Parathyroid Hormone as a Modulator of Skeletal Muscle: Insights into Bone–Muscle and Nerve–Muscle Interactions
Abstract
1. Introduction
2. Methodology
- -
- Original research articles, reviews, and meta-analyses published in English.
- -
- Studies addressing the role or effects of PTH (or its analogs) on skeletal muscle, neuromuscular junctions, or crosstalk between muscle and bone or nerve.
- -
- Studies involving in vivo, in vitro, or clinical investigations.
3. Parathyroid Hormone
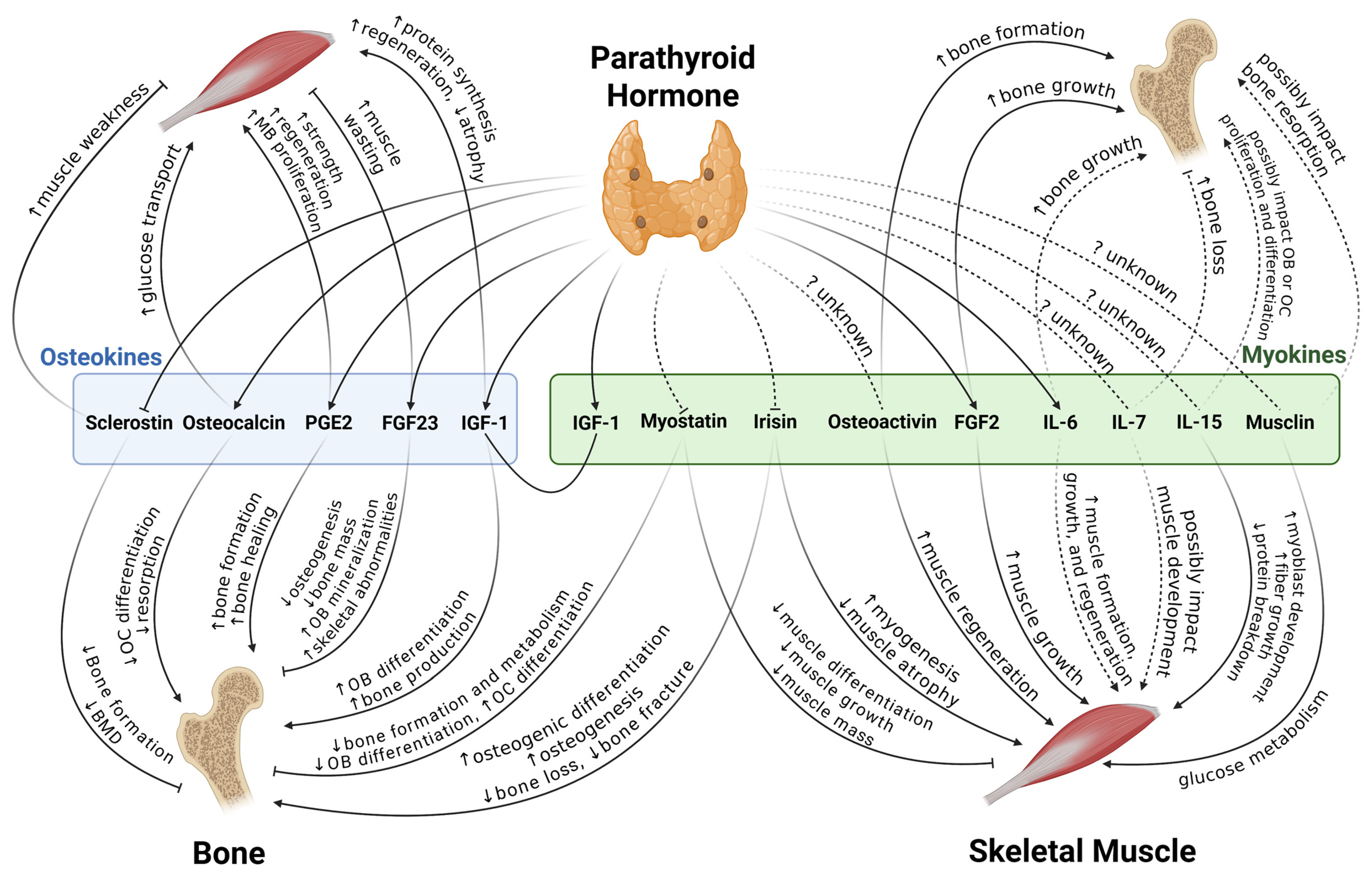
4. Bone and Skeletal Muscle Interactions
4.1. Bone to Muscle
4.1.1. FGF23
4.1.2. PGE2
4.1.3. Osteocalcin
4.1.4. IGF-1
4.1.5. Sclerostin
4.2. Muscle to Bone
4.2.1. Myostatin
4.2.2. IGF-1
4.2.3. Osteoactivin
4.2.4. Interleukins
4.2.5. Irisin
4.2.6. FGF2
4.2.7. Musclin
| Osteokines/Myokines | Effects on Bone | Mechanism/Signaling Involved | Effects on Skeletal Muscle | Mechanism/Signaling Involved |
|---|---|---|---|---|
| FGF23 | In vitro: ↓ BMSCs osteogenesis, ↓ mature osteoblast mineralization [27] | FGFR3-ERK [27] | In vitro: ↓ Muscle cell differentiation [30] | Insulin/IGF-1, klotho [30] |
| Clinical: Serum level associated with low bone mass [28] | Relevant to skeletal muscle wasting [33] | |||
| PGE2 | Regulating both bone resorption and formation processes [34,35] | In vitro: Myogenesis [38] | EP4 receptor [38] | |
| In vivo, in vitro: Muscle regeneration and strength [39] | Muscle-specific stem cells [39] | |||
| Related: Wnt, β-catenin [36], cAMP/PKA [37] | ||||
| Osteocalcin | Glucose metabolism, reproduction, and cognition [41] | In vitro: ↑ Glucose transport [43] Clinical, in vivo: ↑ Muscle uptake [44,45] | ||
| In vitro: ↓ Osteoclasts differentiation [42] | GPRC6A [42] | |||
| IGF-1 | ↑ Osteoblasts differentiation, bone production [47] | ↑ Protein synthesis and regeneration ↓ Muscle atrophy [48] | ||
| Related: PI3K/Akt, MAPK/ERK [46] | ||||
| Sclerostin | Clinical, in vivo, in vitro: ↓ Bone formation [50,51] | Wnt [49] | Clinical: ↑ Muscle weakness [54] | |
| Myostatin | ↓ Bone formation Metabolism [57] | ↓ Muscle mass [55] | ||
| In vitro: ↓ Osteoblastic differentiation [58] | Osteocyte-derived exosomal miR-218 [58] | |||
| ↓ Osteoclast differentiation [59] | RANKL, NFATC1 [59] | |||
| Related: ERK1/2, Wnt, TGF-β1, IGF-1 [55] | ||||
| Osteoactivin | In vivo, in vitro: ↓ Osteoclastogenesis [61] | CD44-ERK [61] | In vivo: Protection from fibrosis [63] | MMP-3, MMP-9 [63] |
| In vivo, in vitro: ↑ Bone formation [66] | TGF-β [66] | |||
| IL-6 | ↑ Bone growth [70,71] | ↑ Formation, growth, regeneration, satellite-cell-dependent myogenesis [69] | ||
| ↑ Bone loss (in several osteolytic diseases) [70,71] | ↑ Protein synthesis and breakdown Engaged with muscle atrophy [69] | |||
| IL-7 | In vivo: ↑ Bone loss [74] | RANKL | Might affect satellite cells [72,73] | |
| IL-15 | Bidirectional regulatory function [79] | Clinical: ↑ Myoblast development, fiber growth; ↓ protein breakdown [78] | ||
| Irisin | In vivo: ↓ Bone loss [84] | In vitro: Mitochondrial biogenesis [82] In vivo: ↑ Myogenesis [83] ↓ Muscle atrophy [84] | ||
| Related: MAPK [85], ERK/STAT, BMP/SMAD [87], Wnt/β-catenin [88] | ||||
| FGF2 | Bone growth [92,93,94] | Clinical, in vivo: ↑ Muscle growth, intramuscular adipogenesis [91] | miR-29a/SPARC [91] | |
| In vivo: Bone marrow MSC Osteogenesis [95] | ERK/Runx2 [95] | |||
| Musclin | Bone resorption [100] | RANKL [100] | Glucose metabolism [96,97] | |
| In vivo: ↑ Physical endurance [98] | Mitochondrial biogenesis | |||
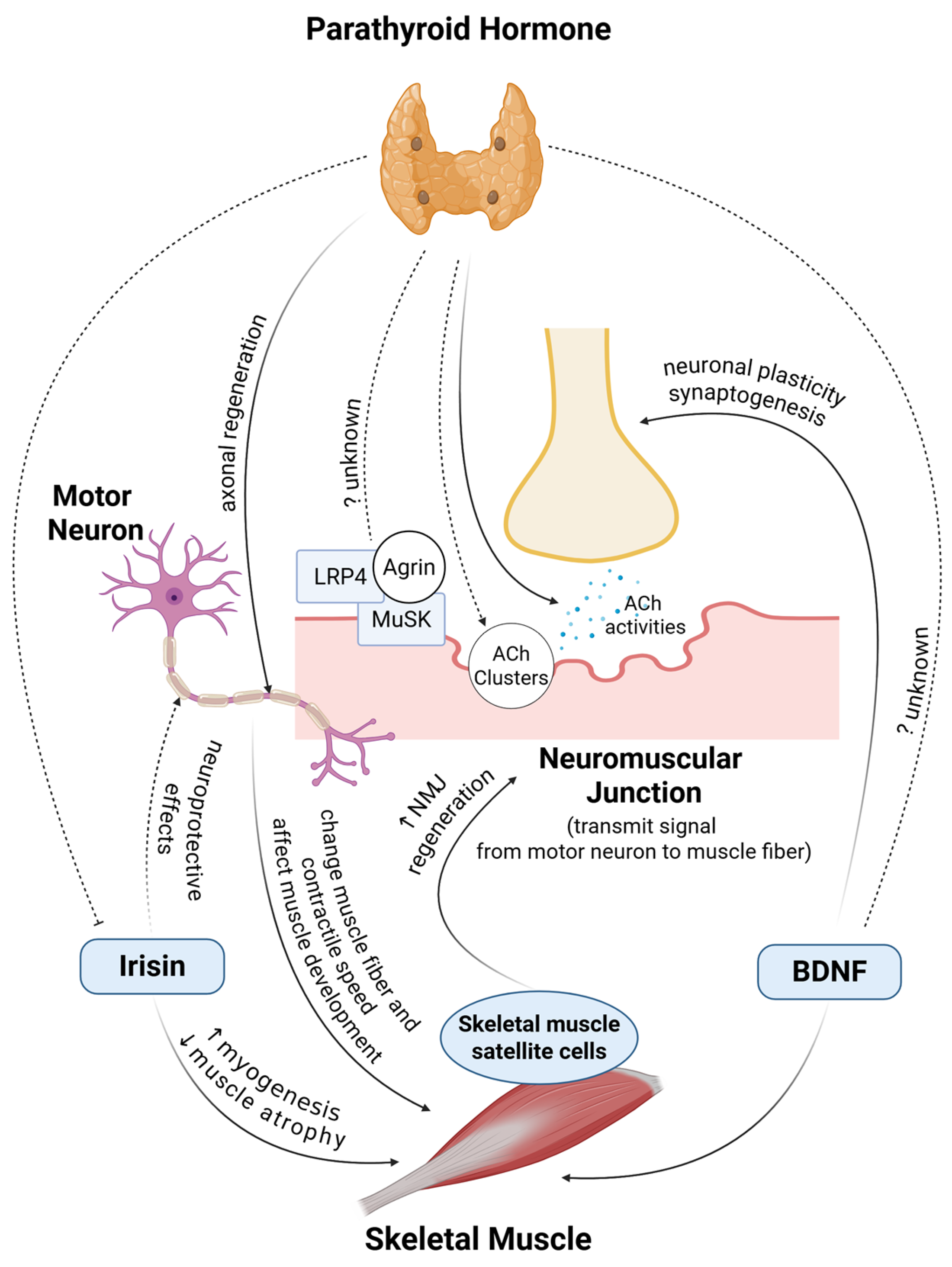
5. Muscle and Nerve Communication
5.1. Neuromuscular Junctions
5.2. Nerve to Muscle
5.3. Muscle to Nerve
| Factors | Effects on Nerve | Mechanism/Signaling Involved | Effects on Skeletal Muscle | Mechanism/Signaling Involved |
|---|---|---|---|---|
| Motor neurons | Clinical: Affecting muscle fiber morphology and phenotype [116,117] In vivo: Differentiation of slow muscles [120]; affects contractile speed of re-innervated muscle [121] | |||
| Gap junctions/NMJs | In vitro: ↑ Myoblast fusion [118] | Intercellular communication [118] | ||
| Poor signal transmission and muscular weakening in aging [123,124] | NMJ deteriorates, mitochondria mechanism [123,124] | |||
| Neural and hormonal influences | ↑ Muscle development [119] | Isogenes [119] | ||
| DOK7 | In vivo: ↑ Muscles and motor activities [125] | ↑ NMJ innervation [125] | ||
| MuSCs | NMJ repair and maintenance [135,136,137,138,139] | Myofiber components, derived factors, associated satellite cells [135,136,137,138,139] | Muscle repair and regeneration [123,128] | |
| BDNF | ↑ Hippocampal neurons, neuronal plasticity, and synaptogenesis ↓ Neuroinflammation [130,131,132] | Supporting muscle regeneration and utilization [129] | ||
| Irisin | In vitro: Regulating astrocytes, neuroprotective effects [133] | Interleukins, COX-2, AKT, NFκB [133] | In vitro: Mitochondrial biogenesis [82] In vivo: ↑ Myogenesis [83] ↓ Muscle atrophy [84] | |
| In vitro: Neural generation and development [134] | Post-neural progenitor formation [134] | |||
6. The Effects of PTH on Skeletal Muscle
7. Parathyroid Hormone, Bone, Nerves, and Skeletal Muscle
7.1. PTH’s Role in the Bone–Muscle Axis
7.2. PTH’s Role in the Nerve–Muscle Axis
| Target of NMJ Components | Study Design | PTH’s Effects | Ref. |
|---|---|---|---|
| Axon/Neuron | In vitro | PTH boosts the mean speed of both anterograde and retrograde organelle traffic on axons | [209] |
| In vivo | PTH (1–34) treatment can affect axonal regeneration by enhancing endogenous BMP-7 in rat Schwann cells | [210] | |
| In vivo | Circulating PTH activates neurons in the subfornical organ | [211] | |
| Acetylcholine activities | In vitro | In the rat superior cervical ganglion, ACh is released when PTH increases and calcitonin is inhibited | [212] |
| In vitro | PTH affects 3H-acetylcholine synthesis in rat parathyroid glands | [213] | |
| In vitro | PTH-induced oxidative stress preserves ACh | [214] |
7.3. PTH and Skeletal Muscle
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| BDNF | Brain-derived neurotrophic factor |
| BMD | Bone mineral density |
| BMP | Bone morphogenetic protein |
| BW | Body weight |
| EVs | Extracellular vesicles |
| exRNAs | Extracellular RNAs |
| FAPs | Fibro-adipogenic progenitors |
| FDA | Food and Drug Administration |
| FGF | Fibroblast growth factor |
| FNDC5 | Fibronectin type III domain-containing 5 |
| FOXO1 | Forkhead box protein O1 |
| GDF | Growth differentiation factor |
| GH | Growth hormone |
| GPRC6A | G protein-coupled receptor family C group 6 member A |
| hSCs | Human skeletal muscle biopsies |
| IGF | Insulin-like growth factor |
| IL | Interleukin |
| MAFbx | Muscle atrophy F-box |
| MB | Myoblast |
| mRNA | Messenger RNA |
| MuRF1 | Muscle-specific RING finger protein 1 |
| MuSCs | Muscle stem cells |
| MuSK | Muscle-specific kinase |
| NMJ | Neuromuscular junction |
| LRP | Low-density lipoprotein receptor-related protein |
| OB | Osteoblast |
| OC | Osteoclast |
| OSE1 | Osteoblast-specific element 1 |
| OVX | Ovariectomy |
| PGE2 | Prostaglandin E2 |
| PTH | Parathyroid hormone |
| PTH1R | Parathyroid hormone 1 receptor |
| PTH2R | Parathyroid hormone 2 receptor |
| PTHrP | Parathyroid hormone-related peptide |
| SNAPs | Soluble NSF Attachment Proteins |
| TGF | Transforming growth factor |
| TPTD | Teriparatide |
| UCP1 | Uncoupling protein 1 |
| VEGF | Vascular endothelial growth factor |
References
- Goltzman, D. Physiology of Parathyroid Hormone. Endocrinol. Metab. Clin. N. Am. 2018, 47, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yuan, H.; Ma, G.; Cao, H. Bone-Muscle Crosstalk under Physiological and Pathological Conditions. Cell. Mol. Life Sci. 2024, 81, 310. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.-J.; Walrand, S.; Coxam, V. Muscle and Bone, Two Interconnected Tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Rubin, D.I. Normal and Abnormal Voluntary Activity. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 160, pp. 281–301. [Google Scholar] [CrossRef]
