How Can Plant-Derived Natural Products and Plant Biotechnology Help Against Emerging Viruses?
Abstract
1. Introduction
1.1. The Persisting Era of Infectious Disease
1.2. Antivirals
2. Life Cycle of (Re)Emerging Viruses—How Can Natural Products Help?
3. Plant Compounds That Target Stages of the Viral Life Cycle
3.1. Phytochemicals Modulating Viral Entry, Attachment, and Fusion
3.2. Phytochemicals Modulating Viral Replication, Protein Synthesis, and Maturation
4. (Re)Emerging Viruses—How Can Plant Biotechnology Help?
4.1. Plant-Derived Vaccines
4.2. Bio-Encapsulation of mRNA Within Plant-Derived Virus-like Particles (VLPs) and Chimeric VLP Production
4.3. Plant-Derived Antibodies Used for Passive Immunotherapy
4.4. Recombinant Cytokines Produced in Plants
4.5. Recombinant Carbohydrate-Binding Proteins with Antiviral Activity Produced in Plants
5. Engineering of Plant Biosynthetic Pathways for Overproduction of Phytochemicals
5.1. Improved Production of Phytochemicals by CRISPR-Cas9 Genome Editing
5.2. Transient Expression of Biosynthetic Enzymes
6. Challenges and Limitations of Plant-Derived Antivirals and Recombinant Proteins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SARS-CoV | Severe Acute Respiratory Syndrome Coronavirus |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| MERS-CoV | Middle East Respiratory Syndrome Coronavirus |
| CoVs | Coronaviruses |
| H1N1 | Hemagglutinin Type 1 and Neuraminidase Type 1 (Influenza A subtype) |
| HIV-1 | Human Immunodeficiency Virus Type 1 |
| mRNA | Messenger Ribonucleic Acid |
| VLPs | Virus-Like Particles |
| IVA | Influenza Virus A |
| DENV | Dengue Virus |
| ZIKV | Zika Virus |
| EBOV | Ebola Virus |
| MPXV | Monkeypox Virus |
| RNA | Ribonucleic Acid |
| RNP | Ribonucleoprotein |
| DNA | Deoxyribonucleic Acid |
| S protein | Spike Protein |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| DPP4 | Dipeptidyl Peptidase 4 |
| TMPRSS2 | Transmembrane Protease Serine 2 |
| NP | Nucleoprotein |
| VP | Viral Protein |
| GP | Glycoprotein |
| sGP | Soluble GP |
| L | Polymerase |
| NTPase | Nucleoside Triphosphatase |
| HA | Hemagglutinin |
| vRNP | Viral Ribonucleoprotein Complex |
| E protein | Envelope Protein |
| CD4 | Cluster of Differentiation 4 |
| CCR5 | C-C Chemokine Receptor Type 5 |
| CXCR4 | C-X-C Chemokine Receptor Type 4 |
| NPC1 | Niemann–Pick C1 Protein |
| E8L | A Viral Envelope Protein from MPXV |
| EGCG | Epigallocatechin Gallate |
| ASA, ASAI | Allium Sativum Lectins |
| IC50 | Half Maximal Inhibitory Concentration |
| FRET | Förster Resonance Energy Transfer |
| Mpro/3CLpro | Main Protease/3-Chymotrypsin-Like Protease (also nsp5) |
| PLpro | Papain-Like Protease (also nsp3) |
| NSPs | Non-Structural Proteins |
| RdRp | RNA-Dependent RNA Polymerase (also nsp12) |
| RTC | Replication–Transcription Complex |
| ER | Endoplasmic Reticulum |
| ERGIC | Endoplasmic Reticulum–Golgi Intermediate Compartment |
| IRES | Internal Ribosome Entry Site |
| RT | Reverse Transcriptase |
| GAGs | Glycosaminoglycans |
| DC-SIGN/L-SIGN | Dendritic Cell-Specific Intercellular Adhesion Molecule-3-Grabbing Non-Integrin/Liver/Lymph Node-Specific ICAM-3-Grabbing Integrin |
| TIM-1 | T-cell Immunoglobulin and Mucin-Domain-Containing-1 |
| PI3K/Akt | Phosphoinositide 3-Kinase/Protein Kinase B pathway |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PMF | Plant Molecular Farming |
| DSP | Downstream Processing |
| HIV | Human Immunodeficiency Virus |
| TMV | Tobacco Mosaic Virus |
| CPMV | Cowpea Mosaic Virus |
| HPV16 | Human Papillomavirus 16 |
| AMV | Alfalfa Mosaic Virus |
| BaMV | Bamboo Mosaic Virus-Based |
| FMDV | Foot-and-Mouth Disease Virus |
| AP 205 | Bacteriophage AP 205 |
| HBV | Hepatitis B Virus |
| HEV | Hepatitis E Virus |
| BTV | Bluetongue Virus |
| AHSV | African Horse Sickness Virus |
| mAb | Monoclonal Antibody |
| KDEL | Retention Signal Sequence in Proteins |
| ΔXF | Deletion of Xylosyl- and Fucosyltransferase (enzyme activity) |
| CTP | Cytidine Triphosphate |
| CCT | CTP:phosphocholine cytidylyltransferase |
| CRISPR/Cas | Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-Associated Protein |
| IgA | Immunoglobulin A |
| INF-α | Interferon Alfa |
| FDA | Food and Drug Administration |
| GRFT | Griffithsin |
| CV-N | Cyanovirin-N |
| EC50 | Half Maximal Effective Concentration |
| STLs | Sesquiterpene Lactones |
| CiGAS | Germacrene A Synthase from Cichorium |
Appendix A
| Compound | Activity | Cell Type Tested | Target | IC50/EC50 | Plant | Reference |
|---|---|---|---|---|---|---|
| SARS-CoV-2 | ||||||
| Cepharantine | Inhibition of pre-entry, entry, and membrane fusion | Calu-3, A549, HEK293T-ACE2, Vero E6 | Blockage of host calcium channels | 0.315 µM | N/A | [23] |
| Hernandezine | Inhibition of pre-entry, entry, and membrane fusion | Calu-3, A549, HEK293T-ACE2, Vero E6 | Blockage of host calcium channels | 0.111 µM | N/A | [23] |
| Neferine | Inhibition of pre-entry, entry, and membrane fusion | Calu-3, A549, HEK293T-ACE2, Vero E6 | Blockage of host calcium channels | 0.946 µM | N/A | [23] |
| ASA | Inhibition of early viral attachment | Vero E6 | N/A | >4 µM | Allium sativum L. | [271] |
| ASA1 | Inhibition of early viral attachment | Vero E6 | N/A | >4 µM | Allium sativum L. | [271] |
| Punicalin | Inhibition of viral entry | N/A | Disruption of spike glycoprotein–host ACE2 interaction | 0.009 µM | Gunnera perpensa | [25] |
| Punicalagin | Inhibition of viral entry | N/A | Disruption of spike glycoprotein–host ACE2 interaction | 0.029 µM | Gunnera perpensa | [25] |
| Epigallocatechin gallate | Inhibition of viral entry | HEK293FT, Caco-2 | Disruption of spike glycoprotein–host ACE2 interaction | 33.9 µM | Camelia sinensis | [28] |
| 4,6-dihydroxyquinoline-2-carboxylic acid | Inhibition of viral entry | Calu-3, HEK293T-ACE2 | Disruption of spike glycoprotein–host ACE2 interaction | 0.07 µM | Ephedra sinica | [26] |
| 4-hydroxy-6-methoxyquinoline-2-carboxylic acid | Inhibition of viral entry | Calu-3, HEK293T-ACE2 | Disruption of spike glycoprotein–host ACE2 interaction | 0.15 µM | Ephedra sinica | [26] |
| 4-hydroxyquinoline-2-carboxylic acid | Inhibition of viral entry | Calu-3, HEK293T-ACE2 | Disruption of spike glycoprotein–host ACE2 interaction | 0.58 µM | Ephedra sinica | [26] |
| Dengue virus | ||||||
| Gossypol | Inhibition of viral attachment | LLC-MK2 | Envelope protein region III | 1.87 µM (DENV-1) 1.89 µM (DENV-2) 3.7 µM/(DENV-3) 2.6 µM/ (DENV-4) | Gossypium spp. | [38] |
| Baicalein | Inhibition of DENV-2 adsorption | Vero E6 | Not established | 7.14 μg/mL | Scutellaria baicalensis | [35] |
| Baicalin | Inhibition of viral adsorption | Vero E6 | Not established | 18.07 μg/mL | Scutellaria baicalensis | [37] |
| Zika virus | ||||||
| Baicalin | Inhibition of viral attachment to host cells | Vero E6 | Envelope E protein | 14 µM | Scutellaria baicalensis | [36] |
| Gossypol | Inhibition of viral attachment to host cells | Vero E6 | Envelope protein region III | 22.2 µM | Gossypium sp. | [38] |
| Curcumin | Inhibition of viral attachment to host cells | HeLa, BHK-21, Vero E6 | Envelope E protein | 1.90 µM | Curcuma longa | [40] |
| (−) Epigallocatechin gallate | Destabilization and dissolution of viral particle | Vero E6 | Viral envelope phospholipids | 21.4 µM | Camellia sinensis | [39] |
| Isoquercitrin | Inhibition of membrane-associated viral particle internalization into A549 cells | A549 | Not established | 15.5 µM | Mangifera indica | [41] |
| HIV-1 | ||||||
| Ajoene | Inhibition of adhesive interactions and fusion of leukocytes | T-lymphoblasts (H9, CEM13) | N/A | 45 µM | Allium sativum L. | [272] |
| “TFmix” (theaflavin; theaflavin-3-gallate; theaflavin-3′-gallate; theaflavin-3-3′-digallate) | Inhibition of viral attachment | H9/HIV-1IIIB cells, MT-2 | Interference with viral gp41 6-helix bundle formation | 6.25 µM | Camellia sinensis assamica | [273] |
| Cassiabrevone | Inhibition of viral attachment | U373-CD4-CXCR4 | Viral gp120-host CD4 binding | 30.96 µM | Cassia abbreviata | [274] |
| Acerosin | Inhibition of viral entry | MT-2 | Surmised blocking of virus–CD4 or CXCR4/CCR5 host cell receptor interaction | 2.7 µM | Artemisia campestris | [275] |
| Xanthomicrol | Inhibition of viral entry | MT-2 | Surmised blocking of virus–CD4 or CXCR4/CCR5 host cell receptor interaction | 17.43 µM | Artemisia campestris | [275] |
| Guibourtinidol-(4α → 8)-epiafzelechin | Inhibition of viral attachment | U373-CD4-CXCR4 | Viral gp120-host CD4 binding | 42.47 µM | Cassia abbreviata | [274] |
| Baicalin | Inhibition of viral fusion | Hos/CD4/CCR5, Hos/CD4/CXCR4 | Broad inhibition of T-cell tropic (X4) and monocyte tropic (R5) HIV-1 Env protein-mediated fusion with host CD4/CXCR4 or CD4/CCR5 | 4 µM | Scutellaria baicalensis | [32] |
| Procyanidin A (pentamer) | Inhibition of viral attachment | PBMC | Blocks binding of viral gp120 to host heparan sulfate | 7 µM | Cinnamomum cassia | [276] |
| Procyanidin A (trimer) | Inhibition of viral attachment | PBMC | Blocks binding of viral gp120 to host heparan sulfate | 7.5 µM | Cinnamomum cassia | [276] |
| Procyanidin A (pentamer) | Inhibition of viral attachment | PBMC | Blocks binding of viral gp120 to CD4 | 21.5 µM (YU2 HIV-1 envelope), 20 µM (MN HIV-1 envelope) | Cinnamomum cassia | [276] |
| Ebola virus | ||||||
| (+) Catechin | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 36.0 µM | Maesa perlarius | [59] |
| Ellagic acid | Inhibition of viral entry | A549, HeLa | Blocking Ebola glycoprotein-mediated entry | 1.4 µM (against pseudovirions in A549 cells) 10.5 µM (against EBOV in HeLa cells) | Rhodiola rosea L. | [277] |
| (−) Epicatechin | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 22.1 µM | Maesa perlarius | [59] |
| Epicatechin gallate | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 2.95 µM | Maesa perlarius | [59] |
| Epigallocatechin | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 5.53 µM | Maesa perlarius | [59] |
| Epigallocatechin-3-gallate | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 2.8 µM | Maesa perlarius | [59] |
| Gallocatechin | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 12.4 µM | Maesa perlarius | [59] |
| Procyanidin B1 | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 0.95 µM | Maesa perlarius | [59] |
| Procyanidin B2 | Inhibition of viral attachment, entry, and fusion | A549, HEK293T | Blocking Ebola glycoprotein | 0.83 µM | Maesa perlarius | [59] |
| Quercetin 3-β-O-d-glucoside | Inhibition of early stages of viral entry | Vero E6 | Not established, surmised involvement of NPC1 endosomal transporter/LDL receptors | 5.3 µM | N/A | [60] |
| Influenza A | ||||||
| Harmalol | Viricidal and antivirus adsorption effects | MDCK | Not established | 0.035 µg/mL | Peganum harmala L. | [56] |
| Harmane | Viricidal and antivirus adsorption effects | MDCK | Not established | 0.033 µg/mL | Peganum harmala L. | [56] |
| Harmaline | Viricidal and antivirus adsorption effects | MDCK | Not established | 0.056 µg/mL | Peganum harmala L. | [56] |
| Strychnine sulfate | Viricidal and antivirus adsorption effects | MDCK | Not established | 0.06 µg/mL | Peganum harmala L. | [56] |
| Pentagalloylglucose | Inhibition of viral adsorption | MDCK | Viral hemagglutinin | 2.51 µM | Phyllanthus emblica Linn | [278] |
| 5,7,3′,4′-tetra-O-methylquercetin | Blocking host cell entry and/or recognition | MDCK | Binding to H1N1 virions | 0.36 µM | Sambucus nigra L. | [54] |
| (±)-dihydromyricetin | Blocking host cell entry and/or recognition | MDCK | Binding to H1N1 virions | 8.7 µM | Sambucus nigra L. | [54] |
| Cyanidin 3-sambubioside | Inhibition of viral adsorption | MDCK | Not established | 252 µg/mL (whole-extract value) | Sambucus nigra L. | [54] |
| Quercetin | Inhibition of viral entry | MDCK | HA2 subunit of influenza hemagglutinin | 7.76 µM (A/Puerto Rico/8/34 (H1N1)), 6.23 µM (A/FM-1/47/1 (H1N1)), 2.74 µM (A/Aichi/2/68 (H3N2)) | N/A | [53] |
| Chikungunya virus | ||||||
| Epigallocatechin gallate | Inhibition of viral entry | HEK293T | Envelope glycoprotein | 12 µM | Camellia Sinensis | [47] |
| Baicalein | Inhibition of viral adsorption | Vero cells | Not established | 103.76 µM | Scutellaria baicalensis | [48] |
| Quercetagetin | Inhibition of viral adsorption | Vero cells | Not established | 25.3 µM | N/A | [48] |
| Curcumin | Affects viral glycoprotein conformation and/or membrane fluidity | HeLa | Viral envelope | 3.89 µM | Curcuma longa | [40] |
| 2-(butoxycarbonyl) benzoic acid (BCB) | Inhibition of viral entry | Vero cells | E1 CHIKV envelope glycoprotein | 2.49 µg/mL vs. Asian isolate 28.62 µg/mL vs. African isolate | Tectona grandis | [50] |
| 3,7,11,15-tetramethyl-1-hexadecanol (THD) | Inhibition of viral entry | Vero cells | E1 CHIKV envelope glycoprotein | 1.66 µg/mL vs. Asian isolate 122.4 µg/mL vs. African isolate | Tectona grandis | [50] |
| Benzene 1-carboxylic acid hexadecanoate (BHCD) | Inhibition of viral entry | Vero cells | E1 CHIKV envelope glycoprotein | 3.04 µg/mL vs. Asian isolate 76.46 µg/mL vs. African isolate | Tectona grandis | [50] |
| Compound | Target | Cell Type Tested | IC50/EC50 | Plant | Reference |
|---|---|---|---|---|---|
| Coronaviruses (SARS-CoV, SARS-CoV-2, MERS-CoV) 3CLPro | |||||
| 3′-(3-Methylbut-2-enyl)-3′,4′,7-trihydroxyflavane | MERS-CoV-3CLPro | N/A | 34.7 µM | Broussonetia papyrifera | [72] |
| 4-Hydroxyisolonchocarpin | MERS-CoV-3CLPro | N/A | 193.7 µM | Broussonetia papyrifera | [72] |
| Broussochalcone A | MERS-CoV-3CLPro | N/A | 36.2 µM | Broussonetia papyrifera | [72] |
| Broussochalcone B | MERS-CoV-3CLPro | N/A | 27.9 µM | Broussonetia papyrifera | [72] |
| Broussoflavan A | MERS-CoV-3CLPro | N/A | 125.7 µM | Broussonetia papyrifera | [72] |
| Isoliquiritigenin | MERS-CoV-3CLPro | N/A | 33.9 µM | Broussonetia papyrifera | [72] |
| Kaempferol | MERS-CoV-3CLPro | N/A | 35.3 µM | Broussonetia papyrifera | [72] |
| Kazinol A | MERS-CoV-3CLPro | N/A | 66.2 µM | Broussonetia papyrifera | [72] |
| Kazinol B | MERS-CoV-3CLPro | N/A | 31.4 µM | Broussonetia papyrifera | [72] |
| Kazinol F | MERS-CoV-3CLPro | N/A | 135.0 µM | Broussonetia papyrifera | [72] |
| Kazinol J | MERS-CoV-3CLPro | N/A | 109.2 µM | Broussonetia papyrifera | [72] |
| Papyriflavonol A | MERS-CoV-3CLPro | N/A | 64.5 µM | Broussonetia papyrifera | [72] |
