Curcumin and Dementia: A Systematic Review of Its Effects on Oxidative Stress and Cognitive Outcomes in Animal Models
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Data Extraction and Management
2.5. Quality Assessment
3. Results
3.1. Study Selection
3.2. Study Risk of Bias Assessment
3.3. Study Characteristics
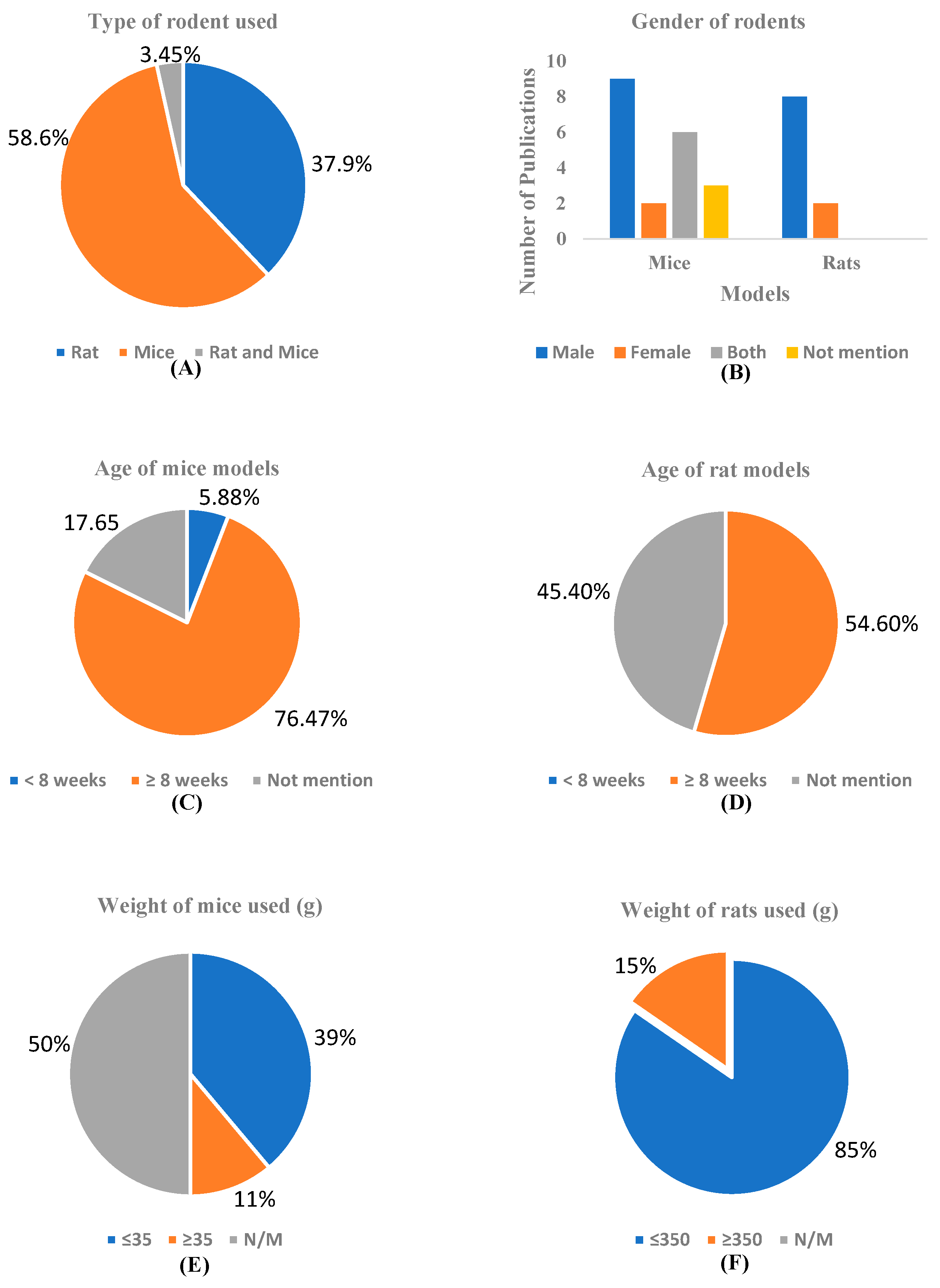
3.3.1. Demographic Data
3.3.2. Animal Models
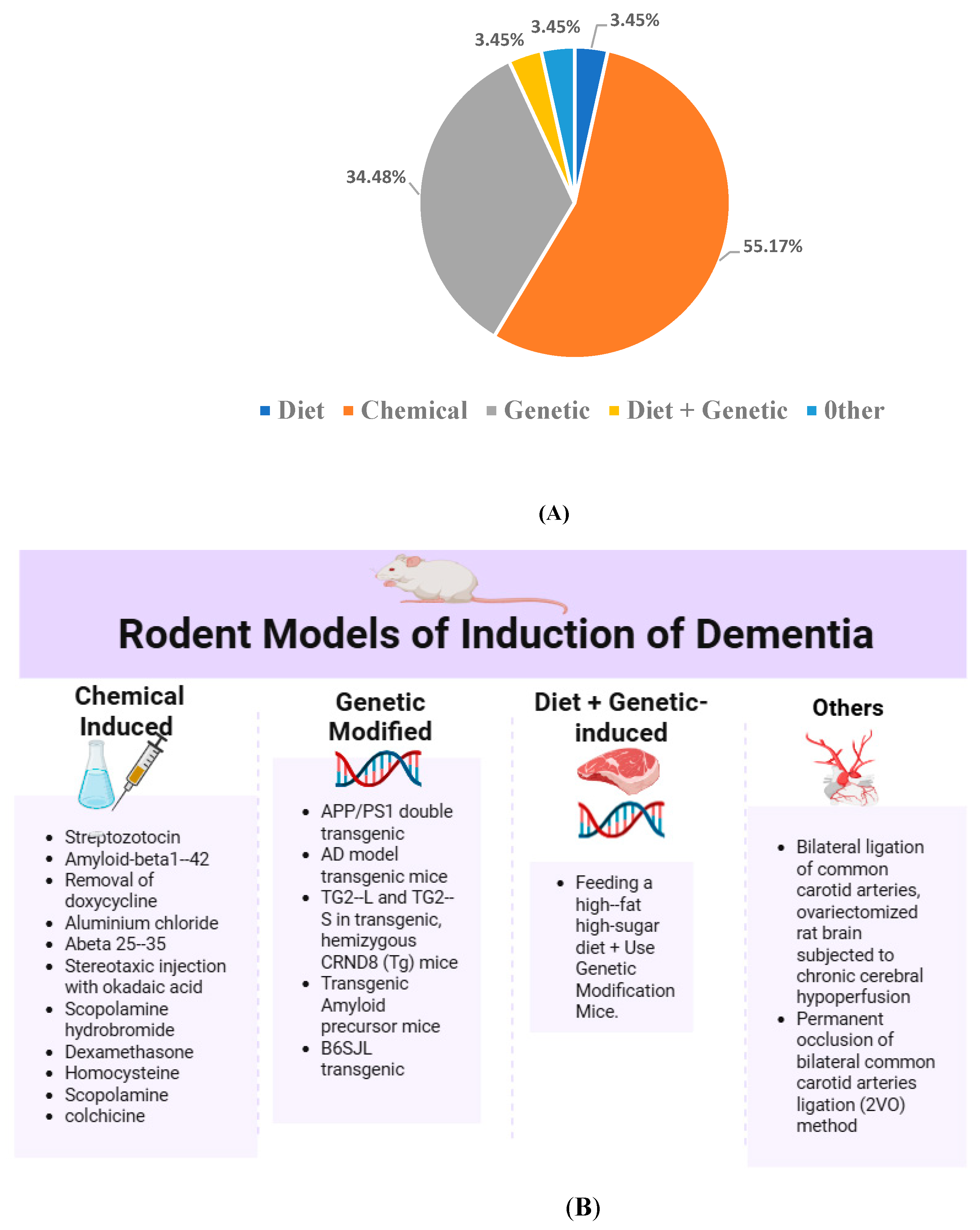
3.3.3. Induction Method of Dementia in Animal Models
- Chemical Induction of Dementia Models
- Genetic Induction
- Dietary Induction
- Combined Diet and Genetic Induction
- Surgical and Other Models
3.4. Intervention Characteristics
3.4.1. Type of Intervention
3.4.2. Dosing Strategies
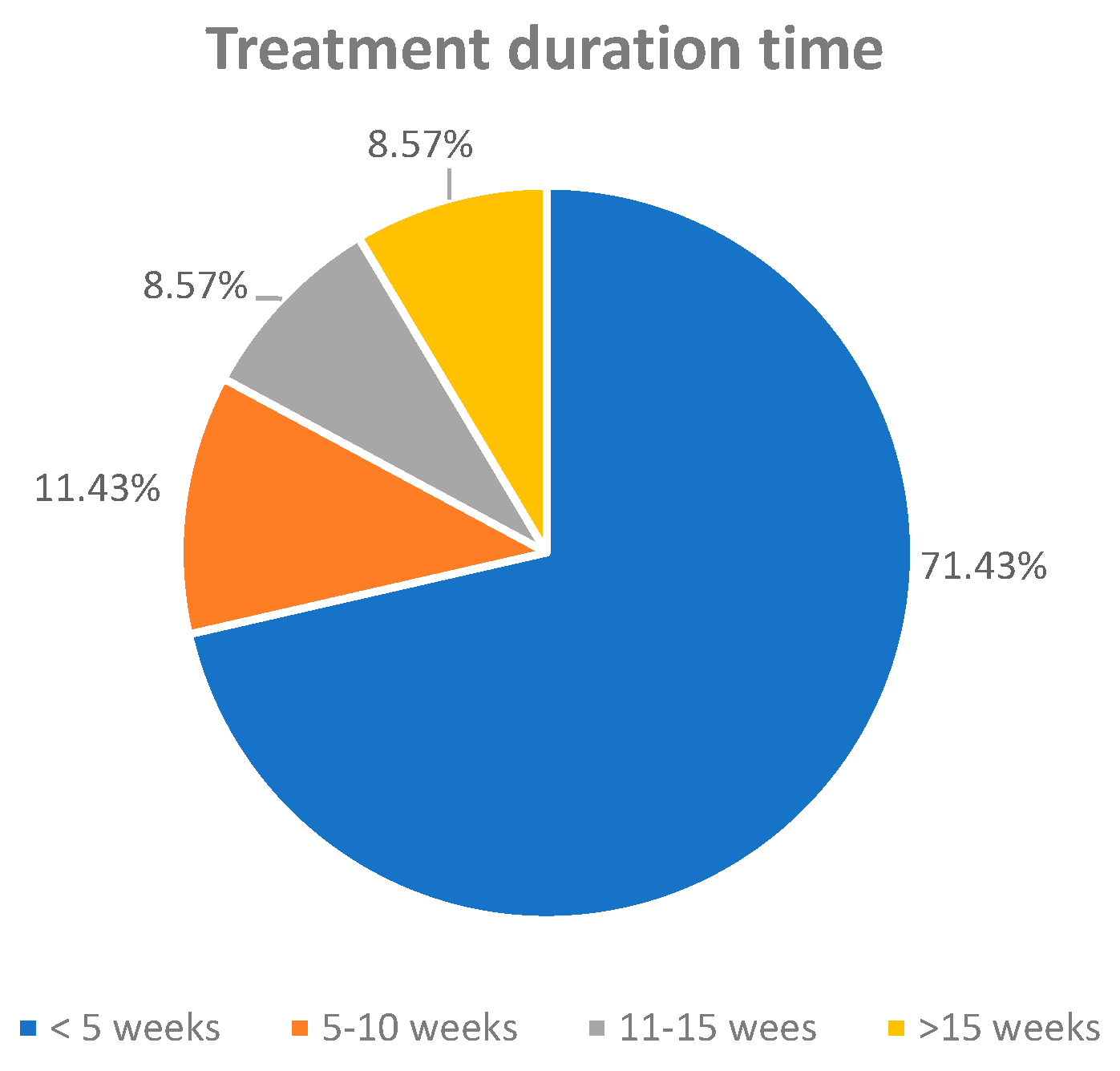
3.4.3. Treatment Duration
3.4.4. Treatment Regimens
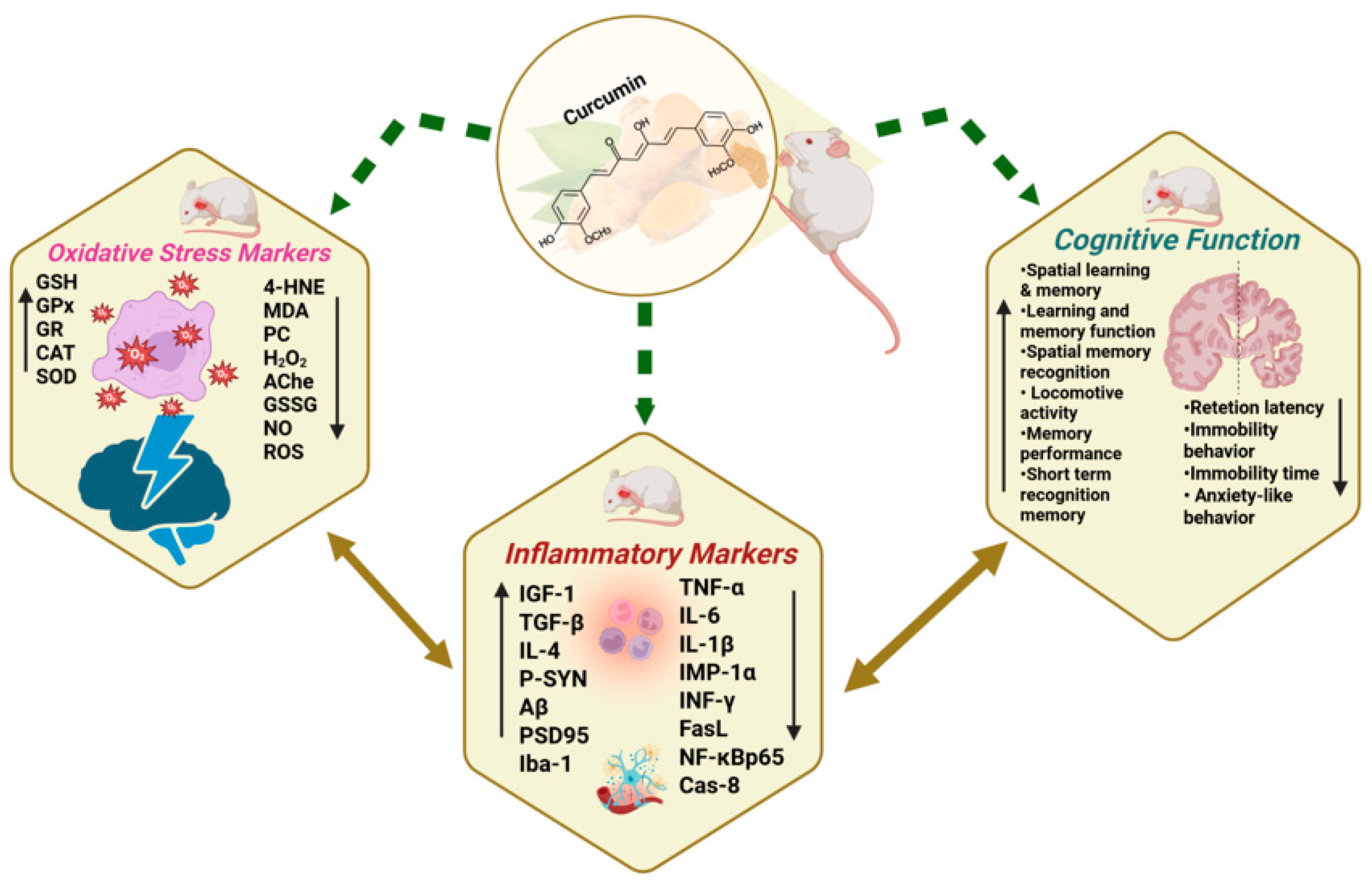
3.5. Antioxidative Effects of Curcumin/Curcuma Longa in Dementia Models
3.6. Protective Effects of Curcumin/Curcuma Longa on Neuroinflammation in Dementia Models
3.7. Ameliorative Effects of Curcumin/Curcuma Longa on Cognitive Functions in Dementia Models
- Cognitive Function Improvements
- Behavioral Improvements Related to Cognition
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1beta |
| TNF-α | Tumor necrosis factor-alpha |
| INF-γ | Interferon-gamma |
| MPO | Myeloperoxidase |
| NFκB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| TBARS | Thiobarbituric acid reactive substances |
| 4-HNE | 4-Hydroxynonenal |
| MDA | Malonaldehyde level |
| H2O2 | Hydrogen Peroxide |
| PC | Protein Carbonyl |
| GSH | Reduced Glutathione |
| GSSG | Oxidized Glutathione |
| GPx | Glutathione Peroxidase |
| GR | Glutathione Reductase |
| Na+-K+ ATPase | Sodium/potassium-dependent ATPase activity |
| Ache | Choline acetyltransferase |
| SOD | Superoxide Dismutase |
| CAT | Catalase |
| ROS | Reactive Oxygen species |
| NO | Nitric oxide |
| NPSH | Non-protein thiol |
| AD | Alzheimer’s disease |
| MRI | Magnetic Resonance Imaging |
| FDG-PET | Fluorodeoxyglucose Positron Emission Tomography |
| CSF | Cerebrospinal Fluid |
| Aβ42 | Amyloid-beta 42 |
| t-Tau | Total Tau |
| p-Tau | Phosphorylated Tau |
| STZ | Streptozotocin |
| Aβ | Amyloid-beta |
| MCI | Mild Cognitive Impairment. |
| AMED | Allied and Complementary Medicine Database |
| LILACS | Latin American and Caribbean Health Sciences Literature |
Appendix A
| Database | Keywords | Results |
|---|---|---|
| PubMed (4 April 2024) | KP-1: (((curcumin[Title/Abstract]) OR (curcuma[Title/Abstract]))) AND ((“in vivo”[Title/Abstract])) OR (rat[Title/Abstract])) OR (mice[Title/Abstract]))) AND (((dementia[Title/Abstract])) AND ((oxidative stress[Title/Abstract]))) | 16 |
| KP-2: (curcumin[Title/Abstract]) OR (curcuma[Title/Abstract]) AND (“in vivo”[Title/Abstract]) OR (rat[Title/Abstract])) OR (mice[Title/Abstract]) AND (dementia[Title/Abstract] OR (frontotemporal dementia[Title/Abstract]) AND (Neurodegeneration[Title/Abstract]) | 10 | |
| KP-3: (((curcumin[Title/Abstract] OR curcuma[Title/Abstract]) AND (rat[Title/Abstract] OR mice[Title/Abstract] OR “in vivo”[Title/Abstract])) AND (dementia[Title/Abstract])) AND (neurodegenerative disease[Title/Abstract] OR neurodegeneration[Title/Abstract] OR neuroinflammation[Title/Abstract]) | 16 | |
| KP-4: (((curcumin[Title/Abstract] OR curcuma[Title/Abstract]) AND (rat[Title/Abstract] OR mice[Title/Abstract] OR “in vivo”[Title/Abstract])) AND (dementia[Title/Abstract])) AND (synaptic[Title/Abstract]) | 4 | |
| KP-5: ((((curcumin[Title/Abstract] OR curcuma[Title/Abstract]) AND (rat[Title/Abstract] OR mice[Title/Abstract] OR “in vivo”[Title/Abstract])) AND (dementia[Title/Abstract]))) AND (cognitive ability[Title/Abstract] OR cognition[Title/Abstract]) (((curcumin[Title/Abstract]) OR (curcuma[Title/Abstract]))) AND ((“in vivo”[Title/Abstract])) OR (rat[Title/Abstract])) OR (mice[Title/Abstract]))) AND ((Cognitive abilities[Title/Abstract])) AND (((dementia[Title/Abstract]))) | 5 | |
| Scopus (3 April 2024) | KS-1: (TITLE-ABS-KEY (curcumin) AND TITLE-ABS-KEY (“in vivo”) OR TITLE-ABS-KEY (rat) OR TITLE-ABS-KEY (mice) AND TITLE-ABS-KEY (dementia) AND TITLE-ABS-KEY (oxidative stress)) | 66 |
| KS -2: (TITLE-ABS-KEY (curcumin) AND TITLE-ABS-KEY (“in vivo”) OR TITLE-ABS-KEY (rat) OR TITLE-ABS-KEY (mice) AND TITLE-ABS-KEY (dementia) OR TITLE-ABS-KEY (frontotemporal dementia) AND TITLE-ABS-KEY (oxidative stress)) | 5 | |