- Lepore, E.; Casola, I.; Dobrowolny, G.; Musarò, A. Neuromuscular Junction as an Entity of Nerve-Muscle Communication. Cells 2019, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, C.; Brandi, M.L. Muscle Physiopathology in Parathyroid Hormone Disorders. Front. Med. 2021, 8, 764346. [Google Scholar] [CrossRef]
- Taterra, D.; Wong, L.M.; Vikse, J.; Sanna, B.; Pękala, P.; Walocha, J.; Cirocchi, R.; Tomaszewski, K.; Henry, B.M. The Prevalence and Anatomy of Parathyroid Glands: A Meta-Analysis with Implications for Parathyroid Surgery. Langenbecks Arch. Surg. 2019, 404, 63. [Google Scholar] [CrossRef]
- Dobolyi, A.; Palkovits, M.; Usdin, T.B. The TIP39–PTH2 Receptor System: Unique Peptidergic Cell Groups in the Brainstem and Their Interactions with Central Regulatory Mechanisms. Prog. Neurobiol. 2010, 90, 29–59. [Google Scholar] [CrossRef]
- Kimura, S.; Yoshioka, K. Parathyroid Hormone and Parathyroid Hormone Type-1 Receptor Accelerate Myocyte Differentiation. Sci. Rep. 2014, 4, 5066. [Google Scholar] [CrossRef]
- Hastings, R.H. Parathyroid Hormone-Related Protein and Lung Biology. Respir. Physiol. Neurobiol. 2004, 142, 95–113. [Google Scholar] [CrossRef]
- Murray, T.M.; Rao, L.G.; Divieti, P.; Bringhurst, F.R. Parathyroid Hormone Secretion and Action: Evidence for Discrete Receptors for the Carboxyl-Terminal Region and Related Biological Actions of Carboxyl-Terminal Ligands. Endocr. Rev. 2005, 26, 78–113. [Google Scholar] [CrossRef]
- Wang, S.P.; Chen, Y.J.; Hsu, C.E.; Chiu, Y.C.; Tsai, M.T.; Hsu, J.T. Intermittent Parathyroid Hormone Treatment Affects the Bone Structural Parameters and Mechanical Strength of the Femoral Neck after Ovariectomy-Induced Osteoporosis in Rats. Biomed. Eng. Online 2022, 21, 6. [Google Scholar] [CrossRef]
- Mannstadt, M.; Clarke, B.L.; Bilezikian, J.P.; Bone, H.; Denham, D.; Levine, M.A.; Peacock, M.; Rothman, J.; Shoback, D.M.; Warren, M.L.; et al. Safety and Efficacy of 5 Years of Treatment with Recombinant Human Parathyroid Hormone in Adults With Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 5136–5147. [Google Scholar] [CrossRef]
- Yamaguchi, D.T.; Kleeman, C.R. The Neuromuscular Manifestations of Primary Hyperparathyroidism in Humans. In New Actions of Parathyroid Hormone; Springer: Boston, MA, USA, 1989; pp. 365–377. [Google Scholar] [CrossRef]
- Cheng, T.C.; Huang, S.H.; Kao, C.L.; Hsu, P.C. Muscle Wasting in Chronic Kidney Disease: Mechanism and Clinical Implications—A Narrative Review. Int. J. Mol. Sci. 2022, 23, 6047. [Google Scholar] [CrossRef]
- Pietrzak, W.S. Foreword. In Mechanical Testing of Orthopaedic Implants; Woodhead Publishing: Cambridge, UK, 2017; pp. xi–xii. [Google Scholar] [CrossRef]
- Digirolamo, D.J.; Kiel, D.P.; Esser, K.A. Bone and Skeletal Muscle: Neighbors With Close Ties. J. Bone Miner. Res. 2013, 28, 1509–1518. [Google Scholar] [CrossRef]
- Kim, T.N.; Choi, K.M. Sarcopenia: Definition, Epidemiology, and Pathophysiology. J. Bone Metab. 2013, 20, 1. [Google Scholar] [CrossRef]
- Bonjour, J.P.; Theintz, G.; Law, F.; Slosman, D.; Rizzoli, R. Peak Bone Mass. Osteoporos. Int. 1994, 4, S7–S13. [Google Scholar] [CrossRef]
- Girgis, C.M.; Mokbel, N.; DiGirolamo, D.J. Therapies for Musculoskeletal Disease: Can We Treat Two Birds with One Stone? Curr. Osteoporos. Rep. 2014, 12, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Bailly, M.; Boscaro, A.; Thomas, T.; Féasson, L.; Costes, F.; Pereira, B.; Hager, J.; Estour, B.; Galusca, B.; Metz, L.; et al. New Insights on Bone Tissue and Structural Muscle-Bone Unit in Constitutional Thinness. Front. Physiol. 2022, 13, 921351. [Google Scholar] [CrossRef]
- Herrmann, M.; Engelke, K.; Ebert, R.; Müller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between Muscle and Bone—Where Physics Meets Biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. The Fibroblast Growth Factor Signaling Pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015, 4, 215–266. [Google Scholar] [CrossRef] [PubMed]
- Beenken, A.; Mohammadi, M. The FGF Family: Biology, Pathophysiology and Therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Moin, M.R.; Das, S.; Sanyal, S. Emerging Roles of Osteocytes in the Regulation of Bone and Skeletal Muscle Mass. J. Mol. Endocrinol. 2024, 74, e240033. [Google Scholar] [CrossRef]
- Simic, P.; Babitt, J.L. Regulation of FGF23: Beyond Bone. Curr. Osteoporos. Rep. 2021, 19, 563–573. [Google Scholar] [CrossRef]
- Lyu, Z.; Li, H.; Li, X.; Wang, H.; Jiao, H.; Wang, X.; Zhao, J.; Lin, H. Fibroblast Growth Factor 23 Inhibits Osteogenic Differentiation and Mineralization of Chicken Bone Marrow Mesenchymal Stem Cells. Poult. Sci. 2023, 102, 102287. [Google Scholar] [CrossRef]
- Shen, J.; Fu, S.; Song, Y. Relationship of Fibroblast Growth Factor 23 (FGF-23) Serum Levels With Low Bone Mass in Postmenopausal Women. J. Cell. Biochem. 2017, 118, 4454–4459. [Google Scholar] [CrossRef]
- Kurpas, A.; Supeł, K.; Idzikowska, K.; Zielińska, M. FGF23: A Review of Its Role in Mineral Metabolism and Renal and Cardiovascular Disease. Dis. Markers 2021, 2021, 8821292. [Google Scholar] [CrossRef] [PubMed]
- Kido, S.; Hashimoto, Y.; Segawa, H.; Tatsumi, S.; Miyamoto, K. Muscle Atrophy in Patients Wirh Ckd Results from Fgf23/Klotho-Mediated Supression of Insulin/Igf-i Signaling. Kidney Res. Clin. Pract. 2012, 31, A44. [Google Scholar] [CrossRef]
- Si, Y.; Kazamel, M.; Benatar, M.; Wuu, J.; Kwon, Y.; Kwan, T.; Jiang, N.; Kentrup, D.; Faul, C.; Alesce, L.; et al. FGF23, a Novel Muscle Biomarker Detected in the Early Stages of ALS. Sci. Rep. 2021, 11, 12062. [Google Scholar] [CrossRef] [PubMed]
- Faul, C.; Amaral, A.P.; Oskouei, B.; Hu, M.C.; Sloan, A.; Isakova, T.; Gutiérrez, O.M.; Aguillon-Prada, R.; Lincoln, J.; Hare, J.M.; et al. FGF23 Induces Left Ventricular Hypertrophy. J. Clin. Investig. 2011, 121, 4393–4408. [Google Scholar] [CrossRef]
- Elsurer Afsar, R.; Afsar, B.; Ikizler, T.A. Fibroblast Growth Factor 23 and Muscle Wasting: A Metabolic Point of View. Kidney Int. Rep. 2023, 8, 1301–1314. [Google Scholar] [CrossRef]
- Blackwell, K.A.; Raisz, L.G.; Pilbeam, C.C. Prostaglandins in Bone: Bad Cop, Good Cop? Trends Endocrinol. Metab. 2010, 21, 294–301. [Google Scholar] [CrossRef]
- Ohshiba, T.; Miyaura, C.; Ito, A. Role of Prostaglandin E Produced by Osteoblasts in Osteolysis Due to Bone Metastasis. Biochem. Biophys. Res. Commun. 2003, 300, 957–964. [Google Scholar] [CrossRef]
- Gupta, A.; Chatree, S.; Buo, A.M.; Moorer, M.C.; Stains, J.P. Connexin43 Enhances Wnt and PGE2-Dependent Activation of β-Catenin in Osteoblasts. Pflugers Arch. 2019, 471, 1235–1243. [Google Scholar] [CrossRef]
- Kitase, Y.; Barragan, L.; Qing, H.; Kondoh, S.; Jiang, J.X.; Johnson, M.L.; Bonewald, L.F. Mechanical Induction of PGE2 in Osteocytes Blocks Glucocorticoid-induced Apoptosis through Both the Β-catenin and PKA Pathways. J. Bone Miner. Res. 2010, 25, 2657–2668. [Google Scholar] [CrossRef]
- Mo, C.; Zhao, R.; Vallejo, J.; Igwe, O.; Bonewald, L.; Wetmore, L.; Brotto, M. Prostaglandin E2 Promotes Proliferation of Skeletal Muscle Myoblasts via EP4 Receptor Activation. Cell Cycle 2015, 14, 1507–1516. [Google Scholar] [CrossRef]
- Ho, A.T.V.; Palla, A.R.; Blake, M.R.; Yucel, N.D.; Wang, Y.X.; Magnusson, K.E.G.; Holbrook, C.A.; Kraft, P.E.; Delp, S.L.; Blau, H.M. Prostaglandin E2 Is Essential for Efficacious Skeletal Muscle Stem-Cell Function, Augmenting Regeneration and Strength. Proc. Natl. Acad. Sci. USA 2017, 114, 6675–6684. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Robey, P.G. The Regulatory Role of Matrix Proteins in Mineralization of Bone. In Osteoporosis, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 235–255. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New Insights into the Biology of Osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Xu, Z.; Wu, F.; Zhang, H.; Yang, C.; Chen, J.; Ding, B.; Sui, X.; Guo, Z.; et al. Undercarboxylated Osteocalcin Inhibits the Early Differentiation of Osteoclast Mediated by Gprc6a. PeerJ 2021, 9, e10898. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.S.; Grams, J.; Walton, R.G.; Liu, J.; Moellering, D.R.; Garvey, W.T. Carboxylated and Uncarboxylated Forms of Osteocalcin Directly Modulate the Glucose Transport System and Inflammation in Adipocytes. Horm. Metab. Res. 2014, 46, 341–347. [Google Scholar] [CrossRef]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galán-Díez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef]
