Beyond the Walls of Troy: A Scoping Review on Pharmacological Strategies to Enhance Drug Delivery Across the Blood–Brain Barrier and Blood–Tumor Barrier
Abstract
1. Introduction
2. Methods
3. Results
3.1. Blood–Brain Barrier
3.2. Endothelial Cells
3.3. Basal Membrane
3.4. Pericytes
3.5. Astrocytes
3.6. Tight Junctions
3.7. Adherens Junctions
3.8. Blood–Tumor Barrier in Primary Tumors
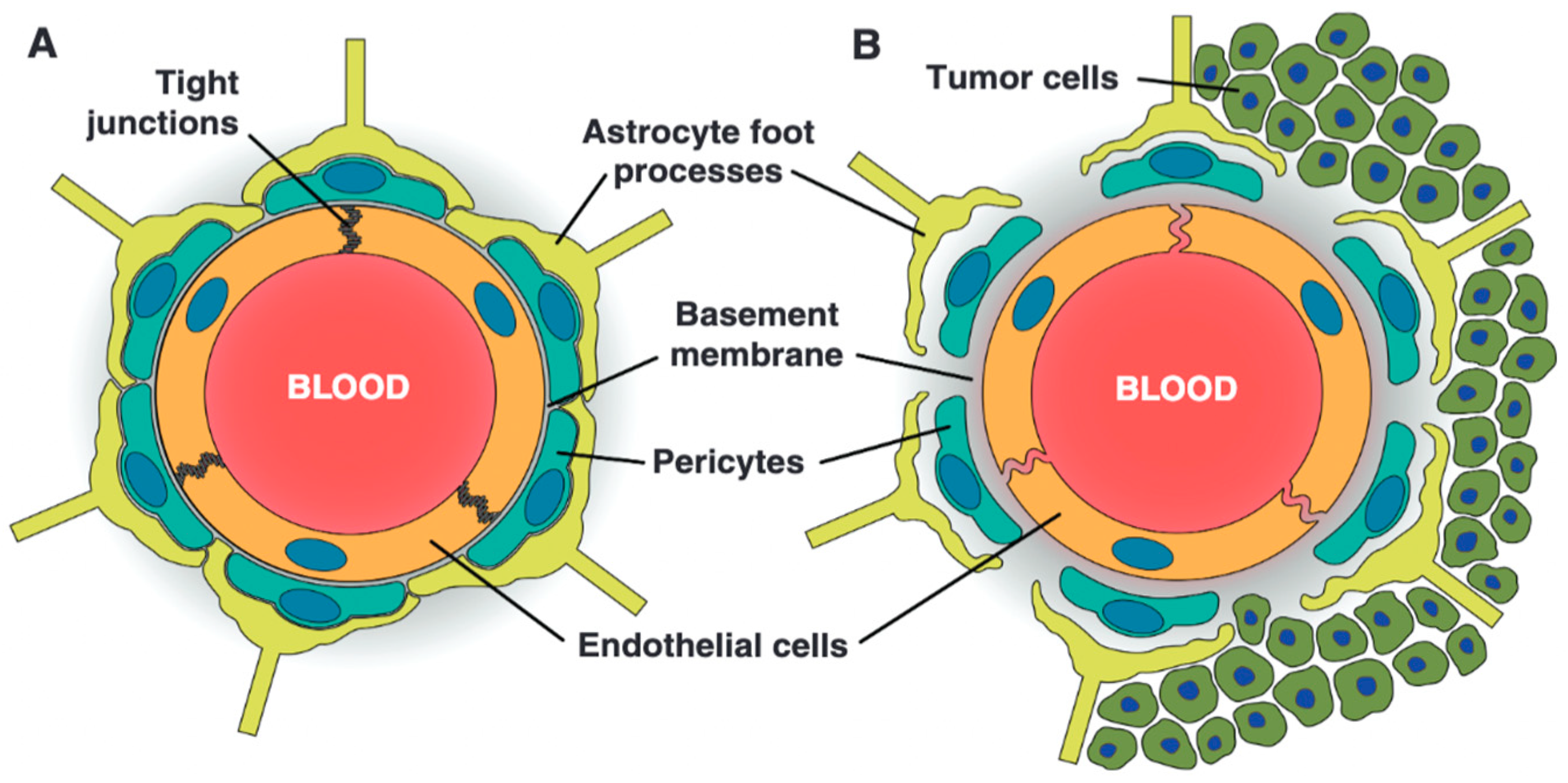
3.9. Blood–Tumor Barrier in Brain Metastasis
3.10. Tumor Microenvironment
3.11. Methods to Enhance Drug Delivery in Brain Tumors
3.12. Bradykinin Receptor
3.13. Adenosine Receptor
3.14. Sphingosine-1-Phosphate Receptor
3.15. Inhibition of Drug Efflux Transporters
3.16. Nanotechnology for Brain Tumor Drug Delivery: Passive and Active Targeting Strategies
3.17. Passive Targeting
3.18. Active Targeting
3.19. Carrier-Mediated Transport
3.20. Glucose Transporter 1
3.21. L-Type Amino Acid Transporter 1
3.22. Choline Transporter-like Protein 1
3.23. Other Transporters
3.24. Adsorptive-Mediated Transcytosis
3.25. Receptor-Mediated Transcytosis
3.26. Transferrin Receptor 1
3.27. Transferrin Conjugates
3.28. Transferrin Receptor 1-Antibodies
3.29. Transferrin Receptor 1-Targeting Peptides
3.30. Ferritin-Based Nanoparticles for Transferrin Receptor 1-Targeted Drug Delivery
3.31. Lactoferrin Receptor
3.32. Low-Density Lipoprotein Receptor Family
3.33. Nicotine Acetylcholine Receptors
3.34. Growth Factor Receptors
3.35. Folate Receptor
3.36. Leptin Receptor
3.37. Scavenger Receptors
3.38. Cell-Mediated Drug Delivery
3.39. Mesenchymal Stem Cells
3.40. Neural Stem Cells
3.41. Macrophages
3.42. Exosomes
3.43. Bridging Therapy: Integrating Neurosurgical Techniques with Pharmacological Strategies
3.44. Osmotic Blood–Brain Barrier Disruption with Intra-Arterial Chemotherapy
3.45. Convection-Enhanced Delivery
3.46. Focused Ultrasound
3.47. Microsurgery
3.48. Conclusions and Future Directions
3.49. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Gould, J. Breaking down the epidemiology of brain cancer. Nature 2018, 561, S40–S41. [Google Scholar] [CrossRef] [PubMed]
- Roh, T.H.; Kim, S.H. Supramaximal Resection for Glioblastoma: Redefining the Extent of Resection Criteria and Its Impact on Survival. Brain Tumor Res. Treat. 2023, 11, 166–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thiruvengadam, M. Radioresistance in brain tumors: Strategies for improved radiotherapy outcomes. Brain Spine 2024, 4, 102912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lassman, A.B.; Pugh, S.L.; Wang, T.J.C.; Aldape, K.; Gan, H.K.; Preusser, M.; Vogelbaum, M.A.; Sulman, E.P.; Won, M.; Zhang, P.; et al. Depatuxizumab mafodotin in EGFR-amplified newly diagnosed glioblastoma: A phase III randomized clinical trial. Neuro Oncol. 2023, 25, 339–350. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tripathy, D.; Tolaney, S.M.; Seidman, A.D.; Anders, C.K.; Ibrahim, N.; Rugo, H.S.; Twelves, C.; Diéras, V.; Müller, V.; Du, Y.; et al. Treatment with Etirinotecan Pegol for Patients with Metastatic Breast Cancer and Brain Metastases: Final Results from the Phase 3 ATTAIN Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1047–1052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pardridge, W.M. A Historical Review of Brain Drug Delivery. Pharmaceutics 2022, 14, 1283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostermann, S.; Csajka, C.; Buclin, T.; Leyvraz, S.; Lejeune, F.; Decosterd, L.A.; Stupp, R. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin. Cancer Res. 2004, 10, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, D.; Smith, Q.R.; Lockman, P.R.; Bronder, J.; Gril, B.; Chambers, A.F.; Weil, R.J.; Steeg, P.S. Brain metastases of breast cancer. Breast Dis. 2007, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Badie, B.; Chen, M.; Liu, A.; Blanchard, S.; Synold, T.W. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: Potential implications for the current approach to chemoradiation. Clin. Cancer Res. 2009, 15, 7092–7098. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Silbergeld, D.L.; Chicoine, M.R. Isolation and characterization of human malignant glioma cells from histologically normal brain. J. Neurosurg. 1997, 86, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Ling, E.A. The circumventricular organs. Histol. Histopathol. 2017, 32, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Pfau, S.J.; Langen, U.H.; Fisher, T.M.; Prakash, I.; Nagpurwala, F.; Lozoya, R.A.; Lee, W.A.; Wu, Z.; Gu, C. Characteristics of blood-brain barrier heterogeneity between brain regions revealed by profiling vascular and perivascular cells. Nat. Neurosci. 2024, 27, 1892–1903. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef] [PubMed]
- Mizee, M.R.; de Vries, H.E. Blood-brain barrier regulation: Environmental cues controlling the onset of barrier properties. Tissue Barriers 2013, 1, e26882. [Google Scholar] [CrossRef] [PubMed]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Fang, F.; Gao, W.; Chen, H.; Wen, J.; Wen, X.; Chen, J. The Structure and Function of the Glycocalyx and Its Connection with Blood-Brain Barrier. Front. Cell Neurosci. 2021, 15, 739699. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brasnjevic, I.; Steinbusch, H.W.; Schmitz, C.; Martinez-Martinez, P.; European NanoBioPharmaceutics Research Initiative. Delivery of peptide and protein drugs over the blood-brain barrier. Prog. Neurobiol. 2009, 87, 212–251. [Google Scholar] [CrossRef] [PubMed]
- Turowski, P.; Kenny, B.A. The blood-brain barrier and methamphetamine: Open sesame? Front. Neurosci. 2015, 9, 156. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial Electrical Resistance Measurement across the Blood-Brain Barrier: A Critical Review of Methods. Micromachines 2021, 12, 685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Zhao, Q.; Zhu, X.; Zhou, L.; Song, P.; Liu, B.; Tian, D.; Chen, Q.; Zhou, J.; Deng, G. Self-Assembled nanoparticles of natural bioactive molecules enhance the delivery and efficacy of paclitaxel in glioblastoma. CNS Neurosci. Ther. 2024, 30, e14528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McDonald, B.; Barth, K.; Schmidt, M.H.H. The origin of brain malignancies at the blood-brain barrier. Cell. Mol. Life Sci. 2023, 80, 282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mathiisen, T.M.; Lehre, K.P.; Danbolt, N.C.; Ottersen, O.P. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia 2010, 58, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Liu, X.Y.; Fagan, A.; Gonzalez-Toledo, M.E.; Zhao, L.R. Ultrastructural changes in cerebral capillary pericytes in aged Notch3 mutant transgenic mice. Ultrastruct. Pathol. 2012, 36, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Kebede, A.A.; Barres, B.A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010, 468, 562–566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Klett, F.; Priller, J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J. Cereb. Blood Flow. Metab. 2015, 35, 883–887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Castro, V.; Toborek, M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J. Cell. Mol. Med. 2012, 16, 2950–2957. [Google Scholar] [CrossRef]
- Ayloo, S.; Lazo, C.G.; Sun, S.; Zhang, W.; Cui, B.; Gu, C. Pericyte-to-endothelial cell signaling via vitronectin-integrin regulates blood-CNS barrier. Neuron 2022, 110, 1641–1655.E6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sweeney, M.D.; Ayyadurai, S.; Zlokovic, B.V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Koulakoff, A.; Roux, L.; Holcman, D.; Rouach, N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010, 11, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Hösli, L.; Zuend, M.; Bredell, G.; Zanker, H.S.; Porto de Oliveira, C.E.; Saab, A.S.; Weber, B. Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep. 2022, 39, 110599. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Iddings, J.A. Astrocyte regulation of cerebral vascular tone. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H609–H619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Filosa, J.A.; Morrison, H.W.; Iddings, J.A.; Du, W.; Kim, K.J. Beyond neurovascular coupling, role of astrocytes in the regulation of vascular tone. Neuroscience 2016, 323, 96–109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janzer, R.C.; Raff, M.C. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature 1987, 325, 253–257. [Google Scholar] [CrossRef]
- Liu, D.; Liao, P.; Li, H.; Tong, S.; Wang, B.; Lu, Y.; Gao, Y.; Huang, Y.; Zhou, H.; Shi, L.; et al. Regulation of blood-brain barrier integrity by Dmp1-expressing astrocytes through mitochondrial transfer. Sci. Adv. 2024, 10, eadk2913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kubotera, H.; Ikeshima-Kataoka, H.; Hatashita, Y.; Allegra Mascaro, A.L.; Pavone, F.S.; Inoue, T. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci. Rep. 2019, 9, 1263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stamatovic, S.M.; Johnson, A.M.; Keep, R.F.; Andjelkovic, A.V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 2016, 4, e1154641. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pfeiffer, F.; Schäfer, J.; Lyck, R.; Makrides, V.; Brunner, S.; Schaeren-Wiemers, N.; Deutsch, U.; Engelhardt, B. Claudin-1 induced sealing of blood-brain barrier tight junctions ameliorates chronic experimental autoimmune encephalomyelitis. Acta Neuropathol. 2011, 122, 601–614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood-brain barrier: Structural and functional aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Martìn-Padura, I.; Lostaglio, S.; Schneemann, M.; Williams, L.; Romano, M.; Fruscella, P.; Panzeri, C.; Stoppacciaro, A.; Ruco, L.; Villa, A.; et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J. Cell Biol. 1998, 142, 117–127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wegmann, F.; Ebnet, K.; Du Pasquier, L.; Vestweber, D.; Butz, S. Endothelial adhesion molecule ESAM binds directly to the multidomain adaptor MAGI-1 and recruits it to cell contacts. Exp. Cell Res. 2004, 300, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, S.; Sasaki, T.; Tanaka, H.; Yan, H.; Ikegami, T.; Kanki, H.; Nishiyama, K.; Beck, G.; Gon, Y.; Okazaki, S.; et al. The tight junction protein occludin modulates blood-brain barrier integrity and neurological function after ischemic stroke in mice. Sci. Rep. 2023, 13, 2892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Li, X.; Qiao, S.; Yang, D.; Li, Z.; Xu, J.; Li, W.; Su, L.; Liu, W. Occludin degradation makes brain microvascular endothelial cells more vulnerable to reperfusion injury in vitro. J. Neurochem. 2021, 156, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Zhou, L.; Agalliu, D.; Cahoy, J.D.; Kaushal, A.; Barres, B.A. The mouse blood-brain barrier transcriptome: A new resource for understanding the development and function of brain endothelial cells. PLoS ONE 2010, 5, e13741. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nitta, T.; Hata, M.; Gotoh, S.; Seo, Y.; Sasaki, H.; Hashimoto, N.; Furuse, M.; Tsukita, S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell Biol. 2003, 161, 653–660. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lv, J.; Hu, W.; Yang, Z.; Li, T.; Jiang, S.; Ma, Z.; Chen, F.; Yang, Y. Focusing on claudin-5: A promising candidate in the regulation of BBB to treat ischemic stroke. Prog. Neurobiol. 2018, 161, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Berndt, P.; Winkler, L.; Cording, J.; Breitkreuz-Korff, O.; Rex, A.; Dithmer, S.; Rausch, V.; Blasig, R.; Richter, M.; Sporbert, A.; et al. Tight junction proteins at the blood-brain barrier: Far more than claudin-5. Cell. Mol. Life Sci. 2019, 76, 1987–2002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uchida, Y.; Sumiya, T.; Tachikawa, M.; Yamakawa, T.; Murata, S.; Yagi, Y.; Sato, K.; Stephan, A.; Ito, K.; Ohtsuki, S.; et al. Involvement of Claudin-11 in Disruption of Blood-Brain, -Spinal Cord, and -Arachnoid Barriers in Multiple Sclerosis. Mol. Neurobiol. 2019, 56, 2039–2056. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Fischmann, A.; Rascher, G.; Duffner, F.; Grote, E.-H.; Kalbacher, H.; Wolburg, H. Claudin-1 and claudin-5 expression and tight junction morphology are altered in blood vessels of human glioblastoma multiforme. Acta Neuropathol. 2000, 100, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Vanlandewijck, M.; He, L.; Mäe, M.A.; Andrae, J.; Ando, K.; Del Gaudio, F.; Nahar, K.; Lebouvier, T.; Laviña, B.; Gouveia, L.; et al. A molecular atlas of cell types and zonation in the brain vasculature. Nature 2018, 554, 475–480, Erratum in Nature 2018, 560, E3. https://doi.org/10.1038/s41586-018-0232-x. [Google Scholar] [CrossRef] [PubMed]
- Wolburg, H.; Wolburg-Buchholz, K.; Kraus, J.; Rascher-Eggstein, G.; Liebner, S.; Hamm, S.; Duffner, F.; Grote, E.H.; Risau, W.; Engelhardt, B. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003, 105, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Castro Dias, M.; Coisne, C.; Lazarevic, I.; Baden, P.; Hata, M.; Iwamoto, N.; Francisco, D.M.F.; Vanlandewijck, M.; He, L.; Baier, F.A.; et al. Claudin-3-deficient C57BL/6J mice display intact brain barriers. Sci. Rep. 2019, 9, 203, Erratum in Sci. Rep. 2019, 9, 10702. https://doi.org/10.1038/s41598-019-43511-0. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castro Dias, M.; Coisne, C.; Baden, P.; Enzmann, G.; Garrett, L.; Becker, L.; Hölter, S.M.; German Mouse Clinic Consortium; Hrabě de Angelis, M.; Deutsch, U.; et al. Claudin-12 is not required for blood-brain barrier tight junction function. Fluids Barriers CNS 2019, 16, 30. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campbell, H.K.; Maiers, J.L.; DeMali, K.A. Interplay between tight junctions & adherens junctions. Exp. Cell Res. 2017, 358, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Tietz, S.; Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corada, M.; Mariotti, M.; Thurston, G.; Smith, K.; Kunkel, R.; Brockhaus, M.; Lampugnani, M.G.; Martin-Padura, I.; Stoppacciaro, A.; Ruco, L.; et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc. Natl. Acad. Sci. USA 1999, 96, 9815–9820. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, Y.; Radice, G.L. N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J. Cell Biol. 2005, 169, 29–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mishra, R.; Singh, S.K. HIV-1 Tat C modulates expression of miRNA-101 to suppress VE-cadherin in human brain microvascular endothelial cells. J. Neurosci. 2013, 33, 5992–6000. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morini, M.F.; Giampietro, C.; Corada, M.; Pisati, F.; Lavarone, E.; Cunha, S.I.; Conze, L.L.; O’Reilly, N.; Joshi, D.; Kjaer, S.; et al. VE-Cadherin-Mediated Epigenetic Regulation of Endothelial Gene Expression. Circ. Res. 2018, 122, 231–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tran, K.A.; Zhang, X.; Predescu, D.; Huang, X.; Machado, R.F.; Göthert, J.R.; Malik, A.B.; Valyi-Nagy, T.; Zhao, Y.Y. Endothelial β-Catenin Signaling Is Required for Maintaining Adult Blood-Brain Barrier Integrity and Central Nervous System Homeostasis. Circulation 2016, 133, 177–186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conacci-Sorrell, M.; Zhurinsky, J.; Ben-Ze’ev, A. The cadherin-catenin adhesion system in signaling and cancer. J. Clin. Investig. 