| KS-3: (TITLE-ABS-KEY (curcumin) AND TITLE-ABS-KEY (“in vivo”) OR TITLE-ABS-KEY (rat) OR TITLE-ABS-KEY (mice) AND TITLE-ABS-KEY (dementia) AND TITLE-ABS-KEY (Neurodegenerative disease) OR TITLE-ABS-KEY (Neurodegeneration) OR TITLE-ABS-KEY (Neuroinflammation) | 66 | |
| KS-4: (TITLE-ABS-KEY (curcumin) AND TITLE-ABS-KEY (“in vivo”) OR TITLE-ABS-KEY (rat) OR TITLE-ABS-KEY (mice) AND TITLE-ABS-KEY (dementia) AND TITLE-ABS-KEY (synaptic dysfunction) OR TITLE-ABS-KEY ((synaptopathies) | 1 | |
| KS-5: (TITLE-ABS-KEY (curcumin) AND TITLE-ABS-KEY (“in vivo”) OR TITLE-ABS-KEY (rat) OR TITLE-ABS-KEY (mice) AND TITLE-ABS-KEY (dementia) AND TITLE-ABS-KEY (Neuropsychological functions) OR TITLE-ABS-KEY (Cognitive abilities) | 3 | |
| AMED (4 April 2024) | KA-1: TITLE curcumin AND AB dementia AND AB mice or rat AND AB oxidative stress | 22 |
| KA-2: TITLE curcumin AND AB mice or rat AND AB dementia OR frontotemporal dementia AND AB neurodegeneration | 6 | |
| KA-3: TITLE curcumin AND AB mice or rat AND AB dementia AND AB neurodegenerative disease OR neurodegeneration OR neuroinflammation | 23 | |
| KA-4: TITLE curcumin AND AB dementia AND AB mice or rat AND AB dementia AND AB synaptic dysfunction OR synaptopathies | 1 | |
| KA-5: TITLE curcumin AND AB dementia AND AB mice or rat AND AB dementia AND AB neuropsychological functions or cognitive ability | 6 | |
| Allied and Complementary Medicine Database (AMED) EBSCO | ||
| LILACS (3 April 2024) | KL-1: Title, abstract, subject: curcumin and rat or mice and dementia and oxidative stress | 30 |
| KL-2: Title, abstract, subject: curcumin and rat or mice and dementia or frontotemporal dementia and neurodegeneration | 12 | |
| KL-3: Title, abstract, subject: curcumin and rat or mice and dementia and neurodegenerative disease or neurodegeneration or neuroinflammation | 34 | |
| KL-4: Title, abstract, subject: curcumin and rat or mice and dementia and synaptic dysfunction or synaptopathies | 16 | |
| KL-5: Title, abstract, subject: curcumin and rat or mice and dementia and cognitive ability | 10 | |
| LILACS, scientific health information from Latin America and the Caribbean countries |
| Author, Year | Methods Used | The Construction of the Induction | |||
|---|---|---|---|---|---|
| Diet | Chemical | Genetic | Other | ||
| Agrawal et al., 2009 [28] | Streptozotocin (STZ)-induced | - | √ | - | - |
| Awasthi et al., 2009 [29] | Streptozotocin (STZ)-induced | - | √ | - | - |
| Bassani et al., 2017 [30] | Streptozotocin (STZ)-induced | - | √ | - | - |
| Campisi et al., 2022 [31] | TG2-L and TG2-S in transgenic, | - | - | √ | - |
| hemizygous, CRND8 (Tg) mice | |||||
| ELBini-Dhouib et al., 2021 [32] | Aluminium chloride induced | - | √ | - | - |
| Fidelis et al., 2018 [33] | Abeta 25–35-induced neurotoxicity | - | √ | - | - |
| Ishrat et al., 2009 [34] | Streptozotocin (STZ)-induced | - | √ | - | - |
| Isik et al., 2008 [35] | Streptozotocin (STZ)-induced) | - | √ | - | - |
| Jearjaroen et al., 2020 [36] | Dexamethasone-induced | - | √ | - | - |
| Khurana et al., 2012 [37] | Colchicine-induced | - | √ | - | - |
| Kim et al., 2019 [38] | B6SJL transgenic | - | - | √ | - |
| Kumar et al., 2023 [39] | Scopolamine-induced | - | √ | - | - |
| Lamichhane et al., 2024 [40] | Feeding a high-fat high-sugar diet, | √ | - | √ | - |
| Use Genetic Modification Mice | |||||
| Li and Yu, 2015 [41] | Permanent occlusion of bilateral | - | - | - | √ |
| caroid arteries ligation (2VO) method | |||||
| Lim et al., 2001 [42] | Transgenic Amyloid precursor mice- | - | √ | - | |
| Özaçmak & Özaçmak, 2010 [43] | Bilateral ligation of carotid arteries, | - | - | - | √ |
| ovariectomized rat brain subjected to | |||||
| chronic cerebral hypoperfusion | |||||
| Patel et al., 2020 [44] | Scopolamine-induced | - | √ | - | - |
| Prathipati et al., 2021 [45] | Homocysteine (HCY)-induced | - | √ | - | - |
| Rinwa et al., 2010 [46] | Streptozotocin (STZ)-induced | - | √ | - | - |
| Samy et al., 2015 [47] | Streptozotocin (STZ)-induced | - | √ | - | - |
| Shao et al., 2023 [48] | Amyloid-beta1-induced | - | √ | - | - |
| Sun et al., 2020 [50] | APP/PS1 double transgenic | - | - | √ | - |
| Spinelli et al., 2015 [49] | AD transgenic mice | - | - | √ | - |
| Sundaram et al., 2017 [51] | p25/Cdk5 hyperactivation-induced by | - | √ | - | - |
| removal of doxycycline in water | |||||
| Tian et al., 2021 [52] | AD model transgenic mice | - | - | √ | - |
| Wang et al., 2014 [53] | Double Transgenic mice | - | - | √ | - |
| Wang et al., 2019 [54] | Stereotaxic injection with | - | √ | - | - |
| okadaic acid (OA) | |||||
| Xu et al., 2020 [55] | APP/PS mouse | - | - | √ | - |
| Zheng et al., 2016 [56] | AD transgenic mice | - | - | √ | - |
