Dietary Flavonoids Vitexin and Isovitexin: New Insights into Their Functional Roles in Human Health and Disease Prevention
Abstract
1. Introduction
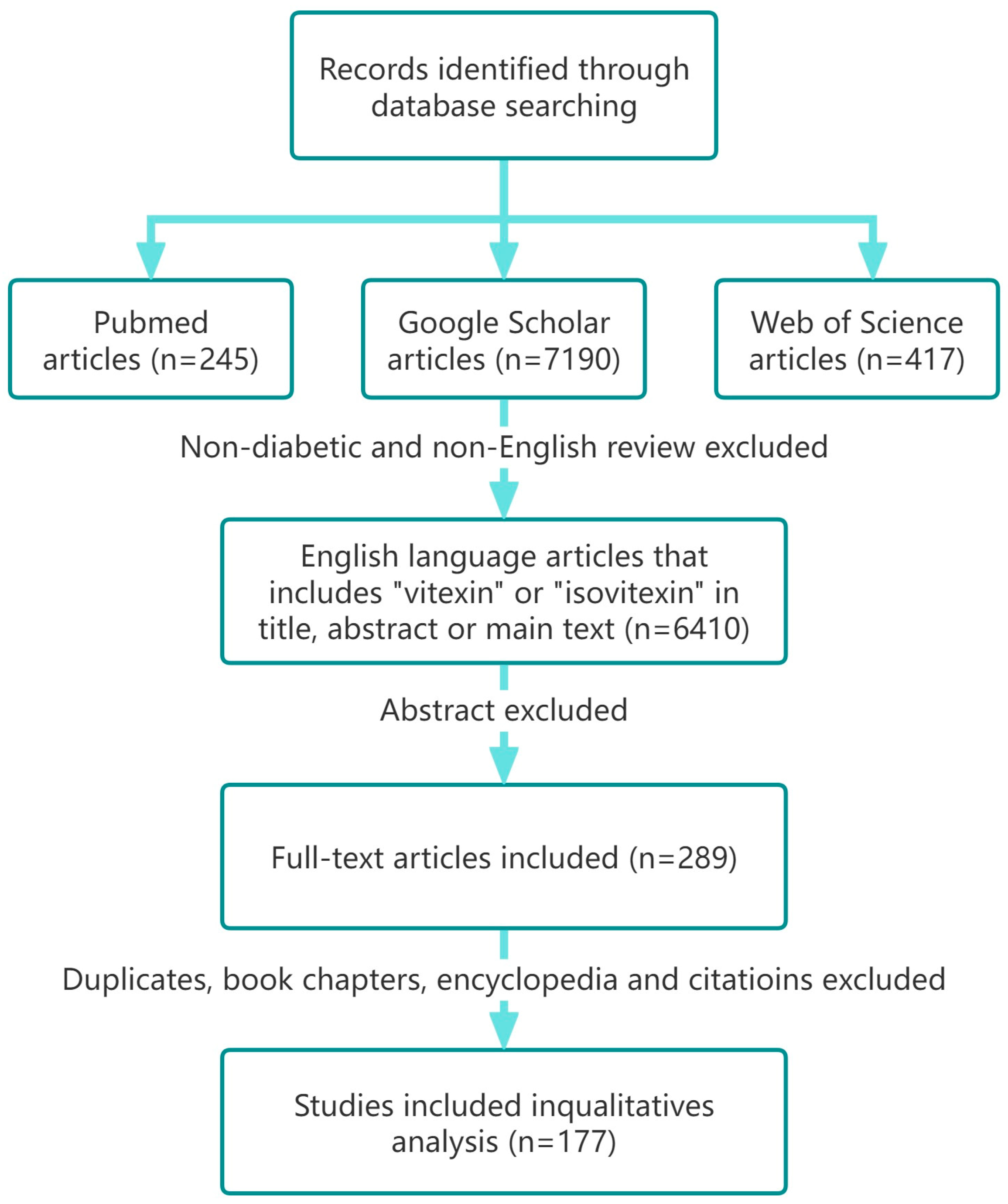
2. Methodology
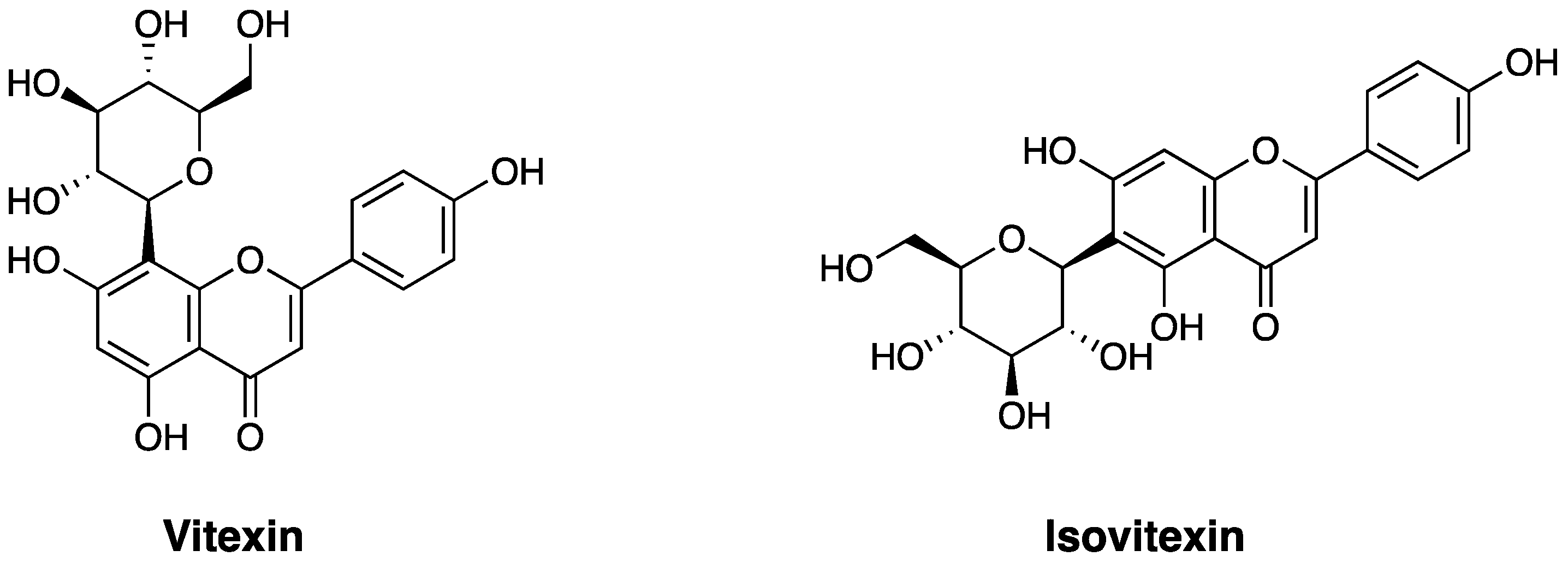
Chemical Structure and Sources
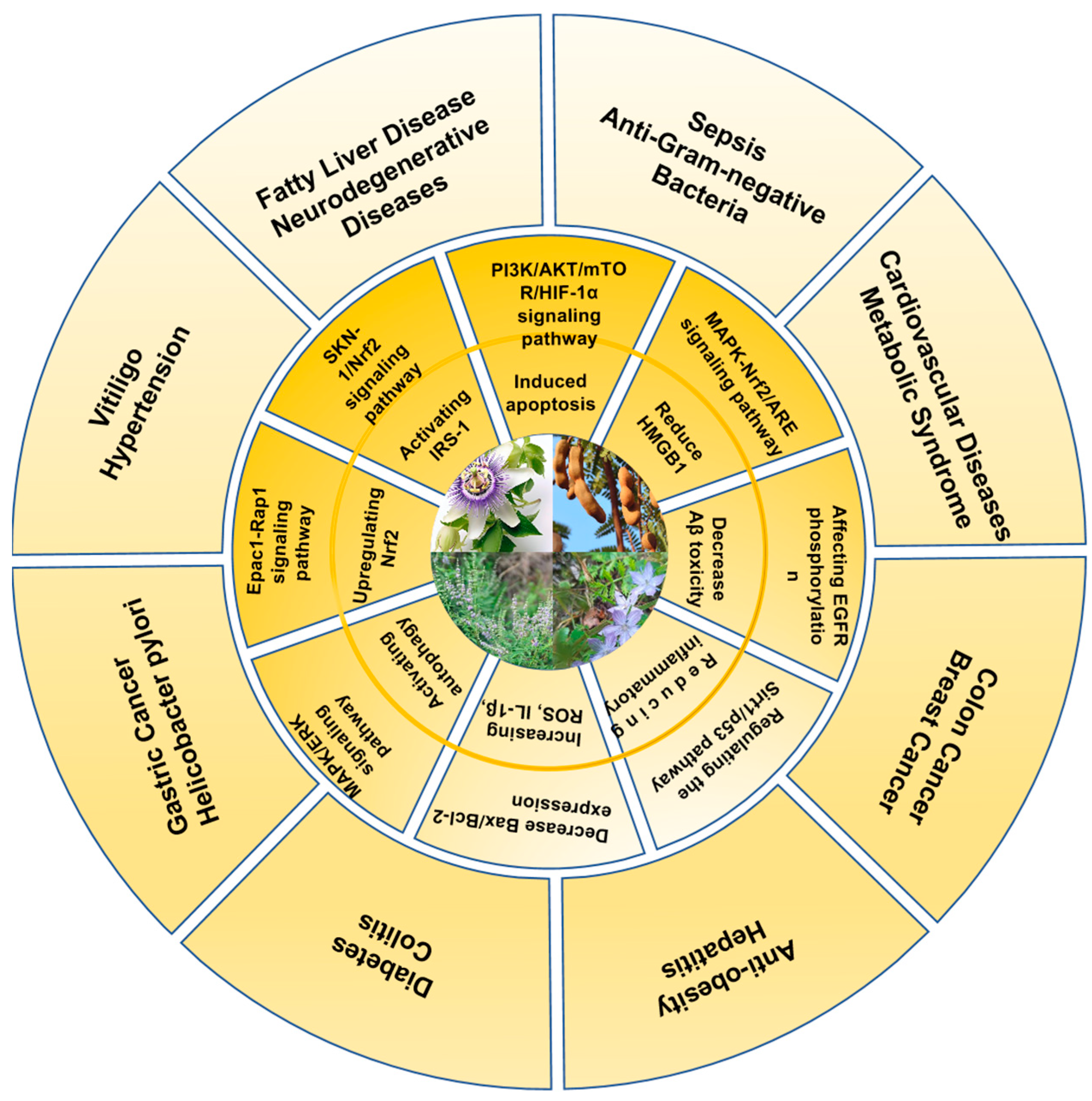
3. Health Promotion Effects of Vitexin and Isovitexin
3.1. Regulating Cardiovascular Protection
3.2. Regulating Blood Sugar and Combating Obesity
3.3. Lowering Blood Cholesterol and Treating Fatty Liver
3.4. Anticancer Effect
3.5. Antioxidant Effect
3.6. Anti-Inflammatory Effect
3.7. Neuroprotective Effect
3.8. Antimicrobial and Antibacterial Effect
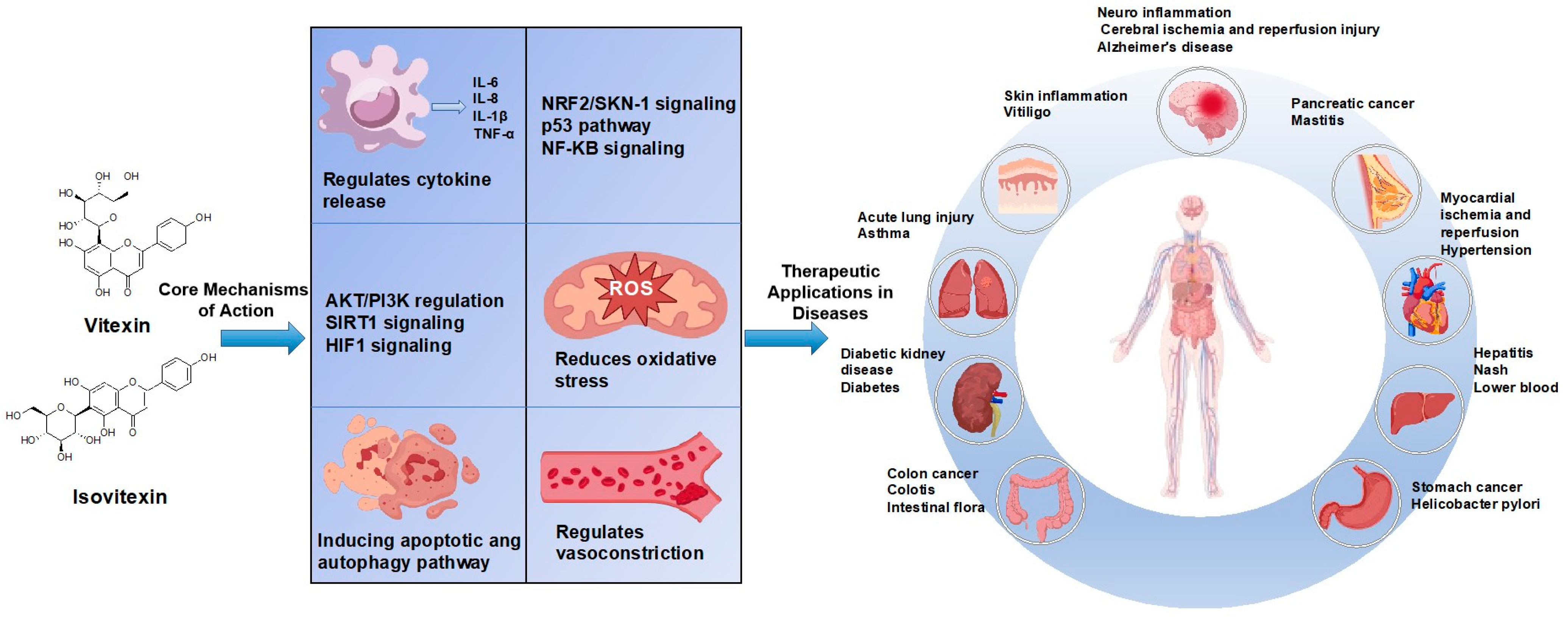
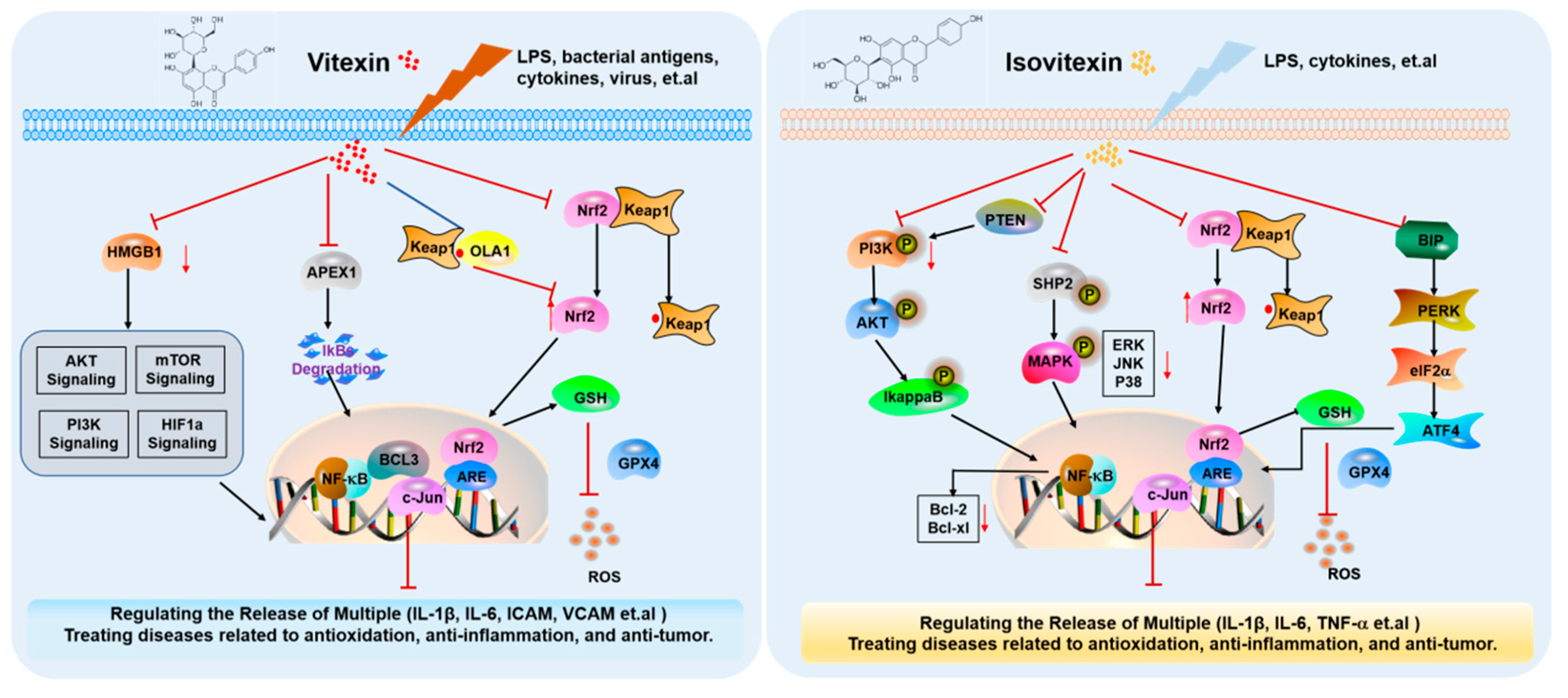
4. Mechanisms
5. Safety Profile and Toxicological Assessment of Vitexin and Isovitexin
6. Absorption and Metabolism of Vitexin and Isovitexin in Human
7. Bioavailability
8. Prospective
9. Conclusions
- Identification of molecular targets involved in neuroprotection using animal models.
- Investigation of potential synergistic effects and structure–activity relationships between vitexin, isovitexin, and other therapeutic agents.
Author Contributions
Funding
Conflicts of Interest
References
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ran, H.; Feng, Q.; Shen, Q.; Zhou, S.; Sun, Y.; Hou, D. Unveiling the differences between vitexin and isovitexin: From the perspective of sources, green advanced extraction technologies, biological activities, and safety. Food Chem. 2025, 415, 144600. [Google Scholar] [CrossRef] [PubMed]
- Abdulai, I.L.; Kwofie, S.K.; Gbewonyo, W.S.; Boison, D.; Puplampu, J.B.; Adinortey, M.B. Multitargeted effects of vitexin and isovitexin on diabetes mellitus and its complications. Sci. World J. 2021, 2021, 6641128. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Wang, X.; Tang, H.; Sun, F.; Zhu, H.; Huang, D.; Dong, L. Vitexin attenuates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction induced by mitochondrial dynamics imbalance. Biomed. Pharmacother. 2020, 124, 109849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ning, T.; Wang, H. Vitexin alleviates inflammation and enhances apoptosis through the regulation of the JAK/STAT/SOCS signaling pathway in the arthritis rat model. J. Biochem. Mol. Toxicol. 2022, 36, e23201. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Li, H.; Yi, J.; Zhang, J.; Che, H.; Cao, J.; Yang, L.; Zhu, C.; Jiang, W. Antioxidant properties of the mung bean flavonoids on alleviating heat stress. PLoS ONE 2011, 6, e21071. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.A.M.; Yariwake, J.H.; McCullagh, M. Distinction of the C-glycosylflavone isomer pairs orientin/isoorientin and vitexin/isovitexin using HPLC-MS exact mass measurement and in-source CID. Phytochem. Anal. 2005, 16, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Praveena, R.; Sadasivam, K.; Kumaresan, R.; Deepha, V.; Sivakumar, R. Experimental and DFT studies on the antioxidant activity of a C-glycoside from Rhynchosia capitata. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 103, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xue, W.; Ding, C.; Wang, C.; Xu, B.; Chen, S.; Zha, B.; Sun, Y.; Zhu, H.; Zhang, J.; et al. Vitexin mitigates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction via Epac1-Rap1 signaling. Oxid. Med. Cell. Longev. 2021, 2021, 9921982. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Yan, B.; Yu, W.Y.; Yao, X.; Ma, X.; Sheng, G.; Ma, Q. Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of FOXO3a. Exp. Ther. Med. 2016, 12, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Huang, J.; Su, Y.; Wang, F.; Kong, H.; Xin, H. Vitexin ameliorates preeclampsia phenotypes by inhibiting TFPI-2 and HIF-1α/VEGF in a l-NAME induced rat model. Drug Dev. Res. 2019, 80, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Choo, C.Y.; Sulong, N.Y.; Man, F.; Wong, T.W. Vitexin and isovitexin from the leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J. Ethnopharmacol. 2012, 142, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Saeting, O.; Chandarajoti, K.; Phongphisutthinan, A.; Hongsprabhas, P.; Sae-Tan, S. Water extract of mungbean (Vigna radiata L.) inhibits protein tyrosine phosphatase-1B in insulin-resistant HepG2 cells. Molecules 2021, 26, 1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jin, S.; Zhang, S.; Li, Y.Y.; Wang, H.; Chen, Y.; Lu, H. Vitexin protects against high glucose-induced endothelial cell apoptosis and oxidative stress via Wnt/β-catenin and Nrf2 signalling pathway. Arch. Physiol. Biochem. 