| Quercetin | MERS-CoV-3CLPro | N/A | 34.8 µM | Broussonetia papyrifera | [72] |
| Quercetin-β-galactoside | MERS-CoV-3CLPro | N/A | 68.0 µM | Broussonetia papyrifera | [72] |
| 3′-(3-Methylbut-2-enyl)-3′,4′,7-trihydroxyflavane | SARS-CoV-3CLPro | N/A | 30.2 µM | Broussonetia papyrifera | [72] |
| 3-isotheaflavin-3-gallate | SARS-CoV-3CLPro | N/A | 7 µM | Camellia sinensis | [80] |
| 4-Hydroxyisolonchocarpin | SARS-CoV-3CLPro | N/A | 202.7 µM | Broussonetia papyrifera | [72] |
| Aloe emodin | SARS-CoV-3CLPro | Vero cells | 132 μM | Isatis Indigotica | [279] |
| Amentoflavone | SARS-CoV-3CLPro | N/A | 8.3 μM | Torreya nucifera | [73] |
| Apigenin | SARS-CoV-3CLPro | N/A | 280.8 μM | Torreya nucifera | [73] |
| beta-sitosterol | SARS-CoV-3CLPro | Vero cells | 115 μM | Isatis indigotica | [279] |
| Betulinic acid | SARS-CoV-3CLPro | Vero E6 | 8.2 μM | Betula pubescens | [280] |
| Broussochalcone A | SARS-CoV-3CLPro | N/A | 88.1 µM | Broussonetia papyrifera | [72] |
| Broussochalcone B | SARS-CoV-3CLPro | N/A | 57.8 µM | Broussonetia papyrifera | [72] |
| Broussoflavan A | SARS-CoV-3CLPro | N/A | 92.4 µM | Broussonetia papyrifera | [72] |
| Chalcone | SARS-CoV-3CLPro | N/A | 11.4 µM | Angelica keiskei | [72] |
| Daidzein | SARS-CoV-3CLPro | Vero cells | 105 μM | Isatis Indigotica | [279] |
| Dihydrotanshinone I | SARS-CoV-3CLPro | N/A | 14.4 µM | Salvia miltiorrhiza | [281] |
| Herbacetin | SARS-CoV-3CLPro | N/A | 33.17 µM | Rhodiola Rosea | [282] |
| Hesperetin | SARS-CoV-3CLPro | Vero cells | 60 μM | Isatis Indigotica | [279] |
| Hirsutanolol | SARS-CoV-3CLPro | N/A | 105.6 µM | Alnus japonica | [283] |
| Hirsutenone | SARS-CoV-3CLPro | N/A | 36.2 µM | Alnus japonica | [283] |
| Indigo | SARS-CoV-3CLPro | Vero cells | 300 μM | Isatis indigotica | [279] |
| Indirubin | SARS-CoV-3CLPro | Vero cells | 293 μM | Isatis indigotica | [279] |
| Isoliquiritigenin | SARS-CoV-3CLPro | N/A | 61.9 µM | Broussonetia papyrifera | [72] |
| Kaempferol | SARS-CoV-3CLPro | N/A | 116.3 µM | Broussonetia papyrifera | [72] |
| Kazinol A | SARS-CoV-3CLPro | N/A | 84.8 µM | Broussonetia papyrifera | [72] |
| Kazinol B | SARS-CoV-3CLPro | N/A | 233.3 µM | Broussonetia papyrifera | [72] |
| Kazinol F | SARS-CoV-3CLPro | N/A | 43.3 µM | Broussonetia papyrifera | [72] |
| Kazinol J | SARS-CoV-3CLPro | N/A | 64.2 µM | Broussonetia papyrifera | [72] |
| Methyl tanshinoate | SARS-CoV-3CLPro | N/A | 21.1 µM | Salvia miltiorrhiza | [281] |
| Oregonin | SARS-CoV-3CLPro | N/A | 129.5 µM | Alnus japonica | [281] |
| Papyriflavonol A | SARS-CoV-3CLPro | N/A | 103.6 µM | Broussonetia papyrifera | [72] |
| Pectolinarin | SARS-CoV-3CLPro | N/A | 37.78 µM | Cirsium heterophyllum | [282] |
| Quercetin | SARS-CoV-3CLPro | N/A | 23.8 μM | Torreya nucifera | [73] |
| Quercetin-β-galactoside | SARS-CoV-3CLPro | N/A | 128.8 µM | Broussonetia papyrifera | [72] |
| Rhoifolin | SARS-CoV-3CLPro | N/A | 27.45 µM | Citrus limon | [282] |
| Rosmariquinone | SARS-CoV-3CLPro | N/A | 21.1 µM | Salvia miltiorrhiza | [281] |
| Rubranol | SARS-CoV-3CLPro | N/A | 144.6 µM | Alnus japonica | [283] |
| Rubranoside A | SARS-CoV-3CLPro | N/A | 102.1 µM | Alnus japonica | [283] |
| Rubranoside B | SARS-CoV-3CLPro | N/A | 105.3 µM | Alnus japonica | [283] |
| Sinigrin | SARS-CoV-3CLPro | Vero cells | 121 μM | Isatis indigotica | [279] |
| Tannic acid | SARS-CoV-3CLPro | N/A | 3 µM | Rhus Coriaria | [80] |
| Tanshinone I | SARS-CoV-3CLPro | N/A | 38.7 µM | Salvia miltiorrhiza | [281] |
| Tanshinone IIA | SARS-CoV-3CLPro | N/A | 89.1 µM | Salvia miltiorrhiza | [281] |
| Tanshinone IIB | SARS-CoV-3CLPro | N/A | 24.8 µM | Salvia miltiorrhiza | [281] |
| Theaflavin | SARS-CoV-3CLPro | N/A | 56.0 µM | Camellia Sinensis | [80] |
| Theaflavin-3,3′-digallate | SARS-CoV-3CLPro | N/A | 9.5 µM | Camellia Sinensis | [80] |
| Baicalein | SARS-CoV-2-3CLPro | N/A | 0.94 μM | Scutellaria baicalensis | [67] |
| Baicalin | SARS-CoV-2-3CLPro | N/A | 6.41 μM | Scutellaria baicalensis | [67] |
| β-carotene | SARS-CoV-2-3CLPro | N/A | 17.54 μM | Vaccinium Oxycoccos | [82] |
| Cyanidin 3-O-galactoside | SARS-CoV-2-3CLPro | N/A | 9.98 μM | Vaccinium Oxycoccos | [82] |
| Epicatechin | SARS-CoV-2-3CLPro | N/A | 12.54 μM | Vaccinium Oxycoccos | [82] |
| Isoschaftoside | SARS-CoV-2-3CLPro | N/A | 30.22 μM | Camellia Sinensis | [74] |
| Kaempferol-3-O-gentiobioside | SARS-CoV-2-3CLPro | N/A | 35.89 μM | Camellia Sinensis | [74] |
| Narcissoside | SARS-CoV-2-3CLPro | N/A | 38.14 μM | Zygophyllum simplex | [74] |
| Rutin | SARS-CoV-2-3CLPro | N/A | 31.26 μM | Fagopyrum tataricum | [74] |
| Vicenin-2 | SARS-CoV-2-3CLPro | N/A | 38.86 μM | Citrus Reticulata | [74] |
| PLPro | |||||
| Kazinol F | MERS-CoV-PLpro | N/A | 39.5 µM | Broussonetia papyrifera | [72] |
| Broussochalcone A | MERS-CoV-PLpro | N/A | 42.1 µM | Broussonetia papyrifera | [72] |
| Broussoflavan A | MERS-CoV-PLpro | N/A | 49.1 µM | Broussonetia papyrifera | [72] |
| Isoliquiritigenin | MERS-CoV-PLpro | N/A | 82.2 µM | Broussonetia papyrifera | [72] |
| Kazinol A | MERS-CoV-PLpro | N/A | 88.5 µM | Broussonetia papyrifera | [72] |
| Kazinol B | MERS-CoV-PLpro | N/A | 94.9 µM | Broussonetia papyrifera | [72] |
| Kazinol J | MERS-CoV-PLpro | N/A | 55.0 µM | Broussonetia papyrifera | [72] |
| 3′-(3-methylbut-2-enyl)-3′,4′,7-trihydroxyflavane | MERS-CoV-PLpro | N/A | 48.8 µM | Broussonetia papyrifera | [72] |
| Cryptotanshinone | SARS-CoV-PLPro | N/A | 0.8 µM | Salvia miltiorrhiza | [281] |
| Diplacone | SARS-CoV-PLPro | N/A | 10.4 µM | Paulownia tomentosa | [284] |
| Broussochalcone A | SARS-CoV-PLPro | N/A | 9.2 µM | Broussonetia papyrifera | [72] |
| Broussoflavan A | SARS-CoV-PLPro | N/A | 30.4 µM | Broussonetia papyrifera | [72] |
| Chalcone | SARS-CoV-PLPro | Vero cells | 1.2 µM | Angelica keiskei | [285] |
| Curcumin | SARS-CoV-PLPro | N/A | 5.7 µM | Curcuma longa | [283] |
| Dihydrotanshinone I | SARS-CoV-PLPro | N/A | 4.9 µM | Salvia miltiorrhiza | [281] |
| 6-geranyl-4′,5,7-trihydroxy-3′,5′-dimethoxyflavanone | SARS-CoV-PLPro | N/A | 13.9 µM | Paulownia tomentosa | [284] |
| Hirsutanonol 5 | SARS-CoV-PLPro | N/A | 7.8 µM | Alnus japonica | [283] |
| Hirsutenone 2 | SARS-CoV-PLPro | N/A | 4.1 µM | Alnus japonica | [283] |
| 4-hydroxyisolonchocarpin | SARS-CoV-PLPro | N/A | 35.4 µM | Broussonetia papyrifera | [72] |
| Isoliquiritigenin | SARS-CoV-PLPro | N/A | 24.6 µM | Broussonetia papyrifera | [72] |
| Kaempferol | SARS-CoV-PLPro | N/A | 16.3 µM | Broussonetia papyrifera | [72] |
| Kazinol A | SARS-CoV-PLPro | N/A | 66.2 µM | Broussonetia papyrifera | [72] |
| Kazinol B | SARS-CoV-PLPro | N/A | 31.4 µM | Broussonetia papyrifera | [72] |
| Kazinol F | SARS-CoV-PLPro | N/A | 27.8 µM | Broussonetia papyrifera | [72] |
| Kazinol J | SARS-CoV-PLPro | N/A | 15.2 µM | Broussonetia papyrifera | [72] |
| 3′-(3-methylbut-2-enyl)-3′,4′,7-trihydroxyflavane | SARS-CoV-PLPro | N/A | 35.8 µM | Broussonetia papyrifera | [72] |
| 3′-O-methyldiplacol | SARS-CoV-PLPro | N/A | 9.5 µM | Paulownia tomentosa | [284] |
| 4′-O-methyldiplacol | SARS-CoV-PLPro | N/A | 9.2 µM | Paulownia tomentosa | [284] |
| 3′-O-methyldiplacone | SARS-CoV-PLPro | N/A | 13.2 µM | Paulownia tomentosa | [284] |
| 4′-O-methyldiplacone | SARS-CoV-PLPro | N/A | 12.7 µM | Paulownia tomentosa | [284] |
| Mimulone | SARS-CoV-PLPro | N/A | 14.4 µM | Paulownia tomentosa | [284] |
| Oregonin | SARS-CoV-PLPro | N/A | 20.1 µM | Alnus japonica | [283] |
| Tanshinone IIA | SARS-CoV-PLPro | N/A | 1.6 µM | Salvia miltiorrhiza | [281] |
| Tomentin A | SARS-CoV-PLPro | N/A | 6.2 µM | Paulownia tomentosa | [284] |
| Tomentin B | SARS-CoV-PLPro | N/A | 6.1 µM | Paulownia tomentosa | [284] |
| Tomentin C | SARS-CoV-PLPro | N/A | 11.6 µM | Paulownia tomentosa | [284] |
| Tomentin D | SARS-CoV-PLPro | N/A | 12.5 µM | Paulownia tomentosa | [284] |
| Tomentin E | SARS-CoV-PLPro | N/A | 5.0 µM | Paulownia tomentosa | [284] |
| Rubranol | SARS-CoV-PLPro | N/A | 12.3 µM | Alnus japonica | [283] |
| Rubranoside A | SARS-CoV-PLPro | N/A | 9.1 µM | Alnus japonica | [283] |
| Rubranoside B | SARS-CoV-PLPro | N/A | 8.0 µM | Alnus japonica | [283] |
| Papyriflavonol A | SARS-CoV PLPro | N/A | 3.7 µM | Broussonetia papyrifera | [72] |
| Quercetin | SARS-CoV PLPro | N/A | 8.6 µM | Broussonetia papyrifera | [72] |
| Quercetin-β-galactoside | SARS-CoV PLPro | N/A | 51.9 µM | Broussonetia papyrifera | [72] |
| Broussochalcone B | SARS-CoV-PLPro | N/A | 11.6 µM | Broussonetia papyrifera | [72] |
| RdRp | |||||
| Amentoflavone | SARS-CoV-2 RdRp | RD cells | 13.17 µM | Selaginella tamariscina | [77] |
| Baicalein | SARS-CoV-2 RdRp | Vero CCL-81 | 4.5 µM | Scutellaria baicalensis | [69] |
| Baicalin | SARS-CoV-2 RdRp | Vero CCL-81 | 9 µM | Scutellaria baicalensis | [69] |
| Corilagin | SARS-CoV-2 RdRp | Vero CCL-81 | 0.13 µM | Caesalpinia coriaria | [286] |
| Luteolin | SARS-CoV-2 RdRp | N/A | 4.6 µM | Apium Graveolens | [287] |
| Lycorine | MERS-CoV RdRp | Vero CCL-81 | 1.41 µM | Lycoris Radiata | [87] |
| Lycorine | SARS-CoV RdRp | Vero CCL-81 | 1.02 µM | Lycoris Radiata | [87] |
| Lycorine | SARS-CoV-2 RdRp | Vero CCL-81 | 0.88 µM | Lycoris Radiata | [87] |
| Quercetin | SARS-CoV-2 RdRp | N/A | 6.9 µM | Alium Cepa | [287] |
| SARS-CoV nsp13-Helicase/ATPase activity | |||||
| Myricetin | nsp13 ATPase | N/A | 2.71 µM | N/A | [78] |
| Scutellarein | nsp13 ATPase | N/A | 0.86 µM | Scutellaria baicalensis | [78] |
| Baicalein | nsp13 ATPase | N/A | 0.47 µM | Scutellaria baicalensis | [288] |
| Baicalein | nsp13 helicase | Vero E6 | 2.9 µM | Scutellaria baicalensis | [70] |
| Dihydro-myricetin | nsp13 helicase | Vero E6 | 25.6 µM | N/A | [70] |
| Diosmetin | nsp13 helicase | Vero E6 | 10.6 µM | Vicia cracca | [70] |
| Ellagic acid | nsp13 ATPase/helicase | N/A | 2.8 µM | N/A | [83] |
| Flavanone | nsp13 helicase | Vero E6 | 0.52 µM | N/A | [70] |
| Flavanone-7-O-glucoside | nsp13 helicase | Vero E6 | 2.88 µM | N/A | [70] |
| (−)-Gallocatechin gallate | nsp13 helicase | N/A | 1.34 µM | Camellia sinensis | [83] |
| Licoflavone C | nsp13 helicase | Vero E6 | 1.34 µM | Genista ephedroides | [70] |
| Kaempferol | nsp13 helicase | Vero E6 | 0.76 µM | N/A | [70] |
| Katacine | nsp13 helicase | N/A | 5.98 µM | Polygonum coriarium | [83] |
| Licoflavone C | nsp13 ATPase (in the presence of BSA, TCEP and polyrA) | Vero E6 | 18.3 µM | Genista ephedroides | [70] |
| Linoleic acid | nsp13 helicase/ATPase | N/A | 4.3 µM | N/A | [289] |
| Myricetin | nsp13 helicase | Vero E6 | 0.41 µM | N/A | [70] |
| Oleic acid | nsp13 helicase/ATPase | N/A | 14 µM | N/A | [289] |
| Gossypol | nsp13 helicase/ATPase | N/A | 1.3 µM | Gossypium spp. | [289] |
| Prunetin | nsp13 helicase | Vero E6 | 11.5 µM | Prunus emarginata | [70] |
| Punicalagin | nsp13 helicase | Vero cells, A549-ACE2 | 0.43 µM | Punica granatum | [83] |
| Quercetin | nsp13 helicase | Vero E6 | 0.53 µM | N/A | [70] |
| Rhodiosin | nsp13 helicase | N/A | 0.48 µM | Rhodiola spp. | [83] |
| Rosmanol | nsp13 helicase | N/A | 8.93 µM | Rosmarinus officinalis L. | [83] |
| Tannic acid | nsp13 helicase | N/A | 1.25 µM | N/A | [83] |
| Wogonin | nsp13 helicase | Vero E6 | 24.9 µM | Scutellaria baicalensis | [70] |
| Dengue virus | |||||
| Sotetsuflavone | NS5 RdRp | N/A | 0.16 µM | Dacrydium araucarioides | [107] |
| Apigenin | NS5 RdRp and restores STAT2 inhibition by NS5 | IFN-I competent Huh7 cells, engineered K562 cell platform | EC50 29.7 µM | N/A | [108] |
| Luteolin | NS5 RdRp and restores STAT2 inhibition by NS5 | IFN-I competent Huh7 cells, engineered K562 cell platform | EC50 9.2 µM | N/A | [108] |
| Bisdemethoxycurcumin | NS2B/NS3 protease (DENV2) | BHK-21 cells | 36.23 µM | Curcuma longa | [110] |
| Curcumin | NS2B/NS3 protease (DENV2) | BHK-21 cells | 66.0 µM | Curcuma longa | [110] |
| Myricetin | NS2B/NS3 protease | N/A | 8.46 µM | N/A | [109] |
| Influenza A | |||||
| Apigenin | Neuraminidase | MDCK | 33 µM | Rhodiola rosea roots | [123] |
| Astragalin | Neuraminidase | MDCK | 38 µM | Rhodiola rosea roots | [123] |
| Cosmosiin | Neuraminidase | MDCK | 47 µM | Rhodiola rosea roots | [123] |
| Demethoxymatteucinol | Neuraminidase | MDCK | 30 µM | Pentarhizidium orientale | [125] |
| Gossypetin | Neuraminidase | MDCK | 3 µM | Rhodiola rosea roots | [123] |
| Herbacetin | Neuraminidase | MDCK | 9 µM | Rhodiola rosea roots | [123] |
| Hispidulin | Neuraminidase | MDCK | 19.83 µM | Salvia plebeia R. Br | [124] |
| 3′-hydroxy-5′-methoxy-6,8-dimethylhuazhongilexone | Neuraminidase | MDCK | 24 µM | Pentarhizidium orientale | [125] |
| Kaempferol | Neuraminidase | MDCK | 11 µM | Rhodiola rosea roots | [123] |
| Linocinamarin | Neuraminidase | MDCK | 44 µM | Rhodiola rosea roots | [123] |
| Luteolin | Neuraminidase | MDCK | 17.96 µM | Salvia plebeia R. Br | [124] |
| Matteucin | Neuraminidase | MDCK | 24 µM | Pentarhizidium orientale | [125] |
| Matteucinol | Neuraminidase | MDCK | 25 µM | Pentarhizidium orientale | [125] |
| Methoxymatteucin | Neuraminidase | MDCK | 25 µM | Pentarhizidium orientale | [125] |
| Nicotiflorin | Neuraminidase | MDCK | 32 µM | Rhodiola rosea roots | [123] |
| Quercetin | Neuraminidase | MDCK | 2 µM | Rhodiola rosea roots | [123] |
| Rosmarinic acid methyl ester | Neuraminidase | MDCK | 16.65 µM | Salvia plebeia R. Br | [124] |
| Rutin | Neuraminidase | MDCK | 34 µM | Rhodiola rosea roots | [123] |
| Rhodiolinin | Neuraminidase | MDCK | 10 µM | Rhodiola rosea roots | [123] |
| Rhodionin | Neuraminidase | MDCK | 32 µM | Rhodiola rosea roots | [123] |
| Rhodiosin | Neuraminidase | MDCK | 57 µM | Rhodiola rosea roots | [123] |
| Nepetin | Neuraminidase | MDCK | 11.18 µM | Salvia plebeia R. Br | [124] |
| 2′,4′dihydroxy-6′-methoxy-3′,5′-dimethylchalcone | Neuraminidase | HEK293, MDCK | 8.23 µM (H1N1) 5.07 µM (H9N2) 7.02 μM (H1N1 WT) 8.84 μM (H1N1-H274Y mutation) | Cleistocalyx operculatus | [126] |
| Myricetin-3′,5′-dimethylether-3-O-β-D-galactopyranoside | Neuraminidase | HEK293, MDCK | 8.86 µM (H1N1) 6.50 µM (H9N2) 7.10 μM (H1N1 WT) 9.34 μM (H1N1-H274Y mutation) | Cleistocalyx operculatus | [126] |
| Berberine | HAE cells, blocks nuclear export of IAV ribonucleoprotein to cytoplasm | MDCK, A549, LET1, HAE | 16 µM | Berberis sp. | [129] |
| HIV-1 | |||||
| Oleanolic acid | Protease | N/A | 10 µg/mL | Xanthoceras sorbifolia | [290] |
| 3-oxotirucalla-7, 24-dien-21-oic acid | Protease | N/A | 20 µg/mL | Xanthoceras sorbifolia | [290] |