2002, 109, 987–991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sebo, D.J.; Ali, I.; Fetsko, A.R.; Trimbach, A.A.; Taylor, M.R. Activation of Wnt/β-catenin in neural progenitor cells regulates blood-brain barrier development and promotes neuroinflammation. Sci. Rep. 2025, 15, 3496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fetsko, A.R.; Sebo, D.J.; Budzynski, L.B.; Scharbarth, A.; Taylor, M.R. IL-1β disrupts the initiation of blood-brain barrier development by inhibiting endothelial Wnt/β-catenin signaling. iScience 2024, 27, 109651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bao, X.; Wu, J.; Xie, Y.; Kim, S.; Michelhaugh, S.; Jiang, J.; Mittal, S.; Sanai, N.; Li, J. Protein Expression and Functional Relevance of Efflux and Uptake Drug Transporters at the Blood-Brain Barrier of Human Brain and Glioblastoma. Clin. Pharmacol. Ther. 2020, 107, 1116–1127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steeg, P.S. The blood-tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K.; et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.S.; Harford, J.B.; Pirollo, K.F.; Chang, E.H. Effective treatment of glioblastoma requires crossing the blood-brain barrier and targeting tumors including cancer stem cells: The promise of nanomedicine. Biochem. Biophys. Res. Commun. 2015, 468, 485–489. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Blethen, K.E.; Arsiwala, T.A.; Fladeland, R.A.; Sprowls, S.A.; Panchal, D.M.; Adkins, C.E.; Kielkowski, B.N.; Earp, L.E.; Glass, M.J.; Pritt, T.A.; et al. Modulation of the blood-tumor barrier to enhance drug delivery and efficacy for brain metastases. Neurooncol. Adv. 2021, 3 (Suppl. S5), v133–v143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Liebner, S.; Dijkhuizen, R.M.; Reiss, Y.; Plate, K.H.; Agalliu, D.; Constantin, G. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018, 135, 311–336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lan, Y.L.; Wang, X.; Lou, J.C.; Ma, X.C.; Zhang, B. The potential roles of aquaporin 4 in malignant gliomas. Oncotarget 2017, 8, 32345–32355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maddison, K.; Bowden, N.A.; Graves, M.C.; Tooney, P.A. Characteristics of vasculogenic mimicry and tumour to endothelial transdifferentiation in human glioblastoma: A systematic review. BMC Cancer 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Li, F.; Yang, Y.T.; Xu, X.D.; Chen, J.S.; Chen, T.L.; Chen, H.J.; Zhu, Y.B.; Lin, J.Y.; Li, Y.; et al. IGFBP2 promotes vasculogenic mimicry formation via regulating CD144 and MMP2 expression in glioma. Oncogene 2019, 38, 1815–1831. [Google Scholar] [CrossRef] [PubMed]
- Soda, Y.; Marumoto, T.; Friedmann-Morvinski, D.; Soda, M.; Liu, F.; Michiue, H.; Pastorino, S.; Yang, M.; Hoffman, R.M.; Kesari, S.; et al. Transdifferentiation of glioblastoma cells into vascular endothelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4274–4280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yano, S.; Shinohara, H.; Herbst, R.S.; Kuniyasu, H.; Bucana, C.D.; Ellis, L.M.; Davis, D.W.; McConkey, D.J.; Fidler, I.J. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000, 60, 4959–4967. [Google Scholar] [PubMed]
- Millar, B.A.; Purdie, T.G.; Yeung, I.; Pond, G.R.; Billingsley, S.; Wong, R.; Haddad, P.; Wong, C.S.; Laperriere, N. Assessing perfusion changes during whole brain irradiation for patients with cerebral metastases. J. Neurooncol. 2005, 71, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Ling, H.W.; Chen, K.M.; Jiang, H.; Zhu, Y.B. Comparison of cerebral blood volume and permeability in preoperative grading of intracranial glioma using CT perfusion imaging. Neuroradiology 2006, 48, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Tabor, J.K.; Onoichenco, A.; Narayan, V.; Wernicke, A.G.; D’Amico, R.S.; Vojnic, M. Brain metastasis screening in the molecular age. Neurooncol. Adv. 2023, 5, vdad080. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adler, O.; Zait, Y.; Cohen, N.; Blazquez, R.; Doron, H.; Monteran, L.; Scharff, Y.; Shami, T.; Mundhe, D.; Glehr, G.; et al. Reciprocal interactions between innate immune cells and astrocytes facilitate neuroinflammation and brain metastasis via lipocalin-2. Nat. Cancer 2023, 4, 401–418. [Google Scholar] [CrossRef] [PubMed]
- Paku, S.; Döme, B.; Tóth, R.; Timár, J. Organ-specificity of the extravasation process: An ultrastructural study. Clin. Exp. Metastasis 2000, 18, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Lorger, M.; Felding-Habermann, B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am. J. Pathol. 2010, 176, 2958–2971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santos, L.; Tomatis, F.; Ferreira, H.R.S.; Almeida, S.F.F.; Ciputra, E.; Sereno, J.; Almeida, R.; Teixeira, P.; Ribeiro, A.S.; Moreira, J.N.; et al. ENPP1 induces blood-brain barrier dysfunction and promotes brain metastasis formation in human epidermal growth factor receptor 2-positive breast cancer. Neuro Oncol. 2025, 27, 167–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuczynski, E.A.; Vermeulen, P.B.; Pezzella, F.; Kerbel, R.S.; Reynolds, A.R. Vessel co-option in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 469–493. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mittapalli, R.K.; Taskar, K.S.; Rudraraju, V.; Gril, B.; Bohn, K.A.; Adkins, C.E.; Roberts, A.; Thorsheim, H.R.; Gaasch, J.A.; et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin. Cancer Res. 2010, 16, 5664–5678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bao, X.; Wu, J.; Jiang, J.; Tien, A.C.; Sanai, N.; Li, J. Quantitative protein expression of blood-brain barrier transporters in the vasculature of brain metastases of patients with lung and breast cancer. Clin. Transl. Sci. 2021, 14, 1265–1271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murrell, D.H.; Hamilton, A.M.; Mallett, C.L.; van Gorkum, R.; Chambers, A.F.; Foster, P.J. Understanding Heterogeneity and Permeability of Brain Metastases in Murine Models of HER2-Positive Breast Cancer Through Magnetic Resonance Imaging: Implications for Detection and Therapy. Transl. Oncol. 2015, 8, 176–184. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Samala, R.; Thorsheim, H.R.; Goda, S.; Taskar, K.; Gril, B.; Steeg, P.S.; Smith, Q.R. Vinorelbine Delivery and Efficacy in the MDA-MB-231BR Preclinical Model of Brain Metastases of Breast Cancer. Pharm. Res. 2016, 33, 2904–2919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Terrell-Hall, T.B.; Nounou, M.I.; El-Amrawy, F.; Griffith, J.I.G.; Lockman, P.R. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017, 8, 83734–83744. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morikawa, A.; Peereboom, D.M.; Thorsheim, H.R.; Samala, R.; Balyan, R.; Murphy, C.G.; Lockman, P.R.; Simmons, A.; Weil, R.J.; Tabar, V.; et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: A prospective study. Neuro Oncol. 2015, 17, 289–295, Erratum in Neuro Oncol. 2015, 17, 1423. https://doi.org/10.1093/neuonc/nov137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saleem, A.; Searle, G.E.; Kenny, L.M.; Huiban, M.; Kozlowski, K.; Waldman, A.D.; Woodley, L.; Palmieri, C.; Lowdell, C.; Kaneko, T.; et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 2015, 5, 30, Erratum in EJNMMI Res. 2017, 7, 74. https://doi.org/10.1186/s13550-017-0323-y. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer. 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akbari, H.; Kazerooni, A.F.; Ware, J.B.; Mamourian, E.; Anderson, H.; Guiry, S.; Sako, C.; Raymond, C.; Yao, J.; Brem, S.; et al. Quantification of tumor microenvironment acidity in glioblastoma using principal component analysis of dynamic susceptibility contrast enhanced MR imaging. Sci. Rep. 2021, 11, 15011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rong, Y.; Durden, D.L.; Van Meir, E.G.; Brat, D.J. ‘Pseudopalisading’ necrosis in glioblastoma: A familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006, 65, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Gatenby, R.A. Metabolism and its sequelae in cancer evolution and therapy. Cancer J. 2015, 21, 88–96. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fantin, J.; Toutain, J.; Pérès, E.A.; Bernay, B.; Mehani, S.M.; Helaine, C.; Bourgeois, M.; Brunaud, C.; Chazalviel, L.; Pontin, J.; et al. Assessment of hypoxia and oxidative-related changes in a lung-derived brain metastasis model by [64Cu][Cu(ATSM)] PET and proteomic studies. EJNMMI Res. 2023, 13, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corroyer-Dulmont, A.; Valable, S.; Fantin, J.; Toutain, J.; Divoux, D.; Chazalviel, L.; Bernier, M.; Bernaudin, M.; Barbier, E.L.; Petit, E. Multimodal evaluation of hypoxia in brain metastases of lung cancer and interest of hypoxia image-guided radiotherapy. Sci. Rep. 2021, 11, 11239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tannock, I.F.; Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 1989, 49, 4373–4384. [Google Scholar] [PubMed]
- Roepe, P.D. Analysis of the steady-state and initial rate of doxorubicin efflux from a series of multidrug-resistant cells expressing different levels of P-glycoprotein. Biochemistry 1992, 31, 12555–12564. [Google Scholar] [CrossRef] [PubMed]
- Huai, Y.; Hossen, M.N.; Wilhelm, S.; Bhattacharya, R.; Mukherjee, P. Nanoparticle Interactions with the Tumor Microenvironment. Bioconjugate Chem. 2019, 30, 2247–2263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unterberg, A.; Wahl, M.; Baethmann, A. Effects of bradykinin on permeability and diameter of pial vessels in vivo. J. Cereb. Blood Flow. Metab. 1984, 4, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Inamura, T.; Black, K.L. Bradykinin selectively opens blood-tumor barrier in experimental brain tumors. J. Cereb. Blood Flow. Metab. 1994, 14, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Kroll, R.A.; Pagel, M.A.; Muldoon, L.L.; Roman-Goldstein, S.; Fiamengo, S.A.; Neuwelt, E.A. Improving drug delivery to intracerebral tumor and surrounding brain in a rodent model: A comparison of osmotic versus bradykinin modification of the blood-brain and/or blood-tumor barriers. Neurosurgery 1998, 43, 879–886, discussion 886–889. [Google Scholar] [CrossRef] [PubMed]
- Inamura, T.; Nomura, T.; Bartus, R.T.; Black, K.L. Intracarotid infusion of RMP-7, a bradykinin analog: A method for selective drug delivery to brain tumors. J. Neurosurg. 1994, 81, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Snodgrass, P.; Dean, R.; Agostino, M.; Hasler, B.; Pink, M.; Xiong, H.; Kim, B.S.; Bartus, R.T. Enhanced delivery of carboplatin into brain tumours with intravenous Cereport (RMP-7): Dramatic differences and insight gained from dosing parameters. Br. J. Cancer 1999, 80, 964–970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Black, K.L.; Cloughesy, T.; Huang, S.C.; Gobin, Y.P.; Zhou, Y.; Grous, J.; Nelson, G.; Farahani, K.; Hoh, C.K.; Phelps, M. Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. J. Neurosurg. 1997, 86, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.; Osborn, C.; Barton, T.; Bleehen, N.M. A phase I study of intravenous RMP-7 with carboplatin in patients with progression of malignant glioma. Eur. J. Cancer 1998, 34, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Lind, M.; Newman, H.; Grant, R.; Hadley, D.M.; Barton, T.; Osborn, C. Phase II studies of RMP-7 and carboplatin in the treatment of recurrent high grade glioma. RMP-7 European Study Group. J. Neurooncol. 1999, 44, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Packer, R.J.; Krailo, M.; Mehta, M.; Warren, K.; Allen, J.; Jakacki, R.; Villablanca, J.G.; Chiba, A.; Reaman, G. A Phase I study of concurrent RMP-7 and carboplatin with radiation therapy for children with newly diagnosed brainstem gliomas. Cancer 2005, 104, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Prados, M.D.; Schold, S.C., Jr.; Fine, H.A.; Jaeckle, K.; Hochberg, F.; Mechtler, L.; Fetell, M.R.; Phuphanich, S.; Feun, L.; Janus, T.J.; et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003, 5, 96–103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warren, K.; Jakacki, R.; Widemann, B.; Aikin, A.; Libucha, M.; Packer, R.; Vezina, G.; Reaman, G.; Shaw, D.; Krailo, M.; et al. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: A report from the Children’s Oncology Group. Cancer Chemother. Pharmacol. 2006, 58, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Massó, S.R.; Erickson, M.A.; Banks, W.A.; Ulrich, H.; Martins, A.H. The Bradykinin B2 Receptor Agonist (NG291) Causes Rapid Onset of Transient Blood-Brain Barrier Disruption Without Evidence of Early Brain Injury. Front. Neurosci. 2021, 15, 791709. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cuddapah, V.A.; Turner, K.L.; Seifert, S.; Sontheimer, H. Bradykinin-induced chemotaxis of human gliomas requires the activation of KCa3.1 and ClC-3. J. Neurosci. 2013, 33, 1427–1440. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montana, V.; Sontheimer, H. Bradykinin promotes the chemotactic invasion of primary brain tumors. J. Neurosci. 2011, 31, 4858–4867. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seifert, S.; Sontheimer, H. Bradykinin enhances invasion of malignant glioma into the brain parenchyma by inducing cells to undergo amoeboid migration. J. Physiol. 2014, 592, 5109–5127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mills, J.H.; Thompson, L.F.; Mueller, C.; Waickman, A.T.; Jalkanen, S.; Niemela, J.; Airas, L.; Bynoe, M.S. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2008, 105, 9325–9330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carman, A.J.; Mills, J.H.; Krenz, A.; Kim, D.G.; Bynoe, M.S. Adenosine receptor signaling modulates permeability of the blood-brain barrier. J. Neurosci. 2011, 31, 13272–13280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grossman, S.A.; Romo, C.G.; Ye, X.; Kral, B.; Strowd, R.E.; Lesser, G.; Raymond, C.; Iacoboni, M.; Desideri, S.; Fisher, J.; et al. Assessing the dose of regadenoson required to transiently alter blood-brain barrier integrity in patients with infiltrating gliomas. Neurooncol. Adv. 2025, 7, vdaf041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, D.G.; Bynoe, M.S. A2A Adenosine Receptor Regulates the Human Blood-Brain Barrier Permeability. Mol. Neurobiol. 2015, 52, 664–678. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, S.; Anders, N.M.; Mangraviti, A.; Wanjiku, T.M.; Sankey, E.W.; Liu, A.; Brem, H.; Tyler, B.; Rudek, M.A.; Grossman, S.A. The effect of regadenoson-induced transient disruption of the blood-brain barrier on temozolomide delivery to normal rat brain. J. Neurooncol. 2016, 126, 433–439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, S.; George, R.T.; Lodge, M.A.; Piotrowski, A.; Wahl, R.L.; Gujar, S.K.; Grossman, S.A. The effect of regadenoson on the integrity of the human blood-brain barrier, a pilot study. J. Neurooncol. 2017, 132, 513–519. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jackson, S.; Weingart, J.; Nduom, E.K.; Harfi, T.T.; George, R.T.; McAreavey, D.; Ye, X.; Anders, N.M.; Peer, C.; Figg, W.D.; et al. The effect of an adenosine A2A agonist on intra-tumoral concentrations of temozolomide in patients with recurrent glioblastoma. Fluids Barriers CNS 2018, 15, 2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yanagida, K.; Liu, C.H.; Faraco, G.; Galvani, S.; Smith, H.K.; Burg, N.; Anrather, J.; Sanchez, T.; Iadecola, C.; Hla, T. Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc. Natl. Acad. Sci. USA 2017, 114, 4531–4536. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Proia, R.L.; Hla, T. Emerging biology of sphingosine-1-phosphate: Its role in pathogenesis and therapy. J. Clin. Investig. 2015, 125, 1379–1387. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hansen, L.; Lohfink, N.; Vutukuri, R.; Kestner, R.I.; Trautmann, S.; Hecht, M.; Wagner, P.V.; Spitzer, D.; Khel, M.I.; Macas, J.; et al. Endothelial Sphingosine-1-Phosphate Receptor 4 Regulates Blood-Brain Barrier Permeability and Promotes a Homeostatic Endothelial Phenotype. J. Neurosci. 2022, 42, 1908–1929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahringer, A.; Fricker, G. ABC transporters at the blood-brain barrier. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.; Stewart, A.J.; Dangerfield, W.; Okiji, S.; Liddle, C.; Bootle, D.; Plumb, J.A.; Templeton, D.; Charlton, P. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001, 61, 749–758. [Google Scholar] [PubMed]