2024, 130, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Yutharaksanukul, P.; Tangpromphan, P.; Tunsagool, P.; Sae-Tan, S.; Nitisinprasert, S.; Somnuk, S.; Nakphaichit, M.; Pusuntisumpun, N.; Wanikorn, B. Effects of purified vitexin and iso-vitexin from mung bean seed coat on antihyperglycemic activity and gut microbiota in overweight individuals’ modulation. Nutrients 2024, 16, 3017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, S.; Wang, H.; Chen, Y. Vitexin ameliorated diabetic nephropathy via suppressing GPX4-mediated ferroptosis. Eur. J. Pharmacol. 2023, 951, 175787. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, Q.; Gong, Y.; Zhuo, C.; Huang, J.; Tang, Q. Vitexin attenuates non-alcoholic fatty liver disease lipid accumulation in high fat-diet fed mice by activating autophagy and reducing endoplasmic reticulum stress in liver. Biol. Pharm. Bull. 2022, 45, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Sun, Q.; Xu, W.; He, Y.; Jin, W.; Yuan, L.; Gao, R. Vitexin ameliorates high fat diet-induced obesity in male C57BL/6J mice via the AMPKα-mediated pathway. Food Funct. 2019, 10, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Luo, W.; Chen, S.; Su, H.; Zhu, W.; Wei, Y.; Qiu, Y.; Long, Y.; Shi, Y.; Wei, J. Isovitexin alleviates hepatic fibrosis by regulating miR-21-mediated PI3K/Akt signaling and glutathione metabolic pathway: Based on transcriptomics and metabolomics. Phytomedicine 2023, 121, 155117. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, L.; Huang, H.; Wen, X.; Zhang, Y.; Zhang, R.; Huang, W. Vitexin inhibits TNBC progression and metastasis by modulating macrophage polarization through EGFR signaling. J. Immunother. 2024, 47, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zheng, Z.H.; Wan, T.; Wu, J.; Liao, C.W.; Sun, X.J. Vitexin inhibits gastric cancer growth and metastasis through HMGB1-mediated inactivation of the PI3K/AKT/mTOR/HIF-1α signaling pathway. J. Gastric Cancer 2021, 21, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, Y.; Shi, H.; Han, C.; Zhu, X.; Wang, C.; Wang, B.; Liu, J.; Shi, Y.; Hua, D. Investigating the molecular mechanism of vitexin targeting CDK1 to inhibit colon cancer cell proliferation via GEO chip data mining, computer simulation, and biological activity verification. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 398, 1637–1652. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shang, L.; Zhou, F.; Wang, S.; Liu, N.; Zhou, M.; Lin, Q.; Zhang, M.; Cai, Y.; Chen, G.; et al. Herba Patriniae and its component isovitexin show anti-colorectal cancer effects by inducing apoptosis and cell-cycle arrest via p53 activation. Biomed. Pharmacother. 2023, 168, 115690. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, X.; Zha, D.; Cai, F.; Zhang, W.; He, Y.; Huang, Q.; Zhuang, H.; Hua, Z. Apigenin potentiates TRAIL therapy of non-small cell lung cancer via upregulating DR4/DR5 expression in a p53-dependent manner. Sci. Rep. 2016, 6, 35468. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, X.; Cao, X.; Liu, L.; Qiu, Y.; Li, X.; Zhou, L.; Ning, Y.; Ren, K.; Cao, J. Isovitexin inhibits stemness and induces apoptosis in hepatocellular carcinoma SK-Hep-1 spheroids by upregulating miR-34a expression. Anticancer Agents Med. Chem. 2020, 20, 1654–1663. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-S.; Tang, X.-Y.; Su, W.; Li, X. Vitexin protects melanocytes from oxidative stress via activating MAPK-Nrf2/ARE pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yu, Z.; Zheng, Y.; Wang, L.; Qin, X.; Cheng, G.; Ci, X. Isovitexin exerts anti-inflammatory and anti-oxidant activities on lipopolysaccharide-induced acute lung injury by inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. Int. J. Biol. Sci. 2016, 12, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, D.; Tu, Y.; Sang, T.; Pan, T.; Lin, H.; Cai, C.; Jin, X.; Wu, F.; Xu, L.; et al. Vitexin attenuates autoimmune hepatitis in mouse induced by syngeneic liver cytosolic proteins via activation of AMPK/AKT/GSK-3β/Nrf2 pathway. Eur. J. Pharmacol. 2022, 917, 174720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, J.; Huang, Z.; Yin, B.; Umar, T.; Yang, C.; Zhang, X.; Jing, H.; Guo, S.; Guo, M.; et al. Vitexin mitigates Staphylococcus aureus-induced mastitis via regulation of ROS/ER stress/NF-κB/MAPK pathway. Oxid. Med. Cell Longev. 2022, 2022, 7977433. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, P.; Qu, X.; Liu, W. Isovitexin alleviates acute gouty arthritis in rats by inhibiting inflammation via the TLR4/MyD88/NF-κB pathway. Pharm. Biol. 2021, 59, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.Y.; Yu, P.R.; Hsu, C.C.; Lin, H.H.; Chen, J.H. The effect of isovitexin on lipopolysaccharide-induced renal injury and inflammation by induction of protective autophagy. Food Chem. Toxicol. 2023, 172, 113581. [Google Scholar] [CrossRef] [PubMed]