- Lin, X.; Parker, L.; McLennan, E.; Hayes, A.; McConell, G.; Brennan-Speranza, T.C.; Levinger, I. Undercarboxylated Osteocalcin Improves Insulin-Stimulated Glucose Uptake in Muscles of Corticosterone-Treated Mice. J. Bone Miner. Res. 2019, 34, 1517–1530. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zugaza, J.L.; Torres Aleman, I. The Signaling Landscape of Insulin-like Growth Factor 1. J. Biol. Chem. 2025, 301, 108047. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, X.; Chen, X.; Wang, Z.; Zheng, S.; Cheng, Y.; Liu, S.; Hao, L. The Role of Insulin-like Growth Factor-1 in Bone Remodeling: A Review. Int. J. Biol. Macromol. 2023, 238, 124125. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT Signaling in Bone Homeostasis and Disease: From Human Mutations to Treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Fulzele, K.; Lai, F.; Dedic, C.; Saini, V.; Uda, Y.; Shi, C.; Tuck, P.; Aronson, J.L.; Liu, X.; Spatz, J.M.; et al. Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J. Bone Miner. Res. 2017, 32, 373–384. [Google Scholar] [CrossRef]
- Fairfield, H.; Falank, C.; Harris, E.; Demambro, V.; McDonald, M.; Pettitt, J.A.; Mohanty, S.T.; Croucher, P.; Kramer, I.; Kneissel, M.; et al. The Skeletal Cell-Derived Molecule Sclerostin Drives Bone Marrow Adipogenesis. J. Cell. Physiol. 2018, 233, 1156–1167. [Google Scholar] [CrossRef]
- Morse, L.R.; Sudhakar, S.; Lazzari, A.A.; Tun, C.; Garshick, E.; Zafonte, R.; Battaglino, R.A. Sclerostin: A Candidate Biomarker of SCI-Induced Osteoporosis. Osteoporos. Int. 2013, 24, 961–968. [Google Scholar] [CrossRef]
- Rudnicki, M.A.; Williams, B.O. Wnt Signaling in Bone and Muscle. Bone 2015, 80, 60–66. [Google Scholar] [CrossRef]
- Hesse, E.; Schröder, S.; Brandt, D.; Pamperin, J.; Saito, H.; Taipaleenmäki, H. Sclerostin Inhibition Alleviates Breast Cancer–Induced Bone Metastases and Muscle Weakness. JCI Insight 2019, 4, e125543. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Ho Lim, J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and Its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef] [PubMed]
- Giannesini, B.; Vilmen, C.; Amthor, H.; Bernard, M.; Bendahan, D. Lack of Myostatin Impairs Mechanical Performance and ATP Cost of Contraction in Exercising Mouse Gastrocnemius Muscle in Vivo. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yi, Q.; Sun, W.; Huang, D.; Zhang, H.; Duan, L.; Shang, H.; Wang, D.; Xiong, J. Molecular Basis and Therapeutic Potential of Myostatin on Bone Formation and Metabolism in Orthopedic Disease. BioFactors 2023, 49, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Peng, Y.; Zhao, W.; Pan, J.; Ksiezak-Reding, H.; Cardozo, C.; Wu, Y.; Pajevic, P.D.; Bonewald, L.F.; Bauman, W.A.; et al. Myostatin Inhibits Osteoblastic Differentiation by Suppressing Osteocyte-Derived Exosomal MicroRNA-218: A Novel Mechanism in Muscle-Bone Communication. J. Biol. Chem. 2017, 292, 11021. [Google Scholar] [CrossRef]
- Dankbar, B.; Fennen, M.; Brunert, D.; Hayer, S.; Frank, S.; Wehmeyer, C.; Beckmann, D.; Paruzel, P.; Bertrand, J.; Redlich, K.; et al. Myostatin Is a Direct Regulator of Osteoclast Differentiation and Its Inhibition Reduces Inflammatory Joint Destruction in Mice. Nat. Med. 2015, 21, 1085–1090. [Google Scholar] [CrossRef]
- Barclay, R.D.; Burd, N.A.; Tyler, C.; Tillin, N.A.; Mackenzie, R.W. The Role of the IGF-1 Signaling Cascade in Muscle Protein Synthesis and Anabolic Resistance in Aging Skeletal Muscle. Front. Nutr. 2019, 6, 462838. [Google Scholar] [CrossRef]
- Sondag, G.R.; Mbimba, T.S.; Moussa, F.M.; Novak, K.; Yu, B.; Jaber, F.A.; Abdelmagid, S.M.; Geldenhuys, W.J.; Safadi, F.F. Osteoactivin Inhibition of Osteoclastogenesis Is Mediated through CD44-ERK Signaling. Exp. Mol. Med. 2016, 48, e257. [Google Scholar] [CrossRef]
- Nikawa, T.; Ishidoh, K.; Hirasaka, K.; Ishihara, I.; Ikemoto, M.; Kano, M.; Kominami, E.; Nonaka, I.; Ogawa, T.; Adams, G.R.; et al. Skeletal Muscle Gene Expression in Space-Flown Rats. FASEB J. 2004, 18, 522–524. [Google Scholar] [CrossRef]
- Furochi, H.; Tamura, S.; Takeshima, K.; Hirasaka, K.; Nakao, R.; Kishi, K.; Nikawa, T. Overexpression of Osteoactivin Protects Skeletal Muscle from Severe Degeneration Caused by Long-Term Denervation in Mice. J. Med. Investig. 2007, 54, 248–254. [Google Scholar] [CrossRef]
- Miyazaki, T.; Miyauchi, S.; Anada, T.; Tawada, A.; Suzuki, O. Chondroitin Sulfate-E Binds to Both Osteoactivin and Integrin AVβ3 and Inhibits Osteoclast Differentiation. J. Cell. Biochem. 2015, 116, 2247–2257. [Google Scholar] [CrossRef]
- Sheng, M.H.-C.; Wergedal, J.E.; Mohan, S.; Lau, K.-H.W. Osteoactivin Is a Novel Osteoclastic Protein and Plays a Key Role in Osteoclast Differentiation and Activity. FEBS Lett. 2008, 582, 1451–1458. [Google Scholar] [CrossRef]
- Abdelmagid, S.M.; Belcher, J.Y.; Moussa, F.M.; Lababidi, S.L.; Sondag, G.R.; Novak, K.M.; Sanyurah, A.S.; Frara, N.A.; Razmpour, R.; Del Carpio-Cano, F.E.; et al. Mutation in Osteoactivin Decreases Bone Formation in Vivo and Osteoblast Differentiation in Vitro. Am. J. Pathol. 2014, 184, 697–713. [Google Scholar] [CrossRef]
- Hamrick, M.W. The Skeletal Muscle Secretome: An Emerging Player in Muscle–Bone Crosstalk. Bonekey Rep. 2012, 1, 60. [Google Scholar] [CrossRef]
- Wueest, S.; Konrad, D. The Controversial Role of IL-6 in Adipose Tissue on Obesity-Induced Dysregulation of Glucose Metabolism. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E607–E613. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Cánoves, P.; Scheele, C.; Pedersen, B.K.; Serrano, A.L. Interleukin-6 Myokine Signaling in Skeletal Muscle: A Double-Edged Sword? FEBS J. 2013, 280, 4131–4148. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, F.; Duplomb, L.; Baud’huin, M.; Brounais, B. The Dual Role of IL-6-Type Cytokines on Bone Remodeling and Bone Tumors. Cytokine Growth Factor Rev. 2009, 20, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A. Influences of the IL-6 Cytokine Family on Bone Structure and Function. Cytokine 2021, 146, 155655. [Google Scholar] [CrossRef]
- Haugen, F.; Norheim, F.; Lian, H.; Wensaas, A.J.; Dueland, S.; Berg, O.; Funderud, A.; Skålhegg, B.S.; Raastad, T.; Drevon, C.A. IL-7 Is Expressed and Secreted by Human Skeletal Muscle Cells. Am. J. Physiol. Cell. Physiol. 2010, 298, C807–C816. [Google Scholar] [CrossRef]
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal Muscle as Potential Central Link between Sarcopenia and Immune Senescence. EBioMedicine 2019, 49, 381–388. [Google Scholar] [CrossRef]
- Toraldo, G.; Roggia, C.; Qian, W.P.; Pacific, R.; Weitzmann, M.N. IL-7 Induces Bone Loss in Vivo by Induction of Receptor Activator of Nuclear Factor ΚB Ligand and Tumor Necrosis Factor α from T Cells. Proc. Natl. Acad. Sci. USA 2003, 100, 125–130. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Roggia, C.; Toraldo, G.; Weitzmann, L.; Pacifici, R. Increased Production of IL-7 Uncouples Bone Formation from Bone Resorption during Estrogen Deficiency. J. Clin. Investig. 2002, 110, 1643–1650. [Google Scholar] [CrossRef]
- Mishra, A.; Sullivan, L.; Caligiuri, M.A. Molecular Pathways: Interleukin-15 Signaling in Health and in Cancer. Clin. Cancer Res. 2014, 20, 2044–2050. [Google Scholar] [CrossRef]
- Nadeau, L.; Aguer, C. Interleukin-15 as a Myokine: Mechanistic Insight into Its Effect on Skeletal Muscle Metabolism. Appl. Physiol. Nutr. Metab. 2018, 44, 229–238. [Google Scholar] [CrossRef]