- Chen, Z.S.; Lee, K.; Walther, S.; Raftogianis, R.B.; Kuwano, M.; Zeng, H.; Kruh, G.D. Analysis of methotrexate and folate transport by multidrug resistance protein 4 (ABCC4): MRP4 is a component of the methotrexate efflux system. Cancer Res. 2002, 62, 3144–3150. [Google Scholar] [PubMed]
- Stacy, A.E.; Jansson, P.J.; Richardson, D.R. Molecular pharmacology of ABCG2 and its role in chemoresistance. Mol. Pharmacol. 2013, 84, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Unadkat, J.D. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport—An update. AAPS J. 2015, 17, 65–82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Gooijer, M.C.; de Vries, N.A.; Buckle, T.; Buil, L.C.M.; Beijnen, J.H.; Boogerd, W.; van Tellingen, O. Improved Brain Penetration and Antitumor Efficacy of Temozolomide by Inhibition of ABCB1 and ABCG2. Neoplasia 2018, 20, 710–720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fellner, S.; Bauer, B.; Miller, D.S.; Schaffrik, M.; Fankhänel, M.; Spruss, T.; Bernhardt, G.; Graeff, C.; Fricker, G. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Investig. 2002, 110, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Borst, P. Looking back at multidrug resistance (MDR) research and ten mistakes to be avoided when writing about ABC transporters in MDR. FEBS Lett. 2020, 594, 4001–4011. [Google Scholar] [CrossRef] [PubMed]
- Bajraktari-Sylejmani, G.; Kamaraj, R.; Theile, D.; Pávek, P.; Weiss, J. The “specific” P-glycoprotein inhibitor zosuquidar (LY335979) also weakly inhibits human organic cation transporters. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 7147–7153. [Google Scholar] [CrossRef] [PubMed]
- Lentzas, A.; de Gooijer, M.C.; Zuidema, S.; Çitirikkaya, C.H.; Venekamp, N.; Beijnen, J.H.; van Tellingen, O. P10.21.A the ABCB1/ABCG2 inhibitor elacridar is a more potent pharmacoenhancer compared to tariquidar for treatment of intracranial tumors with small molecule drugs. Neuro Oncol. 2023, 25 (Suppl. S2), ii67. [Google Scholar] [CrossRef] [PubMed Central]
- Kemper, E.M.; van Zandbergen, A.E.; Cleypool, C.; Mos, H.A.; Boogerd, W.; Beijnen, J.H.; van Tellingen, O. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin. Cancer Res. 2003, 9, 2849–2855. [Google Scholar] [PubMed]
- Hubensack, M.; Müller, C.; Höcherl, P.; Fellner, S.; Spruss, T.; Bernhardt, G.; Buschauer, A. Effect of the ABCB1 modulators elacridar and tariquidar on the distribution of paclitaxel in nude mice. J. Cancer Res. Clin. Oncol. 2008, 134, 597–607. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, E.A.; van Schaik, R.; Edelbroek, P.M.; Redeker, S.; Aronica, E.; Wadman, W.J.; Marchi, N.; Vezzani, A.; Gorter, J.A. Inhibition of the multidrug transporter P-glycoprotein improves seizure control in phenytoin-treated chronic epileptic rats. Epilepsia 2006, 47, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Brouwer, K.R.; McNamara, P.J. GF120918, a P-glycoprotein modulator, increases the concentration of unbound amprenavir in the central nervous system in rats. Antimicrob. Agents Chemother. 2002, 46, 2284–2286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Agarwal, S.; Mittapalli, R.K.; Zellmer, D.M.; Gallardo, J.L.; Donelson, R.; Seiler, C.; Decker, S.A.; Santacruz, K.S.; Pokorny, J.L.; Sarkaria, J.N.; et al. Active efflux of Dasatinib from the brain limits efficacy against murine glioblastoma: Broad implications for the clinical use of molecularly targeted agents. Mol. Cancer Ther. 2012, 11, 2183–2192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lagas, J.S.; van Waterschoot, R.A.; Sparidans, R.W.; Wagenaar, E.; Beijnen, J.H.; Schinkel, A.H. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol. Cancer Ther. 2010, 9, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, R.K.; Mittapalli, R.K.; Elmquist, W.F. Pharmacokinetic assessment of efflux transport in sunitinib distribution to the brain. J. Pharmacol. Exp. Ther. 2013, 347, 755–764. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, F.; de Gooijer, M.C.; Roig, E.M.; Buil, L.C.; Christner, S.M.; Beumer, J.H.; Würdinger, T.; Beijnen, J.H.; van Tellingen, O. ABCB1, ABCG2, and PTEN determine the response of glioblastoma to temozolomide and ABT-888 therapy. Clin. Cancer Res. 2014, 20, 2703–2713. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.K.; Kim, M.J.; Kim, M.S.; Lee, J.H.; Chung, S.J.; Song, J.S.; Chae, Y.J.; Lee, K.R. Role of the efflux transporters Abcb1 and Abcg2 in the brain distribution of olaparib in mice. Eur. J. Pharm. Sci. 2022, 173, 106177. [Google Scholar] [CrossRef] [PubMed]
- de Vries, N.A.; Zhao, J.; Kroon, E.; Buckle, T.; Beijnen, J.H.; van Tellingen, O. P-glycoprotein and breast cancer resistance protein: Two dominant transporters working together in limiting the brain penetration of topotecan. Clin. Cancer Res. 2007, 13, 6440–6449. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Regulation of ABC transporters at the blood-brain barrier. Clin. Pharmacol. Ther. 2015, 97, 395–403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bauer, M.; Zeitlinger, M.; Karch, R.; Matzneller, P.; Stanek, J.; Jäger, W.; Böhmdorfer, M.; Wadsak, W.; Mitterhauser, M.; Bankstahl, J.P.; et al. Pgp-mediated interaction between (R)-[11C]verapamil and tariquidar at the human blood-brain barrier: A comparison with rat data. Clin. Pharmacol. Ther. 2012, 91, 227–233. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreisl, W.C.; Liow, J.S.; Kimura, N.; Seneca, N.; Zoghbi, S.S.; Morse, C.L.; Herscovitch, P.; Pike, V.W.; Innis, R.B. P-glycoprotein function at the blood-brain barrier in humans can be quantified with the substrate radiotracer 11C-N-desmethyl-loperamide. J. Nucl. Med. 2010, 51, 559–566. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wagner, C.C.; Bauer, M.; Karch, R.; Feurstein, T.; Kopp, S.; Chiba, P.; Kletter, K.; Löscher, W.; Müller, M.; Zeitlinger, M.; et al. A pilot study to assess the efficacy of tariquidar to inhibit P-glycoprotein at the human blood-brain barrier with (R)-11C-verapamil and PET. J. Nucl. Med. 2009, 50, 1954–1961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deli, M.A.; Abrahám, C.S.; Niwa, M.; Falus, A. N,N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine increases the permeability of primary mouse cerebral endothelial cell monolayers. Inflamm. Res. 2003, 52 (Suppl. S1), S39–S40. [Google Scholar] [CrossRef] [PubMed]
- Deli, M.A.; Németh, L.; Falus, A.; Abrahám, C.S. Effects of N,N-diethyl-2-[4-(phenylmethyl)phenoxy]ethanamine on the blood-brain barrier permeability in the rat. Eur. J. Pharmacol. 2000, 387, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.R.; Veszelka, S.; Pásztói, M.; Péterfi, Z.A.; Tóth, A.; Rákhely, G.; Cervenak, L.; Ábrahám, C.S.; Deli, M.A. Tesmilifene modifies brain endothelial functions and opens the blood-brain/blood-glioma barrier. J. Neurochem. 2015, 134, 1040–1054. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.; Palazzo, C.; Evrard, B.; Piel, G. Nanocarriers for the treatment of glioblastoma multiforme: Current state-of-the-art. J. Control. Release 2016, 227, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Wang, K.; Stephen, Z.R.; Mu, Q.; Kievit, F.M.; Chiu, D.T.; Press, O.W.; Zhang, M. Temozolomide nanoparticles for targeted glioblastoma therapy. ACS Appl. Mater. Interfaces 2015, 7, 6674–6682. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091–8108. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narsinh, K.H.; Perez, E.; Haddad, A.F.; Young, J.S.; Savastano, L.; Villanueva-Meyer, J.E.; Winkler, E.; de Groot, J. Strategies to Improve Drug Delivery Across the Blood-Brain Barrier for Glioblastoma. Curr. Neurol. Neurosci. Rep. 2024, 24, 123–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iliff, J.J.; Nedergaard, M. Is there a cerebral lymphatic system? Stroke 2013, 44 (Suppl. S1), S93–S95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schumann, T.; König, J.; Henke, C.; Willmes, D.M.; Bornstein, S.R.; Jordan, J.; Fromm, M.F.; Birkenfeld, A.L. Solute Carrier Transporters as Potential Targets for the Treatment of Metabolic Disease. Pharmacol. Rev. 2020, 72, 343–379. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Ohtsuki, S.; Katsukura, Y.; Ikeda, C.; Suzuki, T.; Kamiie, J.; Terasaki, T. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J. Neurochem. 2011, 117, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Cornford, E.M.; Hyman, S.; Cornford, M.E.; Caron, M.J. Glut1 glucose transporter activity in human brain injury. J. Neurotrauma 1996, 13, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Komaki, S.; Sugita, Y.; Furuta, T.; Yamada, K.; Moritsubo, M.; Abe, H.; Akiba, J.; Miyagi, N.; Nakamura, H.; Miyoshi, H.; et al. Expression of GLUT1 in Pseudopalisaded and Perivascular Tumor Cells Is an Independent Prognostic Factor for Patients with Glioblastomas. J. Neuropathol. Exp. Neurol. 2019, 78, 389–397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Salas-Burgos, A.; Iserovich, P.; Zuniga, F.; Vera, J.C.; Fischbarg, J. Predicting the three-dimensional structure of the human facilitative glucose transporter glut1 by a novel evolutionary homology strategy: Insights on the molecular mechanism of substrate migration, and binding sites for glucose and inhibitory molecules. Biophys. J. 2004, 87, 2990–2999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xie, F.; Yao, N.; Qin, Y.; Zhang, Q.; Chen, H.; Yuan, M.; Tang, J.; Li, X.; Fan, W.; Zhang, Q.; et al. Investigation of glucose-modified liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int. J. Nanomed. 2012, 7, 163–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, Y.; Fan, W.; Chen, H.; Yao, N.; Tang, W.; Tang, J.; Yuan, W.; Kuai, R.; Zhang, Z.; Wu, Y.; et al. In vitro and in vivo investigation of glucose-mediated brain-targeting liposomes. J. Drug Target. 2010, 18, 536–549. [Google Scholar] [CrossRef] [PubMed]
- Simpson, I.A.; Appel, N.M.; Hokari, M.; Oki, J.; Holman, G.D.; Maher, F.; Koehler-Stec, E.M.; Vannucci, S.J.; Smith, Q.R. Blood-brain barrier glucose transporter: Effects of hypo- and hyperglycemia revisited. J. Neurochem. 1999, 72, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Anraku, Y.; Kuwahara, H.; Fukusato, Y.; Mizoguchi, A.; Ishii, T.; Nitta, K.; Matsumoto, Y.; Toh, K.; Miyata, K.; Uchida, S.; et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nat. Commun. 2017, 8, 1001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiley, D.T.; Webster, P.; Gale, A.; Davis, M.E. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 8662–8667. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kozlovskaya, L.; Stepensky, D. Quantitative analysis of the brain-targeted delivery of drugs and model compounds using nano-delivery systems. J. Control. Release 2013, 171, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Min, H.S.; Kim, H.J.; Naito, M.; Ogura, S.; Toh, K.; Hayashi, K.; Kim, B.S.; Fukushima, S.; Anraku, Y.; Miyata, K.; et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew. Chem. Int. Ed. Engl. 2020, 59, 8173–8180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donihi, A.C.; Raval, D.; Saul, M.; Korytkowski, M.T.; DeVita, M.A. Prevalence and predictors of corticosteroid-related hyperglycemia in hospitalized patients. Endocr. Pract. 2006, 12, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Peura, L.; Malmioja, K.; Laine, K.; Leppänen, J.; Gynther, M.; Isotalo, A.; Rautio, J. Large amino acid transporter 1 (LAT1) prodrugs of valproic acid: New prodrug design ideas for central nervous system delivery. Mol. Pharm. 2011, 8, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Killian, D.M.; Chikhale, P.J. Predominant functional activity of the large, neutral amino acid transporter (LAT1) isoform at the cerebrovasculature. Neurosci. Lett. 2001, 306, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Youland, R.S.; Kitange, G.J.; Peterson, T.E.; Pafundi, D.H.; Ramiscal, J.A.; Pokorny, J.L.; Giannini, C.; Laack, N.N.; Parney, I.F.; Lowe, V.J.; et al. The role of LAT1 in 18F-DOPA uptake in malignant gliomas. J. Neurooncol. 2013, 111, 11–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedman, H.S.; Colvin, O.M.; Ludeman, S.M.; Schold, S.C., Jr.; Boyd, V.L.; Mulhbaier, L.H.; Bigner, D.D. Experimental chemotherapy of human medulloblastoma with classical alkylators. Cancer Res. 1986, 46, 2827–2833. [Google Scholar] [PubMed]
- Greig, N.H.; Momma, S.; Sweeney, D.J.; Smith, Q.R.; Rapoport, S.I. Facilitated transport of melphalan at the rat blood-brain barrier by the large neutral amino acid carrier system. Cancer Res. 1987, 47, 1571–1576. [Google Scholar] [PubMed]
- Kurpad, S.N.; Friedman, H.S.; Archer, G.E.; McLendon, R.E.; Petros, W.M.; Fuchs, H.E.; Guaspari, A.; Bigner, D.D. Intraarterial administration of melphalan for treatment of intracranial human glioma xenografts in athymic rats. Cancer Res. 1995, 55, 3803–3809. [Google Scholar] [PubMed]
- Chamberlain, M.C.; Prados, M.D.; Silver, P.; Levin, V.A. A phase II trial of oral melphalan in recurrent primary brain tumors. Am. J. Clin. Oncol. 1988, 11, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Rodriguez, M.; Zhao, G.; Yao, T.W.; Fischer, W.N.; Jandeleit, B.; Koller, K.; Nicolaides, T. A Novel Blood-Brain Barrier-Permeable Chemotherapeutic Agent for the Treatment of Glioblastoma. Cureus 2021, 13, e17595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahman, R.; Trippa, L.; Aquilanti, E.; Lee, E.; Arrillaga-Romany, I.; Wiley, J.; Chukwueke, U.; McFaline-Figueroa, J.; Partridge, K.; Beroukhim, R.; et al. Safety Lead-In Results for QBS10072S in the Individual Screening Trial of Innovative Glioblastoma Therapy (INSIGHT) Trial, a Randomized Adaptive Platform Trial for Newly Diagnosed MGMT Unmethylated Glioblastoma. Neuro Oncol. 2024, 26 (Suppl. S8), viii110. [Google Scholar] [CrossRef]
- Kharya, P.; Jain, A.; Gulbake, A.; Shilpi, S.; Jain, A.; Hurkat, P.; Majumdar, S.; Jain, S.K. Phenylalanine-coupled solid lipid nanoparticles for brain tumor targeting. J. Nanopart Res. 2013, 15, 1–11. [Google Scholar] [CrossRef]
