1. Introduction
Soybean is one of the most important crops that accumulates high amounts of protein and oil in its seeds [
1]. Its protein and oil content are about 40 and 20%, respectively. Most of the soybeans produced in the US are primarily used in animal feed. Soybeans are processed to first extract oil and residual protein meal is subjected to additional processing to yield defatted soybean meal. Soybean meal (SBM), with its complete amino acid profile, is an excellent protein source for livestock [
2]. Importantly, soybeans are very productive and potentially more environmentally sustainable, due to their ability to fix nitrogen in association with soil-dwelling rhizobia. Despite these advantages, SBM cannot be directly used for animal feed mixtures, due to the presence of various antinutritional compounds, which reduce animal weight gain [
3,
4,
5].
Among the antinutritional compounds identified in soybeans, proteinase inhibitors pose a challenge due to their interference with the digestive enzymes in animals, specifically trypsin and chymotrypsin [
6,
7,
8]. The two major proteinase inhibitors in soybean seeds are the Kunitz trypsin inhibitor (KTi) and the Bowman–Birk inhibitor (BBi). KTi specifically inactivates trypsin, whereas BBi inhibits both trypsin and chymotrypsin [
6,
7,
8]. Soybeans contain three KTi isoforms, KTi1, KTi2, and KTi3, with KTi3 being the most abundant [
9]. In contrast, five BBi isoforms, A, B, C-II, D-II, and E-1 have been isolated from soybean seeds [
8,
10]. BBi-A and BBi-B exhibit similar inhibitory specificity and amino acid composition [
8]. In silico and quantitative RT-PCR analyses have identified 11 potential BBi genes in the soybean genome [
11], as detailed in
Supplementary Table S1. Among these, three genes,
BBi-A,
BBi-CII, and
BBi-DII, are specifically expressed in seeds, with
BBi-DII transcripts being the most abundant, followed by
BBi-A and
BBi-CII [
11]. Both KTi and BBi proteins can be irreversibly inactivated by heat, although BBi proteins exhibit greater resistance to heat inactivation compared to KTi proteins [
3]. Consequently, soybean seed meal undergoes routine heat processing, which can reduce protein quality and digestibility due to the Maillard reaction [
12].
Germplasm screening has identified soybean accessions with reduced or absent Bowman–Birk (BBi) or Kunitz trypsin inhibitor (KTi) proteins. One such accession, PI 157740, exhibits significantly reduced (~40%) trypsin inhibitor activity [
13]. Molecular cloning and sequencing of this mutant revealed a frameshift mutation in the
KTI3 coding region [
14]. This accession has been extensively backcrossed and subjected to feeding trials, which demonstrated that raw, extruded protein meal lacking KTI3 enhances animal weight gain compared to raw soybeans [
5,
15,
16]. In addition, another PI line (PI 68679) was identified with a frameshift mutation in the
KTI1 coding region. Through conventional hybridization, this line was crossed with the
KTI3-deficient line, generating an experimental soybean line deficient in both KTI1 and KTI3 proteins [
17]. This double mutant exhibited a pronounced reduction in trypsin inhibitor activity. Nevertheless, all mutant lines retained substantial protease inhibitor activity, due to the continued presence of Bowman–Birk inhibitors.
Although progress has been made in reducing the trypsin inhibitor content of soybean seeds, no soybean cultivars have been released that are entirely devoid of trypsin inhibitor activity. This is primarily due to the presence of the Bowman–Birk protease inhibitor (BBi), which inhibits both chymotrypsin and trypsin. To date, no
Glycine max (
G. max) germplasm has been identified with a complete or consistent reduction in seed-expressed
BBi genes. However, Bowman–Birk protease inhibitor mutants have been found in several perennial
Glycine species [
18]. A
Glycine microphylla (
G. microphylla) accession with a single loss-of-function frameshift mutation affecting one of the
BBi genes has been identified [
19]. Due to substantial hybridization barriers between
G. microphylla and
G. max, the BBi-null trait has not been successfully transferred to
G. max.
Efforts to reduce BBi protein levels in soybean seeds have been pursued through genetic engineering. Nelson and colleagues overexpressed a mutated, inactive BBi transgene, which successfully outcompeted endogenous BBi transcripts, leading to a significant reduction in BBi protein levels [
20]. More recently, CRISPR/Cas9 gene editing technology has been employed to introduce mutations in
BBi genes, drastically lowering the protease inhibitor content in soybean seeds [
21]. This study demonstrated that mutations in two highly expressed, seed-specific
BBi genes resulted in substantial reductions in both trypsin and chymotrypsin inhibitor activities. However, even in these CRISPR/Cas9-modified lines, trypsin and chymotrypsin inhibitor activities were not completely eliminated [
21].