- Zolkiffly, S.Z.I.; Stanslas, J.; Abdul Hamid, H.; Mehat, M.Z. Ficus deltoidea: Potential inhibitor of pro-inflammatory mediators in lipopolysaccharide-induced activation of microglial cells. J. Ethnopharmacol. 2021, 279, 114309. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Shi, L. Vitexin improves cerebral ischemia-reperfusion injury by attenuating oxidative injury and ferroptosis via Keap1/Nrf2/HO-1 signaling. Neurochem. Res. 2023, 48, 980–995. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Liu, N.; Jiang, Y.P.; Yang, J.M.; Zheng, J.; Sun, M.; Li, Y.X.; Sun, T.; Wu, J.; Yu, J.Q. Vitexin alleviates streptozotocin-induced sexual dysfunction and fertility impairments in male mice via modulating the hypothalamus-pituitary-gonadal axis. Chem. Biol. Interact. 2019, 297, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Nurdiana, S.; Goh, Y.M.; Ahmad, H.; Dom, S.M.; Syimal’ain Azmi, N.; Noor Mohamad Zin, N.S.; Ebrahimi, M. Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Complement. Altern. Med. 2017, 17, 290. [Google Scholar] [CrossRef]
- Nurdiana, S.; Goh, Y.M.; Hafandi, A.; Dom, S.M.; Nur Syimal’ain, A.; Noor Syaffinaz, N.M.; Ebrahimi, M. Improvement of spatial learning and memory, cortical gyrification patterns and brain oxidative stress markers in diabetic rats treated with Ficus deltoidea leaf extract and vitexin. J. Tradit. Complement. Med. 2017, 8, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, M.; Chang, K.; Fang, C.; Lin, Y.; Tseng, H. Vitexin mitigates haloperidol-induced orofacial dyskinesia in rats through activation of the Nrf2 pathway. Int. J. Mol. Sci. 2024, 25, 10206. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Li, P.; Li, P.; Yan, R.; Zhang, X.Q.; Wang, Y.; Zhang, X.T.; Ye, W.C.; Zhang, Q.W. α-Glucosidase inhibitory effect and simultaneous quantification of three major flavonoid glycosides in Microctis folium. Molecules 2013, 18, 4221–4232. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Wang, H.; Sheng, J.; Zhang, W.; Lei, J.; Gan, W.; Cai, F.; Yang, Y. Vitexin attenuates chronic kidney disease by inhibiting renal tubular epithelial cell ferroptosis via NRF2 activation. Mol. Med. 2023, 29, 147. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Chen, J.; Kim, J.T.; Zhou, Y.; Moon, J.H.; Lee, S.B.; Park, H.J.; Lee, H.J. Suppression of adipogenesis and fat accumulation by vitexin through activation of hedgehog signaling in 3T3-L1 adipocytes. J. Med. Food 2022, 25, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, Z.; Xiao, S.; Du, Q.; Zhang, M.; Song, H.; Zhao, C.; Zheng, L. Protective effect of vitexin against high fat-induced vascular endothelial inflammation through inhibiting trimethylamine N-oxide-mediated RNA m6A modification. Food Funct. 2024, 15, 6988–7002. [Google Scholar] [CrossRef] [PubMed]
- Mackowiak, B.; Fu, Y.; Maccioni, L.; Gao, B. Alcohol-associated liver disease. J. Clin. Investig. 2024, 134, e176345. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Duan, S.; Guan, T.; Yuan, X.; Lin, J.; Hou, S.; Lai, X.; Huang, S.; Du, X.; Chen, S. Vitexin protects against ethanol-induced liver injury through Sirt1/p53 signaling pathway. Eur. J. Pharmacol. 2020, 873, 173007. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, S.; Joshi, A.; Malik, S.; Boppana, R.; Ghaskadbi, S. Vitexin alleviates non-alcoholic fatty liver disease by activating AMPK in high fat diet fed mice. Biochem. Biophys. Res. Commun. 2019, 519, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Ejiyi, C.J.; Qin, Z.; Agbesi, V.K.; Yi, D.; Atwereboannah, A.A.; Chikwendu, I.A.; Bamisile, O.F.; Bakanina Kissanga, G.-M.; Bamisile, O.O. Advancing cancer diagnosis and prognostication through deep learning mastery in breast, colon, and lung histopathology with ResoMergeNet. Comput. Biol. Med. 2024, 185, 109494. [Google Scholar] [CrossRef] [PubMed]