- Wu, W.; Dong, Y.; Gao, J.; Gong, M.; Zhang, X.; Kong, W.; Li, Y.; Zeng, Y.; Si, D.; Wei, Z.; et al. Aspartate-modified doxorubicin on its N-terminal increases drug accumulation in LAT1-overexpressing tumors. Cancer Sci. 2015, 106, 747–756. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, L.; Di, X.; Zhang, S.; Kan, Q.; Liu, H.; Lu, T.; Wang, Y.; Fu, Q.; Sun, J.; He, Z. Large amino acid transporter 1 mediated glutamate modified docetaxel-loaded liposomes for glioma targeting. Colloids Surf. B Biointerfaces 2016, 141, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Teerawonganan, P.; Hasriadi Dasuni Wasana, P.W.; Angsuwattana, P.; Suksamrarn, A.; Nalinratana, N.; Vajragupta, O.; Towiwat, P.; Thitikornpong, W.; Rojsitthisak, P. Synthesis, Cytotoxicity, and Mechanistic Evaluation of Tetrahydrocurcumin-Amino Acid Conjugates as LAT1-Targeting Anticancer Agents in C6 Glioma Cells. Int. J. Mol. Sci. 2024, 25, 11266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Cheng, Q.; Xue, Y.; Yao, K.; Syeda, M.Z.; Xu, J.; Wu, J.; Wang, Z.; Tang, L.; Mu, Q. LAT1-targeted brain delivery of temozolomide and sorafenib for effective glioma therapy. Nano Res. 2023, 16, 9743–9751. [Google Scholar] [CrossRef]
- Lim, Y.N.; Ryu, I.S.; Jung, Y.J.; Helmlinger, G.; Kim, I.; Park, H.W.; Kang, H.; Lee, J.; Lee, H.J.; Lee, K.S.; et al. l-Type amino acid transporter 1-targeting nanoparticles for antisense oligonucleotide delivery to the CNS. Mol. Ther. Nucleic Acids 2024, 35, 102340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, D.D.; Smith, Q.R. Characterization of the blood-brain barrier choline transporter using the in situ rat brain perfusion technique. J. Neurochem. 2001, 76, 1032–1041, Erratum in J. Neurochem. 2001, 77, 704. [Google Scholar] [CrossRef] [PubMed]
- Iwao, B.; Yara, M.; Hara, N.; Kawai, Y.; Yamanaka, T.; Nishihara, H.; Inoue, T.; Inazu, M. Functional expression of choline transporter like-protein 1 (CTL1) and CTL2 in human brain microvascular endothelial cells. Neurochem. Int. 2016, 93, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Zhang, Z.; Tachikawa, M.; Terasaki, T. Quantitative targeted absolute proteomics of rat blood-cerebrospinal fluid barrier transporters: Comparison with a human specimen. J. Neurochem. 2015, 134, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Machová, E.; O’Regan, S.; Newcombe, J.; Meunier, F.M.; Prentice, J.; Dove, R.; Lisá, V.; Dolezal, V. Detection of choline transporter-like 1 protein CTL1 in neuroblastoma x glioma cells and in the CNS, and its role in choline uptake. J. Neurochem. 2009, 110, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, H.; Zhang, Y.; Jiang, X.; Guo, Y.; An, S.; Ma, H.; He, X.; Jiang, C. Choline Derivate-Modified Doxorubicin Loaded Micelle for Glioma Therapy. ACS Appl. Mater. Interfaces 2015, 7, 21589–21601. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Qin, M.; Xu, D.; Wang, L.; Liu, C.; Ren, J.; Zhou, G.; Chen, C.; Yang, F.; Li, Y.; et al. A Bioinspired Platform for Effective Delivery of Protein Therapeutics to the Central Nervous System. Adv. Mater. 2019, 31, e1807557. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Chao, Y.; Zhao, H.; Zhou, X.; Zhang, F.; Zhang, Z.; Li, Z.; Pan, J.; Wang, J.; Chen, Q.; et al. Smart Nanomedicine to Enable Crossing Blood-Brain Barrier Delivery of Checkpoint Blockade Antibody for Immunotherapy of Glioma. ACS Nano 2022, 16, 664–674. [Google Scholar] [CrossRef] [PubMed]
- Inano, A.; Sai, Y.; Nikaido, H.; Hasimoto, N.; Asano, M.; Tsuji, A.; Tamai, I. Acetyl-L-carnitine permeability across the blood-brain barrier and involvement of carnitine transporter OCTN2. Biopharm. Drug Dispos. 2003, 24, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Juraszek, B.; Czarnecka-Herok, J.; Nałęcz, K.A. Glioma cells survival depends both on fatty acid oxidation and on functional carnitine transport by SLC22A5. J. Neurochem. 2021, 156, 642–657. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Hou, Y.; Yao, Q.; Guo, W.; Wang, G.; Wang, M.; Fu, Q.; He, Z.; Ganapathy, V.; Sun, J. L-Carnitine-conjugated nanoparticles to promote permeation across blood-brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Guan, X. Glutathione transporter as a target for brain drug delivery. Med. Chem. Res. 2024, 33, 1281–1291. [Google Scholar] [CrossRef]
- Okamura, T.; Okada, M.; Kikuchi, T.; Wakizaka, H.; Zhang, M.R. Mechanisms of glutathione-conjugate efflux from the brain into blood: Involvement of multiple transporters in the course. J. Cereb. Blood Flow. Metab. 2020, 40, 116–125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Gaillard, P.J.; de Lange, E.C.M.; Hammarlund-Udenaes, M. Targeted brain delivery of methotrexate by glutathione PEGylated liposomes: How can the formulation make a difference? Eur. J. Pharm. Biopharm. 2019, 139, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, P.J.; Appeldoorn, C.C.; Dorland, R.; van Kregten, J.; Manca, F.; Vugts, D.J.; Windhorst, B.; van Dongen, G.A.; de Vries, H.E.; Maussang, D.; et al. Pharmacokinetics, brain delivery, and efficacy in brain tumor-bearing mice of glutathione pegylated liposomal doxorubicin (2B3-101). PLoS ONE 2014, 9, e82331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rip, J.; Chen, L.; Hartman, R.; van den Heuvel, A.; Reijerkerk, A.; van Kregten, J.; van der Boom, B.; Appeldoorn, C.; de Boer, M.; Maussang, D.; et al. Glutathione PEGylated liposomes: Pharmacokinetics and delivery of cargo across the blood-brain barrier in rats. J. Drug Target. 2014, 22, 460–467. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brandsma, D.; Dieras, V.; Altintas, S.; Anders, C.; Arnedos, M.; Gelderblom, H.; Soetekouw, P.; Jager, A.; van Linde, M.; Aftimos, P. P08.03: 2B3-101, glutathione pegylated liposomal doxorubicin, in patients with recurrent high grade gliomas and breast cancer brain metastases. Neuro Oncol. 2014, 16 (Suppl. S2), ii50–ii51. [Google Scholar] [CrossRef] [PubMed Central]
- Brandsma, D.; Kerklaan, B.M.; Diéras, V.; Altintas, S.; Anders, C.K.; Ballester, M.A.; Gelderblom, H.; Soetekouw, P.M.M.B.; Gladdines, W.; Lonnqvist, F.; et al. Phase 1/2A study of glutathione pegylated liposomal doxorubicin (2B3-101) in patients with brain metastases (BM) from solid tumors or recurrent high grade gliomas (HGG). Ann. Oncol. 2014, 25, iv157. [Google Scholar] [CrossRef]
- Zhuo, W.; Wang, W.; Zhou, W.; Duan, Z.; He, S.; Zhang, X.; Yi, L.; Zhang, R.; Guo, A.; Gou, X.; et al. A Targeted and Responsive Nanoprodrug Delivery System for Synergistic Glioma Chemotherapy. Small 2024, 20, e2400630. [Google Scholar] [CrossRef] [PubMed]
- Hervé, F.; Ghinea, N.; Scherrmann, J.M. CNS delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Triguero, D.; Buciak, J.L.; Pardridge, W.M. Cationization of immunoglobulin G results in enhanced organ uptake of the protein after intravenous administration in rats and primate. J. Pharmacol. Exp. Ther. 1991, 258, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wan, J.; Zhang, Q.; She, Z.; Jiang, X. Aclarubicin-loaded cationic albumin-conjugated pegylated nanoparticle for glioma chemotherapy in rats. Int. J. Cancer 2007, 120, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Qi, S.; Zhang, H.; Li, Z.; Wang, K.; Zhu, T.; Ye, R.; Zhang, W.; Huang, G.; Yi, G.Z. Albumin-bound paclitaxel augment temozolomide treatment sensitivity of glioblastoma cells by disrupting DNA damage repair and promoting ferroptosis. J. Exp. Clin. Cancer Res. 2023, 42, 285, Erratum in J. Exp. Clin. Cancer Res. 2023, 42, 314. https://doi.org/10.1186/s13046-023-02905-9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooper, I.; Schnaider-Beeri, M.; Fridkin, M.; Shechter, Y. Albumin-Methotrexate Prodrug Analogues That Undergo Intracellular Reactivation Following Entrance into Cancerous Glioma Cells. Pharmaceutics 2021, 14, 71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, Z.; Du, Y.; Lei, L.; Xia, X.; Wang, X.; Tong, F.; Li, Y.; Gao, H. Co-delivery of ibrutinib and hydroxychloroquine by albumin nanoparticles for enhanced chemotherapy of glioma. Int. J. Pharm. 2023, 630, 122436. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Sun, Q.; Wan, J.; She, Z.; Jiang, X.G. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006, 66, 11878–11887. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.N.; Mehta, R.R.; Yamada, T.; Lekmine, F.; Christov, K.; Chakrabarty, A.M.; Green, A.; Bratescu, L.; Shilkaitis, A.; Beattie, C.W.; et al. Noncationic peptides obtained from azurin preferentially enter cancer cells. Cancer Res. 2009, 69, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Robinson, S.M.; Nath, A. Permeability of the blood-brain barrier to HIV-1 Tat. Exp. Neurol. 2005, 193, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, L.; Zhao, L.; Liao, J.; Zhao, C.; Xu, X.; Wang, F.; Zhang, X. A Novel Blood-Brain Barrier-Penetrating and Vascular-Targeting Chimeric Peptide Inhibits Glioma Angiogenesis. Int. J. Mol. Sci. 2023, 24, 8753. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaraíz-Rodríguez, M.; Talaverón, R.; García-Vicente, L.; Pelaz, S.G.; Domínguez-Prieto, M.; Álvarez-Vázquez, A.; Flores-Hernández, R.; Sin, W.C.; Bechberger, J.; Medina, J.M.; et al. Connexin43 peptide, TAT-Cx43266-283, selectively targets glioma cells, impairs malignant growth, and enhances survival in mouse models in vivo. Neuro Oncol. 2020, 22, 493–504, Erratum in Neuro Oncol. 2021, 23, 1414. https://doi.org/10.1093/neuonc/noaa087. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelaz, S.G.; Jaraíz-Rodríguez, M.; Álvarez-Vázquez, A.; Talaverón, R.; García-Vicente, L.; Flores-Hernández, R.; Gómez de Cedrón, M.; Tabernero, M.; Ramírez de Molina, A.; Lillo, C.; et al. Targeting metabolic plasticity in glioma stem cells in vitro and in vivo through specific inhibition of c-Src by TAT-Cx43266-283. EBioMedicine 2020, 62, 103134, Erratum in EBioMedicine 2021, 74, 103752. https://doi.org/10.1016/j.ebiom.2021.103752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mander, S.; Gorman, G.S.; Coward, L.U.; Christov, K.; Green, A.; Das Gupta, T.K.; Yamada, T. The brain-penetrant cell-cycle inhibitor p28 sensitizes brain metastases to DNA-damaging agents. Neurooncol. Adv. 2023, 5, vdad042. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, Q.; Gallo, J.M. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro Oncol. 2009, 11, 301–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Q.; Rager, J.D.; Weinstein, K.; Kardos, P.S.; Dobson, G.L.; Li, J.; Hidalgo, I.J. Evaluation of the MDR-MDCK cell line as a permeability screen for the blood-brain barrier. Int. J. Pharm. 2005, 288, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Warso, M.A.; Richards, J.M.; Mehta, D.; Christov, K.; Schaeffer, C.; Rae Bressler, L.; Yamada, T.; Majumdar, D.; Kennedy, S.A.; Beattie, C.W.; et al. A first-in-class, first-in-human, phase I trial of p28, a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in patients with advanced solid tumours. Br. J. Cancer 2013, 108, 1061–1070. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhu, Z.; Zhai, Y.; Hao, Y.; Wang, Q.; Han, F.; Zheng, W.; Hong, J.; Cui, L.; Jin, W.; Ma, S.; et al. Specific anti-glioma targeted-delivery strategy of engineered small extracellular vesicles dual-functionalised by Angiopep-2 and TAT peptides. J. Extracell. Vesicles 2022, 11, e12255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, W.; Yin, G.; Pu, X.; Chen, X.; Liao, X.; Huang, Z. Glioma targeted delivery strategy of doxorubicin-loaded liposomes by dual-ligand modification. J. Biomater. Sci. Polym. Ed. 2017, 28, 1695–1712. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Ma, C.; Bai, E.; Yang, K.; Xu, R. Transferrin and cell-penetrating peptide dual-functioned liposome for targeted drug delivery to glioma. Int. J. Clin. Exp. Med. 2015, 8, 1658–1668. [Google Scholar] [PubMed] [PubMed Central]
- Mei, L.; Zhang, Q.; Yang, Y.; He, Q.; Gao, H. Angiopep-2 and activatable cell penetrating peptide dual modified nanoparticles for enhanced tumor targeting and penetrating. Int. J. Pharm. 2014, 474, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.; Gautam, A. CPPsite 2.0: A repository of experimentally validated cell-penetrating peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pardridge, W.M. Receptor-mediated peptide transport through the blood-brain barrier. Endocr. Rev. 1986, 7, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Lui, P.C.; Li, J.Y. Receptor-mediated therapeutic transport across the blood-brain barrier. Immunotherapy 2009, 1, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.R.; Puri, R.K. Interleukin-13 receptor-directed cytotoxin for malignant glioma therapy: From bench to bedside. J. Neurooncol. 2003, 65, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Delorme-Walker, V.D.; Milner, R. β1 integrin is essential for blood-brain barrier integrity under stable and vascular remodelling conditions; effects differ with age. Fluids Barriers CNS 2023, 20, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khoury, N.; Pizzo, M.E.; Discenza, C.B.; Joy, D.; Tatarakis, D.; Todorov, M.I.; Negwer, M.; Ha, C.; De Melo, G.L.; Sarrafha, L.; et al. Fc-engineered large molecules targeting blood-brain barrier transferrin receptor and CD98hc have distinct central nervous system and peripheral biodistribution. Nat. Commun. 2025, 16, 1822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Calzolari, A.; Larocca, L.M.; Deaglio, S.; Finisguerra, V.; Boe, A.; Raggi, C.; Ricci-Vitani, L.; Pierconti, F.; Malavasi, F.; De Maria, R.; et al. Transferrin receptor 2 is frequently and highly expressed in glioblastomas. Transl. Oncol. 2010, 3, 123–134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bailey, S.; Evans, R.W.; Garratt, R.C.; Gorinsky, B.; Hasnain, S.; Horsburgh, C.; Jhoti, H.; Lindley, P.F.; Mydin, A.; Sarra, R.; et al. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry 1988, 27, 5804–5812. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qin, L.; Wang, C.Z.; Fan, H.J.; Zhang, C.J.; Zhang, H.W.; Lv, M.H.; Cui, S.D. A dual-targeting liposome conjugated with transferrin and arginine-glycine-aspartic acid peptide for glioma-targeting therapy. Oncol. Lett. 2014, 8, 2000–2006. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gomes, M.J.; Kennedy, P.J.; Martins, S.; Sarmento, B. Delivery of siRNA silencing P-gp in peptide-functionalized nanoparticles causes efflux modulation at the blood-brain barrier. Nanomedicine 2017, 12, 1385–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Hu, L.; Yin, Q.; Feng, L.; Li, Y. Transferrin-modified c[RGDfK]-paclitaxel loaded hybrid micelle for sequential blood-brain barrier penetration and glioma targeting therapy. Mol. Pharm. 2012, 9, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Li, L.M.; Han, M.; Tang, X.J.; Yao, J.N.; Ying, X.Y.; Li, F.Z.; Gao, J.Q. Characteristics of sequential targeting of brain glioma for transferrin-modified cisplatin liposome. Int. J. Pharm. 2013, 444, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sonali Singh, R.P.; Singh, N.; Sharma, G.; Vijayakumar, M.R.; Koch, B.; Singh, S.; Singh, U.; Dash, D.; Pandey, B.L.; Muthu, M.S. Transferrin liposomes of docetaxel for brain-targeted cancer applications: Formulation and brain theranostics. Drug Deliv. 