| Author, Year | Types of Treatment | Doses of Treatment | Duration (Weeks) |
|---|---|---|---|
| Agrawal et al., 2009 [28] | Curcumin pre-treated | 200 mg/kg | Day 1 to Day 14 (Daily) |
| Curcumin post treated | 200 mg/kg | Day14 to Day20 (Daily) | |
| Awasthi et al., 2009 [29] | Curcumin pre-treated | 10 mg/kg | |
| Curcumin pre-treated | 20 mg/kg | 3 | |
| Curcumin pre-treated | 50 mg/kg | ||
| Curcumin post-treated | 25 mg/kg | 1 | |
| Curcumin post-treated | 50 mg/kg | ||
| Bassani et al., 2017 [30] | Curcumin | 25 mg/kg | |
| Curcumin | 50 mg/kg | 4 (+2 days) | |
| Curcumin | 100 mg/kg | ||
| Campisi et al., 2022 [31] | WT mice with solid lipid nanoparticles-curcumin | 150 mg/kg | 3 |
| Tg mice with solid lipid nanoparticles-curcumin | 150 mg/kg | ||
| ELBini-Dhouib et al., 2021 [32] | curcumin (dissolved in 1mL of corn oil) | 100 mg/kg | 9 (+5 days) |
| 100 mg/kg | Phase 1: 9 (+5 days) + Phase 2: 7 (+4 days) | ||
| Fidelis et al., 2018 [33] | NLCC: curcumin-loaded nanocapsules | 10 mg/mL | Alternative days; Once every 48 h |
| Free curcumin in canola oil | 10 mg/mL | day 2, 4, 6, 8, 10, 12 | |
| Ishrat et al., 2009 [34] | Curcumin | 80 mg/kg | 3 |
| Curcumin | 80 mg/kg | 3 | |
| Isik et al., 2008 [35] | Curcumin in a vehicle using a mixture of 1%sodium carboxy methyl cellulose and 1% Tween-80) | 300 mg/kg | 1 (+3 days) |
| Jearjaroen et al., 2020 [36] | Hexahydrocurcumin (HHC) | HHC (40 mg/kg) | 4 |
| Khurana et al., 2012 [37] | Curcumin | 100 mg/kg | 4 weeks (twice daily) |
| 200 mg/kg | 4 weeks (twice daily) | ||
| 400 mg/kg | 4 weeks (twice daily) | ||
| 100 mg/kg | 4 weeks; started 7 days before colchicine injection; (twice daily) | ||
| 200 mg/kg | 4 weeks; started 7 days before colchicine injection; (twice daily) | ||
| 400 mg/kg | 4 weeks; started 7 days before colchicine injection; (twice daily) | ||
| 100 mg/kg | for 1 week before colchicine injection (twice daily) | ||
| 200 mg/kg | for 1 week before colchicine injection (twice daily) | ||
| 400 mg/kg | for 1 week before colchicine injection (twice daily) | ||
| Kim et al., 2019 [38] | Theracurmin | 100 mg/kg, p.o. once a day | 12 |
| 300 mg/kg, p.o. once a day | |||
| 1000 mg/kg, p.o. once a day | |||
| Kumar et al., 2023 [39] | Curcumin | Curcumin (100 mg/kg) | |
| Curcumin (200 mg/kg) | 3 | ||
| Curcumin and CoQ10 | Curcumin (200 mg/kg) | ||
| and CoQ10 (200 mg/kg) | |||
| Lamichhane et al., 2024 [40] | Curcumin | 1. NCD + CUR gp- curcumin-supplemented (4 g/kg) with normal chow diet, | |
| 2. HFHSD + CUR fed gp-curcumin-supplemented (4 g/kg) with high-fat-high-sugar diet | 14 | ||
| Li and Yu, 2015 [41] | 2VO + Curcumin | 50 mg/kg | 4 (+2 days) |
| 2VO + Curcumin | 100 mg/kg | ||
| Lim et al., 2001 [42] | A low dose of curcumin (mixed with diet) | 160 ppm | 24 |
| A high dose of curcumin (mixed with diet) | 5000 ppm | ||
| Özaçmak & Özaçmak, 2010 [43] | Curcumin mixing with peanut butter | Curcumin (100 mg/kg) | 2 |
| Patel et al., 2020 [44] | Curcumin alone | Curcumin (205 mg/kg) | 1 (+3 days) |
| Curcumin + Lactobacillus rhamnosus | Curcumin (205 mg/kg) + Lactobacillus rhamnosus (1 × 106 CFU) | ||
| Prathipati et al., 2021 [45] | Curcumin | 25 mg/kg | |
| Curcumin | 50 mg/kg | 2 | |
| Curcumin-Solid Lipid Nano Particle | 10 mg/kg | ||
| Curcumin-Solid Lipid Nano Particle | 25 mg/kg | ||
| Rinwa et al., 2010 [46] | Curcumin suspended in 0.5% w/v sodium carboxymethyl cellulose | Curcumin (20 mg/kg, p.o.). The administration of curcumin was continued (administered 30 min before) during acquisition trial conducted from days 1 to 4. The animals were administered vehicle (0.5% w/v CMC, 10 mL/kg, p.o.) only, given 30 min before retrieval trial conducted on day 5” curcumin (20 mg/kg, p.o.) for 14 days and rest of the procedure was same as described as in the above group.” | 2 |
| Samy et al., 2015 [47] | Vehicle + curcumin | 80 mg/kg/day | 12 |
| Erythropoietin | 500 IU/kg | ||
| Combined Curcumin + Erythropoietin | N/M | ||
| Shao et al., 2023 [48] | Curcumin (dissolved in 10% polyethylene glycol) | 150 mg/kg | 1 (+3 days) |
| Spinelli et al., 2015 [49] | Curcumin premixed in soybean oil prior to incorporation into the diet (dietary supplement grade curcumin) | 500 ppm | 24 |
| Dietary supplement grade curcumin | 15 mg/kg | 2 (+1 days) | |
| Sun et al., 2020 [50] | High dose curcumin | 200 mg/kg | 12 |
| Low dose curcumin | 50 mg/kg | 12 | |
| Sundaram et al., 2017 [51] | Curcumin | 4 g/kg (0.8 g curcumin/kg) | 12 |
| Tian et al., 2021 [52] | Curcumin | Low dose: 0.16 g/kg High dose:1 g/kg | 24 |
