, Y.; He, S.; McNamara, W.F.; Chernichenko, N.; Lee, S.-Y.; Barajas, F.; Chen, C.-H.; et al. Schwann cells induce cancer cell dispersion and invasion. J. Clin. Investig. 2016, 126, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Ayala, G.E.; Wheeler, T.M.; Shine, H.D.; Schmelz, M.; Frolov, A.; Chakraborty, S.; Rowley, D. In vitro dorsal root ganglia and human prostate cell line interaction: Redefining perineural invasion in prostate cancer. Prostate 2001, 49, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Peterson, S.C.; Eberl, M.; Vagnozzi, A.N.; Belkadi, A.; Veniaminova, N.A.; Verhaegen, M.E.; Bichakjian, C.K.; Ward, N.L.; Dlugosz, A.A.; Wong, S.Y. Basal cell carcinoma preferentially arises from stem cells within hair follicle and mechanosensory niches. Cell Stem Cell 2015, 16, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Porzio, G.; Adile, C.; Aielli, F.; Cortegiani, A.; Caruselli, A.; Casuccio, A. Pain intensity as prognostic factor in cancer pain management. Pain Pract. 2014, 15, E1–E8. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Albo, D. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 2009, 27, 5131–5137. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Anderson, K.O.; Merriman, K.W.; Todd, K.H.; Shete, S.S.; Hanna, E.Y. Survival patterns in squamous cell carcinoma of the head and neck: Pain as an independent prognostic factor for survival. J. Pain 2014, 15, 1015–1022. [Google Scholar] [CrossRef]
- Halvorson, K.G.; Kubota, K.; Sevcik, M.A.; Lindsay, T.H.; Sotillo, J.E.; Ghilardi, J.R.; Rosol, T.J.; Boustany, L.; Shelton, D.L.; Mantyh, P.W. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005, 65, 9426–9435. [Google Scholar] [CrossRef]
- Özler, S.; Pazarci, P. Anti-tumoral effect of beta-blockers on prostate and bladder cancer cells via mitogen-activated protein kinase pathways. Anticancer Drugs 2022, 33, 384–388. [Google Scholar] [CrossRef]
- Grenda, T.; Grenda, A.; Krawczyk, P.; Kwiatek, K. Botulinum toxin in cancer therapy—Current perspectives and limitations. Appl. Microbiol. Biotechnol. 2022, 106, 485–495. [Google Scholar] [CrossRef]
- Deborde, S.; Wong, R.J. The Role of Schwann Cells in Cancer. Adv. Biol. 2022, 6, e2200089. [Google Scholar] [CrossRef]
- Han, S.; Wang, D.; Huang, Y.; Zeng, Z.; Xu, P.; Xiong, H.; Ke, Z.; Zhang, Y.; Hu, Y.; Wang, F.; et al. A reciprocal feedback between colon cancer cells and Schwann cells promotes the proliferation and metastasis of colon cancer. J. Exp. Clin. Cancer Res. 2022, 41, 348. [Google Scholar] [CrossRef] [PubMed]
- Shurin, G.V.; Kruglov, O.; Ding, F.; Lin, Y.; Hao, X.; Keskinov, A.A.; You, Z.; Lokshin, A.E.; LaFramboise, W.A.; Falo, L.D.; et al. Melanoma-Induced Reprogramming of Schwann Cell Signaling Aids Tumor Growth. Cancer Res. 2019, 79, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.; Saraithong, P.; Curtin, J.G.; Janal, M.N.; Ye, Y. Reciprocal interactions between cancer and Schwann cells contribute to oral cancer progression and pain. Heliyon 2019, 5, e01223. [Google Scholar] [CrossRef] [PubMed]
- Deborde, S.; Gusain, L.; Powers, A.; Marcadis, A.; Yu, Y.; Chen, C.-H.; Frants, A.; Kao, E.; Tang, L.H.; Vakiani, E.; et al. Reprogrammed Schwann Cells Organize into Dynamic Tracks that Promote Pancreatic Cancer Invasion. Cancer Discov. 2022, 12, 2454–2473. [Google Scholar] [CrossRef]
- Demir, I.E.; Boldis, A.; Pfitzinger, P.L.; Teller, S.; Brunner, E.; Klose, N.; Kehl, T.; Maak, M.; Lesina, M.; Laschinger, M.; et al. Investigation of Schwann cells at neoplastic cell sites before the onset of cancer invasion. JNCI J. Natl. Cancer Inst. 2014, 106, dju184. [Google Scholar] [CrossRef]
- Wilcox, M.B.; Laranjeira, S.G.; Eriksson, T.M.; Jessen, K.R.; Mirsky, R.; Quick, T.J.; Phillips, J.B. Characterising cellular and molecular features of human peripheral nerve degeneration. Acta Neuropathol. Commun. 2020, 8, 51. [Google Scholar] [CrossRef]