| Apigenin | Integrase | N/A | 22 µM | Punica granatum | [94] |
| Ellagic acid | Integrase | N/A | 0.075 µM | Punica granatum | [94] |
| Betulinic acid | Integrase | N/A | 96.5 µM | Punica granatum | [94] |
| Kuwanon-L | RT-associated RDDP | TZM-bl (modified HeLa) | 0.99 µM | Xanthocer assorbifolia | [291] |
| Kuwanon-L | RT-associated RNase | TZM-bl (modified HeLa) | 0.57 µM | Xanthocer assorbifolia | [291] |
| Luteolin | Integrase | N/A | 6.5 µM | Punica granatum | [94] |
| Luteolin 7-O-glucoside | Integrase | N/A | 8.5 µM | Punica granatum | [94] |
| Punicalins | Integrase | N/A | 0.09 µM | Punica granatum | [94] |
| Punicalagins | Integrase | N/A | 0.065 µM | Punica granatum | [94] |
| Corilagin | Reverse transcriptase | MT4 T-lymphoid, MAGI cells | 9.3 µM | Phyllanthus amarus | [93] |
| Norisoboldine | Reverse transcriptase | N/A | 153.7 μg/mL | Croton echinocarpus | [292] |
| L-chicoric acid | Reverse transcriptase (presence of heteropolymeric template) | MT-2 | 17 µM | Echinacea purpurea | [92] |
| Geraniin | Reverse transcriptase | MT4 T-lymphoid, MAGI cells | 1.9 µM | Phyllanthus amarus | [93] |
| 1-methoxyoxalyl-3,5-DCQA | Reverse transcriptase (presence of heteropolymeric template) | MT-2 | 7 µM | Echinacea spp. | [92] |
| Apigenin | RT-associated RNase | N/A | 16.1 µM | Punica granatum | [94] |
| Betulinic acid | RT-associated RNase | N/A | 2.0 µM | Punica granatum | [94] |
| Ellagic acid | RT-associated RNase | N/A | 1.4 µM | Punica granatum | [94] |
| Luteolin | RT-associated RNase | N/A | 3.7 µM | Punica granatum | [94] |
| Oleanolic acid | RT-associated RNase | N/A | 6.7 µM | Punica granatum | [94] |
| Punicalins | RT-associated RNase | N/A | 0.18 µM | Punica granatum | [94] |
| Punicalagins | RT-associated RNase | N/A | 0.12 µM | Punica granatum | [94] |
| Ursolic acid | RT-associated RNase | N/A | 5.7 µM | Punica granatum | [94] |
| 3-O-(3′,3′-dimethylsuccinyl) betulinic acid—Bevirimat | p25-to-p24 conversion | PBMC, MT-2 | 10.3 nM | Syzygium claviflorum | [95] |
| Zika virus | |||||
| Astragalin | NS2B-NS3Pro | N/A | 112.0 µM | Phytolacca americana | [101] |
| Epicatechin gallate | NS2B-NS3Pro | N/A | 98.0 µM | Camellia sinensis | [101] |
| Epigallocatechin gallate | NS2B-NS3Pro | N/A | 87.0 µM | Camellia sinensis | [101] |
| Gallocatechin gallate | NS2B-NS3Pro | N/A | 99.0 µM | Camellia sinensis | [101] |
| Luteolin | NS2B-NS3Pro | N/A | 53.0 µM | N/A | [101] |
| Myricetin | NS2B-NS3Pro | N/A | 22.0 µM | N/A | [101] |
| Rutin | NS2B-NS3Pro | N/A | 112.0 µM | N/A | [101] |
| (−)-epigallocatechin-3-gallate | NS3 helicase–ATPase activity | N/A | 2.95 µM | Camellia sinensis | [102] |
| Chikungunya virus | |||||
| Berberine | Host MAPK pathway | HEK293T | 4.5 µM | Anamirta cocculus | [115] |
| Harringtonine | Nsp2 protease | BHK-21 | 0.24 µM | Cephalotaxus harringtonia | [114] |
| Tomatidine | Nsp2 protease | Huh7 | 1.3 µM | Solanum dulcamara | [293] |
| Apigenin | Replicase complex | BHK-21 | 70.8 µM | N/A | [113] |
| Chrysin | Replicase complex | BHK-21 | 126.6 µM | N/A | [113] |
| Naringenin | Replicase complex | BHK-21 | 118.4 µM | N/A | [113] |
| Silybin | Replicase complex | BHK-21 | 92.3 µM | N/A | [113] |
| Withaferin A | Nsp2 protease | BHK-21 | 0.51 µM | Withania somnifera | [294] |
| Prostratin | Not established/replication machinery | Vero cells, BGM, HEL | 8 µM (Vero) 7.6 µM (BGM) 7.1 µM (HEL) | Trigonostemon howii | [116] |
| Baicalein | Replicase complex | Vero cells | 7 µM | Scutellaria baicalensis | [48] |
| Fisetin | Replicase complex | Vero cells | 29.5 µM | N/A | [48] |
| Quercetagetin | Replicase complex | Vero cells | 43.52 µM | N/A | [48] |
| Silymarin | Replicase complex | Vero cells | 16.9 µg/mL | Silybum marianum | [117] |
| Trigocherrierin A | CHIKV-induced cell death | Vero cells | 0.6 µM | Trigonostemon cherrieri | [295] |
References
- Han, J.J.; Song, H.A.; Pierson, S.L.; Shen-Gunther, J.; Xia, Q. Emerging Infectious Diseases Are Virulent Viruses—Are We Prepared? An Overview. Microorganisms 2023, 11, 2618. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.E.; Mahmud, A.S.; Miller, I.F.; Rajeev, M.; Rasambainarivo, F.; Rice, B.L.; Takahashi, S.; Tatem, A.J.; Wagner, C.E.; Wang, L.-F.; et al. Infectious Disease in an Era of Global Change. Nat. Rev. Microbiol. 2022, 20, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Takova, K.; Tonova, V.; Koynarski, T.; Lukov, L.L.; Minkov, I.; Pishmisheva, M.; Kotsev, S.; Tsachev, I.; Baymakova, M.; et al. The Re-Emergence of Hepatitis E Virus in Europe and Vaccine Development. Viruses 2023, 15, 1558. [Google Scholar] [CrossRef] [PubMed]
- Bhadoria, P.; Gupta, G.; Agarwal, A. Viral Pandemics in the Past Two Decades: An Overview. J. Fam. Med. Prim. Care 2021, 10, 2745–2750. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Hong, K.-J. Innovations, Challenges, and Future Prospects for Combination Vaccines Against Human Infections. Vaccines 2025, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.-K.; Trinh, K.T.L. Molecular Farming for Immunization: Current Advances and Future Prospects in Plant-Produced Vaccines. Vaccines 2025, 13, 191. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-Based Expression and Characterization of SARS-CoV-2 Virus-like Particles Presenting a Native Spike Protein. Plant Biotechnol. J. 2022, 20, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Abbas, A.; Lehmann, C.; Rupasinghe, H.P.V. Antiviral and Anti-Inflammatory Plant-Derived Bioactive Compounds and Their Potential Use in the Treatment of COVID-19-Related Pathologies. J. Xenobiotics 2022, 12, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Dubey, N.K.; Sharma, M.; Kharkwal, H.; Bajpai, R.; Srivastava, R. Boosting the Human Antiviral Response in Conjunction with Natural Plant Products. Front. Nat. Prod. 2025, 3, 1470639. [Google Scholar] [CrossRef]
- Riaz, M.; Khalid, R.; Afzal, M.; Anjum, F.; Fatima, H.; Zia, S.; Rasool, G.; Egbuna, C.; Mtewa, A.G.; Uche, C.Z.; et al. Phytobioactive Compounds as Therapeutic Agents for Human Diseases: A Review. Food Sci. Nutr. 2023, 11, 2500–2529. [Google Scholar] [CrossRef] [PubMed]
- Popoola, T.D.; Segun, P.A.; Ekuadzi, E.; Dickson, R.A.; Awotona, O.R.; Nahar, L.; Sarker, S.D.; Fatokun, A.A. West African Medicinal Plants and Their Constituent Compounds as Treatments for Viral Infections, Including SARS-CoV-2/COVID-19. Daru J. Fac. Pharm. Tehran Univ. Med. Sci. 2022, 30, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Atampugbire, G.; Adomako, E.E.A.; Quaye, O. Medicinal Plants as Effective Antiviral Agents and Their Potential Benefits. Nat. Prod. Commun. 2024, 19, 1934578X241282923. [Google Scholar] [CrossRef]
- Thomas, E.; Stewart, L.E.; Darley, B.A.; Pham, A.M.; Esteban, I.; Panda, S.S. Plant-Based Natural Products and Extracts: Potential Source to Develop New Antiviral Drug Candidates. Molecules 2021, 26, 6197. [Google Scholar] [CrossRef] [PubMed]
- BioRender. Available online: https://app.biorender.com/user/signin (accessed on 18 June 2025).
- Wang, S.; Li, W.; Wang, Z.; Yang, W.; Li, E.; Xia, X.; Yan, F.; Chiu, S. Emerging and Reemerging Infectious Diseases: Global Trends and New Strategies for Their Prevention and Control. Signal Transduct. Target. Ther. 2024, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.R. Structure and Classification of Viruses. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 978-0-9631172-1-2. [Google Scholar]
- Kesheh, M.M.; Hosseini, P.; Soltani, S.; Zandi, M. An Overview on the Seven Pathogenic Human Coronaviruses. Rev. Med. Virol. 2022, 32, e2282. [Google Scholar] [CrossRef] [PubMed]
- Schoeman, D.; Fielding, B.C. Coronavirus Envelope Protein: Current Knowledge. Virol. J. 2019, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Munster, V.J.; Koopmans, M.; van Doremalen, N.; van Riel, D.; de Wit, E. A Novel Coronavirus Emerging in China—Key Questions for Impact Assessment. N. Engl. J. Med. 2020, 382, 692–694. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, K.; Huang, S.; Ivanov, K.I.; Yang, S.; Ji, Y.; Zhang, H.; Wu, W.; He, Y.; Zeng, Q.; et al. Anti-SARS-CoV-2 Prodrug ATV006 Has Broad-Spectrum Antiviral Activity against Human and Animal Coronaviruses. Acta Pharm. Sin. B 2025, 15, 2498–2510. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.A.; Saier, M.H. The SARS-Coronavirus Infection Cycle: A Survey of Viral Membrane Proteins, Their Functional Interactions and Pathogenesis. Int. J. Mol. Sci. 2021, 22, 1308. [Google Scholar] [CrossRef] [PubMed]
- He, C.-L.; Huang, L.-Y.; Wang, K.; Gu, C.-J.; Hu, J.; Zhang, G.-J.; Xu, W.; Xie, Y.-H.; Tang, N.; Huang, A.-L. Identification of Bis-Benzylisoquinoline Alkaloids as SARS-CoV-2 Entry Inhibitors from a Library of Natural Products. Signal Transduct. Target. Ther. 2021, 6, 131. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, E.; Vijgen, L.; Pannecouque, C.; Van Damme, E.; Peumans, W.; Egberink, H.; Balzarini, J.; Van Ranst, M. Plant Lectins Are Potent Inhibitors of Coronaviruses by Interfering with Two Targets in the Viral Replication Cycle. Antiviral Res. 2007, 75, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, L.; Moyo, P.; Cassel, J.; Isaacs, F.J.; Salvino, J.M.; Montaner, L.J.; Tietjen, I.; Maharaj, V. Use of Hyphenated Analytical Techniques to Identify the Bioactive Constituents of Gunnera perpensa L., a South African Medicinal Plant, Which Potently Inhibit SARS-CoV-2 Spike Glycoprotein-Host ACE2 Binding. Anal. Bioanal. Chem. 2022, 414, 3971–3985. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhou, Y.; Yang, X.; Zhang, F.; Liu, X.; Yu, B. Active Components in Ephedra Sinica Stapf Disrupt the Interaction between ACE2 and SARS-CoV-2 RBD: Potent COVID-19 Therapeutic Agents. J. Ethnopharmacol. 2021, 278, 114303. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-Y.; Wu, S.-L.; Chen, J.-C.; Li, C.-C.; Hsiang, C.-Y. Emodin Blocks the SARS Coronavirus Spike Protein and Angiotensin-Converting Enzyme 2 Interaction. Antiviral Res. 2007, 74, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Hishiki, T.; Baig, M.S.; Rajpoot, S.; Saqib, U.; Takasaki, T.; Hara, Y. Epigallocatechin Gallate (EGCG) Attenuates Severe Acute Respiratory Coronavirus Disease 2 (SARS-CoV-2) Infection by Blocking the Interaction of SARS-CoV-2 Spike Protein Receptor-Binding Domain to Human Angiotensin-Converting Enzyme 2. PLoS ONE 2022, 17, e0271112. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Chen, Y.; Wang, Y.-C.; Wang, W.-J.; Yang, C.-S.; Tsai, C.-L.; Hou, M.-H.; Chen, H.-F.; Shen, Y.-C.; Hung, M.-C. Tannic Acid Suppresses SARS-CoV-2 as a Dual Inhibitor of the Viral Main Protease and the Cellular TMPRSS2 Protease. Am. J. Cancer Res. 2020, 10, 4538–4546. [Google Scholar] [PubMed]
- Roy, A.V.; Chan, M.; Banadyga, L.; He, S.; Zhu, W.; Chrétien, M.; Mbikay, M. Quercetin Inhibits SARS-CoV-2 Infection and Prevents Syncytium Formation by Cells Co-Expressing the Viral Spike Protein and Human ACE2. Virol. J. 2024, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Cb, W.; Jc, T.; Rw, D. Molecular Mechanisms of HIV Entry. Adv. Exp. Med. Biol. 2012, 726, 223–242. [Google Scholar] [CrossRef]
- Li, B.Q.; Fu, T.; Dongyan, Y.; Mikovits, J.A.; Ruscetti, F.W.; Wang, J.M. Flavonoid Baicalin Inhibits HIV-1 Infection at the Level of Viral Entry. Biochem. Biophys. Res. Commun. 2000, 276, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Agrelli, A.; de Moura, R.R.; Crovella, S.; Brandão, L.A.C. ZIKA Virus Entry Mechanisms in Human Cells. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2019, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Frabasile, S.; Koishi, A.C.; Kuczera, D.; Silveira, G.F.; Verri, W.A.; Duarte Dos Santos, C.N.; Bordignon, J. Corrigendum: The Citrus Flavanone Naringenin Impairs Dengue Virus Replication in Human Cells. Sci. Rep. 2017, 7, 43976. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Teoh, B.-T.; Sam, S.-S.; Wong, P.-F.; Mustafa, M.R.; Abubakar, S. Novel Antiviral Activity of Baicalein against Dengue Virus. BMC Complement. Altern. Med. 2012, 12, 214. [Google Scholar] [CrossRef] [PubMed]
- Oo, A.; Teoh, B.T.; Sam, S.S.; Bakar, S.A.; Zandi, K. Baicalein and Baicalin as Zika Virus Inhibitors. Arch. Virol. 2019, 164, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, E.; Teoh, B.-T.; Sam, S.-S.; Lani, R.; Hassandarvish, P.; Chik, Z.; Yueh, A.; Abubakar, S.; Zandi, K. Baicalin, a Metabolite of Baicalein with Antiviral Activity against Dengue Virus. Sci. Rep. 2014, 4, 5452. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tai, W.; Wang, N.; Li, X.; Jiang, S.; Debnath, A.K.; Du, L.; Chen, S. Identification of Novel Natural Products as Effective and Broad-Spectrum Anti-Zika Virus Inhibitors. Viruses 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.M.; Batista, M.N.; Braga, A.C.S.; Nogueira, M.L.; Rahal, P. The Green Tea Molecule EGCG Inhibits Zika Virus Entry. Virology 2016, 496, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Mounce, B.C.; Cesaro, T.; Carrau, L.; Vallet, T.; Vignuzzi, M. Curcumin Inhibits Zika and Chikungunya Virus Infection by Inhibiting Cell Binding. Antiviral Res. 2017, 142, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Desprès, P.; El-Kalamouni, C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Zhao, Z.; Chai, Y.; Jin, X.; Li, C.; Yuan, F.; Liu, S.; Gao, Z.; Wang, H.; Song, J.; et al. Molecular Basis of Arthritogenic Alphavirus Receptor MXRA8 Binding to Chikungunya Virus Envelope Protein. Cell 2019, 177, 1714–1724.e12. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Wikan, N.; Kuadkitkan, A.; Jaimipuk, T.; Ubol, S.; Pulmanausahakul, R.; Auewarakul, P.; Kasinrerk, W.; Weng, W.-Y.; Panyasrivanit, M.; et al. Identification of Prohibitin as a Chikungunya Virus Receptor Protein. J. Med. Virol. 2012, 84, 1757–1770. [Google Scholar] [CrossRef] [PubMed]