- Khalafi, M.; Maleki, A.H.; Symonds, M.E.; Sakhaei, M.H.; Rosenkranz, S.K.; Ehsanifar, M.; Korivi, M.; Liu, Y. Interleukin-15 Responses to Acute and Chronic Exercise in Adults: A Systematic Review and Meta-Analysis. Front. Immunol. 2023, 14, 1288537. [Google Scholar] [CrossRef]
- Duan, Z.; Yang, Y.; Qin, M.; Yi, X. Interleukin 15: A New Intermediary in the Effects of Exercise and Training on Skeletal Muscle and Bone Function. J. Cell. Mol. Med. 2024, 28, e70136. [Google Scholar] [CrossRef]
- Mahgoub, M.O.; D’Souza, C.; Al Darmaki, R.S.M.H.; Baniyas, M.M.Y.H.; Adeghate, E. An Update on the Role of Irisin in the Regulation of Endocrine and Metabolic Functions. Peptides 2018, 104, 15–23. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the Metabolic Effects of Irisin on Skeletal Muscle in Vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Becerril, S.; Méndez-Giménez, L.; Ramírez, B.; Sáinz, N.; Catalán, V.; Gómez-Ambrosi, J.; Frühbeck, G. Leptin Administration Activates Irisin-Induced Myogenesis via Nitric Oxide-Dependent Mechanisms, but Reduces Its Effect on Subcutaneous Fat Browning in Mice. Int. J. Obes. 2014, 39, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin Prevents and Restores Bone Loss and Muscle Atrophy in Hind-Limb Suspended Mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.Y.; Nie, Y.; Ma, Y.X.; Chen, Y.; Cheng, R.; Yin, W.Y.; Hu, Y.; Xu, W.M.; Xu, L.Z. Irisin Promotes Osteoblast Proliferation and Differentiation via Activating the MAP Kinase Signaling Pathways. Sci. Rep. 2016, 6, 18732. [Google Scholar] [CrossRef]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Comite, M.D.; Mori, G.; et al. The Myokine Irisin Increases Cortical Bone Mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Hu, S.; Chen, C.; He, J.; Sun, J.; Jin, Y.; Zhang, Y.; Zhu, G.; Shi, Q.; Rui, Y. Myokine Irisin Promotes Osteogenesis by Activating BMP/SMAD Signaling via AV Integrin and Regulates Bone Mass in Mice. Int. J. Biol. Sci. 2022, 18, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, K.; Zhao, S.; Geng, T.; Fan, X.; Sun, S.; Zheng, M.; Jin, Q. Irisin Promotes Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells by Activating Autophagy via the Wnt//β-Catenin Signal Pathway. Cytokine 2020, 136, 155292. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wu, H.; Lu, J.; Zhang, R.; Shen, X.; Gu, Y.; Shi, C.; Zhang, Y.; Yuan, W. Irisin Reduces Bone Fracture by Facilitating Osteogenesis and Antagonizing TGF-β/Smad Signaling in a Growing Mouse Model of Osteogenesis Imperfecta. J. Orthop. Transl. 2023, 38, 175–189. [Google Scholar] [CrossRef]
- Floege, J.; Hudkins, K.L.; Eitner, F.; Cui, Y.; Morrison, R.S.; Schelling, M.A.; Alpers, C.E. Localization of Fibroblast Growth Factor-2 (Basic FGF) and FGF Receptor-1 in Adult Human Kidney. Kidney Int. 1999, 56, 883–897. [Google Scholar] [CrossRef]
- Mathes, S.; Fahrner, A.; Ghoshdastider, U.; Rüdiger, H.A.; Leunig, M.; Wolfrum, C.; Krützfeldt, J. FGF-2–Dependent Signaling Activated in Aged Human Skeletal Muscle Promotes Intramuscular Adipogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2021013118. [Google Scholar] [CrossRef]
- Yang, W.; Cao, Y.; Zhang, Z.; Du, F.; Shi, Y.; Li, X.; Zhang, Q. Targeted Delivery of FGF2 to Subchondral Bone Enhanced the Repair of Articular Cartilage Defect. Acta Biomater. 2018, 69, 170–182. [Google Scholar] [CrossRef]
- Poudel, S.B.; Min, C.K.; Lee, J.H.; Shin, Y.J.; Kwon, T.H.; Jeon, Y.M.; Lee, J.C. Local Supplementation with Plant-Derived Recombinant Human FGF2 Protein Enhances Bone Formation in Critical-Sized Calvarial Defects. J. Bone Miner. Metab. 2019, 37, 900–912. [Google Scholar] [CrossRef]
- Huang, G.; Zhao, G.; Xia, J.; Wei, Y.; Chen, F.; Chen, J.; Shi, J. FGF2 and FAM201A Affect the Development of Osteonecrosis of the Femoral Head after Femoral Neck Fracture. Gene 2018, 652, 39–47. [Google Scholar] [CrossRef]
- Qian, X.; Zhang, C.; Chen, G.; Tang, Z.; Liu, Q.; Chen, J.; Tong, X.; Wang, J. Effects of BMP-2 and FGF2 on the Osteogenesis of Bone Marrow-Derived Mesenchymal Stem Cells in Hindlimb-Unloaded Rats. Cell Biochem. Biophys. 2014, 70, 1127–1136. [Google Scholar] [CrossRef]
- Nishizawa, H.; Matsuda, M.; Yamada, Y.; Kawai, K.; Suzuki, E.; Makishima, M.; Kitamura, T.; Shimomura, I. Musclin, a Novel Skeletal Muscle-Derived Secretory Factor. J. Biol. Chem. 2004, 279, 19391–19395. [Google Scholar] [CrossRef] [PubMed]
- Szaroszyk, M.; Kattih, B.; Martin-Garrido, A.; Trogisch, F.A.; Dittrich, G.M.; Grund, A.; Abouissa, A.; Derlin, K.; Meier, M.; Holler, T.; et al. Skeletal Muscle Derived Musclin Protects the Heart during Pathological Overload. Nat. Commun. 2022, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Subbotina, E.; Sierra, A.; Zhu, Z.; Gao, Z.; Koganti, S.R.K.; Reyes, S.; Stepniak, E.; Walsh, S.A.; Acevedo, M.R.; Perez-Terzic, C.M.; et al. Musclin Is an Activity-Stimulated Myokine That Enhances Physical Endurance. Proc. Natl. Acad. Sci. USA 2015, 112, 16042–16047. [Google Scholar] [CrossRef]
- Yasui, A.; Nishizawa, H.; Okuno, Y.; Morita, K.; Kobayashi, H.; Kawai, K.; Matsuda, M.; Kishida, K.; Kihara, S.; Kamei, Y.; et al. Foxo1 Represses Expression of Musclin, a Skeletal Muscle-Derived Secretory Factor. Biochem. Biophys. Res. Commun. 2007, 364, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Bartell, S.M.; Kim, H.N.; Ambrogini, E.; Han, L.; Iyer, S.; Ucer, S.S.; Rabinovitch, P.; Jilka, R.L.; Weinstein, R.S.; Zhao, H.; et al. FoxO Proteins Restrain Osteoclastogenesis and Bone Resorption by Attenuating H2O2 Accumulation. Nat. Commun. 2014, 5, 3773. [Google Scholar] [CrossRef]
- Carlson, B. Mature Skeletal Muscle—An Overview. In Muscle Biology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–33. [Google Scholar] [CrossRef]
- Adrian, E.D. The All-or-none Principle in Nerve. J. Physiol. 1914, 47, 460–474. [Google Scholar] [CrossRef]
- Drukarch, B.; Holland, H.A.; Velichkov, M.; Geurts, J.J.G.; Voorn, P.; Glas, G.; de Regt, H.W. Thinking about the Nerve Impulse: A Critical Analysis of the Electricity-Centered Conception of Nerve Excitability. Prog. Neurobiol. 2018, 169, 172–185. [Google Scholar] [CrossRef]
- Rudolf, R.; Khan, M.M.; Witzemann, V. Motor Endplate—Anatomical, Functional, and Molecular Concepts in the Historical Perspective. Cells 2019, 8, 387. [Google Scholar] [CrossRef]
- Bock, O. Cajal, Golgi, Nansen, Schäfer and the Neuron Doctrine. Endeavour 2013, 37, 228–234. [Google Scholar] [CrossRef]
- Andersson-Cedergren, E. Ultrastructure of Motor End Plate and Sarcoplasmic Components of Mouse Skeletal Muscle Fiber as Revealed by Three-Dimensional Reconstructions from Serial Sections. J. Ultrastruct. Res. 1959, 2, 5–191. [Google Scholar] [CrossRef]
- Hirsch, N.P. Neuromuscular Junction in Health and Disease. Br. J. Anaesth. 2007, 99, 132–138. [Google Scholar] [CrossRef]
- Slater, C.R. The Structure of Human Neuromuscular Junctions: Some Unanswered Molecular Questions. Int. J. Mol. Sci. 2017, 18, 2183. [Google Scholar] [CrossRef]
- Ratliff, W.A.; Saykally, J.N.; Kane, M.J.; Citron, B.A. Neuromuscular Junction Morphology and Gene Dysregulation in the Wobbler Model of Spinal Neurodegeneration. J. Mol. Neurosci. 2018, 66, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Tsim, K.W.K.; Ruegg, M.A.; Escher, G.; Kröger, S.; McMahan, U.J. CDNA That Encodes Active Agrin. Neuron 1992, 8, 677–689. [Google Scholar] [CrossRef] [PubMed]
- DeChiara, T.M.; Bowen, D.C.; Valenzuela, D.M.; Simmons, M.V.; Poueymirou, W.T.; Thomas, S.; Kinetz, E.; Compton, D.L.; Rojas, E.; Park, J.S.; et al. The Receptor Tyrosine Kinase MuSK Is Required for Neuromuscular Junction Formation In Vivo. Cell 1996, 85, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Porter, S.; Froehner, S.C. Characterization and Localization of the Mr = 43,000 Proteins Associated with Acetylcholine Receptor-Rich Membranes. J. Biol. Chem. 1983, 258, 10034–10040. [Google Scholar] [CrossRef]