- Lasser, S.A.; Kurt, F.G.O.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef]
- Jordan, K.R.; Amaria, R.N.; Ramirez, O.; Callihan, E.B.; Gao, D.; Borakove, M.; Manthey, E.; Borges, V.F.; McCarter, M.D. Myeloid-derived suppressor cells are associated with disease progression and decreased overall survival in advanced-stage melanoma patients. Cancer Immunol. Immunother. 2013, 62, 1711–1722. [Google Scholar] [CrossRef]
- Li, X.; Xing, Y.-F.; Lei, A.-H.; Xiao, Q.; Lin, Z.-H.; Hong, Y.-F.; Wu, X.-Y.; Zhou, J. Neutrophil count is associated with myeloid derived suppressor cell level and presents prognostic value for hepatocellular carcinoma patients. Oncotarget 2017, 8, 24380–24388. [Google Scholar] [CrossRef]
- Xue, M.; Zhu, Y.; Jiang, Y.; Han, L.; Shi, M.; Su, R.; Wang, L.; Xiong, C.; Wang, C.; Wang, T.; et al. Schwann cells regulate tumor cells and cancer-associated fibroblasts in the pancreatic ductal adenocarcinoma microenvironment. Nat. Commun. 2023, 14, 4600. [Google Scholar] [CrossRef]
- de Franchis, V.; Petrungaro, S.; Pizzichini, E.; Camerini, S.; Casella, M.; Somma, F.; Mandolini, E.; Carpino, G.; Overi, D.; Cardinale, V.; et al. Cholangiocarcinoma Malignant Traits Are Promoted by Schwann Cells through TGFβ Signaling in a Model of Perineural Invasion. Cells 2024, 13, 366. [Google Scholar] [CrossRef]
- Zhou, Y.; Shurin, G.V.; Zhong, H.; Bunimovich, Y.L.; Han, B.; Shurin, M.R. Schwann Cells Augment Cell Spreading and Metastasis of Lung Cancer. Cancer Res. 2018, 78, 5927–5939. [Google Scholar] [CrossRef] [PubMed]
- García-Reyes, B.; Kuzmanov, I.; Schneider, R.; Schneiker, B.; Efferz, P.; Kalff, J.C.; Wehner, S. Glial cell-derived soluble factors increase the metastatic potential of pancreatic adenocarcinoma cells and induce epithelial-to-mesenchymal transition. J. Cancer Res. Clin. Oncol. 2023, 149, 14315–14327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, Y.; Xu, J.; Wang, Y.; Yang, Y.; Wang, W.; Gu, A.; Han, B.; Shurin, G.V.; Zhong, R.; et al. Schwann cell-derived exosomes promote lung cancer progression via miRNA-21-5p. Glia 2024, 72, 692–707. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Yu, C.; Zhao, Y.; Hu, X.; Wang, H.; He, Y.; Wu, H. Exosomal miR-1228-5p down-regulates DUSP22 to promotes cell proliferation and migration in small cell lung cancer. Life Sci. 2024, 351, 122787. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef]
- Rappl, L.M. Physiological changes in tissues denervated by spinal cord injury tissues and possible effects on wound healing. Int. Wound J. 2008, 5, 435–444. [Google Scholar] [CrossRef]
- Laverdet, B.; Laverdet, B.; Danigo, A.; Girard, D.; Magy, L.; Demiot, C.; Desmoulière, A. Skin innervation: Important roles during normal and pathological cutaneous repair. Histol. Histopathol. 2015, 30, 875–892. [Google Scholar]
- Shu, B.; Xie, J.-L.; Xu, Y.-B.; Lai, W.; Huang, Y.; Mao, R.-X.; Liu, X.-S.; Qi, S.-H. Effects of skin-derived precursors on wound healing of denervated skin in a nude mouse model. Int. J. Clin. Exp. Pathol. 2015, 8, 2660–2669. [Google Scholar]
- Fukai, T.; Takeda, A.; Uchinuma, E. Wound healing in denervated rat skin. Wound Repair Regen. 2005, 13, 175–180. [Google Scholar] [CrossRef]
- Porel, P.; Kaur, M.; Sharma, V.; Aran, K.R. Understanding molecular mechanism of diabetic wound healing: Addressing recent advancements in therapeutic managements. J. Diabetes Metab. Disord. 2025, 24, 76. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Calvo-Enrique, L.; Zhang, M.-D.; Nyengaard, J.R.; Karlsson, P.; Ernfors, P. Demise of nociceptive Schwann cells causes nerve retraction and pain hyperalgesia. Pain 2021, 162, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Tschachler, E.; Reinisch, C.M.; Mayer, C.; Paiha, K.; Lassmann, H.; Weninger, W. Sheet preparations expose the dermal nerve plexus of human skin and render the dermal nerve end organ accessible to extensive analysis. J. Investig. Dermatol. 2004, 122, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Adameyko, I.; Lallemend, F.; Aquino, J.B.; Pereira, J.A.; Topilko, P.; Müller, T.; Fritz, N.; Beljajeva, A.; Mochii, M.; Liste, I.; et al. Schwann Cell Precursors from Nerve Innervation Are a cellular origin of melanocytes in skin. Cell 2009, 139, 366–379. [Google Scholar] [CrossRef]