- Moller-Tank, S.; Kondratowicz, A.S.; Davey, R.A.; Rennert, P.D.; Maury, W. Role of the Phosphatidylserine Receptor TIM-1 in Enveloped-Virus Entry. J. Virol. 2013, 87, 8327–8341. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.A.; Khomandiak, S.; Ashbrook, A.W.; Weller, R.; Heise, M.T.; Morrison, T.E.; Dermody, T.S. A Single-Amino-Acid Polymorphism in Chikungunya Virus E2 Glycoprotein Influences Glycosaminoglycan Utilization. J. Virol. 2014, 88, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Santhosh, S.R.; Tiwari, M.; Lakshmana Rao, P.V.; Parida, M. Assessment of in Vitro Prophylactic and Therapeutic Efficacy of Chloroquine against Chikungunya Virus in Vero Cells. J. Med. Virol. 2010, 82, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.; Sliva, K.; von Rhein, C.; Kümmerer, B.M.; Schnierle, B.S. The Green Tea Catechin, Epigallocatechin Gallate Inhibits Chikungunya Virus Infection. Antiviral Res. 2015, 113, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lani, R.; Hassandarvish, P.; Shu, M.-H.; Phoon, W.H.; Chu, J.J.H.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; Zandi, K. Antiviral Activity of Selected Flavonoids against Chikungunya Virus. Antiviral Res. 2016, 133, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Wintachai, P.; Thuaud, F.; Basmadjian, C.; Roytrakul, S.; Ubol, S.; Désaubry, L.; Smith, D.R. Assessment of Flavaglines as Potential Chikungunya Virus Entry Inhibitors. Microbiol. Immunol. 2015, 59, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, K.; Purushothaman, I.; Rajarajan, S. Spectral Characterisation, Antiviral Activities, in Silico ADMET and Molecular Docking of the Compounds Isolated from Tectona grandis to Chikungunya Virus. Biomed. Pharmacother. 2017, 87, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Dou, D.; Revol, R.; Östbye, H.; Wang, H.; Daniels, R. Influenza A Virus Cell Entry, Replication, Virion Assembly and Movement. Front. Immunol. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-M.; Lee, K.-H.; Seong, B.-L. Antiviral Effect of Catechins in Green Tea on Influenza Virus. Antiviral Res. 2005, 68, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Roschek, B.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry Flavonoids Bind to and Prevent H1N1 Infection in Vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Palamara, A.T.; Nencioni, L.; Aquilano, K.; De Chiara, G.; Hernandez, L.; Cozzolino, F.; Ciriolo, M.R.; Garaci, E. Inhibition of Influenza A Virus Replication by Resveratrol. J. Infect. Dis. 2005, 191, 1719–1729. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, A.; Mahmoud, S.H.; Elshaier, Y.A.M.M.; Shama, N.M.A.; Nasr, N.F.; Ali, M.A.; El-Shazly, A.M.; Mostafa, I.; Mostafa, A. Antiviral Activities of Plant-Derived Indole and β-Carboline Alkaloids against Human and Avian Influenza Viruses. Sci. Rep. 2023, 13, 1612. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, B.S.; Hoenen, T.; Wendt, L. Molecular Insights into the Ebola Virus Life Cycle. Nat. Microbiol. 2024, 9, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.M.; Phan, A.; Bo, Y.; LeBlond, N.D.; Smith, T.K.T.; Laroche, G.; Giguère, P.M.; Fullerton, M.D.; Pelchat, M.; Kobasa, D.; et al. Ebola Virus Triggers Receptor Tyrosine Kinase-Dependent Signaling to Promote the Delivery of Viral Particles to Entry-Conducive Intracellular Compartments. PLoS Pathog. 2021, 17, e1009275. [Google Scholar] [CrossRef] [PubMed]
- Tsang, N.Y.; Li, W.-F.; Varhegyi, E.; Rong, L.; Zhang, H.-J. Ebola Entry Inhibitors Discovered from Maesa Perlarius. Int. J. Mol. Sci. 2022, 23, 2620. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kroeker, A.; He, S.; Kozak, R.; Audet, J.; Mbikay, M.; Chrétien, M. Prophylactic Efficacy of Quercetin 3-β-O-d-Glucoside against Ebola Virus Infection. Antimicrob. Agents Chemother. 2016, 60, 5182–5188. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.; Tennant, P. Chapter 3—Replication and Expression Strategies of Viruses. In Viruses: Molecular Biology, Host Interactions and Applications to Biotechnology; Academic Press: Cambridge, MA, USA, 2018; pp. 55–82. [Google Scholar] [CrossRef]
- Yang, S.J.; Lim, Y. Resveratrol Ameliorates Hepatic Metaflammation and Inhibits NLRP3 Inflammasome Activation. Metabolism. 2014, 63, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Boopathi, S.; Poma, A.B.; Kolandaivel, P. Novel 2019 Coronavirus Structure, Mechanism of Action, Antiviral Drug Promises and Rule out against Its Treatment. J. Biomol. Struct. Dyn. 2021, 39, 3409–3418. [Google Scholar] [CrossRef] [PubMed]
- Jena, N.R. Drug Targets, Mechanisms of Drug Action, and Therapeutics against SARS-CoV-2. Chem. Phys. Impact 2021, 2, 100011. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An Overview of Viral Structure and Host Response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Yao, S.; Zhao, W.; Li, M.; Liu, J.; Shang, W.; Xie, H.; Ke, C.; Gao, M.; Yu, K.; et al. Discovery of Baicalin and Baicalein as Novel, Natural Product Inhibitors of SARS-CoV-2 3CL Protease in Vitro. bioRxiv 2020. [Google Scholar] [CrossRef]
- Su, H.; Yao, S.; Zhao, W.; Li, M.; Liu, J.; Shang, W.; Xie, H.; Ke, C.; Hu, H.; Gao, M.; et al. Anti-SARS-CoV-2 Activities in Vitro of Shuanghuanglian Preparations and Bioactive Ingredients. Acta Pharmacol. Sin. 2020, 41, 1167–1177. [Google Scholar] [CrossRef] [PubMed]
- Zandi, K.; Musall, K.; Oo, A.; Cao, D.; Liang, B.; Hassandarvish, P.; Lan, S.; Slack, R.L.; Kirby, K.A.; Bassit, L.; et al. Baicalein and Baicalin Inhibit SARS-CoV-2 RNA-Dependent-RNA Polymerase. Microorganisms 2021, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Corona, A.; Wycisk, K.; Talarico, C.; Manelfi, C.; Milia, J.; Cannalire, R.; Esposito, F.; Gribbon, P.; Zaliani, A.; Iaconis, D.; et al. Natural Compounds Inhibit SARS-CoV-2 Nsp13 Unwinding and ATPase Enzyme Activities. ACS Pharmacol. Transl. Sci. 2022, 5, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Yuk, H.J.; Ryu, H.W.; Lim, S.H.; Kim, K.S.; Park, K.H.; Ryu, Y.B.; Lee, W.S. Evaluation of Polyphenols from Broussonetia Papyrifera as Coronavirus Protease Inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Ryu, Y.B.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, J.-Y.; Kim, D.; Nguyen, T.T.H.; Park, S.-J.; Chang, J.S.; Park, K.H.; et al. Biflavonoids from Torreya Nucifera Displaying SARS-CoV 3CL(pro) Inhibition. Bioorg. Med. Chem. 2010, 18, 7940–7947. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Chen, Z.; Tao, Y.; Zhang, B.; Wu, X.; Yang, L.; Wang, Q.; Wang, Z. An Integrated Method for Optimized Identification of Effective Natural Inhibitors against SARS-CoV-2 3CLpro. Sci. Rep. 2021, 11, 22796. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Tang, N.; Lai, X.; Zhang, J.; Wen, W.; Li, X.; Li, A.; Wu, Y.; Liu, Z. Insights Into Amentoflavone: A Natural Multifunctional Biflavonoid. Front. Pharmacol. 2021, 12, 768708. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Tan, N.; Zeng, G.; Zhang, Y.; Jia, R. Amentoflavone and Its Derivatives as Novel Natural Inhibitors of Human Cathepsin B. Bioorg. Med. Chem. 2005, 13, 5819–5825. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.W.; Lee, S.; Kim, J.H.; Park, Y.-I.; So, J.; Kim, C.; Cho, C.W.; Park, J. Amentoflavone from Selaginella Tamariscina Inhibits SARS-CoV-2 RNA-Dependent RNA Polymerase. Heliyon 2024, 10, e36568. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-S.; Lee, J.; Lee, J.M.; Kim, Y.; Chin, Y.-W.; Jee, J.-G.; Keum, Y.-S.; Jeong, Y.-J. Identification of Myricetin and Scutellarein as Novel Chemical Inhibitors of the SARS Coronavirus Helicase, nsP13. Bioorg. Med. Chem. Lett. 2012, 22, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.R.; White, H.M.; McCormack, J.D.; Niemeyer, E.D. Catechin Composition, Phenolic Content, and Antioxidant Properties of Commercially-Available Bagged, Gunpowder, and Matcha Green Teas. Plant Foods Hum. Nutr. Dordr. Neth. 2023, 78, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-N.; Lin, C.P.C.; Huang, K.-K.; Chen, W.-C.; Hsieh, H.-P.; Liang, P.-H.; Hsu, J.T.-A. Inhibition of SARS-CoV 3C-like Protease Activity by Theaflavin-3,3′-Digallate (TF3). Evid.-Based Complement. Altern. Med. ECAM 2005, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.H.; Woo, H.-J.; Kang, H.-K.; Nguyen, V.D.; Kim, Y.-M.; Kim, D.-W.; Ahn, S.-A.; Xia, Y.; Kim, D. Flavonoid-Mediated Inhibition of SARS Coronavirus 3C-like Protease Expressed in Pichia Pastoris. Biotechnol. Lett. 2012, 34, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Pillai, U.J.; Cherian, L.; Taunk, K.; Iype, E.; Dutta, M. Identification of Antiviral Phytochemicals from Cranberry as Potential Inhibitors of SARS-CoV-2 Main Protease (Mpro). Int. J. Biol. Macromol. 2024, 261, 129655. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Peng, Y.; Yao, H.; Wang, Y.; Li, J.; Yang, Y.; Lin, Z. Punicalagin as an Allosteric NSP13 Helicase Inhibitor Potently Suppresses SARS-CoV-2 Replication in Vitro. Antiviral Res. 2022, 206, 105389. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.N.; Shipley, P.R. Determination of Anthocyanins in Cranberry Fruit and Cranberry Fruit Products by High-Performance Liquid Chromatography with Ultraviolet Detection: Single-Laboratory Validation. J. Aoac Int. 2011, 94, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Meng, X.; Tan, C.; Tong, Y.; Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Kong, Y.; Ma, Y. Composition and Antioxidant Activity of Anthocyanins from Aronia Melanocarpa Extracted Using an Ultrasonic-Microwave-Assisted Natural Deep Eutectic Solvent Extraction Method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef] [PubMed]
- Kiselova-Kaneva, Y.; Nashar, M.; Roussev, B.; Salim, A.; Hristova, M.; Olczyk, P.; Komosinska-Vassev, K.; Dincheva, I.; Badjakov, I.; Galunska, B.; et al. Sambucus Ebulus (Elderberry) Fruits Modulate Inflammation and Complement System Activity in Humans. Int. J. Mol. Sci. 2023, 24, 8714. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Min, J.S.; Jeon, S.; Lee, J.; Kim, S.; Park, T.; Park, D.; Jang, M.S.; Park, C.M.; Song, J.H.; et al. Lycorine, a Non-Nucleoside RNA Dependent RNA Polymerase Inhibitor, as Potential Treatment for Emerging Coronavirus Infections. Phytomedicine Int. J. Phytother. Phytopharm. 2021, 86, 153440. [Google Scholar] [CrossRef] [PubMed]
- Hillen, H.S.; Kokic, G.; Farnung, L.; Dienemann, C.; Tegunov, D.; Cramer, P. Structure of Replicating SARS-CoV-2 Polymerase. Nature 2020, 584, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-Dependent RNA Polymerase from COVID-19 Virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [PubMed]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 Polymerase Stalling by Remdesivir. Nat. Commun. 2021, 12, 279. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.-S.; Hughes, S.H. HIV-1 Reverse Transcription. Cold Spring Harb. Perspect. Med. 2012, 2, a006882. [Google Scholar] [CrossRef] [PubMed]
- McDougall, B.; King, P.J.; Wu, B.W.; Hostomsky, Z.; Reinecke, M.G.; Robinson, W.E. Dicaffeoylquinic and Dicaffeoyltartaric Acids Are Selective Inhibitors of Human Immunodeficiency Virus Type 1 Integrase. Antimicrob. Agents Chemother. 1998, 42, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Notka, F.; Meier, G.R.; Wagner, R. Inhibition of Wild-Type Human Immunodeficiency Virus and Reverse Transcriptase Inhibitor-Resistant Variants by Phyllanthus Amarus. Antiviral Res. 2003, 58, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Sanna, C.; Marengo, A.; Acquadro, S.; Caredda, A.; Lai, R.; Corona, A.; Tramontano, E.; Rubiolo, P.; Esposito, F. In Vitro Anti-HIV-1 Reverse Transcriptase and Integrase Properties of Punica granatum L. Leaves, Bark, and Peel Extracts and Their Main Compounds. Plants 2021, 10, 2124. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Goila-Gaur, R.; Salzwedel, K.; Kilgore, N.R.; Reddick, M.; Matallana, C.; Castillo, A.; Zoumplis, D.; Martin, D.E.; Orenstein, J.M.; et al. PA-457: A Potent HIV Inhibitor That Disrupts Core Condensation by Targeting a Late Step in Gag Processing. Proc. Natl. Acad. Sci. USA 2003, 100, 13555–13560. [Google Scholar] [CrossRef] [PubMed]
- Laureti, M.; Paradkar, P.N.; Fazakerley, J.K.; Rodriguez-Andres, J. Superinfection Exclusion in Mosquitoes and Its Potential as an Arbovirus Control Strategy. Viruses 2020, 12, 1259. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tan, X.-F.; Thurmond, S.; Zhang, Z.-M.; Lin, A.; Hai, R.; Song, J. The Structure of Zika Virus NS5 Reveals a Conserved Domain Conformation. Nat. Commun. 2017, 8, 14763. [Google Scholar] [CrossRef]
- Zhao, B.; Yi, G.; Du, F.; Chuang, Y.-C.; Vaughan, R.C.; Sankaran, B.; Kao, C.C.; Li, P. Structure and Function of the Zika Virus Full-Length NS5 Protein. Nat. Commun. 2017, 8, 14762. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.G.; Gadea, G.; Desprès, P.; El Kalamouni, C. Chapter 38—Medicinal Plants as Promising Source of Natural Antiviral Substances against Zika Virus. In Zika Virus Impact, Diagnosis, Control, and Models; Martin, C.R., Hollins Martin, C.J., Preedy, V.R., Rajendram, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 397–407. ISBN 978-0-12-820267-8. [Google Scholar]
- Kang, C.; Keller, T.H.; Luo, D. Zika Virus Protease: An Antiviral Drug Target. Trends Microbiol. 2017, 25, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-J.; Nguyen, T.T.H.; Kim, N.M.; Park, J.-S.; Jang, T.-S.; Kim, D. Inhibitory Effect of Flavonoids against NS2B-NS3 Protease of ZIKA Virus and Their Structure Activity Relationship. Biotechnol. Lett. 2017, 39, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, N.; Aarthy, M.; Singh, S.K.; Giri, R. Mechanistic Insights into Zika Virus NS3 Helicase Inhibition by Epigallocatechin-3-Gallate. ACS Omega 2020, 5, 11217–11226. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lao, Z.; Xu, J.; Li, Z.; Long, H.; Li, D.; Lin, L.; Liu, X.; Yu, L.; Liu, W.; et al. Antiviral Activity of Lycorine against Zika Virus in Vivo and in Vitro. Virology 2020, 546, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.; Kuhn, R.J. Structural Proteomics of Dengue Virus. Curr. Opin. Microbiol. 2008, 11, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.R.; Saeedi, B.J.; Campagnola, G.; Geiss, B.J. Analysis of RNA Binding by the Dengue Virus NS5 RNA Capping Enzyme. PLoS ONE 2011, 6, e25795. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.-C.; Chem, Y.-K.; Koo, C.; Mudin, R.N.B.; Amin, F.M.; Lee, K.-S.; Kheong, C.C. 2013 Dengue Outbreaks in Singapore and Malaysia Caused by Different Viral Strains. Am. J. Trop. Med. Hyg. 2015, 92, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Coulerie, P.; Nour, M.; Maciuk, A.; Eydoux, C.; Guillemot, J.-C.; Lebouvier, N.; Hnawia, E.; Leblanc, K.; Lewin, G.; Canard, B.; et al. Structure-Activity Relationship Study of Biflavonoids on the Dengue Virus Polymerase DENV-NS5 RdRp. Planta Med. 2013, 79, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Acchioni, C.; Acchioni, M.; Mancini, F.; Amendola, A.; Marsili, G.; Tirelli, V.; Gwee, C.P.; Chan, K.W.-K.; Sandini, S.; Bisbocci, M.; et al. A Cellular Screening Platform, Stably Expressing DENV2 NS5, Defines a Novel Anti-DENV Mechanism of Action of Apigenin Based on STAT2 Activation. Virology 2023, 583, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dang, M.; Lim, L.; Roy, A.; Song, J. Myricetin Allosterically Inhibits the Dengue NS2B-NS3 Protease by Disrupting the Active and Locking the Inactive Conformations. ACS Omega 2022, 7, 2798–2808. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, A.; Pilankatta, R.; Teramoto, T.; Sajith, A.M.; Nwulia, E.; Kulkarni, A.; Padmanabhan, R. Inhibition of Dengue Virus by Curcuminoids. Antiviral Res. 2019, 162, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Frolov, I.; Frolova, E.I. Molecular Virology of Chikungunya Virus. Curr. Top. Microbiol. Immunol. 2022, 435, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeeusen, K.; Daniel, M.; LaBeaud, D.A.; Gasque, P.; Peeling, R.W.; Stephenson, K.E.; Ng, L.F.P.; Ariën, K.K. Chikungunya Fever. Nat. Rev. Dis. Primer 2023, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of Alphavirus Entry and Replication Identified with a Stable Chikungunya Replicon Cell Line and Virus-Based Assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Thiruchelvan, M.; Lee, R.C.H.; Chen, H.; Chen, K.C.; Ng, M.L.; Chu, J.J.H. Inhibition of Chikungunya Virus Replication by Harringtonine, a Novel Antiviral That Suppresses Viral Protein Expression. Antimicrob. Agents Chemother. 2013, 57, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.P.; Ahola, T. The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef] [PubMed]