| Wang et al., 2014 [53] | Low dose curcumin (160 ppm) | 160 ppm | 24 |
| High dose curcumin (1000 ppm) | 1000 ppm | ||
| Wang et al., 2019 [54] | Curcumine | 100 μg/mL | 1 |
| Exo-curcumine | 100 μg/mL | ||
| Xu et al., 2020 [55] | Bisdemethoxycurcumin | 5 µg/kg | 4 |
| Zheng et al., 2016 [56] | Curcumin | 150 or 300 mg/kg | 7 (+4 days) |
References
- Warren, J.; Collinge, J.; Fox, N.; Mead, S.; Mummery, C.; Rohrer, J.; Rossor, M.; Schott, J.; Weil, R. Cognitive impairment and dementia. In Neurology: A Queen Square Textbook; Wiley Online Library: Hoboken, NJ, USA, 2024; pp. 319–367. [Google Scholar] [CrossRef]
- Birajdar, S.V.; Mulchandani, M.; Mazahir, F.; Yadav, A.K. Dementia and neurodegenerative disorder: An introduction. In Nanomedicine-Based Approaches for the Treatment of Dementia; Academic Press: Cambridge, MA, USA, 2023; pp. 1–36. [Google Scholar] [CrossRef]
- Minett, T.; Brayne, C. Epidemiology of dementia. In Oxford Textbook of Cognitive Neurology and Dementia; Oxford Academic Press: Oxford, UK, 2016; pp. 211–220. [Google Scholar]
- World Health Organization. Global Status Report on the Public Health Response to Dementia; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Health Organization. A Blueprint for Dementia Research; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Pedroza, P.; Miller-Petrie, M.K.; Chen, C.; Chakrabarti, S.; Chapin, A.; Hay, S.; Tsakalos, G.; Wimo, A.; Dieleman, J.L. Global and regional spending on dementia care from 2000–2019 and expected future health spending scenarios from 2020–2050: An economic modelling exercise. eClinicalMedicine 2022, 45, 101337. [Google Scholar] [CrossRef] [PubMed]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and therapeutic perspectives of oxidative stress and neurodegenerative diseases: A narrative review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Houldsworth, A. Role of oxidative stress in neurodegenerative disorders: A review of reactive oxygen species and prevention by antioxidants. Brain Commun. 2024, 6, fcad356. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Schönfeld, P.; Reiser, G. How the brain fights fatty acids’ toxicity. Neurochem. Int. 2021, 148, 105050. [Google Scholar] [CrossRef]
- Jones, K.C. Update on major neurocognitive disorders. Focus 2021, 19, 271–281. [Google Scholar] [CrossRef]
- Petersen, R.C.; Wiste, H.J.; Weigand, S.D.; Fields, J.A.; Geda, Y.E.; Graff-Radford, J.; Knopman, D.S.; Kremers, W.K.; Lowe, V.; Machulda, M.M.; et al. NIA-AA Alzheimer’s disease framework: Clinical characterization of stages. Ann. Neurol. 2021, 89, 1145–1156. [Google Scholar] [CrossRef]
- World Health Organization. Clinical Descriptions and Diagnostic Requirements for ICD-11 Mental, Behavioural and Neurodevelopmental Disorders; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- de Wolf, F.; Ghanbari, M.; Licher, S.; McRae-McKee, K.; Gras, L.; Weverling, G.J.; Wermeling, P.; Sedaghat, S.; Ikram, M.K.; Waziry, R.; et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 2020, 143, 1220–1232. [Google Scholar] [CrossRef]
- Zhang, K.; Mizuma, H.; Zhang, X.; Takahashi, K.; Jin, C.; Song, F.; Gao, Y.; Kanayama, Y.; Wu, Y.; Li, Y.; et al. PET imaging of neural activity, β-amyloid, and tau in normal brain aging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3859–3871. [Google Scholar] [CrossRef]
- Pemberton, H.G.; Collij, L.E.; Heeman, F.; Bollack, A.; Shekari, M.; Salvadó, G.; Alves, I.L.; Garcia, D.V.; Battle, M.; Buckley, C.; et al. Quantification of amyloid PET for future clinical use: A state-of-the-art review. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3508–3528. [Google Scholar] [CrossRef]
- Mankhong, S.; Kim, S.; Lee, S.; Kwak, H.B.; Park, D.H.; Joa, K.L.; Kang, J.H. Development of Alzheimer’s disease biomarkers: From CSF-to blood-based biomarkers. Biomedicines 2022, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Raulin, A.C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, F.R.; D’Anca, M.; Fenoglio, C.; Scarpini, E.; Galimberti, D. Role of oxidative damage in Alzheimer’s disease and neurodegeneration: From pathogenic mechanisms to biomarker discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef]
- Pluta, R.; Furmaga-Jabłońska, W.; Januszewski, S.; Czuczwar, S.J. Post-ischemic brain neurodegeneration in the form of Alzheimer’s disease proteinopathy: Possible therapeutic role of curcumin. Nutrients 2022, 14, 248. [Google Scholar] [CrossRef]
- Flora, S.J. Arsenic-induced oxidative stress and its reversibility. Free. Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef]