2016, 23, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Porru, M.; Zappavigna, S.; Salzano, G.; Luce, A.; Stoppacciaro, A.; Balestrieri, M.L.; Artuso, S.; Lusa, S.; De Rosa, G.; Leonetti, C.; et al. Medical treatment of orthotopic glioblastoma with transferrin-conjugated nanoparticles encapsulating zoledronic acid. Oncotarget 2014, 5, 10446–10459. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, W.; Guo, L. Immobilized transferrin Fe3O4@SiO2 nanoparticle with high doxorubicin loading for dual-targeted tumor drug delivery. Int. J. Nanomed. 2013, 8, 4631–4639. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luo, M.; Lewik, G.; Ratcliffe, J.C.; Choi, C.H.J.; Mäkilä, E.; Tong, W.Y.; Voelcker, N.H. Systematic Evaluation of Transferrin-Modified Porous Silicon Nanoparticles for Targeted Delivery of Doxorubicin to Glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 33637–33649. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Mao, J.; Jiang, Z.; Sun, T.; Hu, Y.; Jiang, Z.; Zhang, C.; Dong, J.; Huang, Q.; Lan, Q. Transferrin-modified Doxorubicin-loaded biodegradable nanoparticles exhibit enhanced efficacy in treating brain glioma-bearing rats. Cancer Biother. Radiopharm. 2013, 28, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.J.; Thayer, M.B.; Kim, C.; Tatarakis, D.; Simon, M.J.; Dial, R.; Nilewski, L.; Wells, R.C.; Zhou, Y.; Afetian, M.; et al. Targeting the transferrin receptor to transport antisense oligonucleotides across the mammalian blood-brain barrier. Sci. Transl. Med. 2024, 16, eadi2245. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Wu, H.; Li, Y.; Huang, Y.; Yao, L.; Chen, X.; Han, X.; Zhou, Y.; Du, Z. Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy. Oncotarget 2017, 8, 74451–74465. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 2018, 9, 1991. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friden, P.M.; Walus, L.R.; Musso, G.F.; Taylor Ma Malfroy, B.; Starzyk, R.M. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood-brain barrier. Proc. Natl. Acad. Sci. USA 1991, 88, 4771–4775. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Portilla-Arias, J.; Ding, H.; Inoue, S.; Konda, B.; Hu, J.; Wawrowsky, K.A.; Shin, P.K.; Black, K.L.; Holler, E.; et al. Temozolomide delivery to tumor cells by a multifunctional nano vehicle based on poly(β-L-malic acid). Pharm. Res. 2010, 27, 2317–2329. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pardridge, W.M.; Buciak, J.L.; Friden, P.M. Selective transport of an anti-transferrin receptor antibody through the blood-brain barrier in vivo. J. Pharmacol. Exp. Ther. 1991, 259, 66–70. [Google Scholar] [CrossRef]
- Ashrafzadeh, M.S.; Akbarzadeh, A.; Heydarinasab, A.; Ardjmand, M. In vivo Glioblastoma Therapy Using Targeted Liposomal Cisplatin. Int. J. Nanomed. 2020, 15, 7035–7049. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramalho, M.J.; Sevin, E.; Gosselet, F.; Lima, J.; Coelho, M.A.N.; Loureiro, J.A.; Pereira, M.C. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int. J. Pharm. 2018, 545, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Gosk, S.; Vermehren, C.; Storm, G.; Moos, T. Targeting anti-transferrin receptor antibody (OX26) and OX26-conjugated liposomes to brain capillary endothelial cells using in situ perfusion. J. Cereb. Blood Flow. Metab. 2004, 24, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Haqqani, A.S.; Thom, G.; Burrell, M.; Delaney, C.E.; Brunette, E.; Baumann, E.; Sodja, C.; Jezierski, A.; Webster, C.; Stanimirovic, D.B. Intracellular sorting and transcytosis of the rat transferrin receptor antibody OX26 across the blood-brain barrier in vitro is dependent on its binding affinity. J. Neurochem. 2018, 146, 735–752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, H.Y.; Wu, S.; Li, Y.; Zhang, W.; Burrell, M.; Webster, C.I.; Shah, D.K. Brain pharmacokinetics of anti-transferrin receptor antibody affinity variants in rats determined using microdialysis. MAbs 2021, 13, 1874121. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petersen, I.; Morrison, J.I.; Petrovic, A.; Babic, N.; Metzendorf, N.G.; Godec, A.; de la Rosa, A.; Rofo, F.; Bondza, S.; Buijs, J.; et al. A shorter linker in the bispecific antibody RmAb158-scFv8D3 improves TfR-mediated blood-brain barrier transcytosis in vitro. Sci. Rep. 2024, 14, 30613. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Morrison, J.I.; Metzendorf, N.G.; Rofo, F.; Petrovic, A.; Hultqvist, G. A single-chain fragment constant design enables easy production of a monovalent blood-brain barrier transporter and provides an improved brain uptake at elevated doses. J. Neurochem. 2023, 165, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Hultqvist, G.; Syvänen, S.; Fang, X.T.; Lannfelt, L.; Sehlin, D. Bivalent Brain Shuttle Increases Antibody Uptake by Monovalent Binding to the Transferrin Receptor. Theranostics 2017, 7, 308–318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Johnsen, K.B.; Bak, M.; Kempen, P.J.; Melander, F.; Burkhart, A.; Thomsen, M.S.; Nielsen, M.S.; Moos, T.; Andresen, T.L. Antibody affinity and valency impact brain uptake of transferrin receptor-targeted gold nanoparticles. Theranostics 2018, 8, 3416–3436. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, P.; Anami, Y.; Gao, P.; Fan, X.; Li, L.; Tsuchikama, K.; Zhang, N.; An, Z. Enhanced anti-angiogenetic effect of transferrin receptor-mediated delivery of VEGF-trap in a glioblastoma mouse model. MAbs 2022, 14, 2057269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keunen, O.; Johansson, M.; Oudin, A.; Sanzey, M.; Rahim, S.A.; Fack, F.; Thorsen, F.; Taxt, T.; Bartos, M.; Jirik, R.; et al. Anti-VEGF treatment reduces blood supply and increases tumor cell invasion in glioblastoma. Proc. Natl. Acad. Sci. USA 2011, 108, 3749–3754. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, J.L.; Dong, Z.X.; Chen, Y.R.; Lin, M.H.; Liu, Y.C.; Roffler, S.R.; Lin, W.W.; Chang, C.Y.; Tzou, S.C.; Cheng, T.L.; et al. pH-Responsive Polyethylene Glycol Engagers for Enhanced Brain Delivery of PEGylated Nanomedicine to Treat Glioblastoma. ACS Nano 2025, 19, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.J.; Atwal, J.K.; Zhang, Y.; Tong, R.K.; Wildsmith, K.R.; Tan, C.; Bien-Ly, N.; Hersom, M.; Maloney, J.A.; Meilandt, W.J.; et al. Therapeutic bispecific antibodies cross the blood-brain barrier in nonhuman primates. Sci. Transl. Med. 2014, 6, 261ra154. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Pang, Z.; Jiang, X. Targeted delivery of nano-therapeutics for major disorders of the central nervous system. Pharm. Res. 2013, 30, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, Y.J.; Zhang, Y.; Kenrick, M.; Hoyte, K.; Luk, W.; Lu, Y.; Atwal, J.; Elliott, J.M.; Prabhu, S.; Watts, R.J.; et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 2011, 3, 84ra44. [Google Scholar] [CrossRef] [PubMed]
- Bien-Ly, N.; Yu, Y.J.; Bumbaca, D.; Elstrott, J.; Boswell, C.A.; Zhang, Y.; Luk, W.; Lu, Y.; Dennis, M.S.; Weimer, R.M.; et al. Transferrin receptor (TfR) trafficking determines brain uptake of TfR antibody affinity variants. J. Exp. Med. 2014, 211, 233–244. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monnier, P.P.; Vigouroux, R.J.; Tassew, N.G. In vivo applications of single chain Fv (variable domain) (scFv) fragments. Antibodies 2013, 2, 193–208. [Google Scholar] [CrossRef]
- Kim, S.S.; Rait, A.; Kim, E.; DeMarco, J.; Pirollo, K.F.; Chang, E.H. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett. 2015, 369, 250–258. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, S.S.; Rait, A.; Kim, E.; Pirollo, K.F.; Chang, E.H. A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme. Nanomedicine 2015, 11, 301–311. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Obrador, E.; Moreno-Murciano, P.; Oriol-Caballo, M.; López-Blanch, R.; Pineda, B.; Gutiérrez-Arroyo, J.L.; Loras, A.; Gonzalez-Bonet, L.G.; Martinez-Cadenas, C.; Estrela, J.M.; et al. Glioblastoma Therapy: Past, Present and Future. Int. J. Mol. Sci. 2024, 25, 2529. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Engler, J.A.; Collawn, J.F.; Moore, B.A. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur. J. Biochem. 2001, 268, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Burkhart, A.; Melander, F.; Kempen, P.J.; Vejlebo, J.B.; Siupka, P.; Nielsen, M.S.; Andresen, T.L.; Moos, T. Targeting transferrin receptors at the blood-brain barrier improves the uptake of immunoliposomes and subsequent cargo transport into the brain parenchyma. Sci. Rep. 2017, 7, 10396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, P.; Xiao, Y.; Di, Q.; Ma, W.; Ma, X.; Wang, Q.; Chen, W. Transferrin receptor-targeted PEG-PLA polymeric micelles for chemotherapy against glioblastoma multiforme. Int. J. Nanomed. 2020, 15, 6673–6687. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, M.; Zeng, F.; Jin, H.; Xu, Q.; Huang, Y. Dual-targeting magnetic PLGA nanoparticles for codelivery of paclitaxel and curcumin for brain tumor therapy. ACS Appl. Mater. Interfaces 2016, 8, 32159–32169. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.; Jiang, M.; Jiang, D.; Feng, X.; Yao, J.; Song, Q.; Chen, H.; Gao, X.; Chen, J. Enhancing Glioblastoma-Specific Penetration by Functionalization of Nanoparticles with an Iron-Mimic Peptide Targeting Transferrin/Transferrin Receptor Complex. Mol. Pharm. 2015, 12, 2947–2961. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.M.; Bu, Y.Z.; Liu, L.; Xie, H.J.; Ju, R.J.; Wu, J.S.; Zeng, F.; Zhao, Y.; Zhang, J.Y.; Lu, W.L. Lipid vesicles containing transferrin receptor binding peptide TfR-T12 and octa-arginine conjugate stearyl-R8 efficiently treat brain glioma along with glioma stem cells. Sci. Rep. 2017, 7, 3487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Youn, P.; Chen, Y.; Furgeson, D.Y. A myristoylated cell-penetrating peptide bearing a transferrin receptor-targeting sequence for neuro-targeted siRNA delivery. Mol. Pharm. 2014, 11, 486–495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, L.; Guo, X.Y.; Yang, T.; Yu, M.Z.; Chen, D.W.; Wang, J.C. Brain tumor-targeted therapy by systemic delivery of siRNA with Transferrin receptor-mediated core-shell nanoparticles. Int. J. Pharm. 2016, 510, 394–405. [Google Scholar] [CrossRef] [PubMed]
- He, G.Z.; Lin, W.J. Peptide-functionalized nanoparticles-encapsulated cyclin-dependent kinases inhibitor seliciclib in transferrin receptor overexpressed cancer cells. Nanomaterials 2021, 11, 772. [Google Scholar] [CrossRef]
- Liu, S.; Guo, Y.; Huang, R.; Li, J.; Huang, S.; Kuang, Y.; Han, L.; Jiang, C. Gene and doxorubicin co-delivery system for targeting therapy of glioma. Biomaterials 2012, 33, 4907–4916. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liang, M.; Wang, Y.; Cui, L.; Gao, C.; Chu, X.; Liu, Q.; Feng, Y.; Gong, W.; Yang, M.; et al. Dual-Modified Novel Biomimetic Nanocarriers Improve Targeting and Therapeutic Efficacy in Glioma. ACS Appl. Mater. Interfaces 2019, 11, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Kim, M.; Lee, Y.; Byun, J.W.; Hwang, D.W.; Lee, M. Systemic delivery of microRNA-21 antisense oligonucleotides to the brain using T7-peptide decorated exosomes. J. Control Release 2020, 317, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhan, C.; Chen, X.; Hou, J.; Xie, C.; Lu, W. Retro-inverso isomer of Angiopep-2: A stable d-peptide ligand inspires brain-targeted drug delivery. Mol. Pharm. 2014, 11, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Xu, B. Inspiration from the mirror: D-amino acid containing peptides in biomedical approaches. Biomol. Concepts. 2016, 7, 179–187. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, M.; Su, D.; Yang, Y.; Qin, L.; Hu, C.; Liu, R.; Zhou, Y.; Yang, C.; Yang, X.; Wang, G.; et al. D-T7 Peptide-Modified PEGylated Bilirubin Nanoparticles Loaded with Cediranib and Paclitaxel for Antiangiogenesis and Chemotherapy of Glioma. ACS Appl. Mater. Interfaces 2019, 11, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Seo, M.; Kim, K.; Kim, A.R.; Lee, H.; Kim, H.S.; Park, C.G.; Cho, S.W.; Kang, J.H.; Joo, J.; et al. Aptamer Nanoconstructs Crossing Human Blood-Brain Barrier Discovered via Microphysiological System-Based SELEX Technology. ACS Nano 2023, 17, 8153–8166. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Wilbanks, B.; Khatua, S.; Maher, L.J., 3rd. Aptamers in neuro-oncology: An emerging therapeutic modality. Neuro Oncol. 2024, 26, 38–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiang, D.; Zheng, C.; Zhou, S.F.; Qiao, S.; Tran, P.H.; Pu, C.; Li, Y.; Kong, L.; Kouzani, A.Z.; Lin, J.; et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics 2015, 5, 1083–1097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, E.L.; Cardle, I.I.; Kacherovsky, N.; Bansia, H.; Wang, T.; Zhou, Y.; Raman, J.; Yen, A.; Gutierrez, D.; Salipante, S.J.; et al. Discovery of a Transferrin Receptor 1-Binding Aptamer and Its Application in Cancer Cell Depletion for Adoptive T-Cell Therapy Manufacturing. J. Am. Chem. Soc. 2022, 144, 13851–13864. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, P.; Zhang, N.; An, Z. Engineering antibody and protein therapeutics to cross the blood-brain barrier. Antib. Ther. 2022, 5, 311–331. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, W.; You, C.; Ma, L.; Li, H.; Ju, Y.; Guo, X.; Shi, S.; Zhang, T.; Zhou, R.; Lin, Y. Enhanced Efficacy of Temozolomide Loaded by a Tetrahedral Framework DNA Nanoparticle in the Therapy for Glioblastoma. ACS Appl. Mater. Interfaces 2019, 11, 39525–39533. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Yao, Z.; Chen, Z.; Zhou, S.; Wang, Z.; Xia, H.; Liu, S.; Wu, Y. TfR Aptamer Enhanced Blood-Brain Barrier Penetration of Biomimetic Nanocomplexes for Intracellular Transglutaminase 2 Imaging and Silencing in Glioma. Small 2022, 18, e2203448. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aime, S.; Frullano, L.; Geninatti Crich, S. Compartmentalization of a gadolinium complex in the apoferritin cavity: A route to obtain high relaxivity contrast agents for magnetic resonance imaging. Angew. Chem. Int. Ed. Engl. 2002, 41, 1017–1019. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Jia, X.; Zhou, M.; Wang, K.; Conde, J.; He, J.; Tian, J.; Yan, X. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano 2018, 12, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lin, Q.; Fu, Y.; Huang, S.; Guo, C.; Li, L.; Wang, L.; Zhang, Z.; Zhang, L. Target delivering paclitaxel by ferritin heavy chain nanocages for glioma treatment. J. Control. Release 2020, 323, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, B.; Li, J.; Guo, Z.; Zhang, C.; Chen, X.; Ma, L.; Wang, Z.; Yang, H.; Li, Y.; et al. Bioengineered protein nanocarrier facilitating siRNA escape from lysosomes for targeted RNAi therapy in glioblastoma. Sci. Adv. 2025, 11, eadr9266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marrocco, F.; Falvo, E.; Mosca, L.; Tisci, G.; Arcovito, A.; Reccagni, A.; Limatola, C.; Bernardini, R.; Ceci, P.; D’Alessandro, G.; et al. Nose-to-brain selective drug delivery to glioma via ferritin-based nanovectors reduces tumor growth and improves survival rate. Cell Death Dis. 2024, 15, 262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, B.; Tang, M.; Yuan, Z.; Li, Z.; Hu, B.; Bai, X.; Chu, J.; Xu, X.; Zhang, X.Q. Targeted delivery of a STING agonist to brain tumors using bioengineered protein nanoparticles for enhanced immunotherapy. Bioact. Mater. 