Since eliminating the BBi would enhance the nutritive value of soybeans for both animal feed and human consumption, developing soybean lines with minimal or no Bowman–Birk protease inhibitors remains a desirable goal. As an alternative to CRISPR/Cas9 gene editing, we have successfully employed RNA interference (RNAi) technology to downregulate the expression of the most abundant BBi genes in soybean seeds. RNAi-generated BBi mutant plants exhibited a drastic reduction in both trypsin and chymotrypsin inhibitor activities. Furthermore, the elimination of the BBi improved the digestibility of soybean seed proteins.
3. Discussion
Our study provides experimental evidence demonstrating that RNAi-generated transgenic soybean lines exhibit significantly reduced protease inhibitor activity. BBi knockdown lines show not only a drastic reduction in chymotrypsin inhibitor activity, but also in trypsin inhibitor activity. This outcome is expected, as BBi inhibits both trypsin and chymotrypsin, two critical digestive enzymes. Western blot analysis confirmed that the BBi knockdown lines failed to accumulate this protein in their seeds, while transcriptomic analysis revealed a substantial downregulation of most seed-specific BBi genes in the knockdown lines. However, the suppression of the most abundant seed-specific BBi genes did not eliminate chymotrypsin inhibitor activity. All the transgenic soybean lines generated in this study exhibited residual chymotrypsin inhibitor activity, albeit at much lower levels than wild-type soybeans. We speculate that the residual chymotrypsin inhibitor activity may be due to the upregulation of minor seed-specific BBi genes. Supporting this hypothesis, our transcriptomic analysis revealed that two BBi genes, Glyma.18G231400 and Glyma.18G231500, which are expressed at extremely low levels in wild-type seeds, exhibited increased expression in the BBi knockdown line. This upregulation could account for the residual chymotrypsin inhibitor activity observed in the BBi knockdown lines.
Previous studies have demonstrated that gene editing technologies can be effectively utilized to reduce the activity of Kunitz trypsin inhibitors (KTIs) and Bowman–Birk inhibitors (BBis) in soybean seeds [
21,
26,
27]. Specifically, CRISPR/Cas9-mediated genome editing was employed to introduce small insertions or deletions within the open reading frames of the
kti1 and
kti3 alleles in the soybean cultivar, Williams 82 [
26]. The resulting
kti1/kti3 double mutants exhibited a marked reduction in KTi and overall trypsin inhibitor (TI) activity compared to wild-type seeds. Importantly, these genetic modifications did not adversely affect plant growth or the days to maturity under greenhouse conditions [
26]. In parallel, recent research targeting
BBi genes using CRISPR/Cas9 revealed dramatic reductions in total trypsin inhibition and chymotrypsin inhibition relative to the wild-type controls [
21,
27]. More importantly, the
BBi knockdown lines also showed potential for improved processing efficiency, as they required less thermal treatment to inactivate residual protease inhibitors [
27], an advantage that could reduce energy costs and preserve protein quality during soybean meal production. Collectively, these findings underscore the promise of genome editing as a powerful strategy to minimize antinutritional factors in soybean seeds, thereby enhancing the nutritional value of soybean-derived food and feed products.