- Bourjot, M.; Delang, L.; Nguyen, V.H.; Neyts, J.; Guéritte, F.; Leyssen, P.; Litaudon, M. Prostratin and 12-O-Tetradecanoylphorbol 13-Acetate Are Potent and Selective Inhibitors of Chikungunya Virus Replication. J. Nat. Prod. 2012, 75, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Lani, R.; Hassandarvish, P.; Chiam, C.W.; Moghaddam, E.; Chu, J.J.H.; Rausalu, K.; Merits, A.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; et al. Antiviral Activity of Silymarin against Chikungunya Virus. Sci. Rep. 2015, 5, 11421. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, T.M.; te Velthuis, A.J. The RNA-Dependent RNA Polymerase of the Influenza A Virus. Future Virol. 2014, 9, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Terrier, O.; Rosa-Calatrava, M. Influenza Viruses and mRNA Splicing: Doing More with Less. mBio 2014, 5, e00070-14. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.S.; Naeve, C.W.; Webster, R.G.; Bootman, J.S.; Newman, R.; Schild, G.C. Alterations in the Hemagglutinin Associated with Adaptation of Influenza B Virus to Growth in Eggs. Virology 1985, 143, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Schmidtke, M.; von Grafenstein, S.; Kirchmair, J.; Liedl, K.R.; Rollinger, J.M. Influenza Neuraminidase: A Druggable Target for Natural Products. Nat. Prod. Rep. 2012, 29, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Gong, J.; Li, M.; Fang, H.; Xu, W. The Medicinal Potential of Influenza Virus Surface Proteins: Hemagglutinin and Neuraminidase. Curr. Med. Chem. 2011, 18, 1050–1066. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Ryu, Y.B.; Park, S.-J.; Kim, J.H.; Kwon, H.-J.; Kim, J.H.; Park, K.H.; Rho, M.-C.; Lee, W.S. Neuraminidase Inhibitory Activities of Flavonols Isolated from Rhodiola Rosea Roots and Their in Vitro Anti-Influenza Viral Activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Quy Ha, T.K.; Lee, C.; Li, W.; Oh, W.-K.; Shim, S.H. Antiviral Activities of Compounds from Aerial Parts of Salvia plebeia R. Br. J. Ethnopharmacol. 2016, 192, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Ha, T.K.Q.; Kang, K.B.; Kim, K.H.; Oh, W.K.; Kim, J.; Sung, S.H. C-Methylated Flavonoid Glycosides from Pentarhizidium Orientale Rhizomes and Their Inhibitory Effects on the H1N1 Influenza Virus. J. Nat. Prod. 2017, 80, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.K.Q.; Dao, T.T.; Nguyen, N.H.; Kim, J.; Kim, E.; Cho, T.O.; Oh, W.K. Antiviral Phenolics from the Leaves of Cleistocalyx Operculatus. Fitoterapia 2016, 110, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.W.; Ha, T.K.Q.; Cho, H.M.; An, J.-P.; Kim, S.K.; Kim, C.-S.; Kim, E.; Oh, W.K. Antiviral Activity of Furanocoumarins Isolated from Angelica Dahurica against Influenza a Viruses H1N1 and H9N2. J. Ethnopharmacol. 2020, 259, 112945. [Google Scholar] [CrossRef] [PubMed]
- Kuzuhara, T.; Iwai, Y.; Takahashi, H.; Hatakeyama, D.; Echigo, N. Green Tea Catechins Inhibit the Endonuclease Activity of Influenza A Virus RNA Polymerase. PLoS Curr. 2009, 1, RRN1052. [Google Scholar] [CrossRef] [PubMed]
- Botwina, P.; Owczarek, K.; Rajfur, Z.; Ochman, M.; Urlik, M.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway. Viruses 2020, 12, 344. [Google Scholar] [CrossRef] [PubMed]
- Mühlberger, E.; Weik, M.; Volchkov, V.E.; Klenk, H.D.; Becker, S. Comparison of the Transcription and Replication Strategies of Marburg Virus and Ebola Virus by Using Artificial Replication Systems. J. Virol. 1999, 73, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.W.; Borek, D.; Luthra, P.; Binning, J.M.; Anantpadma, M.; Liu, G.; Harvey, I.B.; Su, Z.; Endlich-Frazier, A.; Pan, J.; et al. An Intrinsically Disordered Peptide from Ebola Virus VP35 Controls Viral RNA Synthesis by Modulating Nucleoprotein-RNA Interactions. Cell Rep. 2015, 11, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Kolesnikova, L.; Becker, S. Ebola Virus Proteins NP, VP35, and VP24 Are Essential and Sufficient to Mediate Nucleocapsid Transport. Proc. Natl. Acad. Sci. USA 2018, 115, 1075–1080. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Gan, T.; Bai, P.; Wang, X.; Qian, Q.; Zhou, H.; Cheng, Q.; Qiu, Y.; Yin, L.; Zhong, J.; et al. Ebola Virus VP35 Has Novel NTPase and Helicase-like Activities. Nucleic Acids Res. 2019, 47, 5837–5851. [Google Scholar] [CrossRef] [PubMed]
- Corona, A.; Fanunza, E.; Salata, C.; Morwitzer, M.J.; Distinto, S.; Zinzula, L.; Sanna, C.; Frau, A.; Daino, G.L.; Quartu, M.; et al. Cynarin Blocks Ebola Virus Replication by Counteracting VP35 Inhibition of Interferon-Beta Production. Antiviral Res. 2022, 198, 105251. [Google Scholar] [CrossRef] [PubMed]
- Takova, K.; Koynarski, T.; Minkov, G.; Toneva, V.; Mardanova, E.; Ravin, N.; Lukov, G.L.; Zahmanova, G. Development and Optimization of an Enzyme Immunoassay to Detect Serum Antibodies against the Hepatitis E Virus in Pigs, Using Plant-Derived ORF2 Recombinant Protein. Vaccines 2021, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P.; Chikwamba, R.; Koch, M.; Rhodes, J.I.; Groenewald, J.-H. Plant-Made Therapeutics: An Emerging Platform in South Africa. Biotechnol. Adv. 2012, 30, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Aljabali, A.A.A.; Takova, K.; Minkov, G.; Tambuwala, M.M.; Minkov, I.; Lomonossoff, G.P. Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 17575. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Stöger, E.; Bortesi, L. Targeted Genome Editing of Plants and Plant Cells for Biomanufacturing. Transgenic Res. 2021, 30, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Timko, M.P. Improving Protein Quantity and Quality-The Next Level of Plant Molecular Farming. Int. J. Mol. Sci. 2022, 23, 1326. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R. Plant Glycoengineering for Designing Next-Generation Vaccines and Therapeutic Proteins. Biotechnol. Adv. 2023, 67, 108197. [Google Scholar] [CrossRef] [PubMed]
- Thuenemann, E.C.; Lenzi, P.; Love, A.J.; Taliansky, M.; Bécares, M.; Zuñiga, S.; Enjuanes, L.; Zahmanova, G.G.; Minkov, I.N.; Matić, S.; et al. The Use of Transient Expression Systems for the Rapid Production of Virus-like Particles in Plants. Curr. Pharm. Des. 2013, 19, 5564–5573. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Aljabali, A.A.; Takova, K.; Toneva, V.; Tambuwala, M.M.; Andonov, A.P.; Lukov, G.L.; Minkov, I. The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health? Int. J. Mol. Sci. 2023, 24, 1533. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel pEff Vector Based on the Genome of Potato virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Lomonossoff, G.P. When Plant Virology Met Agrobacterium: The Rise of the Deconstructed Clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Lomonossoff, G.P. Extremely High-Level and Rapid Transient Protein Production in Plants without the Use of Viral Replication. Plant Physiol. 2008, 148, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Dukiandjiev, S.; Minkov, I.; Andonov, A. Molecular farming—The production of recombinant subunit hepatitis b vaccine in tobacco plants. In Proceedings of the Balkan Scientific Conference of Biology, Plovdiv, Bulgaria, 19–21 May 2005; pp. 329–338. [Google Scholar]
- Rybicki, E.P. Plant-Based Vaccines against Viruses. Virol. J. 2014, 11, 205. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Takova, K.; Valkova, R.; Toneva, V.; Minkov, I.; Andonov, A.; Lukov, G.L. Plant-Derived Recombinant Vaccines against Zoonotic Viruses. Life 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Team, B.P. Baiya Phytopharm Announces Preliminary Results of the Phase 1 Clinical Trial of Its COVID-19 Vaccine Baiya SARS-CoV-2 Vax 2. Baiya Phytopharm. 2022. Available online: https://baiyaphytopharm.com/baiyaphytopharm-announces-preliminary-results-of-the-phase-1-clinical-trial-of-its-covid-19-vaccine-baiya-sars-cov-2-vax-2/ (accessed on 15 June 2025).
- KBio Inc A Phase I/II, First-in-Human, Observer-Blinded, Randomized, Placebo-Controlled, Parallel Group Study to Evaluate the Safety and Immunogenicity of TAP-COVID-19 SARS-CoV-2 Vaccine with CpG Adjuvant in Healthy Adults Aged 18–49 and 50–85; clinicaltrials.gov, 2023. Available online: https://outbreak.info/resources/NCT04473690 (accessed on 15 June 2025).
- Medicago, GSK Announce Positive Results for Plant-Based COVID-19 Vaccine. Available online: https://www.pharmacytimes.com/view/medicago-gsk-announce-positive-results-for-plant-based-covid-19-vaccine (accessed on 15 June 2025).
- Ramessar, K.; Sabalza, M.; Capell, T.; Christou, P. Maize Plants: An Ideal Production Platform for Effective and Safe Molecular Pharming. Plant Sci. 2008, 174, 409–419. [Google Scholar] [CrossRef]
- Concha, C.; Cañas, R.; Macuer, J.; Torres, M.J.; Herrada, A.A.; Jamett, F.; Ibáñez, C. Disease Prevention: An Opportunity to Expand Edible Plant-Based Vaccines? Vaccines 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Saini, V.; Kohli, D.V. Edible Transgenic Plant Vaccines for Different Diseases. Curr. Pharm. Biotechnol. 2013, 14, 594–614. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.; Chakauya, E.; Shephard, E.; Tanzer, F.L.; Maclean, J.; Lynch, A.; Williamson, A.-L.; Rybicki, E.P. Expression of HIV-1 Antigens in Plants as Potential Subunit Vaccines. BMC Biotechnol. 2008, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating Plant Molecular Farming and Materials Research for Next-Generation Vaccines. Nat. Rev. Mater. 2022, 7, 372–388. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Steele, J.F.C.; Jung, J.-W.; Thuenemann, E.C.; Meshcheriakova, Y.; Lomonossoff, G.P. Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult. Vaccines 2021, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, E.; Broer, I.; D’Aoust, M.-A.; Hitzeroth, I.; Hundleby, P.; Menassa, R.; Oksman-Caldentey, K.-M.; Peyret, H.; Salgueiro, S.; Saxena, P.; et al. Plant Molecular Farming in the Wake of the Closure of Medicago Inc. Nat. Biotechnol. 2023, 41, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Cirilo, T.M.; Grossi de Oliveira, A.L.; Costa Pinto, J.; Rihs, J.B.d.R.; Ruas, A.C.L.; Siqueira, W.F.; de Castro, J.C.; Sernizon Guimarães, N.; de Medeiros Brito, R.M.; Bueno, L.L.; et al. Assessing the Efficacy of Modified Plant Vaccine Antigens in Animal Immunization: A Systematic Review. Food Biosci. 2025, 66, 106178. [Google Scholar] [CrossRef]
- Mogojwe, H. Current and Planned Vaccine Manufacturing in Africa. Available online: https://www.clintonhealthaccess.org/report/current-and-planned-vaccine-manufacturing/ (accessed on 21 September 2023).
- BioApplications Inc. Available online: https://www.bioapplications.global/ (accessed on 15 June 2025).
- NCT05040789. Available online: https://covid-19.cochrane.org/studies/crs-18688748 (accessed on 10 September 2021).