- Nitzan, E.; Pfaltzgraff, E.R.; Labosky, P.A.; Kalcheim, C. Neural crest and Schwann cell progenitor-derived melanocytes are two spatially segregated populations similarly regulated by Foxd3. Proc. Natl. Acad. Sci. USA 2013, 110, 12709–12714. [Google Scholar] [CrossRef]
- Akhtar, S.; Khalid, W.; Rehman, A.; Siddique, K.; Khan, Q.U.A. A Rare Case of Primary Intracerebral Malignant Melanoma. Cureus 2023, 15, e43359. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, Y.; Zhang, J.; Jian, W.; Li, R.; Fu, Q. Atypical primary malignant melanoma originating in the spinal canal: A case report and literature review. Oncol. Lett. 2023, 26, 433. [Google Scholar] [CrossRef]
- Küsters-Vandevelde, H.V.; Küsters, B.; Grunsven, A.C.v.E.; Groenen, P.J.; Wesseling, P.; Blokx, W.A. Primary melanocytic tumors of the central nervous system: A review with focus on molecular aspects. Brain Pathol. 2015, 25, 209–226. [Google Scholar] [CrossRef]
- Diaz, M.J.; Mark, I.; Rodriguez, D.; Gelman, B.; Tran, J.T.; Kleinberg, G.; Levin, A.; Beneke, A.; Root, K.T.; Tran, A.X.V.; et al. Melanoma Brain Metastases: A Systematic Review of Opportunities for Earlier Detection, Diagnosis, and Treatment. Life 2023, 13, 828. [Google Scholar] [CrossRef]
- Krantz, B.A.; Krantz, B.A.; Dave, N.; Komatsubara, K.M.; Marr, B.P.; Carvajal, R.D. Uveal melanoma: Epidemiology, etiology, and treatment of primary disease. Clin. Ophthalmol. 2017, 11, 279–289. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Yang, S.; Wei, S.; Fan, Q.; Liu, J.; Yang, L.; Li, H. Targeting tumor innervation: Premises, promises, and challenges. Cell Death Discov. 2022, 8, 131. [Google Scholar] [CrossRef]
- Blasko, F.; Horvathova, L. The relationship between the tumor and its innervation: Historical, methodical, morphological, and functional assessments—A minireview. Endocr. Regul. 2024, 58, 68–82. [Google Scholar] [CrossRef]
- Balood, M.; Ahmadi, M.; Eichwald, T.; Ahmadi, A.; Majdoubi, A.; Roversi, K.; Lucido, C.T.; Restaino, A.C.; Huang, S.; Ji, L.; et al. Nociceptor neurons affect cancer immunosurveillance. Nature 2022, 611, 405–412. [Google Scholar] [CrossRef]
- Yin, T.; Wang, G.; Wang, L.; Mudgal, P.; Wang, E.; Pan, C.C.; Alexander, P.B.; Wu, H.; Cao, C.; Liang, Y.; et al. Breaking NGF–TrkA immunosuppression in melanoma sensitizes immunotherapy for durable memory T cell protection. Nat. Immunol. 2024, 25, 268–281. [Google Scholar] [CrossRef]
- Kruglov, O.; Vats, K.; Soman, V.; Tyurin, V.A.; Tyurina, Y.Y.; Wang, J.; Williams, L.; Zhang, J.; Carey, C.D.; Jaklitsch, E.; et al. Melanoma-associated repair-like Schwann cells suppress anti-tumor T-cells via 12/15-LOX/COX2-associated eicosanoid production. OncoImmunology 2023, 12, 2192098. [Google Scholar] [CrossRef]
- Huang, C.; Zheng, Y.; Ji, R.; Qiao, L.; Zhang, X.; Lin, H.; Liu, F.; Xu, J.; Li, Y.; Zhang, Z.; et al. GPNMB promotes peripheral nerve regeneration by activating the Erk1/2 and Akt pathways via binding Na(+)/K(+)-ATPase α1 in Schwann cells. Exp. Neurol. 2024, 373, 114687. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, A.C.; Mehta, N.A. A Case of Malignant Melanotic Schwannoma of the Trigeminal Nerve: A Case Report and Review of Literature. Asian J. Neurosurg. 2023, 18, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.; Wakeman, K.; Williams, B.J.; Miller, D.M.; Sak, M.; Abdullaev, Z.; Pacheco, M.C.; Aldape, K.; Lehman, N.L. Malignant melanotic nerve sheath tumor with PRKAR1A, KMT2C, and GNAQ mutations. Free Neuropathol. 2022, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Yosipovitch, G.; Mills, K.C.; Nattkemper, L.A.; Feneran, A.; Tey, H.L.; Lowenthal, B.M.; Pearce, D.J.; Williford, P.M.; Sangueza, O.P.; et al. Association of pain and itch with depth of invasion and inflammatory cell constitution in skin cancer: Results of a large clinicopathologic study. JAMA Dermatol. 2014, 150, 1160–1166. [Google Scholar] [CrossRef]