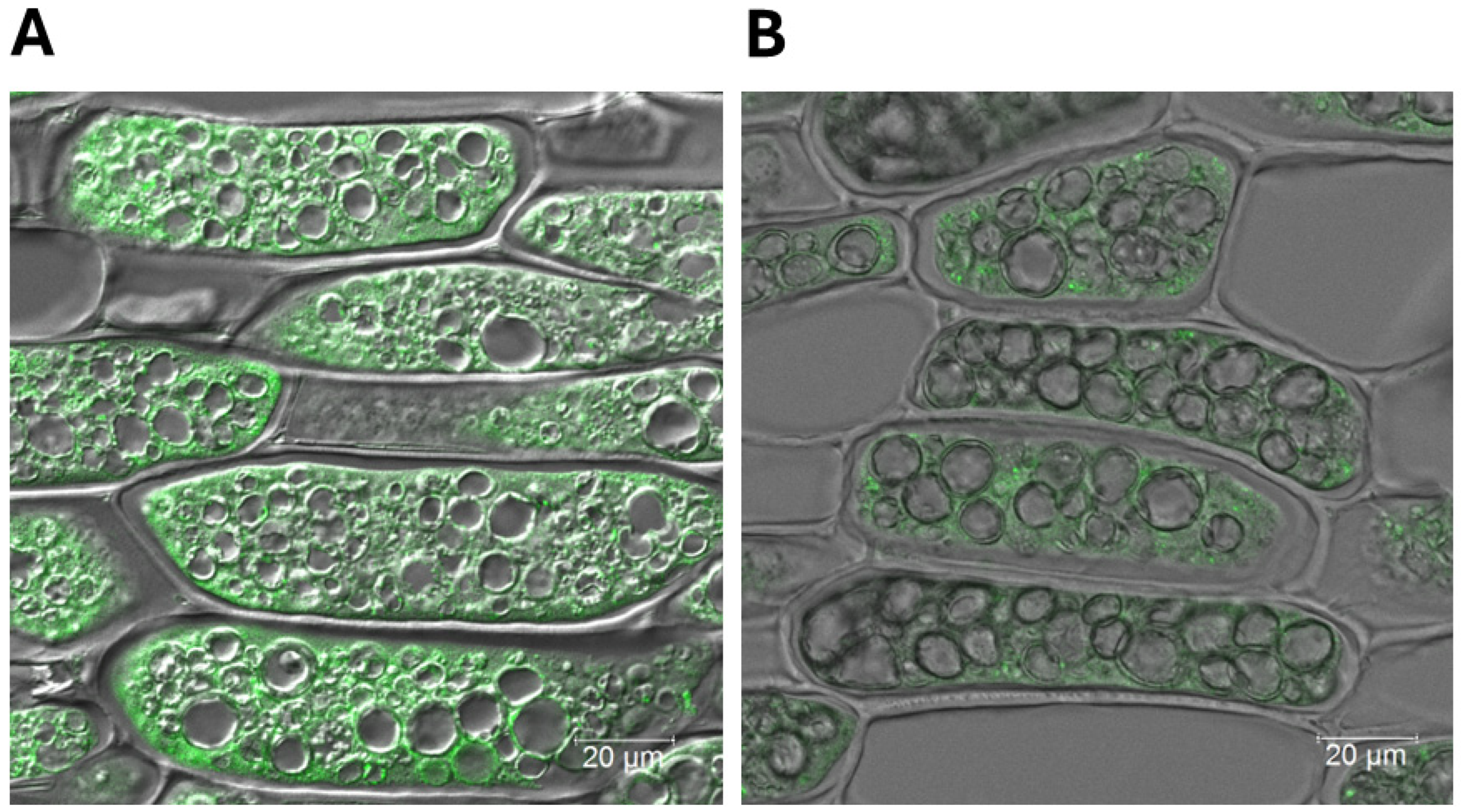
Earlier ultrastructural studies of soybean cotyledons and embryonic axes have shown that the Bowman–Birk inhibitor (BBi) is localized in protein bodies, the nucleus, and, to a lesser extent, the cytoplasm [
28]. However, our confocal fluorescent microscopy study clearly demonstrates that the BBi is exclusively localized in the cytoplasm and not in protein storage vacuoles. Protease inhibitors, including the Kunitz trypsin inhibitor (KTi) and BBi, belong to the 2S albumin fraction, but their subcellular localization in legumes remains incompletely understood. In mung beans, fluorescent antibody studies have shown that protease inhibitors are primarily associated with the cytoplasm [
29]. Similarly, in
Adenanthera pavonina, immunocytochemical localization studies have confirmed that protease inhibitors are predominantly found in the cytoplasm, rather than in protein bodies or protein storage vacuoles [
30]. Based on these findings, together with our own observations, we conclude that protease inhibitors are primarily localized in the cytoplasm of legume seeds.