- Yang, M.; Sun, H.; Lai, H.; Hurtado, J.; Chen, Q. Plant-produced Zika Virus Envelope Protein Elicits Neutralizing Immune Responses That Correlate with Protective Immunity against Zika Virus in Mice. Plant Biotechnol. J. 2017, 16, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Phoolcharoen, W.; Bhoo, S.H.; Lai, H.; Ma, J.; Arntzen, C.J.; Chen, Q.; Mason, H.S. Expression of an Immunogenic Ebola Immune Complex in Nicotiana Benthamiana. Plant Biotechnol. J. 2011, 9, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Huerta, R.; Monreal-Escalante, E.; Govea-Alonso, D.O.; Angulo, C.; Rosales-Mendoza, S. Expression of an Immunogenic LTB-Based Chimeric Protein Targeting Zaire Ebolavirus Epitopes from GP1 in Plant Cells. Plant Cell Rep. 2017, 36, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Aubin, É.; Trépanier, S.; Poulin, J.-F.; Yassine-Diab, B.; ter Meulen, J.; Ward, B.J.; Landry, N. Humoral and Cell-Mediated Immune Responses to H5N1 Plant-Made Virus-like Particle Vaccine Are Differentially Impacted by Alum and GLA-SE Adjuvants in a Phase 2 Clinical Trial. Npj Vaccines 2018, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pillet, S.; Couillard, J.; Trépanier, S.; Poulin, J.-F.; Yassine-Diab, B.; Guy, B.; Ward, B.J.; Landry, N. Immunogenicity and Safety of a Quadrivalent Plant-Derived Virus like Particle Influenza Vaccine Candidate-Two Randomized Phase II Clinical Trials in 18 to 49 and ≥50 Years Old Adults. PLoS ONE 2019, 14, e0216533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lai, H. Plant-Derived Virus-like Particles as Vaccines. Hum. Vaccines Immunother. 2013, 9, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Kheirvari, M.; Liu, H.; Tumban, E. Virus-like Particle Vaccines and Platforms for Vaccine Development. Viruses 2023, 15, 1109. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Kotlyarov, R.Y.; Stuchinskaya, M.D.; Nikolaeva, L.I.; Zahmanova, G.; Ravin, N.V. High-Yield Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope and Receptor Binding Domain of SARS-CoV-2 in Plants Using Viral Vectors. Int. J. Mol. Sci. 2022, 23, 15684. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Vasyagin, E.A.; Kotova, K.G.; Zahmanova, G.G.; Ravin, N.V. Plant-Produced Chimeric Hepatitis E Virus-like Particles as Carriers for Antigen Presentation. Viruses 2024, 16, 1093. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, E.A.; Mardanova, E.S.; Zykova, A.A.; Shuklina, M.A.; Stepanova, L.A.; Tsybalova, L.M.; Ravin, N.V. Chimeric Virus-like Particles of Physalis Mottle Virus as Carriers of M2e Peptides of Influenza a Virus. Viruses 2024, 16, 1802. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, K.; Uttenthal, A.; Jones, T.D.; Xu, F.; Merryweather, A.; Hamilton, W.D.; Langeveld, J.P.; Boshuizen, R.S.; Kamstrup, S.; Lomonossoff, G.P.; et al. Plant-Derived Vaccine Protects Target Animals against a Viral Disease. Nat. Biotechnol. 1997, 15, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.E.; Benko, A.; Doucette, S.A.; Cameron, T.I.; Foster, T.; Hanley, K.M.; McCormick, A.A.; McCulloch, M.; Pogue, G.P.; Smith, M.L.; et al. Protection of Rabbits against Cutaneous Papillomavirus Infection Using Recombinant Tobacco Mosaic Virus Containing L2 Capsid Epitopes. Vaccine 2006, 24, 5516–5525. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.M.; Chichester, J.A.; Mett, V.; Jaje, J.; Tottey, S.; Manceva, S.; Casta, L.J.; Gibbs, S.K.; Musiychuk, K.; Shamloul, M.; et al. A Plant-Produced Pfs25 VLP Malaria Vaccine Candidate Induces Persistent Transmission Blocking Antibodies against Plasmodium Falciparum in Immunized Mice. PLoS ONE 2013, 8, e79538. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lai, H.; Sun, H.; Chen, Q. Virus-like Particles That Display Zika Virus Envelope Protein Domain III Induce Potent Neutralizing Immune Responses in Mice. Sci. Rep. 2017, 7, 7679. [Google Scholar] [CrossRef] [PubMed]
- Ravin, N.V.; Kotlyarov, R.Y.; Mardanova, E.S.; Kuprianov, V.V.; Migunov, A.I.; Stepanova, L.A.; Tsybalova, L.M.; Kiselev, O.I.; Skryabin, K.G. Plant-Produced Recombinant Influenza Vaccine Based on Virus-like HBc Particles Carrying an Extracellular Domain of M2 Protein. Biochem. Biokhimiia 2012, 77, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Mazalovska, M.; Takova, K.; Toneva, V.; Minkov, I.; Peyret, H.; Lomonossoff, G. Efficient Production of Chimeric Hepatitis B Virus-Like Particles Bearing an Epitope of Hepatitis E Virus Capsid by Transient Expression in Nicotiana Benthamiana. Life 2021, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.G.; Mazalovska, M.; Takova, K.H.; Toneva, V.T.; Minkov, I.N.; Mardanova, E.S.; Ravin, N.V.; Lomonossoff, G.P. Rapid High-Yield Transient Expression of Swine Hepatitis E ORF2 Capsid Proteins in Nicotiana Benthamiana Plants and Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope. Plants 2019, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Mazalovska, M.; Toneva, V.; Minkov, I.; Lomonossoff, G. Production of Chimeric Virus-like Particles Bearing M2e Influenza Epitope in Nicotiana Benthamiana Plants. J. Biotechnol. 2015, 208, S109. [Google Scholar] [CrossRef]
- Ponndorf, D.; Meshcheriakova, Y.; Thuenemann, E.C.; Dobon Alonso, A.; Overman, R.; Holton, N.; Dowall, S.; Kennedy, E.; Stocks, M.; Lomonossoff, G.P.; et al. Plant-Made Dengue Virus-like Particles Produced by Co-Expression of Structural and Non-Structural Proteins Induce a Humoral Immune Response in Mice. Plant Biotechnol. J. 2021, 19, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, D.A.; Mokoena, N.B.; Tsekoa, T.L.; Dibakwane, V.S.; O’Kennedy, M.M. Plant-Produced Chimeric Virus-like Particles—A New Generation Vaccine against African Horse Sickness. BMC Vet. Res. 2019, 15, 432. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A. Passive Antibody Administration (Immediate Immunity) as a Specific Defense against Biological Weapons. Emerg. Infect. Dis. 2002, 8, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, A.; Whaley, K.J.; Zeitlin, L. Plant-Derived Monoclonal Antibodies for Prevention and Treatment of Infectious Disease. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, A.; Pauly, M. Monoclonal Antibodies from Plants: A New Speed Record. Proc. Natl. Acad. Sci. USA 2006, 103, 14645–14646. [Google Scholar] [CrossRef] [PubMed]
- Giritch, A.; Marillonnet, S.; Engler, C.; van Eldik, G.; Botterman, J.; Klimyuk, V.; Gleba, Y. Rapid High-Yield Expression of Full-Size IgG Antibodies in Plants Coinfected with Noncompeting Viral Vectors. Proc. Natl. Acad. Sci. USA 2006, 103, 14701–14706. [Google Scholar] [CrossRef] [PubMed]
- Diamos, A.G.; Hunter, J.G.L.; Pardhe, M.D.; Rosenthal, S.H.; Sun, H.; Foster, B.C.; DiPalma, M.P.; Chen, Q.; Mason, H.S. High Level Production of Monoclonal Antibodies Using an Optimized Plant Expression System. Front. Bioeng. Biotechnol. 2020, 7, 472. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, L.; Voglmeir, J. mAbs N-Glycosylation: Implications for Biotechnology and Analytics. Carbohydr. Res. 2022, 514, 108541. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Tekoah, Y.; Rudd, P.M.; Harvey, D.J.; Dwek, R.A.; Spitsin, S.; Hanlon, C.A.; Rupprecht, C.; Dietzschold, B.; Golovkin, M.; et al. Function and Glycosylation of Plant-Derived Antiviral Monoclonal Antibody. Proc. Natl. Acad. Sci. USA 2003, 100, 8013–8018. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Steinkellner, H. Glyco-Engineering in Plants to Produce Human-like N-Glycan Structures. Biotechnol. J. 2012, 7, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, D.-S.; Ko, K. Expression of a Large Single-Chain 13F6 Antibody with Binding Activity against Ebola Virus-Like Particles in a Plant System. Int. J. Mol. Sci. 2020, 21, 7007. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Castilho, A.; Stadlmann, J.; Kunert, R.; Quendler, H.; Gattinger, P.; Jez, J.; Rademacher, T.; Altmann, F.; Mach, L.; et al. Improved Virus Neutralization by Plant-Produced Anti-HIV Antibodies with a Homogeneous Β1,4-Galactosylated N-Glycan Profile*. J. Biol. Chem. 2009, 284, 20479–20485. [Google Scholar] [CrossRef] [PubMed]
- Göritzer, K.; Melnik, S.; Schwestka, J.; Arcalis, E.; Drapal, M.; Fraser, P.; Ma, J.K.-C.; Stoger, E. Enhancing Quality and Yield of Recombinant Secretory IgA Antibodies in Nicotiana Benthamiana by Endoplasmic Reticulum Engineering. Plant Biotechnol. J. 2025, 23, 1178–1189. [Google Scholar] [CrossRef] [PubMed]
- Niemer, M.; Mehofer, U.; Torres Acosta, J.A.; Verdianz, M.; Henkel, T.; Loos, A.; Strasser, R.; Maresch, D.; Rademacher, T.; Steinkellner, H.; et al. The Human Anti-HIV Antibodies 2F5, 2G12, and PG9 Differ in Their Susceptibility to Proteolytic Degradation: Down-Regulation of Endogenous Serine and Cysteine Proteinase Activities Could Improve Antibody Production in Plant-Based Expression Platforms. Biotechnol. J. 2014, 9, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.K.-C.; Drossard, J.; Lewis, D.; Altmann, F.; Boyle, J.; Christou, P.; Cole, T.; Dale, P.; van Dolleweerd, C.J.; Isitt, V.; et al. Regulatory Approval and a First-in-Human Phase I Clinical Trial of a Monoclonal Antibody Produced in Transgenic Tobacco Plants. Plant Biotechnol. J. 2015, 13, 1106–1120. [Google Scholar] [CrossRef] [PubMed]
- Lotter-Stark, H.C.T.; Rybicki, E.P.; Chikwamba, R.K. Plant Made Anti-HIV Microbicides—A Field of Opportunity. Biotechnol. Adv. 2012, 30, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Loos, A.; Van Droogenbroeck, B.; Hillmer, S.; Grass, J.; Pabst, M.; Castilho, A.; Kunert, R.; Liang, M.; Arcalis, E.; Robinson, D.G.; et al. Expression of Antibody Fragments with a Controlled N-Glycosylation Pattern and Induction of Endoplasmic Reticulum-Derived Vesicles in Seeds of Arabidopsis. Plant Physiol. 2011, 155, 2036–2048. [Google Scholar] [CrossRef] [PubMed]
- Grandits, M.; Grünwald-Gruber, C.; Gastine, S.; Standing, J.F.; Reljic, R.; Teh, A.Y.-H.; Ma, J.K.-C. Improving the Efficacy of Plant-Made Anti-HIV Monoclonal Antibodies for Clinical Use. Front. Plant Sci. 2023, 14, 1126470. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, T.; Sack, M.; Arcalis, E.; Stadlmann, J.; Balzer, S.; Altmann, F.; Quendler, H.; Stiegler, G.; Kunert, R.; Fischer, R.; et al. Recombinant Antibody 2G12 Produced in Maize Endosperm Efficiently Neutralizes HIV-1 and Contains Predominantly Single-GlcNAc N-Glycans. Plant Biotechnol. J. 2008, 6, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Bohorova, N.; Grass, J.; Bohorov, O.; Zeitlin, L.; Whaley, K.; Altmann, F.; Steinkellner, H. Rapid High Yield Production of Different Glycoforms of Ebola Virus Monoclonal Antibody. PLoS ONE 2011, 6, e26040. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Davis, K.R. The Potential of Plants as a System for the Development and Production of Human Biologics. F1000Research 2016, 5, 912. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Engle, M.; Fuchs, A.; Keller, T.; Johnson, S.; Gorlatov, S.; Diamond, M.S.; Chen, Q. Monoclonal Antibody Produced in Plants Efficiently Treats West Nile Virus Infection in Mice. Proc. Natl. Acad. Sci. USA 2010, 107, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Jugler, C.; Joensuu, J.; Chen, Q. Hydrophobin-Protein A Fusion Protein Produced in Plants Efficiently Purified an Anti-West Nile Virus Monoclonal Antibody from Plant Extracts via Aqueous Two-Phase Separation. Int. J. Mol. Sci. 2020, 21, 2140. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, J.; Modelska, A.; Figlerowicz, M.; Pniewski, T.; Letellier, M.; Lisowa, O.; Yusibov, V.; Koprowski, H.; Plucienniczak, A.; Legocki, A.B. A Plant-Derived Edible Vaccine against Hepatitis B Virus. FASEB J. 1999, 13, 1796–1799. [Google Scholar] [CrossRef] [PubMed]
- Joung, Y.H.; Park, S.H.; Moon, K.-B.; Jeon, J.-H.; Cho, H.-S.; Kim, H.-S. The Last Ten Years of Advancements in Plant-Derived Recombinant Vaccines against Hepatitis B. Int. J. Mol. Sci. 2016, 17, 1715. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Rattanapisit, K.; Manopwisedjaroen, S.; Thitithanyanont, A.; Phoolcharoen, W. Monoclonal Antibodies B38 and H4 Produced in Nicotiana Benthamiana Neutralize SARS-CoV-2 in Vitro. Front. Plant Sci. 2020, 11, 589995. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, R.; Marusic, C.; Villani, M.E.; Lico, C.; Capodicasa, C.; Andreano, E.; Paciello, I.; Rappuoli, R.; Salzano, A.M.; Scaloni, A.; et al. Production of Two SARS-CoV-2 Neutralizing Antibodies with Different Potencies in Nicotiana Benthamiana. Front. Plant Sci. 2022, 13, 956741. [Google Scholar] [CrossRef] [PubMed]
- Jugler, C.; Sun, H.; Grill, F.; Kibler, K.; Esqueda, A.; Lai, H.; Li, Y.; Lake, D.; Chen, Q. Potential for a Plant-Made SARS-CoV-2 Neutralizing Monoclonal Antibody as a Synergetic Cocktail Component. Vaccines 2022, 10, 772. [Google Scholar] [CrossRef] [PubMed]
- Girard, L.S.; Fabis, M.J.; Bastin, M.; Courtois, D.; Pétiard, V.; Koprowski, H. Expression of a Human Anti-Rabies Virus Monoclonal Antibody in Tobacco Cell Culture. Biochem. Biophys. Res. Commun. 2006, 345, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Pisuttinusart, N.; Rattanapisit, K.; Srisaowakarn, C.; Thitithanyanont, A.; Strasser, R.; Shanmugaraj, B.; Phoolcharoen, W. Neutralizing Activity of Anti-Respiratory Syncytial Virus Monoclonal Antibody Produced in Nicotiana Benthamiana. Hum. Vaccines Immunother. 2024, 20, 2327142. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolny, M. GM Rice Delivers Antibodies against Deadly Rotavirus. Nature 2013. [Google Scholar] [CrossRef]
- Dong, J.-L.; Liang, B.-G.; Jin, Y.-S.; Zhang, W.-J.; Wang, T. Oral Immunization with pBsVP6-Transgenic Alfalfa Protects Mice against Rotavirus Infection. Virology 2005, 339, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Juárez, P.; Presa, S.; Espí, J.; Pineda, B.; Antón, M.T.; Moreno, V.; Buesa, J.; Granell, A.; Orzaez, D. Neutralizing Antibodies against Rotavirus Produced in Transgenically Labelled Purple Tomatoes. Plant Biotechnol. J. 2012, 10, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Esparza, J.F.; Gómez-Lim, M.A. Transient Expression in Cytoplasm and Apoplast of Rotavirus VP6 Protein Fused to Anti-DEC205 Antibody in Nicotiana Benthamiana and Nicotiana Sylvestris. Mol. Biotechnol. 2021, 63, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Krittanai, S.; Rattanapisit, K.; Bulaon, C.J.I.; Pitaksajjakul, P.; Keadsanti, S.; Ramasoota, P.; Strasser, R.; Phoolcharoen, W. Nicotiana Benthamiana as a Potential Source for Producing Anti-Dengue Virus D54 Neutralizing Therapeutic Antibody. Biotechnol. Rep. Amst. Neth. 2024, 42, e00844. [Google Scholar] [CrossRef] [PubMed]
- Dent, M.; Hurtado, J.; Paul, A.M.; Sun, H.; Lai, H.; Yang, M.; Esqueda, A.; Bai, F.; Steinkellner, H.; Chen, Q. Plant-Produced Anti-Dengue Virus Monoclonal Antibodies Exhibit Reduced Antibody-Dependent Enhancement of Infection Activity. J. Gen. Virol. 2016, 97, 3280–3290. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.M.; Paul, M.J.; Pinneh, E.; Shanmugaraj, B.; Ashall, J.; Ramalingam, S.; Hewson, R.; Diamond, M.S.; Fox, J.M.; Ma, J.K.-C. Characterisation of Chikungunya Virus Neutralising Monoclonal Antibodies Expressed in Tobacco Plants. J. Biotechnol. 2025, 406, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Sun, H.; Lai, H.; Neupane, B.; Bai, F.; Steinkellner, H.; Chen, Q. Plant-Produced Anti-Zika Virus Monoclonal Antibody Glycovariant Exhibits Abrogated Antibody-Dependent Enhancement of Infection. Vaccines 2023, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Sirko, A.; Vaněk, T.; Góra-Sochacka, A.; Redkiewicz, P. Recombinant Cytokines from Plants. Int. J. Mol. Sci. 2011, 12, 3536–3552. [Google Scholar] [CrossRef] [PubMed]