2022, 16, 232–248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Cheng, Y.; Yang, Z.; Ji, Q.; Huan, M.; Ye, W.; Liu, M.; Zhang, B.; Liu, D.; Zhou, S. Glioma-targeted oxaliplatin/ferritin clathrate reversing the immunosuppressive microenvironment through hijacking Fe2+ and boosting Fenton reaction. J. Nanobiotechnol. 2024, 22, 93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, D.; Tian, S.; Liu, Y.; Zheng, M.; Yang, X.; Zou, Y.; Shi, B.; Luo, L. Near infrared-activatable biomimetic nanogels enabling deep tumor drug penetration inhibit orthotopic glioblastoma. Nat. Commun. 2022, 13, 6835. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, J.; Zhang, Z.; Jiang, M.; Li, S.; Yuan, H.; Sun, H.; Yang, F.; Liang, H. Developing a Novel Gold(III) Agent to Treat Glioma Based on the Unique Properties of Apoferritin Nanoparticles: Inducing Lethal Autophagy and Apoptosis. J. Med. Chem. 2020, 63, 13695–13708. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhai, M.; Xie, X.; Zhang, Y.; Ma, S.; Li, Z.; Yu, F.; Zhao, B.; Zhang, M.; Yang, Y.; et al. Apoferritin Nanocage for Brain Targeted Doxorubicin Delivery. Mol. Pharm. 2017, 14, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Zhai, M.; Wang, Y.; Zhang, L.; Liang, M.; Fu, S.; Cui, L.; Yang, M.; Gong, W.; Li, Z.; Yu, L.; et al. Glioma targeting peptide modified apoferritin nanocage. Drug Deliv. 2018, 25, 1013–1024. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uchida, M.; Kang, S.; Reichhardt, C.; Harlen, K.; Douglas, T. The ferritin superfamily: Supramolecular templates for materials synthesis. Biochim. Biophys. Acta 2010, 1800, 834–845. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Truffi, M.; Fiandra, L.; Sorrentino, L.; Monieri, M.; Corsi, F.; Mazzucchelli, S. Ferritin nanocages: A biological platform for drug delivery, imaging and theranostics in cancer. Pharmacol. Res. 2016, 107, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gao, H.; Zhang, Y.; Liu, G.; Niu, G.; Chen, X. Functional ferritin nanoparticles for biomedical applications. Front. Chem. Sci. Eng. 2017, 11, 633–646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fillebeen, C.; Descamps, L.; Dehouck, M.P.; Fenart, L.; Benaïssa, M.; Spik, G.; Cecchelli, R.; Pierce, A. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J. Biol. Chem. 1999, 274, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Miao, D.; Jiang, M.; Liu, Z.; Gu, G.; Hu, Q.; Kang, T.; Song, Q.; Yao, L.; Li, W.; Gao, X.; et al. Co-administration of dual-targeting nanoparticles with penetration enhancement peptide for antiglioblastoma therapy. Mol. Pharm. 2014, 11, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Sun, Y.; Zhao, H.; Lan, M.; Gao, F.; Song, C.; Lou, K.; Li, H.; Wang, W. A novel lactoferrin-modified β-cyclodextrin nanocarrier for brain-targeting drug delivery. Int. J. Pharm. 2013, 458, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Agwa, M.M.; Sabra, S. Lactoferrin coated or conjugated nanomaterials as an active targeting approach in nanomedicine. Int. J. Biol. Macromol. 2021, 167, 1527–1543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Asghar, S.; Tian, C.; Hu, Z.; Ping, Q.; Chen, Z.; Shao, F.; Xiao, Y. Lactoferrin/phenylboronic acid-functionalized hyaluronic acid nanogels loading doxorubicin hydrochloride for targeting glioma. Carbohydr. Polym. 2021, 253, 117194. [Google Scholar] [CrossRef] [PubMed]
- Song, M.M.; Xu, H.L.; Liang, J.X.; Xiang, H.H.; Liu, R.; Shen, Y.X. Lactoferrin modified graphene oxide iron oxide nanocomposite for glioma-targeted drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Liu, Y.; Liu, B.; Peng, J.; Tang, Y.; Lu, G.; Shi, H.; Zhu, F. Enhanced anti-glioma efficacy of biodegradable periodic mesoporous organosilica nanoparticles through target delivery of chemotherapeutics. J. Mater. Sci. Mater. Med. 2023, 34, 48. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Su, Z.; Xing, L.; Chen, Y.; Xu, Y.; Yang, F.; Zhang, C.; Ping, Q.; Xiao, Y. Lactoferrin-modified poly(ethylene glycol)-grafted BSA nanoparticles as a dual-targeting carrier for treating brain gliomas. Mol. Pharm. 2014, 11, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.; Duan, W.; Gao, D.; Ma, N.; Zhang, J.; Feng, J.; Li, A. “Guide” of muscone modification enhanced brain-targeting efficacy and anti-glioma effect of lactoferrin modified DTX liposomes. Bioeng. Transl. Med. 2022, 8, e10393. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, N.; Zhang, S.; Zhou, X.; Duan, W.; Gao, D.; Feng, J.; Li, A. Combined integrin αvβ3 and lactoferrin receptor targeted docetaxel liposomes enhance the brain targeting effect and anti-glioma effect. J. Nanobiotechnol. 2021, 19, 446. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Janjua, T.I.; Cao, Y.; Ahmed-Cox, A.; Raza, A.; Moniruzzaman, M.; Akhter, D.T.; Fletcher, N.L.; Kavallaris, M.; Thurecht, K.J.; Popat, A. Efficient delivery of Temozolomide using ultrasmall large-pore silica nanoparticles for glioblastoma. J. Control. Release 2023, 357, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Chen, Y.C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015, 479, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Cheng, S.J. Brain targeted delivery of carmustine using solid lipid nanoparticles modified with tamoxifen and lactoferrin for antitumor proliferation. Int. J. Pharm. 2016, 499, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Zheng, Z.; He, Y.; Zhong, H.; Kang, X.; Shi, M.; Liu, T.; Jiao, Z.; Huang, Y. Antiglioma via regulating oxidative stress and remodeling tumor-associated macrophage using lactoferrin-mediated biomimetic codelivery of simvastatin/fenretinide. J. Control. Release 2018, 287, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Lee, Y.J.; Rajesh, R. Enhanced activity of AZD5582 and SM-164 in rabies virus glycoprotein-lactoferrin-liposomes to downregulate inhibitors of apoptosis proteins in glioblastoma. Biomater. Adv. 2022, 133, 112615. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Feng, L.; Hua, R.; Chen, J.; Gao, H.; Pan, S.; Jiang, X.; Zhang, P. Lactoferrin-conjugated biodegradable polymersome holding doxorubicin and tetrandrine for chemotherapy of glioma rats. Mol. Pharm. 2010, 7, 1995–2005. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tong, Y.; Bai, L.; Ye, L.; Zhong, L.; Duan, X.; Zhu, Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018, 107 Pt A, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Asghar, S.; Yang, L.; Li, H.; Wang, Z.; Ping, Q.; Xiao, Y. Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr. Polym. 2017, 157, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.H.; Lai, Y.H.; Chiu, T.L.; Chen, Y.Y.; Hu, S.H.; Chen, S.Y. Magnetic core-shell nanocapsules with dual-targeting capabilities and co-delivery of multiple drugs to treat brain gliomas. Adv. Healthc. Mater. 2014, 3, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Bhattacharya, D.; Rangaraj, N.; Chakarvarty, S.; Kondapi, A.K.; Rao, N.M. Aurora kinase B siRNA-loaded lactoferrin nanoparticles potentiate the efficacy of temozolomide in treating glioblastoma. Nanomedicine 2018, 13, 2579–2596. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, X.; Yang, H.; Tan, J.; Gong, R.; Mei, C.; Cai, X.; Su, Z.; Kong, F. Lactoferrin/CD133 antibody conjugated nanostructured lipid carriers for dual targeting of blood-brain-barrier and glioblastoma stem cells. Biomed. Mater. 2024, 19, 055041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiao, X.; Zhu, J.; Gao, Z.; Lai, X.; Zhu, X.; Mao, G. Lactoferrin- and RGD-comodified, temozolomide and vincristine-coloaded nanostructured lipid carriers for gliomatosis cerebri combination therapy. Int. J. Nanomed. 2018, 13, 3039–3051. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumari, S.; Ahsan, S.M.; Kumar, J.M.; Kondapi, A.K.; Rao, N.M. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci. Rep. 2017, 7, 6602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sreedhara, A.; Flengsrud, R.; Langsrud, T.; Kaul, P.; Prakash, V.; Vegarud, G.E. Structural characteristic, pH and thermal stabilities of apo and holo forms of caprine and bovine lactoferrins. Biometals 2010, 23, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Kim, H.S.; Lee, D.Y. Gastrointestinally absorbable lactoferrin-heparin conjugate with anti-angiogenic activity for treatment of brain tumor. J. Control. Release 2023, 355, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Song, W.; Zhu, J.; Shao, X.; Yang, C.; Xiong, W.; Wang, B.; Zhao, P.; Chen, M.; Huang, Y. Biomimetic nano-chelate diethyldithiocarbamate Cu/Fe for enhanced metalloimmunity and ferroptosis activation in glioma therapy. J. Control. Release 2024, 368, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, L.; Tian, H.; Wang, Z.; Liu, G.; Duan, X.; Guo, M.; Liu, J.; Zhang, W.; Nice, E.C.; et al. Drug Repurposing-Based Brain-Targeting Self-Assembly Nanoplatform Using Enhanced Ferroptosis against Glioblastoma. Small 2023, 19, e2303073. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, B.; Fenart, L.; Dehouck, M.P.; Pierce, A.; Torpier, G.; Cecchelli, R. A new function for the LDL receptor: Transcytosis of LDL across the blood-brain barrier. J. Cell Biol. 1997, 138, 877–889. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Q.; Zhu, Q.; Miao, T.; Tao, J.; Ju, X.; Sun, Z.; Li, H.; Xu, G.; Chen, H.; Han, L. LRP1-upregulated nanoparticles for efficiently conquering the blood-brain barrier and targetedly suppressing multifocal and infiltrative brain metastases. J. Control. Release 2019, 303, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Mastrantuono, E.; Ghibaudi, M.; Matias, D.; Battaglia, G. The multifaceted therapeutical role of low-density lipoprotein receptor family in high-grade glioma. Mol. Oncol. 2024, 18, 2966–2976. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kurata, T.; Miyazaki, K.; Morimoto, N.; Kawai, H.; Ohta, Y.; Ikeda, Y.; Abe, K. Atorvastatin and pitavastatin reduce oxidative stress and improve IR/LDL-R signals in Alzheimer’s disease. Neurol. Res. 2013, 35, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I.; Meaney, S. Brain cholesterol: Long secret life behind a barrier. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, N.C.; D’Orsi, L.; Sambri, I.; Nusco, E.; Monaco, C.; Spampanato, C.; Polishchuk, E.; Saccone, P.; De Leonibus, E.; Ballabio, A.; et al. A highly secreted sulphamidase engineered to cross the blood-brain barrier corrects brain lesions of mice with mucopolysaccharidoses type IIIA. EMBO Mol. Med. 2013, 5, 675–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, D.; El-Amouri, S.S.; Dai, M.; Kuan, C.Y.; Hui, D.Y.; Brady, R.O.; Pan, D. Engineering a lysosomal enzyme with a derivative of receptor-binding domain of apoE enables delivery across the blood-brain barrier. Proc. Natl. Acad. Sci. USA 2013, 110, 2999–3004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, J.; Xia, Y.; Meng, F.; Ni, D.; Qiu, X.; Zhong, Z. Small, Smart, and LDLR-Specific Micelles Augment Sorafenib Therapy of Glioblastoma. Biomacromolecules 2021, 22, 4814–4822. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Kim, E.H.; Chang, W.S.; Lee, W.S.; Kim, K.H.; Kim, J.K. Enhanced proton treatment with a LDLR-ligand peptide-conjugated gold nanoparticles targeting the tumor microenvironment in an infiltrative brain tumor model. Am. J. Cancer Res. 2022, 12, 198–209. [Google Scholar] [PubMed] [PubMed Central]
- Aina, O.H.; Sroka, T.C.; Chen, M.L.; Lam, K.S. Therapeutic cancer targeting peptides. Biopolymers 2002, 66, 184–199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sun, X.; Mei, H.; Wang, Y.; Liao, Z.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. LDLR-mediated peptide-22-conjugated nanoparticles for dual-targeting therapy of brain glioma. Biomaterials 2013, 34, 9171–9182. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Cao, B.; Snyder, N.R.; Woeppel, K.M.; Eles, J.R.; Cui, X.T. ROS responsive resveratrol delivery from LDLR peptide conjugated PLA-coated mesoporous silica nanoparticles across the blood-brain barrier. J. Nanobiotechnol. 2018, 16, 13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Mauro, P.P.; Cascante, A.; Brugada Vilà, P.; Gómez-Vallejo, V.; Llop, J.; Borrós, S. Peptide-functionalized and high drug loaded novel nanoparticles as dual-targeting drug delivery system for modulated and controlled release of paclitaxel to brain glioma. Int. J. Pharm. 2018, 553, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Bu, G.; Maksymovitch, E.A.; Geuze, H.; Schwartz, A.L. Subcellular localization and endocytic function of low density lipoprotein receptor-related protein in human glioblastoma cells. J. Biol. Chem. 1994, 269, 29874–29882. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Anderson, R.G.; Brown, M.S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 1979, 279, 679–685. [Google Scholar] [CrossRef] [PubMed]
- di Polidoro, A.C.; Cafarchio, A.; Vecchione, D.; Donato, P.; De Nola, F.; Torino, E. Revealing Angiopep-2/LRP1 Molecular Interaction for Optimal Delivery to Glioblastoma (GBM). Molecules 2022, 27, 6696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Regina, A.; Demeule, M.; Tripathy, S.; Lord-Dufour, S.; Currie, J.C.; Iddir, M.; Annabi, B.; Castaigne, J.P.; Lachowicz, J.E. ANG4043, a novel brain-penetrant peptide-mAb conjugate, is efficacious against HER2-positive intracranial tumors in mice. Mol. Cancer Ther. 2015, 14, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Shinohara, T.; Adachi, Y.; Asami, T.; Ohtaki, T. A novel LRP1-binding peptide L57 that crosses the blood brain barrier. Biochem. Biophys. Rep. 2017, 12, 135–139. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, F.C.; Taskar, K.; Rudraraju, V.; Goda, S.; Thorsheim, H.R.; Gaasch, J.A.; Mittapalli, R.K.; Palmieri, D.; Steeg, P.S.; Lockman, P.R.; et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood-brain barrier into brain and experimental brain metastases of breast cancer. Pharm. Res. 2009, 26, 2486–2494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumthekar, P.; Tang, S.C.; Brenner, A.J.; Kesari, S.; Piccioni, D.E.; Anders, C.; Carrillo, J.; Chalasani, P.; Kabos, P.; Puhalla, S.; et al. ANG1005, a Brain-Penetrating Peptide-Drug Conjugate, Shows Activity in Patients with Breast Cancer with Leptomeningeal Carcinomatosis and Recurrent Brain Metastases. Clin. Cancer Res. 2020, 26, 2789–2799. [Google Scholar] [CrossRef] [PubMed]