- Negin, B.P.; Riedel, E.; Oliveria, S.A.; Berwick, M.; Coit, D.G.; Brady, M.S. Symptoms and signs of primary melanoma: Important indicators of Breslow depth. Cancer 2003, 98, 344–348. [Google Scholar] [CrossRef]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Law, W.P.; Pereira, N.; Vaska, K. Perineural spread of recurrent cutaneous melanoma along cervical nerves into the spinal cord. BJR|Case Rep. 2017, 3, 20160122. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Marini, M.; Landini, L.; Souza Monteiro de Araujo, D.; Bartalucci, N.; Trevisan, G.; Bruno, G.; Marangoni, M.; Schmidt, B.L.; Bunnett, N.W.; et al. Peripheral Nerve Resident Macrophages and Schwann Cells Mediate Cancer-Induced Pain. Cancer Res. 2021, 81, 3387–3401. [Google Scholar] [CrossRef] [PubMed]
- Jobling, P.; Pundavela, J.; Oliveira, S.M.; Roselli, S.; Walker, M.M.; Hondermarck, H. Nerve–Cancer Cell Cross-talk: A Novel Promoter of Tumor Progression. Cancer Res. 2015, 75, 1777–1781. [Google Scholar] [CrossRef]
- Scanlon, P.; Tian, J.; Zhong, J.; Silva, I.; Shapiro, R.; Pavlick, A.; Berman, R.; Osman, I.; Darvishian, F. Enhanced immunohistochemical detection of neural infiltration in primary melanoma: Is there a clinical value? Hum. Pathol. 2014, 45, 1656–1663. [Google Scholar] [CrossRef]
- Shiralkar, J.; Anthony, T.; McCallum, G.A.; Durand, D.M.; Chen, S. Neural recordings can differentiate between spontaneously metastasizing melanomas and melanomas with low metastatic potential. PLoS ONE 2024, 19, e0297281. [Google Scholar] [CrossRef]
- Chiu, I.M.; A von Hehn, C.; Woolf, C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012, 15, 1063–1067. [Google Scholar] [CrossRef]
- Mo, R.J.; Han, Z.D.; Liang, Y.K.; Ye, J.H.; Wu, S.L.; Lin, S.X.; Zhang, Y.Q.; Song, S.D.; Jiang, F.N.; Zhong, W.D.; et al. Expression of PD-L1 in tumor-associated nerves correlates with reduced CD8(+) tumor-associated lymphocytes and poor prognosis in prostate cancer. Int. J. Cancer 2019, 144, 3099–3110. [Google Scholar] [CrossRef]
- Truzzi, F.; Marconi, A.; Lotti, R.; Dallaglio, K.; French, L.E.; Hempstead, B.L.; Pincelli, C. Neurotrophins and their receptors stimulate melanoma cell proliferation and migration. J. Investig. Dermatol. 2008, 128, 2031–2040. [Google Scholar] [CrossRef]
- Mizuno, K.; Ueno, Y. Autonomic Nervous System and the Liver. Hepatol. Res. 2017, 47, 160–165. [Google Scholar] [CrossRef]
- Miller, B.M.; Oderberg, I.M.; Goessling, W. Hepatic Nervous System in Development, Regeneration, and Disease. Hepatology 2021, 74, 3513–3522. [Google Scholar] [CrossRef]
- Tomaipitinca, L.; Mandatori, S.; Mancinelli, R.; Giulitti, F.; Petrungaro, S.; Moresi, V.; Facchiano, A.; Ziparo, E.; Gaudio, E.; Giampietri, C. The Role of Autophagy in Liver Epithelial Cells and Its Impact on Systemic Homeostasis. Nutrients 2019, 11, 827. [Google Scholar] [CrossRef]
- Pizaño, M.Y.M.; Arias, M.d.J.L.; Luna, R.M.d.O.; Cárdenas, O.S.; Juárez, J.V.; Ortega, M.H.M. Neuroimmunomodulation of adrenoblockers during liver cirrhosis: Modulation of hepatic stellate cell activity. Ann. Med. 2023, 55, 543–557. [Google Scholar] [CrossRef]
- Serna-Salas, S.A.; Arroyave-Ospina, J.C.; Zhang, M.; Damba, T.; Buist-Homan, M.; Muñoz-Ortega, M.H.; Ventura-Juárez, J.; Moshage, H. α-1 Adrenergic receptor antagonist doxazosin reverses hepatic stellate cells activation via induction of senescence. Mech. Ageing Dev. 2022, 201, 111617. [Google Scholar] [CrossRef]
- Jensen, K.J.; Alpini, G.; Glaser, S. Hepatic nervous system and neurobiology of the liver. Compr. Physiol. 2013, 3, 655–665. [Google Scholar] [CrossRef]