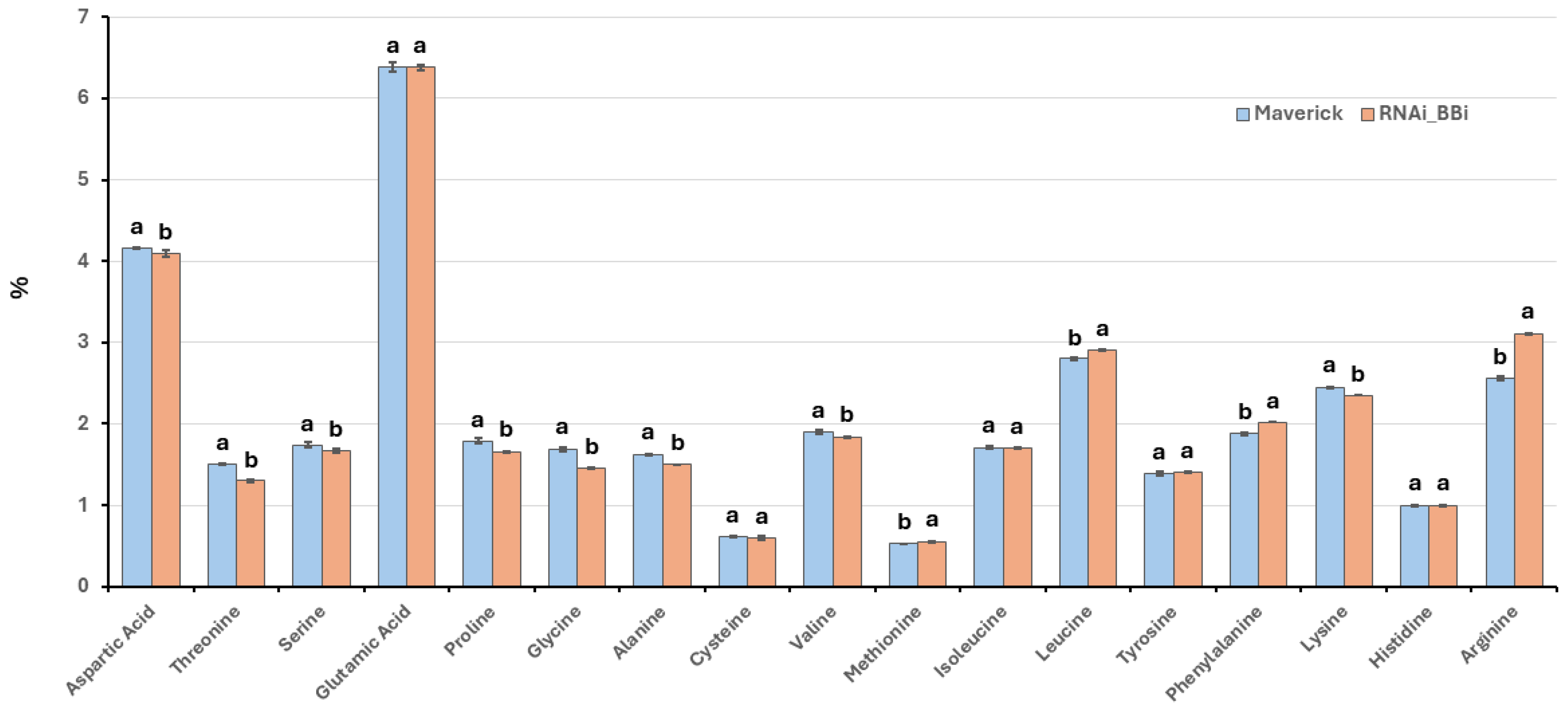
Soybeans, like other legumes, contain relatively low levels of sulfur-containing amino acids, such as cysteine and methionine [
31]. Bowman–Birk inhibitors (BBi) serve as a sulfur amino acid reserve in soybean seeds, as they contain seven disulfide bonds. The amino acid composition of the mature BBi protein, which consists of 71 amino acids, includes 14 cysteine residues [
24,
25]. Thus, eliminating BBi accumulation could theoretically lead to a drastic reduction in the overall cysteine content of the seeds. However, amino acid analysis of
BBi-silenced lines reveals no decrease in the cysteine content of seed proteins compared to wild-type soybeans. This observation suggests that, in the absence of BBi, available cysteine may be incorporated into other cysteine-rich proteins. Previous studies have shown that proteomic rebalancing occurs when the accumulation of abundant seed proteins is disrupted [
32]. A more in-depth proteomic analysis of seed proteins could clarify whether proteomic rebalancing takes place in
BBi-silenced lines.
Proteinase inhibitors are widely recognized for their critical role in defending plants against a broad range of pathogens and insect pests [
33,
34,
35,
36]. Given that the BBi knockdown lines exhibit significantly reduced levels of these inhibitors, it is essential to investigate whether this reduction renders the plants more susceptible to biotic stress. To address this, comprehensive field evaluations will be necessary to assess the performance of the BBi knockdown lines under natural pathogen and pest pressure. These trials should determine whether the reduction in BBi levels leads to agronomic compromise, including yield loss, reduced stress tolerance, or other undesirable traits.