- Najafipour, R.; Momeni, A.M.; Mirmazloomi, Y.; Moghbelinejad, S. Vitexin induces apoptosis in MCF-7 breast cancer cells through the regulation of specific miRNAs expression. Int. J. Mol. Cell Med. 2022, 11, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.S.; Antonini, E.; Palma, F.; Mari, M.; Ninfali, P. Antiproliferative activity of vitexin-2-O-xyloside and avenanthramides on CaCo-2 and HepG2 cancer cells occurs through apoptosis induction and reduction of pro-survival mechanisms. Eur. J. Nutr. 2018, 57, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Mołdoch, J.; Paduch, R.; Strzemski, M.; Szkutnik, J.; Tyszczuk-Rotko, K.; Dresler, S.; Szczepanek, D.; Wójciak, M. Polyphenolic composition of Carlina acaulis L. extract and cytotoxic potential against colorectal adenocarcinoma and cervical cancer cells. Molecules 2023, 28, 6148. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Paul, S.; Jakhar, R.; Khan, I.; Kang, J.I.; Kim, H.M.; Yun, J.W.; Lee, S.J.; Cho, H.J.; Lee, H.G.; et al. Vitexin confers HSF-1 mediated autophagic cell death by activating JNK and ApoL1 in colorectal carcinoma cells. Oncotarget 2017, 8, 112426–112441. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, B.; Yuan, X.; Lu, Y.; Hu, J.; Gao, J.; Lin, J.; Liang, J.; Hou, S.; Chen, S. Vitexin prevents colitis-associated carcinogenesis in mice through regulating macrophage polarization. Phytomedicine 2021, 83, 153489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, J.; Chen, S.; Lin, N.; Xu, S.; Miao, J.; Zhang, J.; Chen, C.; Yuan, X.; Xie, Z.; et al. Discovery of vitexin as a novel VDR agonist that mitigates the transition from chronic intestinal inflammation to colorectal cancer. Mol. Cancer 2024, 23, 196. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, C.; Wu, J.; Du, B.; Liu, Q.; Sui, Y.; Song, C.; Zhang, J.; Tang, H.; Chen, J. Ononin inhibits triple-negative breast cancer lung metastasis by targeting the EGFR-mediated PI3K/Akt/mTOR pathway. Sci. China Life Sci. 2024, 67, 1849–1866. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chang, Y.; Tsai, K.; Shen, C.; Lee, S. Vitexin suppresses high-glucose-upregulated adhesion molecule expression in endothelial cells through Inhibiting NF-κB signaling pathway. ACS Omega 2024, 9, 32727–32734. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liang, T.; Zhang, Y.; Huang, K.; Yang, S.; Lv, H.; Chen, Y.; Zhang, C.; Guan, X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant 2734, anti-inflammatory and gut microbiota modulating properties. Free Radic. Biol. Med. 2021, 171, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Shen, H.; Tian, Z.; Kang, M.; Ma, J.; He, Q.; Wang, J.; Zhang, Y.; Deng, Y.; Wang, D. Protective effect of hawthorn vitexin on the ethanol-injured DNA of BRL-3A hepatocytes. Medicine 2021, 100, e28228. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Li, R.; Xu, T.; Zhang, Z.; Zheng, D.; Xia, Z.; Wu, T.; Pan, S.; Xu, X. Vitexin and isovitexin delayed ageing and enhanced stress-resistance through the activation of the SKN-1/Nrf2 signaling pathway. Int. J. Food Sci. Nutr. 2023, 74, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Sheng, Y.; Tian, Y.; Wang, C. Vitexin regulates heat shock protein expression by modulating ROS levels thereby protecting against heat-stress-induced apoptosis. Molecules 2023, 28, 7639. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Simon, J.E.; Wang, M.; Wu, Y.; Huang, Y.; Wu, Q. Kinkéliba (Combretum micranthum) leaf extract alleviates skin inflammation: In vitro and in vivo study. Molecules 2023, 28, 1791. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Mu, L.; Zhu, L.; Chang, X.; Nie, L.; Wang, L.; Li, G. Lycium barbarum polysaccharides attenuate cardiovascular oxidative stress injury by enhancing the Keap1/Nrf2 signaling pathway in exhaustive exercise rats. Mol. Med. Rep. 2021, 24, 643. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.; Xing, Q.; Li, N.; Deng, Z.; Pan, C.; Liu, X.; Zheng, L. Protective effect of vitexin against IL-17-induced vascular endothelial inflammation through Keap1/Nrf2-dependent signaling pathway. Mol. Nutr. Food Res. 2024, 68, e2300331. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Ghosh, P.; Bishayi, B. Vitexin along with verapamil downregulates efflux pump P-glycoprotein in macrophages and potentiate M1 to M2 switching via TLR4-NF-κB-TNFR2 pathway in lipopolysaccharide treated mice. Immunobiology 2024, 229, 152767. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, S.; Pang, L.; Feng, Z.; Su, H.; Zhu, W.; Wei, J. Isovitexin protects against acute liver injury by targeting PTEN, PI3K and BiP via modification of m6A. Eur. J. Pharmacol. 2022, 917, 174749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, F.; Chen, Z.; Chen, Y.; Yuan, J.; Xiong, Q.; Hou, S.; Huang, S.; Liu, C.; Liang, J. Vitexin protects against dextran sodium sulfate-induced colitis in mice and its potential mechanisms. J. Agric. Food Chem. 2022, 70, 12041–12054. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, L.; Wang, S.; Sun, Q.; Zhang, Y.; Huang, K.; Guan, X. Regulation of gut microbiota and alleviation of DSS-induced colitis by vitexin. Eur. J. Nutr. 2023, 62, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Gao, J.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef] [PubMed]