- Weatherbee, S.D.; Anderson, K.V.; Niswander, L.A. LDL-Receptor-Related Protein 4 Is Crucial for Formation of the Neuromuscular Junction. Development 2006, 133, 4993–5000. [Google Scholar] [CrossRef]
- Frail, D.E.; McLaughlin, L.L.; Mudd, J.; Merlie, J.P. Identification of the Mouse Muscle 43,000-Dalton Acetylcholine Receptor-Associated Protein (RAPsyn) by CDNA Cloning. J. Biol. Chem. 1988, 263, 15602–15607. [Google Scholar] [CrossRef]
- Belotti, E.; Schaeffer, L. Regulation of Gene Expression at the Neuromuscular Junction. Neurosci. Lett. 2020, 735, 135163. [Google Scholar] [CrossRef]
- Lømo, T. Chapter 4 Nerve–Muscle Interactions. In Handbook of Clinical Neurophysiology; IFCN: Milwaukee, WI, USA, 2003; Volume 2, pp. 47–65. [Google Scholar] [CrossRef]
- Tøien, T.; Nielsen, J.L.; Berg, O.K.; Brobakken, M.F.; Nyberg, S.K.; Espedal, L.; Malmo, T.; Frandsen, U.; Aagaard, P.; Wang, E. The Impact of Life-Long Strength versus Endurance Training on Muscle Fiber Morphology and Phenotype Composition in Older Men. J. Appl. Physiol. 2023, 135, 1360–1371. [Google Scholar] [CrossRef]
- Mège, R.M.; Goudou, D.; Giaume, C.; Nicolet, M.; Rieger, F. Is Intercellular Communication Via Gap Junctions Required for Myoblast Fusion? Cell Adhes. Commun. 1994, 2, 329–343. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Molecular Diversity of Myofibrillar Proteins: Gene Regulation and Functional Significance. Physiol. Rev. 1996, 76, 371–423. [Google Scholar] [CrossRef]
- Buller, A.J.; Eccles, J.C.; Eccles, R.M. Differentiation of Fast and Slow Muscles in the Cat Hind Limb. J. Physiol. 1960, 150, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Niven-Jenkins, N.; Vrbová, G. Observations on Neuromuscular Connection between the Vagus Nerve and Skeletal Muscle. Neuroscience 1980, 5, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jiang, J.; Xu, J. Denervation-Related Neuromuscular Junction Changes: From Degeneration to Regeneration. Front. Mol. Neurosci. 2022, 14, 810919. [Google Scholar] [CrossRef]
- Coletti, C.; Acosta, G.F.; Keslacy, S.; Coletti, D. Exercise-Mediated Reinnervation of Skeletal Muscle in Elderly People: An Update. Eur. J. Transl. Myol. 2022, 32, 2022. [Google Scholar] [CrossRef]
- Anagnostou, M.-E.; Hepple, R.T. Mitochondrial Mechanisms of Neuromuscular Junction Degeneration with Aging. Cells 2020, 9, 197. [Google Scholar] [CrossRef]
- Ueta, R.; Sugita, S.; Minegishi, Y.; Shimotoyodome, A.; Ota, N.; Ogiso, N.; Eguchi, T.; Yamanashi, Y. DOK7 Gene Therapy Enhances Neuromuscular Junction Innervation and Motor Function in Aged Mice. iScience 2020, 23, 101385. [Google Scholar] [CrossRef]
- Kedlian, V.R.; Wang, Y.; Liu, T.; Chen, X.; Bolt, L.; Tudor, C.; Shen, Z.; Fasouli, E.S.; Prigmore, E.; Kleshchevnikov, V.; et al. Human Skeletal Muscle Aging Atlas. Nat. Aging 2024, 4, 727–744. [Google Scholar] [CrossRef]
- Blau, H.M.; Cosgrove, B.D.; Ho, A.T.V. The Central Role of Muscle Stem Cells in Regenerative Failure with Aging. Nat. Med. 2015, 21, 854–862. [Google Scholar] [CrossRef]
- Muñoz-Cánoves, P.; Neves, J.; Sousa-Victor, P. Understanding Muscle Regenerative Decline with Aging: New Approaches to Bring Back Youthfulness to Aged Stem Cells. FEBS J. 2020, 287, 406–416. [Google Scholar] [CrossRef]
- Rentería, I.; García-Suárez, P.C.; Fry, A.C.; Moncada-Jiménez, J.; Machado-Parra, J.P.; Antunes, B.M.; Jiménez-Maldonado, A. The Molecular Effects of BDNF Synthesis on Skeletal Muscle: A Mini-Review. Front. Physiol. 2022, 13, 934714. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Pedersen, M.; Krabbe, K.S.; Bruunsgaard, H.; Matthews, V.B.; Febbraio, M.A. Role of Exercise-Induced Brain-Derived Neurotrophic Factor Production in the Regulation of Energy Homeostasis in Mammals. Exp. Physiol. 2009, 94, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2017, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and Molecular Mechanisms Regulating Neuronal Growth by Brain-Derived Neurotrophic Factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Wang, H.; Wang, J.H.; Song, F.; Sun, Y. Irisin Exerts Neuroprotective Effects on Cultured Neurons by Regulating Astrocytes. Mediat. Inflamm. 2018, 2018, 9070341. [Google Scholar] [CrossRef]
- Hashemi, M.S.; Ghaedi, K.; Salamian, A.; Karbalaie, K.; Emadi-Baygi, M.; Tanhaei, S.; Nasr-Esfahani, M.H.; Baharvand, H. Fndc5 Knockdown Significantly Decreased Neural Differentiation Rate of Mouse Embryonic Stem Cells. Neuroscience 2013, 231, 296–304. [Google Scholar] [CrossRef]
- Sanes, J.R.; Marshall, L.M.; McMahan, U.J. Reinnervation of Muscle Fiber Basal Lamina after Removal of Myofibers. Differentiation of Regenerating Axons at Original Synaptic Sites. J. Cell Biol. 1978, 78, 176–198. [Google Scholar] [CrossRef]
- Sanes, J.R.; Lichtman, J.W. Development of the Vertebrate Neuromuscular Junction. Annu. Rev. Neurosci. 1999, 22, 389–442. [Google Scholar] [CrossRef]
- Fox, M.A.; Sanes, J.R.; Borza, D.B.; Eswarakumar, V.P.; Fässler, R.; Hudson, B.G.; John, S.W.M.; Ninomiya, Y.; Pedchenko, V.; Pfaff, S.L.; et al. Distinct Target-Derived Signals Organize Formation, Maturation, and Maintenance of Motor Nerve Terminals. Cell 2007, 129, 179–193. [Google Scholar] [CrossRef]
- Williams, A.H.; Valdez, G.; Moresi, V.; Qi, X.; McAnally, J.; Elliott, J.L.; Bassel-Duby, R.; Sanes, J.R.; Olson, E.N. MicroRNA-206 Delays ALS Progression and Promotes Regeneration of Neuromuscular Synapses in Mice. Science 2009, 326, 1549–1554. [Google Scholar] [CrossRef]
- Liu, W.; Wei-LaPierre, L.; Klose, A.; Dirksen, R.T.; Chakkalakal, J.V. Inducible Depletion of Adult Skeletal Muscle Stem Cells Impairs the Regeneration of Neuromuscular Junctions. eLife 2015, 4, e09221. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garber, A.J. Effects of Parathyroid Hormone on Skeletal Muscle Protein and Amino Acid Metabolism in the Rat. J. Clin. Investig. 1983, 71, 1806–1821. [Google Scholar] [CrossRef]
- Baczynski, R.; Massry, S.G.; Magott, M.; el-Belbessi, S.; Kohan, R.; Brautbar, N. Effect of Parathyroid Hormone on Energy Metabolism Ofskeletal Muscle. Kidney Int. 1985, 28, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Iio, R.; Manaka, T.; Takada, N.; Orita, K.; Nakazawa, K.; Hirakawa, Y.; Ito, Y.; Nakamura, H. Parathyroid Hormone Inhibits Fatty Infiltration and Muscle Atrophy After Rotator Cuff Tear by Browning of Fibroadipogenic Progenitors in a Rodent Model. Am. J. Sports Med. 2023, 51, 3251–3260. [Google Scholar] [CrossRef]
- Romagnoli, C.; Zonefrati, R.; Lucattelli, E.; Innocenti, M.; Civinini, R.; Iantomasi, T.; Brandi, M.L. In Vitro Effects of PTH (1-84) on Human Skeletal Muscle-Derived Satellite Cells. Biomedicines 2023, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Fujimaki, T.; Ando, T.; Hata, T.; Takayama, Y.; Ohba, T.; Ichikawa, J.; Takiyama, Y.; Tatsuno, R.; Koyama, K.; Haro, H. Exogenous Parathyroid Hormone Attenuates Ovariectomy-Induced Skeletal Muscle Weakness in Vivo. Bone 2021, 151, 116029. [Google Scholar] [CrossRef]
- Palermo, A.; Sanesi, L.; Colaianni, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Pedone, C.; Lelli, D.; Brunetti, G.; Mori, G.; et al. A Novel Interplay Between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3088–3096. [Google Scholar] [CrossRef]
- Abboud, M.; Rybchyn, M.S.; Liu, J.; Ning, Y.; Gordon-Thomson, C.; Brennan-Speranza, T.C.; Cole, L.; Greenfield, H.; Fraser, D.R.; Mason, R.S. The Effect of Parathyroid Hormone on the Uptake and Retention of 25-Hydroxyvitamin D in Skeletal Muscle Cells. J. Steroid Biochem. Mol. Biol. 2017, 173, 173–179. [Google Scholar] [CrossRef]