A recent study [
37] compared the protein digestibility of seed proteins in a triple-null soybean mutant, lacking the Kunitz trypsin inhibitor (KTi), agglutinin, and the P34 allergen, to that of the wild type, using an in vitro protein digestibility assay. Contrary to our findings, they reported no significant improvement in the protein digestibility of the triple mutant compared to the corresponding control lines. This discrepancy may be attributed to the presence of the Bowman–Birk inhibitor (BBi) in the triple mutant, despite the absence of the KTi. In soybean seeds, the total protease inhibitor activity results from the combined effects of the KTi and BBi. Previous research has shown that the KTi mutant retains a substantial amount of trypsin inhibitor activity [
17], whereas the BBi mutant exhibits a much more pronounced reduction in both trypsin and chymotrypsin inhibitor activity [
21]. These findings suggest that the BBi is the predominant contributor to protease inhibitor activity in soybean seeds. Therefore, it is reasonable to expect that a soybean BBi mutant would demonstrate improved protein digestibility compared to a KTi mutant.
Soybeans have long been regarded as the “gold standard” against which other protein sources are compared. Their high protein content, coupled with a well-balanced amino acid profile, makes soybeans an ideal protein ingredient in animal feed. However, the full nutritional potential of soybeans is hindered by the presence of several antinutritional factors (ANFs), such as lectins, trypsin inhibitors, and chymotrypsin inhibitors. These ANFs can significantly impair protein digestion and reduce nutrient absorption in the intestine [
38]. In our study, we developed
BBi knockdown soybean lines that show great promise for improving the formulation of animal feed. These lines contain significantly reduced levels of trypsin and chymotrypsin inhibitors, two key ANFs that typically require extensive heat treatment in order to be neutralized. By reducing the levels of these protease inhibitors, the
BBi knockdown lines offer several advantages. They are likely to require minimal heat processing, translating into substantial cost savings during production. Additionally, the absence of protease inhibitors enhances protein digestibility, thereby improving nutrient utilization and boosting animal performance. The results of our in vitro protein digestion analysis further underscore the benefits of the
BBi knockdown lines. Proteins derived from these soybeans are broken down more efficiently into lower molecular weight oligopeptides compared to those from wild-type soybeans. This improved digestibility highlights the potential of
BBi knockdown soybeans as a superior ingredient in animal feed, offering both economic and nutritional benefits.
Efforts to reduce or eliminate protease inhibitors in soybean seeds have long been a key objective for soybean breeders. Previously, we developed a soybean germplasm with the lowest reported levels of trypsin inhibitor in conventional soybeans [
17]. This was achieved through traditional crossing of two soybean PI lines lacking KTi-3 and KTi-1. However, the resulting progeny still exhibited significant trypsin inhibitor activity, due to the presence of Bowman–Birk inhibitor (BBi) proteins. To develop soybean lines completely devoid of protease inhibitors, both the KTi and BBi must be eliminated. Using RNAi technology, we have successfully generated soybean lines with nearly undetectable levels of the BBi in seeds. Moreover, these transgenic lines show significantly reduced trypsin and chymotrypsin inhibitor activities. We are now optimally positioned to combine these two traits, eliminating both the KTi and BBi, into a single soybean line. The creation of soybean lines lacking both KTi and BBi will enhance the seed value for farmers and enable the use of raw soybean meal in swine, poultry, and other animal feed.
4. Materials and Methods
4.1. Chemicals
Most chemicals and reagents used in this study were of analytical grade. Trypsin, chymotrypsin, β-mercaptoethanol, Nα-Benzoyl-DL-arginine p-nitroanilide hydrochloride, and N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acrylamide, bis acrylamide, SDS, TEMED, and ammonium persulfate were purchased from GE healthcare (Piscataway, NJ, USA).
4.2. Construction of RNAi Cassettes to Downregulate Bowman–Birk Inhibitor Genes and for Soybean Transformation
To simultaneously suppress the expression of Bowman–Birk inhibitors (BBi), we constructed an RNAi cassette, using the following procedure. First, the
BBi promoter region (Glyma.16G208900) was amplified from the soybean cultivar Maverick genomic DNA, utilizing the primer pair 5′H3Xh16G_H_Promoter (5′-AAGCTTCTCGAGATTCAACGGTAAATTTATTG-3′), which contains HindIII and XhoI restriction sites, and 16G_H_PromoterBH (5′-GGATCCGTTGTTCTTCAAACTCATCTTTATTAATTG-3′), which contains a BamHI restriction site. The amplified
BBi promoter was cloned into pGEM-T Easy vector and designated as pGBBipro. The promoter region was then excised using HindIII and BamHI and cloned into the corresponding restriction site of the pZPlapha′P binary vector [
21], resulting in pZBBipro.