- Matar, A.; Damianos, J.A.; Jencks, K.J.; Camilleri, M. Intestinal barrier impairment, preservation, and repair: An update. Nutrients 2024, 16, 3494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, Z.; Wang, W.; Wang, L.; Cao, F.; Zhao, L.; Fang, X. Isovitexin inhibits ginkgolic acids-induced inflammation through downregulating SHP2 activation. Front. Pharmacol. 2021, 12, 630320. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.H.; Zhang, X.Q.; Wang, N.D.; Zheng, M.D.; Yan, J. Vitexin protects against ischemia/reperfusion-induced brain endothelial permeability. Eur. J. Pharmacol. 2019, 853, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Yang, Q.; Xiang, Y.; Zhang, Y.; Wan, J.; Liu, S.; Li, N.; Peng, W. Nose to brain drug delivery—A promising strategy for active components from herbal medicine for treating cerebral ischemia reperfusion. Pharmacol. Res. 2020, 159, 104795. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhen, Y.; Wu, X.; Jiang, Q.; Li, X.; Chen, Z.; Zhang, G.; Dong, L. Vitexin protects brain against ischemia/reperfusion injury via modulating mitogen-activated protein kinase and apoptosis signaling in mice. Phytomedicine 2015, 22, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Malar, D.S.; Prasanth, M.I.; Jeyakumar, M.; Balamurugan, K.; Devi, K.P. Vitexin prevents Aβ proteotoxicity in transgenic Caenorhabditis elegans model of Alzheimer’s disease by modulating unfolded protein response. J. Biochem. Mol. Toxicol. 2021, 35, e22632. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, N.K.S.; Almeida, M.R.S.; Pontes, F.M.M.; Barcelos, M.P.; Silva, G.M.; de Paula da Silva, C.H.T.; Cruz, R.A.S.; da Silva Hage-Melim, L.I. Molecular docking, physicochemical properties, pharmacokinetics and toxicity of flavonoids present in Euterpe oleracea Martius. Curr. Comput. Aided Drug Des. 2021, 17, 589–617. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, X.; Li, S.; Li, T. Vitexin-rhamnoside encapsulated with zein-pectin nanoparticles relieved high-fat diet induced lipid metabolism disorders in mice by altering the gut microbiota. Int. J. Biol. Macromol. 2024, 264, 130704. [Google Scholar] [CrossRef] [PubMed]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.S.; Abdel-Aziz, M.M.; Abu-Baker, M.S.; Saad, A.M.; Mohamed, M.A.; Ghareeb, M.A. Antibacterial and potential antidiabetic activities of flavone C-glycosides isolated from Beta vulgaris subspecies cicla L. var. Flavescens (Amaranthaceae) cultivated in Egypt. Curr. Pharm. Biotechnol. 2019, 20, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Sinan, K.I.; Zengin, G.; Mahomoodally, M.F.; Sadeer, N.B.; Etienne, O.K.; Cziáky, Z.; Jekő, J.; Glamocilja, J.; Sokovic, M.; et al. Identification of chemical profiles and biological properties of Rhizophora racemosa G. Mey. extracts obtained by different methods and solvents. Antioxidants 2020, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Król-Kogus, B.; Głód, D.; Hałasa, R.; Krauze-Baranowska, M.; Pobłocka-Olech, L. 2D LC as a tool for standardization of Foenugraeci semen extracts containing compounds with anti-Helicobacter pylori activity. Food Funct. 2021, 12, 2686–2692. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, R.; Li, W.; Du, Q.; Deng, Z.; Fan, Y.; Zheng, L. Identification of OLA1 as a novel protein target of vitexin to ameliorate dextran sulfate sodium-induced colitis with tissue thermal proteome profiling. J. Agric. Food Chem. 2023, 71, 16057–16066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.R.; Yang, F.F.; Cui, Q.; Wang, D.; Zhou, Y.; Li, Y.S.; Zhang, Y.P.; Tang, R.Z.; Yao, W.J.; Tang, R.Z. Vitexin inhibits APEX1 to counteract the flow-induced endothelial inflammation. Proc. Natl. Acad. Sci. USA 2021, 118, e2115158118. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.I.G.; Rios-Santos, F.; Balogun, S.O.; Martins, D.T.d.O. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine 2016, 23, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, S.; Liu, J.; Chen, Y.; Zhong, S.; Lian, D.; Liang, J.; Huang, S.; Hou, S. Vitexin regulates angiogenesis and osteogenesis in ovariectomy-induced osteoporosis of rats via the VDR/PI3K/AKT/eNOS signaling pathway. J. Agric. Food Chem. 2023, 71, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Zhang, Q.; Wang, B.; Zhang, M.; Xu, Y.; Li, S.; Zhao, Y.; Yu, Z. Plasma pharmacokinetics, bioavailability, and tissue distribution of four C-glycosyl flavones from mung bean (Vigna radiata L.) seed extracts in rat by ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2017, 65, 5570–5580. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lv, H.; Chen, Y.; Song, H.; Zhang, Y.; Wang, S.; Luo, L.; Guan, X. N-trimethyl chitosan coated targeting nanoparticles improve the oral bioavailability and antioxidant activity of vitexin. Carbohydr. Polym. 2022, 286, 119273. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, T.; Li, S. Encapsulation of vitexin-rhamnoside based on zein/pectin nanoparticles improved its stability and bioavailability. Curr. Res. Food Sci. 2022, 6, 100419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Xia, X.; Adu-Frimpong, M.; Shen, X.; He, Q.; Rong, W.; Shi, F.; Cao, X.; Ji, H.; et al. Vitexin loaded mixed polymeric micelles: Preparation, optimization, evaluation and anti-osteoporotic effect. Biomed. Mater. 2023, 18, 045005. [Google Scholar] [CrossRef] [PubMed]
- Yoksan, R.; Towongphaichayonte, P. Vitexin-loaded poly(ethylene glycol) methyl ether-grafted chitosan/alginate nanoparticles: Preparation, physicochemical properties and in vitro release behaviors. J. Sci. Food Agric. 2024, 104, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.; Iqbal, A.; Rana, N.F.; Fatima, M.; Maryam, T.; Batool, F.; Rehman, Z.; Menaa, F.; Azhar, S.; Nawaz, A.; et al. A novel Sprague-Dawley rat model presents improved NASH/NAFLD symptoms with PEG coated vitexin liposomes. Int. J. Mol. Sci. 2022, 23, 3131. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Aidhen, I.S. Convenient synthesis of 3-glycosylated isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
| Vitexin/ Isovitexin | Biological Activities | Cell/Animal | Dosage | Mechanisms and Pathways | Targets | References |
|---|---|---|---|---|---|---|
| Vitexin | Myocardial ischemia/reperfusion (I/R) injury | Isolated Sprague-Dawley rat hearts, H9c2 cells | 10 μM | Reducing ROS levels; improving mitochondrial activity, mitochondrial membrane potential, and ATP content; markedly increasing MFN2 expression and reducing the recruitment of Drp1 in mitochondria. | MFN2, Drp1, Epac1-Rap1 | [4] |
| Inhibiting ischemic myocardial mitochondrial dysfunction and reducing cardiomyocyte apoptosis by regulating Epac1-Rap1 signaling. | [9] | |||||
| Vitexin | Protect against DOX-induced acute cardiotoxicity | Rats | 30 mg/kg | Vitexin induced elevated FOXO3a protein expression levels, by suppressing oxidative stress, inflammation, and apoptotic signals. | FOXO3a | [10] |
| Vitexin | Pre-eclampsia | Pregnant rats | 45–60 mg/kg | Decreased sFlt-1, increased PlGF, and alleviated oxidative stress |
HIF-1α,
VEGF | [11] |
| Vitexin/ Isovitexin | Diabetes, overweight | Diabetic rats | 1 mg/kg | Inhibits the negative regulator of insulin signaling, protein tyrosine phosphatase (PTP)-1B, inhibits α-amylase and α-glucosidase. | PTP-1B | [12] |
| Vitexin/ Isovitexin | HepG2 | [13] | ||||
| Vitexin | HUVECs | 5–100 μM | Vitexin disrupted Wnt/β-catenin signaling pathway, vitexin activated nuclear factor-erythroid 2-related factor 2 (Nrf2) in HUVEC under high glucose. | Nrf2 | [14] | |
| Vitexin/ isovitexin | Diabetes, overweight | HepG2 cells within an insulin-resistant system | Regulating glycemia, through changes in anti-hyperglycemic activity and in the gut microbiota in overweight individuals. | [15] | ||
| Vitexin | Diabetic nephropathy | HK-2 cells/DN rat | 0–40 μM | Vitexin could alleviate diabetic nephropathy by attenuated ferroptosis via activating GPX4. | GPX4 | [16] |
| Vitexin | Non-alcoholic fatty liver disease | NAFLD mice | 6 mg/kg | Vitexin degraded lipids in HFD-induced NAFLD mice liver by inducing autophagy and restoring both ER and mitochondrial biological proteins. | [17] | |
| CSHFD mice | 40 mg/kg | Inhibit TLR4/NF-κB signaling and reduce fatty acid synthesis proteins. | [2] | |||
| Vitexin | Obesity | C57BL/6J, 3T3-L1 adipocytes | 30 mg/kg | Vitexin may prevent HFD-induced obesity/adipogenesis via the AMPKα mediated pathway. | AMPKα | [18] |