- Sato, C.; Miyakoshi, N.; Kasukawa, Y.; Nozaka, K.; Tsuchie, H.; Nagahata, I.; Yuasa, Y.; Abe, K.; Saito, H.; Shoji, R.; et al. Teriparatide and Exercise Improve Bone, Skeletal Muscle, and Fat Parameters in Ovariectomized and Tail-Suspended Rats. J. Bone Miner. Metab. 2021, 39, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Grynpas, M.; Mitchell, J. Intermittent PTH Treatment Improves Bone and Muscle in Glucocorticoid Treated Mdx Mice: A Model of Duchenne Muscular Dystrophy. Bone 2019, 121, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Brent, M.B.; Brüel, A.; Thomsen, J.S. PTH (1–34) and Growth Hormone in Prevention of Disuse Osteopenia and Sarcopenia in Rats. Bone 2018, 110, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Komrakova, M.; Stuermer, E.K.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Daub, F.; Martens, T.; Witzenhausen, P.; et al. Effect of Human Parathyroid Hormone HPTH (1–34) Applied at Different Regimes on Fracture Healing and Muscle in Ovariectomized and Healthy Rats. Bone 2010, 47, 480–492. [Google Scholar] [CrossRef]
- Visser, M.; Deeg, D.J.H.; Lips, P. Low Vitamin D and High Parathyroid Hormone Levels as Determinants of Loss of Muscle Strength and Muscle Mass (Sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef]
- Sikjaer, T.; Rolighed, L.; Hess, A.; Fuglsang-Frederiksen, A.; Mosekilde, L.; Rejnmark, L. Effects of PTH(1-84) Therapy on Muscle Function and Quality of Life in Hypoparathyroidism: Results from a Randomized Controlled Trial. Osteoporos. Int. 2014, 25, 1717–1726. [Google Scholar] [CrossRef]
- Gielen, E.; O’neill, T.W.; Pye, S.R.; Adams, J.E.; Wu, F.C.; Laurent, M.R.; Claessens, F.; Ward, K.A.; Boonen, S.; Bouillon, R.; et al. Endocrine Determinants of Incident Sarcopenia in Middle-Aged and Elderly European Men. J. Cachexia Sarcopenia Muscle 2015, 6, 242–252. [Google Scholar] [CrossRef]
- Umakanthan, M.; Li, J.W.; Sud, K.; Duque, G.; Guilfoyle, D.; Cho, K.; Brown, C.; Boersma, D.; Komala, M.G. Prevalence and Factors Associated with Sarcopenia in Patients on Maintenance Dialysis in Australia—A Single Centre, Cross-Sectional Study. Nutrients 2021, 13, 3284. [Google Scholar] [CrossRef]
- Iolascon, G.; Paoletta, M.; Liguori, S.; Curci, C.; Moretti, A. Neuromuscular Diseases and Bone. Front. Endocrinol. 2019, 10, 497297. [Google Scholar] [CrossRef]
- Khan, A.; Bilezikian, J. Primary Hyperparathyroidism: Pathophysiology and Impact on Bone. CMAJ Can. Med. Assoc. J. 2000, 163, 184. [Google Scholar]
- Rolighed, L.; Amstrup, A.K.; Jakobsen, N.F.B.; Sikjaer, T.; Mosekilde, L.; Christiansen, P.; Rejnmark, L. Muscle Function Is Impaired in Patients With “Asymptomatic” Primary Hyperparathyroidism. World J. Surg. 2014, 38, 549–557. [Google Scholar] [CrossRef]
- Diniz, E.T.; Bandeira, F.; Lins, O.G.; Cavalcanti, É.N.B.; De Arruda, T.M.; Januário, A.M.S.; Diniz, K.T.; Marques, T.F.; Azevedo, H. Primary Hyperparathyroidism Is Associated With Subclinical Peripheral Neural Alterations. Endocr. Pract. 2013, 19, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kuro-o, M. Klotho as a Regulator of Fibroblast Growth Factor Signaling and Phosphate/Calcium Metabolism. Curr. Opin. Nephrol. Hypertens. 2006, 15, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Naveh-Many, T. Phosphate and the Parathyroid. Kidney Int. 2009, 75, 898–905. [Google Scholar] [CrossRef]
- Lavi-Moshayoff, V.; Wasserman, G.; Meir, T.; Silver, J.; Naveh-Many, T. PTH Increases FGF23 Gene Expression and Mediates the High-FGF23 Levels of Experimental Kidney Failure: A Bone Parathyroid Feedback Loop. Am. J. Physiol. Renal. Physiol. 2010, 299, 882–889. [Google Scholar] [CrossRef]
- Bakker, A.D.; Joldersma, M.; Klein-Nulend, J.; Burger, E.H. Interactive Effects of PTH and Mechanical Stress on Nitric Oxide and PGE2 Production by Primary Mouse Osteoblastic Cells. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E608–E613. [Google Scholar] [CrossRef]
- Raisz, L.G.; Simmons, H.A. Effects of Parathyroid Hormone and Cortisol on Prostaglandin Production by Neonatal Rat Calvaria in Vitro. Endocr. Res. 1985, 11, 59–74. [Google Scholar] [CrossRef]
- Coetzee, M.; Haag, M.; Claassen, N.; Kruger, M.C. Stimulation of Prostaglandin E2 (PGE2) Production by Arachidonic Acid, Oestrogen and Parathyroid Hormone in MG-63 and MC3T3-E1 Osteoblast-like Cells. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 423–430. [Google Scholar] [CrossRef]
- Yu, X.P.; Chandrasekhar, S. Parathyroid Hormone (PTH 1–34) Regulation of Rat Osteocalcin Gene Transcription. Endocrinology 1997, 138, 3085–3092. [Google Scholar] [CrossRef]
- Boguslawski, G.; Hale, L.V.; Yu, X.P.; Miles, R.R.; Onyia, J.E.; Santerre, R.F.; Chandrasekhar, S. Activation of Osteocalcin Transcription Involves Interaction of Protein Kinase A- and Protein Kinase C-Dependent Pathways. J. Biol. Chem. 2000, 275, 999–1006. [Google Scholar] [CrossRef]
- Jiang, D.; Franceschi, R.T.; Boules, H.; Xiao, G. Parathyroid Hormone Induction of the Osteocalcin Gene: Requirement for an Osteoblast-Specific Element 1 Sequence in the Promoter and Involvement of Multiple Signaling Pathways. J. Biol. Chem. 2004, 279, 5329–5337. [Google Scholar] [CrossRef] [PubMed]
- Canalis, E.; Centrella, M.; Burch, W.; McCarthy, T.L. Insulin-like Growth Factor I Mediates Selective Anabolic Effects of Parathyroid Hormone in Bone Cultures. J. Clin. Investig. 1989, 83, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Linkhart, T.A.; Mohan, S. Parathyroid Hormone Stimulates Release of Insulin-Like Growth Factor-I (IGF-I) and IGF-II from Neonatal Mouse Calvaria in Organ Culture. Endocrinology 1989, 125, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, T.L.; Centrella, M.; Canalis, E. Regulatory Effects of Insulin-Like Growth Factors I and II on Bone Collagen Synthesis in Rat Calvarial Cultures. Endocrinology 1989, 124, 301–309. [Google Scholar] [CrossRef]
- Harvey, A.K.; Yu, X.P.; Frolik, C.A.; Chandrasekhar, S. Parathyroid Hormone-(1–34) Enhances Aggrecan Synthesis via an Insulin-like Growth Factor-I Pathway. J. Biol. Chem. 1999, 274, 23249–23255. [Google Scholar] [CrossRef]
- Miyakoshi, N.; Kasukawa, Y.; Linkhart, T.A.; Baylink, D.J.; Mohan, S. Evidence That Anabolic Effects of PTH on Bone Require IGF-I in Growing Mice. Endocrinology 2001, 142, 4349–4356. [Google Scholar] [CrossRef]
- Bikle, D.D.; Sakata, T.; Leary, C.; Elalieh, H.; Ginzinger, D.; Rosen, C.J.; Beamer, W.; Majumdar, S.; Halloran, B.P. Insulin-Like Growth Factor I Is Required for the Anabolic Actions of Parathyroid Hormone on Mouse Bone. J. Bone Miner. Res. 2002, 17, 1570–1578. [Google Scholar] [CrossRef]
- Wang, Y.; Nishida, S.; Boudignon, B.M.; Burghardt, A.; Elalieh, H.Z.; Hamilton, M.M.; Majumdar, S.; Halloran, B.P.; Clemens, T.L.; Bikle, D.D. IGF-I Receptor Is Required for the Anabolic Actions of Parathyroid Hormone on Bone. J. Bone Miner. Res. 2007, 22, 1329–1337. [Google Scholar] [CrossRef]
- Wang, Y.; Menendez, A.; Fong, C.; Elalieh, H.Z.; Chang, W.; Bikle, D.D. Ephrin B2/EphB4 Mediates the Actions of IGF-I Signaling in Regulating Endochondral Bone Formation. J. Bone Miner. Res. 2014, 29, 1900–1913. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Z.; Elalieh, H.Z.; Nakamura, E.; Nguyen, M.T.; MacKem, S.; Clemens, T.L.; Bikle, D.D.; Chang, W. IGF-1R Signaling in Chondrocytes Modulates Growth Plate Development by Interacting with the PTHrP/Ihh Pathway. J. Bone Miner. Res. 2011, 26, 1437–1446. [Google Scholar] [CrossRef]
- Keller, H.; Kneissel, M. SOST Is a Target Gene for PTH in Bone. Bone 2005, 37, 148–158. [Google Scholar] [CrossRef]
- Bellido, T.; Ali, A.A.; Gubrij, I.; Plotkin, L.I.; Fu, Q.; O’Brien, C.A.; Manolagas, S.C.; Jilka, R.L. Chronic Elevation of Parathyroid Hormone in Mice Reduces Expression of Sclerostin by Osteocytes: A Novel Mechanism for Hormonal Control of Osteoblastogenesis. Endocrinology 2005, 146, 4577–4583. [Google Scholar] [CrossRef]
- Drake, M.T.; Srinivasan, B.; Mödder, U.I.; Peterson, J.M.; McCready, L.K.; Riggs, B.L.; Dwyer, D.; Stolina, M.; Kostenuik, P.; Khosla, S. Effects of Parathyroid Hormone Treatment on Circulating Sclerostin Levels in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2010, 95, 5056–5062. [Google Scholar] [CrossRef]
- Masiukiewicz, U.S.; Mitnick, M.; Grey, A.B.; Insogna, K.L. Estrogen Modulates Parathyroid Hormone-Induced Interleukin-6 Production in Vivo and in Vitro. Endocrinology 2000, 141, 2526–2531. [Google Scholar] [CrossRef] [PubMed]