The coding region of the soybean Bowman–Birk inhibitor (BBi) was amplified from soybean seed total RNA, using primers 5′-CTCTTCACAGCAAAAACAATTAAT-3′ and 5′-CCATTTGAGAGAGCTATTAGTTTTTC-3′. The amplified PCR product was cloned into the pGEM-T Easy vector and designated as pGBBi16G. The sense and antisense BBi fragments were amplified from pGBBi16G, using the primer pair SNd16G_H_RNAi (5′-GTCGACCATATGAGTTTGAAGAACAAC-3′), with introduced SalI and NdeI restriction sites, and 16G_H168_RNAi_XhR (5′-GATATCCTCGAGTGTGCACATGCAGAGATCAC-3′), with introduced EcoRV and XhoI restriction sites. The amplified 168 bp sense and antisense fragments were cloned into pGEM-T Easy vector and designated as pGBBi168RNAi.
The sense fragment was excised from pGBBi168RNAi using NdeI and XhoI, then cloned into the PHK vector, resulting in PHK168iA. The antisense fragment was excised from pGBBi168RNAi using EcoRV and SalI, then cloned into PHK168iA, generating PHK168iAB. Following digestion with XbaI, the entire BBi hairpin sequence was cloned into the corresponding restriction site of the pZBBipro binary vector, designated as pZBBi_168. This final vector (
Supplementary Figure S3) places the
BBi hairpins under the regulatory control of the
BBi promoter, the α′-subunit of the β-conglycinin promoter, and the terminator of the potato proteinase inhibitor gene (PinII). Additionally, the vector contains the barcoding region under the regulatory control of the cauliflower mosaic virus (CaMV) 35S promoter and the 3′ region of the nopaline synthase gene. The RNAi construct was mobilized into a disarmed
Agrobacterium tumefaciens strain, EHA105, via heat-shock transformation.
The soybean transformation was performed, following the protocol described previously [
21]. ‘Maverick’ soybean (Reg. no. CV-372, PI 598124), which was developed by the Missouri and Illinois Agricultural Experiment Stations at the Universities of Missouri and Illinois, respectively, was used for plant transformation. Putative transgenic soybean plants were identified by a leaf paginating assay, using 100 mg of L−1 glufosinate ammonium solution on leaves at three stages [
39]. Herbicide-resistant plants were then cultivated in greenhouses under controlled conditions, maintaining a 16 h day at 28 °C and an 8 h night at 24 °C.
4.3. Protein Extraction and 1-D Electrophoresis
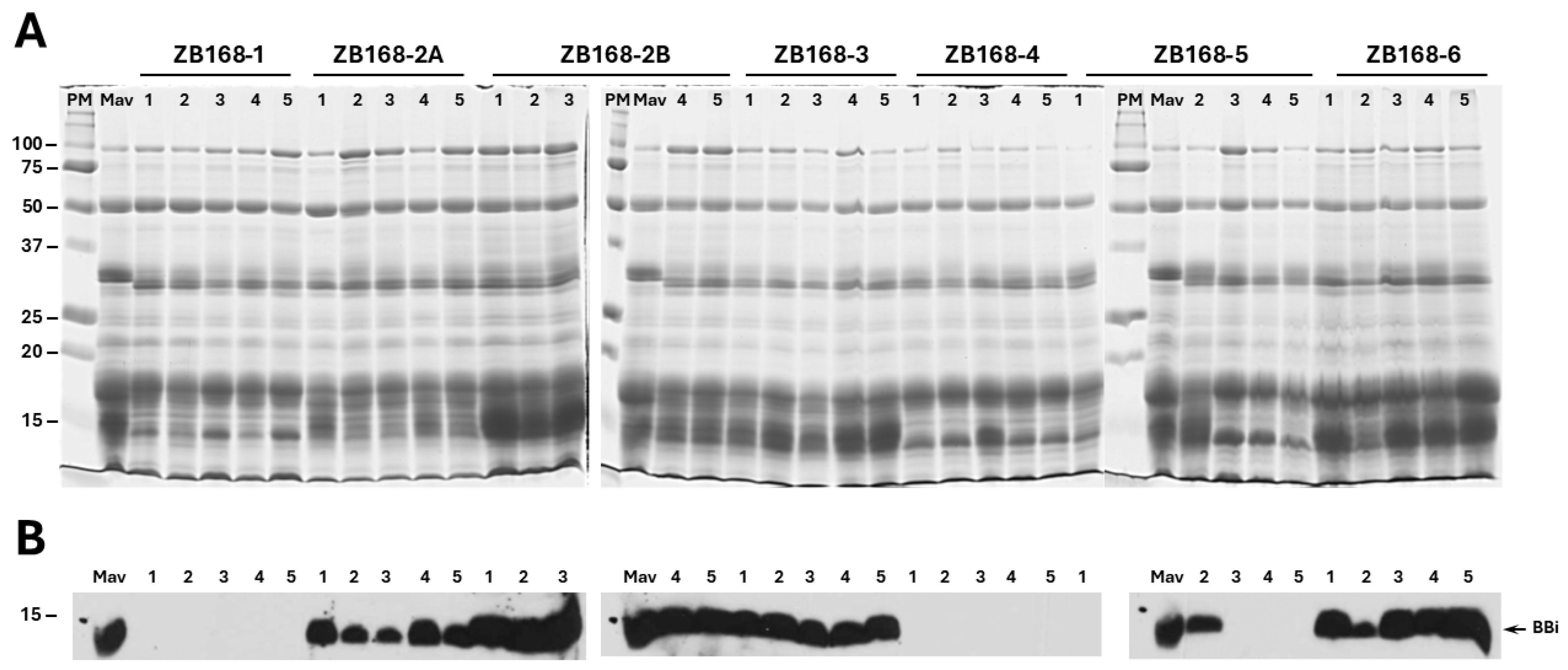
The total soybean seed protein extraction and subsequent separation using 1-D SDS-PAGE were conducted as previously described [
21]. Briefly, dry soybean seeds were ground into a fine powder, using a mortar and pestle. Ten mg of seed powder was transferred into 1.5 mL plastic tubes, to which 1 mL of SDS sample buffer (60 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 5% 2-mercaptoethanol) was added. The tubes were vortexed for 10 min at room temperature, then heated to 100 °C for 5 min, followed by centrifugation at 15,800×