| Isovitexin | Liver fibrosis | Hepatic stellate cell models | Regulation of miR-21, targeting PTEN-Akt signaling and the GSH metabolic pathway. | PTEN | [19] | |
| Vitexin | Triple-negative breast cancer | Vitexin promoted M1 polarization and suppressed M2 polarization, affecting EGFR phosphorylation and downstream signaling. | EGFR | [20] | ||
| Vitexin | Gastric cancer | Nude mice/GC cells | 2 mg/kg 10–160 μM | Vitexin inhibited the malignant progression of GC in vitro and in vivo by suppressing HMGB1-mediated activation of PI3K/Akt/HIF-1α signaling pathway. | HMGB-1 | [21] |
| Vitexin | Colon cancer | HCT-116 cells | 1–300 μM | Inhibit colon cancer HCT-116 cell proliferation by suppressing CDK1/cyclin B expression, leading to cell cycle arrest in the G2/M phase. | [22] | |
| Isovitexin | Colon cancer | Promoted apoptosis and suppressed cell proliferation by activating the p53 signaling pathway. | [23] | |||
| Vitexin/ isovitexin | Non-small cell lung cancer cells | A549/ H1299 cells, nude mice | 1–120 μM | Suppressed NF-κB, AKT and ERK activation. | [24] | |
| Vitexin | A549 cells, nude mice | 0–40 μM | Reduced the levels of p-PI3K, p-Akt, and p-mTOR. | [25] | ||
| Isovitexin | Hepatocarcinoma | SK-Hep-1 cells | Mediated miR-34a upregulation induces apoptosis and suppresses the stemness of SK-SC. | miR-34a | [26] | |
| Vitexin | Vitiligo | human melanocyte PIG1 | 0–40 μM | Protected melanocytes from oxidative stress by activating MAPK-Nrf2/ARE signaling pathway. | Nrf2 | [27] |
| Isovitexin | Acute lung injury | RAW 264.7 cells | 0–50 μM | Inhibiting MAPK and NF-κB and activating HO-1/Nrf2 pathways. | Nrf2 | [28] |
| Vitexin | Autoimmune hepatitis | EAH mice | 5 mg/kg | Vitexin ameliorated hepatic injury in EAH mice through activation of the AMPK/AKT/GSK-3β pathway and upregulation of the Nrf2 gene. | Nrf2 | [29] |
| Vitexin | Mastitis | MAC-T cells | 20 μM | Vitexin inhibited the production of ROS through promoting PPARγ, increased the activity of antioxidant enzymes, and reduced inflammatory cytokines and apoptosis by alleviating ER stress and inactivation MAPKs and NF-κB signaling pathway. | PPARγ | [30] |
| Isovitexin | Acute gouty arthritis | Sprague-Dawley rats | 100 mg/kg | Isovitexin ameliorates joint inflammation in acute GA via the TLR4/MyD88/NF-κB pathway. | TLR4 | [31] |
| Isovitexin | Chronic kidney disease | SV40-MES-13 cells/C57BL/6 mice | 0–50 μM, 5 mg/kg | Ameliorated renal injury, inflammation, and increased protected autophagy by anti-ROS production, anti-inflammation, and anti-pyroptosis. | [32] | |
| Vitexin/ isovitexin | Alzheimer’s disease | Microglial cells | 0.1–100 μg/mL | Mediating the nuclear factor-kappa B (NF-κB) signaling pathway. | NF-κB | [33] |
| Vitexin | Cerebral ischemia/reperfusion | Rat | 50 mg/kg | Protect the neuron cells and brain related with the Keap1/Nrf2/HO-1 signaling pathway. | Keap1, Nrf2 | [34] |
| Pharmacological Effect | Compound | Mechanism/Pathway | Target Molecules | Reference |
|---|---|---|---|---|
| Anti-inflammatory | Vitexin and Isovitexin | Inhibits NF-κB, activates Nrf2/HO-1, AMPK/AKT/GSK-3β | TNF-α, IL-6, IL-1β, Nrf2, ICAM-1, VCAM | [28,29,63] |
| Antioxidant | Isovitexin | Activates HO-1/Nrf2, reduces ROS, inhibits MAPK | ROS, GPx, SOD, HO-1, CAT | [28,32] |
| Anti-cancer | Vitexin | Inhibits PI3K/Akt, promotes apoptosis, suppresses HMGB1, modulates Bcl-2/Bax ratio | HMGB1, caspase-3, CDK1, Bcl-2, Bax | [21,22,25] |
| Hepatoprotective | Vitexin | Modulates Sirt1/p53, reduces apoptosis and lipid accumulation, activates AMPK, enhances IRS-1/AKT signaling | Sirt1, p53, TG, ALT, AST, PPARγ, SREBP-1c | [44,47] |
| Neuroprotective | Vitexin | Regulates HIF-1-α, MAPK, Keap1/Nrf2, AKT/mTOR, reduces inflammation | VEGF, MMPs, Bcl-2, Bax, caspase-3 | [34,71,73] |
| Cardioprotective | Vitexin | Regulates Epac1-Rap1 pathway, FOXO3a, MAPK/ERK | FOXO3a, Epac1, Drp1, MFN2 | [4,9,10] |
| Antidiabetic | Vitexin and Isovitexin | Inhibits α-glucosidase/α-amylase, promotes GLUT4, modulates gut microbiota | PTP-1B, GLUT4, GPx4, Nrf2 | [12,15,16,40] |
| Anti-obesity | Vitexin | Activates AMPKα, inhibits C/EBPα, FAS, activates Hedgehog signaling | AMPKα, C/EBPα, FAS | [17,18,41] |
| Anti-fibrotic | Isovitexin | Suppresses PI3K/Akt, modulates miR-21 and GSH pathway | miR-21, PI3K, GSH, PTEN | [19] |
| Antimicrobial | Vitexin | Inhibits H+/K+-ATPase, suppresses MPO and biofilm formation, interferes with bacterial efflux pumps | MPO, H+/K+-ATPase | [77,79,80] |