- Escudero-Lourdes, C. Toxicity mechanisms of arsenic that are shared with neurodegenerative diseases and cognitive impairment: Role of oxidative stress and inflammatory responses. Neurotoxicology 2016, 53, 223–235. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Houshmand, G.; Kalantar, M.; Khalili, H.R.; Mehrzadi, S.; Goudarzi, M. Neuroprotective effects of gallic acid against neurotoxicity induced by sodium arsenite in rats. Comp. Clin. Pathol. 2020, 29, 621–629. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 18, 343. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Mishra, B.; Tyagi, E.; Nath, C.; Shukla, R. Effect of curcumin on brain insulin receptors and memory functions in STZ (ICV) induced dementia model of rat. Pharmacol. Res. 2010, 61, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, H.; Tota, S.; Hanif, K.; Nath, C.; Shukla, R. Protective effect of curcumin against intracerebral streptozotocin induced impairment in memory and cerebral blood flow. Life Sci. 2010, 86, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Bassani, T.B.; Turnes, J.M.; Moura, E.L.; Bonato, J.M.; Cóppola-Segovia, V.; Zanata, S.M.; Oliveira, R.M.; Vital, M.A. Effects of curcumin on short-term spatial and recognition memory, adult neurogenesis and neuroinflammation in a streptozotocin-induced rat model of dementia of Alzheimer’s type. Behav. Brain Res. 2017, 335, 41–54. [Google Scholar] [CrossRef]
- Campisi, A.; Sposito, G.; Pellitteri, R.; Santonocito, D.; Bisicchia, J.; Raciti, G.; Russo, C.; Nardiello, P.; Pignatello, R.; Casamenti, F.; et al. Effect of unloaded and curcumin-loaded solid lipid nanoparticles on tissue transglutaminase isoforms expression levels in an experimental model of Alzheimer’s disease. Antioxidants 2022, 11, 1863. [Google Scholar] [CrossRef]
- ELBini-Dhouib, I.; Doghri, R.; Ellefi, A.; Degrach, I.; Srairi-Abid, N.; Gati, A. Curcumin attenuated neurotoxicity in sporadic animal model of Alzheimer’s disease. Molecules 2021, 26, 3011. [Google Scholar] [CrossRef]
- Fidelis, E.M.; Savall, A.S.P.; da Luz Abreu, E.; Carvalho, F.; Teixeira, F.E.G.; Haas, S.E.; Sampaio, T.B.; Pinton, S. Curcumin-loaded nanocapsules reverses the depressant-like behavior and oxidative stress induced by β-amyloid in mice. Neuroscience 2019, 423, 122–130. [Google Scholar] [CrossRef]
- Ishrat, T.; Hoda, M.N.; Khan, M.B.; Yousuf, S.; Ahmad, M.; Khan, M.M.; Ahmad, A.; Islam, F. Amelioration of cognitive deficits and neurodegeneration by curcumin in rat model of sporadic dementia of Alzheimer’s type (SDAT). Eur. Neuropsychopharmacol. 2009, 19, 636–647. [Google Scholar] [CrossRef]
- Isik, A.T.; Celik, T.; Ulusoy, G.; Ongoru, O.; Elibol, B.; Doruk, H.; Bozoglu, E.; Kayir, H.; Mas, M.R.; Akman, S. Curcumin ameliorates impaired insulin/IGF signalling and memory deficit in a streptozotocin-treated rat model. Age 2009, 31, 39–49. [Google Scholar] [CrossRef]
- Jearjaroen, P.; Pakdeepak, K.; Tocharus, C.; Chaichompoo, W.; Suksamrarn, A.; Tocharus, J. Inhibitory effect of hexahydrocurcumin on memory impairment and amyloidogenesis in dexamethasone-treated mice. Neurotox. Res. 2021, 39, 266–276. [Google Scholar] [CrossRef]
- Khurana, S.; Jain, S.; Mediratta, P.K.; Banerjee, B.D.; Sharma, K.K. Protective role of curcumin on colchicine-induced cognitive dysfunction and oxidative stress in rats. Hum. Exp. Toxicol. 2012, 31, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Huang, Z.; Goo, N.; Bae, H.J.; Jeong, Y.; Park, H.J.; Cai, M.; Cho, K.; Jung, S.Y.; et al. Theracurmin ameliorates cognitive dysfunctions in 5XFAD mice by improving synaptic function and mitigating oxidative stress. Biomol. Ther. 2019, 27, 327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, A.; Kumar, A.; Kumar, R.; Pal, R.; Sachan, A.K.; Dixit, R.K.; Nath, R. Effect of curcumin and coenzyme Q10 alone and in combination on learning and memory in an animal model of Alzheimer’s disease. Biomedicines 2023, 11, 1422. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, G.; Liu, J.; Lee, S.J.; Lee, D.Y.; Zhang, G.; Kim, Y. Curcumin mitigates the high-fat high-sugar diet-induced impairment of spatial memory, hepatic metabolism, and the alteration of the gut microbiome in Alzheimer’s disease-induced (3xTg-AD) mice. Nutrients 2024, 16, 240. [Google Scholar] [CrossRef]
- Tang, L.; Li, Y. Effects of plant compound curcumin on learning and memory of chronic cerebral hypoperfusion rats. Adv. Mater. Res. 2015, 1120, 853–856. [Google Scholar] [CrossRef]