g for 5 min. The supernatant, representing the total seed protein fraction, was resolved using 13.5% SDS-PAGE gels in a Hoefer SE 260 minigel apparatus (Amersham Biosciences, Piscataway, NJ, USA). Electrophoretic separation was carried out at a constant current of 20 mA per gel. The separated proteins were visualized using Coomassie Blue R-250 solution (0.3% Coomassie Brilliant Blue R-250, 45% methanol, and 10% glacial acetic acid).
4.4. Immunoblot Analysis
The proteins were extracted from the wild-type soybean cultivar ‘Maverick’ and T1:2 seeds of the
BBi-mutated lines were separated using 13.5% SDS-PAGE gels. These proteins were then transferred to nitrocellulose membranes and incubated with TBS (10 mM Tris-HCl, pH 7.5, 500 mM NaCl), supplemented with 5% non-fat dry milk for 1 h at room temperature. After incubation, the nitrocellulose membranes were washed three times with TBST (15 min each) and incubated overnight with antibodies at a 1:10,000 dilution, specifically raised against either purified soybean Kunitz trypsin or Bowman–Birk protease inhibitors [
23]. The specifically bound primary antibodies were detected using anti-rabbit IgG–horseradish peroxidase conjugates and the SuperSignal West Pico kit (Pierce, Rockford, IL, USA).
4.5. Trypsin and Chymotrypsin Inhibitor Assay
To measure the trypsin inhibitor activity in various
BBi-mutated CRISPR/CAS9 lines, the methodology described by Kim and Krishnan, 2024 [
21], was followed. In brief, 20 mg of dry soybean seed powder was placed into a 2 mL Eppendorf tube, then 1 mL of 10 mM NaOH solution was added. This mixture was vigorously agitated, using a vortex mixer, for 10 min at room temperature. The resulting slurry was clarified through centrifugation at 16,000×
g for 10 min, and the clear supernatant was used to determine the KTi activity. The trypsin inhibitor activity was quantified as the trypsin units inhibited (TUI) per mg of the sample, with the results presented as the mean ± SD from three biological replicates.
To assess the chymotrypsin inhibitor activity, soybean seed extracts were used, with N-Succinyl-Ala-Ala-Pro-Phe p-nitroanilide (AAPF) serving as the substrate. The soybean seed extract, which inhibited 40 to 60% of chymotrypsin, was added into a 1.5 mL Eppendorf tube, containing 900 µL of assay buffer (100 mM-Tris-HCL, pH 8.0), 8 µL of α-chymotrypsin (Sigma-Aldrich Company), dissolved in 1 mM HCl solution (0.1 mg/mL), and 8 to 10 µL of seed extract. This mixture was incubated at 37 °C for 5 min. Subsequently, 80 µL of AAPF (1 mg/mL) was added, and the incubation was continued for an additional 10 min at 37 °C. The assay was terminated by adding 500 µL of 30% acetic acid. Absorbance at 410 nm was measured, and the chymotrypsin inhibitor units were calculated based on the reduction in absorbance at 410 nm by 0.1 optical density. The results were expressed as the mean ± SD from three biological replicates. The data from the trypsin and chymotrypsin inhibitor assays were visualized and compared using the JMP statistical software version 16.0 (SAS Institute Inc., Cary, NC, USA). A one-way ANOVA was performed, and significant differences (α = 0.05) between the means were determined using the Tukey–Kramer HSD test.