- Grey, A.; Mitnick, M.A.; Masiukiewicz, U.; Sun, B.H.; Rudikoff, S.; Jilka, R.L.; Manolagas, S.C.; Insogna, K. A Role for Interleukin-6 in Parathyroid Hormone-Induced Bone Resorption in Vivo. Endocrinology 1999, 140, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Safley, S.A.; Villinger, F.; Jackson, E.H.; Tucker-Burden, C.; Cohen, C.; Weber, C.J. Interleukin-6 Production and Secretion by Human Parathyroids. Clin. Exp. Immunol. 2004, 136, 145. [Google Scholar] [CrossRef]
- Szulc, P.; Schoppet, M.; Goettsch, C.; Rauner, M.; Dschietzig, T.; Chapurlat, R.; Hofbauer, L.C. Endocrine and Clinical Correlates of Myostatin Serum Concentration in Men—The STRAMBO Study. J. Clin. Endocrinol. Metab. 2012, 97, 3700–3708. [Google Scholar] [CrossRef] [PubMed]
- De Martino, V.; Pepe, J.; Biamonte, F.; Colangelo, L.; Di Giuseppe, L.; Nieddu, L.; Occhiuto, M.; Minisola, S.; Cipriani, C. Impairment in Muscle Strength and Its Determinants in Primary Hyperparathyroidism: A Study in Postmenopausal Women. Bone 2023, 166, 116604. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid Hormone: Anabolic and Catabolic Actions on the Skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Chang, E.; Donkin, S.S.; Teegarden, D. Parathyroid Hormone Suppresses Insulin Signaling in Adipocytes. Mol. Cell. Endocrinol. 2009, 307, 77–82. [Google Scholar] [CrossRef]
- Hurley, M.M.; Okada, Y.; Xiao, L.; Tanaka, Y.; Ito, M.; Okimoto, N.; Nakamura, T.; Rosen, C.J.; Doetschman, T.; Coffin, J.D. Impaired Bone Anabolic Response to Parathyroid Hormone in Fgf2−/− and Fgf2+/− Mice. Biochem. Biophys. Res. Commun. 2006, 341, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Sabbieti, M.G.; Agas, D.; Xiao, L.; Marchetti, L.; Coffin, J.D.; Doetschman, T.; Hurley, M.M. Endogenous FGF-2 Is Critically Important in PTH Anabolic Effects on Bone. J. Cell. Physiol. 2009, 219, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.M.; Tetradis, S.; Huang, Y.F.; Hock, J.; Kream, B.E.; Raisz, L.G.; Sabbieti, M.G. Parathyroid Hormone Regulates the Expression of Fibroblast Growth Factor-2 MRNA and Fibroblast Growth Factor Receptor MRNA in Osteoblastic Cells. J. Bone Miner. Res. 1999, 14, 776–783. [Google Scholar] [CrossRef]
- Okada, Y.; Montero, A.; Zhang, X.; Sobue, T.; Lorenzo, J.; Doetschman, T.; Coffin, J.D.; Hurley, M.M. Impaired Osteoclast Formation in Bone Marrow Cultures of Fgf2 Null Mice in Response to Parathyroid Hormone. J. Biol. Chem. 2003, 278, 21258–21266. [Google Scholar] [CrossRef] [PubMed]
- Slavin, B.R.; Sarhane, K.A.; von Guionneau, N.; Hanwright, P.J.; Qiu, C.; Mao, H.Q.; Höke, A.; Tuffaha, S.H. Insulin-Like Growth Factor-1: A Promising Therapeutic Target for Peripheral Nerve Injury. Front. Bioeng. Biotechnol. 2021, 9, 695850. [Google Scholar] [CrossRef]
- McGregor, C.E.; English, A.W. The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met. Front. Cell. Neurosci. 2019, 12, 427102. [Google Scholar] [CrossRef]
- Cintron-Colon, A.; Almeida-Alves, G.; Vangyseghem, J.; Spitsbergen, J. GDNF to the Rescue: GDNF Delivery Effects on Motor Neurons and Nerves, and Muscle Re-Innervation after Peripheral Nerve Injuries. Neural Regen. Res. 2022, 17, 748–753. [Google Scholar] [CrossRef]
- Zhang, W.; Cui, Z.; Shen, D.; Gao, L.; Li, Q. Testosterone Levels Positively Linked to Muscle Mass but Not Strength in Adult Males Aged 20–59 Years: A Cross-Sectional Study. Front. Physiol. 2025, 16, 1512268. [Google Scholar] [CrossRef]
- Joassard, O.R.; Durieux, A.C.; Freyssenet, D.G. Β2-Adrenergic Agonists and the Treatment of Skeletal Muscle Wasting Disorders. Int. J. Biochem. Cell Biol. 2013, 45, 2309–2321. [Google Scholar] [CrossRef]
- Rossi, L.; Mota, B.I.; Valadão, P.A.C.; Magalhães-Gomes, M.P.S.; Oliveira, B.S.; Guatimosim, S.; Navegantes, L.C.C.; Miranda, A.S.; Prado, M.A.M.; Prado, V.F.; et al. Influence of Β2-Adrenergic Selective Agonist Formoterol on the Motor Unit of a Mouse Model of a Congenital Myasthenic Syndrome with Complete VAChT Deletion. Neuropharmacology 2024, 260, 110116. [Google Scholar] [CrossRef]
- Chen, C.; Tian, Y.; Wang, J.; Zhang, X.; Nan, L.; Dai, P.; Gao, Y.; Zheng, S.; Liu, W.; Zhang, Y. Testosterone Propionate Can Promote Effects of Acellular Nerve Allograft-Seeded Bone Marrow Mesenchymal Stem Cells on Repairing Canine Sciatic Nerve. J. Tissue Eng. Regen. Med. 2019, 13, 1685–1701. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Song, H.; Shen, J.; Wang, J.; Yang, Y.; Yang, Y.; Cao, J.; Xue, L.; Zhao, F.; Xiao, T.; et al. Functional Role of Skeletal Muscle-Derived Interleukin-6 and Its Effects on Lipid Metabolism. Front. Physiol. 2023, 14, 1110926. [Google Scholar] [CrossRef] [PubMed]
- Kummer, K.K.; Zeidler, M.; Kalpachidou, T.; Kress, M. Role of IL-6 in the Regulation of Neuronal Development, Survival and Function. Cytokine 2021, 144, 155582. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yao, J.; Li, J.; Zhang, J.; Wang, D.; Zuo, H.; Zhang, Y.; Xu, B.; Zhong, Y.; Shen, F.; et al. Irisin Ameliorates Age-Associated Sarcopenia and Metabolic Dysfunction. J. Cachexia Sarcopenia Muscle 2023, 14, 391–405. [Google Scholar] [CrossRef]
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of Irisin in Physiology and Pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- Südhof, T.C. Calcium Control of Neurotransmitter Release. Cold Spring Harb. Perspect. Biol. 2012, 4, a011353. [Google Scholar] [CrossRef]
- Cho, C.H.; Woo, J.S.; Perez, C.F.; Lee, E.H. A Focus on Extracellular Ca2+ Entry into Skeletal Muscle. Exp. Mol. Med. 2017, 49, e378. [Google Scholar] [CrossRef]
- Kaplan, M.M.; Sultana, N.; Benedetti, A.; Obermair, G.J.; Linde, N.F.; Papadopoulos, S.; Dayal, A.; Grabner, M.; Flucher, B.E. Calcium Influx and Release Cooperatively Regulate AChR Patterning and Motor Axon Outgrowth during Neuromuscular Junction Formation. Cell Rep. 2018, 23, 3891–3904. [Google Scholar] [CrossRef]
- Dierdorf, S.F. Hypocalcemia/Hypercalcemia; McEvoy, M.D., Furse, C.M., Eds.; Oxford University Press: Oxford, UK, 2017; Volume 1. [Google Scholar]
- Allen, B.W.; Somjen, G.G.; Sanders, D.B. Effect of Hypocalcemia on Neuromuscular Function in Cats. In Ion Measurements in Physiology and Medicine; Springer: Berlin/Heidelberg, Germany, 1985; pp. 243–248. [Google Scholar]
- Monti, E.; Reggiani, C.; Franchi, M.V.; Toniolo, L.; Sandri, M.; Armani, A.; Zampieri, S.; Giacomello, E.; Sarto, F.; Sirago, G.; et al. Neuromuscular Junction Instability and Altered Intracellular Calcium Handling as Early Determinants of Force Loss during Unloading in Humans. J. Physiol. 2021, 599, 3037–3061. [Google Scholar] [CrossRef]
- Gifondorwa, D.J.; Thompson, T.D.; Wiley, J.; Culver, A.E.; Shetler, P.K.; Rocha, G.V.; Ma, Y.L.; Krishnan, V.; Bryant, H.U. Vitamin D and/or Calcium Deficient Diets May Differentially Affect Muscle Fiber Neuromuscular Junction Innervation. Muscle Nerve 2016, 54, 1120–1132. [Google Scholar] [CrossRef]
- Breuer, A.C.; Atkinson, M.B. Fast Axonal Transport Alterations in Amyotrophic Lateral Sclerosis (ALS) and in Parathyroid Hormone (PTH)-Treated Axons. Cell Motil. Cytoskelet. 1988, 10, 321–330. [Google Scholar] [CrossRef]
- Kokubu, N.; Tsujii, M.; Akeda, K.; Iino, T.; Sudo, A. BMP-7/Smad Expression in Dedifferentiated Schwann Cells during Axonal Regeneration and Upregulation of Endogenous BMP-7 Following Administration of PTH (1-34). J. Orthop. Surg. 2018, 26, 2309499018812953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, N.; Shao, J.; Wang, L.; Lu, W.W. Bidirectional Control of Parathyroid Hormone and Bone Mass by Subfornical Organ. Neuron 2023, 111, 1914–1932.e6. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.E.; Cardinali, D.P. Effect of Parathyroid Hormone and Calcitonin on Acetylcholine Release in Rat Sympathetic Superior Cervical Ganglion. Brain Res. 1994, 650, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.E.; Cardinali, D.P. Effect of Parathyroid Hormone and Calcitonin on Cholinergic Markers in Rat Parathyroid Gland. J. Neuroendocrinol. 1995, 7, 689–693. [Google Scholar] [CrossRef]