- Bonjardim, C.A. Interferons (IFNs) Are Key Cytokines in Both Innate and Adaptive Antiviral Immune Responses—And Viruses Counteract IFN Action. Microbes Infect. 2005, 7, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Tayal, V.; Kalra, B.S. Cytokines and Anti-Cytokines as Therapeutics—An Update. Eur. J. Pharmacol. 2008, 579, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-L.; Lin, Y.-L.; Lee, Y.-L.; Yang, N.-S.; Chan, M.-T. Expression of Bioactive Human Interferon-Gamma in Transgenic Rice Cell Suspension Cultures. Transgenic Res. 2004, 13, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Ohya, K.; Matsumura, T.; Ohashi, K.; Onuma, M.; Sugimoto, C. Expression of Two Subtypes of Human IFN-Alpha in Transgenic Potato Plants. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2001, 21, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Arazi, T.; Slutsky, S.G.; Shiboleth, Y.M.; Wang, Y.; Rubinstein, M.; Barak, S.; Yang, J.; Gal-On, A. Engineering Zucchini Yellow Mosaic Potyvirus as a Non-Pathogenic Vector for Expression of Heterologous Proteins in Cucurbits. J. Biotechnol. 2001, 87, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Arlen, P.A.; Falconer, R.; Cherukumilli, S.; Cole, A.; Cole, A.M.; Oishi, K.K.; Daniell, H. Field Production and Functional Evaluation of Chloroplast-Derived Interferon-Alpha2b. Plant Biotechnol. J. 2007, 5, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Sawahel, W.A. The Production of Transgenic Potato Plants Expressing Human Alpha-Interferon Using Lipofectin-Mediated Transformation. Cell. Mol. Biol. Lett. 2002, 7, 19–29. [Google Scholar] [PubMed]
- Luchakivskaya, Y.; Kishchenko, O.; Gerasymenko, I.; Olevinskaya, Z.; Simonenko, Y.; Spivak, M.; Kuchuk, M. High-Level Expression of Human Interferon Alpha-2b in Transgenic Carrot (Daucus carota L.) Plants. Plant Cell Rep. 2011, 30, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Liu, X.W.; Zhang, H.C.; Shen, F.F.; Wang, G.P. Transient Expression of an Active Human Interferon-Beta in Lettuce. ResearchGate 2007, 112, 258–265. [Google Scholar] [CrossRef]
- Nabi-Afjadi, M.; Heydari, M.; Zalpoor, H.; Arman, I.; Sadoughi, A.; Sahami, P.; Aghazadeh, S. Lectins and Lectibodies: Potential Promising Antiviral Agents. Cell. Mol. Biol. Lett. 2022, 27, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, C. Griffithsin, a Highly Potent Broad-Spectrum Antiviral Lectin from Red Algae: From Discovery to Clinical Application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Kouokam, J.C. Lectins as Promising Therapeutics for the Prevention and Treatment of HIV and Other Potential Coinfections. BioMed Res. Int. 2018, 2018, 3750646. [Google Scholar] [CrossRef] [PubMed]
- Bellande, K.; Lalo, A.; Ligat, L.; Roujol, D.; Jamet, E.; Canut, H. Recombinant N-Glycosylation Isoforms of Legume Lectins: Production and Purification from Nicotiana Benthamiana Leaves Following RuBisCO Depletion. Plant Physiol. Biochem. PPB 2020, 157, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Hoelscher, M.; Tiller, N.; Teh, A.Y.-H.; Wu, G.-Z.; Ma, J.K.-C.; Bock, R. High-Level Expression of the HIV Entry Inhibitor Griffithsin from the Plastid Genome and Retention of Biological Activity in Dried Tobacco Leaves. Plant Mol. Biol. 2018, 97, 357–370. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Vojdani, F.; Buffa, V.; Shattock, R.J.; Montefiori, D.C.; Bakke, J.; Mirsalis, J.; d’Andrea, A.-L.; Hume, S.D.; Bratcher, B.; et al. Scaleable Manufacture of HIV-1 Entry Inhibitor Griffithsin and Validation of Its Safety and Efficacy as a Topical Microbicide Component. Proc. Natl. Acad. Sci. USA 2009, 106, 6099–6104. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Arcalis, E.; Ramessar, K.; Evans, A.; O’Keefe, B.R.; Shattock, R.J.; Medina, V.; Stöger, E.; Christou, P.; Capell, T. Rice Endosperm Is Cost-Effective for the Production of Recombinant Griffithsin with Potent Activity against HIV. Plant Biotechnol. J. 2016, 14, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.; Drake, P.M.; Mahmood, N.; Harman, S.J.; Shattock, R.J.; Ma, J.K.-C. Transgenic Plant Production of Cyanovirin-N, an HIV Microbicide. FASEB J. 2006, 20, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Drake, P.M.W.; de Moraes Madeira, L.; Szeto, T.H.; Ma, J.K.-C. Transformation of Althaea officinalis L. by Agrobacterium Rhizogenes for the Production of Transgenic Roots Expressing the Anti-HIV Microbicide Cyanovirin-N. Transgenic Res. 2013, 22, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, B.R.; Murad, A.M.; Vianna, G.R.; Ramessar, K.; Saucedo, C.J.; Wilson, J.; Buckheit, K.W.; da Cunha, N.B.; Araújo, A.C.G.; Lacorte, C.C.; et al. Engineering Soya Bean Seeds as a Scalable Platform to Produce cyanovirin-N, a non-ARV Microbicide against HIV. Plant Biotechnol. J. 2015, 13, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Evans, A.; Ramessar, K.; Krumpe, L.R.H.; Shattock, R.J.; O’Keefe, B.R.; Christou, P.; Capell, T. Cyanovirin-N Produced in Rice Endosperm Offers Effective Pre-Exposure Prophylaxis against HIV-1BaL Infection in Vitro. Plant Cell Rep. 2016, 35, 1309–1319. [Google Scholar] [CrossRef] [PubMed]
- Sexton, A.; Harman, S.; Shattock, R.J.; Ma, J.K.-C. Design, Expression, and Characterization of a Multivalent, Combination HIV Microbicide. FASEB J. 2009, 23, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Farré, G.; Molinos-Albert, L.M.; Evans, A.; Canela-Xandri, A.; Twyman, R.M.; Carrillo, J.; Ordóñez, R.A.; Shattock, R.J.; O’Keefe, B.R.; et al. Unexpected Synergistic HIV Neutralization by a Triple Microbicide Produced in Rice Endosperm. Proc. Natl. Acad. Sci. USA 2018, 115, E7854–E7862. [Google Scholar] [CrossRef] [PubMed]
- Seber Kasinger, L.E.; Dent, M.W.; Mahajan, G.; Hamorsky, K.T.; Matoba, N. A Novel Anti-HIV-1 Bispecific bNAb-Lectin Fusion Protein Engineered in a Plant-Based Transient Expression System. Plant Biotechnol. J. 2019, 17, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Hilal, B.; Khan, M.M.; Fariduddin, Q. Recent Advancements in Deciphering the Therapeutic Properties of Plant Secondary Metabolites: Phenolics, Terpenes, and Alkaloids. Plant Physiol. Biochem. 2024, 211, 108674. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Du, Z.; Hua, Q.; Kai, G. CRISPR/Cas9-Mediated Targeted Mutagenesis of bZIP2 in Salvia Miltiorrhiza Leads to Promoted Phenolic Acid Biosynthesis. Ind. Crops Prod. 2021, 167, 113560. [Google Scholar] [CrossRef]
- Janda, K.; Gutowska, I.; Geszke-Moritz, M.; Jakubczyk, K. The Common Cichory (Cichorium intybus L.) as a Source of Extracts with Health-Promoting Properties-A Review. Molecules 2021, 26, 1814. [Google Scholar] [CrossRef] [PubMed]
- Cankar, K.; Bundock, P.; Sevenier, R.; Häkkinen, S.T.; Hakkert, J.C.; Beekwilder, J.; van der Meer, I.M.; de Both, M.; Bosch, D. Inactivation of the Germacrene A Synthase Genes by CRISPR/Cas9 Eliminates the Biosynthesis of Sesquiterpene Lactones in Cichorium intybus L. Plant Biotechnol. J. 2021, 19, 2442–2453. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, F.; Wu, Y.; Lu, J.; Gao, X.; Zhu, X.; Yang, J.; Liu, S.; Xiao, G.; Pan, X. Identification of Tanshinone I as Cap-Dependent Endonuclease Inhibitor with Broad-Spectrum Antiviral Effect. J. Virol. 2023, 97, e0079623. [Google Scholar] [CrossRef] [PubMed]
- Langat, R.; Chakrawarti, A.; Klatt, N.R. Cannabis Use in HIV: Impact on Inflammation, Immunity and the Microbiome. Curr. HIV/AIDS Rep. 2025, 22, 19. [Google Scholar] [CrossRef] [PubMed]
- Ross, I.A. (Ed.) Antiviral Activities of Cannabis. In Plant-Based Therapeutics, Volume 1: Cannabis sativa; Springer International Publishing: Cham, Switzerland, 2023; pp. 629–639. ISBN 978-3-031-35155-6. [Google Scholar]
- Liu, X.-F.; Zhu, J.; Ge, S.-Y.; Xia, L.-J.; Yang, H.-Y.; Qian, Y.-T.; Ren, F.-Z. Orally Administered Dendrobium Officinale and Its Polysaccharides Enhance Immune Functions in BALB/c Mice. Nat. Prod. Commun. 2011, 6, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Kui, L.; Chen, H.; Zhang, W.; He, S.; Xiong, Z.; Zhang, Y.; Yan, L.; Zhong, C.; He, F.; Chen, J.; et al. Building a Genetic Manipulation Tool Box for Orchid Biology: Identification of Constitutive Promoters and Application of CRISPR/Cas9 in the Orchid, Dendrobium Officinale. Front. Plant Sci. 2016, 7, 2036. [Google Scholar] [CrossRef] [PubMed]
- Ouassaf, M.; Bourougaa, L.; Bahaz, F.; Alhatlani, B.Y. Exploring the Antiviral Potential of Artemisia Annua Through JAK-STAT Pathway Targeting: A Network Pharmacology Approach. Pharmaceuticals 2024, 17, 1539. [Google Scholar] [CrossRef] [PubMed]
- Qamar, F.; Ashrafi, K.; Singh, A.; Dash, P.K.; Abdin, M.Z. Artemisinin Production Strategies for Industrial Scale: Current Progress and Future Directions. Ind. Crops Prod. 2024, 218, 118937. [Google Scholar] [CrossRef]
- Sinha, S.; Sandhu, K.; Bisht, N.; Naliwal, T.; Saini, I.; Kaushik, P. Ascertaining the Paradigm of Secondary Metabolism Enhancement through Gene Level Modification in Therapeutic Plants. J. Young Pharm. 2019, 11, 337–343. [Google Scholar] [CrossRef]
- Grützner, R.; König, K.; Horn, C.; Engler, C.; Laub, A.; Vogt, T.; Marillonnet, S. A Transient Expression Tool Box for Anthocyanin Biosynthesis in Nicotiana Benthamiana. Plant Biotechnol. J. 2024, 22, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Osbourn, A. Engineering Terpenoid Production through Transient Expression in Nicotiana Benthamiana. Plant Cell Rep. 2018, 37, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.; Orme, A.; El-Demerdash, A.; Owen, C.; Martin, L.B.B.; Misra, R.C.; Kikuchi, S.; Rejzek, M.; Martin, A.C.; Harkess, A.; et al. Elucidation of the Pathway for Biosynthesis of Saponin Adjuvants from the Soapbark Tree. Science 2023, 379, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ding, T.; Chang, H.; Peng, Y.; Li, J.; Liang, X.; Ma, H.; Li, F.; Ren, M.; Wang, W. Plant-Derived Strategies to Fight against Severe Acute Respiratory Syndrome Coronavirus 2. Eur. J. Med. Chem. 2024, 264, 116000. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Tan, H.; Li, Q.; Chen, J.; Gao, S.; Wang, Y.; Chen, W.; Zhang, L. CRISPR/Cas9-Mediated Efficient Targeted Mutagenesis of RAS in Salvia Miltiorrhiza. Phytochemistry 2018, 148, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Bamba, T.; Kondo, A.; Hasunuma, T. Metabolomics-Based Development of Bioproduction Processes toward Industrial-Scale Production. Curr. Opin. Biotechnol. 2024, 85, 103057. [Google Scholar] [CrossRef] [PubMed]
- Chastang, T.; Pozzobon, V.; Taidi, B.; Courot, E.; Clément, C.; Pareau, D. Resveratrol Production by Grapevine Cells in Fed-Batch Bioreactor: Experiments and Modelling. Biochem. Eng. J. 2018, 131, 9–16. [Google Scholar] [CrossRef]
- Rebelo, B.A.; Folgado, A.; Ferreira, A.C.; Abranches, R. Production of the SARS-CoV-2 Spike Protein and Its Receptor Binding Domain in Plant Cell Suspension Cultures. Front. Plant Sci. 2022, 13, 995429. [Google Scholar] [CrossRef] [PubMed]
- Takova, K.; Tonova, V.; Minkov, I.; Mardanova, E.S.; Ravin, N.V.; Kotsev, S.; Pishmisheva, M.; Zahmanova, G. Plant-Based Antigen Production Strategy for SARS-CoV-2 Nucleoprotein and RBD and Its Application for Detection of Antibody Responses in COVID-19 Patients. Appl. Sci. 2025, 15, 786. [Google Scholar] [CrossRef]
- Tusé, D.; McNulty, M.; McDonald, K.A.; Buchman, L.W. A Review and Outlook on Expression of Animal Proteins in Plants. Front. Plant Sci. 2024, 15, 1426239. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Buyel, J.F. Molecular Farming—The Slope of Enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef] [PubMed]
- Kaldis, A.; Uddin, M.S.; Guluarte, J.O.; Martin, C.; Alexander, T.W.; Menassa, R. Development of a Plant-Based Oral Vaccine Candidate against the Bovine Respiratory Pathogen Mannheimia Haemolytica. Front. Plant Sci. 2023, 14, 1251046. [Google Scholar] [CrossRef] [PubMed]
- Pantazica, A.-M.M.; Cucos, L.-M.; Stavaru, C.; Clarke, J.-L.; Branza-Nichita, N. Challenges and Prospects of Plant-Derived Oral Vaccines against Hepatitis B and C Viruses. Plants 2021, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.S.; Chimeno Zoth, S.; Jaton, J.; Gravisaco, M.J.; Pinto, S.; Richetta, M.; Berinstein, A.; Gómez, E. Oral Immunization With Plant-Based Vaccine Induces a Protective Response Against Infectious Bursal Disease. Front. Plant Sci. 2021, 12, 741469. [Google Scholar] [CrossRef] [PubMed]
- Pomatto, M.A.C.; Gai, C.; Negro, F.; Massari, L.; Deregibus, M.C.; Grange, C.; De Rosa, F.G.; Camussi, G. Plant-Derived Extracellular Vesicles as a Delivery Platform for RNA-Based Vaccine: Feasibility Study of an Oral and Intranasal SARS-CoV-2 Vaccine. Pharmaceutics 2023, 15, 974. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral Potential of Garlic (Allium sativum) and Its Organosulfur Compounds: A Systematic Update of Pre-Clinical and Clinical Data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Tatarintsev, A.V.; Vrzheshch, P.V.; Schegolev, A.A.; Yershov, D.E.; Turgiev, A.S.; Varfolomeyev, S.D.; Kornilayeva, G.V.; Makarova, T.V.; Karamov, E.V. Ajoene Antagonizes Integrin-Dependent Processes in HIV-Infected T-Lymphoblasts. AIDS Lond. Engl. 1992, 6, 1215–1217. [Google Scholar]
- Yang, J.; Li, L.; Tan, S.; Jin, H.; Qiu, J.; Mao, Q.; Li, R.; Xia, C.; Jiang, Z.-H.; Jiang, S.; et al. A Natural Theaflavins Preparation Inhibits HIV-1 Infection by Targeting the Entry Step: Potential Applications for Preventing HIV-1 Infection. Fitoterapia 2012, 83, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, X.-W.; Schols, D.; Mori, M.; Botta, B.; Chevigné, A.; Mulinge, M.; Steinmetz, A.; Schmit, J.-C.; Seguin-Devaux, C. Active Components from Cassia Abbreviata Prevent HIV-1 Entry by Distinct Mechanisms of Action. Int. J. Mol. Sci. 2021, 22, 5052. [Google Scholar] [CrossRef] [PubMed]
- Apaza Ticona, L.; Bermejo, P.; Guerra, J.A.; Abad, M.J.; Beltrán, M.; Martín Lázaro, R.; Alcamí, J.; Bedoya, L.M. Ethanolic Extract of Artemisia Campestris Subsp. Glutinosa (Besser) Batt. Inhibits HIV-1 Replication in Vitro through the Activity of Terpenes and Flavonoids on Viral Entry and NF-κB Pathway. J. Ethnopharmacol. 2020, 263, 113163. [Google Scholar] [CrossRef] [PubMed]