4.6. RNA-Seq Analysis
The total RNA was isolated from developing seeds of the BBi mutant (ZB168-1) and the wild-type soybean cultivar ‘Maverick’ at three distinct reproductive stages: early (R5), middle (R6), and late (R7). RNA extraction was performed using the TRIzol® Reagent (Thermo Fisher Scientific, Waltham, MA, USA). For each developmental stage, three biological replicates were included. The total RNA was treated with DNase I (Invitrogen, Carlsbad, CA, USA) to remove any contaminating genomic DNA. The RNAseq libraries were prepared by Azenta Life Sciences (Burlington, MA, USA). The process involved the conversion of single-stranded RNA into double-stranded complementary DNA (cDNA), with the addition of sequencing adapters and barcodes, which are subsequently analyzed using next-generation sequencing (NGS). Data analysis was conducted, and the identification of differentially expressed genes (DEGs) was determined.
4.7. Confocal Immunofluorescence Microscopy Localization of Bowman–Birk Protease Inhibitor
Soybean seeds soaked in water were cut into several small cubes, using a razor blade. The dissected seed samples were immediately fixed, as previously described [
6], in a solution containing 2% paraformaldehyde and 2% glutaraldehyde, in 100 mM cacodylate buffer, pH 7.2. After fixation, the samples were dehydrated through the use of a graded ethanol series (80–95–100%) and embedded in paraffin. The paraffin-embedded samples were then processed for confocal immunofluorescence microscopy. Immunohistochemical localization was conducted on paraffin-embedded sections, utilizing antibodies against Bowman–Birk protease inhibitor peptides, raised in rabbits. Five micrometer sections of paraffin-embedded soybean cotyledons were mounted onto X-tra Plus microscope slides (Leica, Richmond, IL, USA), de-waxed in xylene, rehydrated through the use of graded ethanol concentrations, and finally in water. The sections were incubated for 60 min at room temperature, with a 1:200 dilution of the soybean BBi antibody, followed by a 30 min incubation with Alexa Fluor 488 Plus-conjugated goat anti-rabbit secondary antibody (Invitrogen/Thermo Fisher, Waltham, MA, USA), diluted 1:500. The sections were then cover slipped with a mounting medium containing an antifade reagent and observed under a Leica SP8 laser scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA), with a 20×/NA 0.7 objective, using a 495 nm excitation laser line and a 505–550 nm bandpass.
4.8. Protein, Oil, and Amino Acid Analyses
The protein and oil content were determined using near-infrared spectroscopy (FOSS North America, Eden Prairie, MN, USA). The amino acid analysis of the wild-type (Maverick) and BBi knockdown line (ZB168-1) was performed at the University of Missouri Agriculture Experiment Station Chemical Laboratories, University of Missouri. The amino acids were separated using a Beckman 6300 Amino Acid Analyzer (Beckman Coulter Life Sciences, Indianapolis, IN, USA), equipped with a high-performance, cation exchange resin column. The results are reported as the mean ± standard deviation from three biological replicates.
4.9. In Vitro Protein Digestibility Assay
Seed proteins from the wild-type (Maverick) and BBi knockdown line (ZB168-1) were isolated by extracting 30 mg of dry soybean seed powder with 1 mL of 10 mM Tris-HCl, pH 7.5, in a rotary shaker, for 20 min at room temperature. The slurry was centrifuged at 14,000 rpm for 3 min and the resulting supernatant was saved in separate tubes. To this supernatant, 10 µL of trypsin (10 mg/mL) and 10 µL of chymotrypsin (10 mg/mL) stock solution was added and incubated in rotary shaker, maintained at 37 °C. At intervals of 0, 5, 15, 60, and 120 min, 50 µL of protein solution was removed. To this 10 µL of 6× SDS Laemmli sample buffer was added, mixed thoroughly and placed in a boiling water bath for 5 min. The protein samples were resolved on 13.5% SDS-PAGE gels and the separated proteins were visualized by Coomassie Brilliant Blue staining.