- Gambardella, J.; De Rosa, M.; Sorriento, D.; Prevete, N.; Fiordelisi, A.; Ciccarelli, M.; Trimarco, B.; De Luca, N.; Iaccarino, G. Parathyroid Hormone Causes Endothelial Dysfunction by Inducing Mitochondrial ROS and Specific Oxidative Signal Transduction Modifications. Oxidative Med. Cell. Longev. 2018, 2018, 9582319. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, J.-W.; Lin, J.; Zhu, J.-H.; Lin, Y.-Z.; Wang, X.-X.; Zhou, L.-Y.; Huang, H.-B.; Tian, X.-B. The Stability of Intact Parathyroid Hormone (PTH) in Different Types of Blood Collection Tubes. Clin. Lab. 2022, 68, 463–471. [Google Scholar] [CrossRef]
- Geara, A.S.; Castellanos, M.R.; Bassil, C.; Schuller-Levis, G.; Park, E.; Smith, M.; Goldman, M.; Elsayegh, S. Effects of Parathyroid Hormone on Immune Function. J. Immunol. Res. 2010, 2010, 418695. [Google Scholar] [CrossRef]
- Puliani, G.; Hasenmajer, V.; Simonelli, I.; Sada, V.; Pofi, R.; Minnetti, M.; Cozzolino, A.; Napoli, N.; Pasqualetti, P.; Gianfrilli, D.; et al. Safety and Efficacy of PTH 1-34 and 1-84 Therapy in Chronic Hypoparathyroidism: A Meta-Analysis of Prospective Trials. J. Bone Miner. Res. 2022, 37, 1233–1250. [Google Scholar] [CrossRef]
- Hong, H.; Song, T.; Liu, Y.; Li, J.; Jiang, Q.; Song, Q.; Deng, Z. The Effectiveness and Safety of Parathyroid Hormone in Fracture Healing: A Meta-Analysis. Clinics 2019, 74, e800. [Google Scholar] [CrossRef]
- Qin, W.; Dallas, S.L. Exosomes and Extracellular RNA in Muscle and Bone Aging and Crosstalk. Curr. Osteoporos. Rep. 2019, 17, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, Y.; Chen, Q.; Zhang, H. Exosomal MiRNAs in Muscle-Bone Crosstalk: Mechanistic Links, Exercise Modulation and Implications for Sarcopenia, Osteoporosis and Osteosarcopenia. Metabolism 2025, 170, 156333. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wang, L.; Zhou, Y.; Zhang, P.; Chen, X. Muscle-Derived Extracellular Vesicles Mediate Crosstalk between Skeletal Muscle and Other Organs. Front. Physiol. 2024, 15, 1501957. [Google Scholar] [CrossRef] [PubMed]
- Vrščaj, L.A.; Marc, J.; Ostanek, B. Interactome of PTH-Regulated MiRNAs and Their Predicted Target Genes for Investigating the Epigenetic Effects of PTH (1–34) in Bone Metabolism. Genes 2022, 13, 1443. [Google Scholar] [CrossRef]
- Mohanakrishnan, V.; Balasubramanian, A.; Mahalingam, G.; Partridge, N.C.; Ramachandran, I.; Selvamurugan, N. Parathyroid Hormone-Induced down-Regulation of MiR-532-5p for Matrix Metalloproteinase-13 Expression in Rat Osteoblasts. J. Cell. Biochem. 2018, 119, 6181–6193. [Google Scholar] [CrossRef]
- Evenson, A.; Mitchell, J.; Wei, W.; Poylin, V.; Parangi, S.; Hasselgren, P.O. The Gene Expression and Activity of Calpains and the Muscle Wasting-Associated Ubiquitin Ligases, Atrogin-1 and MuRF1, Are Not Altered in Patients with Primary Hyperparathyroidism. Int. J. Mol. Med. 2006, 18, 471–475. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Khan, A.A.; Silverberg, S.J.; Fuleihan, G.E.H.; Marcocci, C.; Minisola, S.; Perrier, N.; Sitges-Serra, A.; Thakker, R.V.; Guyatt, G.; et al. Evaluation and Management of Primary Hyperparathyroidism: Summary Statement and Guidelines from the Fifth International Workshop. J. Bone Miner. Res. 2022, 37, 2293–2314. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Qian, A.; Li, Y.P. Wnt/β-Catenin Signaling Components and Mechanisms in Bone Formation, Homeostasis, and Disease. Bone Res. 2024, 12, 39. [Google Scholar] [CrossRef]
- Lin, W.; Chow, S.K.H.; Cui, C.; Liu, C.; Wang, Q.; Chai, S.; Wong, R.M.Y.; Zhang, N.; Cheung, W.H. Wnt/β-Catenin Signaling Pathway as an Important Mediator in Muscle and Bone Crosstalk: A Systematic Review. J. Orthop. Transl. 2024, 47, 63–73. [Google Scholar] [CrossRef]
- Lin, F.X.; Gu, H.Y.; He, W. MAPK Signaling Pathway in Spinal Cord Injury: Mechanisms and Therapeutic Potential. Exp. Neurol. 2025, 383, 115043. [Google Scholar] [CrossRef]
- Zhu, W.; Ming, P.; Zhang, S.; Qiu, J. Role of MAPK/JNK Signaling Pathway on the Regulation of Biological Behaviors of MC3T3-E1 Osteoblasts under Titanium Ion Exposure. Mol. Med. Rep. 2020, 22, 4792–4800. [Google Scholar] [CrossRef]
- Brennan, C.M.; Emerson, C.P.; Owens, J.; Christoforou, N. P38 MAPKs—Roles in Skeletal Muscle Physiology, Disease Mechanisms, and as Potential Therapeutic Targets. JCI Insight 2021, 6, e149915. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The Role of Calcium–Calcineurin–NFAT Signaling Pathway in Health and Autoimmune Diseases. Front. Immunol. 2020, 11, 455685. [Google Scholar] [CrossRef]
- Kipanyula, M.J.; Kimaro, W.H.; Etet, P.F.S. The Emerging Roles of the Calcineurin-Nuclear Factor of Activated T-Lymphocytes Pathway in Nervous System Functions and Diseases. J. Aging Res. 2016, 2016, 5081021. [Google Scholar] [CrossRef]
| In Vitro/Ex Vivo | |||
| Treatment | Cell Model | Mechanism | Ref. |
| 100 nM PTH | FAPs and C2C12 coculture |
| [142] |
| PTH (1–84) from 10−6 to 10−12 mol/L | hSCs | Promotes the myogenic differentiation process | [143] |
| 1–1000 nM PTH (1–34) | 3T3-L1, MCF7, C2C12, MC3T3-E1, G8 |
| [144] |
| 10−10 M to 10−8 M PTH (1–34) | C2C12, MC3T3-E1 | r-Irisin leads to a 50% downregulation of PTH-r mRNA expression compared with untreated cells | [145] |
| 0.1 pM, 1 pM, 10 pM and 100 pM PTH (1–34) | C2 | Modulates muscle cell uptake and retention of 25(OH)D3 | [146] |
| 20 nM rat PTH (1–34) | C2C12 and ZHTc6-MyoD | Accelerates myocyte differentiation | [9] |
| PTH (1–34) and PTH (1–84) | Rat skeletal muscle | Increases the release of alanine and glutamine | [140] |
| In vivo | |||
| Treatment | Animal model | Mechanism | Ref. |
| 30 μg/kg teriparatide 3 times a week for 4 or 8 weeks | 9-week-old Sprague Dawley rats |
| [142] |
| 80 μg/kg of PTH (1–34) three times a week for 20 weeks | 8-week-old female WT C57BL/6J mice |
| [144] |
| 30 µg/kg TPTD, 3 days/week | 7-month-old female Wistar rats | Improves bone, skeletal muscle, and fat mass | [147] |
| 150 μg/kg body weight/day of PTH (1–34), daily | 4-week-old C57BL/10ScSn-Dmdmdx/J (Mdx) and C57BL/10SnJ wild-type (WT), male mice |
| [148] |
| PTH (1–34) 60 μg/kg/day, 5 days a week | 12–14-week-old female Wistar rats |
| [149] |
| 60 μg/kg/d of PTH for 59 days | 4-week-old Mdx mice | Improved the muscle strength and histological characteristics of the skeletal muscle | [9] |
| PTH (40 μg/kg BW/day) every other day for 1–35 days | 3-month-old female Sprague–Dawley rats | No impacts on muscle weight or muscle fiber size | [150] |
| 1–84 or 1–34 PTH, 200 U/day, for 4 days | Sprague Dawley rats weighing 150 to 200 g | Decreases energy production, transfer, and utilization | [141] |
| PTH (1–34) and PTH (1–84) | Sprague Dawley rats | In primary hyperparathyroidism and chronic uremia, PTH may directly impact muscle dysfunction and wasting | [140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.-L.; Lee, K.-B.; Moon, Y.J. Parathyroid Hormone as a Modulator of Skeletal Muscle: Insights into Bone–Muscle and Nerve–Muscle Interactions. Int. J. Mol. Sci. 2025, 26, 7060. https://doi.org/10.3390/ijms26157060
Nguyen V-L, Lee K-B, Moon YJ. Parathyroid Hormone as a Modulator of Skeletal Muscle: Insights into Bone–Muscle and Nerve–Muscle Interactions. International Journal of Molecular Sciences. 2025; 26(15):7060. https://doi.org/10.3390/ijms26157060
Chicago/Turabian StyleNguyen, Vinh-Lac, Kwang-Bok Lee, and Young Jae Moon. 2025. "Parathyroid Hormone as a Modulator of Skeletal Muscle: Insights into Bone–Muscle and Nerve–Muscle Interactions" International Journal of Molecular Sciences 26, no. 15: 7060. https://doi.org/10.3390/ijms26157060
APA StyleNguyen, V.-L., Lee, K.-B., & Moon, Y. J. (2025). Parathyroid Hormone as a Modulator of Skeletal Muscle: Insights into Bone–Muscle and Nerve–Muscle Interactions. International Journal of Molecular Sciences, 26(15), 7060. https://doi.org/10.3390/ijms26157060