| Nano Types | Efficacy Tested | Model Type | Results | References |
|---|---|---|---|---|
| Vitexin-loaded bilayer nanoparticles by the assembly of soybean peptides and the coating of goblet cell targeting peptide CSKSSDYQC (CSK) coupled N-trimethyl chitosan (TMC) | The bilayer nanoparticles could protect vitexin from being released in stomach and promote sustained release in intestine | In vitro | Bioaccessibility and bioavailability of vitexin was significantly increased by the bilayer nanoparticles and vitexin exhibited better antioxidant activity after encapsulation. | [87] |
| Encapsulated by the zein-pectin nanoparticles system | Nanoparticles exhibited significant slow-release properties and the highest absorption rate in the duodenal segment of rats | In vivo/In vitro | It provides a theoretical and technical approach for the construction of vitexin delivery system with sustained-release properties and higher bioavailability | [88] |
| Vitexin (Vi)-loaded D-ɑ-tocopherol polyethylene glycol succinate, polyvinylpyrrolidone K30, and sodium cholate-mixed micelles | Vi-MMs exhibited enhanced bioavailability and anti-osteoporotic effect | In vivo | The oral bioavailability of Vi-MMs was increased by 5.6-fold compared to free vitexin. | [89] |
| Vitexin into poly(ethylene glycol) methyl ether-grafted chitosan (mPEG-g-CTS)/alginate (ALG) polyelectrolyte complex nanoparticles. | The gastrointestinal digestion of vitexin increased by encapsulating into mPEG-g-CTS/ALG nanoparticles. | In vitro | Nanoparticles are suitable for oral intestinal-specific delivery systems. | [90] |
| Vitexin-encapsulated liposomes were synthesized by the ‘thin-film hydration’ method | VLP-treated group also showed better results up to some extent. | In vivo | Liposomal encapsulation of vitexin and subsequent PEG coating to be a substantial strategy for treating liver cirrhosis through oral drug delivery. | [91] |
| Vitexin-rhamnoside (VR) and zein-VR-pectin nanoparticles (VRN) | Alleviating chronic inflammation and hepatic injury in HFD mice. | In vivo | Provided new evidence that nanoparticles enhance the bioavailability of vitexin bioactive ingredients. | [76] |
| Vitexin/ Isovitexin | Disease | Model | Change at Genus Levels | Results | References |
|---|---|---|---|---|---|
| Vitexin/ Isovitexin | Overweight | Simulation of Human Gut Model | Adlercreutzia, Terrisporobactel, Promicromonospor, Pseudonocardia, Anaerostipes, Akkermansia, Alistipes, Parabacteroides, Enterocloster, Peptacetobacter, Collinsella, Paraclostridium, Duncaniella, Streptococcus, Gillisia | Industry can use this optimal ratio to formulate more effective functional ingredients for functional foods and create nutraceuticals designed to reduce the risk of T2DM in overweight individuals. | [15] |
| Vitexin | Acute colitis | An acute colitis mice model | Veillonella, Terrisporobacter, Klebsiella, Paeniclostridium, Parabacteroides, Flavonifractor, Blautia | Vitexin could alleviate colitis by regulating gut microbiota and attenuating gut inflammation. | [67] |
| Vitexin | Neuro-inflammation | Mice model | Akkermansia, Lachnospiraceae | Vitexin exerted neural protective effects via anti-oxidant, anti-inflammatory, and gut microbiota modulating properties. | [57] |
| Vitexin | Lipid metabolism disorders | Rombousia and Faecalibaculum | Vitexin can regulate the gut microbiota and thus improve lipid metabolism. | [76] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, W.; Cheng, J.; Xu, B. Dietary Flavonoids Vitexin and Isovitexin: New Insights into Their Functional Roles in Human Health and Disease Prevention. Int. J. Mol. Sci. 2025, 26, 6997. https://doi.org/10.3390/ijms26146997
Yan W, Cheng J, Xu B. Dietary Flavonoids Vitexin and Isovitexin: New Insights into Their Functional Roles in Human Health and Disease Prevention. International Journal of Molecular Sciences. 2025; 26(14):6997. https://doi.org/10.3390/ijms26146997
Chicago/Turabian StyleYan, Weiqi, Junying Cheng, and Baojun Xu. 2025. "Dietary Flavonoids Vitexin and Isovitexin: New Insights into Their Functional Roles in Human Health and Disease Prevention" International Journal of Molecular Sciences 26, no. 14: 6997. https://doi.org/10.3390/ijms26146997
APA StyleYan, W., Cheng, J., & Xu, B. (2025). Dietary Flavonoids Vitexin and Isovitexin: New Insights into Their Functional Roles in Human Health and Disease Prevention. International Journal of Molecular Sciences, 26(14), 6997. https://doi.org/10.3390/ijms26146997