- Drappatz, J.; Brenner, A.; Wong, E.T.; Eichler, A.; Schiff, D.; Groves, M.D.; Mikkelsen, T.; Rosenfeld, S.; Sarantopoulos, J.; Meyers, C.A.; et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin. Cancer Res. 2013, 19, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Dmello, C.; Brenner, A.; Piccioni, D.; Wen, P.Y.; Drappatz, J.; Mrugala, M.; Lewis, L.D.; Schiff, D.; Fadul, C.E.; Chamberlain, M.; et al. Phase II trial of blood-brain barrier permeable peptide-paclitaxel conjugate ANG1005 in patients with recurrent high-grade glioma. Neurooncol. Adv. 2024, 6, vdae186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Costagliola di Polidoro, A.; Zambito, G.; Haeck, J.; Mezzanotte, L.; Lamfers, M.; Netti, P.A.; Torino, E. Theranostic Design of Angiopep-2 Conjugated Hyaluronic Acid Nanoparticles (Thera-ANG-cHANPs) for Dual Targeting and Boosted Imaging of Glioma Cells. Cancers 2021, 13, 503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sahoo, R.K.; Kumar, H.; Jain, V.; Sinha, S.; Ajazuddin Gupta, U. Angiopep-2 Grafted PAMAM Dendrimers for the Targeted Delivery of Temozolomide: In Vitro and In Vivo Effects of PEGylation in the Management of Glioblastoma Multiforme. ACS Biomater. Sci. Eng. 2023, 9, 4288–4301. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Refaat, A.; Gan, P.Y.; Fan, B.; Yu, H.; Thang, S.H.; Drummond, C.J.; Voelcker, N.H.; Tran, N.; Zhai, J. Angiopep-2-Functionalized Lipid Cubosomes for Blood-Brain Barrier Crossing and Glioblastoma Treatment. ACS Appl. Mater. Interfaces 2024, 16, 12161–12174. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sun, S.; Xu, H.; Zhao, Z.; Han, Z.; Jia, J.; Wu, D.; Lu, J.; Liu, H.; Yu, R. Combined Delivery of Temozolomide and siPLK1 Using Targeted Nanoparticles to Enhance Temozolomide Sensitivity in Glioma. Int. J. Nanomed. 2020, 15, 3347–3362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Awad, S.; Araújo, M.; Faria, P.; Sarmento, B.; Martins, C. Chemical engineering of zein with polyethylene glycol and Angiopep-2 to manufacture a brain-targeted docetaxel nanomedicine for glioblastoma treatment. Drug Deliv. Transl. Res. 2024, 14, 3585–3598. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xin, H.; Sha, X.; Jiang, X.; Zhang, W.; Chen, L.; Fang, X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 2012, 33, 8167–8176. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Fei, W.; Tang, H.; Li, C.; Mu, C.; Zheng, H.; Li, F.; Zhu, Z. Angiopep-2-Conjugated “Core-Shell” Hybrid Nanovehicles for Targeted and pH-Triggered Delivery of Arsenic Trioxide into Glioma. Mol. Pharm. 2019, 16, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Liu, Y.F.; Zhang, H.B.; Liu, X.L.; Zhang, H.W.; Huang, B.; Lin, F.; Li, W.H. A Novel ANG-BSA/BCNU/ICG MNPs Integrated for Targeting Therapy of Glioblastoma. Technol. Cancer Res. Treat. 2024, 23, 15330338241281321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.; Yin, H.; Zhang, X. Modification of graphene oxide by angiopep-2 enhances anti-glioma efficiency of the nanoscaled delivery system for doxorubicin. Aging 2020, 12, 10506–10516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Hao, Y.; Li, H.; Zhao, Y.; Meng, D.; Li, D.; Shi, J.; Zhang, H.; Zhang, Z.; Zhang, Y. Co-delivery of doxorubicin and siRNA for glioma therapy by a brain targeting system: Angiopep-2-modified poly(lactic-co-glycolic acid) nanoparticles. J. Drug Target. 2015, 23, 832–846. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, D.; Zhang, H.; Zhao, K.; Hou, W. Preparation and characterization of angiopep-2 functionalized Ginsenoside-Rg3 loaded nanoparticles and the effect on C6 Glioma cells. Pharm. Dev. Technol. 2020, 25, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, L.; Ren, Z.; Yuan, Y.; Yu, J.; Zhang, Y.; Gu, L.; Wang, X.; Wang, Y.; Xu, H.; et al. Stepwise-targeting and hypoxia-responsive liposome AMVY@NPs carrying siYAP and verteporfin for glioblastoma therapy. J. Nanobiotechnol. 2024, 22, 495. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Zheng, M.; Jiao, M.; Yan, C.; Xu, S.; Du, Q.; Morsch, M.; Yin, J.; Shi, B. Polymeric nanoparticle mediated inhibition of miR-21 with enhanced miR-124 expression for combinatorial glioblastoma therapy. Biomaterials 2021, 276, 121036. [Google Scholar] [CrossRef] [PubMed]
- Ruan, W.; Jiao, M.; Xu, S.; Ismail, M.; Xie, X.; An, Y.; Guo, H.; Qian, R.; Shi, B.; Zheng, M. Brain-targeted CRISPR/Cas9 nanomedicine for effective glioblastoma therapy. J. Control. Release 2022, 351, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, W.; Song, T.; Song, M.; Liu, S.; Zhou, J.; Cheng, H.; Ding, Y. Lipid-Polymer Nanoparticles Mediate Compartmentalized Delivery of Cas9 and sgRNA for Glioblastoma Vasculature and Immune Reprogramming. Adv. Sci. 2024, 11, e2309314. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, X.; Zhan, C.; Shen, Q.; Fu, W.; Xie, C.; Gao, J.; Peng, C.; Zheng, P.; Lu, W. A D-peptide ligand of nicotine acetylcholine receptors for brain-targeted drug delivery. Angew. Chem. Int. Ed. Engl. 2015, 54, 3023–3027. [Google Scholar] [CrossRef] [PubMed]
- Zhan, C.; Li, B.; Hu, L.; Wei, X.; Feng, L.; Fu, W.; Lu, W. Micelle-based brain-targeted drug delivery enabled by a nicotine acetylcholine receptor ligand. Angew. Chem. Int. Ed. Engl. 2011, 50, 5482–5485. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Li, L.; Wang, L.; Degos, V.; Maze, M.; Su, H. Alpha-7 nicotinic acetylcholine receptor agonist treatment reduces neuroinflammation, oxidative stress, and brain injury in mice with ischemic stroke and bone fracture. J. Neurochem. 2014, 131, 498–508. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deutch, A.Y.; Holliday, J.; Roth, R.H.; Chun, L.L.; Hawrot, E. Immunohistochemical localization of a neuronal nicotinic acetylcholine receptor in mammalian brain. Proc. Natl. Acad. Sci. USA 1987, 84, 8697–8701. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abbruscato, T.J.; Lopez, S.P.; Mark, K.S.; Hawkins, B.T.; Davis, T.P. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J. Pharm. Sci. 2002, 91, 2525–2538. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Xie, W.; Zhang, Y.; Zhou, S.; Yang, J.; Wang, R.; Sun, Y.; Wang, X.; Xu, J.; Chen, D.; et al. The influx/efflux mechanisms of d-peptide ligand of nAChRs across the blood-brain barrier and its therapeutic value in treating glioma. J. Control. Release 2020, 327, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, R.; Han, L.; Ke, W.; Shao, K.; Ye, L.; Lou, J.; Jiang, C. Brain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticles. Biomaterials 2009, 30, 4195–4202. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Wu, H.; McBride, J.L.; Jung, K.E.; Kim, M.H.; Davidson, B.L.; Lee, S.K.; Shankar, P.; Manjunath, N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhan, C.; Gu, B.; Meng, Q.; Wang, H.; Lu, W.; Hou, H. Targeted brain delivery of itraconazole via RVG29 anchored nanoparticles. J. Drug Target. 2011, 19, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Savari, M.N. Fe3O4@Chitosan@ZIF-8@RVG29, an anti-glioma nanoplatform guided by fixed and activated by alternating magnetic field. Sci. Rep. 2024, 14, 7000. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, H.; Wang, Y.; Wang, H.; Zhang, K.; Liu, Y.; Li, Q.; Li, C.; Wen, Z.; Chen, Z. Biomimetic nanocarriers loaded with temozolomide by cloaking brain-targeting peptides for targeting drug delivery system to promote anticancer effects in glioblastoma cells. Heliyon 2024, 10, e28256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, M.; Xing, H.; Chen, L.; Cui, M.; Zhang, Y.; Qi, L.; Jin, M.; Yang, Y.; Gao, C.; Gao, Z.; et al. Efficient antiglioblastoma therapy in mice through doxorubicin-loaded nanomicelles modified using a novel brain-targeted RVG-15 peptide. J. Drug Target. 2021, 29, 1016–1028. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Liu, W.; Zhang, Z.A.; Han, Y.; Qi, L.L.; Zhang, Y.Y.; Zhang, X.T.; Duan, H.X.; Chen, L.Q.; Jin, M.J.; et al. Efficient Anti-Glioma Therapy Through the Brain-Targeted RVG15-Modified Liposomes Loading Paclitaxel-Cholesterol Complex. Int. J. Nanomed. 2021, 16, 5755–5776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhan, C.; Yan, Z.; Xie, C.; Lu, W. Loop 2 of Ophiophagus hannah toxin b binds with neuronal nicotinic acetylcholine receptors and enhances intracranial drug delivery. Mol. Pharm. 2010, 7, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Q.Y.; Haqqani, A.S.; Leclerc, S.; Liu, Z.; Fauteux, F.; Baumann, E.; Delaney, C.E.; Ly, D.; Star, A.T.; et al. Differential expression of receptors mediating receptor-mediated transcytosis (RMT) in brain microvessels, brain parenchyma and peripheral tissues of the mouse and the human. Fluids Barriers CNS 2020, 17, 47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alata, W.; Yogi, A.; Brunette, E.; Delaney, C.E.; van Faassen, H.; Hussack, G.; Iqbal, U.; Kemmerich, K.; Haqqani, A.S.; Moreno, M.J.; et al. Targeting insulin-like growth factor-1 receptor (IGF1R) for brain delivery of biologics. FASEB J. 2022, 36, e22208. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Gerber, H.P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Saldana, S.M.; Lee, H.H.; Lowery, F.J.; Khotskaya, Y.B.; Xia, W.; Zhang, C.; Chang, S.S.; Chou, C.K.; Steeg, P.S.; Yu, D.; et al. Inhibition of type I insulin-like growth factor receptor signaling attenuates the development of breast cancer brain metastasis. PLoS ONE 2013, 8, e73406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yogi, A.; Hussack, G.; van Faassen, H.; Haqqani, A.S.; Delaney, C.E.; Brunette, E.; Sandhu, J.K.; Hewitt, M.; Sulea, T.; Kemmerich, K.; et al. Brain Delivery of IGF1R5, a Single-Domain Antibody Targeting Insulin-like Growth Factor-1 Receptor. Pharmaceutics 2022, 14, 1452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marin, B.M.; Porath, K.A.; Jain, S.; Kim, M.; Conage-Pough, J.E.; Oh, J.H.; Miller, C.L.; Talele, S.; Kitange, G.J.; Tian, S.; et al. Heterogeneous delivery across the blood-brain barrier limits the efficacy of an EGFR-targeting antibody drug conjugate in glioblastoma. Neuro Oncol. 2021, 23, 2042–2053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, J.; Ran, D.; Xie, C.; Shen, Q.; Wang, S.; Lu, W. EGFR/EGFRvIII Dual-Targeting Peptide-Mediated Drug Delivery for Enhanced Glioma Therapy. ACS Appl. Mater. Interfaces 2017, 9, 24462–24475. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.C.; Liang, C.T. Inhibition of human brain malignant glioblastoma cells using carmustine-loaded catanionic solid lipid nanoparticles with surface anti-epithelial growth factor receptor. Biomaterials 2011, 32, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Mamot, C.; Drummond, D.C.; Greiser, U.; Hong, K.; Kirpotin, D.B.; Marks, J.D.; Park, J.W. Epidermal growth factor receptor (EGFR)targeted immunoliposomes mediate specific and efficient drug delivery to EGFR and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003, 63, 3154–3161. [Google Scholar] [PubMed]
- Banstola, A.; Duwa, R.; Emami, F.; Jeong, J.H.; Yook, S. Enhanced caspase-mediated abrogation of autophagy by temozolomide-loaded and panitumumab-conjugated poly(lactic-co-glycolic acid) nanoparticles in epidermal growth factor receptor overexpressing glioblastoma cells. Mol. Pharm. 2020, 17, 4386–4400. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, B.M.; Antipova, O.M.; Sliman, Y.A.; Samoylenkova, N.S.; Pronin, I.N.; Pavlova, G.V.; Kopylov, A.M. Use of Anti-EGFR Aptamer Construct GR20hh for Controlled Delivery of Doxorubicin into Patient-Derived Glioblastoma Cells. Neurosci. Behav. Phys. 2024, 54, 923–928. [Google Scholar] [CrossRef]
- Kessler, T.; Sahm, F.; Blaes, J.; Osswald, M.; Rübmann, P.; Milford, D.; Urban, S.; Jestaedt, L.; Heiland, S.; Bendszus, M.; et al. Glioma cell VEGFR-2 confers resistance to chemotherapeutic and antiangiogenic treatments in PTEN-deficient glioblastoma. Oncotarget 2015, 6, 31050–31068. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gelfand, M.V.; Hagan, N.; Tata, A.; Oh, W.J.; Lacoste, B.; Kang, K.T.; Kopycinska, J.; Bischoff, J.; Wang, J.H.; Gu, C. Neuropilin-1 functions as a VEGFR2 co-receptor to guide developmental angiogenesis independent of ligand binding. eLife 2014, 3, e03720. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, M.; Lu, L.; Ying, M.; Ruan, H.; Wang, X.; Wang, H.; Chai, Z.; Wang, S.; Zhan, C.; Pan, J.; et al. Enhanced Glioblastoma Targeting Ability of Carfilzomib Enabled by a DA7R-Modified Lipid Nanodisk. Mol. Pharm. 2018, 15, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhai, M.; Chen, Z.; Han, X.; Yu, F.; Li, Z.; Xie, X.; Han, C.; Yu, L.; Yang, Y.; et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017, 24, 1045–1055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Filippo, L.D.; Lobato Duarte, J.; Hofstätter Azambuja, J.; Isler Mancuso, R.; Tavares Luiz, M.; Hugo Sousa Araújo, V.; Delbone Figueiredo, I.; Barretto-de-Souza, L.; Miguel Sábio, R.; Sasso-Cerri, E.; et al. Glioblastoma multiforme targeted delivery of docetaxel using bevacizumab-modified nanostructured lipid carriers impair in vitro cell growth and in vivo tumor progression. Int. J. Pharm. 2022, 618, 121682. [Google Scholar] [CrossRef] [PubMed]
- Venishetty, V.K.; Komuravelli, R.; Kuncha, M.; Sistla, R.; Diwan, P.V. Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles. Nanomedicine 2013, 9, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.; Schriever, S.C.; Feuchtinger, A.; Kyriakou, E.; Baumann, P.; Pfuhlmann, K.; Messias, A.C.; Walch, A.; Tschöp, M.H.; Pfluger, P.T. Fluorescent blood-brain barrier tracing shows intact leptin transport in obese mice. Int. J. Obes. 2019, 43, 1305–1318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banks, W.A. Leptin and the Blood-Brain Barrier: Curiosities and Controversies. Compr. Physiol. 2021, 11, 2351–2369. [Google Scholar] [CrossRef] [PubMed]
- Barrett, G.L.; Trieu, J.; Naim, T. The identification of leptin-derived peptides that are taken up by the brain. Regul. Pept. 2009, 155, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Shao, K.; Huang, R.; Ye, L.; Lou, J.; Jiang, C. A leptin derived 30-amino-acid peptide modified pegylated poly-L-lysine dendrigraft for brain targeted gene delivery. Biomaterials 2010, 31, 5246–5257. [Google Scholar] [CrossRef] [PubMed]