- Lim, G.P.; Chu, T.; Yang, F.; Beech, W.; Frautschy, S.A.; Cole, G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001, 21, 8370–8377. [Google Scholar] [CrossRef]
- Özaçmak, V.H.; Özaçmak-Sayan, H. Curcumin reduces oxidative stress in ovariectomized rat brain subjected to chronic cerebral hypoperfusion. Turk. J. Geriatr. 2010, 13, 160–165. [Google Scholar]
- Patel, C.; Pande, S.; Acharya, S. Potentiation of anti-Alzheimer activity of curcumin by probiotic Lactobacillus rhamnosus UBLR-58 against scopolamine-induced memory impairment in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1955–1962. [Google Scholar] [CrossRef]
- Prathipati, B.; Rohini, P.; Kola, P.K.; Danduga, R.C.S.R. Neuroprotective effects of curcumin loaded solid lipid nanoparticles on homocysteine induced oxidative stress in vascular dementia. Curr. Res. Behav. Sci. 2021, 2, 100029. [Google Scholar] [CrossRef]
- Rinwa, P.; Kaur, B.; Jaggi, A.S.; Singh, N. Involvement of PPAR-gamma in curcumin-mediated beneficial effects in experimental dementia. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 529–539. [Google Scholar] [CrossRef]
- Samy, D.M.; Ismail, C.A.; Nassra, R.A.; Zeitoun, T.M.; Nomair, A.M. Downstream modulation of extrinsic apoptotic pathway in streptozotocin-induced Alzheimer’s dementia in rats: Erythropoietin versus curcumin. Eur. J. Pharmacol. 2016, 770, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Ye, X.; Su, W.; Wang, Y. Curcumin alleviates Alzheimer’s disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J. Chem. Neuroanat. 2023, 134, 102363. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, K.J.; Osterberg, V.R.; Meshul, C.K.; Soumyanath, A.; Unni, V.K. Curcumin treatment improves motor behavior in α-synuclein transgenic mice. PLoS ONE 2015, 10, e0128510. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Z.; Li, X.Y.; Wang, S.; Shen, L.; Ji, H.F. Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer’s disease. Appl. Microbiol. Biotechnol. 2020, 104, 3507–3515. [Google Scholar] [CrossRef]
- Sundaram, J.R.; Poore, C.P.; Sulaimee, N.H.B.; Pareek, T.; Cheong, W.F.; Wenk, M.R.; Pant, H.C.; Frautschy, S.A.; Low, C.M.; Kesavapany, S. Curcumin ameliorates neuroinflammation, neurodegeneration, and memory deficits in p25 transgenic mouse model that bears hallmarks of Alzheimer’s disease. J. Alzheimers Dis. 2017, 60, 1429–1442. [Google Scholar] [CrossRef]
- Tian, M.; Zhou, F.; Teng, Z.; Wang, C.; Zhang, X.; Wang, Y.; Li, Y. Curcumin ameliorates lipid metabolic disorder and cognitive dysfunction via the ABCA1 transmembrane transport system in APP/PS1 double transgenic mice. J. Integr. Neurosci. 2021, 20, 895–903. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur. J. Pharmacol. 2014, 740, 312–320. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, R.; He, D.; Zhou, G.; Wu, H.; Xu, C.; He, B.; Wu, L.; Wang, Y.; Chang, Y.; et al. Bisdemethoxycurcumin inhibits oxidative stress and antagonizes Alzheimer’s disease by up-regulating SIRT1. Brain Behav. 2020, 10, e01655. [Google Scholar] [CrossRef]
- Zheng, K.; Dai, X.; Xiao, N.A.; Wu, X.; Wei, Z.; Fang, W.; Zhu, Y.; Zhang, J.; Chen, X. Curcumin ameliorates memory decline via inhibiting BACE1 expression and β-Amyloid pathology in 5×FAD transgenic mice. Mol. Neurobiol. 2017, 54, 1967–1977. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verma, K.; Nalla, S.; Kulshreshtha, A.; Lall, R.; Prasad, S. Free radicals as a double-edged sword: The cancer preventive and therapeutic roles of curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Pavani, B.; Tiwari, G. Mechanistic Insights into Tau Protein-Mediated Regulation of Oxidative Stress. Chin. J. Appl. Physiol. 2024, 40, e20240028. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Mhillaj, E.; Cuomo, V.; Mancuso, C. The contribution of transgenic and nontransgenic animal models in Alzheimer’s disease drug research and development. Behav. Pharmacol. 2017, 28, 95–111. [Google Scholar] [CrossRef]
- Sinha, S.; Wal, P.; Goudanavar, P.; Divya, S.; Kimothi, V.; Jyothi, D.; Sharma, M.C.; Wal, A. Research on Alzheimer’s Disease (AD) Involving the Use of In Vivo and In Vitro Models and Mechanisms. Cent. Nerv. Syst. Agents Med. Chem. 2025, 25, 123–142. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Borrelli, L.A.; Rozkalne, A.; Hyman, B.T.; Bacskai, B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007, 102, 1095–1104. [Google Scholar] [CrossRef]
- Moore, T.L.; Bowley, B.G.; Shultz, P.L.; Calderazzo, S.M.; Shobin, E.J.; Uprety, A.R.; Rosene, D.L.; Moss, M.B. Oral curcumin supplementation improves fine motor function in the middle-aged rhesus monkey. Somatosens. Mot. Res. 2018, 35, 1–10. [Google Scholar] [CrossRef]