- Oben, J.A.; Roskams, T.; Yang, S.; Lin, H.; Sinelli, N.; Torbenson, M.; Smedh, U.; Moran, T.H.; Li, Z.; Huang, J.; et al. Hepatic fibrogenesis requires sympathetic neurotransmitters. Gut 2004, 53, 438–445. [Google Scholar] [CrossRef]
- Tan, X.; Sivakumar, S.; Bednarsch, J.; Wiltberger, G.; Kather, J.N.; Niehues, J.; de Vos-Geelen, J.; Iersel, L.V.-V.; Kintsler, S.; Roeth, A.; et al. Nerve fibers in the tumor microenvironment in neurotropic cancer—Pancreatic cancer and cholangiocarcinoma. Oncogene 2021, 40, 899–908. [Google Scholar] [CrossRef]
- Crippa, S.; Pergolini, I.; Javed, A.A.; Honselmann, K.C.; Weiss, M.J.; Di Salvo, F.; Burkhart, R.; Zamboni, G.; Belfiori, G.; Ferrone, C.R.; et al. Implications of Perineural Invasion on Disease Recurrence and Survival After Pancreatectomy for Pancreatic Head Ductal Adenocarcinoma. Ann. Surg. 2022, 276, 378–385. [Google Scholar] [CrossRef]
- Qian, X.; Liu, E.; Zhang, C.; Feng, R.; Tran, N.; Zhai, W.; Wang, F.; Qin, Z. Promotion of perineural invasion of cholangiocarcinoma by Schwann cells via nerve growth factor. J. Gastrointest. Oncol. 2024, 15, 1198–1213. [Google Scholar] [CrossRef]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Saricaoglu, Ö.C.; Pfitzinger, P.L.; Teller, S.; Wang, K.; Waldbaur, C.; Kurkowski, M.U.; Wörmann, S.M.; et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut 2016, 65, 1001–1014. [Google Scholar] [CrossRef]
- Yurteri, Ü.; Çifcibaşı, K.; Friess, H.; Ceyhan, G.O.; Istvanffy, R.; Demir, I.E. Schwann Cells in Peripheral Cancers: Bystanders or Promoters? Adv. Biol. 2022, 6, e2200033. [Google Scholar] [CrossRef]
- Mantyh, W.; Jimenez-Andrade, J.; Stake, J.; Bloom, A.; Kaczmarska, M.; Taylor, R.N.; Freeman, K.; Ghilardi, J.; Kuskowski, M.; Mantyh, P. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience 2010, 171, 588–598. [Google Scholar] [CrossRef]
- Sevcik, M.A.; Ghilardi, J.R.; Peters, C.M.; Lindsay, T.H.; Halvorson, K.G.; Jonas, B.M.; Kubota, K.; Kuskowski, M.A.; Boustany, L.; Shelton, D.L.; et al. Anti-NGF therapy profoundly reduces bone cancer pain and the accompanying increase in markers of peripheral and central sensitization. Pain 2005, 115, 128–141. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Han, B.; Zhong, R.; Zhong, H. Schwann cells promote lung cancer proliferation by promoting the M2 polarization of macrophages. Cell. Immunol. 2020, 357, 104211. [Google Scholar] [CrossRef]
- Sousa, S.; Brion, R.; Lintunen, M.; Kronqvist, P.; Sandholm, J.; Mönkkönen, J.; Kellokumpu-Lehtinen, P.-L.; Lauttia, S.; Tynninen, O.; Joensuu, H.; et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015, 17, 101. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Z.; Guo, J.; Li, H.; Zhang, J.; Zhang, B.; Zhou, B.; Feng, Y. Tumor-derived exosomal LINC01812 induces M2 macrophage polarization to promote perineural invasion in cholangiocarcinoma. Cancer Lett. 2025, 617, 217596. [Google Scholar] [CrossRef]
- Luo, C.; Xin, H.; Zhou, Z.; Hu, Z.; Sun, R.; Yao, N.; Sun, Q.; Borjigin, U.; Wu, X.; Fan, J.; et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology 2022, 76, 982–999. [Google Scholar] [CrossRef]
- Deipenbrock, A.; Wilmes, B.E.; Sommermann, T.; Abdo, N.; Moustakas, K.; Raasch, M.; Rennert, K.; Teusch, N.E. Modelling of the multicellular tumor microenvironment of pancreatic ductal adenocarcinoma (PDAC) on a fit-for-purpose biochip for preclinical drug discovery. Lab A Chip 2025, 25, 2168–2181. [Google Scholar] [CrossRef]
- Ali, S.R.; Jordan, M.; Nagarajan, P.; Amit, M. Nerve Density and Neuronal Biomarkers in Cancer. Cancers 2022, 14, 4817. [Google Scholar] [CrossRef]
- Göhrig, A.; Hilfenhaus, G.; Rosseck, F.; Welzel, M.; Moser, B.; Barbone, G.; Kunze, C.A.; Rein, J.; Wilken, G.; Böhmig, M.; et al. Placental growth factor promotes neural invasion and predicts disease prognosis in resectable pancreatic cancer. J. Exp. Clin. Cancer Res. 2024, 43, 153. [Google Scholar] [CrossRef]