- Sedghi Aminabad, N.; Saeedi, Y.; Adiban, J.; Nemati, M.; Shaterabadi, D.; Najafi, F.; Rahbarghazi, R.; Talebi, M.; Zarebkohan, A. Discovery of a Novel Dual Targeting Peptide for Human Glioma: From In Silico Simulation to Acting as Targeting Ligand. Adv. Pharm. Bull. 2024, 14, 453–468. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srimanee, A.; Regberg, J.; Hällbrink, M.; Vajragupta, O.; Langel, Ü. Role of scavenger receptors in peptide-based delivery of plasmid DNA across a blood-brain barrier model. Int. J. Pharm. 2016, 500, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.A.; Day, E.S.; Ko, C.H.; Hurley, L.A.; Luciano, J.P.; Kouri, F.M.; Merkel, T.J.; Luthi, A.J.; Patel, P.C.; Cutler, J.I.; et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci. Transl. Med. 2013, 5, 209ra152. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Sci. Transl. Med. 2021, 13, eabb3945. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akiyama, Y.; Radtke, C.; Kocsis, J.D. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J. Neurosci. 2002, 22, 6623–6630. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Osaka, M.; Honmou, O.; Murakami, T.; Nonaka, T.; Houkin, K.; Hamada, H.; Kocsis, J.D. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 2010, 1343, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Soper, B.W.; Duffy, T.M.; Lessard, M.D.; Jude, C.D.; Schuldt, A.J.; Vogler, C.A.; Levy, B.; Barker, J.E. Transplanted ER-MP12hi20-58med/hi myeloid progenitors produce resident macrophages from marrow that are therapeutic for lysosomal storage disease. Blood Cells Mol. Dis. 2004, 32, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sanberg, P.R.; Li, Y.; Wang, L.; Lu, M.; Willing, A.E.; Sanchez-Ramos, J.; Chopp, M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke 2001, 32, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Aboody, K.S.; Brown, A.; Rainov, N.G.; Bower, K.A.; Liu, S.; Yang, W.; Small, J.E.; Herrlinger, U.; Ourednik, V.; Black, P.M.; et al. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas. Proc. Natl. Acad. Sci. USA 2000, 97, 12846–12851, Erratum in Proc. Natl. Acad. Sci. USA 2001, 98, 777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, R.; Cai, J.; Nathan, C.; Wei, X.; Schleidt, S.; Rosenwasser, R.; Iacovitti, L. Neurogenesis is enhanced by stroke in multiple new stem cell niches along the ventricular system at sites of high BBB permeability. Neurobiol. Dis. 2015, 74, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Aboody, K.S.; Najbauer, J.; Metz, M.Z.; D’Apuzzo, M.; Gutova, M.; Annala, A.J.; Synold, T.W.; Couture, L.A.; Blanchard, S.; Moats, R.A.; et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: Preclinical studies. Sci. Transl. Med. 2013, 5, 184ra59. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hussain, S.F.; Yang, D.; Suki, D.; Aldape, K.; Grimm, E.; Heimberger, A.B. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006, 8, 261–279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood-brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2021, 27, 36–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Henze, A.T.; Mazzone, M. The impact of hypoxia on tumor-associated macrophages. J. Clin. Investig. 2016, 126, 3672–3679. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Georgieva, P.B.; Mathivet, T.; Alt, S.; Giese, W.; Riva, M.; Balcer, M.; Gerhardt, H. Long-lived tumor-associated macrophages in glioma. Neurooncol. Adv. 2020, 2, vdaa127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schreurs, L.D.; Vom Stein, A.F.; Jünger, S.T.; Timmer, M.; Noh, K.W.; Buettner, R.; Kashkar, H.; Neuschmelting, V.; Goldbrunner, R.; Nguyen, P.H. The immune landscape in brain metastasis. Neuro Oncol. 2025, 27, 50–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pang, L.; Qin, J.; Han, L.; Zhao, W.; Liang, J.; Xie, Z.; Yang, P.; Wang, J. Exploiting macrophages as targeted carrier to guide nanoparticles into glioma. Oncotarget 2016, 7, 37081–37091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ding, X.; Sun, X.; Cai, H.; Wu, L.; Liu, Y.; Zhao, Y.; Zhou, D.; Yu, G.; Zhou, X. Engineering Macrophages via Nanotechnology and Genetic Manipulation for Cancer Therapy. Front. Oncol. 2022, 11, 786913. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, M.; Bialasek, M.; Rygiel, T.; Król, M.; Weller, M.; Weiss, T. OS10.4.A A macrophage-based drug delivery platform for glioma treatment. Neuro Oncol. 2022, 24 (Suppl. S2). [Google Scholar] [CrossRef] [PubMed Central]
- Wróblewska, A.; Szczygieł, A.; Szermer-Olearnik, B.; Pajtasz-Piasecka, E. Macrophages as Promising Carriers for Nanoparticle Delivery in Anticancer Therapy. Int. J. Nanomed. 2023, 18, 4521–4539. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, X.; Zhang, Z.; Du, J.; Xue, Y.; Chen, X.; Zhang, J.; Yang, X.; Chang, D.; Xie, J.; Ju, S. Recruiting T-Cells toward the Brain for Enhanced Glioblastoma Immunotherapeutic Efficacy by Co-Delivery of Cytokines and Immune Checkpoint Antibodies with Macrophage-Membrane-Camouflaged Nanovesicles. Adv. Mater. 2023, 35, e2209785. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, H.; Tachikawa, M.; Yagi, Y.; Umetsu, M.; Nurdin, A.; Miyauchi, E.; Watanabe, M.; Uchida, Y.; Terasaki, T. Cluster of Differentiation 46 Is the Major Receptor in Human Blood-Brain Barrier Endothelial Cells for Uptake of Exosomes Derived from Brain-Metastatic Melanoma Cells (SK-Mel-28). Mol. Pharm. 2019, 16, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Shan, S.; Chen, J.; Sun, Y.; Wang, Y.; Xia, B.; Tan, H.; Pan, C.; Gu, G.; Zhong, J.; Qing, G.; et al. Functionalized Macrophage Exosomes with Panobinostat and PPM1D-siRNA for Diffuse Intrinsic Pontine Gliomas Therapy. Adv. Sci. 2022, 9, e2200353. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Zaczyński, A.; Zawadzki, M. Breaking Barriers in Neuro-Oncology: A Scoping Literature Review on Invasive and Non-Invasive Techniques for Blood-Brain Barrier Disruption. Cancers 2024, 16, 236. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinkiewicz, M.; Pinkiewicz, M.; Walecki, J.; Zawadzki, M. A systematic review on intra-arterial cerebral infusions of chemotherapeutics in the treatment of glioblastoma multiforme: The state-of-the-art. Front. Oncol. 2022, 12, 950167. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fortin, D. Drug Delivery Technology to the CNS in the Treatment of Brain Tumors: The Sherbrooke Experience. Pharmaceutics 2019, 11, 248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Charest, G.; Paquette, B.; Fortin, D.; Mathieu, D.; Sanche, L. Concomitant treatment of F98 glioma cells with new liposomal platinum compounds and ionizing radiation. J. Neurooncol. 2010, 97, 187–193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chakraborty, S.; Filippi, C.G.; Burkhardt, J.K.; Fralin, S.; Ray, A.; Wong, T.; Ortiz, R.; Langer, D.J.; Boockvar, J.A. Durability of single dose intra-arterial bevacizumab after blood/brain barrier disruption for recurrent glioblastoma. J. Exp. Ther. Oncol. 2016, 11, 261–267. [Google Scholar] [PubMed]
- Burkhardt, J.K.; Riina, H.A.; Shin, B.J.; Moliterno, J.A.; Hofstetter, C.P.; Boockvar, J.A. Intra-arterial chemotherapy for malignant gliomas: A critical analysis. Interv. Neuroradiol. 2011, 17, 286–295, Erratum in Interv. Neuroradiol. 2011, 17, 506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Uluc, K.; Ambady, P.; McIntyre, M.K.; Tabb, J.P.; Kersch, C.N.; Nerison, C.S.; Huddleston, A.; Liu, J.J.; Dogan, A.; Priest, R.A.; et al. Safety of intra-arterial chemotherapy with or without osmotic blood-brain barrier disruption for the treatment of patients with brain tumors. Neurooncol. Adv. 2022, 4, vdac104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferreira, C.; Ferreira, M.Y.; Singh, F.; Wong, T.; Bokil, S.; Massimo, S.; Cavallaro, J.; Albers, O.; D’Amico, R.; Langer, D.; et al. Superselective Intra-Arterial Cerebral Infusion of Chemotherapeutics After Osmotic Blood-Brain Barrier Disruption in Newly Diagnosed or Recurrent Glioblastoma: Technical Insights and Clinical Outcomes from a Single-Center Experience. J. Neurointerv. Surg. 2025; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Gahide, G.; Vendrell, J.F.; Massicotte-Tisluck, K.; Caux, S.; Deschamps, S.; Noël-Lamy, M.; Belzile, F.; Roy, L.O.; Fortin, D. Safety of Cerebral Intra-Arterial Chemotherapy for the Treatment of Malignant Brain Tumours. J. Clin. Med. 2025, 14, 524. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, S.R.; Chen, M.M.; Ene, C.; Lang, F.F.; Kan, P. Perfusion-guided endovascular super-selective intra-arterial infusion for treatment of malignant brain tumors. J. Neurointerv. Surg. 2022, 14, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.M.; Lang, F.F.; Chen, S.R.; Chen, M.M.; Gumin, J.; Johnson, J.; Burkhardt, J.K.; Kan, P. Advances in endovascular neuro-oncology: Endovascular selective intra-arterial (ESIA) infusion of targeted biologic therapy for brain tumors. J. Neurointerv. Surg. 2020, 12, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Sperring, C.P.; Argenziano, M.G.; Savage, W.M.; Teasley, D.E.; Upadhyayula, P.S.; Winans, N.J.; Canoll, P.; Bruce, J.N. Convection-enhanced delivery of immunomodulatory therapy for high-grade glioma. Neurooncol. Adv. 2023, 5, vdad044. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allard, E.; Passirani, C.; Benoit, J.P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials 2009, 30, 2302–2318. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.S.; Aghi, M.K.; Vogelbaum, M.A.; Bruce, J.N. Convection-enhanced drug delivery for glioblastoma: A review. J. Neurooncol. 2021, 151, 415–427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mehta, A.I.; Choi, B.D.; Ajay, D.; Raghavan, R.; Brady, M.; Friedman, A.H.; Pastan, I.; Bigner, D.D.; Sampson, J.H. Convection enhanced delivery of macromolecules for brain tumors. Curr. Drug Discov. Technol. 2012, 9, 305–310. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bruce, J.N.; Fine, R.L.; Canoll, P.; Yun, J.; Kennedy, B.C.; Rosenfeld, S.S.; Sands, S.A.; Surapaneni, K.; Lai, R.; Yanes, C.L.; et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 2011, 69, 1272–1279, discussion 1279–1280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narsinh, K.H.; Kumar, K.; Bankiewicz, K.; Martin, A.J.; Berger, M.; Clarke, J.; Taylor, J.; Bush, N.A.O.; Molinaro, A.M.; Aghi, M.; et al. A phase I study of convection-enhanced delivery (CED) of liposomal-irinotecan using real-time magnetic resonance imaging in patients with recurrent high-grade glioma. J. Neurooncol. 2025, 172, 219–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, M.C.; Holl, E.K.; Boczkowski, D.; Dobrikova, E.; Mosaheb, M.; Chandramohan, V.; Bigner, D.D.; Gromeier, M.; Nair, S.K. Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci. Transl. Med. 2017, 9, eaan4220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heiss, J.D.; Jamshidi, A.; Shah, S.; Martin, S.; Wolters, P.L.; Argersinger, D.P.; Warren, K.E.; Lonser, R.R. Phase I trial of convection-enhanced delivery of IL13-Pseudomonas toxin in children with diffuse intrinsic pontine glioma. J. Neurosurg. Pediatr. 2019, 23, 333–342. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guisado, D.I.; Singh, R.; Minkowitz, S.; Zhou, Z.; Haque, S.; Peck, K.K.; Young, R.J.; Tsiouris, A.J.; Souweidane, M.M.; Thakur, S.B. A Novel Methodology for Applying Multivoxel MR Spectroscopy to Evaluate Convection-Enhanced Drug Delivery in Diffuse Intrinsic Pontine Gliomas. AJNR Am. J. Neuroradiol. 2016, 37, 1367–1373. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singleton, W.G.B.; Bienemann, A.S.; Woolley, M.; Johnson, D.; Lewis, O.; Wyatt, M.J.; Damment, S.J.P.; Boulter, L.J.; Killick-Cole, C.L.; Asby, D.J.; et al. The distribution, clearance, and brainstem toxicity of panobinostat administered by convection-enhanced delivery. J. Neurosurg. Pediatr. 2018, 22, 288–296, Erratum in J. Neurosurg. Pediatr. 2018, 23, 133. https://doi.org/10.3171/2018.9.PEDS17663a. [Google Scholar] [CrossRef] [PubMed]
- Rechberger, J.S.; Power, E.A.; Lu, V.M.; Zhang, L.; Sarkaria, J.N.; Daniels, D.J. Evaluating infusate parameters for direct drug delivery to the brainstem: A comparative study of convection-enhanced delivery versus osmotic pump delivery. Neurosurg. Focus 2020, 48, E2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stephen, Z.R.; Kievit, F.M.; Veiseh, O.; Chiarelli, P.A.; Fang, C.; Wang, K.; Hatzinger, S.J.; Ellenbogen, R.G.; Silber, J.R.; Zhang, M. Redox-responsive magnetic nanoparticle for targeted convection-enhanced delivery of O6-benzylguanine to brain tumors. ACS Nano 2014, 8, 10383–10395. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anastasiadis, P.; Gandhi, D.; Guo, Y.; Ahmed, A.K.; Bentzen, S.M.; Arvanitis, C.; Woodworth, G.F. Localized blood-brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound. Proc. Natl. Acad. Sci. USA 2021, 118, e2103280118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, K.T.; Chai, W.Y.; Lin, Y.J.; Lin, C.J.; Chen, P.Y.; Tsai, H.C.; Huang, C.Y.; Kuo, J.S.; Liu, H.L.; Wei, K.C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, Y.; Reilly, R.M.; Pezo, R.C.; Trudeau, M.; Sahgal, A.; Singnurkar, A.; Perry, J.; Myrehaug, S.; Pople, C.B.; Davidson, B.; et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci. Transl. Med. 2021, 13, eabj4011. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.Y.; et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef] [PubMed]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.H.; Moore, D.; Lee, C.C.; Su, Y.H. Focused ultrasound for brain metastases: An update on global clinical trials. J. Neurooncol. 2023, 165, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Gould, A.; Luan, Y.; Hou, Y.; Korobova, F.V.; Chen, L.; Arrieta, V.A.; Amidei, C.; Ward, R.; Gomez, C.; Castro, B.; et al. Endothelial response to blood-brain barrier disruption in the human brain. JCI Insight 2024, 10, e187328. [Google Scholar] [CrossRef] [PubMed]
- Sonabend, A.M.; Gould, A.; Amidei, C.; Ward, R.; Schmidt, K.A.; Zhang, D.Y.; Gomez, C.; Bebawy, J.F.; Liu, B.P.; Bouchoux, G.; et al. Repeated blood-brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: A phase 1 trial. Lancet Oncol. 2023, 24, 509–522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Curley, C.T.; Mead, B.P.; Negron, K.; Kim, N.; Garrison, W.J.; Miller, G.W.; Kingsmore, K.M.; Thim, E.A.; Song, J.; Munson, J.M.; et al. Augmentation of brain tumor interstitial flow via focused ultrasound promotes brain-penetrating nanoparticle dispersion and transfection. Sci. Adv. 2020, 6, eaay1344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, G.; Huang, Q.; Wang, F.; Zhang, X.; Hu, J.; Tan, Y.; Huang, N.; Wang, Z.; Wang, Z.; Cheng, Y. Targeted shRNA-loaded liposome complex combined with focused ultrasound for blood brain barrier disruption and suppressing glioma growth. Cancer Lett. 2018, 418, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.Y.; Wong, T.T.; Teng, M.C.; Liu, R.S.; Lu, M.; Liang, H.F.; Wei, M.C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.F.; Lozano, N.; Peeyatu, C.; Ho, Y.; Thompson, L.; Kostarelos, K.; Kisby, T. Surgery transiently disrupts the blood-brain barrier of the glioblastoma resection margin: Opportunities for early and selective postoperative treatment. Neuro-Oncology 2024, 26 (Suppl. S5), v15. [Google Scholar] [CrossRef]
- Liang, F.; You, Q.; Yu, B.; Wang, C.; Yang, Y.; Zhu, L.; He, Z. Neurotransmitter-Mimicking Nanovesicles Facilitate Postoperative Glioblastoma Stem Cell-Specific Treatment for Preventing Tumor Recurrence. Adv. Sci. 2025, 12, e2409713. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef] [PubMed]
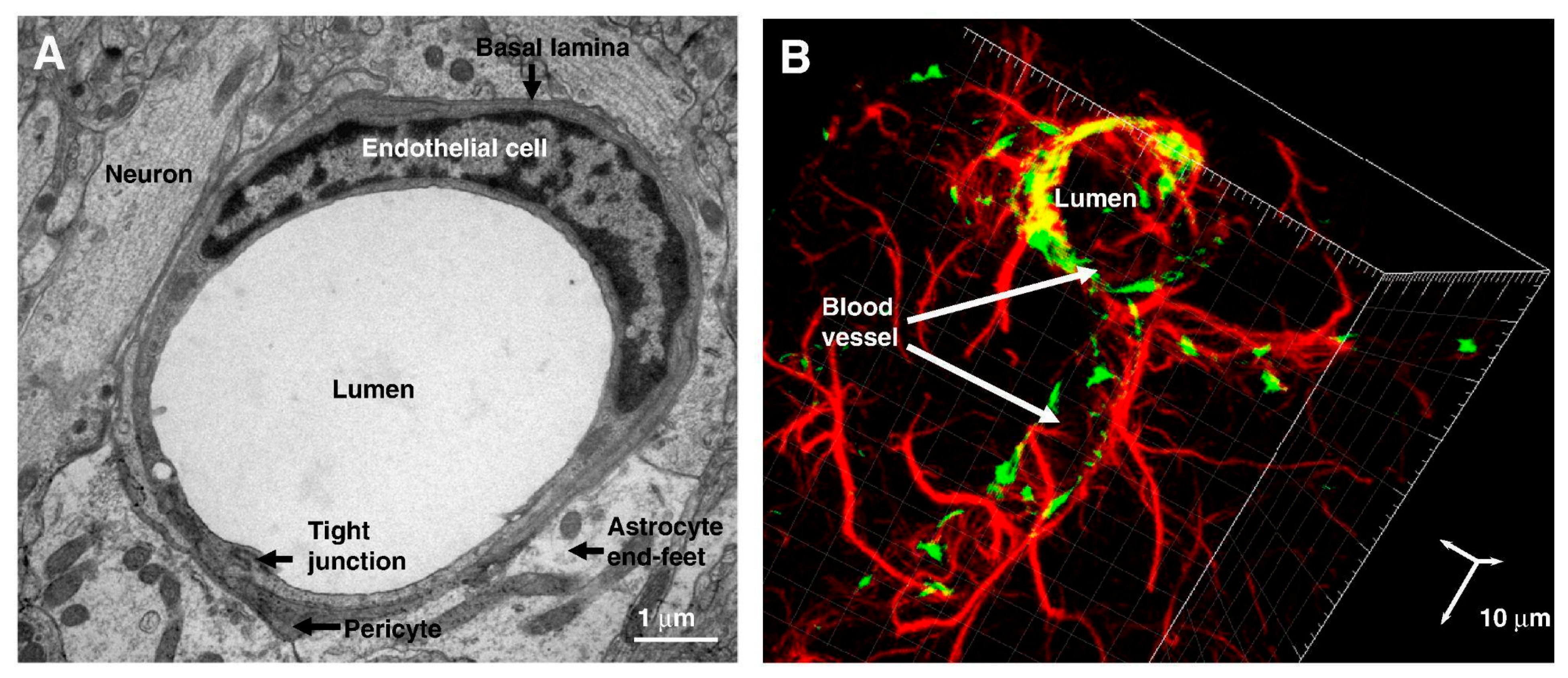
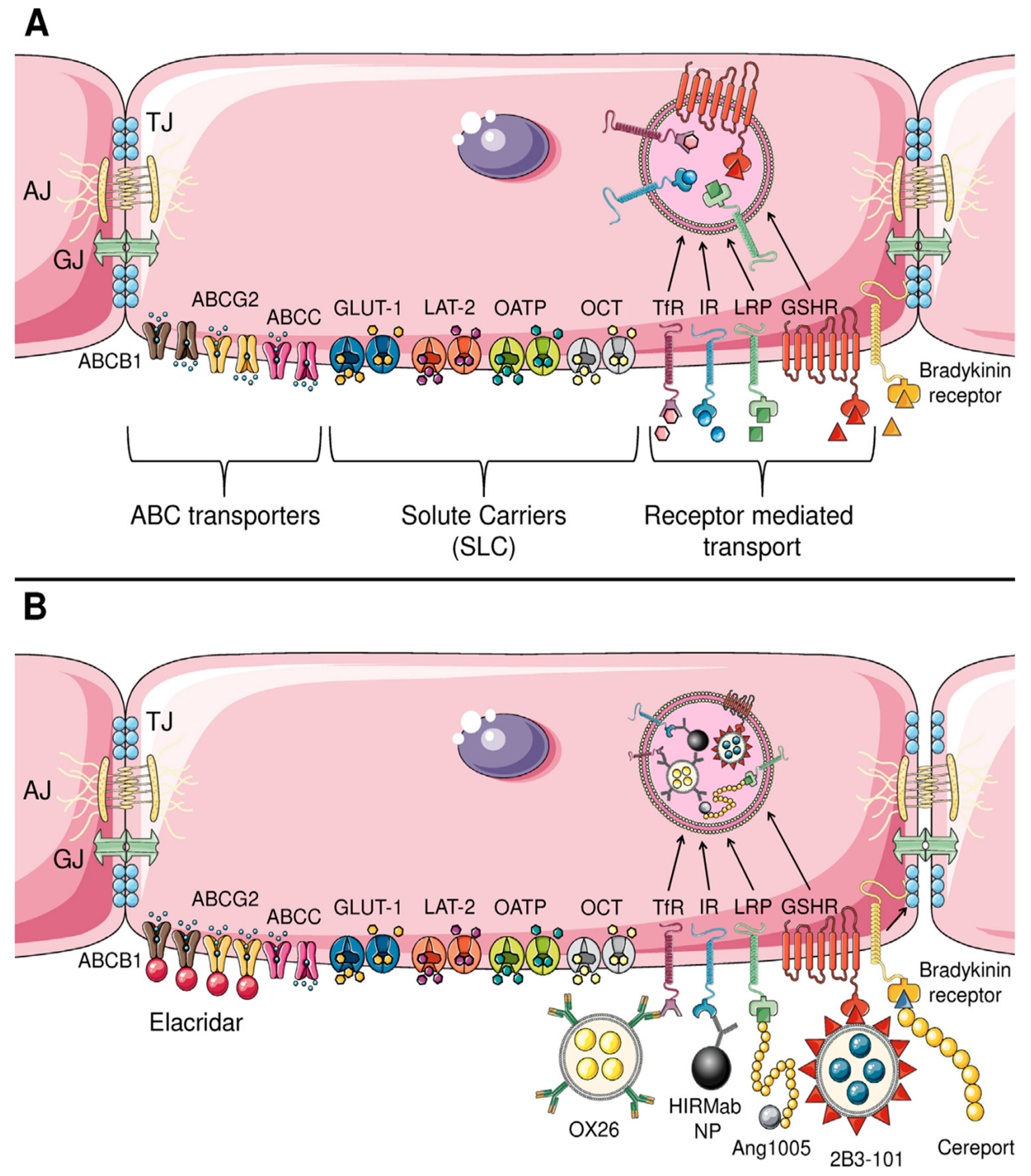
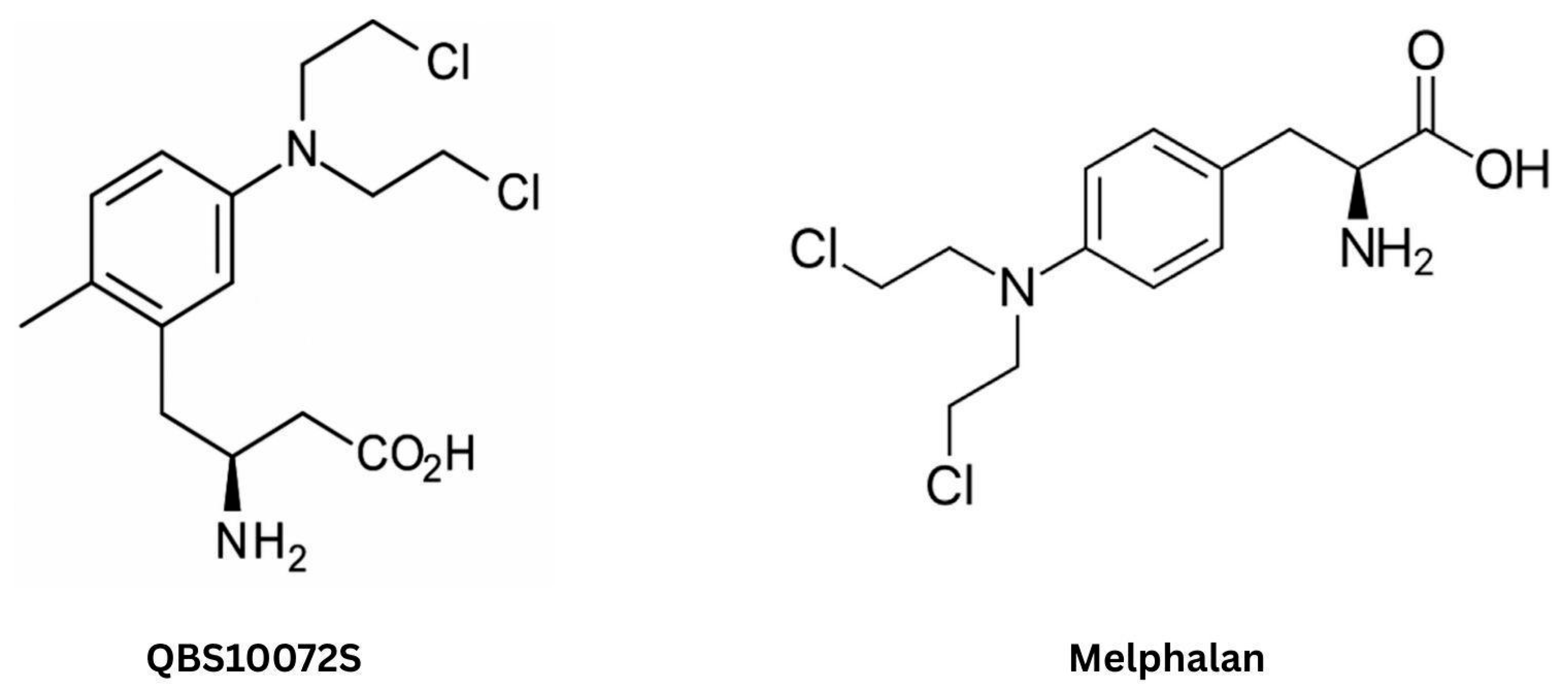
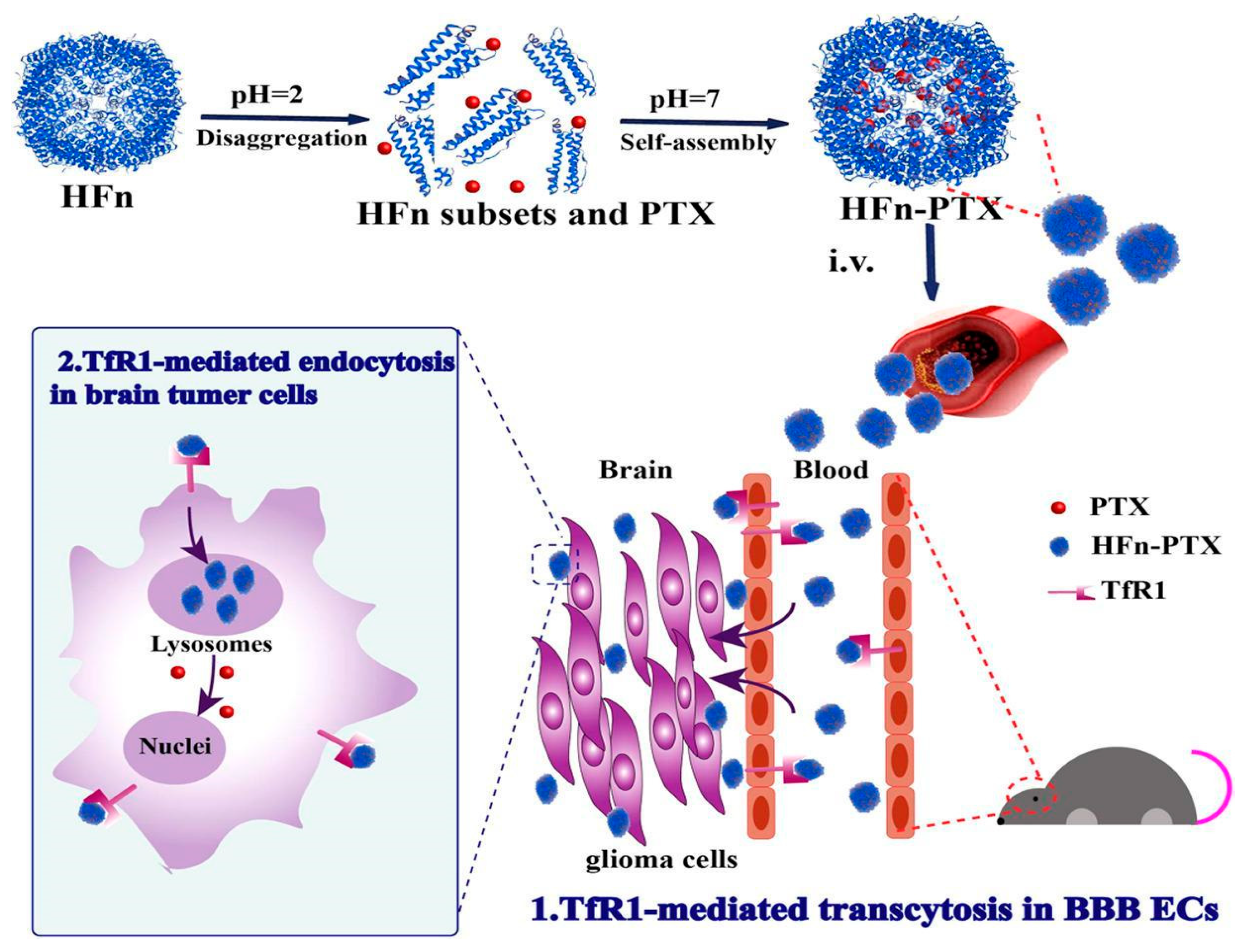
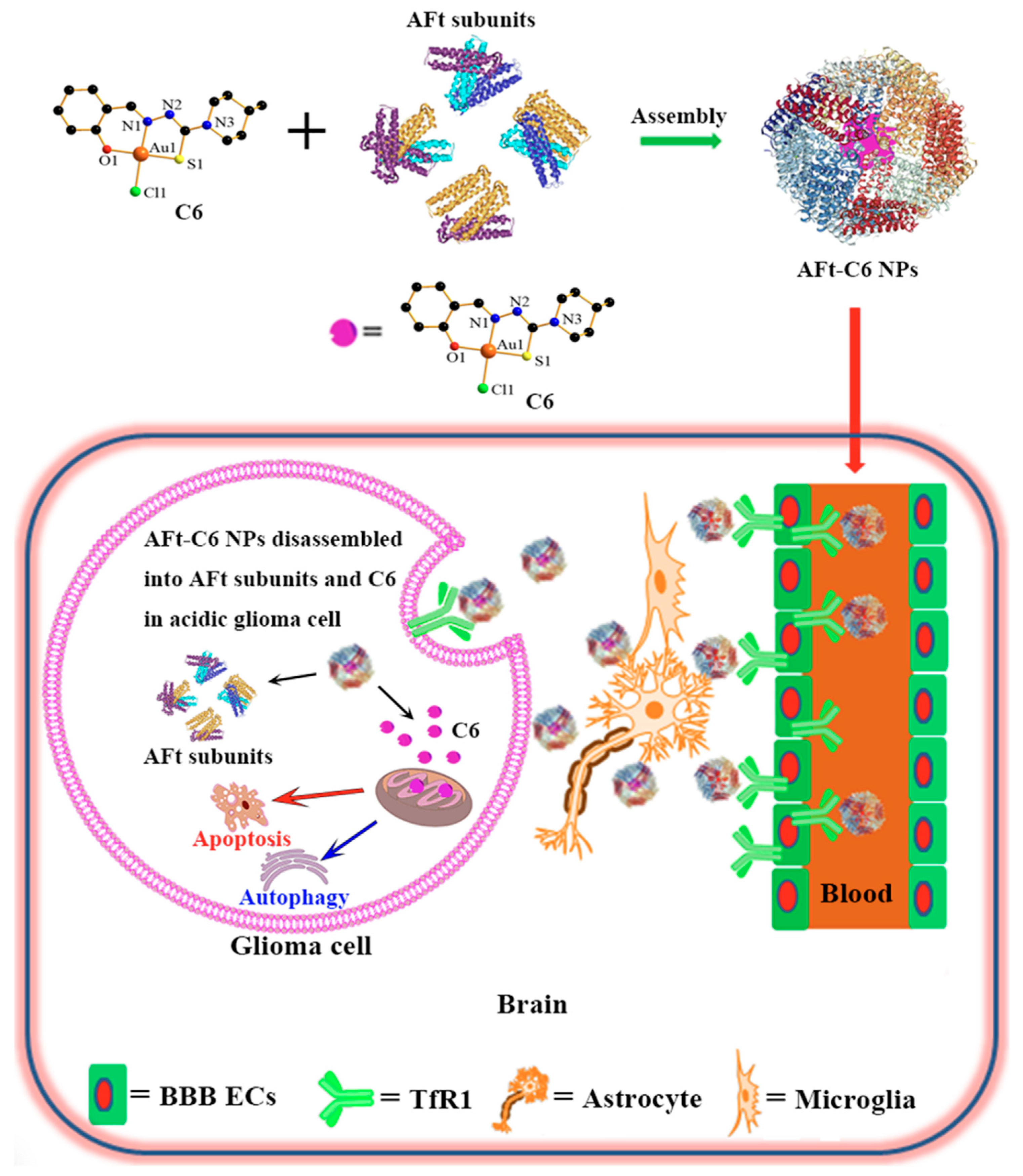

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinkiewicz, M.; Zaczyński, A.; Walecki, J.; Zawadzki, M. Beyond the Walls of Troy: A Scoping Review on Pharmacological Strategies to Enhance Drug Delivery Across the Blood–Brain Barrier and Blood–Tumor Barrier. Int. J. Mol. Sci. 2025, 26, 7050. https://doi.org/10.3390/ijms26157050
Pinkiewicz M, Zaczyński A, Walecki J, Zawadzki M. Beyond the Walls of Troy: A Scoping Review on Pharmacological Strategies to Enhance Drug Delivery Across the Blood–Brain Barrier and Blood–Tumor Barrier. International Journal of Molecular Sciences. 2025; 26(15):7050. https://doi.org/10.3390/ijms26157050
Chicago/Turabian StylePinkiewicz, Miłosz, Artur Zaczyński, Jerzy Walecki, and Michał Zawadzki. 2025. "Beyond the Walls of Troy: A Scoping Review on Pharmacological Strategies to Enhance Drug Delivery Across the Blood–Brain Barrier and Blood–Tumor Barrier" International Journal of Molecular Sciences 26, no. 15: 7050. https://doi.org/10.3390/ijms26157050
APA StylePinkiewicz, M., Zaczyński, A., Walecki, J., & Zawadzki, M. (2025). Beyond the Walls of Troy: A Scoping Review on Pharmacological Strategies to Enhance Drug Delivery Across the Blood–Brain Barrier and Blood–Tumor Barrier. International Journal of Molecular Sciences, 26(15), 7050. https://doi.org/10.3390/ijms26157050