- Akinyemi, A.J.; Oboh, G.; Ogunsuyi, O.; Abolaji, A.O.; Udofia, A. Curcumin-supplemented diets improve antioxidant enzymes and alter acetylcholinesterase genes expression level in Drosophila melanogaster model. Metab. Brain Dis. 2018, 33, 369–375. [Google Scholar] [CrossRef]
- Rahman, M.M.; Noman, M.A.; Hossain, M.W.; Alam, R.; Akter, S.; Kabir, M.M.; Uddin, M.J.; Amin, M.Z.; Syfuddin, H.M.; Akhter, S.; et al. Curcuma longa L. Prevents the Loss of β-Tubulin in the Brain and Maintains Healthy Aging in Drosophila melanogaster. Mol. Neurobiol. 2022, 59, 1819–1835. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, S.A.; Baek, S.M.; Lee, E.Y.; Lee, E.S.; Chung, C.H.; Ahn, C.M.; Park, S.K. Cur2004-8, a synthetic curcumin derivative, extends lifespan and modulates age-related physiological changes in Caenorhabditis elegans. Drug Discov. Ther. 2019, 3, 198–206. [Google Scholar] [CrossRef]
- Coradini, K.; de Andrade, D.F.; Altenhofen, S.; Reolon, G.K.; Nery, L.R.; Silva, N.E.; Vianna, M.R.; Bonan, C.D.; Beck, R.C. Free and nanoencapsulated curcumin prevents scopolamine-induced cognitive impairment in adult zebrafish. J. Drug Deliv. Sci. Technol. 2021, 66, 102781. [Google Scholar] [CrossRef]
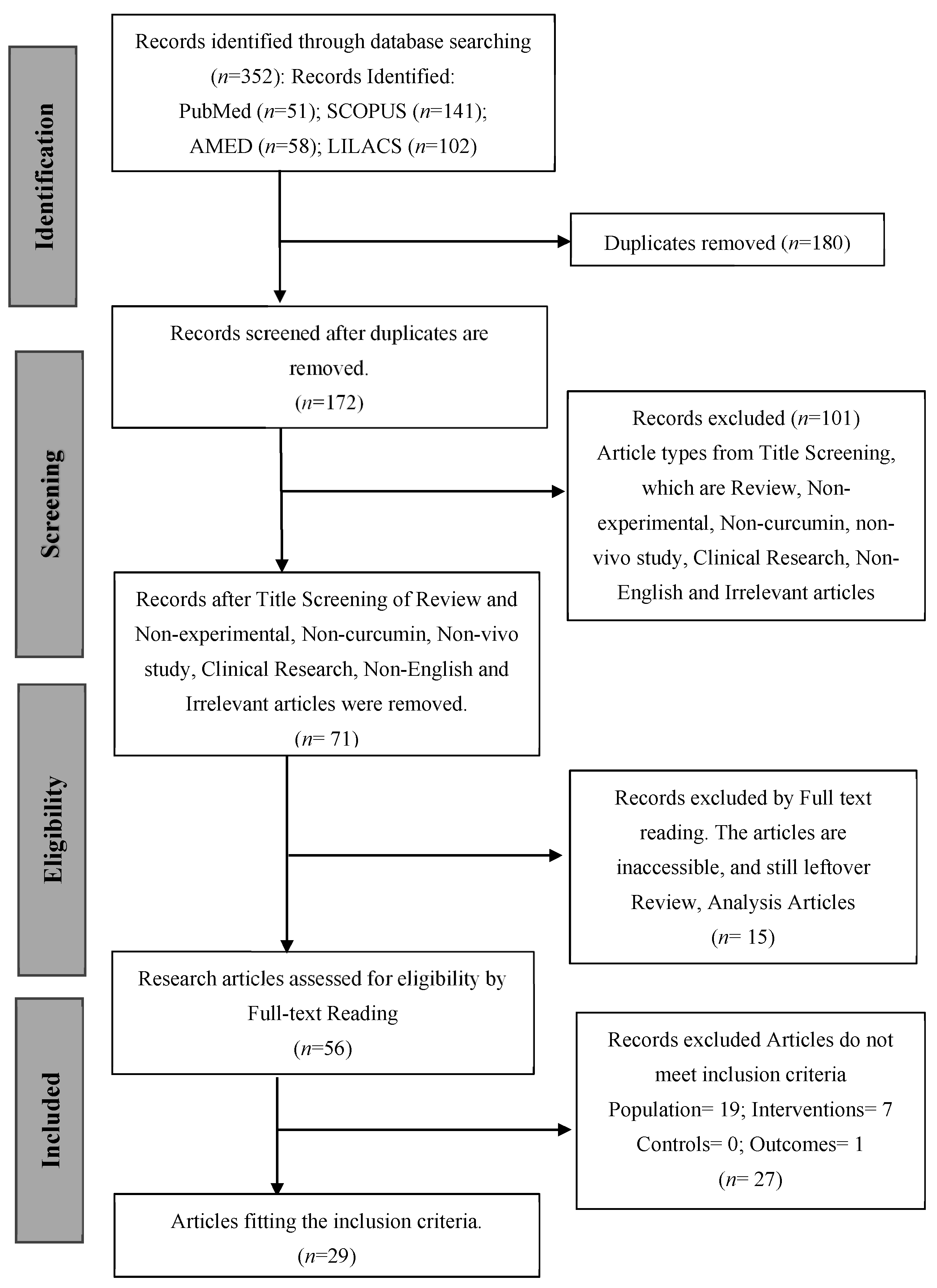
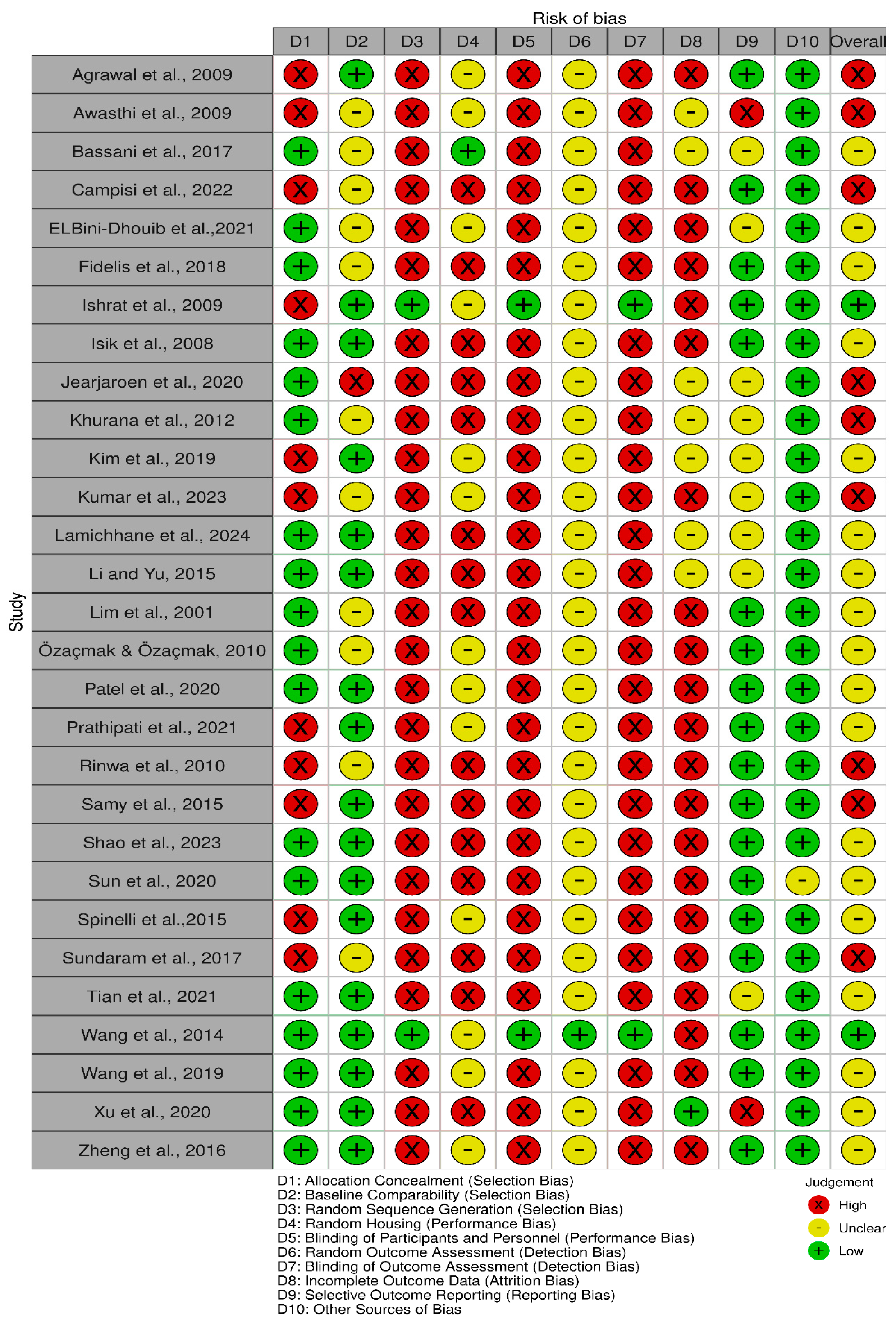
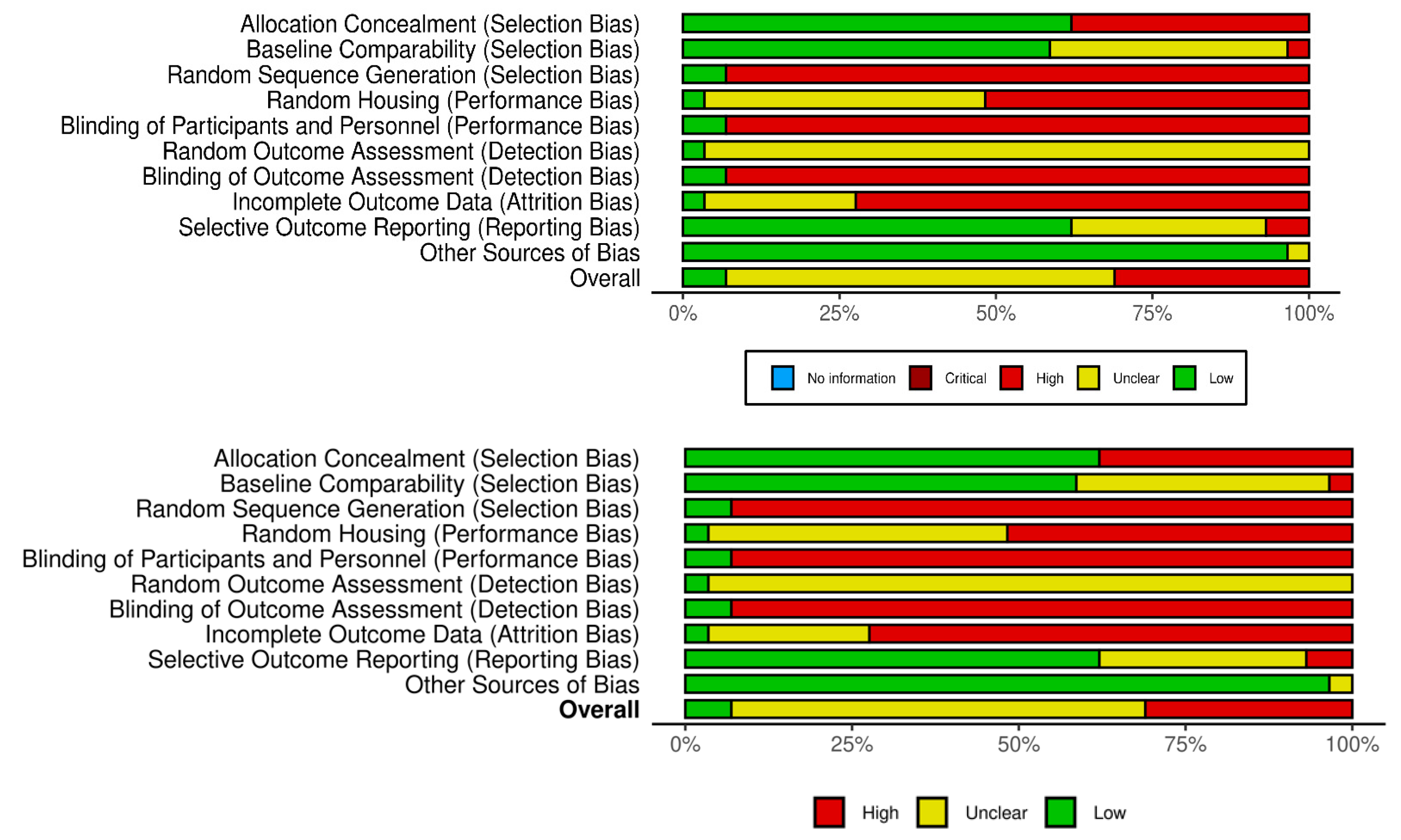
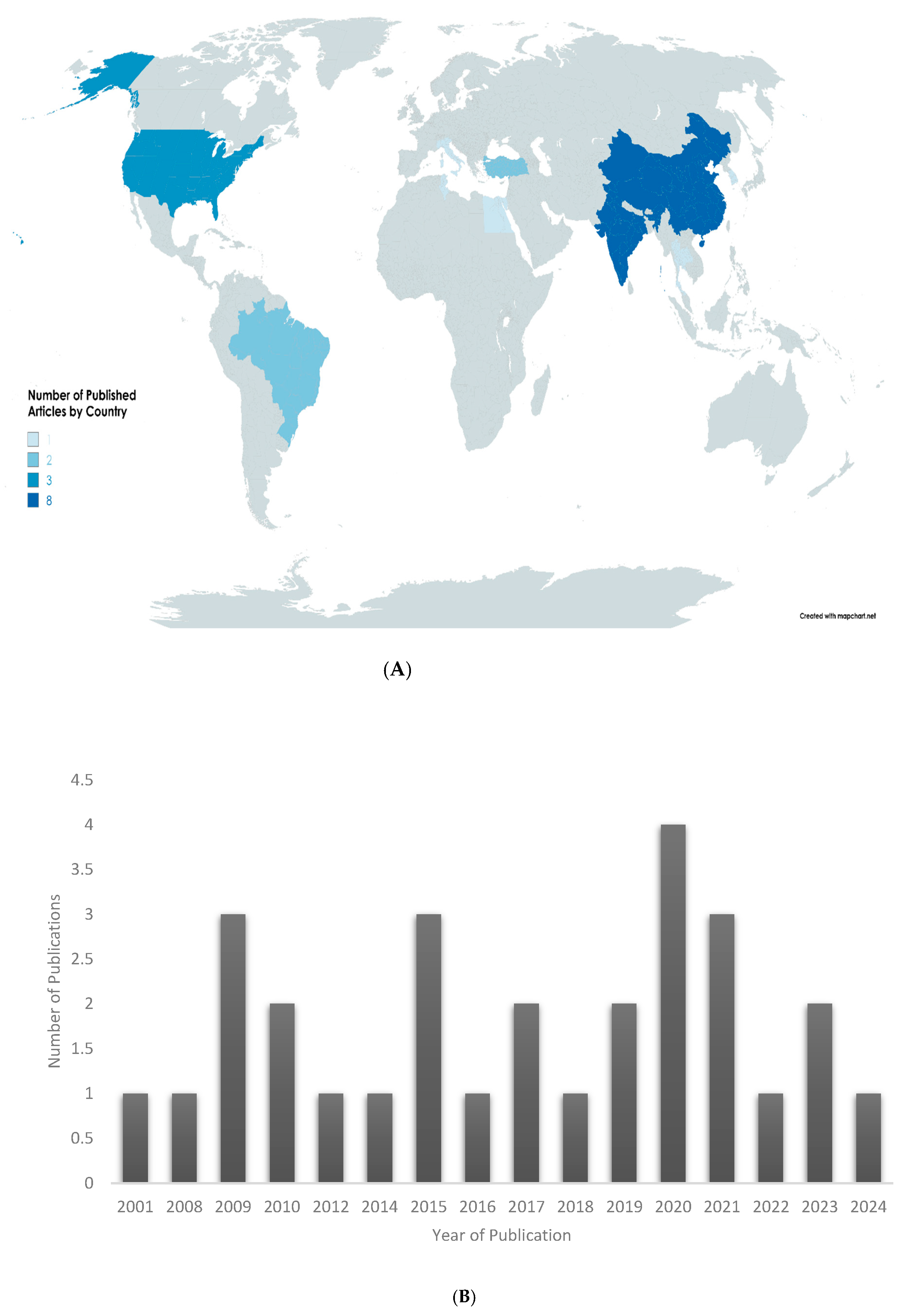
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies in dementia animal models Treatment with Curcumin and its derivatives | Studies conducted in vitro Non-curcumin, clinical research |
| Articles that were published in English | Non-English, Non-experimental |
| Controlled study design | Reviews, commentaries and unpublished studies |
| Studies with one or more of the following: oxidative stress, inflammation and cognitive function in relation to curcumin | Publications without full text access and Studies lacking relevant outcomes |
| Author | Year | Country | No. of Animals | Characteristics of Rodents Used | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Per Group | Types of Rodents | Weight (g) | Age (wk.) | Sex (M/F) | |||
| Agrawal et al. [28] | 2009 | India | 40 | 5 | Sprague-Dawley SD rats | 200–250 | N/M | M |
| Awasthi et al. [29] | 2009 | India | N/M | 6–8 | Swiss albino mice | 25–30 | 8 | M |
| Bassani et al. [30] | 2017 | Brazil | 35 | 6–8 | Wistar rats | 300–340 | 12–16 | M |
| Campisi et al. [31] | 2022 | Italy | 20 | 5 | WT & Tg mice | 20–30 | 16 | N/M |
| El Bini-Dhouib et al. [32] | 2021 | Tunisia | 42 | 6 | Wistar albino rats | 142–148 | N/M | M |
| Fidelis et al. [33] | 2018 | Brazil | N/M | 7–8 | Swiss mice | 40–50 | 12 | M |
| Ishrat et al. [34] | 2009 | India | 40 | 10 | Wistar rats | 470–500 | 52 | M |
| Isik et al. [35] | 2008 | Turkey | 24 | 8, 7, 8 | Wistar rats | 225–275 | 52 | M |
| Jeerajaroe et al. [36] | 2020 | Thailand | 84 | 12 | ICR mice | 35–45 | N/M | M |
| Khurana et al. [37] | 2012 | India | 104 | 8 | Wistar rats | 250–350 | 12–14 | M |
| Kim et al. [38] | 2019 | Korea | N/M | 5–10 | B6SJL mice | N/M | 6–7 | M |
| Kumar et al. [39] | 2023 | India | 42 | 6 | Wistar rats | 160–200 | N/M | F |
| Lamichhane et al. [40] | 2024 | USA | N/M | N/M | 3 × Tg-AD mice, B6129SF2/J mice | N/M | 28 | F |
| Li and Yu [41] | 2015 | China | 70 | N/M | Sprague-Dawley rats | 250–300; 400–450 | 12–24; 72–244 | M; M |
| Lim et al. [42] | 2001 | USA | 28 | 5–9 | APP/PS1 mice | N/M | 40 | M, F |
| Özçamak & Özçamak [43] | 2010 | Turkey | 30 | 10 | Wistar rats | 200–250 | 16–24 | F |
| Patel et al. [44] | 2020 | India | 30 | 6 | Swiss albino mice | 25–30 | N/M | F |
| Prathipati et al. [45] | 2021 | India | 60 | 10 | Sprague-Dawley rats | 230–270 | N/M | M |
| Rinwa et al. [46] | 2010 | India | 80 | 10 | Swiss mice | 20–30 | N/M | M, F |
| Samy et al. [47] | 2015 | Egypt | 32 | 8 | Wistar rats | 220–250 | N/M | M |
| Shao et al. [48] | 2023 | China | 30 | 10 | C57BL/6J mice | 25–30 | 8 | M |
| Spinelli et al. [49] | 2015 | USA | 7 | 4, 3 | Offspring of Syn-GFP male mice and BDF1 female mice | N/M | 12 | M, F |
| Sun et al. [50] | 2020 | China | 15 | 5 | APP/PS1 mice | N/M | 24 | M |
| Sundaram et al. [51] | 2017 | Singapore | N/M | 3 | Offspring mice (p25Tg mice) | N/M | 24 | M, F |
| Tian et al. [52] | 2021 | China | 15 | 5 | APP/PS1, C57BL/6J mice | N/M | 24 | N/M |
| Wang et al. [53] | 2014 | China | 33 | 11 | APP/PS1 Double transgenic mice | N/M | 24 | M, F |