- Guillot, J.; Dominici, C.; Lucchesi, A.; Nguyen, H.T.T.; Puget, A.; Hocine, M.; Rangel-Sosa, M.M.; Simic, M.; Nigri, J.; Guillaumond, F.; et al. Sympathetic axonal sprouting induces changes in macrophage populations and protects against pancreatic cancer. Nat. Commun. 2022, 13, 1985. [Google Scholar] [CrossRef]
- Tan, X.; Bednarsch, J.; Rosin, M.; Appinger, S.; Liu, D.; Wiltberger, G.; Vallejo, J.G.; Lang, S.A.; Czigany, Z.; Boroojerdi, S.; et al. PD-1+ T-Cells Correlate with Nerve Fiber Density as a Prognostic Biomarker in Patients with Resected Perihilar Cholangiocarcinoma. Cancers 2022, 14, 2190. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, L.; Tao, M.; Fu, W.; Xiu, D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin. J. Cancer Res. 2016, 28, 180–186. [Google Scholar] [CrossRef]
- Akiyoshi, H.; Gonda, T.; Terada, T. A comparative histochemical and immunohistochemical study of aminergic, cholinergic and peptidergic innervation in rat, hamster, guinea pig, dog and human livers. Liver 1998, 18, 352–359. [Google Scholar] [CrossRef]
- Ueda, K.; Nakanuma, Y.; Terada, T.; Naito, T.; Matsui, O. Well-differentiated hepatocellular carcinoma showing intrahepatic neural invastion: Autopsied case. Jpn. J. Clin. Oncol. 1991, 21, 143–146. [Google Scholar] [CrossRef]
- Kanda, N.; Fukuda, Y.; Imoto, M.; Koyama, Y.; Nakano, I.; Urano, F. Localization of synaptophysin immunoreactivity in the human liver. Scand. J. Gastroenterol. 1994, 29, 275–279. [Google Scholar] [CrossRef]
- Mandal, S.K.; Yadav, P.; Sheth, R.A. The Neuroimmune Axis and Its Therapeutic Potential for Primary Liver Cancer. Int. J. Mol. Sci. 2024, 25, 6237. [Google Scholar] [CrossRef]
- Liebl, F.; Demir, I.E.; Mayer, K.; Schuster, T.; D’Haese, J.G.; Becker, K.; Langer, R.; Bergmann, F.; Wang, K.; Rosenberg, R.; et al. The impact of neural invasion severity in gastrointestinal malignancies: A clinicopathological study. Ann. Surg. 2014, 260, 900–907; discussion 907–908. [Google Scholar] [CrossRef]
- Bednarsch, J.; Tan, X.; Czigany, Z.; Wiltberger, G.; Buelow, R.D.; Boor, P.; Lang, S.A.; Ulmer, T.F.; Neumann, U.P.; Heij, L.R. Limitations of Nerve Fiber Density as a Prognostic Marker in Predicting Oncological Outcomes in Hepatocellular Carcinoma. Cancers 2022, 14, 2237. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, L.L.; Huan, H.B.; Chen, X.J.; Wen, X.D.; Yang, D.P.; Xia, F. Sympathetic and parasympathetic innervation in hepatocellular carcinoma. Neoplasma 2017, 64, 840–846. [Google Scholar] [CrossRef]
- Wang, X.; Lan, H.; Shen, T.; Gu, P.; Guo, F.; Lin, X.; Jin, K. Perineural invasion: A potential reason of hepatocellular carcinoma bone metastasis. Int. J. Clin. Exp. Med. 2015, 8, 5839–5846. [Google Scholar]
- Amir, M.; Yu, M.; He, P.; Srinivasan, S. Hepatic Autonomic Nervous System and Neurotrophic Factors Regulate the Pathogenesis and Progression of Non-alcoholic Fatty Liver Disease. Front. Med. 2020, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Huang, Z.; Wang, S.; Zhao, Z.; Yi, P.; Chen, Y.; Xiao, M.; Quan, J.; Hu, X. The Hepatic Nerves Regulated Inflammatory Effect in the Process of Liver Injury: Is Nerve the Key Treating Target for Liver Inflammation? Inflammation 2023, 46, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-T. The role of the autonomic nervous system in chemically-induced liver damage and repair—Using the essential hypertensive animal model (SHR). J. Auton. Nerv. Syst. 1995, 51, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Sigala, B.; McKee, C.; Soeda, J.; Pazienza, V.; Morgan, M.; Lin, C.-I.; Selden, C.; Borght, S.V.; Mazzoccoli, G.; Roskams, T.; et al. Sympathetic nervous system catecholamines and neuropeptide Y neurotransmitters Are upregulated in human NAFLD and modulate the fibrogenic function of hepatic stellate cells. PLoS ONE 2013, 8, e72928. [Google Scholar] [CrossRef]