- Connell, B.J.; Chang, S.-Y.; Prakash, E.; Yousfi, R.; Mohan, V.; Posch, W.; Wilflingseder, D.; Moog, C.; Kodama, E.N.; Clayette, P.; et al. A Cinnamon-Derived Procyanidin Compound Displays Anti-HIV-1 Activity by Blocking Heparan Sulfate- and Co-Receptor- Binding Sites on Gp120 and Reverses T Cell Exhaustion via Impeding Tim-3 and PD-1 Upregulation. PLoS ONE 2016, 11, e0165386. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Du, R.; Anantpadma, M.; Schafer, A.; Hou, L.; Tian, J.; Davey, R.A.; Cheng, H.; Rong, L. Identification of Ellagic Acid from Plant Rhodiola rosea L. as an Anti-Ebola Virus Entry Inhibitor. Viruses 2018, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xiong, S.; Xiang, Y.-F.; Guo, C.-W.; Ge, F.; Yang, C.-R.; Zhang, Y.-J.; Wang, Y.-F.; Kitazato, K. Antiviral Activity and Possible Mechanisms of Action of Pentagalloylglucose (PGG) against Influenza A Virus. Arch. Virol. 2011, 156, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Tsai, F.-J.; Tsai, C.-H.; Lai, C.-C.; Wan, L.; Ho, T.-Y.; Hsieh, C.-C.; Chao, P.-D.L. Anti-SARS Coronavirus 3C-like Protease Effects of Isatis Indigotica Root and Plant-Derived Phenolic Compounds. Antiviral Res. 2005, 68, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.-C.; Kuo, Y.-H.; Jan, J.-T.; Liang, P.-H.; Wang, S.-Y.; Liu, H.-G.; Lee, C.-K.; Chang, S.-T.; Kuo, C.-J.; Lee, S.-S.; et al. Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus. J. Med. Chem. 2007, 50, 4087–4095. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Kim, J.H.; Kim, Y.M.; Jeong, H.J.; Kim, D.W.; Park, K.H.; Kwon, H.-J.; Park, S.-J.; Lee, W.S.; Ryu, Y.B. Tanshinones as Selective and Slow-Binding Inhibitors for SARS-CoV Cysteine Proteases. Bioorg. Med. Chem. 2012, 20, 5928–5935. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, S.; Shin, D.H.; Kim, M.-S. Inhibition of SARS-CoV 3CL Protease by Flavonoids. J. Enzyme Inhib. Med. Chem. 2020, 35, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Jeong, H.J.; Kim, J.H.; Kim, Y.M.; Park, S.-J.; Kim, D.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Diarylheptanoids from Alnus Japonica Inhibit Papain-like Protease of Severe Acute Respiratory Syndrome Coronavirus. Biol. Pharm. Bull. 2012, 35, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.K.; Curtis-Long, M.J.; Lee, K.H.; Kim, D.W.; Ryu, H.W.; Yuk, H.J.; Park, K.H. Geranylated Flavonoids Displaying SARS-CoV Papain-like Protease Inhibition from the Fruits of Paulownia Tomentosa. Bioorg. Med. Chem. 2013, 21, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Ko, J.-A.; Kim, D.W.; Kim, Y.M.; Kwon, H.-J.; Jeong, H.J.; Kim, C.Y.; Park, K.H.; Lee, W.S.; Ryu, Y.B. Chalcones Isolated from Angelica Keiskei Inhibit Cysteine Proteases of SARS-CoV. J. Enzyme Inhib. Med. Chem. 2016, 31, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yi, D.; Lei, X.; Zhao, J.; Zhang, Y.; Cui, X.; Xiao, X.; Jiao, T.; Dong, X.; Zhao, X.; et al. Corilagin Inhibits SARS-CoV-2 Replication by Targeting Viral RNA-Dependent RNA Polymerase. Acta Pharm. Sin. B 2021, 11, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Munafò, F.; Donati, E.; Brindani, N.; Ottonello, G.; Armirotti, A.; De Vivo, M. Quercetin and Luteolin Are Single-Digit Micromolar Inhibitors of the SARS-CoV-2 RNA-Dependent RNA Polymerase. Sci. Rep. 2022, 12, 10571. [Google Scholar] [CrossRef] [PubMed]
- Keum, Y.-S.; Lee, J.M.; Yu, M.-S.; Chin, Y.-W.; Jeong, Y.-J. Inhibition of SARS Coronavirus Helicase by Baicalein. Bull. Korean Chem. Soc. 2013, 34, 3187–3188. [Google Scholar] [CrossRef]
- Zeng, J.; Weissmann, F.; Bertolin, A.P.; Posse, V.; Canal, B.; Ulferts, R.; Wu, M.; Harvey, R.; Hussain, S.; Milligan, J.C.; et al. Identifying SARS-CoV-2 Antiviral Compounds by Screening for Small Molecule Inhibitors of Nsp13 Helicase. Biochem. J. 2021, 478, 2405–2423. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Nakamura, N.; Hattori, M.; Kakuda, H.; Qiao, J.; Yu, H. Inhibitory Effects on HIV-1 Protease of Constituents from the Wood of Xanthoceras Sorbifolia. J. Nat. Prod. 2000, 63, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Martini, R.; Esposito, F.; Corona, A.; Ferrarese, R.; Ceresola, E.R.; Visconti, L.; Tintori, C.; Barbieri, A.; Calcaterra, A.; Iovine, V.; et al. Natural Product Kuwanon-L Inhibits HIV-1 Replication through Multiple Target Binding. Chembiochem Eur. J. Chem. Biol. 2017, 18, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Ravanelli, N.; Santos, K.P.; Motta, L.B.; Lago, J.H.G.; Furlan, C.M. Alkaloids from Croton Echinocarpus Baill.: Anti-HIV Potential. S. Afr. J. Bot. 2016, 102, 153–156. [Google Scholar] [CrossRef]
- Troost, B.; Mulder, L.M.; Diosa-Toro, M.; van de Pol, D.; Rodenhuis-Zybert, I.A.; Smit, J.M. Tomatidine, a Natural Steroidal Alkaloid Shows Antiviral Activity towards Chikungunya Virus in Vitro. Sci. Rep. 2020, 10, 6364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.B.; Subramani, C.; Ganesh, K.; Sharma, A.; Basu, B.; Balyan, S.; Sharma, G.; Ka, S.; Deb, A.; Srivastava, M.; et al. Withaferin A Inhibits Chikungunya Virus nsP2 Protease and Shows Antiviral Activity in the Cell Culture and Mouse Model of Virus Infection. PLoS Pathog. 2024, 20, e1012816. [Google Scholar] [CrossRef] [PubMed]
- Bourjot, M.; Leyssen, P.; Neyts, J.; Dumontet, V.; Litaudon, M. Trigocherrierin A, a Potent Inhibitor of Chikungunya Virus Replication. Molecules 2014, 19, 3617–3627. [Google Scholar] [CrossRef] [PubMed]
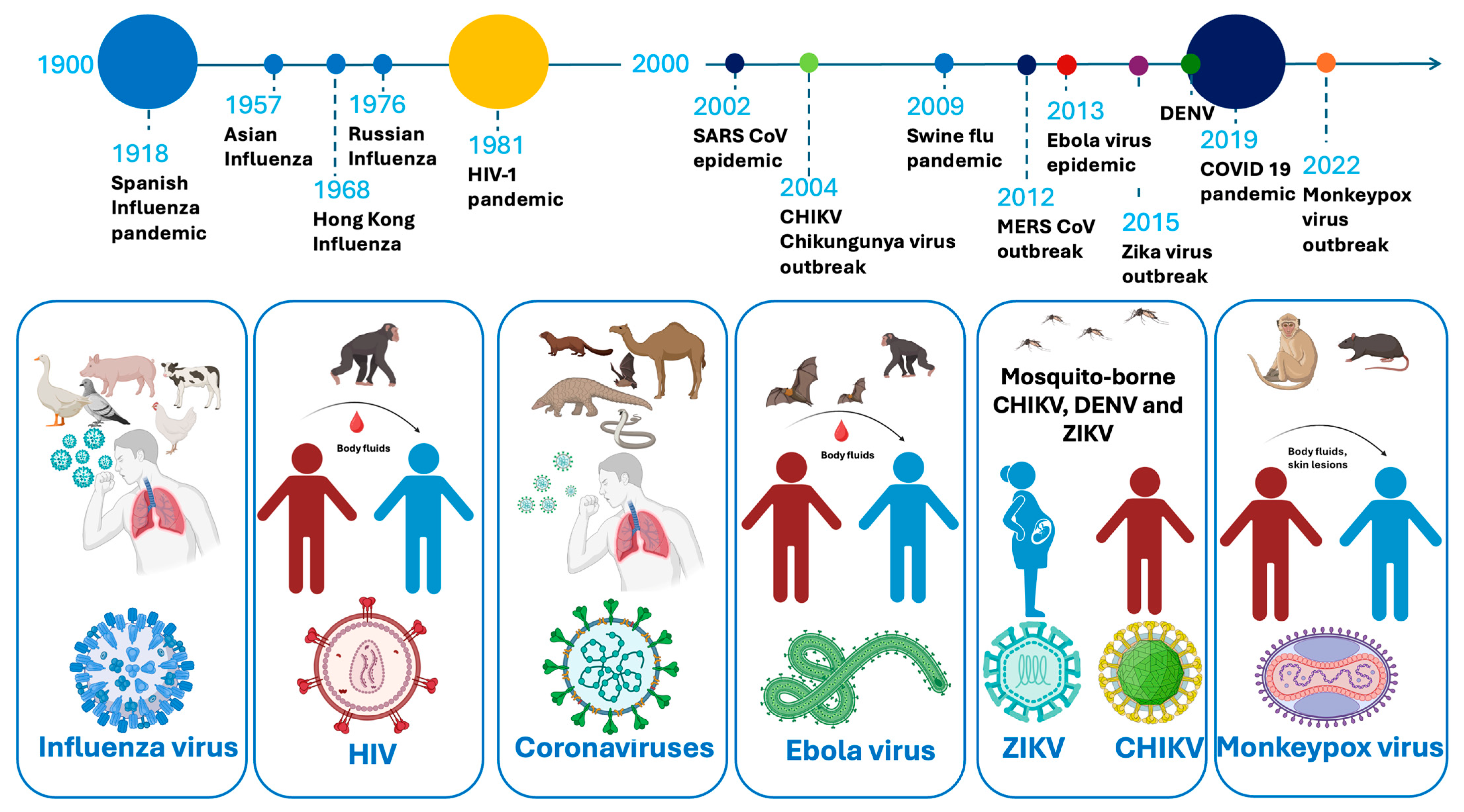

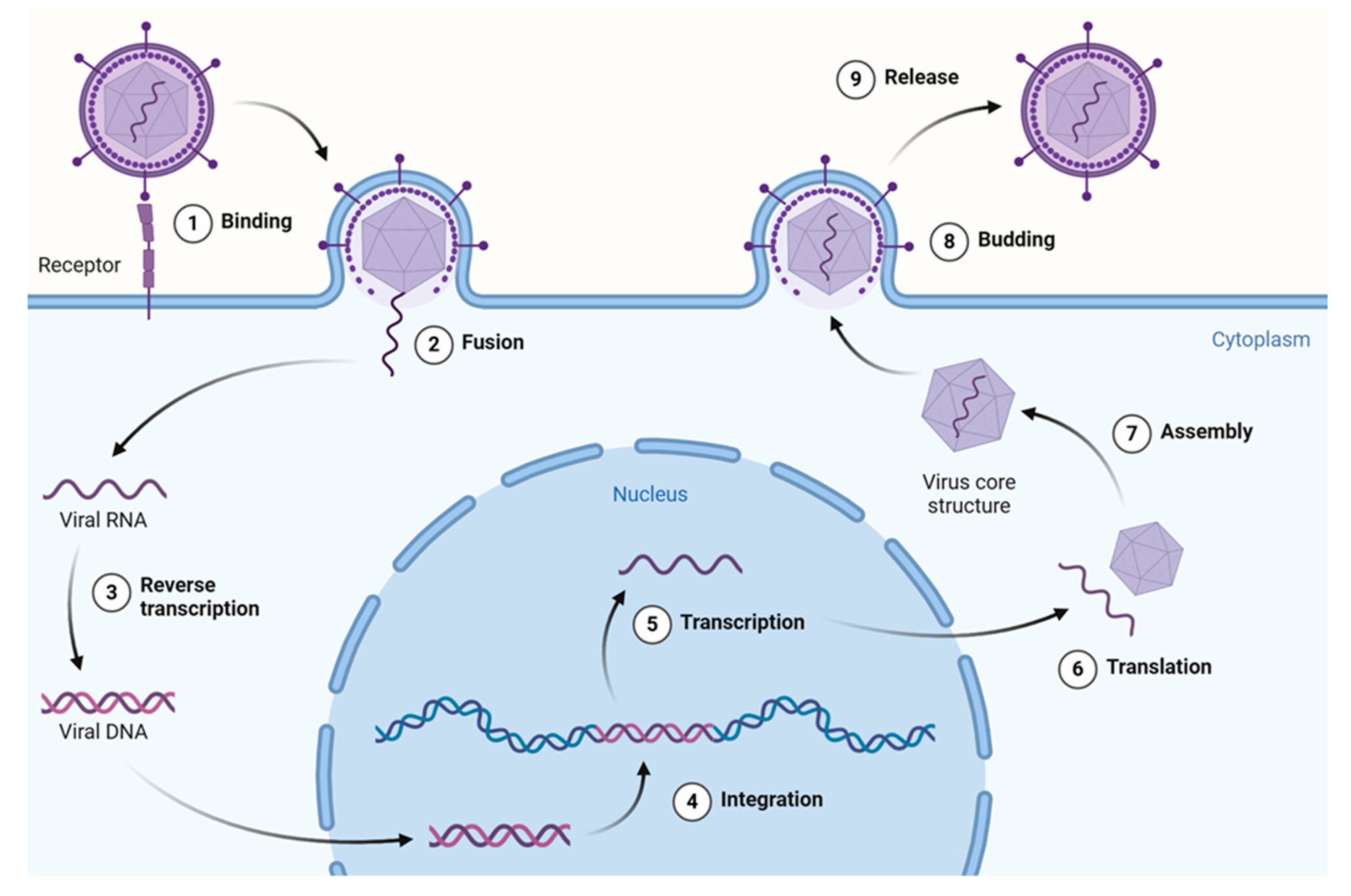
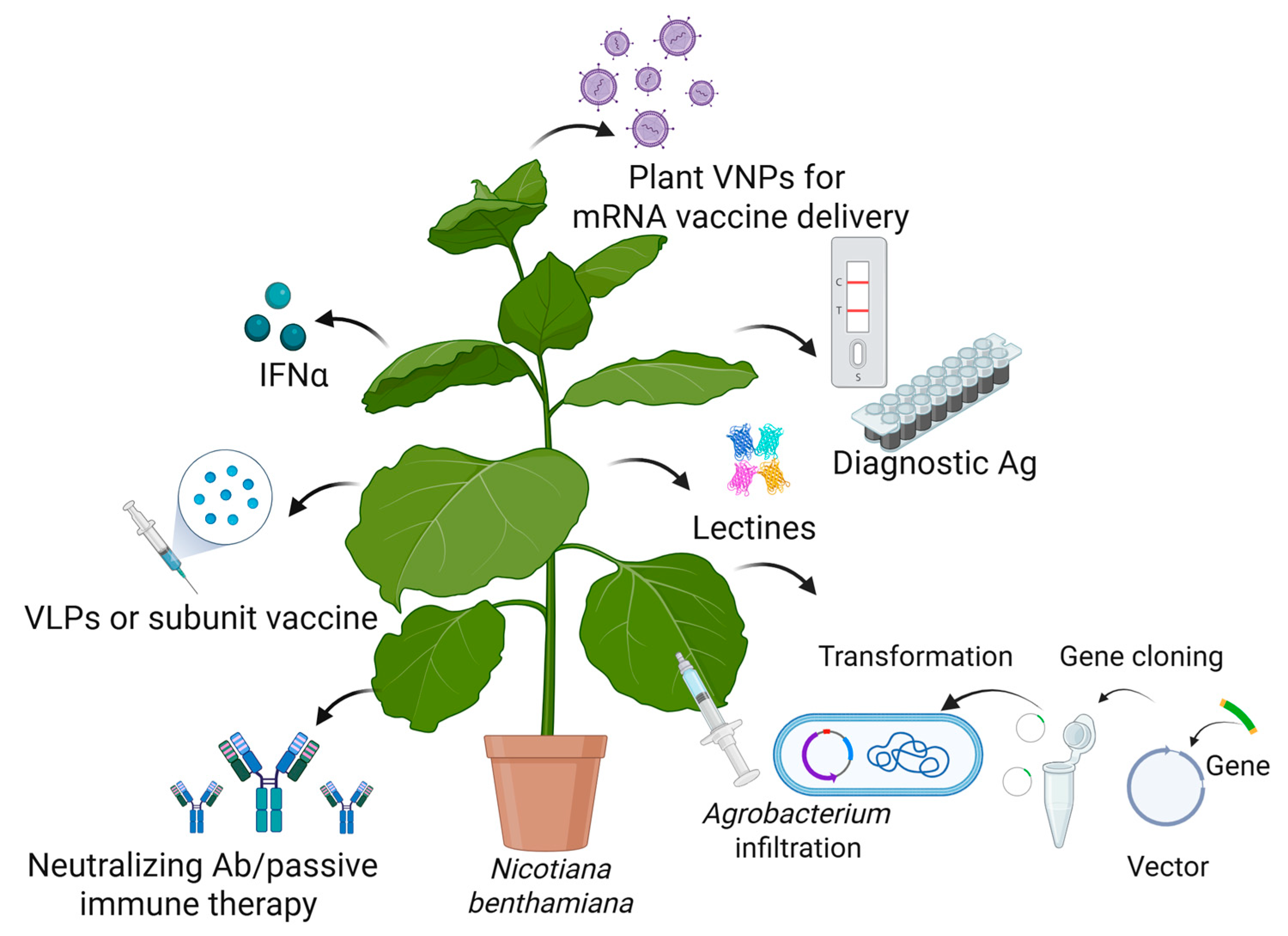
| Virus | Antigen | Production System | Immunogenicity | Company |
|---|---|---|---|---|
| SARS-CoV-2 | Modified VLPs built from S protein | N. benthamiana/transient | Phase-3 clinical trial completed: 71% efficacy rate of VLP plant-based vaccine against all variants of SARS-CoV-2 | Medicago Inc. [162] |
| KBP-201 CoVLPs targeting RBD | N. benthamiana/transient | Phase-1/2 clinical trials | Kentucky Bioprocessing (KBP) [150] | |
| Subunit Vax 1 | N. benthamiana/transient | Phase-1/2 clinical trials | Baiya Phytopharm [149] | |
| Zika virus | Zika virus envelope protein (zE)-based candidate subunit vaccine | N. benthamiana/transient | Elicited potent zE-specific antibodies and cellular immune responses in mice | Academic research [163] |
| Chikungunya virus | VLPs (full-length envelope protein E2) | N. benthamiana/transient | Preclinical, demonstrating feasibility of CHIKV E2 expression in plants | Academic research [157] |
| Ebola virus | Multiepitopic protein Zerola (GP epitopes) | N. tabacum/stable gene expression | No data | Academic research |
| Ebola glycoprotein (GP) in fusion with 6D8 anti-Ebola IgG (6D8 IgG-GP1) | N. benthamiana/transient | 80% survival of immunized mice (SC) against lethal EBV challenge [164] | ||
| EBOV GP1 in fusion with E. coli heat-labile enterotoxin B subunit (LTB-EBOV) | Tobacco/transgenic (nuclear) | IgA and IgG responses induced following oral immunization [165] | ||
| Influenza | VLPs composed only of influenza hemagglutinin (HA)-H5 | N. benthamiana/transient | Elicited potent humoral and cell-mediated immune responses to H5N1 | Medicago Inc. [166] |
| Polyvalent HA VLPs for seasonal flu | N. benthamiana/transient | Successful completion of phase-3 clinical studies for plant-derived VLP quadrivalent flu vaccine | Medicago Inc. [167] |
| Virus Used for VLP Production | Foreign Antigen Displayed on VLPs | Plant/Expression Method | Expression per Fresh Weight | Immune Responses/References |
|---|---|---|---|---|
| CPMV | VP2 capsid protein from mink enteritis virus (MEV) | Vigna unguiculata/ virus transfection | 1 mg/g | A total of 1 mg of the CVPs in mink protected against clinical illness and prevented virus shedding following exposure to virulent MEV [173]. |
| TMV | L2 epitopes from cottontail rabbit papillomavirus and rabbit oral papillomavirus | N. benthamiana/ transient | No data | Rabbits immunized with the chimeric VLPs were protected from developing papillomas [174]. |
| Alfalfa mosaic virus coat protein (AMV) | Pfs25 protein of Plasmodium falciparum | N. benthamiana/ transient | 50 µg/g | In a phase-I study, IgG responses > 3 log10 were observed with a dose of 100 μg [175]. |
| Bamboo mosaic virus-based (BaMV) | Foot-and-mouth disease virus (FMDV) VP1 antigens | N. benthamiana/ transient | No data | No immunization studies have been reported. |
| Bacteriophage AP 205 | Envelope Protein Domain III (EDIII) of WNV | N. benthamiana/ transient | 36 µg/g purified yield | A total of 5 μg of chimeric VLPs elicited IgG responses in immunized mice [175]. |
| Hepatitis B virus (HBV) | Chimeric VLPs based on HBcAg that presented zDIII | N. benthamiana/ transient | 1824 μg/g | They elicit a potent humoral and cellular immune response in mice [176]. |
| Chimeric VLPs presenting M2e of influenza | 1–2% of total soluble protein | Chimeric VLPs have a protective effect against a lethal influenza challenge in mice [177]. | ||
| Chimeric VLPs bearing epitope of HEV capsid | VLP recovery yield of 10 µg/g | No immunization studies have been reported [178]. | ||
| Hepatitis E virus (HEV) | M2e of influenza | N. benthamiana/ transient | 300 µg/g | They do not induce a protective immune response in mice [171,179,180]. |
| RBD of SARS-CoV-2 | 100 µg/g | HEV/RBD chimeric proteins are recognized in human serum from COVID-19 patients [170]. | ||
| Bluetongue virus (BTV) | EDIII of dengue virus and Zika virus | N. benthamiana/ transient | 5–15 µg/g purified yield | They induce a humoral immune response in mice [181]. |
| African horse sickness virus (AHSV) | VP2, VP3, VP5, and VP7 from AHS serotype 1; VP2 and VP5, serotype 7; VP5, serotype 3, and VP2, serotype 6 | N. benthamiana/ transient | No data | They elicit a weak neutralizing humoral immune response in these target animals against homologous AHSV virus [182]. |
| Disease/Pathogen | Antibody | Plant System | Reference |
|---|---|---|---|
| HIV | 2G12; 2F5; b12: b12-CV-N; 10-1074, VRC01; 3BNC117; 4E10 | N. benthamiana, N. tabacum, Z. mays (maize), A. thaliana, O. sativa L. (rice) | [194,195,196,197,198,199,200] |
| Ebola | h-13F6, ZMapp™, anti-Ebola monoclonal antibodies | N. benthamiana, N. tabacum | [191,200,201] |
| West Nile Virus | Hu-E16 mAb, HPA | N. benthamiana | [202,203] |
| Hepatitis B virus | Anti-HB Ab | N. tabacum, S. lycopersicum L. (tomato), S. tuberosum L. (potato), L. sativa L. (lettuce), Banana. cv. Rasthali | [204,205] |
| SARS-CoV-2 | Anti-SARS-CoV-2 Ab, mAbJ08-MUT, mAb675, B38, H4 | N. benthamiana | [206,207,208] |
| Rabies | Anti-rabies Ab | N. benthamiana | [209] |
| Respiratory syncytial virus | Anti-RSV Ab | N. benthamiana | [210] |
| Rotavirus | Anti-Rotavirus Ab | N. benthamiana, S. lycopersicum L., S. tuberosum L., M. sativa L. (Alfalfa), O. sativa L. | [211,212,213,214] |
| Dengue virus | Anti-dengue Ab | N. benthamiana | [215,216] |
| CHIKV | Five anti-chikungunya neutralizing monoclonal antibodies (mAbs) | N. benthamiana | [217] |
| Zika virus | Anti-Zika mAb | N. benthamiana | [218] |
| Plant System/Expression Method | Lectin Yield | Virus Neutralization Activity/Reference |
|---|---|---|
| N. tabacum, stable chloroplast transformation | GRFT up to 5% of TSP of plant; yield of 360 µg/g of fresh weight (FW). | It demonstrated similar anti-HIV activity to GRFT expressed in bacteria [234]. |
| N. benthamiana, transient expression | Final recovery of 30% of in planta level of 1 g/kg. | GRFT-P showed broad-spectrum activity against HIV [235]. |
| Transgenic rice (O. sativa), endosperm | GRFT up to 223 μg/g dry seed W, recovery of 74%. | It binds to HIV glycans with similar efficiency to GRFT produced in E. Coli [236]. |
| N. tabacum, stable transformation | Cyanovirin-N (CV-N) recoverable at levels of 130 ng/mg of FW. | CV-N bound to soluble gp120IIIb in a concentration-dependent manner [237]. |
| Transgenic roots of marshmallow plant (Althaea officinalis L.) | Concentration of CV-N in root tissue of 2.4 lg/g FW. | An ELISA plate coated with gp120 confirmed the functionality of the CV-N [238]. |
| Transgenic soya bean plants | CV-N with yield of 350 μg/g of dry seed weight, 92% purity. | Purified rCV-N is active in anti-HIV assays with an EC50 of 0.82–2.7 nM [239]. |
| Transgenic rice plants (O. sativa), endosperm | Yield up to 10 µg CV-N per gram dry seed. | The crude extracts showed dose-dependent gp120-binding activity [240]. |
| Transgenic N. tabacum plants | Expression of fusion protein consisting of mAb b12/CV-N. | Each moiety of the fusion protein retained its binding ability to gp120 [241]. |
| Transgenic rice plants (O. sativa), endosperm | 2G12, GRFT, and CV-N expressed simultaneously with varying yields. | Extracts of transgenic plants expressing all three proteins showed enhanced in vitro binding to gp120 [242]. |
| N. benthamiana, transient expression | Fusion VRC01Fab–Avaren protein with yield of 40 mg/kg of FW. | NVRC01Fab–Avaren showed stronger HIV-1 neutralization activity [243]. |
| Plant Species | Metabolite(s) | Potential Antiviral Activity | Target Gene Edited/References |
| Salvia miltiorhiza (red sage/Danshen) | Tanshinones | Tanshinone I acts as a cap-dependent endonuclease inhibitor. | CPS1 (tanshinone biosynthesis pathway) [248]. |
| Cannabis sativa (hemp) | THC, THC-free related metabolites | Cannabinoids show potential antiviral activity against HIV and herpes simplex. | CsPDS (to generate THC-free phenotype) [249,250]. |
| Dendrobium officinale (Dendrobium orchid) | Polysaccharides, bibenzyls | Polysaccharides from D. officinale have immune-boosting effects. | C3H, C4H, 4CL, CCR, IRX (metabolic pathway genes) [251,252]. |
| Artemisia annua (sweet wormwood) | Artemisinin | Artemisinin has potential antiviral properties against HIV and HBV. | SQS (Squalene synthase, competitive with artemisinin) [253,254]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahmanova, G.; Takova, K.; Tonova, V.; Minkov, I.; Barbolov, M.; Nedeva, N.; Vankova, D.; Ivanova, D.; Kiselova-Kaneva, Y.; Lukov, G.L. How Can Plant-Derived Natural Products and Plant Biotechnology Help Against Emerging Viruses? Int. J. Mol. Sci. 2025, 26, 7046. https://doi.org/10.3390/ijms26157046
Zahmanova G, Takova K, Tonova V, Minkov I, Barbolov M, Nedeva N, Vankova D, Ivanova D, Kiselova-Kaneva Y, Lukov GL. How Can Plant-Derived Natural Products and Plant Biotechnology Help Against Emerging Viruses? International Journal of Molecular Sciences. 2025; 26(15):7046. https://doi.org/10.3390/ijms26157046
Chicago/Turabian StyleZahmanova, Gergana, Katerina Takova, Valeria Tonova, Ivan Minkov, Momchil Barbolov, Neda Nedeva, Deyana Vankova, Diana Ivanova, Yoana Kiselova-Kaneva, and Georgi L. Lukov. 2025. "How Can Plant-Derived Natural Products and Plant Biotechnology Help Against Emerging Viruses?" International Journal of Molecular Sciences 26, no. 15: 7046. https://doi.org/10.3390/ijms26157046
APA StyleZahmanova, G., Takova, K., Tonova, V., Minkov, I., Barbolov, M., Nedeva, N., Vankova, D., Ivanova, D., Kiselova-Kaneva, Y., & Lukov, G. L. (2025). How Can Plant-Derived Natural Products and Plant Biotechnology Help Against Emerging Viruses? International Journal of Molecular Sciences, 26(15), 7046. https://doi.org/10.3390/ijms26157046