| Wang et al. [54] | 2019 | China | 6 | 3 | Sprague-Dawley rats | 188–218 | 7–8 | M |
| Xu et al. [55] | 2020 | China | 40 | 10 | C57BL/6 mice | N/M | 6–8 | M |
| Zheng et al. [56] | 2016 | China | N/M | ≤5 | Offspring mice (5 × FAD mice) | N/M | 16 | M |
| Studies | Oxidative Stress Markers | Inflammatory Markers | Cognitive Function Parameters |
|---|---|---|---|
| Agrawal et al., 2009 [28] | ↓ MDA, ↓ AChe, ↑GSH | - | ↓ Spatial learning Memory (Escape latency) |
| Awasthi et al., 2009 [29] | ↓ MDA, ↓AChe, ↓ROS, ↓NO↑GSH | - | ↓Spatial learning memory (Escape latency) ↑ Memory and learning |
| Bassani et al., 2017 [30] | - | - | ↓Short-term spatial memory, ↓Anxiety-like behavior ↓Spontaneous locomotion and exploratory behavior ↑Short-term recognition memory |
| Campisi et al., 2022 [31] | - | - | ↑Memory performance |
| ELBini-Dhouib et al., 2021 [32] | ↓ MDA, ↑AChe, ↑SOD, ↑CAT | ↓INF-γ, ↑ IL-4 | ↓ Anxiety-like behavior ↓Mark impairment in memory recognition |
| Fidelis et al., 2018 [33] | ↓ CAT ↑SOD, ↑RS, ↑SOD/CAT, NPSH↔ | - | ↓ Immobility behavior, ↓Immobility time ↑Locomotor activity, |
| Ishrat et al., 2009 [34] | ↓4-HNE/MDA, ↓H2O2, ↓PC, ↑GSSG, ↑GSH, ↑GPx, ↑GR, ↑Na+-K+ ATPase, ↑ChAT | - | ↑ Retention latency, ↑Spatial learning and memory, |
| Isik et al., 2008 [35] | ↓4-HNE | ↓IL-6, ↓NF-κB-p65 | ↑Spatial learning and memory, ↓ Cognitive impairment |
| Jearjaroen et al., 2020 [36] | - | ↑IGF-1 | ↓ Cognitive impairment, |
| Khurana et al., 2012 [37] | ↓MDA, ↓LPO ↑GSH, ↑AChe, | - | ↓ Cognitive impairment ↓Retention latency ↑ Step down latency |
| Kim et al., 2019 [38] | ↓ MDA, ↑GSH, ↑SOD | ↓Iba-1, ↑Synoptophysin, ↑PSD95, | ↑ Recognition memory ↑ Spatial memory |
| Kumar et al., 2023 [39] | ↓ AChe, ↑SOD | ↓TNF-α, | ↓Transfer latency, ↓Anxiety-like behavior ↑%alterations on memory function, |
| Lamichhane et al., 2024 [40] | - | - | ↑Spatial recognition memory, ↔ locomotive behavior |
| Li and Yu, 2015 [41] | - | - | ↓Escape latency, ↑Learning & memory ability |
| Lim et al., 2001 [42] | ↓ PC, | ↓IL-1β | - |
| Özaçmak & Özaçmak, 2010 [43] | ↓ MDA, ↑GSH, | - | - |
| Patel et al., 2020 [44] | ↓MDA, ↑GPx, ↓Ache, ↑SOD, ↑CAT, | - | ↓Memory impairment |
| Prathipati et al., 2021 [45] | ↓ MDA, ↓ AChe, ↑GSH, ↑SOD, ↑CAT, | - | ↓Transfer latency by spatial memory ↑% Spontaneous alterations |
| Rinwa et al., 2010 [46] | ↓ TBAR, ↓ AChe, ↑GSH | - | ↑Spatial learning and memory |
| Samy et al., 2015 [47] | ↓ MDA, ↓TBAR ↑GSH | ↓FasL, ↓Cas-8 | ↑ Spatial, Learning and memory, ↑Retention latency |
| Shao et al., 2023 [48] | ↑SOD | ↓TNF-α, ↓IL-6, ↓IL-1β, | ↑Learning & memory ability, ↑Spatial working memory, |
| Spinelli et al., 2015 [49] | - | ↑P-SYN, | ↑ Motor behavior |
| Sun et al., 2020 [50] | - | - | ↑Spatial learning and memory, |
| Sundaram et al., 2017 [51] | - | ↓TNF-α, ↓IL-1β, ↓MIP-1α ↔ TGF-β, | ↓ Learning memory, |
| Tian et al., 2021 [52] | - | - | ↑Hippocampal-dependent spatial learning and memory ability |
| Wang et al., 2014 [53] | - | - | ↑Memory and cognition function, |
| Wang et al., 2019 [54] | - | - | ↑Spatial learning and memory |
| Xu et al., 2020 [55] | ↑GSH, ↑SOD | - | ↑Spatial learning and memory, ↑Learning & memory function, |
| Zheng et al., 2016 [56] | - | - | ↑Spatial learning and memory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kehinde, S.A.; Lin, W.P.; Lay, B.B.; Phyo, K.Y.; San, M.M.; Pattanayaiying, R.; Chusri, S. Curcumin and Dementia: A Systematic Review of Its Effects on Oxidative Stress and Cognitive Outcomes in Animal Models. Int. J. Mol. Sci. 2025, 26, 7026. https://doi.org/10.3390/ijms26147026
Kehinde SA, Lin WP, Lay BB, Phyo KY, San MM, Pattanayaiying R, Chusri S. Curcumin and Dementia: A Systematic Review of Its Effects on Oxidative Stress and Cognitive Outcomes in Animal Models. International Journal of Molecular Sciences. 2025; 26(14):7026. https://doi.org/10.3390/ijms26147026
Chicago/Turabian StyleKehinde, Samuel Abiodun, Wai Phyo Lin, Bo Bo Lay, Khin Yadanar Phyo, Myat Mon San, Rinrada Pattanayaiying, and Sasitorn Chusri. 2025. "Curcumin and Dementia: A Systematic Review of Its Effects on Oxidative Stress and Cognitive Outcomes in Animal Models" International Journal of Molecular Sciences 26, no. 14: 7026. https://doi.org/10.3390/ijms26147026
APA StyleKehinde, S. A., Lin, W. P., Lay, B. B., Phyo, K. Y., San, M. M., Pattanayaiying, R., & Chusri, S. (2025). Curcumin and Dementia: A Systematic Review of Its Effects on Oxidative Stress and Cognitive Outcomes in Animal Models. International Journal of Molecular Sciences, 26(14), 7026. https://doi.org/10.3390/ijms26147026