- Huan, H.-B.; Wen, X.-D.; Chen, X.-J.; Wu, L.-L.; Zhang, L.; Yang, D.-P.; Zhang, X.; Bie, P.; Qian, C.; Xia, F. Sympathetic nervous system promotes hepatocarcinogenesis by modulating inflammation through activation of alpha1-adrenergic receptors of Kupffer cells. Brain Behav. Immun. 2017, 59, 118–134. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Yu, Y.; Yuan, H.; Xu, H.; Zhu, Q.; Wang, C.; Shi, X. The vagus nerve attenuates fulminant hepatitis by activating the Src kinase in Kupffer cells. Scand. J. Immunol. 2014, 79, 105–112. [Google Scholar] [CrossRef]
- Izumi, T.; Imai, J.; Yamamoto, J.; Kawana, Y.; Endo, A.; Sugawara, H.; Kohata, M.; Asai, Y.; Takahashi, K.; Kodama, S.; et al. Vagus-macrophage-hepatocyte link promotes post-injury liver regeneration and whole-body survival through hepatic FoxM1 activation. Nat. Commun. 2018, 9, 5300. [Google Scholar] [CrossRef]
- Herrero, A.; Toubert, C.; Bedoya, J.U.; Assenat, E.; Guiu, B.; Panaro, F.; Bardol, T.; Cassese, G. Management of hepatocellular carcinoma recurrence after liver surgery and thermal ablations: State of the art and future perspectives. HepatoBiliary Surg. Nutr. 2024, 13, 71–88. [Google Scholar] [CrossRef]
- Nour, M.A.; Kheradmand, F.; Rasmi, Y.; Baradaran, B. Alpha7 nicotinic acetylcholine receptor expression in Sorafenib-resistant Hepatocellular carcinoma cells. Med Oncol. 2022, 39, 165. [Google Scholar] [CrossRef]
- Pérez-Aguilar, B.; Vidal, C.J.; Palomec, G.; García-Dolores, F.; Gutiérrez-Ruiz, M.C.; Bucio, L.; Gómez-Olivares, J.L.; Gómez-Quiroz, L.E. Acetylcholinesterase is associated with a decrease in cell proliferation of hepatocellular carcinoma cells. Biochim. Et Biophys. Acta (BBA)—Mol. Basis Dis. 2015, 1852, 1380–1387. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Norbiato, G.; Chebat, E.; Baldi, G.; Bertora, P.; Vago, T.; Regalia, E.; Colella, G.; Gennari, L. Changes in alpha-1 and beta-2 adrenoceptor density in human hepatocellular carcinoma. Cancer 1991, 67, 2543–2551. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-Q.; Fang, T.; Yu, L.-X.; Lv, G.-S.; Lv, H.-W.; Liang, D.; Li, T.; Wang, C.-Z.; Tan, Y.-X.; Ding, J.; et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1α. J. Hepatol. 2016, 65, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Lee, S.H. Beta-adrenergic receptor blockers and hepatocellular carcinoma survival: A systemic review and meta-analysis. Clin. Exp. Med. 2023, 23, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Saber, S.; Amer, A.E.; Hamad, R.S.; Abdel-Reheim, M.A.; Elmorsy, E.A.; Abdelhamid, A.M. The multifaceted role of beta-blockers in overcoming cancer progression and drug resistance: Extending beyond cardiovascular disorders. FASEB J. 2024, 38, e23813. [Google Scholar] [CrossRef]
- Li, X.-Q.; Peng, W.-T.; Shan, S.; Wu, J.-J.; Li, N.; Du, J.-J.; Sun, J.-C.; Chen, T.-T.; Wei, W.; Sun, W.-Y. β-arrestin2 regulating β2-adrenergic receptor signaling in hepatic stellate cells contributes to hepatocellular carcinoma progression. J. Cancer 2021, 12, 7287–7299. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Chen, C.; Li, L.; Li, J.; Wang, X.; Chu, Q.; Qiu, L.; Ba, Q.; Li, X.; et al. Publisher Correction: Environmental eustress modulates β-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat. Commun. 2021, 12, 6100. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Shi, Y.; Luo, J.; Zhang, Y.; Pan, Z.; Wu, F.; Tian, J.; Yu, W. Comprehensive analysis to identify the neurotransmitter receptor-related genes as prognostic and therapeutic biomarkers in hepatocellular carcinoma. Front. Cell Dev. Biol. 2022, 10, 887076. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Jin, M.-Z.; Zhu, X.-R.; Jin, W.-L. Reclassification of Hepatocellular Cancer With Neural-Related Genes. Front. Oncol. 2022, 12, 877657. [Google Scholar] [CrossRef]
- Guo, D.; Hou, X.; Zhang, H.; Sun, W.; Zhu, L.; Liang, J.; Jiang, X. More expressions of BDNF and TrkB in multiple hepatocellular carcinoma and anti-BDNF or K252a induced apoptosis, supressed invasion of HepG2 and HCCLM3 cells. J. Exp. Clin. Cancer Res. 2011, 30, 97. [Google Scholar] [CrossRef]
- Lam, C.T.; Yang, Z.F.; Lau, C.K.; Tam, K.H.; Fan, S.T.; Poon, R.T. Brain-derived neurotrophic factor promotes tumorigenesis via induction of neovascularization: Implication in hepatocellular carcinoma. Clin. Cancer Res. 2011, 17, 3123–3133. [Google Scholar] [CrossRef] [PubMed]
- Kishibe, K.; Yamada, Y.; Ogawa, K. Production of nerve growth factor by mouse hepatocellular carcinoma cells and expression of TrkA in tumor-associated arteries in mice. Gastroenterology 2002, 122, 1978–1986. [Google Scholar] [CrossRef] [PubMed]
- Rasi, G. Nerve growth factor involvement in liver cirrhosis and hepatocellular carcinoma. World J. Gastroenterol. 2007, 13, 4986–4995. [Google Scholar] [CrossRef] [PubMed]
- Tokusashi, Y.; Asai, K.; Tamakawa, S.; Yamamoto, M.; Yoshie, M.; Yaginuma, Y.; Miyokawa, N.; Aoki, T.; Kino, S.; Kasai, S.; et al. Expression of NGF in hepatocellular carcinoma cells with its receptors in non-tumor cell components. Int. J. Cancer 2005, 114, 39–45. [Google Scholar] [CrossRef]
- He, Y.; Jin, H.; Zhang, X.; Song, J.; Liu, J.; Yan, L.; Xie, H.; Song, J.; Pan, Y.; Wu, K.; et al. The inhibitory effect of p75 neurotrophin receptor on growth of human hepatocellular carcinoma cells. Cancer Lett. 2008, 268, 110–119. [Google Scholar] [CrossRef]
- Miller, S.J.; Rangwala, F.; Williams, J.; Ackerman, P.; Kong, S.; Jegga, A.G.; Kaiser, S.; Aronow, B.J.; Frahm, S.; Kluwe, L.; et al. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 2006, 66, 2584–2591. [Google Scholar] [CrossRef]
- Zhang, S.H.; Shurin, G.V.; Khosravi, H.; Kazi, R.; Kruglov, O.; Shurin, M.R.; Bunimovich, Y.L. Immunomodulation by Schwann cells in disease. Cancer Immunol. Immunother. 2020, 69, 245–253. [Google Scholar] [CrossRef]
| Pro-Tumural Role of SCs | Sympathetic Fibres | Parasympathetic Fibres | Sensory Fibres | Relevant Neurotrophic Molecules | Potential Drugs | References | |
|---|---|---|---|---|---|---|---|
| Melanoma | + | promote tumor growth | undefined | promote/inhibit tumor growth | NGF | β-blocker drugs | [29,39,53,82,83,86,100,146] |
| PDAC | + | inhibit tumor growth | promote tumor growth | promote tumor growth | BDNF; GDNF; NGF | NGF inhibitors | [35,37,38,61,108,109,112,120,121,122] |
| CCA | + | promote tumor growth | undefined | undefined | TGFβ | undefined | [62,108,110] |
| HCC | undefined | promote tumor growth | promote tumor growth | undefined | BDNF; NGF | β-blocker drugs | [131,137,145,147] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giampietri, C.; Pizzichini, E.; Somma, F.; Petrungaro, S.; De Santis, E.; Rahimi, S.; Facchiano, A.; Fabrizi, C. Roles of Peripheral Nerves in Tumor Initiation and Progression. Int. J. Mol. Sci. 2025, 26, 7064. https://doi.org/10.3390/ijms26157064
Giampietri C, Pizzichini E, Somma F, Petrungaro S, De Santis E, Rahimi S, Facchiano A, Fabrizi C. Roles of Peripheral Nerves in Tumor Initiation and Progression. International Journal of Molecular Sciences. 2025; 26(15):7064. https://doi.org/10.3390/ijms26157064
Chicago/Turabian StyleGiampietri, Claudia, Elisa Pizzichini, Francesca Somma, Simonetta Petrungaro, Elena De Santis, Siavash Rahimi, Antonio Facchiano, and Cinzia Fabrizi. 2025. "Roles of Peripheral Nerves in Tumor Initiation and Progression" International Journal of Molecular Sciences 26, no. 15: 7064. https://doi.org/10.3390/ijms26157064
APA StyleGiampietri, C., Pizzichini, E., Somma, F., Petrungaro, S., De Santis, E., Rahimi, S., Facchiano, A., & Fabrizi, C. (2025). Roles of Peripheral Nerves in Tumor Initiation and Progression. International Journal of Molecular Sciences, 26(15), 7064. https://doi.org/10.3390/ijms26157064






